Abstract
Schwann cells (SCs) differentiate into a myelinating cell when simultaneously adhering to an axon destined for myelination and basal lamina. We are interested in defining the signaling pathway activated by basal lamina. Using SC/sensory neuron (N) cocultures, we identified β1 integrin and F-actin as components of a pathway leading to myelin gene expression and myelination (Fernandez-Valle et al., 1994, 1997). Here, we show that focal adhesion kinase (FAK) and paxillin are constitutively expressed by SCs contacting axons in the absence of basal lamina. Tyrosine phosphorylation of FAK and paxillin increases as SCs form basal lamina and differentiate. FAK and paxillin specifically coimmunoprecipitate with β1 integrin in differentiating SC/N cocultures but not SC-only cultures. Paxillin coimmunoprecipitates with FAK and fyn kinase in differentiating SC/N cocultures. A subset of tyrosine-phosphorylated β1 integrin, FAK, and paxillin molecules reside in the insoluble, F-actin-rich fraction of differentiating cocultures. Cytochalasin D, an actin depolymerizing agent, decreases tyrosine phosphorylation of FAK and paxillin and their association with β1 integrin and causes a dose-dependent increase in the abundance of insoluble FAK and paxillin complexes. Collectively, our work indicates that β1 integrin, FAK, paxillin, and fyn kinase form an actin-associated complex in SCs adhering to basal lamina in the presence of axons. This complex may be important for initiating the process of SC differentiation into a myelinating cell.
Keywords: Schwann cells, myelination, basal lamina, β1 integrin, focal adhesion kinase, paxillin, tyrosine phosphorylation, signal transduction
Cell adhesion to extracellular matrix (ECM) regulates gene expression, motility, growth, differentiation, and survival of many cell types, including Schwann cells (SCs), the myelin-forming cell of the peripheral nervous system (Eldridge et al., 1987; Werb et al., 1989; Streuli et al., 1991;Fernandez-Valle et al., 1993; Lin and Bissell, 1993) (for review, seeLukashev and Werb, 1998). The biological effects of ECM are mediated in part by integrins, a large family of heterodimeric transmembrane receptors for ECM proteins (Hynes, 1992). Upon binding specific ligands, integrins cluster and stimulate tyrosine phosphorylation and recruitment of signaling and structural proteins to the plasma membrane at focal adhesions, cell-substratum contact sites (Clark and Brugge, 1995; Burridge and Chrzanowska-Wodnicka, 1996).
Focal adhesion kinase (FAK) and paxillin play critical roles in β1 integrin-dependent signaling (for review, see Zachary and Rozengurt, 1992; Richardson and Parsons, 1995; Guan, 1997). FAK is an intracellular protein tyrosine kinase that rapidly autophosphorylates in response to β1 integrin binding to ECM, growth factor receptor activation, and membrane depolarization (Burridge et al., 1992;Schaller et al., 1992; Siciliano et al., 1996). A direct β1 integrin–FAK association is proposed based on results of in vitro peptide binding studies but has not been demonstrated in cells (Schaller et al., 1995). FAK binds numerous signaling molecules, including Shc, Grb2, and src family kinases (Schlaepfer et al., 1999). FAK plays an essential role during development as FAK knock-out mice suffer lethal mesodermal defects (Ilic et al., 1995) (for review, seeRidyard and Sanders, 1999). Migration studies using FAK null cells suggest that FAK regulates focal adhesion turnover (Ilic et al., 1997). Focal adhesions also serve as nucleating sites for stress fibers. Disruption of actin polymerization with cytochalasin D (CD) inhibits FAK tyrosine phosphorylation but not its plasma membrane localization (Lipfert et al., 1992; Miyamoto et al., 1995). Paxillin is a 68 kDa adapter protein that localizes with β1 integrin, FAK, vinculin, and src family kinases at focal adhesions (Bellis et al., 1995;Turner, 1998). It is phosphorylated after cell activation by ECM, growth factors, and neuropeptides, and during embryogenesis, metastasis, and wound repair (Turner, 1991; Mueller et al., 1992;Rozengurt, 1995; Chen et al., 1998). It contains multiple protein–protein binding motifs including Src homology 2 (SH2), SH3, four LD domain (leucine, aspartate rich), four double-zinc finger LIM domain [LIN-II, ISI-1, MEC-3 (homeodomain proteins)], and tyrosine and serine/threonine phosphorylation sites (Turner and Miller, 1994;Salgia et al., 1995). Paxillin binds FAK, vinculin, and a multitude of structural and signaling proteins and links integrin signaling with mitogen-activated protein kinase and c-Jun N-terminal protein kinase pathways (Turner and Miller, 1994; Brown et al., 1996; Tong et al., 1997).
SCs must synthesize and adhere to basal lamina to differentiate in response to axonal signals (Moya et al., 1980; Eldridge et al., 1987;Fernandez-Valle et al., 1993) (for review, see Bunge and Fernandez-Valle, 1995). In SC/sensory neuron (N) cocultures, SCs begin to assemble basal lamina immediately after ascorbate addition to the serum-containing medium and myelin 3–4 d later (Fernandez-Valle et al., 1998). Ascorbate is an essential cofactor for synthesis of triple helical collagen type IV, the scaffold for assembly of laminin–nidogen and heparan sulfate proteoglycan into basal lamina (Woodley et al., 1983; Aumailley et al., 1987). Purified laminin-1 induces SC myelination in the absence of ascorbate and is likely the “inducing” basal lamina component (Eldridge et al., 1989; Guenard et al., 1995). SCs express several laminin integrin receptors in a developmentally regulated manner. Undifferentiated and early differentiating SCs express α1β1 and α6β1 integrin, and myelinating SCs express α6β4 integrin (Tawil et al., 1990; Einheber et al., 1993; Feltri et al., 1994; Niessen et al., 1994). β1 integrin function-blocking antibody inhibits SC adhesion to nascent basal lamina and myelination in SC/N cocultures (Fernandez-Valle et al., 1994).
Previously, we found that low CD concentrations inhibit SC myelin gene expression and myelination, and we localized FAK to SC juxtamembrane regions at putative focal adhesions (Fernandez-Valle et al., 1997,1998). Here, we provide evidence for formation of a β1 integrin–FAK–paxillin–fyn kinase complex in SCs adhering to axons and basal lamina.
MATERIALS AND METHODS
Antibodies
Rabbit β1 integrin polyclonal antibody was a gift from S. Carbonetto (McGill University, Montreal, Canada) (Tawil et al., 1990,1993), and paxillin 165 antibody was a gift from Chris Turner (State University of New York, Syracuse, NY). Hamster β1 integrin monoclonal antibody (catalog #09871D; PharMingen, San Diego, CA), FAK monoclonal antibody (catalog #F15020; Upstate Biotechnology, Lake Placid, NY), phosphotyrosine (TY-P) antibody (ICN, Costa Mesa, CA), Fyn kinase polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), paxillin and phosphotyrosine antibody conjugated to horseradish peroxidase (HRP) (Transduction Laboratories, Lexington, KY), normal rabbit serum (Sigma, St. Louis, MO), normal hamster IgG1 (PharMingen), normal mouse IgG1 (Sigma), and goat anti-mouse and goat anti-rabbit conjugated to HRP (Promega, Madison, WI) were purchased.
Tissue culture
Primary Schwann cell cultures. The Brockes method (Brockes et al., 1979) was used to isolate SCs from sciatic nerves of embryonic day 21 or newborn Sprague Dawley rats (Harlan Sprague Dawley, Indianapolis, IN). Briefly, sciatic nerves were removed and stripped of connective tissues. After collagenase (Worthington, Lakewood, NJ) and trypsin (Life Technologies, Rockville, MD) dissociation, isolated cells were placed on a 60 mm tissue culture dish (Corning, Corning, NY) in D10 medium consisting of DMEM containing 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, UT). On the following day, 10−5m cytosine arabinoside (Sigma) was added for 5 d to eliminate fibroblasts. Residual fibroblasts were lysed by treatment with Thy 1.1 antibody (103-TIB; American Type Culture Collection, Manassas, VA), followed by guinea pig complement (Life Technologies). SCs were expanded in D10 plus 2 μm forskolin (Sigma) and 20 μg/ml pituitary extract (Biomedical Technologies, Stoughton, MA) on poly-l-lysine-coated (200 μg/ml; Sigma) 100 mm tissue culture dishes. SC cultures were passaged no more than four times before plating SCs onto sensory neuron cultures.
