Abstract
In the present study, we characterized the intrinsic electrophysiological properties and the membrane currents activated by dopamine (DA) D2 and GABAB receptors in midbrain dopaminergic neurons, maintained in vitro in a slice preparation, from wild-type and homozygous weaver(wv/wv) mice. By using patch-clamp techniques, we found that membrane potential, apparent input resistance, and spontaneous firing of wv/wv dopaminergic neurons were similar to those of dopamine-containing cells recorded from nonaffected (+/+) animals.
More interestingly, the wv/wv neurons were excited rather than inhibited by dopamine and the GABAB agonist baclofen. This neurotransmitter-mediated excitation was attributable to the activation of a G-protein-gated inward current that reversed polarity at a membrane potential of approximately −30 mV. We suggest that the altered behavior of the receptor-operatedwv G-protein-gated inwardly rectifying K+ channel 2 (GIRK2) might be related to the selective degeneration of the dopaminergic neurons. In addition, thewv GIRK2 would not only suppress the autoreceptor-mediated feedback inhibition of DA release but could also establish a feedforward mechanism of DA release in the terminal fields.
Keywords: substantia nigra, dopamine, baclofen, inwardly rectifying K+ channels, weaver mouse, electrophysiology, dopamine-related disorders
Weaver (wv) is an autosomic recessive mutation of the mouse G-protein-gated inwardly rectifying K+ channels (GIRK2) mainly associated to postnatal loss of the external granuli of the cerebellum (Rakic et al., 1973) and the dopaminergic cells of the ventral midbrain (Schmidt et al., 1982; Gupta et al., 1987; Triarhou et al., 1988; Smith et al., 1990). With regard to the dopaminergic neurons, it has been shown that they degenerate during the first 3 weeks of life in homozygous wv/wv mutant mice. However, the number of tyrosine hydroxylase (TH)-positive neurons in wv/wv and wild-type (+/+) midbrain is the same at birth (Bayer et al., 1995; Verney et al., 1995), suggesting that the wv gene exclusively targets and impairs postmitotic dopaminergic cells. Consequently, the wv/wv mouse has a lower level of dopamine (DA) in the brain than +/+ mice (Schmidt et al., 1982; Roffler-Tarlov and Graybiel, 1984; Triarhou et al., 1988) and represents a genetic animal model of nigrostriatal deficiency that could mimic the pathophysiology of Parkinson's disease (Simon and Ghetti, 1994).
The wv mutation is a single amino acid substitution (glycine 156 to serine) located in the gene encoding for GIRK2 (Patil et al., 1995). The functional GIRK channel consists of tetramers of five subunits (Kofuji et al., 1996), and only the GIRK1–GIRK3 subunits were found in the CNS. The GIRK channel is the functional target of many neurotransmitters (North, 1989; Liao et al., 1996; Sodickson and Bean, 1998) and regulates neuronal excitability, being permeable to potassium ions (Hille, 1992; Jan and Jan, 1994). Western blotting,in situ hybridization, and immunocytochemistry studies have shown that a strong GIRK2 signal is present in the dopaminergic neurons of the substantia nigra and ventral tegmental area, being the GIRK1 at the background level (Liao et al., 1996; Murer et al., 1997; Inanobe et al., 1999). When expressed in Xenopus oocytes, thewv GIRK2 displays three novel properties: (1) a loss of selectivity for potassium ions and gain of permeability for sodium (Slesinger et al., 1996; Tong et al., 1996) and/or calcium (Silverman et al., 1996; Tucker et al., 1996); (2) a constitutive activation that does not require G-proteins (Navarro et al., 1996); and (3) a blockade by a class of molecules (QX-314, MK-801, verapamil) that do not affect the wild GIRK. Although there is general agreement on the loss of K+ selectivity and on the pharmacology of the wv GIRK2, the gating properties and functional effects on native neurons appear to be very heterogeneous depending on the type of neurons investigated. It is not yet known whether the constitutive activation of the inwardly rectifying K+channels is inherent to the mutated wv GIRK2 channel protein or is an indirect consequence of other cellular properties (Silverman et al., 1996). For instance, the developmental stage ofwv/wv cerebellar granule cells determines the state of the G-protein modulation of the inwardly rectifying current and possibly its tonic activation (Kofuji et al., 1996; Slesinger et al., 1996, 1997; Surmeier et al., 1996; Rossi et al., 1998). On the other hand, the wv GIRK2 is neither tonically active nor G-protein-operated in CA3 hippocampal neurons (Jarolimek et al., 1998). Considering that GIRK2 tetramers are almost selectively present in the membrane of midbrain dopaminergic neurons in which they represent the common functional target of the inhibitory inputs mediated by dopamine D2 and GABAB receptors (Lacey et al., 1988; Kim et al., 1997; Inanobe et al., 1999), we have been interested in studying whether the wv mutation affects the electrical membrane properties and the response to dopamine and GABA of these neurons recorded in slices of mouse mesencephalon.
MATERIALS AND METHODS
Preparation of the tissue. Homozygous (wv/wv) weaver mice were obtained by breeding heterozygous +/wv mice (B6CBA) (Jackson Laboratories, Bar Harbor, ME). They were ∼25% of littermates according to simple mendelian segregation and were identified by their clearly visible motor dysfunction, tremor, and cerebellar atrophy (Rakic and Sidman, 1973). Horizontal slices comprising the substantia nigra and the ventral tegmental area were cut from the ventral mesencephalon of 16- to 20-d-old wv/wv, and +/+ mice were anesthetized with ketamine and killed. The method used has been described previously (Mercuri et al., 1994, 1997). The brain was rapidly removed, and the slices (200- to 250-μm-thick) were obtained by using a vibratome starting from the ventral surface of the midbrain. Slices recovered for at least 1 hr in a holding chamber and then were transferred into a recording chamber. They were completely submerged with a continuously flowing (2.5 ml/min) artificial CSF solution at 34–35°C, pH 7.4. This solution contained (in mm): NaCl 126, KCl 2.5, MgCl2 1.2, NaH2PO4 1.2, CaCl2 2.4, glucose 10, NaHCO3 18, gassed with 95% O2 and 5% CO2, pH 7.4.
Patch-clamp recordings. The recording chamber was mounted on the stage of an upright microscope (Axioscope FS; Zeiss, Oberkochen, Germany) equipped for an infrared video microscopy (Hamamatsu, Hamamatsu City, Japan). Individual dopaminergic neurons were visualized by infrared video imaging and approached by applying positive pressure. Pipettes were made from borosilicate glass (1.5 mm; World Precision Instruments, Sarasota, FL) and pulled with a vertical PP 83 Narishige (Tokyo, Japan) puller. They had a resistance of ≅4 MΩ when filled with a standard solution containing (in mm): K+ gluconate 145, CaCl2 0.1, MgCl2 2, HEPES 10, EGTA 0.75, Mg-ATP 2, and Na3-GTP 0.3, pH 7.3. In a subset of experiments, Na3-GTP was substituted with the nonhydrolizable analog GTP-γ-S (0.6 mm) or with GDP-β-S (0.6 mm). Whole-cell recordings were performed with an Axopatch 1D amplifier (Axon Instruments, Foster City, CA) and series resistances were compensated. Hyperpolarizing voltage steps were delivered from −60 to −120 mV (holding potential of −40 mV, 10 mV increments) and lasted 800 msec. Activation kinetics ofIh were calculated by fitting the current at −120 mV with a first-order exponential decay function.
Because of the presence of the largeIh current in the dopaminergic neurons (Grace and Onn, 1989; Lacey et al., 1989; Johnson and North, 1992;Mercuri et al., 1995, 1997), voltage ramps were preceded by a voltage step of 600 msec from the holding potential of −40 to −120 mV to fully activate the Ih current (see Fig. 4C). The membrane voltage and current were acquired using pClamp and Axioscope softwares (Axon Instruments); data analysis was performed using Origin software (Microcal, Northampton, MA). Cell-attached recordings were made after the giga seal was established, by measuring the number of spontaneous action potentials from the extracellular side of the membrane.
