Abstract
In addition to their involvement in transsynaptic communication in the adult nervous system, neurotransmitters also participate in many developmental events, such as neurite initiation and outgrowth. Although growth cones can release transmitters and are themselves sensitive to exogenously applied neurotransmitters, a direct causal relationship between the release of transmitter from one growth cone and its effect on another has not yet been demonstrated. In this study, we provide evidence that dopamine release from the growth cones of an identified Lymnaea neuron, right pedal dorsal 1 (RPeD1), differentially regulates the growth cone behavior of itsin vivo target and nontarget neurons in vitro. In coculture, RPeD1 growth cones enhanced the rate of growth cone advance from target cells and synaptic connections developed immediately after contact. In contrast, RPeD1 growth cones not only inhibited the rate of growth cone advance from nontarget cells but they also induced growth cone collapse. Using a “sniffer cell” approach, we demonstrated that both RPeD1 growth cones and somata released dopamine, which can be detected at a distance of several hundred micrometers. RPeD1 somata were used to demonstrate that spontaneous release of dopamine also acted as a chemoattractant for target growth cones but as a chemorepellent for nontarget growth cones. These effects were mimicked by exogenous dopamine application, and both RPeD1 growth cone and soma-induced effects were also blocked in the presence of dopamine receptor antagonists. This study emphasizes the importance of transmitter–receptor interactions between growth cones in target cell selection.
Keywords: transmitter, dopamine, growth cone, culture, target selection, regeneration, neurite outgrowth, mollusc
Neuronal growth cones located at the tips of developing neurites are responsible for axonal pathfinding, target cell selection, and specific synapse formation (Lockerbie, 1987;Kater and Shibata, 1994). A variety of molecules assist growth cones in their navigational tasks to ensure that developing neurites are guided toward their appropriate synaptic targets and that specific synapses are formed. These guidance cues include cell surface (Letourneau, 1992) and extracellular matrix (Kuhn et al., 1995) molecules, as well as various diffusible factors, such as neurotrophins (Phelan et al., 1992) and neurotransmitters (for review, see Lipton and Kater, 1989; Lauder, 1993). Neurotransmitter-induced effects can be chemoattractive, resulting in growth cone turning toward the transmitter source (Zheng et al., 1994, 1996), or inhibitory and chemorepulsive, causing growth cone collapse and/or turning away of the growth cone (Haydon et al., 1984; Lankford et al., 1987). In addition to their responsiveness to exogenously applied transmitters, growth cones also contain and release transmitters (Hume et al., 1983; Young and Poo, 1983; Sun and Poo, 1987) before establishing synaptic contacts (Taylor et al., 1990). These findings thus suggest that transmitter–receptor interactions between neuronal growth cones may play a nonsynaptic role during axonal pathfinding and/or target cell selection. However, a direct causal relationship between the release of transmitter from one growth cone and its direct effect on another has not yet been demonstrated.
Because interactions between growth cones of defined presynaptic and postsynaptic neurons are often difficult to investigate in the intact CNS, we have therefore opted to use in vitro isolated neurons of Lymnaea stagnalis. Using identified neurons, we tested whether the specificity of target cell selection in vitro involves transmitter–receptor interactions between growth cones. An identified interneuron, right pedal dorsal 1 (RPeD1) synthesizes (Cottrell et al., 1979), contains (Magoski et al., 1995), and releases (Syed et al., 1996) the transmitter dopamine and makes monosynaptic connections with identified target neurons in the visceral ganglia [visceral I (VI) and visceral J (VJ) cells]. A target cell is defined as a neuron with which RPeD1 makes synaptic contactsin vivo. RPeD1 does not however make synaptic contacts with “nontarget” cells, such as the right parietal B and visceral F cells, although these cells are located near the target cells and have processes in the vicinity of the neurites of RPeD1. When isolated from the central ring ganglia and plated in appropriate cell culture conditions, Lymnaea neurons exhibit robust sprouting, and the pattern of in vivo synaptogenesis is recapitulated in vitro (Syed et al., 1990; Syed and Spencer, 1994). When cultured together, RPeD1 reforms appropriate synaptic connections with target but not nontarget cells (Spencer et al., 1998). Using simultaneous time-lapse video imaging and intracellular recording techniques, we provide the first direct evidence that dopamine release from RPeD1 growth cones exerts differential growth regulatory effects on target and nontarget growth cones, effects that are likely mediated by different dopamine receptors. Transmitter–receptor interactions between developing growth cones may thus play an important role in determining target cell selection that leads to cell–cell recognition and specific synapse formation.
MATERIALS AND METHODS
Cell isolation. Specimens of Lymnaea stagnalis were laboratory bred, kept in well aerated, artificial pond water, and fed on lettuce. All cell culture procedures were performed as described previously (Syed et al., 1990). Briefly, the central ring ganglia were isolated under sterile conditions, and after a number of antibiotic washes and subsequent enzymatic treatment, pinned down in a dissection dish and bathed in high osmolarity defined medium (DM) (Ridgway et al., 1991). The connective tissue sheath surrounding the ganglia was removed using a pair of fine forceps, and identified somata were individually extracted by applying gentle suction through a fire-polished pipette. The isolated cells were either plated directly on poly-l-lysine-coated dishes containing brain-conditioned medium (CM) (Wong et al., 1981) or maintained overnight in hemolymph-coated dishes containing CM (to prevent neuronal adhesion) and subsequently plated on poly-l-lysine-coated dishes.
Electrophysiology. For intracellular recordings, conventional electrophysiological techniques were used as described previously (Syed et al., 1990). Glass microelectrodes (resistance of 20–40 MΩ) were filled with a saturated solution of potassium sulfate (K2SO4), and neurons were impaled using Narishige (Tokyo, Japan) micromanipulators (models M202 and M204). The electrophysiological signals were amplified (Neuro Data amplifier, model IR-283), displayed on a digital storage oscilloscope (Philips PM 3394), and recorded on a Gould chart recorder (model TA240S; Gould Instruments, Valley View, OH).
Chemicals. Dopamine hydrochloride (10−5m; Sigma, Toronto, Canada) was dissolved in normal saline (containing 1% Na-metabisulfate as an antioxidant) and pressure ejected [model 5242 (Eppendorf Scientific, Westbury, NY); 10–30 sec pulses, 6–10 psi, pipette tip diameter of 2–5 μm] directly onto the individual growth cones. The D1 antagonist R(+)-SCH-23390 HCl (Research Biochemicals, Natick, MA) and the D2antagonist sulpiride (Research Biochemicals) were dissolved in distilled water to a stock concentration of 10−2m. Stock solutions were further diluted in either saline or DM and added to the bath for a final concentration of 10−4 or 10−5m.
Dopamine detection from RPeD1 growth cones and somata. To detect dopamine release from RPeD1 somata and growth cones, individually isolated RPeD1 neurons were plated in CM and left overnight to extend neurites. Freshly isolated target somata were subsequently introduced to the culture dish and manipulated in close proximity to either the growth cones or soma of RPeD1 (distance ranging from 50 to 500 μm). Both neurons were impaled with sharp intracellular electrodes, and RPeD1 was injected with depolarizing current to induce spiking activity (10–30 action potentials). Nonsynaptic, electrophysiological responses were then recorded in the target somata and analyzed.
Growth cone–growth cone interactions. Either target or nontarget neurons were plated in close proximity to RPeD1 cells. After outgrowth, the behavior of both target and nontarget growth cones was monitored continuously for several hours, as they reached within a distance of 500 μm from the RPeD1 growth cones. To monitor the rate of growth cone advance in the presence of the dopamine receptor antagonists, these were added to the bath (final concentration of 10−5m) before the target or nontarget growth cones reached the vicinity (500 μm) of the RPeD1 growth cones. The growth cone behavior was monitored for at least 8 hr after the addition of antagonists or until contact with the RPeD1 growth cones was made.
