Abstract
Type A GABA receptors (GABAA) mediate the majority of fast synaptic inhibition in the brain and are believed to be predominantly composed of α, β, and γ subunits. Although changes in cell surface GABAA receptor number have been postulated to be of importance in modulating inhibitory synaptic transmission, little is currently known on the mechanism used by neurons to modify surface receptor levels at inhibitory synapses. To address this issue, we have studied the cell surface expression and maintenance of GABAA receptors. Here we show that constitutive internalization of GABAA receptors in hippocampal neurons and recombinant receptors expressed in A293 cells is mediated by clathrin-dependent endocytosis. Furthermore, we identify an interaction between the GABAA receptor β and γ subunits with the adaptin complex AP2, which is critical for the recruitment of integral membrane proteins into clathrin-coated pits. GABAA receptors also colocalize with AP2 in cultured hippocampal neurons. Finally, blocking clathrin-dependant endocytosis with a peptide that disrupts the association between amphiphysin and dynamin causes a large sustained increase in the amplitude of miniature IPSCs in cultured hippocampal neurons. These results suggest that GABAA receptors cycle between the synaptic membrane and intracellular sites, and their association with AP2 followed by recruitment into clathrin-coated pits represents an important mechanism in the postsynaptic modulation of inhibitory synaptic transmission.
Keywords: GABAA receptor, endocytosis, clathrin, adaptin, mIPSC, AP2, dynamin
GABA type A (GABAA) receptors are the major sites of fast synaptic inhibition in the brain and are also the sites of action for a range of therapeutic agents, including the benzodiazepines and barbiturates (Macdonald and Olsen, 1994; Rabow et al., 1995). GABAA receptors are members of the ligand-gated ion channel superfamily and can be assembled as pentameric hetero-oligomers from multiple subunit classes including α(1–6), β(1–4), γ(1–4), δ, ε, and π (Macdonald and Olsen, 1994;Rabow et al., 1995; Davies et al., 1997; Bonnert et al., 1999). Heterologous expression has revealed that the coexpression of receptor α, β, and γ subunits reproduces many of the physiological and pharmacological properties of neuronal GABAAreceptors (Macdonald and Olsen, 1994; Rabow et al., 1995).
Given the central role that GABAA receptors play as mediators of synaptic inhibition, it is important to understand how receptor number at the cell surface is regulated. Insulin treatment of neurons in culture (Wan et al., 1997) and the kindling model of epileptogenesis (Nusser et al., 1998) have both been shown to increase GABAA receptor surface number. In contrast to these observations, GABAA receptors have also been found to be downregulated by an agonist-dependent mechanism (Barnes, 1996; Tehrani and Barnes, 1997). We have also shown recently that both recombinant and neuronal GABAAreceptors can constitutively recycle between the cell surface and an intracellular endosomal compartment (Connolly et al., 1999a,b). Furthermore, GABAA receptor levels are reduced upon protein kinase C (PKC) activation (Chapell et al., 1998; Connolly et al., 1999b; Kittler et al., 2000). Little is known, however, about the molecular mechanisms responsible for the redistribution and cycling of GABAA receptors between the cell surface and intracellular locations. Dynamin-dependent endocytosis has been shown to be important in the regulation of cell surface levels of a number of integral membrane proteins (Schmid, 1997), including opioid receptors (Chu et al., 1997), the β-adrenergic receptor (Pitcher et al., 1998), and more recently ionotropic glutamate receptors (Carroll et al., 1999;Luscher et al., 1999; Man et al., 2000). Endocytosis of such membrane proteins involves their recruitment into clathrin-coated pits by adaptor proteins. The target protein–adaptor complex is then capable of interacting with other binding partners, including clathrin, the GTPase dynamin, and its binding partner amphiphysin (Marsh and McMahon, 1999), which are key elements of the endocytotic machinery.
Here we show that internalization of GABAAreceptors is mediated by clathrin-dependent endocytosis. Furthermore, GABAA receptors associate with the adaptin complex AP2 and colocalize with AP2 in cultured hippocampal neurons. Importantly, inhibition of endocytosis dramatically affects the miniature IPSC (mIPSC) amplitude, resulting in an increase of function of synaptic GABAA receptors in cultured hippocampal neurons. These results suggest a molecular mechanism that may allow the removal of GABAA receptors from synaptic sites, potentially having a critical role in controlling the efficacy of inhibitory synaptic transmission.
MATERIALS AND METHODS
DNA constructs. Murine α1, β2, γ2S, and γ2L subunit cDNAs were expressed from the mammalian expression vector pGW1 (McDonald et al., 1998). The γ2L and γ2S subunits differ by the presence of eight amino acids within the major intracellular domain of this subunit (Macdonald and Olsen, 1994; Rabow et al., 1995). The subunits were tagged with 9E10 epitope (EQKLISEEDL) between amino acids 4 and 5 as described previously (Connolly et al., 1996).
Cell culture. A293 cells (catalog # CRL 1573; American Type Culture Collection, Manassas, VA) were maintained in DMEM (Life Technologies, Paisley, UK) supplemented with 10% fetal bovine serum. Exponentially growing cells, seeded at 2 × 106 cells/10 cm dish, were transfected by electroporation (400 V; infinity resistance, 125 μF; Gene Electropulser II; Bio-Rad, Hercules, CA) with 10 μg of DNA using equimolar ratios of expression constructs (Connolly et al., 1996). For immunofluorescence studies, cells were plated onto poly-l-lysine–fibronectin (10 μg/ml)-coated coverslips and analyzed 18–36 hr after transfection. Low-density cultures of hippocampal neurons were prepared as described previously (Goslin and Banker, 1991) on poly-l-lysine coated (1 mg/ml) glass coverslips over a glial feeder layer and used at 2–3 weeks in culture.
