Abstract
We studied the role of GABA in adaptive changes in a lateral inhibitory system in the tiger salamander retina. In dark-adapted retinal slice preparations picrotoxin caused a slow enhancement of glycine-mediated IPSCs in ganglion cells. The enhancement of glycinergic IPSCs developed slowly over the course of 5–20 min, even though picrotoxin blocked both GABAA and GABACreceptors within a few seconds. The slow enhancement of glycinergic IPSCs by picrotoxin was much weaker in light-adapted preparations. The slow enhancement of glycinergic inhibitory inputs was not produced by bicuculline, indicating that it involved GABAC receptors. The responses of ganglion cells to direct application of glycine were not enhanced by picrotoxin, indicating that the enhancement was not caused by an action on glycine receptors. In dark-adapted eyecup preparations picrotoxin caused a slow enhancement of glycinergic IPSPs and transient lateral inhibition produced by a rotating windmill pattern, similar to the effect of light adaptation. The results suggest that the glycinergic inhibitory inputs are modulated by an unknown substance whose synthesis and/or release is inhibited in dark-adapted retinas by GABA acting at GABAC receptors.
Keywords: retina, adaptation, GABAC receptor, neuromodulator, glycine, salamander
The vertebrate retina undergoes several types of morphological and physiological adaptive changes during light and dark adaptation. Adaptive changes that occur beyond the photoreceptor level are generally termed “network adaptation” and may involve reorganization of synaptic pathways by several mechanisms such as changes in gap junction conductance or changes in gain at specific types of synapses in the retinal network. An understanding of these mechanisms is important because they may also occur in other parts of the nervous system. In the retina, several of these changes are mediated by the neuromodulator substances dopamine or nitric oxide (Dowling, 1991; Witkovsky and Dearry, 1991; Greenstreet and Djamgoz, 1994; Mills and Massey, 1995). However, there are other adaptive changes in the retina and other neural networks for which the mechanism and the modulatory substance(s) that mediate them are still poorly understood.
One such type of network adaptation in the retina is the modulation of change-sensitive or transient lateral inhibition (TLI), in which ganglion cells are inhibited by changing light stimuli. This inner retinal mechanism, which has been described in both amphibian and mammalian retinas, is mediated by transient (on–off) amacrine cells (Werblin, 1972; Werblin and Copenhagen, 1974; Thibos and Werblin, 1978;Enroth-Cugell and Jakiela, 1980). TLI is weak or absent in dark-adapted retinas, but it gradually becomes enabled over a time course of 5–20 min when the retina is exposed to an adapting light (Cook and McReynolds, 1998). Because the amacrine cells that mediate TLI in salamander retina are glycinergic (Cook et al., 1998), we studied glycinergic inhibition of ganglion cells under different conditions of adaptation. Because salamander ganglion cells receive both GABAergic and glycinergic inputs (Belgum et al., 1984), GABA antagonists were used to isolate the glycinergic inhibition. We found that in dark-adapted retinas picrotoxin, but not bicuculline, caused a slow enhancement of glycinergic inhibition that mimicked the effect of light adaptation. The results suggest that GABA, acting via GABAC receptors, affects the synthesis or release of an unknown substance that modulates glycinergic inhibition in the inner retina.
MATERIALS AND METHODS
Eyecup preparations were made from larval tiger salamanders (Ambystoma tigrinum) obtained from Charles Sullivan, Inc. (Nashville, TN). The care and use of animals were in accordance with the University of Michigan and the Society for Neuroscience policies on the use of animals in research. Details of the preparation, electrical recording, and light stimulation are described in detail elsewhere (Cook et al., 1998). Intracellular voltage recordings were made from on–off ganglion cells using micropipettes filled with 2m potassium acetate (resistance, 300–500 MΩ) and conventional electronics. Light stimuli were a 400-μm-diameter spot in the receptive field center (determined before the experiment) and concentric annuli [inner diameter (i.d.), 1200 or 500 μm; outer diameter (o.d.), 2600 μm]. All light stimuli were 560 nm, the intensity of which was controlled with calibrated neutral density filters and expressed as log quanta·cm−2·sec−1. Retinas were superfused with amphibian Ringer's solution (in mm): NaCl, 110; KCl, 2.5; CaCl2, 1.8; MgCl2, 1.2; glucose, 11; and HEPES buffer, 5, adjusted to pH 7.8 with NaOH; drugs were added by switching to another Ringer's solution containing either strychnine (2–10 μm), 150 μm picrotoxin, or both.
Retinal slice preparations (350–500 μm thick) were made from larval tiger salamander eyes as described in detail elsewhere (Lukasiewicz et al., 1994; Cook et al., 1998). Whole-cell voltage-clamp recordings were made using patch electrodes containing (in mm): cesium gluconate, 99; tetraethylammonium chloride, 8; NaCl, 3.4; MgCl2, 0.4; CaCl2, 0.4; EGTA, 11; and HEPES buffer, 10, adjusted to pH 7.7 with CsOH. The bath solution contained (in mm): NaCl, 112; KCl, 2; CaCl2, 2; MgCl2, 1; glucose, 5; and HEPES buffer, 5, adjusted to pH 7.8 with NaOH. Light stimuli were diffuse flashes of white light whose intensity at the retinal surface was equivalent to 3.6 × 108quanta·cm−2·sec−1at 560 nm. The slice was superfused, and drugs were applied through a large diameter pipette connected to a gravity-driven superfusion system, which permitted rapid switching between control and test solutions. Ganglion cells were identified by the location of their somas in the ganglion cell layer and by large (>1000 pA) inward currents elicited by depolarizing voltage steps. Some cells were also visually identified by inclusion of Lucifer yellow in the recording pipette.
