Abstract
Cellular adhesion to the extracellular matrix is mediated by a diverse class of α/β heterodimeric receptors known as integrins, which transduce signals to activate multiple intracellular signal transduction pathways within the cells. The signaling pathway linking integrins to mediate neuronal process outgrowth is not well understood. Here, we have provided evidence that intracellular signaling by the α1β1 integrin-induced activation of cyclin-dependent kinase 5 (cdk5) is involved in neurite outgrowth and human neurofilament protein H (hNF-H) Lys-Ser-Pro (KSP) tail domain phosphorylation in differentiated human SH-SY5Y cells. The integrin α1 and β1 monoclonal antibodies and BL-1, a specific cdk5 inhibitor, inhibited these effects. We also demonstrated that cdk5 activity and hNF-H KSP tail domain phosphorylation were increased in cdk5/p35 and hNF-H tail domain co-transfected HEK293 cells grown on laminin. This increased hNF-H tail domain phosphorylation was triggered by cdk5 activation. Taken together, these results indicated that cdk5 may play an important role in promoting neurite outgrowth and hNF-H tail KSP domain phosphorylation through the integrin α1β1signaling pathway.
Keywords: cdk5, p35, neurofilament, integrin, matrix, laminin, retinoic acid
During neuronal development, constituents of the extracellular matrix (ECM) components, such as laminin, collagen, fibronectin, vitronectin, and tenascin, are important regulators for neurite extension. Among these ECM components, laminin has been thought to regulate in vitro neurite outgrowth (Manthorpe et al., 1983; Rogers et al., 1983), differentiation (Reh and Radke, 1988), and survival (Calof and Reichardt, 1984; Edgar et al., 1984; Sanes, 1989). Integrins are transmembrane, heterodimeric receptors, which bind ECM molecules and mediate cell adhesion, migration, and nerve regeneration in the nervous system. (Hemler, 1990; Hynes, 1992; Trigg et al., 1998). The integrin family of receptors includes a large number of heterodimeric proteins, which associate into various α and β subunit combinations, thereby producing diverse cellular functions (Hemler, 1990). Integrins containing the β1 subunit, which can associate with at least 10 distinct subunits, are particularly important for neuronal interactions. β1 class integrins have been shown to mediate the interaction in both central and peripheral neurons as well as neuronal cell lines (Reichardt and Tomaselli, 1991; Ruoslahti and Vaheri, 1997). Integrin α3β1 and α1β1 have been identified as the major β1 integrins expressed by PC12 cells (Arregui et al., 1994) and human neuroblastoma cell line SH-SY5Y (Choi et al., 1994). Integrin α1 and β1 have been shown to be upregulated by retinoic acid (RA) during differentiation of neuroblastoma cellsin vitro (Rossino et al., 1991). However, little is known about the mechanism(s) whereby RA and integrin mediate signaling in neurite outgrowth and neurofilament protein H (NF-H) tail domain phosphorylation.
Cyclin-dependent kinase 5 (cdk5) is a multifunctional protein kinase. Although, it associates with cyclins (Xiong et al., 1992; Guidato et al., 1996), its activity has been detected in postmitotic cells because of its association with neuron-specific activators p35, p39, and p67 (Lew et al., 1994; Shetty et al., 1995; Hirooka et al., 1996). In addition to its role in neuronal migration and neurite extension (Ohshima et al., 1996; Chae et al., 1997), cdk5 affects dopamine signaling (Bibb et al., 1999) and exocytosis (Fletcher et al., 1999). Cdk5 activity has also been reported to inhibit fast anterograde axonal transport (Ratner et al., 1998), which may affect neurite outgrowth. Cdk5 phosphorylates neuronal cytoskeletal proteins such as NF-H, NF-M, MAP1B, and tau (Paudel et al., 1993; Shetty et al., 1993; Pigino et al., 1997; Paglini et al., 1998; Patrick et al., 1999; Sharma et al., 1999). Phosphorylation of neurofilament proteins, specifically NF-M and NF-H, has been reported to protect them from proteolysis (Goldstein et al., 1987; Pant, 1988). This may provide stability to axonal structures (Shea and Beermann, 1994).
Neurofilament proteins are among the most highly phosphorylated proteins in the nervous system (Hoffman and Lasek, 1975; Julien and Mushynski, 1983; Hoffman et al., 1984; Nixon et al., 1987; Nixon and Sihag, 1991; Elhanany et al., 1994; Pant and Veeranna, 1995). It has been proposed that phosphorylation of the NF-H and NF-M tail domains increase the total negative charges and the lateral extension of neurofilament side arms, which in turn increase neurofilament spacing, axonal caliber (Hirokawa et al., 1984; de Waegh et al., 1992; Brown and Lasek, 1993; Nakagawa et al., 1995), and conduction velocity of nerve fiber. The extensive phosphorylation of NF-M and NF-H occurs in the Lys-Ser-Pro (KSP) multiple repeat motifs of C-terminal tail domains. The phosphorylation of these motifs is regulated by extracellular signal-regulated kinase 1/2 (Erk1/2), stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase), and cdk5 in vitro (Xu et al., 1992; Elhanny et al., 1994; Giasson and Mushynski, 1996, 1997;Veeranna et al., 1998; Li et al.; 1999a,b). In the human NF-H tail domain, there are 43/44 KSP repeats, of which 32 are KSPXK motifs. Cdk5 has been shown to phosphorylate specifically the serine-threonine sites in Lys-Ser-Pro-X-Lys (KSPXK)-type motifs but not others, e.g., KSPXXK or KSPXXXK, in the tail domain of neurofilaments (Hisanaga et al., 1991; Shetty et al., 1993; Lew et al., 1994; Veeranna et al., 1998). Although Erk1/2 and SAPK are activated by various external and stress stimuli, respectively, activation of cdk5 by external stimuli remains poorly understood. Because human NF-H (hNF-H) has many more KSPXK motifs compared with rat or mouse NF-H, we focused on cdk5 phosphorylation of human NF-H in SH-SY5Y cells.
In this study we have demonstrated that cdk5 activity is elevated, and the hNF-H KSP tail domain phosphorylation is upregulated on integrin α1β1 receptor activation. These effects were inhibited by the integrin α1β1 antibodies and by BL-1, a specific inhibitor of cdk5. We also found that the increased hNF-H phosphorylation was mainly triggered by cdk5 activity in cdk5/hNF-H co-transfected HEK293 cells grown on laminin. These findings indicated that integrin α1β1 signaling pathway-mediated activation of cdk5 is involved in neurite outgrowth and hNF-H KSP tail domain phosphorylation.
MATERIALS AND METHODS
Materials. Anti-phosphorylation-dependent antibody (SMI31) and anti-phosphorylation-independent antibody (SMI33) were obtained from Sternberger Monoclonals Inc. (Baltimore, MD). Other antibodies included an affinity-purified rabbit polyclonal antibody raised against a peptide corresponding to amino acids 2–21 mapping at the N terminus of p35 (N-20; Santa Cruz Biotechnology, Santa Cruz, CA), an affinity-purified rabbit polyclonal antibody raised against a peptide corresponding to amino acid residues 289–307 mapping at the C terminus of p35 (C-19; Santa Cruz Biotechnology), and an affinity-purified rabbit polyclonal antibody raised against a peptide corresponding to amino acid residues 284–291 mapping at the C terminus of cdk5 (C-8; Santa Cruz Biotechnology). The phospho-independent Erk1/2 polyclonal antibody made against peptide 345–358 of the molecule, phospho-Erk1/2 monoclonal antibody prepared against a peptide phosphorylated at Thr202 and Tyr204 (Thr202/Tyr204 Erk1/2), and the MAP kinase kinase (MEK) inhibitor PD098059 were obtained from New England Biolabs (Boston, MA). BL-1 was obtained from Biomol (Plymouth Meeting, PA). Integrin α1 (FB12) and integrin β1 (P4G11) antibodies were obtained from Chemicon (Temecula, CA), and functional blocking anti-β1 (DE9) antibodies were obtained from Upstate Biotechnology (Lake Placid, NY). The pcDNA/Amp eukaryotic expression vector was purchased from Invitrogen (San Diego, CA). Mouse laminin was obtained from Life Technologies (Gaithersburg, MD).
Differentiation of human neuroblastoma SH-SY5Y cells. The human neuroblastoma cell line SH-SY5Y, obtained from Dr. T. Shea (University of Massachusetts, Lowell, MA), was cultured in DMEM supplemented with 10% heat-inactivated fetal calf serum and 2 mml-glutamine. To induce differentiation, the cells were treated with 10 μm RA in the dark for 7 d.
