Abstract
It is widely believed that the brain processes information and stores memories by modifying and stabilizing synaptic connections between neurons. In experimental models of synaptic plasticity, such as long-term potentiation (LTP), the stabilization of changes in synaptic strength requires rapid de novo RNA and protein synthesis. Candidate genes, which could underlie activity-dependent plasticity, have been identified on the basis of their rapid induction in brain neurons. Immediate-early genes (IEGs) are induced in hippocampal neurons by high-frequency electrical stimulation that induces LTP and by behavioral training that results in long-term memory (LTM) formation. Here, we investigated the role of the IEGArc (also termed Arg3.1) in hippocampal plasticity. Arc protein is known to be enriched in dendrites of hippocampal neurons where it associates with cytoskeletal proteins (Lyford et al., 1995).Arc is also notable in that its mRNA and protein accumulate in dendrites at sites of recent synaptic activity (Steward et al., 1998). We used intrahippocampal infusions of antisense oligodeoxynucleotides to inhibit Arc protein expression and examined the effect of this treatment on both LTP and spatial learning. Our studies show that disruption of Arc protein expression impairs the maintenance phase of LTP without affecting its induction and impairs consolidation of LTM for spatial water task training without affecting task acquisition or short-term memory. Thus, Arc appears to play a fundamental role in the stabilization of activity-dependent hippocampal plasticity.
Keywords: neuron; synaptic plasticity, long-term potentiation; long-term memory; hippocampus; oligodeoxynucleotide; immediate-early; gene; spatial memory
Immediate-early genes (IEGs) are rapidly induced in brain neurons in response to patterned synaptic activity (Cole et al., 1989; Dragunow et al., 1989). Initial investigations focused on IEGs that encode transcription factors (Dragunow and Robertson, 1988; Saffen et al., 1988), primarily because these genes had been identified previously in molecular studies of cell growth (Curran et al., 1982; Linzer and Nathans, 1983; Greenberg and Ziff, 1984; Lau and Nathans, 1987). In support of a role for IEGs in synaptic plasticity, IEG induction and long-term potentiation (LTP) in the hippocampus were shown to have similar stimulation intensity thresholds and pharmacology (Cole et al., 1989; Worley et al., 1993). Recent studies have demonstrated that the IEG response is complex and includes, in addition to transcription factors, growth factors, metabolic and signaling enzymes, phosphatases, small GTP-binding proteins, and structural proteins (Lanahan and Worley, 1998). A current goal is to understand the specific contribution of these IEG proteins to activity-dependent synaptic plasticity.
Arc (also termed Arg3.1) is an IEG that was cloned from brain on the basis of its rapid induction after seizure stimulation (Link et al., 1995; Lyford et al., 1995). Arcgene expression is broadly responsive to neuronal activation by physiological stimuli including hippocampal LTP induction in the absence of seizures (Link et al., 1995; Lyford et al., 1995) and by dopamine-dependent mechanisms in the striatum (Fosnaugh et al., 1995;Berke et al., 1998). The Arc protein is enriched in dendrites where it localizes in a distribution that parallels that of F-actin (Lyford et al., 1995). Biochemical studies demonstrated that Arc protein interacts with polymerized crude actin, consistent with a role in activity-dependent changes in dendritic structure. Arc is unique among known IEGs in that its mRNA rapidly distributes throughout the dendritic arbor after induction (Link et al., 1995; Lyford et al., 1995) and localizes to discrete regions that have received direct synaptic stimulation (Steward et al., 1998). We have demonstrated recently that Arc RNA induction in CA1 neurons is specifically linked to the neural encoding process during spatial exploration (Guzowski et al., 1999). Although these data are consistent with the idea that Arc may play an active role in modifying long-term synaptic responses, direct evidence linking Arcgene expression to synaptic plasticity is lacking.
Antisense oligodeoxynucleotides (ODNs), which can block the synthesis of specific proteins (for review, see Ghosh and Cohen, 1992), have been used to elucidate molecular pathways and to examine the contribution of specific genes to neuroplastic mechanisms and behavioral responses (Wahlestedt et al., 1993; Konradi et al., 1994;Wahlestedt, 1994; Ogawa and Pfaff, 1996; Guzowski and McGaugh, 1997). In the present study, we have used antisense ODNs to assess the contribution of Arc to synaptic plasticity in the rat hippocampus and to behavioral memory. The two lines of experiments described here, electrophysiology and behavioral studies, were started independently and proceeded in parallel by the Tucson and Baltimore groups and the Irvine group, respectively. However, because of the congruence of the studies and the strong correspondence of the results, we have included them in one communication. The data presented here indicate that hippocampal Arc protein expression plays a critical role in the stabilization, but not the induction, of LTP and in the consolidation of long-term memory after spatial water task training.
Parts of this paper have been published previously (Lyford et al., 1996; Guzowski et al., 1997).
MATERIALS AND METHODS
Oligodeoxynucleotide design and preparation
ODN pairs were prepared that encoded antisense and scrambled sequence for the Arc mRNA sequence near the translation start site (Lyford et al., 1995). The nucleotide composition of each ODN pair was identical. The scrambled ODNs, which served as controls, did not show significant homology to sequences in the GenBank database. In our initial studies, we examined the usefulness of full phosphorothioate ODNs but determined that they affected baseline synaptic transmission in our in vivo electrophysiological studies. Subsequently, we used chimeric phosphorothioate/phosphodiester ODNs, which contained phosphorothioate linkages on the three terminal bases of both the 5′ and 3′ ends and phosphodiester internal bonds. This nucleotide chemistry was selected because of reports showing that such ODNs retain biochemical specificity, are more stable than unmodified phosphodiester ODNs in vivo, and are less toxic than full phosphorothioate ODNs (Hooper et al., 1994; Widnell et al., 1996). Consistent with these previous reports, the chimeric ODNs did not adversely affect baseline synaptic responses and were used subsequently in all of the studies described here.
For the LTP studies, which were conducted in Tucson, two different sets of 18 mer antisense and scrambled ODNs were used. Reports of successful use of antisense ODNs typically target the region of the translation start site (Ghosh and Cohen, 1992). Accordingly, the sequence of “Arc antisense 1” ODN was from bases 214 to 231, and that of “Arc antisense 2” ODN was from bases 232 to 249 of the published sequence (GenBank accession number U19866; the translation start site is at base 217). Corresponding scrambled-sequence ODNs were prepared in which the third base from each triplet was moved to the first position (designated “scrambled 1” and “scrambled 2,” respectively). The ODNs were synthesized at the DNA Synthesis Core Facility at Johns Hopkins University (Baltimore, MD). The gel filtration-purified ODNs were diluted in artificial CSF.
For the water task experiments, which were conducted in Irvine, the antisense and scrambled ODNs were 20 mers. The sequence of “Arc antisense 3” ODN was the reverse complement of bases 209 to 228 of the published Arc sequence and primarily overlapped Arc antisense 1. The scrambled control ODN (“scrambled 3”) contained the same base composition, but in a randomized order. The gel filtration-purified ODNs (Midland Certified Reagent Company) were resuspended in PBS, pH 7.4, and were further purified on Sephadex G-25 spin columns (Pharmacia, Piscataway, NJ). ODN concentrations were determined spectrophotometrically, and ODN integrity was confirmed by denaturing gel electrophoresis.
