Abstract
GABAA receptor α1 and α2 subunits are expressed differentially with ontogenic period in the brain, but their functional roles are not known. We have recorded GABAAreceptor-mediated IPSCs from laterodorsal (LD) thalamic relay neurons in slices of rat brain at various postnatal ages and found that decay times of evoked IPSCs and spontaneous miniature IPSCs undergo progressive shortening during the first postnatal month. With a similar time course, expression of transcripts and proteins of GABAA receptor α2 subunit in LD thalamic region declined, being replaced by those of α1 subunit. To further address the causal relationship between α subunits and IPSC decay time kinetics, we have overexpressed GABAA receptor α1 subunit together with green fluorescent protein in LD thalamic neurons in organotypic culture using recombinant Sindbis virus vectors. Miniature IPSCs recorded from the LD thalamic neurons overexpressed with α1 subunit had significantly faster decay time compared with control expressed with β-galactosidase. We conclude that the α2-to-α1 subunit switch underlies the developmental speeding in the decay time of GABAergic IPSCs.
Keywords: GABAA receptor, α subunit, thalamus, IPSC, postnatal development, patch clamp, Sindbis virus vector
The thalamocortical tract is the principal pathway transferring sensory information from the periphery to cerebral cortex. Thalamic sensory nuclei send axonal projections to neocortex and receive feedback projections from the same cortical area. Thalamocortical and corticothalamic axons also send collaterals to the reticular thalamic nucleus (RTN), a GABAergic nucleus surrounding the thalamus (Houser et al., 1980), which provides feedback inhibition to thalamic relay neurons thereby playing a pacemaker role for coordination and modulation of thalamocortical rhythms (Steriade and Llinas, 1988).
GABAA receptors are composed of five subunits, each deriving from one of seven identified families: α1–6, β1–3, γ1–3, δ, ε, π, and the recently cloned θ (Bonnert et al., 1999). The majority of native receptors are formed by combinations of αβγ or αβδ subunits (McKernan and Whiting, 1996). During postnatal development, the GABAA receptor α2 and α3 subunits are replaced by α1 subunits in thalamus (Laurie et al., 1992b; Fritschy et al., 1994). However, the functional significance of this subunit switch is not known. In recombinant GABAAreceptors expressed in cultured cells, the decay time of current response after a short pulse application of GABA is faster for the receptors containing the α1 subunit compared with those containing the α2 or α3 subunits (Verdoorn 1994; Gingrich et al., 1995; Lavoie and Twyman, 1996). Because the decay of transmitter-induced current response after washout reflects deactivation of underlying channels and its time course shapes the decay time of synaptic currents, it is possible that GABAergic IPSCs in thalamus acquire fast decay time kinetics during development because of the switch of GABAA receptor α subunits. To test this hypothesis, we first made whole-cell recordings of GABAergic IPSCs from laterodorsal (LD) thalamic neurons visually identified in slices at various postnatal ages of rats. Subsequently, we measured the expression of transcripts and proteins of GABAAreceptor α subunits in LD thalamic nucleus region at various postnatal ages. Finally, we overexpressed α1 subunits in LD thalamic neurons in organotypic culture and examined the decay time of IPSCs. Our results indicate that the decay time of GABAergic IPSCs in thalamus undergoes developmental shortening because of the α2-to-α1 subunit switch of GABAA receptor during the first postnatal month.
MATERIALS AND METHODS
Preparation and solutions. Wistar rats, aged postnatal day 4 (P4) to P40, were decapitated under halothane anesthesia. After isolating the thalamus, sagittal slices of 150–200 μm thickness were cut using a tissue slicer (DTK 1000; Dosaka, Kyoto, Japan). Slices were incubated at 36–37°C for 1 hr in a submerged chamber bubbled with 95% O2–5% CO2. After incubation, slices were kept at room temperature in a moist chamber equilibrated with 95% O2–5% CO2. For experiments, slices were transferred into a superfusing chamber on a stage of upright microscope (Axioskop; Zeiss, Oberkochen, Germany), and LD thalamic neurons were viewed under Nomarski optics with a 40× water immersion objective. The superfusing artificial CSF (aCSF) had the following composition (in mm): NaCl 125, KCl 2.5, NaH2PO4 1.26, NaHCO3 26, glucose 15, CaCl2 2.0, MgCl2 1.0, and lactate 5, pH 7.3 when equilibrated with 95% O2–5% CO2. The aCSF routinely contained strychnine (0.5 μm) (Sigma, St. Louis, MO) to exclude possible contamination of glycinergic IPSCs, and d(−)-2-amino-5-phosphonopentanoic acid (AP-5) (25 μm; Tocris Cookson, Bristol, UK) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 μm; Tocris Cookson) to block glutamatergic EPSCs. All experiments were performed at room temperature (24–26°C).
Recordings and data analyses. Whole-cell recordings of spontaneous and evoked IPSCs were made from the LD thalamic neurons using an EPC-7 amplifier (List Biologic, Campbell, CA). The patch pipettes were pulled from borosilicate glass (GC150F-7.5; Clark Electromedical Instruments, Pangbourne, UK) and filled with the internal solution having the following composition (in mm): CsCl 140, NaCl 4, EGTA 5, HEPES 10, MgATP 2, and Na3GTP 0.3, pH 7.3 adjusted with CsOH. The access resistance of whole-cell recording was typically 20–30 MΩ, and when it exceeded 40 MΩ, these data were discarded. GABAergic IPSCs were evoked by stimulating RTN with a suprathreshold intensity, 300–700 μm away from LD neurons, with a pipette (3–5 μm in tip diameter) filled with 1 m NaCl. Virtually all neurons in RTN are GABAergic (Houser et al., 1980). IPSCs were recorded from LD neurons at the holding potential of −70 mV under voltage clamp. Spontaneous miniature IPSCs were recorded after blocking action potentials with tetrodotoxin (TTX) (1 μm;Sankyo, Tokyo, Japan). The membrane potential was not corrected for the liquid junctional potential between pipette solution and superfusates (+3 mV). Records were low-pass filtered at 5 kHz, digitized at 10 kHz (Dagan LM-12S interface; Dagan Instruments, Minneapolis, MN), and analyzed on a personal computer (Dell 466/LV; Dell Computer Corporation, Round Rock, TX). All values are expressed as means ± SEMs. Data from animals of 1 d difference in age were pooled. Statistical significance was evaluated by Student's unpairedt test.
