Abstract
The temporal and spatial distributions of several growth factors suggest roles in the regulation of neuronal differentiation in the neocortex. Among such growth factors, the insulin-like growth factors (IGF-I and -II) are of particular interest because they are available to neurons from multiple sources under independent control. IGF-I is produced by many neurons throughout the brain and also by cells in the cerebral vasculature. IGF-II is found at high levels in the CSF, and both IGF-I and IGF-II cross the blood–brain barrier. Thus, the IGFs may act as both paracrine and endocrine regulators of neuronal development. As an initial step toward understanding the influence of IGFs in the developing cerebral cortex, the present study examined the effects of IGF-I and of the neurotrophins brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) on the dendritic complexity of layer 2 pyramidal neurons. The results demonstrate that IGF-I increased the branching and total extent of both apical and basal dendrites of pyramidal cells in organotypic slices of rat primary somatosensory cortex. BDNF and NT-3 also enhanced dendritic development, but the two neurotrophins increased the extent of only basal, not apical, dendrites and promoted greater elongation than was seen after IGF-I treatment. These results provide direct evidence that IGF-I can regulate the dendritic elaboration of cortical neurons and indicate that endogenous IGFs may influence dendritic differentiation and the formation of cortical connections. In addition, IGF-dependent regulation of dendritic structure may represent a link between age-related declines in IGFs and cognitive deficits seen in senescence.
Keywords: growth factors, neurotrophins, organotypic slice, neocortex, dendrites, aging
The temporal and spatial distributions of the insulin-like growth factors (IGF-I and -II) and of IGF receptors in the CNS (Bondy, 1991; Garcia-Segura et al., 1991; Bondy et al., 1992; Kar et al., 1993; Niblock et al., 1998) suggest that IGFs may influence the development and plasticity of CNS neurons (Anlar et al., 1999; Torres-Aleman, 1999). Among growth factors, IGFs are of special interest because they are available to neurons from several sources. IGF-I is produced in the liver and released into the bloodstream (Roberts et al., 1986; Hynes et al., 1987). Plasma IGF-I crosses the blood–brain barrier (Reinhardt and Bondy, 1994; Pulford et al., 1999) and may be particularly available via this route in the first few postnatal weeks, before the blood–brain barrier is established fully (Schulze and Firth, 1992). IGF-I also is produced by cortical and other CNS neurons (Lund et al., 1986; Adamo et al., 1988; Bach et al., 1991; Bondy, 1991;Niblock et al., 1998) and by endothelial and smooth muscle cells of the cerebral vasculature (Delafontaine et al., 1991; Sonntag et al., 1999). IGF-II is abundant in the CSF because of synthesis in the choroid plexus and transport from the blood and is produced by glial cells in several neural regions (Lauterio, 1991; LeRoith et al., 1993). Such diverse sources and regulation suggest that IGFs could play multiple roles in the developing CNS, perhaps as general growth-promoting factors and as focal regulators of neuronal differentiation. The observation that IGFs decline in the aging brain (Sonntag et al., 1999) increases further the importance of understanding the influence of the factors on neuronal structure and function.
Several reports suggest that IGFs influence axonal and/or dendritic elaboration. Axonal diameter is increased in the brains of IGF-I-overexpressing mice (Ye et al., 1995), and axonal number and myelination are reduced in IGF-I (−/−) mice (Beck et al., 1995; Cheng et al., 1998). Neuropil volume is increased in IGF-I-overexpressing mice and reduced in mice in which IGF-I signaling is decreased (Gutierrez-Ospina et al., 1996). Beyond studies of transgenic mice, however, assessing the role of IGFs in neuronal differentiationin vivo is difficult. Although IGF-I and II cross the blood–brain barrier (Duffy et al., 1988; Reinhardt and Bondy, 1994, Pulford et al., 1999), systemic effects make it difficult to interpret neuronal changes after peripheral delivery.
In vitro, IGF-I influences the differentiation of dissociated peripheral, cerebellar, and other neurons (Recio-Pinto and Ishii, 1988; Torres-Aleman et al., 1989; Lauterio, 1992; Russo and Werther, 1994), but effects on the development of cortical neurons remain primarily unexplored. This study investigated the effects of IGF-I on dendritic elaboration of layer 2 pyramidal neurons within the primary somatosensory cortex of the rat in which mRNA for IGF-I and its receptor are expressed highly during the period of dendritic elaboration (Bondy, 1991; Bondy et al., 1992). Organotypic cultures provided an accessible and controlled environment, facilitated identification, and labeling of a defined population of cortical neurons, and permitted analysis of the growth of two distinct dendritic compartments, the apical and basal dendrites. The effects of brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT-3) were also examined in these studies. The two neurotrophins have primarily opposite effects on the growth of dendrites of neurons in the deep layers of the ferret visual cortex (McAllister et al., 1995, 1996,1997); thus, it was of interest to assess the effects of BDNF and NT-3 on neurons in supragranular cortex and to compare those effects with the effects of IGF-I.
MATERIALS AND METHODS
Female Sprague Dawley rats with litters were obtained from Zivic-Miller Laboratories (Zelienople, PA) and maintained in the Wake Forest University School of Medicine (WFUSM) animal facility on a 12 hr light/dark schedule in a climate-controlled room. Food and water were available ad libitum. The animal facility at WFUSM is fully accredited by the American Association for Accreditation of Laboratory Animal Care and complies with all Public Health Service–National Institutes of Health and institutional policies and standards for laboratory animal care. All protocols described herein were approved by the institutional Animal Care and Use Committee.
Postnatal day 10 (P10) (day of birth is P0) rat pups were anesthetized, cleaned with 70% ethanol, and decapitated. Before removing the brain, coronal cuts were made rostral to the tectum and rostral to the hippocampal commissure. The isolated tissue block then was removed and placed into cold (4°C), sterile artificial CSF (aCSF) (in mm: 280 NaCl, 5 KCL, 1 MgCl2,24 dextrose, 1 CaCl2, and 10 HEPES, pH 7.2) (McAllister et al., 1995). All tissue preparation was performed under sterile conditions in a laminar flow hood. Each brain was glued with cyanoacrylate adhesive (caudal side down) to the floor of a vibratome cutting chamber and immediately covered with cold aCSF; the bath surrounding the cutting chamber was filled with ice water. Coronal vibratome sections (300 μm) were cut and collected with aCSF on polyethylene terephthalate membrane inserts (one slice per insert) in six-well tissue culture plates (Falcon). Immediately after the slices were collected, the aCSF was removed, and 980 μl of media [50% Basal Medium Eagle, 25% HBSS, 25% horse serum, 10 mm HEPES (Life Technologies, Gaithersburg, MD), 36 mm dextrose, and 25 mm KCl, pH 7.2 (modified from McAllister et al., 1995)] was added to the well under the insert.
After all of the slices for an experiment were collected, trophic factors were added to the culture wells in 20 μl of media. Three trophic factors were tested in each experiment. Slices were incubated with des(1–3) IGF-I (des IGF-I) (200 ng/ml; GroPep, Adelaide, South Australia), BDNF (200 ng/ml; Regeneron Pharmaceuticals, Tarrytown, NY), or NT-3 (200 ng/ml; Regeneron Pharmaceuticals) for 24 hr. Des IGF-I is a naturally occurring post-translational modification of IGF-I that has a significantly reduced affinity for the IGF binding proteins. Des IGF-I was used in all of these experiments to minimize interactions of IGF-I with endogenous and media-derived IGF binding proteins. Because the culture media contained serum, which was likely to contain IGF-I, we measured IGF-I in the prepared media by radioimmunoassay (Sonntag et al., 1992). IGF-I was present in control media at a concentration of 7.5 ng/ml. Thus, all of the cultures were exposed to IGF-I at a level that was <4% of what was added in IGF-I-treated cultures.
Slices were incubated at 37°C in 5% CO2 for 24 hr. The culture period was kept short to minimize changes in slice organization that might secondarily effect dendritic structure; preliminary experiments revealed robust responses to growth factors within that period. After 24 hr in culture, the slices were fixed by immersion in 2.5% paraformaldehyde with 4% sucrose in 0.1m phosphate buffer, pH 7.4, for 35 min at room temperature and then stored in 0.1 m phosphate buffer at 4°C for up to 1 week. To better visualize cortical lamination, slices were stained briefly with a 0.1% solution of 4′,6′-diamidino-2-phenylindole before intracellular injections. Slices then were placed on the modified stage of an Olympus Optical (Tokyo, Japan) BX50-WI microscope, and a sharp electrode was positioned manually at the surface of the tissue under a long-working distance 60× immersion objective. A motorized micromanipulator was used to advance the electrode until a cell was encountered (as evidenced by partial filling of the cell body or dendrites). Cells were filled with a mixture of 3% Lucifer yellow (lithium salt) and 3% Lucifer yellow cadaverine biotin-X (dipotassium salt; Molecular Probes, Eugene, OR) in distilled water (2–8 nA negative current, 600 msec pulse, 1 Hz, for 5–10 min). Slices with filled cells were post-fixed in 4% paraformaldehyde in 0.1m phosphate buffer for at least 24 hr before subsequent processing.
