Abstract
We examined the effects of the neurosteroid pregnenolone sulfate (PS) on GABAA receptor-mediated synaptic currents and currents elicited by rapid applications of GABA onto nucleated outside-out patches in cultured postnatal rat hippocampal neurons. At 10 μm, PS significantly depressed peak responses and accelerated the decay of evoked inhibitory synaptic currents.
In nucleated outside-out patches, PS depressed peak currents and speeded deactivation after 5 msec applications of a saturating concentration of GABA. PS also increased the rate and degree of macroscopic GABA receptor desensitization during prolonged GABA applications. In a paired GABA application paradigm, PS slowed the rate of recovery from desensitization.
In contrast to its prominent effects on currents produced by saturating GABA concentrations, PS had only small effects on peak currents and failed to alter deactivation after brief applications of the weakly desensitizing GABAA receptor agonists taurine and β-alanine. However, when β-alanine was applied for a sufficient duration to promote receptor desensitization, PS augmented macroscopic desensitization and slowed deactivation.
These results suggest that PS inhibits GABA-gated chloride currents by enhancing receptor desensitization and stabilizing desensitized states. This contention is supported by kinetic modeling studies in which increases in the rate of entry into doubly liganded desensitized states mimic most effects of PS.
Keywords: GABA, neurosteroids, desensitization, synapses, outside-out patches, kinetics
GABA mediates much of the fast inhibitory neurotransmission in the mammalian CNS and is believed to participate in the pathophysiology of major neuropsychiatric disorders, including epilepsy, substance abuse disorders, mood disorders, and anxiety (Lambert et al., 1995; Sieghart, 1995). Drugs that enhance GABAergic transmission can be useful clinically as anticonvulsants, anesthetics, muscle relaxants, and anxiolytics (Sieghart, 1995). Agents that inhibit GABAergic function have convulsant, anxiogenic, and perhaps cognitive-enhancing effects.
Given the importance of GABA in CNS synaptic function, there is considerable interest in understanding the mechanisms of endogenous agents that modulate GABA-mediated neurotransmission. The neurosteroids represent a class of molecules that are synthesized in the CNS (Mensah-Nyagan et al., 1999) and have potent effects on GABAA receptors (Lambert et al., 1995, 1996). Some of these steroids, exemplified by (3α,5α)-3-hydroxypregnan-20-one (3α5αP) and 3α, 21-dihydroxy-5α-pregnan-20-one (THDOC), greatly enhance the function of GABAA receptors and increase inhibitory transmission in various brain regions (Lambert et al., 1995). Pregnenolone sulfate (PS) is a neurosteroid that inhibits responses mediated by GABAA receptors (Majewska and Schwartz, 1987; Majewska et al., 1988). Previous studies have shown that PS is a noncompetitive GABAA receptor antagonist that acts, at least in part, by decreasing channel opening frequency (Mienville and Vicini, 1989). This raises the possibility that PS may function as an endogenous modulator of fast inhibitory synaptic transmission. However, the effects of PS on GABA-mediated synaptic transmission are poorly understood at present.
Similar to glutamate acting at fast excitatory synapses, it appears that GABA-mediated IPSCs result from very brief exposure of postsynaptic GABAA receptors to high, possibly saturating, concentrations of transmitter (Maconochie et al., 1994;Jones and Westbrook, 1995). However, the time course of GABA-mediated IPSCs is prolonged relative to the excitatory actions of glutamate at AMPA receptors. The slow time course of GABAergic IPSCs is thought to result, in part, from fast entry of GABAA receptors into desensitized states coupled with fast recovery from desensitization and reopening of ion channels before agonist unbinding (Jones and Westbrook, 1996). The kinetics of GABA-mediated responses and the brief nature of the GABA concentration transient at synapses make it important to use nonstationary experimental approaches to mimic conditions at synapses when studying the effects of neuromodulators. In the present experiments, we examined the effects of PS on inhibitory autaptic currents (IACs) in cultured hippocampal neurons and compared actions on synaptic transmission to effects on GABA currents in membrane patches exposed rapidly and briefly to high concentrations of agonists.
MATERIALS AND METHODS
Hippocampal cultures. Primary microisland cultures of hippocampal cells were prepared from 1- to 3-d-old postnatal albino rats using established methods (Mennerick et al., 1995). Under halothane anesthesia, rats were decapitated, and the hippocampi were dissected and cut into 500-μm-thick transverse slices. The slices were dissociated with 1 mg/ml papain in oxygenated Leibovitz L-15 medium and mechanical trituration in modified Eagle's medium containing 5% horse serum, 5% fetal calf serum, 17 mmd-glucose, 400 μm glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. Isolated cells were plated onto plastic culture dishes at a density of 75 cells/mm2. Before plating, culture dishes were coated with a layer of 0.15% agarose, dried overnight, and sprayed with small droplets of rat tail collagen using a microatomizer (Thomas Scientific, Swedesboro, NJ). To halt glial proliferation, cultures were treated with 10 μm cytosine arabinoside after 3 d in vitro. Experiments were performed in cultures that were 7–14 d old.
Electrophysiology. For synaptic studies, the growth medium was exchanged for a solution containing (in mm): 138 NaCl, 4 KCl, 10 HEPES, 10 d-glucose, 2 CaCl2, and 1 MgCl2, pH 7.25. Osmolarity was maintained at 310–320 mOsm/l by addition of sucrose. Whole-cell voltage-clamp recordings of autaptic currents were performed from neurons on single-neuron microislands using recording pipettes with open tip resistances of 2–5 MΩ. The pipette solution contained (in mm): 140 KCl, 4 NaCl, 5 EGTA, 0.5 CaCl2, 10 HEPES, 2 MgATP, and 0.5 NaGTP, pH 7.25. Neurons were recorded using an EPC-7 patch-clamp amplifier (List Electronics), and series resistance was compensated 70–90% during experiments. Synaptic transmission was activated by stimulating neurons with 0.5 msec voltage steps from −70 to +30 mV at intervals of 20 sec. During experiments, microislands were continuously superfused using a gravity-driven multibarrel system with a common exit port. The tip of this local perfusion system was placed ∼400 μm from the microisland being recorded, and solution flowed at a rate of 0.8–1.5 ml/min. All recordings were done at room temperature (∼22°C) on the stage of a Nikon inverted microscope equipped with phase-contrast optics.