Sensory neuron cultures. Neurons were isolated from cervical dorsal root ganglia of Sprague Dawley rat embryos at 16 d of gestation by dissociation with trypsin. Cells were plated on poly-l-lysine- and laminin-coated (5 μg/coverslip; Life Technologies) 11 mm German glass coverslips (Carolina, Burlington, NC) at a density of 0.8–1 ganglion per coverslip. The growth medium was Eagle's MEM (EMEM) containing 5% human placental serum (a generous gift from Dr. R. Devon, University of Saskatchewan, Saskatchewan, Canada), 50 ng/ml nerve growth factor (a generous gift from Dr. P. Wood, University of Miami, Miami, FL), and 400 mm glucose. Non-neuronal cells were eliminated using one pulse of anti-mitotics (uridine–fluorodeoxyuridine, 10−5m; Sigma). Purified neuron cultures were maintained in CB medium (EMEM containing 5% FBS, 50 ng/ml NGF, and 0.4% glucose) for 7–10 d before seeding with SCs. Additional details of the culture procedure are provided by Kleitman et al. (1991).
SC/neuron cocultures. SCs were removed from culture dishes using trypsin, washed extensively in L-15 containing 10% serum. Approximately 100,000 SCs were seeded onto purified neuron cultures in CB medium and were maintained for 1 week to allow additional SC proliferation in response to axonal mitogens (Wood and Bunge, 1975; Marchionni et al., 1993; Morrissey et al., 1995). To initiate differentiation into myelin-forming cells, cultures were switched from CB (serum-only) to M (serum plus ascorbate) medium (EMEM containing 15% FBS, 50 ng/ml NGF, 50 μg/ml ascorbate, and 0.4% glucose) and were grown for 4–21 d to allow myelination. Other cultures were maintained in CB medium as undifferentiated cultures.
Cytochalasin D treatment. CD-containing medium was prepared from a stock solution of 0.5 mg/ml in DMSO. Cultures were switched to M medium containing 0.25, 0.50, or 0.75 μg/ml CD, or were maintained in CB. Some cultures were switched to M/D medium (M plus 0.075% DMSO). Cultures were fed every other day for 7–8 d before extraction.
Sudan black staining and quantitation
SC/N cultures were grown in serum-only medium for 21 d or in serum-plus ascorbate medium for 4, 7, 14, and 21 d. Cultures were fixed for 10 min in 4% paraformaldehyde in 0.1 msodium phosphate buffer, rinsed several times in buffer, and osmicated in 1% osmium tetroxide–phosphate buffer for an additional 1 hr. Cultures were dehydrated in 25, 50, and 70% ethanol for 5 min each and stained with 0.5% Sudan black in 70% ethanol for 1 hr. Cultures were destained for 1–2 min in 70% ethanol, rehydrated in 50 and 25% ethanol followed by phosphate buffer, and then mounted in glycerin jelly. Myelin counts were determined at 400× magnification by sampling the same 20 coordinates in each culture and counting the number of myelin segments within a defined grid. The counts were adjusted to reflect the number of myelin segments in the total area of the coverslip. The adjusted numbers are an under-representation of the total myelin segments because of difficulty in accurately counting densely myelinated cultures. One culture at each time point from three separate experiments was quantitated, and the mean and SD are provided.
Cell extraction and immunoprecipitation
Cultures were rinsed three times on ice with PBS, pH 7.4 (Life Technologies). Cultures were extracted on ice for 30 min with cold TAN buffer consisting of 10 mm Tris-acetate, pH 8.0, 1.0% NP-40, 100 mm NaCl, 1 mmphenylmethylsulfonyl fluoride (PMSF), 2 mmn-ethylmaleimide, 2 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 mm sodium orthovanadate (Sigma). After scraping, pooled cultures were sonicated for 15 min in an ice-water bath and centrifuged for 15 min at 14,000 rpm at 4°C. Protein concentrations were determined using DC protein assay kit (Bio-Rad, Hercules, CA). For immunoprecipitation, 100–400 μg of total cell extracts were immunoprecipitated with normal mouse IgG1, hamster IgG1, rabbit IgG (1–2 μg of antibody/100 μg of protein), or preimmune rabbit serum for 2 hr, followed by an overnight incubation with protein A-Sepharose or protein G-agarose. The resulting supernatant was incubated with the indicated specific antibodies and were collected as above. Immune complexes were washed twice in TAN extraction buffer, centrifuged through a 60% sucrose cushion, and washed sequentially in solution A (10 mmTris, pH 7.0, 1 mm EDTA, 0.5%Triton X-100, and 1m NaCl), solution B (10 mmTris, pH 7.0, 1 mm EDTA, 0.5% Triton X-100, 0.2m NaCl, and 0.1% SDS), and solution C (10 mm Tris, pH 7.0, 1 mm EDTA, and 0.5% Triton X-100). Samples were solubilized in 2× SDS sample buffer containing 5% β-mercaptoethanol.
The soluble culture fractions were obtained by extraction in 100 μl/coculture of CSK buffer (10 mm PIPES, pH 6.8, 0.5% Triton X-100, 50 mm NaCl, 300 mm sucrose, 2 mm MgCl2, 2 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 mm PMSF), as described by Plopper and Ingber (1993). The remaining insoluble fraction was extracted in 100 μl/culture of TAN buffer. The extracts from 10–20 cultures were pooled and used for Western blot analysis and immunoprecipitations.
Gel electrophoresis and Western blotting
Immunoprecipitated samples were boiled at 100°C for 10 min. Equal protein was electrophoresed on 7.5 or 10% SDS-PAGE gels and transferred to nitrocellulose membranes. Membranes were stained with India Ink (1:1000) in TBS-T (20 mm Tris, pH 7.6, 137 mm NaCl, and 0.1% Tween 20) for 30 min to visualize protein bands. Membranes were blocked in TBS-T containing 5% dried milk, except membranes blotted for phosphotyrosine were blocked in TBS-T containing 4% bovine serum albumin and 1% chicken ovalbumin. After blocking for 1–2 hr, the membranes were rinsed and incubated with primary antibody in TBS-T for 1 hr at the following dilutions: paxillin 1:10,000, FAK 1:500 or 1:1000, β1 integrin 1:1000, and Fyn kinase 1:500. After incubation in primary antibody, membranes were rinsed in TBS-T and incubated with HRP-conjugated goat anti-mouse or goat anti-rabbit (Promega) at 1:15,000 antibody for 30 min. Membranes were rinsed in TBS-T and incubated with ECL reagents (Pierce, Rockford, IL). Controls for Western blotting consisted of identical lanes that were reacted with HRP-conjugated secondary antibody only.
RESULTS
SCs express FAK and paxillin in the absence of axon and basal lamina contact
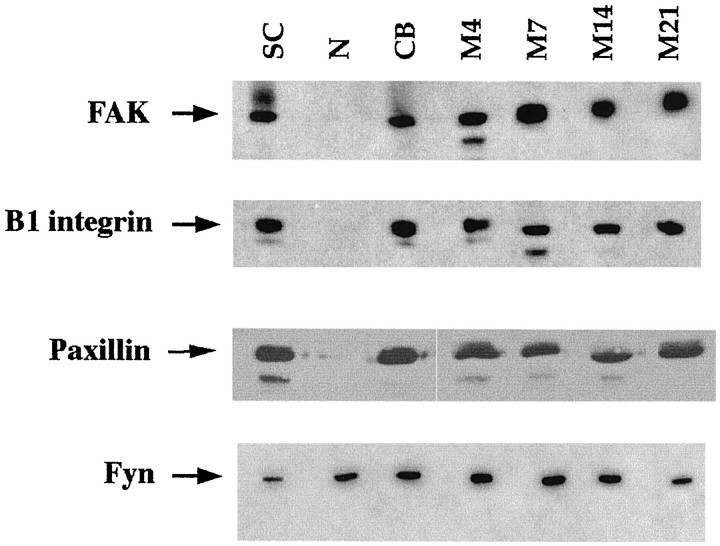
Total protein extracts were prepared from primary cultures of SCs, N, and SC/N cocultures grown in serum-containing but ascorbate-lacking (CB) medium that does not support basal lamina formation or myelination, and serum plus ascorbate (M) medium that promotes basal lamina and myelin formation for 4–28 d. Western blot analysis was performed using equal protein for each culture type (Fig.1). The expression pattern for FAK, paxillin, and β1 integrin is very similar. The three proteins are expressed in all SC-containing cultures, and the level of expression does not vary substantially during SC differentiation. The abundance of paxillin in SCs is much greater than FAK or β1 integrin because one-sixth as much protein was used in paxillin Western blots. Neuron-only cultures express negligible amounts of paxillin, FAK, and β1 integrin compared with SCs. Fyn kinase is expressed in neurons, as well as in SCs, and its expression level does not change during SC differentiation. These results demonstrate that SCs do not require axon or basal lamina contact to synthesize FAK, paxillin, or β1 integrin.
Fig. 1.
