Abstract
1,1′-Dimethyl-4,4′-bipyridinium dichloride (methyl viologen; paraquat), an herbicide that causes depletion of NADPH and generates excessive reactive oxygen species (ROS) in vivo, has been used to screen for ROS-sensitiveDrosophila mutants. One mutant so isolated, namedquiver1(qvr1), has a leg-shaking phenotype. Mutants of the Shaker (Sh),Hyperkinetic (Hk), and ether a go-go (eag) genes, which encode different K+ channel subunits that regulate the A-type K+ current (IA) in different ways, exhibit leg shaking under ether anesthesia and have heightened metabolic rates and shortened life spans. We found thatSh, Hk, and eag mutant flies were all hypersensitive to paraquat. Double-mutant combinations among the three channel mutations andqvr1 had drastically enhanced sensitivity to paraquat. Synaptic transmission at the larval neuromuscular junction was increased in theqvr1 mutant to the level ofSh mutants. Similar to eag Sh double mutants, double mutants of eag andqvr1 showed striking enhancement in synaptic transmission and a wings-down phenotype, the hallmarks of extreme hyperexcitability. Voltage-clamp experiments demonstrated that the qvr1 mutation specifically disrupted the Sh-dependent IAcurrent without altering the other currents [IK, Ca2+-activated fast (ICF) and slow (ICS) currents, andICa] in larval muscles. Several deficiency strains of the qvr locus failed to complementqvr1 and confirmed that ether-induced leg shaking, reduced IA current, and paraquat hypersensitivity map to the same locus. Our results suggest that the qvr gene may encode a novel K+ channel-related polypeptide and indicate a strong link between a voltage-activated K+ current and vulnerability to ROS.
Keywords: Shaker, Hyperkinetic, ether a go-go, quiver, potassium channel, synaptic transmission, paraquat, free radical
A set of well studied mutations has defined a suite of phenotypes associated with defective K+ channels in Drosophila. In different ways, mutations of Shaker (Sh),ether a go-go (eag), and Hyperkinetic(Hk) impair the transient A-type K+ current (IA) in Drosophila muscles (Salkoff and Wyman, 1981; Wu et al., 1983; Wu and Haugland, 1985; Zhong and Wu, 1991; Wang and Wu, 1996) and neurons (Tanouye and Ferrus, 1985;Baker and Salkoff, 1990; Saito and Wu, 1993; Zhao et al., 1995; Yao and Wu, 1999). These genes encode either the pore-forming or auxiliary subunits of Sh-dependent K+channels (Kamb et al., 1988; Pongs et al., 1988; Schwarz et al., 1988;Warmke et al., 1991; Chouinard et al., 1995; Chen et al., 1996). These channel mutations enhance synaptic transmission at the larval neuromuscular junction (Jan et al., 1977; Ganetzky and Wu, 1983, 1985;Wu et al., 1983; Stern and Ganetzky, 1989), suggesting that theSh-dependent IA current has a functional role in terminating neurotransmitter release in the presynaptic terminal. Behavioral analysis has demonstrated thatSh-dependent K+ channels are crucial for the control of the peristaltic locomotion inDrosophila larvae (Wang et al., 1997).
Sh, eag, and Hk mutants are well known for their leg-shaking phenotype (Kaplan and Trout, 1969). However, little attention has been given to the observations that oxygen consumption is increased by Sh, eag, andHk mutations and longevity is inversely related to the enhancement of metabolic rate in these mutant flies (Trout and Kaplan, 1970). Drosophila, like other aerobic organisms, uses several enzymes for reactive oxygen species (ROS) homeostasis (Campell et al., 1986; Mackay and Bewley, 1989; Phillips et al., 1989; Staveley et al., 1990). The superoxide radical is catalytically reduced by superoxide dismutase (SOD) to hydrogen peroxide, which in turn is catalytically reduced to water by catalase (Fridovich, 1995). Genetic tools are available in Drosophila to investigate ROS homeostasis and relevant pathways (Phillips and Hilliker, 1990). 1,1′-Dimethyl-4,4′-bipyridinium dichloride (methyl viologen; paraquat) is an herbicide that generates superoxide in vivo at the expense of NADPH when oxygen is available. Susceptibility to millimolar concentrations of paraquat has been used successfully in screening for mutants in the ROS pathway (Phillips et al., 1989; Humphreys et al., 1993, 1996)
We demonstrated that like quiver (qvr) mutants,Drosophila K+ channel mutantsSh, eag, and Hk were also hypersensitive to paraquat challenge. The EMS-inducedqvr1 mutation, along with several deficiency lines, reduced the amplitude and slowed the kinetics ofIA, like several previously isolated leg-shaking mutants. These results elucidate the physiological roles of the qvr polypeptide and revealed functional similarities among qvr and the known IAK+ channel mutants.Sh-dependent K+ channels are known to be modulated not only by second messenger-dependent processes (Zhong and Wu, 1993b) but also by oxidoreduction (Schlief et al., 1996;Gulbis et al., 1999; J. Chen et al., 2000), which may provide a means to regulate synaptic efficacy. This study may initiate work toward a comprehensive understanding of qvr and K+ channel mutants to shed light on the link between ROS and K+ currents.
MATERIALS AND METHODS
Fly stocks. All flies were raised at room temperature (20–23°C) and fed with standard Drosophila medium. The parental stock qvr+;ry+5, for generating theqvr1 mutant, was originally derived from the wild-type strain Oregon-R and was used in this study as the control. The Canton-S (CS) wild-type strain, used for comparison, is not significantly different from Oregon-R in many physiological aspects examined in this study. The qvr locus was mapped previously to 48A (Humphreys et al., 1996). Df(2R)en-SFX31/CyO (48A1; 48B5-7) and w; Df(2R)en-B,b1pr1/CyO (47E3-6; 48A4-B2) were provided by the Bloomington Stock Center (Bloomington, IN). These two deficiency lines are homozygous lethal and failed to complement theqvr1 mutation in leg-shaking behavior and paraquat hypersensitivity (Humphreys et al., 1996).qvrΔ1-1, qvrΔ1-2,qvrΔ1-3, and qvrΔ1-4are homozygous lethal deficiency lines generated by mobilization and imprecise excision of a nearby P-element P[17en1] (Humphreys, 1996). qvrΔ43-1 is a homozygous lethal deficiency line generated by mobilization and imprecise excision of a nearby P-element P[17en43] (Humphreys, 1996). Except for qvrΔ1-1, all P-element mutagenesis lines failed to complement theqvr1 mutation in leg-shaking behavior in this study. P[17en1] and P[17en43] were kindly provided Dr. Judy Kassis at the Food and Drug Administration Center for Biologics Evaluation and Research.
Sh5,ShM, g sd ShrKO120 (abbreviatedSh120 in the text; see Table1), Hk1,eag1, andnapts1 were originally from the collection of Dr. Seymour Benzer at the California Institute of Technology. ShM is a null allele (Zhao et al., 1995) and eliminatesIA in larval muscles (Wu and Haugland, 1985). Sh5 is a point mutation in the S4–S5 cytoplasmic linker (Gautam and Tanouye, 1990) and alters the voltage dependence of IA(Wu and Haugland, 1985). TheHk2 strain is the original stock described in Kaplan and Trout (1969) and is kindly provided by Dr. Rodney Williamson at the Beckman Research Institute of the City of Hope. The compound mutantseag1Sh120,Hk1eag1, andeag1Sh120napts1 are the same stocks used in previous studies (Budnik et al., 1990). Other compound mutants were generated for this study. Compound mutants were all confirmed by scoring leg-shaking phenotype and electrophysiological phenotype in larval muscles. The semicolon for indication of mutations on separate chromosomes is omitted in the text for simplicity.napts1 is an EMS-induced mutation (Wu et al., 1978), which reduces the expression of sodium channels and is allelic with mle mutations (Kernan et al., 1991). Flies bearing this mutation become paralyzed at 37°C or higher because of the blocking of nerve action potentials.
