Abstract
ATP has been shown to be an important extracellular signaling molecule. There are two subgroups of receptors for ATP (and other purines and pyrimidines): the ionotropic P2X and the G-protein-coupled P2Y receptors. Different subtypes of these receptors have been identified by molecular biology, but little is known about their functional properties in the nervous system. Here we present data for the existence of P2 receptors in Müller (glial) cells of the human retina. The cells were studied by immunocytochemistry, electrophysiology, Ca2+-microfluorimetry, and molecular biology. They displayed both P2Y and P2X receptors. Freshly enzymatically isolated cells were used throughout the study. Although the [Ca2+]i response to ATP was dominated by release from intracellular stores, there is multiple evidence that the ATP-induced membrane currents were caused by an activation of P2X7 receptors. Immunocytochemistry and single-cell RT-PCR revealed the expression of P2X7receptors by Müller cells. In patch-clamp studies, we found that (1) benzoyl-benzoyl ATP (BzATP) was the most effective agonist to evoke large inward currents and (2) the currents were abolished by P2X antagonists; however, (3) long-lasting application of BzATP did not cause an opening of large pores in addition to the cationic channels. By microfluorimetry it was shown that the P2X receptors mediated a Ca2+ influx that contributed a small component to the total [Ca2+]i response. Activation of P2X receptors may modulate the uptake of neurotransmitters from the extracellular space by Müller cells in the retina.
Keywords: Müller cells, glia, P2 receptors, ATP, glutamate uptake, human
Extracellular purines have been described as transmitter molecules in various cells in different tissues. Like various other ligands, ATP is thought to activate several distinct receptor types. The current nomenclature includes the G-protein-coupled P2Y family of receptors and the P2X receptors that are ligand-gated ion channels (Burnstock, 1997). ATP-activated ion channels have been found in neurons and muscle cells (Bean, 1992; Illes and Nörenberg, 1993) and more recently also in several types of glial cells, including astrocytes (Walz et al., 1994), microglia (Walz et al., 1993; Nörenberg et al., 1994), and Schwann cells (Amedee and Despeyroux, 1995). Until now, few data supported the role of purinoceptors in the visual system. Brändle et al. (1998a,b) demonstrated the existence of several types of P2X receptors in the rat retina by means of RT-PCR. The P2X2receptor subunit is expressed by distinct retinal neurons of the rat (Greenwood et al., 1997). A modulating role of endogenous ATP in retinal neurons was demonstrated by Neal and Cunningham (1994).Taschenberger et al. (1999) studied Ca2+-permeable P2X receptors in cultured rat retinal ganglion cells. With regard to retinal glia, Neal et al. (1998) found that P2X receptor agonists increase GABA release from Müller glial cells of the rabbit retina and suggested the existence of P2X receptors on these cells. On the other hand, Keirstead and Miller (1997) as well as Newman and Zahs (1997) stimulated a release of Ca2+ from internal stores in Müller cells from salamander and rat, respectively, probably caused by the activation of P2Y receptors. A study conducted by Liu and Wakakura (1998) revealed increases of intracellular Ca2+ concentration [Ca2+]i in cultured rabbit Müller cells after the application of P2X and P2Y agonists. Taken together, although these studies provide convincing evidence that Müller cells of various species express P2 receptors, detailed investigations of the possible receptor subtype are still missing, and it is unknown whether these receptors are present in Müller cells of humans. Thus, we have combined data from electrophysiology, immunocytochemistry, microfluorimetric recording of intracellular Ca2+, and molecular biology to characterize a P2X-type ATP receptor in Müller cells from the human retina.
MATERIALS AND METHODS
Materials. The following drugs were used: ATP (Serva, Heidelberg, Germany), adenosine 5′-O-(2-thiodiphosphate) trilithium (ADPβS), oxidized ATP, 2′- and 3′-O-(4-benzoyl-benzoyl)-ATP triethylammonium (BzATP), fura-2 AM, Lucifer yellow dilithium, α,β-methylene-ATP dilithium (α,β-meATP), β,γ-methylene-ATP disodium (β,γ-meATP; Sigma, Deisenhofen, Germany), cyclopiazonic acid (CPA), (S)-5-isoquinolinesulfonic acid, 4-[2-[(5-isoquinolinyl-sulfonyl)methylamino]-3-oxo-3-(4-phenyl-1-piperazinyl)-propyl]phenyl ester (KN-62), suramin hexasodium (RBI, Natick, USA), 2-methylthio-ATP tetrasodium (2-MeSATP), pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid tetrasodium (PPADS), thapsigargin (Tocris, Bristol, UK), YO-PRO-1 iodide, ethidium bromide, and Alexa Fluor 488 hydrazide sodium salt (Molecular Probes, Leiden, Netherlands).
Other substances were from Sigma unless indicated otherwise.
Preparation of cells. Human retinal tissue was obtained during vitreoretinal surgery in cases of proliferative vitreoretinopathy when retinectomies were indicated, as well as from organ donors whose eyes served as a source for corneal transplantation. Use of human retinae was approved by the ethics committee of the School of Medicine, University of Leipzig.
The procedure for the preparation of Müller cells has been described previously (Reichenbach and Birkenmeyer, 1984; Francke et al., 1997). Briefly, the retinal tissue was stored for 30 min in Ca2+/Mg2+-free PBS (Seromed, Berlin, Germany) containing 0.2–0.5 mg/ml papain (Boehringer, Mannheim, Germany) at 35°C. After washing with PBS containing 0.1 mg/ml DNase I, the retina was triturated using a 1 ml pipette tip until single Müller cells were dissociated. Isolated cells were stored until use in minimum essential medium (MEM; Serva) on ice.
For dye-filling experiments, the cells were incubated at room temperature (20–24°C) or 37°C in PBS (Ca2+/Mg2+-free) containing the fluorescent dye and BzATP (50 and 100 μm). Under control conditions, cells were kept without BzATP. After the incubation period, cells were investigated using epifluorescent equipment (for all of the fluorescent dyes that were used: excitation bandpass 450–490 nm, emission long-pass 520 nm; additionally for ethidium bromide: excitation 546 nm, emission long-pass 590 nm; Zeiss, Germany). A part of the experiments using the dye Alexa Fluor 488 was performed at a laser scanning microscope (LSM 510, Zeiss, Germany) with an excitation wavelength of 488 nm and a long-pass for emission at 505 nm.
Preparation of antibodies and immunocytochemistry. Specific polyclonal antibodies were prepared to the C-terminal part of the human and rat P2X7 receptor as described previously (Oglesby et al., 1999). The specificity of the antibodies was verified by Western blotting (Gröschel-Stewart et al., 1999;Oglesby et al., 1999).
Preparations of dissociated cells were used for immunocytochemical demonstration of P2X7 receptors. Isolated Müller cells were fixed in 4% paraformaldehyde (20 min), washed in PBS, and air-dried. For blocking and permeabilization, the cells were pretreated in PBS containing 5% normal goat serum (Dianova, Hamburg, Germany), 1% DMSO, and 0.3% Triton X-100 for 1 hr. Afterward, the samples were incubated 12 hr at 4°C with the primary antibodies, diluted 1:400. After washing, the slides were incubated with Cy3-tagged goat anti-rabbit IgG (Dianova) at a 1:500 dilution for 1 hr at room temperature. After a wash step, the specimens were dehydrated and mounted in Entellan (Merck, Darmstadt, Germany). The results were documented by using the LSM 510.
Electrophysiology. For patch-clamp recordings, the cells were suspended in extracellular solution in a recording chamber mounted on the stage of an upright microscope (Axioskop, Zeiss, Germany). Control extracellular solution contained (in mm): NaCl 110, KCl 3, CaCl2 2, MgCl2 1, Na2HPO4 1, glucose 11, HEPES-Tris 10, NaHCO3 25, and was equilibrated to pH 7.4 by continuous bubbling with carbogen gas (95% O2, 5% CO2). If not otherwise indicated, responses to ATP and its analogs were recorded in a solution where Ca2+, Mg2+, and K+ions were replaced by Na+. Removal of K+ was performed to suppress the dominating K+ conductance of the Müller cells. The divalent cations would reduce the concentration of ATP4− (or the corresponding forms of its analogs), which is suggested to be the species active at the P2Z (=P2X7) receptor (Di Virgilio, 1995; Rassendren et al., 1997).
