Abstract
Activation of several G-protein-coupled receptors leads to voltage-dependent (VD) inhibition of N- and P/Q-type Ca2+ channels via G-protein βγ subunits (Gβγ). The purpose of the present study was to determine the ability of different Gβγ combinations to produce VD inhibition of N-type Ca2+ channels in rat superior cervical ganglion neurons. Various Gβγ combinations were heterologously overexpressed by intranuclear microinjection of cDNA and tonic VD Ca2+ channel inhibition evaluated using the whole-cell voltage-clamp technique. Overexpression of Gβ1–Gβ5, in combination with several different Gγ subunits, resulted in tonic VD Ca2+ channel inhibition. Robust Ca2+ channel modulation required coexpression of both Gβ and Gγ. Expression of either subunit alone produced minimal effects. To substantiate the apparent lack of Gβγ specificity, we examined whether heterologously expressed Gβγ displaced native Gβγ from heterotrimeric complexes. To this end, mutant Gβ subunits were constructed that differentially modulated N-type Ca2+ and G-protein-gated inward rectifier K+ channels. Results from these studies indicated that significant displacement does not occur, and thus the observed Gβγ modulation can be attributed directly to the heterologously expressed Gβγ combinations.
Keywords: G-protein, N-type Ca2+ channel, GIRK channel, Gβγ, ion channel modulation, SCG neurons
Inhibition of neuronal Ca2+ channels by G-protein-coupled receptors (GPCR) represents an important mechanism for modulating release of neurotransmitters from presynaptic nerve endings (Dunlap et al., 1995). Although several discrete signaling pathways leading to N-type Ca2+ channel inhibition have been identified (Hille, 1994), the most commonly used and best characterized pathway results from activation of GPCR that couple to pertussis toxin-sensitive G-proteins (Ikeda and Dunlap, 1999). After receptor activation, N-type Ca2+ channels are inhibited by a membrane-delimited pathway that results in a shift of the channels from a “willing” to “reluctant” mode in which a more depolarized membrane potential is required for channel opening (Bean, 1989). Consequently, the resulting Ca2+ channel inhibition is voltage-dependent (VD), i.e., the magnitude of inhibition is dependent on the membrane potential at which channel opening is measured.
Recently, the molecular mechanism underlying VD inhibition of N- and P/Q-type has begun to emerge (Zamponi and Snutch, 1998; Ikeda and Dunlap, 1999). Experiments in which various G-protein subunits were heterologously expressed in neurons or Ca2+ channel-expressing cells demonstrated that the Gβγ, rather than the Gα, component of heterotrimeric G-proteins was responsible for VD inhibition (Herlitze et al., 1996;Ikeda, 1996). Subsequent studies demonstrated that Gβγ interacts with various regions of Ca2+ channel α1 subunits (De Waard et al., 1997; Qin et al., 1997; Zamponi et al., 1997; Furukawa et al., 1998; Canti et al., 1999). Currently, the consensus view of VD inhibition envisions “release” of Gβγ from the Gαβγ heterotrimer after GPCR activation, followed by direct binding of Gβγ to the Ca2+ channel. At depolarized potentials, the Gβγ subunit is believed to unbind from the Ca2+ channel α1subunit thereby relieving the inhibition and producing biophysical alterations, i.e., “kinetic slowing” of activation and “prepulse facilitation,” which are the electrophysiological signatures of the VD pathway.
Given this mechanism, the question arises whether distinct combinations of Gβγ confer specificity in regard to VD N-type Ca2+ channel modulation. Currently, five Gβ subunits (β1–β5) and eleven Gγ subunits (Gγ1–Gγ12; Gγ6 was renamed Gγ2) have been identified from cloning studies (Watson and Arkinstall, 1994; Clapham and Neer, 1997). Although few combinations of Gβ and Gγ are unlikely to participate in modulation because functional Gβγ monomers do not form or expression is highly restricted, there appear to be a large number of potential combinations that could participate in Ca2+ channel modulation. Previously, Ikeda (1996) and Herlitze et al. (1996)reported that expression of Gβ1γ2, Gβ1γ3 or Gβ1γ7, and Gβ2γ3, respectively, produce VD inhibition of N-type Ca2+ channels. Recently, Garcia et al. (1998) reported that overexpression of some Gβ subunits (Gβ1, Gβ2, or Gβ5) but not others (Gβ3 or Gβ4) resulted in N-type Ca2+ channel inhibition. The purpose of the present study was to extend these studies by heterologously overexpressing defined Gβγ combinations and determining which subunit combination(s) produced tonic (i.e., in the absence of GPCR activation) VD inhibition of N-type Ca2+channels in superior cervical ganglion (SCG) neurons. Unlike Garcia et al. (1998), our results indicate that Gβ1–Gβ5-containing heterodimers are capable of producing VD modulation.
MATERIALS AND METHODS
Neuron isolation and cDNA microinjection. Neurons from adult rat SCG were prepared using methods described previously (Ikeda, 1997). Briefly, male Wistar rats (175–225 gm) were killed by decapitation using a laboratory guillotine without previous anesthesia, and the SCG was dissected in chilled HBSS. The ganglia were incubated with 0.6 mg/ml collagenase type D (Boehringer Mannheim, Indianapolis, IN), 0.4 mg/ml trypsin (TRL type; Worthington Biochemical Corp., Lakewood, NJ), and 0.1 mg/ml DNase Type I (Sigma, St. Louis, MO) for 60 min in a water bath shaker at 35°C. After incubation, the dispersed neurons were centrifuged twice for 6 min at 50 ×g and then resuspended in Minimal Essential Medium (Mediatech, Inc., Herndon, VA) supplemented with 10% fetal calf serum (Atlanta Biologicals, Atlanta, GA), 1% glutamine, and 1% penicillin–streptomycin solution (both from Mediatech, Inc.). The neurons were then plated into 35 mm tissue culture plates coated with poly-l-lysine and stored in a humidified incubator containing 5% CO2 in air at 37°C.
Nuclear microinjection of plasmids was performed with an Eppendorf (Madison, WI) 5246 microinjector and 5171 micromanipulator ∼3–5 hr after plating as described previously (Ikeda, 1997; Ruiz-Velasco and Ikeda, 1998). Plasmids coding for human Gβ2 and β3, mouse Gβ4, Gβ5, and Gγ4, and bovine Gβ1, Gγ1, Gγ2, and Gγ3 (all subcloned into the mammalian expression vector, pCI; Promega, Madison, WI) were prepared using anion exchange columns (Qiagen, Chatsworth, CA) and stored in TE buffer (10 mm Tris and 1 mmEDTA, pH 8.0). Human G-protein-gated inward rectifier K+ channel 1 (GIRK1) and GIRK4 (Kir 3.1 and 3.4, respectively) and bovine Gαtr were supplied in pcDNA3.1 (Invitrogen, Carlsbad, CA) and prepared as above. Site-directed mutagenesis of Gβ subunits was performed using the GeneEditor in vitro site-directed mutagenesis kit (Promega) per the manufacturer's instructions. Mutations were confirmed by automated DNA sequencing (ABI 310; Perkin-Elmer, Foster City, CA). Neurons receiving a successful nuclear injection were identified by fluorescence from coexpressed jellyfish green fluorescent protein (pEGFP-N1, 5 ng/μl; Clontech Laboratories, Palo Alto, CA) as described previously (Ruiz-Velasco and Ikeda, 1998).
