Abstract
Sensorimotor gating, measured by prepulse inhibition (PPI) of the startle reflex, is reduced in schizophrenia patients and in rats treated with dopamine agonists. Strain differences in the sensitivity to the PPI-disruptive effects of dopamine agonists may provide insight into the genetic basis for human population differences in sensorimotor gating. We reported strain differences in the sensitivity to the PPI-disruptive effects of the D1/D2 agonist apomorphine in adult rats, with greater sensitivity in Harlan Sprague Dawley (SDH) versus Wistar (WH) rats. However, Kinney et al. (1999) recently reported opposite findings, using Bantin-Kingman Sprague Dawley (SDBK) and Wistar (WBK) rats; in fact, SDBK rats did not exhibit clear apomorphine-induced reductions in sensorimotor gating. These new findings of Kinney et al. (1999) directly conflict with over 15 years of results from our laboratories and challenge interpretations from a large body of literature. The present studies carefully assessed drug effects on sensorimotor gating in SD versus W strains, across rat suppliers (H vs BK). Significantly greater SDH than WH apomorphine sensitivity in PPI measures was observed in both adult and 18 d pups, confirming that these strain differences are both robust and innate. These strain differences in apomorphine sensitivity were not found in adult BK rats. Supplier differences in sensitivity (SDH > SDBK) were also evident in the PPI-disruptive effects of D1 but not D2-family agonists; PPI was clearly disrupted by quinpirole in both SDH and SDBK rats. These findings demonstrate robust, innate, neurochemically specific, and apparently heritable phenotypic differences in an animal model of sensorimotor gating deficits in human neuropsychiatric disorders.
Keywords: apomorphine, dopamine, prepulse inhibition, schizophrenia, startle, strain
Startle reflex magnitude is reduced when the startling stimulus is preceded ∼100 msec by a weak prestimulus (Graham, 1975). Prepulse inhibition (PPI) is an operational measure of sensorimotor gating that is used to study CNS mechanisms that protect the integrity of sensory and cognitive information. PPI is reduced in specific neuropsychiatric disorders characterized by deficient inhibition in sensory, motor, or cognitive domains (Braff et al., 1978, 1992; Cadenhead et al., 1993; Bolino et al., 1994; Swerdlow et al., 1995b; Castellanos et al., 1996; Kumari et al., 1999), and animal models of impaired PPI are used to understand the neural basis for these clinical conditions (cf. Geyer and Braff, 1987; Koch and Schnitzler, 1997; Swerdlow and Geyer, 1998, 1999). Recent studies have focused on the genetic regulation of PPI, as a means of understanding the potential genetic contributions to deficient sensorimotor gating in schizophrenia and other disorders (Ellenbroek et al., 1995; Bullock et al., 1997; Cadenhead et al., 1998). That such a complex phenotype might have an identifiable genetic basis is supported by findings that PPI is profoundly reduced or eliminated in humans with an autosomal-dominant genetic disorder—Huntington's disease (HD) (Swerdlow et al., 1995b)—and in mice transgenic for the HD gene (Carter et al., 1999).
In rats, strain and substrain differences have been reported in both basal levels of PPI and in the sensitivity to the PPI-disruptive effects of specific drugs. For example, PPI is reduced or eliminated by the direct dopamine (DA) agonist apomorphine, but the sensitivity to this effect varies greatly across strains and within strains across rat suppliers (Rigdon, 1990). We recently reported greater sensitivity to the PPI-disruptive effects of apomorphine in adult Sprague Dawley (SD) versus Wistar (W) rats (Harlan Laboratories, USA; SDH vs WH, respectively) (Swerdlow et al., 1997). However, in a recent report in this journal, Kinney et al. (1999) identified the oppositepattern of strain sensitivity (W > SD) in rats from Bantin-Kingman (BK; Hull, UK) and failed to detect any apomorphine-induced reduction of PPI in SDBK rats. This new report byKinney et al. (1999) could be viewed as a failure to replicate over 15 years of studies from our laboratory and other groups, which might fundamentally challenge the interpretation of findings from dozens of scientific reports. Alternatively, these different findings may reflect the fact that subtle genetic differences between supplier substrains impact on substrates responsible for the DAergic regulation of PPI; in this case, such differences might be targets of investigation for understanding the genetic regulation of this complex phenotype. Strain and supplier differences among outbred SD and W rats are found in processes that span numerous domains of neurobiological function (Luedtke et al., 1992; Oliff et al., 1996, 1997; Loscher et al., 1998; Gleason et al., 1999; Turnbull and Rivier, 1999).
The present investigation was designed to accomplish the following five goals: (1) to assess the reliability of SDH versus WH differences in sensitivity to the PPI-disruptive effects of apomorphine; (2) to determine whether these strain differences are “innate” versus “acquired,” by studying SDH and WH rats at the earliest possible developmental time point, after rearing SDH and WH pups under identical conditions; (3) to assess the dopaminergic substrates of this strain difference; (4) to assess under these same testing conditions the reliability of the lack of SDBK versus WBK strain differences in this critical measure, as reported by Kinney et al. (1999); and (5) to begin to understand the neurochemical basis for supplier-based differences in this complex phenotype. The overarching goal was to guide future studies of the genetic basis of a complex phenotype with face, predictive, and construct validity for the loss of sensorimotor gating in schizophrenia (Swerdlow et al., 1994a).
MATERIALS AND METHODS
Experimental animals. A total of 30 SDH rat pups and 29 WH pups (pup weights, 29–45 gm) and 57 adult male SDH rats, 57 adult male WH rats, 64 adult male SDBK rats, and 64 adult male WBK rats (adult weights, 250–325 gm) were used in these experiments. Studies in adults were limited to male rats, on the basis of findings of the estrous cyclicity of the PPI-disruptive effects of apomorphine in adult female rats (Koch, 1998). To match closely the rearing environments of SDH and WH pups, we housed timed pregnant female SDH and WH rats individually and housed pups with their mothers until 5–7 d after birth; at that time rat pups were sexed and redistributed so that each litter was approximately the same size and contained an equal number of male and female pups. Aside from the strain of the nursing female rat, rearing conditions for SDH and WH pups were identical; SDH and WH pups were raised in the same room, on the same cage rack. Adult male rats were housed in same-sex rooms, in groups of two or three. Methods for housing and all behavioral testing were consistent with the substantial literature of startle measures in rodents (cf. Geyer and Swerdlow, 1998). For example, a reversed 12 hr light/dark cycle was used (lights on at 19:00 hr and off at 07:00 hr) for at least 1 week before testing. After arrival from the United Kingdom, BK rats were maintained in the housing facility for at least 2 weeks before behavioral testing. Although Kinney et al. (1999) reported housing lights on at 08:00 hr, the circadian phase in which testing occurred was not clear; in the present study, all testing and drug administration occurred between 10:00 and 17:00 hr. Weiss et al. (1999) recently reported that circadian time does not modify either PPI or its disruption by apomorphine. Rats were handled regularly before any procedures to minimize stress during behavioral testing and were given access to food and water ad libitum except during behavioral testing. Throughout these studies, all efforts were made to minimize animal suffering and to reduce the number of animals used. All experiments conform to guidelines of the National Institutes of Health for the use of animals in biomedical research and were approved by the Animal Subjects Committee at the University of California, San Diego (protocol 0224907).
Drugs. Apomorphine (0.1% ascorbate/saline vehicle; 0.1, 0.25, or 0.5 mg/kg), quinpirole (saline vehicle; 0.1, 0.2, and 0.5 mg/kg), and SKF 82958 (saline vehicle; 0.1, 1.0, and 5.0 mg/kg) were administered subcutaneously to rats immediately before testing (apomorphine) or 10 min before testing (quinpirole and SKF 82958), in a volume of 1 ml/kg.
Apparatus. Startle experiments used four startle chambers (SR-LAB; San Diego Instruments, San Diego, CA) housed in a sound-attenuated room with a 60 dB ambient noise level. Each startle chamber consisted of a Plexiglas cylinder (8.7 cm internal diameter for adults; 3.75 cm internal diameter for pups) resting on a 12.5 × 25.5 cm Plexiglas stand. Acoustic stimuli and background noise were presented via a Radioshack Supertweeter mounted 24 cm above the Plexiglas cylinder. Startle magnitude was detected and recorded as transduced cylinder movement via a piezoelectric device mounted below the Plexiglas stand. Response sensitivities were calibrated (SR-LAB Startle Calibration System) to be nearly identical in each of the four startle chambers (maximum variability < 1% of stimulus range and < 5% of response ranges). Response sensitivities were calibrated for adult and pup chambers separately and recalibrated each time the chambers were changed, always within the < 5% response range. Chambers were also balanced across all experimental groups. Sound levels were measured and calibrated with a sound level meter (Quest Electronics, Oconomowoc, WI), A scale (relative to 20 μN/m2), with a microphone placed inside the Plexiglas cylinder. Methodological details can be found in published material (Geyer and Swerdlow, 1998).
Startle testing procedures. In our testing apparatus, reliable measures of startle could first be obtained in pups at 14 d of age. At 14 d of age, different groups of rat pups were exposed to a brief “matching” startle session, as reported previously (Geyer and Swerdlow, 1998; Martinez et al., 2000). Rat pups were placed in a startle chamber and exposed to 5 min of 70 dB background noise followed by 17 PULSE trials of 40 msec, 120 dB noise bursts and 5 PREPULSE + PULSE trials consisting of a 20 msec, 82 dB (12 dB above background) prepulse followed after 100 msec by a 120 dB pulse (onset to onset). Adult rats were exposed to this matching session 3–7 d before testing. Data from this session were used to assign rat pups and adults to balanced dose groups according to their average PULSE startle magnitude.
