Abstract
The studies reported here were designed to investigate the role of the mutation eat-4 in the response to tap and in habituation in the nematode Caenorhabditis elegans. InC. elegans eat-4 has been found to affect a number of glutamatergic pathways. It has been hypothesized to positively regulate glutaminase activity and therefore glutamatergic neurotransmission. In the eat-4(ky5) loss-of-function worms, there is presumably insufficient glutamate available for sustained transmission. In the experiments reported here eat-4 worms showed no differences from wild-type in the magnitude of response to a single tap, indicating that the neural circuit underlying the response was intact and functional in the mutant worms. However, wheneat-4 worms were given repeated taps the resulting habituation was different from that seen in wild-type worms:eat-4 worms habituate more rapidly and recover more slowly than wild-type worms at all interstimulus intervals tested. In addition, eat-4 worms do not show dishabituation. The same transgene rescues pharyngeal activity defects and both the habituation and dishabituation deficits seen in theeat-4 worms. Our results suggest that neurotransmitter regulation plays a role in habituation and may play a role in dishabituation.
Keywords: C. elegans, habituation, invertebrate learning, glutamate, behavior genetics, sodium dependent inorganic phosphate co-transporter
Habituation to a mechanical tap in Caenorhabditis elegans has been studied on behavioral (Rankin et al., 1990) and neural circuit levels (Wicks and Rankin, 1995, 1996) (see Fig. 1). The most likely sites of plasticity are the chemical synapses between the sensory neurons (the touch cells) and the four pairs of interneurons mediating forward and backward movement (AVA, AVB, AVD, and PVC) (Wicks and Rankin, 1997).
Fig. 1.
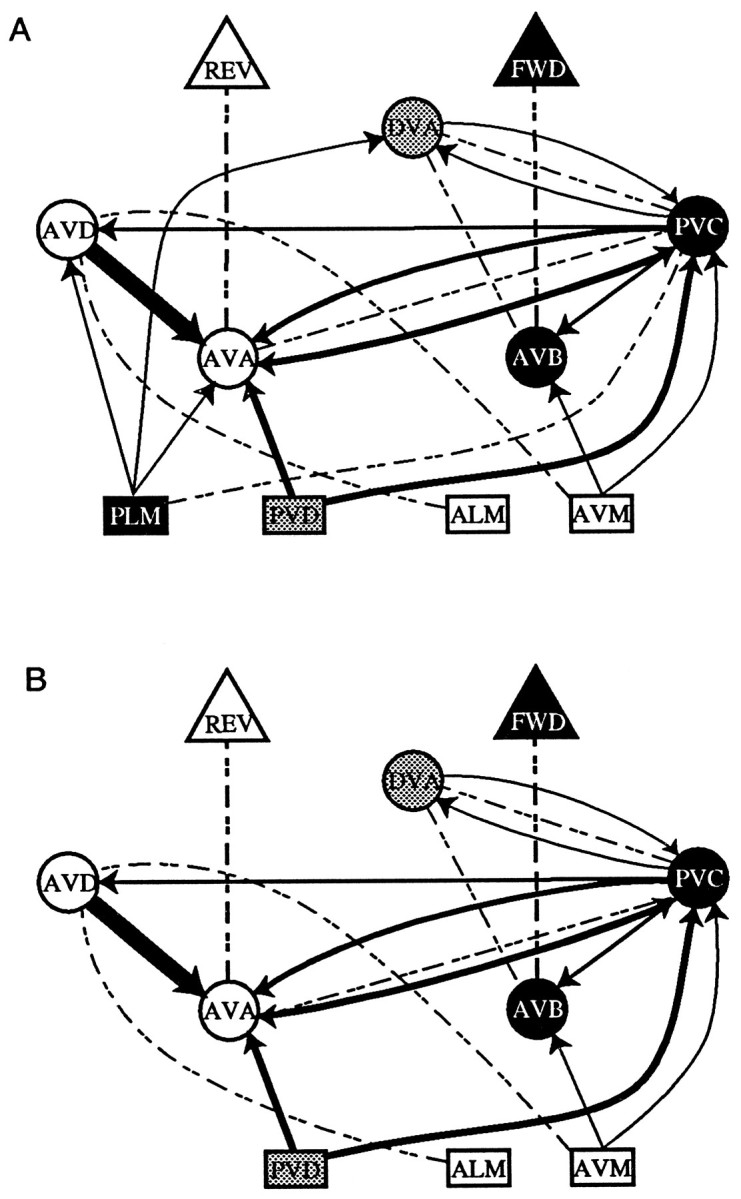
A, The intact neural circuit underlying the response to tap. The two subcircuits are highlighted inwhite, black, orgray. The rectangles represent sensory neurons, the circles represent interneurons, and the triangles represent large pools of motor neurons. Chemical connections are shown in solid lines; electrical connection is shown in dashed lines. The thickness of the line reflects the relative number of synapses between the cells. Cells involved in forward movement are blackwith white lettering. The PLM tail touch neurons electrically excite the PVC interneurons, which activate AVB interneurons to drive a pool of motor neurons to produce forward movement, while simultaneously inhibiting the AVD and AVA interneurons chemically. Cells involved in backward movement are shown inwhite with black lettering. The ALM and AVM head touch neurons excite the AVD interneurons, which stimulate the AVA interneurons that, in turn, stimulate a pool of motor neurons to produce backward movement, while simultaneously inhibiting the AVB and PVC interneurons chemically. Cells shaded in gray(PVD and DVA) are involved in both forward and backward movement. B, The neural circuit for tap once the PLM tail sensory neurons were laser-ablated (modified fromWicks and Rankin, 1995).
Recent evidence has suggested that the chemical synapses from the touch cells onto the interneurons may be glutamatergic. Genes have been isolated that code for three classes of glutamate receptors:glr-1 [homologous to AMPA type (Hart et al., 1995; Maricq et al., 1995)], avr-15 [glutamate-gated Cl-channel (Dent et al., 1997)], and nmr-1 [homologous to NMDA-type channels (Brockie et al., 1997)]. All three receptor types are expressed in one or more of the four ventral cord interneurons (AVA, AVB, AVD, and PVC) that play a central role in the integration of the tap withdrawal response.