Current recordings from slice preparations were digitized at 2 kHz. GABAA- and GABAC-mediated synaptic currents were blocked by the presence of 150 μmpicrotoxin in the superfusate. In some experiments the retina was electrically stimulated by applying zaps (1 msec positive current pulses; 0.1–2 μA) using a constant-current stimulator (Grass S48 with stimulus isolation unit PSIU6) through a Ringer's solution-filled pipette whose tip was located in the outer plexiform layer (OPL) directly over the recording site. The return path for the current was a silver–silver chloride electrode, separate from the recording ground electrode, connected to the bath through an agar bridge filled with 1 mm KCl. The OPL site was used because it produced larger and more consistent responses than when the electrode was located in the inner retina. Focal applications (puffs) of neurotransmitter were made by pressure (5 msec at 5 psi) ejection (Picospritzer) from a pipette containing 0.5 mm glycine or GABA. All drugs were obtained from Sigma (St. Louis, MO).
Fully dark-adapted eyecup and slice preparations were made from animals that had been dark-adapted overnight, with all procedures done in total darkness using infrared illumination. Where indicated, data were obtained from light-adapted preparations that had been previously dark-adapted but exposed to room light before the experiment, so that the degree of light adaptation was variable.
RESULTS
Picrotoxin causes a slow enhancement of glycinergic inputs to ganglion cells in dark-adapted retinal slices
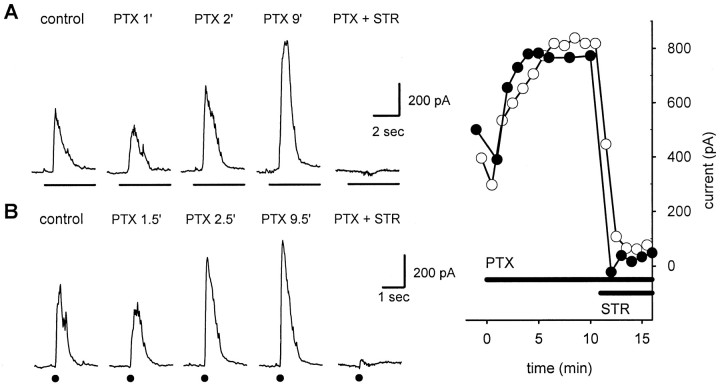
Because light stimuli elicit both GABA- and glycine-mediated responses in ganglion cells, we used picrotoxin to block GABA receptors and isolate glycinergic IPSCs. Figure1A shows whole-cell patch recordings from an on–off ganglion cell in a dark-adapted retinal slice preparation. The cell was voltage clamped at 0 mV to eliminate excitatory currents, and IPSCs were elicited by full-field light stimuli. In the slice preparation the light stimuli produced transient IPSCs at light on and light off, although only the responses at light onset are shown here. Responses to the same light stimulus were recorded in normal Ringer's solution (control), at 1, 2, and 9 min after the onset of continuous superfusion with 150 μmpicrotoxin, and finally after addition of 2 μm strychnine while still in picrotoxin. Immediately after the addition of picrotoxin (PTX 1′) the IPSC was reduced in amplitude because the GABA-mediated component was blocked. The small glycinergic IPSC then slowly increased in amplitude and reached a maximum after 9 min in picrotoxin (PTX 9′). The IPSC in picrotoxin was completely abolished by the subsequent addition of strychnine (PTX + STR). The effects of picrotoxin on the IPSCs at light off (data not shown) were similar.
Fig. 1.
Effects of picrotoxin on inhibitory currents in ganglion cells. Responses are from an on–off ganglion cell in a tiger salamander slice preparation. The cell was voltage clamped at 0 mV to eliminate glutamate-mediated excitatory currents.A, IPSCs elicited by 4 sec full-field light stimuli (indicated by the horizontalbarbeloweachtrace).Traces show IPSCs recorded before application of picrotoxin (control) and 1, 2, and 9 min after onset of superfusion with picrotoxin (PTX 1′, 2′, and9′). Subsequent addition of 2 μmstrychnine in the continued presence of picrotoxin (PTX + STR) completely blocked the enhanced IPSCs, indicating that they were mediated by glycine. The holding current was +42 pA. The light flashes also elicited IPSCs at light off (data not shown); the effects on the off responses were similar to those of the on responses.B, Same as A, except that the IPSCs were evoked by zaps (+0.5 μA; 1 msec) in the outer plexiform layer directly above the recorded ganglion cell. The time of the zap stimulus is indicated by dotbelow each response.Inset,Right, Peak light-evoked (filledcircles) and zap-evoked (opencircles) IPSC amplitudes at additional times.
We also tested the effect of picrotoxin on IPSCs elicited by focal electrical stimulation (zaps) in the outer plexiform layer directly over the recorded ganglion cell. Picrotoxin also caused a slow enhancement of the zap-evoked IPSCs (Fig. 1B). Because the zap electrodes directly stimulate bipolar cells (Higgs and Lukasiewicz, 1999), the slow enhancement of the glycinergic IPSCs was not caused by an action of picrotoxin in the outer retina.
The time course of the slow enhancement of light- and zap-evoked IPSCs by picrotoxin, and their subsequent block by strychnine, is shown in more detail in the Figure 1inset on the right. In other experiments (data not shown) addition of strychnine at any time after the onset of picrotoxin eliminated the IPSCs, indicating that all of these responses were glycinergic.
The time course of the enhancement of glycinergic IPSCs from 16 dark-adapted preparations is summarized in Figure2A(filled circles). Because the amplitudes of the IPSCs and the amount of enhancement were different in different cells, the responses of each cell were normalized to the first IPSC recorded in the presence of picrotoxin (1 min after onset of picrotoxin), which represents the control value for the glycinergic IPSC.
Fig. 2.
