Abstract
The retinal degeneration slow or rdsgene encodes rds/peripherin, an integral membrane glycoprotein in the outer segments of rod and cone photoreceptors. Mice homozygous for a null mutation in rds fail to develop outer segments and undergo subsequent degeneration of photoreceptors by the apoptotic pathway. Mutations in the human RDS gene are responsible for several forms of inherited blindness including autosomal-dominant retinitis pigmentosa and macular degeneration. Here, we examined the effects of ectopic Bcl-2 expression in transgenic photoreceptors on the rate of retinal degeneration in rds mutant mice. We observed an approximately twofold preservation of photoreceptors compared with nontransgenic rds mutant mice at 3 months. Immunoblot analysis showed similar levels of Bcl-2 in 2-, 3-, and 4-week-old transgenic mice. Expression of Bcl-2 in therds mouse did not lead to outer segment formation and did not induce cell death. These results suggest that Bcl-2 expression may be an effective therapeutic strategy in humans with mutations inRDS or other genes that affect the integrity of photoreceptor outer segments.
Keywords: rds mice, rds/peripherin, retinal degeneration, retinitis pigmentosa, Bcl-2, transgene, apoptosis
The mammalian retina is a useful system for studying neuronal degeneration. Mutations in multiple genes have been shown to cause blindness because of photoreceptor cell death (for review, see Molday, 1998; Travis, 1998). An example is the retinal degeneration slow or rds mouse. Photoreceptors in mice homozygous for a null mutation in the rds gene fail to develop outer segments, the site of photon capture and of the reactions of visual transduction (Jansen and Sanyal, 1984). Photoreceptor cell death starts between the second and third postnatal week and is completed at ∼1 year (Sanyal et al., 1980; Nir et al., 1990). A light response can be elicited in rds mice although at a greatly reduced sensitivity (Reuter and Sanyal, 1984). The residual photoactivity is mediated by opsin that is localized in the inner segment and connecting cilium plasma membranes (Nir and Papermaster, 1986).
In humans, over 50 mutations in the RDS gene have been implicated in dominant forms of retinitis pigmentosa (RP) and macular degeneration (for review, see Keen and Inglehearn, 1996; Shastry, 1997). RDS encodes rds/peripherin, a structural glycoprotein in the rims of outer segment disks (Connell et al., 1991; Travis et al., 1991; Kedzierski et al., 1999). In previous studies, we demonstrated complete rescue of the rds phenotype in transgenic mice that express normal rds/peripherin (Travis et al., 1992). Subsequently, we showed that the rds rescue transgene integrated into chromosome X in line 113 mice. Hemizygous females from line 113 showed mosaic expression of the transgene and partial rescue of the rds phenotype, whereas males of this line were completely rescued (Kedzierski et al., 1998).
The events that lead from primary mutation to the onset of cell death are not known for any form of retinal degeneration. In every system studied, however, photoreceptors have been shown to die by the apoptotic pathway (Chang et al., 1993; Lolley et al., 1994;Portera-Cailliau et al., 1994; Tso et al., 1994; Cook et al., 1995;Abler et al., 1996; Molthagen et al., 1996). An important step in apoptosis is the release of cytochrome c from the intermembrane space of mitochondria (Krippner et al., 1996; Li et al., 1997). Members of the Bcl-2 protein family exert antiapoptotic effects by blocking the release of cytochrome c from mitochondria (Kluck et al., 1997; Yang et al., 1997). Bcl-2 is not expressed in mature photoreceptors, although the related protein Bcl-XL is present in these cells (Levin et al., 1997).
Prevention of photoreceptor death by ectopic expression of Bcl-2 was attempted in several mouse models of inherited retinal degeneration. These studies showed either no effect (Joseph and Li, 1996) or limited and short-lived effects (Chen et al., 1996; Tsang et al., 1997). In the current study we examined the effects of Bcl-2 expression on photoreceptor death in rds mutant mice that undergo a relatively slow degeneration, similar to many forms of retinal degenerations in humans. We show that expression of Bcl-2 causes a significant and long-term suppression of photoreceptor cell death in homozygous rds mutants.