Recording electrodes were made from borosilicate glass (Science Products, Hofheim, Germany) and had resistances of 4–6 MΩ if filled with an intracellular solution containing (in mm): NaCl 10, CsCl 130, CaCl2 1, MgCl2 2, EGTA 10, HEPES-Tris 10, pH 7.1. In some experiments with K+ in the extracellular solution, CsCl was replaced by KCl. After a seal was established, the recording chamber was continuously perfused (2 ml/min) with extracellular solution. All agonists and antagonists were applied by the bath perfusion. Experiments were performed at room temperature (20–24°C).
Recordings in the whole-cell configuration of the patch-clamp technique were performed using the patch-clamp amplifier Axopatch 200A (Axon Instruments, Foster City, CA). Currents were low-pass-filtered at 1 kHz with an eight-pole Bessel filter and digitized at 5 kHz using a 12 bit-A/D converter. Voltage command protocols were generated and data analysis was performed with the software ISO 2 (MFK, Niedernhausen, Germany). Unless indicated otherwise, data are given as mean values with SD. For curve fitting the software SigmaPlot (Jandel Scientific, Corte Madera, CA) was used.
Ca2+-microfluorimetry. Isolated Müller cells in MEM (see above) were seeded in the center of glass coverslips coated with poly-l-lysine. After attachment of the cells (15 min), the preparations were loaded with the Ca2+-sensitive fluorescent dye fura-2 AM (5 μm) for 30 min and then incubated in Mg2+-free physiological saline containing (in mm: NaCl 135, KCl 5, CaCl2 0.5, HEPES 10, d-glucose 10, pH 7.2, adjusted with Tris base) for an additional 20–30 min to remove traces of extracellular fura-2 AM and to complete de-esterification. After that, the coverslips were mounted cell-side up into the free bottom of a perfusion chamber (250 μl) placed on the stage of an inverted microscope with epifluorescence optics (Diaphot 200, Nikon, Japan). Throughout the experiments, cells were continuously superfused (at 0.8 ml/min) by means of a roller pump with control and drug-containing solutions, respectively. Different superfusion solutions were selected with a valve bank coupled to several reservoirs. ATP and various P2 receptor agonists were applied for 30 sec (intervals between two applications 10–12 min); Ca2+-free solution (always with 1 mm EGTA) and modulating drugs such as CPA, thapsigargin, or KN-62, were superfused 10 min before and during additional application of the ATP derivatives. Fluorescence ratio measurements were made on selected single Müller cells with a dual wavelength spectrometer. Fura-2 AM fluorescence (over the cell soma), excited alternatively at 340 and 380 nm, was measured at 510/520 nm by a microscope photometer attached to a photomultiplier detection system (Ratiomaster System, Photon Technology International, Wedel, Germany). Data are expressed as ratios of the fluorescence intensities at 340 versus 380 nm. Complete data acquisition, presentation, and analysis were performed computer-controlled, using the software FeliX, Vers. 1.1 (Photon Technology International). All experiments were performed at room temperature in the dark. Background fluorescence was minimal and was thus not corrected.
Molecular biology. Subsequent to electrophysiological characterization of individual Müller cells by measuring the response to BzATP, single-cell RT-PCR was performed. Cytoplasm from the endfoot region was harvested into the patch pipette containing 6 μl intracellular solution (with KCl) by applying negative pressure. Only BzATP-responsive cells with the seal remaining stable during suction were used for further steps. The content of the patch pipette was expelled into a PCR-tube containing 2 μl dNTP/N6 mix (2.5 mm/25 μm), 1 μl dithiothreitol (100 mm), 0.5 μl RNase inhibitor (20 U; Stratagene), and 1 μl 5× first-strand buffer. Subsequently, Superscript-II-reverse transcriptase (0.5 μl; Life Technologies) was added, and after an additional 5 min at room temperature the reverse transcription was performed at 45°C for 1 hr. Controls were performed by advancing the patch pipette into the bath solution and using its content for RT-PCR; in no case were false amplifications obtained.
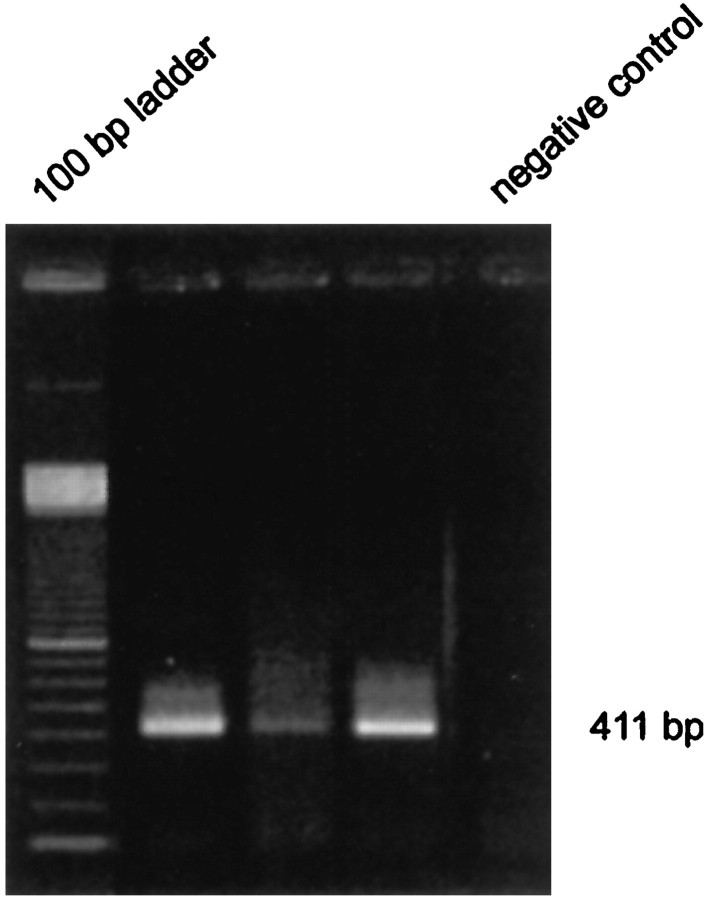
Amplification of the P2X7-specific cDNA fragment was performed by nested hot-start PCR with primers selected by OLIGO 5.0 (MedProbe, Oslo, Norway). The first PCR amplification was performed using the primer pair P2X7 sense (outer) (5′-ATCGTGGAGAATGGAGTGAAG-3′, binding position 249) and P2X7 antisense (5′-GGATGGCAGTGATGGAAC-3′; binding position 840) yielding a 591 bp fragment. PCR was performed on a PTC-200 thermal cycler (MJ Research) under hot-start conditions using 1.5 U AmpliTaq DNA Polymerase (Perkin-Elmer, Norwalk, CT) and each primer in a final concentration of 100 nm. Reaction conditions were as follows: initial denaturation (94°C for 2 min), 40 cycles of denaturation (94°C for 30 sec), annealing (62°C for 30 sec), and a final extension at 72°C for 10 min. In the second PCR amplification, only the sense primer was replaced by P2X7 inner (5′-TGTAAAAAGGGATGGATGGAC-3′; binding position 429). Five microliters of each reaction were amplified for an additional 45 cycles at an annealing temperature of 60°C. The 411 bp amplification product was analyzed by ethidium bromide staining subsequent to agarose gel electrophoresis (1.5%). To verify the identity of amplified DNA, the PCR product was sequenced using BigDye Terminator chemistry (PE Applied Biosystems, Foster City, CA). The sequence was analyzed on a DNA sequencer ABI 377 (PE Applied Biosystems, Foster City, CA).