Immunofluorescence. Cultured hippocampal neurons or A293 cells were fixed in 4% paraformaldehyde and then blocked in PBS containing 10% fetal bovine serum and 0.5% bovine serum albumin. Where appropriate, cells were permeabilized with 0.2% Triton X-100 for 10 min in blocking solution. Subsequent antibody dilutions were performed in blocking solution and washes were in PBS. Antibodies were used at the following: anti-GABAA receptor β2/β3, 10 μg/ml (BD17; Chemicon, Temecula, CA); anti-β1/3, 10 μg/ml (McDonald et al., 1998); and anti-α- and β-adaptin, 1:100 (Sigma, St. Louis, MO). When antibody internalization assays were performed with A293 cells, living cells were incubated with 9E10 (50 μg/ml) for 1 hr on ice in DMEM supplemented with 25 mm HEPES and 0.5% bovine serum albumin, pH7.4 (Connolly et al., 1999b). Excess antibody was removed, and internalization was performed at 37°C. For cultured hippocampal neurons, receptors were labeled at 37°C with BD17 (25 μg/ml) in culture medium. FITC- and Texas Red-conjugated anti-mouse and anti-rabbit secondary antibodies were from Molecular Probes (Eugene, OR) and Jackson ImmunoResearch (West Grove, PA) and used at 1:400. Coverslips were examined using a confocal microscope (MRC1000; Bio-Rad).
Production and purification of fusion proteins. GlutathioneS-transferase (GST) fusion proteins encoding the intracellular domains of the GABAA receptor α1, α2, α6, β1, β3, γ2S, and γ2L subunits were expressed inEscherichia coli and purified as described previously (Smith and Johnson, 1988; Brandon et al., 1999).
Affinity purification “pull-down” assays. Pull-down assays were performed as described previously (Brandon et al., 1999). Precipitated material was then separated by SDS-PAGE and analyzed by Western blotting using the anti-α- and β-adaptin antibodies (1:100). HRP-conjugated anti-mouse and anti-rabbit secondary antibodies for Western blotting were from Jackson ImmunoResearch and used at 1:5000, followed by detection with ECL.
Immunoprecipitation. Brain membranes (500 μg of total protein) were solubilized in a buffer containing: 1% Triton, 150 mm NaCl, 50 mm Tris, pH 7.6, 5 mm EDTA, 5 mm EGTA, 50 mm NaF, 1 mm Na orthovanadate, 1 mm PMSF, and 10 μg/ml leupeptin, pepstatin, and antipain (Brandon et al., 1999). Solubilized material was then exposed to anti-β-adaptin antisera (10 μg) or control IgG covalently coupled to protein G-Sepharose using dimethylpimelimidate (Harlow and Lane, 1988) for 1 hr. Bound material was washed three times in the above buffer before elution with SDS sample buffer. Bound material was then Western blotted using anti-GABAA receptor BD17 (10 μg/ml) mouse monoclonal antibody and ECL. HRP-conjugated anti-mouse and anti-rabbit secondary antibodies for Western blotting were from Jackson ImmunoResearch and used at 1:5000.
Electrophysiology. Hippocampal neurons were transferred to a recording chamber that was mounted on the stage of an inverted microscope (Diaphot 300; Nikon, Tokyo, Japan). The extracellular solution consisted of (in mm): NaCl 120, KCl 3.5, HEPES 10, NaHCO3 23, glucose 11, MgCl2 2, and CaCl2 2.5, pH 7.35 (285–300 mOsm). For mIPSC recording, the extracellular solution was supplemented with tetrodotoxin (TTX) (500 nm), 6 cyano-nitroquinoxaline-2,3-dione (CNQX) (20 μm), and 2-amino-5-phosphonopentanoic acid (APV) (50 μm). The bath solution was continuously oxygenated with a mixture of 95% O2–5% CO2 and perfused at a rate of 2.5 ml/min at 32–33°C. The internal solution consisted of (in mm): CsCl 120, CsOH 25, MgCl2 1, HEPES 10, CaCl2 1, EGTA 11, ATP 4, and GTP 2 (Na+ salt), adjusted to pH 7.3–7.4. In experiments in which GDP-βS was added to the pipette solution, GTP was omitted. Pipettes had a resistance of 3–4 MΩ when filled with these internal solutions. Experiments were performed in the whole-cell patch-clamp configuration using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA). Series resistance and membrane capacitance were partially compensated (70–80%) and monitored throughout. Recordings in which input and series resistance varied by >10% were discarded from the analysis. The holding potential in all experiments was −70 mV. mIPSCs were analyzed off-line using Mini Analysis 4 (Synaptosoft Inc., Leonia, NJ). Peptides P4 (QVPSRPNRAP; a kind gift of Dr. Harvey McMahon, Laboratory of Molecular Biology, Cambridge, UK) and scrambled peptide (SP) (PRAPNSRQPV) were dissolved at 50 μm in the internal solution described above. Data are expressed as mean ± SEM.