Studies examining exogenous applications of agonists were performed using nucleated outside-out patches (Sather et al., 1992; Zhu and Vicini, 1997). For these experiments, the pipette recording solution contained (in mm): 140 CsCl, 4 NaCl, 1 CaCl2, 3.45 Cs4BAPTA, 10 HEPES, and 5 MgATP, pH 7.25. GABAA receptor agonists and drugs were applied to patches from theta tubes using a piezoelectric delivery system (Burleigh Instruments, Fishers, NY). This system rapidly switches between control and experimental solutions and allows complete solution exchange in <1 msec, based on measurements using open patch pipettes. The time course of solution exchange over a nucleated patch is likely to be somewhat slower than this, reflecting the more complex geometry of the patches. The opening of the theta tube was positioned ∼100 μm from the membrane patch. In patch experiments, PS was administered for 10–20 sec before and during GABAA receptor agonist application. We previously found that the effects of PS are reversible (Nilsson et al., 1998). However, drug effects can take >5 min to wash out in isolated patch experiments. Thus, in most experiments we did not attempt to demonstrate reversibility of effect. For experiments using taurine and β-alanine, the sucrose and NaCl concentrations in the extracellular solution were adjusted to maintain constant osmolarity, and 5 μm strychnine was included to block effects of these agonists on glycine receptors. Reagents were purchased from Sigma (St. Louis, MO).
Averages of four to eight traces were used for analysis and display. Currents were filtered at 1–5 kHz using a four-pole Bessel filter and were digitized using pClamp version 6.0 (Axon Instruments, Foster City, CA). Data were analyzed off-line using the pClamp software and IgorPro (Wavemetrics, Lake Oswego, OR). Exponential curve fitting was performed using a simplex algorithm combined with a Chebyshev routine for initial seed estimates. In fitting the time course of desensitization in response to longer agonist applications, the current at the end of the long pulse was taken as the steady-state (offset) level. Unless otherwise noted, results represent mean ± SEM. Statistical differences were determined using two-tailed t tests.
Kinetic modeling. To describe the effects of PS on GABA currents, we used a seven-state kinetic model of GABA receptor function proposed originally by Jones and Westbrook (1995). This model incorporates two independent GABA-binding steps, two open states (from mono-liganded and diliganded receptor states), and two desensitized states. The version of the model used in the present simulations incorporates low probability interactions between the two desensitized states (Jones et al., 1998). During simulated changes in agonist binding and desensitization, rates between desensitized states were adjusted to maintain microscopic reversibility. Simulations were performed using the SCoP software (Simulation Resources, Berrien Springs, MI). Initial parameters used in the model were based on previous studies (Jones and Westbrook, 1995; Jones et al., 1998;Mozrzymas et al., 1999) and were selected to mimic the time course of deactivation to brief pulses of GABA observed experimentally. Parameters used to model β-alanine and taurine were derived fromJones and Westbrook (1995) and Jones et al. (1998), adjusting bothkon andkoff. The decay of simulated responses (deactivation and desensitization) was analyzed using exponential curve fitting as described above.
RESULTS
PS inhibits GABA-mediated inhibitory autaptic currents
GABA-mediated IACs were recorded at −70 mV in solutions containing nearly symmetrical extracellular and intracellular chloride concentrations (ECl, ∼0 mV). Under these conditions, both GABA and glutamate evoke inward synaptic currents at a holding potential of −70 mV. IACs were readily distinguished from glutamate-mediated excitatory autaptic currents (EACs) by their slower time course (decays of hundreds vs tens of milliseconds) and their sensitivity to inhibition by 25 μm bicuculline, a competitive antagonist at GABAA receptors (data not shown).
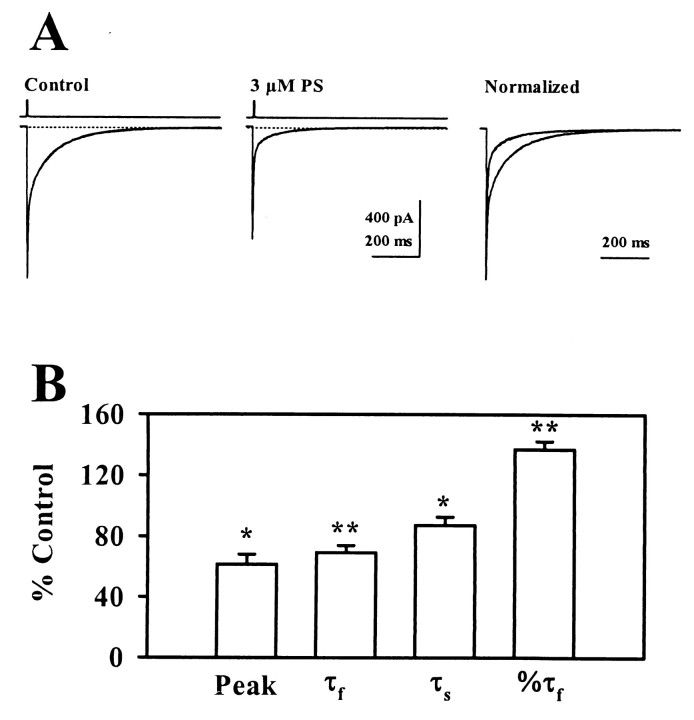
At 10 μm, PS depressed peak IACs by 34.0 ± 3.6% and decreased the total synaptic charge transfer by 62.4 ± 5.2% (n = 7; Fig. 1). The decay of control IACs was described by the sum of two exponentials with time constants of 13.4 ± 1.8 msec and 116.2 ± 16.3 msec (n = 7). After application of 10 μm PS, these time constants were decreased to 7.0 ± 0.9 msec and 43.8 ± 3.3 msec. PS had no effect on the relative contribution of the fast and slow components to the decay of IACs. To highlight the speeding of IAC decay in the presence of PS, Figure 1A shows traces in which peak responses were normalized.