Behavioral testing continued 4 d after the matching session for pups and 2–4 d after the matching session for adults. Eighteen-day-old rat pups were brought to the laboratory in their home cages with their mothers to minimize stress before and after testing. Adult rats were brought to the laboratory in individual cages. In most cases, test sessions were ∼16 min long and consisted of 5 min of 70 dB background followed by five trial types: PULSE noise bursts, PREPULSE trials (20 msec noise bursts 5, 10, or 15 dB above background followed after 100 msec by a PULSE), and NOSTIM trials (stabilimeter recordings obtained when no stimulus was presented). The session consisted of initial and final blocks of 4 PULSE trials, separated by two blocks that included 8 PULSE trials and 15 PREPULSE trials (the latter divided equally among 5, 10, and 15 dB prepulse intensities); NOSTIM trials were interspersed between startle trials. For these NOSTIM trials, stabilimeter readings were recorded during periods in which no stimulus was presented; these trials were used to assess gross motor activity during the test session but were not included in the calculation of intertrial intervals, which were variable and averaged 15 sec. Reflex “habituation” was determined on the basis of the change in startle magnitude from the initial to the final block of PULSE trials. By the use of this design, PPI is measured during a portion of the session in which startle magnitude is relatively constant. In specific cases, modified stimulus parameters were used to test specific hypotheses, as described below.
Locomotor testing procedures. In one experiment, locomotor activity was assessed to examine possible strain differences in the dopaminergic regulation of behaviors other than startle and PPI. Horizontal locomotion was assessed using wire-mesh photocell cages (22 × 35 × 15 cm) fitted with two parallel infrared beams 1 cm above the floor, perpendicular to the long axis of the cage. Consecutive interruption of the two beams was counted as a crossover. Ten identical cages were monitored simultaneously by computer, and the total number of beam breaks and cage crossovers was calculated for each 10 min interval. Animals were familiarized with the cages for 180 min 2–4 d before testing. On testing days, animals were returned to these cages immediately after startle testing, and locomotor activity was monitored for 90 min.
Data analysis. PPI was calculated as a percent reduction in startle magnitude on PREPULSE trials compared with PULSE trials. Any drug effects on the percent PPI prompted separate analyses to assess the relationship of these effects to drug-induced changes in startle magnitude on PULSE and PREPULSE trials. An important issue raised byKinney et al. (1999) and in many previous reports (Mansbach et al., 1988; Davis et al., 1990; Swerdlow and Geyer, 1993) relates to the fact that drug-induced changes in startle magnitude—independent of prepulse effects—can change the amount of percent PPI. Unequivocal changes in sensorimotor gating occur when the reflex-inhibiting effects of prepulses are modified, independent of changes in the startle magnitude on PULSE trials. Thus, for each strain and supplier, data were assessed to determine whether drug-induced changes in the calculated amount of percent PPI reflected actual changes in sensorimotor gating per se.
All startle data were analyzed using an ANOVA with drug treatment (and for pups, sex) as between-subject factors and trial block and trial type as within-subject repeated measures. Locomotor data (both beam breaks and crossovers) were analyzed by ANOVA with drug treatment and strain as between-subject factors and time interval as a within-subject factor. Because analyses of total beam breaks and crossovers yielded identical findings, only crossovers are reported. For analysis of developmental effects on PPI, age (pup vs adult) was a between-group factor. However, because of the obvious (fivefold) differences in recorded startle magnitude between pups and adults, measures involving reflex or motor force (startle magnitude, habituation, and NOSTIM activity) were not combined across age groups. For other comparisons, strains (SD vs W) or suppliers (H vs BK) were used as between-group factors. These studies were designed to assess relatively subtle differences in drug sensitivity across a number of variables (age, strain, supplier, sex, etc.); in an attempt to limit the number of redundant comparison groups and thus the total number of animals used in these studies, and in keeping with the guidelines of the National Institutes of Health, in some cases, the same groups of rats (e.g., H-derived adults) were used in comparisons of both developmental factors (vs H-derived pups), strain differences (SDH vs WH adults), and supplier differences (H vs BK adults). Post hoc comparisons of significant interaction effects and relevant main factor effects were conducted using the Tukey-Kramer and one-factor ANOVA tests. α was set at 0.05. For ease of presentation, unless otherwise stated, several normal parametric effects can be assumed to be statistically significant in all startle analyses: effects of trial block on startle magnitude and effect of prepulse intensity on prepulse inhibition. Also, unless otherwise stated, reported values of the mean percent PPI can be assumed to be collapsed across all prepulse intensities and trial blocks. In most instances, only statistically significant effects, or those relevant to the critical comparisons, will be reported in detail.
RESULTS
The major dependent measure of these studies was PPI; all findings with this measure, in addition to startle magnitude, and the implications of these results in terms of changes in sensorimotor gating are summarized in the text and in Table1. Additional behavioral measures are also reported, because they may influence the interpretation of PPI results.
Table 1.
Schematic summary of drug effects on startle magnitude on PULSE trials, percent PPI, and sensorimotor gating1-a in six groups of rats
| Rat group | Apomorphine | SKF 82958 | Quinpirole | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Startle magnitude | PPI | Gating | Startle magnitude | PPI | Gating | Startle magnitude | PPI | Gating | |
| SDH (pup) | 0 | ⇊ | ⇊ | XXX | XXX | XXX | XXX | XXX | XXX |
| WH (pup) | 0 | ↓ | ↓ | XXX | XXX | XXX | XXX | XXX | XXX |
| SDH (adult) | 0 | ⇊ | ⇊ | 0 | ↓ | ?↓ | 0 | ↓ | ↓ |
| WH (adult) | 0 | ↓ | ↓ | ↑ | ↓ | 0 | ↓ | 0 | 0 |
| SDBK (adult) | ↑ | ↓ | ? | 0 | 0 | 0 | 0 | ↓ | ↓ |
| WBK (adult) | 0 | ↓ | ↓ | XXX | XXX | XXX | XXX | XXX | XXX |
0, No significant change; XXX, not assessed; arrows, direction and magnitude of significant change; ?, ambiguous result, see text.
A change in sensorimotor gating was inferred based on clear reduced startle-inhibiting effects of prepulses, distinct from changes in startle magnitude on pulse trials.
PPI: SDH versus WH strain differences in pups and adults
As reported previously (Martinez et al., 2000), apomorphine significantly reduced PPI in 18-d-old pups; this effect was significant in SDH but not WH pups (Fig.1A). ANOVA revealed a significant effect of strain (F = 9.06; df, 1,43;p < 0.005) and apomorphine (F = 9.85; df, 3,43; p < 0.001) and a significant strain × apomorphine interaction (F = 3.44; df, 3,43;p < 0.025). Basal (vehicle) levels of PPI did not differ significantly between SDH and WH rats. There were no significant effects of sex or interactions of sex with any combination of variables. Post hoc ANOVAs revealed no significant effect of apomorphine in WH rats (F = 2.16; df, 3,21; NS), with no significant interactions, whereas in SDH rats, there was a significant effect of apomorphine (F = 10.12; df, 3,22; p < 0.0003), with no significant interactions. Tukey tests revealed significantly reduced PPI in SDH rats treated with 0.1, 0.25, or 0.5 mg/kg doses of apomorphine (p < 0.05, all comparisons). Although the ANOVA in WH rats revealed no significant main effect of apomorphine, a one-factor ANOVA in WH pups revealed that PPI was reduced significantly by the lowest dose of apomorphine (0.1 mg/kg; F = 5.66; df, 1,10; p < 0.04) but not by higher doses of apomorphine. The different dose sensitivities across strains were revealed by significantly lower PPI in SDH versus WH rats treated with the highest dose (0.5 mg/kg) of apomorphine (F = 17.10; df, 1,12; p < 0.002) and by a trend toward this effect for the middle dose (0.25 mg/kg) of apomorphine (F = 3.75; df, 1,10; p < 0.085), despite the bias against detecting such a difference based on slightly lower baseline (vehicle) PPI levels in WH versus SDH pups [mean (SEM) %PPI, WH = 28.96 (4.44) vs SDH = 33.38 (5.51)].
Fig. 1.

Prepulse inhibition after treatment with apomorphine (vehicle or 0.1, 0.25, or 0.5 mg/kg, s.c.) in 18-d-old pups (A) or adults (B, C). SDH rats were more sensitive to the PPI-disruptive effects of apomorphine, compared with WH rats, and this phenotypic difference was evident in both pups and adults. SDH rats were also more sensitive to the PPI-disruptive effects of apomorphine, compared with SDBK rats. *p < 0.05, posthocTukey comparison after a significant strain × dose interaction for SDH versus WH pups and adults and after a significant main effect of strain in SDH versus SDBK adults. Compared with SDBK rats, WBK rats exhibited relative reduced basal levels of PPI (+; C); this reflected a relative insensitivity to prepulse effects in WBK rats, which could be overcome (#) via modifications of stimulus parameters (C).
Inspection of the raw data (Fig.2A,B) confirmed that these effects of apomorphine in SDH pups clearly reflected a disruption of sensorimotor gating, i.e., the effectiveness of the prepulse in reducing startle magnitude. For example, in comparison with SDH pups treated with vehicle, startle magnitude for PULSE trials in SDH pups treated with apomorphine (0.5 mg/kg) was nonsignificantly reduced [mean (SEM) = 67.99 (15.42) vs 61.52 (9.32), respectively], whereas startle magnitude on 15 dB PREPULSE trials was significantly increased [29.81 (7.80) vs 69.49 (12.89), respectively]. The same comparison in WH pups suggested that the PPI-disruptive effects of apomorphine in this strain were accompanied by increases in startle magnitude on both PULSE trials [mean (SEM) vehicle vs apomorphine = 53.08 (3.07) vs 71.51 (13.89), respectively] and 15 dB PREPULSE trials [mean (SEM) vehicle vs apomorphine = 26.16 (2.23) vs 55.75 (11.06), respectively]. In this respect, apomorphine effects in SDH pups resembled those reported by Kinney et al. (1999) in WBK adults, and apomorphine effects in WH pups resembled those reported by Kinney et al. in SDBK adults [see Kinney et al. (1999), their Fig. 1, p 5646]).
