Abstract
We have used combined membrane capacitance measurements (Cm) and voltage-clamp recordings to examine the mechanisms underlying modulation of stimulus–secretion coupling by a Gi/o-coupled purinoceptor (P2Y) in adrenal chromaffin cells. P2Y purinoceptors respond to extracellular ATP and are thought to provide an important inhibitory feedback regulation of catecholamine release from central and sympathetic neurons. Inhibition of neurosecretion by other Gi/o-protein-coupled receptors may occur by either inhibition of voltage-operated Ca2+ channels or modulation of the exocytotic machinery itself. In this study, we show that the P2Y purinoceptor agonist 2-methylthio ATP (2-MeSATP) significantly inhibits Ca2+ entry and changes inCm evoked by single 200 msec depolarizations or a train of 20 msec depolarizations (2.5 Hz). We found that P2Y modulation of secretion declines during a train such that only ∼50% of the modulatory effect remains at the end of a train. The inhibition of both Ca2+ entry and ΔCm are also attenuated by large depolarizing prepulses and treatment with pertussis toxin. Inhibition of N-type, and to lesser extent P/Q-type, Ca2+channels contribute to the modulation of exocytosis by 2-MeSATP. The Ca2+-dependence of exocytosis triggered by either single pulses or trains of depolarizations was unaffected by 2-MeSATP. When Ca2+ channels were bypassed and exocytosis was evoked by flash photolysis of caged Ca2+, the inhibitory effect of 2-MeSATP was not observed. Collectively, these data suggest that inhibition of exocytosis by Gi/o-coupled P2Y purinoceptors results from inhibition of Ca2+ channels and the Ca2+ signal controlling exocytosis rather than a direct effect on the secretory machinery.
Keywords: P2Y purinoceptor, G-protein-coupled receptor, voltage-operated calcium channels, exocytosis, modulation, presynaptic inhibition, adrenal chromaffin cells
It is now well established that ATP can act as a fast excitatory neurotransmitter by activation of postsynaptic purinoceptors (Edwards et al., 1992; Evans et al., 1992) (for review, see Zimmermann, 1994, Gibb and Halliday, 1996). Recent studies have shown that ATP may also act as a neuromodulator. Activation of presynaptic P2X purinoceptors facilitates neurotransmission (Khakh and Henderson, 1998; Boehm, 1999) (for review, see MacDermott et al., 1999), whereas activation of presynaptic P2Y purinoceptors is thought to inhibit neurotransmission (Von Kügelgen et al., 1989; Boehm, 1999). The mechanism(s) underlying inhibition of neurotransmission by P2Y purinoceptors is unknown. Presynaptic inhibition of neurotransmitter release by other G-protein-coupled receptors (GPCRs), however, is generally thought to involve either changes in membrane excitability and Ca2+ signaling or a direct effect on some component of the release machinery (Hille, 1994; Wu and Saggau, 1997;Miller, 1998). Central to the Ca2+hypothesis for presynaptic inhibition are the observations that neuronal, somatic voltage-operated Ca2+channels are inhibited by Gi/o-proteins (Dolphin, 1998) and that pharmacologically similar channels control exocytosis (Dunlap et al., 1995). It is assumed that the modulatory processes that regulate Ca2+ channels in the soma also occur at release sites. The release machinery hypothesis, on the other hand, arises from the observation that activation of GPCRs inhibits exocytosis when Ca2+ channels are bypassed or blocked (Silinsky, 1986; ManSonHing et al., 1989; Shen and Surprenant, 1990; Lang et al., 1995). Whether modulation of the release machinery contributes significantly to GPCR-dependent inhibition of stimulus-evoked neurotransmitter release is still a matter of debate (Thompson et al., 1993; Miller, 1998).
The aim of this study was to determine the mechanisms underlying modulation of exocytosis by P2Y purinoceptors. The system we chose to study was the adrenal chromaffin cell because it is well established that these cells secrete catecholamines by Ca2+-regulated exocytosis (Neher, 1998) and they express inhibitory P2Y purinoceptors that couple to neuronal Ca2+ channels (Diverse-Pierluissi et al., 1991; Gandia et al., 1993; Currie and Fox, 1996). Moreover, chromaffin cells co-store and release ATP along with catecholamine (Winkler and Westhead, 1980), and it is thought that, as in sympathetic neurons (Von Kügelgen et al., 1994), ATP may act as an autocrine regulator of secretion from these cells (Carabelli et al., 1998). We used combined voltage-clamp and membrane capacitance measurements (Cm) to study the effects of P2Y purinoceptor activation on stimulus–secretion coupling. Our results show that the selective P2Y agonist 2-methylthio ATP (2-MeSATP) inhibited both Ca2+ channels and ΔCm via a voltage-dependent, pertussis toxin (PTX)-sensitive mechanism. The P2Y-mediated inhibition of exocytosis was not associated with a change in the Ca2+-dependence of secretion. When Ca2+ channels were bypassed and exocytosis was stimulated by flash photolysis of nitrophenyl-EGTA (NP-EGTA), 2-MeSATP had no effect on ΔCm. These data provide strong evidence in favor of the Ca2+ hypothesis for presynaptic inhibition by Gi/oPCR and may also explain feedback inhibition of catecholamine release through P2Y purinoceptors.
MATERIALS AND METHODS
Chromaffin cell culture. Chromaffin cells were prepared by collagenase digestion of bovine adrenal glands according to the method of Trifaro and Lee (1981). Adrenal glands from 18- to 24-month-old cows were obtained from a local abattoir and were retrogradely perfused at 25 ml/min for 30 min at 37°C with the digestive enzymes 0.03% collagenase type 2 (Worthington Biochemical Corp., Lakewood, NJ) and 0.0013% DNase I (Boehringer Mannheim, Indianapolis, IN) added to a Locke's solution consisting of (in mm): 154.2 NaCl, 2.6 KCl, 2.2 K2HPO4, 0.85 KH2PO4, 10 glucose, and 5 HEPES, with 0.0005% Phenol Red (Life Technologies, Paisley, UK), pH adjusted to 7.2 with NaOH. After surgical removal of the cortex, the medulla was dissected, cut into small pieces, placed in a trypsinization flask with fresh enzyme solution, and stirred at slow speed for 30 min at 37°C. Cells were washed twice with Earle's Balanced Salt Solution (Life Technologies) and resuspended in DMEM (Life Technologies) supplemented with 44 mm NaHCO3, 15 mm HEPES, 10% fetal calf serum (Life Technologies), 1% glutamine, 1% penicillin–streptomycin solution, 2.5 mg/ml gentamycin, 0.5 mg/ml 5′-fluorodeoxyuridine, and 0.01 mg/ml cytosine-β-δ-arabino-furanoside. Cells were plated on glass coverslips coated with matrigel (Becton Dickinson, Bedford, MA) at a density of ∼800 cells/mm2. Approximately 80% of the media was replaced 24 hr after plating, and cells were maintained for up to 7 d in a humidified atmosphere of 95% O2–5% CO2 at 37°C.
Electrophysiological recordings. A coverslip carrying chromaffin cells was placed in a microperfusion chamber on the stage of an inverted phase-contrast microscope (Diaphot 200; Nikon, Tokyo, Japan). Cells were continuously superfused with an external solution consisting of (in mm): 130 NaCl, 2 KCl, 1 MgCl2, 5 CaCl2, 10 glucose, and 10 HEPES, adjusted to pH 7.2 with NaOH, osmolarity of ∼280 mOsm. In some experiments, cells were superfused with a external solution in which the NaCl was replaced with equimolar tetraethyl ammonium (TEA) chloride. Tetrodotoxin was omitted because it slows Na+ channel-gating kinetics 10-fold, resulting in a contamination of the Cmtrace (Horrigan and Bookman, 1994). Drugs were prepared as stock solutions in double-distilled water, except where stated, and then diluted at least 1000-fold into external solution. Special care was taken to superfuse cells at a high rate (∼ 3 ml/min) throughout the experiment and to select well isolated single cells for recording to avoid compounding effects of endogenously released modulators (Carabelli et al., 1998).
Ionic currents were recorded in perforated patch-clamp configuration using borosilicate glass electrodes coated with Sylgard 184 (Dow Corning, Midland, MI) and fire polished on a microforge to a resistance of 1–2 MΩ. Electrodes were filled with an internal solution consisting of (in mm): 145 Cs-glutamate (Calbiochem, Nottingham, UK), 10 HEPES, 9.5 NaCl, and 0.3 BAPTA (Molecular Probes, Eugene, OR), adjusted to pH 7.2 with CsOH (ICN Biomedicals, Aurora, OH), osmolarity ∼280 mOsm. Gramicidin D (Sigma, Poole, UK) at a final concentration of 9.7 μg/ml (stock solution in DMSO 1080 μg/ml) was used for perforation. Flash photolysis experiments were performed as described by Parsons et al. (1996) using the whole-cell patch-clamp configuration and electrodes filled with an internal solution consisting of (in mm): 110 Cs-glutamate, 40 HEPES, 10 NP-EGTA (Molecular Probes), 7 CaCl2, and 0.3 fura-2 pentapotassium salt (Calbiochem), adjusted to pH 7.2 with CsOH.
For both whole-cell and perforated patch recordings, series resistance was <12 MΩ and compensated (typically >70%) electronically using a patch-clamp amplifier (Axopatch 200B; Axon Instruments, Foster City, CA). Voltage protocol generation and data acquisition were performed using custom data acquisition software (kindly provided by Dr. A. P. Fox, University of Chicago, Chicago, IL) running on a Pentium computer equipped with a Digidata 1200 acquisition board (Axon Instruments). Current traces were low-pass filtered at 5 kHz using the four-pole Bessel filter supplied with the amplifier and digitized at 10 kHz. Current traces were corrected off-line for linear leakage current (typically <10 pA, at −90 mV) using the P4 method. Chromaffin cells were voltage-clamped at −90 mV, andCm was sampled with a resolution of 12 msec using a software-based phase-tracking method as described previously (Fidler and Fernandez, 1989; Seward et al., 1995).Cm measurements were interrupted during voltage-steps and then resumed 40 msec after the stimulus to exclude gating charge artifacts (Horrigan and Bookman, 1994; Chow et al., 1996). Data were stored on the computer hard drive and analyzed off-line using self-written analysis software (Axobasic; Axon Instruments) and commercial software (Origin; Microcal, Northampton, MA). All experiments were performed at ambient temperature (21–25°C).
