Abstract
To investigate the effects of somatostatin (somatotropin release-inhibiting factor, SRIF) on the regulation of SST2A receptors in mammalian brain, we examined how blockade of SRIF release or stimulation by the SRIF analog [d-Trp8]-SRIF would affect the expression and cell surface availability of SST2A receptors in rat brain slices. First, we measured the intensity of SST2A immunoreactivity, using quantitative light microscopic immunocytochemistry, and levels of SST2A mRNA, using semiquantitative RT-PCR, under conditions of acute SRIF release blockade. Incubation of slices from the claustrum or basolateral amygdala, two regions previously shown to contain high concentrations of SST2A receptors, in Ca2+-free Ringer's for 40 min induced a decrease in the intensity of SST2A receptor immunoreactivity and concentration of SST2A mRNA as compared with control values obtained in Ca2+-supplemented Ringer's. These effects were counteracted in a dose-dependent manner by the addition of 10–100 nm [d-Trp8]-SRIF to the Ca2+-free medium. Furthermore, both of these effects were abolished in the presence of the endocytosis inhibitors phenylarsine oxide or hyperosmolar sucrose, suggesting that they were dependent on receptor internalization. Electron microscopic immunogold labeling confirmed the existence of an agonist-induced internalization of SST2A receptors in central neurons. At a high (10 μm), but not at a low (10 nm), concentration of agonist this internalization resulted in a significant decrease in cell surface receptor density, irrespective of the presence of Ca2+ in the medium. Taken together, these results suggest that ligand-induced endocytosis is responsible for rapid transcriptional (increase in SST2A expression) and trafficking (loss of cell surface receptors) events involved in the control of the somatostatinergic signal.
Keywords: somatostatin, endocytosis, receptor, immunocytochemistry, electron microscopy, signaling
Somatostatin (somatotropin release-inhibiting factor, SRIF) is a biogenic peptide widely distributed in brain and periphery (for review, see Patel, 1992). Originally identified on the basis of its ability to inhibit growth hormone secretion from the pituitary (Brazeau et al., 1973), it since has been found to exert a variety of hormonal and neural actions (Epelbaum, 1986). SRIF exists under two forms, derived from the same precursor molecule: a 14-amino-acid short form (SRIF-14) and an N-terminally extended form of the latter, SRIF-28 (for review, seePatel, 1992). Both forms coexist in the brain; however, SRIF-14 is the one that appears to play a predominant role (Johansson et al., 1984;Patel, 1992). Both central and peripheral actions of SRIF are mediated by G-protein-coupled receptors, five of which, designated SST1–5 (Hoyer et al., 1995), have been cloned so far (Bruno et al., 1992; Kluxen et al., 1992; Meyerhof et al., 1992;O'Carroll et al., 1992; Yamada et al., 1992; Yasuda et al., 1992). All of these receptors bind SRIF-14 and SRIF-28 with comparable affinities except for SST5, which exhibits a slightly higher affinity for SRIF-28 than for SRIF-14 (O'Carroll et al., 1992; Hoyer et al., 1994; Reisine and Bell, 1995; Siehler et al., 1998).
The distribution of SRIF binding sites in rat brain was first established by using radioligand binding and receptor autoradiographic techniques (for review, see Krantic et al., 1992). Since then molecular biological studies have demonstrated that all five subtypes of SRIF receptors are expressed in mammalian CNS (Bruno et al., 1993;Raulf et al., 1994; Viollet et al., 1995). Messenger RNAs for SST1–5 receptor subtypes were localized in adult rodent brain by in situ hybridization (Breder et al., 1992;Kaupmann et al., 1993; Kong et al., 1994; Pérez et al., 1994;Señarís et al., 1994; Beaudet et al., 1995; Thoss et al., 1995), and SST receptor proteins were visualized at cellular and subcellular levels by immunocytochemistry (Dournaud et al., 1996;Schindler et al., 1997, 1999; Helboe et al., 1998; Hervieu and Emson, 1998; Händel et al., 1999; Stroh et al., 1999). Most of these studies concur in reporting an extensive brain distribution for SST2 and particularly for its splice variant SST2A, a somewhat more restricted distribution for SST1 and SST3–4, and a limited distribution for SST5.
Recent studies have shown that the interaction of SRIF with its receptors resulted in a temperature- and receptor-dependent internalization of receptor–ligand complexes in cell lines expressing either native (Koenig et al., 1997; Sarret et al., 1999) or recombinant (Hukovic et al., 1996; Hipkin et al., 1997; Nouel et al., 1997; Roth et al., 1997; Stroh et al., 2000b) SRIF receptor subtypes. However, studies on transfected cells have shown major differences between subtypes in both patterns and efficiency of internalization (Hukovic et al., 1996; Nouel et al., 1997; Roth et al., 1997). Briefly, high internalization yields were observed for SST2, SST3, and SST5 receptors, whereas poor internalization yields were found for SST1 and SST4 subtypes (Hukovic et al., 1996; Hipkin et al., 1997; Nouel et al., 1997; Roth et al., 1997; Kreienkamp et al., 1998; Stroh et al., 2000b). SRIF analogs also have been reported to internalize in neurons in primary culture via SST2 and other unidentified (but likely SST3) SST subtypes (Stroh et al., 2000a). There is also immunocytochemical evidence for SRIF-induced internalization of SST2A receptors in human glioma cells (Krisch et al., 1998) and intact rat brain (Dournaud et al., 1998). Little is known, however, about the implication of this internalization process for brain function.
As for other G-protein-coupled receptors, ligand-induced SRIF receptor internalization has been proposed to be involved in receptor desensitization via cell surface receptor downregulation (Hipkin et al., 1997; Beaumont et al., 1998). Recent studies have also raised the possibility that internalization may play a role in transmembrane signaling. Evidence for internalization-induced signaling mainly stems from the study of growth factor and cytokine receptors (for review, seeBevan et al., 1995). However, changes in the duration of inositol phosphate accumulation and associated calcium responses (Griendling et al., 1987; Hunyady et al., 1991), as well as in transcription of receptor mRNA (Souazé et al., 1997), have been linked to impaired G-protein-coupled receptor internalization. Furthermore, ligand-induced receptor internalization recently was shown to be critical for the inhibition of growth hormone expression by SRIF in AtT-20 cells (Sarret et al., 1999).
The aim of the present study was to investigate the consequences of SRIF internalization in rat brain, specifically with regard to cell surface regulation of the SST2A receptor subtype and the potential role of the internalization process in SRIF-induced transcriptional effects. For this purpose we examined, using light and electron microscopic immunocytochemistry and semiquantitative PCR, the expression and distribution of SST2A receptors in rat brain slices exposed to various concentrations of SRIF.
MATERIALS AND METHODS
Slice preparation
Adult male Sprague Dawley rats (200–250 gm) were decapitated, and their brains were removed rapidly and blocked on a vibratome chuck. Coronal sections containing the claustrum and the basolateral nucleus of the amygdala (BLA) were cut at 100 μm thickness and collected in ice-cold oxygenated Ringer's buffer [containing (in mm) 124 NaCl, 5 KCl, 1.2 NaH2PO4, 2.4 CaCl2, 1.5 MgSO4, 26 NaHCO3, and 10 glucose, pH 7.4]. During all of the following procedures the slices were oxygenated continuously with 95%O2/5%CO2 bubbled into the incubation buffer. Three sets of experiments were performed. (1) To determine the effect of agonist stimulation on SST2A receptor expression and distribution, we first equilibrated slices for 40 min at room temperature with Ringer's buffer. Then the slices were transferred for 40 min at 37°C in the same buffer containing (experimental) or not (control) 0.01, 0.1, or 10 μm[d-Trp8]-SRIF, a metabolically resistant analog of SRIF. (2) To investigate the effects of neuronal activity blockade on SST2A receptor expression and distribution, we first equilibrated slices for 40 min at room temperature in Ringer's buffer devoid of CaCl2 and complemented the buffer with 20 mm EDTA (Ca2+-free buffer). Then the slices were transferred to Ca2+-free buffer at 37°C containing (experimental) or not (control) [d-Trp8]-SRIF in the same range of concentrations as above. (3) To investigate the effects of ligand internalization on SST2A receptor expression and distribution, we first equilibrated slices for 15 min in Ca2+-free or Ca2+-supplemented buffer in the presence (experimental) or the absence (control) of the endocytosis inhibitors phenylarsine oxide (PAO; 10 μm) or sucrose 0.4m. Then we incubated them with 0.1 or 10 μm[d-Trp8]-SRIF in the same buffers.