Procedures for electrophysiological recording experiments
Subjects. A total of 23 male retired breeder Fischer-344 rats, obtained from Charles River Laboratories (Wilmington, MA) between the ages of 9 and 11 months (middle-aged), were used for the in vivo LTP experiments. All rats were handled and given health checks, housed individually in 45 × 24 × 21 cm Plexiglas cages, and maintained on food and water available ad libitum and on a reversed 12:12 hr light/dark schedule with lights off at 10 A.M. Recording sessions were conducted in the dark phase of this schedule, and each rat was individually tested at the same time of day over the weeks of recording.
Surgery. Chronic implantations of bilateral recording cannulae and stimulating electrodes in the hilus of the fascia dentata and angular bundle were conducted according to National Institutes of Health guidelines, essentially as described by Barnes et al. (1994). Recording cannulae consisted of a Teflon-insulated (25 μm) 26 ga stainless steel tube, sealed with a 200 μm stylet that was flush with the cannula tip. The cannula or electrode enabled electrical recordings to be made during all phases of the infusion procedure. Stimulating electrodes were constructed of 114 μm Teflon-coated stainless steel wire (Medwire Corporation), with ∼300 μm of insulation removed from the tip. Rats were anesthetized with 40 mg/kg Nembutal (sodium pentobarbital), given Bicilin (30,000 units, i.m.) before the start of surgery to prevent infection, and mounted in a stereotaxic frame. Bone flaps were removed over the recording (3.8 mm posterior and 2.0 mm lateral to bregma) and stimulating (8.1 mm posterior and 4.4 mm lateral) sites, and holes for reference and anchor screws were drilled (see Fig. 1A). Approximately 30 min before the recording cannulae were lowered into place, rats were given 2.5 mg/kg Diazepam to reduce the incidence of afterdischarges caused by this mechanical disturbance. Evoked responses were optimized electrophysiologically to obtain positive-going perforant path–granule cell responses. Postoperative care consisted of a subcutaneous injection of 10 ml of saline, Children's Tylenol for analgesic purposes, and fresh fruit. Rats recovered in a warmed humidified incubator until full righting reflexes returned. All rats were given at least a 1 week recovery period before recording began.
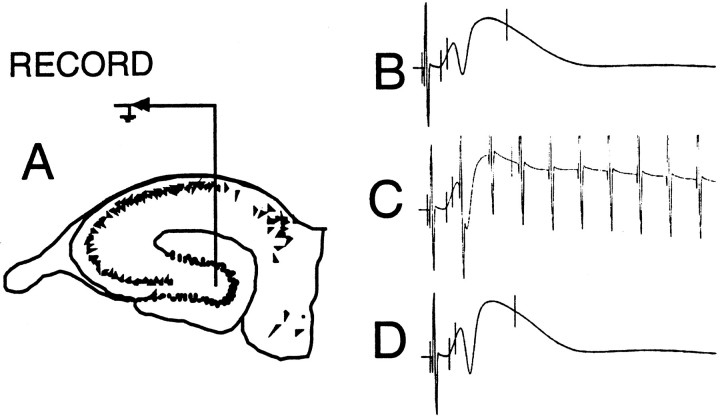
Fig. 1.
A, Schematic illustration of the placement of the chronically implanted infusion cannula/recording electrode in the hilus of the fascia dentata. The stimulating electrode was implanted in the angular bundle to optimize the perforant path–granule cell evoked field potential. B, Example of the extracellular hippocampal evoked response elicited from perforant path stimulation before high-frequency stimulation. C, Example of the response during the high-frequency burst used to induce LTP. D, Example of the response on the day after LTP induction.
Electrophysiological recording. Adaptation to the electrophysiological testing environment commenced by connecting the animal to the recording system and obtaining baseline evoked responses at a rate of 0.1 Hz in each hemisphere (alternating hemispheres). The time elapsed between removal from the colony room and initiation of recording was ∼30 sec; thus 10 responses could be obtained within several minutes, making potential alterations in the evoked response by brain temperature changes (Moser et al., 1993) a negligible factor. A 2.56 sec EEG sample was collected before each stimulus delivery along with responses to the low-frequency test stimulation (200 msec, 300 μA diphasic constant-current stimuli) during the baseline and LTP induction periods. The LTP induction protocol used was 10 repetitions of a 25 msec train (i.e., 10 pulses) delivered at 400 Hz, for a total of 100 stimulus pulses (see Fig. 1C). All responses were digitized at 20 kHz by an 80386 computer and stored on disk for subsequent off-line analysis. The field EPSP was measured as the voltage difference between two cursors set after the EPSP onset and 0.5 msec beyond this, well before the onset of the population spike. To calculate the amount of change observed after LTP induction, the formula (V1 − V0)/V0 was used, with the average amplitude of the EPSP on low-frequency baseline recording days (see Fig. 1B) set as V0 and the EPSP amplitude after LTP-inducing stimulation was delivered set as V1 (see Fig. 1D).
The total consecutive number of days that any given rat was tested ranged up to 128, depending on how quickly the baseline responses stabilized and whether afterdischarges were induced by cannula placement or infusion and “restabilization” of the response was necessary. Eighteen of the 23 rats implanted had good bilateral evoked responses. Because it was critical to compare infusion of the antisense ODN with the scrambled ODN, the final n for the LTP experiment was 18. The other animals were used to test the effects on baseline synaptic transmission of phosphorothioate and hybrid ODNs.
Delivery of Arc antisense and scrambled control oligodeoxynucleotides. To inject the ODN, the stylet was removed from the guide cannula and a 33 ga infusion needle was inserted through the cannula 200 μm beyond the tip in each hemisphere. The infusion needles were attached by polyethylene (PE50) tubing to 10 μl Hamilton syringes, which were controlled by a CMA Microdialysis (CMA100) microinfusion pump. The infusion needles extended 200 μm beyond the cannula. A volume of 0.5 μl of the antisense ODN was delivered at a rate of 0.1 μl/min to one hemisphere, and 0.5 μl of the scrambled antisense was delivered simultaneously at the same rate to the other hemisphere. Nine rats were given the Arcantisense 1 and nine rats the Arc antisense 2 ODN to one hippocampus and the corresponding scrambled ODN to the contralateral hippocampus. The hemisphere that received the antisense ODN was determined randomly. The recording procedure during infusion was as follows: (1) 10 evoked responses (bilateral) were recorded before infusion; (2) the stylet was removed, and 10 responses were recorded; (3) the infusion needle was inserted, and 10 responses were recorded; (4) the infusion pump was started, and 30 responses were recorded from each hemisphere during drug delivery; (5) immediately after the infusion 10 responses were recorded; (6) the infusion needle was withdrawn, and 10 responses were recorded; and (7) the stylet was inserted, and a final 10 responses were evoked and recorded in each hemisphere. After this infusion procedure, the rats were returned to their home cages for 1.5 hr before being given the LTP-inducing stimulation.
The mechanical manipulations involved in performing the infusions of ODNs (i.e., withdrawal of stylet, insertion of infusion needle, infusion of drug, withdrawal of needle, and reinsertion of stylet) caused no observable alteration in baseline synaptic transmission in 52% of treatments (of 124 total infusions). In the cases in which the effects from one of these mechanical manipulations were significant (i.e., afterdischarges resulting in reductions or increases in the amplitude of the evoked response), the procedure was terminated, and the data were not used in the analysis. When baseline responses normalized during the subsequent week, rats that showed change in response size because of mechanical effects were tested again.