Photolysis of caged calcium and [Ca2+]imeasurements. Flashes of UV light were derived from a pulsed arc lamp (TILL Photonics, Planegg, Germany) coupled to the epi-illumination port of an Axiovert 100 inverted microscope equipped with a 40× oil immersion objective with a numerical aperture of 1.3 (Zeiss, Jena, Germany). UV flashes were restricted to a 240 μm spot using the objective, and the cell under investigation was placed in the center of this region. Fluorescence measurements and UV flashes were restricted to the area immediately surrounding the cell using rectangular field stops. To measure [Ca2+]i, the internal solution contained fura-2 (for composition, see above), and the cell was alternately illuminated at 340 and 380 nm using a monochromator (TILL Photonics) controlled by theCm data acquisition software. Emission >430 nm was collected with a photomultiplier tube (TILL Photonics) and sampled at the rate of Cmmeasurements, approximately every 12 msec. Data were stored on a personal computer, and ratios of 340/380 nm were calculated off-line (Axobasic-written software). Calibration of fura-2 was performed by the method of Grynkiewicz et al. (1985).Rmin andRmax were obtained by permeabilizing chromaffin cells with 10 μm ionomycin or 3 μm digitonin in the presence of 10 mm EGTA or 20 mmCa2+, respectively. In control experiments (n = 4) in which cells were loaded with Ca2+-free NP-EGTA internal solution, flashes failed to produce either a rise in fura-2 ratio at 340/380 nm or Cm.
In some perforated patch experiments, cells were loaded with the Ca2+ indicator by addition of 5 μm fura-2 AM (Molecular Probes) to DMEM medium and incubating for 25 min at 37°C. Cells were then washed with fresh DMEM and incubated an additional 15 min at 37°C. TheKd for fura-2 alters according to the surrounding milieu (Grynkiewicz et al., 1985). Because the intracellular environment is unknown in the perforated patch configuration, coupled with difficulty in determiningRmin, fura-2 measurements were not calibrated in these experiments and ratios are given in Results.
Data analysis. ΔCm was measured relative to a 100 fF calibration signal, which was routinely switched in and out of the circuit during the course of a recording. The ΔCm triggered by a single voltage pulse was calculated off-line. “Immediate” ΔCm occurring during a voltage pulse was measured by averaging four Cmpoints immediately before the voltage pulse and subtracting this value from the average of the first four Cmpoints acquired immediately after the voltage pulse. “Slow” ΔCm observed as drifts inCm after membrane repolarization were measured by taking the average of the first fourCm points acquired immediately after the voltage pulse and subtracting them from the average of fourCm points taken at the peak of theCm rise. Slow ΔCm that exceeded twice the restingCm noise (10–20 fF) were considered significant. Unless otherwise indicated, the total ΔCm resulting from a single depolarization was taken as the sum of the immediate response and slow response. The total ΔCm evoked by a train of depolarizations was calculated as the sum of immediate ΔCm for all the pulses in the train plus any drifts in Cm occurring between pulses. The latter was calculated by taking the average of the first four Cm points acquired after a pulse and subtracting them from the average of fourCm points taken before the next voltage pulse.
Ca2+ entry was determined by integration of Ca2+ currents (ICa), and the left limit was set ∼3 msec into the voltage pulse, to exclude the major portion of the contaminating Na+ current. Before integration, currents were leak-subtracted using the P4 method. Statistical significance was determined using either paired or independent Student's t test, as appropriate. All data are expressed as the mean ± SEM. Data reported are taken from 67 cells from 20 separate cultures.
RESULTS
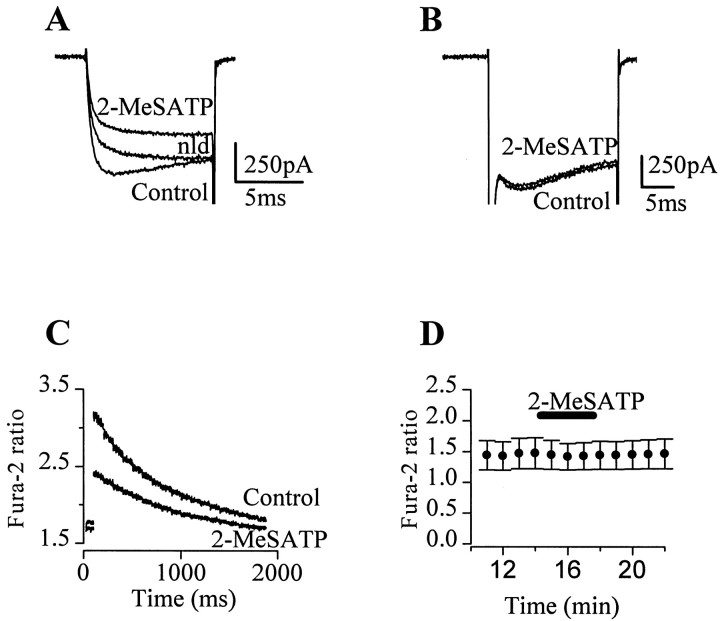
Previous whole-cell patch-clamp studies with Ba2+ as the divalent cation have shown that ATP inhibits ICa in adrenal chromaffin cells (Diverse-Pierluissi et al., 1991; Gandia et al., 1993). For our studies on exocytosis, we used the perforated patch configuration to avoid secretory rundown (Engisch and Nowycky, 1996;Seward and Nowycky, 1996), and we used Ca2+ as the charge carrier to obtain efficient secretion (Seward et al., 1996). In non-neuronal cells and the chromaffin-like PC12 cell line, P2Y purinoceptors are commonly found to be Gq-coupled and reported to increase rather than decrease [Ca2+]i (for review, see Boeynaems et al., 1998). Therefore, in our first series of experiments, we examined the effects of P2Y purinoceptor activation on Ca2+ channels and [Ca2+]i in isolated chromaffin cells under our recording conditions. Superfusion of 2-MeSATP, an ATP analog with preference for metabotropic purinoceptors, caused a reversible inhibition ofICa evoked by step depolarizations to +20 mV from a holding potential of −90 mV (Fig.1A). The IC50 for 2-MeSATP inhibition ofICa was ∼3 nm(A. D. Powell and E. P. Seward, unpublished observations). At a maximally effective concentration (100 nm), 2-MeSATP inhibited the Ca2+ entry integrated over 17 msec by 51 ± 3% (n = 5) and slowed current activation kinetics (Fig. 1A). Similar effects of 2-MeSATP were observed onICa recorded using the whole-cell recording configuration in which 0.3 mm BAPTA was used to buffer [Ca2+]i (47 ± 2% inhibition; n = 4). TheICa inhibition was voltage-dependent because application of a 20 msec prepulse to +120 mV reduced the inhibition of the integrated Ca2+ entry to 14 ± 4% (n = 3). Treatment of chromaffin cells with PTX (250 ng/ml, 24 hr) reduced the effect of 2-MeSATP (100 nm) on integrated Ca2+ entry to 3.5 ± 0.9% (n = 4) (Fig. 1B). In cells loaded with fura-2 AM to monitor [Ca2+]i under perforated patch recording conditions, superfusion with 2-MeSATP did not produce any significant change in basal [Ca2+]i (mean ratio at 340/380 nm excitation before agonist was 1.48 ± 0.24 and in the presence of 2-MeSATP was 1.44 ± 0.24;n = 4) (Fig. 1D), suggesting that there are no functional Ca2+-mobilizing Gq-coupled P2Y purinoceptors or P2X purinoceptors. However, in these same cells, 2-MeSATP did inhibit peak stimulus-evoked [Ca2+]i increases by 47 ± 6% (n = 4) (Fig. 1C). Together, these data indicate that activation of purinoceptors in bovine adrenal chromaffin cells leads to inhibition of Ca2+ entry through Ca2+ channels and that this effect is mediated by a PTX-sensitive Gi/o-protein.
Fig. 1.
P2Y receptor activation inhibitedICa in a voltage-dependent, PTX-sensitive manner. A, B, Superimposed current traces during a 20 msec depolarization to +20 mV from a holding potential of −90 mV in the absence (Control) or presence of 100 nm 2-MeSATP. A,ICa recorded after equimolar replacement of NaCl in the superfusing solution with TEA-Cl to remove contaminating Na+ currents. Under control conditions, 2-MeSATP reduced the maximal current amplitude and slowed the activation kinetics. The effect on kinetics can be seen by normalizing the current in the presence of 2-MeSATP to the control trace (nld).B, Treatment with PTX (250 ng/ml, 24 hr) occluded the inhibitory effect of 2-MeSATP on ICa. The transient current visible during the first 3 msec of the depolarization is a Na+ current, which has been cut off for illustration purposes only. C, D, Effect of 2-MeSATP on [Ca2+]i measured in cells loaded with the Ca2+ indicator fura-2 AM (see Materials and Methods). Data shown are the ratios of emitted fluorescence (510 nm) for excitation at 340 and 380 nm.C, Measurement of cell-averaged [Ca2+]i recorded after a 30 msec depolarization to +20 mV before (Control) and in the presence of 2-MeSATP. D, Superfusion of 2-MeSATP (100 nm) did not induce any changes in the resting [Ca2+]i recorded at −90 mV immediately before a voltage step (data shown is the mean ± SEM for n = 4).