RPeD1 somata as the source of dopamine release. Because both RPeD1 somata and growth cones generated very similar nonsynaptic responses in the target “sniffer cells,” RPeD1 somata were used as a source of spontaneous dopamine release in some parts of the study. Specifically, individually isolated RPeD1 somata were maintained overnight in hemolymph-coated dishes containing CM. This treatment prevented neuronal adhesion to the substrate and resulted in complete resorption of the axon stump. On the subsequent day, the spherical somata of RPeD1 were introduced to the poly-l-lysine-coated culture dishes containing either sprouted target or nontarget cells. A sharp intracellular glass micropipette (dipped in a solution of 2% poly-l-lysine) was used to manipulate an RPeD1 soma (the soma adhered to the pipette immediately on contact and so no impalement was necessary) in close proximity of a growth cone. To determine whether spontaneous dopamine release from an unstimulated RPeD1 soma could induce growth cone turning, RPeD1 was placed within 50–200 μm of either target or nontarget cell growth cones. Growth cone behavior was monitored for 90–180 min for each growth cone. To test for the specificity of growth cone turning either toward or away from the RPeD1 somata, growth cone behavior was also monitored in the presence of dopamine receptor antagonists, which were added before the manipulation of the RPeD1 somata near to the growth cones.
Data collection and statistical analysis. Images were captured using a Hitachi (Tokyo, Japan) CCD camera and were recorded on a Panasonic (Secaucas, NJ) time-lapse video recorder (model AG-6720A). Thirty-five millimeter photographs were taken using a Contax (Toronto, Canada) camera mounted on a Zeiss (Oberkochen, Germany) Axiovert 135 inverted microscope. Growth cones were visually scored for a collapsed morphology, and their rate of advance and turning angles were measured using either the NIH Image program or Adobe Photoshop (Adobe Systems, San Jose, CA). Data were analyzed using Student's t test, and values were expressed as either mean ± SEM or as a percentage.
RESULTS
Growth cone interactions between RPeD1 and target neurons resulted in synapse formation
To monitor growth cone interactions between presynaptic and postsynaptic neurons, individually isolated somata of RPeD1 and the target VI/VJ cells were plated in close proximity to each other. Time-lapse recordings of the growth cone interactions were made, and in some instances, at the same time as intracellular recordings from the somata. The motility and the rate of target growth cone advance were monitored, in either the presence or absence of approaching RPeD1 growth cones.
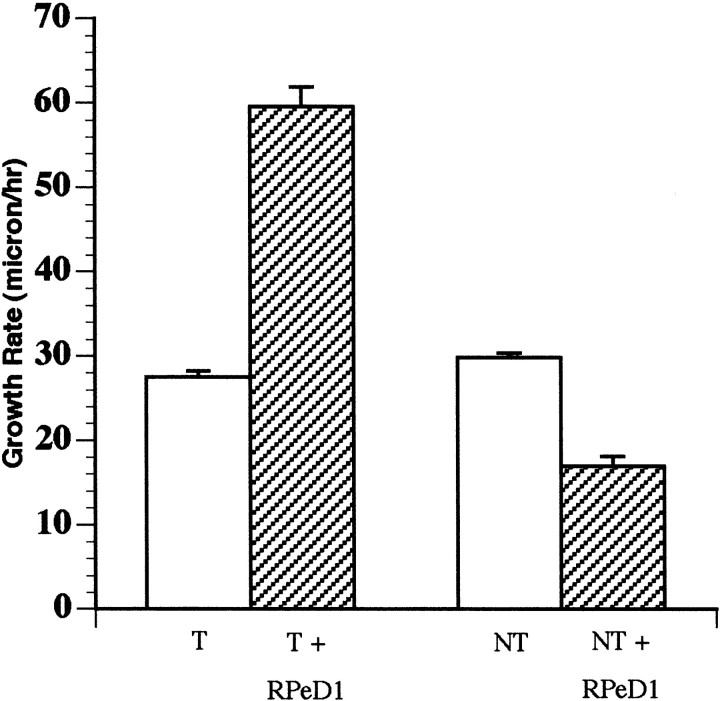
We found that when cultured alone (in the absence of RPeD1), the target cell growth cones advanced at a rate of 27.5 ± 0.7 μm/hr (n = 20) (Fig. 1). However, as target growth cones approached RPeD1 growth cones in coculture (within 300–500 μm), the growth rate increased significantly to 59.5 ± 2.3 μm/hr (n = 34;t test; df = 52; p < 0.0001). This increase in the rate of growth cone advance was observed before any physical contact between the presynaptic and postsynaptic growth cones. In 88% (n = 30 of 34) of cases, the target cell growth cones eventually made physical contact with the RPeD1 growth cones and grew along its neurites (Fig.2A–E). The remaining 12% of target growth cones, on the other hand, either exhibited collapse or turned away from the RPeD1 growth cones (n= 4 of 34).
Fig. 1.
Target and nontarget growth cone motility rates were significantly different when cocultured with RPeD1. Bar graph showing that the growth cone motility rate was significantly increased (p < 0.0001) when target cells were cocultured with RPeD1 (T + RPeD1) compared with when cultured alone (T). The growth cone motility rate, however, was significantly reduced (p< 0.0001) when nontarget cells were cocultured with RPeD1 (NT + RPeD1) compared with when cultured alone (NT). There was no significant difference (p > 0.05) in the growth cone motility rate of target and nontarget cell growth cones cultured alone in the absence of RPeD1.
Fig. 2.
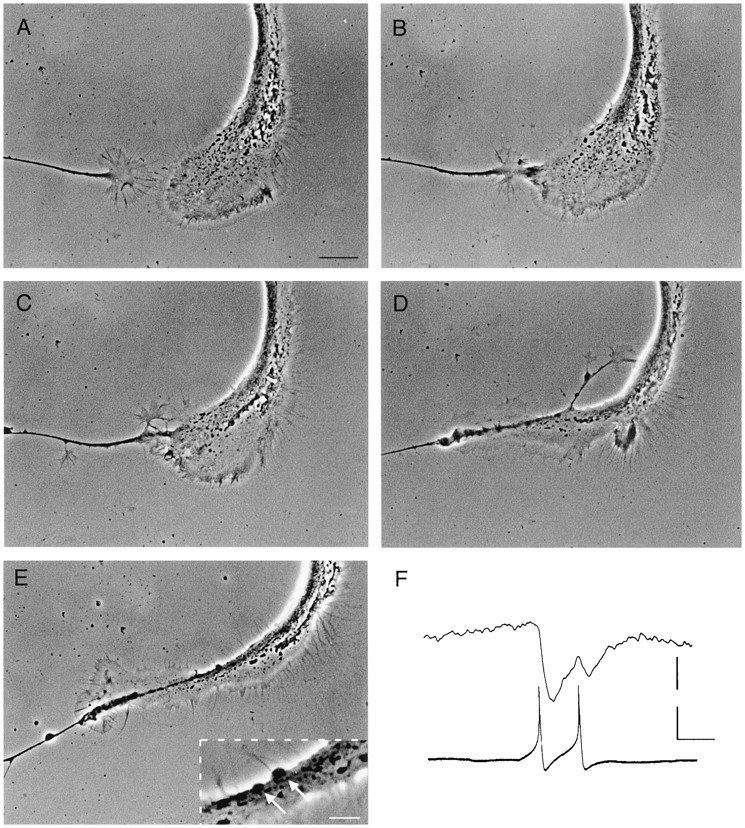
Growth cone interactions between RPeD1 and a target cell resulted in synapse formation. A, A target cell growth cone (left) approached a large RPeD1 growth cone (right), and physical contact followed (B). The target cell growth cone continued to grow along the RPeD1 growth cone (C) and neurite (D), eventually resulting in the appearance of varicosities (E). Inset inE is a magnified area showing the varicosities (arrows). Scale bars: A–E, 20 μm;inset in E, 5 μm. F, Spontaneous action potentials in RPeD1 induced 1:1 IPSPs in the target cell. Calibration: 15 and 20 mV, 200 msec.
To determine whether growth cone interactions between RPeD1 and the target cell growth cones resulted in synapse formation, simultaneous intracellular recordings were made, both before and after growth cone contacts. Electrical stimulation of RPeD1 did not produce a detectable electrophysiological response in the postsynaptic target cell, either before or immediately after physical contact (n = 7). However, 50–60 min after contact, both spontaneous and electrically induced action potentials in RPeD1 produced 1:1 IPSPs in all of the target cells tested (n = 13) (Fig.2F). The RPeD1-induced IPSPs were observed concomitant with the appearance of synaptic varicosities at the sites of cell–cell contact (Fig. 2E). The above data demonstrate that RPeD1 growth cones not only increased the rate of target cell growth cone advance from a distance but that these growth cone interactions also resulted in functional chemical synapses between the cells.