Table 1.
Paraquat sensitivity of Drosophila channel mutants and qvr
| Genotype | 0 mm Paraquat | 10 mm Paraquat | ||
|---|---|---|---|---|
| % Survival | n | % Survival | n | |
| qvr+ | 99 | 259 | 97 | 340 |
| qvr1 | 99 | 238 | 42 | 445 |
| eag1 | 98 | 207 | 48 | 269 |
| Hk1 | 100 | 219 | 48 | 328 |
| Sh5 | 100 | 30 | 34 | 70 |
| Sh120 | 97 | 30 | 32 | 60 |
| napts1 | 80 | 20 | 28 | 50 |
| eag1Sh120 | 80 | 20 | 0 | 50 |
| eag1Sh120;napts1 | 100 | 30 | 12 | 60 |
| eag1Hk1 | Not done | 0 | 30 | |
| eag1;qvr1 | 82 | 33 | 0 | 59 |
| Sh5;qvr1 | 90 | 20 | 0 | 50 |
| Hk1;qvr1 | 93 | 40 | 2 | 90 |
Wings-down frequency. The frequency of wings-down flies was determined in male F1 noncurly flies of the following cross within 72 hr after eclosion: A/Y; qvr1/CyO × XX/Y; qvr1/CyO, where A represents the X chromosome carrying Hk1,Hk2,eag1, f eag4pm,Sh5, g sd Sh120, orShM. CyO is a second chromosome balancer carrying a dominant marker Cy (curly wings), and XX indicates a compound X chromosome, which carriesy and f markers. Flies with noncurly wings in the F1 generation were homozygous forqvr1, whereas those with curly wings were heterozygous for qvr1. Wings-down flies are flightless and sluggish in locomotion (Engel and Wu, 1992).
Paraquat feeding. The procedure for paraquat (from Sigma, St. Louis, MO) feeding was described previously (Humphreys et al., 1993, 1996). Briefly, 0- to 24-hr-old adult male flies were collected and allowed 24 hr to recover from ether anesthesia before being transferred to vials (10 flies/vial) for paraquat exposure. Flies were then exposed for 48 hr at 25°C to filter paper presoaked with paraquat dissolved in 1% sucrose solution. Flies were held in the dark during exposure, because paraquat is light sensitive. The survival rate was determined at the end of the 48 hr exposure period.
Electrophysiology. Dissection of Drosophilathird-instar larvae was performed in Ca2+-free saline to minimize muscle contraction. Excitatory junctional potentials (EJPs) were recorded intracellularly from muscles of abdominal segment 3–5 in third-instar larvae at room temperature (20–25°C) in HL3 saline (Stewart et al., 1994) containing 1 mmCaCl2. For measuring excitatory junctional currents (EJCs), muscle fibers were maintained at −80 mV with two-electrode voltage clamp at 16°C (Wang et al., 1994). A suction pipette with a tip opening of ∼1 μm was used to stimulate the segmental nerve to evoke synaptic transmission. Stimulus pulses of 0.1 msec duration were delivered at a low repetition rate of 0.5 Hz with a stimulator (model S88; Grass Instruments, Quincy, MA). Normally, two discrete EJC amplitudes were evoked at two different thresholds (Jan and Jan, 1976). A stimulus voltage slightly higher than the upper threshold was therefore used. Signals were low-pass filtered at 2 kHz (model 3202R; Krohn-Hite, Avon, MA). Temperature was controlled by a Peltier stage (Cambion, Cambridge, MA) when specified as different from room temperature.
The two-electrode voltage-clamp technique for measuring muscle K+ currents (IA, IK,ICF, and ICS) and Ba2+ currents has been described previously (Singh and Wu, 1989; Haugland and Wu, 1990; Wang and Wu, 1996). In brief, the voltage-gated IA andIK were recorded in Ca2+-free standard saline containing 128 mm NaCl, 2 mm KCl, 4 mm MgCl2, 35 mm sucrose, 5 mm EGTA, and 5 mm HEPES, pH 7.1. The Ca2+-activatedICF andICS were measured in the Ca2+-free standard saline plus 20 mm CaCl2 (Singh and Wu, 1989). This saline was made hypertonic with addition of 353 mm sucrose to prevent muscle contraction, and 1 mm 4-aminopyridine (4-AP) and 100 μm quinidine were added to blockIA andIK (Zhong and Wu, 1991). For experiments measuring the voltage-gated Ca2+ channel, Ba2+ was used as the charge carrier to assess the Ca2+ conductance without activating ICF andICS (Gielow et al., 1995). Ca2+ channel-mediated Ba2+ currents were examined in the Ca2+-free HL3 saline, with 1 mm 4-AP, 50 μm quinidine, and 20 mm tetraethylammonium (TEA) to block K+ currents and with 4 mm BaCl2. A Master-8 programmable stimulator (A.M.P.I., Jerusalem, Israel) and an IBM-compatible computer equipped with PClamp 5.0 (Axon Instruments, Foster City, CA) were used for voltage-pulse generation and data collection. Data analysis was performed off-line on Macintosh computers with AxoGraph 2.0 (Axon Instruments).
RESULTS
Increased paraquat sensitivity and excitability in double mutants
Vigorous leg shaking when ether-anesthetized, a phenotype similar to that of the previously identified K+channel mutants Sh, Hk, and eag, was observed in qvr1 mutant flies (Humphreys et al., 1996). This phenotypic similarity led us to perform a comprehensive test of paraquat sensitivity in those molecularly characterized leg-shaking mutants. As seen in Table1, when exposed to 10 mm paraquat for 48 hr,Sh5,Sh120,Hk1, andeag1 mutant flies had 32–48% survival rates, similar to that seen inqvr1 (42%) but much lower than that of wild-type controls (97%). These numbers for controls and mutants are consistent with those reported previously for some of these alleles (Humphreys et al., 1996).
Double mutants of eag and Sh are even more hyperexcitable than are eag or Sh single mutants in synaptic transmission at the larval neuromuscular junction (Ganetzky and Wu, 1983, 1985; Zhong and Wu, 1993a) and in the adult flight muscle system (Engel and Wu, 1992). To see whether ROS sensitivity was similarly enhanced in double-mutant combinations, we extended the paraquat-feeding study to include various double combinations amongqvr1 and mutations of the three K+ channel genes. A survival rate of 0% was observed in eag1Sh120 double-mutant flies fed with 10 mm paraquat, which was much more extreme than that of any single mutant (Table 1; 48% foreag1; 32% forSh120).Sh5qvr1,Hk1qvr1, andeag1qvr1 double-mutant flies showed 0, 2, and 0% survival rates after exposure to paraquat, lower than that of each single mutant. napts1, a mutation reducing the expression of a Na+channel and suppressing the hyperexcitability ineag1Sh120napts1 triple mutants (Wu and Ganetzky, 1992), lowered the paraquat-induced mortality ineag1Sh120napts1 mutants, despite the fact that the napts1 mutant flies showed a significant lower survival rate compared with that of the wild-type controls (Table 1). These results indicate that hyperexcitability is closely correlated with paraquat hypersensitivity.