Fig. 4.
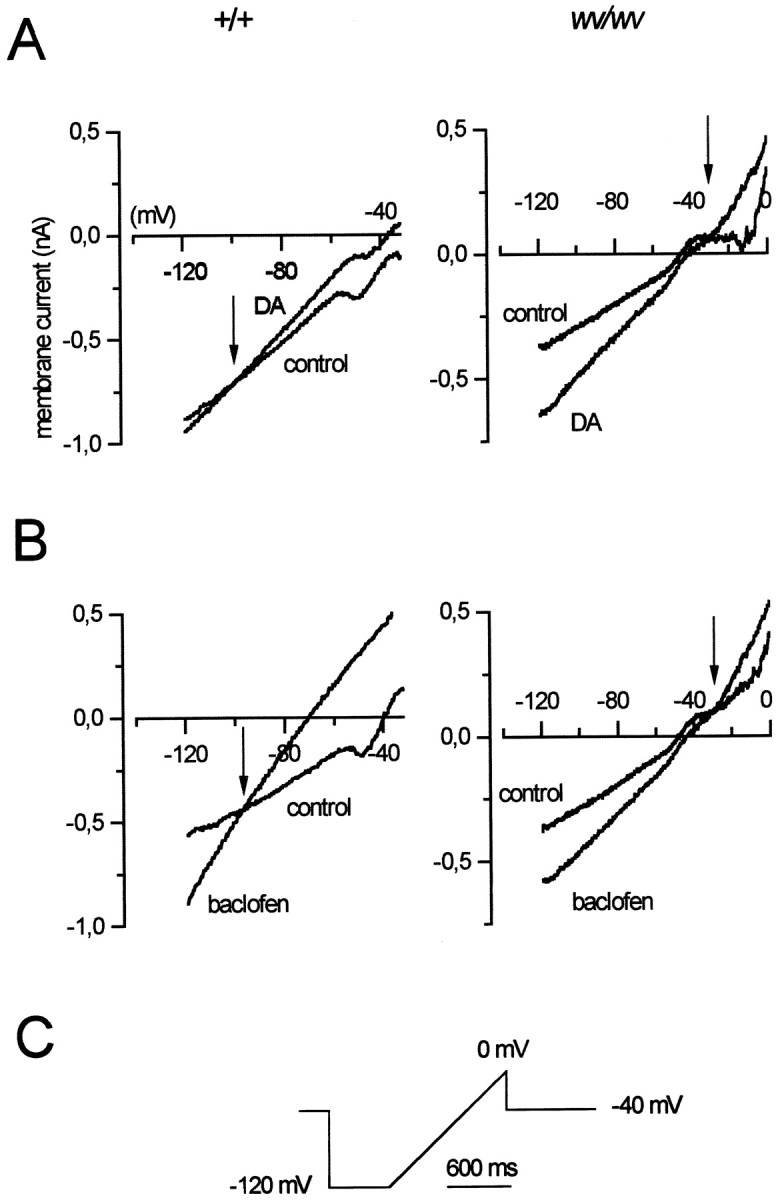
Properties of the DA- and baclofen-induced currents in wv/wv neurons. A, Voltage ramps (from −120 to 0 mV) delivered in control condition and in the presence of DA revealed that the DA-induced (100 μm) current reversed at −86 ± 6 mV (n = 4) in +/+ neurons (one cell is shown in the left), whereas it reversed at −27 ± 3.2 mV (n = 6) inwv/wv neurons (one cell is shown in theright). B, The baclofen-activated (10 μm) current reversed at negative potentials (−87 ± 3 mV, n = 4) in +/+ neurons (one cell is shown in the left), whereas it reversed at −34.1 ± 3.2 mV (n = 6) in wv/wv neurons (one cell is shown in the right). The current traces of thewv/wv neurons were recorded in the presence of TTX (0.5 μm), tetraethylammonium chloride (5 mm), and nifedipine (10 μm) to reduce voltage-dependent sodium, potassium, and calcium conductances. C shows the protocol used to induce the slow depolarizing ramps.
Drug application. Drugs were bath-applied by switching the superfusing solution to one containing a known concentration of drugs. Full exchange of the solution in the recording chamber was achieved within 1 min. l-sulpiride was from Ravizza, CGP 55845A and R-baclofen were from Novartis Pharma Ag (Basel, Switzerland), QX-314 was purchased from Alomone Labs (Jerusalem, Israel), and ZD 7288 was from Tocris Cookson (Bristol, UK). Dopamine, GTP-γ-S, and GDP-β-S were from Sigma (Milano, Italy). In voltage-clamp, experiments we used higher DA concentration to evoke maximal current amplitudes.
Immunohystochemistry. Biocytin (free base, 5 mm; Sigma) was added to the pipette solution to perform post hoc labeling of the recorded cells with TH. Immediately after recording, slices containing biocytin-loaded cells were fixed by immersion in 4% paraformaldehyde in 0.1m PBS overnight at +4°C. The tissue was subsequently immersed in 20% sucrose–10% glycerol in 0.1m PBS for 3 hr at room temperature for cryoprotection. Slices were then frozen and cut to 40-μm-thick sections by a sliding microtome. Sections were incubated with a cocktail of avidin-conjugated fluorescein isothiocyanate (FITC) (1:200; Sigma) and anti-mouse tyrosine hydroxylase monoclonal antibody (1:200) in PBS containing 0.1% Triton X-100 overnight at +4°C. After 10 min rinses in 0.1 m PBS, sections were incubated in a mixture of avidin-conjugated FITC (1:200) and tetramethyl rhodamine (TRITC) (Sigma) -conjugated goat anti mouse IgG 1:50 in PBS containing 0.1% Triton X-100 for 3 hr at room temperature. Sections were then rinsed three times for 10 min in 0.1 m PBS, mounted on slides, and coverslipped with 50% glycerol in PBS. Slides were observed with an epi-illumination fluorescence microscope (Zeiss) and with a confocal laser-scanning microscope (Zeiss LSM 510).
Statistical analysis. The data were presented as mean ± SEM. Statistical difference was determined by paired or unpaired Student's t test at a significance level of 0.05.
RESULTS
Intrinsic properties of native and mutated dopaminergic neurons
To identify the dopaminergic neurons among a heterogeneous population of cells, we considered the electrophysiological parameters already established as “typical” for these cells (Grace and Onn, 1989; Lacey et al., 1989: Johnson and North, 1992; Mercuri et al., 1995, 1997; Richards et al., 1997). In fact, we evaluated the presence of a strong hyperpolarization-activated inward current (Ih), the ability to fire spontaneously in a pacemaker manner, and the value of the resting membrane potential of the presumed dopaminergic neurons from both +/+ and homozygous wv/wv mice. Hyperpolarizing voltage steps (Fig. 1A) from −60 to −120 mV (Vh of −40 mV) evoked an inward current (Ih) in both +/+ and wv/wv neurons. The mean current amplitudes, measured at −120 mV, were 321 ± 46 pA (n = 10) in +/+ and 490 ± 46 pA (n = 16) (unpaired t test,p < 0.05) in wv/wv neurons. Activation kinetics at −120 mV were 145 ± 9 msec in +/+ (n= 10) and 116 ± 8 msec (n = 16) (unpairedt test, p < 0.05) in wv/wvdopaminergic neurons, respectively. Under current-clamp recordings (Fig. 1B), depolarizing current steps elicited regularly firing action potentials, whereas hyperpolarizing pulses activated a characteristic “sag” potential in both +/+ (n = 10) and wv/wv (n = 16) neurons. The mean resting membrane potential (Fig. 1C,left) (holding potential at 0 current) was −47.6 ± 1.5 mV in +/+ (n = 17) and −47.1 ± 1.3 mV inwv/wv (n = 18) (unpaired t test,p = 0.81) neurons. The mean values of the spontaneous firing (Fig. 1C, right) were 2.1 ± 0.12 Hz (n = 7) in +/+ and 2.4 ± 0.2 Hz (n = 15) (unpaired t test, p= 0.35) in wv/wv cells. The apparent input resistance (measured by a small 5 mV hyperpolarizing step) was 410 ± 28 (n = 18) and 515 ± 81 (n = 7) MΩ (unpaired t test, p = 0.12) in +/+ andwv/wv cells, respectively.
