Abstract
In this report, we have investigated the signaling pathways that are activated by, and mediate the effects of, the neuregulins and axonal contact in Schwann cells. Phosphatidylinositol 3-kinase (PI 3-kinase) and mitogen-activated protein kinase kinase (MAPK kinase) are strongly activated in Schwann cells by glial growth factor (GGF), a soluble neuregulin, and by contact with neurite membranes; both kinase activities are also detected in Schwann cell-DRG neuron cocultures. Inhibition of the PI 3-kinase, but not the MAP kinase, pathway reversibly inhibited Schwann cell proliferation induced by GGF and neurites. Cultured Schwann cells undergo apoptosis after serum deprivation and can be rescued by GGF or contact with neurites; these survival effects were also blocked by inhibition of PI 3-kinase. Finally, we have examined the role of these signaling pathways in Schwann cell differentiation in cocultures. At early stages of coculture, inhibition of PI 3-kinase, but not MAPK kinase, blocked Schwann cell elongation and subsequent myelination but did not affect laminin deposition. Later, after Schwann cells established a one-to-one relationship with axons, inhibition of PI 3-kinase did not block myelin formation, but the myelin sheaths that formed were shorter, and the rate of myelin protein accumulation was markedly decreased. PI 3-kinase inhibition had no observable effect on the maintenance of myelin sheaths in mature myelinated cocultures. These results indicate that activation of PI 3-kinase by axonal factors, including the neuregulins, promotes Schwann cell proliferation and survival and implicate PI 3-kinase in the early events of myelination.
Keywords: Schwann cell, PI 3-kinase, MAP kinase, proliferation, survival, myelination, signaling pathways
The development of myelinated nerve fibers in the peripheral nervous system depends on complex interactions between Schwann cells and axons. Axon-associated factors promote the generation of Schwann cells during development via mitogenic (Wood and Bunge, 1975; Salzer et al., 1980a) and trophic (Grinspan et al., 1996;Trachtenberg and Thompson, 1996) effects. Axons also promote the deposition of the basal lamina by Schwann cells, which is required for the ensheathment of axons and the subsequent differentiation of Schwann cells (Bunge et al., 1986). Identification of the axonal signals that regulate Schwann cell development and the intracellular signaling pathways they activate will be important to elucidate the mechanisms involved in these interactions.
The neuregulin family of growth factors plays a crucial role during the early stages of Schwann cell development (Lemke, 1996; Jessen and Mirsky, 1998; Adlkofer and Lai, 2000). Several membrane-bound and secreted isoforms are generated by alternative splicing of at least four genes (Burden and Yarden, 1997; Harari et al., 1999). Neuregulin-1 isoforms promote the proliferation and survival of Schwann cell precursors in cultures (Dong et al., 1995; Syroid et al., 1996). They also promote the proliferation and survival of Schwann cells associated with axons in vitro and in vivo (Morrissey et al., 1995; Grinspan et al., 1996; Trachtenberg and Thompson, 1996). The severe deficiency of Schwann cells in mice in which the neuregulin-1 gene has been inactivated provides striking support for the key role of this growth factor in Schwann cell development (Meyer and Birchmeier, 1995).
Little is known about the signaling pathways by which the neuregulins mediate these diverse biological effects on Schwann cells. Neuregulins bind to receptor tyrosine kinases of the erbB family. After ligand binding, the erbBs undergo dimerization and phosphorylation, thereby recruiting Src homology 2 domain-containing signal-transducing proteins (Burden and Yarden, 1997). These include, among others, mitogen-activated protein kinases (MAP kinases) and phosphatidylinositol 3-kinase (PI 3-kinase), both of which have been implicated in the regulation of cell proliferation, survival, and differentiation in other cell types (Rameh and Cantley, 1999; Widmann et al., 1999). Both PI 3-kinase and MAP kinase activities increased in oligodendrocytes (Canoll et al., 1999) and Schwann cell-dorsal root ganglion (DRG) neuron cocultures (G. Zanazzi, K. K. Teng, R. Kraemer, and J. L. Salzer, personal communication) when treated with glial growth factor (GGF), a soluble neuregulin. Recent studies have also suggested that PI 3-kinase plays a key role in oligodendrocyte and Schwann cell survival in certain settings (Vemuri and McMorris, 1996; Campana et al., 1999; Dong et al., 1999; Weiner and Chun, 1999), consistent with its role in other cells.
We now report that GGF and axonal contact result in a robust activation of PI 3-kinase and that this activation is required for the mitogenic and survival activities of GGF and axons on Schwann cells. MAP kinase kinase (MAPK kinase) is also robustly activated and contributes to the survival mediated by GGF, but it is not required for axon-mediated survival. In a Schwann cell-DRG neuron coculture system, inhibition of PI 3-kinase, but not that of MAPK kinase, also blocks myelination, possibly by blocking initial Schwann cell-axon associations. These results indicate that activation of PI 3-kinase is crucial for the contact-dependent regulation of Schwann cell development by axons.
Parts of this paper have previously appeared in abstract form (Maurel and Salzer, 1999).
MATERIALS AND METHODS
Inhibitors, antibodies, and cell culture materials.The PI 3-kinase inhibitor 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one (LY294002; Sigma,St. Louis, MO) and the MAPK kinase inhibitor U0126 (Promega, Madison, WI ) were prepared in DMSO (Sigma) at stock concentrations of 20 and 10 mm, respectively. Dilutions were made directly in the appropriate culture media, keeping DMSO at a constant final concentration of 0.2% (final LY294002 concentration of 2.5–20 μm; final U0126 concentration of 1–10 μm). Monoclonal antibodies included the fluorescein-conjugated anti-bromodeoxyuridine (BrdU) antibody (Boehringer Mannheim, Indianapolis, IN), the anti-myelin basic protein (MBP) antibody SMI 94, and the anti-neurofilament antibodies SMI 31 and SMI 32 (Sternberger Monoclonals, Lutherville, MD). Rabbit polyclonal antibodies included the anti-Akt, anti-phospho-Akt, anti-MAPK, and anti-phospho-MAPK from New England Biolabs (Beverly, MA), the anti-MBP antibody 644 and an anti-protein zero (P0) antibody (gifts of D. Colman, Mount Sinai Medical Center, New York, NY), and the anti-laminin antibody L-9393 (Sigma). Secondary antibodies were rhodamine-conjugated donkey anti-rabbit IgG, fluorescein-conjugated donkey anti-mouse IgG (Chemicon, Temecula, CA), coumarin-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA),125I-anti-rabbit IgG (ICN Biomedicals, Costa Mesa, CA), and horseradish peroxidase-conjugated goat anti-rabbit IgG (Pharmacia, Piscataway, NJ). Materials used for cell cultures included DMEM (BioWhittaker, Walkersville, MD), MEM, DME/Ham's F12, L15, and l-glutamine (Life Technologies, Gaithersburg, MD), FBS (HyClone, Logan, UT), collagen (Biomedical Technologies, Stoughton, MA), poly-l-lysine, glucose, 5-fluorodeoxyuridine, uridine, insulin, progesterone, putrescine, selenium, ascorbic acid (Sigma), transferrin (Jackson ImmunoResearch), nerve growth factor (NGF; Harlan Bioproducts for Science, Indianapolis, IN), recombinant human GGF (rhGGF2; gift of Cambridge NeuroScience, Cambridge, MA), and FGF-2 (gift of D. Rifkin, New York University Medical Center, New York, NY).
Cell cultures. Cultures of primary rat Schwann cells and DRG neurons were established as described previously (Einheber et al., 1993). Briefly, primary Schwann cells prepared from postnatal day 2 sciatic nerves (Brockes et al., 1979) were expanded in serum-containing D media (DMEM, 10% FBS, and 2 mml-glutamine) supplemented with forskolin and GGF for a period of 7 weeks. Schwann cells were then maintained in D media for at least 3 d before use. Neurons isolated from embryonic day 16 DRGs were plated on a collagen substrate in serum-containing C media (MEM, 10% FBS, 2 mml-glutamine, 0.4% glucose, and 50 ng/ml 2.5 S NGF), at a density of ∼4000 neurons (12 mm glass coverslip) or 40,000 neurons (35 mm plastic dish). Non-neuronal cells were removed by feeding the cultures every 2–3 d alternately with C media or C media supplemented with 5-fluorodeoxyuridine and uridine (both at 10 μm) over a period of 10 d. Neuron cultures were then maintained for 7 d in C media before use.
Neurite membrane preparation. Procedures for isolating neurite membranes were modified from previously described methods (Salzer et al., 1980b). Briefly, DRG neurons from 35-mm-dish cultures were washed once with ice-cold Dulbecco's PBS (dPBS; Life Technologies). The neurites were collected by scraping the tissue with fine forceps and homogenized with 35–40 strokes of a 1 ml Dounce homogenizer (Kontes, Vineland, NJ) in 200 μl of ice-cold dPBS. The volume was brought up to 1.5 ml with ice-cold dPBS, and the homogenate was centrifuged (80 × g; 20 min; 4°C) to remove debris and collagen. The supernatant, diluted up to 7 ml, was then centrifuged at 35,000 × g for 1 hr at 4°C. The supernatant was discarded, and the pellet was resuspended in the appropriate culture media by vortexing.
