Abstract
The activation of cAMP-dependent protein kinase regulates the physiological activity of AMPA-type glutamate receptors. In this study, phosphorylation of the AMPA receptor subunit GluR1 at Ser845 was increased in neostriatal slices by activation of D1-type dopamine receptors and by inhibitors of protein phosphatase 1/protein phosphatase 2A. In contrast, Ser831, a residue which, when phosphorylated by protein kinase C or calcium/calmodulin-dependent kinase II, increases AMPA receptor channel conductance, was unaffected by either D1 or D2 receptor agonists in neostriatal slices. The phosphorylation of Ser845, but not Ser831, was strongly increased in neostriatum in vivo in response to the psychostimulants cocaine and methamphetamine. The effects of dopamine and psychostimulants on the phosphorylation of GluR1 were attenuated in dopamine and cAMP-regulated phosphoproteinMr 32 kDa (DARPP-32) knock-out mice. These results identify DARPP-32 and AMPA-type glutamate receptors as likely essential cellular effectors for psychostimulant actions.
Keywords: dopamine, DARPP-32, methamphetamine, cocaine, protein phosphatase 1, D1 receptor, protein kinase A
Ionotropic glutamate receptors function as ligand-gated ion channels and are believed to play a role in certain forms of learning and memory (Bliss and Collingridge, 1993;Linden, 1994), in the development of psychomotor stimulant sensitization (Wolf, 1998) and in various forms of neurodegenerative disease (Lipton and Rosenberg, 1994). They are classified by their physiological and pharmacological characteristics as members of AMPA, kainate, or NMDA receptor subclasses (Monaghan et al., 1989). AMPA-type glutamate receptors are abundantly expressed on dendritic processes of medium spiny neostriatal neurons (Petralia and Wenthold, 1992) where they mediate a large proportion of the glutamate-induced excitation (Cepeda et al., 1993).
Considerable evidence indicates that AMPA receptors are regulated by protein phosphorylation. For example, activation of cAMP-dependent protein kinase (PKA) in hippocampal (Greengard et al., 1991; Wang et al., 1991; Rosenmund et al., 1994; Kameyama et al., 1998) or neostriatal (Yan et al., 1999) neurons increases AMPA currents recorded from these cells. The notion that these effects occur as the result of phosphorylation of the receptor is supported by the observation that the GluR1 subunit is phosphorylated in response to PKA activation in human embryonic kidney-293 cells (Blackstone et al., 1994; Tan et al., 1994) (but see McGlade-McCulloh et al., 1993). Moreover, a PKA-dependent residue, Ser845, is required for PKA-mediated potentiation of peak current carried by homomeric GluR1 channels (Roche et al., 1996). AMPA receptors also can be regulated by phosphorylation of Ser831after activation of calcium/calmodulin-dependent kinase II (CaMKII) (Barria et al., 1997) or protein kinase C (PKC) leading to potentiation of AMPA currents in hippocampal neurons (Barria et al., 1997; Mammen et al., 1997). Whereas PKA-mediated phosphorylation at Ser845 enhances AMPA currents by increasing channel open time probability (Roche et al., 1996), phosphorylation at Ser831 increases single-channel conductance of AMPA receptors (Derkach et al., 1999). Thus, PKA and CaMKII/PKC signaling cascades control distinct properties of the AMPA receptor by regulating unique GluR1 residues.
Dopamine and cAMP-regulated phosphoproteinMr 32 kDa (DARPP-32); is a neostriatum-enriched substrate for PKA (Walaas et al., 1983; Ouimet et al., 1984). Phosphorylation at Thr34 by PKA converts DARPP-32 into an efficient inhibitor of protein phosphatase 1 (PP1) (Hemmings et al., 1984), a serine/threonine phosphatase enriched in the dendritic spines of neostriatal neurons (Ouimet et al., 1995). Studies using mice lacking the gene for DARPP-32 indicate that DARPP-32 mediates many physiological and behavioral effects of dopamine (Fienberg et al., 1998). A recent study (Yan et al., 1999) showed that whole-cell AMPA currents are enhanced by D1 dopamine receptors via a pathway involving DARPP-32, PP1, and the PP1-targeting protein spinophilin (Allen et al., 1997). A principal goal of the present study was to determine whether regulation of neostriatal GluR1 subunit phosphorylation by this pathway is a plausible mechanism for the physiological control of AMPA receptors by dopamine.
Psychostimulants increase the synaptic availability of dopamine in the brain (Hyman, 1996; Koob and LeMoal, 1997), leading to both acute and long-term plastic changes in dopaminoceptive neurons (Nestler and Aghajanian, 1997). Some of these effects appear to require the activation of glutamate receptors, although the precise role that glutamate plays in addiction is unclear (Wolf, 1998). Thus, a second objective of this study was to investigate the possibility that regulation of AMPA receptor phosphorylation in vivo by psychostimulants might contribute to these effects.
MATERIALS AND METHODS
Preparation and treatment of neostriatal slices. Male C57BL/6 mice (8–12 weeks in age) were killed by decapitation. The brain was rapidly transferred to an ice-cold surface where it was blocked and fixed to the cutting surface of a Vibratome (Ted Pella) maintained at 4°C. The brain was placed in cold, oxygenated (95% O2 and 5% CO2) Krebs' bicarbonate buffer of the following composition (in mm): 125 NaCl, 5 KCl, 26 NaHCO3, 1.5 CaCl2, 1.5 MgSO4, and 10 glucose, pH 7.4. Coronal slices of mouse brain (400 μm in thickness) were cut and pooled in 10 ml of cold buffer. Neostriatal slices were cut from the coronal sections under a dissecting microscope. The slices were pooled, then transferred individually to 4 ml polypropylene tubes containing 2 ml of fresh, cold, oxygenated buffer. The tissue was preincubated for 15 min at 30°C, the buffer was replaced, and tissue sections were preincubated for an additional 30 min. At the end of this second preincubation period, the buffer was replaced with Krebs' buffer or buffer containing the indicated test substances for 30 sec to 60 min. After treatment, the slices were immediately frozen in liquid nitrogen and stored at −80°C until assayed.
In some experiments, neostriatal slices were prepared from C57BL/6 mice (8–10 weeks of age) lacking the gene for DARPP-32 (Fienberg et al., 1998). DARPP-32 knock-out mice and wild-type controls were generated from the offspring of heterozygous mating pairs. All mice were age-matched, and only males were used.
Preparation of GluR1 fusion protein for stoichiometric analysis of Ser845and Ser831 phosphorylation. A bacterial protein comprising the last putative intracellular domain of GluR1 (residues 809–889) fused to glutathione-S-transferase (GST) was prepared as follows: rat brain cDNA was used as a template for PCR amplification of the GluR1 sequence using the primers TGC GTC GAC CGA GTT CTG CTA CAA ATC CC; TTC GCG GCC GCT CCA GTT ACA ATC CTG TG (Operon). The resulting 272 bp fragment was digested withSalI–NotI and ligated in frame with the GST coding sequence contained in a bacterial expression plasmid pGex-4T-2 (Pharmacia, Piscataway, NJ). This plasmid was sequenced to ensure fidelity of PCR amplification and transformed into Escherichia coli strain BL21 DE3. Bacteria were grown at 37°C to an OD600 of 0.5. The temperature was reduced to 30°C, and expression was induced over 2 hr by the addition of isopropylthio-β-galactoside to a final concentration of 0.1 mm. Cells were harvested by centrifugation and lysed in a French press. After clarification of the lysate by centrifugation at 30,000 × g for 20 min, the fusion protein was purified by affinity chromatography on glutathione-agarose beads (Pharmacia), using 5 mm glutathione and 50 mm Tris, pH 8.0, for elution.