Fig. 1.
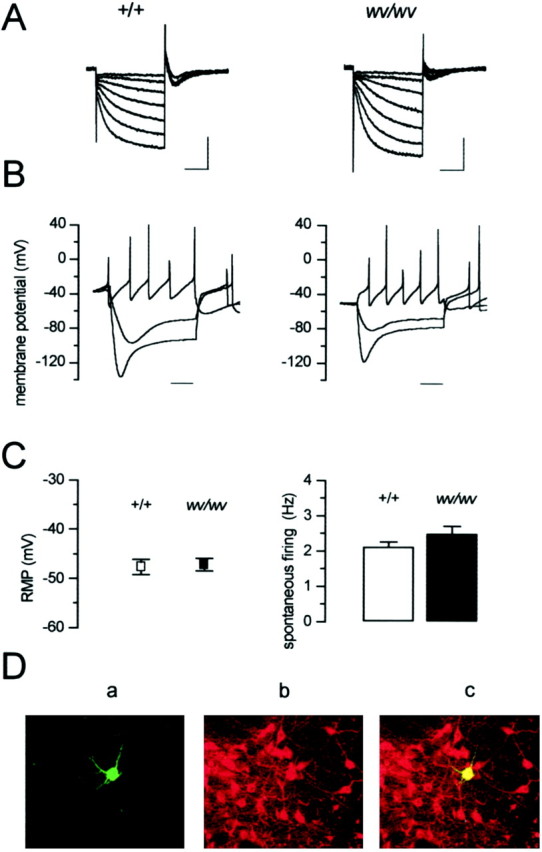
Electrophysiological and immunohistochemical identification of midbrain dopaminergic neurons in +/+ andwv/wv mice. A, Hyperpolarizing voltage steps from −60 to −120 mV (10 mV increments,Vh of −40 mV) activated the mixed cation current (Ih) in both genotypes. Calibration: 250 msec, 500 pA. B, The corresponding current-clamp recording showed that hyperpolarizing pulses (−0.5 and −1 nA) activated the Ih current, thus producing a typical sag potential in both cells taken form the two genotypes. A depolarizing current pulse (0.5 nA) elicited a pacemaker-like sequence of action potentials in both neurons. Action potential amplitudes are clipped because of low sampling rate of the digital interface. Time bar: 100 msec. C,Left, The white square indicates the mean resting membrane potential (RMP) of +/+ dopaminergic cells (−47.6 ± 1.5 mV, n = 17), and theblack square indicates the mean resting membrane potential of wv/wv cells (−47.1 ± 1.3 mV,n = 18); values were not significantly different (p = 0.81). Right, Thecolumns indicate the mean spontaneous firing recorded in cell-attached configuration in +/+ (white) (2.1 ± 0.12 Hz, n = 7) and wv/wv(black) (2.4 ± 0.2 Hz, n = 15) neurons; values were not significantly different (p = 0.35). D, Confocal laser scanning microscope image of a wv/wv neuron loaded with biocytin (5 mm) through the patch pipette showing typical features of a dopaminergic neuron (magnification, 20×):a, biocytin staining as revealed by FITC fluorescence;b, TH immunostaining as revealed by TRITC fluorescence (note that many neurons, including the recorded one, resulted in being TH-positive, within the SNc); c, merged image of the two fluorescent stainings.
Furthermore, we loaded some wv/wv cells showing the electrophysiological properties delineated above with biocityn (5 mm), and we immunostained them with antibodies against TH. Nine of 10 neurons were TH-positive (Fig.1D). Based on these observations, only neurons showing a pronounced Ih and a spontaneous pacemaker activity at rate of ∼2 Hz were considered to be dopaminergic and included in the present study.
Activation of D2 and GABAB receptors mediates excitation of wv/wv dopaminergic neurons
It is well known that dopamine and GABA, by acting on D2 and GABAB receptors, inhibit the firing activity of dopaminergic neurons (Lacey et al., 1988). To test the effects of dopamine on wv/wv dopaminergic neurons without perturbing the intracellular environment with the standard whole-cell configuration, we measured the rate of the spontaneous action potentials in cell-attached mode, in control condition and during the superfusion of dopamine (30 μm) (Fig.2A). Interestingly DA increased the firing frequency of wv/wv neurons from 2.3 ± 0.3 (n = 11) to 4.3 ± 0.4 (n = 11) Hz (paired t test,p < 0.01), whereas it abolished the spontaneous activity of the +/+ neurons (n = 4). In whole-cell current-clamp configuration (Fig. 2B), thewv/wv dopaminergic neurons were depolarized by DA (30 μm), and the number of the action potentials increased (n = 7). In contrast, DA (30 μm) hyperpolarized the membrane and abolished the spontaneous activity of +/+ neurons (n = 4) (Lacey et al., 1988). Whole-cell voltage-clamp experiments (at −40 mV holding potential) showed that the excitatory effect of DA on wv/wvneurons is attributable to the activation of an inward current (58.3 ± 13 pA, n = 7) (Fig. 2C). On the contrary, the inhibitory effect of DA in +/+ neurons is attributable to the activation of an outward current that is caused by the opening of GIRK channels (Lacey et al., 1988; Kim et al., 1997).
Fig. 2.
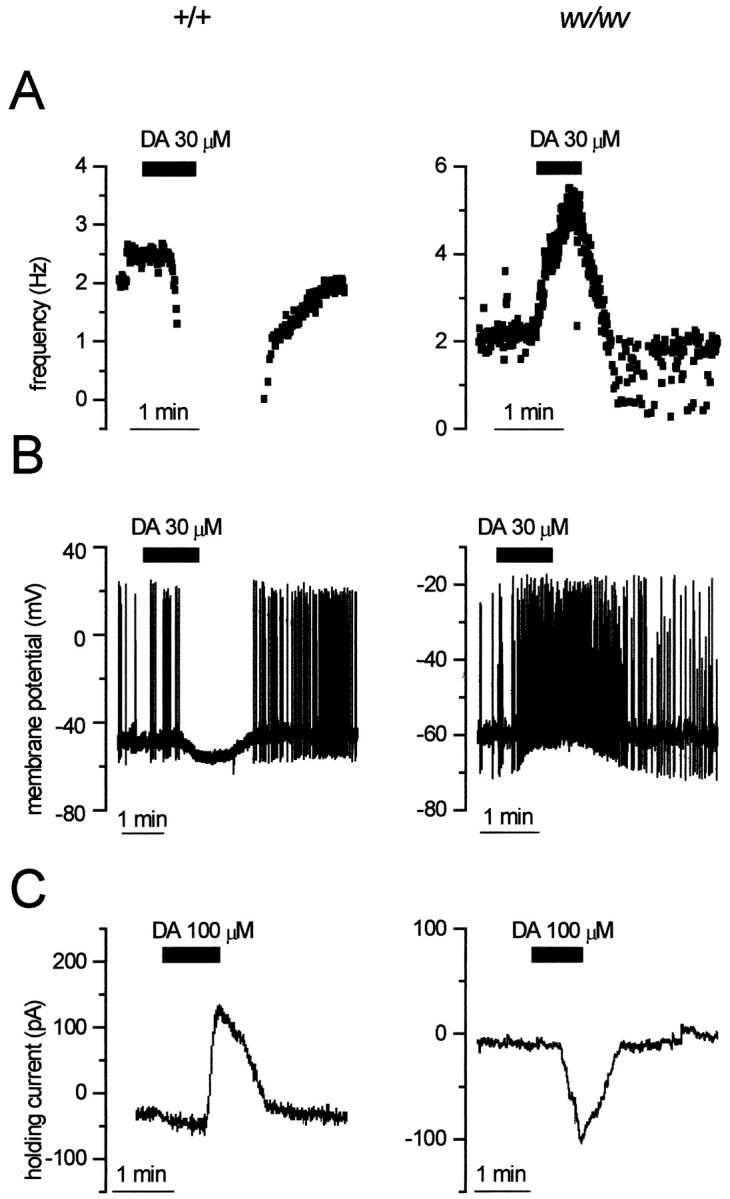
DA mediates inhibition of +/+ and excitation ofwv/wv neurons. A, Cell-attached recordings from a +/+ (left) and wv/wv(right) cell showing the changes of spontaneous firing during the extracellular application of DA (30 μm). DA clearly inhibited the firing of the +/+ neuron, whereas it increased the activity of the wv/wv neuron. B, Whole-cell current-clamp recordings of two dopaminergic cells in which DA caused membrane hyperpolarization–inhibition (+/+) and depolarization–excitation (wv/wv). C, Voltage-clamp recordings (at Vh of −40 mV) showing the activation of outward (+/+) and inward (wv/wv) currents caused by DA (100 μm) in +/+ and wv/wv dopaminergic cells, respectively.
