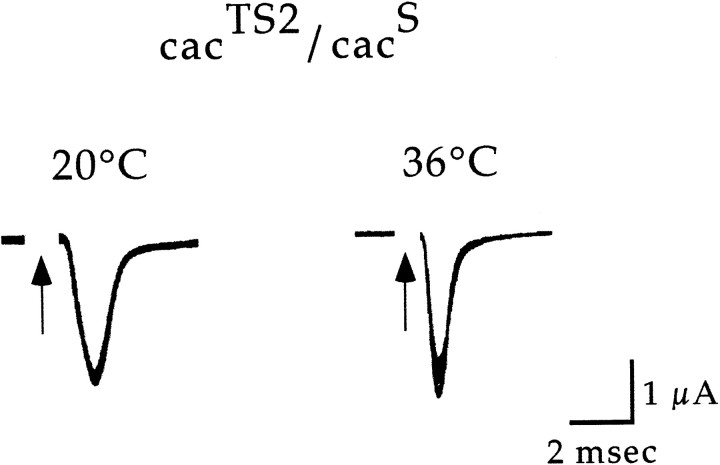Abstract
Neurotransmission at chemical synapses involves regulated exocytosis of neurotransmitter from the presynaptic terminal. Neurotransmitter release is thought to be triggered by calcium influx through specific classes of voltage-gated calcium channels. Here we report genetic and functional analysis implicating a specific calcium channel gene product in neurotransmitter release. We have isolated a temperature-sensitive paralytic allele of the Drosophilacalcium channel α1 subunit gene, cacophony(cac). This mutant, referred to ascacTS2, allows functional analysis of synaptic transmission after acute perturbation of a specific α1 subunit. Electrophysiological analysis at neuromuscular synapses revealed that neurotransmitter release incacTS2 is markedly reduced at elevated temperatures, indicating that cac encodes a primary α1 subunit functioning in synaptic transmission. These observations further define the molecular basis of voltage-gated calcium entry at synapses and provide a new starting point for further genetic analysis of synaptic mechanisms.
Keywords: calcium channel, neurotransmitter release, synaptic transmission, cacophony, Drosophila, temperature-sensitive
A number of studies have implicated specific classes of voltage-gated calcium channels in neurotransmitter release (for review, see Wheeler et al., 1995; Catterall, 1998). These channels are composed of multiple subunits, including α1, the primary structural subunit, as well as α2δ, β, and γ subunits. α1 subunits of the A and B classes have been localized to synaptic terminals (Robitaille et al., 1990; Westenbroek et al., 1995), and heterologous expression shows that their pharmacology resembles that of calcium channels involved in neurotransmitter release. Furthermore, these α1 subunits contain a defined synaptic protein interaction (SYNPRINT) domain that interacts directly with the neurotransmitter release apparatus and may participate in coupling calcium influx to fast synaptic vesicle fusion (Mochida et al., 1996; Rettig et al., 1997; Sheng et al., 1998). Our current understanding of calcium channel function in neurotransmitter release will be further enhanced by complementary genetic analysis defining the in vivofunctions and interactions of specific calcium channel gene products. Here we report analysis of synaptic function in a temperature-sensitive (TS) calcium channel mutant of Drosophila.
To define the physiological roles of specific proteins in synaptic transmission, we have focused on Drosophila mutants exhibiting rapid TS paralysis. These mutants typically develop and function normally at permissive temperature and can be shifted to restrictive temperature to examine the acute functional consequences of perturbing a specific gene product. This approach was pioneered almost 30 years ago (Suzuki et al., 1971; Grigliatti et al., 1973; Siddiqi and Benzer, 1976), and one of the mutants recovered in these early screens was comatose (comt). Our previous work has shown that the comt gene product, a homolog of theN-ethylmaleimide-sensitive fusion protein (Ordway et al., 1994; Pallanck et al., 1995), functions in priming synaptic vesicles for fast, calcium-triggered fusion (Kawasaki et al., 1998;Kawasaki and Ordway, 1999a,b). To broaden our analysis to other gene products functioning in synaptic vesicle trafficking, we performed a genetic screen to identify mutations exhibiting functional interactions with comt (Dellinger et al., 2000). One enhancer of comt was determined to be a TS allele ofcacophony (cac) and has been designatedcacTS2. cac encodes a homolog of voltage-activated calcium channel α1 subunits implicated in neurotransmitter release (Smith et al., 1996).