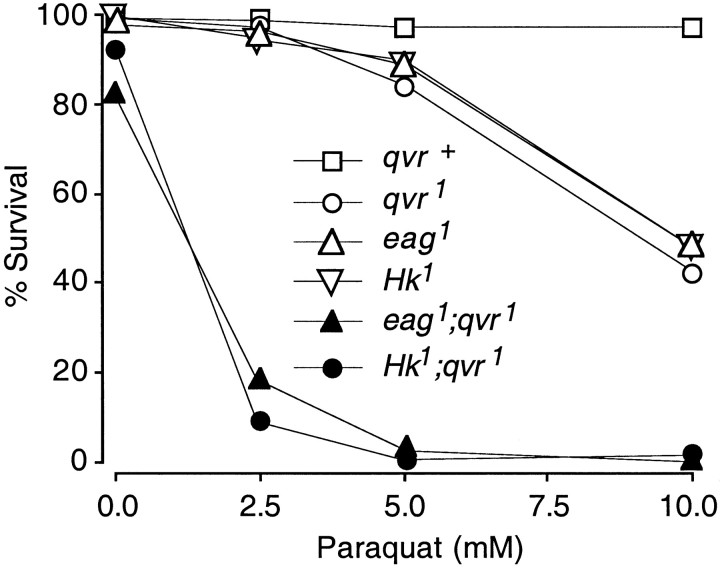
The dosage dependence of survival rate after exposure to paraquat is shown in Figure 1. A noticeable number of double-mutants flies died even without exposure to paraquat. For example, the survival rates for eag1qvr1 andHk1qvr1 in 0 mmparaquat were 82 and 93%, respectively. This could be attributed to the shorter life span of hyperactive flies (Trout and Kaplan, 1970).
Fig. 1.
Paraquat hypersensitivity. Adult flies (24–48 hr old) were fed with paraquat for 48 hr at 25°C in darkness, and the survival rate was determined at the end of the 48 hr period. K+ current mutantseag1 andHk1 were as sensitive to paraquat as was the qvr1 mutant. The double mutants eag1qvr1 andHk1qvr1 were more sensitive to paraquat than were any of the single mutants, indicating synergistic interactions between the qvr1mutation and the K+ current mutations. Forqvr+, n = 259, 259, 293, and 340 (from 0 to 10 mm paraquat); forqvr1, n = 238, 257, 268, and 445; for Hk1,n = 219, 189, 190, and 328; foreag1; n = 207, 250, 250, and 269; for eag1qvr1, n = 33, 44, 37, and 97; for Hk1qvr1, n = 40, 47, 49, and 140.
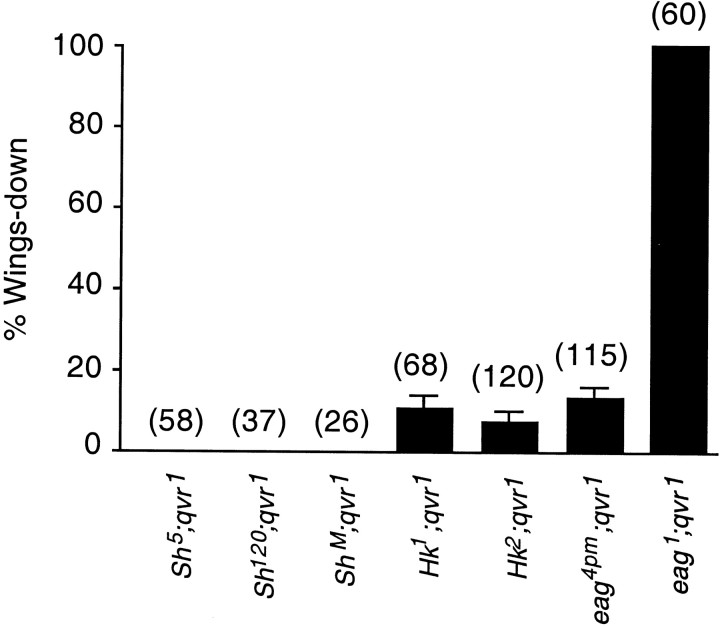
A novel phenotype arose from double mutants ofeag1 andqvr1, similar to the synergistic effects seen in eag Sh double mutants. Wings of the double mutants pointed downward instead of extending horizontally as in normal flies. This “wings-down” phenotype has been studied previously ineag Sh double mutants (Engel and Wu, 1992). It is a hallmark of hyperexcitable mutants (Ganetzky and Wu, 1985) and has been used in mutant screening (Stern and Ganetzky, 1992).
As can be seen in Figure 2, nearly 100% of eag1qvr1 double-mutant flies showed the wings-down phenotype. Hk1qvr1 andeag4pmqvr1 double mutants had 10 and 13% of the flies, respectively, exhibiting the wings-down phenotype. No wings-down flies were observed inSh5qvr1,ShMqvr1, orSh120qvr1, although leg shaking was more vigorous in these double mutants than in Sh orqvr1 alone. Furthermore, no wings-down flies were seen inHk1Sh5 andHk1Sh120 double mutations, in contrast to the 10% wings-down frequency seen in theHk1qvr stock. The sequence of potency for causing the wings-down phenotype iseag1 >eag4pm >Hk1 >Hk2 >ShM (Fig. 2).
Fig. 2.
Wings-down frequency in hyperactive mutants. Nearly 100% of the double-mutanteag1qvr1 flies were wings-down. See Materials and Methods for the determination of wings-down frequency. Error bars represent SD. SD = √(p(1 − p)/n), where pis the wings-down frequency and n is the number of flies. The number of flies examined is indicated aboveeach bar in parentheses.
Synaptic transmission at the larval neuromuscular junction
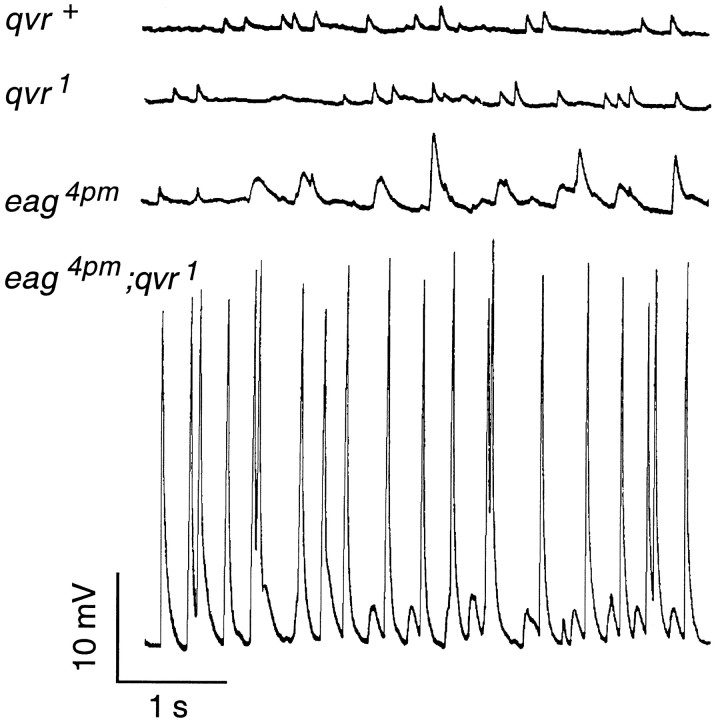
A unique property of eag mutants is that they display spontaneous EJPs, caused by spontaneous firing in the hyperexcitable motor axons (Ganetzky and Wu, 1982), which are different in amplitude and frequency from miniature EJPs (MEJPs). The frequency of spontaneous EJPs is higher in eag1 than ineag4pm (Ganetzky and Wu, 1983). This is correlated to the degree in hyperexcitability conferred by differenteag mutations, with eag1affecting K+ currents in larval muscles more than eag4pm (Zhong and Wu, 1991). The frequency and amplitude of the spontaneous EJPs were drastically increased by the qvr1mutation in eag4pmqvr1 double mutants (Fig.3). However, theqvr1 mutation itself did not cause any noticeable alteration in the amplitude, time course, or frequency of MEJPs. Similar synergistic interaction has been observed betweenSh and eag in double-mutant combinations (Ganetzky and Wu, 1983). This suggests that a presynaptic rather than a postsynaptic alteration conferred by theqvr1 mutation is responsible for the enhancement of the spontaneous EJP phenotype of eag.