RESULTS
GABAA receptor internalization in A293 cells is mediated by clathrin-coated pits
We have shown previously that recombinant GABAA receptors expressed in A293 cells can constitutively endocytose to a transferrin receptor-containing compartment (Connolly et al., 1999a,b). Therefore, we sought to determine whether the endocytosis of GABAAreceptors was mediated by clathrin-coated pits. Internalization of receptors was followed by labeling surface receptors with antibodies to an extracellular 9E10 epitope tag on the N terminus of receptor subunits. These experiments were performed in the absence and presence of 350 mm sucrose because such hypertonic conditions impair clathrin-mediated endocytosis (Heuser and Anderson, 1989;Carroll et al., 1999). A293 cells transiently expressing(9Ε10)α1(9Ε10)β3(9Ε10)γ2L were prebound with 50 μg/ml 9E10 antibody at 4°C and, after removal of excess antibody, cells were incubated at 37°C for 1 hr. In the absence of high sucrose, GABAA receptors internalized efficiently to an intracellular compartment as shown in Figure 1B. However, treatment with 350 mm sucrose strongly inhibited internalization of these α1β3γ2L receptors (Fig. 1C). Receptor αβ subunits have also been shown to endocytose, although to a more peripheral recycling compartment than αβγ constructs (Connolly et al., 1999a,b). Similarly, γ2S subunits have been shown to access the cell surface as nonfunctional monomers and internalize constitutively (Connolly et al., 1999a). High sucrose strongly inhibited the endocytosis of both(9Ε10)α1(9Ε10)β3 receptors (Fig. 1D–F) and(9Ε10)γ2S homomers (Fig. 1G–I) expressed in A293 cells. Therefore, these observations strongly suggest that GABAA receptors composed of α1β3, α1β3γ2S, or homomeric γ2S subunits all internalize in A293 cells via a common mechanism.
Fig. 1.
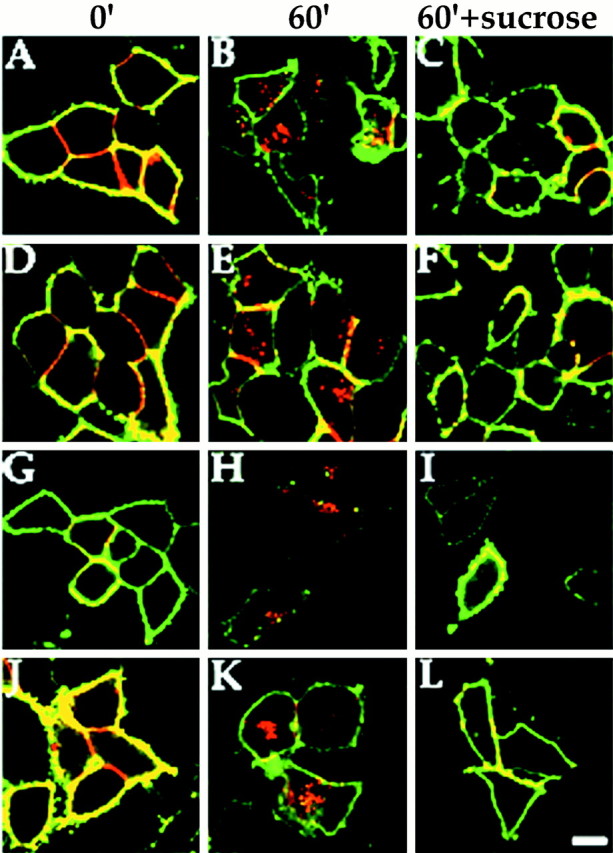
Clathrin-mediated endocytosis of recombinant GABAA receptors. A293 cells expressing α1β3γ2 (A–C, J–L), α1β3 (D–F), or γ2 (G–I) GABAA receptor subunits (all subunits 9E10-tagged) were prebound with anti-9E10 antibody (50 μg/ml) at 4°C for 30 min. Excess antibody was removed, and cells were incubated at 37°C for 60 min. Cells were then fixed, and cell surface receptors were detected in the absence of permeabilization with FITC-conjugated secondary antibody (green signal). The cells were then permeabilized with 0.05% Triton X-100. Internalized antibody was then measured using a secondary antibody conjugated with Texas Red (redsignal). Significant internalization could be detected for all subunit combinations (B, E, H,K) after 60 min compared with 0 min (A, D, G,J). This internalization could be significantly inhibited by treatment with 350 mm sucrose to block clathrin-mediated endocytosis (C, F,I, L). Internalization in the presence of PKC activation by PDBu (K, L) was also blocked by 350 mm sucrose (L). Scale bar, 10 μm.
We have reported previously that GABAA receptor surface levels are reduced upon PKC activation (Connolly et al., 1999b). PKC was found to act by blocking receptor recycling to the cell surface. However, downmodulation of receptor number upon PKC activation is still dependent on the removal of receptors by endocytosis. We therefore investigated whether receptor removal upon PKC activation is also dependent on the same endocytosis mechanism as constitutive endocytosis of receptors. For these experiments, we only analyzed GABAA receptors composed of α1β3γ2L subunits because we have shown previously that PKC-mediated downmodulation of surface receptor number was dependent on the presence of the γ2 subunit (Connolly et al., 1999b). Internalization of α1β3γ2L receptors upon PKC activation with 100 nmphorbol dibutyrate (PDBu) was followed in the presence or absence of 350 mm sucrose (Fig. 1J–L). Similar to constitutive endocytosis, internalization of receptors under conditions of PKC activation (Fig. 1K) was inhibited by blocking clathrin-mediated endocytosis (Fig. 1L).
GABAA receptor internalization in cultured hippocampal neurons is also mediated by clathrin-coated pits
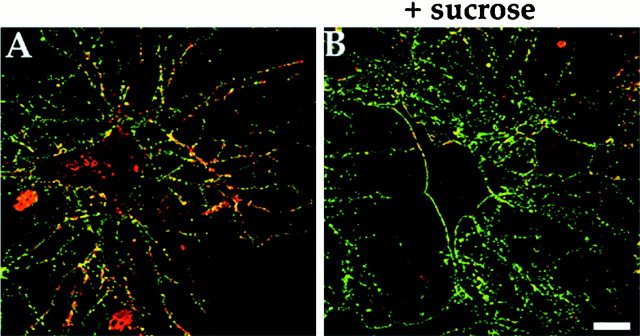
We have shown previously that constitutive endocytosis of GABAA receptors occurs in cultured hippocampal neurons (Connolly et al., 1999b). The involvement of clathrin-coated pits in this process was followed by labeling GABAA receptors on the surface of cultured hippocampal neurons (2 weeks in vitro) with an antibody that recognizes the extracellular N terminus of the β2 and β3 subunits. After incubation, the cells were fixed, and surface receptors were detected using a FITC-conjugated secondary antibody followed by permeabilization and detection of internalized receptors with a Texas Red-conjugated secondary antibody. After antibody feeding, internalized receptors were visualized as the red staining in the cell soma and along dendrites, whereas surface receptors were stainedgreen and yellow (Fig.2A). The treatment of hippocampal neurons with 350 mm sucrose significantly inhibited this internalization of receptors, which could be seen by the large reduction in red staining in the cell soma and along dendrites (Fig. 2B), strongly suggesting that GABAA receptor internalization in neurons is mediated via clathrin-dependent endocytosis.