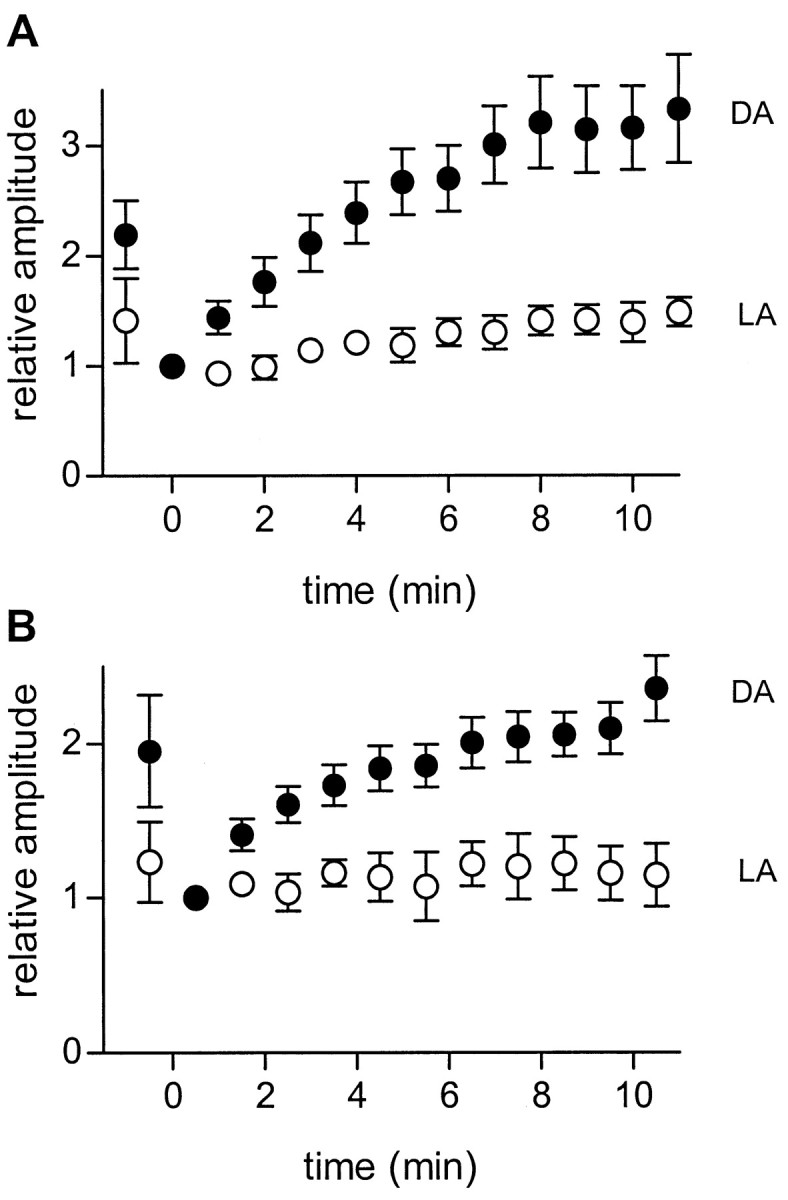
Enhancement of glycinergic IPSCs by picrotoxin is greater in dark- than light-adapted retinas. A, IPSCs elicited by full-field light flashes at different times after onset of continuous superfusion with picrotoxin. Data are averaged from 16 dark-adapted retinas (filledcircles) and 7 light-adapted retinas (opencircles). For each cell, peak IPSC amplitudes were normalized to that of the first response recorded in the presence of picrotoxin, which was recorded within 1 min after switching the superfusate to picrotoxin. Superfusion with picrotoxin begins at t = 0. In 11 of the cells tested (8 dark-adapted, 3 light-adapted) 2 μm strychnine was added after the enhancement had reached a maximum; in all cases strychnine completely blocked the IPSCs, indicating that they were glycinergic. Error bars indicate 1 SEM. The maximum enhancement was 3.57 (± 0.33)-fold in dark-adapted preparations (n = 16) and 1.49 (± 0.15)-fold in light-adapted preparations (n = 7). The probability that this difference was caused by chance was < 0.003 (Student's unpairedt test). B, Same as A, except that the IPSCs were elicited by focal electrical stimuli (+0.5 μA; 1 msec) as described in Figure 1. The maximum enhancement was 2.38 (± 0.20)-fold for dark-adapted preparations (n = 4) and 1.12 (± 0.28)-fold for light-adapted preparations (n = 4). The probability that this difference was caused by chance was < 0.001 (Student's unpaired t test). DA, Dark-adapted; LA, light-adapted.
Similar experiments were done in seven light-adapted preparations (Fig.2A, open circles). In light-adapted preparations the initial reduction of the IPSC was smaller, and there was very little slow enhancement in the continued presence of picrotoxin. This is consistent with the fact that glycinergic inhibition is already enhanced in light-adapted retinas.
Figure 2B shows that the results were similar when the IPSCs were elicited by zaps. In dark-adapted preparations, zap-evoked IPSCs (filled circles) were initially reduced and then slowly enhanced during continuous superfusion with picrotoxin, and this effect was minimal in light-adapted retinas (open circles).
Although picrotoxin caused little slow enhancement of glycinergic IPSCs in light-adapted retinas, it did have a separate, rapid effect on glycinergic IPSCs in some light-adapted preparations. In three of the seven light-adapted ganglion cells, picrotoxin caused a rapid increase in the amplitude and duration of the glycinergic IPSC. An example is shown in Figure 3, which shows light-evoked IPSCs in a light-adapted ganglion cell. In this cell the first IPSC recorded after the addition of picrotoxin was larger and more prolonged than the control IPSC. The increase in duration is shown more clearly in the Figure 3inset on the right, in which the first two traces (control and 1 min after application of picrotoxin) were scaled to the same amplitude and superimposed. Similar rapid changes in amplitude and kinetics of glycinergic responses have been described in light-adapted retinas by others and were attributed to blocking the GABAergic inhibition of glycinergic amacrine cells (Zhang et al., 1997; Roska et al., 1998). This is, however, a separate effect of picrotoxin that is not related to the slow enhancement of glycinergic inhibition described above (see Discussion). After the initial rapid effect of picrotoxin there was little additional increase in IPSC amplitude during the next 17 min, after which it was blocked by the addition of strychnine.