Fig. 3.
Enhancement of nerve activity by theqvr1 mutation. In wild-type neuromuscular junctions of third-instar larvae, MEJPs were observed without nerve stimulation (top trace) as a result of spontaneous quantal release. The qvr1mutant displayed MEJPs similar to that of the wild-type control (second trace from top). However, the amplitude and frequency of the spontaneous EJPs (recognized by amplitudes larger than quantal size) seen ineag4pm mutants were both drastically increased by the qvr1 mutation in theeag4pmqvr1 double mutant (bottom two traces). Experiments were done at room temperature (23°C) in HL3 saline containing 1.0 mm CaCl2 and 20 mm MgCl2.
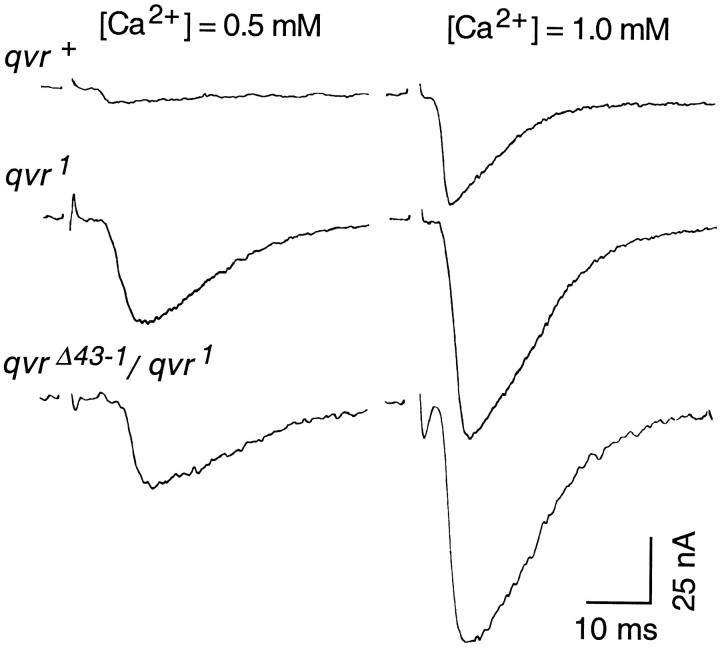
EJCs serve as a quantitative measurement of synaptic transmission, because muscles are held at a constant membrane potential by the voltage-clamp technique to provide a constant driving force and thus avoid nonlinear summation of multiple quantal release in EJP recordings. As described in other species, the amplitude of EJCs follows a fourth-power relationship with external Ca2+ concentration in theDrosophila larval neuromuscular system (Zhong and Wu, 1991;Stewart et al., 1994; Wang et al., 1994). At an external Ca2+ concentration of 0.4 mm, the increase in EJC amplitude caused by theqvr1 mutation [27.2 ± 7.4 nA (EJC ± SD); n = 6] was no greater than that by a null mutation, ShM (32.2 ± 9.1; n = 5), and was not further enhanced inqvr1ShM double mutants (34.6 ± 3.4; n = 5), suggesting that qvr andSh share the same pathway in the regulation of synaptic transmission. The difference betweenqvr1 andqvr+ control is proportionally greater at 0.5 than at 1.0 mm[Ca2+]o (Fig.4). At these Ca2+ concentrations, the EJC amplitudes ofqvr1 andqvr1/qvrΔ43-1mutants did not follow the fourth-power relationship, indicating that defective K+ currents in these mutants could weaken membrane repolarization and cause transmitter release approaching the saturation level at relative lower concentrations. This could be caused by approaching saturation of the glutamate receptors on the postsynaptic membrane at the higher [Ca2+]o, which sets the ceiling of EJCs.
Fig. 4.
Enhanced EJCs in qvr mutations. Muscle membrane potential was voltage-clamped at −80 mV. Experiments were done at 16°C in HL3 saline containing the indicated CaCl2 concentrations and 20 mmMgCl2.
qvr mutations specifically affect theIA current
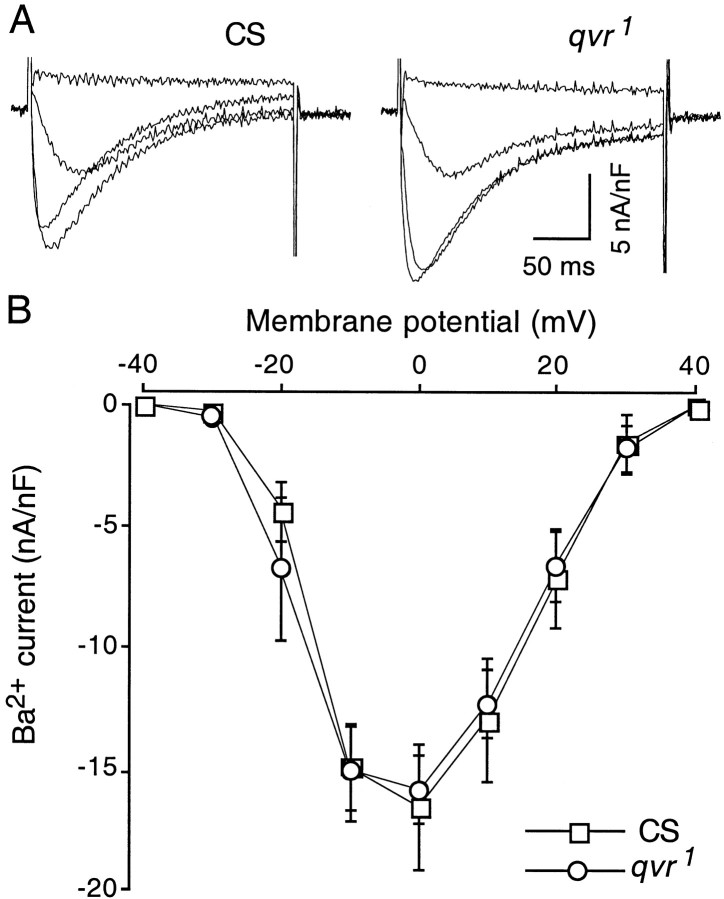
Outward K+ currents inDrosophila larval muscles can be separated into at least four different components: two voltage-dependent currents, a transient (IA) and a delayed rectifier (IK) current, and two Ca2+-activated currents, a fast (ICF) and a slow (ICS) current (Singh and Wu, 1989). Invertebrate muscles generally do not express Na+ channels, and their inward currents are mediated by Ca2+ channels (Schwartz and Stühmer, 1984), which is also true for Drosophilamuscles. We first examined Ca2+ channels for possible defects in qvr1 because of their important role in neurotransmitter release. Quinidine, 4-AP, and TEA were used to block IA andIK (Gielow et al., 1995). Ba2+ ions, which pass through Ca2+ channels with high permeability, were used here as the charge carrier to avoid activating the Ca2+-activated K+ currentsICF andICS. Figure5 shows that the Ca2+ current in larval muscle was not affected by the qvr1 mutation.