At the end of the incubation the slices either were fixed by incubation for 2 hr at room temperature in 0.1 m phosphate buffer (PB), pH 7.4, containing 4% paraformaldehyde and 0.3% glutaraldehyde for SST2A immunogold labeling or were processed immediately for mRNA extraction.
SST2A immunogold labeling
Light microscopy. After several washes in PB the sections were cryoprotected for 30 min in PB containing 25% sucrose and 3% glycerol, permeabilized by quick immersion in isopentane at −70°C followed by liquid nitrogen, and thawed in PB at room temperature. After 30 min of incubation in 0.1 mTris-buffered saline (TBS), pH 7.4, containing 3% normal goat serum (NGS), the slices were incubated for 16 hr at 4°C with a rabbit polyclonal SST2A antibody (R-88; Dournaud et al., 1996; Gu and Schonbrunn, 1997) diluted 1:1500 in TBS containing 0.5% NGS. Then they were rinsed in 0.01 m PBS (0.01m PB, pH 7.4, containing 0.9% NaCl) and incubated for 2 hr in gold-conjugated (1 nm) goat anti-rabbit IgG diluted 1:50 in PBS containing 0.2% gelatin and 0.8% BSA. Sections were post-fixed for 10 min in 2% glutaraldehyde in PBS and washed several times in 0.2m citrate buffer, pH 7.4, after which the immunogold reaction was enhanced by incubation for 7 min in a silver solution (IntenSE M, Amersham, Arlington Heights, IL). The reaction was stopped by washes in citrate buffer, and the sections were mounted on glass slides for light microscopic examination.
The intensity of the light microscopic signal was quantified in the claustrum by computer-assisted microdensitometry, using a Biocom image analysis system (Les Ulis, France). Sections were examined under a Leica Orthoplan microscope equipped with a CCD camera, and nerve cell bodies labeled in the claustrum were outlined. Then labeling densities were measured over individual cells after ensuring that densitometric values were included within the linear portion of the standard gray scale (ranging from 0 to 255). Background values were determined in each section by measuring labeling densities in the corpus callosum, a region devoid of SST2Aimmunostaining, and subtracted from the corresponding totals. For each experimental condition >20 nerve cell bodies/region were recorded from three slices, and measurements were performed in at least three independent experiments (one rat per experiment). Data were averaged for each region and expressed as mean ± SEM. Statistical comparison between different groups was performed with Student'st test.
Electron microscopy. Sections were processed as above, but rather than being mounted on glass slides, they were post-fixed with 2% osmium tetroxide in PB for 40 min, dehydrated in graded ethanols and propylene oxide, and flat-embedded in Epon 812. Ultrathin sections (80 nm) were collected from the surface of blocks including either the claustrum or the BLA, counterstained with lead citrate and uranyl acetate, and examined with a JEOL 100× electron microscope.
Quantitative analysis of the ultrastructural distribution of SST2A immunoreactivity within either region was performed in ultrathin sections by counting gold particles present in the cytoplasm or associated with the plasma membrane of labeled dendrites. For each experimental condition a total of 300–400 grains was counted out of three to four grids from three independent experiments (n = 3 rats). Only dendrites containing at least three gold particles and exhibiting reasonably well preserved morphology (i.e., allowing for unequivocal identification of plasma membranes) were included in the analysis. The proportion of membrane-associated SST2A receptors was expressed as a percentage of the total number of gold particles. Statistical comparisons between groups were performed with Student's ttest.
Quantification of SST2A mRNA
Concentrations of SST2A receptor mRNA were measured by reverse transcription-PCR (RT-PCR) in slices of claustrum incubated or not with [d-Trp8]-SRIF under the same conditions as described above for immunohistochemistry. For this purpose, first the total RNAs were extracted from pooled slices (five slices per condition for each experiment) by homogenization in 1 ml of Trizol reagent (Life Technologies, Gaithersburg, MD) for 5 sec, followed by chloroform extraction and isopropanol precipitation according to the method of Chomczynski and Sacchi (1987). Then RNAs were suspended in H2O-DEPC and frozen at −20°C until use. The total amount of RNA collected from five pooled 100-μm-thick slices routinely ranged between 1 and 3 μg.
Then 2 μg of total RNA per pool was reverse-transcribed with 1 μg of oligo-dT15 primer (reverse transcription system kit, Promega, Madison, WI) and 30 U of AMV reverse transcriptase in a total volume of 20 μl of the supplied buffer. After cDNA synthesis for 1 hr at 42°C the samples were denatured for 5 min at 99°C and chilled on ice. One-tenth of the first-strand cDNA was subjected to 30 cycles of PCR in 25 μl of a final reaction volume containing (in mm) 50 KCl, 10 Tris, pH 9, and 1.5 MgCl2 plus 0.1% Triton X-100, 0.02% BSA, 200 μm dNTPs, 100 ng of sense and antisense primers, and 0.5 U Taq DNA polymerase (Appligene, Heidelberg, Germany). The first cycle was performed at 94°C for 2 min, 52°C for 2 min, and 72°C for 50 sec, and the following cycles were performed at 94°C for 35 sec, 52°C for 40 sec, 72°C for 50 sec, and, for the final extension step, 72°C for 5 min. PCR products were analyzed on a 2% agarose gel.
For amplification of SST2A mRNA, the following sets of oligonucleotides were used: (5′-CCAAGAGGAAAAAGTCAG-3′) as sense primer and (5′-GATACTGGTTTGGAGGTC-3′) as antisense primer, giving rise to a 373 bp band selective for rat SST2A. Plasmid control was generated by amplifying in parallel an XbaI cDNA fragment encoding the mouse SST2A receptor subcloned into the corresponding site of the pCMV-6b expression vector. Internal standards for quantification of SST2AcDNA were generated by amplifying glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA, using the following set of oligonucleotides: (5′-AACCACGAGAAATATGACAAC-3′) as sense primer and (5′-CTCAGTGTAGCCCAGGA-TGCC-3′) as antisense primer, giving rise to a 428 bp band. In all reverse transcription experiments two types of controls were performed: (1) each total RNA sample was subjected to RT in the absence of enzyme to control for intrinsic contamination by genomic DNA, and (2) the reaction was performed on the RT mixture without RNA added to control for contamination during the experiment.
For quantitative analysis the PCR band densities were measured by laser densitometry; ratios between SST2A and GAPDH cDNAs were calculated for each experimental condition. Results were expressed as a percentage of control, using as control the relative amount of cDNA present in slices superfused with Ringer's buffer (containing CaCl2) only. Each value was taken as the mean ± SEM of six independent cDNA measures from two different pools of mRNA.