Electrophysiology and data analysis.Ca2+ and GIRK channel currents were recorded using the whole-cell variant of the patch-clamp technique (Hamill et al., 1981). Patch pipettes were pulled from glass capillaries (Corning 7052; Garner Glass Co., Claremont, CA) on a P-97 Flaming-Brown micropipette puller (Sutter Instrument Co., San Rafael, CA), coated with Sylgard (Dow Corning, Midland, MI) and fire polished on a microforge. Whole-cell currents were acquired with a patch-clamp amplifier (Axopatch 200A or Axopatch 1C; Axon Instruments, Foster City, CA), analog filtered at 1–2 kHz (−3 dB; four-pole Bessel), and digitized using custom designed software (S3) on a Macintosh Quadra 700 computer (Apple Computer, Cupertino, CA) equipped with a 12-bit analog-to-digital converter board (MacADIOS II; G. W. Instruments, Bedford, MA). Cell membrane capacitance and series resistance (80–85%) were electronically compensated. All experiments were performed at room temperature (21–24°C). Data analysis were performed with the Igor (Wavemetrics, Lake Oswego, OR) software package. Graphs and current traces were produced with Igor, StatView (SAS Institute, Inc., Cary, NC) and Canvas (Deneba Software, Miami, FL) software packages. Data are presented as means ± SEM. Statistical analysis were performed with GB-Stat PPC (Dynamic Microsystems, Inc., Silver Spring, MD) software package using the one-way ANOVA, followed by the Newman–Keuls test. p < 0.05 was considered statistically significant.
For recording Ca2+ currents, the pipette solution contained (in mm): 120N-methyl-d-glucamine, 20 tetraethylammonium hydroxide (TEA-OH), 11 EGTA, 10 HEPES, 10 sucrose, 1 CaCl2, 4 Mg-ATP, 0.3 Na2ATP, and 14 Tris creatine phosphate. The pH was adjusted to 7.2 with methanesulfonic acid and HCl (10 mm), and the osmolality was 299–302 mOsm/kg. The external solution consisted of (in mm): 145 TEA-OH, 10 HEPES, 15 glucose, 10 CaCl2, and 0.0003 tetrodotoxin (TTX). The pH was adjusted to 7.4 with methanesulfonic acid, and the osmolality was 319–327 mOsm/kg. For recording GIRK currents, the pipette solution contained (in mm): 135 KCl, 11 EGTA, 1 CaCl2, 2 MgCl2, 10 HEPES, 4 Mg-ATP, and 0.3 Na2ATP. The pH was adjusted to 7.2 with KOH, and the osmolality was 305 mOsm/kg. The GIRK external solution consisted of (in mm): 130 NaCl, 5.4 KCl, 10 HEPES, 10 CaCl2, 0.8 MgCl2, 15 glucose, 15 sucrose, and 0.0003 TTX. The pH was adjusted to 7.4 with NaOH, and the osmolality was 326 mOsm/kg.
Stock solutions (10 mm) of norepinephrine (NE)-bitartrate (Sigma) were prepared in H2O and diluted in the external solution to 10 μm just before use. Application of drugs to the neuron under study was performed by positioning a custom-designed gravity-fed microperfusion system ∼100 μm from the cell as described previously (Ruiz-Velasco and Ikeda, 1998).
RESULTS
Properties of voltage-dependent Ca2+channel inhibition
Kinetic slowing of activation and prepulse facilitation provide a rapid and reliable means of identifying the VD form of Ca2+ channel modulation. Figure1A depicts superimposed Ca2+ current traces recorded from a control (uninjected) neuron in the absence (bottom trace) or presence (top trace) of 10 μm NE. In rat SCG neurons, NE acts via α2-adrenergic receptors (Schofield, 1990) to produce a well characterized VD inhibition. Ca2+ currents were evoked with a voltage protocol consisting of two identical test pulses (+10 mV) separated by a large depolarizing (+80 mV) conditioning pulse (Fig.1A, bottom) (Elmslie et al., 1990). Kinetic slowing is illustrated in the current evoked during the prepulse (i.e., the test pulse preceding the conditioning pulse). Before NE exposure, the Ca2+ current activation phase was rapid, reaching a plateau within the initial 5–10 msec after onset of the test pulse (Fig. 1A,bottom trace). In contrast, after receptor-mediated G-protein activation with NE, the current rising phase was slower and biphasic (Fig. 1A, top trace).
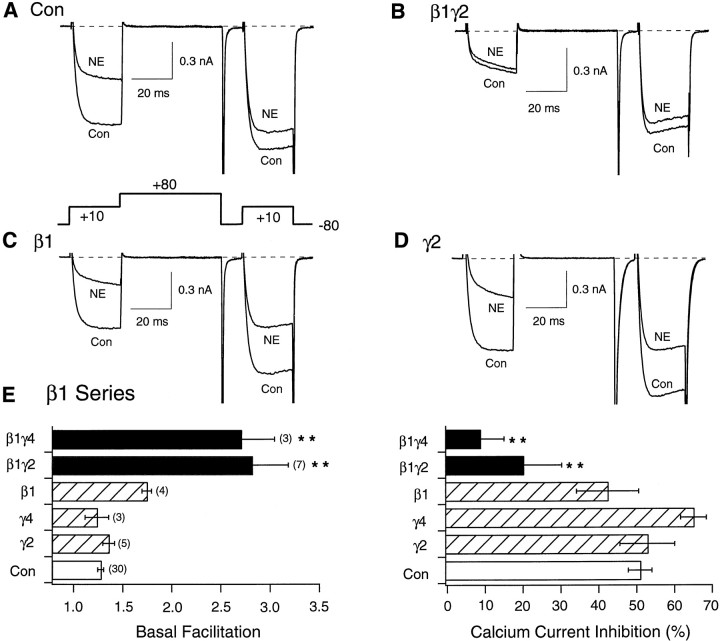
Fig. 1.
Facilitation and NE-mediated inhibition of Ca2+ currents in SCG neurons expressing β1 or Gγ alone or combined. Superimposed Ca2+ current traces evoke with the “double-pulse” voltage protocol (bottom of A) in the absence (bottom traces) and presence (top traces) of 10 μm NE for control (A), Gβ1γ2- (B), Gβ1- (C), and Gγ2-expressing (D) neurons. Currents were evoked every 10 sec. E, Summary graphs of mean ± SEM basal facilitation and Ca2+ current inhibition for neurons expressing Gβ1 alone or combined with Gγ2 and Gγ4 subunits. Final concentration of cDNA injected was 10 ng/μl per subunit. Facilitation was calculated as the ratio of Ca2+ current amplitude determined from the test pulse (+10 mV) occurring after (postpulse) and before (prepulse) the +80 mV conditioning pulse. Ca2+ current inhibition was measured isochronally 10 msec after initiation of the test pulse (+10 mV) in the absence or presence of 10 μm NE. **p < 0.01 versus control. Numbersin parentheses indicate the number of experiments.
A second property of VD inhibition, prepulse facilitation, is evident when the prepulse and postpulse (i.e., current evoked after the condition pulse) current amplitudes are compared. Figure1A shows that, in the absence of NE (bottom trace), the conditioning pulse had a minor, although significant, effect on the postpulse current amplitude (Ikeda, 1991). In the presence of NE, however, the postpulse current was much larger than the prepulse current (relief of NE-mediated inhibition) and displayed normal activation kinetics. The facilitation ratio, a parameter calculated by dividing the postpulse by the prepulse current amplitude, increased dramatically during NE application and thus provided a convenient and reliable measure of VD inhibition. Together, these unique properties (kinetic slowing and increased facilitation ratio) allow VD inhibition to be characterized and measured independently of changes in current amplitude. This strategy was used to determine tonic (i.e., in the absence of agonist) VD inhibition produced after expression of Gβγ subunits.
