Abstract
Dual-probe microdialysis (with HPLC and electrochemical detection) in freely moving rats and single-unit recording in anesthetized rats were used to study the extent to which impulse flow through the ventral tegmental area (VTA) contributes to elevations in nucleus accumbens (NAS) dopamine (DA) evoked by stimulation of the ventral subiculum (VS). During perfusion of artificial extracellular fluid into the VTA, injections of 0.74 μg of the excitatory amino acid NMDA into the VS elevated accumbens DA to >150% of basal values. During intra-VTA perfusion of either 1 μm tetrodotoxin (which blocks impulse flow) or 1 mm kynurenic acid (which blocks excitatory glutamate receptors), injections of NMDA into the VS failed to elevate accumbens DA. Thus, increased impulse flow through VTA DA neurons, mediated by excitatory glutamate inputs to this region, appears critical for VS stimulation to elevate NAS DA. Increased impulse flow through VTA DA neurons was confirmed using single-unit recording in anesthetized rats. Intra-VS NMDA injections increased the firing rates of 45% (14 of 31), decreased the firing rates of 13% (4 of 31), and had no effect on 42% (13 of 31) of VTA DA neurons. Increases in firing rates were evident within 15 min of NMDA injections, a time at which VS NMDA injections elevate accumbens DA in awake animals. The results of the present experiments identify the VTA as a critical site through which outputs from the VS modulate NAS dopaminergic neurotransmission.
Keywords: ventral subiculum, microdialysis, tetrodotoxin, single unit, NMDA, ventral tegmental area, accumbens, dopamine
Dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens septi (NAS) are involved in investigatory behaviors evoked by novel stimuli (Fink and Smith, 1980; Tagzhouti et al., 1985; Schultz and Romo, 1990; Ljungberg et al., 1992) and are thought to be involved in the reinforcement of adaptive investigatory approach evoked by naturally occurring rewards (Wise and Rompré, 1989; Schultz, 1998) and habit-forming drugs (Wise and Bozarth, 1987; Wise, 1996). Both NAS dopamine (DA) and investigatory behavior appear to be influenced by projections from the ventral subiculum (VS) of the hippocampus. Stimulation of the VS elevates NAS DA (Blaha et al., 1997; Brudzynski and Gibson, 1997;Legault and Wise, 1999) and increases investigatory behavior (Yang and Mogenson, 1987; Brudzynski and Gibson, 1997; Legault and Wise, 1999) that is abolished by disruption of dopaminergic transmission (Wu and Brudzynski, 1995; Brenner and Bardgett, 1998). There are at least two mechanisms through which projections from the VS may influence NAS DA. First, glutamate released from direct projections from the VS to the NAS (Walaas, 1981; Christie et al., 1987; Fuller et al., 1987;Totterdell and Smith, 1989; Sesack and Pickel, 1990b) may evoke impulse-independent DA release by actions on dopaminergic terminals (Romo et al., 1986a,b; Glowinski et al., 1988; Blaha et al., 1997;Brudzynski and Gibson, 1997). The idea that glutamate might mediate transmitter release from dopaminergic terminals has been studied largely in the context of prefrontal cortex (PFC) stimulation, which elevates NAS DA (Murase et al., 1993; Taber and Fibiger, 1995; Karreman and Moghaddam, 1996). Although the PFC sends glutamatergic projections to both the NAS (Christie et al., 1987; Fuller et al., 1987; Sesack et al., 1989) and to the VTA (Christie et al., 1985; Sesack et al., 1989;Sesack and Pickel, 1990a), recent microdialysis studies suggest that it is the projections to the VTA that are critical for PFC-evoked elevations in NAS DA (Taber et al., 1995; Karreman and Moghaddam, 1996;Rossetti et al., 1998; You et al., 1998).
Alternately, projections from the VS may indirectly influence dopamine release in the NAS by influencing the firing rates of VTA dopaminergic neurons. Although the VS is not known to project directly to the VTA, we have recently found that NMDA injection into the VS elevates VTA as well as NAS DA (Legault and Wise, 1999). VS-induced elevations in VTA DA are assumed to reflect dendritic transmitter release resulting from increased impulse flow through dopaminergic neurons, raising the possibility that the VS can activate VTA dopaminergic neurons trans-synaptically.
The present experiments were designed to determine if synaptic input to the VTA constitutes a critical link in the circuitry through which the hippocampus modulates NAS DA. First, dual-probe microdialysis was used to determine if elevations in NAS DA evoked by intra-VS injections of NMDA are (1) dependent on impulse-flow through the VTA, and (2) mediated by activation of glutamate receptors in the VTA. In a second experiment, action potentials from VTA dopaminergic neurons were recorded in anesthetized rats, and firing rates were monitored before, during, and after NMDA injections into the VS.
MATERIALS AND METHODS
Subjects
Seventy-one male Long–Evans rats (Charles River, St. Constant, Quebec, Canada) were used for these experiments. The rats, weighing between 300 and 400 gm at the time of surgery, were housed in pairs before and individually after surgery. They were maintained on a 12 hr light/dark cycle. Food and water were available ad libitum.
Microdialysis studies
Surgery. Twenty-six rats were anesthetized with pentobarbital (60 mg/kg, i.p.) and placed in a stereotaxic frame. Each animal was implanted unilaterally with three guide cannulae; an 18 gauge cannula designed to guide a microdialysis probe was aimed at each the NAS and VTA, and a 22 gauge guide cannula was aimed at the VS. To maximize the distance between cannulae, NAS and VS cannulae were implanted with the incisor bar elevated 5 mm above the interaural line, whereas VTA cannulae were implanted with the incisor bar adjusted to set bregma and lambda at the same horizontal level. Coordinates for NAS cannulae were as follows: 3.2 mm anterior to bregma, 2.4 mm lateral to the saggital suture, and 3.0 mm below dura (NAS cannulae were angled 10° toward the midline to avoid penetrating the lateral ventricle; the vertical coordinate refers to the distance along this vector). Coordinates for VS and VTA cannulae were, respectively: anteroposterior (AP), −3.2 mm; lateral (L), 4.8 mm; ventral (V), −6.8 mm; and AP, −5.0 mm; L, 1.1; V, −3.8 mm. Probe cannulae were fitted with obturators that were flush with the cannula tip. Injection cannulae were fitted with obturators that extended 1 mm beyond the cannula tip. Cannula assemblies were secured in place with dental cement, and four stainless steel screws were threaded into the skull. After recovery from anesthesia, each animal was returned to the colony room for at least 7 d before implantation of microdialysis probes.