Other evidence of glutamatergic transmission comes from investigations of eat-4, a gene isolated in a screen for feeding defects in C. elegans (Avery 1993). eat-4has severe defects in nose touch, osmosensory, and volatile odorant responses (Berger et al., 1998). The eat-4 gene was described by Lee et al. (1999) who have shown that EAT-4 is specifically involved in M3 neurons that use glutamate transmission to relax the pharynx at the end of a contraction. EAT-4 shows strong homologies to a brain-specific mammalian (rat) sodium-dependent inorganic phosphate cotransporter (BNPI). Lee et al. (1999)suggest that EAT-4 positively regulates glutaminase activity, which in mammals is required for glutamate synthesis (for review, see Fonnum, 1993). Their hypothesis is that in C. elegans EAT-4 influences glutamate synthesis by modulating the activity of phosphate-activated glutaminase (PAG) by supplying a high intracellular inorganic phosphate concentration. Support for this hypothesis comes from the finding that rat BNPI was localized to the synaptic terminals of neurons and associated preferentially with the membranes of small synaptic vesicles (Bellocchio et al., 1998). Lee et al. (1999) also showed that eat-4 was expressed in the touch cells ALM, AVM, and PLM that transduce the tap. Thus there is both presynaptic and postsynaptic evidence supporting the hypothesis that chemical synapses between the touch cells and the interneurons are glutamatergic.
If the touch cells are glutamatergic and eat-4 interferes with glutamate transmission, then eat-4 worms should show altered responses and altered habituation to tap. The results clearly show that isolated stimulation of a presumably glutamatergic pathway ineat-4 mutants yields normal responses, whereas responses to repeated stimulation are profoundly disrupted. This suggests that an alternative pathway for maintaining glutamatergic transmission in the absence of EAT-4 exists, albeit with a time course that is too slow to maintain a tonic behavior such as pharyngeal pumping. Implications for mechanisms of habituation are discussed.
MATERIALS AND METHODS
Cell designations. All cell classes are described using the classification of White et al. (1986). Unless noted otherwise, all reference to a particular cell class (e.g., ALM) refers to a pair of bilaterally symmetric cells. Reference to a group of animals with the cell class name followed by a negative sign (e.g., PLM−) indicates that all members of the indicated classes were ablated in the group and that all other cell classes were left intact.
Subjects. A total of 397 worms [157 hermaphroditic N2C. elegans Bristol (N2), 160 eat-4(ky5) and 80 transgenic worms] were used. Although there are several alleles of theeat-4 gene known, there were several compelling reasons for choosing the eat-4(ky5) allele for these studies, including the fact that eat-4(ky5) is a loss-of-function allele that is probably a null (L. Avery, personal communication). In the analyses of expression patterns and gene function performed by Lee et al. (1999), eat-4(ky5) was the allele used for cell identification and rescue experiments. In addition, the role of EAT-4 in the touch circuit was tested in eat-4(ky5) using laser ablation studies. Thus it is known that eat-4(ky5) is expressed and functions in the touch cells and therefore might play a role in the response to tap; in addition, the eat-4(ky5)rescue constructs were available to confirm the role of this allele in the behavior.
N2 animals were originally obtained from the Caenorhabditis Genetics Center; eat-4(ky5) animals and transgenic strains were obtained from Leon Avery (University of Texas Southwestern Medical Center). All worms were synchronously grown on Nematode Growth Medium (NGM) agar seeded with Escherichia coli (OP50) as described by Brenner (1974). All testing was performed on 4-d-old adult worms raised at 20°C.
Behavioral testing. All behavioral testing was performed by observing single worms on Petri plates filled with 10 ml of NGM agar, under a stereomicroscope (Wild M3Z, Wild Leitz Canada). All behavior was recorded by a video camera (Panasonic Digital 5100) attached to a VCR (Panasonic AG1960) and monitor (NEC). A time-date generator (Panasonic WJ-810) was used to superimpose a digital stopwatch and time–date display on the video record. Taps (force of 1–2 N) were delivered to the side of the plate as described previously (Rankin and Broster, 1992).
The response to tap was assessed by measuring the magnitude of each animal's response to a single tap. To test for habituation to tap, worms were given 30 tap stimuli at the selected interstimulus interval (ISI; 2, 10, or 60 sec depending on the experiment). To test for spontaneous recovery from habituation, each worm was given single taps 30 sec, 5 min, and 10 min after habituation, and the magnitude of the responses to these taps was measured.
For experiments examining spontaneous behavior, worms were preplated on NGM agar plates for 30 min before 2 min of spontaneous behavior was filmed. Worms were then given 30 taps at a 10 sec ISI followed by a second 2 min of filmed spontaneous behavior. The frequency and magnitude of spontaneous reversals for the 2 min period after habituation were then scored.
To test for dishabituation, worms were first preplated for 24 hr, given 40 tap stimuli at a 10 sec ISI followed 10 sec after tap number 40 by a dishabituating stimulus in the form of a brief train of electric shocks generated by the Grass S88 stimulator. The shocks were delivered using a hand-held spanning electrode with wires placed into the agar on either side of the worm. The train of shocks consisted of 10 msec shocks of 60 V at 10 pulses per second (pps) for 600 msec. The shock was followed 10 sec later by five taps 10 sec apart to assess the effects of shock.
Scoring. In response to tap, animals either reversed (moved backward) through some distance or occasionally accelerated (moved forward more rapidly). In intact animals, a number of responses (usually not more than 10–15% of the total number of responses) are accelerations forward. In previous studies we have shown that the neural circuits underlying backward and forward movements differ and that these responses habituate with different kinetics; therefore, in our analyses of habituation of reversals, accelerations are treated as missing data points. If the animal paused in response to tap, its reversal magnitude was zero. Response magnitude of reversals was quantified by tracing the path of the response using stop-frame video analysis onto acetate sheets. The tracings from the acetate sheets were then scanned into the computer and measured using NIH Image software.
Analysis. For analysis of habituation kinetics and spontaneous recovery curves, reversal magnitude data were standardized by expressing the length of all reversals that occurred in response to tap as a percentage of the mean initial response for that group of worms.
Laser ablation studies. For laser studies, highly synchronous animals were obtained as described in Wicks and Rankin (1995).
Laser pulses were delivered by a VSL-377 nitrogen laser (Laser Science, Cambridge, MA). The beam was directed through a laser dye module (Laser Science) containing a Coumarin 440 dye (Laser Science) that re-emitted with a peak gain of 437 nm. Single-cell ablations were performed under a 100× oil immersion lens mounted on a Zeiss Axioskop equipped with Nomarski (differential interference contrast) optics (Carl ZeissCanada). The beam was directed down through the optics of the microscope with a semi-silvered mirror and targeted into the plane of optical focus with a beam expander (Laser Science).
Single-cell laser ablations were conducted as previously described (Avery and Horvitz, 1989; Wicks and Rankin, 1995). All PLM cells were ablated in early L1, within 3 hr of hatching. All animals were recovered from the microscope slide and placed on individual agar plates seeded with OP50 E. coli within 1 hr of initial anesthesia and placed in a 20°C incubator. Approximately 25% of the animals were remounted without anesthesia 2–3 hr later and checked to ensure that the target cell was destroyed. A laser ablation control group was also run in which intact worms were treated identically to the ablated worms with the exception that the cells were located but not ablated. Behavioral testing of ablation animals was performed on the same plates on which the animals were isolated.
