Abstract
Release of transmitter glutamate implies a drain of α-ketoglutarate from neurons, because glutamate, which is formed from α-ketoglutarate, is taken up by astrocytes. It is generally believed that this drain is compensated by uptake of glutamine from astrocytes, because neurons are considered incapable of de novosynthesis of tricarboxylic acid cycle intermediates, which requires pyruvate carboxylation. Here we show that cultured cerebellar granule neurons form releasable [14C]glutamate from H14CO3− and [1-14C]pyruvate via pyruvate carboxylation, probably mediated by malic enzyme. The activity of pyruvate carboxylation was calculated to be approximately one-third of the pyruvate dehydrogenase activity in neurons. Furthermore, intrastriatal injection of NaH14CO3 or [1-14C]pyruvate labeled glutamate better than glutamine, showing that pyruvate carboxylation occurs in neurons in vivo. This means that neurons themselves to a large extent may support their release of glutamate, and thus entails a revision of the current view of glial–neuronal interactions and the importance of the glutamine cycle.
Keywords: transmitter glutamate, pyruvate carboxylation, CO2 fixation, malic enzyme, anaplerosis, 3-nitropropionic acid
After release from neurons during neurotransmission, glutamate is taken up into astrocytes (Henn et al., 1974; Pines et al., 1992; Danbolt et al., 1998) where some of it is converted to glutamine (Martinez-Hernandez et al., 1977) and some is metabolized by the glial tricarboxylic acid (TCA) cycle (Hassel and Sonnewald, 1995; McKenna et al., 1996). The glutamine is transferred back to neurons and contributes to the replenishment of the neuronal glutamate pool, but this “glutamine cycling” cannot compensate fully for the loss of transmitter glutamate, because not all of the transmitter glutamate is converted to glutamine. Glutamatergic neurotransmission will therefore cause a drain of α-ketoglutarate from the neuronal TCA cycle unless it is compensated via an anaplerotic reaction; a net loss of α-ketoglutarate would reduce the capacity of the neurons for oxidative metabolism and ATP generation, as well as their ability to release glutamate. In the brain the only truly anaplerotic reaction is carboxylation of pyruvate to the TCA cycle intermediates malate or oxaloacetate. This process has been thought to occur in astrocytes and not in neurons, because pyruvate carboxylase, one of the pyruvate-carboxylating enzymes in the brain (Patel, 1974), is only expressed in astrocytes (Yu et al., 1983; Shank et al., 1985;Cesar and Hamprecht, 1995), and because intravenous administration of radiolabeled bicarbonate to rats leads to stronger labeling of glutamine than of glutamate (Waelsch et al., 1964), a sign of astrocytic metabolism (Van den Berg et al., 1969; Hassel et al., 1992). The resulting conclusion has been that astrocytes maintain a net export of glutamine to glutamatergic neurons and that these neurons are completely dependent on astrocytes for neurotransmission. It is suspected, however, that the neuronal uptake of glutamine is too low to account for all the transmitter glutamate being released (Hertz, 1979).
The purpose of the present study was to determine whether pyruvate carboxylation can occur in neurons and whether such carboxylation may support formation of transmitter glutamate. We incubated primary cultures of cerebellar granule cells (neurons) and cerebellar astrocytes with 14C-labeled bicarbonate or [1-14C]pyruvate, and analyzed the radiolabeling of amino acids in the cells. [1-14C]Pyruvate may be metabolized by pyruvate dehydrogenase, in which case the labeled carboxylic group is lost as 14CO2, or via carboxylation, in which case the radiolabeled carboxylic group is conserved in TCA cycle intermediates and related amino acids (Fig.1). Labeling of aspartate, glutamate, and glutamine from [1-14C]pyruvate therefore reflects pyruvate carboxylation. We used 3-nitropropionic acid to block the TCA cycle at the level of succinate dehydrogenase (Alston et al., 1977), leaving pyruvate carboxylation the only route for14C to enter the TCA cycle (Fig. 1). The labeling patterns obtained with [1-14C]pyruvate were compared to those obtained with [2-14C]pyruvate, which labels amino acids via both pyruvate carboxylation and pyruvate dehydrogenase. Furthermore, we injected NaH14CO3, [1-14C]pyruvate, and [2-14C]pyruvate into rat striatum and compared the radiolabeling of amino acids to that obtained with the astrocyte-specific substrate [1-14C]acetate (Hassel et al., 1992,1997; Waniewski and Martin, 1998) to determine whether pyruvate carboxylation occurs in neurons in vivo.
Fig. 1.