Proliferation assays. To investigate the effect of PI 3-kinase and MAPK kinase inhibitors on the proliferation of Schwann cells mediated by soluble growth factors, we plated 50,000 cells in D media onto poly-l-lysine-coated glass coverslips. After 24 hr, forskolin (4 μm) was added to the cultures. The next day, the cultures were treated for 24 hr with either LY294002 or U0126 in fresh D media supplemented with forskolin and GGF (5 ng/ml) or FGF-2 (10 ng/ml). Control cultures were maintained in D media with or without forskolin.
Two approaches were used to investigate the effect of LY294002 and U0126 on the proliferation of Schwann cells induced by contact with neurites. We first examined Schwann cells proliferating in cocultures with DRG neurons. For these studies, 10,000 Schwann cells in C media were seeded onto dissociated DRG neurons. After 24 hr, cultures were fed fresh C media with (treated) or without (control) kinase inhibitors for an additional 24 hr. In other studies, two aliquots of neurite membranes, each equivalent to one-third of a 35 mm DRG neuron culture dish, were centrifuged (200 × g; 10 min; 4°C) at a 24 hr interval on 50,000 Schwann cells cultured in D media alone or supplemented either with LY294002 or U0126. Cells were processed for BrdU immunostaining after an additional 24 hr in culture.
Schwann cell proliferation was assessed using a BrdU nuclear-labeling assay. In all conditions, BrdU was added during the last 4 hr of culture at a final concentration of 20 μm; immunostaining was performed according to the manufacturer's instructions (Boehringer Mannheim). All cultures were mounted on glass slides in Citifluor (Ted Pella, Redding, CA) containing Hoechst at 2 μg/ml and examined by epifluorescence microscopy (Axiophot microscope; Carl Zeiss, Thornwood, NY). BrdU- and Hoechst-labeled nuclei from five to seven random high-power fields per culture were counted. Four to six cultures per condition in two to three separate experiments were analyzed in each study. Typically over 3000 cells were counted per condition. The rate of proliferation of the Schwann cells was determined as the ratio of BrdU- to Hoechst-labeled nuclei (BrdU index).
Survival assays. To investigate the effect of PI 3-kinase and MAPK kinase inhibitors on the survival of Schwann cells mediated by GGF and the neurite membrane preparation, we cultured 30,000 cells in D media for 24 hr. Cells were then washed five times with DMEM and maintained in serum-free media (DMEM and 2 mml-glutamine) alone or supplemented with forskolin (4 μm) and GGF (50 ng/ml) with or without LY294002 or U0126. In other studies neurite membranes were added as described in Proliferation assays, with or without the inhibitors. Cells were treated with fresh media every day, for 3 d (GGF) or 2 d (membrane fraction).
Using the Schwann cell–DRG neuron coculture system, we seeded dissociated DRG neurons with 10,000 Schwann cells in serum-containing C media. After 24 hr, some cultures were fed serum-free media (control), whereas others were switched to serum-free media supplemented with LY294002 or U0126 for 3 d. The serum-free media consisted of DME/Ham's F12, 2 mml-glutamine, 10 μg/ml transferrin, 5 μg/ml insulin, 20 nm progesterone, 100 μm putrescine, 30 nm selenium, and 50 ng/ml NGF.
Schwann cell survival was determined by the TUNEL assay, which was performed with the Apoptosis Detection System (Promega, Madison, WI) according to the manufacturer's instructions. TUNEL and Hoechst-labeled nuclei from 7 to 10 random high-power fields were counted per culture, with four to six cultures per condition in two to three separate experiments, using an Axiophot microscope. The level of apoptosis was determined as the ratio of TUNEL to Hoechst-labeled nuclei (TUNEL index).
Myelination assays. Myelinating Schwann cell–neuron cocultures were prepared by seeding purified DRG neuron cultures with 200,000 Schwann cells in C media. The next day the cultures were switched to, and maintained in, serum-free media for 3 d to allow the Schwann cells to populate the neurites. Basal lamina formation and myelination were then initiated by feeding the cultures C media supplemented with 50 μg/ml ascorbic acid [day 0 (d0)] and maintained for 15 d. To determine the role of PI 3-kinase and MAPK kinase in the process of myelination, kinase inhibitors LY294002 and U0126 were added either at d0 or at d4. Cultures were analyzed for the expression of myelin proteins (MBP and P0) and for laminin, a major basal lamina component, by immunofluorescence and slot blot. Cultures for immunofluorescence microscopy were processed as described previously (Einheber et al., 1997).
Western and slot blot analysis of kinase activities and myelin protein expression. In vivo, full activation of the serine/threonine protein kinase Akt results from the PI 3-kinase-dependent phosphorylation on Thr-308 and Ser-473 residues (Alessi et al., 1996). The phosphorylation of MAP kinase p44/p42 on Thr-202 and Tyr-204 residues depends on the activity of MAPK kinase (Payne et al., 1991). To measure PI 3-kinase and MAPK kinase activities and the efficiency of the LY294002 and U0126 kinase inhibitors on Schwann cells, we therefore analyzed phosphorylation levels of Akt (phospho-Akt) and MAP kinase (phospho-MAPK p44/p42) by Western blotting. Briefly, cultures of Schwann cells were treated either directly with GGF (5 ng/ml; 5 min) or neurite membranes (30 min) at 37°C, or after a preincubation with either kinase inhibitor for 30 min. Controls were from cultures kept in D media. Cells were then lysed in 25 mm Tris buffer, pH 7.4, containing 150 mm NaCl, 1% SDS, 1 mmEDTA, 1 μg/ml aprotinin and leupeptin, 2 mmPMSF, 1 mm orthovanadate, and 2.5 mm sodium pyrophosphate. Protein concentrations were determined by the BCA method (Pierce, Rockford, IL). Ten micrograms of proteins were fractionated by SDS-PAGE, electroblotted onto PROTRAN BA85 nitrocellulose (Schleicher & Schuell, Keene, NH), and probed with anti-Akt, anti-phospho-Akt, anti-MAPK, or anti-phospho-MAPK antibodies. Proteins were visualized by chemiluminescence, which was performed according to the manufacturer's instructions (Pierce).
MBP and P0 levels from myelinating cocultures treated with or without LY294002 were determined by slot blot analysis. Five micrograms of total proteins were directly slot blotted onto nitrocellulose and probed with either anti-MBP 644 or anti-P0 polyclonal antibodies, which were detected with an 125I-anti-rabbit IgG antibody. Quantitation was performed using the PhosphorImager 425 and the ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Statistical analysis. One-way ANOVA, followed by a Bonferroni post-test, was performed with the Prism software package (GraphPad, San Diego, CA), with p < 0.05 considered to be statistically significant.
RESULTS
Mitogen activation of PI 3-kinase is required for the induction of Schwann cell proliferation
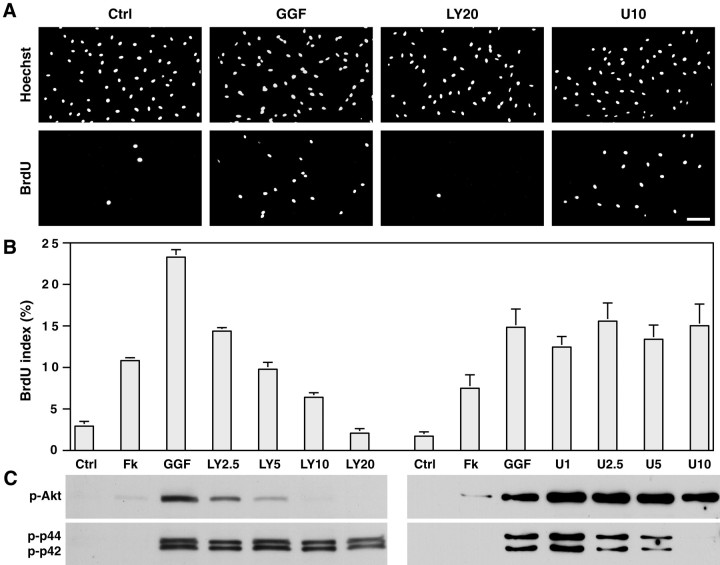
To elucidate whether the PI 3-kinase or the MAP kinase signaling pathways were involved in the induction of Schwann cell proliferation, we treated cells with the neuregulin-1 isoform GGF in the presence or absence of LY294002, a specific inhibitor of PI 3-kinase (Vlahos et al., 1994), or U0126, a specific inhibitor of MAPK kinase (Favata et al., 1998). As shown in Figure1A, the induction of Schwann cell proliferation by GGF, measured by BrdU incorporation, was inhibited by LY294002. In contrast, the MAPK kinase inhibitor U0126 had no effect. The inhibition of Schwann cell proliferation by LY294002 was concentration-dependent as indicated by the progressive decrease in the labeling index with increasing concentrations of this inhibitor (Fig.1B). At the concentration of 20 μm, the proliferation induced by GGF was completely abolished, and the BrdU index was comparable with that of cultures maintained in serum-containing media (Fig.1B, Ctrl). The BrdU index of cells treated with U0126 at concentrations up to 10 μm was not affected.