Measurements of GABAA receptor α subunits mRNAs and proteins. Wistar rats (P5–P40) were decapitated under halothane anesthesia. After isolating the thalamus, LD thalamic nucleus was dissected with a razor blade in chilled aCSF. The mRNAs for the GABAA receptor α subunits in the LD thalamic nucleus were assayed by reverse transcription (RT)-PCR (Sakaguchi et al., 1997). Total RNA was extracted using the acid guanidine phenol chloroform method from LD thalamic nucleus region of three rats at each postnatal day. The cDNAs were synthesized using SuperScript II (Life Technologies, Gaithersburg, MD) and random hexamer as a primer. The RT products were then amplified by PCR using Taq DNA polymerase (Promega, Madison, WI). The subunit cDNAs were amplified with PCR primers common to α1 and α2 followed by digestion with two types of restriction enzymes: one specific for α1 (NsiI) and the other specific for α2 (MfeI). The PCR primers used were CTGGATGGTTA(T/C)GA(C/T)AATCGTCT and ATAA(C/A)CCAGTCCATGGC(C/A)GT. The PCR program consisted of a 5 min initial denaturation at 95°C followed by 20 cycles (95°C, 43 sec; 62°C, 43 sec; 72°C, 43 sec) and 5 min final elongation at 72°C. To ensure the specificity of this PCR method, PCR products were subcloned into pCR2.1 and sequenced. Randomly sampled clones (n = 20) were found to be either α1 or α2 subunit cDNAs. Preparation of plasmid was performed using Qiagen (Hilden, Germany) plasmid mini-kit. After being digested with NsiI or MfeI, the amplified PCR products were subjected to electrophoresis. Amount of each α subunit mRNA was measured with an acrylamide gel (6%) stained with SYBRGreen I (Molecular Probes, Eugene, OR) using FluoroImager 595 (Molecular Dynamics, Sunnyvale, CA).
The expression of GABAA receptor α subunits in LD thalamic nucleus was examined at various postnatal ages by immunoblotting. The LD thalamic nuclei tissue was dissected from three to five rats at each period and was homogenized with 50 mmTris-HCl, pH 8.0, 150 mm NaCl, 1% Nonidet P-40, 2 mm EDTA, 2 μg/ml leupeptin, and 2 μg/ml pepstatin A. The homogenate (10 μg/lane protein) was fractionated on an SDS polyacrylamide gel (7.5%) and electroblotted to Immunobilon-P membrane (Millipore, Bedford, MA) and reacted with anti-GABAA receptor subunit-specific antibodies (Zezula et al., 1991; Tretter et al., 1997). The type of antibodies used are rabbit anti-rat α1 (1–9)P16, α2(416–424)R1, and α3(459–467)R3. The reaction was visualized with the secondary antibody labeled with alkaline phosphatase (Promega) and quantified using a densitometer (GS-700; Bio-Rad, Hercules, CA).
Preparation of Sindbis virus expression vectors.Complementary DNA for the GABAA receptor α1 subunit or lacZ was subcloned into pSinEGdsp plasmid (pSinEGdsp and pSinEGdsp/lacZ were generous gifts from Drs. H. Nawa and M. Kawamura, Niigata University, Niigata, Japan). In this plasmid, two subgenomic promoters were arranged in tandem, and the recombinant enhanced green fluorescence protein (GFP) gene was subcloned downstream of second subgenomic promoter (Namba et al., 1999). Cells infected with this recombinant virus can express GABAA receptor α1 subunit or β-galactosidase (for control) both in combination with GFP. Complementary RNAs transcribed in vitro from the linear plasmids were electroplated into baby hamster kidney 21 cells using SP6 in vitro transcription kit (Invitrogen, San Diego, CA) and Gene Pulser II (Bio-Rad). In vitro packaged particles were harvested for 24 hr after electroplation and stored at −80°C.
Infection of recombinant Sindbis virus in thalamic cells in organotypic culture. Thalamic slices (400 μm thickness) were prepared from P6 or P7 rats and kept in culture as described previously (Stoppini et al., 1991). Slices were kept in culture in a CO2 incubator at 32°C for 4–5 d and subsequently infected with recombinant Sindbis virus using PicoPump (World Precision Instruments, Sarasota, FL) as described by Kantor et al. (1996). One to 2 d after the infection, GABAergic miniature IPSCs were recorded from GFP-positive neurons in LD thalamic nucleus.