GST-GluR1 (35 μm) was preparatively phosphorylated under the following conditions: 10 μg/ml purified PKA in 50 mmTris, pH 8.0, 10 mm magnesium chloride, 0.4 mmEGTA, 50 μm [γ32P] ATP or 1 μg/ml purified CaMKII in 50 mm Tris, pH 8.0, 10 mm magnesium chloride, 0.4 mm EGTA, 2 mm DTT, 1 μm calmodulin, 1.5 mmcalcium chloride, and 50 μm[γ32P]ATP. Reactions were stopped by the addition of Laemmli buffer. Stoichiometry of the phosphorylated fusion proteins was calculated based on PhosphorImager (Molecular Dynamics, Eugene, OR) analysis of phosphate incorporation. Known quantities of these proteins were compared with tissue samples, the levels of phospho-GluR1 and GluR1 were detected by ECL, and the resulting values used to calculate stoichiometries of phosphorylation for the Ser845 and Ser831 sites in neostriatal tissue.
Drug treatment and microwave irradiation of mice. Male C57BL/6 mice (8–12 weeks in age) were injected with vehicle (0.9% NaCl in water) or vehicle containing various concentrations of either methamphetamine HCl (20 or 30 mg/kg, s.c.) or cocaine HCl (10 or 20 mg/kg, i.p.). To insure preservation of phosphoproteins in theirin vivo phosphorylation state, the animals were killed at various times (15–60 min) after injection by focused microwave irradiation (4.5–5 kW for 1.4 sec) using a small-animal microwave (Murimachi Kikai, Tokyo, Japan). The brains were rapidly removed, and the neostriatum was dissected and stored at −80°C until assayed for phosphoprotein levels.
Immunoblotting for phospho-GluR1. Frozen tissue samples were sonicated in 1% SDS. Small aliquots of the homogenate were retained for protein determination by the BCA protein assay method (Pierce, Rockford, IL) using bovine serum albumin as a standard. Equal amounts of protein (50 μg for slice experiments; 250 μg for microwave experiments) were loaded onto 7.5% acrylamide gels. The proteins were separated by SDS-PAGE (Laemmli, 1970), and transferred to nitrocellulose membranes (0.2 μm) (Schleicher and Schuell) by the method of Towbin et al. (1979). Membranes were blocked for 30–60 min in PBS (in mm: 124 NaCl, 4 KCl, 10 Na2HPO4, and 10 KH2PO4, pH 7.2) containing 5% nonfat dry milk and 0.2% Tween 20 (Blotto). The membranes were immunoblotted using antisera that selectively detect either the Ser845-phosphorylated or the Ser831-phosphorylated form of GluR1 (1:200 dilutions for each antibody) (Kameyama et al., 1998), or an antiserum (PharMingen, San Diego, CA; 1:10,000 dilution) that detects the C-terminal region of GluR1, irrespective of phosphorylation state. In some experiments these samples were also immunoblotted with monoclonal antibody 23 (1:750 dilution) (Snyder et al., 1992), a phosphorylation state-specific monoclonal antibody raised against a DARPP-32 peptide containing phospho-Thr34, the site phosphorylated by PKA.
Antibody binding was revealed by incubation with either a goat anti-rabbit horseradish peroxidase-linked IgG or a goat anti-mouse horseradish peroxidase-linked IgG (each at a 1:6000–8000 dilution) (Pierce) and the ECL immunoblotting detection system (Amersham, Arlington Heights, IL). Chemiluminescence was detected by autoradiography using DuPont NEN (Boston, MA) autoradiography film, and bands were quantified by analysis of scanned images by NIH Image 1.52 software. Because the linear range for quantitation of ECL signals by densitometry is limited, several film exposures were obtained for each set of samples to insure that signals were within a density range that allowed accurate quantitation.
In all of the experiments for this study, nitrocellulose membranes were sequentially analyzed for phospho-Ser845GluR1 or for phospho-Ser831 GluR1, and then for total levels of C-terminal GluR1. After probing a membrane for phospho-GluR1, the filter was washed three times for 5 min each in PBS to remove any remaining chemiluminescent reagent. The membrane was then stripped of antibody by incubation at 60°C for 60 min in a buffer containing 100 mm 2-mercaptoethanol, 2% SDS, and 62.5 mm Tris/HCl, pH 6.7. The filter was then washed several times in large volumes of PBS and blocked in Blotto for 30–60 min before immunoblotting with the C-terminal GluR1 antibody.
Data were analyzed by Student's t-test or Mann–WhitneyU test, as indicated, with significance defined asp < 0.05.
RESULTS
Differential phosphorylation of GluR1 in neostriatum by protein kinase activators
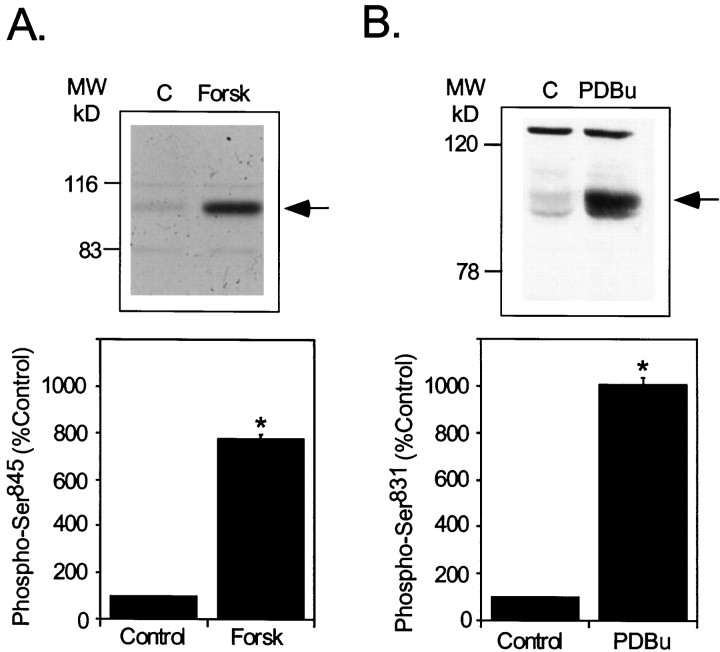
GluR1 is phosphorylated in vitro at Ser845 by PKA (Blackstone et al., 1994) and at Ser831 by PKC (Mammen et al., 1997) or CaMKII (McGlade-McCulloh et al., 1993). Phosphorylation of GluR1 at Ser845, in response to activation of PKA, potentiates AMPA currents by increasing open time probability of the receptor channel (Roche et al., 1996). The phosphorylation of GluR1 at Ser831 in response to activation of CaMKII or PKC increases AMPA receptor function by enhancing channel conductance (Derkach et al., 1999). Treatment of neostriatal slices with forskolin (50 μm), an adenylyl cyclase activator, increased phosphorylation of Ser845 approximately eightfold (776± 20% of control in three experiments) (Fig.1A). The PKC activator phorbol 12,13-dibutyrate (PDBu) (5 μm) (Fig.1B) increased the phosphorylation of GluR1 at Ser831 by an average of 10-fold (1005± 28% of control in three experiments) in neostriatal slices.