MATERIALS AND METHODS
Transgenic mice. Mice carrying a transgene (line B) containing the complete human Bcl-2-coding region downstream of a mouse rhodopsin promoter (Chen et al., 1996) were crossed onto anrds−/− homozygous mutant background on the C57BL/6 strain. The resulting F1 mice were crossed with rds line 113-transgenic mice, also on an rds−/− background, to yield F2 progeny of the desired genotypes. Mice were maintained under a 12 hr dark/light cycle at 40 lux illumination. Tail-cut DNA from mice was analyzed for the presence of the rds transgene by PCR with the following primers: 5′-CCTGGAGTTGCGCTGT and 5′-GTCTTTTT- CATGAAGCACC from the mouse rhodopsin promoter and therds-coding region, respectively (Travis et al., 1989). Mice were analyzed for the presence of the Bcl-2 transgene by PCR with the following primers: 5′-CCTGGAGTTGCGCTGT and 5′-CCTGTTCTCCCAGCGT from the mouse rhodopsin promoter and the human Bcl-2-coding region, respectively.
Microscopy. Mice were killed by cervical dislocation; the eyes were enucleated and placed in 4% formaldehyde and 2% glutaraldehyde in 0.1 m phosphate buffer for fixation. After 30 min, the eyes were bisected along the vertical meridian. The two hemispheres were fixed for an additional 3 hr. The tissue was then treated in 1% OsO4, embedded in araldite. One micrometer sections were prepared for analysis of the outer nuclear layer (ONL) thickness by light microscopy. Thin sections from the same regions of the retina were used for ultrastructural analysis.
Measurement of photoreceptor survival. Light microscopy was used for quantitative assessments of photoreceptor survival. Sections of 1 μm thickness were cut along the superior–inferior meridian. Each section included the full retina from optic nerve head to ora serrata. The number of photoreceptor nuclei within the ONL was counted along the posterior–periphery axis at a distance of 150–1500 μm from the optic nerve head in the superior and inferior hemispheres. Measurements were performed at 150 μm intervals using an eyepiece graticule. Data are expressed as the number of photoreceptor nuclei in a 50 μm retinal length.
Immunoblot analysis of human Bcl-2. Dissected mice retinas were homogenized on ice in 10 mm Tris-HCl, pH 7.5, 1% SDS, 1 mm EDTA, 0.2 mm phenylmethylsulfonyl fluoride, and protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN) and cleared by a 2 min centrifugation in a microfuge at 4°C. The extracts were mixed with 2× Laemmli buffer and incubated at 65°C for 2 min. Samples were resolved by 10% PAGE in 10% 0.75 mm gels and electrotransferred to Immobilon P membranes (Millipore, Bedford, MA) in a transfer buffer containing 25 mm Tris-HCl, 190 mm glycine, 15% methanol, and 0.05% SDS. Blots were preblocked for 1 hr by treating with 1% nonfat dry milk in PBS. The blots were incubated for 2 hr with a mouse monoclonal antibody against human Bcl-2 (Sigma, St. Louis, MO) diluted 1:1000 with PBS containing 1% dry milk. After being washed with PBS containing 0.05% Tween 20 for 1 hr, blots were incubated for an additional hour with goat anti-mouse IgG–horseradish peroxidase conjugate (Bio-Rad, Richmond, CA) diluted 1:10,000 with PBS containing 1% dry milk. Blots were detected with the LumiGLO chemiluminescent substrate (Kirkegaard & Perry, Gaithersburg, MD).
RESULTS
Photoreceptor survival in 3-month-old retinas
We studied photoreceptor survival by light microscopy in 3-month-old mice of the following genotypes: (1) nontransgenicrds−/− homozygotes (rds), (2) Bcl-2-transgenic,rds−/− homozygotes (rds:bcl), (3) rds rescue transgenic, rds−/− homozygotes (rds:113), and (4) Bcl-2 and rds rescue double-transgenic,rds−/− homozygotes (rds:113+bcl). Representative retinal sections from males of these four genotypes are shown in Figure 1. Nontransgenicrds mice showed significant retinal degeneration at 3 months, with reductions in ONL thickness to two to three rows of nuclei (Fig. 1A). Expression of Bcl-2 in rds:bclmice resulted in a partial rescue of retinal degeneration, with retention of five to six rows (Fig. 1B). Expression of normal rds/peripherin in male rds:113 mice prevented retinal degeneration, with retention of eight to nine rows of nuclei (Fig. 1C). Expression of both rds/peripherin and Bcl-2 inrds:113+bcl mice also resulted in full protection from retinal degeneration, with retention of eight to nine rows of nuclei (Fig. 1D).