HEK293 cell culture and transfection. HEK293 cells were obtained from the American Type Culture Collection, cultured in DMEM with 10% calf serum, and supplemented with 100U/ml penicillin and 100 μg/ml streptomycin. Cells were maintained at 37°C in a humidified atmosphere of 5% CO2. The cells were transiently transfected using LipofectAMINE (Life Technologies) according to the manufacturer's instructions. The human NF-H tail domain expression construct, p35, wild-type cdk5, and dominant-negative cdk5 constructs were transfected independently or co-transfected. Twenty-four hours after transfection, the cells were starved in the presence of 0.2% calf serum overnight (to reduce any background stimulation by serum factors), and then the cells were detached and cultured on laminin or poly-l-lysine for another 24 hr with 0.2% calf serum in the presence or absence of BL-1 or PD98059. The cells were fixed for immmunocytochemistry analysis or lysed with lysis buffer for immunoprecipation and Western blot analysis.
Cells grown on laminin-coated dishes. Culture dishes coated with laminin were prepared as described previously (Nojima et al., 1995). In brief, dishes were incubated with PBS containing 10 μg/ml laminin or 100 μg/ml poly-l-lysine at 4°C overnight. After washing three times with PBS, dishes were coated with 1% BSA-PBS by incubating for 1 hr at 37°C. Before plating cells on dishes, differentiated cells were detached by treating with 0.05% trypsin-EDTA. Trypsin inhibitor (1.5 mg/ml) was immediately added to the cell suspension, followed by washing three times with serum-free medium. Cells were then plated onto dishes coated with laminin or poly-l-lysine and incubated at 37°C for the indicated periods in serum-free medium. To test inhibition by anti-α1 and anti-β1antibodies in this work, adherent cells were incubated with antibodies according to the following protocol. After cells were treated with or without RA, the cells were harvested with EGTA and allowed to adhere for 24 hr on dishes coated with laminin (10 μg/ml) or poly-l-lysine (100 μg/ml) in medium without serum. Cells were then incubated for 4 hr at 37°C with anti-α1 (FB12, 1:10) and anti-β1 (DE9, 1:5) (Ji et al., 1998) or the preimmune serum.
Western blot analysis. Cells were harvested by scraping from dishes and lysed in ice-cold lysis buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mmEGTA, 1% Triton X-100, 0.1% SDS, 2.5 mm sodium pyrophosphate, 1 mm β-glycerolphosphate, and 1 mm Na3VO4, supplemented with a mixture of protease inhibitors and 1 mmPMSF) by passing through a 21 gauge needle several times and incubation for 30 min on ice. After centrifugation for 20 min at 13,000 ×g at 4°C, the protein concentrations of the supernatants were determined using BCA protein concentration reagent. An equal amount of total protein (20 μg of protein/lane) was resolved on a 10–20% SDS-polyacrylamide gel and blotted onto a polyvinylidene difluoride membrane for immunoblotting analysis with anti-cdk5 (C-8, 1:200) and P35 (C-20, 1:200) antibodies and phospho-dependent and -independent NF-H antibodies (SMI31, 1:1000; and SMI33, 1:1000). Western blots were performed using the Amersham (Chicago, IL) ECL kit following the manufacturer's instructions.
Immunoprecipitation and kinase assays. Cells were lysed in ice-cold lysis buffer without SDS described as above and immunoprecipitated with an anti-cdk5 (C-8) antibody. The immunoprecipitates were washed twice with lysis buffer and twice with kinase buffer. Kinase activity assays were performed as described previously (Li et al., 1999a). In brief, a total volume of 50 μl of kinase asssay mixture was used, containing 50 mmTris-HCl, pH 7.4, with 1 mm EGTA, 1 mmdithiothretol, 5 mm MgCl2, 0.5 mm microcystin L R, 10 μg of histone H1, and 10 μl of cdk5 immunoprecipitates. The phosphorylation reaction was initiated by the addition of 0.1 mm[γ-32P]ATP and incubated at 30°C for 30 min. The reaction was terminated by spotting 25 μl of the reaction mixture on p81 phosphocellulose pads that were washed five times in 75 mm phosphoric acid followed by rinsing with 95% ethanol. The radioactivity was measured in a liquid scintillation counter. SDS-PAGE and autoradiography assessed the phosphorylated histone H1.
Immunofluorescence staining. After SH-SY5Y cells and transfected HEK293 cells were cultured on coverslips that had been coated with poly-l-lysine or laminin for the indicated times, cells were washed twice in PBS and fixed for 30 min at room temperature in 4% paraformaldehyde, PBS, and 10 mm EGTA and permeabilized (with 25 mm Tris, pH 7.4, 150 mm NaCl, and 0.2% Triton X-100) for 5 min. The coverslips were incubated overnight at 4°C with anti-NF-H phospho-antibody (SMI31; 400× dilution in PBS plus 2% BSA) or cdk5 and p35 antibody (100× dilution) and then washed three times with PBS. Cells were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG and rhodamine-labeled goat anti-rabbit IgG or rhodamine-labeled goat anti-mouse IgG secondary antibody for 2 hr at room temperature. The phospho-NF-H and cdk5 or p35 staining patterns were visualized by confocal microscopy.
Northern blotting. Total RNA from cultured SH-SY5Y cells was isolated with TRIzol reagent (Life Technologies), separated by agarose gel electrophoresis, and transferred onto a nylon membrane. The membrane was hybridized in QuikHyb buffer (Stratagene, La Jolla, CA) containing 32P-labeled cDNA probes specific for p35 and cdk5 (labeled by random priming). RNA loading was determined based on ethidium bromide staining of 28 S ribosomal RNA.
Neurite extension assays. SH-SY5Y neurite extension assays were performed as described previously (Rossino et al., 1990). In brief, 24-well plates were coated with 100 μg/ml poly-l-lysine or 10 μg/ml laminin. Cells were treated with or without RA. After 48 hr, cells were detached from culture dishes by incubation in PBS with 1 mm EGTA and washed twice in serum-free culture medium. Cells (1 × 104 per well) were plated on laminin- or poly-l-lysine-coated dishes and treated with integrin α1 (FB12) and β1 (DE9) antibodies and cdk5 inhibitor BL-1 (10 μm) or left in the absence of these reagents for 24 hr. Adherent cells were fixed with 4% paraformaldehyde, stained with crystal violet, and photographed under phase contrast. Neurites from 120 cells were measured for each sample. Only processes longer than 15 μm (approximately one cell diameter) were counted using NIH Image 1.61 software (Wayne Rasband, National Institutes of Health, Bethesda, MD).
Flow cytometric analysis. The surface expressions of integrin subunits on SH-SY5Y cells were assessed by flow cytometry. For this purpose, cells were grown on coverslips in DMEM and 10% FCS and treated with or without RA. Cells were trypsinized, washed twice in PBS and 2% FCS, and incubated with primary integrin α1 (FB12, 1:100) and β1(P4G11, 1:100) antibodies for 45 min at 4°C. After washing in PBS, cells were incubated in the presence of FITC-conjugated secondary antibodies for 45 min at 4°C. Preparations were then washed again in PBS and analyzed in a FACScan using Lysys II software (Becton Dickinson, San Jose, CA) for determination of integrin expression levels. Cells were sorted on a FACStar Plus (Becton Dickinson).
Data analysis. Data are expressed as mean ± SD. One-way ANOVA followed by the Newman–Keuls test was used as indicated in the figures to determine the statistical significance;p < 0.05 was considered significant.
RESULTS
Laminin-enhanced cdk5 activity and NF-H tail KSP domain phosphorylation in RA-induced differentiated SH-SY5Y cells
Laminin–integrin interactions and cdk5 kinase activity have been implicated in neurite outgrowth of neuronal cells, including RA-induced SH-SY5Y cells and primary cultured cortical neurons (Rossino et al., 1991; Choi et al., 1994; Nikolic et al., 1996; Pigino et al., 1997;Paglini et al., 1998; Sharma et al., 1999). Therefore, we investigated whether laminin-induced cdk5 kinase activity enhances hNF-H tail domain phosphorylation of differentiated SH-SY5Y cells. First we confirmed that RA-induced cell differentiation significantly increased surface expression of integrin α1β1 by flow cytometry. As shown in Figure 1, α1- and β1-integrin surface expression in RA-treated cells exhibited a significant enhancement compared with unstimulated cells. We also confirmed that RA-treated differentiated cells, plated on laminin, displayed a distinctly neuronal phenotype, exhibiting a well developed network of branched neurites, in contrast to cells cultured on poly-l-lysine alone (data not shown; Rossino et al., 1991).
Fig. 1.