RESULTS
GABAergic IPSCs recorded from laterodorsal thalamic neurons
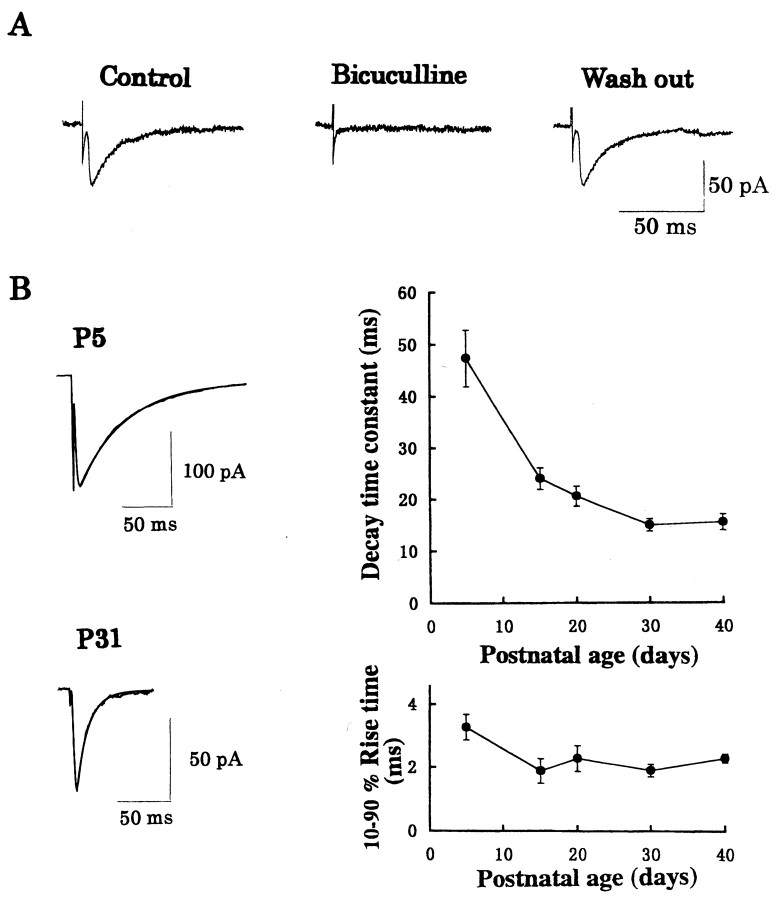
The thalamocortical relay neurons receive GABAergic input from RTN, which is the main source of GABAergic inhibition (Houser et al., 1980). After the whole-cell recording was made from an LD thalamic relay neuron, stimulation applied to RTNevoked synaptic currents in the presence of CNQX and AP-5 (Fig. 1A). These evoked synaptic currents and spontaneous synaptic currents (data not shown) were both blocked by bicuculline (10 μm) in a reversible manner (Fig. 1A), indicating that they were GABAergic IPSCs. The decay time of evoked and spontaneous IPSCs could be fitted by a single exponential time course in most instances (Figs.1B, 2B) as reported for oxytocin neurons (Brussaard et al., 1997) (but see Tia et al., 1996). Although a slight deviation from monoexponential time course was sometimes observed near the tail, we adopted the 100–37% decay time as a decay time constant to simplify comparison across ages in the present study (see also Brickley et al., 1996). As animals matured, the decay time of IPSCs gradually became faster and reached a plateau at approximately P30 (Fig. 1B). The mean decay time of IPSCs was 47.3 ± 5.5 msec at P5 (mean ± SEM;n = 8 cells), whereas the decay time was 15.5 ± 1.6 msec at P30 (n = 11; significantly different, p < 0.001). Rise time (10–90%) of evoked IPSCs also became faster with development, with the mean values being 3.3 ± 0.4 msec at P5 (n = 8) and 2.3 ± 0.1 msec at P30 (n = 11), respectively (p < 0.01).
Fig. 1.
Developmental changes in the kinetics of GABAergic IPSCs in LD thalamic neurons. IPSCs were evoked by RTN stimulation.A, Reversible block of IPSCs by bicuculline (10 μm). Averaged IPSCs (from 10–15 events) at P20 rat are shown. B, Developmental changes in the decay time constant (right top) and 10–90% rise time (right bottom). Each data point and error bar indicate the mean ± SEM from four to seven neurons. Sample records of averaged IPSCs (from 50 consecutive events each) at P5 and P31 are shown. Decay of IPSCs are fitted by monoexponential time courses in this and subsequent figures (Fig. 2B). The decay time constant of IPSCs in the sample records was 40 msec at P5 and 10 msec at P31.
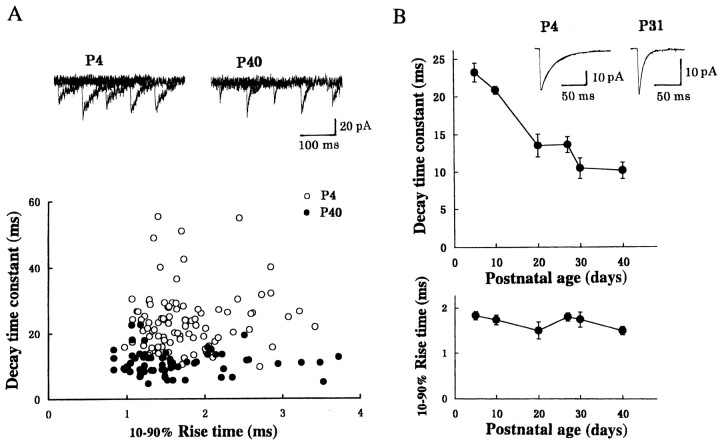
The developmental shortening observed for the decay time of evoked IPSCs may be attributable to a similar change in quantal IPSCs, or it may be attributable to a gain of synchrony in quantal transmitter release in response to a presynaptic action potential. To determine which of these factors is responsible, we have recorded spontaneous miniature IPSCs under TTX (Fig. 2). The miniature IPSC represents the effect of a packet of inhibitory transmitter (Katz, 1969; Takahashi, 1984). When the rise time was plotted against the decay time for individual miniature IPSCs recorded from a neuron at P4 and at P40, no positive correlation was found between them (Fig.2B), suggesting that the decay time is determined independently of the electrotonic distance between the recording and synaptic sites (Takahashi et al., 1992). The decay time of miniature IPSCs at P40 was shorter than that at P4, whereas the range and mean of rise time were comparable between P4 and P40. As shown in Figure2B, the decay time of miniature IPSCs became faster as animals mature, with a similar developmental time course to the evoked IPSCs. However, the mean rise time of miniature IPSCs remained similar during development.
Fig. 2.
