Abstract
Thalamic relay cells respond in two distinct modes, burst and tonic, that depend on a voltage-dependent, low-threshold, transient Ca2+ current (IT), and these modes relay different forms of information to cortex. ITactivation evokes a low-threshold spike (LTS), producing a burst of action potentials. Modulatory inputs from cortex and brainstem are known to activate metabotropic receptors on relay cell dendrites at which the T channels underlying IT may be concentrated. We thus investigated the influence of activating these receptors on the LTS, using current-clamp intracellular recording in anin vitro slice preparation of the cat's lateral geniculate nucleus. We found a strong correlation between LTS amplitude and the number of action potentials evoked in the burst. We then found that activation of either metabotropic glutamate or muscarinic receptors produced a hyperpolarizing shift in the sigmoid relationship between LTS amplitude and the initial holding potentialwithout affecting the maximum LTS amplitude or slope of the relationship. This hyperpolarizing shift in the voltage dependency of LTS amplitude is best explained by space-clamp limitations and significantly more depolarization of T channels near the dendritic location of activated receptors than at the soma. Thus, nonretinal modulatory inputs may have a stronger influence on IT and number of action potentials generated in a burst than previously imagined from somatic recording, because the EPSP amplitudes generated by these inputs at the dendritic location of most T channels are greater than after their electrotonic decay recorded at the soma.
Keywords: thalamus, T channel, IT, burst firing, relay cell, lateral geniculate nucleus, metabotropic glutamate receptor, muscarinic receptor
All thalamic relay cells fire in two distinct modes, tonic and burst, based on the inactivation state of a voltage-dependent Ca2+ current,IT, which operates via T type Ca2+ channels (Llinás and Jahnsen, 1982; Steriade and Llinás, 1988; Sherman and Guillery, 1996). If the cell is more depolarized than approximately −60 mV for >50–100 msec, IT is inactivated, and the cell responds to an excitatory input (e.g., an EPSP) in tonic mode, producing sustained firing of unitary action potentials. However, if the cell is first hyperpolarized below approximately −65 mV for >50–100 msec, IT is de-inactivated, and now a sufficient depolarization will activateIT, leading to a low-threshold Ca2+ spike (LTS) with a burst of 1–10 action potentials riding its crest. Which of these two firing modes is operative plays a significant role in the nature of information relayed to cortex (Sherman, 1996; Reinagel et al., 1999).
For relay cells of the lateral geniculate nucleus, which transmits retinal information to cortex, response mode can be effectively regulated by glutamatergic inputs from cortex and cholinergic inputs from the parabrachial region of the brainstem (for review, see Sherman and Guillery, 1996). Both modulatory pathways produce long-lasting EPSPs in relay cells through activation of metabotropic receptors [metabotropic glutamate receptors (mGluRs) for the corticogeniculate pathway and muscarinic receptors for the parabrachial pathway], thereby switching firing from burst to tonic. Cortical synapses are located on distal dendrites, whereas parabrachial synapses are found more proximally, amid the retinal synapses (Wilson et al., 1984; Erişir et al., 1997). Also, although cortical and retinal synapses are both glutamatergic, only cortical synapses activate metabotropic glutamate receptors, and these are located on peripheral dendrites (McCormick and Von Krosigk, 1992; Godwin et al., 1996). It had been thought that T channels were concentrated on the soma (Llinás and Jahnsen, 1982; Steriade and Llinás, 1988), but recent studies suggest that they are more concentrated in the dendritic tree and thus much nearer to parabrachial and cortical synapses (Zhou et al., 1997; Destexhe et al., 1998).
Modeling thalamic relay cells indicate that potentials, such as EPSPs, generated in dendrites will decay significantly en route to the soma (Bloomfield and Sherman, 1987). If significant numbers of the T channels indeed lie on dendrites, much nearer to cortical and parabrachial inputs than previously thought, this raises the possibility that these inputs could have a correspondingly much greater impact. This is because the EPSP each input generates will depolarize nearby T channels more than it will depolarize the soma. Thus, the effect of such an EPSP on IT would be much greater than predicted from the size of the EPSP recorded in the soma. We reasoned that depolarizing dendrites of geniculate relay cells via agonists to receptors located there would inactivateIT more than equivalent depolarization via current injection into the soma, and such evidence would help to confirm the dendritic location of many T channels. Also, by using agonists to receptors known to be activated normally by cortical or brainstem input (i.e., metabotropic glutamate and muscarinic receptors), we can provide some insights into how cortical and parabrachial inputs serve to control the inactivation ofIT.
MATERIALS AND METHODS
Most of our methods have been described in detail previously (Cox and Sherman, 1999; Zhan et al., 1999) and are briefly outlined below.