Expression of different Gβγ combinations produces VD inhibition
Figure 1B–D illustrates the effects of intranuclear microinjection of β1 and γ2 cDNA (10 ng/μl per subunit) alone or together on Ca2+currents. Neurons previously coinjected with β1γ2 cDNAs displayed dramatic kinetic slowing and prepulse facilitation indicative of large tonic VD inhibition (Fig. 1B) as reported previously (Ikeda, 1996). Consistent with this idea, application of NE failed to produce significant effects, indicating near maximal modulation of the channels by expressed Gβγ. Conversely, previous injection of either Gβ1 (Fig. 1C) or Gγ2 (Fig. 1D) cDNA alone resulted in small and sometimes inconsistent changes (e.g., slightly increased prepulse facilitation) (Fig. 1E) in basal current properties. Moreover, application of NE to Gβ1- or Gγ2-expressing neurons resulted in large inhibitions similar to those observed in uninjected neurons. Figure 1E summarizes the effect of expressing Gβ1, Gγ2, Gγ4, and combinations of these subunits on basal (i.e., in the absence of agonist) facilitation ratio and NE-mediated Ca2+ current inhibition. Clearly, coexpression of Gβ1 with different Gγ subunits produced significantly greater modulatory effect on Ca2+ currents than expression of either subunit alone as indicated by the increased facilitation ratio and attenuation of NE-mediated inhibition (p < 0.01). These results are similar to those obtained previously (Ikeda, 1996), although in the present experiments the concentration of cDNA injected was 10-fold lower than those used in the former study.
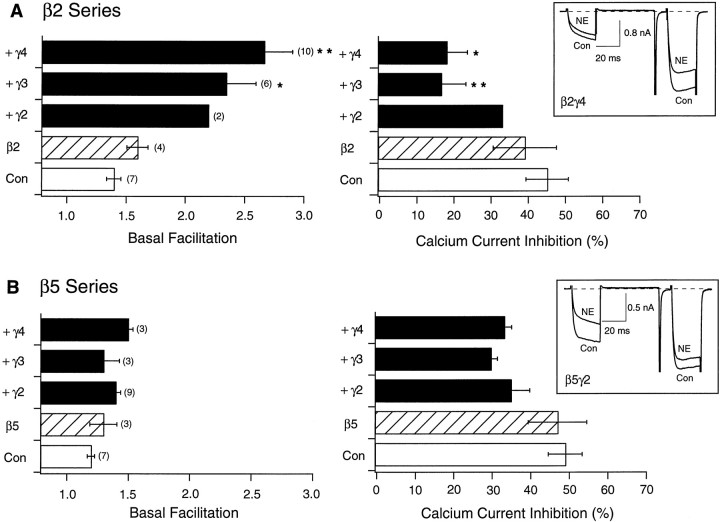
Using this basic experimental paradigm, we next systematically tested the ability of Gβ2–Gβ5, alone and in combination with different Gγ subunits, to produce tonic VD inhibition of N-type Ca2+ channels. Unless otherwise noted, cDNA coding for the various G-protein subunits was injected at a concentration of 10 ng/μl. Figure 2summarizes basal facilitation and NE-mediated Ca2+ current inhibition in SCG neurons previously injected with cDNAs encoding Gβ2 (Fig.2A) or Gβ5 (Fig. 2B) alone or in combination with cDNAs coding for Gγ2–Gγ4. As seen with Gβ1-expressing neurons, expression of either Gβ2 or Gβ5 in the absence of concurrent Gγ expression produced no significant alteration in either basal facilitation ratio or NE-mediated inhibition of Ca2+ currents when compared with uninjected neurons (from the same neuronal preparations). Coexpression of Gβ2 with Gγ subunits, however, resulted in significantly enhanced basal facilitation ratio, decreased NE-mediated inhibition, and obvious kinetic slowing in the absence of agonist (Fig.2A, inset). Conversely, coexpression of Gβ5 with various Gγ subunits failed to produce significant increases in basal facilitation, although small decreases in NE-mediated Ca2+ current inhibition were observed. Increasing the concentration of injected Gβ5 and Gγ2 cDNA to 100 ng/μl per subunit, however, resulted in significant modulation, yet not when expressed alone. Under these conditions, basal facilitation ratios for control and Gβ5- and Gβ5γ2-expressing neurons were 1.23 ± 0.04 (n = 7), 1.19 ± 0.04 (n = 5), and 1.82 ± 0.12 (n= 12; p < 0.05), respectively (data not shown) (Ikeda, 1996).
Fig. 2.
Effect of heterologous overexpression of Gβ2 and β5 alone or with Gγ2, Gγ3, and Gγ4 on facilitation and NE-mediated inhibition of Ca2+ currents.A, B, Summary graphs of mean ± SEM basal facilitation and Ca2+ current inhibition for neurons expressing either β2 or β5 alone and combined with several γ subunits. Final concentration of cDNA injected was 10 ng/μl per subunit. Basal facilitation and Ca2+ current inhibition were calculated as described in Figure1E. Note that scales for both parameters are the same. Numbers in parentheses indicate the number of experiments. Insets show superimposed current traces evoked with the double-pulse voltage protocol (illustrated in Fig. 1D) in the absence or presence of 10 μm NE for β2γ4- and β5γ2-expressing neurons. *p < 0.05 versus control; **p < 0.01 versus control.
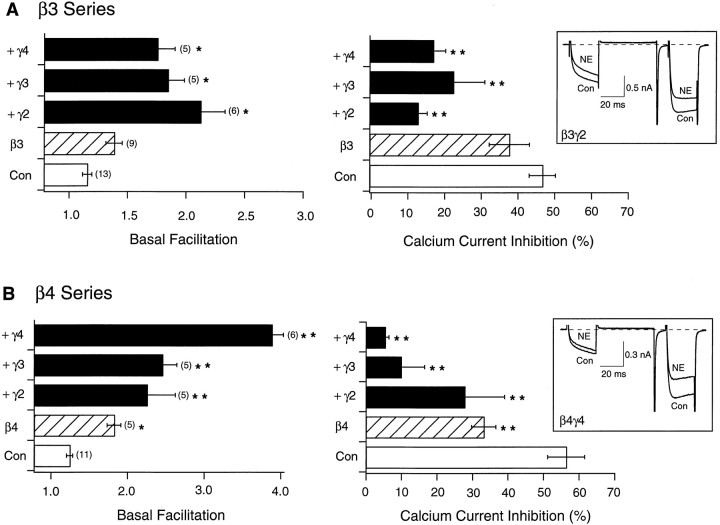
The effects of expressing Gβ3 or Gβ4 alone or together with Gγ2–Gγ4 are summarized in Figure 3. As with the previously tested Gβ subunits, expression of Gβ3 produced significant alterations in basal facilitation ratio and NE-mediated inhibition of Ca2+ current only when coexpressed with a Gγ subunit (Fig. 3A). Conversely, injection of Gβ4 cDNA resulted in a significant increase in basal facilitation ratio and attenuation of NE-mediated inhibition without concurrent injection of Gγ cDNA. Coexpression of Gβ4 with Gγ subunits increased the basal facilitation ratio, an effect especially apparent with Gγ4 (p < 0.01). In fact, the tonic inhibition produced by Gβ4γ4 was the most potent observed in this study as indicated by the large basal facilitation ratio (∼4) and greatly attenuated NE-mediated inhibition (<10%). Expression of Gβ3 or Gβ4 with Gγ subunits produced characteristic kinetic slowing of the Ca2+ current (Fig.3A,B, insets, respectively).
Fig. 3.
Effect of heterologous overexpression of Gβ3 and Gβ4 alone or with Gγ2, Gγ3, and Gγ4 on facilitation and NE-mediated inhibition of Ca2+ currents.A, B, Summary graphs of mean ± SEM basal facilitation and Ca2+ current inhibition for neurons expressing either β3 or β4 alone and combined with several γ subunits. Final concentration of cDNA injected was 10 ng/μl per subunit. Basal facilitation and Ca2+ current inhibition were calculated as described in Figure1E. Note that scales for basal facilitation are different. Numbers in parentheses indicate the number of experiments. Insets show superimposed current traces evoked with the double-pulse voltage protocol (illustrated in Fig.1D) in the absence or presence of 10 μm NE for β3γ2- and β4γ4-expressing neurons. *p < 0.05 versus control; **p< 0.01 versus control.