Fig. 2.

Startle magnitude on PULSE and PREPULSE trials in SDH (A) and WH (B) pups, SDH (C) and WH (D) adults, and SDBK (E) and WBK (F) adults. These raw data correspond to the transformed percent PPI data seen in Figure 1. An unequivocal loss of sensorimotor gating after apomorphine, demonstrated by a reduction in the startle-inhibiting effects of prepulses distinguished from changes in PULSE magnitude, was evident in some groups (SDH pups and adults, WBK adults) but not in others (SDBK adults). See Results for detailed descriptions.
Importantly, although analysis of the percent PPI suggests that both SDH and WH pups were sensitive to the PPI-disruptive effects of the lowest dose of apomorphine (0.1 mg/kg), analysis of raw data revealed significant strain differences even at this dose. The lowest dose of apomorphine resulted in a significant increase in startle magnitude on PREPULSE trials in SDH rats [mean (SEM) vehicle vs apomorphine = 42.64 (4.71) vs 89.86 (5.93), respectively] but not in WH rats [mean (SEM) vehicle vs apomorphine = 37.27 (2.55) vs 36.22 (3.37), respectively] (significant strain × apomorphine interaction,F = 9.30; df, 1,21; p < 0.005). Thus, this dose of apomorphine disrupted the startle-inhibiting effects of prepulses in SDH but not WH pups. The calculated reduction in the percent PPI in WH rats at this low dose of apomorphine actually reflected a nonsignificant reduction in startle magnitude on PULSE trials [mean (SEM) vehicle vs apomorphine = 53.08 (3.07) vs 40.59 (4.60), respectively] rather than a change in startle magnitude on PREPULSE trials. In other words, rather than reflecting an unequivocal disruption of sensorimotor gating, the apparent sensitivity of WH rats to the PPI-disruptive effects of the lowest dose of apomorphine reflected the selective startle magnitude-reducing effects of this dose of apomorphine on PULSE trials (see Discussion).
To examine the developmental course of this strain difference in PPI, ANOVAs were performed with both pups and adult rats. Because of the lack of main or interaction effects of sex on PPI measures in pups, data were collapsed across this variable for pups. Separate analyses, which included only male pups and adults, yielded identical outcomes but are not reported for clarity of presentation. ANOVA revealed significant effects of age (F = 48.57), apomorphine dose (F = 26.14), and rat strain (F = 5.40; df, 1,93; p < 0.025). There was a significant dose × strain interaction (F = 5.57; df, 3,93;p < 0.002) but no other significant interactions. In particular, there was no tendency toward a significant interaction of age × strain × dose (F < 1), confirming that the strain difference in apomorphine sensitivity was independent of the age of the rats. There were no other significant interactions of rat strain with other PPI variables, in any combination. Post hoc comparisons revealed an overall pattern of findings that was essentially identical to that detected in pups alone: PPI was diminished in a dose-dependent manner in SDH rats but reached a “plateau” in WH rats at the lowest dose tested (0.1 mg/kg); significant SDH < WH PPI levels were evident at the 0.25 and 0.5 mg/kg doses (p < 0.05, both comparisons) (Fig.1B). As with pups alone, analyses of raw data across age groups and in adults alone confirmed that the apparent sensitivity of WH rats to the lowest dose of apomorphine (0.1 mg/kg) reflected the selective reduction in PULSE startle magnitude in these rats, whereas the effects of this dose of apomorphine in SDH rats reflected an actual loss of inhibitory effectiveness of prepulses on PULSE trials (Fig.2C,D).
To understand the relative contribution of D1 versus D2 receptor stimulation to the strain differences in sensitivity to the mixed D1 and D2 agonist apomorphine, we tested drug-naive adult SDH and WH rats after treatment with the full D1 agonist SKF 82958 (0–5.0 mg/kg) or the selective D2-family agonist quinpirole (0–0.5 mg/kg). Rats were tested twice, at a 1 week interval. During each week, 16 rats from each strain were tested with one of four doses of either SKF 82958 or quinpirole, with drug order (SKF 82958 vs quinpirole) balanced across test weeks and dose groups randomized across weeks, resulting in 32 rats tested with each drug. For SKF 82958 (Fig.3), ANOVA of PPI revealed a significant effect of SKF 82958 dose (F = 4.42; df, 3,59;p < 0.008) and a significant strain × dose × block interaction. Examination of the data revealed simple monotonic PPI-reducing effects of SKF 82958 in SDH rats (F = 6.39; df, 3,30; p < 0.002) but no significant effect of SKF 82958 on PPI in WH rats (F = 1.30; df, 3,29; NS) (Fig. 3A).
Fig. 3.

Percent prepulse inhibition (A) and startle magnitude (B) after treatment with the full D1 agonist SKF 82958 in adult SDH, WH, and SDBK rats (*p < 0.05, in SDH rats, for 5.0 mg/kg vs vehicle, by posthoc comparison after a significant main effect of dose). See Results for detailed descriptions.
Inspection of the raw data revealed no unambiguous impact of SKF 82958 on sensorimotor gating per se in either SDH or WH rats; changes in startle magnitude on PREPULSE trials generally paralleled drug-induced changes in startle magnitude on PULSE trials. Supporting a gating-disruptive effect in SDH rats was the fact that PPI diminished with increasing doses of SKF 82958, independent of fluctuations in PULSE magnitude (Fig. 3B, top); in fact, the average reflex magnitude on PREPULSE trials increased in SDH rats treated with 1.0 or 5.0 mg/kg SKF 82958 [mean (SEM) vehicle = 61.02 (9.14); 1.0 mg/kg = 72.08 (7.20); 5.0 mg/kg = 83.08 (8.95)], whereas the reflex magnitude on PULSE trials decreased over this dose range [mean (SEM) vehicle = 236.4 (42.5); 1.0 mg/kg = 221.7 (36.9); 5.0 mg/kg = 172.7 (21.2)]. In WH rats, reduced PPI was evident only in conjunction with significant increases in PULSE magnitude (Fig. 3B, middle). It is important to note that although SKF 82958 had no obvious adverse effects on SDH rats, three WH rats responded to SKF 82958 with extreme behavioral excitation (paw treading, hyperactivity, intense high-pitched vocalizations, and some seizure-like activity; high dose,n = 2; middle dose, n = 1) and were eliminated from analyses; one of these rats died within hours of testing.
For quinpirole (Fig. 4), ANOVA of PPI revealed a significant effect of quinpirole dose (F = 7.50; df, 3,59; p < 0.002) but no two- or three-way interactions involving strain and quinpirole dose (Fig.4A). Thus, SDH and WH rats appeared to be approximately equally sensitive to the PPI-disruptive effects of quinpirole (this effect was significant for 0.2 and 0.5 mg/kg quinpirole in SDH rats and for 0.2 mg/kg quinpirole in WH rats). However, inspection of the raw data (Fig. 4B) revealed that this quinpirole-induced disruption of PPI in SDH rats reflected an unequivocal loss of sensorimotor gating (i.e., reduced startle-inhibiting effectiveness of the prepulse) (Fig.4B, top), whereas in WH rats, quinpirole resulted in a dose-dependent reduction in startle magnitude on PULSE trials but had no effect on startle magnitude on PREPULSE trials [for example, at the 0.2 mg/kg dose, which significantly reduced the percent PPI, mean (SEM) startle magnitude was 94.6 (10.5) vs 292.6 (69.0) for vehicle-treated rats] (Fig. 4B,middle).
Fig. 4.
