Abstract
AMPA and NMDA receptors mediate most excitatory synaptic transmission in the CNS. We have developed antibodies that recognize all AMPA or all NMDA receptor variants on the surface of living neurons. AMPA receptor variants were identified with a polyclonal antibody recognizing the conserved extracellular loop region of all four AMPA receptor subunits (GluR1–4, both flip andflop), whereas NMDA receptors were immunolabeled with a polyclonal antibody that binds to an extracellular N-terminal epitope of the NR1 subunit, common to all splice variants. In non-fixed brain sections these antibodies gave labeling patterns similar to autoradiographic distributions with particularly high levels in the hippocampus. Using these antibodies, in conjunction with GluR2-specific and synaptophysin antibodies, we have directly localized and quantified surface-expressed native AMPA and NMDA receptors on cultured living hippocampal neurons during development. Using a quantitative cell ELISA, a dramatic increase was observed in the surface expression of AMPA receptors, but not NMDA receptors, between 3 and 10 d in culture. Immunocytochemical analysis of hippocampal neurons between 3 and 20 d in vitro shows no change in the proportion of synapses expressing NMDA receptors (∼60%) but a dramatic increase (∼50%) in the proportion of them that also express AMPA receptors. Furthermore, over this period the proportion of AMPA receptor-positive synapses expressing the GluR2 subunit increased from ∼67 to ∼96%. These changes will dramatically alter the functional properties of hippocampal synapses.
Keywords: glutamate, AMPA, NMDA, GluR, development, synapse, antibody, histoblot, hippocampal neurons, immunofluorescence, cellular ELISA
Ionotropic glutamate receptors (iGluRs) are the principal excitatory neurotransmitter receptors in the CNS. On the basis of their pharmacology and electrophysiology, iGluRs are classified as AMPA, kainate, and NMDA subtypes (Dingledine et al., 1999). AMPA and NMDA receptors participate in plastic changes in the efficacy of synaptic transmission, such as long-term potentiation (LTP; Bliss and Collingridge, 1993) and long-term depression (LTD; Bear and Abraham, 1996) and in the formation of neural networks during development (Durand et al., 1996). AMPA receptors are composed of four subunits, GluR1–4. NMDA receptors comprise the essential NR1 subunit and one or more of the modulatory NR2 subunits, NR2A-D (Hollmann and Heinemann 1994).
At developmentally early time points many excitatory synapses are thought to be postsynaptically “silent”, possessing functional NMDA but lacking functional AMPA receptors (Isaac et al., 1995, 1997; Liao et al., 1995; Durand et al., 1996; Wu et al., 1996; Li and Zhuo, 1998). Such synapses have been termed “silent synapses” because the NMDA pore is largely blocked at resting membrane potentials (Mayer et al., 1984: Nowak et al., 1984). Following protocols that induce LTP, however, these functionally silent synapses rapidly become AMPA receptor-responsive (Isaac et al., 1995; Liao et al., 1995). One possible mechanism to explain this observation is that a pool of pre-assembled AMPA receptors can be moved from an intracellular compartment to the postsynaptic membrane.
Antibodies that recognize extracellular epitopes and can thus label iGluRs on living neurons are important tools for the study of receptor localization and dynamics. The first such antibody (Molnar et al., 1993) was used to compare the cell surface and intracellular distributions of GluR1 in cultured hippocampal neurons (Richmond et al., 1996) and provided anatomical evidence in support of the “silent synapse” hypothesis. Antibodies against the same region of GluR1 and the N-terminal portion of GluR2 were used to further investigate AMPA receptor localization and dynamics (Mammen et al., 1997; O'Brien et al., 1998b; Carroll et al., 1999; Liao et al., 1999; Lüscher et al., 1999). Using subunit-specific antibodies, however, it is not possible to distinguish between the absence of AMPA receptors and the lack of a particular subunit on the cell surface. For this reason we have used an antibody that recognizes all AMPA receptor subtypes on living neurons. Probes for studying NMDA receptors on living neurons are even more limited. Until now only fluorescently tagged conantokin-G has been used to observe NMDA receptor clusters (Benke et al., 1993;Durand et al., 2000). We have therefore developed an antibody that labels NMDA receptors on living neurons.
Here we describe the characterization and application of these antibodies to study the developmental profile of AMPA and NMDA receptor surface expression. Our results support the view that early in development there are a large proportion of NMDA receptor-only synapses that subsequently acquire AMPA receptors. In addition, we have found a developmental increase in the relative ratio of GluR2 compared to other AMPA receptor subunits on the neuronal surface.
MATERIALS AND METHODS
Pfu DNA polymerase, BamHI, andHindIII were obtained from Stratagene (La Jolla, CA). All oligonucleotides were synthesized by Cruachem (Glasgow, UK). Tissue-culture materials were purchased from Life Technologies (Paisley, Strathclyde, UK). For solid phase peptide synthesis, all reagents were purchased from Novabiochem (Nottingham, UK). All other reagents were from Sigma (Poole, UK).