Fig. 5.
Ca2+ currents in muscle cells were not altered by the qvr1mutation. A, Representative traces of inward currents mediated by Ca2+ channels at membrane potentials from −30 to 0 mV in 10 mV increment.B, I–V curves forqvr1 and CS larvae. The holding potential was −80 mV. Ba2+ (4 mmBaCl2) replaced Ca2+ in the standard saline as the charge carrier to assess the Ca2+ conductance without activatingICF and ICS. Other K+ currents were blocked by 1 mm4-AP, 20 mm TEA, and 50 μm quinidine. Data are the mean ± SEM measured at 11°C.
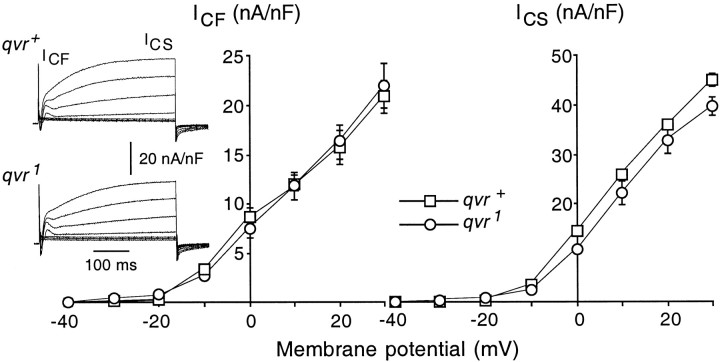
K+ channels are thought to terminate synaptic transmission by a rapid membrane repolarization (Hille, 1992). The paraquat hypersensitivity and the enhanced synaptic transmitter release seen in both qvr mutants and K+ channel mutants suggest that theqvr polypeptide might have a functional role in the modulation of K+ channels. All four K+ currents mentioned above were examined in qvr1 mutant larvae (Figs.6, 7). The Ca2+-activated outward K+ currentsICF andICS were examined in the presence of 20 mm Ca2+, and the saline contained 1 mm 4-AP and 100 μm quinidine to block the voltage-activatedIA andIK. Under these conditions there were no significant differences in the amplitude or kinetics of the outward currents ICF andICS induced by membrane depolarization (Fig. 6).
Fig. 6.
Ca2+-activated K+ currents were not altered by theqvr1 mutation. Traces(left) represent outward currents generated by membrane depolarization to different voltages ranging from −40 to 30 mV at an increment of 10 mV from a holding potential of −80 mV. Standard saline contained 20 mm CaCl2 and 4 mm MgCl2. Voltage-gated K+currents were blocked by 1 mm 4-AP and 100 μmquinidine. Tonicity of the saline was increased by adding 353 mm sucrose to reduce muscle contraction. Data are the mean ± SEM measured at 11°C.
Fig. 7.
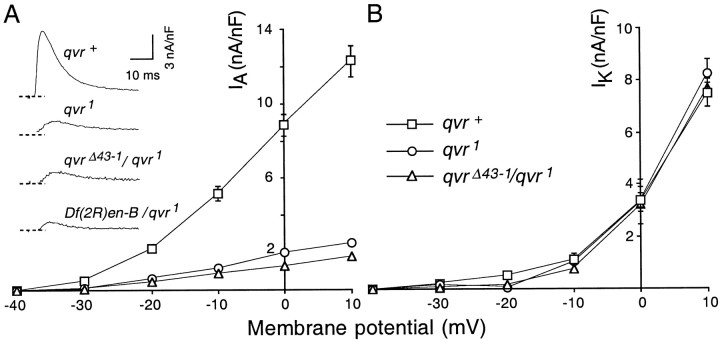
The transient IA and delayed IK currents in qvrmutant muscles. Larval preparations were dissected and recorded in Ca2+-free standard saline containing 14 mm MgCl2 and 5 mm EGTA. The membrane potential was held at −80 mV. A,Right, The amplitude ofIA was drastically reduced byqvr mutations. Left, The activation kinetics of IA was slower inqvr mutants as shown in the representative currenttraces generated by membrane depolarization to +10 mV (see also Table 2, Time to peak). Recordings during the first 3 msec show a capacitive transient and have been omitted for clarity.B, IK was not altered byqvr mutations. I–V curves showIK measured at the end of the depolarization pulse (between 190 and 200 msec after the onset of depolarization) when a plateau was reached. Data are the mean ± SEM.
When Ca2+-free saline is used, onlyIA andIK are activated by a step of depolarizing voltage. IA andIK can be separated physiologically by their different responses to a 2 sec conditioning prepulse from a holding potential of −80 to −20 mV, which inactivatesIA but leavesIK intact when they are assessed by a test pulse delivered 10 msec later (Haugland and Wu, 1990). Figure 7shows that only the IA current was affected by the mutation. The IAcurrent in qvr1 mutants appeared to have a very unstable component that inactivated easily and recovered slowly and incompletely (J. W. Wang and C.-F. Wu, unpublished observations). For simplicity, only the stable and fast-recovery component is presented here. The amplitude of the transientIA was greatly reduced at various membrane potentials as seen in the I–V curve, and the kinetics of IA was slower in theqvr mutations as the time to peakIA was lengthened (Table2; Fig. 7, representativetraces). When larval muscles were depolarized to +10 mV from a holding potential of −80 mV, the average amplitude ofIA for theqvr1 mutant larvae was 2.5 ± 0.3 nA/nF, only 20% of the wild-typeIA current (12.3 ± 0.8 nA/nF) inqvr+ larvae.
Table 2.