An excitation of wv/wv dopaminergic neurons was also observed when they were exposed to the GABABreceptor agonist baclofen (Fig. 3). In fact, in cell-attached configuration (Fig. 3A), baclofen (10 μm) increased the spontaneous activity ofwv/wv neurons from 2.8 ± 0.4 (n = 11) to 5.5 ± 0.5 (n = 11) Hz (paired ttest, p < 0.01), whereas it depressed the spontaneous firing in +/+ neurons (n = 4). In whole-cell current-clamp mode, baclofen mimicked the effects of DA, mediating a depolarization–excitation of wv/wv neurons (n = 7) (Fig. 3B) and a hyperpolarization of +/+ neurons (n = 4). In voltage-clamp recordings, baclofen (10 μm) activated an inward current of 57.5 ± 10.8 pA (n = 7) in wv/wvneurons (Fig. 3C). Conversely, baclofen inhibited the +/+ dopaminergic neurons, activating a classical GIRK-mediated outward current (n = 4) (Lacey et al., 1988).
Fig. 3.
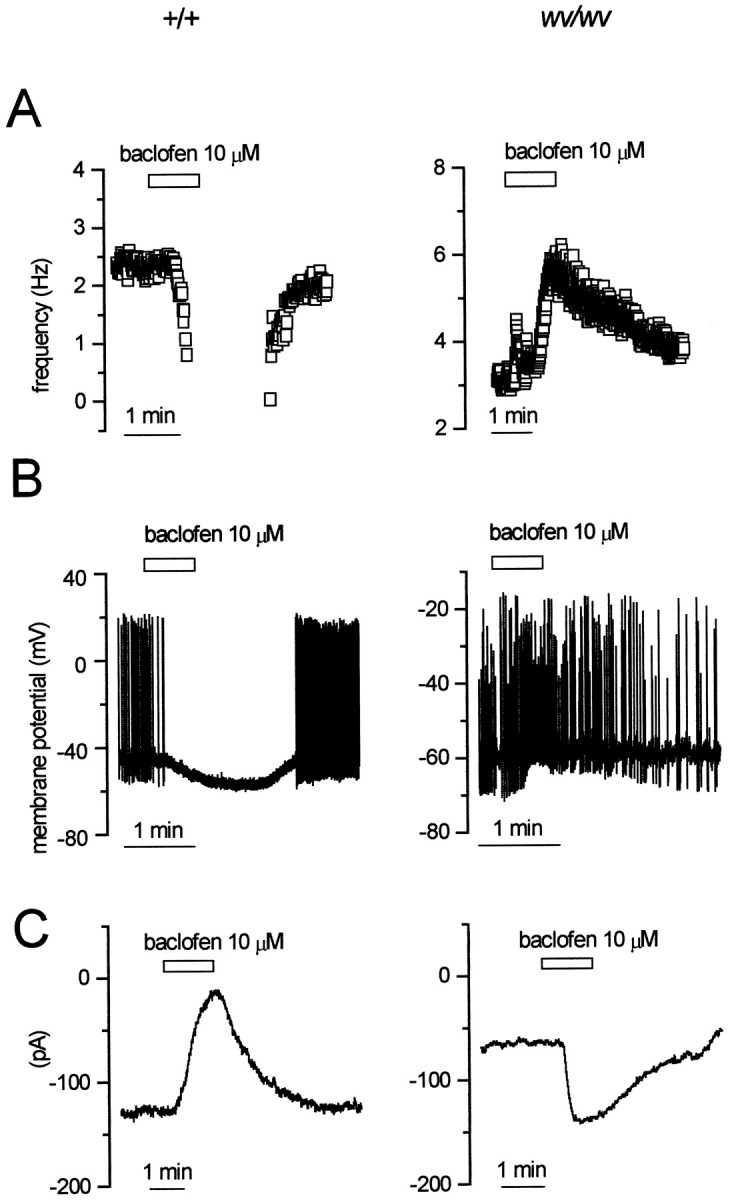
The GABAB agonist baclofen mediates inhibition of +/+ and excitation of wv/wv neurons.A, Cell-attached recordings from a +/+ (left) and a wv/wv (right) neuron showing the modification of the spontaneous firing caused by the GABAB receptor agonist baclofen (10 μm). Note the increase in firing frequency induced by this compound in thewv/wv neuron. B, Whole-cell current-clamp recordings showing the modification in membrane potential and firing activity in +/+ and wv/wv neurons. C, Corresponding voltage-clamp recordings (atVh of −40 mV) showing the changes in membrane current caused by baclofen in a +/+ versus awv/wv neuron. Note that, like dopamine, baclofen activated an inward rather than outward current in wv/wvneurons.