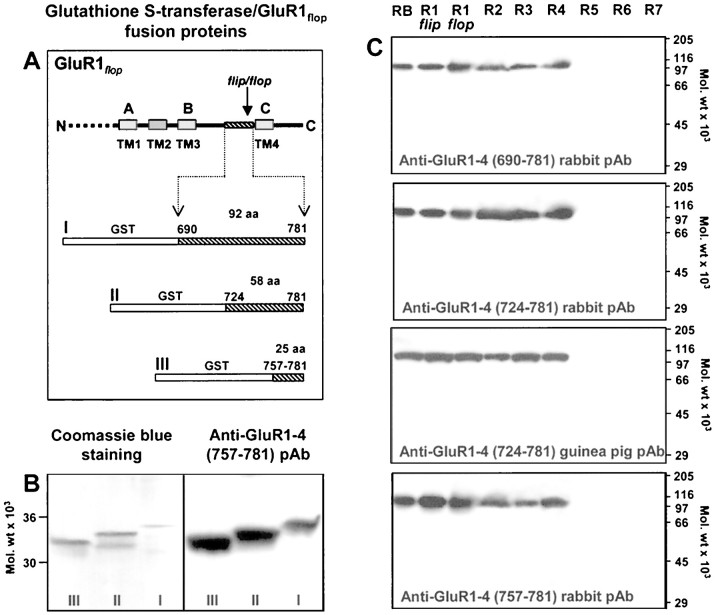
Selection of sequences for antibody production. For production of pan-AMPA antibodies we used 25, 58, and 92 amino acid long segments of the GluR1flop sequence (residues 757–781, 724–781, and 690–781, respectively) preceding the last transmembrane domain (Hollmann et al., 1989). This region shows ∼90% sequence homology with all other AMPA receptor subunits (GluR2–4) and is thought to be extracellular (Cockcroft et al., 1993; Hollmann et al., 1994; Bennett and Dingledine, 1995). In the extracellular N-terminal domain of the NR1 NMDA receptor subunit, a short unique region was identified (residues 436–450 in NR1a). This segment is believed to form a hydrophilic, surface-exposed, and flexible loop linking conserved secondary structures.
Expression and purification of glutathioneS-transferase-GluR1flop fusion proteins. Three glutathione S-transferase (GST) fusion proteins containing 25, 58 and 92 amino acid residues preceding the last membrane-spanning segment of GluR1flop (Hollmann et al., 1989) were produced (Fig.1A). DNA fragments encoding amino acid residues 757–781, 724–781, and 690–781 of GluR1flop were synthesized by PCR. The sequences of the forward primers were: (S1) 5′-GCG GGATCCAAAACAAAAGGCAAATACGCC-3′, (S2) 5′-GCG GGATCCGATTCC-AAAGGCTATGGC-3′, (S3) 5′-GCG GGATCCCAGGGGCTTTTGGAC-3′. A common reverse primer was used for all these PCR reactions (AS 5′-GCGC AAGCTTCAGCTGGTC-TTGTCCTT-GGA-3′). The primers contained sites for BamHI and HindIII (as indicated by the underlined sequences). The reverse primer also incorporated a stop codon at the 3′-end of the amplified sequence. PCR products were purified on a 10% acrylamide gel, digested withBamHI and HindIII, than purified again on a 10% acrylamide gel, and ligated into a BamHI andHindIII-digested pGEX-2TH bacterial expression vector (Pharmacia, Piscataway, NJ). GST fusion proteins were expressed in Escherichia coli strain HB101 competent cells (Promega, Madison, WI). After induction and lysis, GST fusion proteins were purified on a glutathione agarose column and analyzed by SDS-PAGE (Fig.1B). The purified fusion proteins were dialyzed against 20 mm Tris-HCl, pH 7.5, 2 mm MgCl2, and 1 mm DTT and protein concentration was measured with the Lowry et al. (1951) assay. From selected clones, plasmid DNA was prepared, and the DNA sequence was confirmed by di-deoxy DNA sequencing using the Sequenase Quick-Denature plasmid-sequencing kit (United States Biochemical, Cleveland, OH) with a 5′ pGEX sequencing primer (5′-GGGCTGGCAAGCCACGTTTGGTG-3′; Pharmacia).
Fig. 1.