Fig. 2.
Clathrin-mediated endocytosis of GABAAreceptors in cultured hippocampal neurons. Neurons were labeled with anti-GABAA receptor β2/β3 subunit antibody incubated for 30 min at 37°C. Surface receptors were detected in the absence of permeabilization with FITC-conjugated secondary antibody followed by permeabilization and detection of internalized antibody with Texas Red-conjugated secondary antibody. Internalized antibody could be detected after 30 min (A). This internalization was blocked by treatment with 350 mm sucrose to block clathrin-mediated endocytosis (B). Scale bar, 10 μm.
The clathrin adaptor protein AP2 specifically interacts with GABAA receptor β and γ subunit intracellular domains
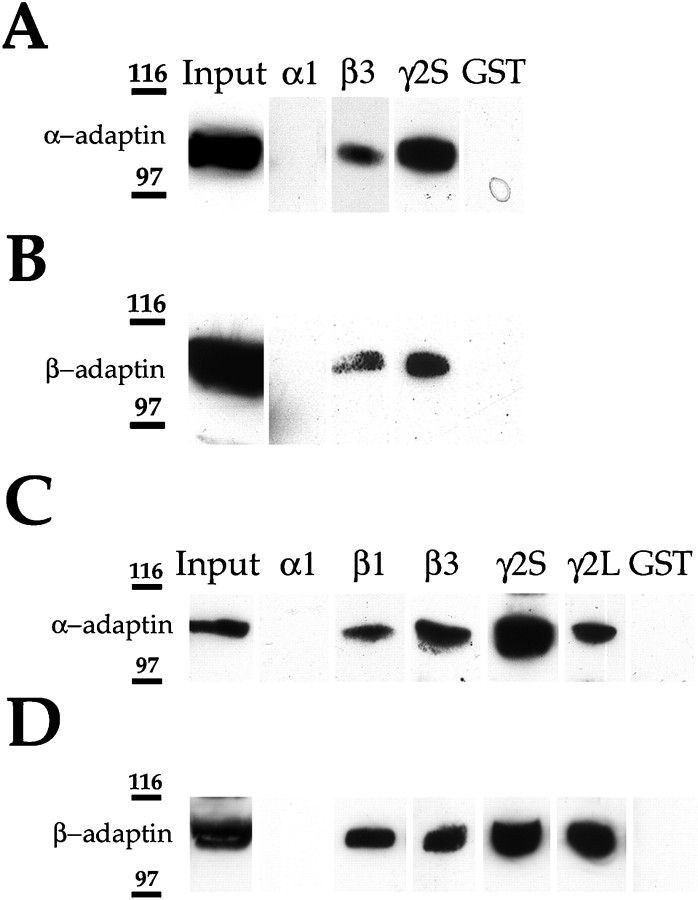
Adaptor complexes have been implicated in the selective recruitment of integral membrane proteins into clathrin-coated pits (Schmid, 1997). After the demonstration of significant clathrin-mediated endocytosis of GABAA receptors, it seemed plausible that this occurred via an interaction between the intracellular domains of receptor subunits with the endocytic adaptor complex AP2, facilitating their recruitment into clathrin-coated vesicles. To examine the interaction of clathrin adaptor proteins with GABAA receptor subunits, we expressed the major intracellular domains of the α, β, and γ2 subunits as soluble GST fusion proteins (Brandon et al., 1999). Purified GST fusion proteins immobilized on glutathione agarose beads were incubated with detergent-solubilized A293 cell extracts. Using this assay, GST-β3 and GST-γ2S were found to interact with α-adaptin (Fig.3A). In contrast, GST-α1 or GST alone were not found to associate with α-adaptin. Interestingly, neither of the intracellular domains of the α2 or α6 subunits appeared to bind α-adaptin using similar methodology (data not shown). To further verify that GABAA receptor β3 and γ2S subunit intracellular domains interact with adaptor proteins as a complex, material bound to the respective fusion proteins was probed with an antisera that recognizes the β-adaptin subunit of AP2. β-Adaptin was found to bind to GST-β3 and GST-γ2S but not GST-α1 or GST alone (Fig. 3B).
Fig. 3.
The intracellular domains of β1, β3, γ2S, and γ2L GABAA receptor subunits bind the adaptin α and β subunits of AP2. α1-GST, β3-GST, γ2S-GST, and GST were incubated with A293 cell extracts. After extensive washing, bound material was resolved by SDS-PAGE and analyzed by immunoblotting with an anti-α (A) or anti-β (B) adaptin antibody. α1-GST, β1-GST, β3-GST, γ2S-GST, γ2L-GST, or GST were incubated with brain extracts. After extensive washing, bound material was resolved by SDS-PAGE and analyzed by immunoblotting with an anti-α (C) or anti-β (D) adaptin antibody.