Slice preparation. We used an in vitro thalamic slice preparation to record intracellularly from cells of the cat's lateral geniculate nucleus. All animals were handled in compliance with approved animal protocols. Briefly, we deeply anesthetized young cats (4–8 weeks old) with a mixture of xylazine (1 mg/kg) and ketamine (25 mg/kg), mounted them in a stereotaxic device, performed a craniotomy to remove a block of tissue containing the lateral geniculate nucleus, and killed the animals with an overdose of sodium pentobarbital. The block was placed in oxygenated cold slicing solution (see below). Slices (300–500 μm thick) were cut through the thalamus coronally or sagittally and placed in a holding chamber for >2 hr before recording. Individual slices were transferred to an interface type recording chamber and continuously superfused with warm oxygenated physiological solution (see below). The tissue was maintained at 33°C for all recordings.
The slicing solution was used throughout the tissue preparation and contained (in mm): 2.5 KCl, 1.25 NaH2PO4, 10.0 MgCl2, 0.5 CaCl2, 26.0 NaHCO3, 11.0 glucose, and 234.0 sucrose. The solution in the holding and recording chambers contained (in mm): 126.0 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2.0 MgCl2, 2.0 CaCl2, 26.0 NaHCO3, and 10.0 glucose, and was gassed with a mixture of 95% O2 and 5% CO2 to a final pH of 7.4. To block conventional Na+/K+ action potentials in many experiments, we bath applied tetrodotoxin (TTX) (0.5–1 μm).
Electrophysiological recordings and pharmacological manipulations. We obtained intracellular recordings in current-clamp mode from the geniculate relay cells. As noted in Results, we used standard sharp electrode intracellular recording techniques in the majority of recordings, but in a subset of cells, we used patch electrodes for whole-cell recordings (Cox and Sherman, 1999). The sharp electrodes had a resistance of 40–80 MΩ and were filled with 1–3 m KAc containing 2–5% neurobiotin or 1–3% biocytin.; the patch electrodes had a resistance of 4–6 MΩ and were filled with (in mm): 117 K-gluconate, 13 KCl, 1.0 MgCl2, 0.07 CaCl2, 0.1 EGTA, 10.0 HEPES, 2.0 Na2-ATP, 0.4 Na-GTP, and 0.5% biocytin. During the recordings, an active bridge circuit was continually monitored and adjusted as needed to balance the voltage drop produced by passing current through the recording electrode. Cells were held at different initial holding potentials for several seconds by injecting current into the cell (i.e., the holding current). The duration of the holding current ensured that any depolarizing sag attributable to Ih would reach an equilibrium, leading to a stable membrane voltage for a sufficient time to create a stable level of IT inactivation. Ca2+ spikes (and Na+/K+ spikes when TTX was not used) were then evoked by depolarizing current steps on top of the initial holding current, the steps ranging from 10 to 1000 pA and having a typical duration of 400 msec.
We accepted only recordings showing a stable resting membrane potential more negative than −50 mV and overshooting action potentials before TTX application. The apparent input resistance was calculated from the slope of the linear portion of the I–V relationship. After each successful recording was completed, neurobiotin or biocytin was iontophoresed into the cell, and the slice was processed with methods routinely used in this laboratory (Tamamaki et al., 1994) to view the labeled cell with the light microscope.
To observe the effects of dendritic depolarization, we bath applied agonists to mGluRs or muscarinic receptors, both of which are found on dendrites (for review, see Sherman and Guillery, 1996). For this, we used the general mGluR agonist (±)-1-aminocyclopentane-trans-1,3-dicarboxylic acid (ACPD) or the general muscarinic agonist carbachol. We used concentrations of these agonists that produced relatively small depolarizations as recorded at the soma (3–5 mV for ACPD and 5–10 mV for carbachol).
Agonists were bath applied, and it thus took time for the agonist to diffuse into (and out of) the slice. We thus always waited at least several minutes for a stable concentration as judged by a stable, depolarized membrane voltage to be attained before recording the effects of agonist application, and we also waited a comparable time before testing effects of washout to determine that the cell returned to control conditions. This slow time course for agonist application and removal meant that, with our methods, we could not effectively test the timing of onset or removal of these effects.
Data analysis and curve fitting. We reported previously that the evoked LTS amplitude varies with the initial holding potential, the LTS amplitude falling with more depolarized holding potentials in a sigmoid manner (Zhan et al., 1999). Therefore, to help quantify our results, we fit these data with a sigmoid curve-fitting algorithm (SigmaPlot; Jandel Scientific, Corte Madera, CA). The formula for a sigmoid is
| Equation 1 |
where X is the holding membrane potential,Y is the corresponding LTS amplitude, and a,b, and m are three free parameters in whicha corresponds to the maximal amplitude, brepresents the downward slope, and m reflects the value of the abscissa at the 50% amplitude point.