Slices containing labeled neurons were reacted histochemically to convert the Lucifer yellow–biotin compound to a stable reaction product. Slices were rinsed in PBS, pH 7.4, infiltrated with dimethyl sulfoxide (5, 10, and 20%), and frozen twice over acetone and dry ice. After the second thawing, slices were rinsed with PBS and incubated in PBS with 10% methanol and 3% H2O2 (30 min) to block endogenous peroxidase activity. Nonspecific binding then was blocked by incubation in 2% bovine serum albumin (BSA) and 0.25% Triton X-100 for 1 hr. Slices were incubated in avidin-biotin complex (ABC; Vector Laboratories, Burlingame, CA) overnight (4°C) with 2% BSA and 0.1% Triton X-100, after which labeled neurons were visualized using 3,3′-diaminobenzidine (0.5 mg/ml) intensified with cobalt chloride (0.03%)and nickel ammonium sulfate (0.2%) (Adams, 1981). The reacted slices were rinsed in PBS, mounted on charged slides, dehydrated through graded ethyl alcohol, cleared in xylene, and coverslipped using Cytoseal (Stephens Scientific).
Figure 1 illustrates a labeled layer 2 pyramidal neuron in a typical slice. A filled cell was chosen for reconstruction and analysis if (1) it was in layer 2 of primary sensory cortex, (2) it had a pyramidal morphology (i.e., a single apical dendrite oriented toward the pia and at least two basal dendrites), and (3) its dendrites appeared to be completely filled. Slides were coded before analysis, and all analyses were completed without knowledge of the experimental condition. A total of 80 cells from 22 slices were reconstructed and analyzed. Somal size was measured in two additional cells in which dendritic filling appeared incomplete.
Fig. 1.
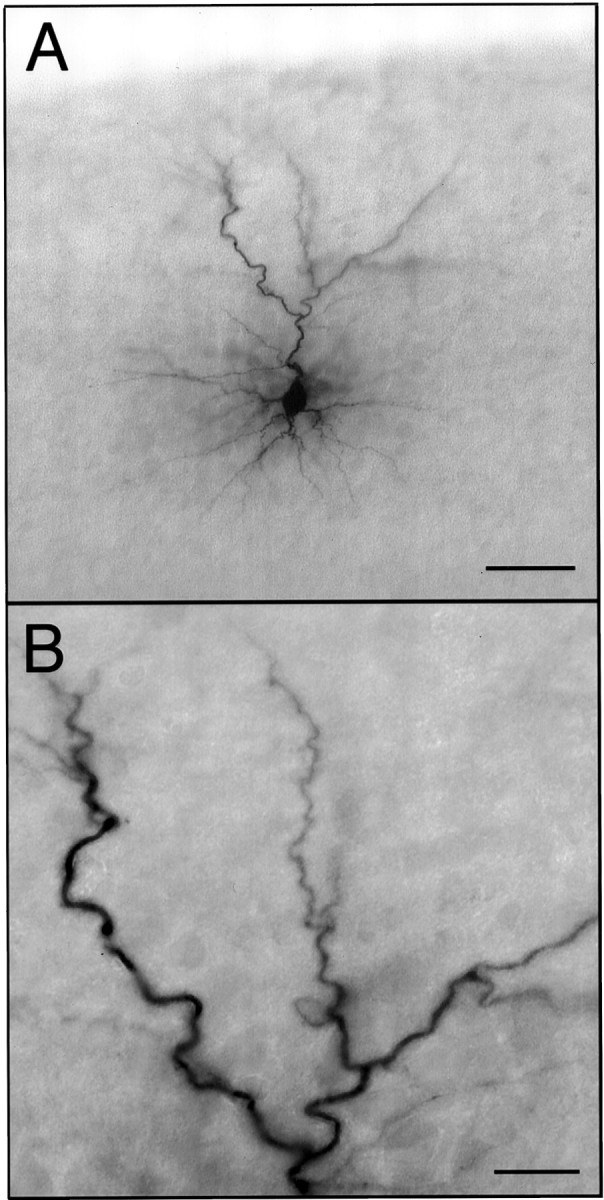
Labeled layer 2 pyramidal neuron.A, The neuron shown is representative of the intracellularly labeled neurons analyzed in this study. The pial surface of the slice is visible at the top of the micrograph.B, A higher-magnification micrograph of a portion of the apical dendritic arbor illustrates the completeness of labeling. Scale bars: A, 50 μm; B, 15 μm.
Labeled neurons were reconstructed in three dimensions using an Olympus Optical BX-50 microscope equipped with a motorized stage, a 100×/1.4 NA PlanApo objective, and the NeuroLucida system for neuronal analysis (MicroBrightField Inc., Colchester, VT). In addition to three-dimensional Sholl analysis (Sholl, 1953), the total number and length of apical and basal dendrites were determined, as well as the number of apical and basal dendritic branch points, number of dendritic segments, and the length of each segment. Branches and segments were ordered progressively from proximal to distal on the dendritic arbor; apical and basal dendrites were analyzed separately. First-order (primary) basal dendrites were those arising from the soma, and first-order apical branches were defined as those arising from the primary apical dendritic shaft. Any two or more branches that arose from a single branch point were considered to be of the same order.
Multiple quantitative analyses were performed to address several specific questions, including whether any of the trophic factors produced changes in fundamental aspects of cell morphology, such as size of the soma and number of primary dendrites. The quantitative measures of the dendritic arbors of neurons in control and trophic factor-treated slices that were obtained using the NeuroLucida system were used to establish the presence and average magnitude of responses to growth factors. In addition, the data were used to analyze where in the dendritic arbor new branching occurred (e.g., in the apical vs the basal arbor, proximally, and/or distally) and the extent to which changes in dendrites arose through increased branching, growth of existing dendritic branches, or both. The last question is not easily assessed quantitatively. When both the number of branches and the dendritic length increase, it is difficult to establish whether the increase in total extent is attributable solely to the addition of new branches or includes increased growth of established branches. In the present study, it was reasoned that increases in the length of dendrite and number of dendritic endings in the outer Sholl analysis spheres in the absence of increased numbers of branch points in those spheres would be prima facie evidence of elongation of exiting dendrites. This issue also was addressed more directly by examining the frequency distributions of the lengths of all terminal and nonterminal dendritic segments under each condition.
The effects of trophic factor treatment on all of these parameters were assessed using the Systat statistical package (SPSS, Chicago, IL). For statistical tests, the n value for each treatment group ranged from 16 to 20 neurons in four to six slices (from the same number of rats) from three separate experiments. In cases in which significant effects of treatment were indicated by ANOVA, the Student–Newman–Keuls multiple comparisons test (SNK) was used to test for effects of each factor on individual variables (e.g., number of branches of a specific order).
RESULTS
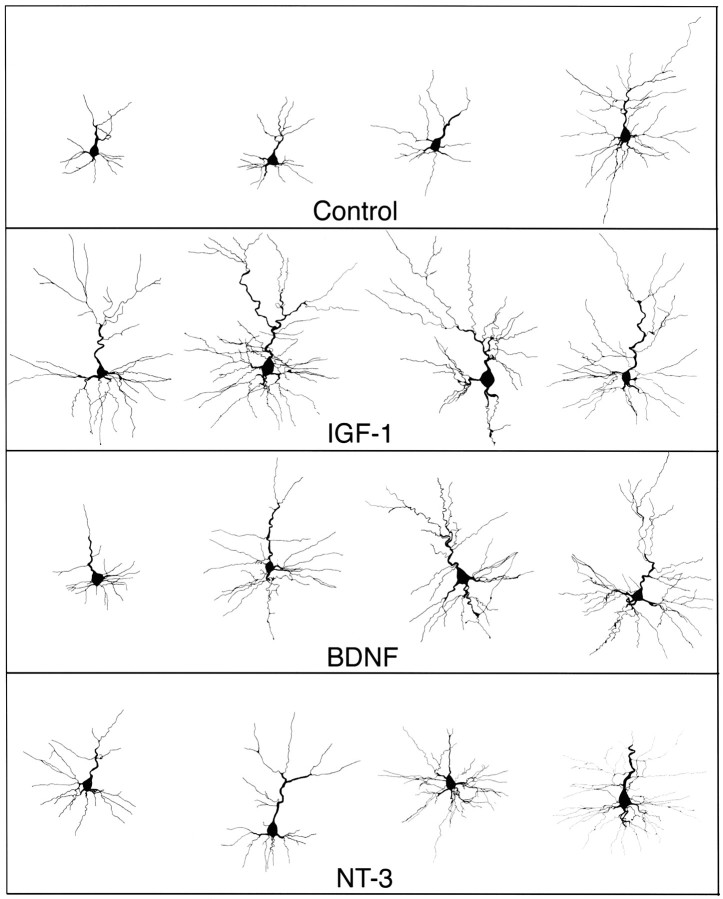
Qualitative observation of filled neurons suggested that there were robust effects of each trophic factor on dendritic arbors. Dendrites in IGF-I and neurotrophin-treated slices were noticeably more elaborate than those in untreated control slices, despite a range of dendritic complexity within each group (Fig.2). Sholl, dendritic length, branch point, and branch order analyses supported the qualitative observations, and ANOVA indicated there were significant and differential effects of IGF-I, BDNF, and NT-3 treatment on dendrites of pyramidal neurons in layer 2. The following sections detail the specific quantitative differences among the trophic factor treatment groups and untreated controls.