P2Y purinoceptor activation inhibits ΔCm evoked by single long depolarizations
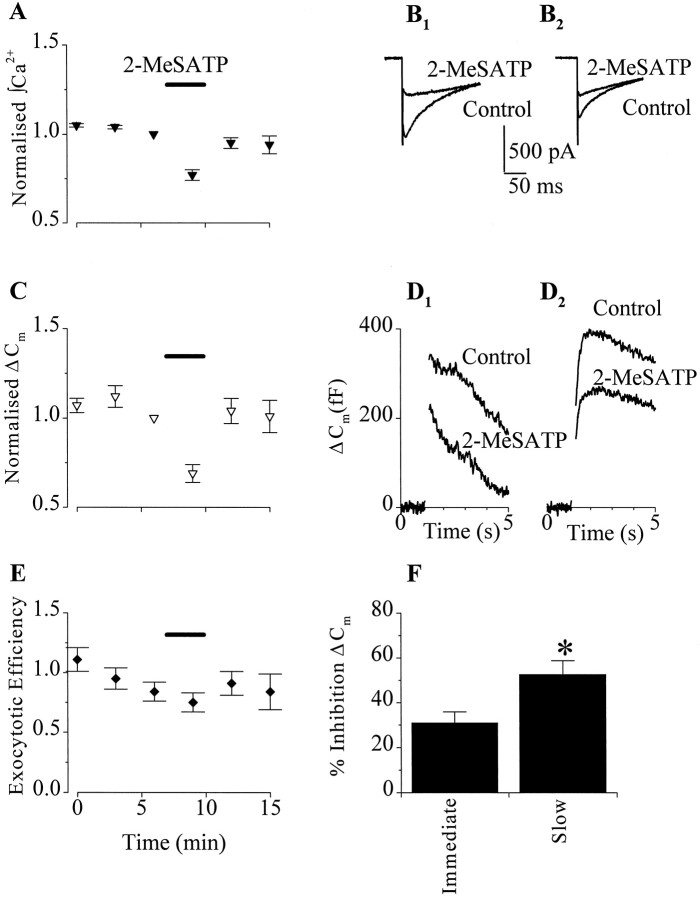
Exocytosis in chromaffin cells and neurons is tightly regulated by Ca2+, with Ca2+ entry through Ca2+ channels providing the trigger for secretory vesicle fusion (Seward and Nowycky, 1996; Neher, 1998). Thus, we predicted that inhibition of Ca2+channels by P2Y purinoceptors in chromaffin cells would be accompanied by a decrease in exocytosis. To measure exocytosis, we combined voltage-clamp recording of Ca2+ channels with Cm measurements to monitor vesicle fusion. Exocytosis was evoked by single long (200 msec) depolarizations to +20 mV from a holding potential of −90 mV once every 3–5 min. Figure 2, Aand C, shows the time course and results of a typical experiment. Empirically, we found that this stimulus protocol produced reproducible stimulus-evokedCm increases without the activity-dependent changes in exocytotic efficiency (Fig.2E) reported previously (Engisch et al., 1997; Smith, 1999). Before agonist application, the mean stimulus-evoked integrated Ca2+ entry over 197 msec was 342 ± 34 × 106 ions (n = 15). The resulting increases in Cmcould be resolved into one or two kinetically distinct phases, depending on the cell. In all cells, Ca2+entry was associated with an immediate increase in ΔCm with a mean secretory rate of 1430 ± 190 fF/sec (n = 15) (Fig.2D). In 6 of these 15 cells, the immediate ΔCm was followed by a smaller, slowCm increase that had a mean rate of 204 ± 56 fF/sec (Fig. 2D2). We found no correlation between the peak amplitude of theICa or integrated Ca2+ entry and the presence or absence of slow Cm increases.
Fig. 2.
P2Y purinoceptor activation inhibited membrane capacitance (Cm) changes evoked by 200 msec depolarizations. A, C,E, Diary plots of the effect of 100 nm2-MeSATP on normalized Ca2+ entry as measured by integrating ICa (A), ΔCm (C), and exocytotic efficiency (ΔCm/Ca2+ entry) (E) (data plotted are the mean ± SEM forn = 15). Both integrated Ca2+entry and ΔCm were normalized to the responses recorded immediately before 2-MeSATP application (horizontal bar). B, Superimposed currents recorded before (Control) and during superfusion with 2-MeSATP from two different chromaffin cells (B1 and B2). Currents were evoked by a 200 msec depolarization to +20 mV from −90 mV. D, ΔCm recorded in response to currents shown in B. Gaps in theCm traces represent when a voltage step was applied. Two kinetically distinct phases ofCm change were observed; inD1, the response consisted solely of an immediate change in Cm and, inD2, an additional slow component of exocytosis that appears as a drift up inCm after the voltage step. 2-MeSATP inhibited both ICa(B1 andB2) and the corresponding immediate and slow ΔCm(D1 and D2).F, Summary of the effect of 2-MeSATP on the immediate (n = 15) and slow (n = 6) ΔCm. *p < 0.02 indicates significant difference between the effect of 2-MeSATP on immediate and slow ΔCm.
Superfusion with 100 nm 2-MeSATP produced a reversible inhibition of stimulus-evoked exocytosis (Fig.2C,D). The inhibition of the immediate and slow ΔCm were, however, significantly different (p = 0.02) (Fig.2F). 2-MeSATP produced a mean inhibition of immediate ΔCm corresponding to 31 ± 5% (n = 15). The inhibition of Ca2+ entry integrated over 197 msec was 22 ± 3%. In cells with pronounced slow exocytosis, 2-MeSATP inhibited the slow ΔCm by 52 ± 6% (n = 6). The larger inhibition of ΔCm compared with Ca2+ entry is consistent with the nonlinear Ca2+-dependence of exocytosis we (see Fig. 5B) and others have measured in chromaffin cells stimulated with long depolarizations (Engisch and Nowycky, 1996).
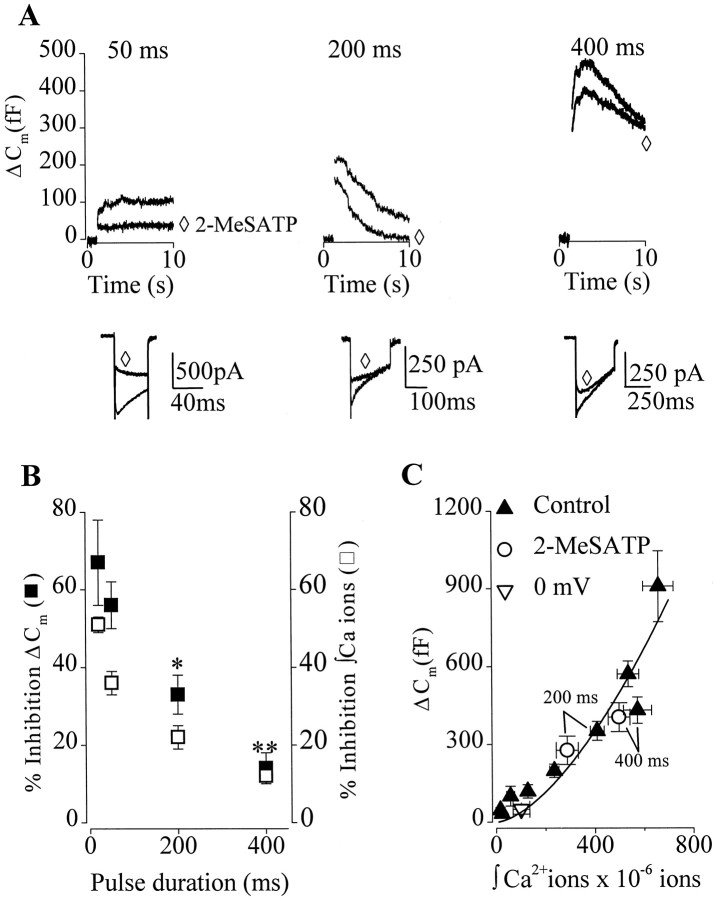
Fig. 5.
P2Y receptor activation did not affect the Ca2+-dependence of Cmchanges evoked by long depolarizations. A, Superimposed traces show the effect of increasing voltage step duration onICa (bottom) and corresponding ΔCm (top) in the absence and presence of 2-MeSATP (⋄). B, Plot of the inhibition of integrated Ca2+ entry (■) and ΔCm (▪) by 2-MeSATP, for 20 (n = 5), 50 (n = 5), 200 (n = 15), and 400 (n = 4) msec step depolarizations to +20 mV. A significant reduction of the effect of 2-MeSATP on integrated Ca2+ entry and ΔCm evoked by longer depolarizations relative to 20 msec are indicated: **p < 0.01; *p < 0.05. C, Plot of mean ΔCm versus mean integrated Ca2+ entry for 10 experiments similar to that illustrated in A. ▴, Data obtained under control conditions with voltage steps to +20 mV of varied durations. Data were binned according to pulse duration (7.5-500 msec).Horizontal error bars represent the SEM of the integrated Ca2+ entry for a given pulse duration. ○, Data obtained with 200 and 400 msec voltage steps to +20 mV (arrows) in the absence and presence of 100 nm 2-MeSATP. ▿, Data obtained under control conditions but with 200 msec depolarizations to 0 mV. The Ca2+-dependence of ΔCmwas calculated according to the method of Engisch and Nowycky (1996). Mean control data were fit to the equation ΔCm = g * (ΔCa2+)n; thesolid line through the data represents the best fit with proportionality constant g = 0.08 and the powern = 1.62. ΔCm recorded in the presence of 2-MeSATP did not significantly deviate from the control Ca2+-dependence curve.
The inhibitory effects of 10 nm 2-MeSATP on Ca2+ entry and ΔCm were less than those produced by 100 nm, and they were reduced in the presence of the purinoceptor antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (100 μm) from 16 ± 3 and 25 ± 7% to 8 ± 4 and 11 ± 2%, respectively (n = 3).