Antibodies raised against different segments of the GluR1flop TM3–TM4 loop can be used to identify all known AMPA receptor subunit proteins. A, Site-directed antibodies were raised against three GST fusion proteins, containing amino acid residues 690–781 (I), 724–781 (II), and 757–781 (III) of GluR1flop. The location of putative membrane-spanning domains [TM1–4 or A–C (Hollmann et al., 1994) are indicated in the figure]. B, The immunostaining of GST-GluR1flop fusion proteins. GST fusion proteins (0.25 μg/lane), containing residues 757–781 (III), 724–781 (II), or 690–781 (I) of the GluR1flop subunit, were either stained with Coomassie blue or immunostained with a polyclonal rabbit antibody raised against the GST-GluR1757–781 fusion protein. C, Characterization of the subunit specificity of antibodies raised against residues 690–781, 724–781, and 757–781 of the GluR1flop AMPA receptor subunit. Membrane proteins (30 μg/lane) from rat brain (RB) and transiently transfected COS-7 cells expressing either GluR1–4 (AMPA) or GluR5–7 (kainate) receptor subunits were immunostained with the indicated antibody (1 μg/ml). All four antibodies cross-react with all of the AMPA receptor subunit proteins (GluR1–4), and both flipand flop alternatively spliced isoforms of GluR1. The kainate receptor subunits (GluR5–7) were unlabeled.
Preparation of synthetic peptides and peptide-carrier protein conjugates for NR1 antibody production. Sequence corresponding to residues 436–450 of NR1a (Moriyoshi et al., 1991) was used to prepare synthetic peptides. This sequence is identical in all known NR1 splice variants (NR1a-g; Moriyoshi et al., 1991). The N-terminal splice site (N1; 190–211) is far from the 436–450 segment and therefore unlikely to interfere with antibody binding. The NR1436–450 peptide was coupled to mercaptosuccinylated ovalbumin carrier protein (Klotz and Heiney, 1962) via the N-terminal cysteine of the peptide, which was conjugated to 5-thio-2-nitrobenzoic acid before coupling.
Immunization and immunoaffinity purification of antibodies.Rabbits (two to four for each fusion protein) were injected subcutaneously with one of the purified GST fusion proteins (containing residues 757–781, 724–781, or 690–781 of GluR1flop), boosted every 4 weeks, and bled 10 d after each boost. Two guinea pigs were injected subcutaneously with the GST fusion proteins containing residues 724–781 (0.1 mg), boosted every 2 weeks, and bled 7 d after each boost. The antisera were preadsorbed using a Sepharose 4B column coupled with the unfused GST protein. Subsequently, GluR1 portion-specific antibodies were affinity-purified with a Sepharose 4B column coupled with the appropriate GST fusion protein containing residues 757–781, 724–781, or 690–781 of GluR1flop. The antibodies were eluted from the columns using a buffer containing 0.1 m glycine-HCl, pH 2.5. The elute was immediately neutralized by addition of 1/10 volume of 1 m Tris-HCl, pH 8.0. The antibody solution was dialyzed against PBS. All rabbits and guinea pigs immunized produced antibodies recognizing the fusion proteins and GluR1–4 subunit proteins in transfected COS-7 cells and rat brain membrane samples (Fig.1B,C).
The ovalbumin-coupled NR1 peptide was coadsorbed with an adjuvant peptide (N-acetylmuramyl-l-alanyl-d-isoglutamine) to colloidal gold particles before immunization of New Zealand white rabbits following previously published protocols (Pow and Crook, 1993). The anti-NR1 antibody was immunoaffinity-purified using the cysteine containing synthetic peptide (5 mg) coupled to activated thiopropyl Sepharose 6B (Pharmacia). Other steps of the purification procedure were performed as described above. Serum antibody titers and the binding activity of the purified antibodies were analyzed using ELISA, as described previously (Molnar et al., 1993).
Dot-blot assay. The subunit specificity of the antibodies raised against the NR1436–450 segment was tested using synthetic peptides representing corresponding residues of NR2A, NR2B, NR2C, and NR2D (residues 439–453, 436–449, 450–464, and 465–477, respectively; Monyer et al., 1992; Cockcroft et al., 1993;Ishii et al., 1993). Nonconjugated synthetic peptides were dissolved in 13 mm sodium carbonate, 35 mm sodium bicarbonate, pH 9.6, and 0.1 μg/dot of each peptide was used to prepare nitrocellulose membranes for immunoreaction. After drying, membranes were immersed in blocking solution (5% nonfat dry milk and 1:50 dilution of normal swine serum in PBS) for 1 hr at 4°C. The membranes were then incubated for 2 hr with 1:200 dilution of the various antisera at room temperature. The bound antibodies were visualized by the enzymatic reaction of the alkaline phosphatase-conjugated anti-rabbit secondary antibody, as described previously (Molnar et al., 1993).
Preparation of membrane fractions from rat brain samples and transfected COS-7 cells. Membrane fractions were prepared from dissected cortical, hippocampal, and cerebellar areas of male Wistar rats. Tissue samples were homogenized in 25 ml of 0.3 msucrose and 20 mm Tris-HCl, pH 7.4, containing the following protease inhibitors: 2 mmdl-dithiothreitol (DTT), 1 μmpepstatin A, 1 mm iodoacetamide, 1 mmphenylmethylsulfonyl fluoride (PMSF), 1 mm1,10-phenanthroline, 2 mm EDTA, and 2 mm EGTA at 2°C with a glass-Teflon homogenizer. The homogenate was centrifuged at 10,000 × g for 15 min, and the microsomal fraction was collected by centrifuging the first supernatant at 200,000 × g for 30 min. All pellets were resuspended in the previously described buffer, snap-frozen in liquid nitrogen, and stored at −70°C until use.