Procedures for behavioral testing experiments
Animals and surgery. Forty-two male Sprague Dawley rats (225–250 gm at arrival; Charles River Laboratories) were used. The rats were individually housed in a temperature (22°C)- and light-controlled vivarium (12:12 hr light/dark cycle with the lights on at 7:00 A.M.), provided access to food and water ad libitum, and acclimatized to laboratory conditions for 1 week before surgery. Under Nembutal general anesthesia (50 mg/kg, i.p.), stainless steel guide cannulae (10.3 mm; 23 ga) targeting the dorsal hippocampus were implanted bilaterally using a stereotaxic frame (Kopf Instruments). The following coordinates were used: anteroposterior = −3.6 mm, mediolateral = ±2.2 mm from bregma, and dorsoventral = −2.5 mm from the skull surface. Postoperative care consisted of a subcutaneous injection of 3 ml of saline and an intramuscular injection of Bicilin (30,000 units). Rats recovered in an incubator until full righting reflexes returned. All rats were given at least a 1 week recovery period before behavioral training began.
Delivery of Arc antisense and scrambled control oligodeoxynucleotides. ODNs were delivered to the dorsal hippocampus of awake, behaving rats via guide cannulae through a 30 ga infusion needle connected to a 10 μl Hamilton syringe by polyethylene tubing. The infusion needles extended 1.7 mm beyond the cannulae. Infusions (1.0 μl) were delivered over 154 sec using a syringe pump (Sage Instruments) to rats either 3 hr before behavioral training (see Fig. 4) or after training (see Fig. 5). The rats were awake and gently restrained during the infusion procedure.
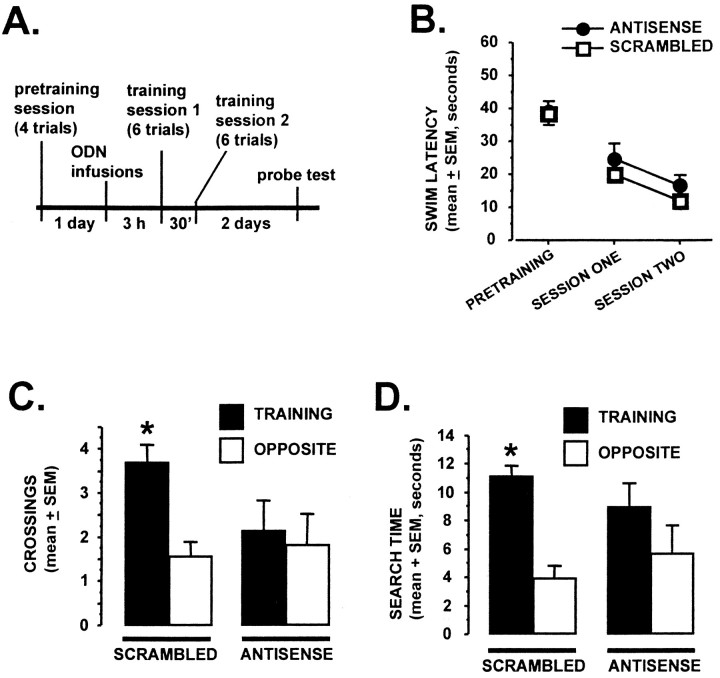
Fig. 4.
Pretraining intrahippocampal infusion ofArc antisense ODNs impairs 48 hr retention test performance, without affecting task acquisition or short-term memory.A, The experimental timeline is shown.Arc antisense 3 ODN or scrambled 3 ODN were administered bilaterally 3 hr before training in the spatial water task (1 nmol in 1 μl; n = 6–7 rats per group). The two training sessions were separated by 30 min. A 90 sec probe test was given 48 hr later. B, Acquisition data from the pretraining and training sessions are shown. No differences were seen for either the pretraining or training sessions between the antisense and scramble ODN groups (p > 0.05, repeated-measures ANOVA).C, D, The platform-crossings (C) and annulus search time (D) retention test measures are shown. Scrambled ODN-treated rats demonstrated a spatial bias for the training location, whereas the Arcantisense ODN-treated rats did not [*p < 0.005, comparing training vs opposite measures within a group (2-tailed pairedt test)].
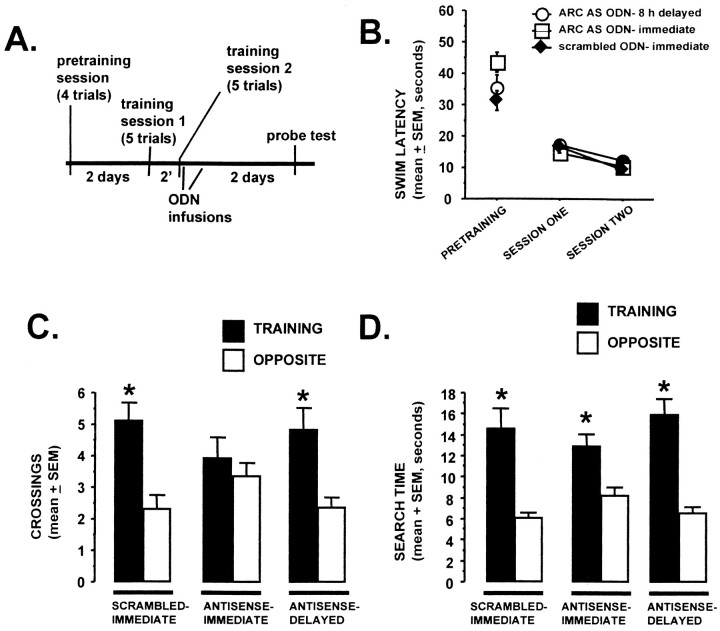
Fig. 5.
Intrahippocampal Arc antisense ODN infusions given immediately but not 8 hr after training impair 48 hr retention test performance. A, The experimental timeline is shown. Three groups of rats were given two water task training sessions separated by 2 min. Separate groups of rats were given bilateral intrahippocampal infusions of either scrambled 3 ODN (n = 8) or Arc antisense 3 ODN (n = 9) immediately after training. The third group received intrahippocampal infusions of Arc antisense 3 ODN 8 hr after training (n = 8). Two days later, all rats were given a 90 sec probe test. B, Acquisition data from the pretraining and two training sessions are shown. All groups performed similarly during the pretraining or training sessions (p > 0.05, repeated-measures ANOVA).C, D, The platform-crossings (C) and annulus search time (D) retention test measures are shown. The groups given scrambled ODN infusions immediately after training and Arc antisense ODNs 8 hr after training demonstrated a spatial bias for the training location on both measures. In contrast, the group given the Arcantisense ODNs immediately after training exhibited impairment on the more stringent retention measure platform crossings but not on the annulus search time measure [*p < 0.02, comparing training vs opposite measures within a group (2-tailed pairedt test)].