RESULTS
eat-4 does not affect the response to a single tap
The response to tap in the intact worm is a balance between two antagonistic response strategies: forward locomotion as a consequence of posterior mechanosensory input (from the pair of PLM touch cells) and backward locomotion as a consequence of anterior mechanosensory input (from the paired ALM and single AVM touch cells) (Wicks and Rankin, 1995). To assess the role of EAT-4 in the response of the touch cells to tap, we compared the tap withdrawal response magnitudes ofeat-4(ky5) animals with those of control worms. In response to tap, wild-type worms swim backward for approximately one worm length in distance before changing direction and swimming forward again. We call this tap–withdrawal response a reversal. Because the tap–withdrawal response in intact worms depends on antagonistic inputs, we tested both intact eat-4 animals andeat-4 animals that had received laser ablations of the posterior touch cells (PLM−; identified aseat-4:: PLM−). These responses were compared with similar groups of wild-type animals. Thus, in this study we are able to assess the function of the chemical synapses from the anterior touch cells ALM right (ALMR), ALM left (ALML), and AVM by looking at both the integrated response and the “pure” reversal response produced by these cells.
In this study, four groups of worms were tested: intact wild-type (n = 20), intact eat-4(ky5)(n = 20), PLM− wild-type (n = 16), andeat-4(ky5) PLM− worms (n = 20). In both PLM− groups the paired PLM tail touch cells were laser-ablated during the L1 larval stage.
If eat-4 were necessary for glutamatergic transmission, then the response to tap would be mediated by only the gap junctions between the touch cells and the interneurons in these mutants (Fig.1). It would be anticipated that the nature of the response in intact worms might be altered by this change. In the absence of both the competing input from the tail sensory neurons and intact chemical neurotransmission, theeat-4:: PLM− animals would only have the benefit of the electrical excitation of backward locomotion, which might alter the response to tap (Fig. 1B).
The mean response size of intact wild-type worms was 534.61 pixels ± 72.032 SEM, whereas the mean response size for intacteat-4 worms was 462.327 ± 56.364 SEM. A comparison of the responses of intact wild-type and eat-4 worms found no significant differences (unpaired t test,t(38) = 0.79, NS). When the tail sensory neurons [PLM left (PLML) and PLM right (PLMR)] were ablated, the mean response size of wild-type:: PLM− was 490.522 ± 55.67 (SEM), and the mean response size for eat-4:: PLM− was 382.08 ± 44.71 (SEM). There were no significant differences in response magnitude between wild-type:: PLM− andeat-4:: PLM− worms (unpaired t test,t(34) = 1.54, NS).
Thus far the eat-4 mutant worms showed no differences in behavior when compared with comparably treated wild-type worms. From this data there does not appear to be a role for the EAT-4 in the response to a single tap stimulus. This suggests that in the absence ofeat-4, chemical transmission is still intact in the tap withdrawal circuit. However, Lee et al. (1999) hypothesized thateat-4 may be required for glutamate transmission. We therefore examined the response to repeated stimulation.
eat-4 mutants show altered habituation to tap
One of the characteristics of habituation and spontaneous recovery from habituation is their sensitivity to the ISI of the habituating stimuli (Groves and Thompson, 1970). At short ISIs, habituation is more rapid and more complete than with long ISIs; however, recovery is also more rapid after habituation at short ISIs than at long ISIs (Rankin and Broster, 1992). This sensitivity of recovery to ISI can be used to show that the decrement in the response is the result of habituation and not fatigue or adaptation. Recovery after either fatigue or adaptation should only be affected by the degree of decrement and should be the same regardless of the ISI of training. Therefore, to look at the effects of an eat-4 mutation on habituation we tested wild-type and eat-4 worms with 30 tap stimuli at three different ISIs: 2, 10, and 60 sec (n = 20 worms per group). To examine spontaneous recovery from habituation, we tested worms from all groups at 30 sec, 5 min, and 10 min after habituation. Both wild-type worms and eat-4 worms habituated at all ISIs and showed some spontaneous recovery at the designated test times.
At both the 10 and 60 sec ISI, the eat-4(ky5) worms show extremely rapid habituation, relatively depressed asymptotic levels, and relatively retarded recovery when compared with wild-type worms (Fig. 2). In addition, eat-4worms appeared to habituate more rapidly and more completely at the 10 sec ISI than at the 60 sec ISI. At the 2 sec ISI, habituation data were not scored before the last response because the early responses last longer than the 2 sec ISI. However, virtually no animals responded to the last stimulus. An examination of spontaneous recovery shows that it was also retarded in eat-4 worms compared with wild type. It is important to note that the recovery responses in both the wild-type worms (Fig. 3, top) and the eat-4 worms (Fig. 3, bottom) showed the sensitivity to ISI described earlier. The eat-4 worms show the most rapid recovery after habituation at the 2 sec ISI and the slowest recovery after habituation at the 60 sec ISI. However, again it is clear that at all ISIs the eat-4 worms recovered more slowly than the wild type. Thus the response decrement seen in theeat-4 worms follows the rules for habituation that are seen not only in intact worms but in all organisms in which habituation has been studied (Groves and Thompson, 1970; Rankin and Broster, 1992).
Fig. 2.
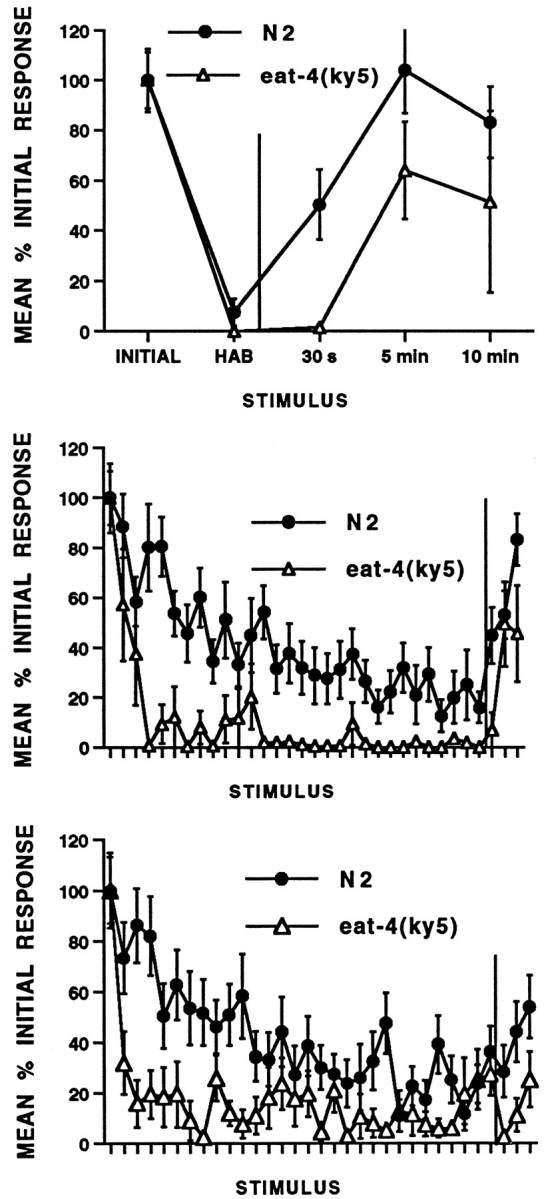
Habituation and spontaneous recovery from habituation for wild-type N2 and eat-4 worms. All ISI worms received 30 stimuli followed by recovery tests at 30 sec, 5 min, and 10 min after habituation (n = 20 per group). At each ISI, habituation was more rapid and complete and recovery slower in eat-4 worms than in wild-type worms.Top, Two-second ISI: mean standardized reversal magnitude (in pixels) for responses to 30 tap stimuli and three recovery tests (after the vertical line; 30 sec, 5 min, and 10 min). eat-4 worms show habituation and rapid but incomplete recovery from habituation. Middle, Ten-second ISI: eat-4 worms show more rapid and complete habituation and slower recovery (after the vertical line) from habituation than wild-type worms.Bottom, Sixty-second ISI: eat-4 worms show more rapid and complete habituation and slower recovery (after thevertical line) from habituation than wild-type worms.
