Abstract
In adult brain, nerve growth factor (NGF) gene expression is generally upregulated by neuronal activity. However, a single episode of hilus lesion (HL)-induced limbic seizures stimulates a biphasic increase in NGF mRNA expression with peaks at 4–6 and 24 hr after lesion and an intervening return to control levels at 10–12 hr after lesion. In vitro studies suggest that NGF transcription is regulated via an activating protein 1 (AP-1) binding site in the first intron of the NGF gene. To examine the relationship between seizure-induced AP-1 binding and NGF gene expression in this paradigm, NGF mRNA levels and AP-1 binding were examined after HL seizures. Furthermore, to gain insight into the functional composition of the AP-1 complex, supershift analysis was performed to characterize which Fos and Jun family members are included in the AP-1-binding complex at the different time points analyzed. Solution hybridization analysis verified the biphasic increase in NGF mRNA content of the dentate gyrus after HL seizures. After an initial increase, AP-1 binding slowly declined in a stepwise manner that encompassed, but did not correspond with, the two phases of NGF mRNA expression. However, supershift analyses demonstrated that the relative contributions of JunD and JunB to the AP-1 complex exhibited positive and negative correlations, respectively, with the phases of increased NGF expression after HL. These results suggest that AP-1 complexes containing JunD promote NGF transactivation and that transient changes in the relative contributions of JunD and JunB to AP-1 binding underlie the biphasic increase in NGF gene expression induced by HL seizures.
Keywords: neurotrophin, nerve growth factor, dentate gyrus, transcription factors, gene expression, JunB, JunD, AP-1
Intense neuronal activity during seizure induces changes in neuronal circuitry and synaptic function (for review, see Ben-Ari and Represa, 1990). These activity-dependent alterations are likely to represent extreme versions of more subtle changes in neuronal structure and function effected by physiological levels of activity. As a consequence, there has been a great deal of interest in the cellular responses to seizures, which may reflect more general mechanisms of functional neuroplasticity. In this light, particularly interesting neuronal responses to seizure are changes in the expression of the nerve growth factor (NGF)-related neurotrophins. The neurotrophins have been shown to induce axonal sprouting and neurotransmitter synthesis (Levi-Montalcini et al., 1954; Olson and Malmfors, 1970; Gnahn et al., 1983) and to potentiate synaptic transmission (Kang and Schuman, 1995; Prakash et al., 1996). Moreover, NGF antibodies retard kindling and block hippocampal sprouting (Holtzman and Lowenstein, 1995; Van der Zee et al., 1995). These data implicate NGF and increases in NGF expression in functional plasticity changes after seizures. However, despite the variety of stimuli that alter expression of NGF (for review, see Lindvall et al., 1994), little is known about the transcriptional mechanisms that mediate these effects.
Results of in vitro studies using NGF promoter-driven constructs suggest that an intronic activating protein 1 (AP-1) binding site directs NGF transcription (Hengerer et al., 1990; Cowie et al., 1994). This is supported by in vivo evidence of increased hippocampal AP-1 binding within an hour after seizure, just before increased NGF gene expression (Sonnenberg et al., 1989a; Pennypacker et al., 1993). However, the chronic increase in hippocampal AP-1 binding activity after kainic acid-induced seizures is not linked to chronically elevated NGF mRNA expression (Gall et al., 1991;Pennypacker et al., 1994), suggesting that the relationship of AP-1 binding to NGF transcription is not straightforward. AP-1 is a dimer most typically composed of both Fos and Jun family proteins, although in a variety of possible combinations (e.g., Fos/JunD, FosB/JunB, etc.). In adult neurons, increases in the expression of mRNAs for the various Fos and Jun proteins follow strikingly different time courses after manipulations including seizures, lesions, and behavioral training (Sonnenberg et al., 1989a; Hengerer et al., 1990; Nikolaev et al., 1992). Consequently, AP-1 composition generally changes with time after stimulation (Hope et al., 1994; Kaminska et al., 1994; Kashihara et al., 1997). In vitro experiments in non-neuronal cells have demonstrated that different AP-1 complexes bind AP-1 sites with different affinities (Ryseck and Bravo, 1991) and can either stimulate or inhibit the expression of a given gene (Schutte et al., 1989; Suzuki et al., 1991). Therefore, although the AP-1 complex was originally considered as a transcriptional activator, the various AP-1 complexes formed after seizure may differentially influence target gene expression.
Recurrent limbic seizures induced by electrolytic hilar lesion placement stimulates biphasic increases in the NGF mRNA content of the dentate gyrus granule cells; NGF mRNA levels are markedly increased by 4–6 hr, return to below control labels at 10 hr, and increase a second time by 24 hr after hilus lesion (HL) placement (Lauterborn et al., 1994). This biphasic profile suggests that seizures induce both positive and negative influences on NGF expression and that the HL paradigm might be particularly useful for analyses of regulatory mechanisms involved. To this end, the present studies used gel shift and supershift analyses to determine whether AP-1 binding levels and/or changes in AP-1 composition corresponds with phases of NGF expression after HL seizures. The results indicate that changes in the composition of complexes bound to the NGF AP-1 site may underlie both positive and negative influences on activity-induced NGF gene expression.
MATERIALS AND METHODS
Animal treatment and tissue collection. For seizure induction, adult male Sprague Dawley rats (Simonsen Labs, Gilmore, CA) were anesthetized (50 mg/kg ketamine and 10 mg/kg xylazine), and an electrolytic lesion was placed in the right dentate hilus (stereotaxic coordinates: 3.8 mm posterior and 2.4 mm lateral to bregma; 3.0 mm ventral to brain surface) using an insulated stainless steel wire and an anodal current (0.8 mA for 7 sec). Such lesions induce bilateral electrographic seizures within the hippocampus and behavioral seizures of the limbic kindling type beginning 2–3 hr after lesion placement and recurring intermittently for 8–10 hr thereafter (Pico and Gall, 1994). Light and electron microscopic studies have detected no secondary cell death outside the immediate field of electrode–lesion placement in HL rats (Pico and Gall, 1989; Bundman et al., 1994), increasing the likelihood that the effects of HL-induced seizures upon AP-1 binding and NGF mRNA expression in the present study are direct consequences of seizure activity. After surgery, rats were monitored for behavioral seizures, with only those exhibiting stage 4 or 5 limbic seizures [i.e., rearing with forelimb clonus, rearing with clonus and falling (Racine, 1972)] included in the study. At 4, 10, and 24 hr after HL experimental seizure, rats and paired controls were deeply anesthetized by isoflurane inhalation and decapitated. Their brains were quickly removed and placed on an ice-cooled stage. The dentate gyrus region was dissected free by first placing the hippocampus on the CA1 alvear surface and then cutting longitudinally along both the hippocampal fissure (visible at the tip of the dentate gyrus) and the ridge at the lateral edge of the external blade of the dentate gyrus. This separates the regio inferior and subicular regions from the central, dentate-enriched sample (henceforth referred to as the dentate gyrus sample), which included the dentate gyrus, some enclosed field CA3c, and the overlying field CA1b. The zone surrounding the visible region of the lesion was removed, and then the remaining dentate gyrus samples from right and left hemispheres were pooled and either frozen on dry ice for subsequent RNA isolation or immediately homogenized for nuclear protein extraction (see below). To verify the absence of seizure-independent effects of lesion placement surgery on NGF mRNA levels, as demonstrated previously over a 96 hr postlesion time course (Lauterborn et al., 1994), a second set of rats was anesthetized, and an electrolytic hilus lesion was placed with a platinum–iridium wire (as above), reproducing the field of ablation made but not inducing seizure activity (Campbell et al., 1984; Pico and Gall, 1994); these rats were killed 4 hr after surgery.