Water task training and testing. The apparatus used for the spatial water task was a black tank (diameter, 1.83 m; height, 0.58 m) filled to a depth of ∼20 cm with water (24 ± 2°C). In this task, rats learn to locate a submerged, and hence hidden, Plexiglas platform (20 × 25 cm) 2 cm below the water's surface. One day (see Fig. 5) or 2 d (see Fig. 4) before the experiment, the rats were given a pretraining water task session of four trials to acclimate them to the demands and stresses of the training procedure. For the pretraining sessions, the platform was in a location different from that used for the actual training. On the day of training, the platform remained in a fixed position throughout training trials and between the two training sessions. The training sessions were separated by 30 min (see Fig. 4) or 2 min (see Fig. 5). A training session consisted of a series of six (see Fig. 4) or five (see Fig. 5) trials with a 20 sec intertrial interval (ITI). For the post-training infusion experiment (see Fig. 5), the intersession interval was reduced to 2 min to shorten the entire training procedure, and the number of trials per session was reduced to five to reduce fatigue that might have been encountered because of the shortened intersession interval. On each trial, the rat started from one of five random positions along the side of the tank. The rat was given 60 sec to find the submerged platform. If a rat did not mount the platform within the 60 sec, it was guided to the platform. The time to mount the platform was recorded as the training latency for each trial. The rat was allowed to remain on the platform for 10 sec before being removed to a holding cage for the ITI. At the end of a training session, the rat was returned to its home cage until the second training session. After completion of the training, the rats were returned to the vivarium until retention testing.
The rats were given 90 sec probe tests 48 hr after training to measure retention. The platform was removed from the tank during the probe test, and the sessions were videotaped for analysis later. Two viewers independently analyzed the videotapes for two measures of spatial bias. The first analysis, platform “crossings,” measured the number of times the rat swam directly over the location where the platform had been during training and an equivalent area located in the opposite area of the tank (see Figs. 4,5, training vs opposite). The second analysis, annulus search time, measured the time the rat spent in a scoring annulus centered over either the initial training location or the opposite location in the tank during the probe trial (see Figs.4,5, training vs opposite). The scoring annulus comprised ∼10% of the total water tank area, and the platform comprised ∼2%.
Confirmation of cannulae placements. After behavioral testing, the majority of the rats were anesthetized and perfused intracardially with saline and then 4% formalin. The brains were sectioned at 80 μm, stained with cresyl violet, and analyzed to confirm that cannulae and infusion needle tracts were within the appropriate region of the dorsal hippocampus. The remaining rats were used for biochemical studies on the efficacy of the Arcantisense ODNs (see Fig. 2 and Immunoblot analysis). One week after testing, these rats were infused with antisense or scrambled control ODNs in different hemispheres and then killed after a delay of 6 hr. For these rats, cannulae placements and infusion sites were confirmed visually from the 1 mm coronal sections used for tissue punches (see Immunoblot analysis). One rat (out of 42) was excluded from further analysis on the basis of these histological findings.
Fig. 2.
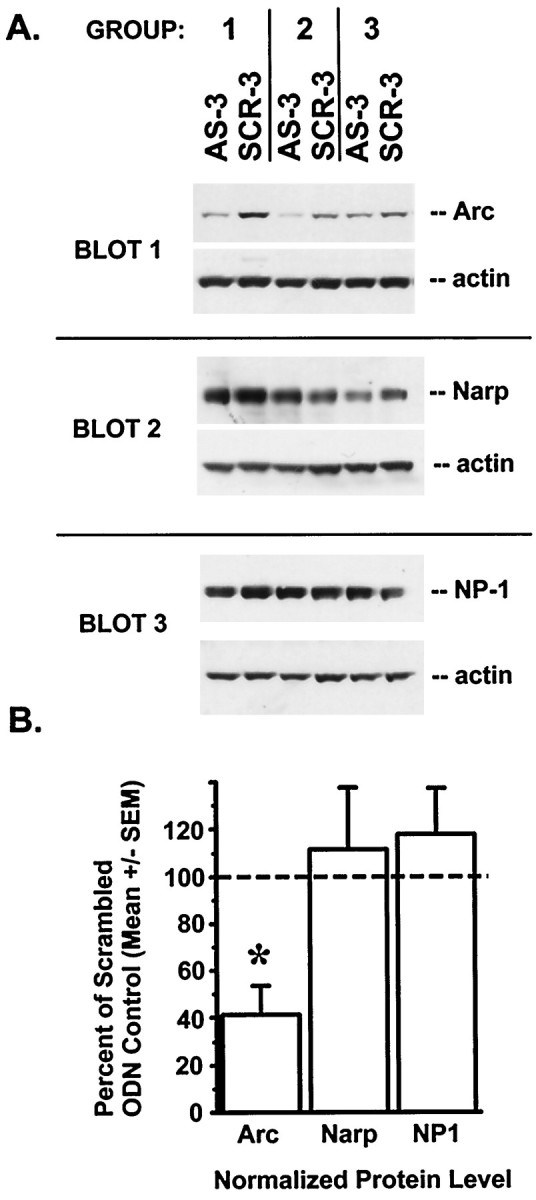
Intrahippocampal Arc antisense ODNs block Arc protein expression. A, Rats received infusions (1 nmol of ODN in 1 μl) of Arc antisense 3 ODN (AS-3) in one hippocampus and scrambled 3 ODN (SCR-3) in the other 6 hr before death and tissue dissection. Protein extracts were prepared as described in Materials and Methods. Group pools were obtained from two to three rats, with theAS-3-treated and SCR-3-treated hemispheres pooled separately. Immunoblot analysis was performed for the synaptic activity regulated and neuron-specific IEG proteins Arc and Narp and for the neuron-specific protein NP1. After detection with either Arc, Narp, or NP1 antibodies, the blots were reprobed with actin antibody. Immunoreactivity levels of the three proteins were normalized to those of actin for each sample. B, Normalized protein levels from Arc antisense ODN samples are expressed as a percentage of normalized Arc levels for the corresponding scrambled ODN samples for the same group. Arc antisense ODN treatment caused a reduction in Arc protein levels (t = −4.84; *p < 0.05), without affecting the levels of either Narp or NP1.
Statistical analysis. Before statistical analyses were performed, the data were subjected to the following outlier analysis: Rats that were >2 SDs from their respective group mean for specific training or testing parameters were excluded from further analysis. These parameters included training session mean, platform-crossings difference (See Figs. 4,5, training vs opposite), and annulus search time difference (see Figs. 4,5, training vs opposite). Two rats (one antisense ODN-treated rat and one scrambled ODN-treated rat) were excluded from the post-training ODN infusion experiment (see Fig. 5) by this analysis, whereas no rats were excluded from the pretraining ODN infusion experiment (see Fig. 4). ANOVA and repeated-measures ANOVA were used to analyze individual trials and trial sessions, respectively. ANOVA was also performed on the probe test measures. Fischer's post hoc tests were used for pairwise comparisons. Spatial bias for the training location was assessed using two-tailed paired t tests to compare training versus opposite probe test measures for a given treatment group. A probability level of <0.05 was accepted as statistical significance for all tests.