Microdialysis procedure. Concentric microdialysis probes were constructed such that a length of dialysis membrane (Hospal AN69; molecular weight cutoff, 40 kDa) extended 4 mm (NAS) or 5 mm (VTA) beyond the tip of a 22 gauge stainless steel shaft that ended flush with the bottom of each guide cannula. Epoxy cement coated the external membrane surface for 1 mm beyond the guide cannula (NAS probes) or 3 mm beyond the guide cannula (VTA probes). Lengths of fused silica tubing (inner diameter, 75 μm; outer diameter, 150 μm) were used for both the fluid inlet and outlet. The fluid inlet terminated at the distal end of the probe near the membrane tip. The fluid outlet originated just inside the probe shaft. Both the inlet and outlet were glued with epoxy cement to the top of the probe shaft. Microdialysis probes were inserted into the brain at least 18 hr before the beginning of any experiment. Each animal was anesthetized with a low dose of sodium pentobarbital (30 mg/kg, i.p.), and probes were inserted and fixed in place with dental cement. Probes were connected to a dual-channel liquid swivel and continuously perfused with a solution of artificial CSF (aCSF) using a microdialysis pump (Harvard); flow rate was set at 1.0 μl/min. The aCSF comprised 2.0 mm Sorenson's phosphate buffer containing (in mm): 145 NaCl, 2.8 KCl, 1.2 MgCl, 1.2 CaCl, and 0.2 ascorbate, pH 7.3–7.4.
Experiments designed to determine the effects of intra-VTA dialysis of TTX (1 μm) or kynurenic acid (KYN, 1 mm) on elevations in NAS DA evoked by intra-VS injections of NMDA were performed over a 2 d period. On day 1, either TTX or KYN was infused into the VTA before, during, and after injections of NMDA into the VS. NAS dialysate samples were first collected during dialysis of aCSF into the VTA. Consecutive 15 min (15 μl) samples were collected until DA content (in picograms) varied by <15% for 90 min (six samples). The mean DA content of the last six samples was defined as baseline. The VTA perfusate (aCSF) was then replaced with either KYN or TTX, and 20 min were then allowed for flow rates to stabilize before collection of the next sample. After this stabilization period, either four (KYN test) or six (TTX test) consecutive samples were then collected from the NAS probe. NMDA (0.74 μg in 0.5 μl, injected in 1 min) or vehicle (aCSF) was then injected into the VS, and NAS dialysate samples were collected for 2 hr. Finally, 2 hr after the NMDA injection, KYN or TTX solutions were replaced with aCSF. Animals that had received intra-VS NMDA injections on day 1 were tested again on day 2. On this day, both NAS and VTA probes were perfused with aCSF. Baseline samples from the NAS were collected as described above; NMDA was then injected into the VS, and NAS dialysate samples were collected for 2 hr. This test allowed us to confirm the effectiveness of the site of injection into the VS.
Analytical procedure. Dialysate samples were analyzed on-line for dopamine content by HPLC with electrochemical detection. Dopamine was isolated using a reverse-phase column (Supelco; supelcosil, 3 mm, LC-18) and quantified using an ESA Coulochem II detector (model 5200) and an analytical cell (ESA model 5011) with two electrodes in series. The potential of the first (oxidizing) electrode was set at 340 mV (500 nA) and second electrode (reduction) was set at −270 mV (5 nA). The mobile phase (in deionized water) consisted of 60 mmNaH2PO4, 3.0 mmascorbate, 15% v/v MEOH, 0.035 mm SDS, and 0.1 mm EDTA with a pH adjusted to 3.5 using NaOH. The detection threshold for DA was at least 0.5 pg.
Single-unit recording of VTA DA neurons
Forty-five rats were used for electrophysiological experiments. Each animal was anesthetized with urethane (1.2 gm/kg, i.p.) and mounted in a stereotaxic frame. The surface of the skull was exposed, and a 22 gauge guide cannula was implanted into the VS at the coordinates previously mentioned. The cannula was secured with wax to two stainless steel screws threaded into the skull. A 28 gauge injection cannula containing an NMDA solution was inserted into the guide cannula and left in place for the remainder of the experiment. The incisor bar was then lowered to set bregma and lambda to the same horizontal plane. The bone and dura above the VTA ipsilateral to the VS injection cannula were removed. A glass micropipette (1–2 μm tip diameter; impedance, 2–7 MΩ at 1000 Hz) filled with Pontamine sky blue (0.2% w/v) was lowered into the VTA by hydraulic microdrive. Dopamine neuron action potentials were identified according to classical criteria of Bunney and Grace (1978): (1) initially positive-going biphasic or triphasic action potentials with a large second negative segment and a duration longer than 2.5 msec; (2) slow, irregular basal firing rate (1–8 Hz); (3) location in the VTA. Dopamine action potentials were isolated from noise using the Cluster Cutting module of the Discovery software package (DataWave Technologies). Once a dopaminergic neuron was identified and isolated, baseline firing was recorded for at least 5 min. Either NMDA or aCSF was then injected into the VS, and action potentials were recorded for another 15 min. In four cases no injection was made, and the cell was recorded for at least 30 min to determine the magnitude of firing rate variation over time.
Drugs and intracranial injections
NMDA was dissolved in aCSF at a concentration of 1.48 μg/μl, and 0.5 μl was injected centrally, at a flow rate of 1 μl/min through a 28 gauge injection cannula. This dose of NMDA was chosen on the basis of a previous study, showing that it increases both DA in the ipsilateral NAS and VTA DA and locomotor activity in awake freely moving rats (Legault and Wise, 1999). Solutions of KYN and TTX were mixed fresh in aCSF and sonicated for at least 20 min immediately before perfusion through microdialysis probes. The concentration of kynurenic acid (KYN, 1 mm) was chosen on the basis of a previous study showing that intra-VTA application of this concentration via dialysis blocks elevations in NAS DA evoked by stimulation of the PFC (You et al., 1998). The concentration of TTX (1 μm) was chosen because it is on the lower end of the range of concentrations that reduce NAS DA when perfused either into the NAS (Westerink et al., 1987) or VTA (Taber et al., 1995; Karreman and Moghaddam, 1996).