Together, these results suggest that Gβ1–Gβ5, in combination with various Gγ subunits, were capable of producing VD modulation of N-type Ca2+ channels. With the exception of Gβ4, coexpression of a Gβ together with a Gγ subunit was required to produce significant effects. At the usual concentration of injected cDNA (10 ng/μl) used in this study, expression of Gβ5, alone or together with Gγ subunits, produced minimal effects. These results are in agreement with some previously reported results (Ikeda, 1996; Delmas et al., 1998) but discrepant in regard to other studies (Herlitze et al., 1996; Garcia et al., 1998). At present, the reason for this discrepancy is unclear. The results are especially puzzling because the preparation used in each of these studies was similar (rat sympathetic neurons).
Does heterologously expressed Gβγ displace native Gβγ?
Meaningful interpretation of the experimental results presented thus far relies on the tacit assumption that heterologously expressed Gβγ were directly responsible for the observed changes in Ca2+ channel properties. The fact that most of the Gβγ combinations tested produced VD inhibition prompted us to investigate a possible alternative interpretation of the data. It was hypothesized that heterologously expressed Gβγ could displace native Gβγ from the G-protein heterotrimer as a result of basal G-protein activation. Under this scenario, the displaced “free” native Gβγ would interact with N-type Ca2+ channels and produce VD inhibition thus leading to interpretive difficulties.
In the absence of overt GPCR stimulation, there appears to be a low level of baseline G-protein activation in SCG neurons. This assumption is based on two previous experimental findings. First, introduction on nonhydrolyzable GTP analogs in SCG neurons (via the patch pipette) results in spontaneous VD inhibition (Ikeda and Schofield, 1989; Ikeda, 1996; Jeong and Ikeda, 1999). Second, a small amount of tonic VD inhibition, as indicated by basal facilitation ratio >1, has been documented in SCG neurons (Ikeda, 1991).
To address the issue of displacement, residues on Gβ were mutated with the goal of imparting properties that would differentiate the actions of heterologously expressed mutant Gβγ from natively expressed wild-type Gβγ. Two separate sets of mutations were developed based on the crystal structure of Gβγ (Wall et al., 1995;Sondek et al., 1996) and previous studies examining the effect of multiple discrete Gβ mutations on effector interaction (Ford et al., 1998; Li et al., 1998). The goal of the Gβ mutagenesis was twofold. First, we desired a Gβ that interacted poorly (as Gβγ) with Gα yet retained the ability to modulate N-type Ca2+ channels. Second, we desired a Gβ (when combined with Gγ) that would differentially modulate two effectors, namely N-type Ca2+ channels and GIRK-type K+ channels, that could be assayed electrophysiologically. The first set of Gβ mutant constructs consisted of a single residue mutation, I80A, that was introduced into Gβ1 and Gβ4. The second set of Gβ mutant constructs consisted of three separate point mutations in Gβ1 (I80A,N88A,K89A) or Gβ4 (L55A,N88A,K89A). These residues (L55, I80, N88, and K89) were chosen because alanine mutations at these sites also seemed to weaken the interaction with Gα based on ADP ribosylation and immunoprecipitation assays (Ford et al., 1998; Li et al., 1998) but preserved interaction with N-type Ca2+ channels. In addition, alanine mutations of residues L55 and I80 appeared to impair GIRK activation (Ford et al., 1998). It was anticipated that both sets of mutations would possess one or more of the desired properties such that the mutant Gβ would modulate N-type Ca2+channels but interact poorly with GIRK channels and Gα.
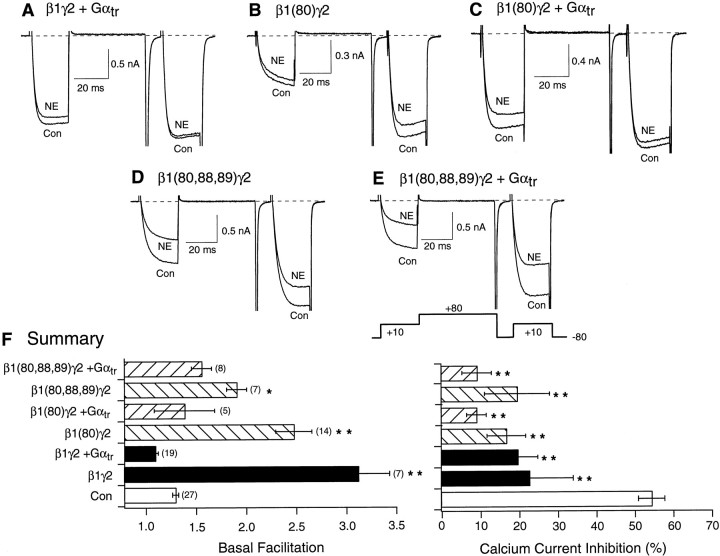
Figure 4 illustrates experiments designed to probe the interaction of heterologously expressed mutant and wild-type Gβ(+Gγ) with a heterologously expressed Gα, transducin (Gαtr). Transducin was chosen as the Gβγ “sink” or buffer because heterotrimers containing Gαtr are thought to couple only to rhodopsin. Expression of Gαtr neutralized the actions of expressed Gβ1γ2 (Fig. 4, compare A, B; Fig.4F, solid bars), consistent with the known high affinity of GDP-bound Gα for Gβγ (Slepak et al., 1995). Expression of either Gβ1(I80A)γ2 (Fig.4B) or Gβ1(I80A,N88A,K89A)γ2 (Fig.4D) resulted in an increased basal facilitation ratio (Fig. 4F, gray bars). Coexpression of Gαtr greatly decreased the basal facilitation resulting from expression of Gβ1(I80A)γ2 (Fig.4C,F, hatched bars) but had a lesser effect on facilitation arising from Gβ1(I80A,N88A,K89A)γ2 expression (Fig. 4E,F,hatched bars). Summary of basal facilitation ratio and NE-mediated Ca2+ current inhibition data for each of these conditions is illustrated in Figure4F. Together, the data suggest that the respective Gβ1 mutants retained the ability to interact with both N-type Ca2+ channels and Gα subunits. In the case of Gβ1(I80A,N88A,K89A), both interactions appeared to be weaker when compared with wild-type Gβ1. However, basal facilitation resulting from this Gβ1 mutant was also attenuated.
Fig. 4.
Effect of heterologous overexpression of mutant Gβ1 and Gαtr on basal facilitation and NE-mediated Ca2+ current inhibition. Superimposed Ca2+ current traces evoke with the double-pulse voltage protocol (bottom of E) in the absence (bottom traces) and presence (top traces) of 10 μm NE for wild-type β1γ2- and Gαtr- (A), β1(80)γ2- (B), β1(80)γ2- and Gαtr- (C), β1(80,88,89)γ2- (D), and β1(80,88,89)γ2 and Gαtr-expressing (E) neurons.F, Summary graphs of mean ± SEM basal facilitation and Ca2+ current inhibition for neurons expressing wild-type and mutant Gβ1γ2 alone or combined with Gαtr. Final concentration of cDNA injected was 10 ng/μl per subunit. Basal facilitation and Ca2+ current inhibition were calculated as described in Figure1E. *p < 0.05 versus control; **p < 0.01 versus control.Numbers in parentheses indicate the number of experiments.