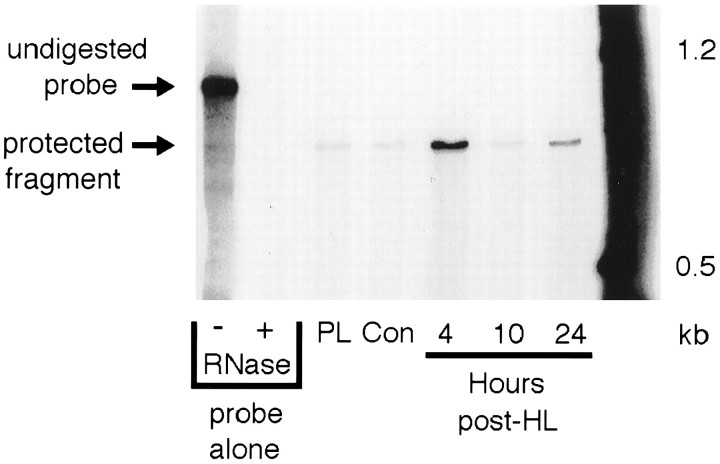
Solution hybridization technique. Dentate gyrus tissue samples (n = 4 animals per group per time point per experiment) were homogenized for total cellular RNA extraction using the protocol for Trizol reagent (Life Technologies, Gaithersburg, MD). RNA pellets were resuspended in 100 μl of diethyl pyrocarbonate-treated H2O and measured spectrophotometrically at OD260nm and OD280nm to determine the yield and purity of RNA. The solution hybridization technique (SHyT) used for measuring specific mRNA species has been described previously (Elliott et al., 1994) and is based on a modified protocol for ribonuclease protection of32P-labeled riboprobes hybridized to complementary RNAs (Hellman et al., 1988; Zhu et al., 1992). In brief, NGF antisense riboprobe was prepared from a construct in which a 771 base pair fragment of the rat NGF 3′ exon was subcloned into a pBluescribe vector (Stratagene, La Jolla, CA). T3 transcription ofPvuII-linearized template with32P-labeled nucleoside triphosphates gives a 947 base antisense riboprobe containing 176 bases of vector linker sequence. Equal amounts of total cellular RNA from control and post-HL time points were individually hybridized with riboprobe in a TES-based hybridization buffer and allowed to incubate at 75°C for 4 hr. After hybridization, samples were digested with RNases A and T1 and then precipitated with trichloroacetic acid (TCA). Hybridized precipitation products were collected over glass microfiber filters (Whatman, Fairfield, NJ) using a 10-place cell harvester (Hoefer/Pharmacia, Piscataway, NJ). Filters were washed three times with 5% TCA, dried, and counted with a standard scintillation counter. Specific hybridization was verified by alternatively running ribonuclease-digested samples on a denaturing polyacrylamide gel and visualizing hybridized fragments autoradiographically (Fig.1). A standard curve was performed for each assay using NGF sense mRNA prepared from in vitrotranscription of the rat cDNA construct used to prepare riboprobe.
Fig. 1.
Representative PAGE gel showing specific hybridization of 32P-labeled NGF antisense riboprobe to total cellular RNA from rat dentate gyrus. Representative gel shown for NGF riboprobe hybridized against 50 μg of total cellular RNA extracted from dentate gyrus subfield dissections of naive control, platinum wire lesion, and experimental seizure (4, 10, and 24 hr after HL) rat hippocampus. After RNase digestion of hybridized samples, protected fragments were run out on a 4% polyacrylamide–8m urea gel and visualized autoradiographically to verify specific hybridization.
Preparation of brain nuclear extracts. After subfield dissection, the dentate gyrus samples (n = 3–5 animals per group per time point per experiment) were processed for nuclear protein extraction based on the protocol of Korner et al. (1989) with modifications. Tissue was added to a glass homogenizer (Wheaton, Millville, NJ) on ice containing 1 ml of buffer A (10 mm HEPES, pH 7.9, 1.5 mmMgCl2, 10 mm KCl, 1 mm dithiothreitol, and the protease inhibitors 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) and transferred to a cold room where the following procedures were performed. Tissue was homogenized by five strokes with a loose pestle, followed by five strokes with a tight pestle. The tissue homogenate was transferred to a 1.5 ml Eppendorf tube and incubated for 15 min after which 55 μl of 10% Nonidet NP-40 was added, followed by 1 min of high-speed microcentrifugation. After discarding the supernatant, the pellet was resuspended in 150 μl of buffer B (20 mm HEPES, pH 7.9, 0.84 m NaCl, 1.5 mmMgCl2, 0.4 mm EDTA, 50% v/v glycerol, 1 mm dithiothreitol, and protease inhibitors as above) and incubated for 15 min. The homogenate was centrifuged at 14,000 rpm in a tabletop microfuge for 15 min, and the supernatant was removed into a fresh Eppendorf tube. Protein content was quantified by the Bradford method (Bradford, 1976).
Electrophoretic mobility shift assay (gel shift) and supershift analysis. Nuclear extracts were subjected to gel shift and supershift analysis according to the protocol of Kaminska et al. (1994)with modifications. AP-1 consensus oligonucleotides (21-mer double-stranded), described in detail below, were32P-labeled with T4 polynucleotide kinase. Fifteen micrograms of protein extract from samples at each experimental time point were combined with 25,000 cpm of labeled probe and buffered with 10 mm HEPES, pH 7.9, 25 mm KCl, 0.5 mm EDTA, 0.25 mg/ml bovine serum albumin, 20 μg/ml poly(dI-dC), and 1 mm dithiothreitol (15 μl total volume) and incubated at room temperature (RT) for 30 min. When supershifting, 200 ng of antibody was mixed with 1 μg of mock or blocking peptide, incubated at 4°C overnight, combined with the protein extract, and then incubated at 4°C for 2 hr before addition of buffer and the radiolabeled oligonucleotide. After the 30 min incubation, 2 μl of 0.1% bromophenol blue was added to each sample before loading on a low ionic strength (0.25× Tris-Borate-EDTA) 4% polyacrylamide gel. Electrophoresis was run for 1.5 hr at 100 V, after which gels were fixed, dried, and exposed to autoradiographic film (X-Omat AR; Eastman Kodak, Rochester, NY). Densitometric evaluation of gel shift bands in the film autoradiograms was performed using the MicroComputer Imaging Device (MCID) system (Imaging Research, St. Catherines, Ontario).