Simplified representation of the tricarboxylic acid cycle showing how 14CO2 from radiolabeled bicarbonate enters the cycle via pyruvate carboxylation (bottom left) and leaves the cycle by decarboxylation of α-ketoglutarate (α-kg; bottom right). Aspartate and glutamate are labeled via oxaloacetate and α-ketoglutarate, respectively. Entry of 14C from [1-14C]pyruvate into the cycle also requires carboxylation, because formation of acetyl∼CoA leads to loss of the labeled carboxylic group as CO2 (top). When the middle carbon of pyruvate is labeled ([2-14C]pyruvate), 14C may enter the cycle via both carboxylation and formation of acetyl∼CoA. Note that for [1-14C]pyruvate to label α-ketoglutarate, the malate formed by pyruvate carboxylation must equilibrate with the symmetrical fumarate to have the 14C randomized between the two carboxylic groups. Without such randomization, the label will be lost as CO2 in the step between citrate and α-ketoglutarate. The asterisk indicates the site of action of 3-nitropropionic acid, an inhibitor of succinate dehydrogenase.
MATERIALS AND METHODS
Cell culture. Primary cultures of cerebellar granule cells and astrocytes were prepared from 8-d-old rats, as previously described (Hertz et al., 1989; Schousboe et al., 1989; Hassel et al., 1995) and grown in culture dishes with a diameter of 90 mm. The concentration of glutamine in the culture medium was 0.2 mm.
14C labeling of amino acids. Radiolabeled compounds were from New England Nuclear (Boston, MA). For radiolabeling of intracellular amino acids, cell cultures were incubated in a buffer consisting of (in mm): NaCl 120, KCl 4, NaH2PO4 1.2, MgCl2 1, CaCl2 1, NaHCO3 25, and glucose 5, pH 7.3. [1-14C]Pyruvate (13 μCi/μmol) or [2-14C]pyruvate (17.5 μCi/μmol) was added to 80 μm. In experiments with radiolabeled bicarbonate, NaH14CO3 (3.4 μCi/μmol) was present at a concentration of 25 mm. When present, 3-nitropropionic acid (Sigma, St. Louis, MO) was added to the incubation medium at a concentration of 1 mm. Cultures were incubated with the radiolabeled substrates for 60 min at 37°C in an atmosphere of 95% air and 5% CO2, at 100% relative humidity; then the cells were washed once with PBS, pH 7.3, before being harvested in 250 μl of 3.5% perchloric acid with 200 μm α-aminoadipate as internal standard. Protein was removed by centrifugation and measured according to Lowry et al. (1951). The supernatant was neutralized with 1 m KOH, and amino acids and their radiolabeling were analyzed as reported (Hassel et al., 1992, 1997).
Glutamate release. For labeling of releasable glutamate, cerebellar granule cell cultures were preincubated with 50 mm sodium fluoroacetate (Sigma) for 2 hr at 37°C to inhibit any astrocytic TCA cycle activity (Swanson and Graham, 1994;Hassel et al., 1997). In this buffer NaCl was 70 mm; the other components were the same as in the above incubation buffer. The cells were then incubated for 30 min at 37°C with [1-14C]pyruvate, 13 μCi/μmol, 200 μm, or NaH14CO3, 6.8 μCi/μmol, 25 mm, in incubation buffer. The cultures were washed twice with incubation buffer without radiolabel (each wash 5 min), then depolarized (5 min) with a buffer containing 56 mm KCl and 68 mm NaCl; the buffer was otherwise identical to the incubation buffer. When CaCl2 was omitted from the wash buffers and the depolarization buffer, it was replaced stoichiometrically by MgCl2.
Enzyme assays. Reagents for the enzyme assays were from Sigma. Malic enzyme, citrate synthase, fumarase, and α-ketoglutarate dehydrogenase were analyzed as described (Hill and Bradshaw, 1969; Hsu and Lardy, 1969; Srere, 1969; Mastrogiacomo et al., 1993) in homogenates of cell cultures obtained by harvesting cells in 1 ml sucrose, 0.32 m, or in 5% homogenates of rat striatum in sucrose, 0.32 m. Succinate dehydrogenase activity was analyzed by formation of formazan from a tetrazolium salt (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide;Kugler et al., 1988); formazan was extracted and assayed spectrophotometrically at 570 nm (Hansen et al., 1989).