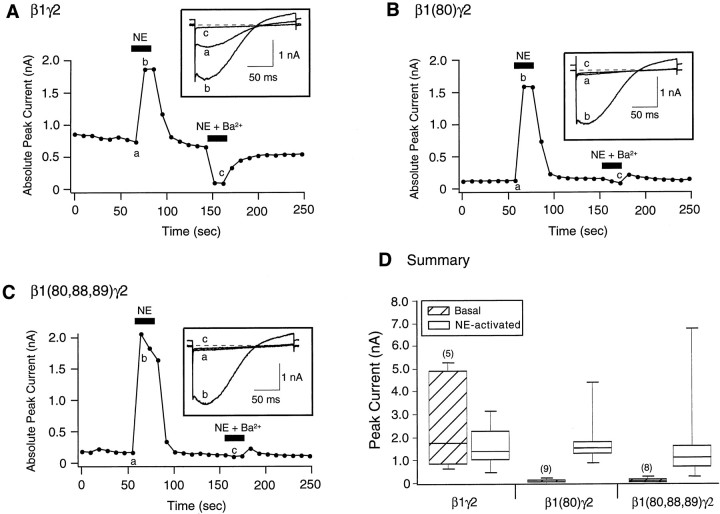
Figure 5 depicts experiments designed to evaluate whether mutant Gβ1 subunits modulate GIRK-type K+ channels. GIRK-type K+ channels are inwardly rectifying channels that are gated by Gβγ binding (Logothetis et al., 1987;Wickman et al., 1994). The rat SCG neurons used in this study do not express native GIRK-type channels. However, functional GIRK channels can be heterologously expressed in SCG neurons (Ruiz-Velasco and Ikeda, 1998; Fernandez-Fernandez et al., 1999), thereby providing a second effector to evaluate Gβγ actions (Wickman and Clapham, 1995; Jan and Jan 1997). Heteromultimeric GIRK1 (Kir 3.1) and GIRK4 (Kir 3.4) channels were expressed in SCG neurons as described previously (Ruiz-Velasco and Ikeda, 1998). GIRK currents were elicited at 0.1 Hz from a holding potential of −60 mV in solutions (see Materials and Methods) designed to support K+ currents. Current amplitude was determined from the peak inward current occurring during a 200 msec voltage ramp from −140 to −40 mV. Figure5A shows GIRK current amplitude as a function of time for a β1γ2-expressing neuron. In the absence of NE, there was a standing inwardly rectifying current (Fig. 5A, inset a) of ∼0.75 nA. Application of NE (10 μm;solid bar) induced an additional 1 nA of inward GIRK current (Fig. 5A, inset b) which reversed after removal of agonist. Application of Ba2+ (1 mm; solid bar), an efficient blocker of GIRK channels, rapidly and reversibly reduced the current to near zero (Fig. 5A, inset c). Similar experiments for Gβ1(I80A)γ2- and Gβ1(I80A,N88A,K89A)γ2-expressing neurons are shown in Figure 5, B and C, respectively. Neither Gβ1 mutant was capable of activating significant GIRK current, as indicated by the low current amplitude, lack of inward rectification in the current trace (Fig.5B,C, inset a), and absence of current inhibition during Ba2+application. However, GIRK currents were still activated after application of NE. Figure 5D summarizes the basal and NE-mediated GIRK current amplitude for Gβ1γ2-, Gβ1(I80A)γ2-, and Gβ1(I80A,N88A,K89A)γ2-expressing neurons. Because of the large scatter in NE-induced GIRK currents (0.2 to 8.8 nA), box plots depicting the 10th, 25th, 50th (median), 75th, and 90th percentiles of the data are shown. The summary data indicate that expression of either Gβ1 mutant (with Gγ2) did not result in the basal activation of GIRK channels as seen with wild-type Gβ1-expressing neurons. However, NE-mediated GIRK current activation, presumably arising from the actions of natively expressed Gβγ, was similar for all three conditions. It has been shown in cardiac myocytes that intracellular Cl−slows the turn-off reaction of GIRK channels leading to a higher sensitivity of GIRK channels to GTP (Nakajima et al., 1992). Unlike Nakajima et al. (1992), in the present study receptor coupling was bypassed such that overexpression of wild-type Gβγ subunits led to basal activation of GIRK channels (Fig.5A,D; see Fig.7A,D). Thus, it is unlikely that the absence of basal GIRK activity in neurons expressing mutant Gβγ subunits was a result of a direct influence of this anion on GIRK channels. Together, these data do not support displacement of endogenous Gβγ by heterologously expressed Gβγ.
Fig. 5.
Effect of heterologous overexpression of wild-type and mutant Gβ1 on GIRK channel activation. Time course of basal and NE-activated GIRK1 and GIRK4 channel currents in β1γ2- (A), β1(80)γ2- (B), and β1(80,88,89)γ2-expressing neurons. Currents were evoked by 200 msec voltage ramps from −140 to −40 mV from a holding potential of −60 mV applied every 10 sec. Filled bars indicate application of 10 μm NE or 1 mm Ba2+and 10 μm NE. Insets show current traces obtained before (a) and after (b) application of NE or NE plus Ba2+ (c). D, Box plot showing the 10th, 25th, 50th (median), 75th, and 90th percentiles of peak GIRK currents before (Basal) and after (NE-activated) external application of 10 μm NE. Both the 10th and 90th percentiles are denoted by shorter lines. Numbers in parentheses indicate the number of experiments.
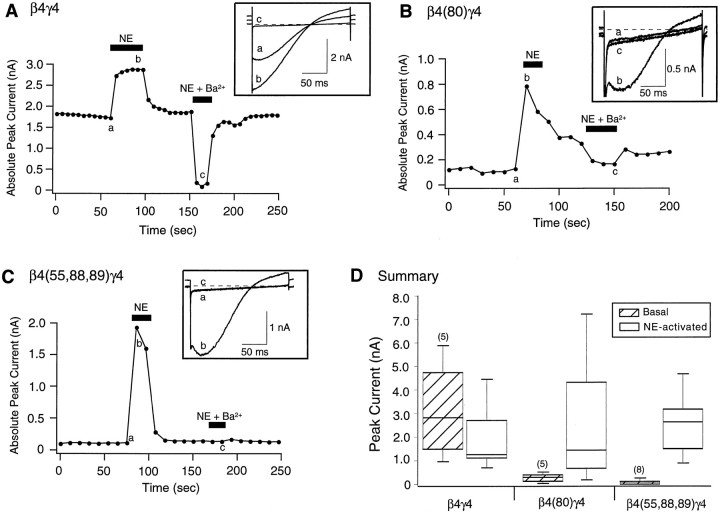
Fig. 7.
Effect of heterologous overexpression of wild-type and mutant Gβ4 on GIRK channel activation. Time course of basal and NE-activated GIRK1 and GIRK4 channel currents in wild-type β4γ4- (A), β4(80)γ4- (B), and β4(55,88,89)γ4-expressing neurons. Currents were evoked by 200 msec voltage ramps from −140 to −40 mV from a holding potential of −60 mV applied every 10 sec. Filled bars indicate application of 10 μm NE or 1 mm Ba2+ and 10 μm NE.Insets show current traces obtained before (a) and after (b) application of NE or NE plus Ba2+(c). D, Box plot showing the 10th, 25th, 50th (median), 75th, and 90th percentiles of peak GIRK currents before (Basal) and after (NE-activated) external application of 10 μm NE. Both the 10th and 90th percentiles are denoted byshorter lines. Numbers in parentheses indicate the number of experiments.
