Abstract
GABA transmission in the deep layers of the superior colliculus/deep mesencephalic reticular formation (deep SC/Me) mediates several motor responses, including those expressed after systemic administration of dopamine agonists. In the present study we examined the role of the deep SC/Me in the modulation of the acoustic startle reflex and its enhancement by the dopamine D1 agonist SKF 82958. Rats were implanted with bilateral cannulas into the deep SC/Me or superficial layers of the SC (super SC) and 1 week later were infused with various compounds. The GABAA antagonist bicuculline (0, 5, and 10 ng) produced a dose- and time-dependent enhancement of startle after infusion into the deep SC/Me, but not the super SC. Infusion of the GABAA agonist muscimol (0.1 μg) into the deep SC/Me, but not the super SC, blocked the enhancement of startle by systemic SKF 82958 (1 mg/kg) but had no effect on baseline startle by itself. This effect was not produced by infusion of the D1 antagonist SCH 23390(1 μg) or the glutamate antagonist NBQX (0.1 μg). Deposits of FluoroGold into the deep SC/Me, combined with immunohistochemistry for glutamic acid decarboxylase (GAD), confirmed a direct GABAergic input from the substantia nigra pars reticulata (SNr) to the deep SC/Me. These results suggest that GABA tone in the deep SC/Me modulates the expression of startle as well as the enhancement of startle by dopamine D1 agonists. On the basis of these data and previous work, we have proposed a striatonigral–tectal–reticular neural pathway mediating the effects of dopamine D1 agonists on startle.
Keywords: startle, superior colliculus, bicuculline, muscimol, SKF 82958, D1 receptor
The acoustic startle reflex in rats is a rapid sensorimotor response elicited by a sudden and intense auditory stimulus (cf. Davis, 1984) and is mediated by a simple neural pathway in the brainstem consisting of cochlear root neurons (CRNs), neurons in the nucleus reticularis pontis caudalis (PnC), and motoneurons in the spinal cord (Lee et al., 1996). The amplitude of this short-latency response can be quantified easily and has been used extensively to study the neurocircuitry and neurochemistry involved in the modulation of reflex/motor behavior (cf. Davis, 1980). Along these lines, we have found that systemic administration of dopamine D1 receptor agonists markedly increases the acoustic startle response (Meloni and Davis, 1999a). This effect is mediated in part by the activation of D1receptors in the substantia nigra pars reticulata (SNr; Meloni and Davis, 1997), a major output structure of the basal ganglia to premotor areas in the midbrain (Graybiel, 1984). Hence, we have been using D1 agonist-induced enhancement of the acoustic startle response to study the neural mechanisms underlying dopaminergic control of motor behavior. In particular, we are interested in how SNr-dependent D1 receptor agonist effects get transmitted to the primary acoustic startle pathway in the brainstem.
In the present study we have focused on the deep layers of the superior colliculus/mesencephalic reticular formation (collectively referred to as the deep SC/Me) as a possible relay between the SNr and the PnC mediating the enhancement of startle by D1agonists. The deep SC/Me is a major target of the SNr (Faull and Mehler, 1978) and has a direct projection to the reticular formation where cells in the PnC are located (Meloni and Davis, 1999b). A number of studies have shown that the nigrotectal projection contains GABA (Di Chiara et al., 1979; Araki et al., 1984), and blockade of GABA transmission in the deep SC/Me elicits a constellation of motor behaviors, including an explosive jumping behavior (Dean et al., 1980;Cools et al., 1983).
On the basis of the above data and the observation that GABA levels are reduced in the deep SC/Me after systemic administration of dopamine agonists (Melis and Gale, 1983), we hypothesized that removal of GABA tone in this area may (1) enhance the acoustic startle response by itself and (2) mediate the enhancement of startle seen after systemic administration of D1 agonists. To test the first hypothesis, we locally infused animals with the GABAA antagonist bicuculline into the deep SC/Me and tested them for their acoustic startle response. To test the second hypothesis, we locally infused animals with the GABAA agonist muscimol into the deep SC/Me in an attempt to restore the putative loss of GABA tone produced by D1 agonist administration and block the enhancement of startle by SKF 82958. We also attempted to confirm a GABAergic input from the SNr to the deep SC/Me, using deposits of a retrograde tracer (FluoroGold) into this area, combined with immunohistochemistry for identification of glutamic acid decarboxylase (GAD), i.e., the enzyme involved in GABA synthesis.
MATERIALS AND METHODS
Animals. The animals were male Sprague Dawley rats (Charles River, Wilmington, MA) weighing between 400 and 450 gm; they were housed in group cages of four rats each until the time of surgery when they were housed singly. Animals were maintained on a 12 hr light/dark cycle (lights on at 7:00 A.M.) with food and water continuously available and were used under conditions consistent with the USDA, Yale University, and National Institutes of Health rules for the care and use of laboratory animals.
Apparatus. Five separate stabilimeters were used to measure the amplitude of the startle response. Each stabilimeter consisted of an 9 × 15 × 15 cm Plexiglas and wire mesh cage suspended between compression springs within a steel frame. Cage movement resulted in displacement of an accelerometer (PCB Piezotronics, Depew, NY) in which the resultant voltage was proportional to the velocity of the cage displacement. The analog output of the accelerometer was amplified and digitized on a scale of 0–4096 units by a MacADIOS II board (GW Instruments, Somerville, MA) interfaced to a Macintosh II computer. Startle amplitude was defined as the peak accelerometer voltage that occurred during the first 200 msec after onset of the startle stimulus. Each stabilimeter was located within a 68.5 × 35.5 × 42 cm ventilated plywood isolation box with the inside temperature monitored and maintained at ∼20°C. All five startle boxes were located in a ventilated sound-attenuated chamber (2.5 × 2.5 × 2 m; Industrial Acoustics, New York, NY). A surveillance camera (Ikegami model ITC-40, Utsunomiya, Japan) was positioned behind each stabilimeter within each box and connected to a TV monitor located outside the Industrial Acoustics isolation chamber. A red light bulb (7.5 W) was located on the floor of the startle box to provide the illumination for the cameras in the otherwise completely dark box.
Startle elicitation. Startle responses were evoked by 50 msec bursts of white noise at various intensities generated by a Lafayette noise generator (model 15011) and delivered through high-frequency speakers (Radio Shack Supertweeters, range 5–40 kHz) located 5 cm from the front of each cage. Constant wideband background noise (60 dB) was produced by another noise generator and delivered through the same speakers. The presentation and sequencing of all stimuli were under the control of a Macintosh II computer. Sound level measurements (SPL) were made with a Brüel & Kjaer model 2235 sound level meter (A scale; random input) with the microphone (Type 4176) located 10 cm from the center of the speaker, which approximates the distance of the rat's ear from the speaker during testing.