In vivo experiments. Male Wistar rats, 3-months-old, were used for stereotactic injection of 1 μCi of [1-14C]pyruvate, [2-14C]pyruvate (specific activities as above), or [1-14C]acetate (2 μCi/μmol), or 2 μCi of NaH14CO3 (6.8 μCi/μmol), as previously described (Hassel et al., 1992). Animals were handled in strict accordance with institutional and national ethical guidelines. Anesthesia was, per kilogram of bodyweight: fentanyl, 0.2 mg; fluanisone, 10 mg (Hypnorm; Janssen Biochimica, Berse, Belgium); and midazolam, 5 mg (Dormicum, Roche Products, Hertforshire, UK) (Hassel et al., 1994). NaH14CO3 was dissolved in double-distilled water to 150 mm, and pH was adjusted to 7.3; the other radiolabeled compounds were dissolved in the buffer described above (0.5 μCi/μl), and 2 μl were injected over 4 min. The tissue was sampled 5 min after completion of the injection and extracted with perchloric acid and KOH (Hassel et al., 1997). All tissue extracts and media that were analyzed for radiolabeling of amino acids were lyophilized to dryness and resuspended in 60 μm double-distilled water before analysis. Amino acids and their radiolabeling were analyzed as previously reported (Hassel et al., 1992, 1997).
RESULTS
Neuronal and astrocytic pyruvate carboxylationin vitro
14C-Labeled sodium bicarbonate labeled aspartate and glutamate more avidly in neuronal cerebellar granule cells than in astrocytes (Table1). In cerebellar granule cells, glutamine was not detectably radiolabeled, whereas in the astrocytes it was strongly labeled. Similarly, incubation with [1-14C]pyruvate led to labeling of amino acids in the neuronal cultures, preferentially of aspartate, in agreement with entry of label into the TCA cycle at the level of malate and oxaloacetate. Again, glutamine was not detectably labeled. In astrocytes, however, glutamine was strongly labeled, as were aspartate and glutamate (Table 1).
Table 1.
Radiolabeling (dpm/mg protein) of amino acids from NaH14CO3, [1-14C]pyruvate or [2-14C]pyruvate in primary cultures of cerebellar granule cells and astrocytes
| Precursor | Specific activity | Aspartate | Glutamate | Glutamine |
|---|---|---|---|---|
| NaH14CO3 | 3.3 mCi/mmol | |||
| Granule cells | 1245 ± 210 | 2071 ± 120 | ND | |
| Astrocytes | 82.5 ± 13.0b | 758 ± 133b | 748 ± 40b | |
| [1-14C]Pyruvate | 13 mCi/mmol | |||
| Granule cells | 6170 ± 515 | 550 ± 50 | ND | |
| Astrocytes | 480 ± 136b | 1261 ± 339 | 778 ± 149b | |
| [2-14C]Pyruvate | 17.5 mCi/mmol | |||
| Granule cells | 23,000 ± 1960 | 176,630 ± 2470 | 5200 ± 1882 | |
| Astrocytes | 4608 ± 1138b | 32,896 ± 4223b | 17,278 ± 26611-a |
Primary cultures of cerebellar granule cells and astrocytes were incubated with NaH14CO3, [1-14C]pyruvate, or [2-14C]pyruvate for 60 min. Radiolabeling of intracellular amino acids was determined. Values are dpm/mg protein. Mean ± SEM of four or five experiments. NaHCO3 was 25 mm, and K+ was 4 mM in all experiments. ND, Not detectable.
p = 0.01;bp < 0.005, Student's t test; significantly different from corresponding value in granule cells.
To compare the carboxylation of pyruvate with its decarboxylation by the pyruvate dehydrogenase complex, we incubated cell cultures with [2-14C]pyruvate. The label in [2-14C]pyruvate can enter the TCA cycle via both carboxylation and dehydrogenation. The labeling of aspartate in the neuronal cultures from [1-14C]pyruvate was approximately one-fourth of that obtained with [2-14C]pyruvate (Table 1). When corrected for differences in the specific activity between the two [14C]pyruvates (13 and 17.5 μCi/μmol for [1-14C]pyruvate and [2-14C]pyruvate, respectively), aspartate labeling with [1-14C]pyruvate was 36% of that obtained with [2-14C]pyruvate, suggesting that pyruvate carboxylation accounts for approximately one-third of the total oxidative metabolism of pyruvate. It should be noted that for [1-14C]pyruvate to label glutamate and glutamine, the malate formed has to equilibrate with fumarate; otherwise the label will be lost as14CO2 in the isocitrate dehydrogenase step of the TCA cycle (between citrate and α-ketoglutarate; Fig. 1). The equilibration of malate with fumarate cannot be assumed to be complete, and therefore the importance of pyruvate carboxylation relative to decarboxylation cannot be assessed by comparing the labeling of glutamate from [1-14C]pyruvate and [2-14C]pyruvate.
The labeling of aspartate and glutamate from [2-14C]pyruvate was approximately five times higher in neurons than in astrocytes, indicating a more active TCA cycle metabolism in neurons (Table 1). [2-14C]Pyruvate labeled glutamine in the neuronal cultures, but only at the expected level of <5% of the labeling of glutamate, in agreement with an astrocytic contamination of neuronal cultures of <5% (Schousboe et al., 1989; Hassel et al., 1995).