Fig. 1.
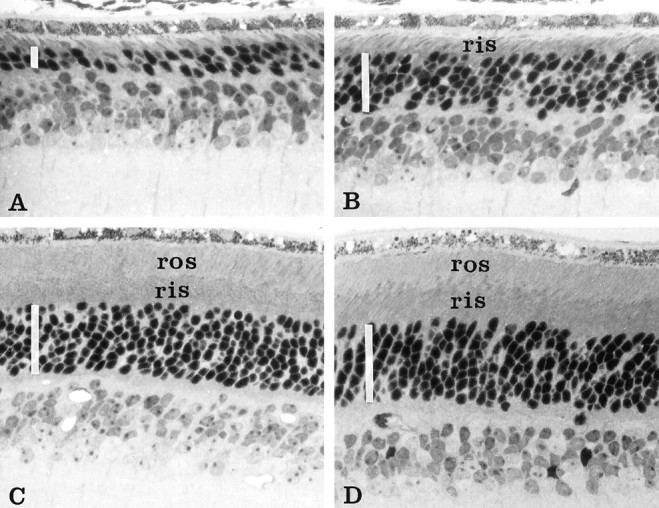
Light micrographs of retinal sections from 3-month-old male mice of different genotypes. The densely stained photoreceptor nuclei layer is indicated in each panel by a white vertical bar. A, Homozygousrds retina (rds). Up to three rows of photoreceptor nuclei are present. B, Homozygousrds retina expressing the Bcl-2 transgene (rds:bcl). Up to six rows of nuclei are present.C, Homozygous rds retina expressing the normal rds/peripherin transgene (rds:113). Up to eight rows of nuclei are present. D, Homozygousrds retina expressing the normal rds/peripherin plus Bcl-2 transgenes (rds:113+bcl). Up to eight rows of nuclei are present. ris, Rod inner segment layer;ros, rod outer segment layer. Magnification, 400×.
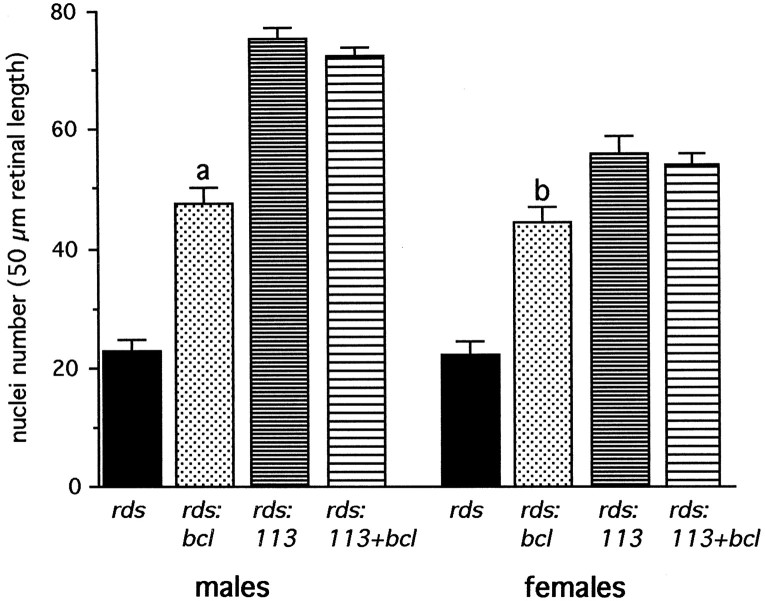
We performed detailed morphometric analysis to define accurately the extent of photoreceptor survival in mice of these four genotypes. Data were collected separately from male and female sibs of seven litters. Figure 2 shows the results of this analysis. Photoreceptor survival was increased by ∼100% in both male and female rds:bcl mice compared with nontransgenicrds mice. However, the effect of Bcl-2 expression on therds phenotype was significantly less than the complete rescue observed in rds:113 males (Kedzierski et al., 1998). Mosaic expression of rds/peripherin in rds:113 females caused partial rescue of the rds phenotype, as described (Kedzierski et al., 1998). Coexpression of Bcl-2 with rds/peripherin inrds:113+bcl females conferred no additional rescue effect.