Oligonucleotides and antibodies. Initial electrophoretic mobility shift assay (EMSA) studies used 21-mer double-stranded oligonucleotides containing the consensus AP-1 site (TGAC/GTCA) from two sources, with the following sequences: pAP1 oligonucleotide, c g c t t g a t g a g t c a g c c g g a a; and NGFAP1 oligonucleotide, g c a t c g g t g a g t c a g g c t g c g.
pAP1 was purchased commercially (Promega, Madison, WI), and NGFAP1 was synthesized in single-strand form (Operon, Alameda, CA) and annealed with its complementary strand in 10 mm Tris, pH 7.5, and 10 mm MgCl2 by heating at 80°C for 15 min and cooling slowly to RT. Although both sequences share the consensus AP-1 site (shown in bold type above), the flanking sequence in pAP1 is that of rat collagenase and that of NGFAP1 is identical to the sequence flanking the AP-1 site in the rat NGF gene (Zheng and Heinrich, 1988). Subsequent supershift studies used only NGFAP1.
Commercial antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) directed against c-Fos (catalog #sc-52), FosB (catalog #sc-48), Fra2 (catalog #sc-171), c-Jun (catalog #sc-45), JunB (catalog #sc-46), and JunD (catalog #sc-74), along with the corresponding blocking peptides for each antibody, were used for supershift analysis. According to manufacturer specifications, all antibodies are rabbit polyclonal IgGs and show no detectable cross-reactivity with other members of the Fos and Jun protein families.
RESULTS
SHyT quantitation of NGF mRNA after HL-induced seizures
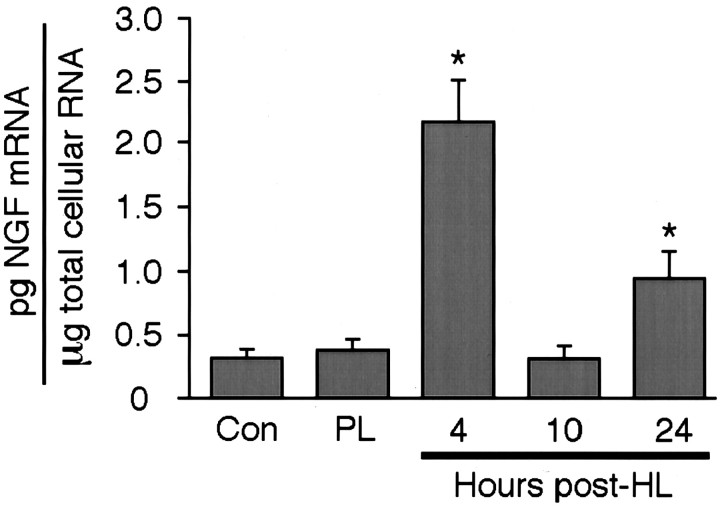
SHyT was used to confirm and quantify changes in NGF mRNA content in the dentate gyrus at a range of time points after placement of a seizure-producing HL. First, preliminary RNase protection analysis was used to verify the presence of changes in NGF mRNA content in the dentate-enriched dissected samples used here. As shown in Figure 1, a single protected band was only faintly evident in samples from the dentate gyrus of naive control rats and from rats with a platinum wire hilar ablation (but no seizure activity). In contrast, in samples from experimental seizure rats, the protected NGF mRNA fragment was greater at both 4 and 24 hr after HL, with an intervening return to control levels at the 10 hr post-HL time point (Fig. 1). Complete SHyT analysis quantified the increases in NGF mRNA content at 4 and 24 hr after lesion at 680 and 300% control values, respectively (Fig.2). In contrast, NGF mRNA levels were not significantly different from control values in samples from rats killed at 10 hr after HL (Fig. 2). As with the RNase protection analysis, NGF mRNA levels in samples from platinum wire lesion control rats killed at 4 hr after HL were also not significantly different from naive control values, indicating that lesion placement in the absence of seizure activity had no substantial effect on NGF mRNA expression.
Fig. 2.
SHyT quantitation of NGF mRNA levels after HL. Fifty micrograms of total cellular RNA were extracted from the dentate gyrus of naive control, platinum wire lesion, and experimental seizure (4, 10, and 24 hr after HL) rats and analyzed for levels of NGF mRNA using the SHyT. Data are the mean ± SEM of values obtained from four independent experiments. *p < 0.05 compared with naive control values; Fisher's PLSD post hoc analysis.
EMSA analysis of AP-1 binding
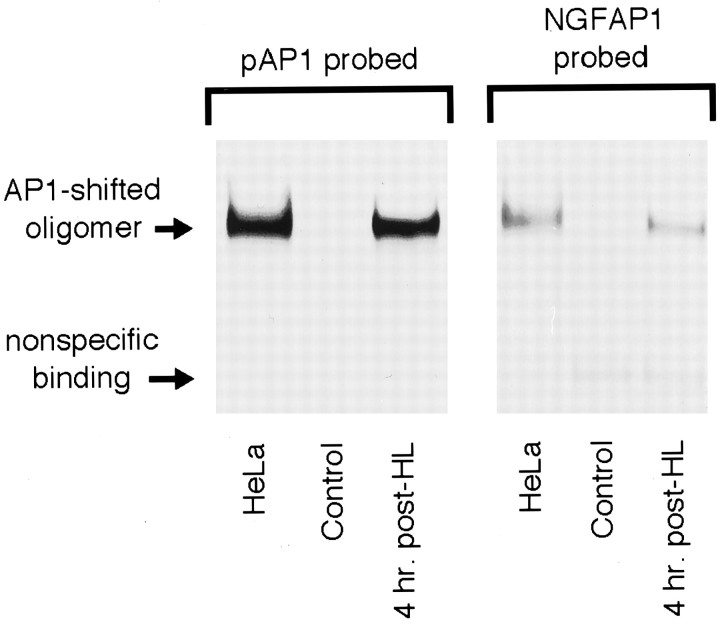
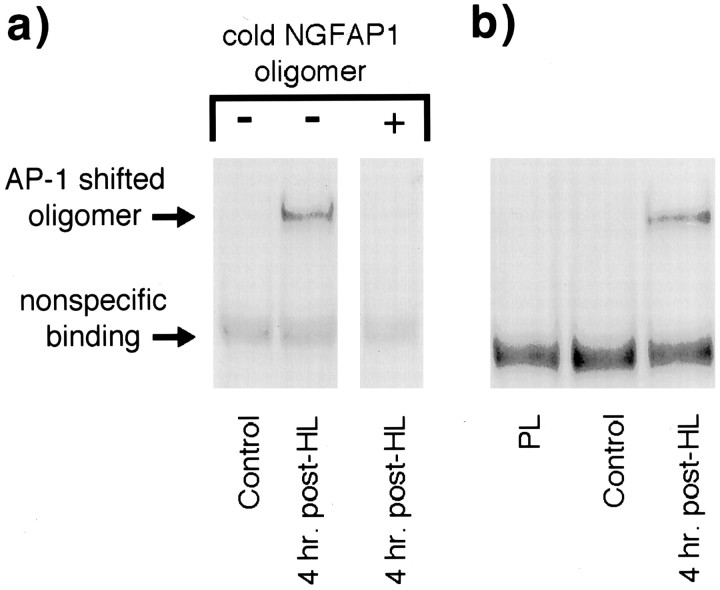
Previous studies have demonstrated that DNA regions flanking an AP-1 site may influence AP-1 binding specificity and affinity (Ryseck and Bravo, 1991), suggesting that an AP-1 site-containing oligonucleotide with random flanking sequence may yield different EMSA results than those obtained with an oligonucleotide with AP-1 flanking sequences found in the NGF gene. To test this possibility, initial EMSA experiments assessed AP-1 binding using both a frequently used, collagenase-derived AP-1 site-containing oligonucleotide (pAP1) and an oligonucleotide duplicating the rat NGF AP-1 site and flanking sequence (NGFAP1). Under identical conditions, both oligonucleotides were used in gel shift analyses of dentate gyrus nuclear extracts purified from control rats and rats killed 4 hr after HL (i.e., 2 hr after seizure onset). Extracts from HeLa cells, known to contain AP-1 binding activity, were assayed in parallel and served as positive controls. As shown in Figure 3, both oligonucleotides formed a single shifted oligomer complex with HeLa cell extract. Moreover, both oligonucleotides formed the same protein–DNA complex (i.e., a band shifted to the exact same position) with dentate gyrus extracts from 4 hr HL rats but not from control rats. Although the amount of complex formed with the NGFAP1 oligonucleotide was markedly less than that obtained with pAP1, these results demonstrate that seizures increase AP-1 protein binding to the AP-1 binding site within the NGF gene and that the NGFAP1 oligonucleotide can be used to assay change in this specific activity over time. Therefore, to maximize the relevance of further analysis to NGF gene expression, subsequent EMSA used NGFAP1 alone. As shown in Figure 4a, the specificity of the AP-1 protein–NGFAP1 oligomer complex was examined by verifying that the addition of excess unlabeled NGFAP1 oligomer blocked complex formation. Moreover, analysis of dentate gyrus extracts from naive control and platinum wire lesion rats showed a common lack of detectable AP-1 binding (Fig. 4b), indicating that, like changes in NGF mRNA content, lesion placement in the absence of seizures does not increase binding activity to the NGFAP1 sequence as evaluated at 4 hr after lesion.