FAK, paxillin, and fyn kinase are constitutively expressed by Schwann cells. Western blot analysis of total protein from extracts of mitogen-stimulated SCs cultures, N cultures, undifferentiated SC/N cocultures grown in CB medium, and differentiating SC/N cocultures grown in M medium for the indicated days. Undifferentiated cocultures grown in CB medium do not contain basal lamina or myelin, but differentiating cocultures grown in M medium contain basal lamina and myelin. Thirty micrograms of protein was used to detect FAK, β1 integrin, and fyn kinase, and 5 μg of protein were used to detect paxillin. The bands were visualized using chemiluminescence. The result shows that equivalent amounts of FAK, β1 integrin, paxillin, and fyn kinase are expressed by SCs under all conditions. Neurons, relative to SCs, express very low levels of β1 integrin, FAK, and paxillin but substantial amounts of fyn kinase.
Representative cocultures were stained with Sudan black, and the number of myelin segments was counted to determine the number of terminally differentiated SCs (Table 1). Myelin abundance in differentiating SC/N cocultures grown in serum plus ascorbate medium increases over 700-fold during the time course. SCs grown with neurons in serum-only medium remain rounded and form few myelin segments.
Table 1.
Quantitation of myelin segments in undifferentiated (CB) and differentiating (M) SC/N cocultures (×103)
| CB | M4 | M7 | M14 | M21 |
|---|---|---|---|---|
| 0 | 0.04 ± 0 | 5.8 ± 3 | 16.2 ± 3 | 27.7 ± 3 |
Values were adjusted to reflect the total number of myelin segments per coculture.
FAK and paxillin are tyrosine-phosphorylated in response to basal lamina formation
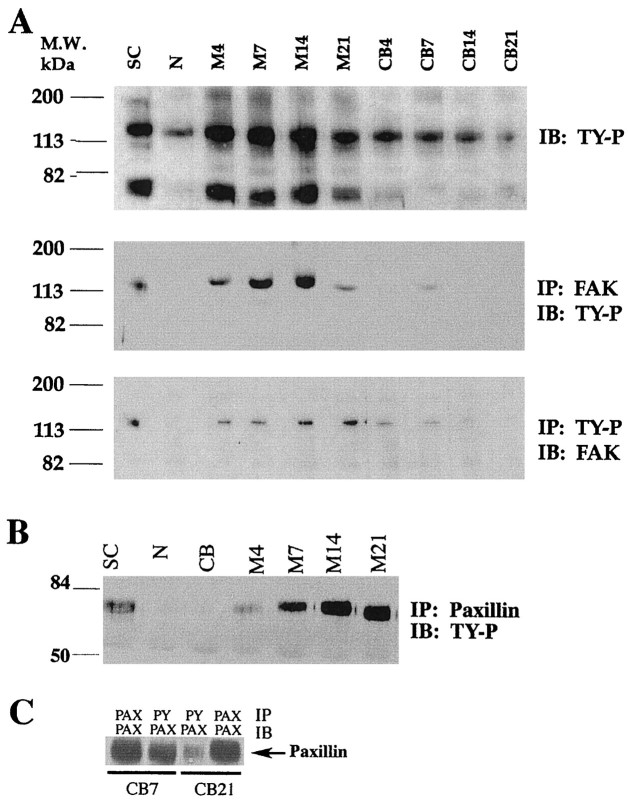
Western blot analysis was performed using phosphotyrosine-HRP-conjugated antibody to identify the major tyrosine-phosphorylated proteins expressed by differentiating SCs (Fig.2A, top panel). Two major bands are observed migrating at ∼125 and 70 kDa in mitogen-stimulated SC cultures and differentiating SC/N cocultures. The 125 kDa band is also present in neuron-only cultures and undifferentiated SC/N cocultures, but its abundance is substantially lower in undifferentiated SC/N cocultures compared with differentiating SC/N cocultures. The 70 kDa band is not observed in neuron-only cultures or undifferentiated SC/N cocultures.
Fig. 2.
Tyrosine phosphorylation of FAK and paxillin is elevated in differentiating SC/N cocultures. A,Top panel, Thirty micrograms of total protein extracts from cultures of SC, N, and SC/N cocultures grown in CB or M medium for 4–21 d were subjected to 7.5% SDS-PAGE and were immunoblotted (IB) with HRP-conjugated TY-P.Middle panel, One hundred micrograms of total protein from the identical extracts were immunoprecipitated (IP) with 1 μg of FAK antibody and were immunoblotted with HRP-conjugated TY-P antibody. Bottom panel, One hundred micrograms of the identical extracts were immunoprecipitated with 1 μg of TY-P antibody and were immunoblotted with FAK antibody. B, Five micrograms of paxillin immunoprecipitates from SC-only (SC), neuron-only (N), 14-d-old undifferentiated (CB), and 4- to 21-d-old differentiating (M) SC/N coculture extracts were immunoblotted with HRP-conjugated TY-P antibody.C, Five micrograms of paxillin immunoprecipitates from 7- and 21-d-old undifferentiated cultures were immunoblotted with paxillin and TY-P antibody. The results show that FAK and paxillin become increasingly tyrosine-phosphorylated with time as increasing numbers of SCs synthesize basal lamina and myelin. The experiments were repeated a minimum of three times.
To determine whether these bands corresponded to FAK and paxillin, culture extracts were immunoprecipitated with FAK and paxillin antibody and were immunoblotted with anti-phosphotyrosine-HRP. Phosphorylated FAK is present in mitogen-stimulated SCs and in differentiating SC/N cocultures and is occasionally detected at very low levels in undifferentiated SC/N cocultures (Fig. 2A, middle panel). The abundance of phosphorylated FAK increases with time of differentiation up to 14 d and then appears to decrease. Similar results are obtained when phosphotyrosine immunoprecipitates are immunoblotted for FAK, except that FAK phosphorylation level remains high at 21 d (Fig. 2A, bottom panel). Phosphorylated paxillin is present in cultures of mitogen-stimulated SCs and increases with time in differentiating SC/N cocultures. Phosphorylated paxillin is not detected in neuron-only cultures or 14-d-old undifferentiated SC/N cocultures (Fig.2B). This suggests that paxillin phosphorylation decreases in SCs contacting axons, because at the time of seeding onto neuron cultures, the mitogen-stimulated SCs contain phosphorylated paxillin. To confirm this observation, 7- and 21-d-old undifferentiated SC/N cocultures were extracted, and equal amounts of paxillin immunoprecipitates were immunoblotted for paxillin and phosphotyrosine. The level of paxillin tyrosine phosphorylation decreases with time of coculture (Fig. 2C). The results suggest that FAK and paxillin tyrosine phosphorylation is stimulated by basal lamina and coincides with the onset of SC differentiation.