Fig. 1.
Effects of protein kinase activators on GluR1 phosphorylation in neostriatal slices. Neostriatal slices were prepared from normal C57BL/6 mice and incubated for 5 min in the absence (Control) or presence of the adenylyl cyclase activator forskolin (A) or the PKC activator PDBu (B). Levels of phospho-Ser845(A) or phospho-Ser831(B) were determined by SDS-PAGE and immunoblotting. Phospho-GluR1 levels were detected, quantitated by densitometry, and expressed as percentage of the level detected in control slices. Arrows (top panels) indicate the position of the phospho-GluR1 bands in representative autoradiograms. Data (bottom panels) are expressed as means ± SEM for three experiments (*p < 0.05 compared with control, Mann–WhitneyU test).
Phosphorylation state-specific antibodies were used to compare the levels of phospho-Ser845 and phospho-Ser831 GluR1 in neostriatal slices with known quantities of GluR1 fusion proteins phosphorylated by PKA or CaMKII, respectively. A low, basal stoichiometry of phosphorylation at Ser845 was measured (∼0.05 mol/mol) in slices, which was increased to ∼0.33 mol/mol after 5 min of forskolin treatment (50 μm). Ser831 was also phosphorylated at a low level in untreated slices (a stoichiometry of 0.06 mol/mol). The stoichiometry of phosphorylation at Ser831was increased to ∼0.26 mol/mol after 5 min of PDBu treatment (5 μm). These data indicate that GluR1 is phosphorylated in intact neostriatal cells at Ser845 or Ser831, after activation of either PKA or PKC/CaMKII, respectively.
Phosphorylation of GluR1 in response to dopamine receptor stimulation
Dopamine receptors are subdivided into a D1 class (i.e., D1, D5 subtype receptors) and a D2 class (i.e., D2, D3, and D4 subtype receptors) with the D1 and D2 subtypes being the most highly enriched in mammalian neostriatum (Sibley and Monsma, 1992). D1 class receptors stimulate adenylyl cyclase and increase cAMP formation (Stoof and Kebabian, 1981). D2 class receptors, via interactions with specific G-proteins, can be coupled to multiple effector systems, including adenylyl cyclase, Ca2+ and K+ channels, and phospholipase C (Huff, 1996). To evaluate the potential contribution of D1-subtype and D2-subtype dopamine receptors to the regulation of GluR1 phosphorylation, we measured the effects of dopamine and selective D1 and D2 agonists and antagonists on phosphorylation at Ser845 and Ser831.
Dopamine increased the phosphorylation of GluR1 at Ser845 (428 ± 68% compared with control) (Fig. 2A). Levels of phosphorylation were maximal at between 2.5 and 5 min, and declined to basal values by 10 min of incubation with dopamine (data not shown). The effect of dopamine was blocked by pretreatment of slices with a D1-type receptor antagonist, SCH23390 (1 μm) (127 ± 21% compared with control). In contrast, the D2-type receptor antagonist sulpiride (1 μm) had no effect on dopamine-stimulated phosphorylation of GluR1 (565 ± 81% compared with control) or on D1 agonist-induced phosphorylation of GluR1 (data not shown). Neither the D1 nor the D2 antagonist alone had a significant effect on the basal level of GluR1 phosphorylation (data not shown) indicating that any endogenously released dopamine was not exerting a significant regulation of this site. The phosphorylation of Ser845 was increased by treatment of slices with SKF81297, a D1 agonist (337 ± 82% of control; Fig. 2A), whereas quinpirole, a D2 agonist, had no effect on basal levels (91 ± 12% of control; Fig.2A). Quinpirole did not reduce phosphorylation of Ser845 induced by a D1 agonist (data not shown). These data indicate that dopamine-induced phosphorylation of GluR1 is most likely mediated by activation of a D1-receptor/PKA-dependent signaling pathway.
Fig. 2.
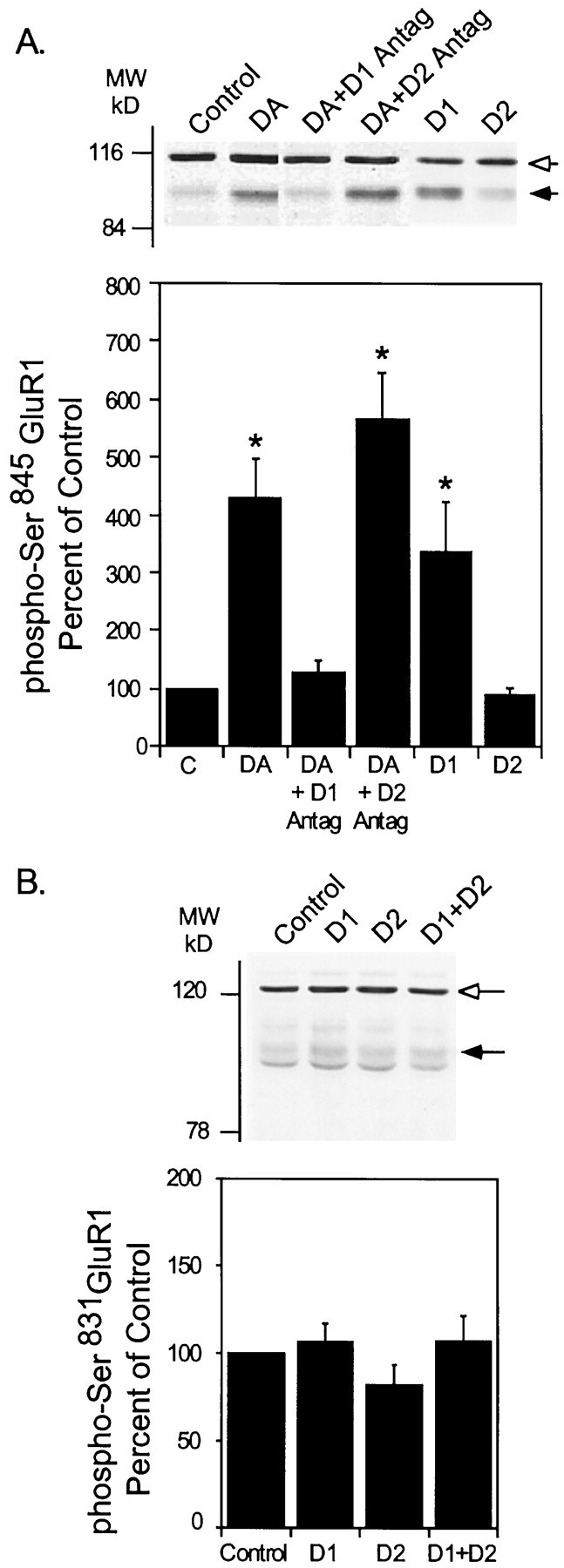
Effect of activation of D1-type or D2-type dopamine receptors on neostriatal GluR1 phosphorylation. Neostriatal slices were prepared from normal C57BL/6 mice. A, At time 0 slices were incubated in the absence or presence of the D1 receptor antagonist SCH23390 (1 μm; D1 Antag) or the D2 receptor antagonist sulpiride (1 μm; D2 Antag), as indicated. At 10 min slices were incubated in normal buffer alone (Control), with dopamine (DA) (100 μm) plus the dopamine uptake inhibitor nomifensine (10 μm), SKF81297 (1 μm) (D1) or quinpirole (1 μm) (D2), with or without SCH23390 or sulpiride for an additional 5 min. B, Slices were incubated with either normal buffer (Control) or with SKF81297 (1 μm) or quinpirole (1 μm) for 5 min. Thesolid arrows indicate the position of phospho-Ser845 GluR1 (A) or phospho-Ser831 GluR1 (B). Theopen arrows indicate cross-reactive bands detected by the phosphorylation state-specific antibody. The intensity of these bands did not change as a function of dopamine agonist or antagonist treatment. Phospho-Ser845 GluR1 (A) or phospho-Ser831 GluR1 (B) was detected in the samples, quantitated by densitometry, and expressed as percentage of the level in control slices. Data are presented as means ± SEM for three experiments (*p < 0.05 compared with control, Mann–Whitney U test).