RESULTS
Intracellular recordings included in the present study were obtained from 29 neurons in the A-laminae of the cat's lateral geniculate nucleus. Of these, 26 were impaled with sharp electrodes, and three were recorded with the whole-cell patch configuration (see Materials and Methods); except for a higher input resistance and larger evoked LTSs for the latter cells, we saw no other obvious differences in our studies of IT related to the recording configuration. All recordings had physiological characteristics (i.e., readily evoked LTSs and the presence ofIh) consistent with relay cells. Anatomical reconstruction of a subset of 11 biocytin-filled cells (data not illustrated) clearly distinguished them as relay cells and not interneurons (Guillery, 1966; Friedlander et al., 1981; Sherman and Friedlander, 1988). Our sample includes cells with both X-like and Y-like morphology (Friedlander et al., 1981), but we saw no correlation between these anatomical features and the effect of the agonists on the LTS.
Relationship of LTS amplitude to burst size
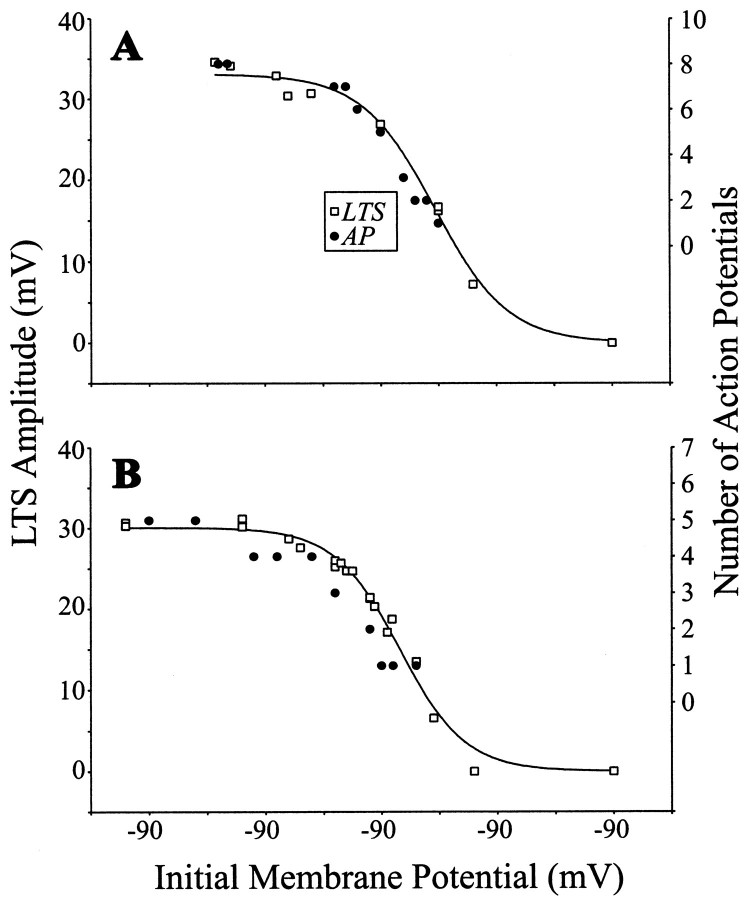
To determine the relationship between the LTS amplitude and size of the evoked burst of action potentials within each of nine cells, we varied the LTS amplitude by using different initial holding membrane potentials before applying a depolarizing pulse to activate an LTS. That is, the more hyperpolarized the initial holding membrane potential, the more de-inactivated ITbecomes and the larger the evoked LTS will be (Zhan et al., 1999). We repeated this before TTX application to determine the number of action potentials per burst and after TTX to eliminate action potentials and thereby obtain clearer measures of LTS amplitude. The relationship between LTS amplitude and Vm during TTX application could readily be fit by a sigmoid function (Eq. 1). Figure 1 shows such an experiment for two representative cells. As shown, the more hyperpolarized the initial holding membrane potential, the larger the evoked LTS and the greater the number of action potentials per burst. Indeed, there is a significant correlation between LTS amplitude (taken from the fitted sigmoid) and the number of action potentials in the associated burst (Fig. 1A, r = 0.99; Fig.1B, r = 0.94; p < 0.001 for each). This type of relationship was seen with each of the other seven cells for which the same experiment was completed; each showed a highly significant correlation between LTS amplitude and the number of action potentials in the burst (r values between 0.93 and 0.99; p < 0.001 for each). This documents the close relationship between LTS amplitude and the size of the burst, which is the actual signal sent to cortex.
Fig. 1.
Comparison of voltage dependence of LTS amplitude and number of action potentials in a burst. LTSs were evoked by small current steps (10–100 pA, 400 msec) from different initial holding membrane potentials before and after 1 μm TTX was applied to the bath. LTS amplitude is defined as the voltage difference between the LTS peak and baseline of extrapolated membrane voltage from subthreshold ohmic responses, and the number of action potentials reflects the number activated via a single LTS (for details, see Zhan et al., 1999). A, Example from geniculate neuron. As shown, there is a close correlation between the number of action potentials per burst (measured before TTX application) and the amplitude of the evoked LTS (measured after TTX application). The curve is the sigmoid fit to the LTS amplitude points (see Materials and Methods). B, Example from another geniculate neuron; conventions as in A.