The cac locus was first identified in a screen for mutants exhibiting altered courtship song (von Schilcher, 1976, 1977) and was subsequently found to be allelic to the nightblind A(nbA) locus (Heisenberg and Götz, 1975; Smith et al., 1998). A synaptic function for cac-encoded calcium channels was suggested by electroretinogram recordings fromcac (nbA) mutants (Heisenberg and Götz, 1975; Smith et al., 1998), by the similarity between cac and α1 subunits previously implicated in synaptic transmission (Smith et al., 1996), and by the genetic interaction ofcacTS2 with comt(Dellinger et al., 2000). Here we report functional analysis demonstrating that cac encodes a primary α1 subunit functioning in neurotransmitter release.
Parts of this work have been reported previously in abstract form (Dellinger et al., 1999; Kawasaki et al., 1999).
MATERIALS AND METHODS
Drosophila lines. Previously isolated caclines were generously provided by Jeffrey C. Hall (Brandeis University, Waltham, MA). The deficiency line Df(1)KA10 was obtained from the Bloomington Stock Center (Indiana University, Bloomington, IN). The left and right limits of this deficiency are 11A01 and 11A07–08, respectively. Wild-type flies were Canton S. All lines used for electrophysiological recording were maintained at 20°C.
Synaptic electrophysiology. For experiments at dorsal longitudinal flight muscle (DLM) neuromuscular synapses, dissection, temperature control, motor axon stimulation, and two-electrode voltage-clamp recordings of synaptic currents were performed as described previously (Kawasaki et al., 1998).
Recordings of intracoxal lateral levator muscle (ICLM) synaptic currents were obtained as follows. A fly was anesthetized with CO2, mounted ventral side up over a hole in an air tube, and secured with wax (Tackiwax Boekel Industries, Feasterville, PA). Air was delivered to the tracheal system using an aquarium pump. The preparation was submerged in recording solution maintained at 20°C. A tungsten knife was used to remove the ventral thoracic cuticle and the coxal muscles, thereby exposing the thoracic ganglion of the CNS. To expose the ICLMs of the first pair of legs, the overlying cuticle was removed from each coxa. The first leg nerve exiting the thoracic ganglion was cut and pulled into a suction electrode for stimulation.
ICLM synaptic currents were recorded by discontinuous single electrode voltage clamp using an AxoClamp-2B amplifier (Axon Instruments, Foster City, CA). Deviations from the command potential did not exceed 5 mV. Glass recording microelectrodes were filled with 3 m KCl. The recording solution consisted of (in mm): 129 NaCl, 2 KCl, 4.0 MgCl2, 1.0 CaCl2, 5 HEPES, and 36 sucrose. The pH was adjusted to 7.0 using NaOH. Stimulation of the first leg nerve was performed using a suction electrode driven by an S-900/S-910 stimulator (Dagan Instruments, Minneapolis, MN). Temperature control was achieved with a TC-202 temperature controller and PDMI microincubator (Medical Systems Corporation, Greenvale, NY).
All recordings at 33 or 36°C were obtained after 5–20 min of exposure to the recording temperature. Recordings at 38°C were obtained after 1–2 min at this temperature.
Data acquisition and analysis. Data were acquired on-line using a Power Macintosh computer (Apple Computers, Cupertino, CA), Pulse software (Heka Electronik, Lambrecht, Germany), and an ITC-16 laboratory interface (Instrutech Corporation, Great Neck, NY). Data were low-pass filtered at 5 or 10 kHz and acquired at 30 kHz. Measurement of synaptic currents was performed using cursor measurements in the data analysis software package IGOR (Wavemetrics, Lake Oswego, OR). Microsoft (Seattle, WA) Excel was used for data tabulation, graphing, and statistical analysis. Data are reported as the mean ± SEM. By the use of an unpaired Student's ttest, statistical significance was assigned to comparisons withp ≤ 0.05
Sequence analysis. The cac α1 subunit sequence (U55776) was aligned to human α1A (U76666), rat α1B (M92905), and rat α1C (M67516) sequences by the Clustal method, using the MegAlign feature of the Lasergene sequence analysis software package (DNAStar Inc., Madison, WI). BLAST sequence similarity searches of theDrosophila genome were performed using the National Center for Biotechnology Information (NCBI) Drosophila genome resources (http://www.ncbi.nlm.nih.gov/PMGifs/Genomes/7227.html). Query nucleotide and protein sequences included those corresponding to the SYNPRINT domain of the above vertebrate α1A and α1B genes [defined as residues 718–963 in α1B (Sheng et al., 1998)], as well as the analogous domain of α1C [residues 753–893 (Wiser et al., 1999)].