D2 agonists have recently been shown to increase intracellular Ca2+ levels in dissociated neostriatal cells (Hernandez et al., 1999), an effect that could result in activation of Ca2+-dependent kinases such as CaMKII. Thus, we evaluated the effects of D1 and D2 receptor activation on Ser831 phosphorylation. In contrast to the robust increase in phosphorylation of Ser845 observed in response to dopamine or a D1-selective agonist (Fig. 2A), neither dopamine, SKF81297 (106 ± 11% of control), quinpirole (82± 12% of control), nor SKF81297 plus quinpirole (107± 15% of control) had a significant effect on Ser831 phosphorylation (Fig.2B).
Regulation of Ser845 phosphorylation: involvement of a DARPP-32/PP1 cascade
The ability of serine/threonine protein phosphatases to regulate the basal phosphorylation of GluR1 in neostriatal cells was examined. Neostriatal slices were incubated with either calyculin A (500 nm) or okadaic acid (1 μm), inhibitors of both PP1 and protein phosphatase 2A (PP2A). Calyculin A increased phospho-Ser845 GluR1 to a level 600± 129% of control after 60 min of incubation (Fig.3A). Similarly, okadaic acid increased phospho-Ser845 GluR1 to a maximal level of 783 ± 102% of control after 60 min. By comparison, cyclosporin A, an inhibitor of calcineurin (protein phosphatase 2B, PP2B), had no significant effect on the basal level of phosphorylated Ser845 GluR1 after a 60 min incubation period (104 ± 4% compared with control; Fig.3A) or on the state of phosphorylation of DARPP-32 at Thr34 (data not shown). Phosphorylation of GluR1 at Ser831 was slightly increased by calyculin A (256 ± 78% of control) in contrast to the absence of effect of okadaic acid (137 ± 36% of control) or cyclosporin A (104 ± 25% of control) (Fig. 3B). However, the effect of calyculin A was not statistically significant (p > 0.05; Mann–Whitney U test, for three experiments). These data indicate that Ser845 is a better substrate than Ser831 for regulation by PP1 and/or PP2A in neostriatal neurons.
Fig. 3.
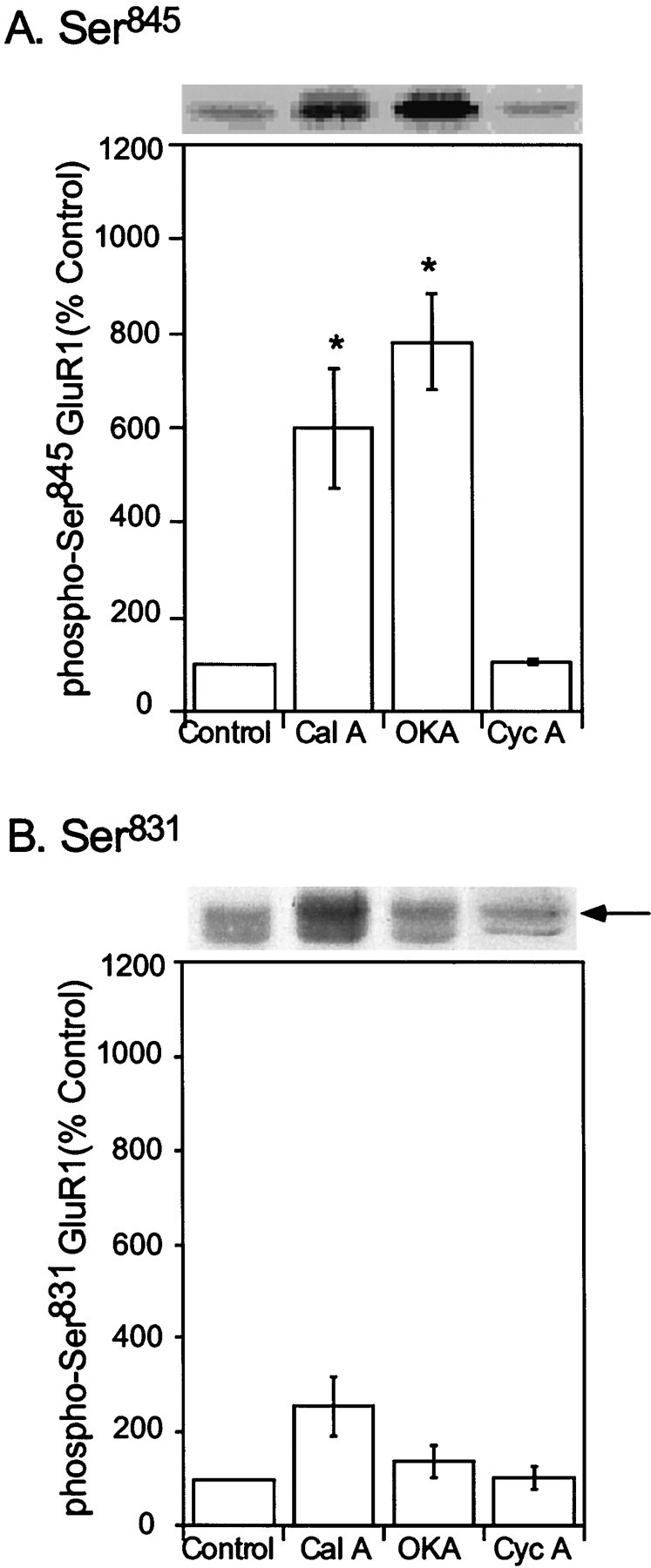
Effects of protein phosphatase inhibitors on the phosphorylation state of neostriatal GluR1 at Ser845and Ser831. Mouse neostriatal slices were incubated for 60 min in the absence (Control) or presence of either okadaic acid (1 μm) (OKA) or calyculin A (0.5 μm) (Cal A), inhibitors of PP1/PP2A, or cyclosporin A (5 μm) (Cyc A), an inhibitor of PP2B. Ser845phospho-GluR1 (A) and Ser831phospho-GluR1 (B) were detected, quantitated by densitometry, and expressed as percentage of the level in control slices. Data are expressed as means ± SEM for three experiments (*p < 0.05 compared with control, Mann–Whitney U test).