Intracerebral cannulation. Rats were anesthetized with Nembutal (50 mg/kg, i.p.) and placed in a Kopf stereotaxic instrument (model 900) with blunt ear bars. The skin was retracted, and bilateral holes were drilled in the skull above the superior colliculus. Stainless steel guide cannulas (22 gauge, 3 mm center to center; Plastics One, Roanoke, VA) with an internal dummy stylette extending 1 mm beyond the guide cannula tip were lowered into the brain, using the following coordinates: −6.3 mm posterior to bregma, ±1.5 mm lateral to the midline, −4.8 mm ventral to dura (deep SC/Me), or −3.0 mm ventral to dura (superficial layers of the superior colliculus; super SC). Three jeweler's screws (0–80) also were placed in the skull to anchor the guide cannulas, and Loctite adhesive (Newington, CT) and dental acrylic were used to cement the cannulas in place. The animals were given 1 week to recover before intracerebral drug infusion effects on startle were tested.
Drug administration. During the intracerebral infusion procedure the rats were placed in individual plastic cages (28 × 17 × 12 cm); their dummy stylettes were removed and replaced with infusion cannulas (28 gauge, 1 mm projection from the tip of the guide cannula; Plastics One) attached to Hamilton microsyringes (10 μl) by polyethylene tubing. A Harvard Apparatus (model 22) infusion pump was used to deliver 0.5 μl/side of either drugs or saline directly into the deep SC/Me or super SC at a rate of 0.2 μl/min for 2.5 min. The infusion cannulas were left in place for 1 min after the infusion, after which time they were removed and the dummy stylettes were replaced.
To test the effects of bicuculline infused into the deep SC/Me on startle, 1 week after surgery we matched animals into three groups (n = 8 each group) having equivalent baseline startle responses, using a startle test identical to the one described below. Then 2 d later each group received bicuculline (0, 5, or 10 ng/side) infused into the deep SC/Me. As an anatomical control, another group of animals (n = 7) also was given a matching session 1 week after surgery and 2 d later received bicuculline (0, 5, or 10 ng/side) infused into the super SC. For the testing of bicuculline in the super SC, drug presentation was given in a counterbalanced order (Latin Square design), with 72 hr separating each of the 3 test days. These doses of bicuculline were chosen on the basis of pilot studies (see below).
A different group of animals (n = 10) received either muscimol (0.1 μg/side) or saline infused into the deep SC/Me, followed by a subcutaneous injection of either SKF 82958 (1 mg/kg) or saline. Drug presentation was given in a counterbalanced order (Latin Square), with 72 hr separating each of the 4 test days. As an anatomical control another group of animals (n = 8) received either muscimol (0.1 μg/side) or saline infused into the super SC, followed by a subcutaneous injection of either SKF 82958 (1 mg/kg) or saline. Drug presentation was given in a counterbalanced order, with 72 hr separating each of the 4 test days. This dose of muscimol was chosen on the basis of a previous study showing a complete blockade of the expression of fear-potentiated startle, with no effect on baseline startle, after local infusion of muscimol (0.1 μg) into the deep SC/Me (Meloni and Davis, 1999b).
As a pharmacological control another group of animals (n = 10) received either SCH 23390 (1 μg/side) or saline infused into the deep SC/Me, followed by a subcutaneous injection of either SKF 82958 (1 mg/kg) or saline. Drug presentation was given in a counterbalanced order, with 72 hr separating each of the 4 test days. This dose of SCH 23390 was chosen on the basis of a previous study showing a complete blockade of SKF 82958-induced enhancement of startle after local infusion of SCH 23390 (1 μg) into the SNr (Meloni and Davis, 1997). Because others have shown that chemical stimulation of neurons in the deep SC with glutamate can elicit defensive motor responses (Dean et al., 1988a), another pharmacological control group was used to test the involvement of glutamate receptors in the deep SC/Me in the enhancement of startle by SKF 82958. These animals (n = 12) were infused with either the AMPA receptor antagonist NBQX (0.1 μg/side) or saline into the deep SC/Me, followed by a subcutaneous injection of either SKF 82958 (1 mg/kg) or saline. Drug presentation was given in a counterbalanced order, with 72 hr separating each of the 4 test days. This dose of NBQX was chosen on the basis of its ability to effectively block the pinna component of acoustic startle (flexion of the ear) when infused into the facial motor nucleus (our unpublished observations).
Drugs. SKF 82958 [(±)-Chloro-APB HBr],R(+)-SCH 23390, NBQX (di-sodium), and (−)-bicuculline methiodide were obtained from Research Biochemicals International (Natick, MA) and muscimol from Sigma (St. Louis, MO). All drugs were dissolved in 0.9% saline.
Behavioral testing. Because the effect of bicuculline infused into the deep SC/Me on startle occurs almost immediately after infusion and lasts for a relatively short time (<20 min as determined in pilot studies), a short test session was used. After the infusion the animals were placed immediately in the startle cages and 1 min later received two habituating startle stimuli (95 dB, 30 sec interstimulus interval). Then the rats were presented with 50 startle stimuli, 10 at each of five different intensities (80, 85, 90, 95, and 100 dB) in a semi-random order with a 30 sec interstimulus interval. In addition to measuring startle, we made baseline activity measurements by sampling cage movement (200 msec duration) 15 sec after the onset of each startle stimulus. This is the same test used to match animals into groups with equivalent startle levels.
First the animals were infused with muscimol, SCH 23390, NBQX, or saline and then immediately were injected subcutaneously with SKF 82958 or saline. Next they were placed in the startle cages and given a test session consisting of a 5 min acclimation period, followed by five habituating startle stimuli (95 dB, 30 sec interstimulus interval). Then the rats were presented with 100 startle stimuli (50 msec duration, 5 msec rise–decay time), 20 at each of five different intensities (80, 85, 90, 95, and 100 dB) in a semi-random order with a 30 sec interstimulus interval. In addition to startle testing, baseline activity measurements were made by sampling cage movement (200 msec duration) 15 sec after the onset of each startle stimulus.
Histology. At the end of each experiment the animals were given an overdose with chloral hydrate and perfused intracardially with 0.9% saline, followed by 10% formalin. The brains were removed from the skull and stored for at least 4 d in a 30% sucrose/formalin solution; subsequently, 40 μm frozen coronal sections were cut through the superior colliculus. Every other brain section was mounted on gelatin-coated slides and stained with cresyl violet. The location of the cannulas in the deep SC/Me and super SC was assessed under a light microscope and transcribed onto atlas sections (Paxinos and Watson, 1997).
Statistical analysis. Startle amplitude data were expressed as the mean ± SEM across startle stimuli at each of the five test intensities or in blocks of two collapsed across intensity for an analysis of drug effects across time. For drug treatment effects, significant differences were evaluated with ANOVA, followed by individual comparisons with Student's t tests. For all analyses, drug treatment and intensity were within-groups factors except for the comparison of doses of bicuculline (0, 5, and 10 ng) in the deep SC/Me where drug treatment was a between-groups factor. Activity level differences were evaluated separately from the startle amplitude data with an ANOVA.