Fig. 3.
Comparison of generic and NGF-like AP-1 site-containing oligomers in the EMSA. Representative gel shift experiment using generic (pAP1) and NGF-like (NGFAP1) AP-1 site-containing oligomer 32P-labeled probes to assess AP-1 binding in nuclear extracts from HeLa cells and naive and 4 hr post-HL rat dentate gyrus. Experiments were repeated three times with similar results.
Fig. 4.
Characterization of the NGFAP1 oligomer probe in EMSA experiments. a, EMSA comparison of dentate gyrus nuclear extracts from naive and 4 hr post-HL rats was performed in the presence of excess unlabeled NGFAP1 probe. Elimination of the AP-1 shifted oligomer band by the cold probe indicates the specificity of the AP-1 protein–NGFAP1 probe interaction (compare with nonspecific binding band, which is unchanged by presence of cold oligomer).b, EMSA comparison of dentate gyrus extracts from naive control, platinum wire lesion, and 4 hr post-HL rats, indicating that surgical technique and lesion placement were not sufficient to induce AP-1 binding. All experiments repeated three times with similar results.
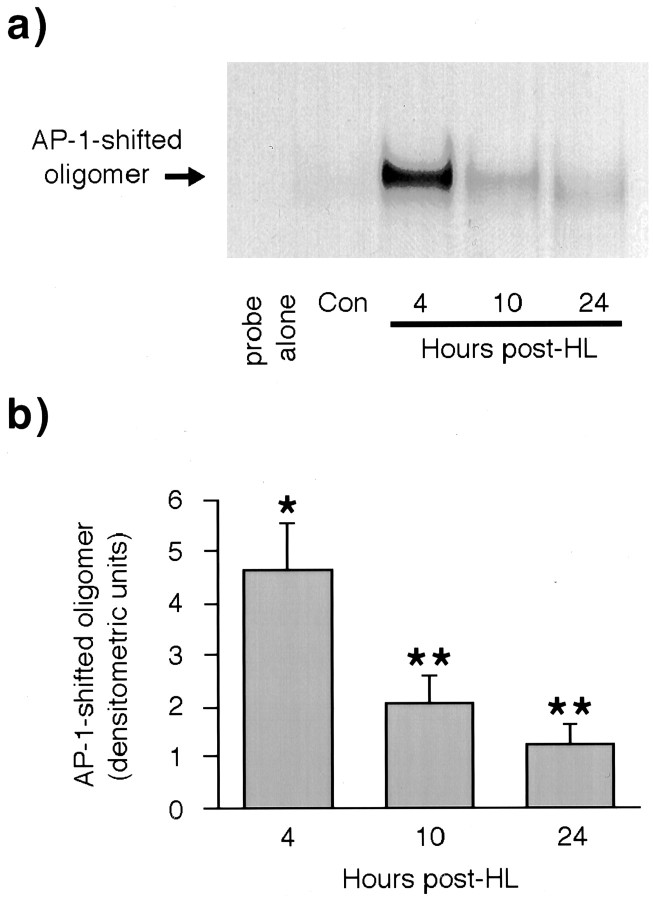
Gel shift analysis was used to determine the relative levels of NGFAP1 binding activity within dentate gyrus nuclear extracts from rats killed 4, 10, and 24 hr after seizure-producing HL placement (Fig.5). As in previous reports of the effects of seizures on AP-1 activity (Sonnenberg et al., 1989a; Pennypacker et al., 1993), NGFAP1 binding was markedly elevated above naive control levels at 4 hr after HL. After this initial rise, AP-1 binding activity declined slowly, falling to 54% of peak values at 10 hr after HL and to 13% of peak values at the 24 hr time point. Note, however, that at all time points the level of AP-1 binding detected is significantly higher than the virtually undetectable degree of AP-1 binding found in control tissue.
Fig. 5.
EMSA analysis of AP-1 binding 4, 10, and 24 hr after HL. a, Representative EMSA gel showing time course of AP-1 binding to NGFAP1 probe using dentate gyrus nuclear extracts from naive rats and rats killed 4, 10, and 24 hr after HL. b, Quantitation of AP-1 binding profile based on densitometric evaluation of EMSA gels from five independent experiments. Bars show group means ± SEM. *p < 0.05 compared with values obtained with extracts from control rats; Fisher's PLSD post hocanalysis. **p < 0.05 compared with values obtained with extracts from control rats and 4 hr post-HL rats; Fisher's PLSDpost hoc analysis.
Supershift analysis of AP-1 complex composition
Results presented above demonstrate that, despite elevated AP-1 binding at 4, 10, and 24 hr after HL, NGF mRNA content is elevated at 4 and 24 hr after lesion but returns to control levels at the 10 hr time point. This suggests that there are changes in the nature of the AP-1 binding activity across these time points and, in particular, that the AP-1 complex bound at 10 hr after HL does not activate NGF mRNA expression. To determine whether there are changes in AP-1 composition associated with positive and negative NGF regulation, supershift analyses of nuclear extracts from each time point were conducted with antibodies against the AP-1 proteins c-Jun, JunB, JunD, c-Fos, FosB, and Fra2. Fra1 was not included because of immunocytochemical evidence that Fra1 expression is most prominent within glial cells in tissue from experimental seizure rats (J. Pinkstaff and C. Gall, personal communication).