Properties of the dopamine- and baclofen-induced inward current
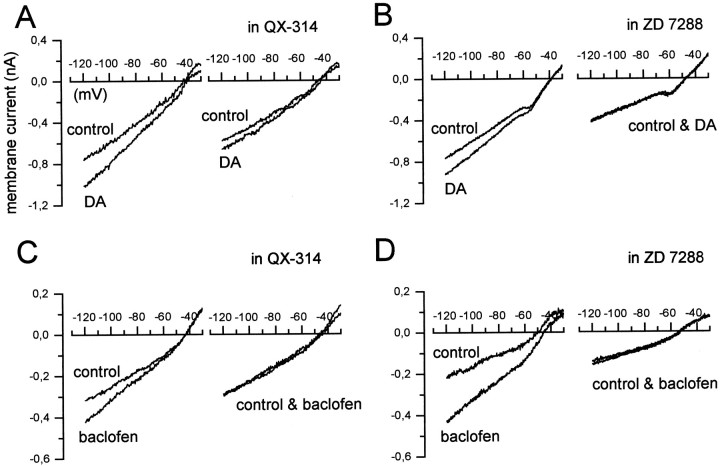
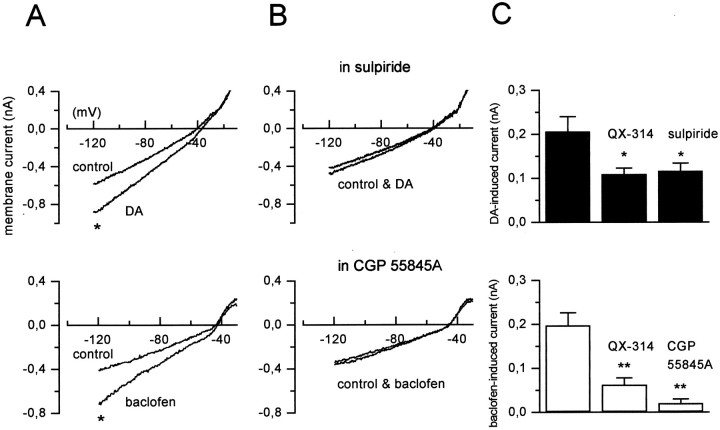
To characterize the I–V relationships of the dopamine- and baclofen-induced inward currents, we performed voltage ramps over a wide range of potentials, between −120 and 0 mV (Fig.4C). The ramps were delivered in control condition and in the presence of DA (100 μm) (Fig. 4A) or baclofen (10 μm) (Fig. 4B). The currents activated by both agonists in wv/wv neurons showed different characteristics compared with those induced in +/+ cells. Indeed, as expected for an increase of a pure potassium conductance (Lacey et al., 1988), the DA- and baclofen-induced currents crossed the control trace around EK at −86 ± 6 (n = 4) and −87 ± 3 (n = 4) mV, respectively, in +/+ neurons. Instead, the DA- and baclofen-induced currents crossed the control trace at −27 ± 3.2 (n = 6) and −34 ± 3.2 (n = 6) mV, respectively, in wv/wv neurons. These values of reversal potential suggest that a cation current is involved in the neurotransmitter-mediated excitation of the wv/wvdopaminergic neurons. Because the Ihis a mixed cationic current, we tested the possibility that both D2 and GABAB receptors could modulate Ih to produce excitation. In the presence of cesium (1–3 mm), a known blocker of Ih, the actions of dopamine and baclofen were not modified (n = 3; data not shown). Conversely, the antiarrhythmic drug ZD 7288 (50 μm), which is also a potent inhibitor ofIh, irreversibly depressed the DA-induced (n = 6) and baclofen-induced (n = 6) inward currents (Fig.5B,D). Furthermore, we tested a more classical cationic blocker, QX-314 (100 μm), on the neurotransmitter-induced inward currents. As already reported in Xenopus oocytes expressing the wv GIRK2 (Kofuji et al., 1996), this drug inhibited the excitatory effects of DA (Figs. 5A,6C) (n = 7) and baclofen (n = 6) (Figs. 5C,6C) on the dopaminergic wv/wv cells without modifying the receptor-operated outward currents in +/+ neurons (data not shown). Furthermore, QX-314 did not change the spontaneous activity and the membrane properties of the wv/wv dopaminergic cells (n = 3 of 3 cells tested; data not shown). The activation of wv GIRK2 by dopamine and baclofen was inhibited by the D2 and GABAB receptor antagonists sulpiride and CGP 55845A (Fig. 6B). In fact, the mean amplitudes of the inward current activated by dopamine and baclofen at −120 mV in control conditions, in the presence of QX-314 (100 μm), sulpiride (3–10 μm), and CGP 55845A (250–500 nm), are shown in Figure 6C. The DA-induced current of 207 ± 33 pA (n = 7) was significantly reduced to 111 ± 13 pA (n = 7) (paired t test, p < 0.05) in the presence of QX-314 and to 117 ± 17 pA (n = 7) (pairedt test, p < 0.05) in the presence of sulpiride (10 μm) (Fig. 6C,top).
Fig. 5.
The cation channel blockers QX-314 and ZD 7288 inhibit the DA- and baclofen-induced inward currents inwv/wv neurons. A, B, The DA-induced (100 μm) current was strongly inhibited by QX-314 (100 μm) and ZD 7288 (50 μm).C, D, QX-314 (100 μm) and ZD 7288 (50 μm) also inhibited the current induced by baclofen (10 μm). Note that the protocol of the voltage ramps in this and the following figures is the same shown in Figure4C.
Fig. 6.
D2 and GABAB receptor antagonists reduce the DA- and baclofen-induced inward currents.A, The inward current induced by both agonists inwv/wv dopaminergic neurons was inhibited by the presence of sulpiride (10 μm; B,top) and CGP 55845A (250 nm;B, bottom). C, The plots show the mean values of DA-induced (top, black columns) and baclofen-induced (bottom,white columns) inward currents at the points indicated by asterisks in A (−120 mV). The DA-induced inward current was 207 ± 33 pA (n= 7; left bar) and was significantly reduced by QX-314 (100 μm) to 111 ± 13 pA (paired ttest, p < 0.05; middle bar) and by sulpiride (10 μm) to 117 ± 17 pA (pairedt test, p < 0.05; right bar). The baclofen-induced inward current of 198 ± 28 pA (n = 6; left bar) was significantly reduced by QX-314 (100 μm) to 63 ± 15 (pairedt test, p < 0.01; middle bar) and by CGP 55845A (250 nm) to 21 ± 8 pA (paired t test, p < 0.01;right bar).
The baclofen-induced current of 198 ± 28 pA (n = 6) was significantly reduced to 63 ± 15 nA (n = 6) (paired t test, p < 0.01) by QX-314 and to 21 ± 8 pA (n = 6) (paired t test,p < 0.01) by CGP 55845A (250 nm) (Fig. 6C, bottom).
D2 and GABAB receptors activatewv GIRK channels in a G-protein-dependent manner
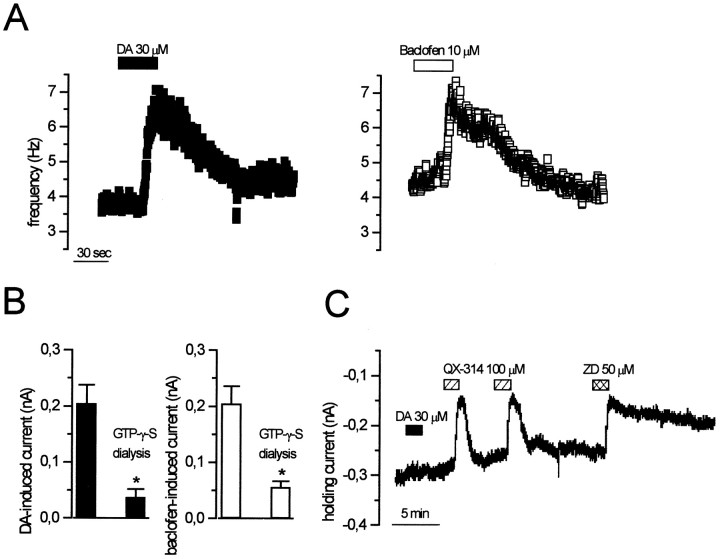
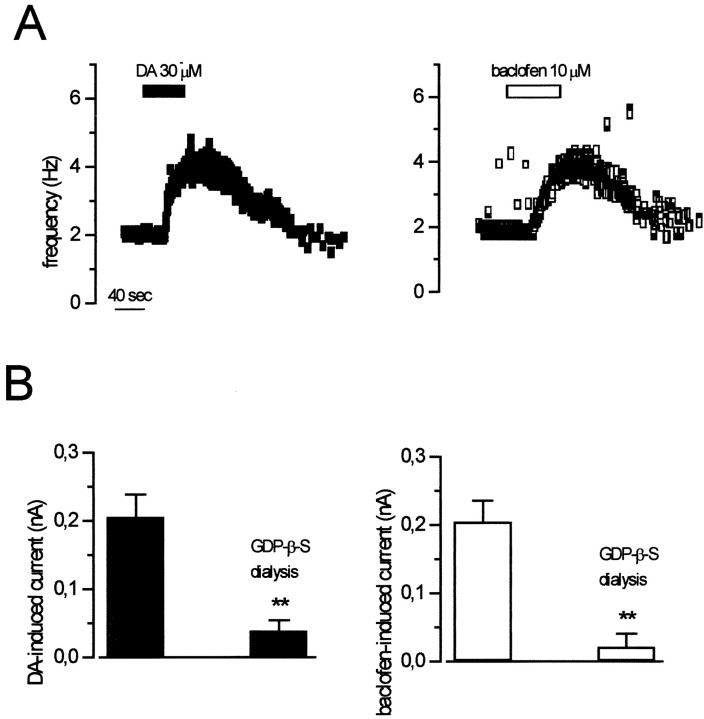
In heterologous expression systems, wv GIRK2 channels not only loose the potassium selectivity but also become constitutively active, being unable to interact with the G-protein (Kofuji et al., 1996, Slesinger et al., 1996). In cells of the CNS, different gating properties of wv GIRK2 have been reported; in fact, some neurons had no functional wv GIRK2 channels (Surmeier et al., 1996; Jarolimek et al., 1998), and others underwent a constitutive activation of GIRK2 (Rossi et al., 1998). To investigate the G-protein dependency of the receptor-operated wv GIRK2, we dialyzed the cytoplasm with a nonhydrolizable GTP analog, GTP-γ-S (0.6 mm). Before breaking the membrane patch for the whole-cell recordings, we tested the DA and baclofen sensitivity of thewv/wv neurons by measuring their firing rate in cell-attached configuration. Both DA (n = 5) and baclofen (n = 5) increased the spontaneous firing of the wv/wv neurons (Fig.7A). Five to 10 min after the membrane rupture, a constitutive inward current developed and reached a plateau of −74 ± 22 pA (n = 5; holding potential at −40 mV). The mean amplitudes of DA- and baclofen-induced currents, at −120 mV, in wv/wv cells loaded with the pipette solution containing GTP-Na3 (control) or GTP-γ-S were significantly different (Fig. 7B). In fact, the DA-induced current of 204 ± 30 pA (n = 10) in GTP-Na3-loaded neurons was reduced to 38 ± 14 pA (n = 5) (unpaired t test,p < 0.05) in GTP-γ-S-loaded neurons. The baclofen-induced current of 205 ± 40 pA (n = 6) in GTP-Na3-loaded neurons was diminished to 57 ± 10 pA (n = 5) (unpaired t test,p < 0.05) in GTP-γ-S-loaded cells. Moreover, we found that the inward current tonically activated by GTP-γ-S (Fig.7C) was reduced in a reversible manner by QX-314 (100 μm) (n = 5) and irreversibly by ZD 7288 (50 μm) (n = 5). These results strongly suggest that the inward current induced by GTP-γ-S is the same as the one generated by DA and baclofen. On the other hand, whereas DA (n = 5) and baclofen (n = 5) excited the wv/wv dopaminergic neurons in cell-attached configuration (Fig.8A), the subsequent intracellular dialysis with the nonhydrolizable GDP analog GDP-β-S (0.6 mm) prevented DA and baclofen effects within a few minutes after membrane rupture (Fig. 8B). In fact, the mean amplitudes of DA-induced current (at −120 mV) of 204 ± 30 pA (n = 10) in GTP-Na3-loaded neurons was significantly reduced to 39 ± 15 pA (n = 5) (unpaired ttest, p < 0.01) in GDP-β-S-loaded neurons. Furthermore, the baclofen-induced currents (at −120 mV) of 205 ± 40 pA (n = 6) in GTP-Na3-loaded neurons was reduced to 21 ± 19 pA (n = 5) (unpaired t test, p < 0.01) in GDP-β-S-loaded neurons.