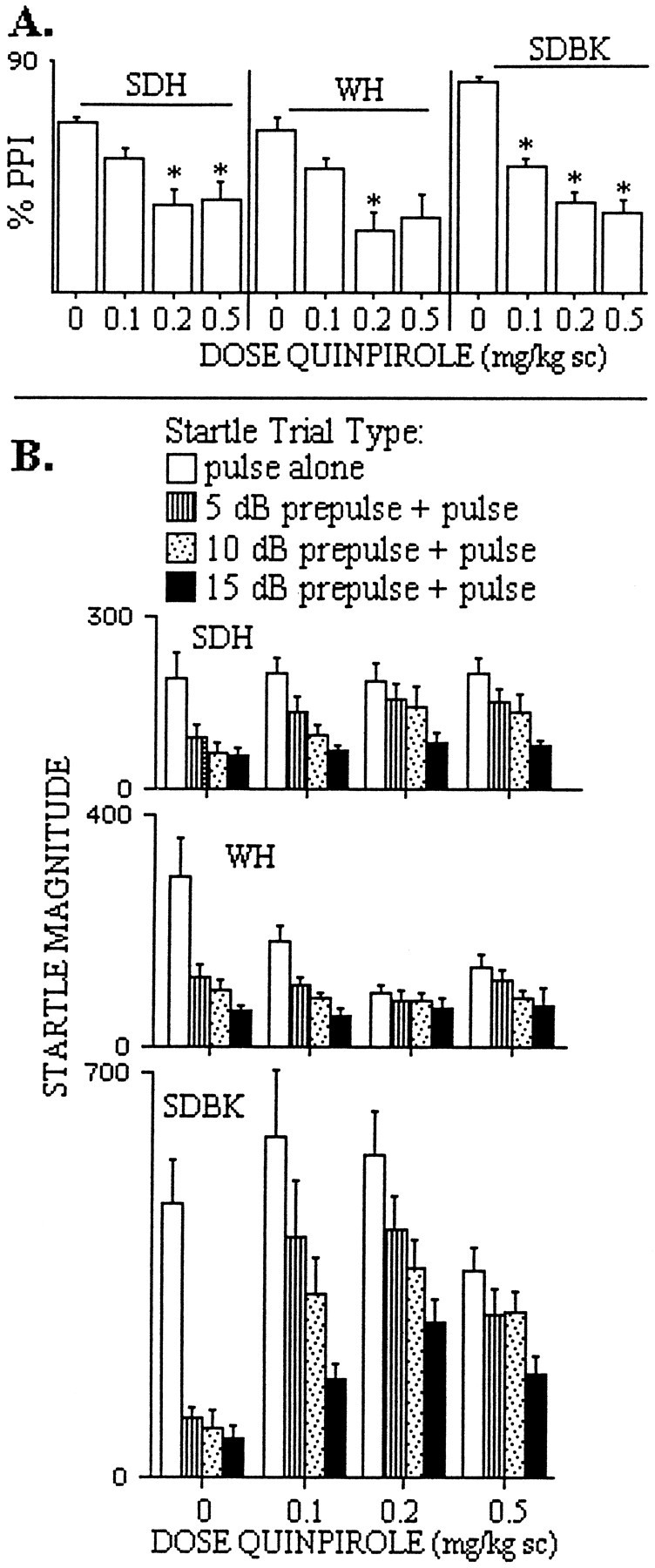
Percent prepulse inhibition (A) and startle magnitude (B) after treatment with the D2-family agonist quinpirole in adult SDH, WH, and SDBK rats (*p < 0.05 vs vehicle, by posthoc comparison after a significant main effect of dose). See Results for detailed descriptions.
To understand further the basis of strain differences in the dopaminergic regulation of PPI and other behaviors, we tested adult SDH and WH rats (n = 50) that had been studied in measures of apomorphine sensitivity again 2 weeks later, after treatment with saline or d-amphetamine (0.5, 1.0, 2.0, or 4.0 mg/kg; n = 4–6 per strain per dose; apomorphine dose history randomized across groups) (Fig.5). Startle testing was followed by locomotor activity measures for 90 min. Despite strain differences in sensitivity to the PPI-disruptive effects of direct DA receptor stimulation, no such differences were evident in the sensitivity to the PPI-disruptive effects of the indirect DA agonist amphetamine (Fig.5A). ANOVA revealed a marginal main effect of amphetamine dose (F = 2.60; df, 4,40; p = 0.051), no significant effect of strain, and no significant strain × dose interaction (F < 1, both comparisons). There was a significant effect of prepulse intensity but no other main or interaction effects. Comparison with saline-treated rats revealed a significant reduction in PPI in rats treated with 4.0 mg/kg amphetamine (F = 12.01; df, 1,18; p < 0.003), with no significant effect of strain or dose × strain interaction (F < 1, both comparisons).
Fig. 5.
Effects of the indirect DA agonist amphetamine on PPI (A) and locomotor activity (B) in SDH and WH rats. Both strains were equally sensitive to the dose-dependent PPI-disruptive effects of amphetamine. Compared with SDH rats, WH rats exhibited more robust locomotor activation after amphetamine, evident at doses that did not produce focused stereotyped behaviors (0.5–2.0 mg/kg).
Rats were moved directly from the startle boxes to photocell activity monitors (Fig. 5B). ANOVA of photocell crossovers revealed a significant effect of amphetamine dose (F = 12.59; df, 4,40; p < 0.0001) and strain (F = 11.94; df, 1,40; p < 0.002) but no significant dose × strain interaction. There was a significant effect of time interval and dose × interval interaction but no other significant two- or three-way interactions. Inspection of the data revealed (1) the expected “inverted-U” dose–response effects of amphetamine, reflecting the tendency of higher doses of amphetamine to produce focused stereotyped behaviors, accompanied by lower crossover counts; 2) the tendency of SDH and WH rats to exhibit maximal locomotor activity at 1.0 and 2.0 mg/kg, respectively; and 3) that the significant effect of strain by ANOVA was entirely a reflection of greater WH versus SDH locomotor activity for locomotor-stimulating doses of amphetamine (e.g., 0.5, 1.0, and 2.0 mg/kg; effect of strain for these doses, F = 10.48; df, 1,22; p< 0.004), rather than differences in baseline (saline) or high-dose amphetamine (4.0 mg/kg) levels of locomotor activity (F< 1, both comparisons). Results were similar whether data were limited to the initial 10–30 min after startle testing or whether beam breaks rather than crossovers were analyzed.
PPI: SDBK versus WBK strain differences in adults
This study was designed to determine whether the findings ofKinney et al. (1999) could be reproduced in our facility. ANOVA revealed significant effects of strain (F = 55.17; df, 1,55; p < 0.0001) and apomorphine (F = 15.07; df, 1,55; p < 0.0001) and a trend toward a strain × apomorphine interaction (F = 2.38; df, 3,55; p < 0.08). There was a significant effect of prepulse intensity and a significant trial block × apomorphine interaction but no other significant main or interaction effects. Inspection of the data (Fig. 1C) revealed more pronounced SDBK than WBK PPI levels in vehicle-treated rats, indicating strain differences in the amount of “baseline” PPI, independent of PPI drug sensitivity.
Inspection of startle magnitude on PULSE and PREPULSE trials (Fig.2E,F) revealed findings similar to those reported by Kinney et al. (1999; see their Figs. 1, 2, p 5646). Apomorphine caused a monotonic dose-dependent increase in startle magnitude on both PULSE and PREPULSE trials in SDBK rats. Although proportionately larger apomorphine-induced increases in startle magnitude were observed on PREPULSE versus PULSE trials in SDBK rats, it would be difficult to argue that these effects reflected a simple disruption of sensorimotor gating per se. In contrast, and consistent with the finding of Kinney et al. (1999), the disruption of PPI in WBK rats reflected a reduction in the effectiveness of the prepulse to inhibit startle, rather than a generalized increase in startle magnitude across all trial types.
PPI: H versus BK supplier differences in adults
Comparison of WH versus WBK rats (Fig. 1B,C) revealed significant effects of supplier (F = 22.35; df, 1,48; p < 0.0001) and apomorphine (F = 11.86; df, 3,48; p < 0.0001) but no significant supplier × apomorphine interaction (F < 1). There was a significant effect of prepulse intensity but no other significant main or interaction effects. Inspection of the data revealed that the significant supplier difference reflected greater WH than WBK levels of PPI at both vehicle and active doses of apomorphine. Thus, these data suggest supplier-based differences in basal PPI levels, rather than apomorphine sensitivity, per se. As noted above, analyses of raw startle magnitudes revealed that these effects of apomorphine on PPI in WBK rats reflected an actual disruption of sensorimotor gating, whereas apomorphine effects on PPI in WH rats appeared to reflect a more complex interaction of drug effects on baseline startle magnitude.
To determine whether the relatively reduced levels of PPI in WBK rats reflected an absolute limitation of this substrain, versus a differential sensitivity to particular stimulus parameters, a second study was performed in drug-naive WBK rats. Startle and PPI were assessed in WBK rats in a session with stimuli designed to elicit maximal PPI levels [only 15 dB prepulse stimuli] and relatively lower startle magnitudes [105 in addition to 120 dB pulse intensities]. As seen in Figure 1C, by the use of these stimulus parameters, PPI levels in WBK approximated those exhibited by WH rats; for example, the mean (SEM) PPI was 58.47 (6.44) for 105 dB startle stimuli (greater than the mean PPI level reported above for WBK rats tested with the “standard” startle session [36.36 (3.13)] but comparable with the mean PPI level that was exhibited by WH rats in that session [59.75 (3.55)]). ANOVA revealed significant effects of apomorphine (F = 3.95; df, 3,28; p < 0.02), with no other significant main or interaction effects. Post hoc Tukey comparisons revealed that PPI was significantly reduced by 0.25 and 0.5 mg/kg doses of apomorphine (p < 0.05, both comparisons). Thus, supplier differences resulting in greater WH than WBK levels of PPI reflected a relatively reduced sensitivity to specific stimulus features in WBK rats, rather than an absolute limitation in their ability to manifest PPI.
Comparison of SDH versus SDBK rats (Fig. 1B,C) revealed significant effects of supplier (F = 6.86; df, 1,49; p < 0.015) and apomorphine (F = 23.14; df, 3,49; p < 0.0001) but no significant supplier × apomorphine interaction (F = 2.04; df, 3,49; NS). There was a significant effect of prepulse intensity, and interactions of apomorphine × intensity and apomorphine × intensity × block, but no other significant main or interaction effects. Inspection of the data revealed that the significant supplier difference was entirely a reflection of relatively lower PPI levels in SDH versus SDBK rats at the 0.25 mg/kg (F = 8.25; df, 1,12; p < 0.015) and 0.5 mg/kg (F = 4.80; df, 1,13; p < 0.05) doses of apomorphine, with nearly equal levels of PPI across suppliers at the vehicle [mean %PPI (SEM), SDH = 67.9 (2.59); SDBK = 71.69 (4.05)] and 0.1 mg/kg [mean %PPI (SEM), SDH = 44.17 (4.54); SDBK = 42.92 (4.16)] doses. As noted above, analyses of raw startle magnitudes revealed that these effects of apomorphine on PPI in SDH rats reflected an actual disruption of sensorimotor gating (Fig. 2C), whereas apomorphine effects on PPI in SDBK rats appeared to reflect a more complex interaction of drug effects on baseline startle magnitude (Fig.2E).