Fig. 3.
Effect of picrotoxin on ganglion cell IPSCs in a light-adapted retinal slice. Left, Details in Figure 1, except that this retina had been light adapted by exposure to background illumination for several minutes. Inset,Right, Superimposed traces of the first two responses (control and PTX 1′) scaled to the same amplitude to better illustrate the difference in time courses.
Picrotoxin rapidly blocks GABA receptors
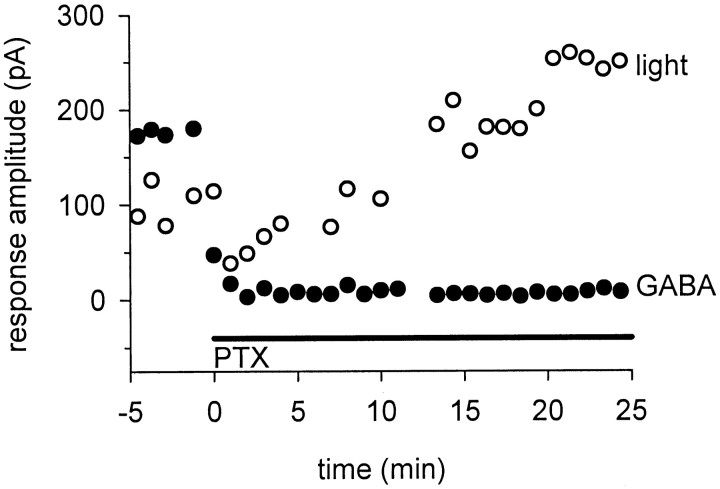
Although picrotoxin caused a slow enhancement of glycinergic inputs to ganglion cells in dark-adapted retinas, this was not caused by a slow action of picrotoxin in blocking GABA receptors. Figure4 shows inhibitory currents elicited by full-field light flashes or puffs of GABA onto the ganglion cell dendrites. The light and puff stimuli were given alternately every 30 sec. The IPSCs elicited by light stimuli (open circles) were slowly enhanced in the presence of picrotoxin as described above, but the responses to direct application of GABA (filled circles) were completely blocked almost immediately after picrotoxin was added.
Fig. 4.
Picrotoxin slowly enhances light-evoked glycinergic responses but rapidly blocks responses to directly applied GABA. Responses are IPSCs evoked by alternating full-field light flashes (opencircles) and puffs of GABA (filledcircles). Picrotoxin (PTX) rapidly blocked the responses to GABA puffs, but the light-evoked IPSCs were initially reduced and then slowly enhanced over the next 20 min. Similar results were seen in all of the four cells tested.
Bicuculline does not cause slow enhancement of glycinergic IPSCs
Because picrotoxin blocks both GABAA and GABAC receptors, we also used bicuculline (150–200 μm), which blocks only GABAA receptors, to test whether the effect of picrotoxin was caused by blocking GABAA or GABAC receptors. Figure5A shows the effects of bicuculline on the light-evoked IPSCs in four ganglion cells. Bicuculline (open circles) rapidly reduced the light-evoked IPSCs but did not cause a subsequent enhancement of these responses. For comparison, the filled circlesshow the IPSCs elicited by the same light stimuli during superfusion with picrotoxin (data from Fig. 2A, filled circles). Bicuculline also failed to enhance zap-evoked IPSCs (Fig. 5B). These results indicate that the enhancement of glycinergic IPSCs by picrotoxin was caused by blocking GABAC receptors.
Fig. 5.
Bicuculline does not enhance glycinergic IPSCs in ganglion cells. A, Light-evoked IPSCs.Opencircles show the amplitude of IPSCs at various times after onset of continuous superfusion with 200 μm bicuculline (BIC). For comparison,filledcircles show the results (from Fig. 2A) obtained during superfusion with 150 μm picrotoxin (PTX). Data were averaged from 16 cells for PTX and 5 cells forBIC. For each cell, peak IPSC amplitudes were normalized to the first response recorded in the presence of picrotoxin.B, Same as A, except that the IPSCs were elicited by zaps (+0.5 μA; 1 msec) as described in Figure 1.