The culturing of COS-7 cells, the transient transfection with cDNA coding for different ionotropic subunits, and the membrane preparation from transfected cells were performed as described previously (McIlhinney and Molnar, 1996). All plasmids were prepared for transfection studies using the Wizard Maxiprep plasmid kit (Promega). Protein concentrations in various membrane fractions were estimated by the procedure of Lowry et al. (1951), using BSA as standard.
SDS-PAGE, electrophoretic transfer of proteins and immunoblot analysis. SDS-PAGE was performed on 7.5 or 10% gels (Laemmli, 1970). Proteins were transferred electrophoretically onto polyvinylidene difluoride microporous membrane (Immobilon; Millipore, Bedford, MA) using an Atto HorizBlot Electrophoretic Transfer Unit with a discontinuous buffer system for 1.5 hr at room temperature, as recommended by the manufacturer (Atto, Tokyo, Japan). Before immunostaining, the Immobilon sheets were blocked overnight at 4°C with 5% nonfat dry milk and 1:50 dilution of normal swine serum in PBS (blocking solution). The proteins on Immobilon sheets were reacted with different affinity-purified antibodies (0.5–1 μg/ml) in blocking solution for 12–16 hr at 4°C. The bound antibodies were detected with either alkaline phosphatase- or horseradish peroxidase-conjugated anti-rabbit or anti-guinea pig IgG secondary antibody, as described previously (Molnar et al., 1993; McIlhinney and Molnar, 1996).
Histoblotting. The distribution of AMPA receptor subunits was analyzed in rat brain, using an in situ blotting technique (histoblot; Tönnes et al., 1999). In brief, horizontal cryostat sections (10 μm) from rat brain were apposed to nitrocellulose membranes moistened with 48 mmTris-base, 39 mm glycine, 2% SDS, and 20% methanol for 15 min at room temperature. After blocking in 3% fish skin gelatin, nitrocellulose membranes were DNase I-treated (5 U/ml), washed, and incubated in 2% SDS, 100 mmβ-mercaptoethanol in 100 mm Tris-HCl, pH 7.0, for 60 min at 45°C to remove adhering tissue residues. After extensive washing, the blots were processed for immunostaining as described for immunoblotting.
Hippocampal cell cultures. Hippocampal cultures were prepared from 3- to 5-d-old rats as previously described (Richmond et al., 1996; Noel et al., 1999). Neurons were used for experiments 3–5, 7–10, and 14–20 d after plating.
Quantification of total and surface-expressed AMPA and NMDA receptor proteins in neuronal cultures using an ELISA-based assay (cell-ELISA). Hippocampal cells were grown on 6-well plates as described above. Cells were fixed using 4% paraformaldehyde in 50 mm phosphate buffer, pH 7.2, for 20 min at room temperature, and then washed three times with PBS. Cells were incubated with 3% H2O2 in PBS for 5 min to minimize endogenous peroxidase activity. Cultures were incubated with blocking solution (5% fetal bovine serum and 1% BSA in PBS) in the presence and absence of 1% Triton X-100 for 30 min, and then with primary antibodies against GluR1–4 or NR1 (1 μg/ml in blocking solution) for 1 hr at room temperature. The addition of 1% Triton X-100 was omitted for detection of surface-expressed proteins. Cells were washed three times with blocking solution, incubated with anti-rabbit peroxidase-conjugated secondary antibody (1:3000 dilution in blocking solution) for 1 hr at 25°C, and washed four times in PBS. Samples in each well were incubated with 0.8 ml of K-Blue substrate (Neogen) for 10 min, after which the colored reaction end product was transferred from the plates into microfuge tubes containing 0.2 ml 1m HCl to stop the reaction. The optical density of samples was determined at 450 nm. Control experiments with preimmune serum, or plates without hippocampal cells, were included routinely to determine background value, which was subtracted from the OD450 readings. After the incubation with K-Blue substrate, cells were washed four times in PBS and solubilized in 0.2 ml 0.5% SDS. Protein concentration was determined with the Pierce (Rockford, IL) BCA protein assay kit using BSA as standard. Each OD450 value of the ELISA reaction was normalized to protein levels. For each experiment four parallel samples were used. The cell ELISA approach was not possible with the anti-GluR2 monoclonal antibody (MAB397; Chemicon, Temecula, CA), because it did not work on paraformaldehyde-fixed cells.