FluoroGold injections and GAD immunohistochemistry.Stereotaxic injections of the retrograde tracer FluoroGold (0.4% in 0.1 m cacodylic acid; Fluorochrome, Englewood, CO) were made in the deep SC/Me, using the coordinates described above. Iontophoretic injections were made by passing a current (4 μA; 4 min on, 4 min off) through the solution contained in a glass micropipette (30 μm tip diameter). After 6 d the rats received stereotaxic injections into the lateral ventricle of 2% colchicine (5 μl; Sigma) dissolved in 0.9% saline. Then 1 d later the rats were overdosed with chloral hydrate and perfused transcardially with a volume of 100 ml of 0.9% saline, followed by a volume of 500 ml of 0.1 mPBS, pH 7.4, containing 2% paraformaldehyde, 0.05% glutaraldehyde, and 0.2% picric acid. After the perfusion the brains were removed and stored for 3–4 d in a 30% sucrose/0.1 m PBS solution. Subsequently, the brains were cut serially from the caudal SC to the rostral SNr in coronal sections of alternating thickness (30 and 15 μm sections) collected in 0.1 m PBS. The 15 μm sections were processed for GAD immunofluorescence histochemistry while alternate 30 μm sections were mounted on gelatin-coated slides and coverslipped with DPX Mountant (Fluka, Milwaukee, WI) for FluoroGold fluorescence microscopy. The other 30 μm sections were mounted on gelatin-coated slides and stained with cresyl violet.
For GAD immunofluorescence histochemistry the sections were incubated in the antibody medium (2% normal goat serum and 1% bovine serum albumin in 0.1 m PBS, pH 7.3) for 30 min, followed by incubation in primary antibody (rabbit anti-glutamate decarboxylase, 1:1000; Chemicon, Temecula, CA) for 3 hr at room temperature. The sections were washed three times in 0.1 m PBS and then incubated in secondary antibody (biotin-SP-conjugated goat anti-rabbit IgG, 1:200; Jackson ImmunoResearch, West Grove, PA) for 2 hr at room temperature. The sections were washed again three times in 0.1m PBS and then incubated for 2 hr at room temperature with avidin-TRITC (Texas Red; Jackson ImmunoResearch) diluted at 1:100 in 0.1 m PBS. The sections were mounted on gelatin-coated slides and coverslipped with DPX Mountant for fluorescence microscopy observed with a Zeiss Axioscope (Oberkochen, Germany) under appropriate filters for FluoroGold fluorescence (excitation/emission peaks = 323/408 nm) and GAD immunofluorescence (excitation/emission peaks = 550/570 nm). Still frame photomicrographs were captured with a Sony DXC5000 digital camera for visualization of FluoroGold retrogradely labeled cells and GAD immunolabeled cells within the SNr in the same brain slice.
RESULTS
Bicuculline in the deep SC/Me
Figure 1Aillustrates the effect of bicuculline (0, 5, and 10 ng) infused into the deep SC/Me on startle. Pilot studies in our laboratory had shown a sensitized startle response after repeated bicuculline infusions into the deep SC/Me. As a result, a between-subjects design was used to examine the effects of different doses of bicuculline infused into the deep SC/Me on startle. These pilot studies also demonstrated a very sensitive dose–response effect of bicuculline in the deep SC/Me; infusion doses higher than 10 ng produced spontaneous and auditory-stimulated explosive motor responses in most animals. In fact, two animals infused with the 10 ng dose of bicuculline into the deep SC/Me showed this explosive behavior and were unable to be tested. The remaining eight animals were sensitive to auditory stimuli in the testing room but were tractable enough to be tested in the startle cages.
Fig. 1.
Dose-dependent enhancement of startle by bicuculline (BIC) infused into the deep layers of the superior colliculus/mesencephalic reticular formation (deep SC/Me), but not the superficial layers of the superior colliculus (super SC), across intensity (A) and time (B).Filled symbols, Deep SC/Me infusions; open symbols, super SC infusions. Square, BIC (10 ng); triangle, BIC (5 ng); circle, saline. Significant differences from saline response: *p < 0.05 and **p < 0.005.
A two-way ANOVA with bicuculline dose (0, 5, and 10 ng) as a between-subjects factor and intensity as a within-subjects factor revealed a significant main effect of bicuculline dose [F(2,21) = 4.8; p < 0.05] and intensity [F(4,21) = 60.4;p < 0.0001]. The bicuculline dose by intensity interaction was not significant. These data indicate that blockade of GABA transmission in the deep SC/Me with the GABAA antagonist bicuculline leads to a dose-dependent enhancement of the acoustic startle response. A one-way ANOVA of activity levels for each dose of bicuculline revealed no significant effects.
Figure 1A also illustrates the effect of bicuculline (0, 5, and 10 ng) infused into the super SC on startle. Pilot studies had not shown a sensitized startle response after repeated bicuculline infusions into the super SC, and a within-subjects design was used to examine the effects of different doses of bicuculline infused into the super SC on startle. A two-way ANOVA with bicuculline dose (0, 5, and 10 ng) and intensity as within-subjects factors revealed a significant main effect of intensity [F(4,24) = 24.4; p < 0.0001]. The main effect of bicuculline dose and the dose by intensity interaction was not significant. These data indicate that blockade of GABA transmission in the super SC with bicuculline has no effect on startle. A one-way ANOVA of activity levels for each dose of bicuculline revealed no significant effects.
Figure 1B illustrates the time course (collapsed across intensity) for the effect of bicuculline (0, 5, and 10 ng) infused into the deep SC/Me on startle. A two-way ANOVA with bicuculline dose as a between-subjects factor and time as a within-subjects factor revealed a significant main effect of bicuculline dose [F(2,21) = 4.8;p < 0.05], time [F(4,21) = 12.9; p < 0.0001], and a dose by time interaction [F(8,84) = 4.9; p < 0.0001]. Individual comparisons revealed a significant, dose-dependent enhancement of startle by bicuculline over the first 15 min of testing. Figure 1B also illustrates the time course of the effect of bicuculline (0, 5, and 10 ng) infused into the super SC. A repeated measures two-way ANOVA revealed no significant effects on startle across time by bicuculline (either dose) infused into the super SC.