Data analysis
For microdialysis experiments, basal DA was estimated by calculating the mean DA content (in picograms) in the six dialysate samples collected before experimental treatments. Each animal's mean baseline was then used to convert the DA content of each of its samples into a percentage of baseline value. For analysis of data from electrophysiological experiments, perievent time histograms with 10 sec bins were generated off-line. For each cell, mean firing rates were calculated for the 5 min preinjection period and for each of the 5 min periods that followed. The 5 min preinjection firing rate was taken as baseline. Postinjection values were then expressed as percentage of baseline firing rate. A cell was considered to have changed its firing rate if there was a minimum of 10% difference from baseline in at least two of the three postinjection periods.
Treatment effects between groups were determined using two-way repeated measures ANOVA with time as a repeated measure, and post hoccomparisons were made using Student's t test. Within-groups treatment effects were analyzed using one-way repeated measures ANOVA with Time as a repeated measure. Post hoc comparisons between baseline and treatment effects were made using Fisher's least significant difference test (LSD) with the level of statistical significance was set at p < 0.05.
Histology
At the end of electrophysiological experiments, the recording site was marked with Pontamine sky blue by passing a 20–40 nA cathodal current through the glass micropipette for 25 min. The brain was removed from each animal and stored in a 10% formalin solution. At the end of microdialysis experiments, each rat was deeply anesthetized by injection of sodium pentobarbital (60 mg/kg) and transcardially perfused with normal saline followed by a 10% formalin solution. All brains were stored in 10% formalin for at least 1 week and subsequently sliced in 20 μm sections with a microtome; recording, microdialysis, and injection sites were localized by examination under low magnification in a light microscope. The locations of microdialysis membranes, VS injection cannulae, and recording pipette tips are represented in Figure 1.
Fig. 1.

Representation of the locations of dialysis membranes, recording pipette tips, and injection cannula tips.Left, Locations of dialysis membranes and VS injection sites. For the sake of clarity, all dialysis and injection sites for animals treated with intra-VTA TTX are represented in theleft hemisphere, and all sites for animals treated with intra-VTA KYN are represented in the right hemisphere.Right, Locations of single-unit recording sites and VS injection sites. The number of lines anddots appears to be less than the total number of animals tested because of overlapping placements.
RESULTS
Effect of TTX perfusion into the VTA
Perfusion of TTX (1 μm) through VTA microdialysis probes decreased NAS DA in all animals. During application of TTX to the VTA, NAS DA levels decreased steadily until, by the fifth post-TTX sample, DA was reduced to <20% of baseline. DA levels remained below 20% of baseline throughout the remainder of the TTX infusion. In animals that were to receive NMDA injections into the VS, the mean baseline value of NAS DA was 0.57 (± 0.038) pg/μl and was decreased to a minimum of 0.074 pg/μl, whereas in animals that were to receive aCSF injections, the mean baseline value of 0.65 (± 0.057) pg/μl was decreased to a minimum of 0.097 pg/μl.
Perfusion of TTX into the VTA prevented the elevations in NAS DA normally induced (day 2 test) by NMDA injections into the VS (Fig.2). During TTX infusions, there were no detectable differences in NAS DA levels between animals that received intra-VS NMDA or intra-VS aCSF injections. Thus, at no point after NMDA injections (administered 90 min after the initiation of TTX perfusion) was NAS DA <20% of baseline.
Fig. 2.
Effect of NMDA injections into the VS on nucleus accumbens dopamine (mean percentage of baseline ± SEM) during TTX perfusion into the VTA (day 1) and during aCSF perfusion into the VTA on day 2. Microdialysis samples were collected every 15 min, and TTX perfusion was initiated after the sixth sample. TTX decreased NAS DA (F(8,19) = 122.0; p= 0.0001). Either NMDA or aCSF was injected into the VS 90 min after the initiation of TTX perfusion (after sample 12). NMDA had no effect on NAS DA; there was no difference NAS DA between animals injected with NMDA or aCSF (F(1,19) = 1.35;p = 0.1604) nor was there a difference between the sample collected immediately before the NMDA injection and any subsequent sample (Fisher's LSD, p > 0.05). Data from the day 2 test are shifted to the right on the abscissa to align with the NMDA injection; the first six symbols represent baseline samples, and subsequent symbols represent samples collected after NMDA injections. Note also that the VTA probe was perfused with aCSF rather than TTX on day 2. Filled symbols indicate significant differences from baseline (Fisher's LSD, p < 0.05).
Each of the animals receiving intra-VS NMDA injections during TTX perfusion into the VTA on day 1 were tested again on day 2 during perfusion of aCSF into the VTA. On day 2, baseline dopamine was 0.57 (± 0.036) pg/μl. NMDA injections increased NAS DA to a maximum of 0.867 pg/μl or 152% of basal values. For two animals that had been tested on day 1, the effectiveness of the VS injection site could not be confirmed because NAS dialysis probes broke between day 1 and day 2; day 1 data were therefore not included in the analyses. For each of these animals NMDA injections failed to elevate NAS DA on day 1. Histological examination revealed that the injection cannula of each of these animals was placed in the VS.