RESULTS
Effect of somatostatin on the distribution and density of SST2A receptor immunoreactivity in the claustrum and basolateral nucleus of the amygdala
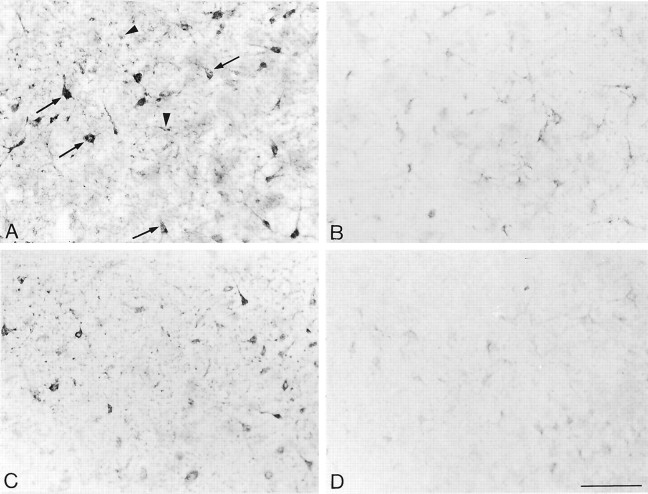
To determine whether exposure of rat brain slices to somatostatin (SRIF) would affect the distribution and/or density of SST2A somatostatin receptor proteins, we examined the effect of [d-Trp8]-SRIF on the light microscopic distribution of SST2Aimmunolabeling in two regions previously documented to exhibit high concentrations of cell surface SST2A receptors, the claustrum and BLA (Dournaud et al., 1996). By light microscopy the baseline SST2A receptor immunoreactivity was intense and mainly associated with neuronal perikarya and dendrites (Fig. 1A). Incubation of the slices for 40 min at 37°C with either 10 or 100 nm[d-Trp8]-SRIF in Ringer's buffer containing 2.4 mm CaCl2 affected neither the intensity nor the pattern of SST2A immunolabeling in either of the two regions that were studied (data not shown). However, removal of Ca2+ from the buffer dramatically reduced both the number and staining intensity of SST2A-immunoreactive elements in both the claustrum (Fig. 1B) and BLA (data not shown). Computer-assisted microdensitometric analysis of the intensity of SST2A immunostaining over neuronal perikarya labeled in the claustrum revealed a 55% reduction in the absence as compared to the presence of extracellular Ca2+ in the incubation medium (Fig.2A).
Fig. 1.
Light microscopic distribution of SST2A receptor immunolabeling in claustrum slices incubated for 40 min at 37°C in Ringer's buffer (A), in Ca2+-free buffer (B), in Ca2+-free buffer containing 100 nm[d-Trp8]-SRIF (C), and in Ca2+-free buffer containing 100 nm[d-Trp8]-SRIF plus 10 μm PAO (D). A, After incubation in Ringer's, intense immunolabeling is observed over nerve cell bodies and their proximal dendrites (arrows). Punctate immunostaining typical of cross-sectioned immunoreactive dendrites is also evident (arrowheads).B, After incubation in Ca2+-free buffer, the intensity of SST2A immunolabeling is reduced dramatically in both cell bodies and surrounding neuropil.C, Addition of 100 nm[d-Trp8]-SRIF to the Ca2+-free incubation medium almost totally reestablishes the level of SST2A immunoreactivity to that seen in Ringer's controls. D, [d-Trp8]-SRIF-induced recovery of SST2A immunoreactivity is totally prevented by addition of the endocytosis inhibitor PAO to the incubation medium. Scale bar, 100 μm.
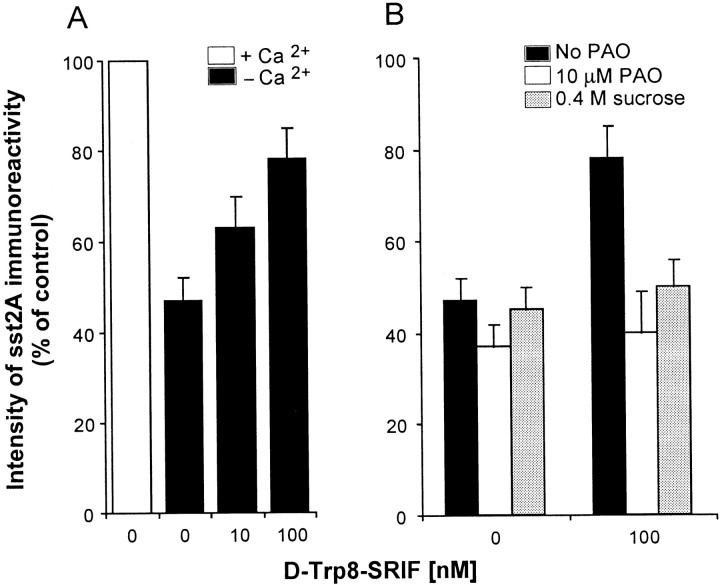
Fig. 2.
A, Effect of exogenous SRIF on the intensity of SST2A immunoreactivity in the claustrum. Slices were incubated in normal or Ca2+-free Ringer's or in Ca2+-free Ringer's containing [d-Trp8]-SRIF (10–100 nm). Density of immunoreactive signal was measured over individual labeled cells with computer-assisted densitometry. Note the substantially higher level of SST2A immunoreactivity in the presence than in the absence of Ca2+ in the buffer and the increase in the intensity of the staining that followed agonist stimulation in Ca2+-free buffer. B, Effect of endocytosis blockers on the increase in SST2Aimmunoreactivity induced by [d-Trp8]-SRIF in Ca2+-free medium. Slices were incubated in Ca2+-free Ringer's containing or not 100 nm [d-Trp8]-SRIF and in the presence or the absence of 10 μm PAO or 0.4m sucrose. Although without effect by themselves, both PAO and sucrose totally inhibited the SRIF-induced increase in SST2A immunoreactivity. Values are the mean ± SEM from three animals.
Hypothesizing that this effect might be the consequence of the inhibition of the Ca2+-dependent release of endogenous SRIF, we examined whether stimulation with exogenous SRIF would counteract for the decrease in SST2Aimmunoreactivity observed after calcium chelation. To this aim, slices from the claustrum and BLA were exposed to increasing concentrations of [d-Trp8]-SRIF under Ca2+-free conditions and were immunostained for SST2A. Such a treatment resulted in a concentration-dependent increase in the intensity of SST2A immunoreactivity in both the claustrum (Figs. 1C, 2A) and the BLA (data not shown). In the claustrum the intensity of SST2Aimmunoreactivity observed after the application of 100 nm[d-Trp8]-SRIF reached 80% of control values recorded in Ca2+-supplemented Ringer's (Fig.2A).
Because it had been shown in various cell lines as well as in neurons in primary culture that stimulation with SRIF resulted in ligand-induced receptor internalization (reviewed in the introductory remarks), we then investigated in slices from the claustrum whether the SRIF-induced increase in SST2A immunolabeling observed under Ca2+-free conditions was affected when endocytosis was blocked with PAO or hyperosmolar sucrose. As shown in Figure 2B, the addition of 10 μm PAO or 0.4 m sucrose in the absence of agonist had no effect on the intensity of SST2A immunolabeling. By contrast, the addition of either PAO or hyperosmolar sucrose in the presence of the agonist (100 nm[d-Trp8]-SRIF) totally abolished the SRIF-induced increase in SST2A immunoreactivity (Figs.1D, 2B).
Effect of somatostatin on SST2A mRNA levels in the claustrum
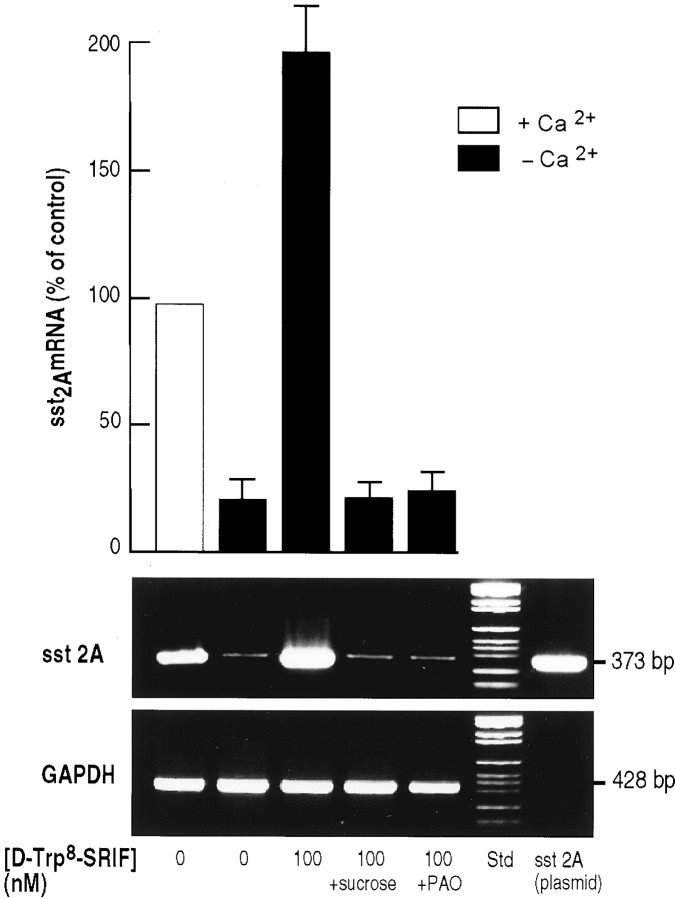
To determine whether the SRIF-induced increase in the intensity of SST2A immunolabeling observed under Ca2+-free conditions resulted from an increase in SST2A expression, we determined the amount of SST2A mRNA present in claustrum slices before and after incubation with SRIF by semiquantitative RT-PCR, using GAPDH as the internal standard. In slices incubated for 40 min at 37°C in the absence of calcium, there was a marked decrease (−80%) in the concentration of SST2A mRNA as compared with controls (Fig. 3). By contrast, slices incubated in calcium-free medium but in the presence of 100 nm [d-Trp8]-SRIF showed a massive increase in SST2A mRNA levels as compared with levels in slices incubated in the absence of SRIF in either Ca2+-free (↑ ninefold) or Ca2+-containing (↑ twofold) medium (Fig.3). However, when the internalization process was blocked by 0.45m sucrose or 10 μm PAO, this SRIF-induced increase in SST2A mRNA was no longer observed (Fig. 3).