Muscimol in the deep SC/Me and the enhancement of startle by SKF 82958
Systemic administration of the dopamine D1receptor agonist SKF 82958 produced a marked enhancement of the acoustic startle response over a range of intensities that was both subthreshold (80 and 85 dB) and suprathreshold (90, 95, and 100 dB) for startle evocation (Fig.2A). The effect of local infusion of muscimol (0.1 μg) into the deep SC/Me on the enhancement of startle by SKF 82958 (1 mg/kg) was analyzed by using the mean startle amplitudes for each drug condition (SAL–SAL, SAL–SKF, MUS–SAL, MUS–SKF) at each intensity (80, 85, 90, 95, and 100 dB) collapsed over the test session. A two-way ANOVA with drug condition and intensity as within-subjects factors revealed a significant main effect of drug condition [F(3,27) = 11.5; p < 0.0001], intensity [F(4,36) = 51.1; p < 0.0001], and a drug condition by intensity interaction [F(12,108) = 8.4; p< 0.0001]. Individual comparisons showed that muscimol (0.1 μg) infused into the deep SC/Me had no effect on baseline startle itself but effectively blocked the enhancement of startle by SKF 82958 at the two highest intensities (95 and 100 dB), but not at the lower intensities (80, 85, and 90 dB). However, because activity levels are elevated significantly in SKF 82958-treated animals infused with muscimol (see below), the increase in animal movement may mask a blockade of SKF 82958-enhanced startle at these subthreshold intensities. This concern is addressed below by examining the effects of muscimol on SKF 82958-enhanced startle across time. In general, these data indicate that pharmacological inactivation of neurons in the deep SC/Me with the GABAA receptor agonist muscimol can block the enhancement of startle by SKF 82958, an effect that is most evident at suprathreshold startle intensities. A separate one-way ANOVA of mean activity levels revealed a significant main effect of drug condition [F(3,27) = 11.8; p < 0.0001]. Individual comparisons showed that activity levels were elevated significantly by muscimol infusion followed by SKF 82958 injections, but not by muscimol or SKF 82958 alone.
Fig. 2.
Effect of muscimol [0.1 μg; muscimol (MUS)] infused into the deep layers of the superior colliculus/mesencephalic reticular formation on the enhancement of startle by systemic SKF 82958 (1 mg/kg; SKF). Graphs are collapsed across intensity (A) and time for suprathreshold (90, 95, and 100 dB; B) and subthreshold (80 and 85 dB) intensities (C) and activity (D). The enhancement of startle by SKF 82958 was blocked by MUS in the deep SC/Me at suprathreshold intensities, but not at subthreshold intensities (but note the blockade over the first 15 min in C). Activity levels were elevated significantly by muscimol in the deep SC/Me and SKF 82958 after 20 min, an effect that may mask blockade at subthreshold intensities. *p < 0.05, **p < 0.005, and ***p < 0.0005 as compared with saline (SAL) response.
Figure 2 also illustrates the effect of muscimol in the deep SC/Me on SKF 82958-enhanced startle across time for suprathreshold (Fig.2B) and subthreshold (Fig. 2C) startle intensities as well as activity levels (Fig. 2D). A two-way ANOVA of startle over time collapsed across suprathreshold intensities (90, 95, and 100 dB) revealed a significant main effect of group [F(3, 27) = 9.6;p < 0.0005] and time [F(9, 81) = 3.6; p < 0.001]. The group by time interaction was not significant. Linear contrast analyses showed that startle was enhanced significantly by SKF 82958 across time in animals that received saline infused into the deep SC/Me [F(1, 27) = 20.1;p < 0.0001], but not in animals that received muscimol infused into the deep SC/Me.
A two-way ANOVA of startle over time collapsed across subthreshold intensities (80 and 85 dB) revealed a significant main effect of group [F(3, 27) = 15.6; p< 0.0001]. The main effect of time and group by time interaction was not significant. Linear contrast analyses showed that startle was enhanced significantly by SKF 82958 across time in animals that received saline infused into the deep SC/Me [F(1, 27) = 32.8; p < 0.0001] as well as those that received muscimol infused into the deep SC/Me [F(1, 27) = 22.8; p< 0.0001]. However, individual comparisons showed that startle was not enhanced significantly by SKF 82958 in muscimol-infused animals versus saline-infused animals over the first 15 min (first two time points).
A two-way ANOVA of activity collapsed over time revealed a significant main effect of group [F(3, 27) = 11.8; p < 0.0001] and a group by time interaction [F(27, 243) = 1.8; p< 0.05]. The main effect of time was not significant. Linear contrast analyses showed that activity was enhanced significantly by SKF 82958 across time in animals that received muscimol infused into the deep SC/Me [F(1, 27) = 29.6;p < 0.0001]. However, individual comparisons showed that activity was not enhanced significantly by SKF 82958 in muscimol-infused animals versus saline-infused animals over the first 15 min (first two time points).
Animals that received muscimol in the deep SC/Me plus SKF 82958 showed an increase in body movement, including frequent repositioning and circling within the startle cage, which accounted for the increase in activity measurements in this treatment group. As a result, this increase in activity would be recorded during subthreshold intensity trials in which there normally is no elicitation of startle (these trials are essentially a measurement of activity levels). A closer examination of the data presented in Figure 2 shows that the response by muscimol/SKF 82958 develops at a similar rate across time in both the subthreshold intensity (Fig. 2C) and activity (Fig.2D) graphs, suggesting that they represent the same phenomenon (i.e., an increase in activity). In fact, over the first 15 min (first two time points) in which activity levels were not elevated significantly, there was a significant blockade of the SKF 82958-enhanced startle at the subthreshold intensities by muscimol in the deep SC/Me. We believe that this effect then becomes masked (after 15 min) by the increase in activity produced by the muscimol/SKF 82958 treatment condition. Furthermore, this increase in activity is not responsible for the blockade of SKF 82958-enhanced startle seen at higher intensities because the animals still show a blockade at the first two time points (Fig. 2B) in which activity levels are not increased significantly (Fig. 2D). Thus, it is not the case that an increase in activity interferes with startle itself or the enhancement of startle by SKF 82958. This assertion is supported by our finding that muscimol infused into the SNr produces a marked increase in activity (circling and stereotyped behavior) as well as an increase in startle (Meloni and Davis, 1997).
As an anatomical control, another group of animals received muscimol (0.1 μg) infused into the super SC (Fig.3A), followed by SKF 82958 injection. A two-way ANOVA revealed a significant main effect of drug condition [F(3,21) = 21.4;p < 0.0001], intensity [F(4,28) = 108.8; p< 0.0001], and a drug condition by intensity interaction [F(12,84) = 9.27; p< 0.0001]. Individual comparisons revealed significant differences from the SAL–SAL condition at each intensity for both the SAL–SKF and MUS–SKF conditions. Furthermore, these two conditions were not significantly different from each other, indicating that muscimol (0.1 μg) infused into the super SC has no effect on the enhancement of startle by SKF 82958. A one-way ANOVA of activity levels revealed a significant increase in activity in the MUS–SKF condition.
Fig. 3.
Anatomical and pharmacological controls.A, Muscimol (0.1 μg; MUS) infused into the superficial layers of the superior colliculus (super SC). B, SCH 23390 (1 μg;SCH) infused into the deep layers of the superior colliculus/mesencephalic reticular formation (deep SC/Me). C, NBQX (0.1 μg) infused into the deep SC/Me. None of these infusions had any effect on the enhancement of startle by systemic SKF 82958 (1 mg/kg; SKF). *p < 0.05, **p < 0.005, and ***p < 0.0005 as compared with saline (SAL) response.