To investigate the specific contribution of PP1 to dopamine-induced phosphorylation of GluR1, we compared Ser845 phosphorylation in wild-type mice and in mice bearing a targeted deletion of the gene for the selective PP1 inhibitor DARPP-32 (Fienberg et al., 1998). These experiments revealed that there was no significant difference in the basal phosphorylation of Ser845 as the result of the DARPP-32 knock-out. Dopamine (100 μm) induced a threefold to fourfold increase in phosphorylation of GluR1 at Ser845 in neostriatal slices from wild-type mice (324 ± 85% of control at 2.5 min and 383± 26% of control at 5 min), but not from DARPP-32 knock-out mice (94 ± 41% of control at 2.5 min and 174± 72% of control at 5 min; Fig.4A). A similar attenuation of dopamine-induced phosphorylation was observed in nucleus accumbens slices (data not shown). These data indicate that inhibition of PP1 is required for dopamine-induced increases in GluR1 phosphorylation. In support of this conclusion, we found that direct, pharmacological inhibition of PP1/PP2A affected GluR1 phosphorylation comparably in wild-type and knock-out slices. In this series of experiments okadaic acid increased Ser845phosphorylation in slices from both wild-type and DARPP-32 knock-out mice (878 ± 210% of control in wild-type mice and 1008± 50% of control in knock-out mice) after 60 min of incubation (Fig. 4B).
Fig. 4.
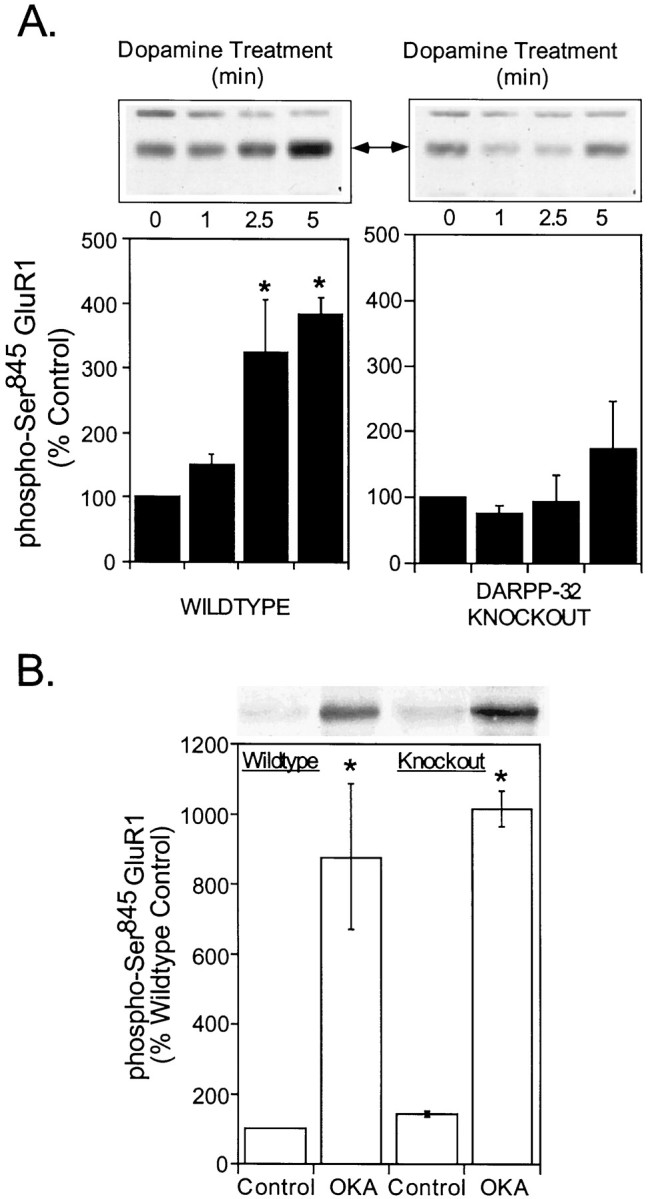
Role of DARPP-32 in the dopamine-induced phosphorylation of neostriatal GluR1 on Ser845.A, Mouse neostriatal slices prepared from wild-type mice (left) or DARPP-32 knock-out mice (right) were incubated in the absence or presence of dopamine (100 μm) plus nomifensine (10 μm) for the indicated times. Samples were immunoblotted for Ser845-phosphorylated GluR1. The level of phospho-GluR1 was quantitated by densitometry, and the data were expressed as percentage of either the wild-type or knock-out control level. The results are expressed as means ± SEM for five experiments (*p < 0.05 compared with 0 time, Mann–Whitney U test). B, Slices from wild-type or knock-out mice were incubated with a maximally effective concentration of the PP1/2A inhibitor, okadaic acid (OKA; 1 μm) for 60 min. The level of phospho-Ser845 GluR1 was quantitated by densitometry, and the data were expressed as percentage of the wild-type control level. Results are expressed as means± SEM for three experiments (*p < 0.05 compared with control, Mann–Whitney U test).
Regulation of GluR1 phosphorylation by cocainein vivo
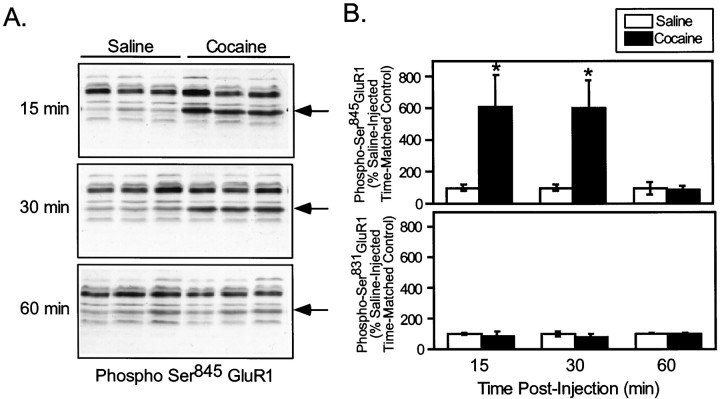
To evaluate the possible involvement of psychostimulants in the control of AMPA receptor phosphorylation in vivo, intact mice were treated with drugs that are known to enhance dopamine availability in the neostriatum, and the effect on the phosphorylation of GluR1 was examined. We first measured the effect of cocaine (20 mg/kg, i.p.), a drug that increases dopamine availability through blockade of the dopamine transporter, on the phosphorylation of GluR1 at Ser845. Cocaine administration produced a behavioral activation in mice characterized by increased locomotor behavior that was evident within 15 min of injection. Cocaine also caused a large increase in the phosphorylation state of GluR1 at Ser845 in neostriatum, as observed 15 min (saline, 100 ± 18%; cocaine, 612 ± 198%) and 30 min (saline, 100 ± 22%; cocaine, 604 ±170%) after injection (Fig. 5). The effect of cocaine treatment on GluR1 phosphorylation rapidly dissipated, as Ser845 phosphorylation returned to the basal level within 60 min (saline, 100 ±4%; cocaine, 89 ± 25%) of drug administration (Fig. 5). In contrast, cocaine treatment did not significantly affect the level of phosphorylation of GluR1 at Ser831 in neostriatum compared with time-matched saline-injected controls at any time point examined (89 ± 30% of control at 15 min; 80± 18% of control at 30 min; and 98 ± 8% of control at 60 min) (Fig. 5). These data indicate that acute cocaine injection selectively regulates phosphorylation of GluR1 at Ser845.
Fig. 5.