We used the same procedure to determine whether GABAA receptors interact with adaptins in brain extracts. GST fusion proteins of the intracellular domains of GABAA receptor α1, β1, β3, γ2S, and γ2L subunits were exposed to detergent-solubilized brain extracts, and associated proteins were resolved by SDS-PAGE and probed with antibodies specific for the α- or β-adaptin subunits. GST-β1, GST-β3, GST-γ2S, and GST-γ2L were found to interact with α-adaptin from brain extracts (Fig. 3C). In contrast, α1-GST or GST alone were not found to associate with α-adaptin (Fig. 3C). GST-β1, GST-β3, GST-γ2S, and GST-γ2L were also found to bind to β-adaptin from brain extracts, whereas no binding could be detected with β-adaptin to α1-GST and GST alone (Fig. 3D).
The interaction of adaptin complexes with neuronal GABAA receptors was further tested via immunoprecipitation. Detergent-solubilized brain membranes were immunoprecipitated with an antibody that recognizes the β-adaptin subunit of the AP2 complex or control nonimmune antisera. Precipitated material was then probed using an antibody that recognizes the GABAA receptor β2 and β3 subunits, components of most neuronal GABAA receptor subtypes (Wisden et al., 1992; Benke et al., 1994). The GABAAreceptor clearly coimmunoprecipitated with anti β-adaptin antisera but not with control IgG (Fig. 4). These results suggest that GABAA receptors can associate with adaptin complexes in brain and that this may be a mechanism to allow the recruitment of receptors to clathrin-coated pits.
Fig. 4.
GABAA receptors immunoprecipitate with AP2 from brain. Detergent-solubilized brain extracts were immunoprecipitated with anti-β-adaptin or control IgG. Bound material was resolved by SDS-PAGE and analyzed by immunoblotting with an anti-GABAA receptor β2/β3 subunit antibody.
GABAA receptors in cultured hippocampal neurons colocalize with AP2 in clathrin-coated pits
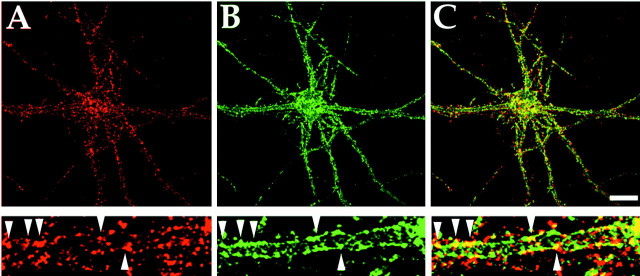
The subcellular distribution of GABAAreceptors with adaptin complexes in cultured hippocampal neurons was analyzed using immunofluorescence and confocal microscopy. Hippocampal neurons that had been maintained in culture for 3 weeks were double-labeled with a rabbit antibody to GABAAreceptor β1 and β3 subunits (McDonald et al., 1998) and a monoclonal β-adaptin antibody and visualized with FITC-conjugated anti-rabbit and Texas Red-conjugated anti-mouse secondary antibodies, respectively. The anti β-adaptin antibody revealed cell surface clusters of fluorescence on the soma and dendrites that represent AP2 in clathrin-coated pits on the plasma membrane (Fig.5A). GABAA receptors exhibited a clustered distribution of receptor expression on the cell soma and dendrites (Fig. 5B). Importantly, colocalization of some GABAA receptor clusters with AP2 complexes, seen as yellow staining in the merged panel, could be detected on the plasma membrane (Fig. 5C).
Fig. 5.
GABAA receptors colocalize with AP2 in clathrin-coated pits in cultured hippocampal neurons. Cultured hippocampal neurons (3 weeks old) were permeabilized and probed with a mouse anti-β-adaptin monoclonal antibody (A) and rabbit anti-GABAA receptor β1/β3 subunit polyclonal antibody (B). Antibodies were visualized with anti-rabbit FITC-conjugated and anti-mouse Texas Red-conjugated secondary antibodies. An enlargement of the same dendrite is shown in each panel. GABAA receptors, which colocalize with AP2, can be seen asyellow clusters in the merged image in C(see arrowheads). Scale bar, 10 μm.
Blocking dynamin-dependent endocytosis results in an increase in the amplitude of mIPSCs
Our biochemical and morphological approaches strongly suggest that GABAA receptors in A293 cells and neurons are undergoing constitutive endocytosis facilitated via association with AP2. To probe the functional significance of this cycling, we examined the effects of compounds that block clathrin-dependent endocytosis on GABAA receptor-mediated mIPSCs. mIPSCs were recorded at −70 mV in the presence of TTX (0.5 μm), APV (50 μm), and CNQX (20 μm). The mIPSCs recorded under these conditions were blocked by bicuculline (20 μm; data not shown), indicating they were mediated via GABAA receptors. To selectively block endocytosis, we used a 10 amino acid peptide (P4; see Materials and Methods) that is known to interfere with the binding of amphiphysin with dynamin (Marks and McMahon, 1998; Wigge and McMahon,1998). The interaction between dynamin and amphiphysin is essential for endocytosis (Wigge et al., 1997; Marsh and McMahon, 1999). Approximately 10 min after the establishment of the whole-cell recording mode, the size of the GABAA mIPSC began to increase and reached a plateau at 1.7- to 2.3-fold of its control value within 40–50 min (Figs. 6,7). In addition to its effect on mIPSC amplitude, the P4 peptide also increased mIPSC frequency (Fig. 6). Although this could possibly result from a higher rate of quantal release (Cohen et al., 1992; Thompson et al., 1993), it could also be fully accounted for by the recruitment of mIPSCs previously below the threshold of detection, owing to an increased number of active receptors (Liao et al., 1995; Wan et al., 1997; Man et al., 2000). As a control, we performed experiments in which neurons were loaded with a control SP, which had no significant effect on GABAA mIPSC amplitude or frequency (Fig. 7). GDP-βS, a less specific inhibitor of dynamin-mediated endocytosis (Luscher et al., 1999), also caused an increase of ∼57 ± 20% of the mIPSCs amplitude within 25–35 min (n = 5; data not shown). Together, these observations suggest an increased number of active postsynaptic GABAA receptors after blockade of endocytosis, which is consistent with the cycling of GABAA receptors between synaptic and intracellular sites.