The maximal activity of malic enzyme in neuronal cultures was 2.5 times greater in the carboxylating than in the decarboxylating direction (p < 0.01; paired t test; Table2). In the astrocytic cultures, the decarboxylating activity was 3.7 times greater than the carboxylating activity (p < 0.01; paired t test; Table 2). The carboxylating activity of malic enzyme in neurons was almost six times higher than the activity of α-ketoglutarate dehydrogenase, which is the rate-limiting enzyme in the cerebral TCA cycle (Lai et al., 1977) and which had the lowest activity of the TCA cycle enzymes measured (Table 2).
Table 2.
Maximal activities of malic enzyme, citrate synthase, α-ketoglutarate dehydrogenase, and fumarase in cultured cerebellar granule cells and astrocytes, and in rat striatum
| Malic enzyme | Citrate synthase | α-Ketoglutarate dehydrogenase | Fumarase | ||
|---|---|---|---|---|---|
| Carboxylating | Decarboxylating | ||||
| Granule cells | 35 ± 5 | 14 ± 3 | 880 ± 60 | 5.9 ± 1.7 | 720 ± 80 |
| Astrocytes | 10 ± 2* | 37 ± 5* | 420 ± 40* | 7.6 ± 0.4 | 230 ± 40* |
| Rat striatum | 7.2 ± 1.2 | 33.7 ± 5.5 | 685 ± 68 | 11.8 ± 0.9 | 141 ± 24 |
Values are nmol/mg protein × min−1, mean ± SEM. *Different from values in cerebellar granule cells; p < 0.005, Student's t test.
Labeling of releasable glutamate via pyruvate carboxylation
Depolarization with and without calcium led to release of 10.6 ± 0.3 and 2.9 ± 0.3 nmol of glutamate in 2 ml of buffer, respectively. During the preceding 5 min wash only 1.2 ± 0.2 nmol of glutamate accumulated in the medium, whether calcium was present or not. Thus, potassium depolarization caused an 8.4-fold increase in glutamate release, of which 73% appeared to be calcium-dependent. The released glutamate had a specific activity of 39.3 ± 3.0 dpm/nmol when NaH14CO3 was the labeled precursor, and 33.4 ± 1.1 dpm/nmol when [1-14C]pyruvate was the labeled precursor. The specific activity of the intracellular glutamate was 31.3 ± 2.6 dpm/nmol and 14.6 ± 0.5 dpm/nmol with NaH14CO3 and [1-14C]pyruvate, respectively. In these experiments the granule cells were pretreated with fluoroacetate (50 mm for 2 hr), a specific inactivator of aconitase of the astrocytic TCA cycle, to eliminate any minor astrocytic contribution to the labeling of glutamate (Swanson et al., 1994; Hassel et al., 1997).
Neuronal pyruvate carboxylation during inhibition of the TCA cycle
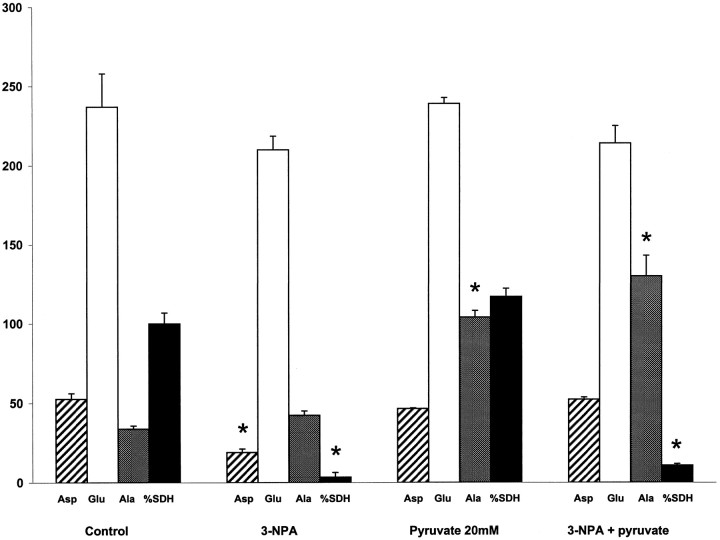
Incubation of cerebellar granule cells with 3-nitropropionic acid (1 mm) led to an inhibition of succinate dehydrogenase of 93.4 ± 2.2% at 15 min, and to almost complete inhibition at 30 min (Fig. 2; compare Fig. 1); this caused the level of aspartate to fall to 36% of control (Fig. 2). Formation of [14C]aspartate from [1-14C]pyruvate was virtually unaffected, however, so that the specific activity of aspartate increased 2.5 times (Table 3). Incubation of cerebellar granule cells with 20 mm sodium pyruvate in the presence of 1 mm 3-nitropropionic acid restored the level of aspartate to control values in spite of a ∼90% inhibition of succinate dehydrogenase (Fig. 2). This result indicates rapidde novo synthesis of malate and oxaloacetate via pyruvate carboxylation during inhibition of succinate dehydrogenase.