RESULTS
A temperature-sensitive synaptic phenotype incacTS2
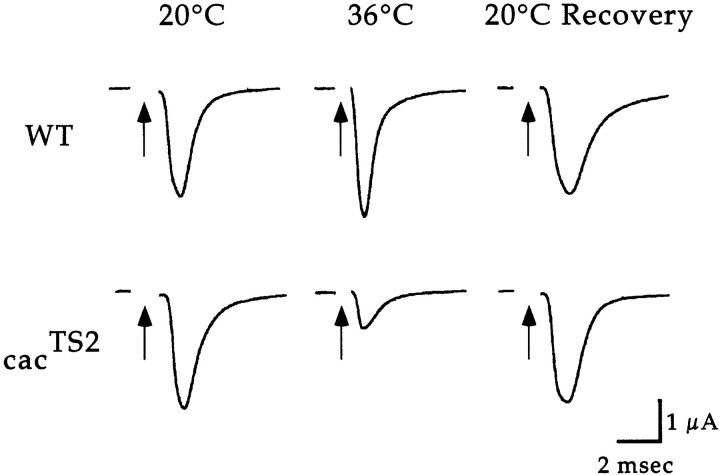
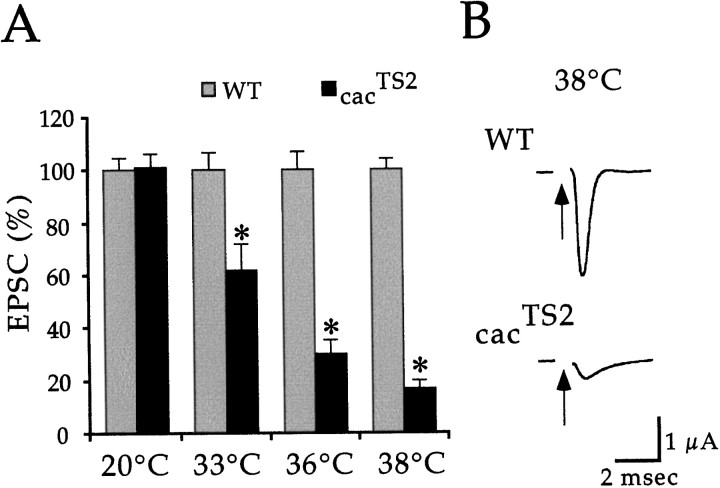
Electrophysiological analysis was performed in wild-type andcacTS2 flies to investigate whethercac-encoded αl subunits function in synaptic transmission. Two-electrode voltage-clamp techniques were used to record synaptic currents at DLM neuromuscular synapses. This technique prevents activation of postsynaptic voltage-gated ion channels and thus records the current passing through ligand-gated neurotransmitter receptor channels. The cacTS2 mutant showed a conditional and reversible electrophysiological phenotype (Fig.1). At 20°C,cacTS2 exhibited wild-type synaptic currents. In contrast, exposure to elevated temperatures produced a marked reduction in the amplitude of the synaptic current with respect to wild type. This phenotype was reversible, showing full recovery upon return to 20°C. The extent of the synaptic current reduction was dependent on temperature (Fig. 2). With respect to wild type, the current amplitude was reduced to 61.5 ± 10.4% (n = 5) at 33°C, 30.1 ± 5.2% (n = 8) at 36°C, and 16.6 ± 3.2% (n = 4) at 38°C.
Fig. 1.
cacTS2 exhibits a conditional and reversible reduction in the synaptic current. DLM synaptic currents evoked by stimulation of the DLM motor axon were recorded at 20 and 36°C, and at 20°C after exposure to 36°C (20°C recovery). The 20°C recovery traces in wild type and cacTS2 were recorded after 1 and 10 min at 20°C, respectively. Axon stimulation is marked by thearrow. In each case, the 36°C and 20°C recovery traces were obtained from the same preparation.
Fig. 2.
The extent of synaptic current reduction incacTS2 is dependent on temperature.A, Peak amplitude measurements of DLM EPSCs fromcacTS2 are shown as a mean percentage of wild-type currents. Error bars indicate SEM, and values significantly different from wild type are marked by anasterisk. B, Sample recordings from wild type and cacTS2 at 38°C.