Antibody recognition of a component of the AP-1 complex typically leads to slower migration, or supershifting, of the AP-1–oligonucleotide band. Alternatively, if the antibody binds its respective AP-1 protein in a manner that prevents DNA binding, the result is a diminution of the AP-1–oligonucleotide band. However, in supershift analyses, there is concern that band diminution can also occur because of nonspecific antibody–AP-1 interactions and, more importantly, that the degree of this nonspecific diminution varies between antibodies. Therefore, to ensure that band diminution in our antibody-treated samples was attributable to specific antibody recognition of AP-1 proteins, control samples were run for each antibody in each experiment in which the antibody was incubated with its respective blocking peptide before addition of nuclear extract, allowing a highly accurate supershift analysis. The importance of these controls is validated by comparing the various antibody control samples in the following supershift data (Figs. 6,7) in which the varying intensities of the respective AP-1 bands reflects the differing amounts of nonspecific antibody–AP-1 interaction. All supershift experiments were repeated a minimum of four times with highly reproducible results. However, even under optimal conditions, the shifted and supershifted bands are too diffuse and nonuniform (i.e., typically being darker at the edges of the lane than in the middle) to be reliably and reproducibly quantified; hence, our evaluation of this experimental data is strictly qualitative.
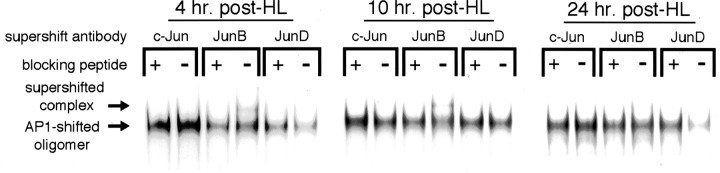
Fig. 6.
Supershift analysis of AP-1 composition with Jun family antibodies after HL. Dentate gyrus nuclear extracts from rats 4, 10, and 24 hr after HL were incubated with antibodies against various Jun family members and then EMSA-probed with 32P-labeled NGFAP1 oligomer. Antibodies were preincubated with antigenic blocking peptide (+ lanes) or mock peptide (−lanes) to control for nonspecific antibody effects on AP-1–NGFAP1 binding. Supershifting and/or diminution of the AP-1-shifted oligomer band by a particular antibody is indicative of participation of the respective protein in the AP-1 complex. Experiments were repeated four times with similar results.
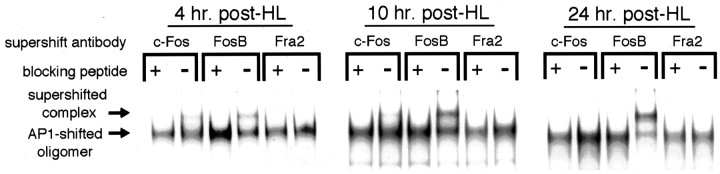
Fig. 7.
Supershift analysis of AP-1 composition with Fos family antibodies after HL. Dentate gyrus nuclear extracts from rats 4, 10, and 24 hr after HL were incubated with antibodies against various Fos family members and then EMSA-probed with 32P-labeled NGFAP1 oligomer. Antibodies were preincubated with antigenic blocking peptide (+ lanes) or mock peptide (−lanes) to control for nonspecific antibody effects on AP-1–NGFAP1 binding. Experiments were repeated four times with similar results.
As shown in Figure 6, supershift analyses indicate that the AP-1 participation of Jun family members was limited to JunB and JunD at experimental time points evaluated. In particular, pretreatment with the JunB antibody in the absence of blocking peptide led to the appearance of a supershifted band that was greatest in samples from the 10 hr postlesion time point, of intermediate density at the 4 hr time point, and just barely detectable in dentate extracts collected at 24 hr after HL. In contrast, pretreatment of nuclear extracts with JunD antibodies did not give rise to a supershifted band but did cause a marked diminution in the density of the AP-1 complex band, indicating a significant contribution of JunD to AP-1 activity in experimental tissue. This diminution was prominent with nuclear extracts from rats killed at 4 and 24 hr after lesion but was not evident at the 10 hr post-HL time point. Finally, pretreatment with c-Jun antibodies did not supershift or diminish AP-1 binding activity at any experimental time point.
Similarly, Fos family participation in the AP-1 complex appeared to be restricted to just two members, c-Fos and FosB, with Fra2 antibodies failing to have any detectable effect on bound AP-1 band intensity or position at any postlesion time point (Fig. 7). With nuclear extracts prepared from rats killed at 4 and 10 hr after HL, pretreatment with c-Fos antibodies gave rise to a modest supershifted band that appeared to be proportionately more dense at the 4 hr time point. This c-Fos containing band was not evident at 24 hr after HL. In contrast, pretreatment with FosB antibodies gave rise to a supershifted band that became progressively more intense from 4 to 10 to 24 hr after lesion.
DISCUSSION
Neurotrophins have been implicated in the development and maintenance of epileptic patterns of neuronal activity and as mediators of physiological processes, such as learning and memory. In a general model applicable to both functions, activity-induced expression of one or more neurotrophins would in turn facilitate synaptic function (Kang and Schuman, 1995; Prakash et al., 1996) and axonal growth and synaptogenesis (Holtzman and Lowenstein, 1995), resulting in a long-term strengthening of circuit function for better (as a cellular strategy for learning) or worse (in the case of epileptogenesis). The cellular underpinnings for this model are the molecular mechanisms responsible for altered neurotrophin expression and/or signaling after intense neuronal activity. To begin investigating this aspect of activity-induced changes in neuron structure and function, the present studies analyzed the transcriptional regulation of one particular neurotrophin, NGF, after HL-induced seizures. In addition to its demonstrated importance in seizure-induced hippocampal sprouting (Holtzman and Lowenstein, 1995; Van der Zee et al., 1995), we focused on NGF in the HL paradigm because of its unique biphasic profile of expression over the 24 hr post-HL period (Lauterborn et al., 1994), suggesting the presence of both positive and negative regulation of transcriptional activity.
The available evidence suggests that seizure-induced NGF expression in the brain is predominantly, if not fully, regulated through AP-1 activation (Zheng and Heinrich, 1988; Hengerer et al., 1990; Cowie et al., 1994). However, previous studies of seizure-induced changes in AP-1 binding and NGF gene expression have been conducted independently, using different techniques and paradigms. Moreover, most of what is known about NGF promoter usage and AP-1 composition and function has come from in vitro manipulations of fibroblasts or other cell lines; this relationship is relatively unstudied under more physiological conditions. To assess the correlation between AP-1 binding and NGF mRNA expression in situ, the present studies performed parallel measures of NGF mRNA expression, AP-1 binding, and AP-1 composition after HL-induced seizures. Initial gel shift results obtained with the prototypical collagenase AP-1 site-containing nucleotide differed significantly from results obtained with an oligonucleotide containing the NGF AP-1 site and flanking sequences. These results are consistent with evidence that sequences flanking the AP-1 site can markedly influence the binding of AP-1 heterodimers (Ryseck and Bravo, 1991) and indicate that analyses of AP-1 binding that is relevant to the regulation of a specific gene, such as that encoding NGF, should use oligonucleotides that replicate sequences within that gene. Therefore, NGF AP-1 oligonucleotides were used exclusively in subsequent EMSA experiments performed on nuclear extracts from experimental seizure rats.