Fig. 2.
Levels of amino acids and activity of succinate dehydrogenase in cerebellar granule neurons incubated with 3-nitropropionic acid, an inhibitor of succinate dehydrogenase (SDH) and 20 mm sodium pyruvate. The SDH activity (% of control) was measured after 30 min of incubation, whereas amino acids (nanomoles per milligram of protein) were measured after 60 min of incubation. Values are mean ± SEM of four or five culture dishes. *Difference from control, p < 0.05, one-way ANOVA, Bonferroni's correction for multiple comparison.Asp, Aspartate; Glu, glutamate;Ala, alanine.
Table 3.
Specific activities of amino acids in cerebellar granule cells incubated with [1-14C]pyruvate or [2-14C]pyruvate in the presence and absence of 3-nitropropionic acid
| Aspartate | Glutamate | Alanine | |
|---|---|---|---|
| [1-14C]Pyruvate, Control | 105 ± 24 | 7.7 ± 2.7 | 134 ± 10 |
| [1-14C]Pyruvate, 3-NPA | 249 ± 36* | 5.7 ± 2.0 | 64.0 ± 20.6* |
Cerebellar granule cells were incubated for 60 min with [1-14C]pyruvate and 3-nitropropionic acid (3-NPA), an inhibitor of succinate dehydrogenase. Values are dpm/nmol, means ± SEM. *Different from control value; p < 0.05, Student's t test.
Neuronal pyruvate carboxylation in vivo
Five minutes after injection of NaH14CO3 into the striatum of anesthetized rats, the specific activity of aspartate was 3.5 times higher than that of glutamate, confirming that the14C entered the TCA cycle via carboxylation of pyruvate to malate and oxaloacetate (Fig. 1). The specific activity of glutamine was ∼60% of that of glutamate, giving a glutamine/glutamate relative specific activity of 0.6 (Table4), which indicates predominantly neuronal pyruvate carboxylation (Van den Berg et al., 1969; Hassel et al., 1992).
Table 4.
Specific activity of cerebral glutamine, aspartate, and alanine relative to that of glutamate after intrastriatal injection of [1-14C]pyruvate, NaH14CO3, [2-14C]pyruvate, or [1-14C]acetate
| Glutamine/ Glutamate | Aspartate/ Glutamate | Aspartate/ Alanine | |
|---|---|---|---|
| NaH14CO3 | 0.59 ± 0.07 | 3.5 ± 0.9 | — |
| [1-14C]Pyruvate | 0.41 ± 0.06 | 4.0 ± 0.5* | 0.8 ± 0.11 |
| [2-14C]Pyruvate | 0.41 ± 0.04 | 0.4 ± 0.1 | 0.5 ± 0.07 |
| [1-14C]Acetate | 2.5 ± 0.3* | 0.3 ± 0.1 | — |
Anesthetized Wistar rats received 1 μCi of NaH14CO3, [1-14C]pyruvate, [2-14C]pyruvate, or [1-14C]acetate stereotactically into the striatum over 4 min, and the tissue was sampled 5 min after completion of the injection. [1-14C]Acetate was included to demonstrate the difference in labeling from a substrate that is metabolized by neurons (bicarbonate, pyruvate) and one that is metabolized by astrocytes (acetate). NaH14CO3 and [1-14C]acetate did not label alanine, hence no value for the aspartate/alanine relative specific activity. Values are means ± SEM of five to seven experiments. *Difference from the values of the other groups; p < 0.05, one-way ANOVA, Bonferroni's method for multiple comparison.
Injection of [1-14C]pyruvate also gave a higher specific activity in glutamate than in glutamine, indicating predominantly neuronal carboxylation of [1-14C]pyruvate, and a four times higher specific activity in aspartate than in glutamate, in agreement with entry of label at the level of malate and oxaloacetate (Table 4). The aspartate–alanine relative specific activity was close to one, suggesting a high activity of neuronal pyruvate carboxylation in vivo, because the injected [1-14C]pyruvate equilibrates rapidly with alanine via transamination. Injection of [2-14C]pyruvate gave the expected low glutamine–glutamate relative specific activity, which indicates neuronal metabolism of pyruvate, but the aspartate/glutamate relative specific activity became lower than one, in agreement with a large flux of radiolabel into the TCA cycle via pyruvate dehydrogenase (Table 4, Fig. 1).