In addition to strain differences in PPI apomorphine sensitivity (SDH > WH and WBK > SDBK), these findings thus suggest supplier-based differences in apomorphine sensitivity among SD rats (SDH > SDBK). To explore the neurochemical basis of this supplier difference, we tested drug-naive SDBK rats as described above for WH and SDH rats, with the full D1 agonist SKF 82958 (Fig. 3) and the D2-family-selective agonist quinpirole (Fig. 4).
As noted above in comparisons of SDH versus WH rats, SDH rats exhibited a significant dose-dependent disruption of PPI after treatment with either SKF 82958 or quinpirole. For SD rats, ANOVA comparing sensitivity to SKF 82958 across suppliers (H vs BK) revealed significant effects of supplier (F = 7.52; df, 1,56;p < 0.009) and SKF 82958 (F = 5.22; df, 1,56; p < 0.004) but no supplier × SKF 82958 interaction (F = 1.22; df, 3,56; NS) (Fig.3A). There was a significant effect of prepulse intensity but no other significant main or interaction effects. Examination of the results revealed that the main effect of supplier could not be accounted for by differences in PPI in rats treated with vehicle [mean %PPI (SEM), SDH = 78.69 (1.86); SDBK = 78.06 (2.34)] but rather reflected lower PPI levels in SDH versus SDBK rats treated with active doses of SKF 82958 (F = 8.46; df, 1,41;p < 0.006 across active doses). Consistent with this, although SKF 82958 significantly reduced PPI in SDH rats (reported above; p < 0.002), there was no significant effect of this drug on PPI in SDBK rats (F < 1) (Fig.3A). Thus, SDH rats were sensitive to the PPI-disruptive effects of the full D1 agonist, whereas SDBK rats were not. Interestingly, SDBK rats exhibited a sensitivity to the adverse effects of SKF 82958 that appeared very similar to what was exhibited by WH rats, with seizure-like activity and intense, high-pitched vocalizations after the high dose in three rats, who were subsequently removed from analyses, one of whom died shortly after testing.
SD rats from both suppliers were sensitive to the PPI-disruptive effects of the D2-family agonist quinpirole (Fig. 4). ANOVA revealed a significant effect of quinpirole dose (F = 21.06; df, 3,58; p < 0.0001), no significant effect of supplier (F < 1), and no significant supplier × quinpirole interaction (F = 1.43; df, 3,58; NS). In addition to a significant effect of prepulse intensity, ANOVA also revealed significant interactions of quinpirole × intensity, trial block × intensity, supplier × trial block × intensity, and quinpirole × trial block × intensity, although there were no interactions that included both supplier and quinpirole factors. These complex interactions reflected the greater impact of quinpirole on PPI elicited by a weak prepulse (5 dB) early in the test session (block 1), as we have reported previously (Wan and Swerdlow, 1993). ANOVA of PPI limited to trial block 1 confirmed the relevant effects described above: a significant effect of quinpirole but no significant effect of supplier or interactions of quinpirole × supplier alone or in combination with any other variable. Separate ANOVAs in SDH and SDBK rats each verified significant effects of quinpirole on PPI (p < 0.01, both comparisons). The magnitude of PPI-disruptive effects of this selective D2 agonist in SDBK rats (Fig. 4A,right) was essentially identical to the effects of those of the mixed D1 and D2 agonist apomorphine (Fig. 1), suggesting that the addition of D1 agonist properties added little “impact” to the PPI-disruptive effects of apomorphine in SDBK rats; this contrasts with the effects of quinpirole on PPI in SDH rats, which had much less of an impact on PPI compared with the mixed D1 and D2 agonist apomorphine in these rats.
Because the studies above were not able to demonstrate clearly that apomorphine-induced disruptions of PPI in SDBK rats did not simply reflect drug-induced increases in startle magnitude, rather than reduced sensorimotor gating per se, we examined both PPI and raw startle magnitude scores on PULSE and PREPULSE trials in quinpirole-treated SDBK rats. In sharp contrast to the effects observed with apomorphine in the present studies (above) and in studies byKinney et al. (1999), quinpirole clearly decreased sensorimotor gating in SDBK rats, independent of changes in startle magnitude on PULSE trials (Fig. 4B, bottom). Thus, although quinpirole resulted in a weak inverted-U pattern of changes in PULSE startle magnitude, startle magnitude on PREPULSE trials was significantly increased by quinpirole at doses that slightly increased (0.1 or 0.25 mg/kg) or decreased (0.5 mg/kg) PULSE startle magnitude. Interestingly, under these conditions of selective D2 receptor stimulation, the “profile” of quinpirole effects on startle scores across trial types in SDBK rats closely resembled the profile produced by apomorphine in WBK rats (Figs. 4B,bottom, vs 2F).
Startle magnitude in SDH and WH pups and adults
Startle magnitude on PULSE trials was analyzed in session blocks 2 and 3, which also contained mixed PREPULSE trials used in the calculation of percent PPI. The effects of apomorphine on startle magnitude were complex in both pups and adults, but importantly, they did not appear to parallel in any systematic way the pattern of drug effects on PPI. In SDH pups, apomorphine tended to elevate startle magnitude at the lower doses (0.1 and 0.25 mg/kg) but not at the highest dose (0.5 mg/kg); in WH pups, startle was reduced by the lowest dose of apomorphine (0.1 mg/kg), increased by the middle dose (0.25 mg/kg), and not affected significantly by the highest dose (0.5 mg/kg). Furthermore, these complex dose effects varied somewhat across sex. ANOVA of startle magnitude revealed a significant effect of trial block (F = 7.17; df, 1,43; p < 0.015), but no other significant main effects, and a significant three-way interaction of apomorphine × block × sex (F= 5.03; df, 3,43; p < 0.005), but no other significant interactions. This interaction reflected a complex pattern. In male pups, the lowest dose (0.1 mg/kg) of apomorphine reduced startle magnitude in block 2, but not block 3, whereas the middle dose (0.25 mg/kg) of apomorphine increased startle in both blocks, and the highest dose (0.5 mg/kg) had no effect on startle magnitude in either block. In female pups, startle magnitude was increased by the low and middle (but not high) doses of apomorphine in block 2. In block 3, there was a simple linear dose relationship, with maximal increases in startle magnitude at the highest dose of apomorphine. The most immediately relevant implication of these findings is that the effects of apomorphine on startle magnitude followed complex dose-, sex-, and time-dependent patterns that were dissociated from the effects of apomorphine on PPI in SDH pups but not in WH pups. In the most obvious example of this dissociation, PPI was maximally disrupted in SDH pups by the highest dose of apomorphine (0.5 mg/kg), which had no significant effect on startle magnitude; in WH pups, the maximal PPI-disruptive effects were observed at the 0.1 mg/kg dose, which also significantly reduced startle magnitude, as noted above.
In adult H rats, ANOVA of apomorphine effects revealed a significant effect of trial block (F = 4.21; df, 1,42;p < 0.05) but no other significant main or interaction effects. Inspection of the dose–response data revealed a U-shaped pattern of a trend toward decreased startle magnitude at the middle, but not the highest, dose of apomorphine in both strains. Again, these dose effects could not be related in any consistent manner to drug effects on PPI. Effects of SKF 82958 and quinpirole on startle magnitude differed significantly across strains; although SDH rats were relatively insensitive to effects of these drugs on startle magnitude, WH rats exhibited a dose-dependent increase in startle magnitude in response to SKF 82958 (F = 3.15; df, 3,29;p < 0.04) and a decrease in startle magnitude in response to quinpirole that approached significance (F= 2.59; df, 3,29; p < 0.075) and was most robust at the middle dose (0.2 mg/kg). In tests with amphetamine (0–4.0 mg/kg), ANOVA of startle magnitude revealed no significant effects of strain or amphetamine dose or any interactions.
Startle magnitude in SDBK and WBK adults
WBK rats exhibited higher baseline startle magnitude than did SDBK rats, and SDBK rats exhibited a clear dose-dependent increase in startle magnitude in response to apomorphine. ANOVA revealed significant effects of strain (F = 12.49; df, 1,55;p < 0.001) and apomorphine (F = 3.85; df, 3,55; p < 0.015) but no significant strain × apomorphine interaction (F = 1.37; df, 3,55; NS). There was a significant effect of trial block and a significant strain × block interaction. Post hoc comparisons revealed significantly higher startle magnitude in WBK versus SDBK rats treated with apomorphine vehicle (F = 12.46; df, 1,14;p < 0.004), confirming that this effect is independent of apomorphine dose. Apomorphine increased startle magnitude in SDBK rats (F = 6.29; df, 3,28; p < 0.003;post hoc Tukey comparison of vehicle vs 0.5 mg/kg,p < 0.05) but not in WBK rats (F = 1.11; df, 3,27; NS). In contrast to the effects of apomorphine, neither the full D1 agonist SKF 82958 nor the selective D2-family agonist quinpirole had significant main or interaction effects on startle magnitude in SDBK rats.