Fig. 2.
Representative sample of reconstructed neurons. The illustrated neurons represent the range of complexity observed in control slices, as well as the increased complexity evident after growth factor treatment
Somal size, number of primary basal dendrites, and length of apical shaft were unchanged
Filled neurons showed no obvious cell shrinkage, hypertrophy, or process degeneration, regardless of treatment condition. Somal area was identical in the four treatment groups (p > 0.1, ANOVA) (Fig. 3A), as was the number of primary basal dendrites (p > 0.3, SNK) (see Fig. 5D, branch order 1). Given that depth from the pial surface (which determines the length of the apical dendritic shaft) significantly influences the extent of the apical dendritic arbor of layer 2 pyramidal neurons, the mean length of the apical dendritic shaft was compared among treatment groups. No significant difference was apparent (p > 0.1, ANOVA) (Fig. 3B); thus, the neurons that were analyzed were located at similar positions within layer 2, and there was no gross change in apical dendritic morphology.
Fig. 3.
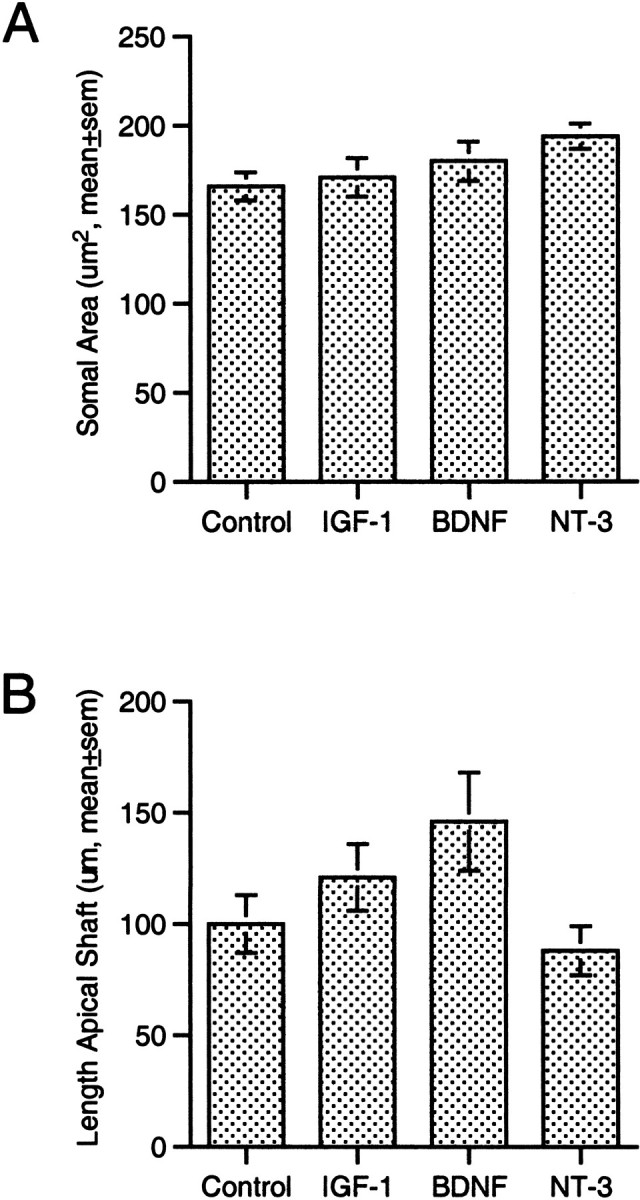
Stability of somal size and length of apical dendritic shaft. None of the growth factors tested elicited a significant change in somal size (A) or in the length of the primary, apical dendritic shaft (B).
Fig. 5.
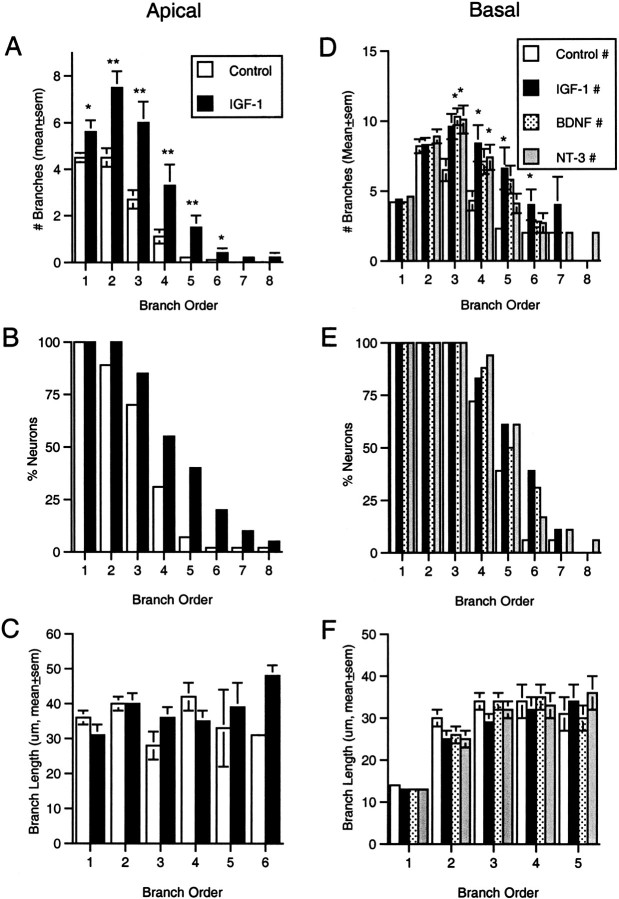
Effects of IGF-I on dendrites by branch order.A, Analysis of the mean number of apical branches of each order for control and IGF-I-treated neurons indicated there were significant effects of treatment and branch order (p < 0.005, ANOVA). Post hoctests demonstrated that IGF-I produced significant increases in the mean number of branches of the first through sixth orders (*p < 0.05, **p < 0.01, SNK).B, Consistent with the increased number of branches at several orders, IGF-I increased the percentage of neurons that included apical dendritic branches of higher orders (fourth through eighth).C, Analysis of the mean length of branches of each order revealed no apparent changes in length in response to IGF-I (the absence of higher-order branches in many neurons, particularly from control slices, precludes statistical analysis like that inA). D, Comparison of the mean number of basal branches at each order within each treatment group demonstrated that there was increased branching (p < 0.005, ANOVA) of intermediate (third through sixth order) branches; IGF-I increased fourth through sixth order branches, NT-3 third and fourth order, and BDNF only third order (*p < 0.05, SNK).E, Consistent with the increased number of branches at intermediate orders, the growth factors increased the percentage of neurons bearing basal branches of the highest orders. F, There was no apparent change in the mean length of basal dendritic branches of any order after treatment with any growth factor.
IGF-I promoted branching of apical and basal dendrites
IGF-I treatment increased the number of branches within, and the total length of, both the apical and basal dendritic arbors. The total number of apical dendritic branches and the total length of the apical arbor in the IGF-I condition were increased ∼75 and 50%, respectively, above the values for untreated controls (p < 0.05, SNK) (Fig.4A–C). Both nonterminal and terminal apical branches increased after IGF-I treatment, from 4.8 ± 0.7 per neuron to 9.6 ±1.5 (mean ± SEM, p < 0.01, SNK) and from 9.3 ± 0.9 to 15.1 ± 1.6 (p < 0.005, SNK), respectively. The effects of IGF-I on basal dendrites appeared to be more modest, with an ∼50% increase in the total number of branches (p < 0.05, SNK) and an apparent 30–40% increase in total dendritic length (Fig.4D–F). The increase in total basal branches included an increase in nonterminal branches from 9.2 ± 0.9 to 15.8 ± 1.9 (p < 0.05, SNK) per neuron and an increase in terminal segments from 13.8 ± 1.2 to 20.5 ± 2.0 (p < 0.05, SNK) per neuron.
Fig. 4.