2-MeSATP inhibition of exocytosis involves N- and P/Q-type Ca2+ channels
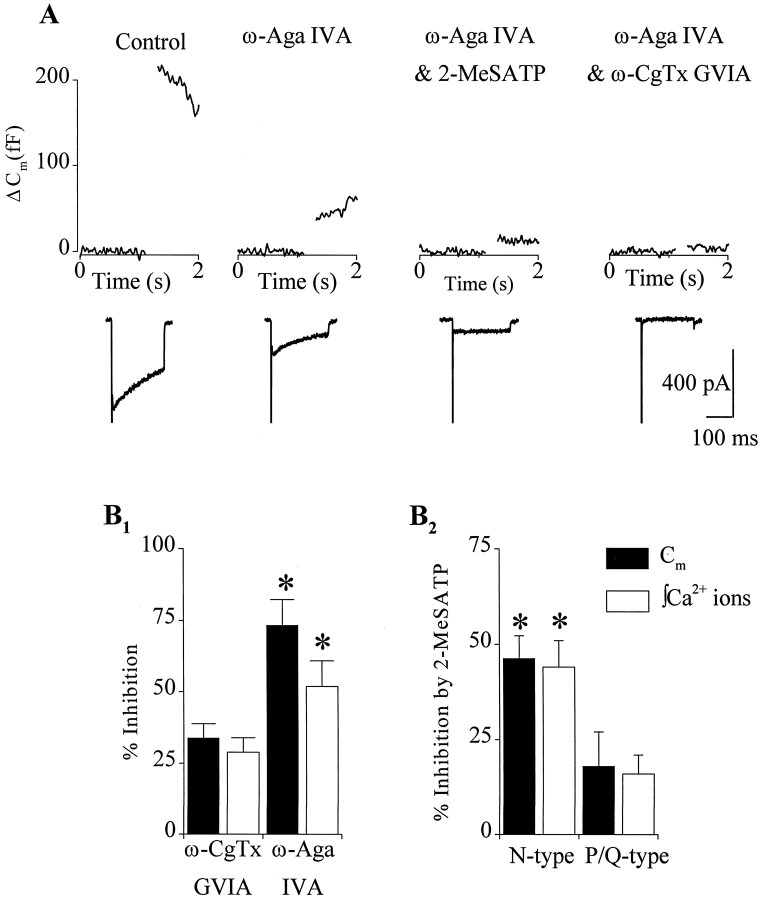
Release of neurotransmitter is often associated with specific subtypes of Ca2+ channels (Dunlap et al., 1995). In chromaffin cells, the role of pharmacologically distinct Ca2+ channels in exocyto-sis depends on stimulus conditions and development (Artalejo et al., 1994;Engisch and Nowycky, 1996; Lomax et al., 1997). We examined the role of different subtypes of Ca2+ channels in mediating the inhibitory effects of 2-MeSATP on exocytosis. Application of ω-conotoxin GVIA (ω-CgTx GVIA) (1 μm) to block N-type Ca2+ channels inhibited Ca2+ entry by 29 ± 5% and inhibited ΔCm evoked by single 200 msec depolarizations by 34 ± 5% (n = 8) (Fig.3B1). Blockade of P/Q-type Ca2+ channels with ω-agatoxin IVA (ω-Aga IVA) (300 nm) inhibited Ca2+ entry by 52 ± 9% and inhibited ΔCm by 73 ± 9% (n = 4) (Fig.3A,B1). Combined application of ω-CgTx GVIA and ω-Aga IVA inhibited Ca2+ entry by 96 ± 1% and inhibited ΔCm by 94 ± 2% (n = 6) (Fig. 3A). No significant contributions to stimulus-evoked exocytosis from a dihydropyridine-sensitive “facilitation” channel were observed, presumably because our studies were performed on adult animals and Ca2+-dependent processes were not perturbed by using barium as the charge carrier. We conclude therefore that, under our recording conditions, Ca2+entry through N- and P/Q-type channels triggers exocytosis. Furthermore, as observed with 2-MeSATP, the inhibition of secretion produced by either toxin was proportional to the inhibition of Ca2+ entry and did not alter the Ca2+-dependence of exocytosis.
Fig. 3.
N- and P/Q-type Ca2+ channels mediated the inhibition of exocytosis by 2-MeSATP. A, Data shown are from a single cell and illustrate the effect of ω-Aga IVA (300 nm), 2-MeSATP (100 nm), and ω-CgTx GVIA (1 μm) on both the Cmchanges (top) and the associatedICa (bottom) recorded in response to 200 msec depolarization to +20 mV from a holding potential of −90 mV. The voltage step is indicated by the gap inCm trace. Both Cmchanges and ICa were inhibited by ω-Aga IVA (A, second panel). The remaining Cm change andICa were inhibited by 2-MeSATP (A, third panel) and could be completely blocked by combined addition of ω-Aga IVA and ω-CgTx GVIA (A, fourth panel).B1, Summary of effects of the Ca2+ channel toxins ω-CgTx GVIA (n = 8) and ω-Aga IVA (n = 4) on integrated Ca2+ entry (open bars) and ΔCm (filled bars). B2, Summary of the effect of 2-MeSATP on integrated Ca2+ entry (open bars) and ΔCm(filled bars) evoked by isolated Ca2+ channel subtypes (N-type, n= 5; P/Q-type, n = 4). ω-CgTx GVIA was used to isolate P/Q-type Ca2+ channels, and ω-Aga IVA was used to isolate N-type Ca2+ channels. Similar results were obtained irrespective of the order in which the toxins were applied.
To investigate the contribution of each subtype of Ca2+ channel to the modulation of secretion by 2-MeSATP, we applied ω-CgTx GVIA (1 μm) or ω-Aga IVA (300 nm) alone to isolate P/Q- or N-type Ca2+ channels, respectively. 2-MeSATP (100 nm) inhibited Ca2+ entry and ΔCm evoked by N-type Ca2+ channels by 46 ± 6 and 44 ± 7% (n = 5) (Fig.3A,B2), whereas Ca2+ entry and ΔCm evoked by P/Q-type Ca2+ channels was inhibited by 18 ± 9 and 16 ± 5% (n = 4), respectively. The inhibitory effects of 2-MeSATP on Ca2+entry and ΔCm involving N-type channels was significantly greater than that involving P/Q-type channels (p < 0.05) (Fig.3B2).
Strong depolarizing prepulses reverse 2-MeSATP inhibition of ΔCm
Modulation of neuronal Ca2+ channels by Gi/o-proteins is thought to occur via a “membrane-delimited” mechanism involving a direct interaction of Gβγ subunits with the Ca2+ channel α subunit (Dolphin, 1998). This interaction is voltage-dependent and can be relieved by strong depolarizing prepulses. We reasoned that, if 2-MeSATP inhibited exocytosis via inhibition of Ca2+channels, then the inhibition of evoked ΔCm should also be attenuated by strong depolarizing prepulses. To test this, the test pulse used to evoke exocytosis was preceded by a 20 msec depolarizing pulse to +120 mV (Fig.4A,B). In the absence of the agonist, application of the prepulse had no significant effect on either ΔCm(98 ± 1% of control) or Ca2+(98 ± 1% of control; n = 5; data not shown) entry. In the presence of 2-MeSATP (100 nm), a depolarizing prepulse, however, reduced the inhibition of both Ca2+ entry and ΔCm from 23 ± 3 and 35 ± 4% to 10 ± 2 and 12 ± 4%, respectively (n= 9) (Fig. 4C). To show that the decreased inhibition by 2-MeSATP observed after a prepulse was not simply a result of receptor desensitization, in three of these cells, superfusion with 2-MeSATP was continued and an additional test pulse without a prepulse was given. The Ca2+ entry and ΔCm response to this second test pulse were inhibited by 16 ± 3 and 31 ± 7%, respectively (n = 3). These experiments demonstrate that the inhibition of exocytosis by 2-MeSATP has the same voltage-dependence as inhibition of Ca2+ channels in adrenal chromaffin cells and support the hypothesis that the inhibition of ΔCm observed is caused by inhibition of Ca2+ entry.
Fig. 4.
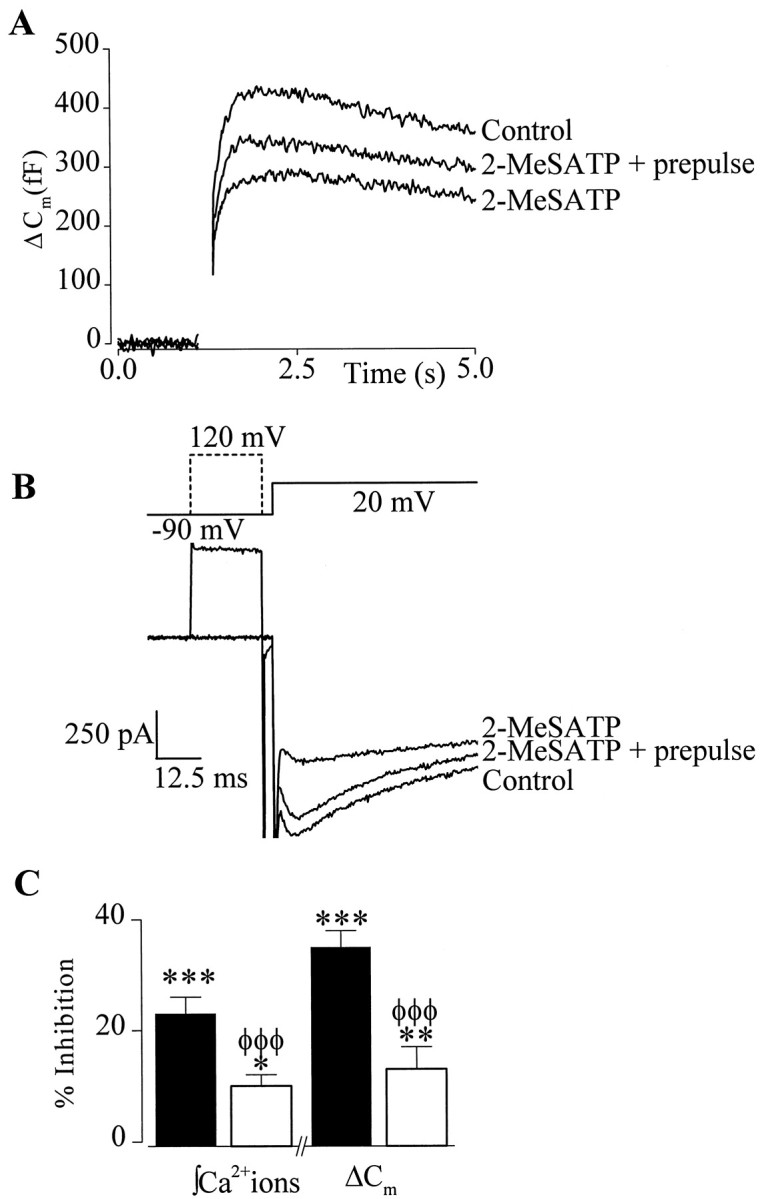
Modulation of both integrated Ca2+ entry and ΔCm was voltage-dependent. A, Superimposed traces from a single cell showing the effect of 2-MeSATP on ΔCmevoked by a 200 msec depolarization to +20 mV from a holding potential of −90 mV with and without a 20 msec depolarizing prepulse.B, Top, Schematic diagram of prepulse (hashed line) voltage protocol. B,Bottom, Superimposed ICa used to evoke ΔCm illustrated inA. Only the first 100 msec of the 200 msec test pulse are shown for clarity. C, Summary of the effects of 2-MeSATP on integrated Ca2+ entry and ΔCm in the presence (open bars) or absence (filled bars) of a depolarizing prepulse (n = 9). Significant effects of 2-MeSATP on Ca2+ entry andCm relative to control are indicated: ***p < 0.005; **p < 0.01; *p < 0.05. Significant differences between responses recorded with and without a prepulse are indicated byφφφp < 0.005.