Effect of acute cocaine on neostriatal GluR1 phosphorylation at Ser845 and Ser831in vivo. Normal C57Bl/6 mice were injected (intraperitoneally) with vehicle alone (Saline) or with vehicle containing cocaine (20 mg/kg) and killed by focused microwave irradiation at 15, 30, or 60 min after injection. Neostriatum was dissected from each brain and analyzed for phospho-Ser845 and phospho-Ser831 GluR1. The arrowsindicate the position of GluR1, as verified by immunoblotting with an antibody against a C-terminal sequence of GluR1. A,Representative experiment showing immunoblots of phospho-Ser845 GluR1 from three individual animals for each treatment condition. B, Quantitation of phospho-Ser845 and phospho-Ser831in three experiments, each analyzed in triplicate (*p < 0.05, compared with saline; Mann–WhitneyU test).
Role of DARPP-32 in cocaine-mediated regulation of Ser845 phosphorylation in vivo
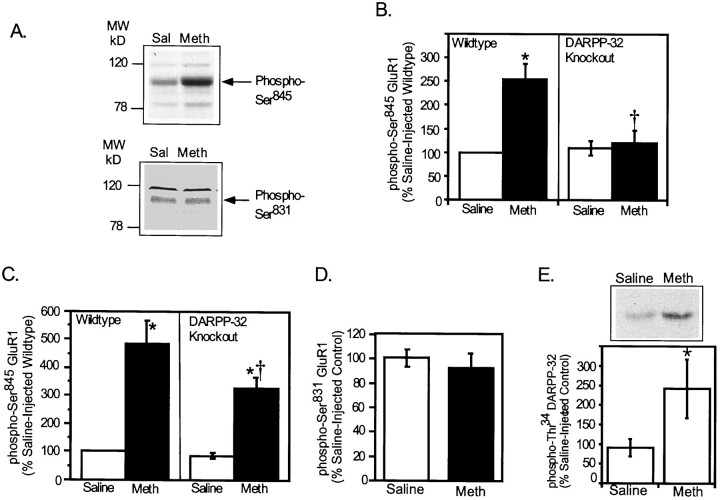
It seemed possible that cocaine-stimulated dopamine release could increase GluR1 phosphorylation by at least two mechanisms. First, dopamine and D1-receptor-mediated activation of PKA would be expected to lead directly to phosphorylation of Ser845. Second, PKA-mediated phosphorylation of DARPP-32 at Thr34 would be expected to inhibit PP1 activity, leading to an increase in phosphorylation state of PP1 substrates, including GluR1. Consistent with this idea, cocaine did induce a severalfold increase in phospho-Thr34 DARPP-32 (605 ±205% of control) (Fig.6A), concurrent with the increase in Ser845 phosphorylation in wild-type mice. A possible role for DARPP-32 in the in vivophosphorylation of GluR1 was further evaluated by measuring cocaine-induced GluR1 phosphorylation in DARPP-32 knock-out mice. The level of GluR1 expressed in neostriatum was unaffected by the genetic deletion of DARPP-32 (data not shown). However, the increase in GluR1 phosphorylation at Ser845 in neostriatum observed after treatment with a low dose of cocaine (10 mg/kg, i.p.) (671 ± 75% of control in this series of experiments) was greatly attenuated in mice lacking DARPP-32 (138 ± 18% of control; Fig. 6A).
Fig. 6.
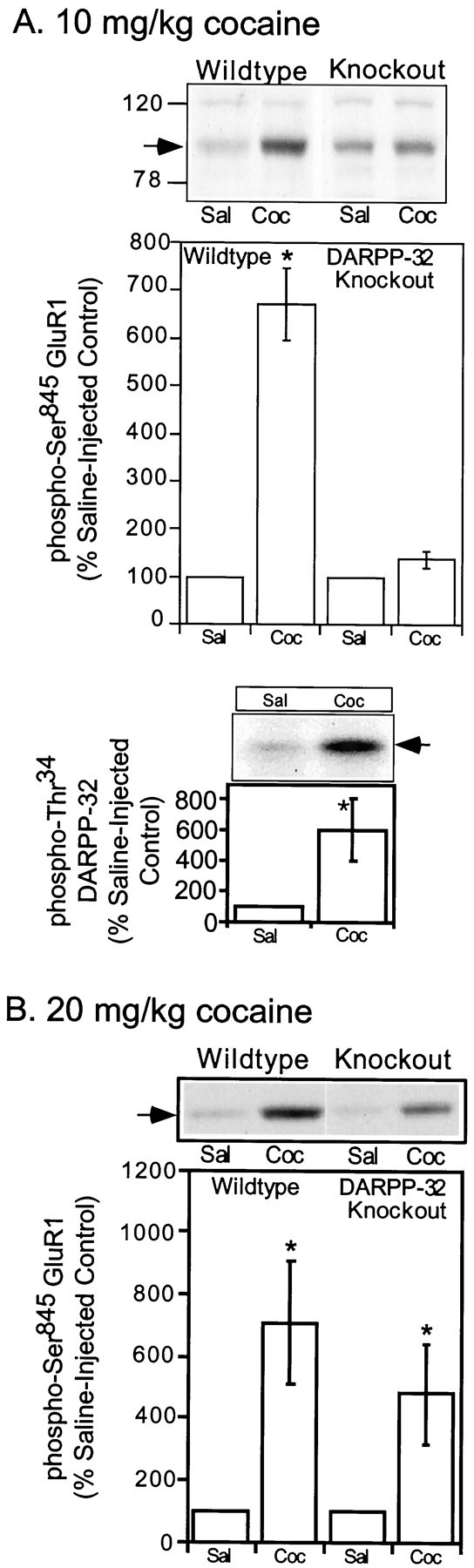
Effect of acute cocaine on in vivophosphorylation of GluR1 at Ser845: comparison of wild-type and DARPP-32 knock-out mice. Wild-type or DARPP-32 knock-out mice were injected (intraperitoneally) with saline vehicle (Sal) or with (10 mg/kg) (A) or (20 mg/kg) (B) cocaine (Coc) and killed by focused microwave irradiation 30 min later. The levels of phospho-Ser845 GluR1 and phospho-Thr34 DARPP-32 were detected in neostriatum by immunoblotting, and the data were quantitated by densitometry.Arrows indicate the position of phospho-GluR1 and phospho-DARPP-32 bands. A, Top, An autoradiogram of a representative experiment shows the level of phospho-GluR1 in saline-injected or cocaine-injected wild-type and DARPP-32 knock-out mice. The bar graph summarizes phospho-Ser845 GluR1 levels in the neostriatum of the wild-type and knock-out mice. Data are expressed as means ± SEM for eight mice per group (*p < 0.01 compared with saline-injected mice; Student's t test).A, Bottom, A typical autoradiogram shows the level of phospho-DARPP-32 in a saline-injected and a cocaine-injected wild-type mouse neostriatum. The bar graph shows phospho-Thr34DARPP-32 levels in the neostriata of mice. Data are expressed as means± SEM for four mice per group (*p< 0.05 compared with saline-injected mice; Mann–WhitneyU test). B, Data are expressed as means± SEM for 3–5 mice per group (*p< 0.05 compared with respective saline-injected controls; Mann–Whitney U test).
A previous report from this laboratory (Fienberg et al., 1998) showed that the effect of DARPP-32 knock-out on cocaine-induced locomotor behavior was dose-dependent. Thus, the increase in locomotor behavior elicited by a low (10 mg/kg), but not by a high (20 mg/kg) dose of cocaine was blocked in DARPP-32 knock-out mice. We examined the dose dependency of the effects of cocaine on GluR1 phosphorylation. A 20 mg/kg concentration of cocaine induced an approximately sevenfold increase in Ser845 phosphorylation (709± 198% of control; Fig. 6B) in wild-type mice. This increase was not significantly reduced in DARPP-32 knock-out mice (479 ± 165% of control; Fig.6B).