RPeD1 growth cones induced the collapse of nontarget cell growth cones in vitro
In vivo, RPeD1 neurites are in close proximity to (Magoski and Bulloch, 1997), but do not form chemical synapses with, many cells (nontargets) in the right parietal and visceral ganglia. Furthermore, when isolated in vitro, RPeD1 does not establish synaptic connections with these cells (Syed and Spencer, 1994). To determine whether target cell selection by RPeD1 is determined in part by growth cone interactions, the nontarget cells were cultured in vitro, and their growth cone behavior was monitored. We found that when cultured alone, the nontarget cell growth cones advanced at a rate of 29.8 ± 0.6 μm/hr (n = 24) (Fig. 1). In coculture experiments however, when the nontarget cell growth cones reached the vicinity of the growth cones of RPeD1 (300–500 μm), their rate of advance was significantly reduced to 16.9 ± 1.2 μm/hr (n = 23; t test; p < 0.0001; df = 45).
On approach to the RPeD1 growth cones, the nontarget cell growth cones collapsed, even before physical contact (n = 37) (Fig.3). In instances in which nontarget cell growth cones did make filopodial contact with the RPeD1 growth cones, they were also induced to collapse (n = 23). However, when physical contacts between RPeD1 and the nontarget growth cones did eventually occur, no electrophysiological detectable synaptic potentials were recorded from the cells, nor were any varicosities observed at the points of contact (data not shown).
Fig. 3.
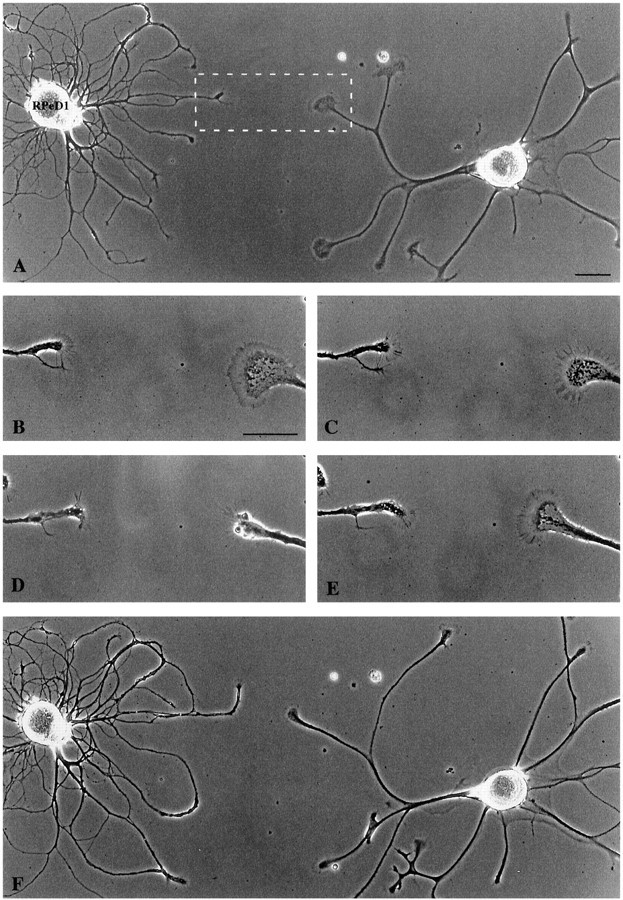
RPeD1 growth cones induced the collapse of a nontarget cell growth cone. A, RPeD1 and a nontarget cell were plated in close proximity and extended neurites over a 24 hr period. Scale bar, 75 μm. The boxed area represents the magnified images in B–E. The nontarget growth cone approached within ∼60 μm of the RPeD1 growth (B, 15 min) and collapsed (C, 30 min). Scale bar, 30 μm. The nontarget cell growth cone was fully collapsed at 60 min (D) and recovered over the next 45 min (E). F, Lower magnification demonstrating that the nontarget growth cone did not contact RPeD1.
Growth cone collapse was often observed before contact with growth cones of RPeD1. We therefore hypothesized that these effects may involve a diffusible substance released from the growth cones of RPeD1. Because RPeD1 growth cones have been shown previously to release dopamine (Syed et al., 1996), we thus postulated that the RPeD1 growth cone-induced effects on target and nontarget cell growth cones were mediated by dopamine. To test this hypothesis directly, we first sought to determine whether dopamine release from the RPeD1 growth cones could be detected at a distance of 50–500 μm.
RPeD1 growth cones and somata released dopamine
To test whether RPeD1 growth cones could release dopamine in cell culture, we first sought to determine whether RPeD1 was spontaneously active and, if so, whether electrical activity would result in transmitter release. RPeD1 neurons were cultured in CM (either as single cells or cocultured with target and nontarget cells), and the intracellular activity of RPeD1 was monitored for several hours. We found that RPeD1 fired spontaneous and prolonged bursts of action potentials throughout the recording period (mean burst duration, 25.8 ± 9.4 sec; interburst interval, 23.7 ± 3.9 sec; number of action potentials, 36.2 ± 11.4; n = 6) (Fig.4A). To test whether action potentials would result in transmitter release, a previously established sniffer cell assay was used (Sun and Poo, 1987; Syed et al., 1996). Specifically, simultaneous intracellular recordings were made from RPeD1 and a target soma, which was held at a distance ranging from 50 to 500 μm away (Fig. 4B). Electrical stimulation of the RPeD1 soma (to produce fewer action potentials than those observed during spontaneously occurring discharges), induced characteristic nonsynaptic, inhibitory responses in the sniffer cells (Fig. 4C). These nonsynaptic responses were reliably detected by the sniffer cell held up to a distance of 300 μm away from either the RPeD1 growth cone (n = 15) or its soma (n = 7). These nonsynaptic responses were completely blocked by the D2 receptor antagonist sulpiride (10−5m;n = 6 of 7) (Fig. 4D) but were unaffected in the presence of the D1 receptor antagonist R(+)-SCH-23390 (10−5m; n = 7; data not shown). These data strongly suggest that both the RPeD1 soma and growth cones released dopamine, which could be detected by the sniffer cell at a distance of several hundred micrometers away.
Fig. 4.
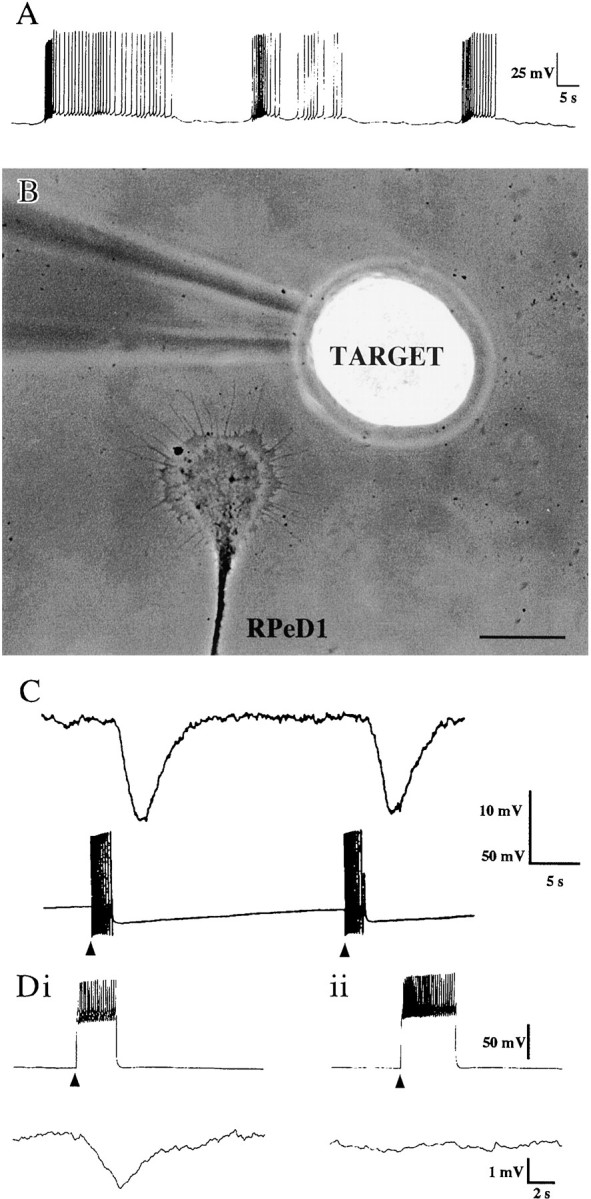
Dopamine release from the RPeD1 growth cone was detected by the sniffer target soma. A, An RPeD1 soma that was plated in CM was spontaneously active and fired bursts of action potentials. B, A target cell soma was juxtaposed near an RPeD1 growth cone. Scale bar, 50 μm. C, After electrical stimulation (arrowheads) of RPeD1 (bottom trace), a compound IPSP was recorded in the target cell (top trace). Di, The stimulation (arrowhead) of RPeD1 (top trace) resulted in a compound IPSP in a target cell (bottom trace). Dii, In the presence of the D2 antagonist sulpiride (10−5m), there was no detectable response in the target cell after RPeD1 stimulation (arrowhead).