Habituation in SDH and WH pups and adults
Habituation was assessed by changes in startle magnitude between blocks 1 and 4, which were the initial and final trial blocks, and consisted only of five consecutive PULSE trials. Apomorphine reduced startle habituation in SDH but not WH pups. ANOVA revealed significant effects of trial block (initial vs final; F = 63.35), an interaction of strain × trial block (F = 15.83; df, 1,43; p < 0.0005), and no other significant main or interaction effects. On the basis of the significant interaction, separate ANOVAs in the two strains revealed significant effects of trial block in both SDH (F = 11.89; df, 1,22; p < 0.003) and WH (F= 52.36) pups. In WH pups, there were no other significant main or interaction effects, whereas in SDH pups, there were significant interactions of trial block × sex and trial block × apomorphine. These interactions reflected less habituation in SDH female versus male pups and a substantial reduction of habituation at all doses of apomorphine. Thus, although mean startle declined ∼77 units between the initial and final PULSE blocks among SDH pups treated with vehicle, it declined only ∼12, 2, and 13 units in SDH pups treated with 0.1, 0.25, and 0.5 mg/kg apomorphine, respectively. Sex differences in habituation in SDH pups appeared to reflect primarily the sex differences in startle magnitude during the initial trial block, rather than a failure of response decrement across the session.
In adult H rats, ANOVA revealed a significant effect of strain (F = 12.50; df, 1,42; p < 0.001) and trial block (F = 55.26), a significant interaction of strain × trial block (F = 10.51; df, 1,42;p < 0.003), but no other significant interactions. The significant interaction reflected substantially greater startle magnitude in the initial trial block in SDH versus WH rats [mean startle magnitude (SEM) = 825.6 (91.3) vs 449.6 (49.8), respectively]; in the context of such extreme differences in initial startle magnitude, it would be difficult to detect a clear strain difference in reflex habituation per se. Analyses of reflex habituation after treatment with SKF 82958 or quinpirole revealed effects on startle magnitude (noted above) but no significant interactions of strain × trial block. In tests with amphetamine (0–4.0 mg/kg), ANOVA of startle habituation revealed no significant effects of strain or amphetamine dose or any interactions.
Habituation in SDBK and WBK adults
ANOVA of startle magnitude in the first and last sequence of PULSE trials revealed significant effects of strain (F = 9.73; df, 1,55; p < 0.003), apomorphine (F = 3.59; df, 3,55; p < 0.02), and trial block (F = 85.55) but no significant interaction effects. Examination of the data revealed dose-dependent increases in startle magnitude in the final block in SDBK rats and an inverted-U dose–response function in block 1 in WBK rats. In SDBK rats, the selective D2-family agonist quinpirole had no significant effects on startle magnitude in either the initial or final trial block [significant effect of trial block (F = 84.10), but not quinpirole, and no significant quinpirole × block interaction].
NOSTIM activity in SDH and WH pups and adults
Apomorphine increases gross motor activity in adult rats, and in the present study, there was a trend toward this effect in pups, which did not differ across strains. ANOVA of NOSTIM activity revealed a near-significant effect of apomorphine (F = 2.76; df, 3,43; p = 0.054) and no significant effect of strain or sex. There were no other significant main or interaction effects. For both SDH and WH pups, NOSTIM activation followed an inverted-U-shaped dose–response function, with maximal effects at the middle dose (0.25 mg/kg) in SDH pups and the lowest dose (0.1 mg/kg) in WH pups;post hoc comparisons revealed that NOSTIM activity at these doses was significantly greater than vehicle levels for both SDH (F = 5.17; df, 1,12; p < 0.05) and WH (F = 8.58; df, 1,10; p < 0.02) pups. As with apomorphine-induced increases in startle magnitude, apomorphine-induced increases in NOSTIM activity were clearly dissociated by dose sensitivity from apomorphine effects on PPI in SDH pups but not in WH pups. In adults, ANOVA of NOSTIM levels revealed no significant or near-significant effects of any variable (allp values > 0.22). Although the D1 agonist SKF 82958 produced no significant changes in NOSTIM activity, the D2 agonist quinpirole produced a dose-dependent increase in NOSTIM activity in SDH (F = 5.35; df, 3,30; p < 0.005) but not WH (F < 1) rats; this strain difference was reflected in a significant dose × strain interaction in the overall ANOVA (F = 4.38; df, 3,59; p < 0.008). In tests with amphetamine (0–4.0 mg/kg), ANOVA revealed greater levels of NOSTIM activity in WH rats (F = 5.84; df, 1,40; p < 0.025) but no significant effects of amphetamine dose or any interactions.
NOSTIM activity in SDBK and WBK adults
ANOVA of NOSTIM activity in BK rats revealed no significant effects of any variables in studies with apomorphine, quinpirole, or SKF 82958.
DISCUSSION
Differences in drug sensitivity across rat strains and substrains may explain apparent discrepancies in reports of the PPI-disruptive effects of DA agonists. Perhaps more important, these differences may reflect relatively subtle genetic drift, which might be manipulated by pharmacogenetic strategies and ultimately serve as targets for quantitative trait loci (QTL) or other approaches for understanding the genetics of complex phenotypes. Because our previous studies (Swerdlow et al., 1997) suggested such strain differences in an animal model with face, predictive, and construct validity for deficient sensorimotor gating in schizophrenia (Swerdlow et al., 1994a), approaches to understanding the genetic basis of this complex phenotype held particular interest. However, speculation of a genetic basis for strain differences in this complex phenotype was tempered by the report ofKinney et al. (1999), which raised the possibility that the apparent apomorphine-induced disruption of PPI in SD rats is actually an artifact of drug-induced changes in startle reflex magnitude. The present study was designed to clarify this conflicting set of findings and to shed light on the biological basis of this potentially important complex phenotype. The results suggest that there are bothstrain and supplier differences in the sensitivity to the sensorimotor gating-disruptive effects of DA agonists. Strain (SDH > WH) differences appear to reflect differences in sensitivity in at least D2, and perhaps D1 and D2, substrates; supplier differences (SDH > SDBK) appear to reflect differences only in D1 substrates.
The present findings demonstrate that in Harlan-derived rats, SD > W strain differences in sensitivity to the PPI-disruptive effects of apomorphine are robust, and evident in 18-d-old identically reared SD and W rat pups, and thus must reflect innate physiological differences that exist by this date. This strain difference does not “develop” during adolescence or adulthood, does not reflect environmental influences that might arise from differences in housing or rearing conditions at the supplier facility (because pups were reared on site under identical conditions), and is not dependent on hormonal changes associated with puberty. These data provide a temporal separation between the substrate(s) responsible for this strain difference and those associated with postpubertal changes in DA function implicated in models of the pathophysiology of schizophrenia (Lipska et al., 1995). Previous reports have identified both the heritability of apomorphine sensitivity in PPI measures (Ellenbroek et al., 1995) and strain differences in the D2 receptor gene locus between SD and W rats (Luedtke et al., 1992). Because the D2 receptor is an important substrate regulating the PPI-disruptive effects of apomorphine (Swerdlow et al., 1994a), it would be a strong candidate for mediating the observed SDH versus WH strain differences in PPI. In this study, there were clear strain differences in sensitivity to the sensorimotor gating-disruptive effects of the D2-family agonist quinpirole (SDH rats were sensitive to these effects, whereas WH rats exhibited changes in PPI that fully reflected drug-induced changes in startle magnitude). These strain differences were accompanied by differences in quinpirole effects on startle magnitude (no effect in SDH rats vs depressed startle in WH rats) and gross motor (NOSTIM) activity (increased in SDH rats vs no change in WH rats). Thus, in Harlan-derived rats, the SD strain exhibits unequivocal sensorimotor gating-disruptive effects of D2 agonists, whereas the W strain does not.
Strain differences in the D1 regulation of sensorimotor gating were not as clearly demonstrated in this study, because the PPI-disruptive effects of the full D1 agonist SKF 82958 (evident in SDH but not WH rats) were less clearly distinguished from drug-induced changes in startle magnitude. At most, findings were suggestive of a weak reduction of sensorimotor gating in response to SKF 82958 in SDH rats. Interestingly, the magnitude of the PPI-disruptive effects of the mixed D1 and D2 agonist apomorphine in SDBK rats (Fig. 1C) was completely matched by that of the D2 agonist quinpirole (Fig.4A, right); it appears that the added D1 activity of apomorphine added little to its PPI-disruptive impact in SDBK rats. In contrast, quinpirole effects on PPI in SDH rats amounted to only approximately one-half of the total impact of apomorphine in these rats; we have reported previously synergistic D1 and D2 interactions in the regulation of PPI in SDH rats (Peng et al., 1990;Wan and Swerdlow, 1993). The precise regulation of PPI and startle magnitude by interacting D1 and D2 substrates differs somewhat across laboratories in published reports (Hoffman and Donovan, 1994; Wan et al., 1996b; Meloni and Davis, 1999), and it is conceivable that some of the inconsistencies across laboratories might reflect subtle differences in this substrate across substrains of rats. For example, although we had not observed startle-potentiating effects of the D1 agonist SKF 82958 previously in SDH rats (Wan et al., 1996b) or such effects in the present study, SKF 82958 clearly potentiated startle in WH rats in the present study, as it has been reported to do by Meloni and Davis (1999) in SD (Charles River) rats. It is not clear how this strain difference may be related to the fact that WH (and SDBK) but not SDH rats exhibited an apparent severe stress-like response after treatment with higher doses of SKF 82958 (hyperarousal and seizure-like activity, occasionally to the point of mortality).
Strain differences among BK rats were evident in baseline levels of PPI (SDBK > WBK) but not in sensitivity to the PPI-disruptive effects of apomorphine. These strain differences in baseline PPI appeared to be a function of sensitivity to specific stimulus parameters rather than an absolute limitation of PPI in WBK rats, because a modification of stimulus parameters resulted in PPI levels in WBK rats that were comparable with those exhibited by SDBK rats.