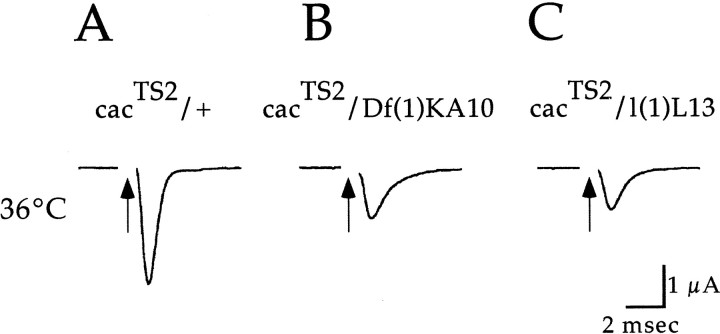
The above results indicate that cac-encoded α1 subunits function in synaptic transmission. Although our previous behavioral analysis shows that the cacTS2,cacS, and l(1)L13mutations are allelic (Dellinger et al., 2000), we performed similar tests using electrophysiological analysis to confirm that the observed synaptic phenotype also maps to the cac locus. The recessive nature of cacTS2 was confirmed in recordings from heterozygouscacTS2/+ flies, which exhibit wild-type synaptic currents at restrictive temperature (Fig.3A). In flies heterozygous forcacTS2 and a deficiency that removescac, the cacTS2 phenotype was observed (Fig. 3B). Finally, complementation tests were performed with other known cac alleles. A previously identified cac lethal mutation, l(1)L13, failed to complement the cacTS2electrophysiological phenotype (Fig. 3C), indicating that this phenotype maps to the cac locus. This was further confirmed in recordings atcacTS2/cacSsynapses as described below.
Fig. 3.
The synaptic current reduction observed incacTS2 mutants maps to thecac locus. DLM synaptic current recordings incacTS2/+ indicate a recessivecacTS2 synaptic phenotype (A). Recordings fromcacTS2/Df(1)KA10(B) and cacTS2/l(1)L13 (C) map the observed synaptic phenotype to thecac locus.
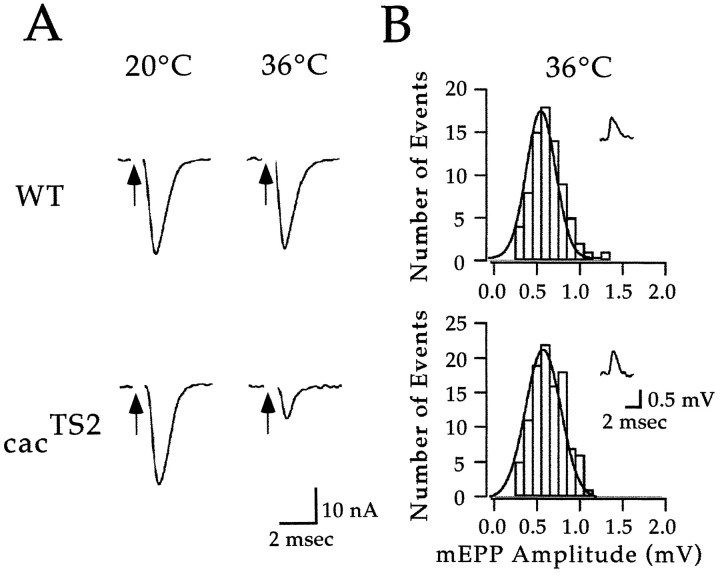
The above results show that a TS mutation in cac results in a conditional reduction in the synaptic current. We considered it likely that the cacTS2 phenotype was presynaptic on the basis that vertebrate homologs of cachave been implicated in neurotransmitter release and that our voltage-clamp analysis should prevent postsynaptic calcium channels from contributing to the recorded currents. To address this point directly, experiments were performed at neuromuscular synapses of the ICLM. This preparation is well suited for recording miniature EPSPs (mEPSPs). Because the amplitude of these mEPSPs reflects the postsynaptic response to a single quantum of neurotransmitter, mEPSPs may be used to monitor postsynaptic sensitivity to neurotransmitter. Voltage-clamp analysis of evoked ICLM synaptic currents confirmed thatcacTS2 exhibits a conditional reduction in the synaptic current as observed at DLM synapses (Fig.4A). Despite a marked reduction in the evoked synaptic current at 36°C,cacTS2 synapses exhibited wild-type mEPSP amplitudes under the same conditions (Fig. 4B). Thus, we conclude that the cacTS2phenotype is presynaptic, resulting from a conditional reduction in neurotransmitter release. In light of the striking reduction of the DLM synaptic current observed in cacTS2at 38°C (Fig. 2), it appears that cac encodes the primary calcium channel α1 subunit responsible for neurotransmitter release at these synapses.