RPeD1-induced effects on target and nontarget growth cones were mimicked by exogenous application of dopamine
To determine whether RPeD1 growth cone-induced effects on target cell growth cones were mimicked by dopamine, it was pressure applied to the target cell growth cones in a pulsatile manner (10–30 sec pulses,10−5m). First, the effect of exogenous dopamine was investigated on the growth rate of the target growth cones. When the pressure pipette containing dopamine was placed in front of an advancing growth cone, there was a 42.4% increase in the growth rate, but this increase was not significant (p > 0.05; n = 9 growth cones from 5 cells). Next, we positioned the pipette at an angle to the advancing growth cone to monitor any changes in the outgrowth direction of the target growth cones. We found that in six of the seven growth cones tested, the exogenous source of dopamine induced positive turning toward the pipette over the monitoring period of 90–120 min (mean angle of turning, 32.8 ± 7.0°) (Fig.5A–C). Although exogenous dopamine did not significantly enhance the growth rate, it did nevertheless induce attractive turning of the target growth cones toward the source of dopamine.
Fig. 5.
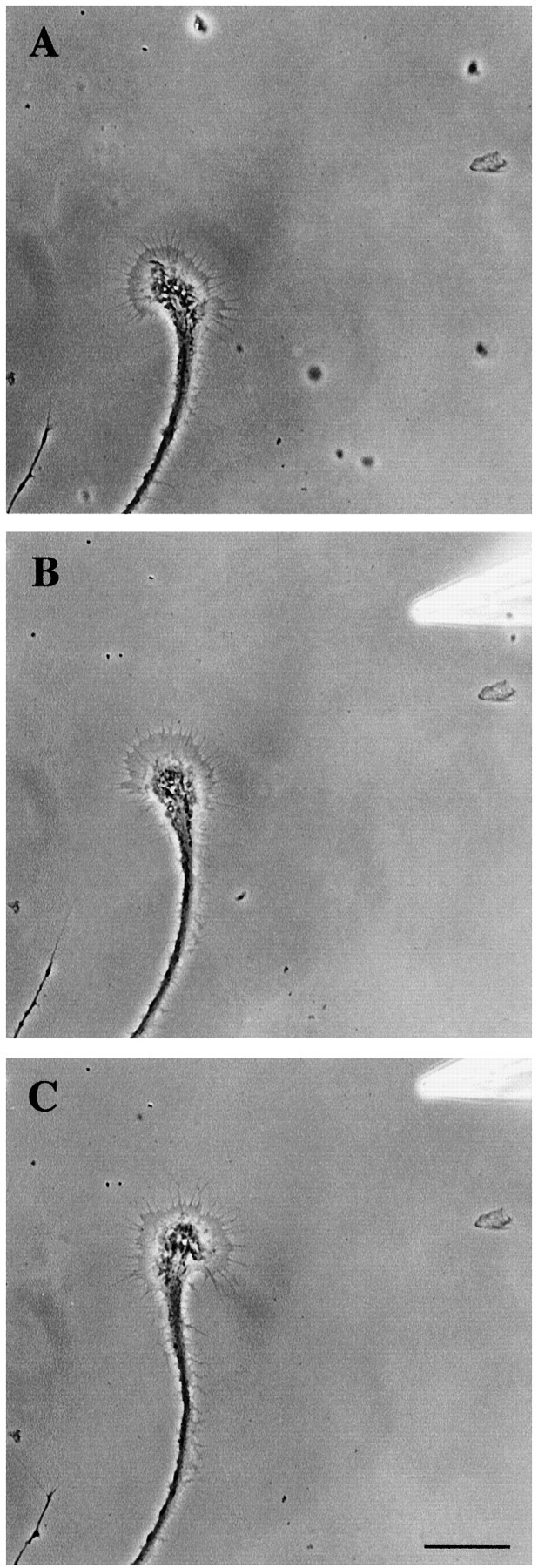
Exogenous dopamine induced the positive turning of a target cell growth cone. The outgrowth of a target cell growth cone was monitored for 60 min (A, 60 min). Exogenous dopamine (10−5m) was pressure applied at an angle to the target growth cone (starting at 60 min and applied in a pulsatile manner for 3 min, repeated 3 times with 10 min intervals).B, At 90 min, the growth cone started to turn toward the dopamine source. C, The target growth cone turned and grew in the direction of the exogenous source of dopamine (135 min). Scale bar, 50 μm.
To define the dopamine-induced, positive turning response of the target growth cones more precisely, we used the RPeD1 soma as an endogenous source of dopamine release. The reason for this is that the RPeD1 soma could be readily manipulated and positioned with ease (compared with unpredictable growth cone interactions), either directly into the path or at an angle to an advancing target growth cone (Fig.6Ai, Bi,Ci). Thus, the effects of endogenously released dopamine could be examined more effectively on a particular growth cone, in the absence of interactions from neighboring RPeD1 growth cones.
Fig. 6.
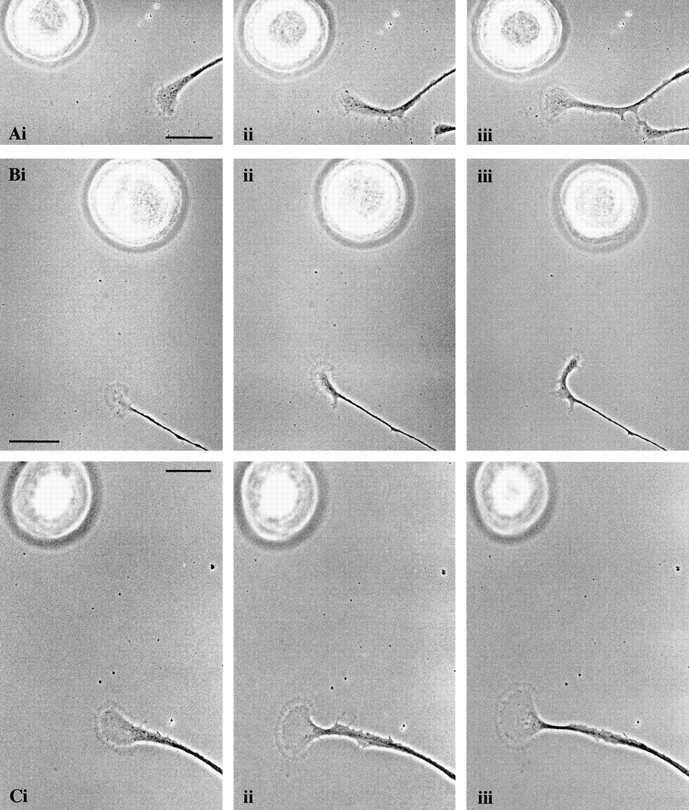
An RPeD1 soma induced the positive turning of a target cell growth cone, which was inhibited in the presence of the D2 antagonist. RPeD1 somata were placed 75–100 μm away (and at an angle) to advancing target cell growth cones (A, Bi, 0 min). The target growth cones began to turn toward the RPeD1 somata (Aii, 90 min;Bii, 60 min) and continued to advance in the direction of RPeD1 (A, Biii, 120 min).C, The D2 antagonist sulpiride (10−5m) was added to the bathing medium before the manipulation of the RPeD1 soma (i, 0 min). In the presence of the dopamine receptor antagonist, the growth cone did not turn (ii, 60 min) and continued to advance on its original path (iii, 90 min). Scale bars:A–C, 50 μm.