Fig. 2.
Quantitation of the number of photoreceptor nuclei. Analysis was performed on 3-month-old male and female retinas of the four genotypes: rds,rds:bcl, rds:113, and rds:113+bcl. Data are presented as means ± SEM (n = 6). Males:ap < 0.001, rds:bclversus rds and rds:113. Females:bp < 0.001, rds:bclversus rds; p < 0.05,rds:bcl versus rds:113.
Photoreceptor survival in 1-month-old retinas
Approximately one-third of photoreceptors are lost during the first postnatal month in rds retinas. We sought to determine whether the preponderance of photoreceptors lost in rds:bclmice occurred during this accelerated cell-death period. Accordingly, we compared the number of photoreceptor nuclei present at 35–38 d inrds and rds:bcl mice. Morphometry was conducted on retinas from rds males and females, five of each, andrds:bcl males and females, four of each. The rdsretinas had an average of 50.8 ± 4.7 photoreceptor nuclei (n = 5) per 50 μm, whereas the rds:bclretinas had 66.6 ± 0.53 nuclei (n = 4) per 50 μm, representing a 31% increase. Thus, the protective effect of Bcl-2 was present during the early phase of cell death. A comparison of these values with the number of nuclei remaining at 3 months (23 ± 1.7 for rds and 47 ± 2.3 forrds:bcl) indicates that photoreceptors continue to die in rds:bcl mice during the ensuing 2 months, although at a slower rate than in rds mice.
Levels of transgenic Bcl-2 expression
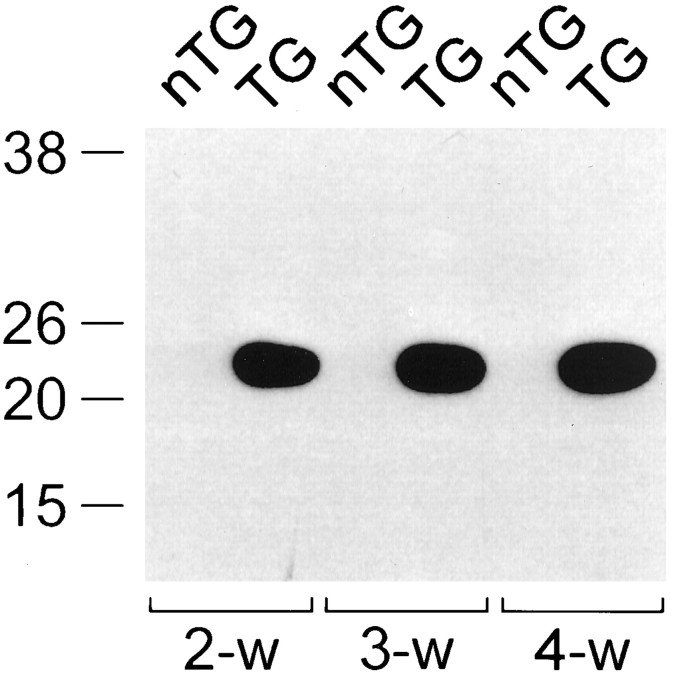
To confirm the levels and stability of Bcl-2 expression, we performed immunoblot analysis on retinal homogenates from transgenic and nontransgenic mice at 2, 3, and 4 weeks of age. These intervals bracket the period of accelerated photoreceptor degeneration in rds mice. No difference was observed in the levels of Bcl-2 in transgenic mice at these different ages (Fig.3). Bcl-2 immunoreactivity was undetectable in the nontransgenic littermates.
Fig. 3.
Immunoblot analysis showing the levels of Bcl-2 in retinas from 2-, 3-, and 4-week-old mice on anrds−/− background. TG denotes Bcl-2 transgenic, and nTG denotes nontransgenic. Note the nearly adult levels of Bcl-2 expression in 2-week-old retinas.w, Week.