Fig. 4.
Recordings of spontaneous and evoked neurotransmitter release at ICLM synapses confirm a presynaptic role for cac-encoded α1 subunits in neurotransmitter release. A, Recordings of evoked synaptic currents from ICLM neuromuscular synapses in wild type (WT) andcacTS2. Similar results were obtained in three experiments for each genotype. B, Histograms of wild-type and cacTS2 mEPSP amplitudes (mEPP Amplitude). The solid linerepresents a Gaussian fit to the data. Insets shown representative mEPSP recordings. Similar results were obtained in three experiments from each genotype.
An activity-dependent and temperature-independent synaptic phenotype in cacS
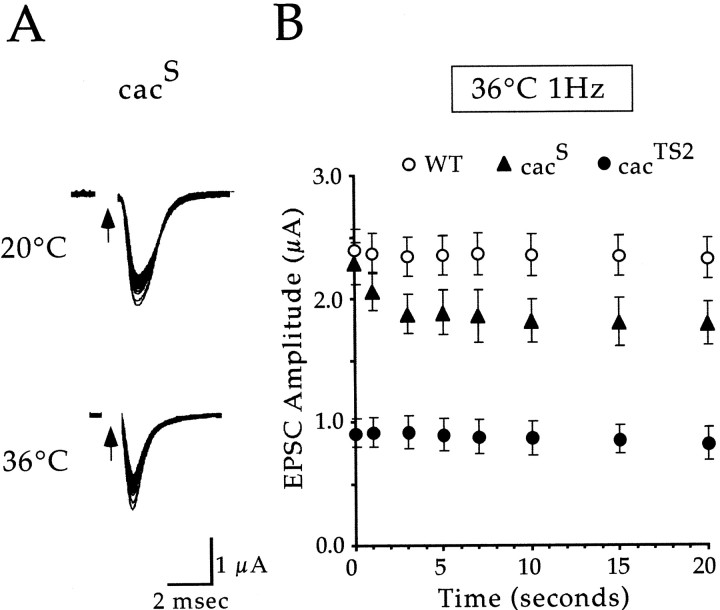
Consistent with the cacTS2phenotype, recordings from the original cac allele,cacS, also showed reduced synaptic currents. However, unlike cacTS2,cacS exhibited a strictly activity-dependent decrease in the synaptic current and no clear dependence on temperature (Fig. 5). Stimulation (1 Hz) at either 20°C or 36°C produced a wild-type synaptic current in response to the first stimulus, followed by reduced amplitude currents in response to subsequent stimuli. The distinct activity dependence of thecacS phenotype is illustrated by comparing cacS,cacTS2, and wild-type synaptic currents during 1 Hz stimulation at 36°C (Fig.5B).
Fig. 5.
cacS exhibits an activity-dependent reduction in the synaptic current. A, Sample DLM synaptic currents fromcacS in response to 1 Hz stimulation at 20 and 36°C. In each case, the first 50 traces were superimposed. Note the activity-dependent reduction in the synaptic current at both temperatures. B, Peak amplitude measurements of DLM EPSCs from wild type (WT),cacS, andcacTS2 are plotted as a function of time during 1 Hz stimulation trains at 36°C. Eachpoint represents the mean ± SEM for four experiments in cacS andcacTS2 and seven experiments in wild type.
As was the case for cacTS2, thecacS phenotype was observed in flies heterozygous for cacS andDf(1)KA10 (data not shown). In addition, recordings obtained fromcacS/cacTS2flies were informative. cacTS2complemented the cacS phenotype at 20°C but failed to complement at 36°C (Fig.6). These results further define the conditional phenotype of cacTS2 (see Discussion).
Fig. 6.
Heteroallelic interactions ofcacTS2 andcacS. DLM synaptic current recordings atcacTS2/cacSsynapses. The cut DLM motor axon was stimulated at 1 Hz, and in each case, the first 50 traces were superimposed. Similar results were obtained in four experiments.