Fig. 1.
Effects of PS on IACs. A, The traces show the effects of 10 μm PS on inhibitory autaptic currents at a holding potential of −70 mV. Therightmost panel shows traces normalized with respect to peak response to highlight effects on the decay time course. Fast transients preceding IACs represent capacitive and ionic currents associated with presynaptic stimulation. B, The graph shows a summary of the effects of 10 μm PS on IACs from seven cells. Peak, Peak amplitude; τf, fast time constant of decay; τs, slow time constant of decay; % τf, relative contribution of the fast phase of decay. Error bars represent mean ± SEM. *p < 0.05; **p < 0.01 by paired t test.
PS speeds deactivation of currents evoked by brief GABA pulses
Previous studies have shown that PS inhibits responses to GABA applied exogenously to neurons, suggesting that postsynaptic actions are the principal mediators of effects on IACs (Majewska et al., 1988;Nilsson et al., 1998). To determine whether effects of PS on GABAA receptor kinetics account for the effects observed on IACs, we examined the effects of PS on responses to rapid applications of GABA onto outside-out patches. In initial pilot experiments, we observed significant rundown of GABA responses in conventional outside-out patches, despite the use of an ATP-regenerating system in pipette recording solutions. To overcome this problem, we performed experiments using nucleated outside-out patches from cultured hippocampal neurons (Sather et al., 1992; Zhu and Vicini, 1997; Berger et al., 1998). As shown in Figure2, nucleated patches exhibited good response stability and allowed collection of control and experimental data in the same patch.
Fig. 2.

GABA responses in nucleated outside-out patches.A, The traces show superimposition of 60 responses of a nucleated patch to 5 msec pulses of 1 mm GABA at a holding potential of −70 mV. The trace at the top in this and subsequent figures shows the response of the open patch pipette to changes in extracellular chloride concentration. B,C, The graphs show the 10–90% decay time (B) and the peak response (C) of the 60 traces displayed inA.
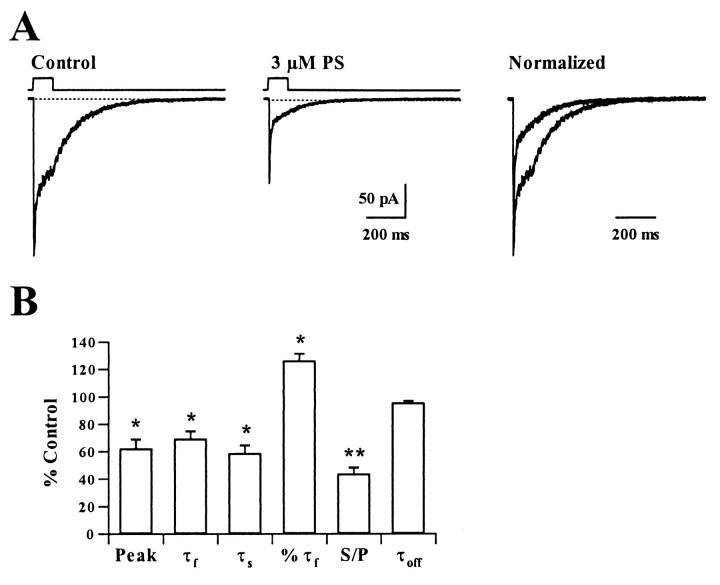
We studied deactivation of GABAA receptors by measuring the decay time course of currents after brief applications of 1 mm GABA. In control patches, 5 msec pulses of 1 mm GABA elicited macroscopic currents that decayed with time courses described by the sum of two exponentials (τfast = 6.0 ± 0.7 msec; τslow = 123.6 ± 16.2 msec, with 53.4 ± 2.1% contributed by the fast component; n = 8). After application of 3 μm PS, peak GABA-mediated currents were depressed to 71.4 ± 7.3% of control (Fig. 3). The deactivation time course was speeded by PS and was described by the sum of two exponentials with time constants of 4.0 ± 0.3 and 99.1 ± 9.3 msec (72.9 ± 2.8% fast component). In contrast to effects on the decay of IACs, PS increased the percentage of the deactivation time course contributed by the fast component (Fig. 3). PS had no effect on the 10–90% rise time of currents activated by 1 mm GABA (0.94 ± 0.07 msec in control and 0.90 ± 0.06 msec in PS;p > 0.05).
Fig. 3.
Effects of PS on deactivation of currents evoked by brief GABA pulses on nucleated outside-out patches.A, The traces depict the effects of 3 μmPS on responses activated by 5 msec applications of 1 mmGABA at a holding potential of −70 mV. The rightmost traces show responses normalized with respect to peak current to demonstrate effects on deactivation time course. B,The graph shows a summary of the effects of 3 μm PS on GABA currents in eight patches. Abbreviations are the same as in Figure1. *p < 0.05; **p < 0.01 by paired t test.
PS enhances macroscopic desensitization of GABA responses
Previous studies have found that the prolonged deactivation of GABA currents after brief applications onto membrane patches reflects not only channel closing but also rapid entry into and exit from desensitized receptor states (Jones and Westbrook, 1995, 1996). Furthermore, certain steroids that enhance GABAAreceptor function may act by altering the kinetics of desensitization (Zhu and Vicini, 1997). To determine whether PS affects macroscopic GABAA receptor desensitization, we used 100 msec applications of 1 mm GABA on nucleated patches. In control applications, the fade in GABA responses during the 100 msec application was described by the sum of two exponentials with time constants of 4.7 ± 0.4 and 80.5 ± 11.2 msec, with 49.7 ± 7.4% of the decay contributed by the fast component (n = 4). After the 100 msec GABA application, the decay of the currents to baseline was described by a single exponential process (τoff = 152.4 ± 12.3 msec) (Fig.4).
Fig. 4.