Fig. 3.
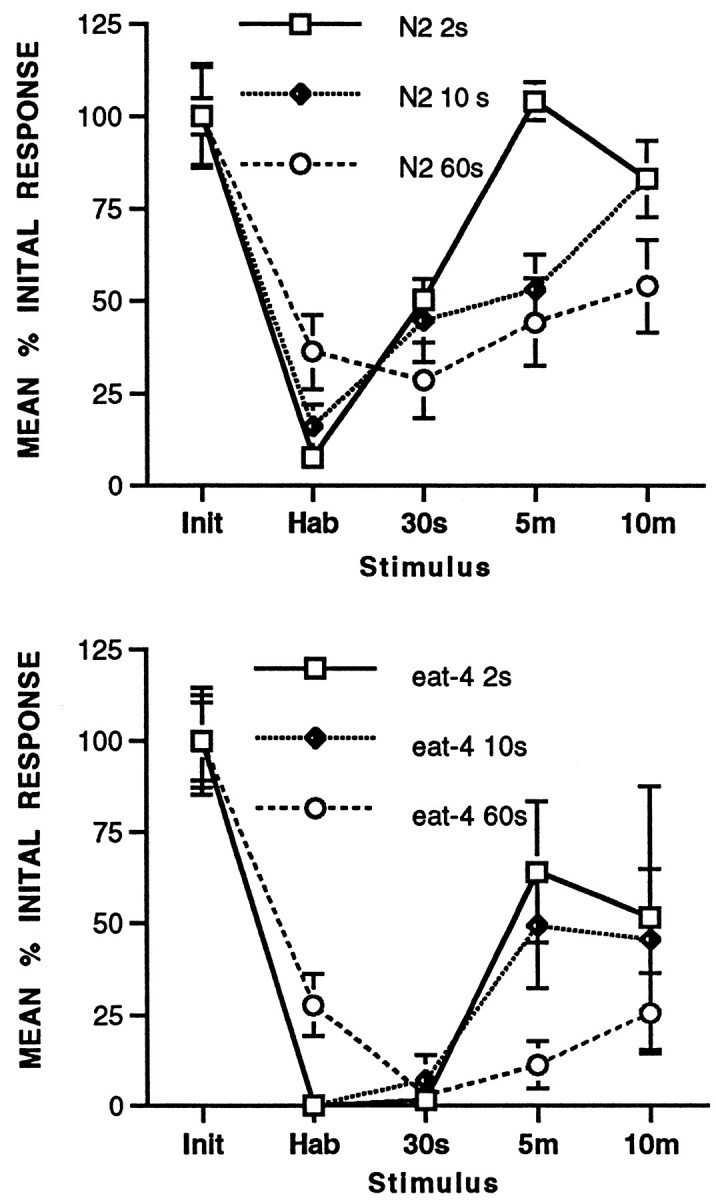
Comparison of recovery at 30 sec, 5 min, and 10 min after habituation at 2, 10, and 60 sec ISIs (redrawn from Fig. 2). Recovery was slower the longer the ISI for both N2 worms andeat-4 worms. Top, Mean initial response (Init), mean of the 30th response (Hab), and 30 sec (30s), 5 min (5m), and 10 min (10m) recovery for N2 wild-type worms. Bottom, Mean initial response (Init), mean of the 30th response (Hab), and 30 sec, 5 min, and 10 min recovery for eat-4worms.
Although EAT-4 does not appear to be required for glutamatergic transmission, it does appear to be required for sustained glutamatergic transmission. These data are consistent with eat-4 acting to facilitate the synaptic function of the touch cells; EAT-4 appears to be required for a time-dependent recovery process after rapid abolishment of synaptic function. This process may involve glutamate synthesis, glutamate reuptake, vesicle recruitment, or vesicle loading.