Developmental changes in the kinetics of spontaneous miniature IPSCs recorded from LD thalamic neurons under TTX. A, The relationship between the decay time constant and 10–90% rise time of individual miniature IPSCs recorded from two LD neurons: one at P4 and the other at P40. In sample records, five traces are superimposed each at P4 and P40. The mean rise time and decay time of miniature IPSCs were 1.8 ± 0.1 and 24 ± 0.9 msec, respectively, at P4 (○; n = 95) but 1.6 ± 0.1 and 11 ± 0.3 msec at P40 (●;n = 96). There was no correlation between the 10–90% rise time and decay time constant at both P4 and P40 (r < 0.09). B, Developmental changes in the decay time constant (top) and rise time (10–90%; bottom) of miniature IPSCs. Sample records are averaged miniature IPSCs (from 50 events each) at P4 and P31, with their decay time fitted by monoexponential time courses. The decay time constant was 23 msec at P4 and 8.4 msec at P31.
Time course of α subunit expression in LD thalamic neurons
Studies on recombinant GABAA receptors indicate that the deactivation time of GABA-induced currents is faster if the receptor contains the α1 subunit (Verdoorn 1994; Gingrich et al., 1995) instead of the α2 (Lavoie and Twyman, 1996) or α3 (Verdoorn 1994; Gingrich et al., 1995) subunits. We therefore examined the time course of the expression of α subunit mRNAs in LD thalamic nucleus region using RT-PCR. After amplification with a common primer, the amount of α1 or α2 subunit mRNAs was determined using the restriction enzymes NsiI and Mfel, which cleave α1 and α2 subunit PCR products, respectively (for details, see Materials and Methods). To ensure the linearity of the PCR method for the quantification of relative amount of nucleic acids, we have applied the method to a mixture of cDNAs encoding α1 or α2 subunit in variable known proportions (Fig. 3A). The relative amounts of α1 and α2 subunit cDNAs measured after PCR were as expected. We then measured α subunit mRNAs at various postnatal ages (Fig. 3B). During postnatal development, expression of α1 subunit mRNAs increased concomitantly with a decrease in α2 subunit mRNAs. At approximately P17, the expression of α1 subunit mRNAs equaled that of α2 subunit mRNAs (Fig. 3C).
Fig. 3.
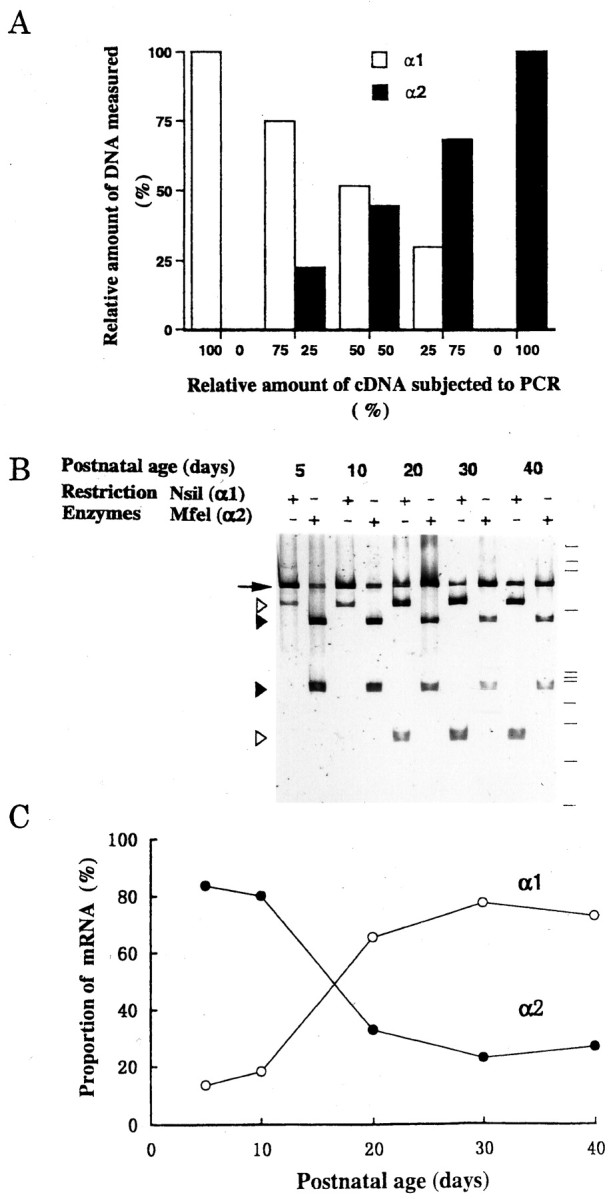
Developmental changes in the expression of mRNAs encoding GABAA receptor α1 and α2 subunits.A, Evaluation of RT-PCR assay. The indicated amounts of cDNAs (0–100 fg each) of α1 and α2 subunits subcloned into pCR2.1 were added together to a PCR reaction mixture, and the amplified PCR products were quantified (see Materials and Methods). Ordinate indicates the amount of digested fractions of α1 DNA (open bars) and α2 DNA (filled bars) relative to the total PCR product (α1 + α2). Data from one experiment.B, Expression of GABAA receptor subunit mRNAs in LD thalamic nucleus at each postnatal day (P5–P40).Open or filled arrowheads indicate PCR products digested by NsiI (α1-specific) orMfeI (α2-specific), respectively. Anarrow indicates PCR products that remained undigested. Size markers (from top to bottom) correspond to 1353, 1087, 872, 603, 310, 281, 271, 234, 194, 118, and 72 bp, respectively. C, Relative amounts of α1 and α2 subunit mRNAs expressed at each postnatal day. Ordinate indicates the proportion of α1 (○) or α2 (●) subunit mRNA relative to the total amount (α1 + α2). Data points are mean values derived from two experiments.