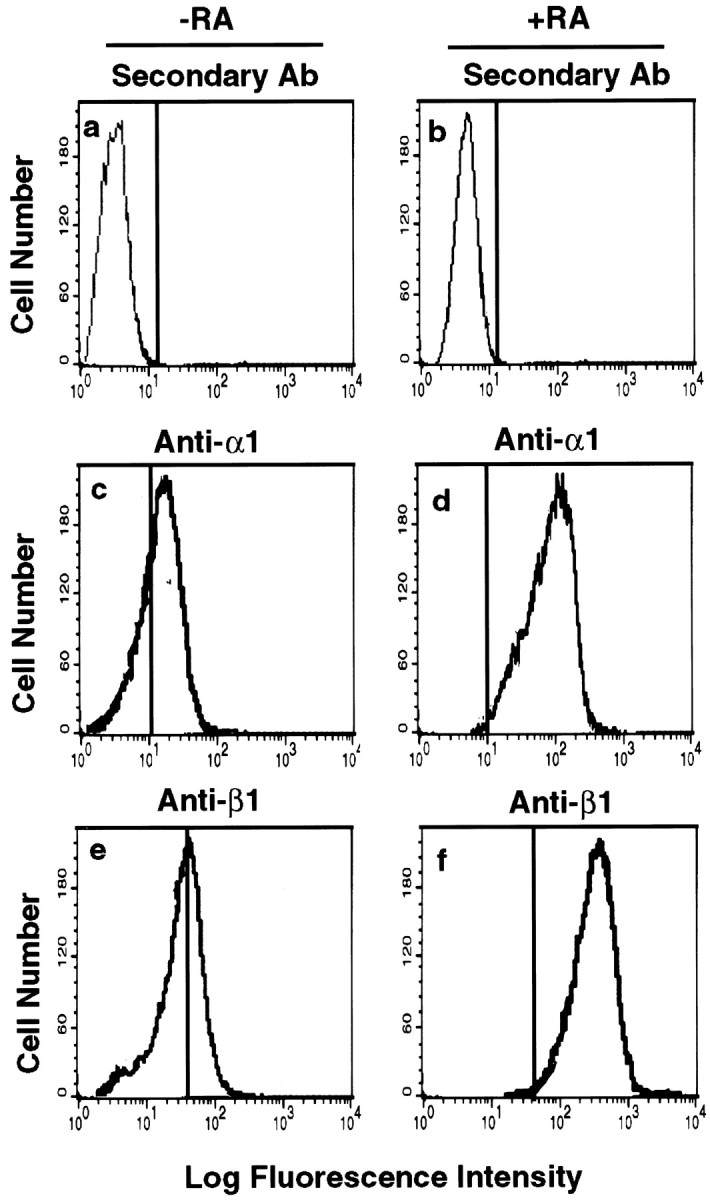
RA-induced increase in integrin α1β1 expression in SH-SY5Y human neuroblastoma cells. The surface expression of integrin subunits on SH-SY5Y cells was assessed by flow cytometry. Cells were grown on coverslips in DMEM plus 10% FCS and treated with RA (10 μm; b, d, f) or without RA (a, c, e) for 7 d. Cells were analyzed as described in Materials and Methods. Fluorescence intensity corresponds to the integrin α1β1 expression levels. a, b, Anti-mouse IgG alone; c, d, integrin anti-α1 antibody (Ab) FB12; e, f, integrin anti-β1 antibody P4G11.
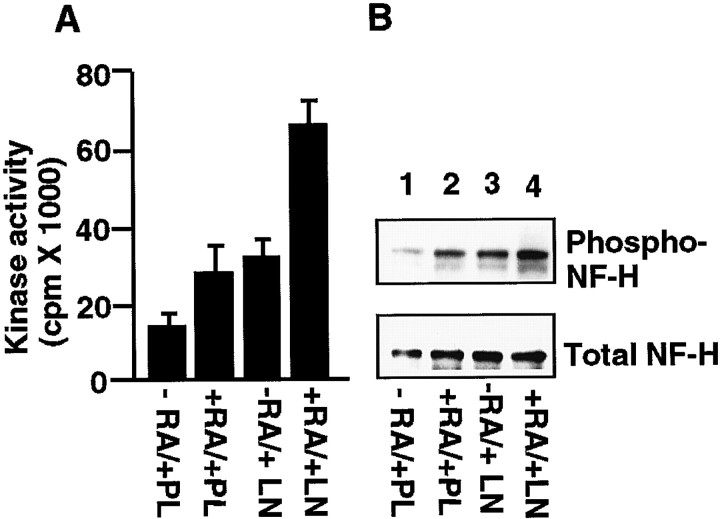
To determine the effect of laminin on the cdk5 kinase activity in the differentiated cells, lysates of SH-SY5Y cells treated with or without RA and maintained on poly-l-lysine or laminin were immunoprecipitated with cdk5 antibody. These immunoprecipitates were assayed for their ability to phosphorylate histone H1 as described in Materials and Methods. As shown in Figure2A, the cdk5 activity was significantly increased after RA treatment when maintained on laminin compared with cells grown on poly-l-lysine.
Fig. 2.
RA- and laminin-induced cdk5 activity and NF-H KSP tail domain phosphorylation in SH-SY5Y human neuroblastoma cells.A, Cells were treated with RA (10 μm) or without for 7 d and then grown on poly-l-lysine or laminin for 24 hr. Cell extracts were immunoprecipitated with cdk5 antibody, and the immunoprecipitates were assayed for their ability to phosphorylate histone H1. B, Equal amounts of protein from cell lysates were used in each case for Western blot analysis using anti-phospho-dependent NF-H tail domain antibody SMI31 and anti-phospho-independent NF-H antibody SMI33. PL, Poly-l-lysine; LN, laminin.
To examine whether increased cdk5 activity correlated with increased phosphorylation of the NF-H KSP tail domain in differentiated SH-SY5Y cells, we performed Western blot analysis of cell lysates grown on laminin or poly-l-lysine. Western blots of NF-H tail domain phosphorylation were detected with SMI31, a monoclonal antibody that recognizes a phosphate-dependent epitope in the tail domain of NF-M and NF-H (Sternberger and Sternberger, 1983; Lee et al., 1988). As shown in Figure 2B, hNF-H tail domain phosphorylation was significantly enhanced by laminin in differentiated SH-SY5Y cells (compare lanes 3, 4 with lanes 1, 2). The levels of total NF-H were not altered by laminin (Fig. 2B, lanes 3, 4), although RA increased the level of total NF-H expression in cells grown on poly-l-lysine dishes.
Laminin-induced cdk5 activity and NF-H tail domain phosphorylation were inhibited by anti-α1β1-integrin antibodies and cdk5 inhibitor BL-1
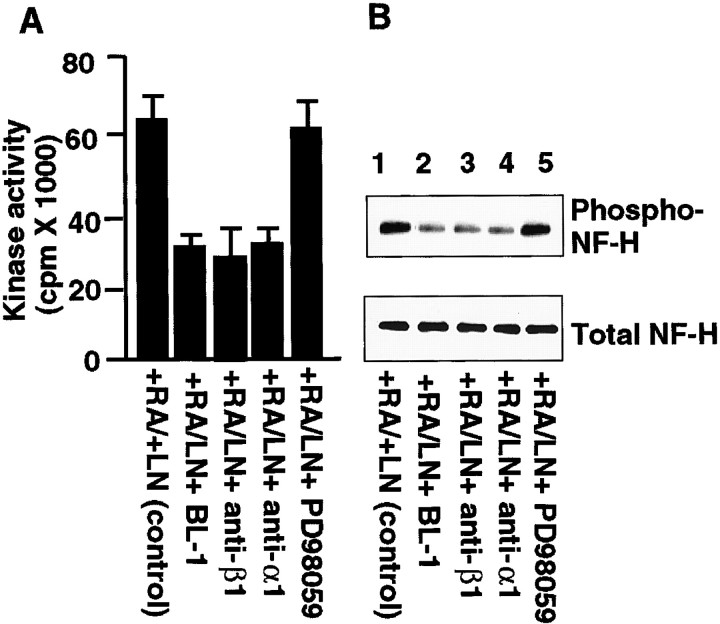
Experiments by Choi et al. (1994) indicate that α1β1 function is required for neurite outgrowth on laminin in SH-SY5Y cells, and Rossino et al. (1991) suggested that α1β1 is the major laminin receptor in RA-treated SH-SY5Y cells. To investigate whether laminin-induced cdk5 kinase activity is specifically caused by the interaction of laminin with integrin α1β1 in differentiated SH-SY5Y cells, we tested the effects of anti-β1- and α1-integrin antibodies, BL-1, a specific cdk5 inhibitor, and PD98059, a specific inhibitor of MEK (Alessi et al., 1995), for their ability to inhibit the laminin-induced cdk5 activity. MEK is an upstream activator of mitogen-activated protein kinase (MAPK; Erk1/2) (Alessi et al., 1995). As shown in Figure3A, the laminin-induced cdk5 kinase activity was significantly reduced after treatment with anti-α1- and -β1-integrin antibodies or BL-1, but the MEK inhibitor had no significant effect.