As a pharmacological control, another group of animals received infusions of the dopamine D1 receptor antagonist SCH 23390 (1 μg) into the deep SC/Me, followed by SKF 82958 injection (Fig. 3B). A two-way ANOVA revealed a significant main effect of drug condition [F(3,27) = 18.7; p < 0.0001], intensity [F(4,36) = 57.3; p < 0.0001], and a drug condition by intensity interaction [F(12,108) = 9.8; p< 0.0001]. Individual comparisons revealed significant differences from the SAL–SAL condition at each intensity for both the SAL–SKF and SCH–SKF conditions. Furthermore, these two conditions were not significantly different from each other, indicating that SCH 23390 (1 μg) infused into the deep SC/Me has no effect on the enhancement of startle by SKF 82958. A one-way ANOVA of activity levels revealed no significant effects.
To test the involvement of glutamate transmission in the deep SC/Me on the enhancement of startle by SKF 82958, we infused another group of animals with the AMPA receptor antagonist NBQX, followed by SKF 82958 injection (Fig. 3C). A two-way ANOVA revealed a significant main effect of drug condition [F(3,33) = 12.1; p < 0.0001], intensity [F(4,44) = 35.5;p < 0.0001], and a drug condition by intensity interaction [F(12,132) = 9.8; p < 0.0001]. Individual comparisons revealed significant differences from the SAL–SAL condition at each intensity for both the SAL–SKF and NBQX–SKF conditions. Furthermore, these two conditions were not significantly different from each other, indicating that NBQX (0.1 μg) infused into the deep SC/Me has no effect on the enhancement of startle by SKF 82958. A one-way ANOVA of activity levels revealed no significant effects.
Cannula placement
Figure 4, A andB, shows representative photomicrographs of cannula placements in the deep SC/Me and super SC, respectively. The shaded areas in the lower panels of Figure 4, A and B, summarize the location of cannula tips in the deep SC/Me and super SC, respectively, for all the animals used in this study. Cannulas in the deep SC/Me were located predominantly in the regions between the deep and intermediate white layers of the superior colliculus and the underlying deep mesencephalic area. Cannulas in the superficial SC were located predominantly in the superficial gray layer and optic nucleus layer of the superior colliculus.
Fig. 4.

Representative digital photomicrographs showing bilateral cannula placement in the deep layers of the superior colliculus/mesencephalic reticular formation (deep SC/Me;A) and superficial layers of the superior colliculus (super SC; B). Shaded areas in thelower panels summarize the location of cannulas in the deep SC/Me and super SC from animals used in this study. Serial plates are from the atlas of Paxinos and Watson (1997) and are listed in millimeters posterior to bregma. Scale bar, 2 mm.
FluoroGold injections and GAD immunohistochemistry
Five animals received unilateral iontophoretic injections of the retrograde tracer FluoroGold into the deep SC/Me, as summarized in the illustration in Figure 5A. These deposits were restricted to the deep white layers of the superior colliculus with some spread of the tracer into the deep mesencephalic area. Retrogradely labeled cells were found throughout the rostrocaudal extent of the ipsilateral SNr, mainly in the dorsolateral division (Fig. 5B). Figure 5C is a representative digital photomicrograph of retrogradely labeled cells in the rostral SNr after a deposit of FluoroGold into the ipsilateral deep SC/Me. Many of the retrogradely labeled cells (marked with arrows in Fig.5C) are shown in the photomicrograph in Figure 5Das being immunoreactive for GAD, indicating a GABAergic input from the SNr to the deep SC/Me.
Fig. 5.
A, Serial plates through the midbrain (from Paxinos and Watson, 1997) showing the extent of a deposit of FluoroGold (black area) in the deep layers of the superior colliculus/mesencephalic reticular formation (deep SC/Me).B, Retrogradely labeled cells (indicated by black dots) were found throughout the rostrocaudal extent of the ipsilateral substantia nigra pars reticulata (SNr), primarily in the dorsolateral division. Shown are digital images (10×) of FluoroGold-filled cells (C) and GAD-positive cells (D). Arrows mark retrogradely labeled cells, most of which also contain GAD, indicating a GABAergic input from the SNr to the deep SC/Me. Scale bar, 50 μm.Numbers are in millimeters posterior to bregma.
DISCUSSION
The major findings of this study are that (1) local infusion of the GABAA antagonist bicuculline into the deep SC/Me produces a dose-dependent enhancement of startle, (2) local infusion of the GABAA agonist muscimol into the deep SC/Me blocks the enhancement of startle by the dopamine D1 agonist SKF 82958, and (3) the SNr is a source of GABA innervation to the deep SC/Me. Taken together, these results add to a body of work showing that GABAergic transmission at the level of the deep SC/Me is an important component in the regulation and expression of motor behaviors. Because the deep SC/Me is not part of the primary acoustic startle pathway (Lingenhöhl and Friauf, 1994; Lee et al., 1996), the results of the present study suggest that the deep SC/Me exerts a modulatory influence on this reflex. A recent report from our laboratory has identified a heavy ipsilateral innervation from the deep SC/Me to the part of the PnC critical for startle (Meloni and Davis, 1999b). Similar pathways have been described before (Redgrave et al., 1987; Yasui et al., 1994) and are believed to mediate a wide range of motor-related behaviors (Dean et al., 1988b). Thus, we believe that this tectal–reticular pathway could provide a direct neural connection relaying effects in the deep SC/Me to the acoustic startle circuit to affect this behavior.
Bicuculline in the deep SC/Me and startle
The enhancement of startle by bicuculline in the deep SC/Me is consistent with a number of other studies that have shown that blockade of GABA transmission in the deep SC/Me elicits an array of motor behaviors (Imperato and Di Chiara, 1981; Redgrave et al., 1981), including a hyperactive escape response (Dean et al., 1980; Cools et al., 1983; Shehab et al., 1995). These data suggest that the deep SC/Me is regulated by GABA innervation, presumably from the SNr (Deniau and Chevalier, 1984), and that removal of this inhibition can produce dramatic motor behaviors, including a markedly enhanced startle response. Because SNr neurons are tonically active (Deniau et al., 1978; Gulley et al., 1999), they would maintain a constant level of GABA tone in the deep SC/Me. Phasic removal of this GABA tone at the level of the deep SC/Me, pharmacologically (with local infusion of GABA antagonists) or by inhibition of the SNr (via D1agonist-induced activation of GABAergic striatonigral neurons), may mediate the expression of these motor responses (Chevalier and Deniau, 1990), including an increase in the acoustic startle response. Moreover, the fact that GABAergic SNr neurons projecting to the deep SC/Me are tonically active also would explain why local infusion of muscimol into the deep SC/Me had no effect by itself on baseline startle amplitude.