P2Y purinoceptor activation does not alter the Ca2+-dependence of ΔCm
To test further the hypothesis that inhibition of Ca2+ entry accounts for inhibition of ΔCm, we examined the effect of increasing pulse duration on 2-MeSATP inhibition of stimulus–secretion coupling. During long depolarizations, agonist-dependent inhibition of Ca2+ channels is overcome (Dolphin, 1998), and therefore, an attenuation of the effects of 2-MeSATP on exocytosis are expected. The time-dependence of the modulation of ΔCm and Ca2+ entry by 2-MeSATP are shown in Figure5, A and B. Increasing pulse duration from 20 to 400 msec significantly reduced (p < 0.01) the inhibition of both ΔCm and Ca2+ entry from 67 ± 11 and 51 ± 2% (n = 5) to 14 ± 4 and 12 ± 3% (n = 4), respectively. Moreover, the small inhibition remaining with 400 msec depolarization was reversed by depolarizing prepulses to +120 mV (n = 4; data not shown). The results from these experiments support the role of Ca2+ channels in agonist-dependent inhibition of exocytosis.
Inhibition of exocytosis by a GPCR may also result from a direct decrease in the Ca2+ sensitivity of the secretory machinery or a decrease in the probability of release. The concomitant inhibition of ΔCm and Ca2+ entry, coupled with the lack of significant effect of 2-MeSATP on exocytotic efficiency (Fig.2E), provide some evidence that the Ca2+ sensitivity of the secretory machinery was not changing. To test this further, we examined the Ca2+-dependence of ΔCm using the method described byEngisch and Nowycky (1996). Exocytosis was evoked by single pulses of varying duration, first under control conditions and then in the presence of 2-MeSATP (100 nm) (Fig.5A). The control data from 10 cells was plotted and fit with an allometric function ΔCm =g * (ΔCa2+)n, and the best fit was obtained with a proportionality constant g= 0.0842 and power n = 1.62 (Fig. 5C). The power value obtained by fitting data from individual cells with the allometric function ranged from n = 1.01 to 3.49, meann = 1.68 ± 0.29. Similar variability and values for n were reported by Engisch and Nowycky (1996). What accounts for this variability is at present unknown; however, it may involve differences in the amounts of endogenous Ca2+ buffers and size of the readily releasable pool of vesicles. Thus, in cells in which endogenous Ca2+ buffering is high, the relationship may be more linear because of a requirement to saturate the buffer before exocytosis can proceed. Similarly, in cells in which the size of the readily releasable pool of vesicles is small, the secretory response measured in response to long depolarizations may be dominated by release from the reserve pool. The Ca2+-dependence of vesicle recruitment from the reserve pool to the readily releasable pool is essentially linear (Neher, 1998). None the less, data obtained by varying Ca2+ entry by changing the step potential (n = 4) (Fig. 5C, open triangle) or application of Ca2+ channel toxins (data not shown) did not deviate from the allometric fit, demonstrating that the Ca2+-dependence of exocytosis was unaffected by simply reducing Ca2+ influx. Similarly, exocytosis evoked by 200 or 400 msec depolarizations in the presence of 2-MeSATP also overlays the control allometric fit (Fig.5C, open circles). These experiments provided evidence that the inhibition of exocytosis observed with 2-MeSATP was not caused by a decrease in the Ca2+-dependence of exocytosis.
2-MeSATP does not modulate exocytosis evoked by flash-released Ca2+
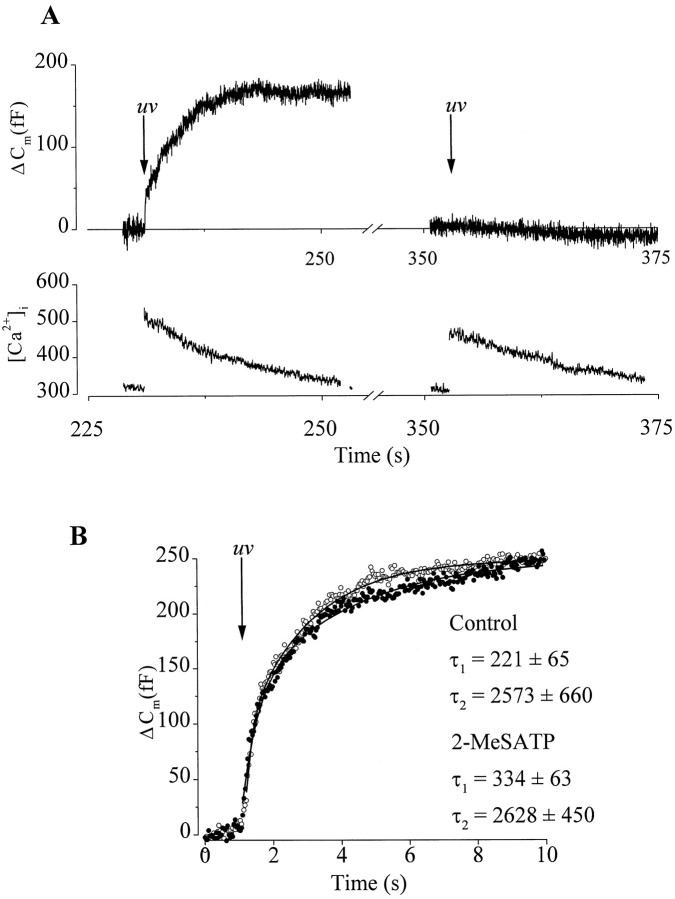
To determine whether 2-MeSATP inhibited the secretory machinery in chromaffin cells, we bypassed Ca2+channels and used flash photolysis of caged Ca2+ to provide the trigger for exocytosis. We hypothesized that, if 2-MeSATP decreased either the Ca2+-dependence or the probability of release, then the rate of ΔCm evoked by photolyzed Ca2+ should also be decreased relative to control. In these experiments, we used NP-EGTA as the photolabile Ca2+ chelator because, in contrast to (1-(2-nitro-4,5-dimethoxyphenyl)-1,2-diaminoethane-N,N,N′,N′-tetra-acetic; dimethoxynitrophenamine)-nitrophen, it did not produce a loading transient and produced smaller increases in [Ca2+]i, thus avoiding exocytosis of nonsecretory granules (Neher and Zucker, 1993; Parsons et al., 1996; Xu et al., 1998). Figure6A shows a typical whole-cell recording of ΔCm and [Ca2+]i from a chromaffin cell loaded with NP-EGTA Ca2+and fura-2. After 3 min of dialysis a UV flash was given to elevate [Ca2+]i, which produced a biphasic increase in ΔCm(Fig. 6B). Because the [Ca2+]i could not be recorded during the flash, the estimated peak [Ca2+]i was calculated by fitting the decay of the fura-2 signal with a monoexponential curve. The [Ca2+]i evoked by a single flash measured by this method peaked at 3.7 ± 1.2 μm (n = 12), well below the 100 μm[Ca2+]i reported to evoke nonsecretory granule fusion (Xu et al., 1998). The mean exocytotic rate measured during 1 sec immediately after the flash was 99 ± 14 fF/sec (n = 9), significantly less than the rate evoked by a single 200 msec depolarization (∼1000 fF/sec). Previous studies have shown that the rate of secretion is proportional to [Ca2+]i and the high exocytotic rates typically produced by depolarizations require [Ca2+]i >50 μm (Neher and Zucker, 1993). Thus, the relatively slow secretory rates observed with photoreleased Ca2+ in our studies are consistent with the small increases in [Ca2+]i measured (∼ 4 μm) and are in good agreement with the rates obtained by Heinemann et al. (1994).
Fig. 6.
P2Y receptor activation did not modulate secretion evoked by flash photolysis of caged Ca2+. A, Cm traces (top trace) and corresponding [Ca2+]i (bottom trace) recorded in response to the first flash (80 J, 500 μsec) given ∼240 sec after establishing the whole-cell configuration (left panel) and a second flash of the same intensity given 120 sec later to the same cell (right panel).B, Kinetic analysis of two example ΔCm recorded in response to the first flash after establishing the whole-cell configuration, either under control conditions (○) or in the presence of 100 nm2-MeSATP (●). For clarity of illustration, only every third point is shown. The solid line drawn through the data represents a double-exponential fit obtained using theCm point immediately after the UV flash and the plateau level as limits. The mean time constants displayed are for both control conditions (n = 5) and in the presence of 100 nm 2-MeSATP (n = 4).
Under control conditions, we observed rapid secretory rundown such that subsequent flashes failed to produce reproducible ΔCm, despite comparable increases in [Ca2+]i (Fig.6A), suggesting that refilling of the readily releasable pool was impaired in whole-cell recording (see also Seward and Nowycky, 1996). This prevented the examination of the effects of P2Y purinoceptor activation on flash-evoked ΔCm within a single cell. Therefore, we compared secretory rates recorded from matched (same culture) control cells and cells superfused with 100 nm2-MeSATP for 2 min before the first UV flash. 2-MeSATP did not significantly affect the rates (Fig. 6B), nor did it inhibit the extent of ΔCm (control, 194 ± 56 fF, n = 6; 2-MeSATP, 140 ± 49 fF,n = 4) evoked by photolysis of NP-EGTA, providing further evidence against a direct inhibitory action of P2Y purinoceptors on the secretory machinery of chromaffin cells.