Fig. 3.
Cdk5 activity and NF-H tail domain phosphorylation are inhibited by anti-α1β1 functional blocking antibodies and cdk5 inhibitor BL-1 in RA-treated SH-SY5Y cells grown on laminin. A, Cell lysates were prepared from RA-treated SH-SY5Y cells cultured on laminin in the presence of anti-β1 (DE9) and anti-α1 (FB12) in the absence of antibodies or treated with the cdk5 inhibitor BL-1 (10 μm) or PD98059, a specific MEK inhibitor (50 μm), for 24 hr. Immunoprecipitates obtained with cdk5 antibody were assayed for their ability to phosphorylate histone H1.B, Both total NF-H and phospho-NF-H tail domain were detected in cell lysates as described in A by Western blot analysis using anti-NF-H C-terminal phospho-independent antibody SMI33 for total NF-H (bottom panel) and monoclonal phospho-specific antibody SMI31 for phosphorylated NF-H KSP tail domain (top panel). Equal amounts of protein were loaded in each lane. PL, Poly-l-lysine;LN, laminin.
Next we determined whether hNF-H tail domain phosphorylation triggered by laminin in differentiated SH-SY5Y cells was mainly attributable to cdk5 phosphorylation. We performed Western blot analysis using lysates from the cultured cells treated with or without functional blocking anti-α1 and β1antibodies. As shown in Figure 3B, laminin-induced hNF-H tail KSP domain phosphorylation was significantly reduced by anti-α1 and β1. Because we have shown previously that both cdk5 and MAP kinase (Erk1/2) phosphorylate rat NF-M and NF-H tail domains (Veeranna et al., 1998; Li et al., 1999a,b; Sharma et al., 1999), we compared laminin-induced hNF-H tail domain phosphorylation in the presence of BL-1 and PD98059. As shown in Figure 3, the cdk5 inhibitor BL-1 significantly inhibited laminin-induced human NF-H tail domain phosphorylation, but PD98059 had no significant effect under these conditions. These effects were consistent with laminin induced-cdk5 activation (Fig. 3A), suggesting that laminin interaction with integrin α1β1triggers cdk5 kinase activation and is involved in human NF-H tail domain phosphorylation in differentiated SH-SY5Y neuroblastoma cells.
Laminin induced an increase in expression of p35 but not cdk5 in differentiated SH-SY5Y cells
The above data show that laminin enhanced cdk5 activity in differentiated SH-SY5Y cells. We investigated the possibility that this increase in kinase activity is attributable to elevation of the expression of cdk5 or p35. The levels of cdk5 and p35 mRNA and protein were analyzed by Northern (Fig.4A) and Western (data not shown) blots. We found that laminin and RA increased the expression of p35 transcripts but had no significant effect on cdk5 expression (Fig. 4A). Laminin or RA only caused some increase (Fig. 4A, a, lanes 2, 3), but in the presence of RA, laminin produced higher expression of p35 mRNA (Fig. 4A, a, lane 4). We also found that the increase in expression of p35 was inhibited by integrin anti-α1 and -β1 functional blocking antibodies (Fig.4A, a, lanes 5, 6), suggesting that laminin increased cdk5 activity by upregulating p35 expression through the integrin α1β1 pathway in differentiated SH-SY5Y cells. These results are consistent with studies reported in rat cerebellar macroneurons (Pigino et al., 1997;Paglini et al., 1998). To determine whether increased expression of p35 was correlated with an increase in hNF-H tail domain phosphorylation in SH-SH5Y cells treated with RA and maintained on laminin or poly-l-lysine, we performed immunofluoresence staining for p35 and hNF-H using polyclonal anti-p35 antibody (C-19) and monoclonal anti-NF-H antibody (SMI31). SMI31 recognizes highly phosphorylated NF-H. As shown in Figure 4B, we found that laminin induced higher p35 expression, which correlated with neurite extension and hNF-H tail domain phosphorylation.
Fig. 4.

Analysis of cdk5 and p35 expression induced by laminin in RA-treated SH-SY5Y neuroblastoma cells. A, SH-SY5Y cells treated with RA for 7 d and cultured on poly-l-lysine or laminin for 24 hr. Total RNA was isolated using TRIzol reagent, separated by agarose gel electrophoresis, and transferred onto a nylon membrane. The membrane was hybridized in QuikHyb buffer containing 32P-labeled cDNA probes specific for p35 (a, b) and cdk5 (c, d).b, d, Quantification of p35 and cdk5 mRNA expression, respectively, in control and treated cells under different conditions. Data represent mean ± SD of three experiments showna and c. B, SH-SY5Y cells were treated with RA (10 μm) for 7 d, and then cells were detached and plated on dishes coated with poly-l-lysine (a, c, e) or with laminin (b, d, f) for 24 hr. Cells were fixed and stained with monoclonal SMI31 antibody, which recognizes phosphorylated NF-H, and polyclonal anti p35 antibody (C-19, 1:50). FITC-conjugated goat anti-mouse IgG (c–f) and rhodamine-labeled goat anti-rabbit IgG (a, b, e, f) secondary antibodies (Sigma, 1:100) were used. Images were obtained using a Zeiss (Thornwood, NY) LSM 410 laser scanning confocal microscope. PL, Poly-l-lysine; LN, laminin.
Laminin-induced cdk5 activity correlated with neurite outgrowth in RA-treated SH-SY5Y cells
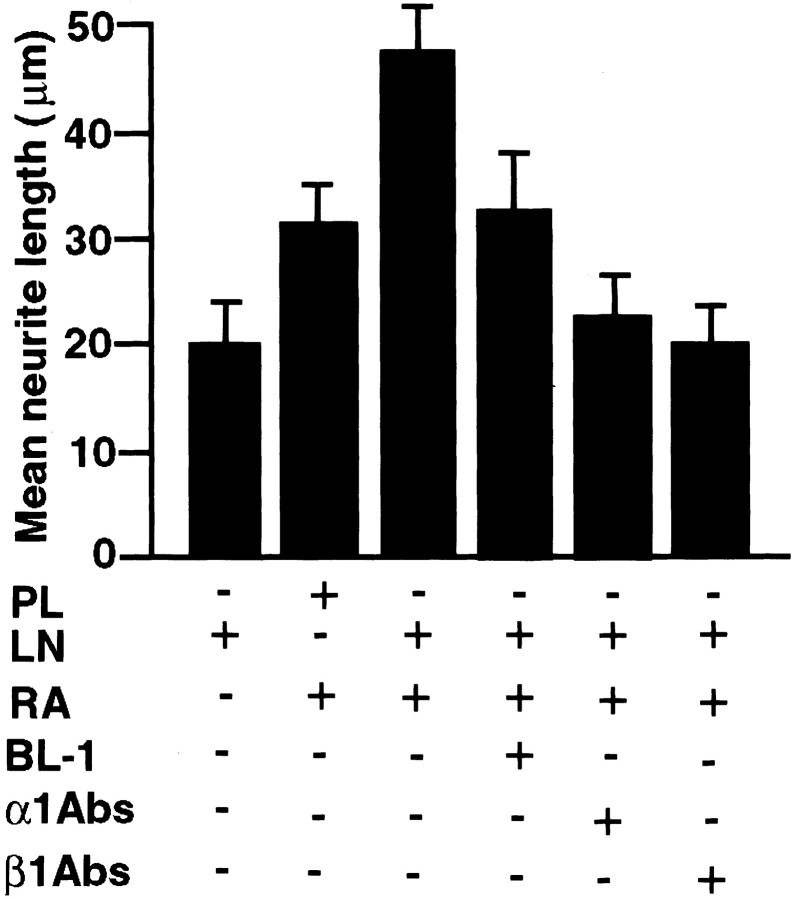
To investigate whether the laminin-induced cdk5 activity is related to neurite outgrowth in RA-treated SH-SY5Y cells, we quantified the neurite outgrowth in the presence of anti-α1 and -β1antibodies or BL-1, the cdk5 inhibitor (Fig.5). Cells were treated with or without RA for 7 d. Cells were detached with EGTA, plated for 24 hr in serum-free medium on laminin- or poly-l-lysine-coated dishes, and treated with or without anti-α1β1antibodies or BL-1. As reported previously (Rossino et al., 1991), we found that laminin and RA together caused a larger increase in outgrowth of neurites compared with laminin or RA alone (Fig. 5). We showed that the integrin α1β1 antibodies and cdk5 inhibitor BL-1 (Fig. 5) inhibited this effect. The α1 and β1 antibodies appeared more effective in reducing neurite outgrowth compared with BL-1 (Fig. 5). However, both integrin α1β1 antibodies and the cdk5 inhibitor BL-1 (Fig. 3) inhibited the cdk5 activity almost equally. These differences may be attributable to the fact that integrins activate other proline-directed kinases (e.g., MAPKs) than cdk5, which are involved in neurite outgrowth (Walowitz and Roth, 1999). Integrin α1β1antibodies are known to block specifically integrin-activated kinase pathways. On the other hand, BL-1, a specific cdk5 inhibitor, will inhibit cdk5 activity alone. This predicts a much less effctive role of BL-1 compared with integrin α1β1 antibodies in the laminin-induced neurite outgrowth. The data shown in Figure 5 are consistent with this prediction.