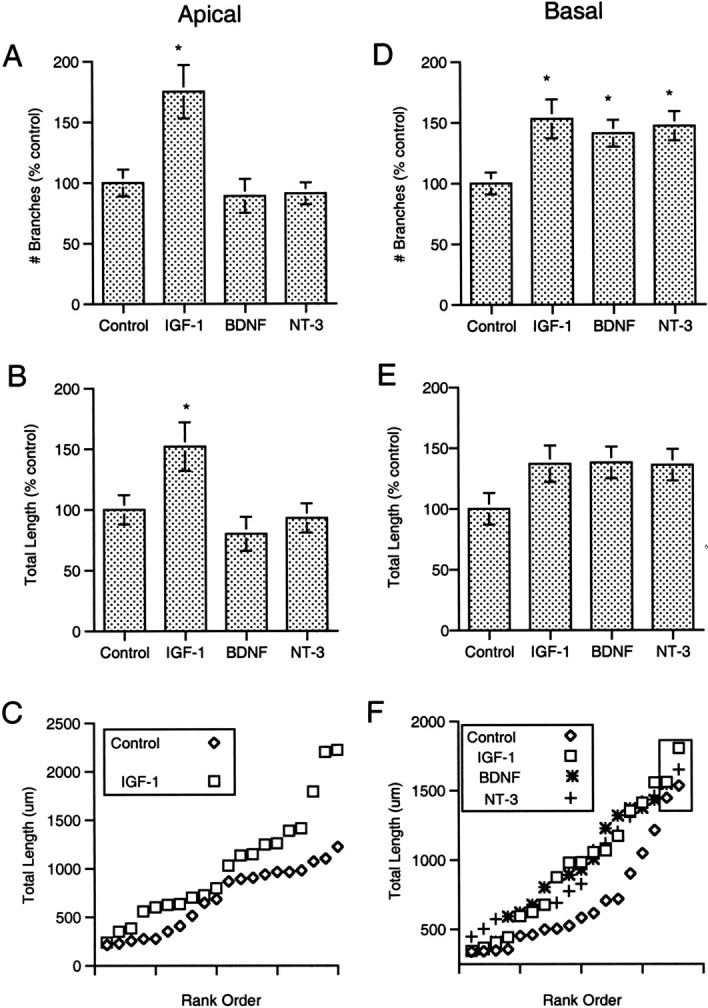
Quantitative effects of growth factors on dendritic branching and total dendritic length. A, IGF-I elicited a 75% increase in the mean number of branches in the apical dendritic arbor (*p < 0.05, SNK).B, IGF-I produced a 50% increase in the mean total length of the apical arbor (p < 0.05, SNK).C, Plotting the total apical dendritic length for each control and IGF-I-treated neuron against its rank (within the treatment group) revealed the wide range in measured values and suggested that even the largest layer 2 neurons responded to IGF-I treatment with an increase in apical dendritic length. D, Each of the growth factors tested elicited an increase in the mean number of branches within the basal dendritic arbor (p< 0.05, SNK). E, It appeared each factor also produced an increase in total basal dendritic length. When all neurons in each group were compared, however, the differences failed to reach statistical significance (p > 0.05, ANOVA).F, Plotting the total length for each neuron against its rank indicated there was an upper limit on basal length at which the values for control and treated neurons converged. After eliminating the two greatest values in each group (e.g., neurons that were presumably already near the limit before treatment; values in box), a second ANOVA indicated a significant effect of treatment, andpost hoc tests demonstrated significant increases in mean length in response to each factor (p < 0.05, SNK).
When the total number of branches of each order was quantified for the apical arbor, it was apparent that IGF-I produced significant increases (compared with untreated controls or neurotrophin-treated slices) in the number of branches of the first through the sixth orders (p < 0.05, SNK) (Fig.5A). Thus, new segments were formed throughout much of the dendritic tree. Within the basal dendritic arbor, the increased branching was restricted to intermediate-order (fourth, fifth, and sixth) branches (p < 0.05, SNK) (Fig. 5D). In addition to increased numbers of branches of specific orders, the increased branching of apical and basal dendrites in response to IGF-I was apparent in the increased percentage of neurons possessing dendrites of the highest orders (Fig.5B,E). Analyses of the mean length of individual dendritic segments (Figs.5C,F) revealed no significant increase in segment lengths in response to IGF-I for any branch order in either apical and basal dendrites.
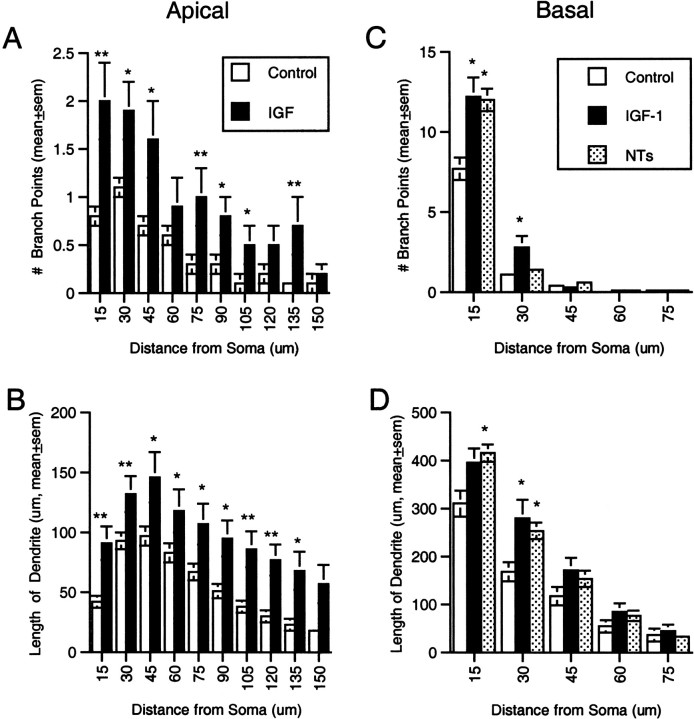
Three-dimensional Sholl analysis of the apical dendrites after IGF-I treatment revealed significant increases in both the number of dendritic branches (p < 0.005, ANOVA) (Fig.6A) and dendritic length (p < 0.005, ANOVA) (Fig.6B) throughout much of the dendritic arbor. Increases in both metrics (p < 0.05, SNK) were apparent from the region immediately surrounding the soma out to ∼135 μm or more from the cell body. Because there was no region of the arbor in which an increase in dendritic length was evident in the absence of increased branching, the increase in total length of the apical arbor may have arisen solely from the formation and growth of new segments.
Fig. 6.
Three-dimensional Sholl analysis of dendrites.A, Comparison between control and IGF-I-treated neurons of the mean number of apical branches within each analysis sphere revealed a significant effect of treatment (p < 0.005, ANOVA) with increases in length out to 135 μm from the soma (*p < 0.05, **p < 0.01, SNK). B, Similar analysis of the length of dendrite within each analysis sphere indicated IGF-I increased dendritic length over the same area.C, Comparison among control and growth factor-treated neurons of the mean number of basal branches within each analysis sphere also revealed a significant effect of growth factor treatment (p < 0.005, ANOVA). IGF-I increased branching out to 30 μm from the soma (*p < 0.05, SNK) but BDNF and NT-3 only out to 15 μm (*p < 0.05, SNK). D, IGF-I, BDNF, and NT-3 each increased dendritic length out to 30 μm from the soma. Thus, for the two neurotrophins, there was increased length in regions of the arbor without increased branching, suggesting there was elongation of preexisting dendritic branches. For the analysis in Cand D, data from BDNF- and NT-3-treated neurons (which were statistically identical with respect to the length of basal dendrites) were combined to increase statistical power.
BDNF and NT-3 increased basal dendritic branching and length
Neither BDNF nor NT-3 altered the total length of, or number of branches within, the apical dendritic arbor (Fig.4A,B). Each increased the number of basal dendritic branches, however, as well as the total basal dendritic length (p < 0.05, SNK) (Fig.4D,E). Like IGF-I, the neurotrophins promoted formation of both nonterminal and terminal branches in the basal arbor. The number of nonterminal segments increased from 9.2 ± 0.9 per neuron for controls to 14.1± 1.2 for BDNF and 14.5 ± 1.3 for NT-3 (p < 0.05, SNK); terminal segments increased from 13.8 ± 1.2 to 18.6 ± 1.3 for BDNF and 19.5± 1.5 for NT-3 (p < 0.05, SNK). Thus, the average magnitudes of the responses to the neurotrophins were similar and were comparable with the effects of IGF-I on branch number and total length for basal dendrites.
Although the effects of IGF-I and the two neurotrophins on total length of the basal dendritic arbor appeared to be striking and consistent (Fig. 4E), the apparent effect of trophic factor treatment did not reach statistical significance (0.1 >p > 0.05, ANOVA). Subsequent examination of the range and distribution of measured values for each condition suggested this was not simply a result of large variance in total length. Ranking and comparing the total basal dendritic lengths for control and treated neurons suggested there was a ceiling effect; the highest values for each group converged at an upper limit for basal dendritic length (Fig.4F). After elimination of the two highest values from each group (e.g., those at the presumed upper limit for dendritic length), reanalysis of the remaining values indicated there was significant effect of each of the three trophic factors on total basal dendritic length (p < 0.05, SNK) (Fig.4F), despite the incumbent decrease in the number of observations.