Fig. 1.
Activation of PI 3-kinase by GGF is required for the induction of Schwann cell proliferation. A, B,Schwann cells, maintained for 24 hr in serum-containing media supplemented with 4 μm forskolin, were incubated an additional 24 hr with 5 ng/ml GGF, without (GGF) or with either the PI 3-kinase inhibitor LY294002 at 2.5–20 μm (LY2.5–LY20) or the MAPK kinase inhibitor U0126 at 1–10 μm (U1–U10). Controls were cultures maintained in serum-containing media without (Ctrl) or with forskolin (Fk). LY294002, in contrast to U0126, inhibits the GGF-induced incorporation of BrdU into Schwann cell nuclei (A) in a concentration-dependent manner (B; mean ± SEM from 3 separate experiments). Scale bar, 100 μm. C, PI 3-kinase and MAPK kinase activities were assessed on Western blots with polyclonal antibodies recognizing phosphorylated Akt (p-Akt) and MAP kinase p44/p42 (p-p44, p-p42), downstream effectors of PI 3-kinase and MAPK kinase, respectively. GGF strongly activates both PI 3-kinase and MAPK kinase, which are specifically inhibited by their respective inhibitor. The inhibition of PI 3-kinase activity correlates with the inhibition in BrdU incorporation.
GGF induced a robust activation of both PI 3-kinase and MAPK kinase in the Schwann cells, as evidenced by the increased phosphorylation of Akt and MAP kinase p44/p42, respectively, detected by Western blot analysis (Fig. 1C). No measurable activity of either kinase was present in quiescent Schwann cells maintained in D media, and only low levels of phospho-Akt were detected in the presence of forskolin. In the presence of LY294002, GGF-induced phospho-Akt levels progressively decreased in a dose-dependent response, whereas the levels of phospho-MAPK p44/p42 were not affected, indicating the specificity of this inhibitor. This finding also indicates that in Schwann cells, activation of MAP kinase by GGF is direct and does not result from the protein kinase activity associated with PI 3-kinase itself (Bondeva et al., 1998). Conversely, U0126 resulted in a concentration-dependent decrease in the levels of phospho-MAPK with no effect on phospho-Akt levels. Another MAPK kinase inhibitor, PD98059, which also had no effect on Schwann cell proliferation, also inhibited phosphorylation of MAP kinase p44/p42 although not as completely as U0126 (data not shown).
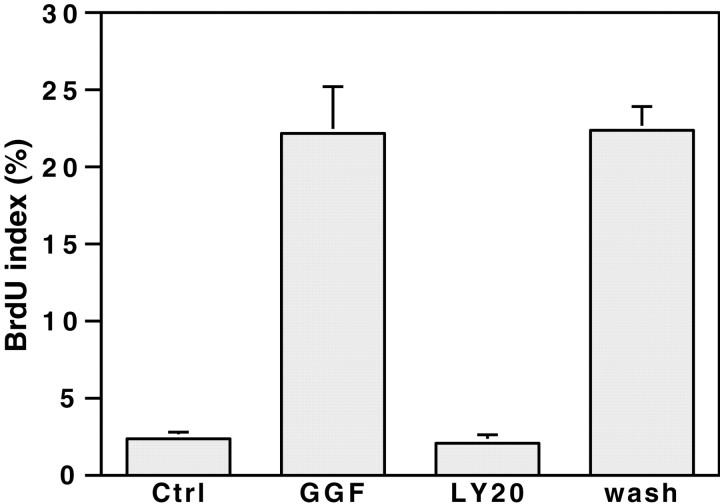
Because LY294002 is a reversible inhibitor of PI 3-kinase, we next examined whether the inhibition of Schwann cell proliferation is reversible. Schwann cells, cultured for 24 hr in the presence of LY294002 and GGF, were briefly rinsed to remove the inhibitor and then maintained on GGF-supplemented media for an additional 24 hr. Removal of the inhibitor completely reversed the inhibition of Schwann cell proliferation, resulting in a BrdU index comparable with that of Schwann cells that had not been treated with the PI 3-kinase inhibitor (Fig. 2). These results indicate that the inhibition of Schwann cell proliferation by LY294002 was not caused by a nonspecific, cytotoxic effect.
Fig. 2.
The inhibition of BrdU incorporation by PI 3-kinase is reversible. Schwann cells, maintained for 24 hr in serum-containing media supplemented with 4 μm forskolin, were then incubated 24 hr with 5 ng/ml GGF, without (GGF) or with the PI 3-kinase inhibitor LY294002 at 20 μm (LY20). A set of cultures was kept in serum-containing media without forskolin (Ctrl). LY294002 was removed by washing the treated cultures five times with DMEM, and cultures were then maintained an additional 24 hr in GGF- and forskolin-supplemented serum-containing media (wash). All the other cultures were identically washed but subsequently maintained in the media they were in before washing. Cells incorporated BrdU comparably with cultures never treated with the PI 3-kinase inhibitor (mean ± SEM from 3 separate experiments).
Schwann cells proliferate in response to a number of other soluble growth factors. We examined whether FGF-2 (basic FGF) that, in combination with forskolin is a Schwann cell mitogen (Davis and Stroobant, 1990), also mediates its effects via PI 3-kinase activation. FGF-2 was an equally potent activator of both PI 3-kinase and MAPK kinase (data not shown). Inhibition of the PI 3-kinase activation by LY294002 resulted in a dose-dependent inhibition of BrdU incorporation similar to that observed with GGF (data not shown). Both of the MAPK kinase inhibitors U0126 and PD98059 specifically inhibited MAPK kinase activity but had no effect on Schwann cell proliferation. These results support a general role of PI 3-kinase activation in Schwann cell proliferation.
Axons promote Schwann cell proliferation via PI 3-kinase
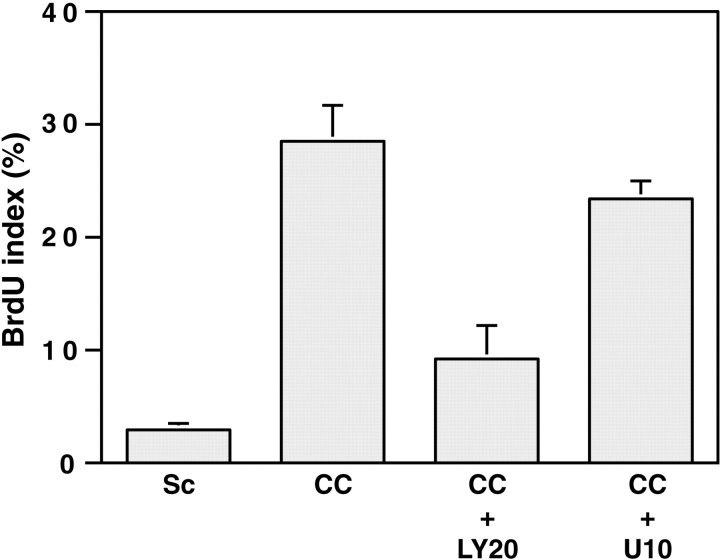
We next determined whether the mitogenic activity of neurites also depends on PI 3-kinase activity. Schwann cells maintained in serum-containing media proliferate slowly (Fig.3). When cocultured with DRG neurons, the labeling index increased by almost 10-fold, consistent with the known mitogenic activity of DRG neurons. The addition of LY294002 at 20 μm to the culture media resulted in a striking decrease (∼67%) in the incorporation of BrdU by the Schwann cells, whereas the MAPK kinase inhibitor (10 μm) had a negligible effect on proliferation that was not statistically significant.
Fig. 3.
Neurons promote Schwann cell proliferation via PI 3-kinase. Ten thousand Schwann cells were seeded onto purified DRG neuron cultures in serum-containing media. Twenty-four hours later, cultures were fed fresh media without (CC) or with LY294002 at 20 μm (CC +LY20) or U0126 at 10 μm (CC+ U10) for an additional 24 hr. Controls were Schwann cells maintained in serum-containing media for 48 hr (Sc). The cultures were then processed for BrdU immunostaining. Values represent the mean BrdU index ± SEM from three separate experiments.
These results suggest that the axonal induction of Schwann cell proliferation was dependent on PI 3-kinase activation. Consistent with this possibility, PI 3-kinase activity was detected in the coculture system (data not shown), although it was not clear whether this activity and its inhibition by LY294002 were primarily associated with Schwann cells, DRG neurons, or both. We therefore added neurite membranes to cultures of Schwann cells that, from previous studies (Salzer et al., 1980a), were known to increase proliferation substantially. We first determined the effect of neurite membranes on Schwann cell signaling pathways. To synchronize membrane addition, we centrifuged the membranes onto Schwann cell monolayers. This resulted in a dramatic increase in the levels of phospho-Akt (Fig.4B); as expected, this activation was inhibited by the PI 3-kinase inhibitor but not by the MAPK kinase inhibitor. Neurite membranes also induced a significant increase in the phosphorylation of MAP kinase p44/p42 that was inhibited by the MAPK kinase, but not by the PI 3-kinase, inhibitor.