Fig. 5.
Evaluation of neurite outgrowth of human SH-SY5Y neuroblastoma cells in the presence and absence of α1β1 antibodies or BL-1. Cells were treated with or without RA as described in Materials and Methods and then detached with EGTA and plated on coverslips coated with 10 μg/ml laminin or 100 μg/ml poly-l-lysine in medium without serum in the presence and absence of α1 (FB12) and β1 (DE9) antibodies or BL-1 for 24 hr. After fixation and staining with crystal violet, five randomly selected fields were photographed in each sample. Processes were measured in 120 cells per sample, and only those longer than 15 μm were scored.PL, Poly- l-lysine; LN, laminin. α1Abs, anti-integrin α1antibody; β1Abs, anti-integrin β1antibody.
cdk5 is a major kinase phosphorylating human NF-H KSP tail domain in response to laminin
Previous studies have shown that cdk5 phosphorylates the rat NF-H in transfected cells (Guidato et al., 1996; Sun et al., 1996); however, there have been no studies on integrin α1β1-mediated human NF-H phosphorylation. In this study, we have shown that integrin α1β1-mediated human NF-H tail KSP domain phosphorylation correlated with activation of cdk5 in differentiated human SH-SY5Y cells. The evidence, however, is indirect. To demonstrate direct evidence that integrin-mediated activation of cdk5 results in hNF-H tail KSP domain phosphorylation, we co-transfected a cdk5/p35 complex with a full-length hNF-H tail domain expression construct containing 32 KSP repeats (Fig.6A) into HEK293 cells. The HEK293 cells are known to express integrin α1 and β1 (Bodary and McLean, 1990). The cells were transfected, cultured for 48 hr, and then lysed. The cell lysate was subjected to Western blot analysis to determine the expression of hNF-H tail domain, cdk5, and p35 proteins (Fig. 6B). It is clear that there are no endogenous p35 and neurofilament proteins, but endogenous cdk5 is present in these cells (Fig. 6B, compare lanes 1, 2). The effects of laminin-induced cdk5 activity and hNF-H tail domain phosphorylation were studied in these transfected cells. It was found that the cdk5 kinase activity was significantly enhanced with an increase in hNF-H tail domain phosphorylation in the transfected cells grown on laminin compared with poly-l-lysine (Fig. 6C,D).
Fig. 6.

Analysis of cdk5, p35, and human NF-H tail domain protein expression, cdk5 activity, and NF-H tail domain phosphorylation in transfected HEK293 cells. HEK293 cells were transiently co-transfected with the following expression constructs: vector only, cdk5, p35, and human NF-H tail domain. A, Schematic representation of the human and rat NF-H tail KSPXK repeats.B, Analysis of cdk5, p35, and hNF-H tail domain protein expression by Western blot. After co-transfection of hNF-H tail domain with cdk5 and p35 for 48 hr, the cell lysates were prepared and subjected to Western blot analysis using anti-cdk5 (C-8), anti-p35, and SMI31 antibodies. Equal amounts of protein were used in each case.Lane 1, Transfection of vector only; lane 2, co-transfection of cdk5, p35, and hNF-H tail domain expression construct. C, HEK293 cells co-transfected with cdk5, p35, and human NF-H tail domain. After transfection for 24 hr, cells were starved overnight and then detached and plated on poly-l-lysine or laminin for an addtional 24 hr. The cells were fixed and incubated with monoclonal anti-phospho-dependent NF-H antibody SMI31 (1:500), followed by rhodamine-labeled goat anti-mouse IgG secondary antibody. Images were obtained using a Zeiss LSM 410 laser scanning confocal microscope. a, Cells grown on poly-l-lysine; b, cells grown on laminin. D, Analysis of cdk5 kinase activity usingin vitro kinase assay. After HEK293 cells were transfected with hNF-H tail domain, wild-type cdk5, and p35 for 24 hr, cells were starved overnight and then detached and plated on poly-l-lysine (lane 2) or laminin (lanes 1, 3) for an addtional 24 hr. Cell lysates were immunoprecipitated with cdk5 antibody and subjected to kinase activity assay using histone H1 as a substrate. Lane 1, Transfection of vector only, grown on laminin; lane 2, co-transfection of hNF-H tail domain with cdk5 and p35, cells grown on poly-l-lysine; lane 3, co-transfection as shown in lane 2, cells grown on laminin. Data represent mean ± SD of three experiments.
To detemine whether the hNF-H KSP tail domain phosphorylation was mainly through cdk5 or by laminin-induced Erk1/2 activity, the HEK293 cells were co-transfected with hNF-H tail domain and wild-type cdk5/p35 in the presence or absence of BL-1 or PD98059. In addition, cells were also co-transfected with hNF-H tail domain and dominant-negative cdk5 and p35 or only cdk5. As shown in Figure7, dominant-negative cdk5 or cells treated with BL-1 significantly reduced both the laminin-induced increased cdk5 activity and hNF-H KSP tail domain phosphorylation, but PD98059 appeared to be less effective. The cells transfected with only cdk5 (no P35) showed reduced levels of phospho-hNF-H tail domain protein, although laminin induced Erk1/2 activation (Fig.7A). These findings indicate that laminin-induced cdk5 activity is more effective than Erk1/2 in phosphorylating the hNF-H KSP tail domain.
Fig. 7.

Cdk5 is the major kinase phosphorylating hNF-H tail domain in co-transfected HEK293 cells. A, Western blot analysis of Erk1/2 phosphorylation and hNF-H tail domain phosphorylation. HEK293 cells were co-transfected with hNF-H tail domain and wild-type cdk5/p35 (a–c) in the presence of BL-1 (b) or PD98059, an MEK inhibitor (c), hNF-H tail domain and dominant-negative cdk5/p53 (d), and hNF-H tail domain and cdk5 only (e). After transfection for 24 hr, cells were starved overnight and then detached and cultured on laminin for an additional 24 hr. The cell lysates were subjected to Western blot analysis using phospho-Erk1/2 (top row) and total Erk1/2 (second row) antibodies. Phosphorylated NF-H tail domain (third row) and total NF-H tail domain (bottom row) were detected using SMI31 and SMI33 antibodies. B, HEK293 cells were co-transfected with hNF-H tail domain and wild-type cdk5/p35 (a–c) in the presence of BL-1 (b) or PD98059 (c), hNF-H tail domain and dominant-negative p35 (d), and hNF-H tail domain and cdk5 only (e). After transfection for 24 hr, cells were starved overnight and then detached and cultured on laminin for additional 24 hr; then cells were lysed, and lysates were immunoprecipitated with anti-cdk5 antibody and subjected to in vitro kinase assay using hitone H1 as a substrate. Data represent mean ± SD of three experiments. C, Immunocytochemical analysis of hNF-H tail domain phosphorylation. Cells were treated as described in A and fixed with 4% paraformaldehyde and PBS. Cells were stained with monoclonal SMI31 antibody (1:500), followed by rhodamine-labeled goat anti-mouse IgG secondary antibody (Sigma, 1:100). Images were obtained using a ZeissLSM 410 laser scanning confocal microscope.