Effects of PS on GABA-mediated macroscopic desensitization. A, The traces show the effects of 3 μm PS on currents activated by 100 msec applications of 1 mm GABA on outside-out patches at a holding potential of −70 mV. The desensitization time course was evaluated by fitting a biexponential decay to the response during the GABA application. Therightmost traces again show responses normalized with respect to peak current to highlight changes in the time course and degree of receptor desensitization. B, The bar graph shows a summary of the effect of 3 μm PS on the rate and degree of desensitization to 100 msec pulses of 1 mm GABA in four patches. Abbreviations are the same as those in Figure 1 with the addition of S/P, ratio of steady-state current to peak current; and τoff, time constant describing the decay time constant after removal of drugs. *p< 0.05; **p < 0.01 by paired ttest.
At 3 μm, PS speeded the time course of desensitization by significantly decreasing both the fast and slow time constants and by increasing the relative contribution of the fast component of decay (τfast = 3.3 ± 0.2 msec; τslow = 46.7 ± 4.3 msec; 61.9 ± 7.2% fast; Fig. 4). PS also diminished the peak response and increased the overall degree of desensitization during the 100 msec GABA application (control: peak − steady-state current = 449.2 ± 145.8 pA − 197.5 ± 67.4 pA; PS: peak − steady-state current = 162.6 ± 22.0 pA − 31.3 ± 7.6 pA). PS appeared to have no significant effect on the time course of offset of the GABA current after the 100 msec application (Fig.4B). However, the small amplitude of steady-state currents in the presence of PS made evaluation of the offset time course difficult.
PS slows recovery of GABA receptors from paired-pulse desensitization
Because the decay of GABA-mediated synaptic currents is thought to reflect, in part, entry into and exit from desensitized states (Jones and Westbrook, 1995), it is possible that PS speeds the decay of GABA responses by fostering receptor entry into desensitized states and stabilizing desensitized states. To test this, we examined the effects of PS on recovery from GABA receptor desensitization after brief applications onto nucleated patches. In control trials, a 5 msec pulse of 1 mm GABA promoted significant desensitization of responses as measured by a second 5 msec GABA pulse administered at intervals ranging from 50 to 4000 msec after the conditioning GABA pulse (Fig. 5). GABA receptors recovered from this brief pulse desensitization with a time course described by the sum of two exponentials with time constants of 136.6 ± 19.6 and 2545 ± 236.7 msec (n = 5). In the presence of 10 μm PS, recovery of GABA currents from paired-pulse depression was prolonged (Fig. 5B), with time constants of 388.4 ± 43.2 and 4207.7 ± 274.5 msec (Fig.5C).
Fig. 5.
Effects of PS on recovery from paired-pulse desensitization of GABA responses. A, B,The traces show responses produced by paired 5 msec applications of 1 mm GABA separated by 50, 80, 130, 200, 400, 800, 2000, and 4000 msec under control conditions (A) and in the presence of 10 μm PS. C, The graph shows the percentage of recovery from desensitization induced by the conditioning (first) pulse at different intervals. The percentage of recovery was calculated as [(peak 2 − peak 1)/(peak 1 − onset 2)] × 100. Peak 1 and peak 2 are the peak amplitudes of the first and second responses, and onset 2 is the value of the current at the start of the second response. Each data point represents the mean of five patches. The solid lines are fit the equation: % recovery = [100 − A1exp(−IPI/τ1) − A2exp(−IPI/τ2)] where τ1 and τ2 are the fast and slow time constants of recovery, and A1 and A2 are the amplitudes of the two components. In the fits shown, τ1 = 146.7 msec (41%) and τ2 = 2639 (29%) for control, and τ1 = 422.7 (33%) and τ2 = 4105.9 msec (43%) for PS.
PS does not alter deactivation after brief pulses of weakly desensitizing GABAA receptor agonists
The data in Figures 4 and 5 suggest that a major effect of PS on the decay of IACs may occur via changes in GABA receptor desensitization. To examine the relative contribution of effects on binding, unbinding and desensitization, we studied effects of PS on responses to GABAA receptor agonists that show little or no desensitization during brief exposures. Previous studies have shown that taurine (Zhu and Vicini, 1997) and β-alanine (Jones and Westbrook, 1995) are low-affinity agonists at GABAA receptors that exhibit fast monoexponential deactivation and little paired-pulse desensitization after brief applications. The single exponential deactivation process most likely represents channel closing and agonist unbinding in the absence of significant receptor desensitization. After a 5 msec application of 20 mm taurine, deactivation was well described by a single exponential time course with a time constant of 4.3 ± 0.4 msec (n = 7) (Fig. 6). At 10 μm, PS had little effect on peak responses produced by taurine and no effect on the time course of deactivation (τ = 4.5 ± 0.4 msec). Similar effects were observed on peak responses and deactivation kinetics of responses gated by 5 msec applications of 100 mm β-alanine (4.2 ± 0.4 msec in control and 4.3 ± 0.4 msec in PS; Fig.7A).
Fig. 6.
Effects of PS on short pulses of taurine.A, The traces show currents activated by 5 msec pulses of 20 mm taurine administered to nucleated patches at a holding potential of −70 mV in the presence of 5 μmstrychnine under control conditions and in the presence of 10 μm PS. The rightmost panel shows traces normalized with respect to peak response. B, The graph shows a summary of the effects of 10 μm PS on taurine responses in seven patches.
Fig. 7.
Effects of PS on short and longer pulses of β-alanine. A, The traces show currents activated by 100 mm β-alanine in the absence and presence of 10 μm PS. The rightmost panel shows responses normalized with respect to peak current. B, In the same nucleated patch, a 200 msec application of β-alanine produces a small amount of desensitization. In the presence of 10 μm PS, desensitization is enhanced. The normalized traces highlight the change in desensitization, and the inset shows that PS prolongs the decay of β-alanine currents after agonist and drug removal.C, The graph shows the effects of 10 μm PS on peak responses and deactivation to 5 msec applications of 100 mm β-alanine. D, The graph shows the effects of PS on peak responses, the time constant of desensitization (τ), the ration of steady-state to peak currents (S/P), and deactivation τoff in response to 200 msec applications of β-alanine.