Fig. 6.
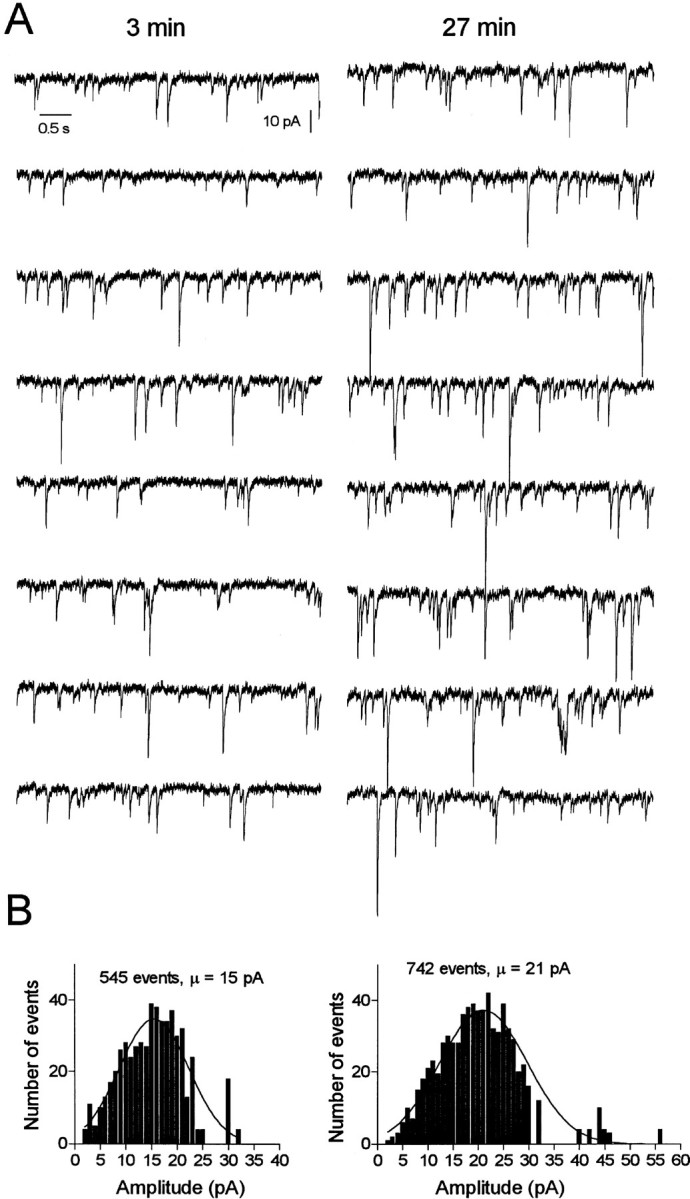
Blocking endocytosis increases the amplitude of GABAA-mediated mIPSCs in hippocampal neurons.A, Consecutive traces of mIPSCs selected 3 (left) and 27 (right) min after achieving whole-cell recording with pipette solution containing the endocytosis-blocking P4 peptide (50 μm).B, Histograms showing amplitude distributions of a 4 min recording of mIPSCs starting at the time indicated inA.
Fig. 7.
Time course of the effects of P4 endocytosis block on GABAA-mediated mIPSCs. Normalized mIPSC amplitude plotted against time after break-in with an internal solution containing either 50 μm P4 (●) or 50 μmSP control (○). The amplitude of the mIPSCs was calculated by averaging individual mIPSCs every 1 min recording. Eachpoint represents the mean ± SEM of four to five cells. The time on the x-axis represents the time after patch breakthrough. Inset, Single exponential decay of mIPSCs selected 3 (a) and 40 (b) min after break-in with the P4 peptide. No change in kinetics was detected during the enhancement of mIPSCs by the P4 peptide.
DISCUSSION
GABAA receptors are of central importance in mediating fast synaptic inhibition in the brain, and it is of fundamental importance to understand how these channels are regulated. One mechanism that could have a significant effect on regulating the strength of synaptic inhibition is by altering the number of receptors at the cell surface in GABAergic synapses. There has been growing evidence that GABAA receptor cell surface number can be dynamically regulated. Insulin treatment (Wan et al., 1997) and the kindling model of epileptogenesis (Nusser et al., 1998) have been shown to increase GABAA receptor surface number, whereas agonist treatment (Barnes, 1996; Tehrani and Barnes, 1997) and kinase activation (Moss and Smart, 1996; Chapell et al., 1998; Connolly et al., 1999b) can also influence receptor activity. However, the precise mechanisms that underlie these changes in GABAA receptor activity remain to be elucidated.
Here we show that GABAA receptors in both recombinant and neuronal preparations are removed from the plasma membrane by clathrin-mediated endocytosis. Internalization of recombinant GABAA receptors composed of αβ or αβγ subunits expressed in A293 cells was blocked by hypertonic sucrose, a classical inhibitor of clathrin-mediated endocytosis (Heuser and Andersen, 1989; Carroll et al., 1999). Interestingly, in recombinant systems, the trafficking itineraries of αβ versus αβγ containing subunits differ, with αβγ containing receptors able to access a later endosomal microtubule-dependent compartment than αβ receptors (Connolly et al., 1999a,b). However, our results suggest that the initial internalization of these differing types of GABAA receptors is via clathrin-coated pits. Furthermore, the PKC-induced downregulation of GABAA receptors in A293 cells (Chapell et al., 1998; Connolly et al., 1999) was also blocked by the high-sucrose protocol, suggesting that the kinase-induced reduction in receptor number is also dependent on the same mechanism of internalization. Our observations in A293 cells are consistent with the localization of the γ2 subunit to coated pits as determined by electron microscopy at steady state in this system (Connolly et al., 1999a). Similarly, endocytosis of neuronal GABAA receptors in cultured hippocampal neurons was also inhibited by blocking clathrin-mediated endocytosis.