Although the animals were generally more active (sniffing and licking), there was no significant increase in activity levels with bicuculline infused into the deep SC/Me in the present study. Others, however, have shown an increase in circling behavior with unilateral infusion of GABA antagonists into the deep SC (Geula and Asdourian, 1984; Speller and Westby, 1996). Because unilateral blockade of GABA transmission in the deep SC/Me may produce hemispheric asymmetries and thus circling behavior, the bilateral bicuculline infusions used in the present study may have avoided this condition. However, both the expression of circling behavior and the enhancement of startle by bicuculline in the deep SC/Me show similar short-duration time courses with effects that rapidly decline to baseline by 15 min after infusion (Speller and Westby, 1996).
Muscimol in the deep SC/Me and the enhancement of startle by SKF 82958
The results of the present study also suggest that the deep SC/Me is involved in the enhancement of startle by the dopamine D1 agonist SKF 82958. This finding is consistent with a number of studies showing a reduction in dopamine agonist-stimulated behaviors after lesions of the deep SC (Pope et al., 1980; Redgrave et al., 1980; Morelli et al., 1981; Kilpatrick et al., 1982) Because blockade of GABA transmission in the deep SC/Me elicits motor behaviors resembling those seen after the administration of dopamine agonists, including an enhancement of startle (present study), we addressed the possibility that removal of GABA tone at this level may mediate the enhancement of startle by SKF 82958. This hypothesis is supported by the observation that GABA levels in the deep SC are reduced after systemic administration of dopamine agonists (Melis and Gale, 1983). We found that pharmacological inactivation of neurons in the deep SC/Me with local infusion of muscimol blocked the enhancement of startle by SKF 82958 at high intensities for the duration of the 1-hr-long test. At the lower intensities the enhancement of startle by SKF 82958 was blocked by muscimol in the deep SC/Me over the first 15 min after SKF 82958 injection, after which time a significant increase in activity may have obscured this blockade. Imperato and Di Chiara (1981) have observed a similar phenomenon in which muscimol infused into the mesencephalic reticular formation had no effect on behavior alone but blocked certain components of apomorphine-induced behaviors (sniffing, licking) with a concomitant expression of hypermotility. Because we observed the increase in activity produced by muscimol/SKF 82958 treatment to develop gradually over 20 min, it is possible that muscimol may be diffusing outside the deep SC/Me to a more caudal area of the mesencephalic reticular formation described by the studies ofImperato and Di Chiara (1981).
The use of a series of controls in the present study supports the anatomical and pharmacological specificity of a GABA-mediated mechanism at the level of the deep SC/Me involved in the enhancement of startle by SKF 82958. Whereas muscimol infused into the deep SC/Me blocked SKF 82958-enhanced startle, this same dose of muscimol (0.1 μg) had no effect when infused into the superficial SC, another area shown to contain GABAA receptors (Pinard et al., 1990). Likewise, infusion of the dopamine D1antagonist SCH 23390 into the deep SC/Me had no effect on the enhancement of startle by SKF 82958. The superior colliculus of the rat has been shown to contain a small population of dopamine receptors (Weller et al., 1987) and receives a dopamine-containing input from the substantia nigra (Takada et al., 1988). However, unlike the complete blockade of SKF 82958-enhanced startle seen after infusion of this dose of SCH 23390 (1 μg) into the SNr (Meloni and Davis, 1997), it appears that D1 receptors in the deep SC/Me play no role in the enhancement of startle by SKF 82958. We examined the effects of the AMPA receptor antagonist NBQX in the deep SC/Me because of a report of an excitatory drive from the cerebellum to the deep SC (Westby et al., 1993) and the report that local infusion of glutamate into this area can elicit various motor responses (Dean et al., 1988a). However, we found no effect of NBQX in the deep SC/Me on the enhancement of startle by SKF 82958. These data also suggest that the blockade seen with muscimol is not attributable to presynaptic inhibition (via GABAA receptors) of a glutamatergic drive in the deep SC/Me. However, further studies are needed to test the involvement of NMDA and metabotropic glutamate receptors in the deep SC/Me in the enhancement of startle by SKF 82958.
A model of dopamine D1 agonist-enhanced startle
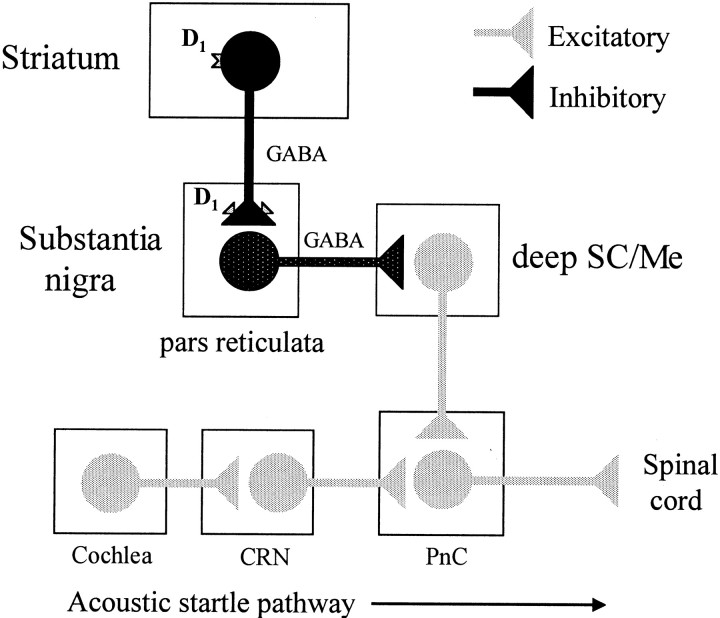
A major aim of the research in our laboratory has been to identify the neural circuits and mechanisms underlying dopaminergic modulation of the startle reflex. On the basis of the results of the present study, together with previous work from our laboratory implicating the SNr in SKF 82958-enhanced startle (Meloni and Davis, 1997), we have proposed a striatonigral–tectal–reticular pathway mediating the effects of dopamine D1 agonists on startle. According to this model, illustrated in Figure6, activation of GABA-containing striatonigral neurons by SKF 82958 acting at D1receptors in the striatum (Hernández-López et al., 1997) would provide the drive needed for GABA release in the SNr (Biggs et al., 1995). In addition, systemic SKF 82958 also would activate D1 receptors located on the terminals of these neurons where it is believed they act to facilitate GABA release in the SNr (Floran et al., 1990; Aceves et al., 1992; Radnikow and Misgeld, 1998; Matuszewich and Yamamoto, 1999). This leads to an inhibition of the tonically active output neurons of the SNr that contain GABA and a disinhibition of nigral targets such as the deep SC/Me. Because the SNr does not project directly to the startle pathway (our unpublished observations), disinhibition of a putative excitatory input from the deep SC/Me to the startle circuit at the level of the PnC (Meloni and Davis, 1999b) could account for the increase in startle. In addition, by restoring GABA tone in the deep SC/Me with muscimol, we prevent this SNr-mediated disinhibition and block the enhancement of startle by SKF 82958. Thus, this model fits the proposal by Chevalier and Deniau (1990) that GABAergic disinhibition at the level of the deep SC/Me serves as mechanism for the expression of basal ganglia-generated behaviors, including the enhancement of startle by dopamine D1 agonists.