DISCUSSION
Previous studies have shown that hNF-H is a better substrate compared with rat or mouse NF-H for cdk5 (Pant and Veeranna, 1995). This is basically attributable to the higher number of KSPXK repeats (32 times) in hNF-H compared with that (10 times) in rat NF-H (Fig.6A). KSPXK is the consensus sequence for cdk5. Cdk5 selectively phosphorylates KSPXK motifs in NF-H, other proteins, and peptides with similar sequences (Shetty et al., 1993). The other motifs, e.g., KSPXXXK (repeats 41 times in rat), are not phosphorylated by cdk5 under similar conditions (Shetty et al., 1993; Veeranna et al., 1998). Therefore, in this study we used human SH-SY5Y cells and HEK293 cells transfected with a construct containing 32 KSPXK repeats in the tail domain of hNF-H to study their phosphorylation mechanisms. Human neuroblastoma SH-SY5Y cell lines, useful model systems to study the phenotypic properties of peripheral neurons, express high levels of hNF-H (Sharma et al., 1999). These cell lines are derived from a neural crest tumor of early childood, contain mostly undifferentiated neuroblast-like cells, and undergo differentiation when treated with all-trans-RA. It has been reported that cdk5 can phosphorylate NF-H in RA-treated SH-SY5Y cells (Sharma et al., 1999). On the basis of the data presented in this work, we propose that integrin α1β1 is involved in regulating neurite outgrowth and hNF-H tail domain phosphorylation through activation of cdk5. This study demonstrates that laminin elevated cdk5 activity in RA-differentiated SH-SY5Y cells. The increase in laminin-induced cdk5 activity was associated with an increase in neurite extension and hNF-H tail domaon phosphorylation. These results are in agreement with those reported for rat primary cerebellar neurons in culture (Pigino et al., 1997; Paglini et al., 1998).
Integrin α1 and β1 represent the major integrin complexes of the human neuroblastoma cell line SH-SY5Y (Rossino et al., 1991) and of rat PC12 cells (Rossino et al., 1990; Tomaselli et al., 1990). Integrin α1β1 has been shown to act as a dual laminin–collagen receptor in neural cells (Ignatius and Reichardt, 1988; Turner and lier, 1989; Lein et al., 1991) and in a variety of non-neuronal cells, including HEK293 cell lines (Bodary and McLean, 1990; Forsberg et al., 1990; Hall et al., 1990). In the nervous system, laminin is produced by Schwann cells and astrocytes (Bunge et al., 1989) and is a basal membrane component in the PNS and in selected regions of the CNS (Liesi, 1985). Integrin α1β1 has been shown to mediate neurite extension and nerve regeneration on laminin substrate (Turner and Flier, 1989; Tomaselli et al., 1990; Toyota et al., 1990). Our results show that laminin interaction with the integrin receptor α1β1 induces an upregulation of p35, the cdk5 regulator. This in turn activates cdk5 and leads to neurite outgrowth and other cytoskeletal proteins, including hNF-H tail domain phosphorylation.
Neurite outgrowth is a complex process that requires adhesion to the substratum as well as active neurite outgrowth. Rates of neurite outgrowth will depend on a number of adhesion molecules such as integrins and cadherins. It is possible that a large number of such molecules triggering signaling pathways are involved in human neurofilament phosphorylation and other cytoskeletal proteins such as MAPs (Paglini et al., 1998; this study) through activation of cdk5 activity. The role of neurofilament phosphorylation in axonal outgrowth is not clear. NF-H knock-out mice exhibit no significant difference in the number of neurofilaments within large axons and no effect on axonal elongation or targeting in peripheral motor and sensory axons. The efficiency of survival of these neurons appeared to be reduced (Rao et al., 1998). However, in another study of NF-H-null mice, the axonal caliber of both large- and small-diameter myelinated axons was reduced (Elder et al., 1998). These findings suggest that NF-H alone may not be important for neurite extension but may play a role in the maintenance and stabilization of axonal structures (Pant, 1988; Shea and Beermann, 1994; Lin and Szaro, 1995). The structural details of C-terminal tail domains, particularly in the NF-H subunits, vary from species to species (Pant and Veeranna, 1995). In cells, with human NF-H the expression of NF-H phosphorylation in axons may behave as a reporter for cdk5-induced neurite outgrowth. We propose that the integrin α1β1 signaling pathway upregulates p35 and activates cdk5, which in turn affects neurite outgrowth and cytoskeletal protein phosphorylation, including the NF-H tail domain. This may help stabilize the axonal structures (Shea and Beermann, 1994).
Although all-trans RA has been shown to alter a variety of signaling pathways, including regulation of cell differentiation (Durston et al., 1989; Barres et al., 1994; Chambon, 1994; Dupin and Le Douarin, 1995), the mechanisms of activation of these pathways are not well understood. The finding that integrin-mediated cdk5 activation is involved in neurite outgrowth and NF-H tail domain phosphorylation in differentiated SH-SY5Y cells supports the idea that the RA could initiate a program of neuronal differentiation in neuroblastoma cell lines by increasing integrin α1β1 expression and interacting with laminin, resulting in the activation of cdk5. This in turn affects neurite outgrowth and NF-H tail domain phosphorylation. This is also supported by the finding that laminin regulates transcription of a set of genes that affect p35 mRNA expression (Paglini et al., 1998; this work).
The phosphorylation of NF-H tail domains is topographically regulated: they are highly phosphorylated in the axonal compartment, but little or no phosphorylation occurs in the cell body. In many neuronal pathologies, however, such as amyotrophic lateral sclerosis (Manetto et al., 1988; Munoz et al., 1988; Sobue et al., 1990), Alzheimer's disease (Cork et al., 1986; Zhang et al., 1989), and Parkinson's disease (Forno et al., 1986; Pollanen et al., 1994), hyperphosphorylation of KSP repeats occurs abnormally in perikarya. Cdk5 has been shown to phosphorylate one type of KSP repeat motif (KSPXK) in cytoskeletal proteins. In human NF-H there are large numbers of KSPXK repeats (Fig. 6A). The laminin-mediated human NF-H KSP tail domain phosphorylation and phosphorylation of other cytoskeletal proteins with KSPXK motifs through activation of cdk5 may provide a new insight into the mechnisms involved in neuronal pathologies.
Footnotes
We thank Dr. Philip Grant for excellent suggestions and critically reading this manuscript. We also thank Dr. Thomas Shea (University of Massachusetts, Lowell, MA) for providing human SH-SY5Y neuroblastoma cell lines. We thank Dr. Carolyn Smith in the National Institute of Neurological Diseases and Stroke Light Microscopy Facility for assistance with confocal microscopy.
Correspondence should be addressed to Dr. Harish C. Pant, Laboratory of Neurochemistry, National Institute of Neurological Diseases and Stroke, National Institutes of Health, Building 36, Room 4D20, 9000 Rockville Pike, Bethesda, MD 20892-4130. E-mail: hcp@codon.nih.gov.