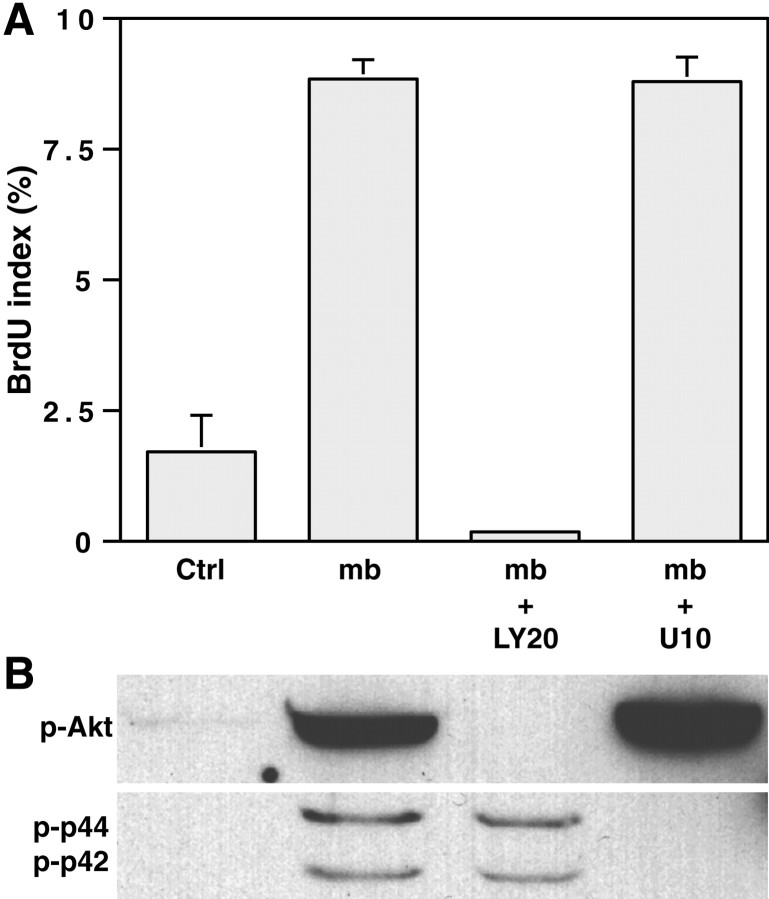
Fig. 4.
Neurite membranes promote Schwann cell proliferation via PI 3-kinase. A, Two aliquots of a neurite membrane preparation were centrifuged onto Schwann cell cultures at a 24 hr interval in serum-containing media without kinase inhibitors (mb) or supplemented with LY294002 at 20 μm (mb + LY20) or U0126 at 10 μm (mb + U10). BrdU incorporation was assessed after an additional 24 hr in culture. Controls were Schwann cells maintained in serum-containing media (Ctrl). B, Schwann cells, in culture for 24 hr in serum-containing media, were preincubated for 30 min with either inhibitor before centrifuging the neurite membrane fraction onto the cells. Lysates were made after 30 min of contact with the membranes. Contact with the neurite membranes strongly activates PI 3-kinase, and MAPK kinase to a lesser extent, in the Schwann cells. The inhibition of PI 3-kinase, but not that of MAPK kinase (B), correlates with the inhibition of the neurite membrane-induced incorporation of BrdU in Schwann cell nuclei (A; mean ± SEM from 2 separate experiments).p-Akt, Phosphorylated Akt; p-p44, p-p42, phosphorylated MAP kinase p44/p42.
We next assayed the effect of neurite membranes on Schwann cell proliferation. We found a fivefold increase in the BrdU-labeling index of Schwann cells treated with membranes; this increase was dependent on the activity of PI 3-kinase but not that of MAPK kinase (Fig.4A). Thus, LY294002 completely abolished the increase in BrdU incorporation, whereas the MAPK kinase inhibitor U0126 had no effect. Taken together, these results indicate that contact-dependent axonal mitogens, including the neuregulins, activate both PI 3-kinase and MAP kinase pathways and that Schwann cell proliferation specifically requires PI 3-kinase activation.
Schwann cell survival promoted by soluble GGF requires PI 3-kinase
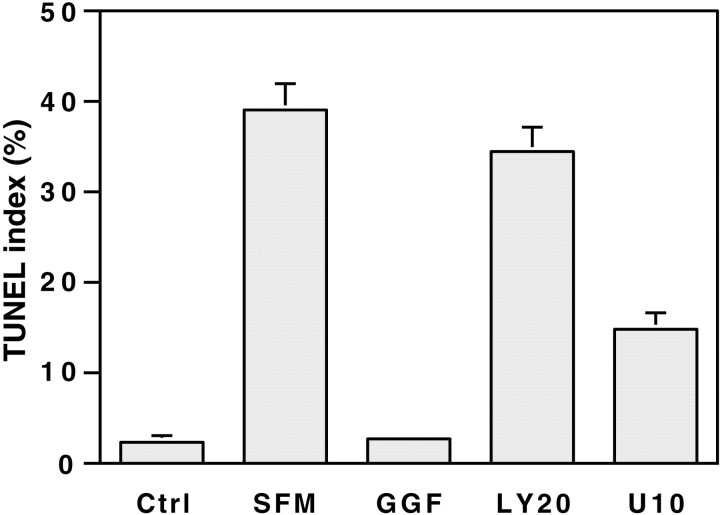
Postnatal Schwann cells cultured in a serum-free media undergo programmed cell death that can be blocked by soluble neuregulins (Syroid et al., 1996). To determine whether neuregulins promote Schwann cell survival via the PI 3-kinase pathway, we examined the effects of the pharmacological inhibitors. When cells were maintained in media containing serum, few cells underwent apoptosis as assessed by either TUNEL assay (Fig. 5) or visualization of nuclei by Hoechst staining. There was a striking increase in the amount of programmed cell death when cells were placed in unsupplemented, serum-free media for a period of 3 d; this increase was prevented by the addition of GGF (50 ng/ml). The GGF-promoted survival of Schwann cells was completely inhibited by the PI 3-kinase inhibitor, whereas addition of the MAPK kinase inhibitor in the culture media had a partial effect, reducing the GGF-mediated survival by 57%. Inhibition of both pathways at the same time by LY294002 and U0126 did not have a cumulative effect because the TUNEL index was similar to that observed with the PI 3-kinase inhibitor alone (data not shown). Interestingly, serum appears to support Schwann cell survival by other pathways. Thus there was no increase in the number of TUNEL cells when Schwann cells maintained in serum-containing media were treated with both kinase inhibitors (data not shown).
Fig. 5.
Schwann cell survival promoted by GGF requires PI 3-kinase. Schwann cells were cultured for 3 d in either serum-containing media (Ctrl) or serum-free media (SFM). In some cultures, the serum-free media were supplemented with 4 μm forskolin and 50 ng/ml GGF, without (GGF) or with 20 μmLY294002 (LY20) or 10 μm U0126 (U10). Apoptotic cells were detected by the TUNEL assay. Values represent the mean BrdU index ± SEM from three separate experiments.
Axons promote Schwann cell survival via contact-dependent signals that require PI 3-kinase
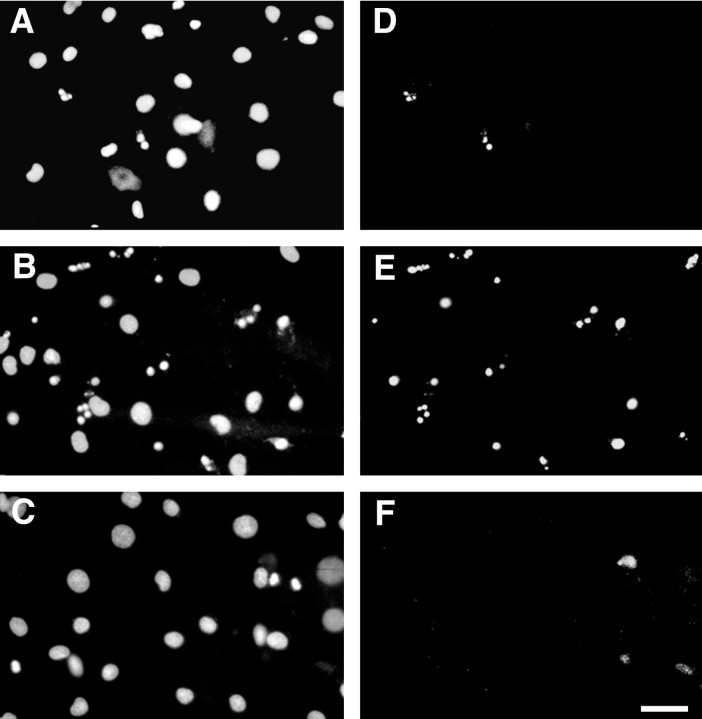
Axon-dependent signals have been shown to promote the survival of Schwann cell precursors and early postnatal Schwann cells (Dong et al., 1995; Trachtenberg and Thompson, 1996). We therefore examined whether the PI 3-kinase and/or the MAP kinase pathways are involved in the survival of Schwann cells promoted by neurons. Schwann cell-neuron cocultures maintained in serum-free media exhibited very few apoptotic cells (Fig. 6A,D). However, when treated with LY294002 at 20 μm, a large number of Schwann cells underwent apoptosis as indicated by pyknotic nuclear fragments that were TUNEL positive (Fig.6B,E). No increase in nuclear fragmentation or in the number of TUNEL Schwann cell nuclei was observed in cocultures treated with the MAPK kinase inhibitor (Fig. 6C,F). These findings suggest that PI 3-kinase is required for neuron-promoted Schwann cell survival.