Parallel studies on Gβ4
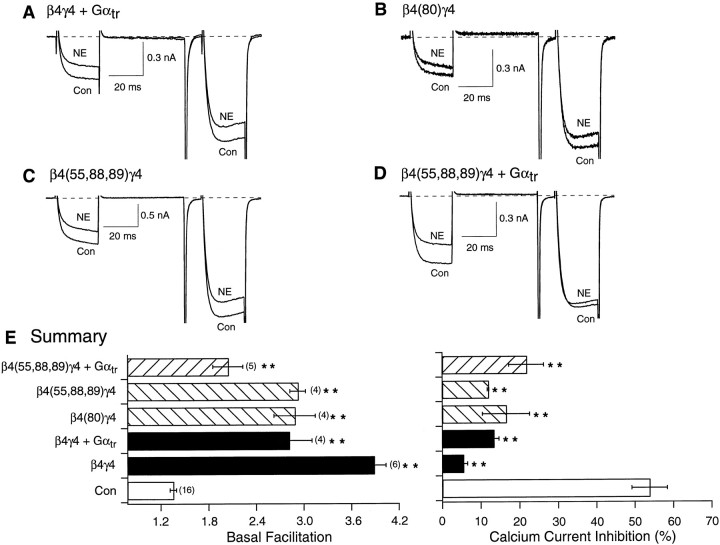
While this work was in progress, a similar study was published byGarcia et al. (1998) in which expression of Gβ3 or Gβ4 (alone or together with Gγ) was reported to produce negligible affects on N-type Ca2+ channels of rat SCG neurons. Because in the current study expression of β4γ4 resulted in the greatest modulatory effect on Ca2+currents (Fig. 3B), we undertook additional studies to further validate our results. Figure6A shows Ca2+ current traces from a neuron expressing β4γ4 and Gαtr in the absence and presence of NE. In contrast to analogous experiments performed with Gβ1, expression of Gαtr was unable to ablate the Gβ4γ4–mediated effects as evidenced by the significant residual basal facilitation (Fig. 6E, solid bars). Whether this differential effect arises from factors innate to the interaction between the various subunits or differences in expression levels remains to be determined. Expression of the Gβ4 mutants β4(I80A) and β4(L55A,N88A,K89A), concurrently with Gγ4, produced large increases in basal facilitation (Fig.6B,C, gray bars). Similar to wild-type Gβ4γ4-expressing neurons, coexpression of Gαtr reduced, but did not eliminate, basal facilitation resulting from expression of β4(L55A,N88A,K89A)γ4 (Fig.6D,E, hatched bars).
Fig. 6.
Effect of heterologous overexpression of mutant Gβ4 and Gαtr on basal facilitation and NE-mediated Ca2+ current inhibition. Superimposed Ca2+ current traces evoked with the double-pulse voltage protocol (shown in Fig. 4E) in the absence (bottom traces) and presence (top traces) of 10 μm NE for wild-type β4γ4 and Gαtr- (A), β4(80)γ4- (B), β4(55,88,89)γ4 (C), and β4(55,88,89)γ4 and Gαtr-expressing (D) neurons.E, Summary graphs of mean ± SEM basal facilitation and Ca2+ current inhibition for neurons expressing wild-type and mutant Gβ4γ4 alone or combined with Gαtr. Final concentration of cDNA injected was 10 ng/μl per subunit. Basal facilitation and Ca2+ current inhibition were calculated as described in Figure1E. **p < 0.01 versus control. Numbers in parentheses indicate the number of experiments.
Figure 7 shows the effects of expressing wild-type and mutant Gβ4 (along with Gγ4) on GIRK channels expressed in SCG neurons. As observed for β1γ2, expression of β4γ4 resulted in significant basal GIRK channel activation (Fig.7A,D) as indicated by the large inwardly rectifying current present in the absence of agonist (Fig.7A, inset a) and the large block of current after Ba2+ exposure (Fig. 7A,inset c). Application of NE resulted in the recruitment of additional GIRK current (Fig. 7A, inset b). Conversely, expression of either β4(I80A)γ4 or β4(L55A,N88A,K89A)γ4 failed to activate GIRK channels as exemplified by the lack of significant current in the absence of NE and the minimal effect of Ba2+ application. Application of NE, however, produced large increases in GIRK current, verifying the successful expression of the channels. The data also indicate that Gβ4 containing Gβγ were capable of activating GIRK-type K+ channels. Together, these data strengthen the argument that heterologously expressed Gβγ do not significantly displace native Gβγ. Consequently, the VD Ca2+ channel modulation produced by expression of Gβ4 likely arose from direct actions of the expressed proteins.
DISCUSSION
Three main conclusions can be drawn from the results. First, heterologous expression of Gβ together with Gγ are required for optimal modulation of N-type Ca2+channels. Second, all five known Gβ subunits, when coexpressed with various Gγ subunits, are capable of producing VD inhibition of N-type Ca2+ channels. Third, heterologous expression of Gβγ does not result in significant displacement of native Gβγ from heterotrimeric complexes.
Coordinated expression of Gβ and Gγ results in optimal modulation
Four of the five Gβ subunits tested produced no significant alteration in basal facilitation ratio when expressed alone. In addition, expression of several Gγ subunits in isolation produced little effect. The exception to this finding, Gβ4, significantly enhanced basal facilitation, even in the absence of concurrent Gγ expression. In all cases, however, it was clear that coinjection of cDNAs coding for both subunits resulted in a much greater modulation of Ca2+ channels when compared with neurons expressing only a single component of Gβγ dimer. It should be pointed out that, although Gβ and Gγ are transcribed from separate genes, the expressed proteins likely assemble into a functional monomer. In vitro studies have demonstrated that strong denaturants are required for Gβγ dissociation once assembly has taken place (Schmidt and Neer, 1991). Because Gγ subunits appear to be required for proper folding of the Gβ subunit (Clapham and Neer, 1997), it seems unlikely that “unpartnered” Gβ would possess significant physiological function. The modest effects produced by expression of either Gβ or Gγ alone can be ascribed to pairing with a natively expressed cognate subunit to form functional Gβγ dimers.
The apparent pairing of Gβ3 with several different Gγ subunits (Fig. 4) requires comment. Based on a tryptic digestion assay, Ray et al. (1995) inferred that Gβ3 failed to form dimers with several Gγ subunits, including some used in this study. However, a recent report from the same laboratory (Richardson and Robishaw, 1999) demonstrated that Gβ3 isolated from Sf9 insect cells formed functional dimers with Gγ4, Gγ5, and Gγ11 in vitro. Hence, the determination as to whether various Gβγ combinations form functional dimers relies on the assay used.
Multiple Gβγ combinations produce VD Ca2+channel inhibition
Our results suggest that Gβ1–Gβ5, when coexpressed with several different Gγ subunits, are capable of producing VD inhibition of N-type Ca2+ channels. In general, expression of Gβ1–Gβ4 with Gγ produced qualitatively similar effects. Basal facilitation ratios increased from ∼1.3 in uninjected cells to near 2–3 in Gβγ-expressing neurons. In all cases, NE-mediated Ca2+ channel inhibition was occluded, although to varying degrees. Given the high degree of sequence homology shared among Gβ1–Gβ4 (∼80%), the results were not surprising. Although minor quantitative differences were noted after expression of Gβ1–Gβ4, the absence of a method for quantifying expressed protein levels precludes interpretation of these differences.
In two cases, however, the magnitude of difference in basal facilitation ratios was deserving of comment. First, expression of β5 with Gγ, at the standard cDNA concentration (10 ng/μl), clearly produced the weakest effects (Fig. 2B). In fact, the concentration of cDNA injected had to be increased 10-fold to obtain statistically significant results (see Results). Although this difference in apparent “potency” could arise from differences in protein expression levels, it should be noted that Gβ5 appears to be unique among the Gβ family in several ways: (1) Gβ5 shares only 53% homology with β1–β4 (Yan et al., 1996; Clapham and Neer, 1997); (2) Gβ5-containing Gβγ subunits form heterotrimers only with members of the Gq/11 family of Gα subunits (Fletcher et al., 1998); and (3) Gβ5 interacts with members of the regulators of G-protein signaling family that contain a GGL domain (Snow et al., 1998; Makino et al., 1999). Given these unique properties, we speculate that the weak effects of Gβ5 arise from factors inherent to this molecule.