Solution hybridization analysis verified the biphasic profile of NGF mRNA expression in the dentate gyrus of HL seizure rats; NGF mRNA levels were significantly increased at 4 and 24 hr after lesion, with an intervening return to control values at the 10 hr postlesion time point. In contrast to this pattern, but in agreement with results of studies using a variety of seizure paradigms (Sonnenberg et al., 1989a,b; Hope et al., 1994; Pennypacker et al., 1994), binding to the NGF AP-1 site was significantly elevated by 4 hr after HL and remained elevated through 10 and 24 hr after seizure induction. One obvious explanation for the lack of correlation between NGF AP-1 binding and NGF transcriptional activity, as seen most particularly at the 10 hr time point, is that AP-1 binding does not influence NGF transcription to the extent suggested from in vitro studies. However, an alternate and more likely explanation is that changes in the composition of AP-1 heterodimers occurring with time after seizure onset underlie alterations in AP-1 function and, most particularly, NGF transactivation potential.
There is evidence that the composition of AP-1 binding changes with time after depolarization and seizures. Among the Fos family members, c-Fos generally predominates at early time points and is replaced with Fos-related antigens, or Fras, at later intervals (Sonnenberg et al., 1989b, Szekely et al., 1990) (for review, see Morgan and Curran, 1991). Similarly, after kainate-induced seizures, JunB and JunD are major components of AP-1 binding at early and late time points, respectively (Kaminska et al., 1994). It has been shown that changes in AP-1 composition can increase or suppress transactivation of target genes in a number of systems (Schutte et al., 1989; Suzuki et al., 1991; Hsu et al., 1993). To see whether changes in the composition of NGF AP-1 protein binding correlate with phases of NGF expression in HL rats, supershift experiments were performed using antibodies to AP-1 proteins reported previously to be induced by seizures.
Although both c-Jun and Fra2 are induced by seizures (Sonnenberg et al., 1989a,c; Gass et al., 1992), neither was detected in complexes binding the NGF AP-1 site in HL rats. With c-Jun, it is possible that expression is transiently increased before 4 hr after HL, the earliest time point examined. After pentylenetetrazole-induced seizures, c-jun mRNA levels rise within 1 hr, decline by 4 hr, and approach control levels by 6 hr of treatment (Sonnenberg et al., 1989c). However, changes in c-Jun protein content would be expected to occur later (Dragunow et al., 1992; Gass et al., 1992) and to overlap time points examined. Indeed, immunostaining for c-Jun is elevated in hippocampus of HL rats through 10 hr after seizure (C. Gall, unpublished observations). This raises the alternative possibility that c-Jun and Fra2 levels are indeed sufficient to make significant contributions to AP-1 complex formation in HL rats but that these particular dimers have relatively low affinity for the NGF AP-1 site. Composition-dependent AP-1 binding affinities have been demonstrated previously for different Fos/Jun family heterodimersin vitro (Ryseck and Bravo, 1991). Differences in dimer affinity might also explain the apparent discrepancy between c-jun message expression and c-Jun detectability in the AP-1 complex after kainic acid-induced seizures (Kaminska et al., 1994).
Unlike c-Jun, JunB and JunD contributed to NGF AP-1 binding in a time-dependent manner. In HL rats, the greater proportion of JunD-containing AP-1 at time points when NGF mRNA levels are increased, and the converse domination by JunB-containing AP-1 when NGF expression is minimal, suggests that JunD-containing dimers activate NGF transcription, whereas JunB-containing dimers do not. This hypothesis is consistent with evidence that JunB can inhibit activation by c-Jun- and JunD-containing dimers (Schutte et al., 1989; Kobierski et al., 1991; Ryseck and Bravo, 1991; Hsu et al., 1993).
Changes in the relative involvement of c-Fos and FosB in NGF AP-1 were also observed over time after HL placement. Both c-Fos and FosB stimulate transcription from AP-1 sites, so the transition from an AP-1 complex containing c-Fos to one containing FosB does not immediately suggest an alteration in AP-1 function. However, future investigations into the relative contributions of full-length FosB and truncated FosB, the latter of which has been shown to be capable of suppressing AP-1 activity (Mumberg et al., 1991; Nakabeppu and Nathans, 1991; Yen et al., 1991), may provide further insight into the effects of this transition.
To conclude, the present results demonstrate that, after HL seizures, there are changes in the composition of dimers binding the NGF AP-1 site that correlate with, and potentially subserve, time-dependent changes in NGF transcription. In particular, it is hypothesized that predominant involvement of JunD and JunB in binding to the NGF AP-1 site accounts for activation and inhibition of NGF transcription, respectively, and specifically for biphasic increases in NGF expression in the dentate gyrus of HL rats. From the uniform pattern of basal and postseizure NGF and Fos/Jun mRNA and protein expression throughout stratum granulosum (Gall and Isackson, 1989; Kiessling et al., 1993;Lanaud et al., 1993; Beer et al., 1998), it is expected that changes in AP-1 composition described here occur in a similar synchronous manner throughout the granule cell layer. However, the possibility remains that transient changes in the expression of different AP-1 proteins by other minority cell types within the dentate gyrus (e.g., hilar and CA3c neurons) may have contributed to changes in AP-1 composition demonstrated in the present supershift analyses. Thus, to both extend the present analysis and begin testing the proposed antagonistic roles of JunB and JunD in AP-1 transactivation of NGF, studies determining the effects of deleting specific Fos and Jun proteins (e.g., FosB, JunB) (Mandelzys et al., 1997) on AP-1 binding and NGF transcription after seizure will be particularly informative. Moreover, the complexity of AP-1 function necessitates the consideration of alternate mechanisms, such as changes in the phosphorylation state of AP-1 proteins, a modification that has been shown to affect AP-1 binding and transcriptional activity (Kobierski et al., 1991; Bannister et al., 1994; Gruda et al., 1994) and may be influenced by NGF itself (Morgan and Curran, 1986). Other nontraditional uses of the AP-1 promoter could also be influencing NGF transcription, including binding of heterodimers containing members of the Maf and activating transcription factor/cAMP response element-binding protein families of proteins (Hai and Curran, 1991; Kerppola and Curran, 1994). These and other mechanisms are not necessarily mutually exclusive and may work in concert with the reported alterations in AP-1 composition to precisely regulate NGF gene expression after both physiological and pathological levels of neuronal activity.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS26748 to C.M.G.
Correspondence should be addressed to Robert C. Elliott, Department of Neurology, Box 0435, University of California, San Francisco, San Francisco, CA 94143-0435. E-mail: rce@itsa.ucsf.edu.
Dr. Elliott's present address: Department of Neurology, University of California, San Francisco, San Francisco, CA 94143.