Ultrastructural analysis
We examined the effects of Bcl-2 expression on the cellular organization of photoreceptors by electron microscopy. Inrds:bcl retinas, photoreceptors completely lacked outer segments (Fig. 4A), similar to those observed in nontransgenic rds mice (Jansen and Sanyal, 1984; Nir and Papermaster, 1986). Thus, the protective effect of Bcl-2 expression on photoreceptor death was not mediated by a correction of the outer segment phenotype in rds mice. The antiapoptotic effect of Bcl-2 is manifested in the increased number of photoreceptor nuclei. In the rds:113+bcl male retina, the expression of the Bcl-2 transgene had no adverse effect on the ultrastructure of outer segments (Fig. 4B).
Fig. 4.
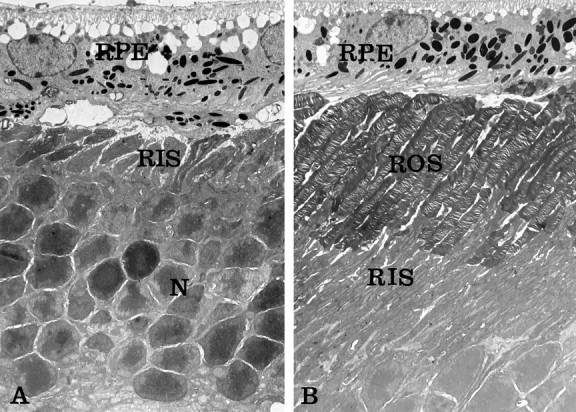
Electron micrographs of 3-month-old mice.A, Homozygous rds retina expressing Bcl-2 (rds:bcl). Note the absence of outer segments and the abnormal position of photoreceptor rod inner segments (RIS) against the retinal pigment epithelium (RPE). The effect of Bcl-2 on photoreceptor survival is evident by the presence of a significant number of photoreceptor nuclei (N). B, Homozygousrds retina expressing both normal rds/peripherin and Bcl-2 transgenes (rds:113+bcl). Note the normal morphology of intact rod outer segments (ROS) adjacent to the RPE. The expression of Bcl-2 did not adversely affect photoreceptor organization. Magnification: A, 3200×; B, 2900×.
DISCUSSION
In the present study, we examined the effect of Bcl-2 expression on photoreceptor degeneration in rds mutant mice. At 3 months, mice expressing the Bcl-2 transgene retained approximately twice the number of photoreceptors as did nontransgenic rdsmice. This is in contrast to previous studies on mice with null mutations in the genes for the β- and γ-subunits of cGMP phosphodiesterase (PDE) and dominant mutations K269E and S334ter in the gene for rhodopsin. Expression of Bcl-2 in these mice resulted in minor and transient increased photoreceptor survival (Chen et al., 1996;Joseph and Li, 1996; Tsang et al., 1997).
In the rd mouse, a mutation in the β-subunit of PDE results in accumulation of cGMP with severe metabolic consequences, leading to early photoreceptor death. Apoptosis starts at postnatal day 7 (P7), and by P20 almost all rods die (Carter-Dawson et al., 1978; Farber et al., 1988; Portera-Cailliau et al., 1994). In thePdegtm1/Pdegtm1mouse with a mutation in the γ-subunit of PDE, almost all cells die between P14 and P21 (Tsang et al., 1996). In the opsin S334ter mutant, as in the rd mouse, the mutation leads to the onset of apoptosis at P8 and completion by P21 (Chen et al., 1996). In contrast to these mutants, cell death in the rds retina starts at P14–P17 and progresses slowly for a full year (Sanyal et al., 1980;Nir et al., 1990). The increased effect of Bcl-2 expression on the preservation of photoreceptors in rds mice may be caused by the slower pace of apoptosis in this mutation. In assessing the significance of the present data it should be noted that the rate of cell death in rds mice more closely resembles the rate observed in human RP and other forms of progressive neuronal degeneration.