To further elucidate the mechanism by which GABAAreceptors are selectively recruited to clathrin-coated pits, we investigated whether these receptors could associate with proteins implicated in the recruitment of integral membrane proteins to clathrin-coated pits. GABAA receptors were found to associate, via their intracellular loops, with the adaptin complex AP2, expressed in both cell lines and brain. Interestingly, association of adaptin complexes was found to occur only with the intracellular loops of the GABAA receptor β and γ subunits but not with α subunits. This is an intriguing result but may be explained by the fact that α subunits are incapable of accessing the cell surface as monomers in both cell lines and neurons (Connolly et al., 1996; Gorrie et al., 1997). They should therefore only be present at the cell surface as heteromeric receptors complexed with β and γ subunits (Connolly et al., 1996, 1999; Taylor et al., 2000). Therefore, the detection of GABAA receptors in clathrin-coated vesicles in the brain, as demonstrated in previous studies (Tehrani and Barnes, 1993; Tehrani et al., 1997), may be facilitated by the interaction of receptor β and γ subunits with adaptins as demonstrated in this study.
To verify our biochemical observations demonstrating the association of GABAA receptors with the AP2 adaptin complex, we could also detect colocalization of AP2 and GABAAreceptors at a number of sites on the plasma membrane of cultured hippocampal neurons. As would be expected, only a subset of receptors were colocalized with adaptin complexes because the majority would be expected to be associated with the inhibitory postsynaptic scaffold. To further investigate the role of endocytosis and recycling in regulating the number of synaptic GABAA receptors, we analyzed the effects of loading neurons with reagents that block endocytosis. We loaded cultured hippocampal neurons with a peptide that blocks endocytosis by disrupting the interaction between dynamin and amphiphysin, an interaction that is essential for clathrin-coated pit-mediated endocytosis (Wigge et al., 1997; Marks and McMahon, 1998;Luscher et al., 1999; Marsh and McMahon, 1999). This resulted in a significant “run up” in the amplitude of GABAA-mediated mIPSCs, consistent with an accumulation of surface GABAA receptors. These results confirm that synaptic GABAA receptors undergo constitutive endocytosis by a dynamin-dependent mechanism. This cycling of GABAA receptors may function to regulate the number of receptors in the inhibitory postsynaptic domain thereby allowing the neuron to regulate the efficacy of inhibitory synaptic transmission. These results are also consistent with our observation that these receptors associate with the AP2 adaptin complex. AP2 complexes have been implicated in the recruitment of a growing number of plasma membrane proteins into clathrin-coated pits to allow their internalization (Schmid, 1997), including ionotropic glutamate receptors (Man et al., 2000). It is therefore likely that the association between GABAA receptors and AP2 serves to recruit these receptors into coated pits to allow their removal via the endocytic pathway. Similar to the internalization of ionotropic glutamate receptors, it remains to be discovered whether internalization of GABAA receptors occurs by regulation of the interaction with adaptin complexes or whether this internalization occurs to all receptors “by default” directly upon release from the postsynaptic scaffolds that link these receptors to the cytoskeleton. It is clear that clathrin-mediated endocytosis of GABAA receptors could be an important means of regulating the levels of cell surface expression of these receptors and therefore of modulating their function. However, it will be of importance to discover the signaling pathways neurons use to control GABAA receptor endocytosis. Downregulation of GABAA receptor function has been observed previously by prolonged treatment with GABA, barbiturates, benzodiazepines, and neurosteroids (Barnes, 1996) and has also been shown recently to be essential for the E-S coupling component of long-term potentiation (Lu et al., 2000). Clathrin-mediated endocytosis of GABAA receptors dependent on their recruitment to clathrin-coated pits via association with adaptin complexes is therefore likely to be an important mechanism for regulating the strength of inhibitory synaptic transmission and synaptic plasticity.
Footnotes
This work was supported by the Medical Research Council and the Wellcome Trust. We thank Dr. Harvey McMahon for the dynamin peptide and Dr. Werner Sieghart for the GABAA receptor β3 antibody. We thank Drs. Andres Couve, Fiona Bedford, Mark Marsh, and Alberto Ramos for helpful discussions.
Correspondence should be addressed to Dr. Stephen J. Moss, Medical Research Council Laboratory of Molecular Cell Biology, University College, Gower Street, London WC1E 6BT, UK. E-mail:steve.moss@ucl.ac.uk.