REFERENCES
- 1.Bannister AJ, Brown HJ, Sutherland JA, Kouzarides T. Phosphorylation of the c-Fos and c-Jun HOB1 motif stimulates its activation capacity. Nucleic Acids Res. 1994;22:5173–5176. doi: 10.1093/nar/22.24.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beer J, Mielke K, Zipp M, Zimmerman M, Herdegen T. Expression of c-jun, junB, c-fos, fra-1 and fra-2 mRNA in the rat brain following seizure activity and axotomy. Brain Res. 1998;794:255–266. doi: 10.1016/s0006-8993(98)00233-9. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ari Y, Represa A. Brief seizure episodes induce long-term potentiation and mossy fibre sprouting in the hippocampus. Trends Neurosci. 1990;13:312–318. doi: 10.1016/0166-2236(90)90135-w. [DOI] [PubMed] [Google Scholar]
- 4.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Bundman MC, Pico RM, Gall CM. Ultrastructural plasticity of the dentate gyrus granule cells following recurrent limbic seizures: I. Increase in somatic spines. Hippocampus. 1994;4:601–610. doi: 10.1002/hipo.450040510. [DOI] [PubMed] [Google Scholar]
- 6.Campbell KA, Bank B, Milgram NW. Epileptogenic effects of electrolytic lesions in the hippocampus: role of iron deposition. Exp Neurol. 1984;86:506–514. doi: 10.1016/0014-4886(84)90085-2. [DOI] [PubMed] [Google Scholar]
- 7.Cowie A, Ivanco TL, Fahnestock M. Mouse NGF promoter upstream sequences do not affect gene expression in mouse fibroblasts. Mol Brain Res. 1994;27:58–62. doi: 10.1016/0169-328x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 8.Dragunow M, Yamada N, Bilkey DK, Lawlor P. Induction of immediate-early gene proteins in dentate granule cells and somatostatin interneurons after hippocampal seizures. Mol Brain Res. 1992;13:119–126. doi: 10.1016/0169-328x(92)90051-c. [DOI] [PubMed] [Google Scholar]
- 9.Elliott RC, Inturrisi CE, Black IB, Dreyfus CF. An improved method detects differential NGF and BDNF gene expression in response to depolarization in cultured hippocampal neurons. Mol Brain Res. 1994;26:81–88. doi: 10.1016/0169-328x(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 10.Gall C, Isackson P. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989;245:758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- 11.Gall C, Murray K, Isackson PJ. Kainic acid-induced seizures stimulate increased expression of nerve growth factor mRNA in rat hippocampus. Mol Brain Res. 1991;9:113–123. doi: 10.1016/0169-328x(91)90136-l. [DOI] [PubMed] [Google Scholar]
- 12.Gass P, Herdegen T, Bravo R, Kiessling M. Induction of immediate early gene encoded proteins in the rat hippocampus after bicuculline-induced seizures: differential expression of KROX-24, Fos and Jun proteins. Neuroscience. 1992;48:315–324. doi: 10.1016/0306-4522(92)90493-l. [DOI] [PubMed] [Google Scholar]
- 13.Gnahn H, Hefti F, Heumann R, Schwab ME, Thoenen H. NGF-mediated increase of choline acetyltransferase (ChAT) in the neonatal rat forebrain: evidence for a physiological role of NGF in the brain? Dev Brain Res. 1983;9:45–52. doi: 10.1016/0165-3806(83)90107-4. [DOI] [PubMed] [Google Scholar]
- 14.Gruda MC, Kovary K, Metz R, Bravo R. Regulation of Fra-1 and Fra-2 phosphorylation differs during the cell cycle of fibroblasts and phosphorylation in vitro by MAP kinase affects DNA binding activity. Oncogene. 1994;9:2537–2547. [PubMed] [Google Scholar]
- 15.Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellman W, Suzuki F, Ohkubo H, Nakanishi S, Ludwig G, Ganten D. Angiotensinogen gene expression in extrahepatic rat tissues: application of a solution hybridization assay. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:327–331. doi: 10.1007/BF00173408. [DOI] [PubMed] [Google Scholar]
- 17.Hengerer B, Lindholm D, Heumann R, Ruther U, Wagner EF, Thoenen H. Lesion-induced increase in nerve growth factor mRNA is mediated by c-Fos. Proc Natl Acad Sci USA. 1990;87:3899–3903. doi: 10.1073/pnas.87.10.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtzman D, Lowenstein D. Selective inhibition of axon outgrowth by antibodies to NGF in a model of temporal lobe epilepsy. J Neurosci. 1995;15:7062–7070. doi: 10.1523/JNEUROSCI.15-11-07062.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hope BT, Kelz MB, Duman RS, Nestler EJ. Chronic electroconvulsive seizure (ECS) treatment results in expression of a long-lasting AP-1 complex in brain with altered composition and characteristics. J Neurosci. 1994;14:4318–4328. doi: 10.1523/JNEUROSCI.14-07-04318.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu JC, Cressman DE, Taub R. Promoter-specific trans-activation and inhibition mediated by JunB. Cancer Res. 1993;53:3789–3794. [PubMed] [Google Scholar]
- 21.Kaminska B, Filipkowski RK, Zurkowska G, Lason W, Przewlocki R, Kaczmarek L. Dynamic changes in the composition of the AP-1 transcription factor DNA-binding activity in rat brain following kainate-induced seizures and cell death. Eur J Neurosci. 1994;6:1558–1566. doi: 10.1111/j.1460-9568.1994.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 22.Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 23.Kashihara K, Sato K, Akiyama K, Okada S, Ishihara T, Hayabara T, Shomori T. Temporal pattern of AP-1 DNA-binding activity in the rat hippocampus following a kindled seizure. Neuroscience. 1997;80:753–761. doi: 10.1016/s0306-4522(97)00133-4. [DOI] [PubMed] [Google Scholar]
- 24.Kerppola TK, Curran T. Maf and Nrl can bind to AP-1 sites and form heterodimers with Fos and Jun. Oncogene. 1994;9:675–684. [PubMed] [Google Scholar]
- 25.Kiessling M, Stumm G, Xie Y, Herdegen T, Aguzzi A, Bravo R, Gass P. Differential transcription and translation of immediate early genes in the gerbil hippocampus after transient global ischemia. J Cereb Blood Flow Metab. 1993;13:914–924. doi: 10.1038/jcbfm.1993.114. [DOI] [PubMed] [Google Scholar]
- 26.Kobierski LA, Chu H-M, Tan Y, Comb MJ. cAMP-dependent regulation of proenkephalin by JunD and JunB: positive and negative effects of AP-1 proteins. Proc Natl Acad Sci USA. 1991;88:10222–10226. doi: 10.1073/pnas.88.22.10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korner M, Rattner A, Mauxion F, Sen R, Citri Y. A brain-specific transcription factor. Neuron. 1989;3:563–572. doi: 10.1016/0896-6273(89)90266-3. [DOI] [PubMed] [Google Scholar]
- 28.Lanaud P, Maggio R, Gale K, Grayson DR. Temporal and spatial patterns of expression of c-fos, zif/268, c-jun, and jun-B mRNAs in rat brain following seizures evoked from deep prepiriform cortex. Exp Neurol. 1993;119:20–31. doi: 10.1006/exnr.1993.1003. [DOI] [PubMed] [Google Scholar]