Effects of ACPD on LTS amplitude
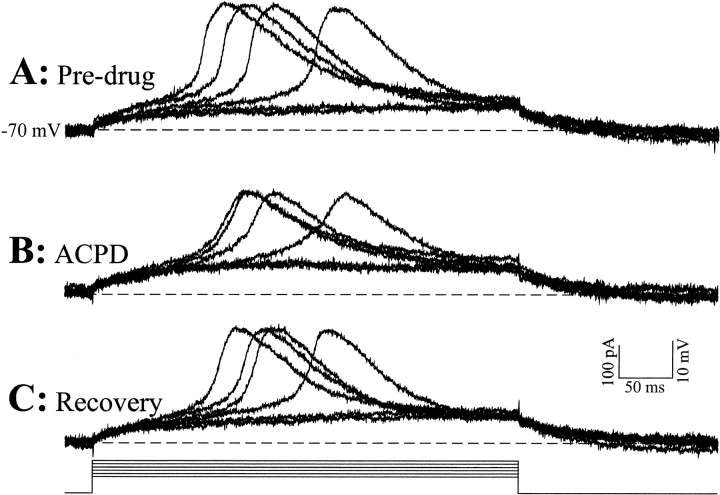
All remaining experiments reported here were performed in the presence of TTX to provide measures of LTS amplitude uncontaminated by action potentials. Figures 2 and3 illustrate the main observations of this study. We have shown previously that the LTS is activated in an all-or-none manner (Zhan et al., 1999). This means that, for any given level of IT inactivation, which itself is determined by the membrane potential at which the cell was held at the time the LTS is activated, the LTS amplitude is constant over a wide range of suprathreshold stimuli. However, because different levels of IT inactivation occur with different initial holding potentials, the LTS amplitude will vary with the holding potential (Fig. 1). Figure 2 shows the all-or-none property of the LTS for a given initial holding potential of −70 mV at the soma. Small incremental depolarizing current steps (10 pA steps) were either subthreshold, evoking an ohmic response, or suprathreshold, evoking an LTS, and increasing suprathreshold currents did not evoke larger LTSs. There is an effect of current intensity on LTS latency for just suprathreshold currents; this has been documented previously (Zhan et al., 1999) and is not further considered here. Note that the LTS is evoked in an all-or-none manner before and during bath application of the general mGluR agonist ACPD (50 μm), which slightly depolarized the cell (1 mV). Thus, ACPD does not affect this feature of the LTS, although ACPD application does slightly diminish the evoked LTS amplitude, even though current injection was used to bring the soma to the same level of polarization (−70 mV) as before ACPD application. Such a reduction of LTS amplitude by ACPD was a feature we consistently observed in these relay cells.
Fig. 2.
The general metabotropic glutamate receptor agonist ACPD reduces overall amplitude of LTSs, which are activated in an all-or-none manner. From an initial membrane potential of −70 mV and in the presence of TTX (1μm), small amplitude current steps (50 and 60 pA) evoke ohmic responses. With increasing current amplitudes (70–100 pA, 10 pA increments), all-or-none LTSs are evoked. A, LTSs evoked before application of ACPD. B, LTSs evoked in the presence of 50 μm ACPD, which produces a steady depolarization of 1 mV. Note that the same current protocols as in A evoke smaller amplitude LTSs. C, LTSs evoked after the washout of the ACPD. The LTS amplitudes recover to the predrug levels inA. Note that, in all three conditions, the initial membrane potential was adjusted using steady current injection to −70 mV, and this cell had an initial resting membrane potential of −64 mV.
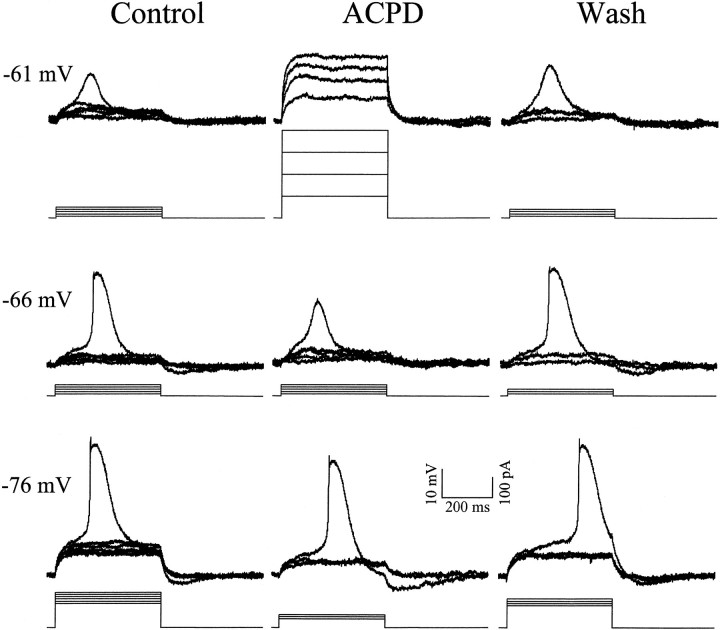
Fig. 3.