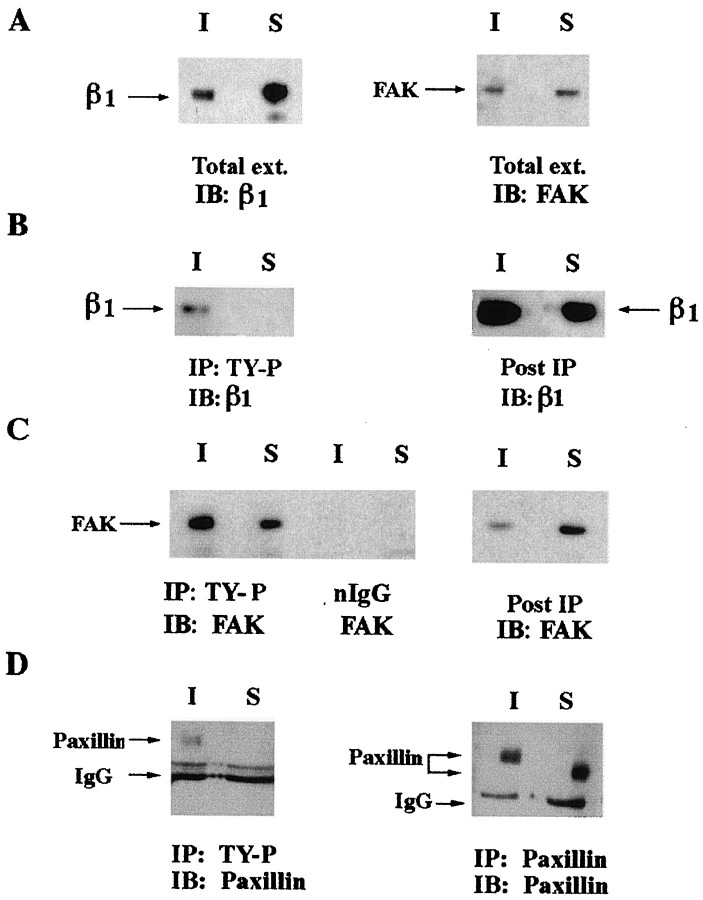
Tyrosine-phosphorylated FAK, paxillin, and β1 integrin localize to the cytoskeleton in differentiating SCs
Studies were conducted to determine the distribution of β1 integrin, FAK, and paxillin in differentiating SCs. Soluble and insoluble fractions of 10-d-old differentiating SC/N cocultures were prepared and used in Western blot analysis. β1 integrin and FAK are present in both the soluble and insoluble fractions (Fig.3A). FAK is evenly distributed between both fractions, whereas the abundance of soluble β1 integrin is ∼120% greater than insoluble β1 integrin. Soluble and insoluble fractions of 10-d-old differentiating SC/N cocultures were immunoprecipitated with phosphotyrosine antibody and were immunoblotted for β1 integrin and FAK. β1 integrin is not detected in phosphotyrosine immunoprecipitates from the soluble fraction but is present in phosphotyrosine immunoprecipitates from the insoluble fraction (Fig. 3B). β1 integrin is present in the post-immunoprecipitated insoluble and soluble fractions. Therefore, only a small fraction of β1 integrin contains phosphotyrosine, and this subset of β1 integrin molecules is confined to the insoluble compartment of differentiating SC/N cocultures. Tyrosine-phosphorylated FAK is present in both the insoluble and soluble fractions; however, the insoluble fraction contains 1.5- to 2-fold more FAK than the soluble fraction (Fig. 3C). Control immunoprecipitations with normal IgG do not contain FAK. After immunoprecipitation, the remaining insoluble and soluble fractions were immunoblotted for FAK. The insoluble fraction is nearly depleted of FAK by one round of phosphotyrosine immunoprecipitation, whereas FAK is abundant in the soluble fraction and is most likely unphosphorylated. Tyrosine-phosphorylated paxillin is observed in the insoluble, but not the soluble, fraction of differentiating SC/N cocultures (Fig. 3D). Paxillin is present in both fractions, and tyrosine-phosphorylated insoluble paxillin migrates more slowly than soluble paxillin lacking tyrosine phosphorylation (Fig. 3D). Undifferentiated SC/N cocultures were not examined because FAK is not tyrosine-phosphorylated in this culture condition (Fig.2B,C, CB lanes).
Fig. 3.
Tyrosine-phosphorylated β1 integrin, FAK, and paxillin colocalize in the insoluble compartment of differentiating SC/N cocultures. A, Thirty micrograms of soluble (S) and insoluble (I) fractions of 10-d-old differentiating SC/N cocultures were immunoblotted (IB) with β1 integrin antibody (left) and FAK antibody (right). B, Four hundred micrograms of soluble and insoluble fractions of 14-d-old differentiating SC/N cocultures were immunoprecipitated (IP) with anti-TY-P antibody and were immunoblotted (IB) with β1 integrin antibody (left). Thirty micrograms of the post-immunoprecipitated extract were immunoblotted with β1 integrin antibody (right). C, Four hundred micrograms of soluble (S) and insoluble (I) extracts of 14-d-old differentiating SC/N cocultures were immunoprecipitated with phosphotyrosine antibody and normal IgG and were immunoblotted with FAK antibody (left). Thirty micrograms of the post-immunoprecipitated extracts were immunoblotted for FAK (right).D, The soluble (S) and insoluble (I) fractions of 14-d-old differentiating SC/N cocultures were immunoprecipitated with paxillin and phosphotyrosine antibodies. Twenty micrograms of phosphotyrosine immunoprecipitate was immunoblotted with paxillin antibody (left). Five micrograms of paxillin immunoprecipitates (IP) were immunoblotted (IB) with paxillin antibody (right). The results indicate the tyrosine-phosphorylated β1 integrin, FAK, and paxillin are bound to the cytoskeleton in differentiating SC/N cocultures.
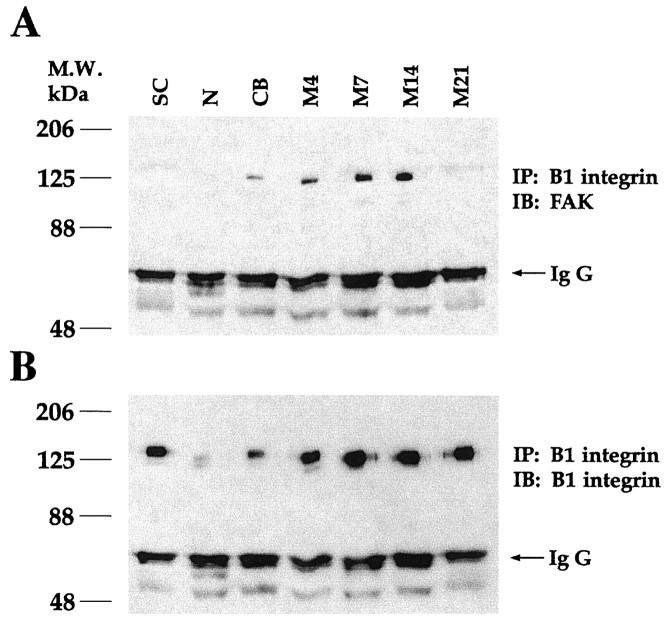
β1 integrin antibody coimmunoprecipitates FAK from differentiating SC/N cocultures
Coimmunoprecipitation experiments were performed to determine whether FAK and β1 integrin associated during SC differentiation. FAK is not detected in β1 integrin immunoprecipitates from SC-only or neuron-only cultures (Fig.4A). A small amount of FAK is detected in β1 integrin immunoprecipitates from undifferentiated 14-d-old SC/N cocultures (CB). Increasing amounts of FAK are detected in β1 integrin immunoprecipitates from 4-, 7-, and 14-d-old differentiating SC/N cocultures (M4–M14) grown in ascorbate-containing medium. FAK is not detected in the β1 integrin immunoprecipitates from 21-d-old differentiating SC/N cocultures or when normal hamster IgG or protein A-Sepharose beads alone are used for immunoprecipitation (data not shown). Aliquots of the identical β1 integrin immunoprecipitates were run simultaneously on a separate gel and were immunoblotted with anti-β1 integrin (Fig.4B). Equal loading of all samples is seen in the equivalent levels of IgG present in all lanes in both gels. Although both β1 integrin and FAK are expressed in SC-only cultures (Fig. 1), FAK does not coimmunoprecipitate with β1 integrin. This suggests that an FAK and β1 integrin complex forms as SCs contact axons and becomes more abundant as SCs assemble basal lamina.
Fig. 4.
β1 integrin antibody coimmunoprecipitates FAK. Two hundred micrograms of total protein extracts of SC, N, and undifferentiated SC/N cocultures grown for 14 d in CB medium and differentiating SC/N cocultures grown in M medium for 4–21 d (M4–M21) were immunoprecipitated (IP) with 1 μg of hamster β1 integrin antibody. The immunoprecipitates were divided in half, and 100 μg of each sample was loaded onto two gels. The samples were resolved by 7.5% SDS-PAGE. β1 immunoprecipitates were immunoblotted (IB) with FAK antibody in A and mouse β1 integrin antibody in B. IgG from each sample is depicted by an arrow and shows equal loading in eachlane. The results show that β1 integrin and FAK coimmunoprecipitate in SC/N cocultures. This is a representative result of three similar experiments.
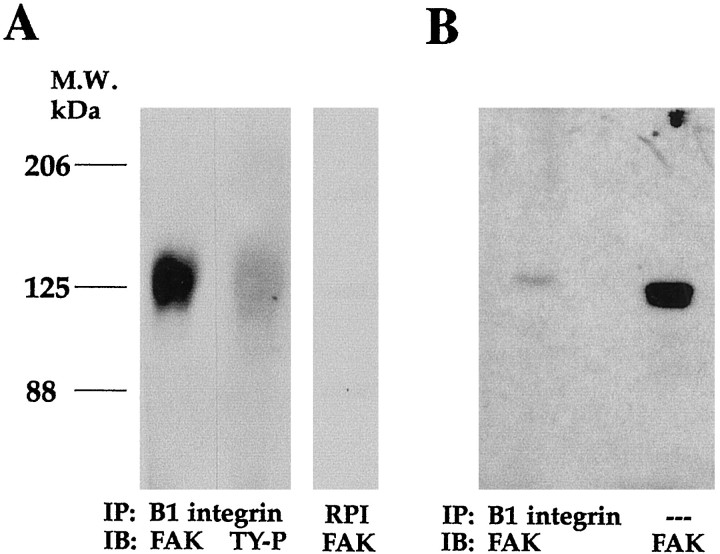
To determine whether β1 integrin-associated FAK was tyrosine-phosphorylated, duplicate samples of extracts from 7-d-old differentiating SC/N cocultures were immunoprecipitated with a rabbit β1 integrin antibody and were subjected to FAK and phosphotyrosine Western blot analysis. FAK is present in β1 integrin immunoprecipitates, as is an identically migrating phosphotyrosine-containing protein (Fig.5A). Immunoprecipitations performed with normal rabbit serum do not contain FAK. This suggests that tyrosine-phosphorylated FAK and β1 integrin associate. A measure of the extent to which FAK molecules associate with β1 integrins was obtained by comparing the amount of FAK present in 20 μg of total protein extract with the amount present in a β1 integrin immunoprecipitation from 200 μg of total protein. FAK Western blot analysis demonstrates that much less FAK is present in the β1 integrin immunoprecipitate than in the total extract of 7-d-old differentiating SC/N cocultures (Fig. 5B). Quantitation of the blot and correction for protein loading suggests that ∼4% of FAK molecules in the differentiating SC/N coculture at this time point associate with β1 integrin.