A key feature of the rds phenotype is absent outer segments (Jansen and Sanyal, 1984; Nir and Papermaster, 1986), because of loss of the structural protein rds/peripherin (Travis et al., 1989, 1991;Connell et al., 1991). Not surprisingly, Bcl-2 expression did not reverse the rds phenotype of absent outer segments (Fig. 4) because expression of this antiapoptotic protein does not correct the inherent genetic mutations leading to the structural defect. The protective effect of Bcl-2 on photoreceptor survival must be by an alternative mechanism. Because Bcl-2 was shown to protect against diverse cytotoxic insults (Adams and Cory, 1998), it is not certain what type of stress triggers apoptosis in the rds retina. One possibility is that the absence of outer segments in rdsmice may predispose photoreceptors to oxygen toxicity. The loss of outer segments might be accompanied by the loss of the cGMP-gated cation channels and a dramatic reduction in cation influx. This may “unload” the Na+/K+ ATPase pumps, resulting in significantly reduced oxygen consumption by inner segment mitochondria and a much flatter pO2gradient across the distal retina. Also, with the loss of outer segments, the photoreceptor cell bodies are physically closer to the oxygen-rich choriocapillaris. These two effects may conspire to produce toxic levels of oxygen in the microenvironment of rdsphotoreceptor cell bodies. The association between apoptotic cell death and the presence of reactive oxygen species is well established. Bcl-2 was shown to prevent neuronal cell death by decreasing the net generation of oxygen species (Kane et al., 1993). Overexpression of Bcl-2 suppressed lipid peroxidation completely (Hockenbery et al., 1993). Also, in a study of Bcl-2-deficient mice, enhanced oxidative stress and susceptibility to oxidants were found (Hochman et al., 1998). Thus, Bcl-2 may be particularly effective in preventing cell death attributable to oxidative damage.
Although expression of Bcl-2 in rds:bcl mice resulted in a near doubling of surviving photoreceptors compared with that in nontransgenic rds mutants, only ∼60% of the photoreceptors were protected from degeneration compared with fully rescued rds:113 male controls. This persistent cell loss was probably not caused by direct Bcl-2 toxicity, because we observed similar numbers of photoreceptors in rds:113 andrds:113+bcl mice. In this study we used a low-expressing Bcl-2-transgenic line (line B), which did not cause photoreceptor degeneration on an otherwise wild-type genetic background (Chen et al., 1996). Higher levels of Bcl-2, or additional members of the Bcl family (besides Bcl-XL), may be required for complete protection. Alternatively, it may be impossible fully to prevent photoreceptor degeneration attributable to mutations in a critical gene by expression of proteins that inhibit apoptosis.
We showed in a previous study that female mice hemizygous for the 113 transgene displayed a mosaic pattern of normal rds/peripherin expression, both by in situ hybridization and outer segment ultrastructure, because of random inactivation of chromosome X (Kedzierski et al., 1998). However, retinal degeneration in these mice was uniform, with no mosaic pattern of cell loss. This observation indicates a non-cell-autonomous component of photoreceptor degeneration in rds mice (Kedzierski et al., 1998). Before the current study, we anticipated that Bcl-2 expression in rds:113+bclhemizygous females may preferentially protect photoreceptors that express the rds rescue transgene, because these cells are entirely normal in the context of transgenic male or homozygous female retinas. This was not observed, however. Expression of Bcl-2 inrds:113+bcl hemizygous females resulted in no additional protection against photoreceptor degeneration compared with that inrds:113 hemizygous females (Fig. 2). The degree of rescue inrds:113 hemizygous females was higher here than in our previous study (Kedzierski et al., 1998). This may be attributable to strain effects on the time of X-chromosome inactivation, which would affect the coarseness of the mosaic.
In summary, Bcl-2 had the greatest protective effect on unrescuedrds photoreceptors. Because rds photoreceptors fail to form outer segments (Jansen and Sanyal, 1984; Nir and Papermaster, 1986), we suggest that the major activity of Bcl-2 is to protect against oxygen-mediated toxicity. Shortening of outer segments is an ultrastructural feature of multiple inherited retinal degenerations, including several that affect humans (Travis, 1998). The results of this study suggest that somatic gene therapy involving the delivery of Bcl-2 to diseased photoreceptors may slow the progression in some forms of human inherited blindness.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant EY10286 to I.N., NIH Grant EY08043 and a grant from the Foundation Fighting Blindness to G.H.T., and NIH Grants EY12155 and EY12703 and grants from the Ruth and Milton Steinbach Fund and Research to Prevent Blindness to J.C. We thank Annemarie Brown and Roxana Radu for their excellent technical support.