Immunoblot analysis
Deeply anesthetized rats that had been given an overdose of sodium pentobarbital (200 mg, i.p.) were briefly perfused with phosphate buffer, pH 7.4, and their brains were rapidly removed. Tissue punches near the infusion sites, comprising cells principally from the dentate gyrus and CA1, were taken with a glass pipet (with an inner diameter of 1 mm) from 1-mm-thick coronal brain slices. Tissue was sonicated in 0.1 m phosphate buffer, pH 7.4 (containing 10% glycerol, 5 mm EDTA, 20 μm leupeptin, 0.1 mmNα-p-tosyl-l-lysine chloromethyl ketone (TLCK), and 1 mm PMSF). Protein concentration was determined by a modified Bradford assay (Bio-Rad, Hercules, CA). Twenty micrograms of protein were heated in sample buffer with reducing agent (NOVEX), loaded and run on 4–12% NuPAGE gels (NOVEX), and then electroblotted to nitrocellulose membranes for immunoblot analysis. Blots were incubated with diluted primary antibody overnight at 4°C. Immunoreactive species were detected by chemiluminescence (SuperSignal; Pierce, Rockford, IL). Blots were first processed to detect Arc immunoreactivity. After this, blots were directly reprobed with antibody to actin. Separate blots using the same protein extracts were processed to determine Narp (Tsui et al., 1996) and NP1 (Schlimgen et al., 1995) levels; as with Arc, blots were then reprobed with actin antibody after detection of either Narp or NP1. SeeBlue Markers (NOVEX) were run on all gels to ensure that the immunoreactive bands were of the correct relative mobility. The following rabbit polyclonal antibodies and dilutions were used: anti-Arc [1:1000 (Lyford et al., 1995)], anti-Narp [1:1000 (O'Brien et al., 1999)], anti-NP1 (1:5000; generated in P.F.W.'s laboratory), and anti-actin (1:200; Sigma, St. Louis, MO; catalog #A2066).
For quantitation of immunoblot results, appropriately exposed films were scanned and converted into TIF files for quantitative analysis using NIH IMAGE software. Control experiments were performed to ensure the linearity of the assay with increasing protein loads. IEG immunoreactivity levels were normalized using the actin immunoreactivity value for that sample.
RESULTS
Delivery of phosphorothioate and hybrid ODNs to the hippocampus of awake rats
Our initial studies examined the effect of ODNs on acute synaptic transmission in vivo. Figure1 shows a schematic representation of the placement of the chronically implanted infusion cannula/recording electrode used and examples of the extracellular evoked responses before and after tetanic stimulation. Because natural phosphodiester bonds are metabolically labile in vivo, we first preparedArc antisense and scrambled control ODNs containing phosphorothioate linkages. The phosphorothioate linkage is resistant to nuclease cleavage and the resulting ODNs are more stable than are phosphodiester-linked ODNs in vivo (Szklarczyk and Kaczmarek, 1995). However, infusions of phosphorothioate ODNs were followed by an immediate reduction in the amplitude of the evoked response. In each of three independent trials, synaptic responses were reduced by 58% within 5 min. Antisense and scrambled control ODNs were equally effective in reducing synaptic transmission. The magnitude of the block was dose dependent, and ∼80% of the response was blocked after infusion of 0.5 μl of 1 mm ODN solution. Synaptic responses showed no recovery for at least several hours and recovered gradually over the subsequent several days. These studies indicate that phosphorothioate ODNs produce a rapid and sequence-independent inhibition of synaptic transmission. Similar findings using phosphorothioate ODNs have been reported by other investigators (Abraham et al., 1997).
We next examined hybrid ODNs in which three nucleotides at both the 5′ and 3′ ends possess phosphorothioate linkages and the remaining nucleotides are linked by phosphodiester bonds. This ODN chemistry is resistant to exonuclease degradation and is less toxic (Hooper et al., 1994; Widnell et al., 1996; Hebb and Robertson, 1997). In contrast to the phosphorothioate ODNs, hybrid ODNs did not alter synaptic transmission elicited at low frequency. Infusions of hybrid ODNs from 0.5 to 1 μl (at a concentration of 1.0 mm) did not produce acute changes in synaptic responses. Moreover, synaptic responses recorded 24 hr after infusion remained stable. For this reason, the hybrid ODN chemistry was used in all subsequent electrophysiological and behavioral experiments.
Arc antisense ODNs block Arc protein expression in the hippocampus
We determined the specificity and efficacy of the Arcantisense ODNs by immunoblot analysis. Individual rats received infusions of Arc antisense ODNs in one hippocampus and scrambled ODNs in the other hippocampus. The infusions of antisense and scrambled ODNs were alternated between right and left hemispheres for different rats. Our previous studies demonstrated that infused ODNs remained localized in the dorsal hippocampus and did not diffuse into the adjacent hemisphere (Guzowski and McGaugh, 1997). The rats were returned to their cages after the infusion procedure. Six hours later, the rats were sacrificed, and tissue punches were taken near the infusion sites. Because Arc is present in a subset of hippocampal neurons in control animals and is regulated by natural synaptic activity (Lyford et al., 1995; Guzowski et al., 1999), this infusion and sacrificing schedule provides a strong functional test for the ability of the Arc antisense ODNs to inhibit Arc protein expression under physiological conditions. Arc antisense ODN protein extracts from two or three rats were pooled, and likewise, the scrambled ODN extracts from the same rats were pooled for immunoblot analysis, which was performed as described in Materials and Methods. This infusion and pooling protocol was performed with three groups of rats. Normalized Arc protein levels were decreased by 58% in theArc antisense-treated hemispheres as compared with the scrambled ODN hemispheres within the same rats (Fig.2; t = −4.84; df = 2; p < 0.05).
To confirm that the observed decrease in Arc protein levels in the antisense ODN-treated hemispheres was not caused by a general effect on synaptic activity, we also measured the protein levels of another IEG known to be regulated by synaptic activity, Narp (Tsui et al., 1996). In striking contrast to Arc, Narp levels were not affected by theArc antisense ODN treatment (Fig. 2; t = 0.46; df = 2; p > 0.05). As a further control, we confirmed that the expression of another neuron-specific gene, NP1, was not altered by antisense ODN infusion (Fig. 2; t = 0.44; df = 2; p > 0.05). These data provide strong evidence that the Arc antisense ODNs specifically inhibit Arc protein expression, without affecting the levels of other neuronal proteins.
Arc antisense ODNs selectively block LTP maintenance but not induction
The experimental design sought to administer a single dose of specific ODN that would block the transient induction of Arc protein after a high-frequency stimulus. Induction of Arc mRNA and protein in the dentate gyrus after a maximal electroconvulsive shock peaks within 1–3 hr and returns to baseline by 8 hr (Lyford et al., 1995; Wallace et al., 1998). Similar kinetics was confirmed in preliminary studies after LTP-inducing high-frequency (HF) stimuli. The timing of the ODN delivery relative to administration of the conditioning stimulus was selected to provide sufficient time for uptake into neurons while being short enough that the ODNs might remain active during the time of induction. Significant intracellular accumulation of ODNs occurs within 15 min of intracerebral ODN infusion, and even the relatively labile phosphodiester ODNs are readily detected in neurons 4 hr later (Ogawa et al., 1995). On the basis of these general parameters, we established a protocol in which the antisense and scrambled control ODNs were administered 1.5 hr before the LTP-inducing stimulus.