Regulation of GluR1 phosphorylation by methamphetaminein vivo
Methamphetamine-HCl, a substituted analog ofd-amphetamine with profound abuse potential, also increases dopamine availability in the neostriatum. The effect of systemic injection of methamphetamine on the in vivo phosphorylation of GluR1 in mouse neostriatum was examined. Injection of mice with methamphetamine (i.e., 20 or 30 mg/kg, s.c.) induced behavioral activation, eliciting intense grooming behavior and hypersensitivity to touch. Treatment of mice with methamphetamine (20 mg/kg, s.c.) increased phospho-Ser845 levels by twofold to threefold in neostriatum 30 min after drug injection (254± 32% of saline-injected wild-type) (Fig.7B). In contrast, methamphetamine treatment had no effect on the level of phospho-Ser831 (93 ± 12% of saline-injected control) (Fig. 7D). This dose of methamphetamine also increased the phosphorylation of DARPP-32 on Thr34 by more than twofold in the neostriatum of mice (270 ± 82% of control) (Fig.7E). The increase in phospho-Ser845 induced by this concentration of methamphetamine was fully blocked in DARPP-32 knock-out mice (120 ± 28% of saline-injected wild type) (Fig. 7B). A higher concentration of methamphetamine (30 mg/kg, s.c.) induced a fourfold to fivefold increase in Ser845 phosphorylation in the neostriatum of wild-type mice compared to vehicle-treated controls (486± 80% of saline-injected wild types) (Fig. 7C). The higher concentration of methamphetamine induced a significant increase in Ser845 phosphorylation even in the DARPP-32 knock-out mice (325 ± 40% of saline-injected knock-out mice) (Fig. 7C). The level of Ser845 phosphorylation that was observed in the knock-out mice, however, was significantly reduced compared to wild-type mice (Fig. 7C).
Fig. 7.
Effect of acute methamphetamine administration onin vivo phosphorylation of GluR1 at Ser845 and Ser831 in wild-type and DARPP-32 knock-out mice. Wild-type (A–E) or DARPP-32 knock-out (B, C) mice were injected with vehicle or methamphetamine (Meth) 20 mg/kg (subcutaneously) (B, D, E) or 30 mg/kg (A, C) and killed by focused microwave irradiation 30 min later. Neostriatal phospho-Ser845GluR1 (A–C) and phospho-Ser831 GluR1 (A, D) and phospho-Thr34 DARPP-32 (E) were detected by immunoblotting and quantitated by densitometry. Data are expressed as percentage of values for saline-injected wild-type mice and represent means ± SEM for nine (B, C), three (D), or eight animals (E) per group (*p < 0.05 compared with saline-injected mice; †p < 0.05, compared with wild-type Meth alone; Students' ttest).
DISCUSSION
Using neostriatal slices, we have demonstrated that dopamine stimulates phosphorylation of GluR1 at Ser845 through activation of D1-type dopamine receptors and stimulation of adenylyl cyclase and PKA. We have also demonstrated that psychostimulants rapidly and reversibly increase the phosphorylation of GluR1 at Ser845 in the neostriatum in vivo. Estimates of the stoichiometry of GluR1 phosphorylation indicate that a substantial proportion of the GluR1 is phosphorylated at Ser845 in response either to dopamine in slices or to cocaine and methamphetaminein vivo. These data indicate that regulation of the state of phosphorylation of GluR1 represents a probable mechanism for control of AMPA receptor currents by dopamine and psychostimulants. Results from a previous study involving a Ser845-Ala mutation of GluR1 indicated that phosphorylation of GluR1 at Ser845 was required to potentiate peak currents carried by homomeric GluR1 receptors (Roche et al., 1996). In addition, PKA agonists reversed synaptic depression of AMPA currents in hippocampal slices by increasing phosphorylation at Ser845 (Kameyama et al., 1998; Lee et al., 1998). Taken together, these data suggest that dopamine-induced, PKA-mediated, phosphorylation of Ser845 in slices and in vivo is likely to enhance glutamatergic transmission through AMPA receptors.
AMPA receptor currents are also modulated by phosphorylation of GluR1 at Ser831 (Barria et al., 1997; Derkach et al., 1999). This residue, which is phosphorylated in vitroby PKC or CaMKII (McGlade-McCulloh et al., 1993; Blackstone et al., 1994), is also phosphorylated to a low but measurable stoichiometry in untreated neostriatum. However, the phosphorylation of this site was not regulated in response to dopaminergic activity in slices orin vivo with either wild-type mice (Fig.2B) or in DARPP-32 knock-out mice (data not shown). The data further suggest that dopamine and D1 agonists regulate AMPA receptor currents through the selective phosphorylation of Ser845. The proportion of GluR1 subunits phosphorylated at Ser831 is greatly increased by activation of PKC (Fig. 1B) or CaMKII signaling pathways (our unpublished observations), suggesting that both Ser845 and Ser831 can serve to mediate neurotransmitter-specific effects on AMPA receptors.
Dopamine-induced phosphorylation at Ser845in slices and psychostimulant-induced phosphorylation of Ser845 in vivo were reduced in the neostriatum of DARPP-32 knock-out mice. These data are consistent with the loss of D1-mediated enhancement of whole-cell AMPA currents in neostriatal neurons from DARPP-32 knock-out mice (Yan et al., 1999). Taken together, the results indicate that a pathway involving PKA and DARPP-32/PP1 functionally regulates AMPA receptors in basal ganglia neurons. It is unlikely that the deficits in psychostimulant action seen in the absence of DARPP-32 are attributable to a reduced ability to release dopamine. This conclusion is supported by a recent study in which dopamine overflow in neostriatum was measured by amperometry in response to stimulation of the medial forebrain bundle. Stimulation-evoked dopamine release in vivo was found to be equivalent in wild-type and DARPP-32 knock-out mice (F. Gonon, A. Fienberg, and P. Greengard, unpublished observations). In addition, deletion of the DARPP-32 gene does not significantly affect either the density of D1 receptors or their affinity for dopamine ligands (P. Svenningsson, B. Fredholm, A. Fienberg, and P. Greengard, unpublished observations). Moreover, Ser845phosphorylation is increased comparably in slices from wild-type and DARPP-32 knock-out mice in response either to forskolin, which induces PKA activity (G. Snyder, unpublished observations), or to drugs that inhibit PP1/PP2A activity (Fig. 4B). Together, the results indicate that the loss of GluR1 regulation is caused by a loss of ability to regulate PP1 activity.
The phosphorylation of Ser845 elicited by either cocaine or methamphetamine is blocked in DARPP-32 knock-out mice at low but not at high concentrations of these drugs. These data are consistent with previous reports (Fienberg et al., 1998, Snyder et al., 1998) showing that DARPP-32 knock-out blocks responses to low (physiological) levels of activation of transmission, but not to high (often supraphysiological) levels of activation. For example, cocaine-induced locomotor activation in mice induced by a 10 mg/kg dose of drug is fully blocked in DARPP-32 knock-out mice, whereas activation induced by a 20 mg/kg cocaine injection is unaffected by DARPP-32 knock-out (Fienberg et al., 1998).