Fig. 5.
β1 integrin-associated FAK is tyrosine-phosphorylated. A, Total extracts of differentiating SC/N cocultures (M7) were immunoprecipitated (IP) with rabbit β1 integrin antiserum, were separated on 6% SDS-PAGE, and were immunoblotted (IB) with FAK antibody or HRP-conjugated TY-P antibody.RPI, Normal rabbit preimmune serum was used as a control. B, Rabbit β1 integrin immunoprecipitates from 200 μg of total extracts of 7-d-old differentiating (M7) SC/N cocultures (left lane) and 20 μg of the identical total extract (right lane) were separated on 7.5% SDS-PAGE and were immunoblotted with FAK antibody. The results suggest that phosphorylated FAK associates with β1 integrin and that only a very small fraction of FAK molecules associate with β1 integrin.
Paxillin associates with β1 integrin, FAK, and fyn kinase in differentiating SC/N cocultures
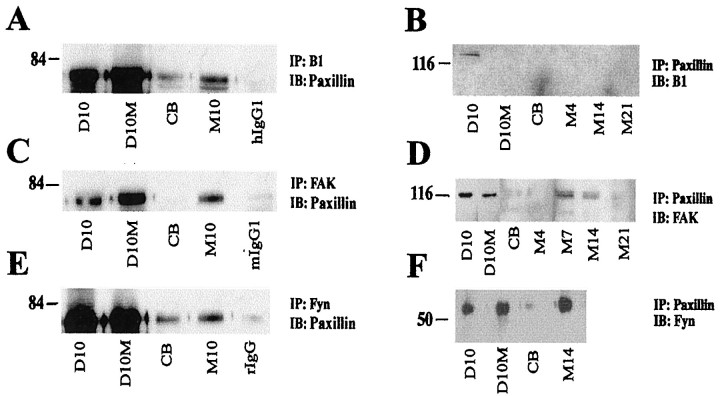
Reciprocal coimmunoprecipitation studies were performed to determine whether paxillin associated with β1 integrin and to identify other paxillin-associated proteins in SC-only and SC/N coculture extracts. Paxillin is detected in large amounts in β1 integrin immunoprecipitates of SC-only cultures, whether grown in the presence or absence of mitogens (Fig.6A). The abundance of β1 integrin-associated paxillin decreases dramatically when SCs are cocultured with axons in the absence of ascorbate but increases if SCs are allowed to differentiate for 10 d in ascorbate-containing medium (Fig. 6A). In the reciprocal coimmunoprecipitation using paxillin antibody, β1 integrin is detected only in SCs grown in the absence of mitogens (Fig.6B). FAK and paxillin reciprocally coimmunoprecipitate from SC-only cultures and differentiating SC/N cocultures but are not detected in undifferentiated SC/N cocultures (Fig. 6C,D). Paxillin and fyn kinase also reciprocally coimmunoprecipitate from SC-only and differentiating SC/N cocultures (Fig. 6E,F). Lesser amounts of paxillin–fyn complexes are observed in undifferentiated SC/N cocultures.
Fig. 6.
Paxillin interacts with β1, FAK, and fyn kinase. Total protein extracts were prepared from SC cultures grown in the absence (D10) and presence (D10M) of mitogens, and undifferentiated (CB) and differentiating (M) SC/N cocultures grown for the indicated days. A, C,E, Equal amounts of protein from each culture type were immunoprecipitated (IP) with the indicated antibody and were immunoblotted (IB) with paxillin antibody. Controls include isotype-matched antibody for mouse (mIgG), hamster (hIgG), and rabbit (rIgG) antibodies. B, Equal amounts of total protein extracts from the various culture paradigms were immunoprecipitated (IP) with paxillin antibody and were immunoblotted (IB) with the indicated antibody. The results indicate that, in SCs cultured alone, paxillin interacts with β1 integrin, FAK, and fyn kinase. In SCs cocultured with neurons, the association of paxillin with β1 integrin, FAK, and fyn kinase is higher in cocultures containing basal lamina than in cocultures lacking basal lamina.
Cytochalasin D inhibits FAK and paxillin tyrosine phosphorylation and interaction with β1 integrin
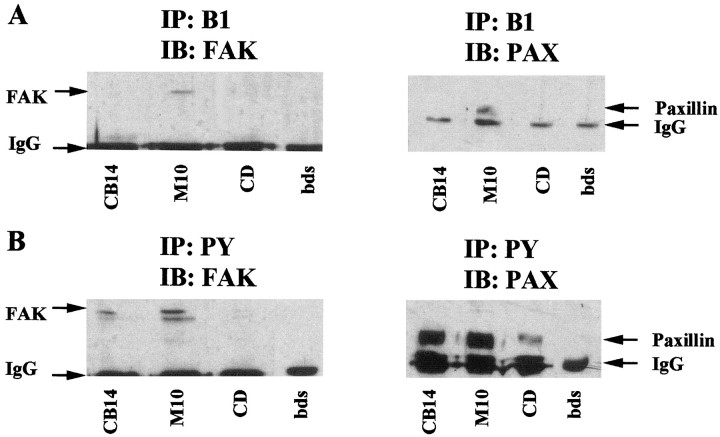
CD disrupts actin polymerization and inhibits FAK tyrosine phosphorylation and focal adhesion formation in other cells (Lipfert et al., 1992). When added to SC/N cocultures, CD inhibits myelin-specific gene expression and SC differentiation into myelinating cells (Fernandez-Valle et al., 1997). We investigated whether the inhibitory effect of 0.5 μg/ml CD on SC differentiation was attributable to disruption of β1 integrin interaction with FAK and paxillin. SC/N cocultures grown for 10 d in the absence of ascorbate and in ascorbate-containing medium with and without CD were extracted and immunoprecipitated with β1 integrin and phosphotyrosine antibodies and were immunoblotted for FAK and paxillin. FAK and paxillin are present in β1 immunoprecipitates of differentiating SC/N cocultures but are not detected in β1 integrin immunoprecipitates from undifferentiated and CD-treated SC/N cocultures (Fig.7A). Exposure to CD resulted in a significant decrease in phosphorylated FAK and paxillin compared with the levels present in undifferentiated and differentiating SC/N cocultures (Fig. 7B). The band below FAK is a nonspecific band recognized by the HRP-conjugated secondary antibody.
Fig. 7.
CD inhibits FAK and paxillin tyrosine phosphorylation and β1 integrin association. SC/N cocultures were grown for 10 d in CB medium, M medium with and without 0.5 μg/ml CD. Three hundred micrograms of total protein extracts for each culture condition were immunoprecipitated with β1 integrin and phosphotyrosine antibody. Samples were electrophoresed on 10% SDS-PAGE and were immunoblotted with FAK and paxillin antibody.A, Two hundred micrograms of β1 immunoprecipitate from each culture condition was immunoblotted with FAK antibody (left panel). Forty micrograms of β1 immunoprecipitate from each culture condition was immunoblotted with paxillin antibody (right panel). B, Thirty-five micrograms of phosphotyrosine immunoprecipitates from each culture condition was immunoblotted with FAK (left panel) and paxillin (right panel) antibodies. β1 integrin associates with FAK and paxillin in differentiating cocultures but not in CD-treated cocultures. FAK and paxillin tyrosine phosphorylation is lower in CD-treated cocultures than in differentiating cocultures.