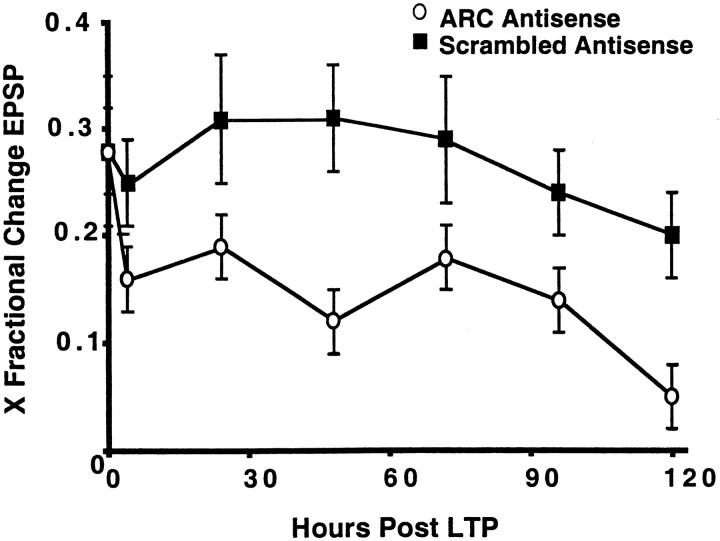
HF synaptic stimulation was delivered to awake, behaving rats, and the evoked response amplitudes were recorded 20 min after, 4 hr after, and subsequently daily for as long as several weeks until responses returned to prestimulation levels. Because there was no statistically significant difference between Arc antisense 1 andArc antisense 2 ODNs on the attenuation of LTP maintenance [F(1,14) = 0.03; p > 0.05], the data from these two treatments are pooled below. As shown in Figure 3, the initial amplitude of LTP did not differ between antisense and scrambled ODN-treated hemispheres, and the LTP amplitude and decay in the scrambled ODN-treated hemispheres were similar to that of other reports in chronically prepared rats (Barnes et al., 1994). However, LTP decayed more rapidly in the Arc antisense ODN-treated hemispheres and was several SEMs below that of the scrambled ODN-treated hemispheres at 5 d. For example, on day 5, the mean LTP remaining in the nine rats that obtained Arc antisense 1 was 4% (± 0.02), and was 7% (± 0.04) in the nine rats that obtained Arcantisense 2. Differences between the antisense and scrambled ODN-treated hemispheres were detected as early as 4 hr after LTP induction.
Fig. 3.
Arc antisense ODN treatment impairs the maintenance of LTP. The mean ± SEM change in EPSP amplitude measured immediately after LTP induction, at 4 hr, and then at 24 hr intervals for 5 d in the 18 rats that had stable data for both scrambled and antisense infusions. Note that there was no difference in LTP magnitude between treatments initially but that LTP decayed more rapidly in the Arc antisense ODN-treated hemispheres over subsequent days.
Intrahippocampal Arc antisense ODN infusions specifically impair long-term memory consolidation
Fluorescent in situ hybridization studies show thatArc RNA is expressed in a discrete population of cells in the granule cell layer and hilus of the dentate gyrus and in the pyramidal cell layer of the hippocampus (CA1–CA3) and that this expression is increased in a spatial exploration paradigm (Guzowski et al., 1999). This observation, combined with the above finding that disruption of Arc expression interferes with the maintenance of LTP, suggests that Arc may play a central role in cellular neuroplastic mechanisms of the hippocampal formation. Consequently, ODN-mediated disruption of Arc expression should interfere with hippocampal function. We tested this hypothesis by examining the effect of intrahippocampal infusions of Arc antisense or scrambled ODNs on the learning of, and memory for, the hippocampal-dependent, spatial version of the Morris water task (Morris et al., 1982).
Two experiments of similar design were conducted. For each experiment, the rats underwent stereotaxic surgery to implant guide cannulae targeting the dorsal hippocampus. The rats were given at least 1 week to recover from surgery and were then briefly handled on 3 different days. One or two days before experimental training, the rats received a pretraining session of four trials to familiarize them with the task demands. In this pretraining session, the submerged platform was in a location different from that used on the day of the experiment.
For the first experiment, the rats received bilateral infusions of either Arc antisense 3 ODN or scrambled 3 ODN into the dorsal hippocampus 3 hr before behavioral training (n = 6–7 rats per group; infusions of 1 nmol of ODN in 1 μl). In a previous study, we showed that 1 μl of ODN infused at the same stereotaxic coordinates remained localized to the dorsal hippocampus, without diffusing into the ventral hippocampus or into other structures (Guzowski and McGaugh, 1997). Three hours after ODN infusions, the rats were trained in the hidden platform, or spatial, version of the Morris water task. This training consisted of two training sessions with a 30 min interval between the sessions. Each session consisted of six training trials with an intertrial interval of 20 sec. No differences were observed between the two groups during the pretraining or training sessions as revealed by repeated-measures ANOVA.
The rats were given a 90 sec probe test 2 d later. Two measures of spatial bias were recorded (platform crossings and annulus search time—see Materials and Methods), and both showed the same result—the scrambled ODN group exhibited a spatial bias for the training location during the probe test (Fig. 4; pairedt test; platform crossings, t = 4.4; df = 6; p < 0.005; annulus search time, t= 7.1; df = 6; p < 0.001), whereas theArc antisense ODN-treated rats did not (platform crossings,t = 0.3; df = 5; p > 0.05; annulus search time, t = 1.4; df = 5;p > 0.05).
In the second experiment, we used a post-training ODN infusion strategy. We reasoned that because intraneuronal accumulation of ODNs can be seen 15 min after intracerebral infusion (Ogawa et al., 1995) and Arc RNA accumulates in the cytoplasm and dendrites within 30–120 min of neuronal stimulation (Wallace et al., 1998;Guzowski et al., 1999), Arc antisense ODNs given after training could block Arc protein expression induced by recent experience. The rats were given two spatial water task training sessions of five trials each separated by 2 min. Immediatelyafter the second training session, separate groups of rats were given bilateral infusions of either Arc antisense 3 ODN or scrambled 3 ODN (1 nmol of ODN in 1 μl; n = 8–9 rats per group). The time between the two training sessions was shortened to 2 min in this experiment to minimize the total time of training, such that infusions of Arc antisense ODNs after training might be most effective. A third group received Arcantisense ODN infusions 8 hr after training (n = 8 rats). Based on the rapid induction of Arc RNA expression after spatial learning (Guzowski et al., 1999) (J. F. Guzowski and J. L. McGaugh, unpublished observations), it was predicted that the 8 hr-delayed infusion of Arc antisense ODN should not affect LTM consolidation. Because infusions were given after training, concerns of ODN effects on task acquisition were eliminated, and, as expected, all groups performed similarly during the training trials.
The rats were then given a retention probe test 2 d later. Probe tests were again analyzed for spatial bias. Probe trial performance of the scrambled ODN (immediate) and Arc antisense ODN (8 hr-delayed) groups was similar—both groups exhibited a selective bias for the training location over the opposite location (Fig.5; scrambled-immediate, platform crossings, t = 4.4; df = 7; p < 0.01; annulus search time, t = 4.6; p< 0.01; antisense-delayed, platform crossings, t = 3.6; df = 8; p < 0.01; annulus search time,t = 5.3; p < 0.001). In contrast, theArc antisense (immediate) group did not exhibit a spatial bias for the training location on the more stringent measurement of platform crossings (Fig. 5; t = 0.7; df = 7;p > 0.05). The Arc antisense ODN (immediate) group did, however, exhibit a spatial bias on the less stringent annulus search time measure (Fig. 5;t = 3.1; p < 0.01). Although the magnitude of this bias (training vs opposite) was less than that for the scrambled ODN (immediate) and Arc antisense ODN (8 hr-delayed) groups, it was not statistically significant. Overall, these results are consistent with those seen with the pretraining ODN infusions.