Fig. 6.
Neurons promote Schwann cell survival via PI 3-kinase. Schwann cells cocultured for 3 d with neurons in serum-free media without kinase inhibitors (A, D), with 20 μm LY294002 (B, E), or with 10 μm U0126 (C, F) were analyzed for apoptosis by Hoechst staining (A–C) and TUNEL assay (D–F). Schwann cells in contact with neurons exhibit very few apoptotic cells (A, D). When treated with the PI 3-kinase inhibitor, numerous Schwann cells undergo apoptosis, as indicated by pyknotic nuclear fragments that are labeled in the TUNEL assay (B, E). No nuclear fragmentation and TUNEL cells were observed in cocultures treated with the MAPK kinase inhibitor (C, F). Scale bar, 50 μm.
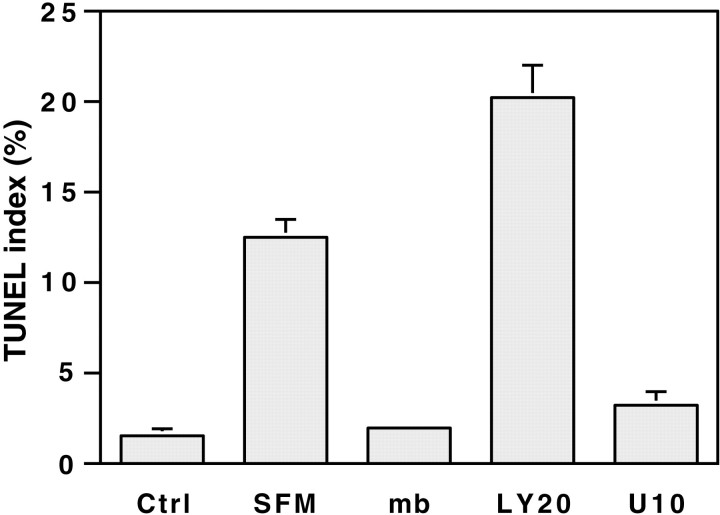
These studies do not distinguish whether Schwann cell survival was mediated by cell contact or the release of soluble factors by neurons. They also do not distinguish whether the primary effect of the inhibitor is on neurons, Schwann cells, or both. Although we did not notice an increase in the number of TUNEL neurons in cocultures treated with the PI 3-kinase inhibitor (data not shown), it remained possible that a decreased viability of the neurons indirectly affected the survival of the Schwann cells. To address these issues, we determined whether neurite membranes could prevent Schwann cells from apoptosis after serum withdrawal. The results are shown in Figure7. The eightfold increase in the TUNEL index observed after serum withdrawal over a period of 2 d was completely abolished by the addition of neurite membranes. The PI 3-kinase inhibitor LY294002 (20 μm), but not the MAPK kinase inhibitor U0126 (10 μm), totally inhibited the survival promoted by the addition of neurite membranes. These results strongly suggest that neurons promote Schwann cell survival via contact-dependent signals that activate the PI 3-kinase pathway.
Fig. 7.
Axons promote Schwann cell survival via contact-dependent signals that require PI 3-kinase. Schwann cells were cultured for 2 d in either serum-containing media (Ctrl) or serum-free media (SFM). In some cultures, the serum-free media were supplemented by centrifuging two aliquots of neurite membranes at 24 hr intervals, without (mb) or with 20 μm LY294002 (LY20) or 10 μmU0126 (U10). Apoptotic cells were detected by the TUNEL assay. Values represent the mean BrdU index ± SEM from two separate experiments.
PI 3-kinase is required for initial events of myelination
In addition to regulating Schwann cell proliferation and survival, axons promote their differentiation. To determine whether PI 3-kinase and/or MAPK kinase were involved in the axonal induction of Schwann cell differentiation, we analyzed the effects of PI 3-kinase and MAPK kinase inhibitors on myelination in DRG neuron-Schwann cell cocultures. Specifically, cocultures were treated with LY294002 (20 μm) or U0126 (10 μm) beginning on day 0 or day 4 after addition of ascorbate and maintained for up to 15 d. Myelination was then assessed by immunostaining for MBP; results are shown in Figure 8. Unlike control cultures, which myelinated extensively (Fig.8A), inhibition of PI 3-kinase beginning at day 0 completely blocked myelination in treated cultures (Fig.8B). In contrast, myelin sheaths were present in cultures when PI 3-kinase was inhibited beginning 4 d after the addition of ascorbic acid (Fig. 8C). Inhibition of MAPK kinase beginning on either day had no effect on the progress of myelination (data not shown). In separate studies, we confirmed the presence of both PI 3-kinase and MAPK kinase activities in the coculture system (Zanazzi, Teng, Kraemer, and Salzer, personal communication) and their specific inhibition by LY294002 and U0126, respectively (data not shown).
Fig. 8.
Inhibition of PI 3-kinase perturbs myelination. Myelinating Schwann cell–DRG neuron cocultures were set up as described in Materials and Methods. A–C, Immunostaining of 11-d-old cocultures for MBP, not treated with the PI 3-kinase inhibitor (A) or incubated with 20 μm LY294002 from day 0 (B; onset of myelination) or from day 4 (C; 4 d after the onset of myelination). The inhibition of PI 3-kinase from day 0 to day 11 completely inhibits myelination (B). In contrast, inhibition of PI 3-kinase from day 4 to day 11 does not prevent the appearance of myelin sheaths. However, these sheaths seem shorter in length than those in untreated cultures of the same age.D–F, Corresponding fields counterstained with Hoechst. Scale bar, 50 μm.
Because Schwann cell proliferation requires PI 3-kinase, the absence of myelination might have resulted from insufficient numbers of Schwann cells to segregate bundles of axons efficiently and promote myelination. To address this possibility, we established cocultures with an excess of Schwann cells (2 × 106 instead of 2 × 105 cells). In the presence of LY294002, the cocultures still failed to myelinate, whereas untreated companion cultures myelinated normally (data not shown). These results suggest that PI 3-kinase inhibition affects axon-Schwann cell interactions required for myelination that are distinct from proliferation. We also did not detect an increase in Schwann cell apoptosis in the LY294002-treated cocultures (data not shown), presumably because of serum factors that are present in myelinating media.
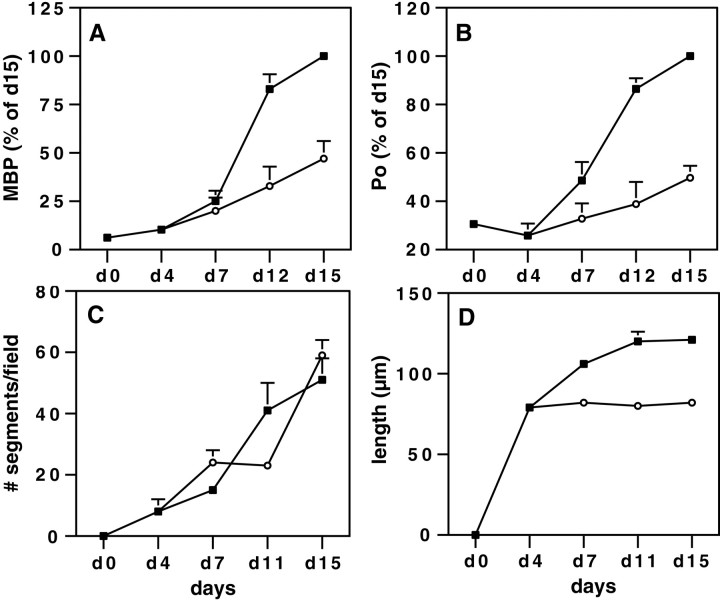
Although MBP-positive segments were present in cocultures treated with the PI 3-kinase inhibitor beginning at d4, the segments that did form appeared to be of shorter length compared with those of untreated cultures of the same age (Fig. 8, compare A, C). We therefore quantitated MBP and P0 protein levels and counted the number and length of MBP-positive segments at various times during the course of myelination (Fig. 9). In control cultures, the amount of MBP and P0 dramatically increased during the first 15 d of culture in myelinating media (Fig.9A,B). When the cultures were treated with LY294002 from day 0 to day 15, the accumulation of both MBP and P0 was completely inhibited with no increase over that of day 0 cultures (data not shown). In contrast, MBP and P0 continued to accumulate in cocultures treated with LY294002 from day 4, although the amount was reduced 50% compared with that of controls at day 15. In these cocultures, the number of MBP-positive segments was similar to that of untreated cultures of the same age (Fig. 9C). However their length was much shorter (Fig. 9D). In control cocultures, myelinated segments averaged 79 μm in length at day 4, 106 μm at day 7, and 120 μm at day 11 and did not increase significantly at later times. When the 4-d-old cocultures were maintained in media supplemented with the PI 3-kinase inhibitor, the myelinated segments that formed remained at 80 μm at 7 d and beyond.