RPeD1 somata were maintained overnight in hemolymph-coated dishes containing CM (to prevent adhesion to the substrate) and subsequently transferred to the culture dish containing the target cells and manipulated in the vicinity of the target growth cones using a sharp-tipped pipette. We found that an unstimulated RPeD1, placed at an angle to a target growth cone, induced positive turning toward the RPeD1 soma (n = 8 growth cones from 7 cells; mean angle of turning, 36.9 ± 10.3°) (Fig. 6Aii, iii,Bii, iii). Addition of the D2 receptor antagonist sulpiride (10−5m) to the bathing medium was found to block this turning response (n = 6 of 7) (Fig.6Cii–iii). We therefore conclude that the turning response of the target growth cones was induced by spontaneous release of dopamine from the RPeD1 somata. These data therefore show that exogenously applied dopamine (pressure applied) and its spontaneous release from an RPeD1 soma exerted similar chemoattractive effects on the target cell growth cones.
We next sought to determine whether the RPeD1 growth cone-induced effects on the nontarget growth cones were also mimicked by exogenous dopamine. Dopamine (10−5m) was pressure applied (in a pulsatile manner) to the growth cones of nontarget cells, and their behavior was monitored. Pressure application of dopamine induced the collapse and halted the extension of all growth cones tested (n = 29) (Fig.7A–C), whereas pressure application of the vehicle solution (saline and 1% Na-metabisulfate) did not affect growth cone motility or morphology (n = 5 of 5; data not shown). To determine whether growth cone responses of the nontarget cells to exogenous dopamine could also be mimicked by spontaneous dopamine release from the RPeD1 soma, it was introduced to the culture dish and manipulated as described above. We found that, in all instances, the RPeD1 soma placed several hundred micrometers away from the nontarget cell growth cone (Fig.8Ai) induced turning away (n = 7 growth cones from 6 cells; mean angle of turning, 43.3 ± 15.5°) (Fig. 8Aii) and/or collapse (n = 8 of 8) (Fig. 8Aiii) of the nontarget growth cones. In no instances was contact between the nontarget growth cones and the RPeD1 soma observed (n = 8 of 8). To demonstrate further that these inhibitory and chemorepulsive effects were indeed mediated by endogenous dopamine release from RPeD1, the above experiments were performed in the presence of the D1 receptor antagonist R(+)-SCH-23390 (10−5m). We found that, in the presence of R(+)-SCH-23390, the RPeD1 soma failed to exert the chemorepulsive effects (n = 11 of 11). That is, the nontarget growth cones did not turn away from the RPeD1 soma (Fig.8Bi, ii) and continued to advance, often resulting in physical contact with RPeD1 (Fig.8Biii).
Fig. 7.
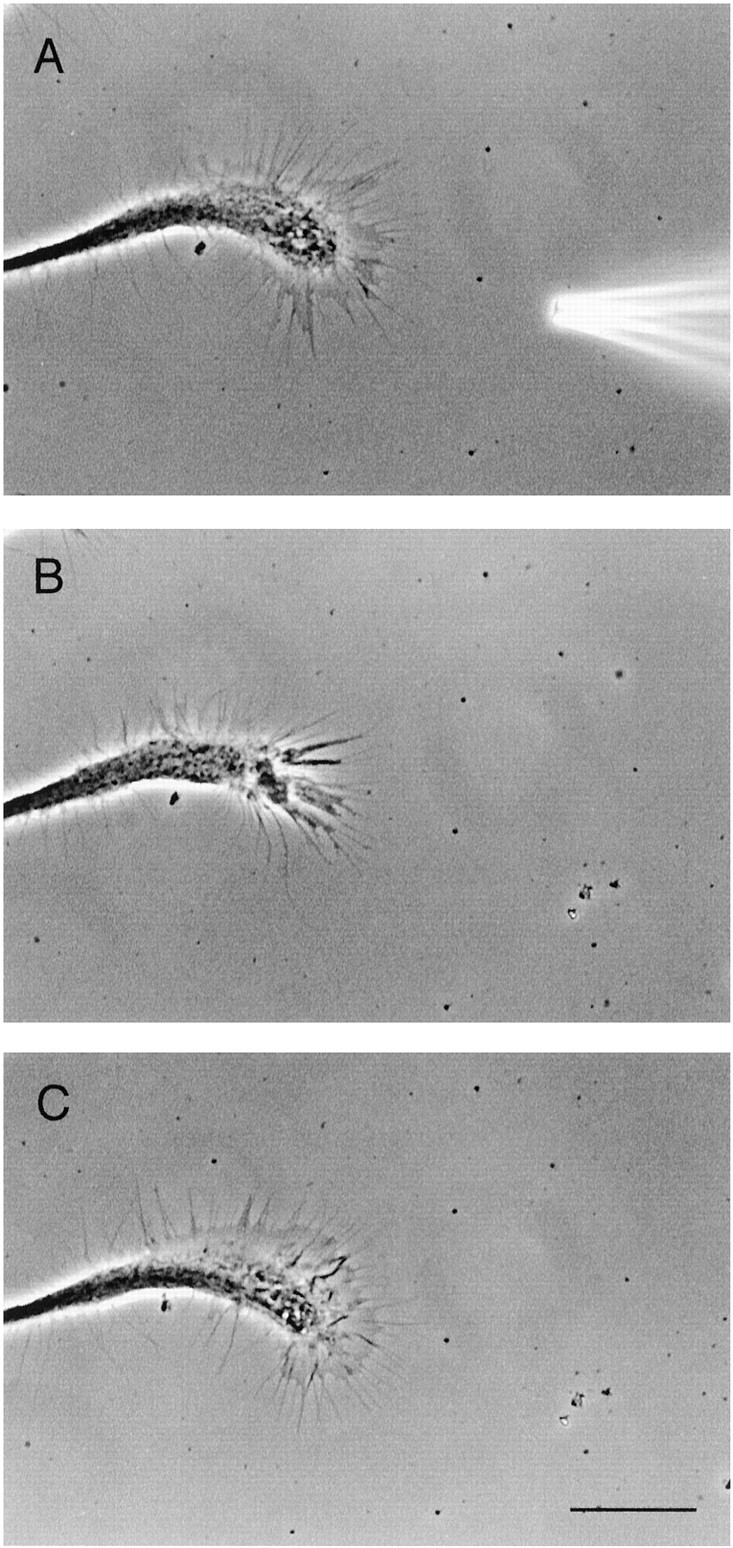
Exogenous dopamine application induced the collapse of the nontarget cell growth cone. A, Exogenous dopamine (10−5m) was applied via a pressure pipette placed in close proximity to the nontarget growth cone. B, Immediately after application, the growth cone collapsed and retracted over a short distance. C, After removal of dopamine, the morphology of the growth cone recovered fully. Scale bar, 30 μm. Frames are 15 min apart.
Fig. 8.
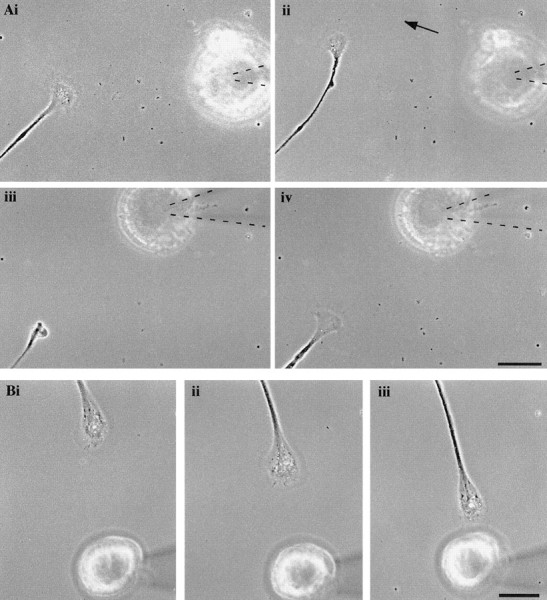
An RPeD1 soma induced the turning away and collapse of a nontarget growth cone, which was prevented by the D1 antagonist. A, An RPeD1 soma was placed in the path of a nontarget growth cone (i, 0 min), which subsequently turned away from RPeD1 after 30 min (ii). The RPeD1 soma was again manipulated using a pipette (highlighted bydashed lines) directly into the path of the growth cone (movement indicated by arrow). This resulted in the collapse of the nontarget growth cone (iii, 70 min).iv, Recovery of the growth cone at 110 min. Scale bar, 50 μm. B, The D1 receptor antagonist was added to the bath, and an RPeD1 soma was placed in the path of a different nontarget growth cone (i, 0 min). In the presence of the antagonist, the nontarget growth cone continued to advance (ii, 60 min) until contact with the RPeD1 soma occurred at 120 min (iii). Scale bar, 50 μm.