Taken together, we propose the following interpretation of these data: that PKA increases phosphorylation of Ser845 by at least two mechanisms: (1) by directly phosphorylating Ser845, and (2) by increasing phosphorylation of DARPP-32 at Thr34, leading to inhibition of PP1 activity. Thus, our data suggest that high concentrations of psychostimulants (i.e., 30 mg/kg methamphetamine or 20 mg/kg cocaine) provide sufficient activation of PKA to sustain PKA-mediated phosphorylation of GluR1 in the absence of PP1 inhibition. The results obtained at the lower concentrations of psychostimulants support a role for PKA-dependent phosphorylation of DARPP-32 at Thr34 as an obligatory component of pathways that mediate a variety of biochemical, physiological, and behavioral effects of dopamine in neostriatal neurons (Fienberg et al., 1998; Greengard et al., 1999). However, a recent study (Bibb et al., 1999) has demonstrated that DARPP-32 is phosphorylated at Thr75 by a cyclin-dependent kinase family member, cdk5, converting the protein into a PKA inhibitor. It will be important in future studies to evaluate the possible contribution of the regulation of Thr75 of DARPP-32 to the control of AMPA channels by dopamine and psychostimulants.
Psychostimulants are known to regulate various biochemical indices of glutamate function including glutamate release, metabolism, and receptor expression (Wolf, 1998). Most of these changes do not occur in response to acute drug use, but appear only after repeated drug presentation, indicating that they represent indirect, long-term adaptive responses. The present results show that the regulation of AMPA receptor current through phosphorylation is likely to be a rapid, acute, and dramatic response to neostriatal dopamine release by psychostimulant drugs. The enhancement of AMPA receptor currents by these drugs represents an attractive mechanism by which glutamate receptors and their signaling pathways might be recruited to mediate a variety of effects, such as increased immediate early gene (IEG) expression (Konradi et al., 1996), which may eventually lead to sensitization. In support of this idea, AMPA or NMDA receptor antagonists block amphetamine-induced IEG expression (Konradi et al., 1996) and prevent the development of behavioral sensitization to amphetamine (Karler et al., 1989; Wolf, 1998).
Footnotes
This work was supported by United States Public Health Service Grants DA10044 and MH40899 (P.G.). We thank Peter Ingrassia and Stacey Galdi for excellent technical assistance.
Correspondence should be addressed to Dr. Gretchen L. Snyder, Laboratory of Molecular and Cellular Neuroscience, The Rockefeller University, Box 296, 1230 York Avenue, New York, NY 10021. E-mail:snyderg@rockvax.rockefeller.edu.
REFERENCES
- 1.Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci USA. 1997;94:9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 3.Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai L-H, Kwon YT, Girault J-A, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 4.Blackstone CD, Murphy TH, Moss SJ, Baraban JM, Huganir RL. Cyclic AMP and synaptic activity-dependent phosphorylation of AMPA-preferring glutamate receptors. J Neurosci. 1994;14:7585–7593. doi: 10.1523/JNEUROSCI.14-12-07585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bliss TVP, Collingridge GL. Synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 6.Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fienberg AA, Hiroi N, Mermelstein PG, Song W-J, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn S-P, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault J-A, Nestler EJ, Greengard P. DARPP-32: Regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;271:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 9.Greengard P, Jen J, Nairn AC, Stevens CF. Enhancement of the glutamate response by cAMP-dependent protein kinase in hippocampal neurons. Science. 1991;253:1135–1138. doi: 10.1126/science.1716001. [DOI] [PubMed] [Google Scholar]
- 10.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 11.Hemmings HC, Jr, Greengard P, Tung HYL, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez S, Day M, Surmeier DJ. Activation of D2 dopamine receptors decreases L-type calcium currents in striatal medium spiny neurons. Soc Neurosci Abstr. 1999;25:1654. [Google Scholar]
- 13.Huff RM. Signal transduction pathways modulated by the D2 subfamily of dopamine receptors. Cell Signal. 1996;8:453–459. doi: 10.1016/s0898-6568(96)00074-5. [DOI] [PubMed] [Google Scholar]
- 14.Hyman S. Addiction to cocaine and amphetamine. Neuron. 1996;16:901–904. doi: 10.1016/s0896-6273(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 15.Kameyama K, Lee H-K, Bear MF, Huganir RL. Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron. 1998;21:1163–1175. doi: 10.1016/s0896-6273(00)80633-9. [DOI] [PubMed] [Google Scholar]
- 16.Karler R, Calder LD, Chaudhry IA, Turkanis SA. Blockade of “reverse tolerance” to cocaine and amphetamine by MK-801. Life Sci. 1989;45:599–606. doi: 10.1016/0024-3205(89)90045-3. [DOI] [PubMed] [Google Scholar]
- 17.Konradi C, Leveque J-C, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci. 1996;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koob GF, LeMoal M. Drug abuse: hedonistic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Lee H-K, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21:1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- 21.Linden DJ. Long-term synaptic depression in the mammalian brain. Neuron. 1994;12:457–472. doi: 10.1016/0896-6273(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 22.Lipton SA, Rosenberg PA. Mechanisms of disease: excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 23.Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- 24.McGlade-McCulloh E, Yamamoto H, Tan S-E, Brickey DA, Soderling TR. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature. 1993;362:640–642. doi: 10.1038/362640a0. [DOI] [PubMed] [Google Scholar]
- 25.Monaghan DT, Bridges RJ, Cotman CW. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the nervous system. Ann Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- 26.Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 27.Ouimet CC, Miller PE, Hemmings HC, Jr, Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci. 1984;4:114–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouimet CC, da Cruz e Silva E, Greengard P. The alpha and gamma 1 isoforms of protein phosphatase 1 are highly and specifically concentrated in dendritic spines. Proc Natl Acad Sci USA. 1995;92:3396–3400. doi: 10.1073/pnas.92.8.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- 30.Roche KW, O-Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 31.Rosenmund C, Carr DW, Bergeson SE, Nilaver G, Scott JD, Westbrook GL. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature. 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 32.Sibley DR, Monsma FJ., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992;13:61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- 33.Snyder GL, Girault J-A, Chen JYC, Czernik AJ, Kebabian JW, Nathanson JA, Greengard P. Phosphorylation of DARPP-32 and protein phosphatase inhibitor-1 in rat choroid plexus: regulation by factors other than dopamine. J Neurosci. 1992;12:3071–3083. doi: 10.1523/JNEUROSCI.12-08-03071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder GL, Fienberg AA, Huganir RL, Greengard P (1998) A dopamine/D1 receptor/PKA/DARPP-32/protein phosphatase-1 pathway regulates dephosphorylation of theN-methyl-d-aspartate receptor J Neurosci 10297–10303. [DOI] [PMC free article] [PubMed]
- 35.Stoof JC, Kebabian JW. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981;294:366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- 36.Tan SE, Wenthold RJ, Soderling TR. Phosphorylation of AMPA-type glutamate receptors by calcium/calmodulin-dependent protein kinase II and protein kinase C in cultured hippocampal neurons. J Neurosci. 1994;14:1123–1129. doi: 10.1523/JNEUROSCI.14-03-01123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Towbin H, Staehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walaas SI, Aswad DW, Greengard P. A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature. 1983;301:69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- 39.Wang LY, Salter MW, McDonald JF. Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science. 1991;253:1132–1135. doi: 10.1126/science.1653455. [DOI] [PubMed] [Google Scholar]
- 40.Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 41.Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P. Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]