Fig. 3.
PCR amplification of SST2A mRNAs extracted from claustrum slices incubated for 40 min at 37°C in Ca2+-supplemented Ringer's (lane 1), in Ca2+-free Ringer's (lane 2), in Ca2+-free Ringer's containing 100 nm[d-Trp8]-SRIF (lane 3), in Ca2+-free Ringer's containing 100 nm [d-Trp8]-SRIF and 0.4 m sucrose (lane 4), and in Ca2+-free Ringer's containing 100 nm[d-Trp8]-SRIF and 10 μmPAO (lane 5). PCR reactions were performed on mRNAs reverse-transcribed with specific SST2A receptor primers. The predicted size of amplified fragments was 373 bp (plasmid control). GAPDH mRNAs were transcribed in parallel (target size, 428 bp) and used as an internal standard for quantitation. SST2A mRNA levels, expressed as a percentage of control (Ringer's buffer), are depicted above the corresponding gel bands. All values are the mean ± SEM of triplicate determinations from two independent experiments.
Effect of somatostatin on the ultrastructural distribution of SST2A receptor immunoreactivity in the claustrum and basolateral nucleus of the amygdala
To determine whether SST2A receptors in the claustrum and BLA internalized on ligand exposure, we examined the subcellular distribution of SST2A receptor protein by electron microscopic immunocytochemistry both before and after the stimulation of brain slices with [d-Trp8]-SRIF.
In conformity with earlier data (Dournaud et al., 1998) the bulk of SST2A-immunoreactive receptors detected in both of these regions was associated with dendritic shafts (Fig.4). Only rare immunogold particles were found over neuronal perikarya, axon terminals, or dendritic spines. Consequently, quantitative analyses were restricted to dendrites.
Fig. 4.
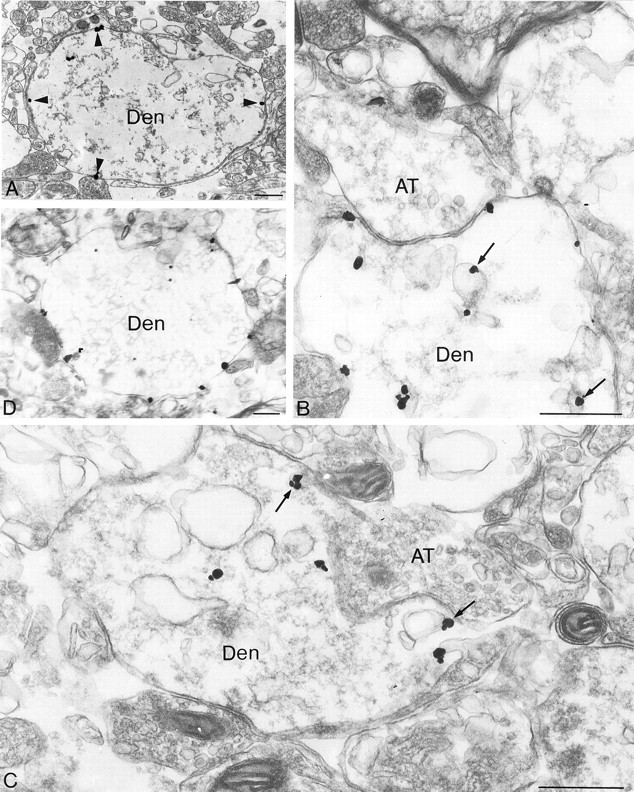
Electron microscopic localization of SST2A receptors, using silver-enhanced immunogold in claustrum slices. Slices were incubated for 40 min at 37°C in Ca2+-supplemented Ringer's in the absence (A, control) or in the presence (B–D) of 10 μm[d-Trp8]-SRIF. A, Under control conditions a high proportion of immunogold particles is associated with dendritic plasma membranes (arrowheads).B, C, In the presence of 10 μm [d-Trp8]-SRIF the bulk of gold particles is intracellular. Several are seen in association with the membrane of endosomes (arrows).D, When [d-Trp8]-SRIF stimulation is performed in the presence of PAO, the distribution of immunoreactive SST2A receptors is comparable to that seen in controls. Den, Dendrite; AT, axon terminal. Scale bars: A, D, 1 μm; B, C, 0.5 μm.
Under baseline conditions SST2A immunolabeling was associated mostly with the internal, cytoplasmic face of dendritic plasma membranes (Fig. 4A). The distribution of immunogold particles along these plasma membranes was rather homogeneous, and no enrichment at postsynaptic sites was observed (Fig.4A). In both claustrum and BLA ∼65% of gold particles associated with dendrites overlaid the plasma membrane (Fig.5). The remaining 35% were intracellular and usually were associated with small-size vesicles (average diameter of 50 nm). Occasionally, intracellular gold particles also were observed in association with the cytoplasmic side of larger vesicles (average diameter of 150 nm) exhibiting the morphological features of endosomes (data not shown).
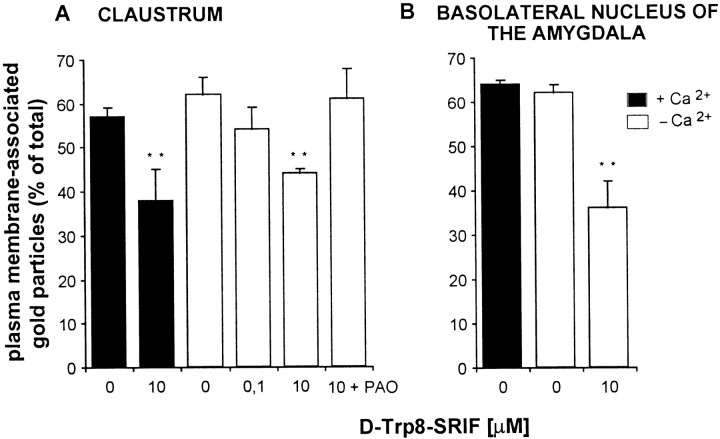
Fig. 5.
Effect of SRIF stimulation on the subcellular distribution of SST2A receptors in dendrites of the claustrum and basolateral amygdala. Slices were incubated with or without [d-Trp8]-SRIF (10 nm–10 μm) in Ca2+-free or Ca2+-supplemented Ringer's. Dendrite-associated immunogold particles were counted and classified as either membrane-associated or intracellular; results are expressed as membrane-associated/total. In the absence of agonist, ∼60% of gold particles were associated with dendritic plasma membranes in both cerebral regions, irrespective of the Ca2+concentration in the medium. Addition of agonist induced a decrease in the percentage of receptors associated with plasma membranes, again both in the presence and absence of Ca2+. The agonist-induced decrease in surface receptors was prevented by adding 10 μm PAO to the incubation medium. Values are the mean ± SEM from three animals; **p < 0.01.