Effect of the general metabotropic glutamate receptor agonist ACPD on LTS amplitude in a geniculate neuron. All responses are in the presence of 1 μm TTX.Left column, Voltage dependency of LTS amplitude in control conditions. As the initial holding membrane potential of the cell is hyperpolarized from −61 to −76 mV, there is an increase in LTS amplitude from 11 (−61 mV), to 26 (−66 mV), to 32 (−76 mV) mV.Middle column, Voltage dependency of LTS amplitude in presence of 40 μm ACPD. At −61 mV, the LTS is completely absent and cannot be evoked by larger amplitude current steps (100 pA increments). At −66 mV, the LTS is 16 mV, much smaller than that evoked from −66 mV in control conditions. At −76 mV, LTS amplitude is 32 mV, comparable with the maximum amplitude evoked in control conditions. Right column, Voltage dependency of LTS amplitude after 65 min washout of ACPD. Now the LTS amplitudes are comparable with those in control conditions, being 12, 26, and 33 mV from top to bottom. The initial resting membrane potential of the cell was −63 mV. Because of the increased input resistance with ACPD, less current was injected to achieve comparable depolarization, and thus the protocol of current injection differed with and without ACPD present.
Figure 3 shows representative responses from another relay neuron at three different initial holding membrane potentials (−61, −66, and −76 mV) achieved by injecting differing steady-state currents into the soma. Given this all-or-none feature of the LTS and for simplicity, only a single evoked LTS is shown for each series of traces. In control conditions at −76 mV, incremental current steps of 10 pA evoked ohmic responses at lower intensities until a threshold was reached so that, at suprathreshold intensities, an all-or-none LTS was evoked. At a more depolarized membrane potential (−66 mV), the LTS is slightly smaller because IT is less de-inactivated, and at −61 mV, the LTS amplitude is significantly reduced becauseIT becomes even less de-inactivated. We next bath applied the general mGluR agonist ACPD (40 μm), which in this cell, produced a small depolarization recorded at the soma (see Materials and Methods). After returning the membrane potential back to pre-ACPD levels with intracellular current injection, we measured the amplitude of LTSs triggered by similar depolarizing current steps (middle column). At −76 mV membrane potential, the amplitude of the LTS is very similar to control conditions. However, at −66 mV, the LTS amplitude is dramatically reduced, and it is nonexistent at −61 mV. Larger depolarizing current steps (100–400 pA) did not evoke any sign of an LTS at −61 mV. This reduction in LTS amplitude was reversible after washout of the ACPD (right column).
In other words, ACPD would activate mGluRs, which are concentrated on distal dendrites (Godwin et al., 1996), and the depolarization seen there would be greater than that recorded at the soma. If the T channels underlying IT were located in dendrites distal enough to be at least partially unclamped, they would see more depolarization and thus be less de-inactivated than would any at the soma or very proximal dendrites.
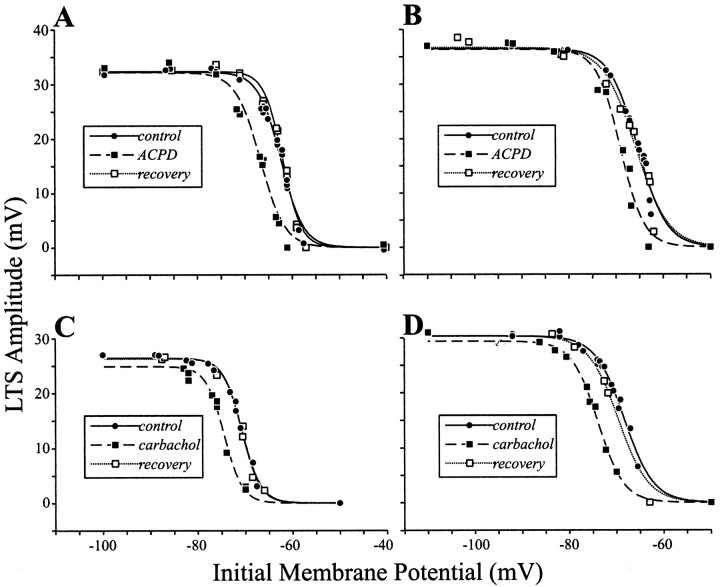
The reduction in LTS amplitude was observed in all cells tested (n = 15), and these effects reversed after a 14–36 min wash. Figure 4, A andB, illustrates for two representative examples the ACPD-mediated suppression at various initial holding membrane potentials. In control conditions, LTS amplitude is clearly voltage-dependent. The data points for the initial holding membrane voltage dependence of LTS amplitude are fitted with sigmoid curves (Eq.1). Because of the time needed for washing out of the drugs to achieve recovery of effects, we did not systematically test each neuron with multiple drug concentrations. However, for three cells, two tested with ACPD and one with carbachol (see below), we were able to demonstrate that larger doses of agonist produced more somatic depolarization and larger effects on LTS amplitude as described above (data not shown). From these data, it appears that ACPD produces a hyperpolarizing shift in the voltage dependence of LTS amplitude (see also Discussion).