DISCUSSION
The principal findings of this study are that ODN-mediated disruption of Arc protein expression impairs the stabilization of activity-dependent changes in synaptic efficacy and memory consolidation. Delivery of the Arc antisense ODNs into the hippocampus of the awake, behaving rat did not inhibit the initial induction of LTP; however, the magnitude of synaptic enhancement was reduced within 4 hr compared with responses in the contralateral hippocampus of the same rat that received a scrambled control ODN. Similarly, intrahippocampal infusion of Arc antisense ODNs did not affect the ability of rats to learn the spatial water task but did impair LTM as assessed 48 hr later. The finding that Arcantisense ODNs delivered immediately after training, but not 8 hr later, caused a similar impairment in the 48 hr probe test performance confirms that the antisense ODNs specifically affected time-limited memory consolidation processes. The lack of effect of theArc antisense ODNs at 8 hr after training is consistent with the rapid and transient time course of Arc induction; experience-dependent Arc RNA expression in the hippocampus occurs rapidly and returns to baseline levels by 2 hr after behavioral training (Guzowski et al., 1999) (Guzowski and McGaugh, unpublished observations).
The specificity of the antisense ODN effects on LTP and LTM is supported by several lines of evidence. For the LTP studies, two independent, and nonoverlapping, sets of antisense and control ODNs provided identical results—for each set, treatment with the antisense ODN did not affect LTP induction but led to a more rapid decay of the enhanced response as compared with the scrambled control ODN. The effect of the Arc antisense ODNs was robust and remained statistically significant throughout the time course of the decay of LTP over 4 d. In the behavioral studies, the lack of effect of the antisense ODN on initial task acquisition or short-term performance in the pretraining infusion experiment (Fig. 4) demonstrates that theArc antisense ODN did not impair hippocampal function at the time of training. The effect of the antisense ODN on the probe test performance suggests either a specific effect on memory consolidation or a delayed toxic effect of the antisense ODN on memory retrieval mechanisms. The absence of an effect of the Arc antisense ODNs when infused 8 hr after training provides an important control that mitigates against a delayed toxic effect. The fact that theArc antisense ODN was only effective in impairing the 48 hr retention performance when administered immediately, but not 8 hr, after training confirms that the antisense ODN had the predicted time-limited effect on memory consolidation.
In many ways, the in vivo LTP preparation provides an ideal system in which to apply antisense technology to the study of synaptic plasticity. In naïve rats, Arc protein is naturally expressed in a small subset of granule cell neurons of the normal hippocampus (∼1 of 100) but is rapidly induced in the entire population of these neurons after seizure or synaptic stimulation that induces LTP (Lyford et al., 1995). In contrast to other uses of the ODN approach, in which a constitutively expressed protein is targeted and reductions in expression are limited by the rate of protein turnover, the current application needs only to block stimulus-induced increases. Electrophysiological recordings can be used to monitor continuously the effects of the ODN on the population of neurons that receive the most direct application of the ODN. Because IEG levels are not increased until after the initial events essential to the initiation of LTP, it is anticipated that they do not play a role in this process. Indeed, LTP induction in the hippocampus is not blocked by inhibitors of either RNA or protein synthesis, nor was it blocked by the Arcantisense ODNs. Accordingly, preservation of LTP induction serves as an anticipated control for physiological viability of the tissue. In principle, the same approach pertains for use of antisense technology to examine the contribution of other IEGs to long-term synaptic plasticity. Like Arc, many of these proteins appear to be expressed at low levels naturally in granule cell neurons, and their induction after a synaptic stimulus might be blocked with a single administration of antisense ODNs. Our current estimates are that perhaps 30 IEGs are induced rapidly in hippocampal neurons in association with LTP (Lanahan and Worley, 1998). This antisense strategy might be useful for identifying those that are most important for durable plastic changes in the brain.
For the reasons described above, the anatomical and temporal specificity of antisense ODN approaches is also well suited to investigating the role of different IEGs in learning and memory processes. For example, the distinction between defects in memory consolidation and retrieval cannot be drawn, at present, from experiments using mice with germ-line null mutations (knock-out mice). Although advances in transgenic approaches now allow some degree of temporal control (Mayford et al., 1996) and region-specific null mutations (Tsien et al., 1996), no current approach can provide the combination of precise temporal and anatomical resolution. Using the temporal precision afforded by antisense ODN infusions, we have shown that disruption of Arc expression within the dorsal hippocampus impairs LTM consolidation processes and does not affect task acquisition, short-term memory, or memory retrieval in the spatial water task.
Although disruption of many different genes has been linked to disruption of LTP, most of these genes or proteins are implicated in the induction phase of LTP. By contrast, Arc plays a selective role in LTP maintenance. How might Arc contribute to LTP maintenance and memory consolidation? Our current understanding of Arc suggests that it possesses two functional domains. The C-terminal half of Arc shows modest homology to spectrin and interacts with structural proteins (Lyford et al., 1995). Arc protein also interacts with calcium and calmodulin-dependent protein kinase type II (CaMKII) (G. L. Lyford, A. Chowdhurry, and P. F. Worley, unpublished observations). The mRNAs of both Arc andCaMKII are present in neuronal dendrites where they are hypothesized to be locally synthesized, perhaps in response to specific forms of synaptic input. In hippocampal granule cell dendrites,CaMKII mRNA is constitutively present whereas ArcmRNA is only transiently present after plasticity-inducing stimuli. Thus, Arc may function as an anchoring or targeting protein for CaMKII or to modulate the activity or substrate specificity of CaMKII (G. L. Lyford and P. F. Worley, unpublished observations).
Arc has many interesting properties that should provide insight into mRNA and protein targeting and mechanisms that contribute to synapse-specific effects of an IEG. Our studies demonstrate that Arc is essential for LTP maintenance and memory consolidation processes. In many model systems, including LTP, Arc is one of the most dynamically regulated IEGs. Arc transcription is also induced several-fold in CA1 and in cortical neurons in response to such natural stimuli as exposure to a novel environment and in spatial-learning paradigms (Guzowski et al., 1999) (Guzowski and McGaugh, unpublished observations). Furthermore, Arc is strongly induced in the striatum in response to dopaminergic agonists including cocaine (Fosnaugh et al., 1995; Berke et al., 1998). Thus,Arc likely plays a fundamental role in many natural functions of neurons in the brain.
Footnotes
This work was supported by National Institutes of Health Grants AG 09219, MH 01227, MH 53603, MH 01152, and MH 12526. We thank M. Papapavlou, K. Nguyen, and G. Rao for assistance with various aspects of these experiments.
Correspondence should be addressed to Dr. C. A. Barnes at the above address. E-mail: carol@nsma.arizona.edu.
Dr. Guzowski's present address: Division of Neural Systems, Memory, and Aging, University of Arizona, Tucson, AZ 85724-5115.