Immunofluorescence staining. The specificity of the purified anti-GluR1–4 antibodies was analyzed using transiently transfected COS-7 cells expressing AMPA receptor subunits. The anti-NR1 antibody was tested using immunohistochemistry on human embryonic kidney (HEK) 293 cells transiently cotransfected with NR1 and NR2A subunits. The transfection and immunostaining procedure was performed as described previously (McIlhinney and Molnar, 1996; McIlhinney et al., 1996, 1998). Whereas untransfected COS-7 or HEK 293 cells showed no staining, transiently transfected COS-7 and HEK 293 cells showed specific surface staining with the anti-GluR1–4 or NR1 antibodies, respectively.
For immunofluorescence experiments with living hippocampal neurons, the cells were washed twice with a HEPES-buffered saline (HBS; 119 mm NaCl, 5 mm KCl, 2 mmCaCl2, 2 mmMgCl2, 25 mm HEPES, 30 mmglucose, and 0.5 μm TTX) heated to 37°C. The cells were labeled with the following antibodies at 20°C in 5% dialyzed BSA in HBS: (1) rabbit or guinea pig polyclonal antibody to the conserved extracellular loop regions of all four GluR1–4 subunits (100 μg/ml), (2) rabbit polyclonal antibody directed against the N-terminal extracellular domain of NR1 (100 μg/ml), or (3) mouse monoclonal antibody to the N-terminal domain (amino acid residues 175–430) of GluR2 (100 μg/ml; MAB397, Chemicon; Vissavajjhala et al., 1996). We have verified that this antibody remains specific for GluR2 during the immunofluorescence staining procedure (at 100 μg/ml concentration) using HEK 293 cells transiently transfected with either GluR2 or the closely related GluR1 AMPA receptor subunit (data not shown).
Incubation with the primary antibody lasted 1.5 hr at room temperature. After washing for at least 1 hr, the cells were fixed with methanol (−20°C for 1–2 min). For the localization of synapses, immunostained cells were first fixed and permeabilized with methanol followed by a second immunostaining with a mouse monoclonal antibody (Boehringer Mannheim, Mannheim, Germany; 2 μg/ml) or rabbit polyclonal antibody (Biogenesis; 57 μg/ml) to synaptophysin. The primary antibodies against GluR1–4, GluR2, NR1, and synaptophysin were visualized using the appropriate fluorochrome-conjugated secondary antibody (Oregon green 488 goat anti-rabbit IgG antibody, 2–20 μg/ml, Molecular Probes, Eugene, OR; Texas Red goat anti-mouse IgG antibody, 10 μg/ml, Jackson ImmunoResearch, West Grove, PA; Texas Red goat anti-guinea pig IgG antibody, 10 μg/ml, Rockland, Gilbertsville, PA) in HBS with 5% BSA, and 0.2% goat serum was then applied for 1.5 hr at 4°C. Coverslips were mounted in Vectashield mounting medium (Vector Laboratories, Peterborough, UK).
Images were captured on a Leica (Heidelberg, Germany) TCS-NT confocal laser-scanning microscope attached to a DM RBE epifluorescence microscope using a 63× lens, (NA 1.32; Leica, Heidelberg, Germany). The 488 and 568 nm laser bands of a Kr-Ar laser were used for dual dye excitation and fluorescein isothiocyanate/tetramethylrhodamine isothiocyanate filters for fluorescence emission. With the imaging conditions used, there was no detectable bleedthrough of fluorescence from one channel to the other when we studied single-labeled specimens. Microscope settings were adjusted so that imaging conditions for both red and green channels were kept constant. For quantitative analysis, confocal images from the red (488 nm) and green (568 nm) channels were merged using Adobe Photoshop software (Adobe Systems, San Jose, CA). Twice the level of background was then subtracted from the merged image to define the receptor clusters. Therefore clusters (or puncta) were designated as discrete regions with more than twice the fluorescence intensity of background. Clusters were ∼1–2.5 μm in size. Only puncta lying along processes interpreted as dendrites were counted. We have excluded regions from quantification where the clear identification of neuronal processes was ambiguous. Synapses (defined as synaptophysin-positive puncta) on dendritic processes were considered glutamate receptor-positive if they were directly apposed (or within 2 pixels) of GluR1–4, GluR2, or NR1 puncta. Similarly, if NR1-positive puncta were directly apposed or within 2 pixels of GluR1–4 or GluR2-positive puncta, they were taken to be colocalized. GluR2 or GluR1–4 puncta were then expressed as a ratio of synaptophysin or NR1 puncta in the same neurons. For statistical analysis, independent group ttests were used.
The intensity of background fluorescence was no more than 7–13% of the mean values for puncta, for all antibodies at all three developmental ages. Changing the criterion of the quantification in our double-immunolabeling experiments (e.g., using 1–3 times the background as threshold) did not alter the relative ratios of immunopositive puncta between the green and red channels. We have previously compared quantitatively the distribution of GluR1–4 and NR1 puncta on paraformaldehyde-fixed and living neurons (Noel et al., 1999), and we have also performed acid-stripping of antibody-labeled cells, as described by Carroll et al. (1999). These data demonstrate that neither antibody-induced aggregation nor internalization of receptors are significant factors in our living cell-staining protocols. Comparison with the GluR2 antibody (Chemicon) have not been made, because it did not work on paraformaldehyde-fixed neurons (Noel et al., 1999).