Fig. 7.
D2 and GABABreceptors couple to wv GIRK2 in a G-protein-dependent manner. A, Recordings of the spontaneous firing of twowv/wv neurons in cell-attached configuration with a pipette solution containing the GTP analog GTP-γ-S (0.6 mm). Note that both DA (left) and baclofen (right) induced an increase of the spontaneous firing before the rupture of the membrane patches. B, Theblack columns show the amplitude of the DA-induced current (at −120 mV) (204 ± 30 pA, n= 10) in control conditions and during the dialysis with GTP-γ-S (38 ± 14 pA, n = 5) (p < 0.05, unpaired data). The white columns show the amplitude of the baclofen-induced current (at −120 mV) (205 ± 40 pA, n = 6) in control condition and during the intracellular dialysis with GTP-γ-S (57 ± 10, n = 5) (p < 0.05, unpaired data). C, GTP-γ-S induced a tonic inward current that was reversibly blocked by QX-314 and irreversibly inhibited by ZD 7288. Note that, under this condition, the response to DA application was not observed.
Fig. 8.
GDP-β-S prevents the DA- and baclofen-induced inward currents. A, Cell-attached recordings of twowv/wv neurons with a pipette containing the GDP analog GDP-β-S (0.6 mm) showing the increase of the spontaneous firing caused by DA and baclofen. B,Left, black columns, In whole-cell configuration, the DA-induced current (at −120 mV) was 204 ± 30 pA (n = 10) and was significantly reduced by GDP-β-S to 39 ± 15 pA (n = 5,p < 0.01, unpaired data). Right,white columns, The baclofen-induced current (at −120 mV) was 205 ± 40 pA (n = 6) and was significantly reduced by GDP-β-S to 21 ± 19 pA (n = 5, p < 0.01, unpaired data).
DISCUSSION
The present study demonstrates that the wvmutation reverses the functional effects (inhibition into excitation) mediated by activation of dopamine D2 and GABAB receptors in dopaminergic neurons of the ventral midbrain (identified physiologically and by TH immunoreactivity) and provides the first evidence of a D2- and GABAB-mediated excitation in native neurons of the CNS.
The membrane properties of wv/wv dopaminergic neurons are not affected by the mutation
There is a general agreement that the wv GIRK2 looses selectivity for potassium ions. In fact, the permeability and gating properties of the wv GIRK2 have been extensively investigated in heterologous expression systems in which it has been shown that the mutated channel becomes permeable to sodium (Navarro et al., 1996; Slesinger et al., 1996) and eventually to calcium ions (Silverman et al., 1996; Tucker et al., 1996). In addition, this channel appears to be constitutively opened (Kofuji et al., 1996). Consequently, there is a leakage entry of sodium into the cells that can be reduced by channel blockers such as QX-314, MK-801, and verapamil. A constitutive activation of the wv GIRK2 was also observed in cultured cerebellar granule cells (Kofuji et al., 1996) and in putative granule cells in the postmigratory position inwv/wv cerebellar slices (Rossi et al., 1998). A similar constitutively opened wv GIRK2 conductance, sensitive to QX-314, has been described recently in substantia nigrawv/wv neurons (Liss et al., 1999). In fact, the dopaminergic cells were found to be tonically depolarized, having no pacemaker activity. Among the surviving cells within the substantia nigra compacta (SNc) of the wv/wv mice, we found a cell population whose resting potential, apparent input resistance, and spontaneous firing discharge were indistinguishable from those of the dopaminergic neurons recorded in +/+ mice. Thus, our results strongly suggest that the mutated GIRK2 channels do not regulate the resting properties of native wv/wv dopaminergic neurons. This is supported by the following arguments: (1) a depolarized resting membrane potential, a decrease in input resistance, and a loss of spontaneous pacemaker activity should be expected if a tonic wv inward conductance is present; and (2) the spontaneous firing activity should be affected by the wv GIRK2 blocker QX-314 if the channel is operative during the resting state of the neurons.
The membrane responses of dopaminergic neurons to dopamine and GABA are modified by the wv mutation
Among the characteristics that identify the dopaminergic neurons in the ventral midbrain, an inhibitory response to dopamine is an important criterion (Mercuri et al., 1992; White, 1996). Inwv/wv, mice we found neurons displaying spontaneous firing, resting membrane potential, apparent input resistance, and firing rate comparable with the ones recorded in wild animals. A more pronouncedIh (Mercuri et al., 1995) was also found in wv/wv cells. This might depend on either compensatory changes of the hyperpolarization-activated channels or a reduced apparent inhibition caused by DA and GABA (Watts et al., 1996). Interestingly, the cellular responses to dopamine and the GABAB agonist baclofen were opposite to those observed in control animals. In fact, both dopamine and baclofen, instead of inhibiting the wv/wv dopaminergic cells by activating a G-protein-dependent outward current (Lacey et al., 1988;Kim et al., 1997), caused an excitation that was generated by an inward current. The obtained null potential (approximately −30 mV) and the sensitivity of this current to cationic channel blockers support the hypothesis that DA and baclofen activate wv GIRK2 channels, which are permeable to sodium and potassium ions (Kofuji et al., 1996). The loss of selectivity of the wv GIRK2 to potassium ions has been already demonstrated in other systems expressing the homomericwv GIRK2 (Kofuji et al., 1996; Navarro et al., 1996;Slesinger et al., 1996). Nevertheless, it is worth mentioning that the responses to baclofen were lost in wv/wv hippocampal (Jarolimek et al., 1998) and cerebellar granule cells (Slesinger et al., 1997). The lack of neurotransmitter action in these cells without a gain of function might depend on the compensatory effect of other GIRK family members (Hou et al., 1999).