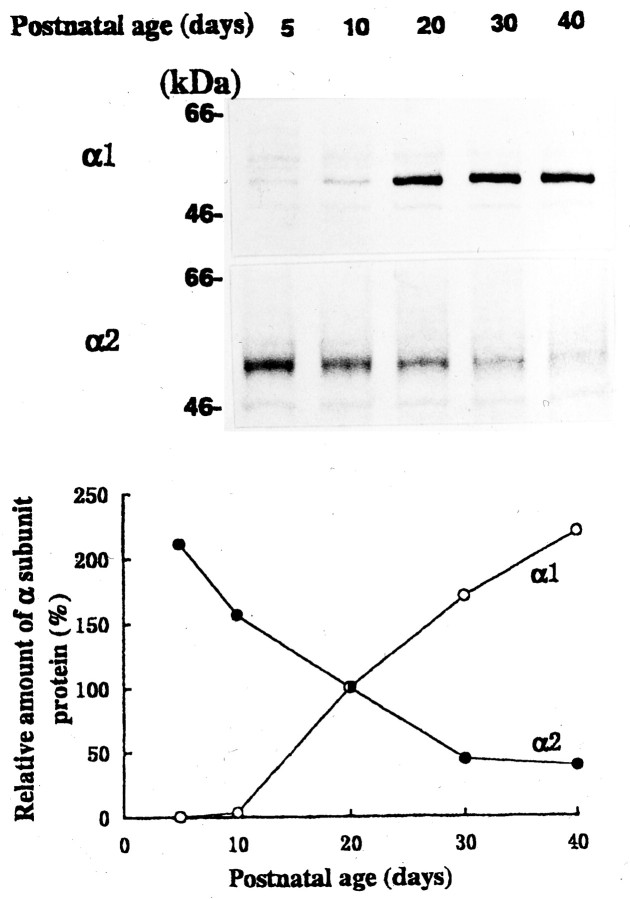
We next measured α subunit proteins by immunoblot analysis using subunit-specific antibodies. As illustrated in Figure4, the α1 subunit immunoreactivity was low at the early postnatal age but increased as animals matured. In contrast, the α2 subunit immunoreactivity was high at P5 but declined with development. The α3 subunit immunoreactivity was barely detectable in the LD thalamic tissue within the period examined (data not shown).
Fig. 4.
Developmental changes in the α1 and α2 subunit proteins of GABAA receptor in LD thalamic nucleus.Top, Immunoblot of GABAA receptor α1 and α2 subunits at different postnatal days. Mobilities of molecular mass markers are indicated. Bottom, Relative amounts of immunoreactive α1 (○) and α2 (●) subunits at various postnatal days. Mean values of two experiments each for α1 and α2 subunits are normalized to those at P20.
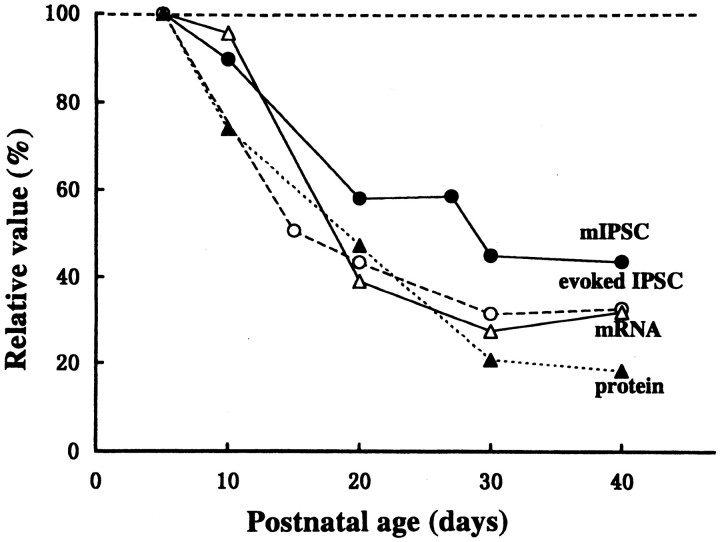
We compared the developmental time course between the decay time of IPSCs and GABAA receptor α subunits by normalizing individual values to those at P5. The relative amount of α2 subunit transcripts or proteins was expressed as a proportion to the sum of α1 and α2 subunits [α2/(α1 + α2)] at various postnatal ages. As illustrated in Figure 5, the developmental time course is similar between the decay time of IPSCs and the relative amount of α subunit transcripts and proteins. The minimal decay time of evoked and miniature IPSCs is reached at approximately P30, when the relative amount of α2 subunit transcript and protein also reached the minimal value.
Fig. 5.
Developmental changes in the IPSC decay time and the expression of GABAA receptor α subunit. The decay time constants of evoked (○) and spontaneous miniature (●) IPSCs, and the relative amounts of transcripts (▵) and proteins (▴) expressed as α2/(α1+ α2) at various postnatal ages normalized to the values at P5 are compared.
Causal relationship between the α1 subunits and IPSC decay time
To obtain more direct evidence for the role of α subunits in the IPSC decay time kinetics, we have overexpressed GABAA receptor α1 subunit in thalamic neurons in organotypic culture using the Sindbis virus-mediated gene transfer. We constructed recombinant Sindbis virus vectors containing α1 subunit and GFP and infected thalamic neurons in organotypic culture and incubated for 1–2 d. Once thalamic slices prepared from P6 rats were kept in organotypic culture, to our surprise, none of the transcripts for α1 or α2 subunits underwent a developmental change up to 15 d after culture (data not shown). Consistent with these observations but in contrast to the normal development, after 6–7 d in culture, miniature IPSCs became slightly longer in both the 10–90% rise time (2.2 ± 0.2 msec; n = 5) and decay time constant (38.0 ± 2.1 msec; n = 5) (Fig. 2). These results suggest that unknown factor(s) responsible for GABAA receptor subunit switch, such as developmental cues (Beattie and Siegel, 1993) or neuronal activity (Mellor et al., 1998), might be missing in the organotypic culture.