Fig. 6.
A model of dopamine D1agonist-enhanced startle. On the basis of the results of the present study, together with previous work from our laboratory, we have proposed a striatonigral–tectal–reticular pathway mediating the effects of dopamine D1 agonists on startle. According to this model (after Chevalier and Deniau, 1990), the activation of striatonigral D1 receptors (on the cell bodies and terminals of these neurons) by SKF 82958 facilitates GABA release in the substantia nigra pars reticulata (SNr). This in turn leads to an inhibition of the tonically active (indicated bystippling) GABA-containing output neurons of the SNr and a disinhibition of nigral targets such as the deep layers of the superior colliculus/mesencephalic reticular formation (deep SC/Me). Disinhibition of a putative excitatory input from the deep SC/Me to the acoustic startle circuit at the level of the nucleus reticularis pontis caudalis (PnC) could account for the increase in startle. Behaviorally, the model is supported by the following observations: (1) both SCH 23390 and bicuculline infused into the SNr have no effect on baseline startle but block the enhancement of startle by SKF 82958 (Meloni and Davis, 1997), (2) local infusion of muscimol into the SNr increases startle (Meloni and Davis, 1997), (3) local infusion of bicuculline into the deep SC/Me increases startle (present study), and (4) local infusion of muscimol into the deep SC/Me has no effect on baseline startle but blocks the enhancement of startle by SKF 82958 (present study). CRN, Cochlear root neurons.
Footnotes
This work was supported by National Institute of Mental Health Grants MH-57250 and MH-47840, Research Scientist Award MH-00004 to M.D., and the Woodruff Foundation. We thank Changjun Shi for his assistance with the immunohistochemical techniques.
Correspondence should be addressed to Dr. Edward Meloni, Emory University, Department of Psychiatry, 1639 Pierce Drive, Suite 4000, Atlanta, GA 30322. E-mail: emeloni@emory.edu.
REFERENCES
- 1.Aceves J, Floran B, Martinez-Fong D, Benitez J, Sierra A, Flores G. Activation of D1 receptors stimulates accumulation of γ-aminobutyric acid in slices of the pars reticulata of 6-hydroxydopamine-lesioned rats. Neurosci Lett. 1992;145:40–42. doi: 10.1016/0304-3940(92)90198-g. [DOI] [PubMed] [Google Scholar]
- 2.Araki M, McGeer PL, McGeer EG. Presumptive γ-aminobutyric acid pathways from the midbrain to the superior colliculus studied by a combined horseradish peroxidase–γ-aminobutyric acid transaminase pharmacohistochemical method. Neuroscience. 1984;13:433–439. doi: 10.1016/0306-4522(84)90241-0. [DOI] [PubMed] [Google Scholar]
- 3.Biggs CS, Fowler LJ, Whitton PS, Starr MS. Impulse-dependent and tetrodotoxin-sensitive release of GABA in the rat's substantia nigra measured by microdialysis. Brain Res. 1995;684:172–178. doi: 10.1016/0006-8993(95)00281-t. [DOI] [PubMed] [Google Scholar]
- 4.Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990;13:277–280. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- 5.Cools AR, Ellenbroek BA, Van Den Heuvel CM. Picrotoxin microinjections into the brain: a model of abrupt withdrawal “jumping” behaviour in rats not exposed to an opiate? Eur J Pharmacol. 1983;90:237–243. doi: 10.1016/0014-2999(83)90243-1. [DOI] [PubMed] [Google Scholar]
- 6.Davis M. Neurochemical modulation of sensory–motor reactivity: acoustic and tactile startle reflexes. Neurosci Biobehav Rev. 1980;4:241–263. doi: 10.1016/0149-7634(80)90016-0. [DOI] [PubMed] [Google Scholar]
- 7.Davis M. The mammalian startle response. In: Eaton RC, editor. Neural mechanisms of startle behavior. Plenum; New York: 1984. pp. 287–351. [Google Scholar]
- 8.Dean P, Redgrave P, Souki W, Lewis G. Behavioural effects of microinjections of picrotoxin into rat superior colliculus and their modulation by intranigral 6-OHDA. Br J Pharmacol. 1980;70:145P. [Google Scholar]
- 9.Dean P, Mitchell IJ, Redgrave P. Responses resembling defensive behaviour produced by microinjection of glutamate into superior colliculus of rats. Neuroscience. 1988a;24:501–510. doi: 10.1016/0306-4522(88)90345-4. [DOI] [PubMed] [Google Scholar]
- 10.Dean P, Redgrave P, Mitchell IJ. Organization of efferent projections from superior colliculus to brainstem in rat: evidence for functional output channels. Prog Brain Res. 1988b;75:27–36. doi: 10.1016/s0079-6123(08)60463-x. [DOI] [PubMed] [Google Scholar]
- 11.Deniau JM, Chevalier G. Synaptic organization of the basal ganglia: an electroanatomical approach in the rat. In: Evered D, O'Connor M, editors. Functions of the basal ganglia, Ciba Foundation Symposium 107. Pitman; London: 1984. pp. 48–63. [DOI] [PubMed] [Google Scholar]
- 12.Deniau JM, Hammond C, Riszk A, Feger J. Electrophysiological properties of identified output neurons of the rat substantia nigra (pars compacta and pars reticulata): evidences for the existence of branched neurons. Exp Brain Res. 1978;32:409–422. doi: 10.1007/BF00238711. [DOI] [PubMed] [Google Scholar]
- 13.Di Chiara G, Porceddu ML, Morelli M, Mulas ML, Gessa GL. Evidence for a GABAergic projection from the substantia nigra to the ventromedial thalamus and to the superior colliculus of the rat. Brain Res. 1979;176:273–284. doi: 10.1016/0006-8993(79)90983-1. [DOI] [PubMed] [Google Scholar]
- 14.Faull RLM, Mehler WR. The cells of origin of nigrotectal, nigrothalamic, and nigrostriatal projections in the rat. Neuroscience. 1978;3:989–1002. doi: 10.1016/0306-4522(78)90119-7. [DOI] [PubMed] [Google Scholar]
- 15.Floran B, Aceves J, Sierra A, Martinez-Fong D. Activation of D1 dopamine receptors stimulates the release of GABA in the basal ganglia of the rat. Neurosci Lett. 1990;116:136–140. doi: 10.1016/0304-3940(90)90399-t. [DOI] [PubMed] [Google Scholar]
- 16.Geula C, Asdourian D. Circling and bodily asymmetry induced by injection of GABA agonists and antagonists into the superior colliculus. Pharmacol Biochem Behav. 1984;21:853–858. doi: 10.1016/s0091-3057(84)80064-7. [DOI] [PubMed] [Google Scholar]
- 17.Graybiel AM. Neurochemically specified subsystems in the basal ganglia. In: Evered D, O'Connor M, editors. Functions of the basal ganglia, Ciba Foundation Symposium 107. Pitman; London: 1984. pp. 114–143. [DOI] [PubMed] [Google Scholar]