For comparison, [1-14C]acetate, which is known to enter astrocytes selectively and rapidly (Hassel et al., 1992;Hassel and Sonnewald, 1995; Waniewski and Martin, 1998), was injected into rat striatum. In contrast to14C-labeled pyruvate, [1-14C]acetate led to a higher specific activity of glutamine than of glutamate, confirming that the metabolism of the injected [14C]pyruvates took place primarily in neurons.
The maximal carboxylating activity of malic enzyme in rat striatum was ∼60% of the α-ketoglutarate dehydrogenase activity, and 20% of the decarboxylating activity of the enzyme (Table2).
DISCUSSION
The present study shows that neurons are capable of pyruvate carboxylation in vivo as well as in vitro. Thein vitro results show that neuronal pyruvate carboxylation leads to de novo synthesis of TCA cycle intermediates and that it supports formation of transmitter glutamate. Neuronal pyruvate carboxylation was highly active, accounting for approximately one-third of the oxidative metabolism of pyruvate in vitro. Although the activity of neuronal carboxylation in vivo is difficult to calculate accurately, the high radiolabeling of aspartate with [1-14C]pyruvate relative to that of alanine in rat striatum suggests that the in vivo activity is high as well. This means that glutamatergic neurons, or at least subpopulations of glutamatergic neurons, to a large extent may be independent of glutamine from astrocytes for the formation of transmitter glutamate, because the neurons themselves are capable of compensating for the loss of α-ketoglutarate inherent in glutamatergic neurotransmission. The present findings probably explain the long-standing enigma of how loss of glutamate during neurotransmission can exceed the neuronal uptake of glutamine (Hertz, 1979).
The finding that radiolabeled bicarbonate is primarily metabolized by neurons when injected intracerebrally (this study) and by astrocytes when injected intravenously (Waelsch et al., 1964) is probably explained by the mode of administration. When injected intravenously, the bicarbonate enters the brain by passing through the astrocytic end feet that surround brain capillaries, thereby being exposed primarily to the astrocytic pyruvate-carboxylating enzymes, whereas the intracerebral administration bypasses this astrocytic interphase. The previous finding that the enzyme pyruvate carboxylase has a strictly astrocytic expression in the brain (Yu et al., 1983; Shank et al., 1985; Cesar and Hamprecht, 1995) has been a main reason for ascribing all cerebral pyruvate carboxylation to astrocytes. However, mitochondrial malic enzyme was recently reported to be expressed in neurons (Vogel et al., 1998). The authors anticipated a decarboxylating role for the enzyme, although in one study it was shown to possess significant carboxylating activity (Salganicoff and Koeppe, 1968). The view of astrocytes as the only pyruvate-carboxylating compartment in the brain apparently received support from a study on14CO2 fixation in cultured neurons and astrocytes (Kaufman and Driscoll, 1992), which found a much higher14CO2 fixation (i.e., carboxylation) in astrocytes. In that study the radiolabeled metabolites were not identified, however, making it impossible to deduce by which enzymatic pathway the carboxylation occurred. Furthermore, the unusually low concentration (5 mm) of radiolabeled bicarbonate used may have favored astrocytic pyruvate carboxylase, because Km for bicarbonate is 1 mm for pyruvate carboxylase (Scrutton et al., 1969) and 13 mm for malic enzyme (Hsu and Lardy, 1969).
Tracer studies alone cannot clearly distinguish between net synthesis and exchange, and malic enzyme, which was shown to be a likely mediator of the neuronal pyruvate carboxylation in this study, is a reversible enzyme (Hsu and Lardy, 1969) that allows exchange of radiolabeled for unlabeled pyruvate or bicarbonate. The finding that a high concentration of pyruvate reversed the depletion of aspartate caused by 3-nitropropionic acid indicates that neuronal pyruvate carboxylation provides a net synthesis of TCA cycle intermediates, a conclusion that is supported by the observation that malic enzyme in cultured neurons (in contrast to the astrocytic isozyme) was more active in the carboxylating than in the decarboxylating direction. The effect of pyruvate supplementation on aspartate levels in the presence of 3-nitropropionic acid should not be taken to imply that pyruvate carboxylation subserves the replenishment of the aspartate pool rather than of the glutamate pool. Like the avid14C-labeling of aspartate from [1-14C]pyruvate and NaH14CO3, this effect merely reflects the biochemical proximity of aspartate formation and pyruvate carboxylation; the levels of aspartate and glutamate depend on the integrity of the same TCA cycle (Fig. 1).