The lack of effects of PS on responses to brief pulses of taurine and β-alanine suggest that PS has its most prominent effect on GABAA receptors under conditions in which the receptors desensitize. To test this, we examined the effects of 10 μm PS on 200 msec applications of β-alanine. During longer pulses of β-alanine there is some degree of desensitization (steady-state current/peak current = 0.58 ± 0.04; τ = 127.5 ± 16.3 msec; n = 6; Fig. 7B). When patches are treated with PS, the longer pulses of β-alanine show enhanced desensitization (steady-state/peak current = 0.22 ± 0.02; τ = 71.6 ± 5.9 msec; n = 6). Additionally, after termination of the β-alanine pulse, the decay of the currents is prolonged in the presence of PS (τoff = 11.4 ± 1.7 msec in control and 25.5 ± 3.4 msec in PS), supporting the hypothesis that entry into desensitized states prolongs deactivation of GABAA receptor-mediated responses.
Kinetic modeling of PS effects on GABAA receptors
The effects of PS on deactivation and desensitization of GABAA receptors are consistent with the importance of rapid desensitization in determining the deactivation time course of GABA responses. To summarize, the principal effects of PS on GABAA receptors include: (1) a decrease in peak GABA currents, (2) speeding of fast and slow phases of deactivation after brief pulses of GABA coupled with an increase in the percentage of the decay accounted for by the fast phase, (3) speeding of the fast and slow phases of desensitization during longer applications of GABA with increased contribution of the fast phase of desensitization, (4) slowing of recovery from desensitization, and (5) little effect on peak currents and deactivation in response to brief applications of weakly desensitizing GABAAreceptor agonists. A seven-state model of GABA receptor function proposed by Jones and Westbrook (1995) has been useful in describing the role of receptor desensitization in the slow decay of GABA responses after brief exposures to high agonist concentrations (Fig.8A). We used this seven-state model to determine the effects of changing binding, channel gating, and desensitization steps on simulated responses to 5 or 200 msec applications of 1 and 10 mm GABA. Our goal in these simulation studies was to determine whether we could replicate the effects of PS by changing only one kinetic parameter.
Fig. 8.
Kinetic modeling of PS effects on GABA and β-alanine currents. A, The left panelshows simulated responses to 5 msec applications of 1 mmGABA (solid trace) and the effects of threefold changes in either d2 (the fast desensitization rate, dashed traces) or d1 plus d2 (dotted traces). The right panel shows the effects of changes in d2 or d1 plus d2 on simulated responses to 200 msec applications of GABA. The inset in theleft panel shows the seven-state model used for simulations. Rate constants used in the modeling were derived fromMozrzymas et al. (1999) and Jones et al. (1998), withkon = 15 mm/msec−1,koff = 0.3 msec−1, α1 = 1.1 msec−1, β1 = 0.2 msec−1, α2 = 0.3 msec−1, β2 = 10 msec−1,d1 = 0.013 msec−1,r1 = 0.00013 msec−1, d2 = 2.0 msec−1, r2 = 0.045 msec−1, p = 0.0222 mm/msec−1, q = 0.002 msec−1. Abbreviations used in the model areR, unbound receptor; AR,A2R, bound states of the receptor with one or two agonist molecules; AR*, A2R*, open states of the ion channel;AD, A2D, desensitized receptor states. B, The panels show the effects of threefold changes in d2 and d1 plus d2 on simulated responses to 5 (left panel) and 200 msec (right panel) applications of 20 mm β-alanine. The effects of β-alanine were simulated using the approach outlined by Jones and Westbrook (1995) and Jones et al. (1998) withkon = 0.075 mm/msec−1 and koff = 10 msec−1. In these simulations, the rate constant,p, was adjusted to maintain microscopic reversibility.
Using rate constants derived from previous studies (Jones and Westbrook, 1995, Jones et al., 1998; Mozrzymas et al., 1999), control simulations exhibited biexponential deactivations with time constants of 10–16 and 100–200 msec after 5 msec applications of 1 or 10 mm GABA (Table 1). Longer (100–200 msec) simulated applications exhibited biexponential desensitization with time constants of 10–15 and ∼50 msec. We found that increasing the rate constant of entry into the fast desensitized state (d2) by threefold provided the best mimic for the overall effects of PS on GABA and β-alanine/taurine currents. Increases in d2 depressed peak simulated GABA currents and speeded fast deactivation, while speeding both the fast and slow components of desensitization and increasing the overall degree of desensitization (Fig. 8, Table 1). However, increases in d2 failed to mimic the speeding of the slow component of deactivation seen with PS and, in fact, slowed slow deactivation. Increases in d2 effectively mimicked the effects of PS on β-alanine/taurine currents, producing only small effects on peak currents and deactivation in response to brief agonist applications. Additionally, increases in d2 mimicked the effects of PS on paired-pulse desensitization, increasing the overall degree of desensitization at brief intervals and slowing recovery from desensitization (Fig. 9).
Table 1.