After thalamic organotypic cultures were infected with the Sindbis virus vectors for 1–2 d, green fluorescence became detectable in a subset of neurons overexpressed with GFP and GABAA receptor α1 subunit (Fig.6A). When the whole-cell recording was made from these neurons, fluorescence intensity rapidly declined because of washout of GFP by the patch pipette solution. As illustrated in Figure6B, miniature IPSCs recorded from LD thalamic neurons overexpressed with GFP and GABAA receptor α1 subunit had a significantly faster decay time compared with control infected with GFP and β-galactosidase constructs, whereas their rise times were comparable. The mean decay time constant of IPSCs recorded from neurons overexpressed with α1 subunit was 22.5 ± 1.3 msec (n = 7 cells), which was significantly faster (p < 0.01) than that in control neurons expressed with GFP–β-galactosidase (36.1 ± 4.1 msec;n = 6) or those unexposed to Sindbis virus infection (38.0 ± 2.1 msec; n = 5). There was no significant difference in the 10–90% rise time of miniature IPSCs between them: 2.2 ± 0.1 msec (n = 7) in α1 subunit-overexpressed neurons and 2.9 ± 0.5 msec (n = 6) in neurons with GFP–β-galactosidase or 2.3 ± 0.2 msec (n = 5) in neurons unexposed to Sindbis virus (p > 0.1). These results suggest that the newly synthesized α1 subunits combine with other endogenous GABAA receptor subunits and form functional subsynaptic receptors, thereby shortening the decay time of GABAergic IPSCs.
Fig. 6.
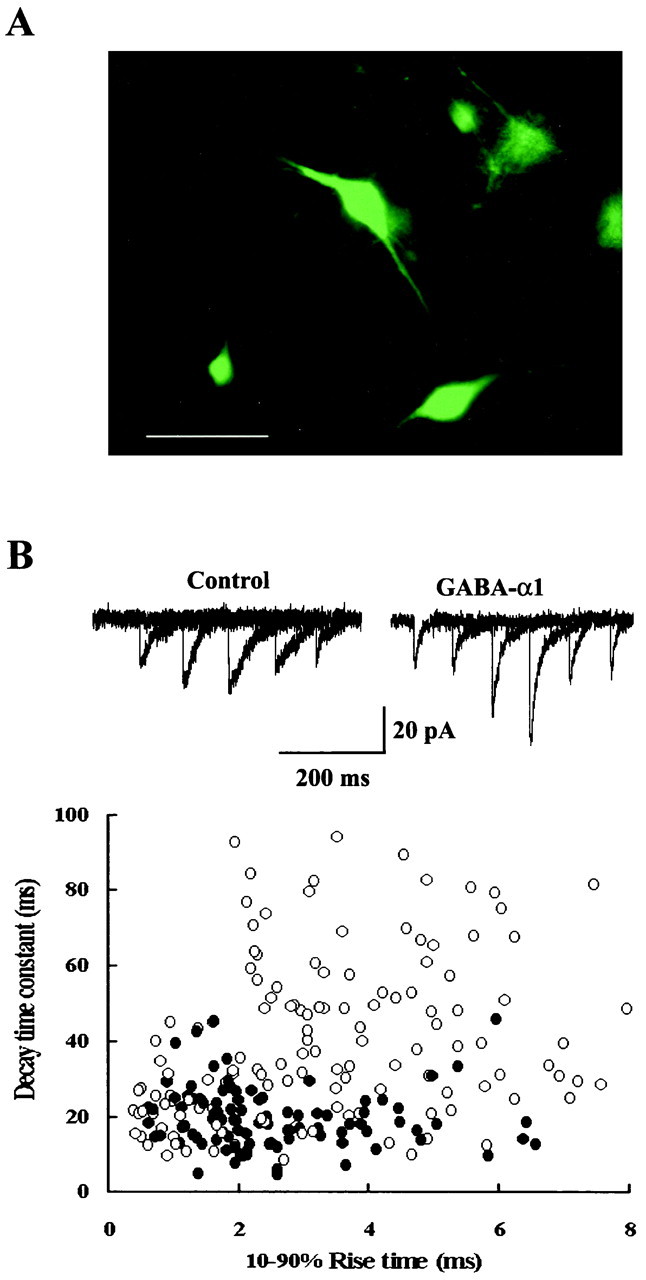
Overexpression of GABAA α1 subunit in LD thalamic neurons in organotypic culture shortens the decay time of miniature IPSCs. A, LD thalamic neurons overexpressed with the GABAA α1 subunit visualized with GFP transferred using recombinant Sindbis virus vector. Scale bar, 100 μm.B, The relationship between the decay time constant and the 10–90% rise time of individual miniature IPSCs recorded under TTX from two GFP-labeled LD neurons in different organotypic cultures: one overexpressed with GABAA α1 subunit (○) and the other with β-galactosidase instead of α1 subunit (control; ●). Five traces are superimposed in each sample record.
DISCUSSION
IPSC decay time and GABA receptor subunit composition
Our results demonstrate that the decay time of evoked and spontaneous miniature GABAergic IPSCs recorded from LD thalamic neurons becomes faster as animals mature during the first postnatal month. If transmitter binds with receptors only once, both the decay time of synaptic currents and the deactivation time of transmitter-induced currents are determined by the mean burst length of the transmitter-gated ion channels, the affinity of the receptor for transmitter and desensitization (Jones and Westbrook, 1995; Jones et al., 1998). In recombinant GABAA receptors expressed in cultured cells, deactivation time of GABA-induced current response is faster when the receptors contain the α1 subunit instead of the α2 or α3 subunits (Verdoorn 1994; Gingrich et al., 1995;Lavoie and Twyman, 1996). These suggest that a developmental switch from α2 or α3 subunit to α1 subunit may accelerate the IPSC decay time. Indeed, our present study has demonstrated that the decay time of LD thalamic IPSCs becomes faster during postnatal development as α2 subunit is replaced by α1 subunits. Furthermore, overexpression of α1 subunits in thalamic neurons in organotypic culture accelerated the IPSC decay time with no effect on the rise time. Thus, we conclude that the α2-to-α1 subunit switch of GABAAreceptor underlies the developmental shortening of the decay of IPSCs in LD thalamic neurons. Interestingly, the reversed switch from α1 to α2 subunit has been reported to be associated with slowing in the decay time of IPSCs in oxytocin neurons during pregnancy (Brussaard et al., 1997).