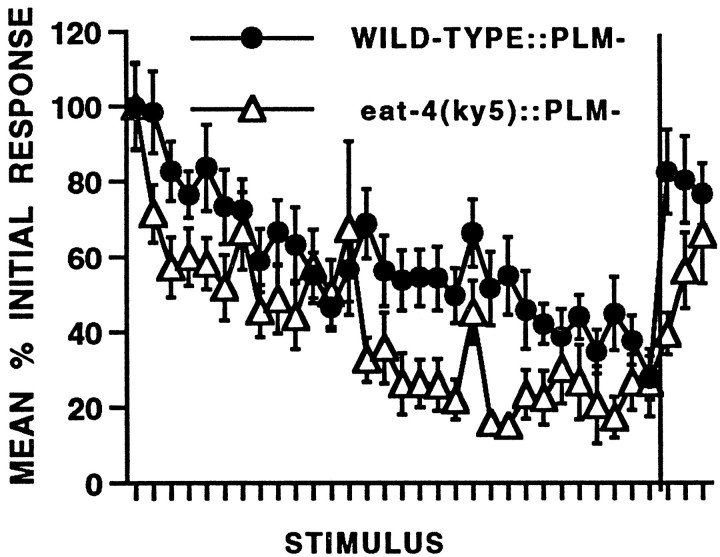
As described above, the tap withdrawal response is a compound behavior composed of two competing responses (Wicks and Rankin, 1995). The kinetics of the habituation curve of the intact worm are the result of the two different habituation patterns seen in worms in which the opposing circuits have been selectively laser-ablated (Wicks and Rankin, 1996). In worms in which the tail touch cells (PLM) have been ablated, the response habituates more slowly than it does in wild-type worms. In contrast, the acceleration response to tap in worms in which the ALM and AVM head touch cells have been ablated appears to facilitate before it begins to habituation and is less habituated after 30 stimuli than are the “pure” reversals in the PLM− group. To investigate the role of eat-4 in habituation in a single pure response, we compared habituation of wild-type (n= 16) and eat-4 worms (n = 20) with the tail touch PLM neurons that were ablated. This ablation eliminates the competing forward responses and we are left with pure reversals. The results of this experiment (Fig. 4) show that in both wild-type and eat-4 ablated worms, habituation is slower and less complete than in intact worms. Again,eat-4:: PLM− worms habituate more and recover more slowly than do wild-type:: PLM− worms. Because there is some residual responding throughout the habituation training in theeat-4:: PLM animals, this suggests that the electrical connections from ALM and AVM are sufficient to mediate a response in the absence of competing inputs from PLM. In intacteat-4 worms, both the forward and backward circuit are activated by electrical connections between the sensory neurons and the interneurons; however, very few stimulations lead to rapid depletion of neurotransmitter that eliminates chemical modulation of the circuits. The electrical circuits may then simply cancel each other out, leading to no behavioral response to the tap. Thus the habituation curve of intact eat-4 worms drops rapidly to zero. In contrast, in eat-4:: PLM− worms only the interneurons for reversals are electrically activated by the repeated taps: there is no competing circuit to cancel the reversal input, and so the worm continues to reverse to repeated taps. However, we still see a decrement in the response of eat-4:: PLM− worms to repeated stimulation. One possibility is that the mutation may not eliminate glutamate but may just decrease dramatically the amount available. In intact eat-4 worms the competition between the two circuits masks any residual glutamate component of the response. Ineat-4:: PLM− worms a gradual decrease in the release of this residual glutamate may be responsible for the habituation seen. The alternative explanation is that the electrical connection between the ALM and AVM sensory neurons onto the interneurons also shows response decrement with repeated stimulation. Further experiments with mutations affecting both chemical and electrical synapses will be useful in exploring these possibilities.
Fig. 4.
Habituation and spontaneous recovery from habituation of wild-type (n = 16) andeat-4 (n = 20) worms that have had the tail sensory neurons (PLM) laser-ablated. Mean percentage initial response is shown for responses to 30 stimuli at a 10 sec ISI followed by recovery tests at 30 sec, 5 min, and 10 min shown after thevertical line. Habituation is more rapid and spontaneous recovery slower in eat-4:: PLM− than in wild-type:: PLM− worms.
Habituation of the tap withdrawal response does not affect spontaneous reversal frequency or magnitude in eat-4worms
Wicks and Rankin (1997) showed that in intact wild-type worms habituation to tap did not affect other behaviors mediated by the interneurons and motor neurons of the tap withdrawal circuit; they found no difference in either the frequency or magnitude of spontaneous reversals after habituation to tap. If the response decrement seen after repeated stimulation in eat-4 worms were the result of fatigue, it would be predicted that after habituation there should be a decrease in either the frequency or magnitude of spontaneous reversals. In this experiment the frequency of spontaneous reversals was recorded for 2 min before and 2 min after habituation training with 30 stimuli at a 10 sec ISI in eat-4 worms (n = 20). The results showed that there were 3.45 ± 0.51 (SEM) spontaneous reversals over 2 min before habituation and 2.45 ± 0.55 (SEM) spontaneous reversals in the 2 min after habituation. A comparison of the mean frequency of spontaneous reversals before and after habituation showed no significant differences (paired ttest, t(19) = 1.218, NS). The mean magnitude of spontaneous reversals before habituation was 289.09 ± 50.22 pixels (SEM), whereas the mean magnitude after habituation was 212.98 ± 23.27 pixels (SEM). There were no significant differences between the mean magnitude of spontaneous reversals before or after habituation for eat-4 worms (paired ttest, t(14) = 1.622, NS). This experiment rules out motor fatigue as an explanation for the decrement seen in the response after repeated taps in eat-4 worms.
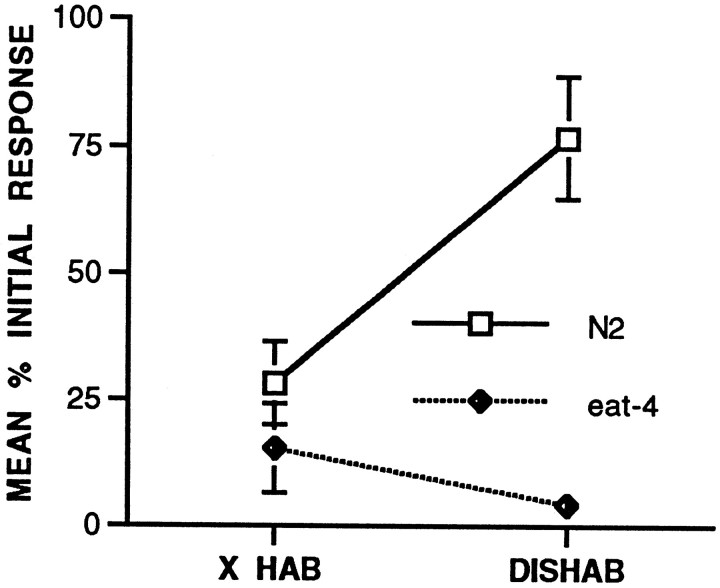
eat-4 worms do not show dishabituation after shock
One of the properties of habituation as described by Groves and Thompson (1970) is the ability of a novel or noxious stimulus to facilitate the decremented response. C. elegans shows dishabituation of the habituated tap withdrawal response after a brief electric shock delivered to the agar on which it moves (Rankin et al., 1990). In this experiment, worms were given 40 tap stimuli at a 10 sec ISI and then a brief electric shock to the agar on either side of the worm. To measure dishabituation, the mean of the five responses before shock was compared with the first response after shock for both the N2 worms (n = 21) and the eat-4 worms (n = 20). The results (Fig.5) showed that there was a significant increase in responses for the N2 worms after shock (t(20) = 3.88, p = 0.0009); however, there was no significant increase for theeat-4 worms (t(19) = 1.29, NS). Although Groves and Thompson (1970) consider dishabituation to be a key feature in distinguishing habituation from fatigue, it is possible that the sensitivity of spontaneous recovery to ISI of training is a better feature. The feature is clearly a property of the same or related mechanisms as those involved in habituation. In contrast, we know little about the relationship between the processes of habituation and dishabituation. It is not known whether dishabituation is simply the reversal of the process of habituation or whether it is a separate facilitatory process superimposed on habituation that then wears off while habituation is undergoing spontaneous recovery. The data from this experiment witheat-4 show that although worms are still capable of habituation and recovery from habituation in an ISI-dependent manner, dishabituation is absent.