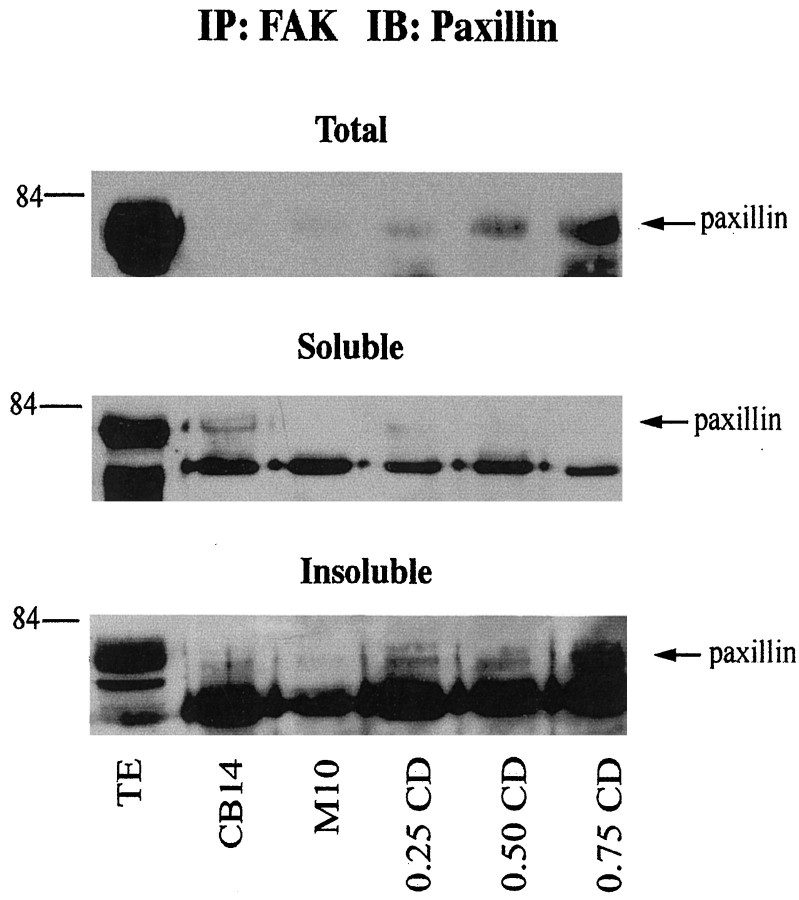
We also examined FAK and paxillin interactions in CD-treated SC/N cocultures. Extracts were prepared from soluble and insoluble fractions of undifferentiated, differentiating, and CD-treated cocultures and were immunoprecipitated for FAK and immunoblotted for paxillin (Fig.8). Increasing amounts of paxillin coimmunoprecipitates with FAK as the CD concentration increases. The FAK–paxillin complex is found in the insoluble, actin-rich fraction of CD-treated cultures.
Fig. 8.
CD causes an increase in the abundance of insoluble FAK–paxillin complexes in SC/N cocultures. SC/N cocultures were grown for 10 d in CB medium, serum and ascorbate medium without CD (M10), and with 0.25, 0.50, and 0.75 μg/ml CD. Equal protein from total, soluble, and insoluble culture extracts were immunoprecipitated with FAK antibody and were immunoblotted with paxillin antibody. TE lanes represent samples of the total, soluble, and insoluble extracts from M10 cultures. An increasing amount of paxillin coimmunoprecipitates with FAK as CD concentration increases in SC/N cocultures. The FAK–paxillin complex is found in the insoluble fraction of the cocultures.
DISCUSSION
We use a well characterized in vitro myelination model to examine the possibility that FAK participates in signaling from β1 integrins after basal lamina adhesion. We provide evidence for formation of a β1 integrin–FAK–paxillin–fyn kinase complex and for FAK and paxillin tyrosine phosphorylation in differentiating SC/N cocultures. Moreover, we demonstrate that CD, shown previously to inhibit SC differentiation, inhibits β1 integrin association with FAK and paxillin and tyrosine phosphorylation of both proteins. These findings support the role of FAK and paxillin as intermediates in β1 integrin-dependent signaling in SCs adhering to basal lamina and axons.
FAK and paxillin are tyrosine-phosphorylated in differentiating SC/N cocultures
FAK and paxillin are constitutively expressed by isolated SCs (Fig. 1), Therefore SCs do not require axon or basal lamina contact to synthesize FAK and paxillin. The expression pattern for FAK, paxillin, and β1 integrin are similar in SCs. Sensory neurons express β1 integrin, FAK, and paxillin at extremely low levels compared with SCs. Only fyn kinase is expressed by sensory neurons at comparable levels to SCs. Previous immunogold labeling studies indicate that sensory neurons do not increase β1 integrin and FAK expression after SC contact (Fernandez-Valle et al., 1994, 1998). Retinal and hippocampal neurons express FAK and proline-rich tyrosine kinase 2 (PYK2), an FAK family member, but the expression levels have not been compared with glial cells, as was done here (Burgaya et al., 1995; Grant et al., 1995).
There is a time-dependent increase in FAK and paxillin tyrosine phosphorylation in cocultures containing basal lamina until days 14–21. This is evident by comparing the levels of phosphorylated FAK and paxillin in CB lanes that represent cocultures lacking basal lamina with M4–M21 lanes that represent cocultures containing basal lamina (Fig. 2). Because cocultures are maintained for 7 d in ascorbate-free medium to allow SC proliferation in response to axons, the basal FAK phosphorylation level at the time ascorbate is added is shown in CB7 lanes. SCs begin to assemble basal lamina immediately after ascorbate supplementation and several days before initiating myelination, which increases slowly with time (Fernandez-Valle et al., 1998). The apparent decrease in FAK tyrosine phosphorylation at 21 d is consistent with the absence of FAK in β1 integrin immunoprecipitations at 21 d shown in Figure4A. The number of mature, myelinating SCs is likely greater than the number of newly differentiating SCs in 21-d-old cultures. Therefore, FAK and paxillin tyrosine phosphorylation begins during the time of rapid basal lamina formation and the onset of differentiation and may be involved in triggering differentiation rather than during active myelination or in maintenance of the myelinating phenotype.
FAK and paxillin are also phosphorylated in SCs grown in the presence of forskolin and pituitary extract used to stimulate SC proliferation before seeding on neuron cultures. Mitogens induce FAK and paxillin tyrosine phosphorylation in other cells (Rankin and Rozengurt, 1994) (for review, see Rozengurt, 1995). FAK is dephosphorylated upon SC contact with axons because very little FAK remains phosphorylated 4 d after seeding onto neurons (Fig. 2A,CB4). Paxillin appears to be slowly dephosphorylated because a small amount of phosphorylated paxillin is present in phosphotyrosine immunoprecipitates from 21-d-old cocultures lacking basal lamina (Fig. 2C). These results demonstrate that paxillin tyrosine phosphorylation increases with time in cocultures forming basal lamina and myelin and decreases with time in cocultures that lack basal lamina and myelin.
A β1 integrin–FAK–paxillin–fyn kinase complex forms in differentiating SCs
FAK and β1 integrin do not coimmunoprecipitate from SC-only culture extracts, although both proteins are expressed and small amounts of tyrosine-phosphorylated FAK is present (Figs. 1, 2).
Coimmunoprecipitation of β1 integrin and FAK has not been documented in isolated cells regardless of the substrate, ECM peptide, or integrin antibody used to activate cells. Here, β1 integrin and FAK coimmunoprecipitate only when SCs are cocultured with neurons under conditions that promote basal lamina assembly. β1 integrin mediates adhesion of newly forming basal lamina to the SC surface (Fernandez-Valle et al., 1994). It is likely that a β1 integrin–FAK association is detected because SCs bind native basal lamina and differentiate.
Several controls indicate that the β1 integrin–FAK coimmunoprecipitation is specific. First, the interaction is observed with two β1 integrin function-blocking antibodies, a hamster monoclonal antibody, and a well characterized rabbit antiserum (Figs.4, 5). Second, the amount of coimmunoprecipitated FAK increases with time for 14 d as the amount of differentiating SCs and phosphorylated FAK increases. Third, FAK is not immunoprecipitated by preimmune rabbit serum, normal hamster IgG, or protein A-Sepharose beads from differentiating SC/N cocultures (Fig. 5 and data not shown). Lastly, β1 integrin antibody does not coimmunoprecipitate vinculin (data not shown), and silver-stained gels reveal only immunoglobulin bands and a band migrating at 130 kDa, consistent with β1 integrin (data not shown). This indicates that focal adhesions are solubilized in our extraction buffer. In the reciprocal experiment, FAK immunoprecipitation, and β1 integrin immunoblotting, only weak positive results were obtained in 4- and 7-d-old differentiating cocultures (data not shown). This could be attributable to the small fraction of FAK molecules associated with β1 integrin (Fig.5B) or to epitope masking when FAK is bound to β1 integrin. The small amount of FAK observed in β1 integrin immunoprecipitates of 14-d-old undifferentiated SC/N cocultures is the greatest amount observed in five experiments (Fig. 4). It is likely that the FAK–β1 integrin complex derives from differentiating SCs in the cocultures. We have localized FAK to the plasma membrane adjacent to basal lamina patches in differentiating and myelinating SCs (Fernandez-Valle et al., 1998). The abundance of β1 integrin is higher in differentiating SCs than in myelinating SCs that preferentially express β4 integrin (Einheber et al., 1993; Feltri et al., 1994; Fernandez-Valle et al., 1994). Axons are infrequently labeled in immunogold studies, and neurons express very low levels of FAK and β1 integrin relative to SCs (Fig. 1).