Incubation of brain slices for 40 min at 37°C in Ca2+-supplemented Ringer's buffer in the presence of 100 nm[d-Trp8]-SRIF resulted in a slight but nonsignificant decrease in the proportion of membrane-associated gold particles within the claustrum (Fig.5A). At a higher concentration of agonist (10 μm) this reduction was substantially more robust (30%) and statistically significant (p< 0.01) in both the claustrum (Fig. 5A) and the BLA (Fig.5B). In turn, a higher proportion of immunoreactive SST2A receptors was associated with endosome-like vesicles after than before stimulation with the agonist in both regions (see Fig. 4B,C). These SRIF-induced changes in SST2A receptor distribution were no longer observed when the incubations were performed in the presence of the endocytosis inhibitor PAO (see Figs. 4D,5A).
To determine whether blocking neuronal activity (including endogenous SRIF release) would affect the ultrastructural distribution of SST2A receptor immunoreactivity, we incubated brain slices as above in a Ca2+-free Ringer's solution containing 20 mm EDTA. In neither of the two brain regions that were examined did the removal of calcium from the Ringer's buffer affect the proportion of SST2A receptors associated with the plasma membrane (Fig. 5), despite its decreasing the overall density of SST2A immunoreactivity (see above). Furthermore, the addition of 10 μm[d-Trp8]-SRIF to the incubation medium under these calcium-free conditions resulted in the same degree of cell surface receptor loss as observed in the presence of calcium in either the claustrum or the BLA (Fig. 5).
DISCUSSION
The present study demonstrates that exposure of rat brain slices to SRIF regulates the expression and availability of SST2A receptors in a concentration-dependent manner. This regulation is rapid (<40 min), involves both transcriptional and trafficking events, and is blocked by endocytosis inhibitors, suggesting that it requires internalization of receptor–ligand complexes.
The specificity of the SST2A antibodies used in the present experiments previously was characterized in details in heterologous transfection systems as well as in rat brain and pituitary (Dournaud et al., 1996, 1998; Gu and Schonbrunn, 1997; Mezey et al., 1998). Accordingly, the regional distribution of immunoreactive SST2A receptors detected here in rat brain slices was correlated closely with that of both SRIF binding sites, as visualized by autoradiography that used SST2-preferring ligands (Schoeffter et al., 1995;Holloway et al., 1996), and of SST2A mRNA, as detected by in situ hybridization that used either ribo- or oligonucleotide probes (Pérez et al., 1994; Señarís et al., 1994; Beaudet et al., 1995). It also conformed to that previously observed by immunohistochemistry within the same regions using either the same (Dournaud et al., 1996, 1998) or a different (Schindler et al., 1997) SST2A antibody.
A striking finding of the present study was that the intensity of SST2A immunoreactivity, expressed in terms of overall staining density, was significantly lower in both the claustrum and basolateral amygdala when the slices were incubated in the absence than in the presence of extracellular Ca2+. By light microscopy this decrease was found to be attributable to a reduction in both the number of immunolabeled neurons and neuronal processes and in the intensity of immunoreactivity within immunolabeled cells. Furthermore, measurement by quantitative PCR of SST2A mRNA present in incubated slices indicated that this decrease in SST2A receptor protein was correlated with a decrease in SST2A mRNA levels. It is unclear whether the latter was attributable to a reduction in transcription or an increase in mRNA degradation, because both processes were shown to be involved in receptor regulation in the case of other G-protein-coupled receptors (Collins et al., 1989; Souazé et al., 1997). In any event, the present results indicate that the expression of SST2A receptors in neurons is under the regulation of Ca2+-dependent processes.
One of the physiological events most likely to play a role in SST2A regulation and clearly impaired in Ca2+-free medium is the release of endogenous SRIF from axon terminals present within the slices. We therefore investigated whether compensating the loss of endogenous SRIF release with exogenous SRIF would reestablish the levels of SST2A receptors to control levels, i.e., to levels observed in slices incubated in the presence of Ca2+. Incubation of slices from both claustrum and BLA with a metabolically resistant SRIF analog in the absence of Ca2+resulted in a concentration-dependent increase in both SST2AmRNA, as measured by quantitative PCR, and SST2Aimmunoreactivity, as quantified by computer-assisted microdensitometry. Such an upregulation of SST2A receptors by SRIF was not totally unexpected because earlier studies had demonstrated an increase in SST2A mRNA in GH3 pituitary cells after 2 hr of stimulation by SRIF (Bruno et al., 1994) as well as within the arcuate nucleus of the hypothalamus 3 hr after systemic administration of the SRIF analog octreotide (Tannenbaum et al., 1995). More recently, long-term continuous release of octreotide for 7 d in tumor-bearing, severe combined immunodeficient (SCID) mice also was shown to increase SST2A expression in tumor cells (Froidevaux et al., 1999). In these experiments the upregulatory effects of SRIF likely were mediated by stimulation of the SST2 receptor itself, because octreotide is known to be relatively selective for the SST2 subtype (Raynor et al., 1993). The effects observed in our slice preparation, however, could be attributable to stimulation of any of the SRIF receptors because [d-Trp8]-SRIF was reported to bind to all five subtypes (Raynor et al., 1993; Patel and Srikant, 1994). It is likely, however, that SST2 was involved predominantly, given the paucity of other SST receptor subtypes demonstrated to date in either the claustrum or the BLA (Hervieu and Emson, 1998; Händel et al., 1999; Schindler et al., 1999; Stroh et al., 1999).
A striking feature of the present results is the fact that the agonist-induced increase in SST2A expression observed in the absence of Ca2+was entirely prevented by the addition of PAO or hyperosmolar sucrose to the incubation medium. Both of these compounds are well documented endocytosis inhibitors acting by impeding the formation of clathrin-coated pits (Koenig and Edwardson, 1997). Admittedly, PAO also was reported to inhibit tyrosine phosphatases (Kleinert et al., 1998;Mahoubi et al., 1998) and thereby could affect cell signaling because activation of SST2 receptors was shown to inhibit cell proliferation by a mechanism involving the stimulation of the protein-tyrosine phosphatase SHP-1 (Lopez et al., 1997). However, the fact that the increase in SST2A receptor expression observed in the present study was abolished not only with PAO but also with hyperosmolar sucrose suggests that this transcriptional effect is really dependent on receptor internalization. Indeed, treatment with hyperosmolar sucrose has never, to our knowledge, been reported to affect intracellular signaling cascades. Furthermore, it was shown specifically not to affect forskolin-induced stimulation of adenylate cyclase (Sarret et al., 1999).
We previously demonstrated that, in the pituitary cell line AtT-20, exposure to SRIF induced a decrease in growth hormone expression as measured by using quantitative PCR (Sarret et al., 1999). In this earlier study, as in the present one, the agonist-induced transcriptional effect was totally abolished in the presence of hyperosmolar sucrose, suggesting that it was dependent on internalization of receptor–ligand complexes. Similarly, chronic stimulation with a nondegradable analog of neurotensin was reported to increase NT1 receptor mRNA in HT29 cells in a PAO-sensitive manner (Souazé et al., 1997). Internalization-dependent transcriptional effects therefore may prove a wider occurrence than previously was suspected.
Somatostatin has been documented to promote receptor-mediated internalization in a variety of cell lines (Koenig et al., 1997; Sarret et al., 1999). Studies in transfected cells have shown the efficacy of this internalization process to vary widely among SRIF receptor subtypes, SST2, SST3, and SST5 providing for the most efficient endocytosis and SST1 and SST4 providing for very poor internalization (Hukovic et al., 1996; Hipkin et al., 1997; Nouel et al., 1997; Roth et al., 1997; Kreienkamp et al., 1998;Stroh et al., 2000b). Recent studies from our laboratory have shown that SRIF internalized efficiently within neurons in primary culture and that this internalization was clathrin-dependent and mediated in part by the SST2 receptor subtype (Stroh et al., 2000a). The present electron microscopic results confirm that agonist stimulation promotes internalization of SST2Areceptors in central neurons. This internalization is sensitive to treatment with both PAO and hyperosmolar sucrose, suggesting that it is clathrin-mediated. The net result of this internalization is a decrease in the density of cell surface receptors and a concomitant increase in the association of intracellular receptors with endosomes. This loss of cell surface receptors was observed whether or not Ca2+was present in the extracellular milieu, in keeping with earlier studies demonstrating that clathrin-mediated endocytosis is a Ca2+-independent process (Vandenbulcke et al., 2000). Surprisingly, however, the SRIF-induced reduction in cell surface labeling was apparent only at high doses of SRIF (10 μm), whereas the affinity of SST2Areceptors for [d-Trp8]-SRIF is in the nanomolar range (Raynor et al., 1993). Accordingly, agonist-induced internalization of these receptors was found in other model systems to proceed within the nanomolar concentration spectrum (Hipkin et al., 1997; Koenig et al., 1997; Nouel et al., 1997). It therefore appears that relatively high concentrations of agonist are needed to downregulate cell surface receptors efficiently, presumably because at lower concentrations the recruitment of spare receptors to the membrane and/or recycling of internalized receptors compensate for the loss of cell surface ones.