Fig. 4.
Reduction of LTS amplitude by ACPD and carbachol. Shown for each cell is the relationship between LTS amplitude and initial holding membrane potential during the control condition, during drug application, and recovery after 14–36 min of wash. Each set of data points is fit with a sigmoid curve.A, B, Effect of ACPD for two different geniculate neurons. After the application of ACPD (50 μm), there is an obvious reduction in LTS amplitude, and the effect is reversible after washout of ACPD. C,D, Effect of carbachol for two other geniculate neurons. The conventions for these plots are the same as in A andB, except that bath application of 20 μmcarbachol was used. Note that carbachol produced a reversible effect on the LTS amplitude that is similar to the ACPD effect.
Effects of carbachol on LTS amplitude
We also investigated the effect of cholinergic muscarinic receptor activation on LTS amplitudes by bath applying the general muscarinic agonist carbachol (20–50 μm). As noted in Materials and Methods, carbachol application only slightly depolarized the soma (5–10 mV). Carbachol application provided an effect on the voltage dependency of the LTS that was qualitatively similar to that seen with ACPD application. Two representative examples are shown in Figure 4,C and D, and this same effect was seen in all five cells tested; three of these were also among the 15 cells tested with ACPD application. Again, after fitting the data points to sigmoid functions, the main effect of carbachol application is to produce a hyperpolarizing shift in the voltage dependence of the LTS amplitude.
Quantification of ACPD and carbachol effects
As illustrated in Figure 4, the voltage dependence of LTS amplitude could be fitted by a sigmoid function (Eq. 1), and we did this for all 17 cells for which we studied the actions of ACPD and carbachol on LTS amplitude. As shown in Equation 1, the sigmoid function consists of three independent parameters, a,b, and m; a corresponds to the maximal amplitude, b represents the downward slope, and mreflects the value of the abscissa in millivolts at the 50% amplitude point. In Figure5A–C, these independent variables have been plotted for each of the 17 cells both before and during drug application (there are 20 points in each panel, because three of the cells were studied for both ACPD and carbachol effects). Figure 5A shows that the maximum amplitude (a) was not significantly reduced by either ACPD or carbachol (p > 0.1, paired Student's ttests). Similarly, Figure 5B shows that neither ACPD nor carbachol had a significant effect on the slope parameter, b(p > 0.1). The clear effect of ACPD and carbachol was on the 50% measure, m (Fig. 5C). All data points are below the unity line, indicating that the two agonists consistently shifted m in the hyperpolarizing direction. The reduction of m was statistically significant (p < 0.001 for the ACPD experiments andp < 0.01 for the carbachol experiments). These analyses indicate that the chief effect of ACPD and carbachol was to shift the voltage dependency of the evoked LTS in the hyperpolarizing direction (i.e., a shift in m) and had relatively little effect on the overall amplitude (i.e., a) or the rate of change with voltage (i.e., b) of the evoked LTS.
Fig. 5.
Population data for effects of ACPD and carbachol. A total of 17 cells were studied, but for three, both ACPD and carbachol was tested, and thus there are 20 data points in the scatter plots. A–C, Independent variables from sigmoid curve fitting for dependency of LTS amplitude on initial holding membrane potential before and during ACPD or carbachol application (see Materials and Methods for details of these variables).D, Effect of ACPD or carbachol on LTS amplitude measured from the individual data points before curve fitting. We crudely estimated the initial holding potential needed to evoke an LTS of 50% the maximum value obtained (from one of the most hyperpolarized holding potentials) by linearly extrapolating from the data points on either side of this 50% value. This provided both the initial holding potential for the 50% LTS amplitude and the abscissa values of the 50% LTS amplitude. During drug application, we used the same initial holding potential estimated for the 50% LTS amplitude at control conditions and crudely determined the evoked LTS amplitude from this holding potential, again by linear extrapolation; this provided the ordinate values. Note that, for every cell, the effect of ACPD or carbachol was to reduce this estimate of the LTS amplitude evoked from this initial holding membrane potential.
Figure 5D shows another analysis reflecting the effects of the drugs on LTS amplitude that was independent of sigmoid curve fitting. Here, we calculated the corresponding initial holding membrane potential during control (i.e., predrug) conditions from the interpolated 50% value of LTS amplitude (i.e., the midpoint between the maximum and minimum LTS value at any holding potential). We then estimated via interpolation the value of the LTS amplitude at the same initial holding membrane potential during drug application. We found, in agreement with Figure 5C, that the metabotropic agonist application reduced the LTS amplitude at this membrane potential for all cells (p < 0.001 for the ACPD experiments and p < 0.01 for the carbachol experiments).