Effect of picrotoxin on excitatory inputs to ganglion cells
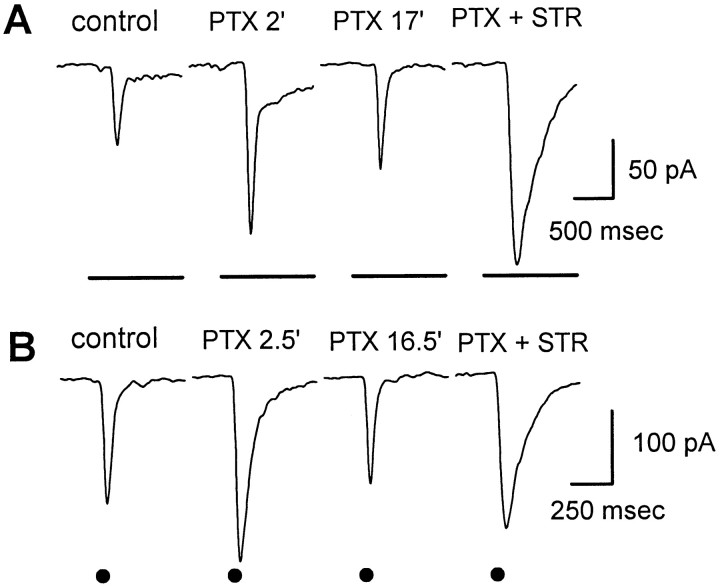
Because GABAC receptors are present on salamander bipolar cell terminals (Lukasiewicz and Werblin, 1994) it is possible that the slow enhancement of glycinergic inputs to ganglion cells in the presence of picrotoxin was caused by removal of tonic inhibition onto bipolar terminals, which would cause increased release of glutamate from bipolar terminals onto glycinergic amacrine cells. To test this possibility we looked for an effect of picrotoxin on glutamate release from bipolar cells by measuring the EPSCs in ganglion cells. To isolate EPSCs the ganglion cells were clamped at the reversal potential for chloride, which eliminated GABA- and glycine-mediated inhibitory currents in the recorded cell. Figure6A shows the effect of picrotoxin on EPSCs elicited by full-field light stimuli. Picrotoxin caused an initial increase in EPSC amplitude (t = 2 min), but after that the EPSCs gradually became smaller in amplitude and shorter in duration (t = 17 min). However, the decrease in EPSC amplitude and duration was reversed when strychnine was added (PTX + STR), suggesting that the slow decrease in EPSC was caused by slowly increasing glycinergic inhibition of bipolar terminals. Similar results were seen in four of the six ganglion cells in which light- and zap-evoked EPSCs were studied (Fig.6B). Thus the slow enhancement of glycinergic IPSCs in picrotoxin was not caused by an increase in glutamate release from bipolar cells.
Fig. 6.
Effect of picrotoxin on excitatory currents in an on–off ganglion cell. The cell was voltage clamped at the chloride reversal potential (−65 mV) to eliminate GABA- and glycine-mediated inhibitory currents. Other details are as described in Figure 1.A, EPSCs that were elicited by 4 sec full-field light stimuli. After the onset of picrotoxin the EPSCs were initially enhanced (PTX 2′) but then gradually declined (PTX 17′). Subsequent addition of 2 μmstrychnine in the continued presence of picrotoxin (PTX + STR) partially reversed the slow decline in EPSC amplitude caused by picrotoxin, indicating that the slowly developing suppression of the EPSC was mediated by glycine. The holding current was −52 pA. The effects on the off responses (data not shown) were similar.B, Same as A, except that the EPSCs were elicited by zaps (+0.5 μA; 1 msec) as described in Figure 1.
Picrotoxin does not increase ganglion cell sensitivity to glycine
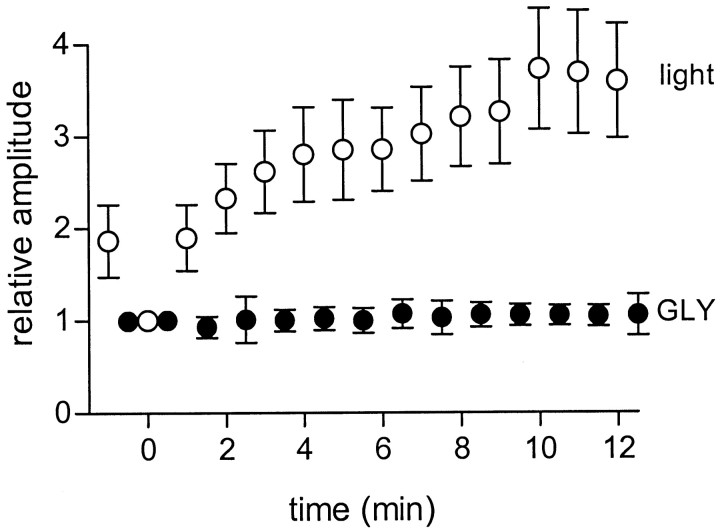
Figure 7 compares the effect of picrotoxin on ganglion cell IPSCs elicited by full-field light stimuli and by puffs of glycine onto the ganglion cell dendrites. Responses are averaged from four cells that were alternately stimulated by light and glycine puffs. After application of picrotoxin the IPSCs elicited by light stimuli (open circles) were initially reduced and then slowly enhanced to nearly twice that size over the time course of several minutes. However, the responses to puffs of glycine (filled circles) were not affected by picrotoxin.
Fig. 7.
Picrotoxin does not affect the ganglion cell response to direct application of glycine. Datapoints indicate the average peak amplitude of IPSCs elicited by full-field illumination (opencircles) and puffs of glycine (filledcircles) in four cells at various times after onset of continuous superfusion with picrotoxin att = 0. For each cell, responses were normalized to the first response after the application of picrotoxin in each cell.GLY, Glycine.
The enhancement of glycinergic inhibition induced by picrotoxin does not involve dopamine
Because dopamine modulates several different retinal changes associated with light adaptation we wanted to determine whether it is involved in the picrotoxin-induced enhancement of glycinergic inhibition, particularly because this enhancement is similar to that observed during light adaptation. We therefore tested the effects of dopamine and dopamine antagonists on the picrotoxin-induced enhancement of glycinergic inhibition of ganglion cells in the slice preparation. Dopamine (20 μm) caused an immediate enhancement of light-evoked IPSCs (Fig.8A) in two of the four cells tested, probably because of its upmodulation of glutamate release from bipolar cells (Wellis and Werblin, 1995). However, in all four cells there was no further enhancement during the next 15 min, and the IPSCs were dramatically reduced by the subsequent addition of picrotoxin, indicating that they were mainly GABAergic and that dopamine had not caused any enhancement of glycinergic input. After the addition of picrotoxin the small, glycinergic IPSC was slowly enhanced over the next 15 min, which was similar to the effect of picrotoxin in the absence of dopamine (compare Fig. 1).
Fig. 8.