The fact that a SRIF-induced decrease in cell surface receptor density was observed both in the presence and in the absence of Ca2+ and hence was irrespective of the overall concentration of receptor proteins suggests that the regulation of cell surface receptor availability and the regulation of SST2A receptor expression are dissociated events. Indeed, under baseline conditions (i.e., under normal extracellular Ca2+concentrations) the transcriptional effects of SRIF have reached saturation, whereas exposure to the agonist can still decrease cell surface receptor densities. This is not to say, however, that the two events are not linked functionally. Indeed, it is tempting to speculate that the SRIF-induced upregulation of SST2A receptor proteins serves to compensate for the loss of cell surface receptors caused by internalization.
Taken together, the present data support the notion that dynamic transcriptional (increase in SST2A expression) and trafficking (loss of cell surface receptors) events are involved in the control of the somatostatinergic signal. A critical finding is that both of these events appear to be dependent on ligand-induced receptor internalization. Agonist-induced endocytosis has long been known to play a key role in G-protein-coupled receptor desensitization, including that of SST2 receptors, via cell surface receptor sequestration and downregulation (Hipkin et al., 1997;Beaumont et al., 1998). The implication of receptor endocytosis in transcriptional effects is less well established in the case of G-protein-coupled receptors, although long admitted for tyrosine kinase receptor-mediated signaling (for review, see Bevan et al., 1995). Recent studies have suggested that internalization may be mandatory for the activation of mitogen-activated protein kinase pathway by β-adrenergic receptors (Luttrell et al., 1999). Whether there are similar mechanisms, or others, involving either endosome signaling (Bevan et al., 1995) or translocation of internalized receptors or of fragments thereof to the nucleus (Jans, 1994; Laduron, 1994) remains to be determined. It even may be that the internalized ligand, which in transfected cells is rapidly segregated from the acidic endosomal milieu and targeted to a juxtanuclear compartment linked to the trans-Golgi network (our unpublished observations), may be acting on intracellular secondary receptors such as the Ku autoantigen. Indeed, this intracellular protein was documented to bind with high affinity a variety of SRIF analogs (including SRIF-28 and octreotide and therefore probably also [d-Trp8]-SRIF) and to activate protein phosphatase 2A and DNA-dependent protein kinase (Reyl-Desmars et al., 1989; Le Romancer et al., 1994; Sadji et al., 1999).
Footnotes
This work was supported by Medical Research Council of Canada Grant MT-7366 to A.B. and National Institutes of Health Grant DK32234 to A.S. H.B. was funded by Institut National de la Santé et de la Recherche Médicale Fonds de la Recherche en Santé du Québec and Jeanne Timmins research fellowships. We thank Mariette Houle for excellent technical assistance and Naomi Takeda for her help with the preparation of this manuscript.
Correspondence should be addressed to Dr. Alain Beaudet, Montreal Neurological Institute, McGill University, 3801 University Street, Montréal, Québec H3A 2B4, Canada. E-mail:mcin@musica.mcgill.ca.
REFERENCES
- 1.Beaudet A, Greenspun D, Raelson J, Tannenbaum GS. Patterns of expression of SSTR1 and SSTR2 somatostatin receptor subtypes in the hypothalamus of the adult rat: relationship to neuroendocrine function. Neuroscience. 1995;65:551–561. doi: 10.1016/0306-4522(94)00486-o. [DOI] [PubMed] [Google Scholar]
- 2.Beaumont V, Hepworth MB, Luty JS, Kelly E, Henderson G. Somatostatin receptor desensitization in NG108-15 cells. J Biol Chem. 1998;273:33174–33183. doi: 10.1074/jbc.273.50.33174. [DOI] [PubMed] [Google Scholar]
- 3.Bevan AP, Burgess JW, Drake PG, Shaver A, Bergeron JJ, Posner BI. Selective activation of the rat hepatic endosomal insulin receptor kinase. Role for the endosome in insulin signaling. J Biol Chem. 1995;270:10784–10791. doi: 10.1074/jbc.270.18.10784. [DOI] [PubMed] [Google Scholar]
- 4.Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 5.Breder CD, Yamada Y, Yasuda K, Seino S, Safer CB, Bell GI. Differential expression of somatostatin receptor subtypes in brain. J Neurosci. 1992;12:3920–3934. doi: 10.1523/JNEUROSCI.12-10-03920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruno JF, Xu Y, Song J, Berelowitz M. Molecular cloning and functional expression of a brain-specific somatostatin receptor. Proc Natl Acad Sci USA. 1992;89:11151–11155. doi: 10.1073/pnas.89.23.11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruno JF, Xu Y, Song J, Berelowitz M. Tissue distribution of somatostatin receptor subtype messenger ribonucleic acid in the rat. Endocrinology. 1993;133:2561–2567. doi: 10.1210/endo.133.6.8243278. [DOI] [PubMed] [Google Scholar]
- 8.Bruno JF, Xu Y, Berelowitz M. Somatostatin regulates somatostatin receptor subtype mRNA expression in GH3 cells. Biochem Biophys Res Commun. 1994;202:1738–1743. doi: 10.1006/bbrc.1994.2136. [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Collins S, Bouvier M, Bolanowski MA, Caron MG, Lefkowitz RJ. cAMP stimulates transcription of the β2-adrenergic receptor gene in response to short-term agonist exposure. Proc Natl Acad Sci USA. 1989;86:4853–4857. doi: 10.1073/pnas.86.13.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dournaud P, Gu YZ, Schonbrunn A, Mazella J, Tannenbaum GS, Beaudet A. Localization of the somatostatin receptor SST2A in rat brain using a specific anti-peptide antibody. J Neurosci. 1996;16:4468–4478. doi: 10.1523/JNEUROSCI.16-14-04468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dournaud P, Boudin H, Schonbrunn A, Tannenbaum GS, Beaudet A. Interrelationships between somatostatin SST2A receptors and somatostatin-containing axons in rat brain: evidence for regulation of cell surface receptors by endogenous somatostatin. J Neurosci. 1998;18:1056–1071. doi: 10.1523/JNEUROSCI.18-03-01056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epelbaum J. Somatostatin in the central nervous system: physiology and pathological modifications. Prog Neurobiol. 1986;27:63–100. doi: 10.1016/0301-0082(86)90012-2. [DOI] [PubMed] [Google Scholar]
- 14.Froidevaux S, Hintermann E, Torok M, Macke HR, Beglinger C, Eberle AN. Differential regulation of somatostatin receptor type 2 (SST2) expression in AR4-2J tumor cells implanted into mice during octreotide treatment. Cancer Res. 1999;59:3652–3657. [PubMed] [Google Scholar]
- 15.Griendling KK, Delafontaine P, Rittenhouse SE, Gimbrone MA, Jr, Alexander RW. Correlation of receptor sequestration with sustained diacylglycerol accumulation in angiotensin II-stimulated cultured vascular smooth muscle cells. J Biol Chem. 1987;262:14555–14562. [PubMed] [Google Scholar]