RESULTS
Development and characterization of antibodies against AMPA and NMDA receptor subunits
To investigate the distribution of AMPA receptor proteins in living cells, polyclonal antibodies were raised against three fusion proteins derived from the TM3-TM4 linker region of the GluR1flop subunit (Fig. 1A,B). A small segment of this region differs in two alternatively spliced versions of AMPA receptor subunits termed flip andflop (Sommer et al., 1990). The amino acid sequence of the TM3-TM4 regions used for immunization shows ∼90% similarity in all cloned AMPA receptor subunits (Cockcroft et al., 1993; Hollmann and Heinemann 1994). The affinity-purified polyclonal antibodies detected all three GST fusion proteins incorporating amino acid residues 757–781, 724–781, or 690–781 of GluR1flop AMPA receptor subunit on immunoblots, whereas no cross-reactivity was observed to GST (data not shown). In immunoblots of rat brain membranes, each of the antibodies specifically recognized a single band with an apparent size of 110 kDa (Fig. 1C), corresponding to the molecular weight of AMPA receptor subunit proteins taking into account subunit glycosylation. COS-7 cells expressing individual subunits showed that antibodies raised against the conserved TM3-TM4 linker recognize all AMPA receptor subunits (GluR1–4 flipand flop), with no cross-reactivity with the related kainate receptor subunits GluR5–7 (Fig. 1C). Furthermore, in vitro translated GluR1–4 subunits were also recognized in bothflip and flop variants (data not shown). All immunoreactivity was blocked by preadsorbing the antibody with 100 μg/ml of the protein used for immunization, and no specific staining was detected by replacing the antibody with the preimmune serum (data not shown).
To study the expression pattern of NMDA receptor proteins, antipeptide antibodies were raised against the NR1 subunit, which is an essential component of all known NMDA receptor heterooligomers (Monyer et al., 1992; Hollmann and Heinemann, 1994). Antibodies were produced against synthetic peptides representing N-terminal residues 436–450 of the NR1a subunit, a region that is present in all splice variants (NR1a-g). All of the immunized rabbits responded to the antigen after the first boosting injection, as determined by dot-blot assays with the nonconjugated peptide (Fig.2A), and ELISA with the BSA-conjugated peptide (data not shown). The immune sera selectively labeled the synthetic peptide used for immunization but did not recognize the corresponding peptide sequences of other NMDA receptor subunits (Fig. 2A). The specificity of antibodies was further tested on immunoblots of membranes prepared from different regions of the rat brain and NR1a cDNA-transfected COS-7 cells (Fig. 2B). Antibodies raised against residues 436–450 of NR1 reacted with a major band on immunoblots of brain samples and NR1a-expressing COS-7 cells with the molecular weight of 115 kDa, which closely approximates that expected for this subunit on the basis of amino acid sequence taking into account subunit glycosylation. The antibodies did not label membranes from COS-7 cells expressing either of the related NR2A-D NMDA receptor subunits (data not shown). Preincubation of the antipeptide antibodies with the respective synthetic peptide (100 μg of peptide/ml) blocked the specific labeling (Fig. 2B). The anti-NR1 antibodies stained the surface of intact HEK 293 cells expressing recombinant NR1a and NR2A subunit-containing receptors (Fig. 2C, left panel). No signal was obtained with nontransfected HEK 293 cells (Fig. 2C, right panel).
Fig. 2.