The present findings also confirm the differential development of processes mediating basal levels of PPI, versus those responsible for the dopaminergic regulation of PPI. Thus, although basal PPI levels clearly increase across rat development, as reported previously (Martinez et al., 2000), the ability of apomorphine to cause a dose-dependent disruption of PPI is fully evident by day 18. We reported this developmental course previously for apomorphine effects on PPI and have observed a similar time course for the PPI-disruptive effects of phencyclidine (Martinez et al., 2000). The ability to detect drug effects on PPI at an early age should facilitate rapid and economical “through-put” in pharmacogenetic studies (Ellenbroek and Cools, 1998; Swerdlow and Geyer, 1998). The present studies suggest a lack of sex differences in apomorphine sensitivity in rat pups, and thus female pups may also be suitable for such early developmental characterization of a PPI drug sensitivity “phenotype.” This is not the case with adult female rats, which exhibit estrous cyclicity in their sensitivity to the PPI-disruptive effects of apomorphine that might complicate a simple assessment of a drug sensitivity phenotype (Koch, 1998).
Rigdon (1990) and others (Varty and Higgins, 1994; Hitchcock et al., 1999) reported differences in sensitivity to the PPI-disruptive effects of apomorphine not only across strains but within strains, across suppliers. The present findings confirm differences in apomorphine sensitivity in this measure among SD rats obtained from H and BK suppliers and, in this manner, may reconcile a large literature of PPI results with the recent report by Kinney et al. (1999), which demonstrated strain differences in sensitivity to the PPI-disruptive and startle magnitude-potentiating effects of apomorphine in BK-derived rats that were entirely opposite to the findings in Harlan-derived rats reported by our group previously (Swerdlow et al., 1997) and in the present study.
Perhaps more important, Kinney et al. (1999) reported that calculations suggesting an apomorphine disruption of PPI in SDBK rats were misleading, in that they reflected changes in “basic startle amplitude” rather than in sensorimotor gating per se. Without evidence to the contrary, this finding challenged the interpretation of a large body of previous reports of the apomorphine-disruptive effects of PPI in SD rats. The present results provide compelling evidence of the sensitivity of Harlan-derived SD rats to the PPI-disruptive effects of apomorphine, which are independent of drug-induced changes in startle magnitude. This sensitivity appears to differ across suppliers, with more SDH than SDBK sensitivity evident in the PPI-disruptive effects of the mixed D1 and D2 agonist apomorphine and the selective D1 agonist SKF 82958 but with comparable sensitivity in SDH and SDBK rats to the PPI-disruptive effects of the selective D2-family agonist quinpirole. Partially confirming the findings of Kinney et al. (1999), in the present study, the PPI-disruptive effects of apomorphine in SDBK rats could not be easily dissociated from drug-induced changes in startle amplitude, whereas WBK rats more clearly demonstrated an apomorphine-induced reduction in the sensorimotor gating of startle. However, unlike the response to the mixed D1 and D2 agonist apomorphine, SDBK rats responded to quinpirole with a clear disruption of sensorimotor gating, which was independent of drug-induced changes in startle magnitude. The present studies did not specifically test whether SDBK versus WBK strain differences in apomorphine sensitivity reflected differences within a particular DA receptor subtype, but the pattern of response of SDBK rats treated with a selective D2 agonist closely resembled the pattern exhibited by WBK rats in response to the mixed D1 and D2 agonist apomorphine.
Although sensitivity to the PPI-disruptive effects of apomorphine varies with rat strain and supplier, it is extremely stable within strain and supplier. In >15 reports from our groups since 1988, including >30 experimental variations and nearly 1000 rats, apomorphine has always resulted in a significant disruption of PPI, with near-total elimination of PPI at subcutaneous doses of ∼0.5 mg/kg or less (Mansbach et al., 1988; Caine et al., 1991, 1995;Swerdlow et al., 1991a,b, 1994a,b, 1995a, 1996, 1997; Swerdlow and Geyer, 1993; Lipska et al., 1995; Wan and Swerdlow, 1996; Wan et al., 1996a,b; Kodsi and Swerdlow, 1997; Hart et al., 1998; Geyer et al., 1999; Martinez et al., 2000). These studies included various stimulus parameters and modalities, and in some cases, near-total disruption of PPI occurred with doses as low as 0.2 mg/kg (Swerdlow et al., 1994a; Caine et al., 1995; Martinez et al., 2000). Obviously, such stability is an important feature for the use of behavioral measures as phenotypic markers of gene expression.
Genetic differences in the dopaminergic regulation of sensorimotor gating may create some difficulties in reconciling findings across laboratories and suppliers. Importantly, however, the differences in apomorphine sensitivity of PPI across strains and within strains—across suppliers—may be crucially valuable in identifying the genetic basis for this phenotype. We have determined recently that the F1 PPI/apomorphine phenotype of an SDH × WH cross is that of the parental WH strain; backcrosses of extremes (low and high sensitivity) of F1 to SDH rats are in progress (Swerdlow et al., 2000). Interstrain and intrastrain differences in this complex phenotype, particularly among inbred strains, may be suitable candidates for QTL analyses and other genome-mapping strategies to identify candidate genes of relevance to schizophrenia and novel antipsychotic treatment strategies. Thus, the apparent inconsistencies in PPI findings across different populations of rats may prove to be its greatest asset as an investigative tool.
Footnotes
This research was supported in part by the National Institute of Mental Health Grants MH-01436, MH-53484, and MH-42228 and by the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Centers. M.A.G. has an equity interest in San Diego Instruments, Inc.
Correspondence should be addressed to Dr. Neal R. Swerdlow, Department of Psychiatry, 0804, University of California, San Diego, School of Medicine, 9500 Gilman Drive, La Jolla, CA 92093-0804. E-mail:nswerdlow@ucsd.edu.
REFERENCES
- 1.Bolino F, Di Michele V, Di Cicco L, Manna V, Daneluzzo E, Casacchia M. Sensorimotor gating and habituation evoked by electro-cutaneous stimulation in schizophrenia. Biol Psychiatry. 1994;36:670–679. doi: 10.1016/0006-3223(94)91176-2. [DOI] [PubMed] [Google Scholar]
- 2.Braff D, Stone C, Callaway E, Geyer MA, Glick ID, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 3.Braff D, Grillon C, Geyer M. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- 4.Bullock AE, Slobe BS, Vazquez V, Collins AC. Inbred mouse strains differ in the regulation of startle and prepulse inhibition of the startle response. Behav Neurosci. 1997;111:1353–1360. doi: 10.1037//0735-7044.111.6.1353. [DOI] [PubMed] [Google Scholar]
- 5.Cadenhead KS, Geyer MA, Braff DL. Impaired startle prepulse inhibition and habituation in schizotypal personality disordered patients. Am J Psychiatry. 1993;150:1862–1867. doi: 10.1176/ajp.150.12.1862. [DOI] [PubMed] [Google Scholar]
- 6.Cadenhead KS, Geyer MA, Swerdlow NR, Shafer K, Diaz M, Clementz BA, Braff DL. Sensorimotor gating deficits in schizophrenic patients and their relatives. Biol Psychiatry Abstr. 1998;43:10S. [Google Scholar]
- 7.Caine SB, Geyer MA, Swerdlow NR. Carbachol infusion into the dentate gyrus disrupts sensorimotor gating of the startle reflex in rats. Psychopharmacology (Berl) 1991;105:347–354. doi: 10.1007/BF02244429. [DOI] [PubMed] [Google Scholar]
- 8.Caine SB, Geyer MA, Swerdlow NR. Effects of D3/D2 dopamine receptor agonists and antagonists on prepulse inhibition of acoustic startle in the rat. Neuropsychopharmacology. 1995;12:139–145. doi: 10.1016/0893-133X(94)00071-7. [DOI] [PubMed] [Google Scholar]
- 9.Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellanos FX, Fine EJ, Kaysen DL, Kozuch PL, Hamburger SD, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette's syndrome and ADHD: preliminary results. Biol Psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 11.Davis M, Mansbach RS, Swerdlow NR, Braff DL, Geyer MA. Apomorphine disrupts prepulse inhibition of acoustic startle in rats. Psychopharmacology (Berl) 1990;102:1–4. doi: 10.1007/BF02245735. [DOI] [PubMed] [Google Scholar]
- 12.Ellenbroek BA, Cools AR. The neurodevelopment hypothesis of schizophrenia: clinical evidence and animal models. Neurosci Res Commun. 1998;22:127–136. [Google Scholar]
- 13.Ellenbroek BA, Geyer MA, Cools MA. The behavior of APO-SUS rats in animal models with construct validity for schizophrenia. J Neurosci. 1995;15:7604–7611. doi: 10.1523/JNEUROSCI.15-11-07604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geyer MA, Braff DL. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr Bull. 1987;13:643–668. doi: 10.1093/schbul/13.4.643. [DOI] [PubMed] [Google Scholar]