RESULTS
Immunocytochemistry
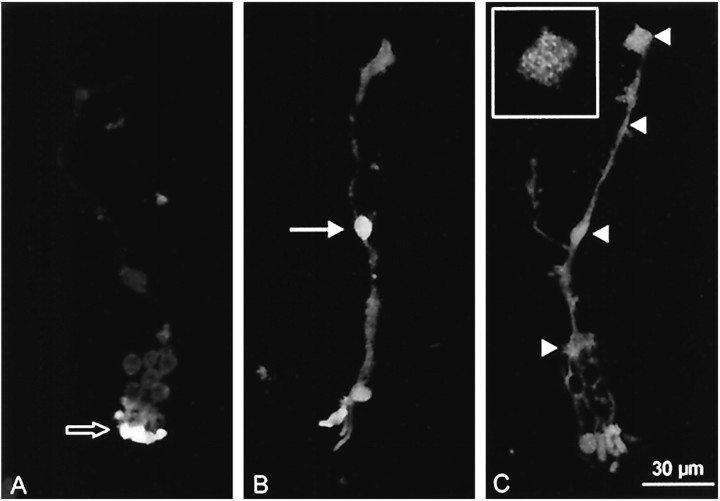
Dissociated Müller cells were subjected to anti-P2X7 receptor immunocytochemistry (Fig.1). Although no specific signal was found in negative controls (omission of the primary antibody) (Fig.1A), prominent immunoreactivity was found in the somata of all Müller cells (Fig. 1B,arrow); it should be noted that there was considerable autofluorescence in substructures of the photoreceptor cells that often adhered to the Müller cells (Fig. 1A,empty arrow). The dominant somatic (nuclear) anti-human P2X7 receptor label in the Müller cells was accompanied by a rather weak immunoreactivity in their cytoplasmic membranes. An antibody was available against a C-terminal amino acid sequence of the rat P2X7 receptor differing by only one amino acid from that of the human receptor. Thus, we also applied the antibody directed against the rat receptor (Fig.1C). This antibody revealed specific labeling in dissociated Müller cells. In comparison to the anti-human P2X7 receptor immunoreactivity, the somatic/nuclear label was less strong, but distinct punctate label was observed along the cytoplasmic membranes (Fig. 1C,arrowheads). The endfoot of the cell is shown at a higher magnification in Figure 1C (inset). The label was uniformly distributed along the entire cell membrane.
Fig. 1.
Immunocytochemistry for P2X7 receptors in enzymatically dissociated Müller cells. Vitread endfeet are located at the top of the photographs, and distal processes with adhering photoreceptors are orientated downward. A negative control was performed by omission of the primary antibody. Autofluorescence of photoreceptor cells is clearly visible (empty arrow). B, C, Immunolabeled cells with a primary antibody against human P2X7 receptor (B) and against rat P2X7 receptor (C). Strong labeling in the somatic region was found with the human-specific antibody (B, arrow), whereas use of the rat-specific antibody resulted in a more uniform label distributed over the whole cell (C, arrowheads). Theinset in C shows the endfoot of the cell at a higher magnification to demonstrate the punctate labeling pattern.
BzATP-evoked inward currents and depolarizations in human Müller cells
When ATP was applied to isolated human Müller cells, small inward currents were evoked. The current amplitude was 63 ± 43 pA (n = 13) with 1 mm ATP (see Fig.4C). Significantly larger currents were evoked by application of the P2X7 agonist BzATP. When applied at various concentrations, BzATP evoked inward currents in all Müller cells investigated (n = 271).
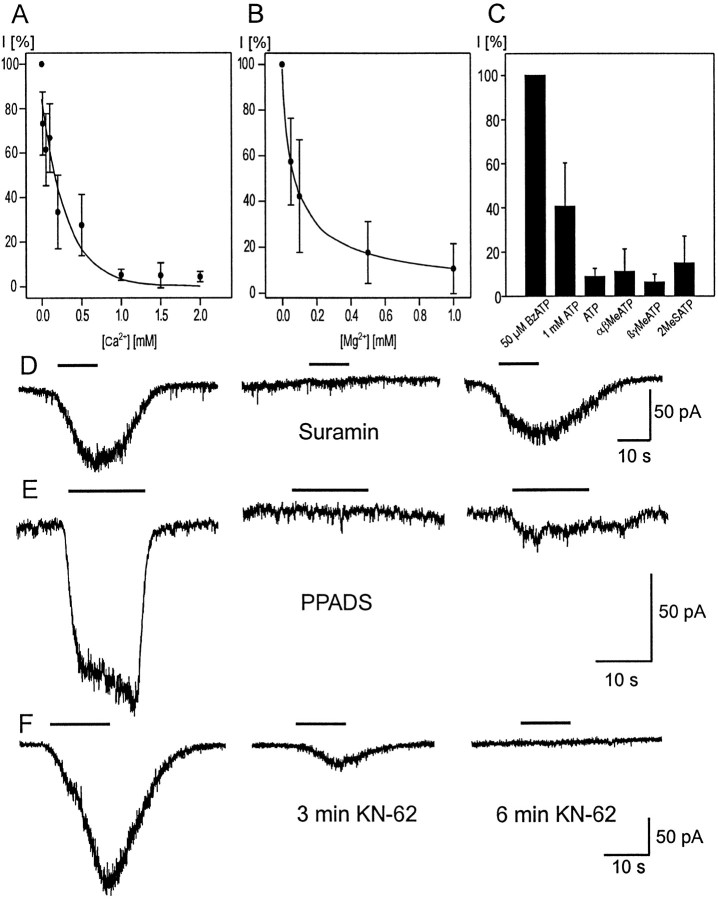
Fig. 4.
Pharmacological effects. A, B BzATP was applied in a concentration of 25 μm in the presence of different concentrations of Ca2+(A) and Mg2+(B). The number of cells examined for each concentration was between 4 and 10. The inward current recorded in the absence of the divalent cations served as the reference value (100% in each cell). Dose–effect curves were analyzed by fitting the data to the Hill equation. The IC50 was 70 μm for Mg2+ and 84 μm for Ca2+. C, BzATP (50 μm), ATP (1 mm and 100 μm), α, β-meATP, β,γ-meATP, and 2-MeSATP (100 μm each) were applied to human Müller cells (n between 7 and 13). The inward current recorded under BzATP was set to 100%, and the other currents are given as relative values. All agonists except BzATP evoked only small inward currents when 100 μm was used, whereas the mean value of inward currents under 1 mm ATP amounted to ∼40% of the control value. D, Suramin (200 μm) was sufficient to block the current evoked by 25 μm BzATP completely and reversibly. E, PPADS (100 μm) blocked the current evoked by 50 μm BzATP; application of BzATP after 25 min wash revealed only a partial recovery. F, The isoquinoline KN-62 (1 μm) strongly reduced the BzATP-evoked current after 3 min incubation time, whereas BzATP (20 μm) caused no effect after 6 min KN-62 incubation. There was no recovery after 10 min wash (data not shown). Bars inD–F indicate application of BzATP.
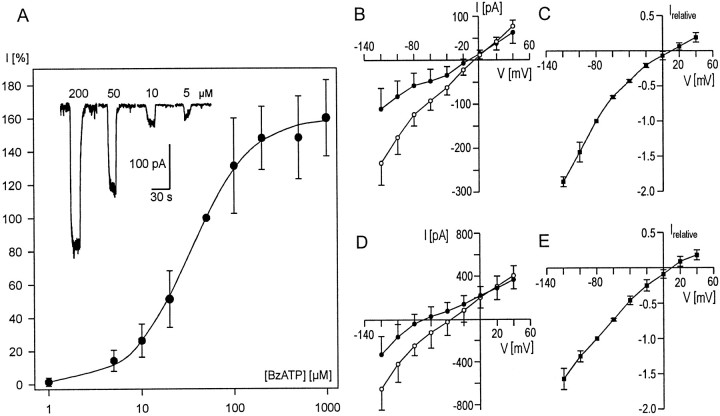
The amplitude of these inward currents increased in a concentration-dependent manner up to 1 mm BzATP (Fig.2A) (K+-free conditions). A concentration of 5 μm BzATP was necessary to evoke a measurable inward current, whereas application of 1 μmBzATP resulted in an increase in the noise (n = 3), or in very small inward currents (n = 3), or had no effect (n = 3). The mean amplitude was 190 ± 181 pA with 50 μm BzATP (n = 120). For individual cells, this value ranged between 20 and 896 pA.