Alteration of IA in qvrdeficiency lines
| Genotype | IA (nA/nF) | Time to peak (msec) | n |
|---|---|---|---|
| qvr+ | 12.3 | 7.2 | 8 |
| qvr1 | 2.5 | 11.9 | 5 |
| qvrΔ1-1/qvr1 | 9.3 | 8.0 | 3 |
| qvrΔ1-2/qvr1 | 1.2 | 10.8 | 2 |
| qvrΔ1-3/qvr1 | 1.6 | 10.6 | 2 |
| qvr1-4/qvr1 | 1.3 | 11.3 | 2 |
| qvrΔ43-1/qvr1 | 1.8 | 11.6 | 5 |
| Df(2R)en-B/qvr1 | 1.8 | 10.4 | 2 |
| Df(2R)en-SFX31/qvr1 | 1.9 | 11.7 | 2 |
The EMS mutagenesis of the second chromosome yielded only one paraquat-hypersensitive leg-shaking allele,qvr1. To attribute the observed physiological phenotype to the qvr locus defined on the basis of paraquat hypersensitivity, we examined two deficiencies,Df(2R)en-B and Df(2R)en-SFX31, that cover a chromosome region that containsqvr1. In addition, we generated five new deficiency lines from the mobilization of two P-elements that map near the qvr locus. All of these deficiency lines are homozygous lethal. Four deficiency lines designatedqvrΔ1-1, qvrΔ1-2,qvrΔ1-3, and qvrΔ1-4were obtained by mobilizing the P-element in P[17en1]. TheqvrΔ43-1 mutation was obtained by mobilizing the P-element in P[17en43]. All of these deficiency lines except qvrΔ1-1 failed to complement the qvr1 mutation in the leg-shaking behavioral test. As shown in Table 2, heterozygotes between these deficiencies and qvr1 showed a reduction in IA amplitude and slowerIA kinetics as indicated by the time to peak IA. These heterozygotes, exceptqvrΔ1-1/qvr1, did not show a significantly different amplitude ofIA from that of theqvr1 mutation. These results establish that the physiological phenotype comaps with the leg-shaking and paraquat hypersensitivity to the qvr locus.
DISCUSSION
In this study we present a genetic and physiological characterization of a novel leg-shaking mutation,qvr1. The observed paraquat hypersensitivity in Sh, Hk, and eagmutant flies may be related to the shorter life span and increased metabolic rate in these hyperactive mutants (Trout and Kaplan, 1970), which could increase ROS production and thus confers paraquat hypersensitivity. The measurement of survival rate in double mutants suggests that the hypersensitivity to paraquat is closely related to membrane hyperexcitability. It should be noted that the Cu/Zn superoxide dismutase mutationcSODn108 or exposure of wild-type flies to 1 mm paraquat did not alter the conductance or kinetics of the IAcurrent in larval muscles (Wang and Wu, unpublished observations) and that the enzymatic levels of catalase or cSOD are normal inqvr1 mutant flies (Humphreys, 1996). These results suggest that general disturbance in ROS homeostasis per se does not alter IA currents. Similar to the eag Sh double mutants, double mutants ofeag and qvr1 showed a wings-down phenotype, the hallmark of extreme hyperexcitability. However, the mutation cSODn108, when combined with Sh5,Hk1, oreag1, did not generate any wings-down double-mutant flies (J. M. Humphreys, A. J. Hilliker, and J. P. Phillips, unpublished observations), suggesting that the wings-down phenotype may be caused by hyperexcitability instead of an increase in the ROS level. These observations raise an interesting possibility that a defect inIA K+channels can disrupt K+ ion homeostasis and in turn results in excessive ROS. This could be confirmed in the future by measuring the ROS level in all of these K+ channel mutants.
Null mutations of the Sh gene eliminate theIA current (Wu et al., 1983), whereas the major component of IK inDrosophila muscles is abolished by a deficiency in theShab locus (Tsunoda and Salkoff, 1995; Singh and Singh, 1999). Deletion of the slowpoke gene removesICF current (Elkins et al., 1986;Komatsu et al., 1990). In contrast to these mutations of K+ channel α subunits, null mutations of the β subunit modify but do not abolishIA (Wang and Wu, 1996; Yao and Wu, 1999). Furthermore, the specific effect of qvr mutations onIA current instead of a more global effect on K+ currents parallels the phenotype of Hk mutations. Mutation of qvrdisrupted the modulation of but did not eliminateIA. The phenotypic similarities of physiological hyperexcitability and leg-shaking behavior betweenqvr and the other K+ channel mutants Sh, Hk, and eag suggest that the qvr gene might encode a novel K+ channel-related polypeptide. Heterozygotes between several deficiencies andqvr1 showed phenotypes similar to that of the qvr1 homozygote in the amplitudes of IA and EJC, suggesting that qvr1 may be a null mutation.
The molecular cloning and physiological characterization of theSh, eag, and Hk genes have served to point out the complex molecular machinery required for the proper functioning of K+ channels. On the basis of the reduction of all four muscle K+currents, IA,IK,ICF, andICS, in eag mutations (Zhong and Wu, 1991) and a multiplicity of modulation sites by protein kinases and cyclic nucleotides on the eag polypeptide (Warmke et al., 1991; Griffith et al., 1994), it has been hypothesized that the eag polypeptide interacts with other K+ α channel subunits of theSh family to confer channel modulation (Zhong and Wu, 1993a). Interacting channel aggregates or heteromultimeric channel assemblies can therefore increase the functional diversity of K+ currents. Coexpression ofeag and Sh in the Xenopus oocyte has subsequently confirmed an interaction between gene products ofeag and Sh (Chen et al., 1996; M. L. Chen et al., 2000). The intricacy of K+ channel function is further increased by the β subunit encoded by theHk gene (Chouinard et al., 1995), which modulates the properties of the IA channel in conductance and kinetics (Wang and Wu, 1996; Yao and Wu, 1999). The rich modulation seen in the IA channel appears to be reasonable for its important role in regulating the delay in initiation and frequency coding of action potentials (Connor and Stevens, 1971; Zhao and Wu, 1997; Yao and Wu, 1999). A comprehensive study of the qvr gene by mutational analysis will lead to a more complete picture of the intricate molecular mechanism underlying the wide-ranging function of K+ channels. Apparently, the lack of proper qvr function could lead to unstable Sh channels. In qvr mutants, the amplitude of Sh IA was highly use dependent. It had a component that was very easily inactivated and recovered very slowly after being inactivated. Therefore, IA in qvr muscle declined quickly to a steady-state level during repeated activation (see Results; Fig. 7). This property may have important functional implications that can only be elucidated in further physiological experiments using in vivo preparations. It is not likely that the expression of Sh channels is affected by theqvr mutation because the Sh current after full recovery displayed nearly normal amplitude. With further information from molecular cloning and the availability of highly specificSh antibodies, some of the above issues may be resolved in a more definitive manner.
Recent studies have demonstrated that oxidation of amino residues in K+ channels can modify their kinetic and gating properties. In particular, several cloned channels of theSh family have been shown to be regulated by oxidation (Schlief et al., 1996; J. Chen et al., 2000). Future experiments to sequence the qvr gene may elucidate the molecular mechanism of the qvr gene. The relevant biochemical or physiological pathways will be important in understanding the link between neuronal excitability and ROS homeostasis and the pathology of diseases that have been correlated to abnormal levels of ROS (Rosen et al., 1993; Busciglio and Yankner, 1995; Youdim and Riederer, 1997).
Footnotes
This work was supported by National Institutes of Health Grants NS 18500 and NS 26528 to C.-F.W. and a grant from the Medical Research Council of Canada to J.P.P. and A.J.H. We thank Mr. Peter Taft for assistance in genetic experiments, Dr. Rodney Williamson for providing the Hk2 stock, and Drs. Jeff Engel and Christopher Rodesh for comments on this manuscript.
Correspondence should be addressed to Dr. Jing W. Wang, Howard Hughes Medical Institute, Columbia University, 701 West 168th Street, Hammer Health Sciences, 10th Floor, New York, NY 10032.
Dr. Humphreys's present address: School of Biological Sciences and Applied Chemistry, Seneca College of Applied Arts and Technology, North York, Ontario, M3J 3M6 Canada.