- 16.Gu YZ, Schonbrunn A. Coupling specificity between somatostatin receptor SST2A and G-proteins: isolation of the receptor–G-protein complex with a receptor antibody. Mol Endocrinol. 1997;11:527–537. doi: 10.1210/mend.11.5.9926. [DOI] [PubMed] [Google Scholar]
- 17.Händel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, Wolf G, Höllt V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- 18.Helboe L, Stidsen CE, Moller M. Immunohistochemical and cytochemical localization of the somatostatin receptor subtype SST1 in the somatostatinergic parvocellular neuronal system of the rat hypothalamus. J Neurosci. 1998;18:4938–4945. doi: 10.1523/JNEUROSCI.18-13-04938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hervieu G, Emson PC. The localization of somatostatin receptor 1 (SST1) immunoreactivity in the rat brain using an N-terminal specific antibody. Neuroscience. 1998;85:1263–1284. doi: 10.1016/s0306-4522(98)00024-4. [DOI] [PubMed] [Google Scholar]
- 20.Hipkin RW, Friedman J, Clark RB, Eppler CM, Schonbrunn A. Agonist-induced desensitization, internalization, and phosphorylation of the SST2A somatostatin receptor. J Biol Chem. 1997;272:13869–13876. doi: 10.1074/jbc.272.21.13869. [DOI] [PubMed] [Google Scholar]
- 21.Holloway S, Feniuk W, Kidd EJ, Humphrey PPA. A quantitative autoradiographical study on the distribution of somatostatin SST2 receptors in the rat central nervous system using [125I]-BIM-23027. Neuropharmacology. 1996;35:1109–1120. doi: 10.1016/s0028-3908(96)00082-2. [DOI] [PubMed] [Google Scholar]
- 22.Hoyer D, Lubbert H, Bruns C. Molecular pharmacology of somatostatin receptors. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:441–453. doi: 10.1007/BF00173012. [DOI] [PubMed] [Google Scholar]
- 23.Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PPA, O'Carroll AM, Patel YC, Schonbrunn A, Taylor JE. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995;16:86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- 24.Hukovic N, Panetta R, Kumar U, Patel YC. Agonist-dependent regulation of cloned human somatostatin receptor types 1–5 (HSSTR1–5): subtype selective internalization or upregulation. Endocrinology. 1996;137:4046–4049. doi: 10.1210/endo.137.9.8756582. [DOI] [PubMed] [Google Scholar]
- 25.Hunyady L, Merelli F, Baukal AJ, Balla T, Catt KJ. Agonist-induced endocytosis and signal generation in adrenal glomerulosa cells. A potential mechanism for receptor-operated calcium entry. J Biol Chem. 1991;266:2783–2788. [PubMed] [Google Scholar]
- 26.Jans DA. Nuclear signaling pathways for polypeptide ligands and their membrane receptors? FASEB J. 1994;8:841–847. doi: 10.1096/fasebj.8.11.8070633. [DOI] [PubMed] [Google Scholar]
- 27.Johansson O, Hökfelt T, Elde RP. Immunohistochemical distribution of somatostatin-like immunoreactivity in the central nervous system of the adult rat. Neuroscience. 1984;13:265–339. doi: 10.1016/0306-4522(84)90233-1. [DOI] [PubMed] [Google Scholar]
- 28.Kaupmann K, Bruns C, Hoyer D, Seuwen K, Lubbert H. Distribution and second messenger coupling of four somatostatin receptor subtypes expressed in brain. FEBS Lett. 1993;331:53–59. doi: 10.1016/0014-5793(93)80296-7. [DOI] [PubMed] [Google Scholar]
- 29.Kleinert H, Wallerath T, Fritz G, Ihrig-Biedert I, Rodriguez-Pascual F, Geller DA, Forstermann U. Cytokine induction of NO synthase II in human DLD-1 cells: roles of the JAK-STAT, AP-1, and NF-κB-signaling pathways. Br J Pharmacol. 1998;125:193–201. doi: 10.1038/sj.bjp.0702039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kluxen FW, Bruns C, Lubbert H. Expression cloning of a rat brain somatostatin receptor cDNA. Proc Natl Acad Sci USA. 1992;89:4618–4622. doi: 10.1073/pnas.89.10.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koenig JA, Edwardson JM. Endocytosis and recycling of g-protein-coupled receptors. Trends Pharmacol Sci. 1997;18:276–287. doi: 10.1016/s0165-6147(97)01091-2. [DOI] [PubMed] [Google Scholar]
- 32.Koenig JA, Edwardson JM, Humphrey PPA. Somatostatin receptors in Neuro2A neuroblastoma cells: ligand internalization. Br J Pharmacol. 1997;120:52–59. doi: 10.1038/sj.bjp.0700859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong H, DePaoli AM, Breder CD, Yasuda K, Bell GI, Reisine T. Differential expression of messenger RNAs for somatostatin receptor subtypes SSTR1, SSTR2 and SSTR3 in adult rat brain: analysis by RNA blotting and in situ hybridization histochemistry. Neuroscience. 1994;59:175–184. doi: 10.1016/0306-4522(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 34.Krantic S, Quirion R, Uhl G. Somatostatin receptors. In: Björklund A, Hökfelt T, Kuhar MJ, editors. Handbook of chemical neuroanatomy, Chap 11, Neuropeptide receptors in the CNS. Elsevier; Amsterdam: 1992. pp. 321–346. [Google Scholar]
- 35.Kreienkamp HJ, Roth A, Richter D. Rat somatostatin receptor subtype 4 can be made sensitive to agonist-induced internalization by mutation of a single threonine (residue 331). DNA Cell Biol. 1998;17:869–878. doi: 10.1089/dna.1998.17.869. [DOI] [PubMed] [Google Scholar]
- 36.Krisch B, Feindt J, Mentlein R. Immunoelectron microscopic analysis of the ligand-induced internalization of the somatostatin receptor subtype 2 in cultured human glioma cells. J Histochem Cytochem. 1998;46:1233–1242. doi: 10.1177/002215549804601103. [DOI] [PubMed] [Google Scholar]
- 37.Laduron PM. From receptor internalization to nuclear translocation. New targets for long-term pharmacology. Biochem Pharmacol. 1994;47:3–14. doi: 10.1016/0006-2952(94)90431-6. [DOI] [PubMed] [Google Scholar]
- 38.Le Romancer M, Reyl-Desmars F, Cherifi Y, Pigeon C, Bottari S, Meyer O, Lewin MJ. The 86-kDa subunit of autoantigen Ku is a somatostatin receptor regulating protein phosphatase-2A activity. J Biol Chem. 1994;269:17464–17468. [PubMed] [Google Scholar]
- 39.Lopez F, Esteve JP, Buscail L, Delesque N, Saint-Laurent N, Theveniau M, Nahmias C, Vaysse N, Susini C. The tyrosine phosphatase SHP-1 associates with the SST2 somatostatin receptor and is an essential component of SST2-mediated inhibitory growth signaling. J Biol Chem. 1997;272:24448–24454. doi: 10.1074/jbc.272.39.24448. [DOI] [PubMed] [Google Scholar]
- 40.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. β-Arrestin-dependent formation of β2 adrenergic receptor–Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 41.Mahoubi K, Young W, Ferreri NR. Tyrosine phosphatase-dependent/tyrosine kinase-independent induction of nuclear factor-κB by tumor necrosis factor-α: effects on prostaglandin endoperoxide synthase-2 mRNA accumulation. J Pharmacol Exp Ther. 1998;285:862–868. [PubMed] [Google Scholar]
- 42.Meyerhof W, Wulfsen I, Schonrock C, Fehr S, Richter D. Molecular cloning of a somatostatin-28 receptor and comparison of its expression pattern with that of a somatostatin-14 receptor in rat brain. Proc Natl Acad Sci USA. 1992;89:10267–10271. doi: 10.1073/pnas.89.21.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mezey E, Hunyady B, Mitra S, Hayes E, Liu Q, Schaeffer J, Schonbrunn A. Cell-specific expression of the SST2A and SST5 somatostatin receptors in the rat anterior pituitary. Endocrinology. 1998;139:414–419. doi: 10.1210/endo.139.1.5807. [DOI] [PubMed] [Google Scholar]