Throughout postnatal development, the rise time of miniature IPSCs remained similar, whereas that of evoked IPSCs was significantly slow at P5. Concomitantly, the decay time of IPSCs was significantly slower than that of miniature IPSCs. Presumably, asynchronous release of quantal packets of transmitter may make the rise and decay times of evoked IPSCs slower than those of miniature IPSCs. Another possible reason for the slower decay of evoked IPSCs relative to miniature IPSCs during the early postnatal period may be the inefficient clearance of transmitter from synaptic cleft. If the clearance of transmitter by diffusion or uptake is delayed, transmitter will bind with receptors more than once, thereby slowing the IPSC decay. This effect will be greater for evoked IPSCs than for miniature IPSCs because of a greater amount of transmitter released during evoked IPSCs (Thompson and Gahwiler, 1992).
Although α2 subunit is replaced by α1 subunit with development in thalamus, α2 subunits remain in adulthood in other brain regions (Laurie et al., 1992a; Wisden et al., 1992). Cerebral neocortex is one such region, and in fact GABAergic IPSCs recorded from pyramidal cells in visual cortex at P1 had a similar decay time to that at P16 (A. Momiyama and T. Takahashi, unpublished observation). In cerebellar granule cells, the decay time of GABAergic IPSCs becomes shorter with development (Brickley et al., 1996; Tia et al., 1996), and this change has been attributed to the α6 subunit (Tia et al., 1996), which is expressed specifically in cerebellar granule cells at the late postnatal period (Laurie et al., 1992b). However, the IPSC decay time becomes faster with development, even in mutant mice lacking α6 subunit (Brickley et al., 1999). Because the transcript of α2 subunit is known to be replaced by that of α1 subunit in developing cerebellum (Laurie et al., 1992b), the α2-to-α1 subunit switch may contribute to the decay time acceleration of cerebellar IPSCs. In thalamus, expressions of mRNAs of α4, α5, β2, or β3 subunits are also developmentally regulated, with mRNAs of α5 or β3 subunits declining but α4 and β2 subunit mRNAs increasing during the first 2 postnatal weeks (Laurie et al., 1992b). Because no kinetic comparison has been made for the recombinant GABAA receptors containing these subunits, it remains to be seen whether these subunits may additionally contribute to the developmental change in the decay time kinetics of thalamic IPSCs.
The developmental shortening of the decay time has been known for other types of synaptic currents. At the mammalian neuromuscular junction, the γ subunit of nicotinic acetylcholine receptor is replaced by the ε subunit with development, thereby speeding the decay time of end plate currents (Mishina et al., 1986). Similarly, in the spinal cord, the decay time of glycinergic IPSCs becomes faster with postnatal development as the fetal form α2 subunit is replaced by the adult form α1 subunit (Takahashi et al., 1992). Also, the decay time of NMDA receptor-mediated excitatory synaptic currents in cerebellar granule cells becomes faster with development because of a switch of the NMDA receptor ε subunit from ε2 to ε1 (Takahashi et al., 1996). Thus, together with our present results, the developmental shortening of decay time attributable to the subunit switch is a common feature among ion channel receptors.
Functional significance of the developmental change in the kinetics of IPSCs
The IPSC decay time can determine the time course of postsynaptic neuronal excitability and its prolongation can cause sedative effects (Tanelian et al., 1993; Franks and Lieb, 1994), whereas its shortening can produce anxiety and seizures (Worms and Lloyd, 1981). GABAergic neurons in RTN are involved in the generation of synchronized activity in thalamocortical networks, and this gamma or “40 Hz” rhythm increases when the subject pays attention and it disappears with a loss of consciousness (Jefferys et al., 1996). Drugs that slow the decay of GABAergic IPSCs lower the frequency of oscillation (Whittington et al., 1995). Thus, duration of GABAergic IPSCs is an important factor controlling the generation of activity in the thalamocortical system, which is reflected in waking, slow-wave sleep, and generalized seizures (Kim et al., 1997). We propose that developmental shortening of the IPSC decay time may support fast rhythmic oscillations, thereby contributing to the maintenance of high level consciousness in mature animals.
Footnotes
This work was supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Sciences, and Culture of Japan Grant 05454681 and Research for the Future Program by The Japan Society for the Promotion of Sciences (JSPS) to T.T., and a Travel Grant by the JSPS-Centre National de la Recherche Scientifique Exchange Program to C.V.R. We thank Kyoko Matsuyama and Kiyomi Kagami for technical assistance, and Toshiya Manabe, Akiko Momiyama, David Saffen, and Yoshinori Sahara for comments on this manuscript. We also thank Norman Davidson and Erin Schuman for technical instructions on the virus method, and Meiko Kawamura and Hiroyuki Nawa for viral vectors.
Correspondence should be addressed to Tomoyuki Takahashi, Department of Neurophysiology, University of Tokyo Faculty of Medicine, Tokyo 113–0033, Japan. E-mail: ttakahas-tky@umin.u-tokyo.ac.jp.
Dr. Van Renterghem's present address: Institut National de la Santé et de la Recherche Médicale U464, Lab Neurobiologie des Canaux Ioniques, IFR Jean Roche Faculte de Médecine, Secteur Nord, Marseille Cedex 20, France.