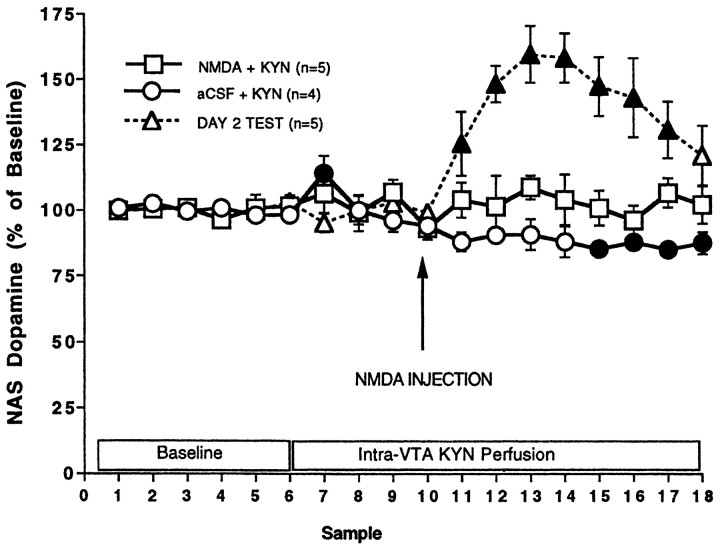
Effect of KYN perfusion into the VTA
Perfusion of KYN through VTA microdialysis probes prevented the elevation of NAS DA by intra-VS NMDA injections (Fig.3). In animals that received NMDA injections, the mean basal value of NAS DA was 0.48 (± 0.027) pg/μl, whereas in animals that received aCSF, the mean basal value was 0.52 (± 0.016) pg/μl. At no point after NMDA injections (administered 60 min after the initiation of KYN perfusion into the VTA) were NAS DA levels different from baseline. In animals that received aCSF injections during KYN perfusion there was a small but reliable decrease in NAS DA. There was no difference in NAS DA between animals receiving KYN + VS NMDA injections and those receiving KYN + aCSF (treatment,F(1,17) = 5.246; p > 0.05).
Fig. 3.
Effect of NMDA injections into the VS on nucleus accumbens dopamine (mean percentage of baseline ± SEM) during perfusion of KYN into the VTA (day 1) and during aCSF perfusion into the VTA on day 2. Microdialysis samples were collected every 15 min, and KYN perfusion was initiated after the sixth sample. One hour later (after the tenth sample) either NMDA or aCSF was injected into the VS. NMDA had no effect on NAS DA; there was no difference between baseline and post-NMDA samples (F(4,17) = 0.478;p = 0.9181). KYN caused a slight decrease in NAS DA in animals injected with aCSF (F(3,17)= 3.996; p = 0.006). Data from the day 2 test are shifted to the right on the abscissa to align with the NMDA injection; the first six symbols represent baseline samples, and subsequent symbols represent samples collected after NMDA injections. Note also that the VTA probe was perfused with aCSF rather than KYN on day 2. Filled symbols indicate significant differences from baseline (Fisher's LSD, p < 0.05).
Each animal tested on day 1 during intra-VTA perfusion of KYN was tested again on day 2 during perfusion of aCSF into the VTA. Injections of NMDA into the VS on day 2 increased NAS DA. Baseline dopamine values on day 2 were 0.65 (± 0.024) pg/μl. After NMDA injections, NAS DA peaked 1.4 pg/μl or 160% of baseline. For two animals, the effectiveness of VS injection sites could not be confirmed by a day 2 test because NAS microdialysis probes broke between day 1 and day 2. Although histological examination revealed cannula placements in the VS, data from these animals was not included in the statistical analyses. Data from an additional three animals that received VTA-KYN and NMDA injections were also excluded. For one animal the VTA microdialysis probe was anterior to the VTA; in this animal KYN infusion failed to block NMDA-induced elevations in NAS dopamine. For the other two animals, NMDA injections on both day 1 and day 2 failed to elevate NAS DA. Histological analysis revealed that injection cannula were placed anterior to the VS bordering the posterior basolateral amygdala (data not shown).
Single-unit recording of VTA dopaminergic neurons
Injection of 0.74 μg of NMDA into the VS increased the firing rates of 45% (14 of 31), decreased the firing rates of 13% (4 of 31), and failed to produce sustained alterations in the firing rates of 42% (13 of 31) of VTA DA neurons (Table 1). Perievent time histograms, each from a representative neuron in which intra-VS NMDA injections increased firing rate, decreased firing rate, or produced no sustained effect in firing rate are shown in Figure4. A total of 35 cells were recorded after NMDA injections into the VS. Only data from cells that were recorded for at least 15 min after NMDA injections were included in statistical analyses for changes in firing rate. This criterion was set for electrophysiological data to coincide with the timing of the first microdialysis sample collected after NMDA injections in the present and in previous experiments (Legault and Wise, 1999). Complete data sets were obtained from 26 cells. For the other nine cells, the firing rates became erratic, action potentials distorted, and the cells were lost before the 15 min postinjection period was completed. However, for five of these cells, action potentials were recorded for at least 10 min after NMDA injections. The effects of NMDA on the firing rates of these cells are included in the descriptive statistics (total of 31 cells).
Table 1.
Mean firing rates, Hz (SEM), of VTA DA neurons before and after NMDA injections into the VS
| Baseline | 5 min | 10 min | 15 min | |
|---|---|---|---|---|
| Increase % of baseline | 3.36 (0.48) | 4.06(0.59) 120(7.7) | 4.74(0.63) 147(8.0) | 4.34(0.50) 136(13.0) |
| Decrease % of baseline | 3.47 (0.68) | 2.59(0.74) 64(12.0) | 2.67(0.92) 59(19.0) | 2.36(0.78) 53(17.0) |
| No Effect % of baseline | 3.96 (0.53) | 4.01(0.54) 101(1.5) | 3.93(0.53) 98(1.4) | 3.93(0.52) 99(2.0) |
| Controls % of baseline | 4.4 (0.47) | 4.35(0.45) 99.5(1.8) | 4.27(0.45) 96.8(1.4) | 4.27(0.44) 99(1.6) |
Fig. 4.

Perievent time histograms (15 sec bins) from three individual VTA dopaminergic neurons recorded for 5 min before and for 15 min after injection of NMDA or aCSF into the VS. The NMDA injection is indicated by the triangle. NMDA typically caused a brief inhibition in the firing rates of dopaminergic neurons followed by either a increase in firing rate (A), a decrease in firing rate (B), or no net effect when averaged over 15 min (C).