DISCUSSION
The availability of cacTS2, a calcium channel mutant exhibiting rapid temperature-sensitive paralysis, has provided a unique opportunity to examine the physiological role of a specific calcium channel gene product in synaptic transmission. Here we report genetic and functional analysis in two cac alleles,cacTS2 andcacS, indicating that cacencodes a primary calcium channel α1 subunit functioning in neurotransmitter release.
Behavior
The present results provide further functional characterization of the cac locus, first identified in a screen for mutations affecting the male courtship song (von Schilcher, 1976, 1977). The courtship song is produced by a patterned beating of the wings, and this pattern, as well as the wing-beat amplitude, are altered incacS mutants (von Schilcher, 1976,1977; Smith et al., 1998). The results presented here suggest that impairment of neurotransmitter release at central synapses may contribute to altered song patterning incacS. Given thatcac-encoded α1 subunits function at flight muscle neuromuscular synapses, peripheral synaptic defects may influence the song phenotype as well.
cacS also exhibits motor defects at elevated temperatures, including a lack of coordinated movement, spinning behavior, and ultimately paralysis after long exposures (Peixoto and Hall, 1998). Although the basis of this temperature-dependent behavior incacS remains unclear, spontaneous neural activity generally increases at elevated temperatures (cf. Kawasaki and Ordway, 1999a) and thus may produce a more severe activity-dependent reduction in synaptic transmission.
The cacS synaptic phenotype
A distinctly activity-dependent and temperature-independent phenotype was observed at cacSsynapses. The activity-dependent reduction in the synaptic current is of interest in light of the molecular lesion identified incacS. The mutation maps to the sixth transmembrane segment of the third repeat (IIIS6), converting a highly conserved phenylalanine to an isoleucine (Smith et al., 1998). Because S6 segments have been implicated in fast inactivation of calcium (Zhang et al., 1994; Hering et al., 1998), sodium (Rojas et al., 1991; McPhee et al., 1995; Cannon, 1996), and potassium (Hoshi et al., 1991) channels, these results raise the possibility that altered inactivation contributes to the activity dependence of thecacS phenotype. Heterologous expression of wild-type and mutant forms of the cac gene, followed by analysis of the resulting calcium currents, is expected to further address this issue. Similarly, molecular characterization ofcacTS2 is expected to reveal the structural basis of the TS phenotype in this mutant.
Heteroallelic interactions ofcacTS2 andcacS
IncacTS2/cacSflies, cacTS2 complements the activity-dependent synaptic phenotype ofcacS at 20°C. However, shifting the temperature to 36°C does not produce a reduction in the synaptic current as observed in cacTS2 but rather reveals the activity-dependent current reduction characteristic of cacS channels. Thus, it appears that the population of α1 subunits mediating fusion of a synaptic vesicle may contain a mixture ofcacTS2 andcacS subunits and that each subpopulation can support a wild-type level of neurotransmitter release. These observations suggest substantial redundancy in calcium channel function at sites of synaptic vesicle fusion.
cac-encoded α1 subunits lack homology to defined SYNPRINT domains
The cac amino acid sequence is most closely related to the vertebrate α1A and α1B subunits (Smith et al., 1996), which contain a SYNPRINT domain within the second intracellular loop. However, alignments of these sequences (see Materials and Methods) indicate that this SYNPRINT domain is absent from the cacα1 subunit, producing a gap at this position in the cacsequence. Similar results were obtained in alignments of cacwith the rat α1C subunit, which contains a different synaptic protein binding domain at this position (Wiser et al., 1999). The above results are consistent with BLAST queries of the Drosophila genome (Adams et al., 2000) using either of the above synaptic protein binding sequences (see Materials and Methods), which reveal no homologous domains. Given the primary role of the cac gene product in neurotransmitter release, the absence of a conserved synaptic protein binding sequence suggests either a novel interaction domain or an alternative mechanism for fast coupling of calcium influx to synaptic vesicle fusion.
The importance of synaptic calcium channels is reflected by thein vivo consequences of their genetic disruption. In addition to the synaptic and behavioral phenotypes observed incac mutants, mutations in homologous α1 subunit genes give rise to several genetic disorders in humans and mice (Hess, 1996;Miller, 1997). Thus, synaptic calcium channels play important roles in behavior, synaptic transmission, and human disease. The results presented here further define the molecular mechanisms of voltage-gated calcium entry at synapses and a provide a new starting point for genetic analysis of synaptic mechanisms in this model system (Brooks et al., 2000).