Fig. 2.
Dose and voltage dependence of BzATP-evoked currents. A, Dose–response curve of BzATP in human Müller cells. For each cell, the inward current after application of 50 μm BzATP was set as 100% (absolute value: 405 ± 242 pA, n = 15); inward currents at the other concentrations are given relative to this value. Data are mean values with SDs; for each concentration at least six cells were recorded. A fit of the data to the Hill equation resulted in an EC50 of 33.9 μm. The insetshows original current responses from one cell to the indicated concentrations of BzATP. Recordings were performed at a holding potential of −80 mV. B–E, Current–voltage (I–V) relationships of BzATP-evoked currents. Whole-cell currents were recorded at different holding potentials both under control conditions (●) and after application of 50 μm BzATP (○). The difference between the currents under BzATP and under control conditions (▪) is given in relative values to normalize the different amplitudes. For normalization, the difference current at a holding potential of −80 mV was set to a relative value of −1 for each cell. Experiments were performed under K+-free conditions (B, C) and in K+-containing solutions (D,E); data are from four and seven cells, respectively. The reversal potential of the difference (BzATP-evoked) current is approximately +10 mV under both conditions.
Recordings of BzATP-induced currents at different holding potentials revealed a reversal potential of approximately +10 mV (n = 4) (Fig.2B,C). The same reversal potential was obtained when K+ was present in both the pipette (130 mm) and the extracellular solution (3 mm; n = 7) (Fig.2D,E). BzATP-induced currents did not inactivate and were stable during application times of several minutes (n = 8) (Fig.3A). Moreover, repeated application of BzATP for ∼10 sec with intervals of 0.5–1 min evoked currents of constant amplitudes (n = 4; data not shown).
Fig. 3.
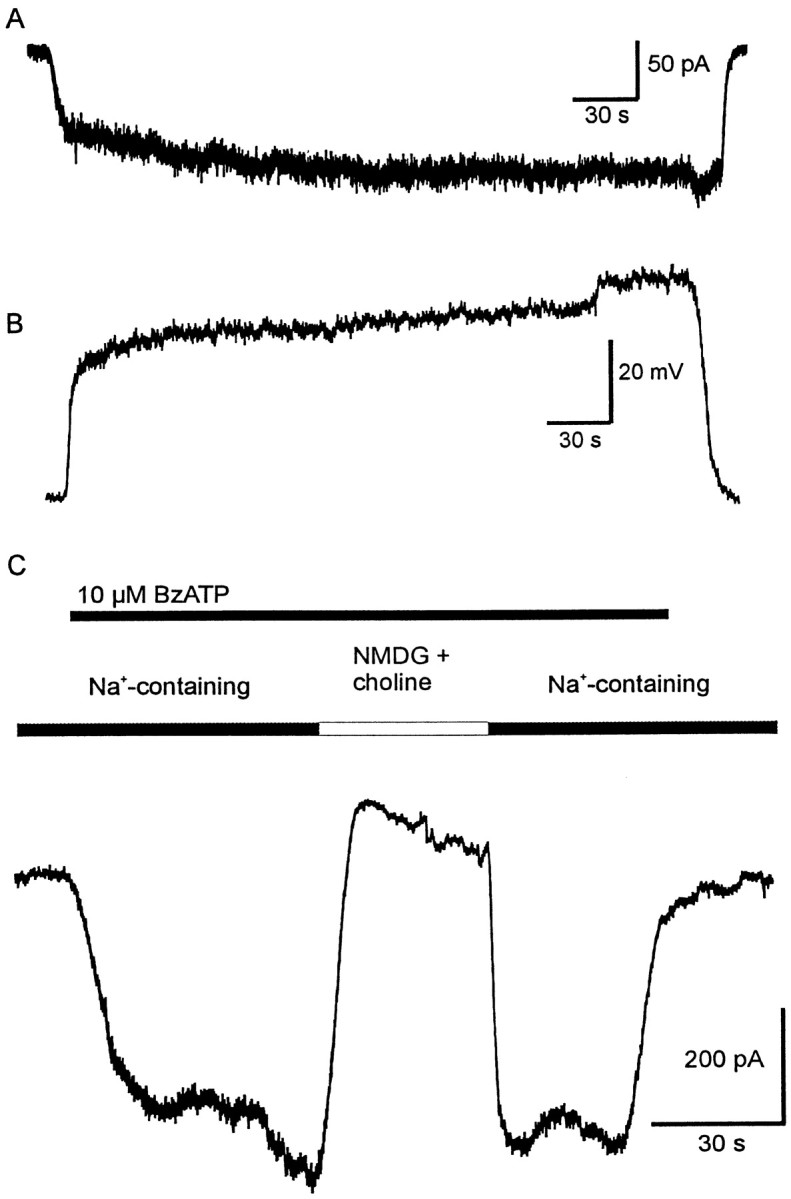
Effects of prolonged application of BzATP.A, Application of 20 μm BzATP causes an inward current with an almost constant amplitude over 5 min application time; the recovery was complete. The holding potential was −80 mV. B, Application of 50 μm BzATP in the current-clamp mode caused a reversible depolarization by ∼50 mV; the resting membrane potential of the cell was −65 mV. Both cells were recorded in K+-containing solutions. The prolonged application did not cause any major increase of current or depolarization. C, BzATP was applied as indicated by thetop line. If the extracellular solution contained Na+ as the main cation (141 mm), an inward current was evoked. Replacement of Na+ byN-methyl-d-glucamine (116 mm) and choline (25 mm) resulted in the disappearance of the current. This effect was reversible.
The effect of BzATP application on the resting membrane potential of Müller cells was investigated under current-clamp conditions. Because under K+-free conditions in both intracellular and extracellular solutions the membrane potential was close to the reversal potential of the BzATP-evoked current, the cells depolarized by only 5 ± 1 mV (n = 5). In contrast, in the presence of K+ the cells depolarized by 27 ± 12 mV (n = 8; the resting membrane potential for these cells was −69 ± 12 mV) (Fig.3B).
To examine which species of ions were carrying these currents, we replaced extracellular Na+ ions withN-methyl-d-glucamine and choline. This prevented the inward current at negative holding potentials (Fig.3C), whereas outward currents could be evoked by BzATP at positive holding potentials (n = 9; data not shown). It is concluded that the currents are carried mainly by Na+ (inward) and Cs+ or K+(outward) ions.
The effects of divalent cations on the BzATP-evoked current were examined by applying extracellular Ca2+and Mg2+ in concentrations from nominally 0 up to 2 and 1 mm, respectively, i.e., in concentrations corresponding to or below physiological levels (Fig.4A,B). Both ion species decreased the currents at low concentrations and were able to prevent the flux of currents almost completely at physiological concentrations.
Pharmacology
There are some P2 receptor agonists that exhibit a relative specificity for certain receptor subtypes. We examined α,β-meATP and β,γ-meATP, which are potent agonists for several types of P2X receptors, and 2-MeSATP, which is active at several P2X and P2Y receptors (Burnstock, 1997). All three ATP analogs (100 μm) caused small inward currents. For a better comparison, the amplitudes of the responses to the three agonists are given relative to the response to 50 μm BzATP (Fig.4C).