Fig. 5.
Dishabituation test. Both wild-type N2 (n = 21) and eat-4(n = 20) worms received 40 tap stimuli at a 10 sec ISI followed by a brief shock and then five taps to assess the effects of shock. Mean response magnitude (in pixels) for the final five responses of the habituation series before the shock and the mean response magnitude for the first response after the shock for wild-type and eat-4 worms is shown. Wild-type worms showed significant dishabituation; eat-4 worms did not.
A genetic construct that rescues the eat-4 pharyngeal phenotype also rescues both the habituation and the dishabituation deficits
In their study of the role of eat-4 on pharyngeal pumping, Lee et al. (1999) mapped, cloned, and sequencedeat-4 and produced genetic constructs that rescued the pharyngeal muscle relaxation deficit of the eat-4(ky5)mutant allele. Lee et al. (1999) showed that the eat-4 gene rescuing activity was localized to a 6.9 kb region of cosmid ZK512. A strain (DA1242) was made using cosmid rescue with cosmid ZK512 that restored the wild-type feeding phenotype. A second strain was made using a minigene constructed from the region of ZK512 thought to contain eat-4 by fusing a SacI–PstI (22339–24738) genomic fragment from ZK512 (the presumptive 5′ regulatory sequence of eat-4) to a 2.2 kb cDNA clone (yk32h2), which codes for a polypeptide of 563 amino acids. Transformation with this minigene led to partial rescue of the pharyngeal muscle deficit, with partial but incomplete restoration of M3 motor neuron activity (Lee et al., 1999). In the construction of the transgenic strains, lin-15 was used as a coinjection marker to transform eat-4(ky5);lin-15(n765ts) mutant worms. We obtained transformed DA1241 and DA1242 strains to test whether these genetic constructs would also rescue the habituation deficit seen in our studies.
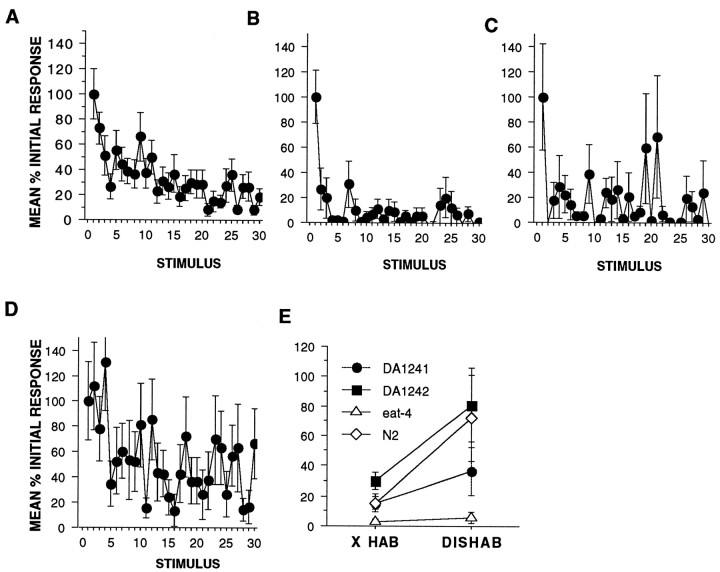
The results of habituation training with 30 tap stimuli at a 10 sec ISI using wild-type, eat-4, DA1241, and DA1242 worms (n = 20 per strain) can be seen in Figure6A–D. Again, eat-4 worms habituated more rapidly and more completely than did wild-type worms (Fig.6A,B). Both transgenic strains showed a high level of variability (as can be seen from the large standard error bars on Fig. 6C,D). This variability is not unexpected from transgenic worms because the transgene has not been integrated into the germline but takes the form of an extrachromosomal array in which the number of copies of the transgene expressed per individual worm and per cell may vary a great deal. Thus the phenotypic expression of transgenes also varies between individuals. The DA1241 worms (Fig. 6C) show more responding and greater variability in response magnitude than the eat-4worms. Although the DA1242 worms also showed greater variability than the wild-type worms, the kinetics of the habituation curve were similar to the wild-type worms (Fig. 6D). It may be that the slightly slower habituation in the DA1242 worms was caused by overexpression of eat-4, but further studies would be needed to confirm or refute this (Fig. 6D). Based on the similarity between the habituation kinetics of the DA1242 worms and the wild-type worms, we suggest that the DA1242 worms reflect a rescue of the eat-4 habituation phenotype.
Fig. 6.
Genetic constructs carrying theeat-4 gene can rescue the behavioral phenotype of the eat-4 worms (n = 20 per group). A, Habituation curves showing responses as a mean percentage of initial response for wild-type N2 worms habituated with 30 stimuli at a 10 sec ISI. B, Habituation curves showing responses as a mean percentage of initial response foreat-4 worms habituated with 30 stimuli at a 10 sec ISI. The eat-4 worms habituate more rapidly and completely than N2 worms. C, Habituation curve showing responses as a mean percentage of initial response for strain DA1241. There was more responding and greater variability in responses in DA1241 worms when compared with eat-4 worms. D, Habituation curve showing responses as a mean percentage of initial response for strain DA1242. Although there is more variability in the DA1242 responses, this figure clearly shows that the transgene has rescued theeat-4 habituation phenotype. E, Test for dishabituation in wild-type, eat-4, DA1241, and DA1242 strains. Mean percentage initial response magnitude (in pixels) for the final five responses of the habituation series before the shock (X HAB) and the mean response magnitude for the first response after the shock (DISHAB) are shown for wild-type, eat-4, Da1241, and DA1242 worms. There was significant dishabituation for wild-type and DA1242 worms and not foreat-4 and DA1241 worms. Thus in DA1242, the transgene has rescued both the habituation and dishabituation phenotypes seen ineat-4 worms.
The results of habituation and dishabituation training with 40 tap stimuli at a 10 sec ISI followed by a brief electric shock and then five stimuli at a 10 sec ISI using wild-type, eat-4, DA1241, and DA1242 worms (n = 20 per strain) can be seen in Figure 6E. A one-tail t test comparing the last five responses before shock (X HAB) with the first response after shock for eat-4 and wild-type worms (DISHAB) replicated the earlier observation that wild-type worms dishabituate (t(19) = −1.9,p = 0.03) whereas eat-4 worms do not (t(19) = −0.59, NS). When the same comparisons were made for the two transgenic strains, the DA1241 worms did not show significant dishabituation (t(19) = −1.28, NS), but the DA1242 worms did show significant dishabituation (t(19) = −1.9, p = 0.03). Thus the transgene in DA1242 worms rescued the dishabituation deficit seen in eat-4 worms.