It is noteworthy that the neurotransmitter-operated wv GIRK2 is not only sensitive to QX-314 but also to the bradycardic agent ZD 7288, a blocker of the mixed sodium/potassium currentIh. This observation rises the possibility that dopamine and baclofen might modulate theIh in the wv/wvdopaminergic cells. However, this is not the case because, when theIh was blocked by extracellular cesium (Mercuri et al., 1995), dopamine and baclofen still induced an inward current in the wv/wv dopaminergic cells. Moreover, it appears that the inward current caused by dopamine and baclofen is attributable to the activation of D2 and GABAB receptors, respectively. In fact, antagonists such as sulpiride (D2) and CGP 55845A, (GABAB) specifically inhibited the effects of these neurotransmitters. The observation that the DA-induced inward current was not completely blocked by sulpiride and QX 314 suggests that this catecholamine might have additional non-D2, non-GIRK2 actions. Further experiments will be necessary to address this problem.
An additional important conclusion of our results is that the neurotransmitter activation of the mutated GIRK2 channel uses, as in control condition, a G-protein. Indeed, intracellular dialysis with GTP-γ-S or GDP-β-S prevented the neurotransmitter-mediated inward currents. In agreement with the involvement of a G-protein in the activation of the wv GIRK2, the intracellular dialysis of the nonhydrolizable analog of GTP, GTP-γ-S, caused a sustained inward current, which determined membrane depolarization, loss of spontaneous activity, and occluded DA- and baclofen-induced responses. The fact that the inward current caused by GTP-γ-S was reversibly inhibited by QX-314 and irreversibly blocked by ZD 7288 also suggests that it is produced by G-protein-regulated opening of the wv GIRK2 channels.
Conclusions
In the present paper, we provide evidence that wv GIRK2 are specifically operated by D2 and GABAB receptors in a G-protein-dependent manner to induce depolarization of native dopaminergic neurons. It is likely that the aberrant receptor-activated cationic entry throughout thewv GIRK2 could result in activity-dependent cell death, attributable to severe depolarization and sodium/calcium accumulation. Moreover, the presence of synaptic excitation instead of a K+-dependent inhibition failing to counterbalance the depolarizing drive sustained by the release of excitatory amino acids (Jensen et al., 1999) certainly contributes to initiate sodium/calcium-dependent intracellular processes, which lead to degeneration of wv/wv dopaminergic cells (Bertolino and Llinas, 1992; Choi, 1995; Verney et al., 1995; Oo et al., 1996). Although the mechanisms producing neuronal degeneration are not known yet, our results suggest that they can be related to the tone of dopamine and GABA in the ventral mesencephalon that regulates a deviate function of the wv GIRK2 channels (Liao et al., 1996). In addition, the mutation of the GIRK2 channel, by reversing the functional effects of dopamine, would not only impair the autoreceptor-mediated control of neurotransmitter release but also enhance, with a feedforward mechanism, the level of this catecholamine in the terminal fields. The altered behavior of the spared dopaminergic cells, as a consequence of DA autoreceptors stimulation, could account for the enhanced fractional release of dopamine in the striatum ofwv/wv mice (Richter et al., 1995). Thus, the observed hyperactivity of the wv/wv mice could, at least in part, depend on the dysfunction of the autoreceptor-mediated control of the dopaminergic neurons.
Footnotes
This work was supported by Telethon Grant E.747 to N.B.M. We thank Dr. Marco Molinari and Dr. Maria Teresa Viscomi for their advice in hystochemistry experiments, Dr. Nicola Berretta for help in the discussion, and Mauro Federici for technical assistance.
Correspondence should be addressed to Dr. Nicola B. Mercuri, Laboratory of Experimental Neurology, Fondazione Santa Lucia, Istituto di Ricorvero e Cura a Carattere Scientifico, Via Ardeatina 306, 00179 Rome, Italy. E-mail: mercurin@med.uniroma2.it.
REFERENCES
- 1.Bayer SA, Willis KV, Triarhou LC, Verina T, Thomas JD, Ghetti B. Selective vulnerability of late-generated dopaminergic neurones of the substantia nigra in weaver mutant mice. Proc Natl Acad Sci USA. 1995;92:9137–9140. doi: 10.1073/pnas.92.20.9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertolino M, Llinas RR. The central role of voltage-activated and receptor-operated calcium channels in neuronal cells. Annu Rev Pharmacol Toxicol. 1992;32:399–421. doi: 10.1146/annurev.pa.32.040192.002151. [DOI] [PubMed] [Google Scholar]
- 3.Choi DW. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- 4.Grace A, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurones recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta AM, Felten DL, Ghetti B. Selective loss of monoaminergic neurones in the weaver mutant mice: an immunocytochemical study. Brain Res. 1987;402:379–382. doi: 10.1016/0006-8993(87)90050-3. [DOI] [PubMed] [Google Scholar]
- 6.Hille B. Ionic channels of excitable membranes. Ed 2. Sinauer; Sunderland, MA: 1992. [Google Scholar]
- 7.Hou P, Yan S, Tang W, Nelson DJ. The inwardly rectifying K+ channel submit GIRK1 rescues the GIRK2 weaver phenotype. J Neurosci. 1999;19:8327–8336. doi: 10.1523/JNEUROSCI.19-19-08327.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inanobe A, Yoshimoto Y, Horio Y, Morishige KI, Hibino H, Matsumoto S, Tokunaga Y, Maeda T, Hata Y, Takai Y, Kurachi Y. Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci. 1999;19:1006–1017. doi: 10.1523/JNEUROSCI.19-03-01006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jan LY, Jan YN. Potassium channels and their evolving gates. Nature. 1994;371:119–122. doi: 10.1038/371119a0. [DOI] [PubMed] [Google Scholar]
- 10.Jarolimek W, Bäurle J, Misgeld U. Pore mutation in a G-protein-gated inwardly rectifying K+ channel subunit causes loss of K+-dependent inhibition in weaver hippocampus. J Neurosci. 1998;18:4001–4007. doi: 10.1523/JNEUROSCI.18-11-04001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen P, Surmeier DJ, Goldowitz D. Rescue of cerebellar granule cells from death in weaver NR1 double mutants. J Neurosci. 1999;19:7991–7998. doi: 10.1523/JNEUROSCI.19-18-07991.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol (Lond) 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KM, Nakajima S, Nakajima Y. Dopamine and GABA receptors in cultured substantia nigra neurons: correlation of electrophysiology and immunocytochemistry. Neuroscience. 1997;78:759–769. doi: 10.1016/s0306-4522(96)00585-4. [DOI] [PubMed] [Google Scholar]