The RPeD1 growth cone-induced collapse of the nontarget growth cones was prevented in the presence of the dopamine antagonist
The above data showed that exogenously applied dopamine and its spontaneous release from the soma induced inhibitory (growth cone collapse) and/or chemorepulsive (turning) effects on the nontarget growth cones. To test whether the RPeD1 growth cone-induced effects on the nontarget growth cones also involved dopamine release from the RPeD1 growth cones (and activation of the D1-like receptor), growth cone interactions between the neurons were examined in the presence of the D1 antagonist. In coculture experiments, RPeD1 growth cones induced the collapse of nontarget cell growth cones (Fig.9A,B) and, immediately after the initial collapse, the D1 receptor antagonist was bath perfused (Fig.9C). Within 10 min of antagonist addition, the nontarget cell growth cones recovered from their collapse, continued to advance, and made physical contacts with the RPeD1 growth cones (n = 7) (Fig. 9D–F). It is important to note that previous studies have demonstrated that nontarget cell growth cones collapsed up to 10 times before physical contact with RPeD1 growth cones and neurites (Spencer et al., 1998). Based on these observations, we hypothesized that, in the presence of the D1 receptor antagonist, the RPeD1 growth cones would also fail to exert any initial inhibitory effects on the nontarget cells. To test this possibility, the growth cone interactions between the RPeD1 and nontarget growth cones were also monitored in the continued presence of the D1 receptor antagonist. We found that, in the presence of R(+)-SCH-23390, no initial growth cone collapse was observed (n = 12), and physical contact was made between the nontarget and RPeD1 growth cones (n = 10 of 12). Simultaneous electrophysiological recordings made from both RPeD1 and the nontarget somata did not reveal synaptic connections between the cells (tested up to 24 hr after contact). These data strongly suggest that dopamine regulates cellular functions in the nontarget cells that are distinct from those involved in transsynaptic communication.
Fig. 9.
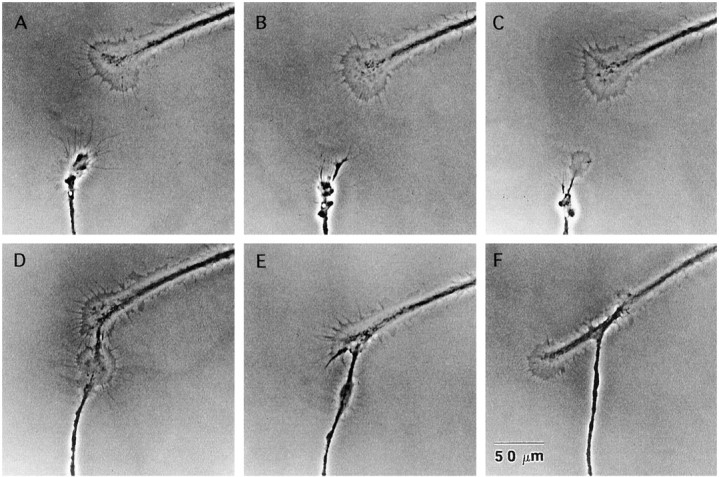
The RPeD1 growth cone-induced collapse of the nontarget growth cone was prevented by the D1 receptor antagonist. The nontarget cell growth cone (bottom) collapsed on approach to an RPeD1 growth cone (A,B). The D1 receptor antagonist was bath perfused (C), and the growth cone recovered and advanced toward the RPeD1 growth cone, making physical contact (D–F). Frames are 15 min apart.
The RPeD1 growth cone-induced effects on the motility rate of target and nontarget growth cones were blocked by the dopamine antagonists
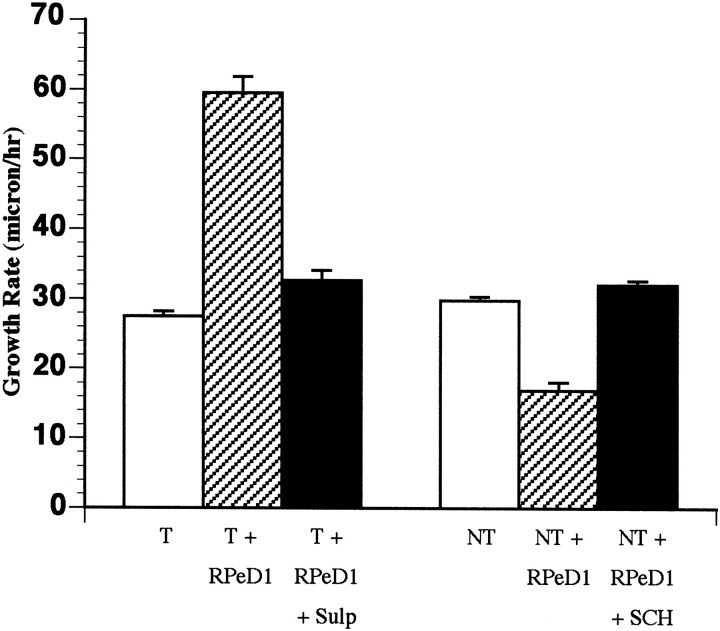
To investigate whether the effects of RPeD1 growth cones on both target and nontarget growth cone motility rates were also mediated by dopamine, the growth cone interactions were analyzed in the presence of the dopamine receptor antagonists. RPeD1 was cocultured with either target or nontarget cells, and the growth cone interactions were monitored in the presence of sulpiride and R(+)-SCH-23390, respectively (Fig. 10). In sulpiride (10−5m), the target cell growth cones advanced at rate of 32.7 ± 1.4 μm/hr (n = 8 growth cones from 8 cells). These values were significantly different (t test; p < 0.0001; df = 40) from those obtained when cocultured with RPeD1 in the absence of the antagonist. Thus, the RPeD1 growth cone-induced enhancement of target growth cone rate was inhibited in the presence of the dopamine receptor antagonist. Similarly, the RPeD1 growth cone-induced inhibitory effects on the motility rates of the nontarget growth cones were undetectable in the presence of R(+)-SCH-23390 (n = 20 growth cones from 10 cells). That is, the nontarget cell growth cones advanced at a rate of 32 ± 0.6 μm/hr in the presence of the dopamine receptor antagonist, which was significantly different from previous values obtained in coculture with RPeD1 in the absence of the antagonist (t test;p < 0.0001; df = 41). These data show that both the growth permissive and inhibitory effects of RPeD1 growth cones on the target and nontarget cell growth cones, respectively, were prevented by the dopamine receptor antagonists and strongly suggest that the effects were mediated by endogenously released dopamine from the growth cones of RPeD1.
Fig. 10.
The RPeD1-induced changes in growth cone motility rates were blocked in the presence of the dopamine receptor antagonists. Bar graph showing that the RPeD1-induced changes in growth cone motility rates of both target and nontarget cells were blocked in the presence of the D2 and D1 receptor antagonists, respectively.
DISCUSSION
This study provides evidence that transmitter–receptor interactions between growth cones of identified Lymnaeaneurons may play an important role in determining the specificity of target cell selection in the nervous system. We demonstrated that spontaneous release of dopamine (as well as exogenous application) acted as a chemoattractant and exerted positive turning effects on the target cells of RPeD1. Other in vitro studies have demonstrated that neuronal growth cones can detect gradients of chemoattractants, which include neurotrophins (Gallo et al., 1997; Ming et al., 1997a) and neurotransmitters (Zheng et al., 1994, 1996). These previous data, together with ours, suggest that chemoattractant molecules may attract incoming neurites from their synaptic partners over distances of hundreds of micrometers (Zheng and Kuffler, 2000) and may facilitate target cell selection and synapse formation during development. This hypothesis is consistent with previous studies that have demonstrated the release of various chemotropic molecules (Pini, 1993), such as netrins (Colamarino and Tessier-Lavigne, 1995;Tessier-Lavigne and Goodman, 1996) and semaphorins (Messersmith et al., 1995; Puschel, 1996) in the developing nervous system. In addition to its role as a chemoattractant in our study, dopamine also served as a chemorepellent for nontarget growth cones. Netrin-1 has also been shown to act as both a chemoattractant and chemorepellent (Colamarino et al., 1995). Furthermore, different members of the semaphorin protein family act as attractive (Sema3C) and repulsive (Sema3A) guidance molecules for cortical neurons (Bagnard et al., 1998, 2000), effects that are also mediated by different classes of receptors (Bashaw and Goodman, 1999).