Like IGF-I, BDNF and NT-3 increased branching of intermediate-order segments in the basal arbor (p < 0.05, SNK) (Fig. 5D). The percentage of neurons possessing higher-order branches was increased (Fig. 5E), and there was no significant change in mean segment length at any branch order (Fig.5F). After treatment with BDNF or NT-3, increased branching was evident only in the innermost Sholl analysis sphere; i.e., within 15 μm of the cell body (p < 0.05, SNK) (Fig. 6D). Dendritic length was increased over a larger portion of the dendritic arbor, out to 30 μm from the cell body (p < 0.05, SNK) (Fig.6C).
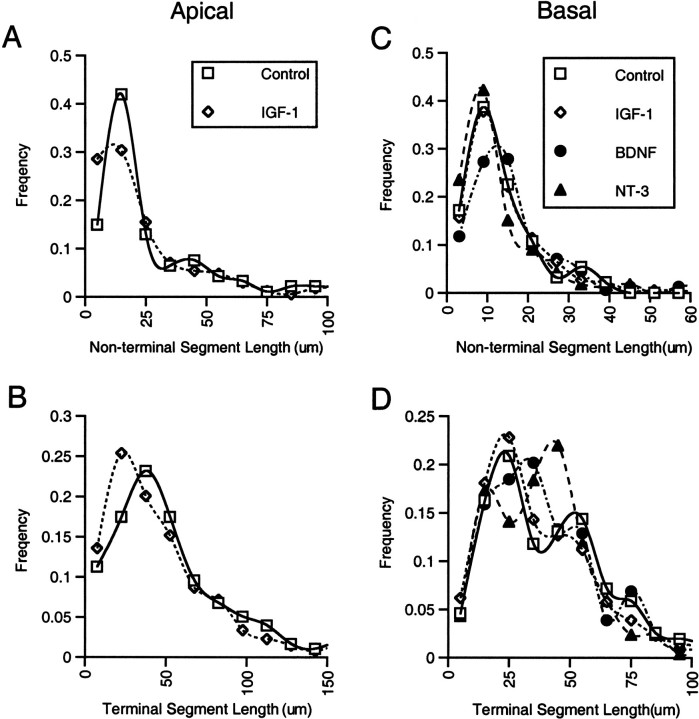
The increase in dendritic length in portions of the arbor in which there was no increase in branch number suggested that BDNF and NT-3 promoted elongation of previously established dendritic segments. This issue was addressed further by examining the measured lengths for all segments in each dendritic arbor for every neuron in each treatment group. Dendritic segments ending at branch points (nonterminal) were analyzed separately from terminal segments. For the apical dendrites of control and IGF-I-treated neurons, the distributions of lengths of nonterminal segments had similar peaks at ∼20 μm (Fig.7A). Comparable plots of the lengths of terminal segments in the apical arbor revealed an increased population of shorter segments after IGF-I treatment (Fig.7B), suggesting that little growth of existing segments accompanied the formation of new terminal segments. Analysis of basal, nonterminal dendritic segments revealed relatively similar distributions for control and growth factor-treated neurons, with peaks at 10–15 μm (Fig. 7C). In contrast, the distributions of lengths of terminal segments in the basal dendritic arbor indicated there was an increased proportion of longer terminal segments after treatment with BDNF or NT-3 (Fig. 7D), providing additional evidence for elongation of terminal segments in response to the two neurotrophins but not in response to IGF-I.
Fig. 7.
Effects of growth factors on the lengths of branching versus terminal dendritic segments. The frequency distributions of the lengths of individual dendritic segments were determined independently for apical and basal segments that ended at branch points (nonterminal, A and C) or as terminal branches (B and D). IGF-I produced no increase in the length of nonterminal (A) or terminal (B) apical dendritic branches. The distribution of terminal segment lengths was shifted toward shorter segments, indicating that little, if any, growth of preexisting segments occurred along with the formation of the new branches that formed in response to IGF-I. Similar analysis of the effects of the growth factors on the length of basal dendritic segments (C and D) revealed a distinct shift toward larger values in the distribution of lengths of terminal branches in response to BDNF and NT-3. This suggests that an increase in the length of dendritic segments accompanied the increased branching of basal dendrites in response to the neurotrophins but not in response to IGF-I.
Layer 2 pyramidal neurons appeared to respond uniformly to growth factor treatment
It cannot be determined by looking at mean measures of dendritic extent under different treatment conditions whether all, or only a subpopulation, of neurons responded to growth factor treatment. As an initial approach to this issue, the frequency distributions of measured values for total dendritic length and for the number of branch points was examined for all of the neurons analyzed. IGF-I appeared to produce a consistent shift in measures of apical dendritic extent, with no indication that only a subpopulation of neurons responded (Fig.8A). Similarly, with respect to basal dendritic changes, growth factor treatment (IGF-I, BDNF, or NT-3) appeared to shift the distribution of measured values toward larger values without appreciably changing the shape of the distribution (Fig. 8B).
Fig. 8.
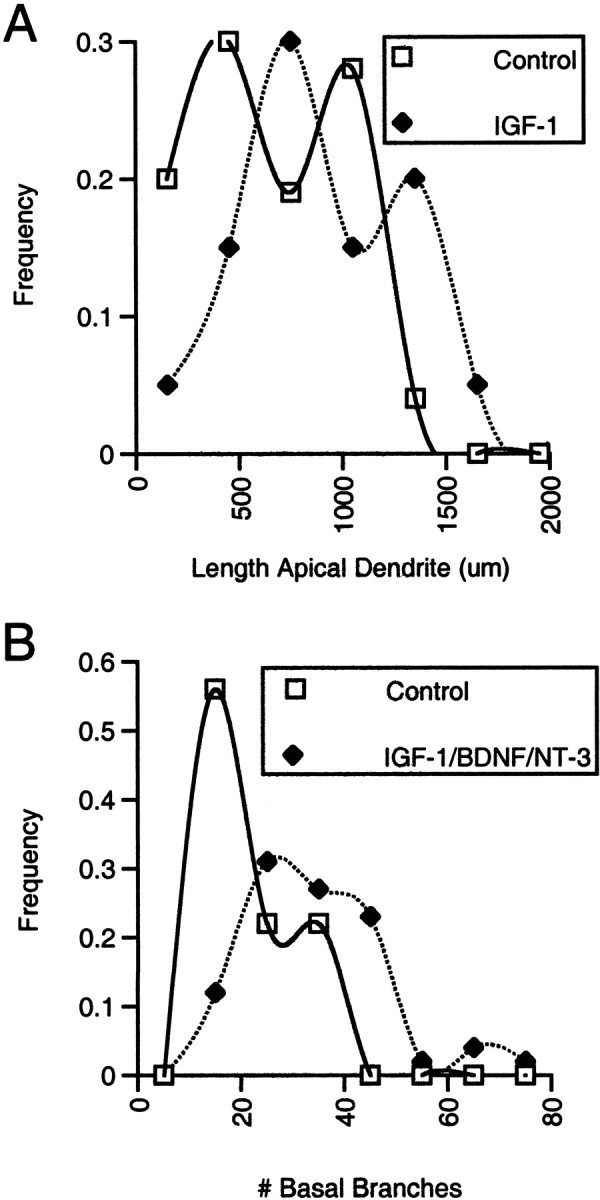
Effects of growth factors on the distributions of dendritic lengths and branch numbers. A, Analysis of the frequency distributions of total apical dendritic lengths for neurons in slices not exposed to IGF-I suggests that the group comprises one population of smaller neurons with mean dendritic length of ∼500 μm and a second population of larger neurons with mean length of ∼1000 μm. Measures from control and BDNF- or NT-3-treated slices, which were statistically identical with respect to apical length and branch number, were combined to provide a smoother distribution (each of the 3 individual distributions also suggested 2 peaks). Comparison of the distribution of dendritic lengths for neurons in IGF-I-treated slices indicated IGF-I elicited a similar increase in the total length of each of the two populations of neurons. Comparable analysis of apical branch points (rather than length) revealed a similar bimodal distribution in untreated slices and increase in response to IGF-I (data not shown).B, The frequency distribution for the number of basal branch points appeared to be unimodal in control slices; the distribution was shifted toward larger values in slices treated with neurotrophic factors. Data from IGF-I-, BNDF-, and NT-3-treated slices, which showed identical increases in the mean number of branches and similar distributions, were combined for the distribution inB. The distributions of basal dendritic lengths in control and treated slices showed similar shapes and changes in response to treatment (data not shown).