Fig. 9.
LY294002-treated cocultures have shorter myelin sheaths. Myelinating cocultures were maintained for up to 15 d, without LY294002 (▪) or with 20 μm LY294002 added from day 4 (○). Cultures were analyzed at different days (d0 to d15) for the presence of the myelin proteins MBP (A) and P0 (B) (expressed as the percent of the total amount present in 15-d-old controls) and for the number (C) and length (D) of the myelin sheaths. Values represent the mean ± SEM of five (A, B), three (C; 54 high-power fields), or two (D; >1000 segments measured for each time point) separate experiments.
Finally we examined the effects of LY294002 on well established, myelinated cultures. Interestingly, chronic inhibition of PI 3-kinase in 5-week-old myelinating cultures had no apparent adverse effects, such as demyelination, suggesting that PI 3-kinase activity is not required for the long-term maintenance of mature myelin sheaths (data not shown).
Effect of PI 3-kinase inhibition on laminin deposition
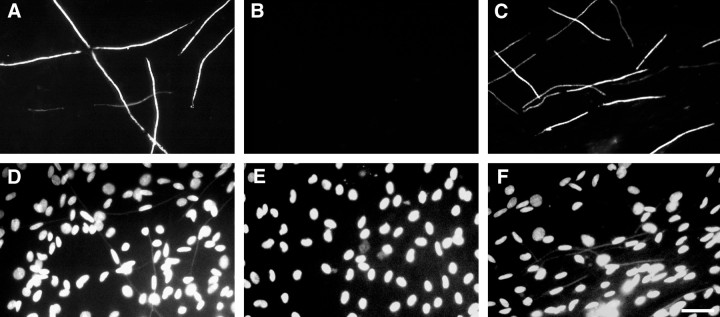
The addition of ascorbate to the culture media initiates the assembly of a basal lamina by the Schwann cell, which is required for these cells to ensheathe or myelinate axons (Eldridge et al., 1987). Failure of myelination in the LY294002-treated cocultures could therefore reflect either a lack of basal lamina deposition or an inhibition of the signaling from the basal lamina to the Schwann cells. We have started to address this possibility by immunostaining cocultures treated with LY294002 for laminin, a major component of the basal lamina (Cornbrooks et al., 1983). In control cultures, robust laminin expression was associated with the outer surface of Schwann cells oriented along axons in an apparent one-to-one relationship (Fig.10A,D), with a centrally located oval nucleus. Treatment of cocultures with LY294002 from day 0 had a striking effect on the organization of the cocultures (Fig. 10B,E). In particular, elongated Schwann cells in an apparent unitary association with axons were not observed. Schwann cells were, however, brightly stained for laminin, demonstrating a fibrillar pattern of expression over their surface that suggests that the extracellular matrix is deposited normally. In agreement, preliminary analysis by Western blotting indicates continued laminin expression in the presence of LY294002 (data not shown). In contrast, the laminin staining of cocultures treated with LY294002 from day 4 was indistinguishable from that of controls (Fig.10C,F). The MAPK kinase inhibitor had no apparent effect on the myelinating cultures, which were comparable with controls at all time points (data not shown).
Fig. 10.
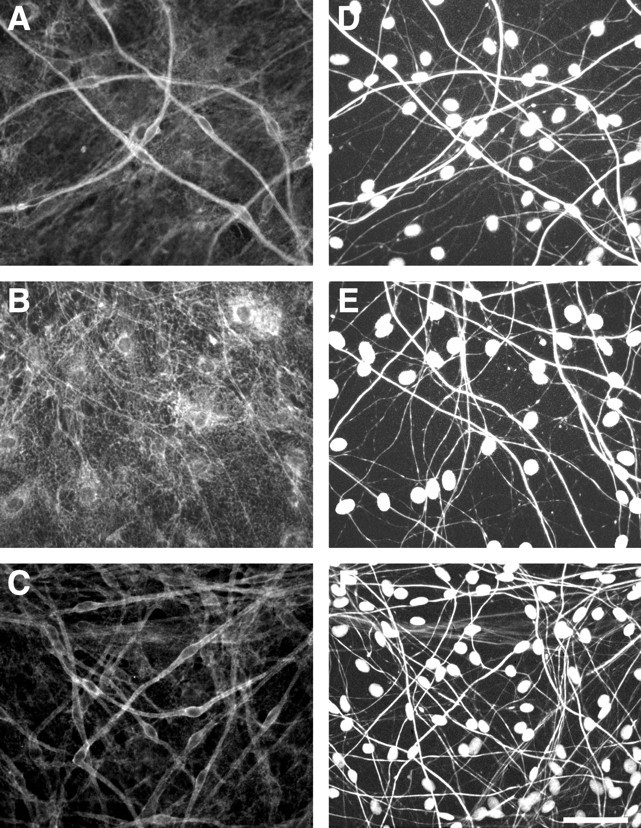
PI 3-kinase does not affect laminin expression. Myelinating cocultures were maintained for up to 11 d, without LY294002 (A, D) or with 20 μm LY294002 added from day 0 (B, E) or from day 4 (C, F). Cultures were immunostained for laminin (A–C) and neurofilament (D–F). Schwann cell nuclei are counterstained with Hoechst. Scale bar, 50 μm.
DISCUSSION
The intracellular signaling pathways activated by axonal contact that regulate Schwann cell development and differentiation have been elusive. In this study, we have shown that both the PI 3-kinase and MAP kinase pathways are potently activated by GGF and by axonal contact. Using specific pharmacological inhibitors, we have also demonstrated that PI 3-kinase but not MAPK kinase activation is crucial for the generation and initial differentiation of Schwann cells. These results are considered further below.
PI 3-kinase is a key signaling pathway in the generation of Schwann cells
A major finding of this study is that activation of PI 3-kinase by axons and neuregulins is required for Schwann cell proliferation and survival. We demonstrated that PI 3-kinase is robustly activated in Schwann cells after treatment with GGF and neurite membranes, consistent with the presence of multiple binding sites for the p85 adaptor of PI 3-kinase on erbB3. To investigate the role of this activation, we used LY294002 as a specific inhibitor of PI 3-kinase (Vlahos et al., 1994). Although we cannot exclude the possibility of an effect on other signaling pathways, the absence of a discernable effect on MAP kinase activation and the dose-dependent inhibition of Akt phosphorylation at micromolar concentrations (Fig. 1) underscore the known specificity of this inhibitor. Inhibition of PI 3-kinase activation with LY294002 completely blocked Schwann cell proliferation induced by either GGF or neurite membranes, whereas inhibition of MAPK kinase had no effect. Similarly, inhibition of PI 3-kinase activity in the cocultures also substantially inhibited Schwann cell proliferation although significant residual proliferation persisted. Because inhibition of the MAP kinase pathway had no significant effect on Schwann cell proliferation in the cocultures, other signaling pathways, distinct from PI 3-kinase and MAPK kinase, may account for this persistent proliferation and contribute to normal Schwann cell proliferation.
PI 3-kinase activity is also required for the trophic effects of GGF and neurite membranes on Schwann cells. Thus, inhibition of PI 3-kinase reversed the effects of GGF, DRG neurons, and neurite membranes on Schwann cell survival (Figs. 5-7). These results are consistent with the key role the PI 3-kinase pathway plays in the survival of many cells, including the trophic effects of other growth factors on Schwann cells (Campana et al., 1999; Delaney et al., 1999; Weiner and Chun, 1999), and with a recent report using the LY294002 inhibitor to demonstrate that PI 3-kinase is required for the neuregulin-promoted survival of murine Schwann cells (Dong et al., 1999).