- 14.Kofuji P, Hofer M, Millen KJ, Millonig JH, Davidson N, Lester HA, Hatten ME. Functional analysis of the weaver mutant GIRK2 K+ channel and rescue of weaver granule cells. Neuron. 1996;16:941–952. doi: 10.1016/s0896-6273(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 15.Lacey MG, Mercuri NB, North RA. On the potassium conductance increase activated by GABAB and D2 receptors in rat substantia nigra neurones. J Physiol (Lond) 1988;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989;9:1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao YJ, Jan YN, Jan LJ. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci. 1996;16:7137–7150. doi: 10.1523/JNEUROSCI.16-22-07137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liss B, Neu A, Roeper J. The weaver mouse gain-of-function phenotype of dopaminergic neurons is determined by coactivation of wvGIRK2 and K-ATP channels. J Neurosci. 1999;19:8839–8848. doi: 10.1523/JNEUROSCI.19-20-08839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercuri NB, Calabresi P, Bernardi G. The electrophysiological actions of dopamine and dopaminergic drugs on neurones of the substantia nigra pars compacta and ventral tegmental area. Life Sci. 1992;51:711–718. doi: 10.1016/0024-3205(92)90479-9. [DOI] [PubMed] [Google Scholar]
- 20.Mercuri NB, Bonci A, Johnson SW, Stratta F, Calabresi P, Bernardi G. Effects of anoxia on rat midbrain dopamine neurons. J Neurophysiol. 1994;71:1165–1173. doi: 10.1152/jn.1994.71.3.1165. [DOI] [PubMed] [Google Scholar]
- 21.Mercuri NB, Bonci A, Calabresi P, Stefani A, Bernardi G. Properties of the hyperpolarization-activated cation current (Ih) in rat midbrain dopaminergic neurons. Eur J Neurosci. 1995;7:462–469. doi: 10.1111/j.1460-9568.1995.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 22.Mercuri NB, Saiardi A, Bonci A, Picetti R, Calabresi P, Bernardi G, Borrelli E. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience. 1997;79:323–327. doi: 10.1016/s0306-4522(97)00135-8. [DOI] [PubMed] [Google Scholar]
- 23.Murer G, Adelbrecht C, Lauritzen I, Lesage F, Lazdunski M, Agid Y, Raisman-Vozari R. An immunocytochemical study on the distribution of two G-protein-gated inward rectifier potassium channels (GIRK2 and GIRK4) in the adult rat brain. Neuroscience. 1997;80:345–357. doi: 10.1016/s0306-4522(97)00001-8. [DOI] [PubMed] [Google Scholar]
- 24.Navarro B, Kennedy ME, Velimirovic B, Bhat D, Peterson AS, Clapham DE. Nonselective and Gβγ-insensitive weaver K+ channels. Science. 1996;272:1950–1953. doi: 10.1126/science.272.5270.1950. [DOI] [PubMed] [Google Scholar]
- 25.North RA. Drug receptors and the inhibition of nerve cells. Twelfth Gaddum memorial lecture. Br J Pharmacol. 1989;98:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oo TF, Blazeski R, Harrison SM, Henchcliffe C, Mason CA, Roffler-Tarlov SK, Burke RE. Neuron death in the substantia nigra of weaver mouse occurs late in development and is not apoptotic. J Neurosci. 1996;16:6134–6145. doi: 10.1523/JNEUROSCI.16-19-06134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patil N, Cox DR, Bhat D, Faham M, Myers RM, Peterson AS. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat Genet. 1995;11:126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- 28.Rakic R, Sidman RL. Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J Comp Neurol. 1973;152:103–132. doi: 10.1002/cne.901520202. [DOI] [PubMed] [Google Scholar]
- 29.Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–557. doi: 10.1016/s0306-4522(97)00093-6. [DOI] [PubMed] [Google Scholar]
- 30.Richter JA, Bare DJ, Yu H, Ghetti B, Simon JR. Dopamine transporter-dependent and -independent endogenous dopamine release from weaver mouse striatum in vitro. J Neurochem. 1995;64:191–198. doi: 10.1046/j.1471-4159.1995.64010191.x. [DOI] [PubMed] [Google Scholar]
- 31.Roffler-Tarlov S, Graybiel AM. Weaver mutant has differential effects on the dopamine-containing innervation of the limbic and non-limbic striatum. Nature. 1984;307:62–66. doi: 10.1038/307062a0. [DOI] [PubMed] [Google Scholar]
- 32.Rossi P, De Filippi G, Armano S, Taglietti V, D'Angelo E. The weaver mutation causes a loss of inward rectifier current regulation in premigratory granule cells of the mouse cerebellum. J Neurosci. 1998;18:3537–3547. doi: 10.1523/JNEUROSCI.18-10-03537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt MJ, Sawyer BD, Perry KW, Foreman MM, Ghetti B. Dopamine deficiency in the weaver mutant mouse. J Neurosci. 1982;2:376–380. doi: 10.1523/JNEUROSCI.02-03-00376.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silverman SK, Kofuji P, Dougherty DA, Davidson N, Lester HA. A rigenerative link in the ionic fluxes through the weaver potassium channel underlies the pathophysiology of the mutation. Proc Natl Acad Sci USA. 1996;93:15429–15434. doi: 10.1073/pnas.93.26.15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon JR, Ghetti B. The weaver mutant mouse as a model of nigrostriatal dysfunction. Mol Neurobiol. 1994;9:183–189. doi: 10.1007/BF02816118. [DOI] [PubMed] [Google Scholar]
- 36.Slesinger PA, Patil N, Liao YJ, Jan YN, Jan LY, Cox DR. Functional effects of the mouse weaver mutation on G-protein-gated inwardly rectifying K+ channels. Neuron. 1996;16:321–331. doi: 10.1016/s0896-6273(00)80050-1. [DOI] [PubMed] [Google Scholar]
- 37.Slesinger PA, Stoffel M, Jan YN, Jan LY. Defective gamma-aminobutyric acid type B receptor-activated inwardly rectifying K+ currents in cerebellar granule cells isolated from weaver and Girk2 null mutant mice. Proc Natl Acad Sci USA. 1997;94:12210–12217. doi: 10.1073/pnas.94.22.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith MW, III, Cooper TR, Joh TH, Smith DE. Cell loss and class distribution of TH-I cells in the substantia nigra of the neurological mutant, weaver. Brain Res. 1990;510:242–250. doi: 10.1016/0006-8993(90)91374-p. [DOI] [PubMed] [Google Scholar]
- 39.Sodickson DL, Bean BP. Neurotransmitter activation of inwardly rectifying potassium current in dissociated hippocampal CA3 neurons: interactions among multiple receptors. J Neurosci. 1998;18:8153–8162. doi: 10.1523/JNEUROSCI.18-20-08153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surmeier DJ, Merlmestein PG, Goldowitz D. The weaver mutation of GIRK2 results in a loss of inwardly rectifying K+ current in cerebellar granule cells. Proc Natl Acad Sci USA. 1996;93:11191–11195. doi: 10.1073/pnas.93.20.11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong Y, Wei J, Zhang S, Strong JA, Dlouhy SR, Hodes ME, Ghetti B, Yu L. The weaver mutation changes the ion selectivity of the affected inwardly rectifying potassium channel GIRK2. FEBS Lett. 1996;390:63–68. doi: 10.1016/0014-5793(96)00632-1. [DOI] [PubMed] [Google Scholar]
- 42.Triarhou LC, Norton J, Ghetti B. Mesencephalic dopamine cell deficit involves areas A8, A9 and A10 in weaver mice. Exp Brain Res. 1988;70:256–265. doi: 10.1007/BF00248351. [DOI] [PubMed] [Google Scholar]
- 43.Tucker SJ, Pessia M, Moorhouse AJ, Gribble F, Ashcroft FM, Maylie J, Adelman JP. Heteromeric channel formation and Ca2+-free media reduce the toxic effect of the weaver Kir 3.2 allele. FEBS Lett. 1996;390:253–257. doi: 10.1016/0014-5793(96)00635-7. [DOI] [PubMed] [Google Scholar]
- 44.Verney C, Febvret-Muzerelle A, Gaspar P. Early postnatal changes of the dopaminergic mesencephalic neurons in the weaver mutant mouse. Brain Res Dev Brain Res. 1995;89:115–119. doi: 10.1016/0165-3806(95)00106-n. [DOI] [PubMed] [Google Scholar]
- 45.Watts AE, Williams JT, Henderson G. Baclofen inhibition of the hyperpolarization-activated cation current, Ih, in rat substantia nigra zona compacta neurons may be secondary to potassium current activation. J Neurophysiol. 1996;76:2262–2270. doi: 10.1152/jn.1996.76.4.2262. [DOI] [PubMed] [Google Scholar]
- 46.White FJ. Synaptic regulation of mesocorticolimbic dopamine neurons. Annu Rev Neurosci. 1996;19:405–436. doi: 10.1146/annurev.ne.19.030196.002201. [DOI] [PubMed] [Google Scholar]