Pharmacological studies of P2X receptors have been limited by the lack of highly specific antagonists. Suramin, a blocker of P2 receptors (but also of other receptor types), markedly reduced the BzATP-evoked inward currents. At a concentration of 100 μm, suramin reduced the effect of 50 μm BzATP to 15 ± 24% (n = 5) of the control, whereas 200 μm suramin blocked the effect of 25 μm BzATP completely but reversibly (n = 3) (Fig. 4D). A second antagonist, PPADS, thought to be more selective for P2X than for P2Y receptors, was tested at concentrations up to 100 μm. PPADS at 100 μmblocked the effect of 50 μm BzATP completely in one cell (Fig. 4E), whereas in four other cells and with lower concentrations of PPADS (20, 50, and 75 μm;n = 18) the current was reduced to <40%. The currents showed hardly any recovery after several minutes of washout of PPADS. Moreover, oxidized ATP, an inhibitor of the P2Z receptor in mouse macrophages (Murgia et al., 1993), was able to suppress the currents evoked by BzATP. Cells were incubated at room temperature in extracellular solution containing 200 μm oxidized ATP. Currents evoked by BzATP were reduced to 21 ± 10 pA (n = 5; control value: 75 ± 44 pA, n = 4) after incubation times <2 hr and were totally abolished after incubation for >2 hr (n = 4; control value: 100 ± 43 pA,n = 4). Recently, a new potent antagonist was described for the P2Z receptor in human lymphocytes, the isoquinoline derivative KN-62 (Gargett and Wiley, 1997). The current evoked in Müller cells by BzATP under control conditions was recorded before application of 1 μm KN-62. After incubation for up to 3 min, the BzATP-induced currents were strongly reduced to 25 ± 24% (n = 11). A complete block of the BzATP effects (between 10 and 25 μm) was seen after an incubation for up to 6 min (n = 6), whereas another cell still displayed a very small response of 2.5% of the original amplitude after a 9 min incubation. Despite washout times of up to 15 min, no recovery was observed (n = 11) (Fig.4F). Moreover, a concentration of 100 nm KN-62 was tested. After an incubation time of 9 ± 1.5 min, the inward current evoked by 25 μm BzATP was reduced to 13 ± 7% (n = 4).
Effects of BzATP application on glial glutamate transporter currents
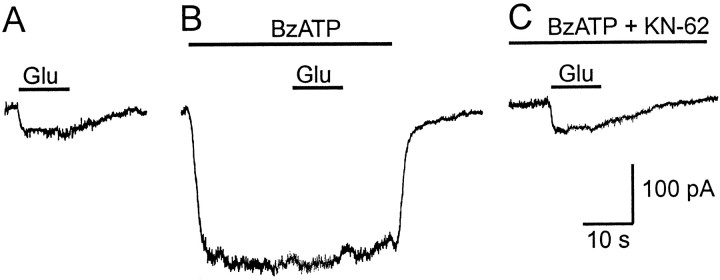
To determine whether the P2X receptors on human Müller cells are able to modify the glial transmitter recycling mechanisms, we recorded currents mediated by the glutamate transporter in the absence and presence of BzATP. For these experiments the pipette solution contained 130 mm K+, and K+ (3 mm) was also present in the extracellular solution because the glutamate transporter of Müller cells depends on intracellular K+ (Barbour et al., 1988). Application of BzATP (10 and 20 μm) significantly reduced the inward current evoked by 100 μm glutamate (Wilcoxon test,p = 0.0002, n = 19) (Fig.5B) to 45.9 ± 30.5 and 38.1 ± 31.3%, respectively. Pretreatment of the cells with 1 μm KN-62 (5 min) suppressed the effect of BzATP, whereas an additional application of glutamate evoked an inward current that was not decreased compared with the current under control conditions (n = 4) (Fig. 5C). 2-MeSATP (100 μm) failed to exert any effect on the transporter-mediated current (n = 4).
Fig. 5.
Effect of the P2X receptor on the glutamate uptake current. Glutamate (100 μm) was applied as indicated onto a Müller cell clamped at −80 mV. A, Under control conditions, an inward current was evoked due to the activation of the glutamate transporter. B, When glutamate application was repeated in the presence of BzATP (10 μm), the amplitude of the glutamate transporter current was strongly reduced. C, Pretreatment with KN-62 (1 μm) suppressed the BzATP-induced current, resulting in a glutamate-evoked inward current similar to A.
Ca2+ microfluorimetry
The existence of receptors for ATP was also demonstrated by fluorescence ratio measurements of intracellular Ca2+ concentrations ([Ca2+]i) using single-cell fura-2 AM microfluorimetry. Repeated application of ATP or the P2X7 receptor agonist BzATP for 30 sec at intervals of 10–12 min evoked Ca2+signals of consistent amplitudes. However, there was a considerable cell-to-cell variability in the magnitude of the responses. The increase in [Ca2+]i was transient in most cells after ATP application, whereas it was more sustained, or even double-peaked, after BzATP (Fig.6A). In contrast to what had been observed for the transmembrane currents, 10 μm ATP was more effective than 100 μm BzATP in inducing increases in [Ca2+]i (Fig.6A). The respective mean peak increases of Δ fluorescence ratio were 1.67 ± 0.42 and 1.22 ± 0.17 (direct comparison in six cells, p < 0.05, paired t test). It is noteworthy that at lower concentrations of BzATP (10 μm) some cells did not respond at all, whereas others revealed a small but clear [Ca2+]i increase that was sensitive to 1 μm KN-62 (reduction of mean peak amplitude to 32.6 ± 10.5%, n = 3). The P2Y1 agonist ADPβS at concentrations of 1 and 10 μm induced clear [Ca2+]i increases (n = 3), whereas the P2X1,3receptor agonist α,β-meATP showed no (1 μm) or only very weak (10 μm) effects (n = 3) (Fig. 6B).
Fig. 6.
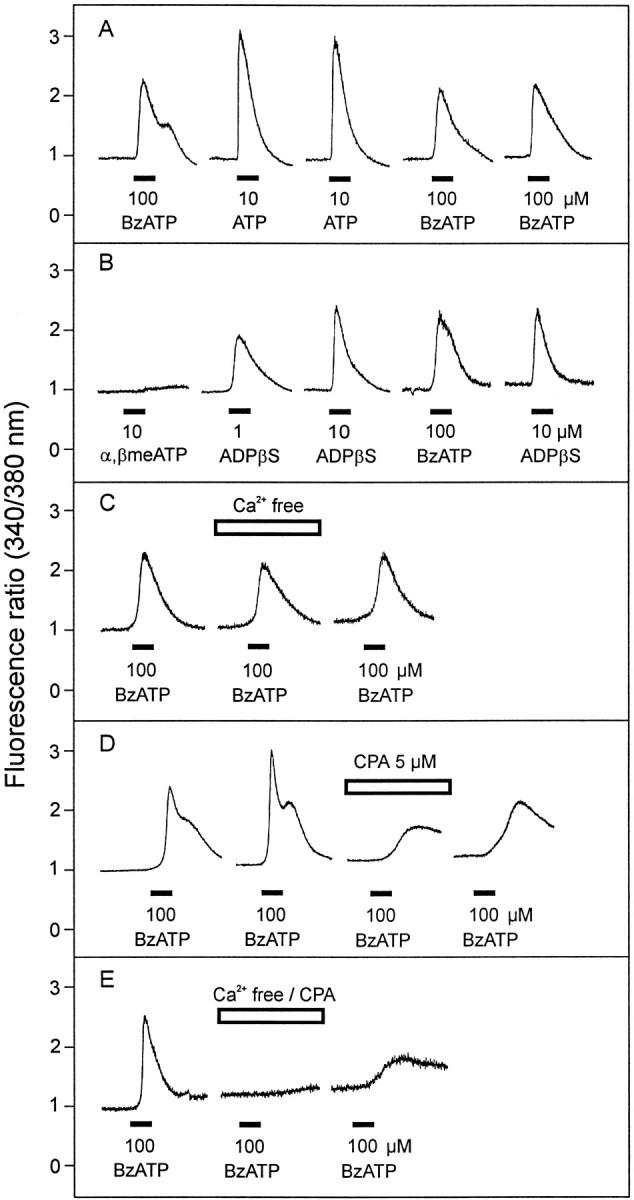
Intracellular Ca2+ responses elicited by ATP and various P2 receptor agonists in dissociated human Müller cells, based on fluorescence ratio measurements using fura-2 AM microfluorimetry. Agonists were applied with the superfusion flow for 30 sec, as indicated by the bars. A, ATP (10 μm) evoked fast and transient [Ca2+]i rises in most Müller cells tested, whereas BzATP (100 μm) showed more sustained responses, sometimes with a second increase.B, The P2Y1 receptor agonist ADPβS evoked clear [Ca2+]i rises, whereas α, β-meATP, a P2X1,3 receptor agonist, failed to elicit a clear response in two of three cells. C, In Ca2+-free solution, the response to BzATP was only slightly diminished. D, Depletion of intracellular Ca2+ stores with cyclopiazonic acid (CPA, 5 μm) markedly reduced the response to BzATP, but BzATP was still able to elicit an increase in [Ca2+]i. E, Exposure of the cells to Ca2+-free solution in combination with CPA (5 μm) abolished the Ca2+ response elicited by BzATP.