A number of downstream effectors of PI 3-kinase have been identified that are candidates to promote Schwann cell proliferation and survival. Of particular interest is the proto-oncogene Akt, which is activated by PI 3-kinase-dependent phosphorylation and by its phosphorylated lipid products. Akt prevents apoptosis in many cells (Franke et al., 1997); indeed, a constitutively active form of Akt was shown recently to rescue Schwann cells from apoptosis induced by serum factor withdrawal (Weiner and Chun, 1999). We have monitored PI 3-kinase activity in these studies by following the levels of phospho-Akt, demonstrating that its phosphorylation is dramatically upregulated by GGF and axonal contact (Figs. 1, 4). Together these results support a role of Akt in the contact-dependent regulation of Schwann cell proliferation and/or apoptosis by neurons that is mediated by the neuregulins. Akt is a serine/threonine kinase with at least five known targets that have been implicated in the control of the apoptotic machinery including BAD (Datta et al., 1997), caspase-9 (Cardone et al., 1998), glycogen synthase kinase-3β (GSK-3β) (Pap and Cooper, 1998), CRE-binding protein (CREB) (Du and Montminy, 1998), and FKHRL1, a member of the Forkhead family of transcription factors (Brunet et al., 1999). CREB is directly phosphorylated by Akt on Ser-133 (Du and Montminy, 1998). Of note, neuregulin also induces CREB phosphorylation at Ser-133 in Schwann cells (Tabernero et al., 1998), potentially via activation of CREB kinase (Rahmatullah et al., 1998), although MAP kinase could also be involved in its phosphorylation (Bonni et al., 1999). Another potential downstream effector of PI 3-kinase is protein kinase C (PKC) (Nakanishi et al., 1993; Chou et al., 1998) that has been implicated in Schwann cell proliferation induced by neurite membranes (Saunders and DeVries, 1988) and GGF (Yoshimura et al., 1993); whether this effect of PKC is PI 3-kinase dependent is not yet known.
MAP kinase has a dispensable role in Schwann cell proliferation and survival
In addition to PI 3-kinase, MAP kinase is also robustly activated by neurite membranes and, as reported previously (Kim et al., 1997), by GGF. Despite its central role in the mitogenic and trophic effects of many growth factors, inhibition of MAPK kinase with two different pharmacological inhibitors had surprisingly little effect on Schwann cell proliferation, survival, or myelination. Although we did not observe a role of the MAP kinase pathway in Schwann cell proliferation, it may partially mediate the survival effects of GGF (see Fig. 5). Thus inhibition of the MAPK kinase reduced the survival promoted by GGF, although not as effectively as blocking the activity of PI 3-kinase. There may have also been a small effect of MAP kinase on the survival promoted by neurite membranes (see Fig. 7), although the results were not statistically significant. Other investigators have found that the MAP kinase pathway mediates Schwann cell survival promoted by certain growth factors (Campana et al., 1999), in particular autocrine survival factors that promote the survival of Schwann cells late in the postnatal period after they lose their perinatal dependence on axonal signals (Meier et al., 1999). These results suggest that Schwann cells may undergo a transition from a dependence on axonal signals mediated by the PI 3-kinase pathway to a dependence on autocrine growth factors mediated by MAP kinase activation. Additional studies will be needed to examine this possibility and the potential role of MAP kinase activation in other aspects of Schwann cell biology not investigated here, including expression of specific cytokines (Nagamoto-Combs et al., 1999).
Role of the PI 3-kinase pathway in Schwann cell differentiation
These studies also indicate that the formation of the myelin sheath is dependent on PI 3-kinase activity. Myelination proceeds in a series of distinctive stages (Webster, 1992). These include the segregation of a large caliber axon into a one-to-one relationship with the Schwann cell, growth of the inner Schwann cell membrane around the axon, compaction of the loosely spiraled lamellae by extrusion of the Schwann cell cytoplasm, and subsequent maturation of the myelin sheath by further radial and longitudinal expansion. Results reported here suggest that PI 3-kinase has important roles at multiple stages of myelination. Of particular note, inhibition of PI 3-kinase at the earliest stages of coculture completely blocked myelin formation (Fig.8), and Schwann cells failed to orient along axons in an apparent one-to-one relationship (Fig. 10). These results suggest that the initial events of myelination (i.e., axon segregation and Schwann cell elongation) require PI 3-kinase. As Schwann cells ensheathe and segregate axons, they undergo extensive morphological reorganization that requires rearrangements of the actin cytoskeleton (Fernandez-Valle et al., 1997). Inhibition of PI 3-kinase might block these initial morphological events by blocking the Rac- and Rho-dependent effector pathways involved in cytoskeletal reorganization (Reif et al., 1996;Han et al., 1998). In the future, electron microscopy will be required to determine precisely at which of these distinctive stages of myelination PI 3-kinase is required.
We considered, as an alternative possibility, that PI 3-kinase might be necessary for the synthesis of the basal lamina. Basal lamina formation, which is dependent on axonal signals, is essential for the initial events of Schwann cell ensheathment and myelination (Bunge et al., 1986; Fernandez-Valle et al., 1993). Inhibition of PI 3-kinase, potentially, might have blocked the axonal signal required for the expression of, or the secretion of, basal lamina components; the latter possibility is consistent with known roles of PI 3-kinase in vesicle trafficking and exocytosis (Rameh and Cantley, 1999). Immunostaining for laminin in treated cocultures indicates that at least this key component of the basal lamina is appropriately expressed, suggesting that the block in myelination is not secondary to failure of basal lamina formation. These results also suggest that other signaling pathways mediate the axonal induction of basal lamina synthesis. It is important to note that Schwann cell interactions with the basal lamina are mediated, in part, via the β1 integrins (Fernandez-Valle et al., 1994), which are known to activate several intracellular signaling pathways including PI 3-kinase (Kumar, 1998; Giancotti and Ruoslahti, 1999). Our results therefore do not distinguish whether the inhibitor interferes with normal myelination by blocking PI 3-kinase signaling activated by the axon, by the basal lamina, or by both. Additional studies will be necessary to determine how PI 3-kinase is activated in these early myelinating cocultures and the precise role of PI 3-kinase in initiating myelination.
At later stages of differentiation, PI 3-kinase seems to be required for the maturation but not the maintenance of myelin sheaths. Thus, cocultures in which PI 3-kinase was inhibited beginning on day 4, a time when Schwann cells have assembled a basal lamina and begun to segregate axons (Fernandez-Valle et al., 1998), were able to form normal numbers of myelin sheaths, but the internodes did not elongate appropriately over time. The accumulation of the myelin proteins MBP and PO was also markedly reduced in such cultures, consistent with the shorter length of the myelinated sheaths and raising the possibility that PI 3-kinase-regulated transcription factors (i.e., CREB and FKHRL1) might be involved in myelin gene expression. In contrast, in mature cultures, myelinating Schwann cells no longer seem to require PI 3-kinase activity. In particular, no adverse effects, such as demyelination, were observed in chronically inhibited mature cocultures, consistent with a transition to a PI 3-kinase-independent state.
In summary, our results strongly implicate PI 3-kinase in the contact-dependent regulation of Schwann cell development by axon signals, including the neuregulins. In the future, it will be of interest to determine whether PI 3-kinase activity is also required for the proliferation of Schwann cells during Wallerian degeneration, which may be mediated in part via erbB receptor activation (Kwon et al., 1997), and for the neuregulin regulation of oligodendrocyte progenitor proliferation and survival, which is similarly associated with robust activation of PI 3-kinase (Canoll et al., 1999). Finally, these studies suggest that other signaling pathways promote later events of myelination and myelin maintenance that have yet to be identified.
Footnotes
This work was supported by the National Institutes of Health Grant NS 26001 (J.L.S.) and the National Multiple Sclerosis Society Grant FA 1302-A-1 (P.M.). We thank G. Zanazzi and S. Einheber for helpful discussions and critical reviews of this manuscript and M. Marchionni and Cambridge Neuroscience for providing rhGGF2.
Correspondence should be addressed to Dr. Patrice Maurel, Department of Cell Biology, New York University Medical Center, 550 First Avenue, New York, NY 10016. E-mail: maurep01@endeavor.med.nyu.edu.