Dr. Phillips's present address: Department of Biology, York University, Toronto, Ontario, M3J 1P3 Canada.
REFERENCES
- 1.Baker K, Salkoff L. The Drosophila Shaker gene codes for a distinctive K+ current in a subset of neurons. Neuron. 1990;2:129–140. doi: 10.1016/0896-6273(90)90449-p. [DOI] [PubMed] [Google Scholar]
- 2.Budnik V, Zhong Y, Wu C-F. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busciglio J, Yankner BA. Apotosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature. 1995;378:776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- 4.Campell SD, Hilliker AJ, Phillips JP. Cytogenetic analysis of the cSOD microregion in Drosophila melanogaster. Genetics. 1986;112:205–215. doi: 10.1093/genetics/112.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Avdonin V, Ciorba MA, Heinemann SH, Hoshi T. Acceleration of P/C-type inactivation in voltage-gated K+ channels by methionine oxidation. Biophys J. 2000;78:174–187. doi: 10.1016/S0006-3495(00)76583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen ML, Hoshi T, Wu C-F. Heteromulteric interactions among K+ channel subunits from Shaker and eag families in Xenopus oocytes. Neuron 17 1996. 535 542 M. L. [DOI] [PubMed] [Google Scholar]
- 7.Chen ML, Hoshi T, Wu CF (2000) Sh and Eag K+channel subunit interaction in frog oocytes depends on level and time of expression. Biophys J, in press. [DOI] [PMC free article] [PubMed]
- 8.Chouinard SW, Wilson GF, Schlimgen AK, Ganetzky B. A potassium channel β subunit related to the aldo-keto reductase superfamily is encoded by the Drosophila Hyperkinetic locus. Proc Natl Acad Sci USA. 1995;92:6763–6767. doi: 10.1073/pnas.92.15.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor JA, Stevens CF. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol (Lond) 1971;213:31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkins T, Ganetzky B, Wu C-F. A Drosophila mutation that eliminate a calcium-dependent potassium current. Proc Natl Acad Sci USA. 1986;83:8415–8419. doi: 10.1073/pnas.83.21.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel JE, Wu C-F. Interactions of membrane excitability mutations affecting potassium and sodium currents in the flight and giant fiber escape systems of Drosophila. J Comp Physiol [A] 1992;171:93–104. doi: 10.1007/BF00195964. [DOI] [PubMed] [Google Scholar]
- 12.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 13.Ganetzky B, Wu C-F. Drosophila mutants with opposing effects on nerve excitability: genetic and spatial interactions in repetitive firing. J Neurophysiol. 1982;47:501–514. doi: 10.1152/jn.1982.47.3.501. [DOI] [PubMed] [Google Scholar]
- 14.Ganetzky B, Wu C-F. Neurogentic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet. 1983;1:17–28. doi: 10.3109/01677068309107069. [DOI] [PubMed] [Google Scholar]
- 15.Ganetzky B, Wu C-F. Genes and membrane excitability in Drosophila. Trends Neurosci. 1985;8:322–326. [Google Scholar]
- 16.Gautam M, Tanouye MA. Alteration of potassium channel gating: molecular analysis of the Drosophila Sh5 mutation. Neuron. 1990;5:67–73. doi: 10.1016/0896-6273(90)90034-d. [DOI] [PubMed] [Google Scholar]
- 17.Gielow ML, Gu G-G, Singh S. Resolution and pharmacological analysis of the voltage-dependent calcium channels of Drosophila larval muscles. J Neurosci. 1995;15:6085–6093. doi: 10.1523/JNEUROSCI.15-09-06085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffith LC, Wang J, Zhong Y, Wu C-F, Greenspan RJ. Calcium/calmodulin-dependent protein kinase II and potassium channel subunit eag similarly affect plasticity in Drosophila. Proc Natl Acad Sci USA. 1994;91:10044–10048. doi: 10.1073/pnas.91.21.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulbis JM, Mann S, MacKinnon R. Structure of a voltage-dependent K+ channel beta subunit. Cell. 1999;97:943–952. doi: 10.1016/s0092-8674(00)80805-3. [DOI] [PubMed] [Google Scholar]
- 20.Haugland FN, Wu C-F. A voltage-clamp analysis of gene-dosage effects of the Shaker locus on larval muscle potassium currents in Drosophila. J Neurosci. 1990;10:1357–1371. doi: 10.1523/JNEUROSCI.10-04-01357.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hille B. Ionic channels of excitable membrane. Sinauer; Sunderland, MA: 1992. [Google Scholar]
- 22.Humphreys JM. PhD thesis. University of Guelph; 1996. Genetic analysis of reative oxygen metabolism in Drosophila melanogaster and identification of a new neurological mutant. [Google Scholar]
- 23.Humphreys JM, Hilliker AJ, Phillips JP. Paraquat selection identifies X-linked oxygen defense genes in Drosophila melanogaster. Genome. 1993;36:162–165. doi: 10.1139/g93-021. [DOI] [PubMed] [Google Scholar]
- 24.Humphreys JM, Duyf B, Joiner M-LA, Phillips JP, Hilliker AJ. Genetic analysis of oxygen defense mechanisms in Drosophila melanogaster and identification of a novel behavioral mutant with a Shaker phenotype. Genome. 1996;39:749–757. doi: 10.1139/g96-094. [DOI] [PubMed] [Google Scholar]
- 25.Jan LY, Jan YN. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol (Lond) 1976;262:215–236. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jan YN, Jan LY, Dennis MJ. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond [Biol] 1977;198:87–108. doi: 10.1098/rspb.1977.0087. [DOI] [PubMed] [Google Scholar]
- 27.Kamb A, Tseng-Crank J, Tanouye MA. Multiple products of the Drosophila Shaker gene may contribute to potassium channel diversity. Neuron. 1988;1:421–430. doi: 10.1016/0896-6273(88)90192-4. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan WD, Trout WE. The behavior of four neurological mutants of Drosophila. Genetics. 1969;61:399–409. doi: 10.1093/genetics/61.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kernan MJ, Kuroda MI, Kreber R, Baker BS, Ganetzky B. napts, a mutant affecting sodium channel activity in Drosophila, is an allele of mle, a regulator of X chromosome transcription. Cell. 1991;66:949–959. doi: 10.1016/0092-8674(91)90440-a. [DOI] [PubMed] [Google Scholar]
- 30.Komatsu A, Singh S, Rathe P, Wu C-F. Mutational and gene dosage analysis of calcium-activated potassium channels in Drosophila: correlation of micro- and macroscopic currents. Neuron. 1990;4:313–321. doi: 10.1016/0896-6273(90)90105-o. [DOI] [PubMed] [Google Scholar]
- 31.Mackay WJ, Bewley GC. The genetics of catalase in Drosophila melanogaster: isolation and characterization of acatalasemic mutants. Genetics. 1989;122:643–652. doi: 10.1093/genetics/122.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips JP, Hilliker AJ. Genetic analysis of oxygen defense mechanisms in Drosophila melanogaster. Adv Genet. 1990;28:43–71. doi: 10.1016/s0065-2660(08)60523-4. [DOI] [PubMed] [Google Scholar]