2-MeSATP acts through Gi/o-proteins to inhibit ΔCm evoked by a train of depolarizing pulses
In situ chromaffin cells secrete catecholamines, ATP, and peptides most efficiently in response to splanchnic nerve stimulation and bursts of action potentials (Douglas and Poisner, 1966; Edwards et al., 1980). To determine whether P2Y receptor activation inhibited exocytosis evoked by bursts of action potentials, we examined the effects of 2-MeSATP on ΔCm evoked by trains of short-duration depolarizations. A control train of 20 depolarizations of 20 msec duration given at 2.5 Hz evoked entry of 541 ± 42 × 106 Ca2+ions and ΔCm of 265 ± 31 fF in total, and an overall mean exocytotic efficiency of 0.64 ± 0.2 fF/106 Ca2+ions. Similar responses were evoked by subsequent control trains of depolarizations (mean exocytotic efficiency 0.52 ± 0.7 fF/106 Ca2+ions; n = 23; p = 0.92), showing that exocytosis did not undergo activity-dependent changes with these relatively mild stimuli (Engisch et al., 1997; Smith, 1999). Both the ΔCm and Ca2+ entry in response to a train of depolarizations were significantly inhibited by 100 nm 2-MeSATP (Fig.7A,B); thus, total Ca2+ entry was reduced to 435 ± 34 × 106Ca2+ ions and total ΔCm to 222 ± 31 fF (n = 23). Inhibition of Ca2+ entry and ΔCm were, however, more pronounced early in the train, reaching a maximum of 54 ± 6% on the fourth pulse and a minimum of 18 ± 5% on the 20th pulse (n = 23) (Fig. 7B).
Fig. 7.
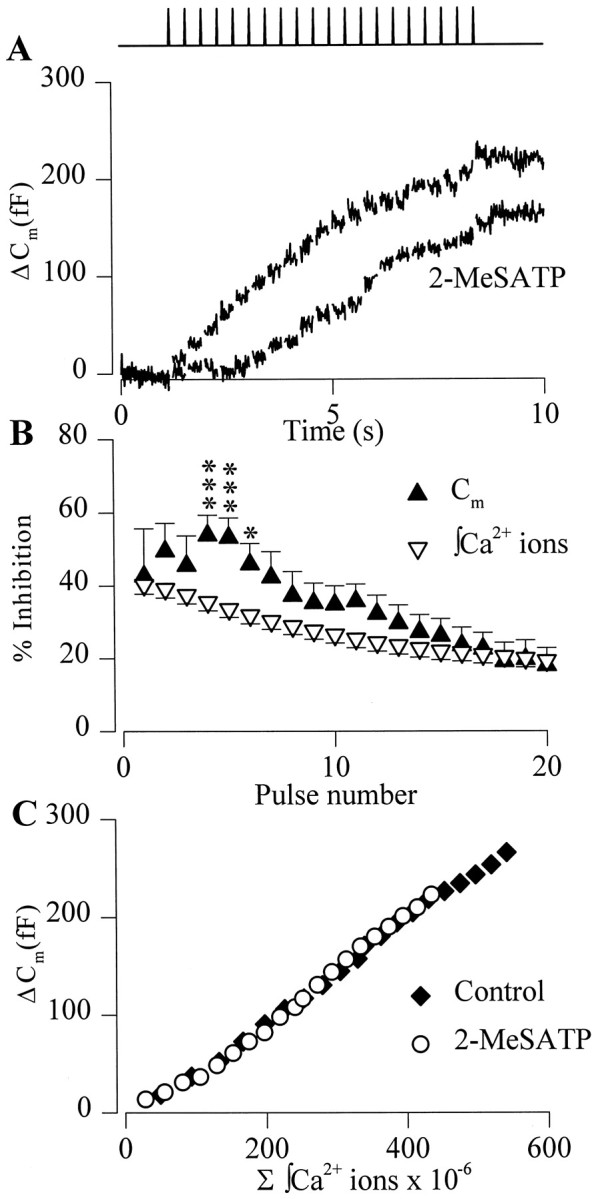
P2Y receptor activation inhibited ΔCm evoked by a train of depolarizing pulses. A, Superimposed Cmtraces recorded from a single cell in response to a train of twenty 20 msec depolarizing pulses to +20 mV from −90 mV. A control train (top trace) evoked an appreciable change inCm, and superfusion of 2-MeSATP (bottomtrace) inhibited the ΔCm in response to a subsequent train. Gaps in the traces and schematic in the top panelindicate timing of the pulses. B, Summary of the mean ± SEM inhibition of integrated Ca2+ entry (▿) and ΔCm (▴) by 2-MeSATP for each pulse in the train of depolarizations. Significant differences between inhibition of integrated Ca2+ entry and ΔCm changes are shown: ***p < 0.005; *p < 0.05 (n = 23). C, Plot of the mean Ca2+-dependence of ΔCmmeasured in response to a train of depolarizing pulses in the absence (♦) and presence (○) of 2-MeSATP (n = 23). Error bars are omitted for clarity.
Neither the exocytotic efficiency (0.51 ± 0.6 fF/106 Ca2+ions; p = 0.98) nor the Ca2+-dependence of secretion evoked by trains of depolarizations was altered by 2-MeSATP (Fig. 7C), suggesting that the mechanisms involved in inhibition of secretion evoked by single long pulses or trains of depolarizations are similar. We next examined the voltage-dependence of the inhibition by giving a 20 msec depolarizing prepulse to +120 mV before every test pulse in the train. In the absence of depolarizing prepulses, 2-MeSATP inhibited both Ca2+ entry and ΔCm by 35 ± 4 and 45 ± 8%, respectively (mean inhibition for pulses four to eight of a train,n = 4). In the same cells, a depolarizing prepulse preceding each test pulse reduced the inhibitory effect of 2-MeSATP on Ca2+ entry to −2 ± 4% and ΔCm to −17 ± 12%. Finally, we examined the second messenger system mediating the inhibitory effects of 2-MeSATP on stimulus–secretion coupling in chromaffin cells. Pretreatment with PTX (250 ng/ml, 24 hr) reduced the inhibitory effect of 2-MeSATP (100 nm) on Ca2+ entry to −2 ± 2.5% and ΔCm to −8 ± 14% (n = 4). Collectively, these data show that 2-MeSATP inhibition of exocytosis evoked by single or trains of depolarizations was mediated by a Gi/o-coupled P2Y purinoceptor modulating Ca2+ entry through Ca2+ channels.
DISCUSSION
Given the ubiquity of ATP in secretory vesicles (Zimmermann, 1994) and the profusion of P2 purinoceptors in the nervous system (North and Barnard, 1997), the potential for ATP to play a major modulatory role in neurotransmission is high. In the sympathetic nervous system, the inhibitory effects of presynaptic purinoceptors on neurotransmission have been well documented (Von Kügelgen et al., 1989; Boehm, 1999). A lack of selective agonists and antagonists, coupled with the inaccessibility of the majority of mammalian nerve terminals, have, however, made investigation of the mechanisms underlying inhibition of transmitter release by ATP difficult. In this study, we have used combined voltage-clamp recording andCm measurements to investigate the effects of purinoceptors on exocytosis in adrenal chromaffin cells. The results show that 2-MeSATP, a potent agonist at P2Y purinoceptors, inhibited exocytosis through an effect on N- and P/Q-type Ca2+ channels. The inhibition of stimulus–secretion coupling was mediated by Gi/o-proteins and was voltage-dependent. Activation of P2Y purinoceptors had no effects downstream of Ca2+ entry on the secretory machinery. The results of this study strongly support the hypothesis that inhibition of presynaptic Ca2+ channels plays the major role in presynaptic inhibition of elicited neurotransmitter release by Gi/oPCRs (Wu and Saggau, 1997) and that this is the mechanism underlying negative feedback modulation of sympathetic transmitter release by P2Y purinoceptors.
Inhibition of neuronal Ca2+ channels by GPCR is thought to represent a ubiquitous mode of modulation of neuronal function (Hille, 1994). The most widely studied and best understood pathway is a membrane-delimited pathway involving a direct interaction of Gβγ subunits with the Ca2+ channel pore-forming α1 subunit (Page et al., 1998; Jeong and Ikeda, 1999). A characteristic property of this signaling pathway is its voltage-dependence in that the inhibition is relieved by strong depolarizations (Dolphin, 1998). In agreement with previous studies in chromaffin cells (Diverse-Pierluissi et al., 1991; Currie and Fox, 1996), we show that P2Y purinoceptors inhibit N- and P/Q-type Ca2+ channels by a PTX-sensitive, voltage-dependent pathway, strongly suggesting that the membrane-delimited pathway is involved (Figs. 1, 3, 4). A functional link between the P2Y-mediated suppression of evokedICa and secretion is shown by the inhibition of evoked ΔCm (Figs. 2,3, 6). In agreement with previous studies (Currie and Fox, 1997;Zamponi and Snutch, 1998), we also find a greater inhibition of Ca2+ entry through N-type over P/Q-type channels, and this is paralleled by a greater inhibition of secretion supported by N-type channels. Paradoxically, we found that N-type channels contributed less than P/Q-type channels to secretion evoked by long depolarizations. The lower sensitivity of P/Q-type channels to modulation by 2-MeSATP suggests either a lower efficacy of interaction between Gi/o-proteins and P- and/or Q-type channels (Bourinet et al., 1999) or preferential compartmentalization of P2Y purinoceptors with N-type channels (Carabelli et al., 1998). Such differential coupling between a GPCR and exocytosis will have significant functional consequences in neurons in which there is tight coupling between subtypes of Ca2+ channels and transmitter release (for review, see Caterall, 1999) and could provide a mechanism whereby cotransmitters stored in separate pools of vesicles may be independently modulated (Von Kügelgen, 1996).