DISCUSSION
When equal somatic depolarization of geniculate relay cells is produced by current injection alone versus current injection plus dendritic depolarization, the latter produces more inactivation ofIT and thus a smaller LTS. Thus, activation of depolarizing inputs onto dendrites of these relay cells has effects on IT inactivation that would be underestimated by conventional somatic recording. We have also shown a monotonic relationship between LTS amplitude and number of action potentials in the evoked burst, which is important, because only the action potentials are transmitted to cortex.
Any inputs that depolarize dendrites sufficiently can affectIT in this manner, whether by activating metabotropic or ionotropic receptors. In this study, we have explicitly tested the effects of activating metabotropic receptors located on dendrites, and these receptors would normally be activated by cortical or parabrachial axons. We have not explicitly tested effects of ionotropic receptors, but below we argue why, in more physiological conditions, activation of metabotropic receptors is more appropriate for controlling ITinactivation.
There are at least two mechanisms that may be considered to explain our observations of the effects of metabotropic receptor agonists onIT inactivation; activation of these receptors causes a voltage-independent inactivation ofIT, or the amount ofIT inactivation is strictly voltage-dependent, but the depolarization of many T channels on dendrites near the activated postsynaptic receptors is greater than the depolarization recorded at the soma because of the space-clamp problem. With the first explanation, metabotropic receptor activation would lead to an overall reduction in the evoked LTS amplitude as a function of the initial holding potential, thereby producing an overall reduction of the LTS at all holding membrane potentials, including the most hyperpolarized at which IT would be maximally de-inactivated. In terms of the fitted sigmoid curves, the chief result would be a reduction of the variable a with little or no effect on variables b and m. With the second explanation, metabotropic receptor activation would depolarize many T channels in the dendritic tree more than the soma, so that with somatic recording we would consistently underestimate the extent of IT inactivation. This would result in a hyperpolarizing shift in the dependency of LTS amplitude on the initial holding membrane potential, which, in terms of the fitted sigmoid curves, would result in a systematic reduction in the variable m and little systematic effect on aor b. Figure 5A–C shows that, in every cell tested, activation of either metabotropic receptor type reduces the variable m with no consistent effects on variablesa or b. Our data thus strongly support the second explanation. This also supports the previous conclusion that many T channels are present in the dendrites of relay cells (Zhou et al., 1997; Destexhe et al., 1998).
There is anatomical evidence that the cholinergic input to relay cell dendrites is more proximally located than is the cortical input and related location of metabotropic glutamate receptors (Godwin et al., 1996; Erişir et al., 1997). This implies that there would be less of a space-clamp problem during recording of effects of ACPD application than with carbachol, and we should thus see more of a hyperpolarizing shift in the voltage dependency of the evoked LTS (or reduction of the variable m) with ACPD. However, we were unable to test this hypothesis for technical reasons. We have insufficient information to know how to balance the concentrations of the two agonists to produce a similar depolarization at their receptor sites, so any direct comparison of agonist effects is impractical at present. Nonetheless, it is interesting to note that we did achieve large effects and shifts in the variable m with carbachol despite the relatively proximal dendritic location of cholinergic synapses and presumably thus muscarinic receptors (Erişir et al., 1997).
Although we depolarized dendrites with agonists to metabotropic and not ionotropic receptors, we do not argue from our data alone ionotropic receptors cannot also control IT. Indeed, any appropriate depolarization of the dendrites should have a similar effect on IT, regardless of the receptor type involved in mediating the EPSP. Having said this, there is another reason to suspect that activation of metabotropic receptors is more effective in inactivatingIT than is equivalent activation of ionotropic receptors, because inactivation ofIT is a complex function of voltage and time (Huguenard and McCormick, 1992; McCormick and Huguenard, 1992;Zhan et al., 1999), requiring depolarization to be sustained for ≥50–100 msec. The rapid EPSPs generated by activation of ionotropic glutamate or nicotinic receptors may well activateIT, but these EPSPs are too fast to sustain effective inactivation of ITwithout considerable temporal summation. In contrast, the sustained EPSPs produced via the metabotropic receptors seem ideally suited temporally to inactivate IT. It is interesting in this context that McCormick and Von Krosigk (1992)showed that activation of metabotropic glutamate receptors on relay cells via stimulation of corticothalamic axons effectively converts the firing mode of the cell from burst to tonic.
Our results support the notion that many T channels are located on dendrites with the results that parabrachial and corticogeniculate synapses depolarize distal dendrites much more strongly than they depolarize the soma and axon hillock. This, in turn, suggests that these inputs may have a stronger role to play in effects on T channels and thus response mode than they do in direct effects on action potential initiation (for a detailed discussion of this and other possible roles of these inputs, see Sherman and Guillery, 1996). Likewise, GABAergic terminals also synapse on dendrites throughout the tree (for review, see Sherman and Guillery, 1996), suggesting that their effects on T channels might also be stronger than suggested via conventional somatic recordings, although in this case the hyperpolarizing effects of GABA would tend to de-inactivate the T channels. Indeed, perhaps the major role of many nonretinal inputs to relay cells is to control response mode through depolarization or hyperpolarization of dendritic T channels.