- 29.Lauterborn JC, Isackson PJ, Gall CM. Seizure-induced increases in NGF mRNA exhibit different time courses across forebrain regions and are biphasic in hippocampus. Exp Neurol. 1994;125:22–40. doi: 10.1006/exnr.1994.1003. [DOI] [PubMed] [Google Scholar]
- 30.Levi-Montalcini R, Meyer RH, Hamburger V. In vitro experiments on the effects of mouse sarcomas 180 and 37 on the spinal and sympathetic ganglia of the chick embryo. Cancer Res. 1954;14:49–57. [PubMed] [Google Scholar]
- 31.Lindvall O, Kokaia Z, Bengzon J, Elmer E, Kokaia M. Neurotrophins and brain insults. Trends Neurosci. 1994;17:490–496. doi: 10.1016/0166-2236(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 32.Mandelzys A, Gruda MA, Bravo R, Morgan JI. Absence of a persistently elevated 37 kDa Fos-related antigen and AP-1-like DNA-binding activity in the brains of kainic acid-treated fosB null mice. J Neurosci. 1997;17:5407–5415. doi: 10.1523/JNEUROSCI.17-14-05407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan JI, Curran T. The role of ion flux in the control of c-Fos expression. Nature. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- 34.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 35.Mumberg D, Lucibello FC, Schuermann M, Muller R. Alternative splicing of fosB transcripts results in differentially expressed mRNAs encoding functionally antagonistic proteins. Genes Dev. 1991;5:1212–1223. doi: 10.1101/gad.5.7.1212. [DOI] [PubMed] [Google Scholar]
- 36.Nakabeppu Y, Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991;64:751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- 37.Nikolaev E, Kaminska B, Tischmeyer W, Matthies H, Kaczmarek L. Induction of expression of genes encoding transcription factors in the rat brain elicited by behavioral training. Brain Res Bull. 1992;28:479–484. doi: 10.1016/0361-9230(92)90050-8. [DOI] [PubMed] [Google Scholar]
- 38.Olson L, Malmfors T. Growth characteristics of adrenergic nerves in the adult rat. Fluorescence, histochemical, and 3H-noradrenaline uptake studies using tissue transplanted to the anterior chamber of the eye. Acta Physiol Scand Suppl. 1970;348:1–111. [PubMed] [Google Scholar]
- 39.Pennypacker KR, Walczak DD, Thai L, Fannin R, Mason E, Douglass J, Hong JS. Kainate-induced changes in opioid peptide genes and AP-1 protein expression in the rat hippocampus. J Neurochem. 1993;60:204–211. doi: 10.1111/j.1471-4159.1993.tb05839.x. [DOI] [PubMed] [Google Scholar]
- 40.Pennypacker KR, Thai L, Hong J-S, McMillian MK. Prolonged expression of AP-1 transcription factors in the rat hippocampus after systemic kainate treatment. J Neurosci. 1994;14:3998–4006. doi: 10.1523/JNEUROSCI.14-07-03998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pico RM, Gall CM. Continuities between outer nuclear membrane and granular endoplasmic reticulum increase in hippocampal neurons during seizure-induced protein synthesis. Brain Res. 1989;497:387–392. doi: 10.1016/0006-8993(89)90286-2. [DOI] [PubMed] [Google Scholar]
- 42.Pico RM, Gall CM. Hippocampal epileptogenesis produced by electrolytic iron deposition in the rat dentate gyrus. Epilepsy Res. 1994;19:27–36. doi: 10.1016/0920-1211(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 43.Prakash N, Cohen-Cory S, Frostig R. Rapid and opposite effects of BDNF and NGF on the functional organization of the adult cortex in vivo. Nature. 1996;381:702–706. doi: 10.1038/381702a0. [DOI] [PubMed] [Google Scholar]
- 44.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 45.Ryseck R-P, Bravo R. c-Jun, JunB, and JunD differ in their binding affinities to AP-1 and CRE consensus sequences: effect of Fos proteins. Oncogene. 1991;6:533–542. [PubMed] [Google Scholar]
- 46.Schutte J, Viallet J, Nau M, Segal S, Fadorko J, Minna J. Jun-B inhibits and c-Fos stimulates the transforming and transactivating activities of c-Jun. Cell. 1989;59:987–997. doi: 10.1016/0092-8674(89)90755-1. [DOI] [PubMed] [Google Scholar]
- 47.Sonnenberg JL, Macgregor-Leon PF, Curran T, Morgan JI. Dynamic alterations occur in the levels and composition of transcription factor AP-1 complexes after seizure. Neuron. 1989a;3:359–365. doi: 10.1016/0896-6273(89)90260-2. [DOI] [PubMed] [Google Scholar]
- 48.Sonnenberg JL, Mitchelmore C, Macgregor-Leon PF, Hempstead J, Morgan JI, Curran T. Glutamate receptor agonists increase the expression of Fos, Fra, and AP-1 DNA binding activity in the mammalian brain. J Neurosci Res. 1989b;24:72–80. doi: 10.1002/jnr.490240111. [DOI] [PubMed] [Google Scholar]
- 49.Sonnenberg JL, Rauscher IIIFJ, Morgan JI, Curran T. Regulation of proenkephalin by Fos and Jun. Science. 1989c;246:1622–1625. doi: 10.1126/science.2512642. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki T, Okuno H, Yoshida T, Endo T, Nishina H, Iba H. Difference in transcriptional regulatory function between c-Fos and Fra-2. Nucleic Acids Res. 1991;19:5537–5542. doi: 10.1093/nar/19.20.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szekely AM, Costa E, Grayson DR. Transciptional program coordination by N-methyl-d-aspartate-sensitive glutamate receptor stimulation in primary cultures of cerebellar neurons. Mol Pharmacol. 1990;38:624–633. [PubMed] [Google Scholar]
- 52.Van der Zee CEEM, Rashid K, Le K, Moore K-A, Stanisz J, Diamond J, Racine RJ, Fahnestock M. Intraventricular administration of antibodies to nerve growth factor retards kindling and blocks mossy fiber sprouting in adult rats. J Neurosci. 1995;15:5316–5323. doi: 10.1523/JNEUROSCI.15-07-05316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yen J, Wisdom RM, Tratner I, Verma IM. An alternative spliced form of FosB is a negative regulator of transcriptional activation and transformation by Fos proteins. Proc Natl Acad Sci USA. 1991;88:5077–5081. doi: 10.1073/pnas.88.12.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng M, Heinrich G. Structural and functional analysis of the promoter region of the nerve growth factor gene. Mol Brain Res. 1988;3:133–140. doi: 10.1016/0169-328x(88)90058-7. [DOI] [PubMed] [Google Scholar]
- 55.Zhu Y-S, Branch AD, Robertson HD, Huang TH, Franklin SO, Inturrisi CE. Time course of enkephalin mRNA and peptides in cultured rat adrenal medulla. Mol Brain Res. 1992;12:173–180. doi: 10.1016/0169-328x(92)90081-l. [DOI] [PubMed] [Google Scholar]