REFERENCES
- 1.Barnes EM., Jr Use dependent regulation of GABAA receptors. Int Rev Neurobiol. 1996;39:54–70. doi: 10.1016/s0074-7742(08)60663-7. [DOI] [PubMed] [Google Scholar]
- 2.Benke D, Fritschy JM, Trzeciak A, Bannwarth W, Mohler H. The distribution, prevalence and drug binding profile of GABAA receptor subtypes differing in the β subunit variant. J Biol Chem. 1994;269:27100–27107. [PubMed] [Google Scholar]
- 3.Bonnert TP, McKernan RM, Farrar S, le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Brown N, Wafford KA, Whiting PJ. Theta, a novel gamma-aminobutyric acid type A receptor subunit. Proc Natl Acad Sci USA. 1999;96:9891–9896. doi: 10.1073/pnas.96.17.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandon N, Uren J, Kittler JT, Wang H, Olsen R, Parker PJ, Moss SJ. Subunit specific association of protein kinase C and the receptor for activated C kinase with γ-aminobutyric acid type A receptors. J Neurosci. 1999;19:9228–9234. doi: 10.1523/JNEUROSCI.19-21-09228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci USA. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapell R, Bueno OF, Alvarez-Hernandez X, Robinson LC, Leidenheimer NJ. Activation of protein kinase C induces gamma-aminobutyric acid type A receptor internalization in Xenopus oocytes. J Biol Chem. 1998;273:32595–32601. doi: 10.1074/jbc.273.49.32595. [DOI] [PubMed] [Google Scholar]
- 7.Chu P, Murray S, Lissu D, von Zastrow M. Delta and kappa opioid receptors are differentially regulated by dynamin dependent endocytosis when activated by the same alkaloid. J Biol Chem. 1997;272:27124–27130. doi: 10.1074/jbc.272.43.27124. [DOI] [PubMed] [Google Scholar]
- 8.Cohen GA, Doze VA, Madison DV. Opioid inhibition of GABA release from presynaptic terminals of the rat hippocampus. Neuron. 1992;9:325–335. doi: 10.1016/0896-6273(92)90171-9. [DOI] [PubMed] [Google Scholar]
- 9.Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Connolly CN, Uren JM, Thomas P, Gorrie GH, Gibson A, Smart TG, Moss SJ. Subcellular localization and endocytosis of homomeric gamma2 subunit splice variants of gamma-aminobutyric acid type A receptors. Mol Cell Neurosci. 1999a;13:259–271. doi: 10.1006/mcne.1999.0746. [DOI] [PubMed] [Google Scholar]
- 11.Connolly CN, Kittler JT, Thomas P, Uren JM, Brandon NJ, Smart TG, Moss SJ. Cell surface stability of gamma-aminobutyric acid type A receptors. Dependence on protein kinase C activity and subunit composition. J Biol Chem. 1999b;274:36565–36572. doi: 10.1074/jbc.274.51.36565. [DOI] [PubMed] [Google Scholar]
- 12.Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABA(A) receptor subunit. Nature. 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 13.Gorrie GH, Vallis Y, Stephenson FA, Whitfield J, Browning B, Smart TG, Moss SJ. Assembly of GABAA receptors composed of α1 and β2 subunits in both cultured neurons and fibroblasts. J Neurosci. 1997;17:6587–6596. doi: 10.1523/JNEUROSCI.17-17-06587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goslin K, Banker G. Culturing nerve cells, Chap 13 (Banker G, Goslin K, eds), pp 251–282. MIT; Cambridge, MA: 1991. [Google Scholar]
- 15.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1988. [Google Scholar]
- 16.Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kittler JT, Wang J, Connolly CN, Vicini S, Smart TG, Moss SJ (2000) Analysis of GABAA receptor assembly in mammalian cell lines and hippocampal neurons using γ2 subunit green fluorescent protein chimeras. Mol Cell Neurosci, in press. [DOI] [PubMed]
- 18.Liao D, Hessler NA, Malinow R. Activation of a postsynaptically silent synapses during pairing-induced LTP in a CA1 region of a hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 19.Lu YM, Mabsuy IM, Kandel ER, Roder J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- 20.Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 22.Man YH, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 23.Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- 24.Marsh M, McMahon HT. The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- 25.McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG. Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci. 1998;1:23–28. doi: 10.1038/223. [DOI] [PubMed] [Google Scholar]
- 26.Moss SJ, Smart TG. Modulation of amino acid activated ligand gated ion channels by protein phosphorylation. Int Rev Neurosci. 1996;39:1–42. doi: 10.1016/s0074-7742(08)60662-5. [DOI] [PubMed] [Google Scholar]
- 27.Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- 28.Pitcher JA, Freedman NJ, Lefkowitz RJ. G-protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 29.Rabow LE, Russek SJ, Farb DH. From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse. 1995;21:189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- 30.Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 31.Smith DB, Johnson KS. Single step purification of polypeptides expressed in E. coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 32.Taylor P, Connolly CN, Gorrie GH, Smart TG, Moss SJ. Identification of residues with GABAA receptor α subunits that mediate specific assembly with receptor β subunits. J Neurosci. 2000;20:1297–1306. doi: 10.1523/JNEUROSCI.20-04-01297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tehrani MH, Barnes EM., Jr Identification of GABAA/benzodiazepine receptors on clathrin-coated vesicles from rat brain. J Neurochem. 1993;60:1755–1761. doi: 10.1111/j.1471-4159.1993.tb13400.x. [DOI] [PubMed] [Google Scholar]
- 34.Tehrani MH, Barnes EM., Jr Sequestration of gamma-aminobutyric acid A receptors on clathrin-coated vesicles during chronic benzodiazepine administration in vivo. J Pharmacol Exp Ther. 1997;283:384–390. [PubMed] [Google Scholar]
- 35.Tehrani MH, Baumgartner B, Barnes EM., Jr Clathrin-coated vesicles from bovine brain contain uncoupled GABAA receptors. Brain Res. 1997;776:195–203. doi: 10.1016/s0006-8993(97)01037-8. [DOI] [PubMed] [Google Scholar]
- 36.Thompson SM, Copogna M, Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 1993;16:222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- 37.Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- 38.Wigge P, McMahon HT. The amphiphysin family of proteins and their role in endocytosis at the synapse. Trends Neurosci. 1998;21:339–344. doi: 10.1016/s0166-2236(98)01264-8. [DOI] [PubMed] [Google Scholar]
- 39.Wigge P, Kohler K, Vallis Y, Doyle CA, Owen D, Hunt SP, McMahon HT. Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis. Mol Biol Cell. 1997;8:2003–2015. doi: 10.1091/mbc.8.10.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;3:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]