In a second series of experiments, the effects of Ca2+-free solution (with 1 mmEGTA, superfusion of the cells for 10 min) was examined on ATP- and BzATP-induced increases in [Ca2+]i. Surprisingly, both the ATP (10 μm) and the BzATP responses (100 μm) (Fig. 6C) were only weakly diminished by removal of extracellular Ca2+ (reduction of mean peak amplitudes to 80.6 ± 8.3%, n = 5, and 78.4 ± 11.4%,n = 6, respectively; p < 0.05 compared with the response in control solution). On the other hand, after superfusion (10 min) with 400 nm thapsigargin (data not shown) or 5 μm CPA (Fig.6D), two specific inhibitors of the endoplasmatic Ca2+-ATPase producing a depletion of intracellular Ca2+ stores, the response to 100 μm BzATP was markedly suppressed but still clearly detectable. Under these conditions, the signals displayed a long latency and increased only slowly (reduction of mean peak amplitude after CPA to 28.8 ± 2.0%, n = 6;p < 0.01). Finally, the combined superfusion of Ca2+-free solution with additional 5 μm CPA antagonized very efficiently the Ca2+ response elicited by 100 μm BzATP (Fig. 6E) (reduction of mean peak amplitudes to 7.9 ± 1.5% vs control before drug application, n = 3).
It should be noted that these experiments are complicated by the fact that although Ca2+ reduces the BzATP-evoked inward current (Fig. 4), it must be present in the extracellular solution if a Ca2+ influx is to be measured by microfluorimetry. Thus, the relatively small [Ca2+]i responses are not easily comparable to the electrophysiologically recorded inward currents, which are mainly caused by the influx of Na+.
Dye filling
There are several studies demonstrating that the activation of P2Z or P2X7 receptors results in the opening of a nonselective membrane pore permeable to molecules up to 900 Da (Steinberg et al., 1987; Surprenant et al., 1996). To investigate the existence of similar pores in human Müller cells after application of BzATP, we used the fluorescent dyes Lucifer yellow [0.1% (MW): 443 for the anion], YO-PRO-1 (10 μm, MW: 375 for the cation), ethidium bromide (20 μm, MW: 314 for the cation), and Alexa Fluor 488 (10 μm, MW: 547 for the anion). Suspensions of isolated Müller cells were incubated in one of the dye solutions for up to 15 min with or without BzATP (50 and 100 μm). After incubation in Lucifer yellow and subsequent wash, ∼50% of the cells (n = 49 andn = 50, respectively) contained the fluorescent dye, regardless of whether BzATP was present or not. In contrast to Lucifer yellow, which is a naturally fluorescing dye, YO-PRO-1 and ethidium bromide display fluorescence only after binding to nucleic acids in the nucleus. Even under control conditions, both dyes were able to stain the nuclei of isolated Müller cells to a certain degree. Additional application of BzATP did not result in any unequivocal increase of the staining intensity (data not shown).
Although Rassendren et al. (1997) investigated pore formation by rat P2X7 receptors by recording YO-PRO-1 uptake at room temperature, other studies suggest a temperature dependence of the pore formation (Steinberg et al., 1987; Nuttle and Dubyak, 1994). For this reason, we examined the uptake of the fluorescent dye Alexa Fluor 488 into isolated Müller cells both at room temperature and at 37°C. This dye was used because we found in preliminary experiments that it did not fill Müller cells in the retinal tissue. Cells were incubated in PBS containing 10 μm of the dye with or without BzATP (50 μm). After a 15 min incubation and subsequent washing, the fluorescence of the cells was examined. In no instance were strongly fluorescent cells found. About 25% of the Müller cells at room temperature and 50% of the cells at 37°C displayed a weak fluorescence (compared with the strong fluorescence of some neuronal cells that were probably damaged by the isolation procedure), regardless of whether BzATP was present or not (n = 20 for each condition). The remaining Müller cells were not fluorescent at all. For control reasons, we observed the uptake of Alexa Fluor 488 by Müller cells after application of the permeabilizing agent digitonin (50 μg/ml). After this treatment the dye entered the cells, which thus acquired bright fluorescence.
In summarizing these results we conclude that application of BzATP does not cause any marked opening of membrane pores large enough to allow fluorescent dyes to enter the cells faster than under control conditions.
Expression of P2X7 receptor mRNA
A total of seven Müller cells were investigated for expression of mRNA encoding P2X7 receptors with single-cell RT-PCR using P2X7-specific primers. Before harvesting cytoplasm, inward currents evoked by application of BzATP were recorded in the whole-cell configuration. In three of these cells, a 411 bp amplification product was detected by gel electrophoresis (Fig. 7). Homology with the known P2X7 gene sequence was verified by sequence analysis.
Fig. 7.
Expression of P2X7 receptor mRNA revealed by single-cell RT-PCR. The PCR product with 411 bp length was found in three individual Müller cells in an ethidium bromide-stained 1.5% agarose gel after electrophoresis. The negative control was performed as described in Materials and Methods.
DISCUSSION
P2 receptors in Müller cells
Here we provide evidence that human Müller cells express P2 receptors. This finding is in accordance with reports on amphibian (Keirstead and Miller, 1997) and nonprimate mammalian (Newman and Zahs, 1997; Liu and Wakakura, 1998; Neal et al., 1998) Müller cells. We confirm that activation of P2Y receptors causes large [Ca2+]i increases (Newman and Zahs, 1997). A predominance of P2Y receptors is indicated by the observation that the increase of [Ca2+]i is only slightly diminished in the absence of extracellular Ca2+ and the clear effects of the P2Y agonist ADPβS. The effect of BzATP under Ca2+-free conditions might be explained by an interaction at the P2Y2 receptor, as has been discussed by Humphreys et al. (1998). Nevertheless, a significant rise of [Ca2+]i during application of BzATP after depletion of intracellular Ca2+-stores favors the existence of P2X receptors in human Müller cells. These receptors could be permeable for Ca2+ (Bean, 1992) or may cause a depolarization attributable to an influx of Na+. Subsequently, Ca2+ would flow into the cell through voltage-activated Ca2+ channels (Puro et al., 1996).
Several subtypes of P2X receptors might be active in Müller cells (Liu and Wakakura, 1998). We found that not only BzATP but also other agonists evoked inward currents. 2-MeSATP was shown to evoke inward currents on P2X7 receptors in microglial cells with a higher EC50 than BzATP (Chessell et al., 1997); however, these authors recorded only very small effects of α,β-meATP. Therefore the inward currents in human Müller cells caused by α,β-meATP (and β,γ-meATP) might be attributable to a P2X receptor type other than P2X7, expressed at a minor rate. This is also in accordance with our immunocytochemical observations (unpublished data). The present study is focused on the demonstration and characterization of P2X7receptors. Because the pharmacological identification of the receptor is hampered by the low specificity of the available drugs, we tried to detect the presence of a P2X7 gene product by single-cell RT-PCR. P2X7 mRNA was found in >40% of the cells investigated, providing the first evidence of P2X7 gene expression in human Müller cells. The negative result in the remaining cells might be attributable to methodological reasons (e.g., degradation of mRNA during preparation or incomplete harvesting of the cytoplasm), because the BzATP-evoked currents were very similar in all cells studied.
P2X7 receptors: pharmacological characterization
We show that human Müller cells possess a functional P2X-type receptor for extracellular ATP and its analog BzATP. Several lines of evidence indicate that this receptor corresponds to the human P2X7 receptor. First, specific antibodies directed to the human P2X7 receptor revealed strong and specific immunoreactivity. Second, the P2X7 receptor mRNA was detected in Müller cells by RT-PCR.