DISCUSSION
This study provides the most direct evidence to date that one or more of the insulin-like growth factors regulates dendritic elaboration in the cerebral cortex. IGFs, IGF receptors, and insulin receptors are all present in the neocortex during the period of rapid dendritic growth (Bondy, 1991; Garcia-Segura et al., 1991; Bondy et al., 1992;Kar et al., 1993), and IGF-I, IGF-II, and insulin previously have been shown to stimulate neurite outgrowth from dissociated neurons (Recio-Pinto and Ishii, 1988; Torres-Aleman et al., 1989; Caroni and Grandes, 1990). The most straightforward interpretation of the current results is that des IGF-I acted through the IGF-I receptor, and the neurotrophins through their associated Trk receptors, to directly promote dendritic growth. Nevertheless, because insulin, IGF-I, and IGF-II can each activate heterologous as well as homologous receptors, (with ∼100-fold less efficacy) (Recio-Pinto and Ishii, 1984; Werner and LeRoith, 1995), we cannot rule out the possibility that effects of IGF-I were mediated in part by insulin receptors. In addition, because neurotrophic factors might influence neuronal or glial survival or other aspects of the in vitro environment, it is also possible that effects of such factors on dendritic elaboration observed in this and previous studies were secondary to changes in cell survival or slice health. Several findings argue against that interpretation in the present study. First, although there is accumulating evidence for laminar differences in neuronal survival in cultured slices, neuronal survival in layer 2 is not influenced by any of the factors used in this study (Niblock et al., 1997). In addition, the differential regulation of apical and basal dendrites observed in this study suggests a direct rather than a general, indirect regulation of dendritic elaboration. In fact, there was no correlation between apical and basal dendritic extent, even in control neurons. Finally, if dendritic extent was primarily a function of slice health, then one would expect neurons within a single cortical slice to be more similar in dendritic extent than neurons in different slices. This was not the case. Every slice under each condition contained neurons with a range of dendritic extents, with values above and below the mean for the given metric and treatment. Thus, although there is not as yet unequivocal evidence that the neurotrophic factors acted directly to promote dendritic branching and growth, there is no evidence to the contrary.
Regulation of dendritic growth
Only specific aspects of neuronal morphology were affected by IGF-I, BDNF, and NT-3. Somal size did not change; thus, there was no overall stimulation of cell growth. Moreover, the number of primary basal dendrites remained constant, in contrast to previous studies of the ferret visual cortex that demonstrated changes in the number of primary dendrites in response to neurotrophins (McAllister et al., 1995). Those studies examined neurons at an earlier age, when layers 2 and 3 are still forming. Although one cannot discount differences among species (rat vs ferret), cortical areas (S1 vs visual cortex), or cortical layers (layer 2 vs layers 4–6), it seems likely that the most fundamental aspects of dendritic architecture, such as the number of primary dendrites, are plastic only very early in the differentiation of cortical neurons (Petit et al., 1988).
Together, the results of the present study demonstrate differential regulation of dendritic growth by IGF-I, BDNF, and NT-3 and suggest that these factors could play specific, compartmentalized roles in regulating dendritic elaboration. The data reveal differential control of the growth of major compartments of the dendritic arbor, demonstrating that IGF-I promotes elaboration of both apical and basal dendrites and that BDNF and NT-3 influence only basal dendrites. Similarly independent control of apical and basal dendrites was reported previously for regulation by neurotrophins of dendritic development in visual cortex (McAllister et al., 1995, 1996, 1997) in which BDNF and NT-3 elicited primarily opposite effects on deep pyramidal neurons. The present study demonstrates that the two neurotrophins do not always act in such opposition, but rather produce qualitatively and quantitatively similar effects on supragranular neurons.
These results demonstrate additional specificity of regulation within individual dendritic compartments. IGF-I promotes branching of lower- and higher-order dendrites in both proximal and distal portions of the apical arbor. BDNF and NT-3 increase basal dendritic extent only proximally and affect only intermediate-order branches. Such specificity also extends to the nature of dendritic growth. IGF-I, BDNF, and NT-3 each significantly increased branching, but the two neurotrophins produced more elongation of previously existing branches than did IGF-I. Thus, distinct aspects of dendritic elaboration may be regulated quite specifically by different trophic factors. Given accumulating evidence that dendrites actively process afferent inputs (Johnston et al., 1996; Yuste and Tank, 1996; Sejnowski, 1997) and the expectation that the location of newly formed synapses may be as important as their number, changes in specific dendritic compartments in response to different growth factors might well produce equally distinct changes in neuronal function.
These observations raise interesting and challenging questions about the cellular and molecular mechanisms by which trophic factors influence dendritic elaboration and by which effects are targeted to specific domains within the dendritic arbor. With regard to promotion of dendritic growth, studies from several laboratories demonstrate that IGFs can influence cytoskeletal changes like those that must underlie dendritic modifications (discussed in Ishii, 1993). For example, expression of α- and β-tubulin and neurofilament proteins increases in response to insulin and the IGFs (Mill et al., 1985; Fernyhough et al., 1989; Ishii et al., 1989; Wang et al., 1992). IGF-I also promotes tyrosine phosphorylation of the focal adhesion proteins focal adhesion kinase (FAK) and paxillin (Leventhal et al., 1997; Kim and Feldman, 1998). FAK and paxillin are important in cytoskeletal remodeling and stabilization within growth cones and may play similar roles during dendritic growth (Burridge et al., 1992; Miyamoto et al., 1995;Leventhal et al., 1997). In addition to these links between growth factor signaling and cytoskeletal regulation, several recent studies provide clues to the means by which effects of neurotrophins could be targeted specifically to basal dendrites, as observed here. Accumulating evidence suggests a link between the expression of cell surface gangliosides and the growth of basal dendrites (Walkley et al., 1998; Zervas and Walkley, 1999). Gangliosides appear to potentiate signaling by Trk receptors (Ferrari et al., 1995; Mutoh et al., 1995;Farooqui et al., 1997); thus, a selective association of gangliosides with basal dendrites might render those dendrites particularly sensitive to the growth-promoting effects of neurotrophins.
IGF-I in the developing cortex
Whatever the underlying cellular and molecular mechanisms, the observation that individual neurotrophic factors regulate specific aspects of dendritic architecture of a given neuron, within specific compartments of the dendritic arbor, may provide important insight into the observation that most neurons in the developing CNS produce, are exposed to, and may respond to a wide variety of trophic agents. Within the peripheral nervous system (PNS), in apparent contrast, a particular cell population often appears to depend on a single neurotrophic factor for survival or differentiation (discussed in Snider, 1994). In the PNS, a critical factor often is produced in a restricted location, acts locally, and is relatively inaccessible to the developing processes of other neuronal populations. Within the CNS, however, the regulation of neuronal development by neurotrophic factors appears to be less singular. A given neuronal population within the cerebral cortex is exposed to, and may respond to, multiple neurotrophic factors at key points in development. These observations beg the obvious question of whether multiple neurotrophic factors have redundant or distinct roles in regulating the development of a given population of neurons. The present results argue against redundancy and for specific regulation of distinct aspects of neuronal differentiation.
The broad effects of IGF-I on dendritic elaboration, combined with its availability to neurons from the vasculature, make IGF-I an attractive candidate as a molecular mediator for regionally differential growth of the neocortex in response to differences in levels of neural and metabolic activity (Riddle et al., 1992, 1993). Because blood vessels are elaborated in more electrically and metabolically active areas of the cortex, the supply of vascularly derived IGF-I to neurons in those areas would increase. This, perhaps in concert with neurally derived IGF-I, could promote dendritic elaboration, increase neuropil volume, and contribute to local growth. Such a model is consistent with the observation that the cortex and somatosensory barrel field are increased in size in IGF-I-overexpressing mice and decreased in mice in which IGF-I signaling is blocked (Gutierrez-Ospina et al., 1996).
Insulin-like growth factors in the aging brain
Several aspects of the regulation of IGF signaling indicate that IGFs may influence neuronal structure and function throughout the life-span. IGF-I levels in the brain are highest during the early postnatal period and then decrease to a lower level that is maintained throughout most of adulthood (Bondy, 1991; Bach et al., 1991;Garcia-Segura et al., 1991; Niblock et al., 1998). Interestingly, IGF-I levels decline precipitously in aged animals, coincident with declines in cerebral vascularization and cognitive ability (Sonntag et al., 1997, 1999). IGF-II is also present in the developing and adult brain (Bondy et al., 1992; LeRoith et al., 1993) and declines in the aging brain (Hammerman, 1987; Kitraki et al., 1993; Dore et al., 1997). Accumulating evidence suggests the relationship between the age-related decrease in IGF levels and cognitive decline is more than coincidental. IGF-I, delivered intracerebroventricularly, significantly improves the cognitive function of aged animals and ameliorates age-related cerebrovascular deficits (Markowska et al., 1998). In addition, blocking IGF-I signaling in young rats produces cognitive deficits similar to those seen in normal aging (Thornton et al., 1999). Ongoing studies will establish whether the cognitive changes that follow manipulation of IGF-I signaling reflect normal roles of IGF-I (and/or IGF-II) in modulating neural function and whether behavioral effects of IGF-I are mediated by neuroanatomical modulation like that demonstrated here.