The data presented in this study also provide evidence that a chemoattractive or repellent molecule, such as dopamine, may significantly change the rate of growth cone motility. For instance, release of transmitter from the RPeD1 growth cones approximately doubled the motility rate of target growth cones, whereas the motility rate of the nontarget cell growth cones was significantly reduced. A similar, semaphorin-induced regulation of growth cone advance rate was also observed recently in cortical neurons. Specifically, this study showed that Sema3A reduced the average rate of cortical growth cones by approximately half, whereas in the presence of Sema3C, the rate of growth cone advance was significantly enhanced (Bagnard et al., 2000). Our studies therefore demonstrate that the neurotransmitter-induced effects on growth cone behavior are in many ways similar to those exerted by other chemoattractants and repellents in the vertebrate nervous system. Our data, however, provide the first direct evidence that neurotransmitter release from a growth cone acts as a growth regulatory signal in a cell-specific manner.
Endogenous dopamine, released from the RPeD1 growth cones produced changes in growth rate, whereas release from the RPeD1 soma induced growth cone turning (of both target and nontarget growth cones). Both of these growth regulatory effects were, however, inhibited in the presence of the dopamine receptor antagonists, indicating that they were indeed mediated by dopamine. It is unclear as to why the endogenously released transmitter produced different effects and also why exogenously applied dopamine mimicked the soma-induced turning of target growth cones but had no significant effect on the rate of growth cone advance. We postulate, however, that these differing effects of dopamine may have resulted from either varying quantities of transmitter released (or applied) or differential diffusion gradients.
Neurotransmitter-induced growth cone collapse has been suggested previously to act as a growth “arrest” signal that precedes synapse formation (Lauder, 1993). Our data support an additional mechanism, that the neurotransmitter-induced collapse may prevent contact between neurons. Transmitters may thus act as long-range signaling molecules by reducing growth cone motility and/or inducing growth cone collapse and preventing contact between inappropriate synaptic partner cells. The neurites of the nontarget cells lie in close proximity to the neurites of RPeD1 in vivo. It is therefore possible that dopamine release during development of the Lymnaea nervous system may play a role in preventing physical contact and/or synapse formation between the inappropriate synaptic partners. Our sniffer cell assay (target soma) was able to reliably detect dopamine release from either the RPeD1 somata or its growth cones at a distance of up to 300 μm but not at 500 μm. It is likely, however, that growth cones are more sensitive to gradients of diffused transmitters than somata, and our data suggest that they detect transmitters at distances of up to 500 μm. Collapse of the nontarget growth cones was also observed after physical contact with RPeD1, and hence the involvement of membrane-bound molecules in contact-mediated collapse cannot be ruled out.
How is it possible that a specific molecule can exert opposing effects, on either growth cones of different cell types or the same growth cone? The specificity of a growth cone response to any given cue (Mueller, 1999) is thought to depend on both the nature and extent of the second messenger cascades that it activates (Kuhn et al., 1999; Rose and Chiba, 1999). Although many different signaling cascades are involved in the regulation of growth cone behavior, the most important of these appear to involve cytosolic-free calcium (Mattson and Kater, 1987;Kater and Mills, 1991), G-proteins (Igarashi et al., 1993; Spencer et al., 1998), cAMP (Mattson et al., 1988; Lohof et al., 1992, Ming et al., 1997b), cyclic nucleotides (Song et al., 1998), and Ca/calmodulin-dependent protein kinases (Zheng et al., 1994). The precise signaling mechanisms responsible for the differential effects of dopamine on both target and nontarget growth cones are currently unknown. Because the dopamine-induced growth-permissive and -suppressive effects on both target and nontarget cells were prevented by the D2 and D1antagonists, respectively, the specificity of the growth cone response to dopamine may be achieved at either the level of the transmitter receptor or the second messenger cascade.
Neurotransmitters may also exert their morphogenic effects by altering the electrophysiological properties (firing rate or membrane potential), which might in turn alter internal Ca2+ homeostasis. Exogenous dopamine does not generate an electrophysiological response in these nontarget cells, suggesting that a change in membrane potential alone could not account for the dopamine-induced modulation of growth cone behavior. We have demonstrated previously that, when challenged with a nontarget cell that possesses functional dopamine receptors and exhibits sensitivity to exogenous dopamine, RPeD1 did not form a synaptic connection with this neuron (Feng et al., 1997). These data suggest that the mere presence of functional dopamine receptors on growth cones is not sufficient for synapse formation. Rather, it is likely that these transmitter receptors serve a developmental and/or regenerative function as opposed to a transsynaptic communicative role. This notion is supported by previous studies that have shown that various transmitters (such as GABA) and their receptors can serve many developmental functions in addition to their involvement in synaptic physiology (Cherubini et al., 1991).
If transmitter–receptor interactions are important for axonal pathfinding and target cell selection in the developing nervous system, what are the mechanisms underlying such interactions? Because the millions of growth cones en route to their targets are believed to encounter a highly complex environment containing many different guidance cues, it is difficult to envisage how a single developing neuron and its growth cones would express receptors for each and every neurotransmitter that it might encounter. It is feasible, however, that the expression of various receptors might be differentially regulated. Consistent with this idea are data that show that receptors for dopamine (Lankford et al., 1987) and serotonin (Daval et al., 1987) are expressed only transiently on certain neurons during development. In addition to transient receptor expression, it is possible that nonsynaptic expression of receptors may occur during neurite outgrowth and development. That is, receptors that are not coupled to membrane ion channels but to cytoskeletal components may play a role in neurite guidance and pathfinding. Our data, which demonstrated that a nontarget cell growth cone responded to dopamine in the absence of an electrophysiological signal, is consistent with this notion.
In summary, this study provides evidence that transmitter– receptor interactions between growth cones of specific neurons play an important role in growth regulation, which leads to target cell recognition and subsequent synapse formation. We propose that transmitters released from growth cones may act as distant cell–cell recognition signals, such that diffused transmitter from a presynaptic neuron might attract growth cones from its potential synaptic partners, while repelling the growth cones of nontarget cells. Together with previous studies, our data thus underscore the importance of transmitters and their receptors in target cell selection during development.
Footnotes
This work was supported by the Medical Research Council of Canada and the Natural Sciences and Engineering Research Council of Canada. G.E.S. is an Alberta Heritage Foundation for Medical Research and Neuroscience Canada Foundation Fellow. We thank W. Zaidi for technical assistance.
Correspondence should be addressed to G. E. Spencer, Departments of Cell Biology and Anatomy, Physiology and Biophysics, Health Sciences Centre, 3330 Hospital Drive N.W., Calgary, Alberta, Canada T2N 4N1. E-mail: gspencer@ucalgary.ca.