Kinetic modeling of PS effects on GABAAreceptors
| Parameter | Peak response | Fast component (msec) | Slow component (msec) | % Fast |
|---|---|---|---|---|
| Deactivation (5 msec application) | ||||
| Control | 0.74 | 11.8 | 125 | 50 |
| ↑d2 | 0.52 | 7.6 | 200 | 64 |
| ↓r2 | 0.74 | 14.9 | 217 | 79 |
| ↓β2 | 0.47 | 7.4 | 91 | 69 |
| ↑α2 | 0.60 | 5.4 | 91 | 76 |
| ↑koff | 0.74 | 11.2 | 53 | 65 |
| ↓kon | 0.73 | 11.6 | 123 | 48 |
| ↑d1 + ↑d2 | 0.52 | 7.6 | 200 | 64 |
| Desensitization (200 msec application) | ||||
| Control | 0.74 → 040 | 12.0 | 47.6 | 92 |
| ↑d2 | 0.52 → 0.19 | 7.7 | 19.6 | 97 |
| ↓r2 | 0.74 → 0.19 | 16.9 | — | — |
| ↓β2 | 0.47 → 0.17 | 7.5 | 47.6 | 97 |
| ↑α2 | 0.60 → 0.17 | 5.6 | 43.4 | 98 |
| ↑koff | 0.74 → 0.38 | 11.9 | 129.8 | 83 |
| ↓kon | 0.73 → 0.38 | 12.0 | 120.5 | 83 |
| ↑d1 + ↑d2 | 0.52 → 0.19 | 7.7 | 43.4 | 94 |
| β-Alanine deactivation (5 msec application) | ||||
| Control | 0.25 | 5.6 | ||
| ↑d2 | 0.21 | 6.3 | ||
| ↓r2 | 0.25 | 5.3 | ||
| ↓β2 | 0.12 | 4.0 | ||
| ↑α2 | 0.14 | 3.0 | ||
| ↑d1 + ↑d2 | 0.20 | 6.3 |
Parameters for control simulations were derived from Mozrzymas et al. (1999) and Jones et al. (1998), adjusting the rate between d1 and d2 to maintain microscopic reversibility (Fig. 8). The Parameter column presents control simulations and simulations using 3× changes in the rate constants. Peak responses represent the peak open channel probability. The deactivation data represent the decay of responses to 1 mm GABA after a 5 msec simulated application. In desensitization simulations the peak response column represents peak → steady-state responses during a 200 msec simulated 1 mmGABA application. The values underlined in the table represent effects that differed qualitatively from the observed experimental actions of PS. The effects of β-alanine were modeled by decreasing kon and increasing koff, as described in Jones and Westbrook (1995) and Jones et al. (1998).
Fig. 9.

Effects of changes in d2 and d1 plus d2 on simulated paired applications of GABA. A, B, The traces show the early time course of recovery from simulated 5 msec applications of 1 mm GABA in control conditions (A) and in the presence of a threefold increase in d2 (B). C, The plot displays the percentage of recovery of peak responses as a function of interpulse interval. The solid lines represent the best fit of a biexponential recovery process to the simulated responses. The time course of recovery was slowed by increases in d2 (squares) or d1 plus d2 (triangles). In the fits shown, τ1 = 132 msec (control), 227 msec (d2) and 213 msec (d1 plus d2), and τ2 = 9.3 sec (control), 13.0 sec (d2) and 13.5 sec (d1 plus d2), with % τ1 = 59% (control), 40% (d2), and 31% (d1 plus d2).
Because increases in d2 do not completely replicate the findings with PS (notably failing to speed slow deactivation), we examined whether changing other kinetic parameters either alone or in combination with changes in d2 provided better mimics for PS. We found that changes in recovery from fast desensitization (r2) or changes in slow desensitization (d1, r1) did not improve the replication of PS effects seen with changes in d2 alone. Similarly changes in binding rates or channel opening and closing rates (β2, α2) failed to provide better mimics for PS than simple changes in d2 alone (Table 1). Although changes in opening or closing rates mimicked responses to brief GABA pulses in the presence of PS, crucial effects of PS on low-affinity agonists were not replicated. Table 1 summarizes the effects of altering various rate constants; values that qualitatively deviate from actual effects of PS are highlighted for clarity.
A previous study found that the actions of another noncompetitive GABA receptor antagonist, chlorpromazine, are best modeled by changes in GABA binding and unbinding steps (Mozrzymas et al., 1999). We found that changes in binding steps had little effect on peak currents and did not mimic the effects of PS on deactivation or desensitization. Therefore, unlike chlorpromazine, PS does not appear to act by altering GABA binding.
Because PS not only enhances desensitization of GABA responses, but also slows recovery from desensitization, we used the seven-state model to examine the effects of changes in rate constants on paired responses to 5 msec applications of GABA. Consistent with our experimental data, we found increases in d2 (with or without changes in d1) promoted greater degrees of desensitization and slowed recovery from desensitization (Fig. 9). Changes in channel opening/closing promoted faster recovery from desensitization.
DISCUSSION
GABAA receptor-mediated IPSCs typically exhibit complex kinetics with at least two components of decay. These include a fast phase with a time constant of 10–20 msec and a slower phase with a time constant of ≥100 msec (Jones and Westbrook, 1995;Galarreta and Hestrin, 1997). Some studies suggest more complicated decays that are characterized by three components with time constants of 5–10, 30–60, and 100–200 msec (Maconochie et al., 1994;Zorumski et al., 1998). This complex time course can be mimicked by brief (1–5 msec) applications of high concentrations of GABA onto membrane patches, suggesting that the kinetics of postsynaptic GABAA channels govern IPSC decay (Maconochie et al., 1994; Jones and Westbrook 1995; Galarreta and Hestrin, 1997;Zorumski et al., 1998). Present evidence also suggests that the time course of GABA-mediated IPSCs is influenced strongly by the kinetics of GABAA receptor desensitization (Celentano and Wong, 1994; Jones and Westbrook, 1995). Desensitized states are thought to buffer receptors in bound conformations that make it possible for channels to reopen before GABA unbinds. The fast phase of desensitization limits the open probability of the channels, influences peak synaptic currents, and contributes to the fast component of IPSC decay. The slow component of decay may result from reopening of GABA channels after exit from desensitized states (Jones and Westbrook, 1995). An alternative hypothesis, based on the effects of a novel class of GABA modulators, is that the slower phase of IPSC decay results partly from subsaturating GABA concentrations and monoliganded openings of GABAA receptors (Hill et al., 1998). It is also possible that heterogeneity among GABAAreceptors expressed in the hippocampus contributes to the complex kinetics of IPSCs (Banks and Pearce, 2000). On the other hand, recombinant GABAA receptors composed of defined subunit combinations also give rise to currents with complex decay kinetics (Lavoie et al., 1997; Haas and Macdonald, 1999).
Using rapid applications of agonists onto membrane patches and a seven-state kinetic model, Jones and Westbrook (1995) described the role of rapid desensitization in the prolonged decay of GABA-mediated IPSCs. Although there are limitations in the seven-state model, this approach has also been useful for interpreting the effects of physiological and pharmacological manipulations of GABA receptor function. For example, inhibitors of protein phosphatase 2B (calcineurin) speed the time course of GABA-mediated IPSCs (Jones and Westbrook, 1997). This effect may result primarily from an increase in the microscopic GABA-unbinding rate, although calcineurin inhibitors also increase macroscopic desensitization of GABA receptors. Other studies have found that the effects of the noncompetitive GABAA receptor antagonist chlorpromazine are best modeled by decreases in GABA binding and increases in unbinding, resulting in decreased stability of bound states (Mozrzymas et al., 1999).