DISCUSSION
eat-4 is a mutation that does not affect initial response to tap but is required for sustained activity
The eat-4 mutation does not affect the response to a single tap; however, it has a marked effect on the responses to repeated stimulation. eat-4 worms show more rapid and complete habituation and slower recovery than wild-type worms at all ISIs tested. This was true for both the intact response that is an integration of two competing responses and the pure reversal response seen in PLM− worms.
A number of converging lines of evidence suggest that the touch cells use glutamate as their neurotransmitter. These include investigation of the eat-4 gene (Lee et al., 1999), localization of a mammalian homolog of eat-4 to glutamatergic terminals in rat (Bellocchio et al., 1998), as well as the localized expression of a number of genes for glutamate receptors in the postsynaptic interneurons of the touch circuit [glr-1 (Hart et al., 1995; Maricq et al., 1995), avr-15 (Dent et al., 1997), andnmr-1 (Brockie et al., 1997)]. The data reported here largely support the hypothesis by Lee et al. (1999) that EAT-4 is required for glutamatergic neurotransmission. However, these data specifically highlight the fact that rather than being absolutely required for glutamatergic neurotransmission, EAT-4 is only required for sustained synaptic activity. Strictly, eat-4(ky5)produces learning deficits: the response to tap is intact, but habituation and dishabituation of the tap withdrawal response are broadly affected. Habituation and spontaneous recovery from habituation are both altered and yet still retain their characteristic sensitivity to ISI (short ISIs produce faster habituation and more rapid recovery than long ISIs do); this suggests that although the mutation affects one cellular mechanism of habituation, other mechanisms are intact. The finding that dishabituation is not present ineat-4 worms and is rescued in the transgenic strain DA1242 suggests that the mutation alters a specific component of dishabituation in the sensory neurons. Gingrich and Byrne (1985)hypothesized that dishabituation might involve the mobilization of neurotransmitter from intracellular stores to the terminal. If there is a decrease in the amount of neurotransmitter available in the sensory neurons of eat-4 worms, it is not surprising that the process of dishabituation is not evident. It may be that some aspect of EAT-4 function is necessary for dishabituation to occur.
Mechanisms of habituation
The little that is known about the mechanisms of habituation in multicellular organisms comes largely from work inDrosophila and Aplysia. In Drosophila, analyses of habituation in the giant fiber escape pathway using memory mutants such as dunce and rutabaga have shown that dunce, which increases cAMP levels, shows an increase in habituation rates, whereas rutabaga, which causes a decrease in cAMP levels, decreases the rate of habituation (Engel and Wu, 1996). Engel and Wu (1998) have also shown altered habituation with mutations in various K+ channels, with some enhancing and some slowing habituation. In studies of habituation in Aplysia,Bailey and Chen (1988) have shown that there are fewer synaptic vesicles in the active zones of sensory neurons from habituated animals than from the terminals of nonhabituated animals. This suggests that an aspect of habituation involves regulation of vesicles at the active zones. In a study on spontaneous and evoked release of neurotransmitter from cultured Aplysia sensory neurons, Eliot et al. (1994)have shown that there is no change in the size or frequency of spontaneous neurotransmitter release after decrement at long ISIs and that there is a transient decrease in spontaneous release frequency after short ISIs. In contrast, there is a marked decrease in release magnitude in response to simulation at both long and short ISIs. Eliot et al. (1994) suggest that different mechanisms may govern spontaneous and evoked release of neurotransmitter.
Thus we have evidence that the mechanisms of habituation may involve regulation of cell metabolism and transmitter availability and release but very little data that directly address the cellular mechanisms underlying habituation. The disruption of a sodium–inorganic phosphate cotransporter, mammalian homologs of which are specifically found at small neuronal vesicles (Bellocchio et al., 1998), suggests one way in which mechanisms may affect synaptic decrement. Lee et al. (1999)suggest that the eat-4 mutation affects transmission by reducing the available inorganic phosphate at the terminal. This, in turn, downregulates the activity of an enzyme (a PAG) involved in the catalysis of glutamine precursors into glutamate. The localization data intriguingly suggest that this conversion may occur only when vesicles fuse to the plasma membrane during release, for at that time a sufficient sodium gradient may exist for Pi import. If this is the case, then glutamate synthesis and loading may be regulated by activity at the synapse and occur on very fast time scales. Furthermore, low resting levels of inorganic phosphate would allow the PAG to synthesize glutamate only inefficiently. Sustained release would be impossible, and thus tonic behaviors that rely on such impaired synapses would be profoundly affected, whereas phasic behaviors, such as the tap withdrawal response, would be relatively unaffected. Only responses to repeated stimulation would be affected.
Implications from behavioral studies
Often, behavioral characteristics of a phenomenon can guide research into cellular mechanisms. In habituation paradigms, shorter ISIs produce more rapid response decrement and recovery from habituation than longer ISIs (Groves and Thompson, 1970; Rankin and Broster, 1992). This is apparent at both the behavioral level and the level of individual neurons. By using changes in the magnitude of the EPSPs in gill motor neurons of Aplysia to measure homosynaptic depression, Byrne (1982) showed that the decrement of the motor neuron EPSP was more rapid and recovered faster when the sensory cell was stimulated at short ISIs than when the sensory cell was stimulated at longer ISIs. Byrne (1982) hypothesized that short ISIs might activate a facilitatory process such as intracellular calcium buildup, which facilitates transmitter release that leads to the rapid spontaneous recovery. During long ISIs, no such buildup would occur because the intrinsic buffering systems of the cells would eliminate free calcium; thus recovery would be slower. It is interesting to note that in the experiments reported here witheat-4 worms, the recovery from the very short ISI (2 sec) was faster than that at longer ISIs but did not show the very rapid facilitated recovery that we see in the wild-type worms. If EAT-4 regulates synaptic transmission by modulating levels of Pi as described above, then an elevation of the levels of inorganic phosphate that would result during heavy synaptic activity rather than elevated calcium levels per se may be responsible for this well characterized transient facilitation seen at short interstimulus intervals during habituation studies.
Long and short ISIs recruit distinct processes during habituation.Rankin and Broster (1992) showed that the ISI of habituation is the most important determinant of the spontaneous recovery rate inC. elegans once habituation is at asymptotic levels. Neither relative level of habituation nor number of stimuli played large roles in determining the rate of recovery. The data suggest that after relatively few stimuli, ISI somehow directs the rate of recovery from habituation, or rephrased, that the ISI of stimulation differentially affects the cells and that the mechanisms of habituation at different ISIs are not identical. A hypothesized cellular correlate of this effect is that the elevated levels of Pi, which might be a consequence of a burst of sustained rapid activity (short ISI), would persist and direct a rapid recovery of available glutamate despite long previous training at a longer ISI. eat-4 has a similar effect at all ISIs investigated; other as yet unidentified genes may preferentially play a role in only long or only short ISIs.