Footnotes
This work was supported by National Science Foundation Grant IBN-9514485. We gratefully acknowledge several members of the lab, Bonnie Dellinger, Missy Hazen, and Kamal Tilakaratne for participating in aspects of this work. We thank Jeffrey C. Hall (Brandeis University) for providing several cac stocks and for stimulating discussions. Deficiency stocks were obtained from the Bloomington Stock Center.
Correspondence should be addressed to Richard W. Ordway, Department of Biology, 208 Mueller Laboratory, Penn State University, University Park, PA 16802. E-mail: rwo4@psu.edu.
REFERENCES
- 1.Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 2. Brooks IM, Felling R, Ordway RW. Genetic modifiers of cacTS2, a temperature-sensitive paralytic calcium channel mutant of Drosophila. 2000. Abstract presented at the meeting of the 41th Annual Drosophila Research Conference, Pittsburgh, PA.
- 3.Cannon SC. Sodium channel defects in mytonia and periodic paralysis. Annu Rev Neurosci. 1996;19:141–164. doi: 10.1146/annurev.ne.19.030196.001041. [DOI] [PubMed] [Google Scholar]
- 4.Catterall WA. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1998;24:307–323. doi: 10.1016/s0143-4160(98)90055-0. [DOI] [PubMed] [Google Scholar]
- 5. Dellinger BB, Felling R, McKinney C, Kawasaki F, Ordway RW. Temperature-sensitive paralytic mutants reveal in vivo functional interactions between NSF and calcium channel proteins in synaptic transmission. 1999. Abstract presented at the 40th Annual Drosophila Research Conference, Seattle, WA.
- 6.Dellinger BB, Felling R, Ordway RW. Genetic modifiers of the Drosophila NSF mutant, comatose, include a temperature-sensitive paralytic allele of the calcium channel α1 subunit gene, cacophony. Genetics. 2000;155:203–211. doi: 10.1093/genetics/155.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grigliatti TA, Hall L, Rosenbluth R, Suzuki DT. Temperature-sensitive mutations in Drosophila melanogaster. XIV. A selection of immobile adults. Mol Genet. 1973;120:107–114. doi: 10.1007/BF00267238. [DOI] [PubMed] [Google Scholar]
- 8.Heisenberg M, Götz KG. The use of mutations for the partial degradation of vision in Drosophila melanogaster. J Comp Physiol. 1975;98:217–241. [Google Scholar]
- 9.Hering S, Berjukow S, Aczél S, Timin EN. Ca2+ channel block and inactivation: common molecular determinants. Trends Pharmacol Sci. 1998;19:439–443. doi: 10.1016/s0165-6147(98)01258-9. [DOI] [PubMed] [Google Scholar]
- 10.Hess EJ. Migraines in mice? Cell. 1996;87:1149–1151. doi: 10.1016/s0092-8674(00)81809-7. [DOI] [PubMed] [Google Scholar]
- 11.Hoshi T, Zagotta WN, Aldrich RW. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991;7:547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki F, Ordway RW. The Drosophila NSF protein, dNSF1, plays a similar role at neuromuscular and some central synapses. J Neurophysiol. 1999a;82:123–130. doi: 10.1152/jn.1999.82.1.123. [DOI] [PubMed] [Google Scholar]
- 13. Kawasaki F, Ordway RW. A new model synapse preparation allows direct demonstration of a strictly presynaptic role for the Drosophila NSF protein, dNSF1. 1999b. Abstract presented at the 40th Annual Drosophila Research Conference, Seattle, WA.
- 14.Kawasaki F, Mattiuz AM, Ordway RW. Synaptic physiology and ultrastructure in comatose mutants define an in vivo role for NSF in neurotransmitter release. J Neurosci. 1998;18:10241–10249. doi: 10.1523/JNEUROSCI.18-24-10241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawasaki F, Felling R, Ordway RW. A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. 1999. Abstract presented at the 1999 Cold Spring Harbor Meeting on Neurobiology of Drosophila, Cold Spring Harbor, NY.