Rapid applications of GABA to outside-out patches have also been used to study the actions of neurosteroids that potentiate the actions of GABA at GABAA receptors. Zhu and Vicini (1997)found that THDOC, a neurosteroid that prolongs IPSCs, preferentially slowed slow deactivation, but did not alter fast deactivation after brief applications of GABA to outside-out patches. Similar effects on deactivation of GABAA receptors were observed with another steroid, 3α5αP, that enhances GABA-mediated responses and prolongs inhibitory synaptic currents (Zorumski et al., 1998). THDOC did not alter desensitization to longer applications of 1 mm GABA, but, like PS, slowed recovery from macroscopic desensitization induced by GABA (Zhu and Vicini, 1997). Interestingly, THDOC had no effect on deactivation of short, nondesensitizing pulses of taurine, but augmented peak taurine currents. THDOC slowed deactivation of taurine responses when taurine was administered long enough to cause receptor desensitization and, somewhat surprisingly, accelerated fast desensitization during long pulses of taurine. These results indicate that THDOC has complex actions on GABAA receptor function that differ qualitatively from the effects of PS. The differences in effects on deactivation and desensitization likely result from structural differences between THDOC and PS that include a 3α- versus 3β-conformation and the presence of a hydroxyl group versus a sulfate group at the 3-position. Also, present evidence strongly suggests that GABA-enhancing steroids and GABA-inhibiting steroids act at different sites on GABAA receptors (Park-Chung et al., 1999).
The present studies indicate that PS accelerates the time course of IACs and both the fast and slow components of deactivation of currents evoked by brief pulses of GABA onto nucleated outside-out patches. PS also increases the rate and degree of macroscopic desensitization of GABA receptors and increases the relative contribution of the fast phase of desensitization. PS prolongs the time that GABA channels spend in desensitized states because PS slows recovery from desensitization after brief GABA pulses. Consistent with the effects of PS on GABA receptor desensitization, the steroid fails to alter the time course of deactivation after brief applications of taurine/β-alanine, although PS promotes desensitization during longer applications of β-alanine. At synapses, the net effect of PS is to depress peak currents and to speed the time course of decay, limiting overall synaptic charge transfer and GABAergic inhibition. In comparing the effects of PS on synaptic currents to effects on membrane patches, it is important to consider possible differences between synaptic and extrasynaptic GABAA receptors. In particular, differences between synaptic and extrasynaptic receptors could account for differences in the effects of PS on the fast component of decay (Brickley et al., 1999; Banks and Pearce, 2000) and the small differences in decay of IACs versus nucleated patch responses.
Using the seven-state GABA receptor model, we found that increases in the rate constant governing channel entry into a doubly liganded desensitized state mimics most effects of PS. The major action unaccounted for by the model is the effect of PS on the slow component of deactivation after brief pulses of GABA. Changes in other rate constants, particularly those governing diliganded channel opening and closing, also mimic effects of PS on deactivation and macroscopic desensitization. However, increases in the microscopic desensitization rate, and not changes in channel opening and closing, provide the best mimic for effects on β-alanine/taurine responses and on recovery from paired-pulse desensitization. Caution must be used in interpreting these simulation studies because the seven-state model is likely to oversimplify receptor behavior. The model effectively describes the time course of deactivation after brief pulses of agonist but underestimates the contribution of the slow phase of desensitization. Furthermore, it is possible that additional states are required to describe receptor kinetics fully, particularly if deactivation consists of more than two components (Maconochie et al., 1994; Zorumski et al., 1998). Nevertheless, the seven-state model has proven useful heuristically and is helpful for comparison to previous studies.
Results obtained with the weakly desensitizing GABAA receptor agonists taurine and β-alanine strongly suggest that the most prominent effects of PS occur under conditions in which GABAA receptors desensitize. At 3–10 μm, PS has prominent effects on peak responses, deactivation, and desensitization to saturating GABA concentrations, but little effect on responses to brief pulses of taurine or β-alanine. However, when β-alanine was applied long enough to drive receptor desensitization, PS increases both the rate and degree of fade in response. After long β-alanine pulses, PS also prolonged the time course of deactivation, a finding that is consistent with the hypothesis that desensitization prolongs the time course of agonist-gated currents (Jones and Westbrook, 1995).
Previous studies have reported variable potencies for PS against GABA responses with IC50 values ranging from 1–10 μm to ∼80 μm (Majewska et al., 1988, 1990; Park-Chung et al., 1999; Shen et al., 1999). In studies using a low (∼EC10) concentration of GABA, we previously found an IC50 value of ∼80 μm for both PS and its unnatural enantiomer (Nilsson et al., 1998). Our present results using a saturating concentration of GABA indicate a lower IC50 value in the range of 3–10 μm. Because GABAA receptor desensitization is concentration-dependent (Celentano and Wong, 1994), these observations, like the experiments with low potency agonists, suggest that receptor desensitization is important in determining the potency of PS. Given that brain concentrations of PS are in the mid- to high nanomolar range with micromolar concentrations likely in localized cellular compartments (Baulieu and Robel, 1990; Corpechot et al., 1997), it appears that the ability of PS to modulate GABAA receptors at low micromolar concentrations could significantly influence GABAergic inhibition in the CNS, possibly contributing to the proposed roles of PS in wakefulness, cognition, and sexual function (Baulieu and Robel, 1990; Majewska, 1992).
Footnotes
This work was supported by National Institutes of Health Grants GM47969 (C.F.Z. and D.F.C.), a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (S.M.), and the Bantly Foundation (C.F.Z.). We thank Ann Benz and Jianxin Que for technical assistance and Drs. Matthew Jones, Joe Henry Steinbach, Chris Lingle, and Alex Evers for helpful discussions.