Hypothesized mechanisms of habituation
The data from research on Aplysia and C. elegans, as well as behavioral data from various organisms, suggest that there may be a large number of cellular processes underlying the “simple” form of learning that we call habituation. It may be that different stimulus protocols (i.e., ISIs) may recruit overlapping subsets of these cellular processes, so that they would both activate some basic set of systems, but that different ISIs might differentially recruit additional nonoverlapping cellular processes. In the case of eat-4, long and short ISIs were similarly affected by the mutation, and so an eat-4-mediated process would fit into the category of overlapping processes. However, transmitter depletion is not the only cellular process involved in habituation, because other characteristics of the phenomenon are still intact (i.e., sensitivity to ISI).
The role of eat-4 in regulating the amount of glutamate at the terminal and its effects on habituation, spontaneous recovery, and dishabituation are consistent with what we know about the mechanisms of habituation. The regulation of transmitter at the terminal and/or regulation of release processes are the most likely ways cells could operate to produce the behavioral changes we see in habituation. Further investigations of mutations in these basic processes may shed additional light on other mechanisms involved in habituation.
Footnotes
This work was supported by an NSERC scholarship to S.R.W. and Natural Sciences and Engineering Research Council of Canada and Human Frontiers of Science operating grants to C.H.R. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. We acknowledge Leon Avery for suggesting that we look at eat-4, for many useful conversations, and for supplying the rescued worms. Some of the experiments reported here were run and/or scored by Natasha Ghosh, Richard Faber, James Dai, Erin Phillip, Sylvia Chen, Carina Fu, and Anthony Chau.
Correspondence should be addressed to Dr. C. H. Rankin, Department of Psychology, University of British Columbia. Vancouver, British Columbia V6T-1Z4, Canada. E-mail: crankin@cortex.psych.ubc.ca.
Dr. Wicks's current address: Division of Molecular Biology, Netherlands Cancer Institute, Amsterdam 1066 CX, The Netherlands.
REFERENCES
- 1.Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avery L, Horvitz HR. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron. 1989;3:473–485. doi: 10.1016/0896-6273(89)90206-7. [DOI] [PubMed] [Google Scholar]
- 3.Bailey CH, Chen M. Long-term memory in Aplysia modulates the total number of varicosities of single identified sensory neurons. Proc Natl Acad Sci USA. 1988;85:2373–2377. doi: 10.1073/pnas.85.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J Neurosci. 1998;18:8648–8659. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger AJ, Hart AC, Kaplan JM. G-alpha(s)-induced neurodegeneration in Caenorhabditis elegans. J Neurosci. 1998;18:2871–2880. doi: 10.1523/JNEUROSCI.18-08-02871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner S. The genetics of the nematode Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brockie PJ, Madsen DM, Maricq AV. Genetic analysis of two C. elegans putative NMDA receptor subunits nmr-1 and nmr-2. Soc Neurosci Abstr. 1997;23:936. [Google Scholar]
- 8.Byrne JH. Analysis of synaptic depression contributing to habituation of gill-withdrawal reflex in Aplysia californica. J Neurophysiol. 1982;48:431–438. doi: 10.1152/jn.1982.48.2.431. [DOI] [PubMed] [Google Scholar]
- 9.Dent JA, Davis MW, Avery L. avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J. 1997;16:5867–5879. doi: 10.1093/emboj/16.19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliot LS, Kandel ER, Hawkins RD. Modulation of spontaneous transmitter release during depression and posttetanic potentiation of Aplysia sensory-motor neuron synapses isolated in culture. J Neurosci. 1994;14:3280–3292. doi: 10.1523/JNEUROSCI.14-05-03280.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engel JE, Wu CF. Altered habituation of an identified escape circuit in Drosophila memory mutants. J Neurosci. 1996;16:3486–3499. doi: 10.1523/JNEUROSCI.16-10-03486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel JE, Wu CF. Genetic dissection of functional contributions of specific potassium channel subunits in habituation of an escape circuit in Drosophila. J Neurosci. 1998;18:2254–2267. doi: 10.1523/JNEUROSCI.18-06-02254.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonnum F. Regulation of the synthesis of the transmitter glutamate pool. Prog Biophys Mol Biol. 1993;60:47–57. doi: 10.1016/0079-6107(93)90012-9. [DOI] [PubMed] [Google Scholar]
- 14.Gingrich KJ, Byrne JH. Simulation of synaptic depression, posttetanic potentiation, and presynaptic facilitation of synaptic potentials from sensory neurons mediating gill-withdrawal reflex in Aplysia. J Neurophysiol. 1985;53:652–669. doi: 10.1152/jn.1985.53.3.652. [DOI] [PubMed] [Google Scholar]
- 15.Groves PM, Thompson RF. Habituation: a dual-process theory. Psychol Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- 16.Hart AC, Sims S, Kaplan JM. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature. 1995;378:82–85. doi: 10.1038/378082a0. [DOI] [PubMed] [Google Scholar]
- 17.Lee RYN, Sawin ER, Chalfie M, Horvitz HR, Avery L. EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate co-transporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J Neurosci. 1999;19:159–167. doi: 10.1523/JNEUROSCI.19-01-00159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maricq AV, Peckol E, Driscoll M, Bargmann CI. Mechanosensory signaling in C. elegans mediated by the GLR-1 glutamate receptor. Nature. 1995;378:78–81. doi: 10.1038/378078a0. [DOI] [PubMed] [Google Scholar]
- 19.Rankin CH, Broster BS. Factors affecting habituation and recovery from habituation in the nematode Caenorhabditis elegans. Behav Neurosci. 1992;106:239–242. doi: 10.1037//0735-7044.106.2.239. [DOI] [PubMed] [Google Scholar]
- 20.Rankin CH, Beck CDO, Chiba CM. Caenorhabditis elegans: a new model system for the study of learning and memory. Behav Brain Res. 1990;37:89–92. doi: 10.1016/0166-4328(90)90074-o. [DOI] [PubMed] [Google Scholar]
- 21.White JE, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 22.Wicks SR, Rankin CH. Integration of mechanosensory stimuli in Caenorhabditis elegans. J Neurosci. 1995;15:2434–2444. doi: 10.1523/JNEUROSCI.15-03-02434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wicks SR, Rankin CH. The integration of antagonistic reflexes revealed by laser ablation of identified neurons determines habituation kinetics of the Caenorhabditis elegans tap withdrawal response. J Comp Physiol [A] 1996;179:675–685. doi: 10.1007/BF00216131. [DOI] [PubMed] [Google Scholar]
- 24.Wicks SR, Rankin CH. The effects of tap withdrawal response habituation on other withdrawal behaviors: the localization of habituation in C. elegans. Behav Neurosci. 1997;111:1–12. doi: 10.1037//0735-7044.111.2.342. [DOI] [PubMed] [Google Scholar]