Third, a higher affinity for BzATP than for ATP (and other analogs) is thought to be characteristic for the P2X7receptor (Burnstock, 1997). Bianchi et al. (1999) demonstrated a high potency of BzATP for activation of P2X receptors other than P2X7, especially of P2X1. However, both this receptor type and the P2X3receptor were shown to display a fast desensitization, which has never been found in Müller cells.
Fourth, the currents evoked by BzATP were blocked by relatively high concentrations of suramin and PPADS. This is in accordance with data ofRassendren at al. (1997) who determined IC50values of 92 and 62 μm, respectively, for the human P2X7 receptor. Finally, KN-62 has been described as a potent antagonist at the human P2Z receptor (Gargett and Wiley, 1997). Humphreys et al. (1998) compared the effects of KN-62 on P2X7 receptors. Interestingly, ATP effects were suppressed only on the human but not on the rat receptor.
The current-decreasing effects of Mg2+ and Ca2+ are well known and were demonstrated for the P2X7 receptor from both rats and humans (Surprenant et al., 1996; Michel et al., 1999). This effect is ascribed to a reduction of the concentration of fully ionized ATP4-, which is chelated by divalent cations. However, a direct effect on the receptor cannot be ruled out (Virginio et al., 1997). Independent of the mechanism, the effect of divalent cations may have a substantial physiological impact. Normal physiological concentrations of these ions must strongly diminish the receptor currents. There remains the question whether sufficiently high extracellular ATP concentrations may be achieved to activate this receptor in Müller cells in the living retina. This should certainly be possible under pathological conditions, e.g., when ATP is released by dying cells, or during physiological light-induced extracellular [Ca2+] decreases (Livsey et al., 1990; Gallemore et al., 1994).
Channel permeability and pore formation
Usually, P2X receptors are described as nonspecific cation channels permeable for Na+ and K+ as well as for Ca2+ (Soto et al., 1997). Activation of the P2Z or P2X7 receptor has been shown to result in the formation of a pore permeable to molecules as large as 900 Da, not only in macrophages (Steinberg et al., 1987) and microglial cells (Ferrari et al., 1996; Chessell et al., 1997) but also in cultured rat cerebral astrocytes (Ballerini et al., 1996). By contrast, we found no evidence for a similar pore formation. Although this type of experiment is impeded in Müller cells by their tendency to accumulate various exogenous dyes even without stimulation of any receptors (Reichenbach et al., 1995), it can be clearly stated that dye accumulation in Müller cells was not significantly increased by BzATP application. Furthermore, the substitution of extracellular Na+ by organic cations (195 Da) resulted in the disappearance of the inward current. This in accordance with similar findings of other authors. Petrou et al. (1997) demonstrated that expression of the rat P2X7 receptor inXenopus oocytes does not cause pore formation. Rassendren et al. (1997) investigated inward currents and the uptake of the dye YO-PRO-1 in HEK cells expressing the rat and the human P2X7 receptor. The membrane permeability to large cations was much lower in the human receptor, and the YO-PRO-1 uptake amounted to only ∼20% compared with cells transfected with the rat receptor. It was demonstrated by these authors that differences in the C-terminal domain of the receptor proteins are responsible for these alterations.
Moreover, the pore formation may not only differ among species but may also depend on the cell type. Humphreys et al. (1998) demonstrated pore formation in a murine macrophage cell line but failed to detect this effect in a murine thymocyte cell line. A similar result was obtained by Markwardt et al. (1997), who described purinoceptors in human lymphocytes with agonist binding characteristics of the P2Z receptor but lacking pore formation.
Functional implications
Müller cells may be exposed to extracellular ATP in different ways. It has long been known that ATP is released into the mammalian eye by stimulation of the trigeminal nerve (Maul and Sears, 1979); because the endfeet of Müller cells are facing the vitreous body, their receptors could be stimulated by any of the events caused by nerve stimulation, such as hyperemia or increased intraocular pressure. On the other hand, there is substantial neuronal purinergic transmission in the retina (Peral and Pintor, 1998) that also may stimulate glial cells.
In any case, it can be stated that human Müller cells express functional P2X7 receptors, which mediate rather small cation currents that are inwardly directed at resting membrane potential and can produce depolarizations. Activation of these receptors causes, at best, moderate Ca2+influxes but no opening of large-conductance pores. Preliminary experiments on Müller cells from pathologically altered retinae indicate that there is no significant increase in the probability of pore formation when compared with cells from healthy retinae (T. Pannicke and F. Faude, unpublished data). It is therefore highly improbable that the P2X7 receptors of human Müller cells may act as “suicide triggers” as proposed for other cell types (Ferrari et al., 1997). They also do not seem to play a major role in the control of the [Ca2+]i, at least compared with the dominant effects of the P2Y receptors. There remains the question of their physiological function.
One of the few ATP effects studied on Müller cells is a net “release” of GABA by reducing GABA uptake after activation of P2X receptors (Neal et al., 1998). Because the GABA uptake of Müller cells depends on both the transmembrane Na+ gradient and the membrane potential, and because BzATP causes Na+ influx and membrane depolarization, it is feasible that a modulation of extracellular [GABA] may be a function of the P2X7 receptor in Müller cells. We investigated the effect on the glutamate uptake, because GABA activates a receptor in human Müller cells (Reichelt et al., 1997) that might interfere with the registration of the uptake current. Concentrations of BzATP lower than the EC50 were able to reduce the glutamate uptake current by >50%. Because the experiments were performed in the voltage-clamp mode, this result can be explained by a reduction of the Na+gradient. In vivo this effect should even be enhanced by an additional depolarization.
Footnotes
This work was supported by the Bundesministerium für Bildung, Forschung und Technologie (BMB+F), Interdisciplinary Center for Clinical Research at the University of Leipzig (01KS9504, Project C5), by Deutscher Akademischer Austauschdienst (DAAD) 313/ARC-pz, and by the Deutsche Forschungsgemeinschaft (PA 615/1-1, AL 414/31). We thank Dr. Knut Krohn (Interdisciplinary Center for Clinical Research at the University of Leipzig, Grant Z3) for sequencing the DNA fragment, Dr. Dagmar Müller for helpful discussion of the single-cell PCR experiments, Dr. Ute Gröschel-Stewart for methodological support, and Roy Jordan for critical reading of this manuscript.
Correspondence should be addressed to Dr. Thomas Pannicke, Paul-Flechsig-Institute for Brain Research, Department of Neurophysiology, University of Leipzig, Jahnallee 59, D-04109 Leipzig, Germany. E-mail: pant@medizin.uni-leipzig.de.