Of the 14 cells that increased firing, 10 were recorded for the entire 15 min session without incident. For these 10 cells, the mean firing rate over the 15 min session was increased to 156% (± 15.0) of baseline (time, F(9,27) = 4.257;p = 0.0138). The mean firing rates of these cells at each of the three postinjection intervals (5 min each) was greater than those of control cells (treatment × time interaction,F(3,51) = 14.3, p = 0.0001; Fisher's LSD, p < 0.05). For three cells that increased firing rate after NMDA injections, the dopamine agonist quinpirole (200 μg/kg, i.p.) was injected 10 min after NMDA. Within 10 min of the quinpirole injection, there was a gradual decrease in firing rate characteristic of dopaminergic neurons (data not shown). Finally, NMDA appeared to stimulate one cell into depolarization block; within 5 min of the NMDA injection there was a rapid increase in the firing rate of this cell (from 4.7 to approximately 15 Hz) followed by complete suppression of activity. Quinpirole (200 μg/kg, i.p.) was injected 10 min after NMDA, and within 5 min firing was reinstated. The mean firing rate in the first 5 min after the reinstatement firing was 160% of baseline and gradually declined over the next 10 min (data not shown).
For the four cells in which NMDA decreased firing by >10% of baseline firing rate, the mean firing rate over the 15 min session was 59% (SEM ± 8.9%) of baseline. Decreases in firing rates were sustained throughout the recording session, however they did not attain statistical significance (main effect of time, F = 3.11, p = 0.81) likely because of the small sample size (n = 4). For the 13 cells in which intra-VS NMDA injections evoked <10%, changes in the mean firing rate was 99.6% (SEM ± 0.92%) of baseline. The firing rates of cells recorded after intra-VS injection of aCSF were not different from those of cells recorded without injections; data from these control groups were pooled. Over the 15 min session the mean firing of control cells was 96.6% of baseline. Within each of the three 5 min periods, the firing rate of no single control cell differed from its baseline firing rate by >10%. Nonetheless, relative to baseline, there was a statistically reliable decrease in the firing rates of control cells over the recording period with a mean decrease to 96% of baseline (F(3,24) = 2.97; p < 0.05).
NMDA often caused complex, multiphasic changes in the firing patterns of some VTA DA neurons. Often, NMDA injections resulted in a biphasic response; there was an initial inhibition followed by a return to basal firing rate or a sustained increase in firing rate. In three cells, NMDA injections produced triphasic effects, with either increases or decreases in firing rate occurring between changes in the opposite direction. Thus, although NMDA failed to cause uniform sustained changes in the mean firing rates of many (42%) neurons recorded for 15 min, these neurons were not necessarily unresponsive to NMDA.
DISCUSSION
The present study confirms that chemical stimulation of the VS elevates NAS DA (Legault et al., 1995; Brudzynski and Gibson, 1997;Legault and Wise, 1999). The present results add to evidence that the hippocampus can modulate dopaminergic transmission by demonstrating that VS stimulation can increase impulse flow through VTA dopaminergic neurons and by demonstrating that impulse flow through VTA dopaminergic neurons is critical for VS stimulation to increase NAS DA. Intra-VS injections of NMDA failed to increase NAS DA when impulse flow through dopaminergic neurons was disrupted by perfusion of TTX into the VTA (on day 1) but increased NAS DA during perfusion of aCSF the VTA (on day 2). VS-evoked elevations in NAS DA were comparable in both magnitude and duration, as we have previously reported (Legault and Wise, 1999).
Electrophysiological recordings confirmed that injections of NMDA into the VS increases the firing rates of at least a subpopulation of identified VTA dopaminergic neurons. The firing rates of ∼50% of dopaminergic neurons were elevated by NMDA injections. These elevations were sustained for a minimum of 15 min, a time during which the same dose of NMDA increases locomotor activity and elevates both NAS and VTA DA collected from microdialysis samples in freely moving rats (Legault and Wise, 1999). The present electrophysiological results are in partial agreement with a recent report from Todd and Grace (1999) in which the number and firing rates of dopaminergic neurons were recorded during multiple electrode descents into the VTA after intra-VS injections of NMDA (0.75 μg, comparable to the dose used in the present study) or TTX (1 μm). In the study of Todd and Grace (1999), NMDA injections increased the number of electrophysiologically identified VTA dopaminergic neurons, whereas TTX injections decreased the number of identified dopaminergic neurons and their firing rates. Thus, both the present study and that of Todd and Grace (1999) demonstrate that the activity of VTA dopaminergic neurons is modulated by the VS. Furthermore, decreases in the activity of dopaminergic neurons associated with TTX injections provide evidence that the facilitory effects of NMDA on dopaminergic neuronal activity were mediated by activation, rather than by depolarization inactivation, of projections from the VS. Unlike the present study, however, Todd and Grace (1999) failed to obtain increases in the firing rates of dopaminergic neurons. Although there is no obvious explanation for this discrepancy between these two studies, it may be relevant that the basal firing rates of dopaminergic neurons reported by Todd and Grace (1999) (∼4.5 Hz) was higher than the basal firing rate reported here and was, in fact, comparable to the augmented firing rates of dopaminergic neurons evoked by NMDA injections.
In addition to monotonic increases in the firing rates of dopaminergic neurons, NMDA injections into the VS frequently caused a transient initial inhibition in firing rates and less frequently caused multiphasic alternations between increases and decreases in firing rates. These multiphasic effects of VS stimulation on dopaminergic neurons are consistent with the effects of electrical stimulation of the VS, which has been reported to cause brief inhibition followed by a long-latency increase in the burst-firing of VTA dopaminergic neurons (Harden and Grace, 1995). The complex responses of dopaminergic neurons to VS stimulation suggest that the VS can modulate both inhibitory and excitatory inputs to VTA DA neurons. Modulation of both inhibitory and excitatory inputs to the VTA may account for the failure to obtain increases in the firing rates in some of the neurons recorded. Convergence of offsetting inputs to a recorded neuron may have resulted in no net increase in firing rate. Misalignment of the VS injection site with respect to VS outputs that converge on the recorded neuron might also account for some of the variability in NMDA-induced responses; it is possible that had dopaminergic neurons been recorded for longer periods, the NMDA would have spread into a region of the VS that gave rise to outputs that ultimately converged on and stimulated the recorded neuron. Nonetheless, it is clear from the present electrophysiological study and a previous microdialysis study (Legault and Wise, 1999) that NMDA injections into the VS have a net excitatory effect on VTA dopaminergic transmission, increasing dopaminergic cell firing and increasing both somatodendritic and terminal DA release.