- 15.Geyer MA, Swerdlow NR. Measurement of startle response, prepulse inhibition, and habituation. In: Crawley JN, Skolnick P, editors. Current protocols in neuroscience. Wiley; New York: 1998. pp. 8.7.1–8.7.15. [DOI] [PubMed] [Google Scholar]
- 16.Geyer MA, Krebs-Thomson K, Varty GB. The effects of M100907 in pharmacological and developmental animal models of prepulse inhibition deficits in schizophrenia. Neuropsychopharmacology. 1999;21:S134–S142. [Google Scholar]
- 17.Gleason TC, Dreiling JL, Crawley JN. Rat strain differences in response to galanin on the Morris water task. Neuropeptides. 1999;33:265–270. doi: 10.1054/npep.1999.0044. [DOI] [PubMed] [Google Scholar]
- 18.Graham F. The more or less startling effects of weak prestimuli. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 19.Hart S, Zreik M, Carper R, Swerdlow NR. Localizing haloperidol effects on sensorimotor gating in a predictive model of antipsychotic potency. Pharmacol Biochem Behav. 1998;61:113–119. doi: 10.1016/s0091-3057(98)00079-3. [DOI] [PubMed] [Google Scholar]
- 20.Hitchcock JM, Selk DE, Wettstein JG, Rush DK. Intrastrain differences in the disruption of prepulse inhibition in rats by PCP, DOI, and 7-OH-DPAT. Schizophr Res. 1999;36:115. [Google Scholar]
- 21.Hoffman DC, Donovan H. D1 and D2 dopamine receptor antagonists reverse prepulse inhibition deficits in an animal model of schizophrenia. Psychopharmacology (Berl) 1994;115:447–453. doi: 10.1007/BF02245567. [DOI] [PubMed] [Google Scholar]
- 22.Kinney GG, Wilkinson LO, Saywell KL, Tricklebank MD. Rat strain differences in ability to disrupt sensorimotor gating are limited to the dopaminergic system, specific to prepulse inhibition, and unrelated to changes in startle amplitude or nucleus accumbens dopamine receptor sensitivity. J Neurosci. 1999;19:5644–5653. doi: 10.1523/JNEUROSCI.19-13-05644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch M. Sensorimotor gating changes across the estrous cycle in female rats. Physiol Behav. 1998;64:625–628. doi: 10.1016/s0031-9384(98)00098-5. [DOI] [PubMed] [Google Scholar]
- 24.Koch M, Schnitzler HU. The acoustic startle response in rats—circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- 25.Kodsi MH, Swerdlow NR. Regulation of sensorimotor gating by pallidal efferent projections. Brain Res Bull. 1997;43:219–228. doi: 10.1016/s0361-9230(96)00440-6. [DOI] [PubMed] [Google Scholar]
- 26.Kumari V, Soni W, Sharma T. Normalization of information processing deficits in schizophrenia with clozapine. Am J Psychiatry. 1999;156:1046–1051. doi: 10.1176/ajp.156.7.1046. [DOI] [PubMed] [Google Scholar]
- 27.Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology (Berl) 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- 28.Loscher W, Cramer S, Ebert U. Differences in kindling development in seven outbred and inbred rat strains. Exp Neurol. 1998;154:551–559. doi: 10.1006/exnr.1998.6948. [DOI] [PubMed] [Google Scholar]
- 29.Luedtke RR, Artymyshyn RP, Monks BR, Molinoff PB. Comparison of the expression, transcription and genomic organization of D2 dopamine receptors in outbred and inbred strains of rat. Brain Res. 1992;584:45–54. doi: 10.1016/0006-8993(92)90876-b. [DOI] [PubMed] [Google Scholar]
- 30.Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology (Berl) 1988;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- 31.Martinez Z, Oostwegel J, Geyer M, Swerdlow NR. Ontogeny of phencyclidine and apomorphine effects on prepulse inhibition (PPI). Pharmacol Biochem Behav. 2000;65:449–457. doi: 10.1016/s0091-3057(99)00217-8. [DOI] [PubMed] [Google Scholar]
- 32.Meloni EG, Davis M. Enhancement of the acoustic startle response in rats by the dopamine D1 receptor agonist SKF 82958. Psychopharmacology (Berl) 1999;144:373–380. doi: 10.1007/s002130051020. [DOI] [PubMed] [Google Scholar]
- 33.Oliff HS, Marek P, Miyazaki B, Weber E. The neuroprotective efficacy of MK-801 in focal cerebral ischemia varies with rat strain and vendor. Brain Res. 1996;731:208–212. doi: 10.1016/0006-8993(96)00582-3. [DOI] [PubMed] [Google Scholar]
- 34.Oliff HS, Coyle P, Weber E. Rat strain and vendor differences in collateral anastomoses. J Cereb Blood Flow Metab. 1997;17:571–576. doi: 10.1097/00004647-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Peng RY, Mansbach RS, Braff DL, Geyer MA. A D2 dopamine receptor agonist disrupts sensorimotor gating in rats. Implications for dopaminergic abnormalities in schizophrenia. Neuropsychopharmacology. 1990;3:211–218. [PubMed] [Google Scholar]
- 36.Rigdon G. Differential effects of apomorphine on prepulse inhibition of acoustic startle reflex in two rat strains. Psychopharmacology (Berl) 1990;102:419–421. doi: 10.1007/BF02244115. [DOI] [PubMed] [Google Scholar]
- 37.Swerdlow NR, Geyer MA. Clozapine and haloperidol in an animal model of sensorimotor gating deficits in schizophrenia. Pharmacol Biochem Behav. 1993;44:741–744. doi: 10.1016/0091-3057(93)90193-w. [DOI] [PubMed] [Google Scholar]
- 38.Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull. 1998;24:285–302. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- 39.Swerdlow NR, Geyer MA. Neurophysiology and neuropharmacology of short lead interval startle modification. In: Dawson ME, Schell A, Boehmelt A, editors. Startle modification: implications for neuroscience, cognitive science, and clinical science. Cambridge UP; Cambridge, UK: 1999. pp. 114–133. [Google Scholar]
- 40.Swerdlow NR, Caine B, Geyer MA. Opiate-dopamine interactions in the neural substrates of acoustic startle gating in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1991a;15:415–426. doi: 10.1016/0278-5846(91)90072-9. [DOI] [PubMed] [Google Scholar]
- 41.Swerdlow NR, Keith VA, Braff DL, Geyer MA. Effects of spiperone, raclopride, SCH 23390 and clozapine on apomorphine inhibition of sensorimotor gating of the startle response in the rat. J Pharmacol Exp Ther. 1991b;256:530–536. [PubMed] [Google Scholar]
- 42.Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994a;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- 43.Swerdlow NR, Zisook D, Taaid N. Seroquel (ICI 204,636) restores prepulse inhibition of acoustic startle in apomorphine-treated rats: similarities to clozapine. Psychopharmacology (Berl) 1994b;114:675–678. doi: 10.1007/BF02245001. [DOI] [PubMed] [Google Scholar]
- 44.Swerdlow NR, Lipska BK, Weinberger DR, Braff DL, Jaskiw GE, Geyer MA. Increased sensitivity to the gating-disruptive effects of apomorphine after lesions of the medial prefrontal cortex or ventral hippocampus in adult rats. Psychopharmacology (Berl) 1995a;122:27–34. doi: 10.1007/BF02246438. [DOI] [PubMed] [Google Scholar]
- 45.Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR. Impaired prepulse inhibition of acoustic and tactile startle in patients with Huntington's disease. J Neurol Neurosurg Psychiatry. 1995b;58:192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swerdlow NR, Bakshi V, Geyer MA. Seroquel restores sensorimotor gating in phencyclidine (PCP)-treated rats. J Pharmacol Exp Ther. 1996;279:1290–1299. [PubMed] [Google Scholar]
- 47.Swerdlow NR, Varty GB, Geyer MA. Discrepant findings of clozapine effects on prepulse inhibition of startle: is it the route or the rat? Neuropsychopharmacology. 1997;18:50–56. doi: 10.1016/S0893-133X(97)00110-3. [DOI] [PubMed] [Google Scholar]
- 48.Swerdlow NR, Martinez ZA, Hanlon FM, Auerbach P, Platten A, Braff DL, Geyer MA (2000) Towards the genetics of a complex phenotype: strain analyses of drug effects on startle gating. Biol Psychiatry, in press.
- 49.Turnbull AV, Rivier CL. Sprague-Dawley rats obtained from different vendors exhibit distinct adrenocorticotropin responses to inflammatory stimuli. Neuroendocrinology. 1999;70:186–195. doi: 10.1159/000054475. [DOI] [PubMed] [Google Scholar]
- 50.Varty GB, Higgins GA. Differences between three rat strains in sensitivity to prepulse inhibition of acoustic startle response: influence of apomorphine and phencyclidine pretreatment. J Psychopharmacol (Oxf) 1994;8:148–156. doi: 10.1177/026988119400800302. [DOI] [PubMed] [Google Scholar]
- 51.Wan FJ, Swerdlow NR. Intra-accumbens infusion of quinpirole impairs sensorimotor gating of acoustic startle in rats. Psychopharmacology (Berl) 1993;113:103–109. doi: 10.1007/BF02244341. [DOI] [PubMed] [Google Scholar]
- 52.Wan FJ, Swerdlow NR. The basolateral amygdala regulates sensorimotor gating of acoustic startle in rats. Neuroscience. 1996;76:715–724. doi: 10.1016/s0306-4522(96)00218-7. [DOI] [PubMed] [Google Scholar]
- 53.Wan FJ, Caine SB, Swerdlow NR. The ventral subiculum modulation of prepulse inhibition is not mediated via D2 dopamine or nucleus accumbens non-NMDA glutamate activity. Eur J Pharmacol. 1996a;314:9–18. doi: 10.1016/s0014-2999(96)00535-3. [DOI] [PubMed] [Google Scholar]
- 54.Wan FJ, Taaid N, Swerdlow NR. Do D1/D2 interactions regulate prepulse inhibition in rats? Neuropsychopharmacology. 1996b;14:265–274. doi: 10.1016/0893-133X(95)00133-X. [DOI] [PubMed] [Google Scholar]
- 55.Weiss IC, Feldon J, Domeney A. Circadian time does not modify the prepulse inhibition response or its attenuation by apomorphine. Pharmacol Biochem Behav. 1999;64:501–505. doi: 10.1016/s0091-3057(99)00100-8. [DOI] [PubMed] [Google Scholar]