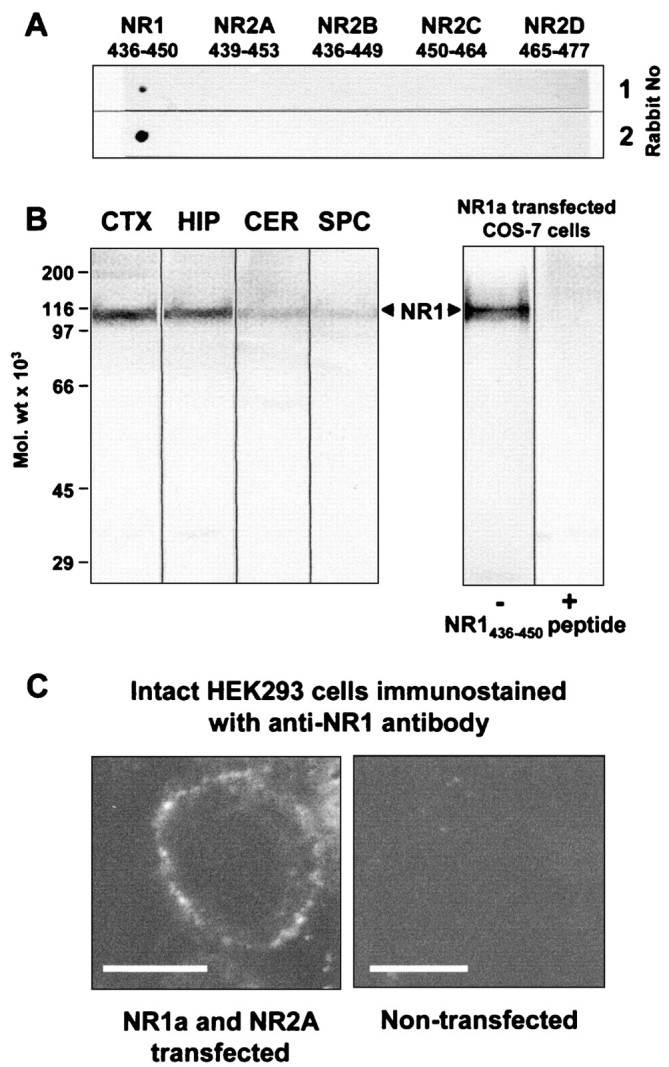
Characterization of the NR1 antibody using synthetic peptides, rat brain membrane fractions, and transiently transfected COS-7 and HEK 293 cells. A, Dot-blot assay of antipeptide NR1 antibody specificity using synthetic peptides corresponding to sequences of different NMDA receptor subunits. Immune sera from two rabbits (1:200 dilution) reacted only with the NR1 peptide, confirming the sequence specificity of these anti-NR1 antibodies. B, Immunoblot analysis of NR1 subunit proteins in rat brain and transiently transfected COS-7 cell membrane preparations. Aliquots of cerebral cortical (CTX), hippocampal (HIP), cerebellar (CER), spinal cord (SPC), and NR1a-transfected COS-7 cell membrane fractions (50 μg of protein/lane) were prepared, as described previously (Molnar et al., 1993; McIlhinney and Molnar, 1996). The bound antibodies were detected by reaction with alkaline phosphatase-conjugated anti-rabbit IgG. The preincubation of the antibody with the NR1 (436–450) peptide (1 μg/ml) blocked the labeling. C, Immunofluorescence staining of intact nonpermeabilized HEK 293 cells after transient transfection with NR1a and NR2A subunits. Transfected cells showed specific surface staining with the anti-NR1 antibody, whereas untransfected cells showed no immunostaining. Scale bars, 10 μm.
Immunochemical characterization of regional expression of AMPA and NMDA receptor proteins in unfixed rat brain
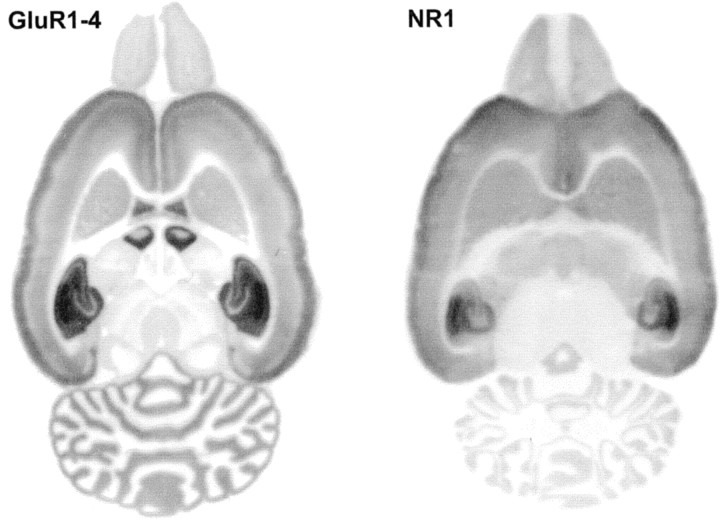
The regional distribution of GluR1–4 and NR1 immunoreactivity was analyzed on horizontal sections of whole unfixed adult rat brains blotted onto nitrocellulose membranes for immunostaining, as described by Tönnes et al. (1999) (Fig. 3). The preparation of tissue samples for immunocytochemistry often requires fixation that introduces covalent modification of the proteins, which could alter the antibody-binding sites. The cross-linked molecules could therefore hinder the access of antibody to epitopes. Direct transfer of proteins onto immobilizing membrane gives much improved accessibility for immunochemical analysis, and it is particularly useful for the testing of antibodies, because it retains the anatomical localization of different brain regions (Benke et al., 1995; Wenzel et al., 1997; Tönnes et al., 1999). Protein images on the nitrocellulose membranes were immunostained with the purified GluR1–4 and NR1 subunit-specific antibodies using conventional immunoblotting. Both GluR1–4 and NR1 immunoreactivities were predominant in the hippocampal formation (particularly in the CA1 region) and in the neocortex. The strongest GluR1–4 staining was found in the neuropil layers of the hippocampal formation, in the superficial layers of neocortex, and in the cerebellar molecular layer. Moderate levels of labeling was found in deeper layers of the neocortex, in the striatum, and in the olfactory bulb (Fig. 3, left panel). The histoblot labeling pattern obtained with the NR1-specific antibody (Fig. 3, right panel) was in agreement with the immunoblot analysis (Fig. 2B). The strongest labeling was in the hippocampal formation, where the highest reactivity was observed in the strata radiatum and oriens of CA1 and in the dentate molecular layer. Weaker reactivity was seen in the CA1 stratum pyramidale and in the dentate granule cell layer and in the CA3 region. In the neocortex the strongest NR1 staining was observed in the superficial layers. Cerebellar NR1 staining was relatively weak and was restricted to the granule cell layer (Fig. 3, right panel).