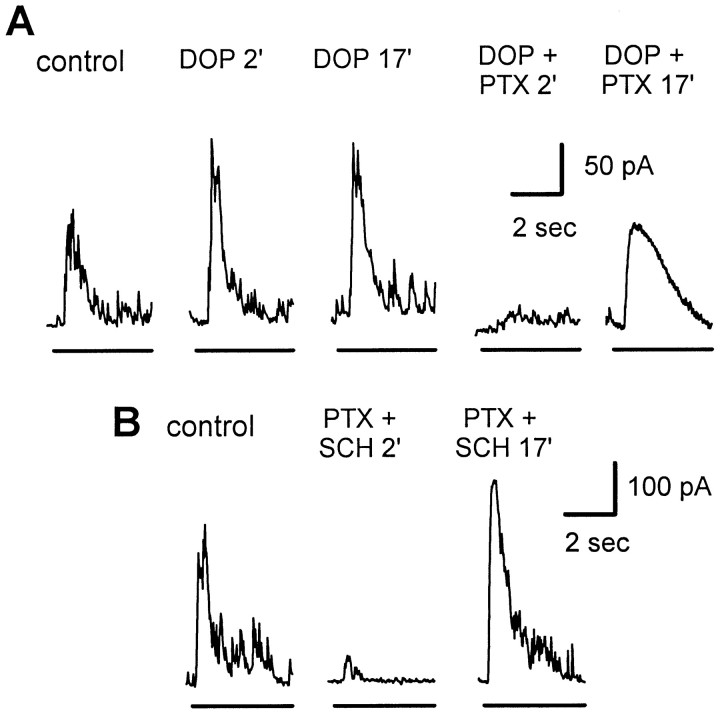
Effect of dopamine and dopamine antagonists on the ability of picrotoxin to cause slow enhancement of glycinergic IPSCs. Light stimuli and recording conditions are as described in Figure 1.A, Addition of 20 μm dopamine caused a rapid increase in IPSC amplitude within 2 min (DOP 2′), but there was no further increase after 15 additional minutes in dopamine (DOP 17′). The initial enhancement of the IPSC by dopamine was seen in only two of the four cells tested; in all four cells the mean enhancement was 1.13 (± 0.32)-fold (p = 0.65). After 18 min in dopamine, addition of 150 μm picrotoxin caused an immediate strong reduction in the IPSC (DOP + PTX 2′). In the continued presence of PTX the IPSC was slowly enhanced (DOP + PTX 17′). The mean enhancement by PTX was 4.02 (± 1.48)-fold (p = 0.02;n = 4). B, Addition of 15 μm SCH23390 and 150 μmPTXcaused an immediate reduction of the IPSC (SCH + PTX 2′), but in the continued presence of PTX andSCH the response slowly became enhanced (SCH + PTX 17′). In the five cells tested, the maximum enhancement byPTX in the presence of SCH was 4.42 (± 1.24)-fold (p = 0.02). In bothA and B the enhanced IPSC inPTX was completely blocked by 2 μmstrychnine in all cells (data not shown).
The dopamine antagonist SCH23390 did not prevent the enhancement of glycinergic IPSCs by picrotoxin (Fig. 8B). In the presence of SCH23390 (15 μm), picrotoxin caused an initial reduction of the light-evoked IPSC, followed by a gradual enhancement of the IPSC over the next 15 min (n = 5). This was similar to the effect of picrotoxin added to control Ringer's solution (compare Fig. 1).
Picrotoxin causes a slow enhancement of glycinergic inhibition and TLI in ganglion cells in dark-adapted eyecup preparations
The slow enhancement of glycinergic IPSCs by picrotoxin in dark-adapted retinal slice preparations suggests that GABAC receptors may be involved in the slow enhancement of TLI by adapting light in intact retinas. To test this possibility we investigated whether picrotoxin could mimic the ability of an adapting light to enhance TLI in dark-adapted eyecup preparations. The results of one such experiment are shown in Figure9A.
Fig. 9.
Effect of picrotoxin on transient lateral inhibition and glycinergic IPSPs in on–off ganglion cells in eyecup preparations. A, Effect of 150 μmpicrotoxin (PTX) on TLI in a dark-adapted eyecup. TLI was measured as the suppression of the response to a small test spot in the receptive field center by rotation of a broken annulus (windmill) pattern. B, Effect of picrotoxin on the transient IPSP elicited at the onset of an annulus (i.d., 500 μm; o.d., 2600 μm) in a light-adapted eyecup. Traces show the IPSP response in control Ringer's solution and at 2 and 10 min after onset of continuous superfusion with 150 μmpicrotoxin. The horizontallinebelow the responsetracesindicates the initial portion of the 4 sec light stimulus.
TLI was measured as the percent suppression of a test response (to a small spot flashed in the receptive field center) by the rotation of a concentric windmill (broken annulus) stimulus, as described in detail elsewhere (Cook and McReynolds, 1998). In the dark-adapted retina, rotation of the windmill did not cause suppression of the test response. A 2 min exposure to picrotoxin caused a transient decrease in the suppressive effect of the rotating windmill, followed by a gradual increase in suppression, which reached a maximum after 8 min and then declined again. A second application of picrotoxin produced essentially the same effect. In this experiment we used a brief exposure to picrotoxin to verify that the effect was reversible, because picrotoxin was difficult to wash out after prolonged application. The effect of continuous superfusion with picrotoxin (data not shown) was similar (n = 9 cells) except that the suppression remained high rather than declining again. The maximum amount of suppression and the time course of its development (2–12 min in 9 cells) were quite variable, even with continuous exposure to picrotoxin.