- 16.McPhee JC, Ragsdale DS, Scheuer T, Catterall WA. A critical role for transmembrane segment IVS6 of the sodium channel α subunit in fast inactivation. J Biol Chem. 1995;270:12025–12034. doi: 10.1074/jbc.270.20.12025. [DOI] [PubMed] [Google Scholar]
- 17.Miller RJ. Calcium channels prove to be a real headache. Trends Neurosci. 1997;20:189–192. doi: 10.1016/s0166-2236(96)01037-5. [DOI] [PubMed] [Google Scholar]
- 18.Mochida S, Sheng Z-H, Carl B, Kobayashi H, Catterall WA. Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-type Ca2+ channels. Neuron. 1996;17:781–788. doi: 10.1016/s0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 19.Ordway RW, Pallanck L, Ganetzky B. Neurally expressed Drosophila genes encoding homologs of the NSF and SNAP secretory proteins. Proc Natl Acad Sci USA. 1994;91:5715–5719. doi: 10.1073/pnas.91.12.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallanck L, Ordway RW, Ganetzky B. A Drosophila NSF mutant. Nature. 1995;376:25. doi: 10.1038/376025a0. [DOI] [PubMed] [Google Scholar]
- 21.Peixoto AA, Hall JC. Analysis of temperature-sensitive mutants reveals new genes involved in the courtship song of Drosophila. Genetics. 1998;148:827–838. doi: 10.1093/genetics/148.2.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rettig J, Heinemann C, Ashery U, Sheng Z-H, Yokoyama CT, Catterall WA, Neher E. Alteration of Ca2+ dependence of neurotransmitter release by disruption of Ca2+ channel/syntaxin interaction. J Neurosci. 1997;17:6647–6656. doi: 10.1523/JNEUROSCI.17-17-06647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robitaille R, Adler EM, Charlton MP. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990;5:773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- 24.Rojas CV, Wang J, Schwartz LS, Hoffman EP, Powell BR, Brown RH., Jr A Met-to-Val mutation in the skeletal muscle Na+ channel α-subunit in hyperkalaemic periodic paralysis. Nature. 1991;354:387–389. doi: 10.1038/354387a0. [DOI] [PubMed] [Google Scholar]
- 25.Sheng Z-H, Westenbroek RE, Catterall WA. Physical link and functional coupling of presynaptic calcium channels and the synaptic vesicle docking/fusion machinery. J Bioenerg Biomembr. 1998;30:335–345. doi: 10.1023/a:1021985521748. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqi O, Benzer S. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1976;73:3253–3257. doi: 10.1073/pnas.73.9.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith LA, Wang X, Peixoto AA, Neumann EK, Hall LM, Hall JC. A Drosophila calcium channel α1 subunit gene maps to a genetic locus associated with behavioral and visual defects. J Neurosci. 1996;16:7868–7879. doi: 10.1523/JNEUROSCI.16-24-07868.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith LA, Peixoto AA, Kramer EM, Villella A, Hall JC. Courtship and visual defects of cacophony mutants reveal functional complexity of a calcium-channel α1 subunit in Drosophila. Genetics. 1998;149:1407–1426. doi: 10.1093/genetics/149.3.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki DT, Grigliatti T, Williamson R. Temperature-sensitive mutations in Drosophila melanogaster. VII. A mutation (paraTS) causing reversible adult paralysis. Proc Natl Acad Sci USA. 1971;68:890–893. doi: 10.1073/pnas.68.5.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Schilcher F. The behavior of cacophony, a courtship song mutant in Drosophila melanogaster. Behav Biol. 1976;17:187–196. doi: 10.1016/s0091-6773(76)90444-2. [DOI] [PubMed] [Google Scholar]
- 31.von Schilcher F. A mutation which changes courtship song in Drosophila melanogaster. Behav Genet. 1977;7:251–259. doi: 10.1007/BF01066278. [DOI] [PubMed] [Google Scholar]
- 32.Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TV, Snutch TP, Catterall WA. Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheeler DB, Randall A, Sather WA, Tsien RW. Neuronal calcium channels encoded by the α1A subunit and their contribution to excitatory synaptic transmission in the CNS. Prog Brain Res. 1995;105:65–78. doi: 10.1016/s0079-6123(08)63284-7. [DOI] [PubMed] [Google Scholar]
- 34.Wiser O, Trus M, Hernández A, Renström E, Barg S, Rorsman P, Atlas D. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc Natl Acad Sci USA. 1999;96:248–253. doi: 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J-F, Ellinor PT, Aldrich RW, Tsien RW. Molecular determinants of voltage-dependent inactivation in calcium channels. Nature. 1994;372:97–100. doi: 10.1038/372097a0. [DOI] [PubMed] [Google Scholar]