In contrast to the results obtained with Gβ5, expression of Gβ4γ4 resulted in an unusually large basal facilitation (Fig.3B). This observation seemed significant for two reasons. First, of the limited Gβγ combinations tested in this study, expression of Gβ4γ4 represented the clearest case in which the contribution of a Gγ seemed to make a significant difference in regard to basal facilitation. The increase in basal facilitation produced by pairing Gβ4 with Gγ4 cannot be ascribed solely to differences in expression levels because coexpression of Gγ4 did not greatly impact the effects of other Gβ subunits. Thus, the identity of the Gγ component may influence the relative potency of a given Gβγ subunit. Given this finding, the interpretation of Gβ potency should probably be framed within the context of the particular Gγ paired with the Gβ. Second, although expression of Gβ4γ4 resulted in the largest basal facilitation ratio observed in this study, another study reported that expression of Gβ4 did not produce significant effects (Garcia et al., 1998). Some possibilities for this discrepancy are discussed below.
While our work was in progress, the aforementioned group published a similarly designed study that addressed questions identical to those posed here. Although the results of both studies are comparable in several aspects, two observations do not appear immediately reconcilable. Garcia et al. (1998) found that (1) coexpression of Gγ did not enhance Gβ effects and (2) expression of Gβ3 or Gβ4 (with and without Gγ) did not significantly modulate N-type Ca2+ channels. These data meshed well with yeast two-hybrid data (presented in the same manuscript) demonstrating that Gβ3 and Gβ4, in contrast to Gβ1, Gβ2, and Gβ5, failed to interact with the domain I-II linker of Ca2+ channel α1B subunits. It should be pointed out, however, that additional Gβγ interaction domains on Ca2+ channel α1 subunits have been identified, including regions on the N and C termini (Zhang et al., 1996; Qin et al., 1997; Page et al., 1998) (for review, see Dolphin, 1998). Therefore, the absence of protein–protein interaction between Gβ3 or Gβ4 and the domain I-II linker region does not preclude the possibility that, under in situ conditions, multiple regions combine to form a high-affinity binding “pocket” for Gβγ (Yamada et al., 1998). Because a nearly identical system was used byGarcia et al. (1998) and the present work, plausible explanations accounting for such large discrepancies are limited. It should be noted that the original Gβ4 cDNA clone (M. I. Simon, California Institute of Technology, Pasadena, CA) that we obtained lacked a start codon, presumably as a result of a spurious mutation that occurred during propagation of the plasmid. Positive results with Gβ4 were obtained only after inserting a “new” start codon into the clone using the PCR. This same clone was used by Garcia et al. (1998) (B. Hille, personal communication) and likely accounts for the lack of channel modulation seen in this study. In regard to the Gβ3 results, the level of protein expression may account for discrepant results.
The effects of heterologously expressed Gβγ do not arise from displacement
tk;2A potential factor confounding meaningful interpretation of our data was the notion that heterologous Gβγ might, during basal Gα GDP–GTP exchange, displace native Gβγ from heterotrimeric complexes. To examine this possibility, two strategies based on Gβ mutagenesis were pursued. First, we sought to develop a Gβ that would not complex with Gα–GDP but would retain the ability to modulate N-type Ca2+ channels. The lack of Gα interaction would render the “displacement hypothesis” moot, thereby simplifying data interpretation. Unfortunately, none of these mutations appeared to completely eliminate Gα interaction based on the ability of heterologously expressed Gαtr to reverse the effects of Gβγ expression on basal facilitation ratio (Figs. 4F, 6F). A second strategy to investigate displacement was based on the idea of distinguishing the effects of heterologously expressed Gβγ from native Gβγ by examining differential effector interactions. GIRK-type K+ channels have been extensively studied in regard to activation by Gβγ (Wickman and Clapham, 1995). We and others (Ruiz-Velasco and Ikeda, 1998; Fernandez-Fernandez et al., 1999) have demonstrated that functional GIRK-type K+ channels can be heterologously expressed in SCG neurons, thus providing a second Gβγ “detector” in these neurons. As exemplified by the Gβ1(I80A)γ2 data, this strategy appeared to achieve our goals. Both the single (I80A) and triple (I80A, N88A, and K89A) mutations ablated tonic GIRK activation (Fig. 5) but retained the ability to induce Ca2+ channel facilitation (Fig. 4). Moreover, NE-mediated GIRK activation remained intact in the Gβ mutant expressing neurons, thus suggesting that (1) native Gβγ was associated with heterotrimeric complexes, i.e., not displaced, and (2) the mutant Gβγ did not block GIRK activation. Similar results with Gβ4 confirmed that these findings were not restricted to a single Gβ subtype. In this regard, a yeast two-hybrid study, analogous to the one mentioned above performed on Ca2+channel domains, suggested that only Gβ1 and Gβ2 interacted with the β1A domain on GIRK1 (Yan and Gautam, 1996). Hence, protein–protein interactions targeted at single domains may not be predicative of functional channel interactions in situ. Together, these data render the notion of Gβγ displacement untenable and strengthen the argument that heterologously expressed Gβγ directly influence N-type Ca2+channel function.
Footnotes
This work was supported by National Institutes of Health Grant GM 56180 (S.R.I.) with a research supplement for V.R.-V. We thank Marina King for excellent technical assistance. We are grateful to the following for providing cDNA clones: Drs. N. Gautam (Washington University, St. Louis, MO), D. E. Logothetis (Mount Sinai School of Medicine, New York, NY), and M. I. Simon (California Institute of Technology, Pasadena, CA).
Correspondence should be addressed to Dr. Stephen R. Ikeda, Laboratory of Molecular Physiology, Guthrie Research Institute, One Guthrie Square, Sayre, PA 18840. E-mail: sikeda@inet.guthrie.org.