Correspondence should be addressed to Dr. Izhak Nir, Department of Pharmacology, University of Texas Health Science Center, San Antonio, TX 78284. E-mail: nir@uthscsa.edu.
REFERENCES
- 1.Abler AS, Chang CJ, Ful J, Tso MO, Lam TT. Photic injury triggers apoptosis of photoreceptor cells. Res Commun Mol Pathol Pharmacol. 1996;92:177–189. [PubMed] [Google Scholar]
- 2.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 3.Carter-Dawson LD, LaVail MM, Sidman RL. Different effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- 4.Chang G-Q, Hao Y, Wong F. Apoptosis: final common pathway of photoreceptor death in rd, rds and rhodopsin mutant mice. Neuron. 1993;11:595–605. doi: 10.1016/0896-6273(93)90072-y. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Flannery JG, LaVail MM, Steinberg RH, Xu J, Simon MI. bcl-2 overexpression reduces apoptotic photoreceptor cell death in three different retinal degenerations. Proc Natl Acad Sci USA. 1996;93:7042–7047. doi: 10.1073/pnas.93.14.7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connell G, Bascom R, Molday R, Reid D, McInnes RR, Molday RS. Photoreceptor peripherin is the normal product of the gene responsible for retinal degeneration in the rds mouse. Proc Natl Acad Sci USA. 1991;88:732–736. doi: 10.1073/pnas.88.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook B, Lewis GP, Fisher SK, Adler R. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995;36:990–996. [PubMed] [Google Scholar]
- 8.Farber DB, Sunmin P, Yamashita C. Cyclic GMP-phosphodiesterase of rd retina: biosynthesis and content. Exp Eye Res. 1988;46:363–374. doi: 10.1016/s0014-4835(88)80026-5. [DOI] [PubMed] [Google Scholar]
- 9.Hochman A, Sternin H, Gorodin S, Korsmeyer S, Ziv I, Melamed E, Offen D. Enhanced oxidative stress and altered antioxidants in the brain of Bcl-2 deficient mice. J Neurochem. 1998;71:741–748. doi: 10.1046/j.1471-4159.1998.71020741.x. [DOI] [PubMed] [Google Scholar]
- 10.Hockenbery DM, Oltvai ZN, Yin X-M, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 11.Jansen HG, Sanyal S. Development and degeneration of retina in rds mutant mice: electron microscopy. J Comp Neurol. 1984;224:71–84. doi: 10.1002/cne.902240107. [DOI] [PubMed] [Google Scholar]
- 12.Joseph RM, Li T. Overexpression of Bcl-2 or Bcl-XL transgenes and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 1996;37:2434–2446. [PubMed] [Google Scholar]
- 13.Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, Ord T, Bredesen DE. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 14.Kedzierski W, Bok D, Travis GH. Non-cell-autonomous photoreceptor degeneration in rds mutant mice mosaic for expression of a rescue transgene. J Neurosci. 1998;18:4076–4082. doi: 10.1523/JNEUROSCI.18-11-04076.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kedzierski W, Weng J, Travis GH. Analysis of the rds/peripherin–rom1 complex in transgenic photoreceptors that express a chimeric protein. J Biol Chem. 1999;274:29181–29187. doi: 10.1074/jbc.274.41.29181. [DOI] [PubMed] [Google Scholar]
- 16.Keen TJ, Inglehearn CF. Mutations and polymorphisms in the human peripherin-RDS gene and their involvement in inherited retinal degeneration. Hum Mutat. 1996;8:297–303. doi: 10.1002/(SICI)1098-1004(1996)8:4<297::AID-HUMU1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 18.Krippner A, Matsuno-Yagi A, Gottlieb RA, Babior BM. Loss of function of cytochrome c in Jurkat cells undergoing fas-mediated apoptosis. J Biol Chem. 1996;271:21629–21636. doi: 10.1074/jbc.271.35.21629. [DOI] [PubMed] [Google Scholar]
- 19.Levin LA, Schlamp CL, Spieldoch RL, Geszvain KM, Nickells RW. Identification of the bcl-2 family of genes in the rat retina. Invest Ophthalmol Vis Sci. 1997;38:2545–2553. [PubMed] [Google Scholar]