The importance of increased impulse flow through dopaminergic neurons for elevations in NAS DA induced by VS stimulation was demonstrated by the effects of TTX perfusion into the VTA. Perfusion of TTX into the VTA reduced dopamine in the NAS to ∼20% of basal levels and prevented the elevations in NAS dopamine normally evoked by stimulation of the VS. The reduction in NAS DA during perfusion of TTX into the VTA is in agreement with previous reports that basal extracellular DA in the striatum or NAS depends on the conduction of impulses (through voltage-gated sodium channels) from dopaminergic cell bodies and along the dopaminergic axons ascending the medial forebrain bundle (Keefe et al., 1992; Karreman and Moghaddam, 1996; Morari et al., 1996). The failure of VS stimulation to elevate NAS DA during blockade of impulse flow through VTA dopaminergic neurons suggests that the hippocampus influences dopaminergic transmission primarily by increasing impulse flow through dopaminergic neurons rather than by evoking glutamate-mediated impulse-independent dopamine release from terminals in the NAS.
An alternative to the suggestion that NMDA injections into the VS elevate NAS DA by causing glutamate-induced impulse-independent DA release (Brudzynski and Gibson, 1997) is that glutamate acts presynaptically to enhance impulse-evoked transmitter release from dopaminergic terminals (cf. Grace, 1995). This possibility was addressed by experiments in which KYN was perfused into the VTA during NMDA injections into the VS. Blockade of ionotropic glutamate receptors on dopaminergic cell bodies by application of KYN does not, like TTX, abolish impulse flow through dopaminergic neurons but shifts impulse activity from burst-firing to pacemaker-like firing (Grenhoff et al., 1988; Charlety et al., 1991). Thus, during perfusion of KYN into the VTA, extracellular DA in the NAS was slightly reduced, by ∼15%. These results are consistent with previous reports that blockade of ionotropic glutamate receptors in the VTA causes small reductions in NAS DA (Taber et al., 1995; Karreman and Moghaddam, 1996). Nonetheless, if glutamate could act on dopaminergic terminals to augment impulse-dependent transmitter release, then stimulation of subiculo-accumbens projections should still have elevated NAS DA. However, perfusion of KYN into the VTA completely abolished NMDA-induced elevations in NAS DA.
The present experiments support the conclusion that the primary mechanism through which VS stimulation elevates NAS DA is by increasing impulse flow through VTA dopaminergic neurons. In apparent discrepancy with these results are those of Blaha et al. (1997), who reported that the electrical stimulation of the VS elevated the dopamine-like voltammetric signal recorded from the NAS and that these elevations were blocked by injections of glutamate receptor antagonists into the NAS. On the basis of their results, Blaha et al. (1997) concluded that VS-evoked elevations in NAS dopamine were mediated by glutamatergic actions on dopaminergic terminals. In the present study, the only indication that stimulation of subiculo-accumbens glutamatergic inputs to the NAS might augment DA release was that intra-VTA KYN slightly decreased NAS DA in animals receiving aCSF injections, whereas there was no decrease in NAS DA in animals that received NMDA injections. However, differences in NAS DA between NMDA-injected and aCSF-injected animals were small and not statistically significant. Thus, although the present study cannot rule out the possibility of glutamate-mediated transmitter release from dopaminergic terminals, they do suggest that the influence of such a phenomenon on VS-evoked elevations in NAS dopamine is minimal and is not detected by microdialysis. Indeed, for exogenously applied glutamate to evoke impulse-independent elevations in NAS DA in a range detectable by microdialysis, high concentrations of glutamate (>1 mm) are required (Moghaddam et al., 1990;Keefe et al., 1992; Westerink et al., 1992), and such concentrations appear to evoke DA release by causing spreading depression (Moghaddam et al., 1990; Svensson et al., 1994).
Although the circuitry through which VS stimulation activates VTA DA neurons remains to be determined, the experiments involving intra-VTA perfusion of KYN suggest a glutamatergic link terminating in the VTA. One possible circuit involves projections from the hippocampus to the PFC (Swanson, 1981; Jay and Witter, 1991). Electrical stimulation of the VS evokes excitatory responses in PFC neurons (Laroche et al., 1990; Jay et al., 1995) and at least some VS-activated PFC neurons project to the VTA (Jay et al., 1995). Injections of NMDA into the VS induce FOS in the PFC (Klarner et al., 1998), suggesting that the PFC is activated by conditions similar to those used in the present study. The PFC, in turn, provides well-characterized glutamatergic inputs to the VTA (Sesack and Pickel, 1990a; Rossetti et al., 1998). Moreover, the microdialysis and electrophysiological data reported in the present study parallel those from studies of PFC-evoked elevations in NAS DA. Activation the PFC elevates NAS and striatal DA and, as was the case with the present experiments involving VS stimulation, elevations in NAS DA evoked by PFC stimulation are blocked by intra-VTA perfusion of TTX (Karreman and Moghaddam, 1996) or of glutamate antagonists (Taber et al., 1995; Karreman and Moghaddam, 1996; You et al., 1998). The similar characteristic of PFC and VS-evoked elevations in NAS DA would be expected if such a common circuit were involved.
Subiculo-accumbens glutamate has been suggested to influence goal-directed behaviors by interactions with dopaminergic inputs to the NAS (Yang and Mogenson, 1987; Burns et al., 1993; Burns et al., 1996;Hitchcott and Phillips, 1997). Dysfunctional interactions between these two inputs to the NAS have been suggested to be important in the pathophysiology of schizophrenia (Gray et al., 1991; O'Donnell and Grace, 1998). Until recently, the study of interactions between hippocampal outputs and NAS DA has focused primarily on the direct hippocampal projection to the NAS. This focus has been based largely on the hypothesis that glutamate augments or induces transmitter release from dopaminergic terminals in this region. The results of the present study, in contrast, indicate that the VTA is an important site through which outputs from the VS ultimately modulate NAS DA by modulating mesolimbic impulse flow.
Footnotes
This work was supported by the National Institute on Drug Abuse and Fonds pour la Formation de Chercheurs et l'Aide a la Recherche (Quebec).
Correspondence should be addressed to Mark Legault, Centre de Recherche Fernand Seguin, Université de Montréal, 7331 rue Hochelaga, Montréal, H1N 3V2 Quebec, Canada. E-mail:Mark.Legault@CRFS.Umontreal.ca.