- 33.Phillips JP, Campell SD, Michaud D, Charbonneau M, Hilliker AJ. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci USA. 1989;86:2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pongs O, Kecskemethy N, Muller R, Krah-Jentgens I, Baumann A, Kiltz HH, Canal I, Llamazares S, Ferrus A. Shaker encodes a family of putative potassium channel proteins in the nervous system of Drosophila. EMBO J. 1988;7:1087–1096. doi: 10.1002/j.1460-2075.1988.tb02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung W-Y, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH., Jr Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 36.Saito M, Wu C-F. Ionic channels in cultured Drosophila neurons. In: Pichon Y, editor. Comparative molecular neurobiology. Birkhauser; Basel: 1993. pp. 366–389. [DOI] [PubMed] [Google Scholar]
- 37.Salkoff L, Wyman R. Genetic modification of potassium channels in Drosophila Shaker mutants. Nature. 1981;293:228–230. doi: 10.1038/293228a0. [DOI] [PubMed] [Google Scholar]
- 38.Schlief T, Schönherr R, Heinemann SH. Modification of C-type inactivating Shaker potassium channels by chloramine-T. Pflügers Arch. 1996;431:483–493. doi: 10.1007/BF02191894. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz LM, Stühmer W. Voltage-dependent sodium channels in an invertebrate striated muscle. Science. 1984;225:523–525. doi: 10.1126/science.6330898. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz TL, Tempel BL, Papazian DM, Jan Y-N, Jan LY. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988;331:137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- 41.Singh A, Singh S. Unmasking of a novel potassium current in Drosophila by a mutation and drugs. J Neurosci. 1999;19:6838–6843. doi: 10.1523/JNEUROSCI.19-16-06838.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh S, Wu C-F. Complete separation of four potassium currents in Drosophila. Neuron. 1989;2:1325–1329. doi: 10.1016/0896-6273(89)90070-6. [DOI] [PubMed] [Google Scholar]
- 43.Staveley BE, Phillips JP, Hilliker AJ. Phenotypic consequence of copper-zinc superoxide dismutase overexpression in Drosophila melanogaster. Genome. 1990;31:867–872. doi: 10.1139/g90-130. [DOI] [PubMed] [Google Scholar]
- 44.Stern M, Ganetzky B. Altered synaptic transmission in Drosophila Hyperkinetic mutants. J Neurogenet. 1989;5:215–228. doi: 10.3109/01677068909066209. [DOI] [PubMed] [Google Scholar]
- 45.Stern M, Ganetzky B. Identification and characterization of inebriated, a gene affecting neuronal excitability in Drosophila. J Neurogenet. 1992;8:157–172. doi: 10.3109/01677069209083445. [DOI] [PubMed] [Google Scholar]
- 46.Stewart BA, Atwood HL, Renger JJ, Wang J, Wu C-F. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol [A] 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- 47.Tanouye MA, Ferrus A. Action potentials in normal and Shaker mutant Drosophila. J Neurogenet. 1985;2:253–271. doi: 10.3109/01677068509102322. [DOI] [PubMed] [Google Scholar]
- 48.Trout WE, Kaplan WD. A relation between longevity, metabolic rate, and activity in Shaker mutants of Drosophila melanogaster. Exp Gerontol. 1970;5:83–92. doi: 10.1016/0531-5565(70)90033-1. [DOI] [PubMed] [Google Scholar]
- 49.Tsunoda S, Salkoff L. The major delayed rectifier in both Drosophila neurons and muscle is encoded by Shab. J Neurosci. 1995;15:5209–5221. doi: 10.1523/JNEUROSCI.15-07-05209.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Renger JJ, Griffith LC, Greenspan RJ, Wu C-F. Concomitant alterations of physiological and developmental plasticity in Drosophila CaM kinase II-inhibited synapses. Neuron. 1994;13:1373–1384. doi: 10.1016/0896-6273(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 51.Wang JW, Wu C-F. In vivo functional role of the Drosophila Hyperkinetic β subunit in gating and inactivation of Shaker K+ channels. Biophys J. 1996;71:3167–3176. doi: 10.1016/S0006-3495(96)79510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang JW, Sylwester AW, Reed D, Wu D-AJ, Soll DR, Wu C-F. Morphometric description of the wandering behavior in Drosophila larvae: aberrant locomotion in Na+ and K+ channel mutants revealed by computer-assisted motion analysis. J Neurogenet. 1997;11:231–254. doi: 10.3109/01677069709115098. [DOI] [PubMed] [Google Scholar]
- 53.Warmke J, Drysdale R, Ganetzky B. A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science. 1991;252:1560–1562. doi: 10.1126/science.1840699. [DOI] [PubMed] [Google Scholar]
- 54.Wu C-F, Ganetzky B. Neurogenetic studies of ion channels in Drosophila. In: Narahashi T, editor. Ion channels, Vol 3. Plenum; New York: 1992. pp. 261–314. [DOI] [PubMed] [Google Scholar]
- 55.Wu C-F, Haugland FN. Voltage-clamp analysis of membrane currents in larval muscle fibers of Drosophila: alteration of potassium currents in Shaker mutants. J Neurosci. 1985;5:2626–2640. doi: 10.1523/JNEUROSCI.05-10-02626.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu C-F, Ganetzky B, Jan LY, Jan Y-N, Benzer S. A Drosophila mutant with a temperature-sensitive block in nerve conduction. Proc Natl Acad Sci USA. 1978;75:4047–4051. doi: 10.1073/pnas.75.8.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu C-F, Ganetzky B, Haugland FN, Liu A-X. Potassium currents in Drosophila: different components affected by mutations of two genes. Science. 1983;220:1076–1078. doi: 10.1126/science.6302847. [DOI] [PubMed] [Google Scholar]
- 58.Yao W-D, Wu C-F. Auxiliary Hyperkinetic β subunit of K+ channel: regulation of firing properties and K+ currents in Drosophila neurons. J Neurophysiol. 1999;81:2472–2484. doi: 10.1152/jn.1999.81.5.2472. [DOI] [PubMed] [Google Scholar]
- 59.Youdim MBH, Riederer P. Understanding Parkinson's disease. Sci Am. 1997;276:52–59. doi: 10.1038/scientificamerican0197-52. [DOI] [PubMed] [Google Scholar]
- 60.Zhao M-L, Wu C-F. Alterations in frequency coding and activity dependence of excitability in cultured neurons of Drosophila memory mutants. J Neurosci. 1997;17:2187–2199. doi: 10.1523/JNEUROSCI.17-06-02187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao M-L, Sable EO, Iverson LE, Wu C-F. Functional expression of Shaker K+ channels in cultured Drosophila “giant” neurons derived from Sh cDNA transformants: distinct properties, distribution, and turnover. J Neurosci. 1995;15:1406–1418. doi: 10.1523/JNEUROSCI.15-02-01406.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhong Y, Wu C-F. Alteration of four identified K+ currents in Drosophila muscle by mutations in eag. Science. 1991;252:1562–1564. doi: 10.1126/science.2047864. [DOI] [PubMed] [Google Scholar]
- 63.Zhong Y, Wu C-F. Modulation of different K+ currents in Drosophila: a hypothetical role for the Eag subunit in multimeric K+ channels. J Neurosci. 1993a;13:4669–4679. doi: 10.1523/JNEUROSCI.13-11-04669.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhong Y, Wu C-F. Differential modulation of potassium currents by cAMP and its long-term and short-term effects: dunce and rutabaga mutants of Drosophila. J Neurogenet. 1993b;9:15–27. doi: 10.3109/01677069309167273. [DOI] [PubMed] [Google Scholar]