- 18.Gulley JM, Kuwajima M, Mayhill E, Rebec GV. Behavior-related changes in the activity of substantia nigra pars reticulata neurons in freely moving rats. Brain Res. 1999;845:68–76. doi: 10.1016/s0006-8993(99)01932-0. [DOI] [PubMed] [Google Scholar]
- 19.Hernández-López S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imperato A, Di Chiara G. Behavioral effects of GABA agonists and antagonists infused in the mesencephalic reticular formation-deep layers of superior colliculus. Brain Res. 1981;224:185–194. doi: 10.1016/0006-8993(81)91131-8. [DOI] [PubMed] [Google Scholar]
- 21.Kilpatrick IC, Collingridge GL, Starr MS. Evidence for the participation of nigrotectal γ-aminobutyrate-containing neurones in striatal and nigral-derived circling in the rat. Neuroscience. 1982;7:207–222. doi: 10.1016/0306-4522(82)90161-0. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y, Lopez DE, Meloni EG, Davis M. A primary acoustic startle circuit: obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis. J Neurosci. 1996;16:3775–3789. doi: 10.1523/JNEUROSCI.16-11-03775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lingenhöhl K, Friauf E. Giant neurons in the rat reticular formation: a sensorimotor interface in the elementary acoustic startle circuit? J Neurosci. 1994;14:1176–1194. doi: 10.1523/JNEUROSCI.14-03-01176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matuszewich L, Yamamoto BK. Modulation of GABA release by dopamine in the substantia nigra. Synapse. 1999;32:29–36. doi: 10.1002/(SICI)1098-2396(199904)32:1<29::AID-SYN4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Melis MR, Gale K. Effects of dopamine agonists on γ-aminobutyric acid (GABA) turnover in the superior colliculus: evidence that nigrotectal GABA projections are under the influence of dopaminergic transmission. J Pharmacol Exp Ther. 1983;226:425–431. [PubMed] [Google Scholar]
- 26.Meloni E, Davis M. Involvement of the substantia nigra pars reticulata in D1 dopamine receptor agonist facilitation of the acoustic startle response in rats. Soc Neurosci Abstr. 1997;23:1856. [Google Scholar]
- 27.Meloni EG, Davis M. Enhancement of the acoustic startle response in rats by the dopamine D1 receptor agonist SKF 82958. Psychopharmacology. 1999a;144:373–380. doi: 10.1007/s002130051020. [DOI] [PubMed] [Google Scholar]
- 28.Meloni EG, Davis M. Muscimol infused into the deep layers of the superior colliculus/mesencephalic reticular formation blocks expression but not acquisition of fear-potentiated startle in rats. Behav Neurosci. 1999b;113:1152–1160. doi: 10.1037//0735-7044.113.6.1152. [DOI] [PubMed] [Google Scholar]
- 29.Morelli M, Imperato A, Porceddu ML, Di Chiara G. Role of dorsal mesencephalic reticular formation and deep layers of superior colliculus in turning behavior elicited from the striatum. Brain Res. 1981;215:337–341. doi: 10.1016/0006-8993(81)90513-8. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 3rd Ed. Academic; New York: 1997. [Google Scholar]
- 31.Pinard R, Richards JG, Lanoir J. Subcellular localization of GABAA/benzodiazepine receptor-like immunoreactivity in the superficial gray layer of the rat superior colliculus. Neurosci Lett. 1990;120:212–216. doi: 10.1016/0304-3940(90)90041-7. [DOI] [PubMed] [Google Scholar]
- 32.Pope SG, Dean P, Redgrave P. Dissociation of d-amphetamine-induced locomotor activity and stereotyped behavior by lesions of the superior colliculus. Psychopharmacology. 1980;70:297–302. doi: 10.1007/BF00427890. [DOI] [PubMed] [Google Scholar]
- 33.Radnikow G, Misgeld U. Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J Neurosci. 1998;18:2009–2016. doi: 10.1523/JNEUROSCI.18-06-02009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redgrave P, Dean P, Donohoe TP, Pope SG. Superior colliculus lesions selectively attenuate apomorphine-induced oral stereotypy: a possible role for the nigrotectal pathway. Brain Res. 1980;196:541–546. doi: 10.1016/0006-8993(80)90422-9. [DOI] [PubMed] [Google Scholar]
- 35.Redgrave P, Dean P, Souki W, Lewis G. Gnawing and changes in reactivity produced by microinjections of picrotoxin into the superior colliculus of rats. Psychopharmacology. 1981;75:198–203. doi: 10.1007/BF00432187. [DOI] [PubMed] [Google Scholar]
- 36.Redgrave P, Mitchell IJ, Dean P. Descending projections from the superior colliculus in rat: a study using orthograde transport of wheatgerm-agglutinin conjugated horseradish peroxidase. Exp Brain Res. 1987;68:147–167. doi: 10.1007/BF00255241. [DOI] [PubMed] [Google Scholar]
- 37.Shehab S, Simkins M, Dean P, Redgrave P. The dorsal midbrain anticonvulsant zone. III. Effects of efferent pathway transections on suppression of electroshock seizures and defense-like reactions produced by local injections of bicuculline. Neuroscience. 1995;65:697–708. doi: 10.1016/0306-4522(94)00517-9. [DOI] [PubMed] [Google Scholar]
- 38.Speller JM, Westby GWM. Bicuculline-induced circling from the rat superior colliculus is blocked by GABA microinjection into the deep cerebellar nuclei. Exp Brain Res. 1996;110:425–434. doi: 10.1007/BF00229142. [DOI] [PubMed] [Google Scholar]
- 39.Takada M, Li ZK, Hattori T. Dopaminergic nigrotectal projection in the rat. Brain Res. 1988;457:165–168. doi: 10.1016/0006-8993(88)90070-4. [DOI] [PubMed] [Google Scholar]
- 40.Weller ME, Rose S, Jenner P, Marsden CD. In vitro characterization of dopamine receptors in the superior colliculus of the rat. Neuropharmacology. 1987;26:347–354. doi: 10.1016/0028-3908(87)90187-0. [DOI] [PubMed] [Google Scholar]
- 41.Westby GWM, Collinson C, Dean P. Excitatory drive from the cerebellar neurons to the superior colliculus in the rat: an electrophysiological mapping study. Eur J Neurosci. 1993;5:1378–1388. doi: 10.1111/j.1460-9568.1993.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 42.Yasui Y, Tsumori T, Ando A, Domoto T, Kayahara T, Nakano K. Descending projections from the superior colliculus to the reticular formation around the motor trigeminal nucleus and the parvicellular reticular formation of the medulla oblongata in the rat. Brain Res. 1994;656:420–426. doi: 10.1016/0006-8993(94)91489-3. [DOI] [PubMed] [Google Scholar]