Dr. Wise's present address: Chief, Behavioral Neuroscience Branch, Intramural Research Program, National Institute on Drug Abuse, Bethesda, MD 20892.
Dr. Legault's present address: Centre de Recherche Fernand Seguin, Université de Montréal, Montréal, Quebec, Canada, H1N 3V2.
REFERENCES
- 1.Blaha CD, Yang CR, Floresco SB, Barr A, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci. 1997;9:902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DM, Bardgett ME. Haloperidol blocks increased locomotor activity elicited by carbachol infusion into the ventral hippocampal formation. Pharmacol Biochem Behav. 1998;60:759–764. doi: 10.1016/s0091-3057(98)00053-7. [DOI] [PubMed] [Google Scholar]
- 3.Brudzynski SM, Gibson CJ. Release of dopamine in the nucleus accumbens caused by stimulation of the subiculum in freely moving rats. Brain Res Bull. 1997;42:303–308. doi: 10.1016/s0361-9230(96)00290-0. [DOI] [PubMed] [Google Scholar]
- 4.Bunney BS, Grace AA. Acute and Chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sciences. 1978;23:1715–1728. doi: 10.1016/0024-3205(78)90471-x. [DOI] [PubMed] [Google Scholar]
- 5.Burns LH, Robbins TW, Everitt BJ. Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of d-amphetamine. Behav Brain Res. 1993;55:167–183. doi: 10.1016/0166-4328(93)90113-5. [DOI] [PubMed] [Google Scholar]
- 6.Burns LH, Annett L, Kelley AE, Everitt BJ, Robbins TW. Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: implications for limbic striatal interactions. Behav Neurosci. 1996;110:60–73. doi: 10.1037//0735-7044.110.1.60. [DOI] [PubMed] [Google Scholar]
- 7.Charlety P, Grenhoff J, Chergui K, De la Chapelle B, Buda M, Svensson T, Chouvet G. Burst firing of mesencephalic dopamine neurons is inhibited by somatodendritic application of kynurenate. Acta Physiol Scand. 1991;142:105–112. doi: 10.1111/j.1748-1716.1991.tb09134.x. [DOI] [PubMed] [Google Scholar]
- 8.Christie MJ, Bridge S, James LB, Beart PM. Excitotoxin lesions suggest an aspartatergic projection from rat medial prefrontal cortex to ventral tegmental area. Brain Res. 1985;333:169–172. doi: 10.1016/0006-8993(85)90140-4. [DOI] [PubMed] [Google Scholar]
- 9.Christie MJ, Sikes RW, Stephenson JA, Cook CJ, Beart PM. Excitatory amino acid projections to the nucleus accumbens septi in the rat: a retrograde transport study using D [3H] aspartate and [3H] GABA. Neuroscience. 1987;22:425–440. doi: 10.1016/0306-4522(87)90345-9. [DOI] [PubMed] [Google Scholar]
- 10.Fink JS, Smith GP. Mesolimbicortical dopamine terminal fields are necessary for normal locomotor and investigatory exploration in rats. Brain Res. 1980;199:359–384. doi: 10.1016/0006-8993(80)90695-2. [DOI] [PubMed] [Google Scholar]
- 11.Fuller TA, Russchen FT, Price JLP. Sources of presumptive glutamatergic/aspartergic afferents to the rat ventral striatopallidal region. J Comp Neurol. 1987;258:317–338. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- 12.Glowinski J, Chéramy A, Romo R, Barbieto L. Presynaptic regulation of dopaminergic transmission in the striatum. Cell Mol Neurobiol. 1988;8:7–17. doi: 10.1007/BF00712906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grace AA. The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 1995;37:111–129. doi: 10.1016/0376-8716(94)01066-t. [DOI] [PubMed] [Google Scholar]
- 14.Gray JA, Feldon J, Rawlins JNP, Hemsely DR, Smith AD. The neuropsychology of schizophrenia. Behav Brain Sci. 1991;14:1–84. [Google Scholar]
- 15.Grenhoff J, Tung C, Svensson T. The excitatory amino acid antagonist kynurenate induces pacemaker-like firing of dopamine neurons in rat ventral tegmental area in vivo. Acta Physiol Scand. 1988;134:567–568. doi: 10.1111/j.1748-1716.1998.tb08535.x. [DOI] [PubMed] [Google Scholar]
- 16.Harden DG, Grace AA. Hippocampal activation suppresses VTA dopamine cell firing: a potential role for hippocampal regulation of phasic DA release. Soc Neurosci Abstr. 1995;21:1660. [Google Scholar]
- 17.Hitchcott PK, Phillips GD. Amygdala and hippocampus control dissociable aspects of drug-associated conditioned rewards. Psychopharmacology. 1997;131:187–195. doi: 10.1007/s002130050283. [DOI] [PubMed] [Google Scholar]
- 18.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- 19.Jay TM, Glowinski J, Thierry AM. Inhibition of hippocampoprefrontal cortex excitatory responses by the mesocortical DA system. NeuroReport. 1995;6:1845–1848. doi: 10.1097/00001756-199510020-00006. [DOI] [PubMed] [Google Scholar]
- 20.Karreman M, Moghaddam B. The prefrontal cortex controls release of dopamine in the medial striatum: an effect mediated by the ventral tegmental area. J Neurochem. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- 21.Keefe K, Zigmond M, Abercrombie E. Extracellular dopamine in striatum: influence of nerve impulse activity in medial forebrain bundle and local glutamatergic input. Neuroscience. 1992;47:325–332. doi: 10.1016/0306-4522(92)90248-z. [DOI] [PubMed] [Google Scholar]
- 22.Klarner A, Koch M, Schnitzler H-U. Induction of FOS-protein in the forebrain and disruption of sensorimotor gating following N-methyl-d-aspartate infusion into the ventral hippocampus of the rat. Neuroscience. 1998;84:443–452. doi: 10.1016/s0306-4522(97)00475-2. [DOI] [PubMed] [Google Scholar]
- 23.Laroche S, Jay TM, Thierry AM. Long-term potentiation in the prefrontal cortex following stimulation of the hippocampal CA1/subicular region. Neurosci Lett. 1990;114:184–190. doi: 10.1016/0304-3940(90)90069-l. [DOI] [PubMed] [Google Scholar]
- 24.Legault M, Wise R A. Injections of N-methyl-d-aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse. 1999;31:241–249. doi: 10.1002/(SICI)1098-2396(19990315)31:4<241::AID-SYN1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Legault M, Rossetti Z, Wise RA. The effect of NMDA injections into the ventral subiculum on nucleus accumbens dopamine. Soc Neurosci Abstr. 1995;21:368. [Google Scholar]