In light-adapted eyecup preparations, TLI was already enhanced, and no slow development of TLI was observed when picrotoxin was added. However, in light-adapted retinas picrotoxin did have a rapid effect on ganglion cell IPSPs, similar to the rapid effect of picrotoxin on IPSCs in light-adapted slices. An example is shown in Figure 9B, in which picrotoxin caused a rapid increase in both the amplitude and duration of the transient IPSP elicited by an annular light stimulus. These changes occurred within 2 min after addition of picrotoxin, but there was no further increase even after 10 min in the continued presence of picrotoxin. Picrotoxin had similar effects on the IPSPs elicited by annular stimuli in three other ganglion cells. In all cases the IPSPs were blocked by strychnine, indicating that they were glycinergic.
DISCUSSION
In dark-adapted retinas picrotoxin caused a slow enhancement of glycinergic inhibition that is similar in action and time course to the effect of light adaptation. Picrotoxin slowly enhanced glycinergic inhibition and TLI in ganglion cells in the eyecup and also slowly enhanced both light- and electrically evoked glycinergic IPSCs in ganglion cells in the slice. The time courses of these actions in the eyecup and slice were similar. The slow decline in ganglion cell EPSC amplitudes during superfusion with picrotoxin and their rapid recovery after subsequent addition of strychnine suggest that picrotoxin also enhances glycinergic feedback to bipolar terminals. The fact that bicuculline did not produce these effects indicates that the enhancement of glycinergic activity was caused by blocking GABAC receptors, although the possibility that the enhancement requires blocking both GABAC and GABAA receptors cannot be ruled out.
The enhancement of glycinergic inhibition was very slow compared with the block of GABA receptors by picrotoxin. The immediate block of ganglion cell responses to GABA puffs indicates that GABAA receptors were rapidly blocked. Because GABAergic feedback to bipolar cell terminals in salamander retina is mediated mainly by GABAC receptors (Lukasiewicz et al., 1994; Dong and Werblin, 1998) the rapid enhancement of EPSCs indicates that GABAC receptors were also rapidly blocked. Other studies have also shown that GABACreceptors are rapidly blocked by picrotoxin (C. R. Shields and P. D. Lukasiewicz, unpublished observations) (see also Feigenspan and Bormann, 1994). The very slow time course of the enhancement of glycinergic inhibition relative to the blocking of GABA receptors suggests that the modulation of glycinergic activity by GABA is indirect.
Where does the modulation of glycinergic inhibition by GABA occur?
The fact that picrotoxin does not enhance the responsiveness of glycine receptors on ganglion cells indicates that the output of the glycinergic amacrine cells is enhanced. This could be caused by an increase in bipolar cell output to glycinergic amacrine cells or by some change in the glycinergic amacrine cells themselves. Two findings suggest that the modulation does not result from increased glutamate release from bipolar terminals. First, prolonged exposure to picrotoxin caused a slow decrease in the amplitude of the EPSCs, which reflects a decrease in glutamate release from bipolar terminals, even though it slowly enhanced glycinergic IPSCs during the same time period. Second, dopamine did not enhance glycinergic IPSCs, even though it increases glutamate release from bipolar cells (Wellis and Werblin, 1985). Therefore, although we cannot rule out the possibility that the excitatory input to glycinergic amacrine cells is from a separate population of bipolar terminals that are not affected by dopamine and do not receive glycinergic feedback, it seems likely that the site of modulation is the glycinergic amacrine cell. The cellular mechanism that is modulated could be the glycinergic amacrine cell's responsiveness to glutamate, its ability to release glycine, or some intervening step. Recording from glycinergic transient amacrine cells in dark- and light-adapted preparations, and in the presence and absence of picrotoxin, may provide additional information about the cellular mechanism involved.
How might blocking GABAC receptors cause changes in the glycinergic amacrine cells? One possibility is that these cells have an unusual type of picrotoxin-sensitive GABA receptor that has slow metabotropic effects. Although metabotropic actions have been described at some “ionotropic” receptors (Wang et al., 1997; Kawai and Sterling, 1999), no such actions have been reported at picrotoxin-sensitive GABA receptors. A more likely possibility is that the modulation is mediated by another, unknown substance and that GABA may act to suppress the synthesis or release of this substance. In such a scheme, the GABAC receptors that control the modulation could be located on the modulatory neurons or on neurons that provide input to these cells. Blocking GABACreceptors on bipolar terminals could also increase release of a modulatory substance by increasing excitatory input to the neurons that release the substance, but again this would require a separate population of bipolar terminals that were not affected by dopamine and did not receive glycinergic feedback.
The slow time course of the enhancement of glycinergic inhibition could be attributable to the time required for synthesis of the modulatory substance or activation of its release mechanism. It is also possible that the action of the substance on its target cells, presumably glycinergic amacrine cells, is slow. The identity of the postulated modulator substance is unknown. Although dopamine modulates several different types of retinal changes associated with light adaptation, it does not seem to be involved in the slow modulation of glycinergic inhibition. No other substances have yet been tested in this regard, and identification of the modulatory substance may be difficult. Possible candidates include nitric oxide, serotonin, or one of the several neuropeptides that have been found in retinal neurons, particularly amacrine cells, but have no clearly defined functional role.
What causes the rapid enhancement of glycinergic IPSCs by picrotoxin in light-adapted retinas?
In three of the seven experiments in light-adapted retinas the addition of picrotoxin caused an immediate increase in the amplitude and duration of glycinergic IPSCs. Similar changes in the amplitude and kinetics of glycinergic responses by GABA antagonists in light-adapted preparations were attributed to blocking GABAAreceptors on glycinergic amacrine cells (Zhang et al., 1997; Roska et al., 1998). Blocking GABAC receptors on bipolar terminals, which increases excitatory input from bipolar cells to third-order neurons (Lukasiewicz and Werblin, 1994; Dong and Werblin, 1998), could also cause a rapid increase in glycinergic IPSCs. The fact that picrotoxin never caused a rapid enhancement of glycinergic IPSCs in dark-adapted retinas supports the idea that in dark-adapted retinas the glycinergic amacrine cells are suppressed by a separate action of GABA.