REFERENCES
- 1.Alessi DG, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD098059 is a specific inhibitor of the activation of mitogen activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Arregui CO, Carbonetto S, McKerracher L. Characterization of neural cell adhesion sites: point contacts are the sites of interaction between integrins and the cytoskeleton in PC12 cells. J Neurosci. 1994;14:6967–6977. doi: 10.1523/JNEUROSCI.14-11-06967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1008. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- 4.Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 5.Bodary SC, McLean JW. The integrin beta 1 subunit associates with the vitronectin receptor alpha v subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990;265:5938–5941. [PubMed] [Google Scholar]
- 6.Brown A, Lasek RJ. Neurofilaments move apart freely when released from the circumferential constraint of the axonal plasma membrane. Cell Motil Cytoskeleton. 1993;26:313–324. doi: 10.1002/cm.970260406. [DOI] [PubMed] [Google Scholar]
- 7.Bunge MB, Bunge RP, Kleitman N, Dean AC. Role of peripheral nerve extracellular matrix in Schwann cell function and in neurite regeneration. Dev Neurosci. 1989;11:348–360. doi: 10.1159/000111911. [DOI] [PubMed] [Google Scholar]
- 8.Calof AL, Reichardt LF. Motoneurons purified by cell sorting respond to two distinct activities in myotube-conditioned medium. Dev Biol. 1984;106:194–210. doi: 10.1016/0012-1606(84)90075-7. [DOI] [PubMed] [Google Scholar]
- 9.Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- 10.Chambon P. The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol. 1994;5:115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- 11.Choi ES, Rettig WJ, Wayner EA, Srour ML, Clegg DO. Functional identification of integrin laminin receptors that mediate process outgrowth by human SY5Y neuroblastoma cells. Neurosci Res. 1994;37:475–488. doi: 10.1002/jnr.490370407. [DOI] [PubMed] [Google Scholar]
- 12.Cork LC, Sternberger NH, Sternberger LA, Casanova MF, Struble RG, Price DL. Phosphorylated neurofilament antigens in neurofibrillary tangles in Alzheimer's disease. J Neurophathol Exp Neurol. 1986;45:56–64. doi: 10.1097/00005072-198601000-00005. [DOI] [PubMed] [Google Scholar]
- 13.de Waegh SM, Lee VM, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992;68:451–463. doi: 10.1016/0092-8674(92)90183-d. [DOI] [PubMed] [Google Scholar]
- 14.Dupin E, Le Douarin NM. Retinoic acid promotes the differentiation of adrenergic cells and melanocytes in quail neural crest cultures. Dev Biol. 1995;168:529–548. doi: 10.1006/dbio.1995.1100. [DOI] [PubMed] [Google Scholar]
- 15.Durston AJ, Timmermans JP, Hage WJ, Hendriks HF, de Vries NJ, Heideveld M, Nieuwkoop PD. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989;340:140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- 16.Edgar D, Timpl R, Thoenen H. The heparin-binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984;3:1463–1468. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elder GA, Friedrich VL, Jr, Kang C, Bosco P, Gourov A, Tu PH, Zhang B, Lee VM, Lazzarini RA. Requirement of heave neurofilament subunite in the development of axons with large calibers. J Cell Biol. 1998;143:195–205. doi: 10.1083/jcb.143.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elhanany E, Jaffe H, Link WT, Shetty DM, Gainer H, Pant HC. Identification of endogenously phosphorylated KSP sites in the high molecular weight rat neurofilament protein. J Neurochem. 1994;63:2324–2335. doi: 10.1046/j.1471-4159.1994.63062324.x. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher AI, Shuang R, Giovannucci DR, Zhang L, Bittner MA, Stuenkel EL. Regulation of exocytosis by cyclin-dependent kinase 5 via phosphorylation of Munc18. J Biol Chem. 1999;274:4027–4035. doi: 10.1074/jbc.274.7.4027. [DOI] [PubMed] [Google Scholar]
- 20.Forno LS, Sternberger LA, Sternberger NH, Sterefiling AM, Swanson K, Eng LF. Reaction of Lewy bodies with antibodies to phosphorylated and non-phosphorylated neurofilaments. Neurosci Lett. 1986;64:253–258. doi: 10.1016/0304-3940(86)90337-x. [DOI] [PubMed] [Google Scholar]
- 21.Forsberg E, Paulsson M, Timpl R, Johansson S. Characterization of a laminin receptor on rat hepatocytes. J Biol Chem. 1990;265:6376–6381. [PubMed] [Google Scholar]
- 22.Giasson BI, Mushynski WE. Aberrant stress-induced phosphorylation of perikaryal neurofilaments. J Biol Chem. 1996;271:30404–30409. doi: 10.1074/jbc.271.48.30404. [DOI] [PubMed] [Google Scholar]
- 23.Giasson BI, Mushynski WE. Study of proline-directed protein kinases involved in phosphorylation of the heavy neurofilament subunit. J Neurosci. 1997;17:9466–9472. doi: 10.1523/JNEUROSCI.17-24-09466.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein ME, Sternberger NH, Sternberger LA. Phosphorylation protects neurofilaments against proteolysis. J Neuroimmunol. 1987;14:149–160. doi: 10.1016/0165-5728(87)90049-x. [DOI] [PubMed] [Google Scholar]
- 25.Guidato S, Tsai LH, Woodgett J, Miller CC. Differential cellular phosphorylation of neurofilament heavy side-arms by glycogen synthase kinase-3 and cyclin-dependent kinase-5. J Neurochem. 1996;66:1698–1706. doi: 10.1046/j.1471-4159.1996.66041698.x. [DOI] [PubMed] [Google Scholar]
- 26.Hall DE, Reichardt LF, Crowley E, Holley B, Moezzi H, Sonnenberg A, Damsky CH. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990;110:2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemler ME. VLA proteins in the integrin family. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 28.Hirokawa N, Glicksman MA, Willard MB. Organization of mammalian neurofilament polypeptides within the neuronal cytoskeleton. J Cell Biol. 1984;98:1523–1536. doi: 10.1083/jcb.98.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirooka K, Tomizawa K, Matsui H, Tokuda M, Itano T, Hasegawa E, Wang JH, Hatase O. Developmental alteration of the expression and kinase activity of cyclin-dependent kinase 5 (Cdk5)/p35nck5a in the rat retina. J Neurochem. 1996;67:2478–2483. doi: 10.1046/j.1471-4159.1996.67062478.x. [DOI] [PubMed] [Google Scholar]
- 30.Hisanaga S, Kusubata M, Okumura E, Kishimoto T. Phosphorylation of neurofilament H subunit at the tail domain by CDC2 kinase dissociates the association to microtubules.the association to microtubules. J Biol Chem. 1991;266:21798–21803. [PubMed] [Google Scholar]
- 31.Hoffman PN, Lasek RJ. The slow component of axonal transport: identification of major structural polypeptides of the axon and their generality among mammalian neurons. J Cell Biol. 1975;66:351–366. doi: 10.1083/jcb.66.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman PN, Griffin JW, Price DL. Control of axonal caliber by neurofilament transport. J Cell Biol. 1984;99:705–714. doi: 10.1083/jcb.99.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 34.Ignatius MJ, Reichardt LF. Identification of a neuronal laminin receptor: a Mr 200K/120K integrin heterodimer that binds laminin in a divalent cation-dependent manner. Neuron. 1988;1:713–725. doi: 10.1016/0896-6273(88)90170-5. [DOI] [PubMed] [Google Scholar]
- 35.Ji YZ, Wolf JP, Jouannet P, Bomsel M. Human gamete fusion can bypass beta1 integrin requirement. Hum Reprod. 1998;13:682–689. doi: 10.1093/humrep/13.3.682. [DOI] [PubMed] [Google Scholar]
- 36.Julien J-P, Mushynski WE. The distribution of phosphorylation sites among identified proteolytic fragments of mammalian neurofilaments. J Biol Chem. 1983;258:4019–4025. [PubMed] [Google Scholar]
- 37.Lee VM-Y, Otvos L, Carden MJ, Hollosi M, Dietzschold B, Lazzarini RA. Identification of the major multiphosphorylation site in mammalian neurofilamentss. Proc Natl Acad Sci USA. 1988;85:1998–2002. doi: 10.1073/pnas.85.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lein PJ, Higgins D, Turner DC, Flier LA, Terranova VP. The NC1 domain of type IV collagen promotes axonal growth in sympathetic neurons through interaction with the alpha 1 beta 1 integrin. J Cell Biol. 1991;113:417–428. doi: 10.1083/jcb.113.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lew J, Huang Q-Q, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 40.Li BS, Veeranna, Grant P, Pant HC. Calcium influx and membrane depolarization induce phosphorylation of neurofilament (NF-M) KSP repeats in PC12 cells. Brain Res Mol Brain Res. 1999a;70:84–91. doi: 10.1016/s0169-328x(99)00142-4. [DOI] [PubMed] [Google Scholar]
- 41.Li BS, Veeranna, Gu J, Grant P, Pant HC. Activation of mitogen-activated protein kinases (Erk1 and Erk2) cascade results in phosphorylation of NF-M tail domains in transfected NIH 3T3 cells. Eur J Biochem. 1999b;262:211–217. doi: 10.1046/j.1432-1327.1999.00372.x. [DOI] [PubMed] [Google Scholar]