REFERENCES
- 1.Bagnard D, Lohrum M, Uziel D, Puschel AW, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125:5043–5053. doi: 10.1242/dev.125.24.5043. [DOI] [PubMed] [Google Scholar]
- 2.Bagnard D, Thomasset N, Lohrum M, Puschel AW, Bolz J. Spatial distributions of guidance molecules regulate chemorepulsion and chemoattraction of growth cones. J Neurosci. 2000;20:1030–1035. doi: 10.1523/JNEUROSCI.20-03-01030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashaw GJ, Goodman CS. Chimeric axon guidance receptors: the cytoplasmic domains of slit and netrin receptors specify attraction versus repulsion. Cell. 1999;97:917–926. doi: 10.1016/s0092-8674(00)80803-x. [DOI] [PubMed] [Google Scholar]
- 4.Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- 5.Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. doi: 10.1016/0092-8674(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 6.Cottrell GA, Abernethy KB, Barrand MA. Large amine-containing neurones in the central ganglia of Lymnaea stagnalis. Neuroscience. 1979;4:685–689. doi: 10.1016/0306-4522(79)90145-3. [DOI] [PubMed] [Google Scholar]
- 7.Daval G, Verge D, Becerril A, Gozlan H, Spampinato U, Hamon M. Transient expression of 5HT1A receptor binding sites in some areas of the rat CNS during postnatal development. Int J Dev Neurosci. 1987;5:171–180. doi: 10.1016/0736-5748(87)90027-x. [DOI] [PubMed] [Google Scholar]
- 8.Feng ZP, Klumperman J, Lukowiak K, Syed NI. In vitro synaptogenesis between the somata of identified Lymnaea neurons requires protein synthesis but not extrinsic growth factors or substrate adhesion molecules. J Neurosci. 1997;17:7839–7849. doi: 10.1523/JNEUROSCI.17-20-07839.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallo G, Lefcort FB, Letourneau PC. The trkA receptor mediates growth cone turning toward a localized source of nerve growth factor. J Neurosci. 1997;17:5445–5454. doi: 10.1523/JNEUROSCI.17-14-05445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haydon PG, McCobb DP, Kater SB. Serotonin selectively inhibits growth cone motility and synaptogenesis of specific identified neurons. Science. 1984;226:561–564. doi: 10.1126/science.6093252. [DOI] [PubMed] [Google Scholar]
- 11.Hume RI, Role LW, Fischbach GD. Acetylcholine release from growth cones detected with patches of acetylcholine receptor-rich membranes. Nature. 1983;305:632–634. doi: 10.1038/305632a0. [DOI] [PubMed] [Google Scholar]
- 12.Igarashi M, Strittmatter SM, Vartanian T, Fishman MC. Mediation by G proteins of signals that cause collapse of growth cones. Science. 1993;259:77–79. doi: 10.1126/science.8418498. [DOI] [PubMed] [Google Scholar]
- 13.Kater SB, Mills LR. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11:891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kater SB, Shibata A. The unique and shared properties of neuronal growth cones that enable navigation and specific pathfinding. J Physiol (Paris) 1994;83:155–163. doi: 10.1016/0928-4257(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn TB, Schmidt MF, Kater SB. Laminin and fibronectin guideposts signal sustained but opposite effects to passing growth cones. Neuron. 1995;14:275–285. doi: 10.1016/0896-6273(95)90285-6. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn TB, Brown MD, Wilcox CL, Raper JA, Bamburg JR. Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of Rac1. J Neurosci. 1999;19:1965–1975. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lankford K, DeMello FG, Klein WL. A transient embryonic dopamine receptor inhibits growth cone motility and neurite outgrowth in a subset of avian retina neurons. Neurosci Lett. 1987;75:169–174. doi: 10.1016/0304-3940(87)90292-8. [DOI] [PubMed] [Google Scholar]
- 18.Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- 19.Letourneau PC. Integrins and N-cadherin are adhesive molecules involved in growth cone migration. In: Letourneau PC, Kater SB, Macagno ER, editors. The nerve growth cone. Raven; New York: 1992. pp. 181–193. [Google Scholar]
- 20.Lipton SA, Kater SB. Neurotransmitter regulation of neuronal outgrowth, plasticity and survival. Trends Neurosci. 1989;12:265–270. doi: 10.1016/0166-2236(89)90026-x. [DOI] [PubMed] [Google Scholar]
- 21.Lockerbie RO. The neuronal growth cone: a review of its locomotory, navigational and target recognition capabilities. Neuroscience. 1987;20:719–729. doi: 10.1016/0306-4522(87)90235-1. [DOI] [PubMed] [Google Scholar]
- 22.Lohof AM, Quillan M, Dan Y, Poo M-M. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J Neurosci. 1992;12:1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magoski NS, Bulloch AG. Localization, physiology and modulation of a molluskan dopaminergic synapse. J Neurobiol. 1997;33:247–264. [PubMed] [Google Scholar]
- 24.Magoski NS, Bauce LG, Syed NI, Bulloch AG. Dopaminergic transmission between identified neurons from the mollusk, Lymnaea stagnalis. J Neurophysiol. 1995;74:1287–1300. doi: 10.1152/jn.1995.74.3.1287. [DOI] [PubMed] [Google Scholar]
- 25.Mattson MP, Kater SB. Calcium regulation of neurite elongation and growth cone motility. J Neurosci. 1987;7:4034–4043. doi: 10.1523/JNEUROSCI.07-12-04034.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattson MP, Taylor-Hunter A, Kater SB. Neurite outgrowth in individual neurons of a neuronal population is differentially regulated by calcium and cAMP. J Neurosci. 1988;8:1704–1711. doi: 10.1523/JNEUROSCI.08-05-01704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron. 1995;14:949–959. doi: 10.1016/0896-6273(95)90333-x. [DOI] [PubMed] [Google Scholar]
- 28.Ming GL, Lohof AM, Zheng JQ. Acute morphogenic and chemotropic effects of neurotrophins on cultured embryonic Xenopus spinal neurons. J Neurosci. 1997a;17:7860–7871. doi: 10.1523/JNEUROSCI.17-20-07860.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997b;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 30.Mueller BK. Growth cone guidance: first steps towards a deeper understanding. Annu Rev Neurosci. 1999;22:351–388. doi: 10.1146/annurev.neuro.22.1.351. [DOI] [PubMed] [Google Scholar]
- 31.Phelan AK, Sherr EH, Aletta JM, Greene LA. Regulation of growth cone formation and function by neurotrophic factors. In: Letourneau PC, Kater SB, Macagno ER, editors. The nerve growth cone. Raven; New York: 1992. pp. 151–166. [Google Scholar]
- 32.Pini A. Chemorepulsion of axons in the developing mammalian nervous system. Science. 1993;261:95–98. doi: 10.1126/science.8316861. [DOI] [PubMed] [Google Scholar]
- 33.Puschel AW. The semaphorins: a family of axonal guidance molecules? Eur J Neurosci. 1996;8:1317–1321. doi: 10.1111/j.1460-9568.1996.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 34.Ridgway RL, Syed NI, Lukowiak K, Bulloch AGM. Nerve growth factor (NGF) induces sprouting of specific neurons of the snail, Lymnaea stagnalis. J Neurobiol. 1991;22:377–390. doi: 10.1002/neu.480220406. [DOI] [PubMed] [Google Scholar]
- 35.Rose D, Chiba A. A single growth cone is capable of integrating simultaneously presented and functionally distinct molecular cues during target recognition. J Neurosci. 1999;19:4899–4906. doi: 10.1523/JNEUROSCI.19-12-04899.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 37.Spencer GE, Klumperman J, Syed NI. Neurotransmitters and neurodevelopment: role of dopamine in neurite outgrowth, target selection and specific synapse formation. Perspect Dev Neurobiol. 1998;5:451–467. [PubMed] [Google Scholar]
- 38.Sun Y-A, Poo M-M. Evoked release of acetylcholine from the growing embryonic neuron. Proc Natl Acad Sci USA. 1987;84:2540–2544. doi: 10.1073/pnas.84.8.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Syed NI, Spencer GE. Target cell selection and specific synapse formation by identified Lymnaea neurons in vitro. Neth J Zool. 1994;44:327–338. [Google Scholar]
- 40.Syed NI, Bulloch AGM, Lukowiak K. In vitro reconstruction of the respiratory central pattern generator of the mollusk Lymnaea stagnalis. Science. 1990;250:282–285. doi: 10.1126/science.2218532. [DOI] [PubMed] [Google Scholar]
- 41.Syed N, Richardson P, Bulloch A. Ciliary neurotrophic factor, unlike nerve growth factor, supports neurite outgrowth but not synapse formation by adult Lymnaea neurons. J Neurobiol. 1996;29:293–303. doi: 10.1002/(SICI)1097-4695(199603)29:3<293::AID-NEU2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Taylor J, Docherty M, Gordon-Weeks PR. GABAergic growth cones: release of endogenous γ-aminobutyric acid precedes the expression of synaptic vesicle antigens. J Neurochem. 1990;54:1689–1699. doi: 10.1111/j.1471-4159.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 43.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 44.Wong RG, Hadley RD, Kater SB, Hauser GC. Neurite outgrowth in molluscan organ and cell cultures: the role of conditioning factor(s). J Neurosci. 1981;1:1008–1021. doi: 10.1523/JNEUROSCI.01-09-01008.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young SH, Poo M-M. Spontaneous release of transmitter from growth cones of embryonic neurones. Nature. 1983;305:634–637. doi: 10.1038/305634a0. [DOI] [PubMed] [Google Scholar]
- 46.Zheng JQ, Felder M, Connor JA, Poo M-M. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- 47.Zheng JQ, Wan J-J, Poo M-M. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J Neurosci. 1996;16:1140–1149. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng M, Kuffler DP. Guidance of regenerating motor axons in vivo by gradients of diffusible peripheral nerve-derived factors. J Neurobiol. 2000;42:212–219. [PubMed] [Google Scholar]