Association of paxillin with β1 integrin, FAK, and fyn kinase was much greater in differentiating (M) cocultures compared with undifferentiated (CB) cocultures (Fig. 6). The associations observed in undifferentiated cocultures could result from residual interactions occurring in the mitogen-treated SCs added to neuron cultures. The complex observed in mitogen-treated (D10M) and untreated (D10) SCs disassembles when SCs contact axons for an extended period of time in the absence of basal lamina. The undifferentiated cocultures are typically 14-d-old.
The respective binding domains on FAK and paxillin have been identified. The NH2 terminus of paxillin contains LD domains involved in binding paxillin binding sequence 1 (PBS1) and PBS2 domains in the C terminus of FAK (Brown et al., 1996). Paxillin is proposed to target FAK to focal adhesions, but in other studies, FAK reaches focal adhesions in the absence of paxillin binding (Hildebrand et al., 1993; Tachibana et al., 1995). LIM domains in paxillin are involved in targeting paxillin to the plasma membrane, but the precise mechanism is unknown (Brown et al., 1998).
The actin cytoskeleton is essential for formation of a β1 integrin complex in differentiating SCs
We demonstrate that a subset of tyrosine-phosphorylated β1 integrin, FAK, and paxillin molecules binds the cytoskeleton in differentiating SC/N cocultures (Figs. 3, 8). Tyrosine-phosphorylated β1 integrin and paxillin are found exclusively in the insoluble fraction, whereas tyrosine-phosphorylated FAK is more abundant in the insoluble than the soluble fraction. β1 integrin is tyrosine-phosphorylated in v-src transformed cells (Hirst et al., 1986; Horvath et al., 1990). Soluble and insoluble paxillin differed in electrophoretic mobility, possibly because of tyrosine phosphorylation differences. Paxillin is also heavily phosphorylated on serine and threonine and is reported to shuttle from focal adhesions to a trans-Golgi–endosomal network (Brown et al., 1998;Norman et al., 1998).
CD inhibits FAK tyrosine phosphorylation and integrin-dependent signaling in other cells (Lipfert et al., 1992). CD, at 0.5 μg/ml, inhibits SC myelination in vitro but not adhesion to basal lamina or elongation (Fernandez-Valle et al., 1997). Here, we observe that CD inhibits tyrosine phosphorylation of FAK and paxillin and coimmunoprecipitation of FAK and paxillin with β1 integrin and causes an increase in the abundance of insoluble FAK–paxillin complexes (Figs. 7, 8). A small amount of FAK and paxillin coimmunoprecipitate from the soluble fraction of undifferentiated cocultures. This association is not detected when total culture extract is used (compareCB lanes in total and soluble fractions). This is likely a residual association occurring in the mitogen-treated SCs that are added to neuron cultures (Fig. 6C,D). An FAK–paxillin complex appears to become insoluble in differentiating (M) cocultures, although only low levels are observed compared with the amount in CD-treated cocultures. It is possible that FAK and paxillin require F-actin for targeting to the plasma membrane at developing focal adhesions. Src-related kinases bind both FAK and paxillin, and targeting of src kinase to the cell periphery requires F-actin (Fincham et al., 1996).
Our work, interpreted in the context of what is known about focal adhesion assembly, suggests that SC adhesion to nascent basal lamina by β1 integrins leads to F-actin-dependent integrin aggregation and recruitment of FAK and paxillin. FAK autophosphorylates and recruits fyn kinase, which could bind and phosphorylate paxillin and activate further downstream signaling events. The precise function of this complex during SC differentiation is unknown. Techniques are being developed to produce stable cell lines of FAK null SCs for use in SC/N cocultures to further elucidate this pathway.
Footnotes
This work was supported by Public Health Service Grant NSRO1–34499 to C.F.V. and by the State of Florida. We thank Dr. Sal Carbonetto for the generous antibody gift, Dr. Patrick Wood for NGF, Dr. Ric Devon for human placenta serum, and Drs. Naomi Kleitman and Patrick Wood for critical comments on this manuscript. The paxillin work completed by D.B. constitutes a Masters thesis.
Correspondence should be addressed to Cristina Fernandez-Valle, Department of Molecular Biology and Microbiology, 12722 Research Parkway, University of Central Florida, Orlando, FL 32826. E-mail:cfernand@pegasus.cc.ucf.edu.
REFERENCES
- 1.Aumailley M, Nurcombe V, Edgar D, Paulson M, Timpl R. The cellular interactions of laminin fragments. J Biol Chem. 1987;262:11532–11538. [PubMed] [Google Scholar]
- 2.Bellis SL, Miller JT, Turner CE. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J Biol Chem. 1995;270:17437–17441. doi: 10.1074/jbc.270.29.17437. [DOI] [PubMed] [Google Scholar]
- 3.Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Rev. 1979;165:105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- 4.Brown MC, Perrotta JA, Turner CE. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J Cell Biol. 1996;135:1109–1123. doi: 10.1083/jcb.135.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown MC, Perrotta JA, Turner CE. Serine and threonine phosphorylation of the paxillin LIM domains regulates paxillin focal adhesion localization and cell adhesion to fibronectin. Mol Biol Cell. 1998;9:1803–1816. doi: 10.1091/mbc.9.7.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunge RP, Fernandez-Valle C. Basic biology of the Schwann cell. In: Kettenmen H, Ransom B, editors. Neuralglia cells. Oxford UP; New York: 1995. pp. 44–57. [Google Scholar]
- 7.Burgaya F, Menegon A, Menegoz M, Valtora F, Girault JA. Focal adhesion kinase in the rat central nervous system. Eur J Neurosci. 1995;7:1810–1821. doi: 10.1111/j.1460-9568.1995.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 8.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility and signalling. Ann Rev Cell Dev Biol. 1996;12:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 9.Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen HC, Chan PC, Tang MJ, Cheng CH, Chang TJ. Tyrosine phosphorylation of focal adhesion kinase stimulated by hepatocyte growth factor leads to mitogen-activate protein kinase activation. J Biol Chem. 1998;273:25777–25782. doi: 10.1074/jbc.273.40.25777. [DOI] [PubMed] [Google Scholar]
- 11.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 12.Einheber S, Milner TA, Giancotti F, Salzer JL. Axonal regulation of Schwann cell integrin expression suggests a role for α6β4 in myelination. J Cell Biol. 1993;123:1223–1236. doi: 10.1083/jcb.123.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eldridge CF, Bunge MB, Bunge RP, Wood PM. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol. 1987;105:1023–1034. doi: 10.1083/jcb.105.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eldridge CF, Bunge MB, Bunge RP. Differentiation of axon related Schwann cells in vitro. II. Control of myelin formation by basal lamina. J Neurosci. 1989;9:625–638. doi: 10.1523/JNEUROSCI.09-02-00625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feltri ML, Scherer SS, Nemni R, Kamholz J, Vogelbacker H, Scott MO, Canal N, Quaranta V, Wrabetz L. Beta 4 integrin expression in myelinating Schwann cells is polarized, developmentally regulated and axonally dependent. Development. 1994;120:1287–1301. doi: 10.1242/dev.120.5.1287. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Valle C, Fregien N, Wood PM, Bunge MB. Expression of the protein zero myelin gene in axon-related Schwann cells is linked to basal lamina formation. Development. 1993;119:867–880. doi: 10.1242/dev.119.3.867. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Valle C, Gwynn L, Wood PM, Carbonetto S, Bunge MB. Anti-β1 integrin antibody inhibits Schwann cell myelination. J Neurobiol. 1994;25:1207–1226. doi: 10.1002/neu.480251004. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Valle C, Gorman D, Gomez AM, Bunge MB. Actin plays a role in both changes in cell shape and gene-expression associated with Schwann cell myelination. J Neurosci. 1997;17:241–250. doi: 10.1523/JNEUROSCI.17-01-00241.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Valle C, Wood PM, Bunge MB. Localization of focal adhesion kinase in differentiating Schwann cell/neuron cultures. Microsc Res Tech. 1998;41:416–430. doi: 10.1002/(SICI)1097-0029(19980601)41:5<416::AID-JEMT8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Fincham VJ, Unlu M, Brunton VG, Pitts JD, Wyke JA, Frame MC. Translocation of src kinase to the cell periphery is mediated by the actin cytoskeleton under the control of the rho family of small G proteins. J Cell Biol. 1996;135:1551–1564. doi: 10.1083/jcb.135.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant SGN, Karl KA, Kiebler MA, Kandel ER. Focal adhesion kinase in the brain: novel subcellular localization and specific regulation by fyn tyrosine kinase in mutant mice. Gene Dev. 1995;9:1909–1921. doi: 10.1101/gad.9.15.1909. [DOI] [PubMed] [Google Scholar]