REFERENCES
- 1.Beattie CE, Siegel RE. Developmental cues modulate GABAA receptor subunit mRNA expression in cultured cerebellar granule neurons. J Neurosci. 1993;13:1784–1792. doi: 10.1523/JNEUROSCI.13-04-01784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJS, Brown N, Wafford KA, Whiting PJ. θ, a novel γ-aminobutyric acid type A receptor subunit. Proc Natl Acad Sci USA. 1999;96:9891–9896. doi: 10.1073/pnas.96.17.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brickley SG, Farrant M, Wisden B, Cull-Candy SG. The role of α6- and δ-containing GABAA receptors in the generation of tonic inhibition in cerebellar granule cells. J Physiol (Lond) 1999;518P:140. [Google Scholar]
- 5.Brussaard AB, Kits KS, Baker RE, Willems WPA, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABAA receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- 6.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 7.Fritschy J-M, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the α-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol (Lond) 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houser CR, Vaughn JE, Barber RP, Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980;200:341–354. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- 10.Jefferys JGR, Traub RD, Whittington MA. Neuronal networks for induced “40 Hz” rhythms. Trends Neurosci. 1996;19:202–208. doi: 10.1016/s0166-2236(96)10023-0. [DOI] [PubMed] [Google Scholar]
- 11.Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 12.Jones MV, Sahara Y, Dzubay JA, Westbrook GL. Defining affinity with the GABAA receptor. J Neurosci. 1998;18:8590–8604. doi: 10.1523/JNEUROSCI.18-21-08590.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantor DB, Lanzrein M, Stary J, Sandoval GM, Smith WB, Sullivan BM, Davidson N, Schuman EM. A role for endothelial NO synthase in LTP revealed by adenovirus-mediated inhibition and rescue. Science. 1996;274:1744–1748. doi: 10.1126/science.274.5293.1744. [DOI] [PubMed] [Google Scholar]
- 14.Katz B. The release of neural transmitter substances. Liverpool UP; Liverpool, UK: 1969. [Google Scholar]
- 15.Kim U, Sanches-Vives MV, McCormick DA. Functional dynamics of GABAergic inhibition in the thalamus. Science. 1997;278:130–134. doi: 10.1126/science.278.5335.130. [DOI] [PubMed] [Google Scholar]
- 16.Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992a;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor submit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992b;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavoie AM, Twyman RE. Direct evidence for diazepam modulation of GABAA receptor microscopic affinity. Neuropharmacology. 1996;35:1383–1392. doi: 10.1016/s0028-3908(96)00077-9. [DOI] [PubMed] [Google Scholar]
- 19.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 20.Mellor JR, Merlo D, Jones A, Wisden W, Randall AD. Mouse cerebellar granule cell differentiation: electrical activity regulates the GABAA receptor α6 subunit gene. J Neurosci. 1998;18:2822–2833. doi: 10.1523/JNEUROSCI.18-08-02822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986;321:406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- 22.Namba H, Kawamura M, Okada M, Otsu Y, Nawa H. Sindbis virus vector carrying GFP as a reporter gene: electrophysiological and cell biological evaluation in the neocortical neuron. Soc Neurosci Abstr. 1999;25:209. [Google Scholar]
- 23.Sakaguchi T, Okada M, Kuno M, Kawasaki K. Dual mode of N-methyl-d-aspartate-induced neuronal death in hippocampal slice cultures in relation to N-methyl-d-aspartate receptor properties. Neuroscience. 1997;76:411–423. doi: 10.1016/s0306-4522(96)00403-4. [DOI] [PubMed] [Google Scholar]
- 24.Steriade M, Llinas RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 25.Stoppini L, Buchs P-A, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi T. Inhibitory miniature synaptic potentials in rat motoneurons. Proc R Soc Lond B Biol Sci. 1984;221:103–109. doi: 10.1098/rspb.1984.0025. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi T, Momiyama A, Hirai K, Hishinuma F, Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992;9:1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T, Feldmeyer D, Suzuki N, Onodera K, Cull-Candy SG, Sakimura K, Mishina M. Functional correlation of NMDA receptor ε subunits expression with the properties of single-channel and synaptic currents in the developing cerebellum. J Neurosci. 1996;16:4376–4382. doi: 10.1523/JNEUROSCI.16-14-04376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanelian DL, Kosek P, Mody I, Maclver MB. The role of the GABAA receptor/chloride channel complex in anesthesia. Anesthesiology. 1993;78:757–776. doi: 10.1097/00000542-199304000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Thompson SM, Gahwiler BH. Effects of the GABA uptake inhibitor tiagabine on inhibitory synaptic potentials in rat hippocampal slice cultures. J Neurophysiol. 1992;67:1698–1701. doi: 10.1152/jn.1992.67.6.1698. [DOI] [PubMed] [Google Scholar]
- 31.Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor α6 subunit. J Neurosci. 1996;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verdoorn TA. Formation of heteromeric γ-aminobutyric acid type A receptors containing two different α subunits. Mol Pharmacol. 1994;45:475–480. [PubMed] [Google Scholar]
- 34.Whittington MA, Traub RD, Jefferys JGR. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 35.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worms P, Lloyd KG. Functional alterations of GABA synapses in relation to seizures. In: Morselli PL, Lloyd KG, Loscher W, Meldrum B, Reynolds EH, editors. Neurotransmitters, seizures and epilepsy. Raven; New York: 1981. pp. 37–48. [Google Scholar]
- 37.Zezula J, Fuchs K, Sieghart W. Separation of α1, α2 and α3 subunits of the GABAA-benzodiazepine receptor complex by immunoaffinity chromatography. Brain Res. 1991;563:325–328. doi: 10.1016/0006-8993(91)91556-g. [DOI] [PubMed] [Google Scholar]