REFERENCES
- 1.Amedee T, Despeyroux S. ATP activates cationic and anionic conductances in Schwann cells cultured from dorsal root ganglia of the mouse. Proc R Soc Lond B Biol Sci. 1995;259:277–284. doi: 10.1098/rspb.1995.0041. [DOI] [PubMed] [Google Scholar]
- 2.Ballerini P, Rathbone MP, Di Iorio P, Renzetti A, Giuliani P, D'Alimonte I, Trubiani O, Ciccarelli R. Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. NeuroReport. 1996;7:2533–2537. doi: 10.1097/00001756-199611040-00026. [DOI] [PubMed] [Google Scholar]
- 3.Barbour B, Brew H, Attwell D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature. 1988;335:433–435. doi: 10.1038/335433a0. [DOI] [PubMed] [Google Scholar]
- 4.Bean BP. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992;13:87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, Jarvis MF, van Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- 6.Brändle U, Guenther E, Irrle C, Wheeler-Schilling TH. Gene expression of the P2X receptors in the rat retina. Mol Brain Res. 1998a;59:269–272. doi: 10.1016/s0169-328x(98)00159-4. [DOI] [PubMed] [Google Scholar]
- 7.Brändle U, Kohler K, Wheeler-Schilling TH. Expression of the P2X7-receptor subunit in neurons of the rat retina. Mol Brain Res. 1998b;62:106–109. doi: 10.1016/s0169-328x(98)00254-x. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 9.Chessell IP, Michel AD, Humphrey PPA. Properties of the pore-forming P2X7 purinoceptor in mouse NTW8 microglial cells. Br J Pharmacol. 1997;121:1429–1437. doi: 10.1038/sj.bjp.0701278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, Di Virgilio F. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J Immunol. 1996;156:1531–1539. [PubMed] [Google Scholar]
- 12.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Collo G, Buell G, Di Virgilio F. ATP-mediated cytotoxicity in microglial cells. Neuropharmacology. 1997;36:1295–1301. doi: 10.1016/s0028-3908(97)00137-8. [DOI] [PubMed] [Google Scholar]
- 13.Francke M, Pannicke T, Biedermann B, Faude F, Wiedemann P, Reichenbach A, Reichelt W. Loss of inwardly rectifying potassium currents by human retinal glial cells in diseases of the eye. Glia. 1997;20:210–218. doi: 10.1002/(sici)1098-1136(199707)20:3<210::aid-glia5>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Gallemore RP, Li J-D, Govardovski VI, Steinberg RH. Calcium gradients and light-evoked calcium changes outside rods in the intact cat retina. Vis Neurosci. 1994;11:753–761. doi: 10.1017/s0952523800003059. [DOI] [PubMed] [Google Scholar]
- 15.Gargett CE, Wiley JS. The isoquinoline derivative KN-62 a potent antagonist of the P2Z-receptor of human lymphocytes. Br J Pharmacol. 1997;120:1483–1490. doi: 10.1038/sj.bjp.0701081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenwood D, Yao WP, Housley GD. Expression of the P2X2 receptor subunit of the ATP-gated ion channel in the retina. NeuroReport. 1997;8:1083–1088. doi: 10.1097/00001756-199703240-00004. [DOI] [PubMed] [Google Scholar]
- 17.Gröschel-Stewart U, Bardini M, Robson T, Burnstock G. Localisation of P2X5 and P2X7 receptors by immunohistochemistry in rat stratified squamous epithelia. Cell Tissue Res. 1999;296:599–605. doi: 10.1007/s004410051321. [DOI] [PubMed] [Google Scholar]
- 18.Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor. Mol Pharmacol. 1998;54:22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]
- 19.Illes P, Nörenberg W. Neuronal ATP receptors and their mechanism of action. Trends Pharmacol Sci. 1993;14:50–54. doi: 10.1016/0165-6147(93)90030-n. [DOI] [PubMed] [Google Scholar]
- 20.Keirstead SA, Miller RF. Metabotropic glutamate receptor agonists evoke calcium waves in isolated Müller cells. Glia. 1997;21:194–203. [PubMed] [Google Scholar]
- 21.Liu Y, Wakakura M. P1-/P2-purinergic receptors on cultured rabbit retinal Müller cells. Jpn J Ophthalmol. 1998;42:33–40. doi: 10.1016/s0021-5155(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 22.Livsey CT, Huang B, Xu J, Karwoski CJ. Light-evoked changes in extracellular calcium concentration in frog retina. Vision Res. 1990;30:853–861. doi: 10.1016/0042-6989(90)90054-o. [DOI] [PubMed] [Google Scholar]
- 23.Markwardt F, Lohn M, Bohm T, Klapperstück M. Purinoceptor-operated cationic channels in human B lymphocytes. J Physiol (Lond) 1997;498:143–151. doi: 10.1113/jphysiol.1997.sp021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maul E, Sears M. ATP is released into the rabbit eye by antidromic stimulation of the trigeminal nerve. Invest Ophthalmol Vis Sci. 1979;18:256–262. [PubMed] [Google Scholar]
- 25.Michel AD, Chessell IP, Humphrey PPA. Ionic effects on human recombinant P2X7 receptor function. Naunyn-Schmiedebergs Arch Pharmacol. 1999;359:102–109. doi: 10.1007/pl00005328. [DOI] [PubMed] [Google Scholar]
- 26.Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993;268:199–203. [PubMed] [Google Scholar]
- 27.Neal MJ, Cunningham JR. Modulation by endogenous ATP of the light-evoked release of ACh from retinal cholinergic neurones. Br J Pharmacol. 1994;113:1085–1087. doi: 10.1111/j.1476-5381.1994.tb17106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neal MJ, Cunningham JR, Dent Z. Modulation of extracellular GABA levels in the retina by activation of glial P2X-purinoceptors. Br J Pharmacol. 1998;124:317–322. doi: 10.1038/sj.bjp.0701841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science. 1997;275:844–847. doi: 10.1126/science.275.5301.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nörenberg W, Langosch JM, Gebicke-Haerter PJ, Illes P. Characterization and possible function of adenosine 5′-triphosphate receptors in activated rat microglia. Br J Pharmacol. 1994;111:942–950. doi: 10.1111/j.1476-5381.1994.tb14830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuttle LC, Dubyak GR. Differential activation of cation channels and non-selective pores by macrophage P2z purinergic receptors expressed in Xenopus oocytes. J Biol Chem. 1994;269:13988–13996. [PubMed] [Google Scholar]
- 32.Oglesby IB, Lachnit WG, Burnstock G, Ford APDW. Subunit specificity of polyclonal antisera to the carboxy terminal regions of P2X receptors, P2X1 through P2X7. Drug Dev Res. 1999;47:189–195. [Google Scholar]
- 33.Peral A, Pintor J. Purinergic transmission in the retina. Neurosci Res Commun. 1998;23:129–137. [Google Scholar]
- 34.Petrou S, Ugur M, Drummond RM, Singer JJ, Walsh JV. P2X7 purinoceptor expression in Xenopus oocytes is not sufficient to produce a pore-forming P2Z-like phenotype. FEBS Lett. 1997;411:339–345. doi: 10.1016/s0014-5793(97)00700-x. [DOI] [PubMed] [Google Scholar]
- 35.Puro DG, Hwang J-J, Kwon O-J, Chin H. Characterization of an l-type calcium channel expressed by human retinal Müller (glial) cells. Mol Brain Res. 1996;37:41–48. doi: 10.1016/0169-328x(96)80478-5. [DOI] [PubMed] [Google Scholar]
- 36.Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor, P2X7: cloning and expression of a human cDNA. J Biol Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- 37.Reichelt W, Pannicke T, Biedermann B, Francke M, Faude F. Comparison between functional characteristics of healthy and pathological human retinal Müller glial cells. Surv Ophthalmol. 1997;42:S105–S117. doi: 10.1016/s0039-6257(97)80033-1. [DOI] [PubMed] [Google Scholar]
- 38.Reichenbach A, Birkenmeyer G. Preparation of isolated Müller cells of the mammalian (rabbit) retina. Z Mikrosk-Anat Forsch. 1984;98:789–792. [PubMed] [Google Scholar]
- 39.Reichenbach A, Grimm D, Mozhaiskaja N, Distler C. Visualization of Müller (retinal glial) cells by bulk filling with procion yellow. J Brain Res. 1995;36:305–311. [PubMed] [Google Scholar]
- 40.Soto F, Garcia-Guzman M, Stühmer W. Cloned ligand-gated channels activated by extracellular ATP (P2X receptors). J Membr Biol. 1997;160:91–100. doi: 10.1007/s002329900298. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg TH, Newman AS, Swanson JA, Silverstein SC. ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987;262:8884–8888. [PubMed] [Google Scholar]
- 42.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 43.Taschenberger H, Jüttner R, Grantyn R. Ca2+-permeable P2X receptor channels in cultured rat retinal ganglion cells. J Neurosci. 1999;19:3353–3366. doi: 10.1523/JNEUROSCI.19-09-03353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Virginio C, Church D, North RA, Surprenant A. Effects of divalent cation, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology. 1997;36:1285–1294. doi: 10.1016/s0028-3908(97)00141-x. [DOI] [PubMed] [Google Scholar]
- 45.Walz W, Ilschner S, Ohlemeyer C, Banati R, Kettenmann H. Extracellular ATP activates a cation conductance and a K+ conductance in cultured microglial cells from the mouse brain. J Neurosci. 1993;13:4403–4411. doi: 10.1523/JNEUROSCI.13-10-04403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walz W, Gimpl G, Ohlemeyer C, Kettenmann H. Extracellular ATP-induced currents in astrocytes: involvement of a cation channel. J Neurosci Res. 1994;38:12–18. doi: 10.1002/jnr.490380104. [DOI] [PubMed] [Google Scholar]