REFERENCES
- 1.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 2.Canti C, Page KM, Stephens GJ, Dolphin AC. Identification of residues in the N terminus of α1B critical for inhibition of the voltage-dependent calcium channel by Gβγ. J Neurosci. 1999;19:6855–6864. doi: 10.1523/JNEUROSCI.19-16-06855.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clapham DE, Neer EJ. G Protein βγ Subunits. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 4.De Waard M, Liu H, Walker D, Scott VES, Gurnett CA, Campbell KP. Direct binding of G-protein βγ complex to voltage-dependent calcium channels. Nature. 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 5.Delmas P, Brown DA, Dayrell M, Abogadie FC, Caulfield MP, Buckley NJ. On the role of endogenous G-protein βγ subunits in N-type Ca2+ current inhibition by neurotransmitters in rat sympathetic neurones. J Physiol (Lond) 1998;506:319–329. doi: 10.1111/j.1469-7793.1998.319bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolphin AC. Mechanisms of modulation of voltage-dependent calcium channels by G proteins. J Physiol (Lond) 1998;50:3–11. doi: 10.1111/j.1469-7793.1998.003bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 8.Elmslie KS, Zhou W, Jones SW. LHRH and GTP-γ-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990;5:75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Fernandez JM, Wanaverbecq N, Halley P, Caulfield MP, Brown DA. Selective activation of heterologously expressed G protein-gated K+ channels by M2 muscarinic receptors in rat sympathetic neurones. J Physiol (Lond) 1999;515:631–637. doi: 10.1111/j.1469-7793.1999.631ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher JE, Lindorfer MA, DeFilippo JM, Yasuda H, Guilmard M, Garrison JC. The G protein β5 subunit interacts selectively with the Gqα subunit. J Biol Chem. 1998;273:636–644. doi: 10.1074/jbc.273.1.636. [DOI] [PubMed] [Google Scholar]
- 11.Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shekter LR, Rosal R, Weng G, Yang CS, Iyengar R, Miller RJ, Jan LY, Lefkowitz RJ, Hamm HE. Molecular basis for interactions of G protein βγ subunits with effectors. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa T, Miura R, Mori Y, Strobeck M, Suzuki K, Ogihara Y, Asano T, Morishita R, Hashii M, Higashida H, Yoshii M, Nukada T. Differential interactions of the C terminus and the cytoplasmic I-II loop of neuronal Ca2+ channels with G-protein α and βγ subunits. II. Evidence for direct binding. J Biol Chem. 1998;273:17595–17603. doi: 10.1074/jbc.273.28.17595. [DOI] [PubMed] [Google Scholar]
- 13.Garcia DE, Li B, Garcia-Ferreiro RE, Hernandez-Ochoa EO, Yan K, Gautam N, Catterall WA, Mackie K, Hille B. G-protein β-subunit specificity in the fast membrane-delimited inhibition of Ca2+ channels. J Neurosci. 1998;18:9163–9170. doi: 10.1523/JNEUROSCI.18-22-09163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cell and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 15.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 16.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda SR. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol (Lond) 1991;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda SR. Heterologous expression of receptors and signaling proteins in adult mammalian sympathetic neurons by microinjection. In: Challis RA, editor. Methods in Molecular Biology. Humana; Totowa, NJ: 1997. pp. 191–202. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda SR, Dunlap K. Voltage-dependent modulation of N-type calcium channels: role of G protein subunits. Adv Second Messenger Phosphoprotein Res. 1999;33:131–151. doi: 10.1016/s1040-7952(99)80008-1. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda SR, Schofield GG. Somatostatin blocks a calcium current in rat sympathetic ganglion neurones. J Physiol (Lond) 1989;409:221–240. doi: 10.1113/jphysiol.1989.sp017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jan LY, Jan YN. Voltage-gated and inwardly rectifying potassium channels. J Physiol (Lond) 1997;505:267–282. doi: 10.1111/j.1469-7793.1997.267bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong SW, Ikeda SR. Sequestration of G-protein βγ subunits by different G-protein α subunits blocks voltage-dependent modulation of Ca2+ channels in rat sympathetic neurons. J Neurosci. 1999;19:4755–4761. doi: 10.1523/JNEUROSCI.19-12-04755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Sternweis PM, Charnecki S, Smith TF, Gilman AG, Neer EJ, Kozasa T. Sites for Gα binding on the G protein β subunit overlap with sites for regulation of phospholipase Cβ and adenylyl cyclase. J Biol Chem. 1998;273:16265–16272. doi: 10.1074/jbc.273.26.16265. [DOI] [PubMed] [Google Scholar]
- 25.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 26.Makino ER, Handy JW, Li T, Arshavsky VY. The GTPase activating factor for transducin in rod photoreceptors is the complex between RGS9 and type 5 G protein β subunit. Proc Natl Acad Sci USA. 1999;96:1947–1952. doi: 10.1073/pnas.96.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima T, Sugimoto T, Kurachi Y. Effect of anions on the G protein-mediated activation of the muscarinic K+ channel in the cardiac atrial cell membrane. Intracellular chloride inhibition of the GTPase activity of GK. J Gen Physiol. 1992;99:665–682. doi: 10.1085/jgp.99.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page KM, Canti C, Stephens GJ, Berrow NS, Dolphin AC. Identification of the amino terminus of neuronal Ca2+ channel α1 subunits α1B and α1E as an essential determinant of G-protein modulation. J Neurosci. 1998;18:4815–4824. doi: 10.1523/JNEUROSCI.18-13-04815.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Direct interaction of Gβγ with a C-terminal Gβγ-binding domain of the Ca2+ channel α1 subunit is responsible for channel inhibition by G protein-coupled receptors. Proc Natl Acad Sci USA. 1997;94:8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray K, Kunsch C, Bonner LM, Robishaw JD. Isolation of cDNA clones encoding eight different human G protein γ subunits including three novel forms designated γ4, γ10, and γ11 subunits. J Biol Chem. 1995;270:21765–21771. doi: 10.1074/jbc.270.37.21765. [DOI] [PubMed] [Google Scholar]
- 31.Richardson M, Robishaw JD. The α2A-adrenergic receptor discriminates between Gi heterotrimers of different βγ subunit composition in Sf9 insect cell membranes. J Biol Chem. 1999;274:13525–13533. doi: 10.1074/jbc.274.19.13525. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Velasco V, Ikeda SR. Heterologous expression and coupling of G protein-gated inwardly rectifying K+ channels in adult rat sympathetic neurons. J Physiol (Lond) 1998;513:761–773. doi: 10.1111/j.1469-7793.1998.761ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt CJ, Neer EJ. In vitro synthesis of G protein βγ dimers. J Biol Chem. 1991;266:4538–4544. [PubMed] [Google Scholar]
- 34.Schofield GG. Norepinephrine blocks a calcium current of adult rat sympathetic neurons via an α2-adrenoceptor. Eur J Pharmacol. 1990;180:37–47. doi: 10.1016/0014-2999(90)90590-3. [DOI] [PubMed] [Google Scholar]
- 35.Slepak VZ, Katz A, Simon MI. Functional analysis of a dominant negative mutant of Gαi2. J Biol Chem. 1995;270:4037–4041. doi: 10.1074/jbc.270.8.4037. [DOI] [PubMed] [Google Scholar]
- 36.Snow BE, Krumins AM, Brothers GM, Lee SF, Wall MA, Chung S, Mangion J, Arya S, Gilman AG, Siderovski DP. A G protein γ subunit-like domain shared between RGS11 and other RGS proteins specifies binding to Gβ5 subunits. Proc Natl Acad Sci USA 1998. 1998;95:13307–13312. doi: 10.1073/pnas.95.22.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB. Crystal structure of a G-protein βγ dimer at 2.1A resolution. Nature. 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- 38.Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. The structure of the G protein heterotrimer Giα1β1γ2. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 39.Watson S, Arkinstall S. The G-protein linked receptor facts book. Academic; San Diego: 1994. G protein γ subunits. pp. 350–355. [Google Scholar]
- 40.Wickman K, Clapham DE. Ion channel regulation by G proteins. Physiol Rev. 1995;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- 41.Wickman KD, Iniguez-Lluhi JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein βγ-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994;368:255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- 42.Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacol Rev. 1998;50:723–760. [PubMed] [Google Scholar]
- 43.Yan K, Gautam N. A domain on the G protein β subunit interacts with both adenylyl cyclase 2 and the muscarinic atrial potassium channel. J Biol Chem. 1996;271:17597–17600. doi: 10.1074/jbc.271.30.17597. [DOI] [PubMed] [Google Scholar]
- 44.Yan K, Kalyanaraman V, Gautam N. Differential ability to form the G protein βγ complex among members of the β and γ subunit families. J Biol Chem. 1996;271:7141–7146. doi: 10.1074/jbc.271.12.7141. [DOI] [PubMed] [Google Scholar]
- 45.Zamponi GW, Snutch TP. Modulation of voltage-dependent calcium channels by G proteins. Curr Opin Neurobiol. 1998;8:351–356. doi: 10.1016/s0959-4388(98)80060-3. [DOI] [PubMed] [Google Scholar]
- 46.Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature. 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- 47.Zhang JF, Ellinor PT, Aldrich RW, Tsien RW. Multiple structural elements in voltage-dependent Ca2+ channels support their inhibition by G proteins. Neuron. 1996;17:991–1003. doi: 10.1016/s0896-6273(00)80229-9. [DOI] [PubMed] [Google Scholar]