The results from this study demonstrate that the inhibition of exocytosis by a Gi/oPCR can be fully accounted for by the inhibition of Ca2+ channels. Three lines of evidence support this hypothesis. First, the Ca2+-dependence of secretion for both stimulus parameters is unaffected by activation of P2Y purinoceptors (Figs. 5, 7). Second, strong depolarizing prepulses relieve the voltage-dependent inhibition of Ca2+channels by 2-MeSATP and, in parallel, the inhibition of exocytosis (Fig. 4). We do not believe that the voltage-dependent relief of secretory inhibition observed in chromaffin cells is caused by a Ca2+-independent, voltage-dependent enhancement of exocytosis similar to that described for fast neurotransmitter systems (Linial et al., 1997; Mochida et al., 1998) because, under our control conditions, strong depolarizing prepulses did not enhance ΔCm per se. The final piece of evidence for the lack of effect of P2Y purinoceptor activation on the secretory machinery comes from the flash photolysis experiments in which exocytosis was evoked independently of Ca2+ channels (Fig. 6). Our data contradicts conclusions from a previous study in which it was suggested that ATP can inhibit the exocytotic machinery in chromaffin cells (Lim et al., 1997). The discrepancy between the two studies may arise from either differences between signaling systems of chromaffin cells from two species or differences in the methods used. Lim and coworkers (1997) used the whole-cell patch-clamp recording technique and examined the rates of exocytosis after slow infusion of buffered Ca2+ solutions into the cells. Differences in chromaffin cell secretory properties have been reported with secretory rundown, complicating data interpretation (von Ruden and Neher, 1993; Seward et al., 1995; Engisch and Nowycky, 1996). Interestingly, the results from our study are in close agreement with results from a central synapse (Takahashi et al., 1996) and peptidergic terminals (Rusin et al., 1997), suggesting that G-protein regulation of voltage-operated Ca2+ channels is the predominant mechanisms underlying presynaptic inhibition of evoked release.
Exocytosis evoked by long depolarizations in chromaffin cells consists of two kinetically distinct components, referred to as immediate and slow exocytosis (Horrigan and Bookman, 1994). P2Y purinoceptor activation inhibited the slow phase of exocytosis to a significantly greater extent than the immediate phase (Fig. 2). A similar observation has been previously reported for opioid inhibition of exocytosis in the neurohypophysis (Rusin et al., 1997). The two kinetically distinct phases of exocytosis are thought to represent release of vesicles with varying degrees of readiness depending on their location in the secretory pathway; thus, immediate exocytosis is caused by fusion of readily releasable vesicles docked to Ca2+channels, and slow release is caused by fusion of readily releasable vesicles responding to diffused Ca2+(Thomas et al., 1990; Horrigan and Bookman, 1994; Chow et al., 1996;Seward and Nowycky, 1996). Alternatively, slow release may represent exocytosis of vesicles from the reserve pool, which are also regulated by diffused Ca2+. Release evoked by diffused Ca2+ is expected to involve multiple subtypes of Ca2+ channels and their associated Gi/o-proteins, and therefore, consistent with our results, the inhibition of slow release should be correlated to the inhibition of the total Ca2+ entry. On the other hand, if immediate ΔCm are associated with chromaffin granules preferentially colocalized with a subtype of Ca2+ channel, then modulation of immediate secretion would depend on the efficacy of coupling between Gi/o-proteins and that particular channel subtype. The relatively small inhibition of immediate exocytosis observed with 2-MeSATP more closely matches the inhibition of P/Q-type than N-type Ca2+ channels, which would be expected if granules are preferentially docked to P/Q-type channels. Support of granule docking to P/Q-type channels comes from a previous study (Lara et al., 1998), as well as our own observations that ω-Aga IVA is a more potent inhibitor of exocytosis than ω-CgTx GVIA. Alternatively, a reduced effectiveness of P2Y purinoceptors to inhibit immediate exocytosis may result from saturation of the Ca2+ sensor (Rusin et al., 1997). We do not believe this to be the case because, if the fusion machinery were saturated then at short depolarizations in which release occurs predominantly from the readily releasable pool, the inhibition of secretion by 2-MeSATP should be occluded. This was not observed; indeed, inhibition was greatest with short depolarizations (Fig.5B). Although the mechanisms underlying the reduced inhibition of immediate release require further investigation, a consequence of this phenomenon will be that, in vivo, released ATP would be more effective at inhibiting asynchronous release than synchronous release and thus would serve to maintain a tight temporal correlation between action potential firing and evoked transmitter release.
Catecholamine secretion from chromaffin cells is most efficiently triggered by high-frequency stimulation of the splanchnic nerve and bursts of action potentials (Edwards, 1982; Zhou and Misler, 1995). We found that P2Y modulation of secretion declines during a train of short depolarizations such that ∼50% of the modulatory effect remains at the end of a train. There are two possible explanations for the loss of effect. First, this study (Fig. 7B) and other studies (Seward et al., 1995; Engisch and Nowycky, 1996) show that significant inactivation of Ca2+ channels occurs during a train of depolarizations. It is possible that the loss of modulatory effect is caused by inactivation of the Ca2+ channels modulated by 2-MeSATP. Alternatively, recent studies have shown that voltage-dependent modulation of Ca2+ channels by Gβγ subunit is strongly dependent on the frequency at which the channels are activated, with high-frequency trains reversing inhibition (Brody et al., 1997; Park and Dunlap, 1998). It seems unlikely, however, that this is occurring in our experiments because reinhibition of N- and P/Q-type channels by G-proteins has a time constant of ∼100 msec in chromaffin cells (Currie and Fox, 1997), which is significantly faster than the 400 msec interpulse duration used in our studies. Therefore, at the high stimulus frequencies that can occur in vivo when an animal is in danger and the fight-or-flight response is activated, purinoceptor inhibition of catecholamine secretion may be virtually abolished by a combination of these two mechanisms.
Chromaffin cells costore and secrete various neuromodulators with catecholamines, including ATP (Winkler and Westhead, 1980). The functional role of ATP in secretory vesicles has recently become the focus of much interest. Evidence suggesting that ATP forms part of an autocrine inhibitory loop controlling Ca2+channels has been provided by several studies (Albillos et al., 1996;Currie and Fox, 1996; Carabelli et al., 1998). The results of this study show that P2Y purinoceptors inhibit stimulus-evoked exocytosis in chromaffin cells as a direct consequence of Gi/o-protein inhibition of Ca2+ channels and the Ca2+ signals regulating vesicle fusion rather than a direct effect on the exocytotic machinery. Presynaptic P2Y purinoceptors in the brain and periphery may act in a similar manner to regulate exocytosis of catecholamines, as well as other transmitters.
Footnotes
This work was supported by a project grant to E.P.S. from the Wellcome Trust and a studentship to A.D.P. from the Medical Research Council of the United Kingdom. We thank Drs. Graeme Henderson, Neil V. Marrion, and Mark Wall for helpful discussion of the work and manuscript.
Correspondence should be addressed to Dr. Elizabeth P. Seward, Department of Pharmacology, University of Bristol, University Walk, Bristol, BS8 1TD, UK. E-mail: liz.seward@bris.ac.uk.
REFERENCES
- 1.Albillos A, Gandía L, Michelena P, Gilabert JA, Del Valle M, Carbone E, García AG. The mechanism of calcium channel facilitation in bovine chromaffin cells. J Physiol (Lond) 1996;494:687–695. doi: 10.1113/jphysiol.1996.sp021524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artalejo CR, Adams ME, Fox AP. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature. 1994;367:72–76. doi: 10.1038/367072a0. [DOI] [PubMed] [Google Scholar]
- 3.Boehm S. ATP stimulates sympathetic transmitter release via presynaptic P2X purinoceptors. J Neurosci. 1999;19:737–746. doi: 10.1523/JNEUROSCI.19-02-00737.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeynaems J-M, Communi D, Janssens R, Motte S, Robaye B, Pirotton S. Nucleotide receptors coupling to the phospholipase C signaling pathway. In: Turner JT, Weisman GA, Fedan JS, editors. The P2 nucleotide receptors. Humana; Totowa, NJ: 1998. pp. 169–183. [Google Scholar]
- 5.Bourinet E, Soong TW, Sutton KG, Slaymaker SJ, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of α 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci. 1999;2:407–415. doi: 10.1038/8070. [DOI] [PubMed] [Google Scholar]
- 6.Brody DL, Patil PG, Mulle JG, Snutch TP, Yue DT. Bursts of action potential waveforms relieve G-protein inhibition of recombinant P/Q-type Ca2+ channels in HEK 293 cells. J Physiol (Lond) 1997;499:637–644. doi: 10.1113/jphysiol.1997.sp021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carabelli V, Carra I, Carbone E. Localized secretion of ATP and opioids revealed through single Ca2+ channel modulation in bovine chromaffin cells. Neuron. 1998;20:1255–1268. doi: 10.1016/s0896-6273(00)80505-x. [DOI] [PubMed] [Google Scholar]
- 8.Catterall WA. Interactions of presynaptic Ca2+ channels and SNARE proteins in neurotransmitter release. Ann NY Acad Sci. 1999;868:144–159. doi: 10.1111/j.1749-6632.1999.tb11284.x. [DOI] [PubMed] [Google Scholar]
- 9.Chow RH, Klingauf J, Heinemann C, Zucker RS, Neher E. Mechanisms determining the time course of secretion in neuroendocrine cells. Neuron. 1996;16:369–376. doi: 10.1016/s0896-6273(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 10.Currie KP, Fox AP. ATP serves as a negative feedback inhibitor of voltage-gated Ca2+ channel currents in cultured bovine adrenal chromaffin cells. Neuron. 1996;16:1027–1036. doi: 10.1016/s0896-6273(00)80126-9. [DOI] [PubMed] [Google Scholar]