There is little doubt that glutamine from astrocytes may act as a precursor for neuronal glutamate and GABA (Hamberger et al., 1979;Hassel et al., 1997). However, it was recently shown that glutaminase, which converts glutamine into glutamate in neurons, is present at much lower levels in some glutamatergic pathways than in others (Laake et al., 1998). This finding supports our view that transmitter glutamate may be formed via enzymatic pathways that do not involve glutamine metabolism. The anaplerotic ability of neurons may allow for greater flexibility in the neuronal response to a varying metabolic demand, whether this is caused by the need for extra ATP or rapid neurotransmitter formation during neuronal activity.
Footnotes
The assistance of Mr. Gunnar Skogan in preparing cell cultures is gratefully acknowledged.
Correspondence should be addressed to Bjørnar Hassel, Norwegian Defense Research Institute, Division for Environmental Toxicology, P.O. Box 25, N-2027 Kjeller, Norway. E-mail: bjornar.hassel@ffi.no.
REFERENCES
- 1.Alston TA, Mela L, Bright HJ. 3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase. Proc Natl Acad Sci USA. 1977;74:3767–3771. doi: 10.1073/pnas.74.9.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesar M, Hamprecht B. Immunocytochemical examination of neural rat and mouse primary cultures using monoclonal antibodies raised against pyruvate carboxylase. J Neurochem. 1995;64:2312–2318. doi: 10.1046/j.1471-4159.1995.64052312.x. [DOI] [PubMed] [Google Scholar]
- 3.Danbolt NC, Chaudhry FA, Dehnes Y, Lehre KP, Levy LM, Ullensvang K, Storm-Mathisen J. Properties and localization of glutamate transporters. Prog Brain Res. 1998;116:23–43. doi: 10.1016/s0079-6123(08)60428-8. [DOI] [PubMed] [Google Scholar]
- 4.Hamberger A, Chiang GH, Nylén ES, Scheff SW, Cotman CW. Glutamate as a CNS transmitter. I. Evaluation of glucose and glutamine as precursors for the synthesis of preferentially released glutamate. Brain Res. 1979;168:513–530. doi: 10.1016/0006-8993(79)90306-8. [DOI] [PubMed] [Google Scholar]
- 5.Hansen M, Nilsen SE, Berg K. Re-examination and future development of a precise and rapid dye for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 6.Hassel B, Sonnewald U. Glial formation of pyruvate and lactate from TCA cycle intermediates. Implications for the inactivation of transmitter amino acids? J Neurochem. 1995;65:2227–2234. doi: 10.1046/j.1471-4159.1995.65052227.x. [DOI] [PubMed] [Google Scholar]
- 7.Hassel B, Paulsen RE, Johnsen A, Fonnum F. Selective inhibition of glial cell metabolism in vivo by fluorocitrate. Brain Res. 1992;576:120–124. doi: 10.1016/0006-8993(92)90616-h. [DOI] [PubMed] [Google Scholar]
- 8.Hassel B, Grini Iversen E, Fonnum F. Neurotoxicity of albumin in vivo. Neurosci Lett. 1994;167:29–32. doi: 10.1016/0304-3940(94)91020-0. [DOI] [PubMed] [Google Scholar]
- 9.Hassel B, Westergaard N, Schousboe A, Fonnum F. Metabolic differences between primary cultures of astrocytes and neurons from cerebellum and cerebral cortex. Effects of fluorocitrate. Neurochem Res. 1995;20:413–420. doi: 10.1007/BF00973096. [DOI] [PubMed] [Google Scholar]
- 10.Hassel B, Bachelard HS, Jones P, Fonnum F, Sonnewald U. Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. J Cereb Blood Flow Metab. 1997;17:1230–1238. doi: 10.1097/00004647-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Henn FA, Goldstein MN, Hamberger A. Uptake of the neurotransmitter candidate glutamate by glia. Nature. 1974;249:663–664. doi: 10.1038/249663a0. [DOI] [PubMed] [Google Scholar]
- 12.Hertz L. Functional interactions between neurons and astrocytes. I. Turnover and metabolism of putative amino acid transmitters. Prog Neurobiol. 1979;13:277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- 13.Hertz L, Juurlink BH, Hertz E, Fosmark H, Schousboe A. Preparation of primary cultures of mouse (rat) astrocytes. In: Shahar A, de Vellis J, Vernandakis A, Haber B, editors. A dissection and tissue culture manual for the nervous system. Alan R. Liss; New York: 1989. pp. 105–108. [Google Scholar]
- 14.Hill RL, Bradshaw RA. Fumarase. In: Lowenstein JM, editor. Methods in enzymology, Vol 13. Academic; New York: 1969. pp. 91–99. [Google Scholar]