- 22.Guan JL. Role of focal adhesion kinase in integrin signaling. Int J Biochem Cell Biol. 1997;29:1085–1096. doi: 10.1016/s1357-2725(97)00051-4. [DOI] [PubMed] [Google Scholar]
- 23.Guenard V, Gwynn LA, Wood PM. Transforming growth factor-β blocks myelination but not ensheathment of axons by Schwann cells in vitro. J Neuroscience. 1995;15:419–428. doi: 10.1523/JNEUROSCI.15-01-00419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildebrand JD, Schaller MD, Parsons JT. Identification of sequences required for the efficient localization of the focal adhesion kinase pp125FAK to cellular focal adhesions. J Cell Biol. 1993;123:993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirst R, Horwitz A, Buck C, Rohrschneider LR. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci USA. 1986;83:6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horvath AR, Elmore MA, Kellie S. Differential tyrosine-specific phosphorylation of integrin in Rous sarcoma virus transformed cells with differing transformed phenotypes. Oncogene. 1990;5:1349–1357. [PubMed] [Google Scholar]
- 27.Hynes RO. Integrins versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 28.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, Aizawa S. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 29.Ilic D, Damsky C, Yamamoto T. Focal adhesion kinase: at the crossroads of signal transduction. J Cell Science. 1997;110:401–407. doi: 10.1242/jcs.110.4.401. [DOI] [PubMed] [Google Scholar]
- 30.Kleitman N, Wood PM, Bunge RP. Tissue culture methods for the study of myelination. In: Banker G, Goslin K, editors. Culturing nerve cells. MIT; Cambridge, MA: 1991. pp. 337–377. [Google Scholar]
- 31.Lin CQ, Bissell MJ. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- 32.Lipfert L, Haimovich B, Schaller MD, Cobb BS, Parsons JT, Brugge JT. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukashev ME, Werb Z. ECM signaling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8:437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- 34.Marchionni MA, Goodearl ADJ, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, Wroblewski D, Lynch C, Baldassare M, Hiles I, Davis JB, Hsuan H, Totty NF, Otsu M, McBurney RN, Waterfield MD, Stroobant P, Gwynne D. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:791–805. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 35.Miyamoto S, Teramoto H, Coso O, Gutkind J, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signalling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrissey TK, Levi AD, Nuijens A, Sliwkowski MX, Bunge RP. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc Natl Acad Sci USA. 1995;92:1431–1435. doi: 10.1073/pnas.92.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moya F, Bunge MB, Bunge RP. Schwann cells proliferate but fail to differentiate in defined medium. Proc Natl Acad Sci USA. 1980;77:6902–6906. doi: 10.1073/pnas.77.11.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller SC, Yeh Y, Chen W-T. Tyrosine phosphorylation of membrane proteins mediates cellular invasion by transformed cells. J Cell Biol. 1992;119:1309–1325. doi: 10.1083/jcb.119.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niessen CM, Cremona O, Daams H, Ferraresi S, Sonnenberg A, Marchinsio PC. Expression of the integrin β6β4 in peripheral nerves: localization in Schwann cells and perineural cells and different variants of the β4 subunit. J Cell Sci. 1994;107:543–552. doi: 10.1242/jcs.107.2.543. [DOI] [PubMed] [Google Scholar]
- 40.Norman JC, Jones D, Barry ST, Holt MR, Cockcroft S, Critchley DR. ARF1 mediates paxillin recruitment to focal adhesions and potentiates Rho-stimulated stress fiber formation in intact and permeabilized Swiss 3T3 fibroblasts. J Cell Biol. 1998;143:1981–1995. doi: 10.1083/jcb.143.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plopper G, Ingber DE. Rapid induction and isolation of focal adhesion complexes. Biochem Biophys Res Commun. 1993;2:571–578. doi: 10.1006/bbrc.1993.1662. [DOI] [PubMed] [Google Scholar]
- 42.Rankin S, Rozengurt E. Platelet-derived growth factor modulation of focal adhesion kinase (p125FAK) and paxillin tyrosine phosphorylation in Swiss 3T3 cells. J Biol Chem. 1994;269:704–710. [PubMed] [Google Scholar]
- 43.Richardson A, Parsons JT. Signal transduction through integrins: a central role for focal adhesion kinase? BioEssays. 1995;17:229–236. doi: 10.1002/bies.950170309. [DOI] [PubMed] [Google Scholar]
- 44.Ridyard M, Sanders EJ. Potential roles for focal adhesion kinase in development. Anat Embryol. 1999;199:1–7. doi: 10.1007/s004290050203. [DOI] [PubMed] [Google Scholar]
- 45.Rozengurt E. Convergent signaling in the action of integrins neuropeptides growth factors and oncogenes. Cancer Surv. 1995;24:81–96. [PubMed] [Google Scholar]
- 46.Salgia R, Li JL, Lo SH, Brunkhorst B, Kansas GS, Sobhany ES, Sun Y, Pisick E, Hallek M, Ernst T, Tantravahi R, Chen LB, Griffin JD. Molecular cloning of human paxillin, a focal adhesion protein phosphorylated by P210BCR/ABL. J Biol Chem. 1995;270:5039–5047. doi: 10.1074/jbc.270.10.5039. [DOI] [PubMed] [Google Scholar]
- 47.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK, a structurally distinct protein tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaller MD, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking β integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlaepfer DD, Hauck CR, Sieg JD. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 50.Siciliano JC, Toutant M, Derkinderen P, Sasaki T, Girault JA. Differential regulation of proline-rich tyrosine kinase 2/cell adhesion kinase β (PYK2/CAKβ) and pp125FAK by glutamate and depolarization in rat hippocampus. J Biol Chem. 1996;271:28942–28946. doi: 10.1074/jbc.271.46.28942. [DOI] [PubMed] [Google Scholar]
- 51.Streuli CH, Bailey N, Bissell M. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell–cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tachibana K, Sato T, D'Avirro N, Morimoto C. Direct association of pp125FAK with paxillin, the focal adhesion-targeting mechanism of pp125FAK. J Exp Med. 1995;182:1089–1100. doi: 10.1084/jem.182.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tawil NJ, Houde M, Blacher R, Esch F, Reichardt LF, Turner DC, Carbonetto S. α1β1 integrin heterodimer functions as a dual laminin/collagen receptor in neural cells. Biochemistry. 1990;29:6540–6544. doi: 10.1021/bi00479a028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tawil NJ, Wilson P, Carbonetto S. Integrins in point contacts mediate cell spreading: factors that regulate integrin accumulation in point contacts vs. focal contacts. J Cell Biol. 1993;120:261–271. doi: 10.1083/jcb.120.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tong X, Salgia R, Li JL, Griffin JD, Howley PM. The bovine papillomavirus E6 protein binds to the LD motif repeats of paxillin and blocks its interaction with vinculin and the focal adhesion kinase. J Biol Chem. 1997;272:33373–33376. doi: 10.1074/jbc.272.52.33373. [DOI] [PubMed] [Google Scholar]
- 56.Turner C. Molecules in focus: paxillin. Int J Biochem Cell Biol. 1998;30:955–959. doi: 10.1016/s1357-2725(98)00062-4. [DOI] [PubMed] [Google Scholar]
- 57.Turner CE. Paxillin is a major phosphotyrosine-containing protein during embryonic development. J Cell Biol. 1991;115:201–207. doi: 10.1083/jcb.115.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turner CE, Miller JT. Primary sequence of paxillin contains putative SH2 and SH3 domain binding motifs and multiple LIM domains: identification of a vinculin and pp125Fak-binding region. J Cell Sci. 1994;107:1583–1591. doi: 10.1242/jcs.107.6.1583. [DOI] [PubMed] [Google Scholar]
- 59.Werb Z, Tremble PM, Behrendsen O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wood PM, Bunge RP. Evidence that sensory axons are mitogenic for Schwann cells. Nature. 1975;256:662–664. doi: 10.1038/256662a0. [DOI] [PubMed] [Google Scholar]
- 61.Woodley DT, Rao CN, Hassell JR, Liotta LA, Martin GR, Kleinman HK. Interactions of basement membrane components. Biochem Biophys Acta. 1983;761:278–283. doi: 10.1016/0304-4165(83)90077-6. [DOI] [PubMed] [Google Scholar]
- 62.Zachary I, Rozengurt E. Focal adhesion kinase (pp125FAK): a point of convergence in the action of neuropeptides, integrins and oncogenes. Cell. 1992;71:891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]