REFERENCES
- 1.Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–111. doi: 10.1002/(sici)1098-1136(20000115)29:2<104::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 3.Bondeva T, Pirola L, Bulgarelli-Leva G, Rubio I, Wetzker R, Wymann MP. Bifurcation of lipid and protein kinase signals of PI3Kγ to the protein kinases PKB and MAPK. Science. 1998;282:293–296. doi: 10.1126/science.282.5387.293. [DOI] [PubMed] [Google Scholar]
- 4.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 5.Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- 6.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 7.Bunge RP, Bunge MB, Eldridge CF. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Annu Rev Neurosci. 1986;9:305–328. doi: 10.1146/annurev.ne.09.030186.001513. [DOI] [PubMed] [Google Scholar]
- 8.Burden S, Yarden Y. Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron. 1997;18:847–855. doi: 10.1016/s0896-6273(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 9.Campana WM, Darin SJ, O'Brien JS. Phosphatidylinositol 3-kinase and Akt protein kinase mediate IGF-1- and prosaptide-induced survival in Schwann cells. J Neurosci Res. 1999;57:332–341. [PubMed] [Google Scholar]
- 10.Canoll PD, Kraemer R, Teng KK, Marchionni MA, Salzer JL. GGF/neuregulin induces a phenotypic reversion of oligodendrocytes. Mol Cell Neurosci. 1999;13:79–94. doi: 10.1006/mcne.1998.0733. [DOI] [PubMed] [Google Scholar]
- 11.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 12.Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen CS, Newton AC, Schaffhausen BS, Toker A. Regulation of protein kinase Cζ by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 13.Cornbrooks CJ, Carey DJ, McDonald JA, Timpl R, Bunge RP. In vivo and in vitro observations on laminin production by Schwann cells. Proc Natl Acad Sci USA. 1983;80:3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 15.Davis JB, Stroobant P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. J Cell Biol. 1990;110:1353–1360. doi: 10.1083/jcb.110.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delaney CL, Cheng HL, Feldman EL. Insulin-like growth factor-1 prevents caspase-mediated apoptosis in Schwann cells. J Neurobiol. 1999;41:540–548. doi: 10.1002/(sici)1097-4695(199912)41:4<540::aid-neu9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen KR. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 18.Dong Z, Sinanan A, Parkinson D, Parmantier E, Mirsky R, Jessen KR. Schwann cell development in embryonic mouse nerves. J Neurosci Res. 1999;56:334–348. doi: 10.1002/(SICI)1097-4547(19990515)56:4<334::AID-JNR2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 20.Einheber S, Milner TA, Giancotti F, Salzer JL. Axonal regulation of Schwann cell integrin expression suggests a role for α6β4 in myelination. J Cell Biol. 1993;123:1223–1236. doi: 10.1083/jcb.123.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, Salzer JL. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eldridge CF, Bunge MB, Bunge RP, Wood PM. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol. 1987;105:1023–1034. doi: 10.1083/jcb.105.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Valle C, Fregien N, Wood PM, Bunge MB. Expression of the protein zero myelin gene in axon-related Schwann cells is linked to basal lamina formation. Development. 1993;119:867–880. doi: 10.1242/dev.119.3.867. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Valle C, Gwynn L, Wood PM, Carbonetto S, Bunge MB. Anti-β1 integrin antibody inhibits Schwann cell myelination. J Neurobiol. 1994;25:1207–1226. doi: 10.1002/neu.480251004. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Valle C, Gorman D, Gomez AM, Bunge MB. Actin plays a role in both changes in cell shape and gene-expression associated with Schwann cell myelination. J Neurosci. 1997;17:241–250. doi: 10.1523/JNEUROSCI.17-01-00241.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Valle C, Wood PM, Bunge MB. Localization of focal adhesion kinase in differentiating Schwann cell/neuron cultures. Microsc Res Tech. 1998;41:416–430. doi: 10.1002/(SICI)1097-0029(19980601)41:5<416::AID-JEMT8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 28.Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 29.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 30.Grinspan JB, Marchionni MA, Reeves M, Coulaloglou M, Scherer SS. Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: neuregulin receptors and the role of neuregulins. J Neurosci. 1996;16:6107–6118. doi: 10.1523/JNEUROSCI.16-19-06107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 32.Harari D, Tzahar E, Romano J, Shelly M, Pierce JH, Andrews GC, Yarden Y. Neuregulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene. 1999;18:2681–2689. doi: 10.1038/sj.onc.1202631. [DOI] [PubMed] [Google Scholar]
- 33.Jessen KR, Mirsky R. Origin and early development of Schwann cells. Microsc Res Tech. 1998;41:393–402. doi: 10.1002/(SICI)1097-0029(19980601)41:5<393::AID-JEMT6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 34.Kim HA, DeClue JE, Ratner N. cAMP-dependent protein kinase A is required for Schwann cell growth: interactions between the cAMP and neuregulin/tyrosine kinase pathways. J Neurosci Res. 1997;49:236–247. [PubMed] [Google Scholar]
- 35.Kumar CC. Signaling by integrin receptors. Oncogene. 1998;17:1365–1373. doi: 10.1038/sj.onc.1202172. [DOI] [PubMed] [Google Scholar]
- 36.Kwon YK, Bhattacharyya A, Alberta JA, Giannobile WV, Cheon K, Stiles CD, Pomeroy SL. Activation of ErbB2 during Wallerian degeneration of sciatic nerve. J Neurosci. 1997;17:8293–8299. doi: 10.1523/JNEUROSCI.17-21-08293.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemke G. Neuregulins in development. Mol Cell Neurosci. 1996;7:247–262. doi: 10.1006/mcne.1996.0019. [DOI] [PubMed] [Google Scholar]
- 38.Maurel P, Salzer JL. Schwann cell proliferation and survival promoted by axonal contact and GGF/neuregulin require PI 3-kinase activation. Soc Neurosci Abstr. 1999;25:482. [Google Scholar]
- 39.Meier C, Parmantier E, Brennan A, Mirsky R, Jessen KR. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J Neurosci. 1999;19:3847–3859. doi: 10.1523/JNEUROSCI.19-10-03847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 41.Morrissey TK, Levi AD, Nuijens A, Sliwkowski MX, Bunge RP. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc Natl Acad Sci USA. 1995;92:1431–1435. doi: 10.1073/pnas.92.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagamoto-Combs K, Vaccariello SA, Zigmond RE. The levels of leukemia inhibitory factor mRNA in a Schwann cell line are regulated by multiple second messenger pathways. J Neurochem. 1999;72:1871–1881. doi: 10.1046/j.1471-4159.1999.0721871.x. [DOI] [PubMed] [Google Scholar]
- 43.Nakanishi H, Brewer KA, Exton JH. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-triphosphate. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- 44.Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 45.Payne DM, Rossomando AJ, Martino P, Erickson AK, Her JH, Shabanowitz J, Hunt DF, Weber MJ, Sturgill TW. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahmatullah M, Schroering A, Rothblum K, Stahl RC, Urban B, Carey DJ. Synergistic regulation of Schwann cell proliferation by heregulin and forskolin. Mol Cell Biol. 1998;18:6245–6252. doi: 10.1128/mcb.18.11.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 48.Reif K, Nobes CD, Thomas G, Hall A, Cantrell DA. Phosphatidylinositol 3-kinase signals activate a selective subset of Rac/Rho-dependent effector pathways. Curr Biol. 1996;6:1445–1455. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- 49.Salzer JL, Williams AK, Glaser L, Bunge RP. Studies of Schwann cell proliferation. II. Characterization of the stimulation and specificity of the response to a neurite membrane fraction. J Cell Biol. 1980a;84:753–766. doi: 10.1083/jcb.84.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salzer JL, Bunge RP, Glaser L. Studies of Schwann cell proliferation. III. Evidence for the surface localization of the neurite mitogen. J Cell Biol. 1980b;84:767–778. doi: 10.1083/jcb.84.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saunders RD, DeVries GH. Schwann cell proliferation is accompanied by enhanced inositol phospholipid metabolism. J Neurochem. 1988;50:876–882. doi: 10.1111/j.1471-4159.1988.tb02994.x. [DOI] [PubMed] [Google Scholar]
- 52.Syroid DE, Maycox PR, Burrola PG, Liu N, Wen D, Lee KF, Lemke G, Kilpatrick TJ. Cell death in the Schwann cell lineage and its regulation by neuregulin. Proc Natl Acad Sci USA. 1996;93:9229–9234. doi: 10.1073/pnas.93.17.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tabernero A, Stewart HJS, Jessen KR, Mirsky R. The neuron-glia signal β-neuregulin induces sustained CREB phosphorylation on Ser-133 in cultured rat Schwann cells. Mol Cell Neurosci. 1998;10:309–322. [PubMed] [Google Scholar]
- 54.Trachtenberg JT, Thompson WJ. Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature. 1996;379:174–177. doi: 10.1038/379174a0. [DOI] [PubMed] [Google Scholar]
- 55.Vemuri GS, McMorris FA. Oligodendrocytes and their precursors require phosphatidylinositol 3-kinase signaling for survival. Development. 1996;122:2529–2537. doi: 10.1242/dev.122.8.2529. [DOI] [PubMed] [Google Scholar]
- 56.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 57.Webster HdeF. Development of peripheral nerve fibers. In: Dyck PJ, Thomas PK, Griffin J, Low PA, Poduslo JF, editors. Peripheral neuropathy. Saunders; Philadelphia: 1992. pp. 243–266. [Google Scholar]
- 58.Weiner JA, Chun J. Schwann cell survival mediated by the signaling phospholipid lysophosphatidic acid. Proc Natl Acad Sci USA. 1999;96:5233–5238. doi: 10.1073/pnas.96.9.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 60.Wood PM, Bunge RP. Evidence that sensory axons are mitogenic for Schwann cells. Nature. 1975;256:662–664. doi: 10.1038/256662a0. [DOI] [PubMed] [Google Scholar]
- 61.Yoshimura T, Goda S, Kobayashi T, Goto I. Involvement of protein kinase C in the proliferation of cultured Schwann cells. Brain Res. 1993;617:55–60. doi: 10.1016/0006-8993(93)90612-q. [DOI] [PubMed] [Google Scholar]