- 26.Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- 27.Moghaddam B, Gruen R, Roth R, Bunney B, Adams R. Effect of l-glutamate on the release of striatal dopamine: in vivo dialysis and electrochemical studies. Brain Res. 1990;518:55–60. doi: 10.1016/0006-8993(90)90953-9. [DOI] [PubMed] [Google Scholar]
- 28.Morari M, O'Connor W, Darvelid M, Ungerstedt U, Bianchi C, Fuxe K. Functional neuroanatomy of the nigrostriatal and striatonigral pathways as studied with dual probe microdialysis in the awake rat - I. Effects of perfusion with tetrodotoxin and low-calcium medium. Neuroscience. 1996;72:79–87. doi: 10.1016/0306-4522(95)00557-9. [DOI] [PubMed] [Google Scholar]
- 29.Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- 30.O'Donnell P, Grace AA. Dysfunctions in multiple interrelated systems as the neurobiological bases of schizophrenic symptom clusters. Schizophrenia Bull. 1998;24:267–283. doi: 10.1093/oxfordjournals.schbul.a033325. [DOI] [PubMed] [Google Scholar]
- 31.Romo R, Chéramy A, Godeheu G, Glowinski J. In vivo presynaptic control of dopamine release in the cat caudate nucleus I. Opposite changes in neuronal activity and release evoked from thalamic motor nuclei. Neuroscience. 1986a;19:1067–1079. doi: 10.1016/0306-4522(86)90123-5. [DOI] [PubMed] [Google Scholar]
- 32.Romo R, Chéramy A, Godeheu G, Glowisnki J. In vivo presynaptic control of dopamine release in the cat caudate nucleus III. Further evidence for the implication of corticostriatal glutamatergic neurons. Neuroscience. 1986b;19:1091–1099. doi: 10.1016/0306-4522(86)90125-9. [DOI] [PubMed] [Google Scholar]
- 33.Rossetti ZL, Marcangione C, Wise RA. Increase of extracellular glutamate and expression of Fos-like immunoreactivity in the ventral tegmental area in response to electrical stimulation of the prefrontal cortex. J Neurochem. 1998;70:1503–1511. doi: 10.1046/j.1471-4159.1998.70041503.x. [DOI] [PubMed] [Google Scholar]
- 34.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabelled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. Brain Res. 1990a;506:166–168. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- 36.Sesack SR, Pickel VM. In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res. 1990b;527:266–279. doi: 10.1016/0006-8993(90)91146-8. [DOI] [PubMed] [Google Scholar]
- 37.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with phaseolus vulgaris leucoagglutinin. J Neural Transm. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 38.Svensson TH, Zhang J, Johannessen K, Engle JA. Effect of local infusion of glutamate analogues into the nucleus accumbens of rats: an electrochemical and behavioral study. Brain Res. 1994;643:155–161. doi: 10.1016/0006-8993(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 39.Swanson LW. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- 40.Taber MT, Fibiger HC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J Neurosci. 1995;15:3896–3904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- 42.Taghzouti K, Louilot S, Herman J, LeMoal M, Simon H. Alternation behavior, spatial discrimination and reversal disturbances following 6-hydroxydopamine lesions in the nucleus accumbens of the rat. Behav Neural Biol. 1985;44:354–363. doi: 10.1016/s0163-1047(85)90640-5. [DOI] [PubMed] [Google Scholar]
- 43.Todd CL, Grace AA. Modulation of ventral tegmental area dopamine cell activity by the ventral subiculum and entorhinal cortex. Ann NY Acad Sci. 1999;877:688–690. doi: 10.1111/j.1749-6632.1999.tb09302.x. [DOI] [PubMed] [Google Scholar]
- 44.Totterdell S, Smith AD. Convergence of hippocampal and dopaminergic input onto identified neurons in the nucleus accumbens of the rat. J Chem Neuroanat. 1989;2:285–298. [PubMed] [Google Scholar]
- 45.Walaas I. Biochemical evidence for overlapping neocortical and allocortical glutamate projections to the nucleus accumbens and rostral caudatoputamen. Neuroscience. 1981;6:399–405. doi: 10.1016/0306-4522(81)90132-9. [DOI] [PubMed] [Google Scholar]
- 46.Westerink BHC, Tuntler J, Damama G, Rollema H, deVries JB. The use of tetrodotoxin for the characterization of drug-enhanced dopamine release in conscious rats studied by brain dialysis. Naunyn Schmiedebergs Arch Pharmacol. 1987;336:502–507. doi: 10.1007/BF00169306. [DOI] [PubMed] [Google Scholar]
- 47.Westerink B, Santiago M, deVries J. The release of dopamine form nerve terminals and dendrites of nigrostriatal neurons induced by excitatory amino acids in the conscious rat. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:523–529. doi: 10.1007/BF00168943. [DOI] [PubMed] [Google Scholar]
- 48.Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 49.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 50.Wise RA, Rompré P-P. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 51.Wu M, Brudzynski SM. Mesolimbic dopamine terminals and locomotor acitivity induced from the subiculum. NeuroReport. 1995;6:1601–1604. doi: 10.1097/00001756-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Yang CR, Mogenson GJ. Hippocampal signal transmission to the pedunculopontine nucleus and its regulation by dopamine D2 receptors in the nucleus accumbens: An electrophysiological and behavioral study. Neuroscience. 1987;23:1041–1055. doi: 10.1016/0306-4522(87)90179-5. [DOI] [PubMed] [Google Scholar]
- 53.You Z-B, Tzschentke TM, Brodin E, Wise RA. Electrical stimulation of the prefrontal cortex increases cholecystokinin, glutamate, and dopamine release in the nucleus accumbens: an in vivo microdialysis study in freely moving rats. J Neurosci. 1998;18:6492–6500. doi: 10.1523/JNEUROSCI.18-16-06492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]