REFERENCES
- 1.Abraham WC, Logan B, Thompson VL, Williams JM, Tate WP. Sequence-independent effects of phosphorothiolated oligonucleotides on synaptic transmission and excitability in the hippocampus in vivo. Neuropharmacology. 1997;36:345–352. doi: 10.1016/s0028-3908(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 2.Barnes CA, Jung MW, McNaughton BL, Korol DL, Andreasson K, Worley PF. LTP saturation and spatial learning disruption: effects of task variables and saturation levels. J Neurosci. 1994;14:5793–5806. doi: 10.1523/JNEUROSCI.14-10-05793.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR. A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci. 1998;18:5301–5310. doi: 10.1523/JNEUROSCI.18-14-05301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- 5.Curran T, Peters G, Van Beveren C, Teich NM, Verma IM. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982;44:674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dragunow M, Robertson HA. Seizure-inducible c-fos protein(s) in mammalian neurons. Trends Pharmacol Sci. 1988;9:5–6. doi: 10.1016/0165-6147(88)90229-5. [DOI] [PubMed] [Google Scholar]
- 7.Dragunow M, Abraham WC, Goulding M, Mason SE, Robertson HA, Faull RL. Long-term potentiation and the induction of c-fos mRNA and proteins in the dentate gyrus of unanesthetized rats. Neurosci Lett. 1989;101:274–280. doi: 10.1016/0304-3940(89)90545-4. [DOI] [PubMed] [Google Scholar]
- 8.Fosnaugh JS, Bhat RV, Yamagata K, Worley PF, Baraban JM. Activation of arc, a putative “effector” immediate early gene, by cocaine in rat brain. J Neurochem. 1995;64:2377–2380. doi: 10.1046/j.1471-4159.1995.64052377.x. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh MK, Cohen JS. Oligodeoxynucleotides as antisense inhibitors of gene expression. Prog Nucleic Acid Res Mol Biol. 1992;42:79–126. doi: 10.1016/s0079-6603(08)60574-7. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg ME, Ziff L. Stimulation of 3T3 cells induces transcription of the c-fos protooncogene. Nature. 1984;331:433–437. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- 11.Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci USA. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzowski JF, Hittner JM, Barnes CA, Worley PF, McGaugh JL. Behavioral regulation of the immediate-early gene Arc and its role in memory consolidation. Soc Neurosci Abstr. 1997;23:822.11. [Google Scholar]
- 13.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific induction of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 14.Hebb MO, Robertson HA. End-capped antisense oligodeoxynucleotides effectively inhibit gene expression in vivo and offer a low-toxicity alternative to fully modified phosphorothioate oligodeoxynucleotides. Brain Res Mol Brain Res. 1997;47:223–228. doi: 10.1016/s0169-328x(97)00048-x. [DOI] [PubMed] [Google Scholar]
- 15.Hooper ML, Chiasson BJ, Robertson HA. Infusion into the brain of an antisense oligonucleotide to the immediate-early gene c-fos suppresses production of fos and produces a behavioral effect. Neuroscience. 1994;63:917–924. doi: 10.1016/0306-4522(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 16.Konradi C, Cole RL, Heckers S, Hyman SE. Amphetamine regulates gene expression in rat striatum via transcription factor CREB. J Neurosci. 1994;14:5623–5634. doi: 10.1523/JNEUROSCI.14-09-05623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanahan A, Worley P. Immediate-early genes and synaptic function. Neurobiol Learn Mem. 1998;70:37–43. doi: 10.1006/nlme.1998.3836. [DOI] [PubMed] [Google Scholar]
- 18.Lau LF, Nathans D. Expression of a set of growth-related immediate early genes in BALB/c3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci USA. 1987;84:1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linzer DIH, Nathans D. Growth-related changes in specific mRNAs of cultured mouse cells. Proc Natl Acad Sci USA. 1983;80:4271–4275. doi: 10.1073/pnas.80.14.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 22.Lyford GL, Stevenson GD, Barnes CA, Worley PF. Injection of Arc antisense oligonucleotides selectively blocks hippocampal LTP maintenance in vivo. Soc Neurosci Abstr. 1996;22:734.3. [Google Scholar]
- 23.Mayford M, Bach ME, Huang Y-Y, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 24.Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 25.Moser E, Mathiesen I, Andersen P. Association between brain temperature and dentate field potentials in exploring and swimming rats. Science. 1993;259:1324–1326. doi: 10.1126/science.8446900. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa S, Pfaff DW. Application of antisense DNA method for the study of molecular bases of brain function and behavior. Behav Genet. 1996;26:279–292. doi: 10.1007/BF02359384. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa S, Brown HE, Okano HJ, Pfaff DW. Cellular uptake of intracerebrally administered oligodeoxynucleotides in mouse brain. Regul Pept. 1995;59:143–149. doi: 10.1016/0167-0115(95)00096-t. [DOI] [PubMed] [Google Scholar]
- 29.Saffen DW, Cole AJ, Worley PF, Christy BA, Ryder K, Baraban JM. Convulsant-induced increase in transcription factor messenger RNAs in rat brain. Proc Natl Acad Sci USA. 1988;85:7795–7799. doi: 10.1073/pnas.85.20.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlimgen AK, Helms JA, Vogel H, Perin MS. Neuronal pentraxin, a secreted protein with homology to acute phase proteins of the immune system. Neuron. 1995;14:519–526. doi: 10.1016/0896-6273(95)90308-9. [DOI] [PubMed] [Google Scholar]
- 31.Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 32.Szklarczyk A, Kaczmarek L. Antisense oligodeoxyribonucleotides: stability and distribution after intracerebral injection into rat brain. J Neurosci Methods. 1995;60:181–187. doi: 10.1016/0165-0270(95)00010-r. [DOI] [PubMed] [Google Scholar]
- 33.Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 34.Tsui C, Copeland NG, Gilbert DJ, Jenkins NA, Barnes CA, Worley PF. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;16:2463–2478. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wahlestedt C. Antisense oligonucleotide strategies in neuropharmacology. Trends Pharmacol Sci. 1994;15:42–46. doi: 10.1016/0165-6147(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 36.Wahlestedt C, Pich EM, Koob GF, Yee F, Heilig M. Modulation of anxiety and neuropeptide Y-Y1 receptors by antisense oligodeoxynucleotides. Science. 1993;259:528–531. doi: 10.1126/science.8380941. [DOI] [PubMed] [Google Scholar]
- 37.Wallace C, Lyford G, Worley P, Steward O. Differential intracellular sorting of immediate early gene mRNAs depends on signals in the mRNA sequence. J Neurosci. 1998;18:26–35. doi: 10.1523/JNEUROSCI.18-01-00026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widnell KL, Self DW, Lane SB, Russell DS, Vaidya VA, Miserendino MJD, Rubin CS, Duman RS, Nestler EJ. Regulation of CREB expression: in vivo evidence for a functional role in morphine action in the nucleus accumbens. J Pharmacol Exp Ther. 1996;276:306–315. [PubMed] [Google Scholar]
- 39.Worley PF, Bhat RV, Baraban JM, Erickson CA, McNaughton BL, Barnes CA. Thresholds for synaptic activation of transcription factors in hippocampus: correlation with long-term enhancement. J Neurosci. 1993;13:4776–4786. doi: 10.1523/JNEUROSCI.13-11-04776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]