Network adaptation in the retina has been studied for over 20 years, but the neural basis for many of the adaptive changes is still not well understood. Here we have demonstrated that adaptive changes in a specific neural circuit in the inner retina are controlled by GABAC receptors. Other aspects of this adaptation, including the identity of the postulated neuromodulator substance, have yet to be determined.
Footnotes
This work was supported by National Institutes of Health Research Grants EY01653 and EY08922 and Core Grants EY07003 and EY02687.
Correspondence should be addressed to Dr. Paul B. Cook, Department of Physiology, University of Michigan Medical School, Ann Arbor, MI 48109-0622. E-mail: pcook@umich.edu.
REFERENCES
- 1.Belgum JH, Dvorak DR, McReynolds JS. Strychnine blocks transient but not sustained inhibition in mudpuppy retinal ganglion cells. J Physiol (Lond) 1984;354:273–286. doi: 10.1113/jphysiol.1984.sp015375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook PB, McReynolds JS. Modulation of sustained and transient lateral inhibitory mechanisms in the mudpuppy retina during light adaptation. J Neurophysiol. 1998;79:197–204. doi: 10.1152/jn.1998.79.1.197. [DOI] [PubMed] [Google Scholar]
- 3.Cook PB, Lukasiewicz PD, McReynolds JS. Action potentials are required for the lateral transmission of glycinergic transient inhibition in the amphibian retina. J Neurosci. 1998;18:2301–2308. doi: 10.1523/JNEUROSCI.18-06-02301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong CJ, Werblin FS. Temporal contrast enhancement via GABAC feedback at bipolar terminals in the tiger salamander retina. J Neurophysiol. 1998;79:2171–2180. doi: 10.1152/jn.1998.79.4.2171. [DOI] [PubMed] [Google Scholar]
- 5.Dowling JE. Retinal neuromodulation: the role of dopamine. Vis Neurosci. 1991;1:87–97. doi: 10.1017/s0952523800010968. [DOI] [PubMed] [Google Scholar]
- 6.Enroth-Cugell C, Jakiela HG. Suppression of cat retinal ganglion cell responses by moving patterns. J Physiol (Lond) 1980;302:49–72. doi: 10.1113/jphysiol.1980.sp013229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feigenspan A, Bormann J. Differential pharmacology of GABAA and GABAC receptors on rat retinal bipolar cells. Eur J Pharmacol. 1994;288:97–104. doi: 10.1016/0922-4106(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 8.Greenstreet EH, Djamgoz MB. Nitric oxide induces light-adaptive morphological changes in retinal neurons. NeuroReport. 1994;6:109–112. doi: 10.1097/00001756-199412300-00029. [DOI] [PubMed] [Google Scholar]
- 9.Higgs M, Lukasiewicz PD. Glutamate uptake limits synaptic excitation of retinal ganglion cells. J Neurosci. 1999;19:3691–3700. doi: 10.1523/JNEUROSCI.19-10-03691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai F, Sterling P. AMPA receptor activates a G-protein that suppresses a cGMP-gated current. J Neurosci. 1999;19:2954–2959. doi: 10.1523/JNEUROSCI.19-08-02954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukasiewicz PD, Werblin FS. A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. J Neurosci. 1994;14:1213–1223. doi: 10.1523/JNEUROSCI.14-03-01213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukasiewicz PD, Maple BR, Werblin FS. A novel GABA receptor on bipolar cell terminals in the tiger salamander retina. J Neurosci. 1994;14:1202–1212. doi: 10.1523/JNEUROSCI.14-03-01202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills SL, Massey SC. Differential properties two gap junctional pathways made by AII amacrine cells. Nature. 1995;377:734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- 14.Roska B, Nemeth E, Werblin FS. Response to change is facilitated by a three-neuron disinhibitory pathway in the tiger salamander retina. J Neurosci. 1998;18:3451–3459. doi: 10.1523/JNEUROSCI.18-09-03451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thibos LN, Werblin FS. The properties of surround antagonism elicited by rotating windmill patterns in the mudpuppy retina. J Physiol (Lond) 1978;278:101–116. doi: 10.1113/jphysiol.1978.sp012295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Small DL, Stanimirovic DB, Morley P, Durkin JP. AMPA receptor-mediated regulation of a Gi-protein in cortical neurons. Nature. 1997;389:502–504. doi: 10.1038/39062. [DOI] [PubMed] [Google Scholar]
- 17.Wellis DP, Werblin FS. Dopamine modulates GABAC receptors mediating inhibition of calcium entry into and transmitter release from bipolar cell terminals in tiger salamander retina. J Neurosci. 1995;15:4748–4761. doi: 10.1523/JNEUROSCI.15-07-04748.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werblin FS. Lateral interactions at inner plexiform layer of a vertebrate retina: antagonistic responses to change. Science. 1972;175:1008–1010. doi: 10.1126/science.175.4025.1008. [DOI] [PubMed] [Google Scholar]
- 19.Werblin FS, Copenhagen DR. Control of retinal sensitivity. III. Lateral interactions at the inner plexiform layer. J Gen Physiol. 1974;63:88–110. doi: 10.1085/jgp.63.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witkovsky P, Dearry A. Functional roles of dopamine in the vertebrate retina. Prog Retinal Res. 1991;11:247–292. [Google Scholar]
- 21.Zhang J, Jung CS, Slaughter MM. Serial inhibitory synapses in retina. Vis Neurosci. 1997;14:553–563. doi: 10.1017/s0952523800012219. [DOI] [PubMed] [Google Scholar]