- 42.Liesi P. Laminin-immunoreactive glia distinguish regenerative adult CNS systems from non-regenerative ones. EMBO J. 1985;4:2505–2511. doi: 10.1002/j.1460-2075.1985.tb03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin W, Szaro BG. Neurofilaments help maintain normal morphologies and support elongation of neurites in Xenopus laevis cultured embryonic spinal cord neurons. J Neurosci. 1995;15:8331–8344. doi: 10.1523/JNEUROSCI.15-12-08331.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manetto V, Stermberger NH, Perry G, Sternberger LA, Gambetti P. Phosphorylatio of neurofilaments is altered in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1988;47:642–653. doi: 10.1097/00005072-198811000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Manthorpe M, Engvall E, Ruoslahti E, Longo FM, Davis GE, Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983;97:1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munoz DG, Greene C, Perl DP, Selkoe DJ. Accumulation of phosphorylated neurofilaments in anterior horn motorneurons of amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1988;47:9–18. doi: 10.1097/00005072-198801000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Nakagawa T, Chen J, Zhang Z, Kanai Y, Hirokawa N. Two distinct functions of the carboxyl-terminal tail domain of NF-M upon neurofilament assembly: cross-bridge formation and longitudinal elongation of filaments. J Cell Biol. 1995;129:387–395. doi: 10.1083/jcb.129.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- 49.Nixon RA, Sihag RK. Neurofilament phosphorylation: a new look at regulation. Trends Neurosci. 1991;14:501–506. doi: 10.1016/0166-2236(91)90062-y. [DOI] [PubMed] [Google Scholar]
- 50.Nixon RA, Lewis SE, Marotta CA. Posttranslational modification of neurofilament proteins by phosphate during axoplasmic transport in retinal ganglion cell neurons. J Neurosci. 1987;7:1145–1158. doi: 10.1523/JNEUROSCI.07-04-01145.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, Sato T, Tachibana K, Morimoto C, Yazaki Y, Hirai H. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. J Biol Chem. 1995;270:15398–15402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- 52.Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paglini G, Pigino G, Kunda P, Morfini G, Maccioni R, Quiroga S, Ferreira A, Caceres A. Evidence for the participation of the neuron-specific CDK5 activator P35 during laminin-enhanced axonal growth. J Neurosci. 1998;18:9858–9869. doi: 10.1523/JNEUROSCI.18-23-09858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pant HC. Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem J. 1988;256:665–668. doi: 10.1042/bj2560665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pant H, Veeranna C. Neurofilament phosphorylation. Biochem Cell Biol. 1995;73:575–592. doi: 10.1139/o95-063. [DOI] [PubMed] [Google Scholar]
- 56.Patrick G, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 57.Paudel HK, Lew J, Ali Z, Wang JH. Brain proline-directed protein kinase phosphorylates tau on sites that are abnormally phosphorylated in tau associated with Alzheimer's paired helical filaments. J Biol Chem. 1993;268:23512–23518. [PubMed] [Google Scholar]
- 58.Pigino G, Paglini G, Ulloa L, Avila J, Caceres A. Analysis of the expression, distribution and function of cyclin dependent kinase 5 (cdk5) in developing cerebellar macroneurons. J Cell Sci. 1997;110:257–270. doi: 10.1242/jcs.110.2.257. [DOI] [PubMed] [Google Scholar]
- 59.Pollanen MS, Bergeron C, Weyer L. Characterization of a shared epitope in cortical Lewy body fibrils and Alzheimer paired helical filaments. Acta Neuropathol (Berl) 1994;88:1–6. doi: 10.1007/BF00294352. [DOI] [PubMed] [Google Scholar]
- 60.Rao MV, Houseweart MK, Williamson TL, Crawford TO, Folmer J, Cleveland DW. Neurofilament-dependent radial growth of motor axons and axonal organization of neurofilaments does not require the neurofilament heavy subunit (NF-H) or its phosphorylation. J Cell Biol. 1998;143:171–181. doi: 10.1083/jcb.143.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ratner N, Bloom GS, Brady ST. A role for cyclin-dependent kinase(s) in the modulation of fast anterograde axonal transport: effects defined by olomoucine and the APC tumor suppressor protein. J Neurosci. 1998;18:7717–7726. doi: 10.1523/JNEUROSCI.18-19-07717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reh TA, Radke K. A role for the extracellular matrix in retinal neurogenesis in vitro. Dev Biol. 1988;129:283–293. doi: 10.1016/0012-1606(88)90375-2. [DOI] [PubMed] [Google Scholar]
- 63.Reichardt LF, Tomaselli KJ. Extracellular matrix molecules and their receptors: functions in neural development. Annu Rev Neurosci. 1991;14:531–570. doi: 10.1146/annurev.ne.14.030191.002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogers SL, Letourneau PC, Palm SL, McCarthy J, Furcht LT. Neurite extension by peripheral and central nervous system neurons in response to substratum-bound fibronectin and laminin. Dev Biol. 1983;98:212–220. doi: 10.1016/0012-1606(83)90350-0. [DOI] [PubMed] [Google Scholar]
- 65.Rossino P, Gavazzi I, Timpl R, Aumailley M, Abbadini M, Giancotti F, Silengo L, Marchisio PC, Tarone G. Nerve growth factor induces increased expression of a laminin-binding integrin in rat pheochromocytoma PC12 cells. Exp Cell Res. 1990;189:100–108. doi: 10.1016/0014-4827(90)90262-9. [DOI] [PubMed] [Google Scholar]
- 66.Rossino P, Defilippi P, Silengo L, Tarone G. Up-regulation of the integrin alpha 1/beta 1 in human neuroblastoma cells differentiated by retinoic acid: correlation with increased neurite outgrowth response to laminin. Cell Regul. 1991;2:1021–1033. doi: 10.1091/mbc.2.12.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruoslahti E, Vaheri A. Cell-to-cell contact and extracellular matrix. Curr Opin Cell Biol. 1997;9:605–607. doi: 10.1016/s0955-0674(97)80112-3. [DOI] [PubMed] [Google Scholar]
- 68.Sanes JR. Extracellular matrix molecules that influence neural development. Annu Rev Neurosci. 1989;12:491–516. doi: 10.1146/annurev.ne.12.030189.002423. [DOI] [PubMed] [Google Scholar]
- 69.Sharma M, Sharma P, Pant HC. CDK-5-mediated neurofilament phosphorylation in SHSY5Y human neuroblastoma cells. J Neurochem. 1999;73:79–86. doi: 10.1046/j.1471-4159.1999.0730079.x. [DOI] [PubMed] [Google Scholar]
- 70.Shea TB, Beermann ML. Respective roles of neurofilaments, microtubules, MAP1B, and tau in neurite outgrowth and stabilization. Mol Biol Cell. 1994;5:863–875. doi: 10.1091/mbc.5.8.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shetty KT, Link WT, Pant HC. Cdk2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: isolation and characterization. Proc Natl Acad Sci USA. 1993;90:6844–6848. doi: 10.1073/pnas.90.14.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shetty KT, Kaech S, Link WT, Jaffe H, Flores CM, Wray S, Pant HC, Beushausen S. Molecular characterization of a neuronal-specific protein that stimulates the activity of Cdk5. J Neurochem. 1995;64:1988–1995. doi: 10.1046/j.1471-4159.1995.64051988.x. [DOI] [PubMed] [Google Scholar]
- 73.Sobue G, Hashizume Y, Yasuda T, Mukai E, Kumagai T, Mitsuma T, Trojanowski JQ. Phosphorylated high molecular weight neurofilament protein in lower motor neurons in amyotrophic lateral sclerosis and other neurodegenerative diseases involving ventral horn cells. Acta Neuropathol (Berl) 1990;79:402–408. doi: 10.1007/BF00308716. [DOI] [PubMed] [Google Scholar]
- 74.Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun D, Leung CL, Liem RKH. Phosphorylation of the high molecular weight neurofilament protein (NF-H) by Cdk5 and p35. J Biol Chem. 1996;271:14245–14251. doi: 10.1074/jbc.271.24.14245. [DOI] [PubMed] [Google Scholar]
- 76.Tomaselli KJ, Hall DE, Flier LA, Gehlsen KR, Turner DC, Carbonetto S, Reichardt LF. A neuronal cell line (PC12) expresses two β1-class integrins-α1β1 and α3β1-that recognize different neurite outgrowth-promoting domains in laminin. Neuron. 1990;5:651–662. doi: 10.1016/0896-6273(90)90219-6. [DOI] [PubMed] [Google Scholar]
- 77.Toyota B, Carbonetto S, David S. A dual laminin/collagen receptor acts in peripheral nerve regeneration. Proc Natl Acad Sci USA. 1990;87:1319–1322. doi: 10.1073/pnas.87.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trigg DJ, O'Grady KM, Bhattacharyya T, Reinke M, Toriumi DM. Peripheral nerve regeneration: comparison of laminin and acidic fibroblast growth factor. Am J Otolaryngol. 1998;19:29–32. doi: 10.1016/s0196-0709(98)90062-x. [DOI] [PubMed] [Google Scholar]
- 79.Turner DC, Flier LA. Receptor-mediated active adhesion to the substratum is required for neurite outgrowth. Dev Neurosci. 1989;11:300–312. doi: 10.1159/000111908. [DOI] [PubMed] [Google Scholar]
- 80.Veeranna, Amin ND, Ahn NG, Jaffe H, Winters CA, Grant P, Pant HC. Mitogen-activated protein kinases (Erk1,2) phosphorylated Lys-Ser-Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J Neurosci. 1998;18:4008–4021. doi: 10.1523/JNEUROSCI.18-11-04008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walowitz JL, Roth JA. Activation of ERK1 and ERK2 is required for manganese-induced neurite outgrowth in rat pheochromocytoma (PC12) cells. J Neurosci Res. 1999;57:847–854. [PubMed] [Google Scholar]
- 82.Xiong Y, Zhang H, Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992;71:505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- 83.Xu Z-S, Liu WS, Willard MB. Identification of six phosphorylation sites in the COOH-terminal of the rat neurofilament NF-M. J Biol Chem. 1992;267:4467–4471. [PubMed] [Google Scholar]
- 84.Zhang H, Sternberger NH, Rabinstein LJ, Herman MH, Binder LI, Sternberger LA. Abnormal processing of multiple proteins in Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:8045–8049. doi: 10.1073/pnas.86.20.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]