- 44.Nouel D, Gaudriault G, Houle M, Reisine T, Vincent JP, Mazella J, Beaudet A. Differential internalization of somatostatin in COS-7 cells transfected with SST1 and SST2 receptor subtypes: a confocal microscopic study using novel fluorescent somatostatin derivatives. Endocrinology. 1997;138:296–306. doi: 10.1210/endo.138.1.4834. [DOI] [PubMed] [Google Scholar]
- 45.O'Carroll AM, Lolait SJ, Konig M, Mahan LC. Molecular cloning and expression of a pituitary somatostatin receptor with preferential affinity for somatostatin-28. Mol Pharmacol. 1992;42:939–946. [PubMed] [Google Scholar]
- 46.Patel YC. General aspects of the biology and function of somatostatin. In: Weil C, Müller EE, Thorner MO, editors. Somatostatin. Springer; Berlin: 1992. pp. 1–16. [Google Scholar]
- 47.Patel YC, Srikant BS. Subtype selectivity of peptide analogs for all five cloned human somatostatin receptors (HSSTR1–5). Endocrinology. 1994;135:2814–2817. doi: 10.1210/endo.135.6.7988476. [DOI] [PubMed] [Google Scholar]
- 48.Pérez J, Rigo M, Kaupmann K, Bruns C, Yasuda K, Bell GI, Lubbert H, Hoyer D. Localization of somatostatin (SRIF) SSTR-1, SSTR-2, and SSTR-3 receptor mRNA in rat brain by in situ hybridization. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:145–160. doi: 10.1007/BF00169831. [DOI] [PubMed] [Google Scholar]
- 49.Raulf F, Pérez J, Hoyer D, Bruns C. Differential expression of five somatostatin receptor subtypes, SSTR1–5, in the CNS and peripheral tissue. Digestion. 1994;55:46–53. doi: 10.1159/000201201. [DOI] [PubMed] [Google Scholar]
- 50.Raynor K, Murphy WA, Coy DH, Raylor JE, Moreau J-P, Yasuda K, Bell GI, Reisine T. Cloned somatostatin receptors: identification of subtype-selective peptides and demonstration of high-affinity binding of linear peptides. Mol Pharmacol. 1993;43:838–844. [PubMed] [Google Scholar]
- 51.Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocr Rev. 1995;16:427–442. doi: 10.1210/edrv-16-4-427. [DOI] [PubMed] [Google Scholar]
- 52.Reyl-Desmars F, Le Roux S, Linard C, Benkouka F, Lewin MJ. Solubilization and immunopurification of a somatostatin receptor from the human gastric tumoral cell line HGT-1. J Biol Chem. 1989;264:18789–18795. [PubMed] [Google Scholar]
- 53.Roth A, Kreienkamp HJ, Nehring RB, Roosterman D, Meyerhof W, Richter D. Endocytosis of the rat somatostatin receptors: subtype discrimination, ligand specificity, and delineation of carboxy-terminal positive and negative sequence motifs. DNA Cell Biol. 1997;16:111–119. doi: 10.1089/dna.1997.16.111. [DOI] [PubMed] [Google Scholar]
- 54.Sadji Z, Le Romancer M, Hervatin F, Lewin MJ, Reyl-Desmars F. Somatostatin analogs stimulate DNA-dependent protein kinase activity in human gastric tumoral cell line HGT-1. Life Sci. 1999;65:2829–2835. doi: 10.1016/s0024-3205(99)00552-4. [DOI] [PubMed] [Google Scholar]
- 55.Sarret P, Nouel D, Dal Farra C, Vincent JP, Beaudet A, Mazella J. Receptor-mediated internalization is critical for the inhibition of the expression of growth hormone by somatostatin in the pituitary cell line AtT-20. J Biol Chem. 1999;274:19294–19300. doi: 10.1074/jbc.274.27.19294. [DOI] [PubMed] [Google Scholar]
- 56.Schindler M, Sellers LA, Humphrey PPA, Emson PC. Immunohistochemical localization of the somatostatin SST2A receptor in the rat brain and spinal cord. Neuroscience. 1997;76:225–240. doi: 10.1016/s0306-4522(96)00388-0. [DOI] [PubMed] [Google Scholar]
- 57.Schindler M, Humphrey PPA, Löhrke S, Friauf E. Immunohistochemical localization of the somatostatin SST2B receptor splice variant in the rat central nervous system. Neuroscience. 1999;90:859–874. doi: 10.1016/s0306-4522(98)00483-7. [DOI] [PubMed] [Google Scholar]
- 58.Schoeffter P, Pérez J, Langenegger D, Schupbach E, Bobirnac I, Lubbert H, Bruns C, Hoyer D. Characterization and distribution of somatostatin SS-1 and SRIF-1 binding sites in rat brain: identity with SSTR-2 receptors. Eur J Pharmacol. 1995;289:163–173. doi: 10.1016/0922-4106(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 59.Señarís RM, Humphrey PPA, Emson PC. Distribution of somatostatin receptors 1, 2, and 3 mRNA in rat brain and pituitary. Eur J Neurosci. 1994;6:1883–1896. doi: 10.1111/j.1460-9568.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 60.Siehler S, Seuwen K, Hoyer D. [125I][Tyr3]octreotide labels human somatostatin SST2 and SST5 receptors. Eur J Pharmacol. 1998;348:311–320. doi: 10.1016/s0014-2999(98)00159-9. [DOI] [PubMed] [Google Scholar]
- 61.Souazé F, Rostène W, Forgez P. Neurotensin agonist induces differential regulation of neurotensin receptor mRNA. Identification of distinct transcriptional and post-transcriptional mechanisms. J Biol Chem. 1997;272:10087–10094. doi: 10.1074/jbc.272.15.10087. [DOI] [PubMed] [Google Scholar]
- 62.Stroh T, Kreienkamp HJ, Beaudet A. Immunohistochemical distribution of the somatostatin receptor subtype 5 in the adult rat brain: predominant expression in the basal forebrain. J Comp Neurol. 1999;412:69–82. doi: 10.1002/(sici)1096-9861(19990913)412:1<69::aid-cne5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 63.Stroh T, Jackson AC, Dal Farra C, Schonbrunn A, Vincent JP, Beaudet A (2000a) Receptor-mediated internalization of somatostatin in rat cortical and hippocampal neurons. Synapse, in press. [DOI] [PubMed]
- 64.Stroh T, Jackson AC, Sarret P, Dal Farra C, Vincent JP, Kreienkamp HJ, Mazella J, Beaudet A. Intracellular dynamics of SST5 receptors in transfected COS-7 cells: maintenance of cell surface receptors during ligand-induced endocytosis. Endocrinology. 2000b;141:354–365. doi: 10.1210/endo.141.1.7259. [DOI] [PubMed] [Google Scholar]
- 65.Tannenbaum GS, Turner J, Guo F, Beaudet A. Homologous up-regulation of somatostatin receptor subtype SSTR2 expression in rat arcuate nucleus in vivo. Soc Neurosci Abstr. 1995;21:1994. [Google Scholar]
- 66.Thoss VS, Pérez J, Duc D, Hoyer D. Embryonic and postnatal mRNA distribution of five somatostatin receptor subtypes in the rat brain. Neuropharmacology. 1995;34:1673–1688. doi: 10.1016/0028-3908(95)00135-2. [DOI] [PubMed] [Google Scholar]
- 67.Vandenbulcke F, Nouel D, Vincent JP, Mazella J, Beaudet A (2000) Ligand-induced internalization of neurotensin in transfected COS-7 cells: differential intracellular trafficking of ligand and receptor. J Cell Sci, in press. [DOI] [PubMed]
- 68.Viollet C, Faivre-Bauman A, Zhang J, Llorens-Cortes C, Loudes C, Kordon C, Epelbaum J. Differential expression of somatostatin receptors by quantitative PCR in the rat brain. C R Acad Sci III. 1995;318:851–857. [PubMed] [Google Scholar]
- 69.Yamada Y, Post SR, Wang K, Tager HS, Bell GI, Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci USA. 1992;89:251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yasuda K, Rens-Domiano S, Breder CD, Law SF, Saper CB, Reisine T, Bell GI. Cloning of a novel somatostatin receptor, SSTR3, coupled to adenylylcyclase. J Biol Chem. 1992;267:20422–20428. [PubMed] [Google Scholar]