Conclusion
In summary, we have observed that IGF-I treatment leads to a general increase in apical and basal dendritic branching in layer 2 of rat primary somatosensory cortex. BDNF and NT-3 influence only basal dendrites but promote both elongation and branching of those dendrites. These results provide evidence that cortical dendritic differentiation within a single neuronal population can be regulated by several trophic factors, with different factors contributing uniquely to specific aspects of dendritic growth. Thus, although widespread production of trophic factors in the cortex may suggest redundancy, the individual factors, in fact, may have distinct roles in the establishment of cortical connections. In addition to supporting a significant role for IGF-1 in the developing brain, these observations suggest that the age-related decrease in IGF levels, through associated changes in dendritic structure, could contribute to the cognitive decline that accompanies senescence.
Footnotes
This work was supported by National Institutes of Health Grant 1 PO1 AG 11370 (J.K.B.-B and D.R.R.) and was done in partial fulfillment of the requirements for the PhD degree in the Program of Neuroscience, Wake Forest University School of Medicine (M.M.N.). We thank Rhonda Ingram and Dr. Inglis Miller for their assistance and Regeneron Pharmaceuticals, Inc. for providing BDNF and NT-3.
Correspondence should be addressed to D. R. Riddle, Department of Neurobiology and Anatomy, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1010. E-mail:driddle@wfubmc.edu.
REFERENCES
- 1.Adamo M, Werner H, Farnsworth W, Roberts CT, Raizada M, LeRoith D. Dexamethasone reduces steady state insulin-like growth factor I messenger ribonucleic acid levels in rat neuronal and glial cells in primary culture. Endocrinology. 1988;123:2565–2570. doi: 10.1210/endo-123-5-2565. [DOI] [PubMed] [Google Scholar]
- 2.Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- 3.Anlar B, Sullivan KA, Feldman EL. Insulin-like growth factor-1 and central nervous system development. Horm Metab Res. 1999;31:120–125. doi: 10.1055/s-2007-978708. [DOI] [PubMed] [Google Scholar]
- 4.Bach MA, Shen-Orr Z, Lowe WL, Roberts CT, LeRoith D. Insulin-like growth factor I mRNA levels are developmentally regulated in specific regions of the rat brain. Brain Res Mol Brain Res. 1991;10:43–48. doi: 10.1016/0169-328x(91)90054-2. [DOI] [PubMed] [Google Scholar]
- 5.Beck KD, Powell-Braxton L, Widmar HR, Valverde J, Hefti F. IGF-I gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995;14:717–730. doi: 10.1016/0896-6273(95)90216-3. [DOI] [PubMed] [Google Scholar]
- 6.Bondy CA. Transient IGF-I gene expression during the maturation of functionally related central projection neurons. J Neurosci. 1991;11:3442–3455. doi: 10.1523/JNEUROSCI.11-11-03442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bondy C, Werner H, Roberts CT, Jr, LeRoith D. Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors I and II. Neuroscience. 1992;46:909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- 8.Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caroni P, Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol. 1990;110:1307–1317. doi: 10.1083/jcb.110.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng CM, Joncas G, Reinhardt RR, Farrer R, Quarles R, Janssen J, McDonald MP, Crawley JN, Powell-Braxton L, Bondy CA. Biochemical and morphometric analyses show that myelination in the insulin-like growth factor I null brain is proportionate to its neuronal composition. J Neurosci. 1998;18:5673–5681. doi: 10.1523/JNEUROSCI.18-15-05673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delafontaine P, Bernstein KE, Alexander RW. Insulin-like growth factor I gene expression in vascular cells. Hypertension. 1991;17:693–699. doi: 10.1161/01.hyp.17.5.693. [DOI] [PubMed] [Google Scholar]
- 12.Dore S, Kar S, Rowe, Quirion R. Distribution and levels of [125I]IGF-II and [125I]insulin receptor binding sites in the hippocampus of aged memory-unimpaired and -impaired rats. Neuroscience. 1997;80:1033–1040. doi: 10.1016/s0306-4522(97)00154-1. [DOI] [PubMed] [Google Scholar]
- 13.Duffy KR, Partridge WM, Rosenfeld RG. Human blood–brain barrier insulin-like growth factor receptor. Metabolism. 1988;37:136–140. doi: 10.1016/s0026-0495(98)90007-5. [DOI] [PubMed] [Google Scholar]
- 14.Farooqui T, Franklin T, Pearl DK, Yates AJ. Ganglioside GM1 enhances induction by nerve growth factor of a putative dimer of TrkA. J Neurochem. 1997;68:2348–2355. doi: 10.1046/j.1471-4159.1997.68062348.x. [DOI] [PubMed] [Google Scholar]
- 15.Fernyhough P, Mill JF, Roberts JL, Ishii DN. Stabilization of tubulin mRNAs by insulin and insulin-like growth factor I during neurite formation. Mol Brain Res. 1989;6:109–120. doi: 10.1016/0169-328x(89)90044-2. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari G, Anderson BL, Stephens RM, Kaplan DR, Greene LA. Prevention of apoptotic neuronal death by GM1 ganglioside. J Biol Chem. 1995;270:3074–3080. doi: 10.1074/jbc.270.7.3074. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Segura LM, Perez J, Pons S, Rejas MT, Torres-Aleman I. Localization of insulin-like growth factor I (IGF-I) immunoreactivity in the developing and adult rat brain. Brain Res. 1991;560:167–174. doi: 10.1016/0006-8993(91)91228-s. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez-Ospina G, Calikoglu AS, Ye P, D'Ercole AJ. In vivo effects of insulin-like growth factor-I on the development of sensory pathways: analysis of the primary somatic sensory cortex (S1) of transgenic mice. Endocrinology. 1996;137:5484–5492. doi: 10.1210/endo.137.12.8940375. [DOI] [PubMed] [Google Scholar]
- 19.Hammerman MR. Insulin-like growth factors and aging. Endocrinol Metab Clin North Am. 1987;16:995–1011. [PubMed] [Google Scholar]
- 20.Hynes MA, Van Wyck JJ, Brooks PJ, D'Ercole AJ, Jansen J, Lund PK. Growth hormone dependence of somatomedin-C/insulin-like growth factor I and insulin-like growth factor II messenger ribonucleic acids. Mol Endrocrinol. 1987;1:233–242. doi: 10.1210/mend-1-3-233. [DOI] [PubMed] [Google Scholar]
- 21.Ishii DN. Neurobiology of insulin and insulin-like growth factors. In: Loughlin S, Fallon J, editors. Neurotrophic factors. Academic; San Diego: 1993. pp. 415–442. [Google Scholar]
- 22.Ishii DN, Glazner GW, Wang C, Fernyhough P. Neurotrophic effects and mechanism of insulin, insulin-like growth factors, and nerve growth factor in spinal cord and peripheral neurons. In: LeRoith D, Raizada MK, editors. Molecular and cellular biology of insulin-like growth factors and their receptors. Plenum; New York: 1989. pp. 403–425. [Google Scholar]
- 23.Johnston D, Magee JC, Colbert CM, Christie BR. Active properties of neuronal dendrites. Annu Rev Neurosci. 1996;19:165–186. doi: 10.1146/annurev.ne.19.030196.001121. [DOI] [PubMed] [Google Scholar]
- 24.Kar S, Chabot J-G, Quirion R. Quantitative autoradiographic localization of 125I insulin-like growth factor I, 125I insulin-like growth factor II, and 125I insulin receptor binding sites in developing and adult rat brain. J Comp Neurol. 1993;333:375–397. doi: 10.1002/cne.903330306. [DOI] [PubMed] [Google Scholar]
- 25.Kim B, Feldman EL. Differential regulation of focal adhesion kinase and mitogen activated protein kinase tyrosine phosphorylation during insulin-like growth factor-I-mediated cytoskeletal reorganization. J Neurochem. 1998;71:1333–1336. doi: 10.1046/j.1471-4159.1998.71031333.x. [DOI] [PubMed] [Google Scholar]
- 26.Kitraki E, Bozas E, Philippidis H, Stylianopoulou F. Aging-related changes in IGF-II and c-fos gene expression in the rat brain. Int J Dev Neurosci. 1993;11:1–9. doi: 10.1016/0736-5748(93)90029-d. [DOI] [PubMed] [Google Scholar]
- 27.Lauterio TJ. Regulation and physiological function of insulin-like growth factors in the central nervous system. In: Raizada MK, Le Roith D, editors. Molecular biology and physiology of insulin and insulin-like growth factors. Plenum; New York: 1991. pp. 419–430. [DOI] [PubMed] [Google Scholar]