- 15.Hsu RY, Lardy HA. Malic enzyme. In: Lowenstein JM, editor. Methods in enzymology, Vol 13. Academic; New York: 1969. pp. 230–235. [Google Scholar]
- 16.Kaufman EE, Driscoll BF. Carbon dioxide fixation in neuronal and astroglial cells in culture. J Neurochem. 1992;58:258–262. doi: 10.1111/j.1471-4159.1992.tb09304.x. [DOI] [PubMed] [Google Scholar]
- 17.Kugler P, Vogt S, Gehm M. Quantitative succinate dehydrogenase histochemistry in the hippocampus of aged rats. Histochemistry. 1988;88:299–307. doi: 10.1007/BF00570287. [DOI] [PubMed] [Google Scholar]
- 18.Laake JH, Takum Y, Eide J, Torgner IA, Roberg B, Kvamme E, Ottersen OP. Postembedding immunogold labelling reveals subcellular localization and pathway-specific enrichment of phosphate-activated glutaminase in rat cerebellum. Neuroscience. 1998;88:1137–1151. doi: 10.1016/s0306-4522(98)00298-x. [DOI] [PubMed] [Google Scholar]
- 19.Lai JCK, Walsh JM, Dennis SC, Clark JB. Synaptic and non-synaptic mitochondria from rat brain: isolation and characterization. J Neurochem. 1977;28:625–631. doi: 10.1111/j.1471-4159.1977.tb10434.x. [DOI] [PubMed] [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- 22.Mastrogiacomo F, Bergeron C, Kish SJ. Brain α-ketoglurate dehydrogenase complex activity in Alzheimer's disease. J Neurochem. 1993;61:2007–2014. doi: 10.1111/j.1471-4159.1993.tb07436.x. [DOI] [PubMed] [Google Scholar]
- 23.McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR. Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem. 1996;66:386–393. doi: 10.1046/j.1471-4159.1996.66010386.x. [DOI] [PubMed] [Google Scholar]
- 24.Patel MS. The relative significance of CO2-fixing enzymes in the metabolism of rat brain. J Neurochem. 1974;22:717–724. doi: 10.1111/j.1471-4159.1974.tb04285.x. [DOI] [PubMed] [Google Scholar]
- 25.Pines G, Danbolt NC, Bjørås M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner B. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 26.Salganicoff L, Koeppe RE. Subcellular distribution of pyruvate carboxylase, diphosphopyridine nucleotide and triphosphopyridine nucleotide isocitrate dehydrogenase, and malate enzyme in rat brain. J Biol Chem. 1968;243:3416–3420. [PubMed] [Google Scholar]
- 27.Schousboe A, Meier E, Drejer J, Hertz L. Preparation of primary cultures of mouse (rat) cerebellar granule cells. In: Shahar A, de Vellis J, Vernandakis A, Haber B, editors. A dissection and tissue culture manual for the nervous system. Alan R. Liss; New York: 1989. pp. 203–206. [Google Scholar]
- 28.Scrutton MC, Olmsted MR, Utter MF. Pyruvate carboxylase from chicken liver. In: Lowenstein JM, editor. Methods in enzymology, Vol 13. Academic; New York: 1969. pp. 235–249. [Google Scholar]
- 29.Shank RP, Bennett GS, Freytag SO, Campbell GL. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res. 1985;329:364–367. doi: 10.1016/0006-8993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- 30.Srere P. Citrate synthase. In: Lowenstein JM, editor. Methods in enzymology, Vol 13. Academic; New York: 1969. pp. 3–11. [Google Scholar]
- 31.Swanson RA, Graham SH. Fluorocitrate and fluoroacetate effects on astrocyte metabolism in vitro. Brain Res. 1994;664:94–100. doi: 10.1016/0006-8993(94)91958-5. [DOI] [PubMed] [Google Scholar]
- 32.Van den Berg CJ, Krzalic LJ, Mela P, Waelsch H. Compartmentation of glutamate metabolism in brain. Biochem J. 1969;113:281–290. doi: 10.1042/bj1130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogel R, Jennemann G, Seitz J, Wiesinger H, Hamprecht B. Mitochondrial malic enzyme: purification from bovine brain, generation of an antiserum, and immunocytochemical localization in neurons of rat brain. J Neurochem. 1998;71:844–852. doi: 10.1046/j.1471-4159.1998.71020844.x. [DOI] [PubMed] [Google Scholar]
- 34.Waelsch H, Berl S, Rossi CA, Clarke DD, Purpura DP. Quantitative aspects of CO2 fixation in mammalian brain in vivo. J Neurochem. 1964;11:717–728. doi: 10.1111/j.1471-4159.1964.tb06117.x. [DOI] [PubMed] [Google Scholar]
- 35.Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu ACH, Drejer J, Hertz L, Schousboe A. Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J Neurochem. 1983;41:1484–1487. doi: 10.1111/j.1471-4159.1983.tb00849.x. [DOI] [PubMed] [Google Scholar]