- 20.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 21.Lolley RN, Rong H, Craft CM. Linkage of photoreceptor degeneration by apoptosis with inherited defect in phototransduction. Invest Ophthalmol Vis Sci. 1994;35:358–362. [PubMed] [Google Scholar]
- 22.Molday SM. Photoreceptor membrane proteins, phototransduction, and retinal degenerative diseases. Invest Ophthalmol Vis Sci. 1998;39:2493–2513. [PubMed] [Google Scholar]
- 23.Molthagen M, Schachner M, Bartsch U. Apoptotic cell death of photoreceptor cells in mice deficient for the adhesion molecule on glia (AMOG, the beta 2-subunit of the Na, K-ATPase). J Neurocytol. 1996;25:243–255. doi: 10.1007/BF02284800. [DOI] [PubMed] [Google Scholar]
- 24.Nir I, Papermaster DS. Immunocytochemical localization of opsin in the inner segment and ciliary plasma membrane of photoreceptors in retinas of rds mutant mice. Invest Ophthalmol Vis Sci. 1986;27:836–840. [PubMed] [Google Scholar]
- 25.Nir I, Agarwal N, Papermaster DS. Opsin gene expression during early and late phases of retinal degeneration in rds mice. Exp Eye Res. 1990;51:257–267. doi: 10.1016/0014-4835(90)90022-m. [DOI] [PubMed] [Google Scholar]
- 26.Portera-Cailliau C, Sung C-H, Nathans J, Adler R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc Natl Acad Sci USA. 1994;91:974–978. doi: 10.1073/pnas.91.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuter JH, Sanyal S. Development and degeneration of retina in rds mutant mice: the electroretinogram. Neurosci Lett. 1984;48:231–237. doi: 10.1016/0304-3940(84)90024-7. [DOI] [PubMed] [Google Scholar]
- 28.Sanyal S, De Ruiter A, Hawkins RK. Development and degeneration of retina in rds mutant mice: light microscopy. J Comp Neurol. 1980;194:193–207. doi: 10.1002/cne.901940110. [DOI] [PubMed] [Google Scholar]
- 29.Shastry BS. Signal transduction in the retina and inherited retinopathies. Cell Mol Life Sci. 1997;53:419–429. doi: 10.1007/s000180050050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Travis GH. Mechanism of cell death in the inherited retinal degenerations. Am J Hum Genet. 1998;62:503–508. doi: 10.1086/301772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Travis GH, Brennan MB, Danielson PE, Kozak CA, Sutcliffe JG. Identification of a photoreceptor-specific mRNA encoded by the gene responsible for retinal degeneration slow (rds). Nature. 1989;338:70–73. doi: 10.1038/338070a0. [DOI] [PubMed] [Google Scholar]
- 32.Travis GH, Sutcliffe JG, Bok D. The retinal degeneration slow (rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron. 1991;6:61–70. doi: 10.1016/0896-6273(91)90122-g. [DOI] [PubMed] [Google Scholar]
- 33.Travis GH, Groshan KR, Lloyd M, Bok D. Complete rescue of photoreceptor dysplasia and degeneration in transgenic retinal degeneration slow (rds) mice. Neuron. 1992;9:113–119. doi: 10.1016/0896-6273(92)90226-4. [DOI] [PubMed] [Google Scholar]
- 34.Tsang SH, Gouras P, Yamashita CK, Kjeldbye H, Fisher J, Farber DB, Goff SP. Retinal degeneration in mice lacking the g-subunit of the rod cGMP phosphodiesterase. Science. 1996;272:1026–1029. doi: 10.1126/science.272.5264.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsang SH, Chen J, Kjeldbye H, Li W, Simon MI, Gouras P, Goff SP. Retarding photoreceptor degeneration in Pdegtm1/Pdegtm1 mice by an apoptosis suppressor gene. Invest Ophthalmol Vis Sci. 1997;38:943–950. [PubMed] [Google Scholar]
- 36.Tso MOM, Zhang C, Abler AS, Chang CJ, Wong F, Chang GQ, Lam TT. Apoptosis leads to photoreceptor degeneration in inherited retinal dystrophy of RCS rats. Invest Ophthalmol Vis Sci. 1994;35:2693–2699. [PubMed] [Google Scholar]
- 37.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]