- 28.Lauterio TJ. The effects of IGF-I and IGF-II on cell growth and differentiation in the central nervous system. Adv Exp Med Biol. 1992;321:31–36. doi: 10.1007/978-1-4615-3448-8_4. [DOI] [PubMed] [Google Scholar]
- 29.LeRoith D, Werner H, Faria TN, Kato H, Adamo M, Roberts CT., Jr Insulin-like growth factor receptors. Implications for nervous system function. Ann NY Acad Sci. 1993;692:22–32. doi: 10.1111/j.1749-6632.1993.tb26202.x. [DOI] [PubMed] [Google Scholar]
- 30.Leventhal PS, Shelden EA, Kim B, Feldman EL. Tyrosine phosphorylation of paxillin and focal adhesion kinase during insulin-like growth factor-I-stimulated lamellipodial advance. J Biol Chem. 1997;272:5214–5218. doi: 10.1074/jbc.272.8.5214. [DOI] [PubMed] [Google Scholar]
- 31.Lund PK, Moats-Staats BM, Hynes MA, Simmons JG, Jansen M, D'Ercole AJ, Van Wyk JJ. Somatomedin-C/insulin-like growth factor-I and insulin-like growth factor-II mRNAs in rat fetal and adult tissues. J Biol Chem. 1986;261:14539–14544. [PubMed] [Google Scholar]
- 32.Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-I (IGF-I) ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- 33.McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 34.McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- 35.McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 36.Mill JF, Chao MV, Ishii DN. Insulin, insulin-like growth factor II, and nerve growth factor effects on tubulin mRNA levels and neurite formation. Proc Natl Acad Sci USA. 1985;82:7126–7130. doi: 10.1073/pnas.82.20.7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mutoh T, Tokuda A, Miyadai T, Hamaguchi M, Fujika N. Ganglioside GM1 binds to the trk protein and regulates receptor function. Proc Natl Acad Sci USA. 1995;92:5087–5091. doi: 10.1073/pnas.92.11.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niblock MM, Riddle DR, Brunso-Bechtold JK. Laminar variation in neuronal viability in a slice preparation of neonatal rat brain. Soc Neurosci Abstr. 1997;23:1236. [Google Scholar]
- 40.Niblock MM, Brunso-Bechtold JK, Lynch CD, Ingram RL, McShane T, Sonntag WE. Distribution and levels of IGF-I mRNA across the life span in the Brown Norway X Fischer 344 rat. Brain Res. 1998;804:79–86. doi: 10.1016/s0006-8993(98)00645-3. [DOI] [PubMed] [Google Scholar]
- 41.Petit TL, LeBoutillier JC, Gregario A, Libstug H. The pattern of dendritic development in the cerebral cortex of the rat. Brain Res. 1988;469:209–219. doi: 10.1016/0165-3806(88)90183-6. [DOI] [PubMed] [Google Scholar]
- 42.Pulford BE, Whalen LR, Ishii DN. Peripherally administered insulin-like growth factor-I preserves hindlimb reflex and spinal cord noradrenergic circuitry following a central nervous system lesion in rats. Exp Neurol. 1999;159:114–123. doi: 10.1006/exnr.1999.7143. [DOI] [PubMed] [Google Scholar]
- 43.Recio-Pinto E, Ishii DN. Effects of insulin, insulin-like growth factor II and nerve growth factor on neurite outgrowth in cultured human neuroblastoma cells. Brain Res. 1984;302:323–334. doi: 10.1016/0006-8993(84)90246-4. [DOI] [PubMed] [Google Scholar]
- 44.Recio-Pinto E, Ishii DN. Insulin and insulin-like growth factor receptors regulating neurite formation in cultured human neuroblastoma cells. J Neurosci Res. 1988;19:312–320. doi: 10.1002/jnr.490190306. [DOI] [PubMed] [Google Scholar]
- 45.Reinhardt RR, Bondy CA. Insulin-like growth factors cross the blood–brain barrier. Endocrinology. 1994;135:1753–1761. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- 46.Riddle DR, Richards A, Zsuppan F, Purves D. Growth of rat somatic sensory cortex and its constituent parts during postnatal development. J Neurosci. 1992;12:3509–3524. doi: 10.1523/JNEUROSCI.12-09-03509.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riddle DR, Gutierrez G, Zheng D, White LE, Richards A, Purves D. Differential metabolic and electrical activity in the somatic sensory cortex of juvenile and adult rats. J Neurosci. 1993;13:4193–4213. doi: 10.1523/JNEUROSCI.13-10-04193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts CT, Brown AL, Graham DE, Seelig S, Berry S, Gabbay KH, Rechler MM. Growth hormone regulates the abundance of insulin-like growth factor I RNA in adult rat liver. J Biol Chem. 1986;261:10025–10028. [PubMed] [Google Scholar]
- 49.Russo VC, Werther GA. Des (1–3) IGF-I potently enhances differentiated cell growth in olfactory bulb organ culture. Growth Factors. 1994;11:301–311. doi: 10.3109/08977199409011003. [DOI] [PubMed] [Google Scholar]
- 50.Schulze C, Firth JA. Interendothelial junctions during blood–brain barrier development in the rat: morphological changes at the level of individual tight junctional contacts. Dev Brain Res. 1992;69:85–95. doi: 10.1016/0165-3806(92)90125-g. [DOI] [PubMed] [Google Scholar]
- 51.Sejnowski TA. The year of the dendrite. Science. 1997;275:178–179. doi: 10.1126/science.275.5297.178. [DOI] [PubMed] [Google Scholar]
- 52.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–407. [PMC free article] [PubMed] [Google Scholar]
- 53.Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 54.Sonntag WE, Lenham JE, Ingram RL. Effects of aging and dietary restriction on tissue protein synthesis: relationship to plasma insulin-like growth factor-1. J Gerontol. 1992;47:B159–B163. doi: 10.1093/geronj/47.5.b159. [DOI] [PubMed] [Google Scholar]
- 55.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 56.Sonntag WE, Lynch CD, Bennett SA, Khan AS, Thornton PL, Cooney PT, Ingram RL, McShane T, Brunso-Bechtold JK. Alterations in insulin-like growth factor (IGF)-I gene and protein expression and type I IGF receptors in the brains of aging rats. Neuroscience. 1999;88:269–279. doi: 10.1016/s0306-4522(98)00192-4. [DOI] [PubMed] [Google Scholar]
- 57.Thornton PL, Ng A, Khan A, Sonntag WE. Insulin-like growth factor 1 modulates learning and memory. Soc Neurosci Abstr. 1999;25:1064. [Google Scholar]
- 58.Torres-Aleman I. Insulin-like growth factors as mediators of functional plasticity in the adult brain. Horm Metab Res. 1999;31:114–119. doi: 10.1055/s-2007-978707. [DOI] [PubMed] [Google Scholar]
- 59.Torres-Aleman I, Naftolin F, Robbins RJ. Growth promoting effects of IGF-I on fetal hypothalamic cell lines under serum-free culture conditions. Int J Dev Neurosci. 1989;7:195–202. doi: 10.1016/0736-5748(89)90069-5. [DOI] [PubMed] [Google Scholar]
- 60.Torres-Aleman I, Pons S, Arevelo MA. The insulin-like growth factor I system in the rat cerebellum: developmental regulation and role in neuronal survival and differentiation. J Neurosci Res. 1994;39:117–126. doi: 10.1002/jnr.490390202. [DOI] [PubMed] [Google Scholar]
- 61.Walkley SU, Siegel DA, Dobrenis K, Zervas M. GM2 ganglioside as a regulator of pyramidal neuron dendritogenesis. Ann NY Acad Sci. 1998;845:188–199. doi: 10.1111/j.1749-6632.1998.tb09671.x. [DOI] [PubMed] [Google Scholar]
- 62.Wang C, Li Y, Wible B, Angelides KJ, Ishii DN. Effects of insulin and insulin-like growth factors on neurofilament mRNA and tubulin mRNA content in human neuroblastoma SH-SY5Y cells. Mol Brain Res. 1992;13:289–300. doi: 10.1016/0169-328x(92)90212-t. [DOI] [PubMed] [Google Scholar]
- 63.Werner H, LeRoith D. Insulin-like growth factor 1 receptor: structure, signal transduction, and function. Diabetes Rev. 1995;3:28–37. [Google Scholar]
- 64.Ye P, Carson J, D'Ercole AJ. In vivo actions of insulin-like growth factor I (IGF-I) on brain myelination: studies of IGF-I and IGF binding protein-1 (IGFBP-1) transgenic mice. J Neurosci. 1995;15:7344–7356. doi: 10.1523/JNEUROSCI.15-11-07344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuste R, Tank DW. Dendritic integration in mammalian neurons, a century after Cajal. Neuron. 1996;16:701–716. doi: 10.1016/s0896-6273(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 66.Zervas M, Walkley SU. Ferret pyramidal cell dendritogenesis: changes in morphology and ganglioside expression during cortical development. J Comp Neurol. 1999;413:429–448. doi: 10.1002/(sici)1096-9861(19991025)413:3<429::aid-cne6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]