- 11.Currie KPM, Fox AP. Comparison of N- and P/Q-type voltage-gated calcium channel current inhibition. J Neurosci. 1997;17:4570–4579. doi: 10.1523/JNEUROSCI.17-12-04570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diverse-Pierluissi M, Dunlap K, Westhead EW. Multiple actions of extracellular ATP on calcium currents in cultured bovine chromaffin cells. Proc Natl Acad Sci USA. 1991;88:1261–1265. doi: 10.1073/pnas.88.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolphin AC. Mechanisms of modulation of voltage-dependent calcium channels by G proteins. J Physiol (Lond) 1998;506:3–11. doi: 10.1111/j.1469-7793.1998.003bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas WW, Poisner AM. On the relation between ATP splitting and secretion in the adrenal chromaffin cell: extrusion of ATP (unhydrolyzed) during release of catecholamines. J Physiol (Lond) 1966;183:249–256. doi: 10.1113/jphysiol.1966.sp007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 16.Edwards AV. Adrenal catecholamine output in response to stimulation of the splanchnic nerve in bursts in the conscious calf. J Physiol (Lond) 1982;327:409–419. doi: 10.1113/jphysiol.1982.sp014239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards AV, Furness PN, Helle KB. Adrenal medullary responses to stimulation of the splanchnic nerve in the conscious calf. J Physiol (Lond) 1980;308:15–27. doi: 10.1113/jphysiol.1980.sp013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- 19.Engisch KL, Nowycky MC. Calcium dependence of large dense-cored vesicle exocytosis evoked by calcium influx in bovine adrenal chromaffin cells. J Neurosci. 1996;16:1359–1369. doi: 10.1523/JNEUROSCI.16-04-01359.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engisch KL, Chernevskaya NI, Nowycky MC. Short-term changes in the Ca2+ –exocytosis relationship during repetitive pulse protocols in bovine adrenal chromaffin cells. J Neurosci. 1997;17:9010–9025. doi: 10.1523/JNEUROSCI.17-23-09010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans RJ, Derkach V, Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- 22.Fidler N, Fernandez JM. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989;56:1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandia L, Garcia AG, Morad M. ATP modulation of calcium channels in chromaffin cells. J Physiol (Lond) 1993;470:55–72. doi: 10.1113/jphysiol.1993.sp019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibb AJ, Halliday FC. Fast purinergic transmission in the central nervous system. Semin Neurosci. 1996;8:225–232. [Google Scholar]
- 25.Grynkiewicz G, Peonie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 26.Heinemann C, Chow RH, Neher E, Zucker RS. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys J. 1994;67:2546–2557. doi: 10.1016/S0006-3495(94)80744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hille B. Modulation of ion-channel by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 28.Horrigan FT, Bookman RJ. Releasable pools and the kinetics of exocytosis in adrenal chromaffin cells. Neuron. 1994;13:1119–1129. doi: 10.1016/0896-6273(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 29.Jeong S-W, Ikeda SR. Sequestration of G-protein βγ subunits by different G-protein α subunits blocks voltage-dependent modulation of Ca2+ channels in rat sympathetic neurons. J Neurosci. 1999;19:4755–4761. doi: 10.1523/JNEUROSCI.19-12-04755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khakh BS, Henderson G. ATP receptor-mediated enhancement of fast excitatory neurotransmitter release in the brain. Mol Pharmacol. 1998;54:372–378. doi: 10.1124/mol.54.2.372. [DOI] [PubMed] [Google Scholar]
- 31.Lang J, Nishimoto I, Okamoto T, Regazzi R, Kiraly C, Weller U, Wollheim CB. Direct control of exocytosis by receptor-mediated activation of the heterotrimeric GTPases Gi and Go or by the expression of their active Gα subunits. EMBO J. 1995;14:3635–3644. doi: 10.1002/j.1460-2075.1995.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lara B, Gandia L, Martinez-Sierra R, Torres A, Garcia AG. Q-type Ca2+ channels are located closer to secretory sites than L-type channels: functional evidence in chromaffin cells. Pflügers Arch. 1998;435:472–478. doi: 10.1007/s004240050541. [DOI] [PubMed] [Google Scholar]
- 33.Lim W, Kim SJ, Yan HD, Kim J. Ca2+ -channel-dependent and -independent inhibition of exocytosis by extracellular ATP in voltage-clamped rat adrenal chromaffin cells. Pflügers Arch. 1997;435:34–42. doi: 10.1007/s004240050481. [DOI] [PubMed] [Google Scholar]
- 34.Linial M, Ilouz N, Parnas H. Voltage-dependent interaction between the muscarinic ACh receptor and proteins of the exocytic machinery. J Physiol (Lond) 1997;504:251–258. doi: 10.1111/j.1469-7793.1997.251be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lomax RB, Michelena P, Nunez L, Garcia-Sancho J, Garcia AG, Montiel C. Different contributions of L- and Q-type Ca2+ channels to Ca2+ signals and secretion in chromaffin cell subtypes. Am J Physiol. 1997;272:C476–C484. doi: 10.1152/ajpcell.1997.272.2.C476. [DOI] [PubMed] [Google Scholar]
- 36.MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 37.ManSonHing H, Zoran MJ, Lukowiak K, Haydon PG. A neuromodulator of synaptic transmission acts on the secretory apparatus as well as on ion channels. Nature. 1989;341:237–239. doi: 10.1038/341237a0. [DOI] [PubMed] [Google Scholar]
- 38.Miller RJ. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- 39.Mochida S, Yokoyama CT, Kim DK, Itoh K, Catterall WA. Evidence for a voltage-dependent enhancement of neurotransmitter release mediated via the synaptic protein interaction site of N-type Ca2+ channels. Proc Natl Acad Sci USA. 1998;95:14523–14528. doi: 10.1073/pnas.95.24.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 41.Neher E, Zucker RS. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron. 1993;10:21–30. doi: 10.1016/0896-6273(93)90238-m. [DOI] [PubMed] [Google Scholar]
- 42.North RA, Barnard EA. Nucleotide receptors. Curr Opin Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- 43.Page KM, Canti C, Stephens GJ, Berrow NS, Dolphin AC. Identification of the amino terminus of neuronal Ca2+ channel α1 subunits α1B and α1E as an essential determinant of G-protein modulation. J Neurosci. 1998;18:4815–4824. doi: 10.1523/JNEUROSCI.18-13-04815.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park D, Dunlap K. Dynamic regulation of calcium influx by G-proteins, action potential waveform, and neuronal firing frequency. J Neurosci. 1998;18:6757–6766. doi: 10.1523/JNEUROSCI.18-17-06757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parsons TD, Ellis-Davies GCR, Almers W. Millisecond studies of calcium-dependent exocytosis in pituitary melanotrophs: comparison of the photolabile calcium chelators nitrophenyl-EGTA and DM-nitrophen. Cell Calcium. 1996;19:185–192. doi: 10.1016/s0143-4160(96)90019-6. [DOI] [PubMed] [Google Scholar]
- 46.Rusin KI, Giovannucci DR, Stuenkel EL, Moises HC. κ-opioid receptor activation modulates Ca2+ currents and secretion in isolated neuroendocrine nerve terminals. J Neurosci. 1997;17:6565–6574. doi: 10.1523/JNEUROSCI.17-17-06565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seward EP, Nowycky MC. Kinetics of stimulus-coupled secretion in dialyzed bovine chromaffin cells in response to trains of depolarizing pulses. J Neurosci. 1996;16:553–562. doi: 10.1523/JNEUROSCI.16-02-00553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seward EP, Chernevskaya NI, Nowycky MC. Exocytosis in peptidergic nerve terminals exhibits two calcium-sensitive phases during pulsatile calcium entry. J Neurosci. 1995;15:3390–3399. doi: 10.1523/JNEUROSCI.15-05-03390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seward EP, Chernevskaya NI, Nowycky MC. Ba2+ ions evoke two kinetically distinct patterns of exocytosis in chromaffin cells, but not neurohypophysial nerve terminals. J Neurosci. 1996;16:1370–1379. doi: 10.1523/JNEUROSCI.16-04-01370.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen KZ, Surprenant A. Mechanisms underlying presynaptic inhibition through alpha2-adrenoceptors in guinea-pig submucosal neurones. J Physiol (Lond) 1990;431:609–628. doi: 10.1113/jphysiol.1990.sp018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silinsky EM. Inhibition of transmitter release by adenosine: are Ca2+ currents depressed or are the intracellular effects of Ca2+ impaired? Trends Pharmacol. 1986;7:180–185. [Google Scholar]
- 52.Smith C. A persistent activity-dependent facilitation in chromaffin cells is caused by Ca2+ activation of protein kinase C. J Neurosci. 1999;19:589–598. doi: 10.1523/JNEUROSCI.19-02-00589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- 54.Thomas P, Surprenant A, Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990;5:723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- 55.Thompson SM, Capogna M, Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 1993;16:222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- 56.Trifaro JM, Lee RWH. Characterization of anti-actin antibodies and their use in immunocytochemical studies on the localization of actin in adrenal chromaffin cells in culture. Neuroscience. 1981;6:2087–2108. doi: 10.1016/0306-4522(81)90048-8. [DOI] [PubMed] [Google Scholar]
- 57.Von Kügelgen I. Modulation of neural ATP release through presynaptic receptors. Semin Neurosci. 1996;8:247–257. [Google Scholar]
- 58.Von Kügelgen I, Schoffel E, Starke K. Inhibition by nucleotides acting at presynaptic P2-receptors of sympathetic neuro-effector transmission in the mouse isolated vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:522–532. doi: 10.1007/BF00260607. [DOI] [PubMed] [Google Scholar]
- 59.Von Kügelgen I, Kurz K, Starke K. P2-purinoceptor-mediated autoinhibition of sympathetic transmitter release in mouse and rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:125–132. doi: 10.1007/BF00169828. [DOI] [PubMed] [Google Scholar]
- 60.von Ruden L, Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993;262:1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]
- 61.Winkler H, Westhead EW. The molecular organisation of adrenal chromaffin granules. Neuroscience. 1980;5:1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]
- 62.Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 63.Xu T, Binz T, Neimann H, Neher E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nat Neurosci. 1998;1:192–200. doi: 10.1038/642. [DOI] [PubMed] [Google Scholar]
- 64.Zamponi GW, Snutch TP. Modulation of voltage-dependent calcium channels by G proteins. Curr Opin Neurobiol. 1998;8:351–356. doi: 10.1016/s0959-4388(98)80060-3. [DOI] [PubMed] [Google Scholar]
- 65.Zhou Z, Misler S. Action potential-induced quantal secretion of catecholamines from rat adrenal chromaffin cells. J Biol Chem. 1995;270:3498–3505. [PubMed] [Google Scholar]
- 66.Zimmermann H. Signalling via ATP in the nervous system. Trends Neurosci. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]