Footnotes
This research was supported by United States Public Health Service Grants EY03038 and EY06884. We also thank Susan Van Horn for her expert technical assistance.
Correspondence should be addressed to S. M. Sherman, Department of Neurobiology, State University of New York, Stony Brook, NY 11794-5230. E-mail: s.sherman@sunysb.edu.
REFERENCES
- 1.Bloomfield SA, Hamos JE, Sherman SM. Passive cable properties and morphological correlates of neurones in the lateral geniculate nucleus of the cat. J Physiol (Lond) 1987;383:653–692. doi: 10.1113/jphysiol.1987.sp016435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox CL, Sherman SM. Glutamate inhibits thalamic reticular neurons. J Neurosci. 1999;19:6694–6699. doi: 10.1523/JNEUROSCI.19-15-06694.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Destexhe A, Neubig M, Ulrich D, Huguenard J. Dendritic low-threshold calcium currents in thalamic relay cells. J Neurosci. 1998;18:3574–3588. doi: 10.1523/JNEUROSCI.18-10-03574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erişir A, Van Horn SC, Bickford ME, Sherman SM. Immunocytochemistry and distribution of parabrachial terminals in the lateral geniculate nucleus of the cat: a comparison with corticogeniculate terminals. J Comp Neurol. 1997;377:535–549. [PubMed] [Google Scholar]
- 5.Friedlander MJ, Lin C-S, Stanford LR, Sherman SM. Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol. 1981;46:80–129. doi: 10.1152/jn.1981.46.1.80. [DOI] [PubMed] [Google Scholar]
- 6.Godwin DW, Van Horn SC, Erişir A, Sesma M, Romano C, Sherman SM. Ultrastructural localization suggests that retinal and cortical inputs access different metabotropic glutamate receptors in the lateral geniculate nucleus. J Neurosci. 1996;16:8181–8192. doi: 10.1523/JNEUROSCI.16-24-08181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillery RW. A study of Golgi preparations from the dorsal lateral geniculate nucleus of the adult cat. J Comp Neurol. 1966;128:21–50. doi: 10.1002/cne.901280104. [DOI] [PubMed] [Google Scholar]
- 8.Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol. 1992;68:1373–1383. doi: 10.1152/jn.1992.68.4.1373. [DOI] [PubMed] [Google Scholar]
- 9.Llinás R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297:406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- 10.McCormick DA, Huguenard JR. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992;68:1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- 11.McCormick DA, Von Krosigk M. Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc Natl Acad Sci USA. 1992;89:2774–2778. doi: 10.1073/pnas.89.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinagel P, Godwin DW, Sherman SM, Koch C. Encoding of visual information by LGN bursts. J Neurophysiol. 1999;81:2558–2569. doi: 10.1152/jn.1999.81.5.2558. [DOI] [PubMed] [Google Scholar]
- 13.Sherman SM. Dual response modes in lateral geniculate neurons: mechanisms and functions. Visual Neurosci. 1996;13:205–213. doi: 10.1017/s0952523800007446. [DOI] [PubMed] [Google Scholar]
- 14.Sherman SM, Friedlander MJ. Identification of X versus Y properties for interneurons in the A-laminae of the cat's lateral geniculate nucleus. Exp Brain Res. 1988;73:384–392. doi: 10.1007/BF00248231. [DOI] [PubMed] [Google Scholar]
- 15.Sherman SM, Guillery RW. The functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- 16.Steriade M, Llinás R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 17.Tamamaki N, Uhlrich DJ, Sherman SM. Morphology of physiologically identified retinal X and Y axons in the cat's thalamus and midbrain as revealed by intra-axonal injection of biocytin. J Comp Neurol. 1994;354:583–607. doi: 10.1002/cne.903540408. [DOI] [PubMed] [Google Scholar]
- 18.Wilson JR, Friedlander MJ, Sherman SM. Fine structural morphology of identified X- and Y-cells in the cat's lateral geniculate nucleus. Proc R Soc Lond B Biol Sci. 1984;221:411–436. doi: 10.1098/rspb.1984.0042. [DOI] [PubMed] [Google Scholar]
- 19.Zhan XJ, Cox CL, Rinzel J, Sherman SM. Current clamp and modeling studies of low threshold calcium spikes in cells of the cat's lateral geniculate nucleus. J Neurophysiol. 1999;81:2360–2373. doi: 10.1152/jn.1999.81.5.2360. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Q, Godwin DW, O'Malley DM, Adams PR. Visualization of calcium influx through channels that shape size burst and tonic firing modes of thalamic relay cells. J Neurophysiol. 1997;77:2816–2825. doi: 10.1152/jn.1997.77.5.2816. [DOI] [PubMed] [Google Scholar]