Correspondence should be addressed to Charles F. Zorumski, Department of Psychiatry, Washington University School of Medicine, 4940 Children's Place, St. Louis, MO 63110. E-mail:zorumskc@psychiatry.wustl.edu.
Dr. Shen's present address: Auditory Physiology Laboratory, Northwestern University, Department of Neurobiology and Physiology, Evanston, IL 60208.
REFERENCES
- 1.Banks MI, Pearce RA. Kinetic differences between synaptic and extrasynaptic GABAA receptors in CA1 pyramidal cells. J Neurosci. 2000;20:937–948. doi: 10.1523/JNEUROSCI.20-03-00937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baulieu E-E, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- 4.Berger T, Schwarz C, Kraushaar U, Monyer H. Dentate gyrus basket cell GABAA receptors are blocked by Zn2+ via changes of their desensitization kinetics: an in situ patch-clamp and single-cell PCR study. J Neurosci. 1998;18:2437–2448. doi: 10.1523/JNEUROSCI.18-07-02437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celentano JJ, Wong RKS. Multiphasic desensitization of the GABAA receptor in outside-out patches. Biophys J. 1994;66:1039–1050. doi: 10.1016/S0006-3495(94)80885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corpechot C, Collins BE, Carey MP, Tsouros A, Robel P, Fry JP. Brain neurosteroids during the mouse estrous cycle. Brain Res. 1997;766:276–280. doi: 10.1016/s0006-8993(97)00749-x. [DOI] [PubMed] [Google Scholar]
- 7.Galarreta M, Hestrin S. Properties of GABAA receptors underlying inhibitory synaptic currents in neocortical pyramidal neurons. J Neurosci. 1997;17:7220–7227. doi: 10.1523/JNEUROSCI.17-19-07220.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas KF, Macdonald RL. GABAA receptor subunit γ2 and δ subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol (Lond) 1999;514:27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill MW, Reddy PA, Covey DF, Rothman SM. Contribution of subsaturating GABA concentrations to IPSCs in cultured hippocampal neurons. J Neurosci. 1998;18:5103–5111. doi: 10.1523/JNEUROSCI.18-14-05103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 11.Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- 12.Jones MV, Westbrook GL. Shaping IPSCs by endogenous calcineurin activity. J Neurosci. 1997;15:7626–7633. doi: 10.1523/JNEUROSCI.17-20-07626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones MV, Sahara Y, Dzubay JA, Westbrook GL. Defining affinity with the GABAA receptor. J Neurosci. 1998;18:8590–8604. doi: 10.1523/JNEUROSCI.18-21-08590.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:293–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 15.Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroid modulation of native and recombinant GABAA receptors. Cell Mol Neurobiol. 1996;16:155–174. doi: 10.1007/BF02088174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on α-subunit isoform. Biophys J. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maconochie DJ, Zempel JM, Steinbach JH. How quickly can GABAA receptors open? Neuron. 1994;12:61–71. doi: 10.1016/0896-6273(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 18.Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- 19.Majewska MD, Schwartz RD. Pregnenolone-sulfate: an endogenous antagonist of the γ-aminobutyric acid receptor complex in brain? Brain Res. 1987;404:355–360. doi: 10.1016/0006-8993(87)91394-1. [DOI] [PubMed] [Google Scholar]
- 20.Majewska MD, Mienville J-M, Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci Lett. 1988;90:279–284. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- 21.Majewska MD, Demirgoren S, London ED. Binding of pregnenolone sulfate to rat brain membranes suggests multiple sites of steroid action at the GABAA receptor. Eur J Pharmacol. 1990;189:307–315. doi: 10.1016/0922-4106(90)90124-g. [DOI] [PubMed] [Google Scholar]
- 22.Mennerick S, Que J, Benz A, Zorumski CF. Passive and synaptic properties of hippocampal neurons grown in microcultures and in mass cultures. J Neurophysiol. 1995;73:320–332. doi: 10.1152/jn.1995.73.1.320. [DOI] [PubMed] [Google Scholar]
- 23.Mensah-Nyagan AG, Do-Rego J-L, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol Rev. 1999;51:63–81. [PubMed] [Google Scholar]
- 24.Mienville J-M, Vicini S. Pregnenolone sulfate antagonizes GABAA receptor-mediated currents via a reduction of channel opening frequency. Brain Res. 1989;489:190–194. doi: 10.1016/0006-8993(89)90024-3. [DOI] [PubMed] [Google Scholar]
- 25.Mozrzymas JW, Barberis A, Michalak K, Cherubini E. Chlorpromazine inhibits miniature GABAergic currents by reducing the binding and by increasing the unbinding rate of GABAA receptors. J Neurosci. 1999;19:2474–2488. doi: 10.1523/JNEUROSCI.19-07-02474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilsson KR, Zorumski CF, Covey DF. Neurosteroid analogues. 6. The synthesis and GABAA receptor pharmacology of enantiomers of dehydroepiandrosterone sulfate, pregnenolone sulfate and (3α,5β)-3-hydroxypregnane-20-one sulfate. J Med Chem. 1998;41:2604–2613. doi: 10.1021/jm980148h. [DOI] [PubMed] [Google Scholar]
- 27.Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate γ-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- 28.Sather W, Dieudonne S, MacDonald JF, Ascher P. Activation and desensitization of N-methyl-d-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol (Lond) 1992;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen W, Mennerick S, Zorumski EC, Covey DF, Zorumski CF. Pregnenolone sulfate and dehydroepiandrosterone sulfate inhibit GABA-gated chloride currents in Xenopus oocytes expressing picrotoxin-insensitive GABAA receptors. Neuropharmacology. 1999;38:267–271. doi: 10.1016/s0028-3908(98)00172-5. [DOI] [PubMed] [Google Scholar]
- 30.Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 31.Zhu WJ, Vicini S. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J Neurosci. 1997;17:4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zorumski CF, Mennerick S, Covey DF. Enantioselective modulation of GABAergic synaptic transmission by steroids and benz[e]indenes in hippocampal microcultures. Synapse. 1998;29:162–171. doi: 10.1002/(SICI)1098-2396(199806)29:2<162::AID-SYN7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]