Fig. 3.
Regional distribution of AMPA and NMDA receptor proteins in rat brain. AMPA and NMDA receptor protein distribution was analyzed on adult rat brain histoblots using affinity-purified anti-GluR1–4 (0.5 μg/ml) and anti-NR1 (1 μg/ml) antibodies. The bound antibodies were visualized using an alkaline phosphatase-conjugated anti-rabbit antibody.
Quantification of developmental changes in total and surface-expressed AMPA and NMDA receptor proteins in CA3-CA1 primary neuronal cultures
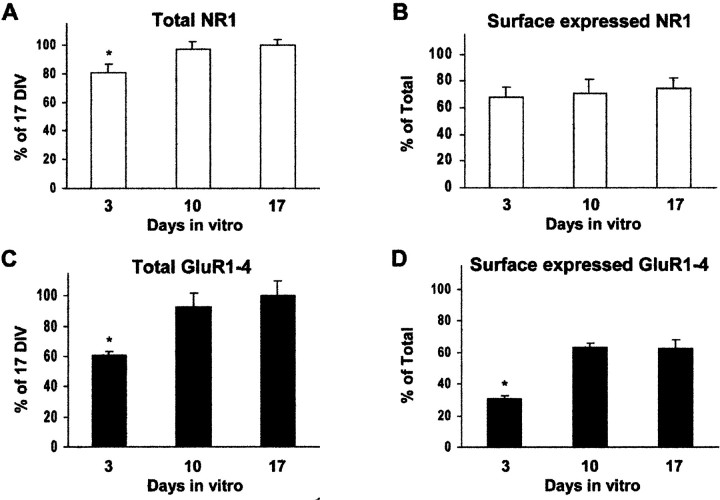
Quantitative analysis of the surface expressed and total GluR1–4 and NR1 subunit proteins was performed by cell-ELISA on 3-, 10-, and 17-d-old populations of cultured hippocampal neurons (Fig.4). We measured the levels of surface-expressed and total immunoreactivity for the GluR1–4 and NR1 antibodies using intact and Triton X-100 permeabilized, paraformaldehyde-fixed neurons. The paraformaldehyde fixation prevented the detachment of cells from the cell culture plate throughout the experiment. The integrity of the plasma membrane in paraformaldehyde-fixed cells was confirmed, using an antibody specific to the intracellular C-terminal domain of GluR2/3 AMPA receptor subunits (Chemicon). This antibody was unable to interact with the intracellular C-terminal domain of GluR2/3 in paraformaldehyde-fixed neurons without Triton X-100 permeabilization (data not shown). In each of the neuronal cultures, immunoreactivity was normalized to protein levels, as described in Materials and Methods.
Fig. 4.
Quantification of developmental changes in surface-expressed and total AMPA and NR1 immunoreactivity on cultured hippocampal neurons using cell ELISA. Immunoreactivity for the NR1 NMDA receptor subunit (A, B) and GluR1–4 AMPA receptor subunits (C, D) was compared in paraformaldehyde-fixed, nonpermeabilized (surface-expressed,B,D) and 1% Triton X-100 permeabilized (total, A,C) cells after 3, 10, or 17 d in vitro using an ELISA-based assay. The total immunoreactivity for NR1 and GluR1–4 subunits (A,C) was expressed as the percentage of the immunoreactivity obtained after 17 d in culture. The surface immunoreactivity for NR1 and GluR1–4 subunits (B,D) was expressed as the percentage of the total immunoreactivity after 3, 10, or 17 d in vitro. For each experiment, a minimum of three parallel samples was used. *p < 0.001 compared with samples in other age groups.
Total NR1 expression increased by ∼15% between days 3 and 10 in culture, but then remained essentially unchanged. Between days 3 and 17 the surface expression of NR1 protein was relatively constant at ∼70% (Fig. 4A,B).
In contrast, we observed an ∼1.5 fold increase in the total AMPA receptor subunit protein expression between days 3 and 10 in culture. This increase then stabilized between days 10 and 17 in culture (Fig.4C). The surface expression of AMPA receptor subunit proteins increased approximately twofold between days 3 and 10 in culture and then remained essentially the same (∼63%) until day 17 (Fig. 4D).
Our cell ELISA data indicate that ∼60% of AMPA receptors and ∼70% of NMDA receptors are surface-expressed in hippocampal neurons after 10 d in culture. This is consistent with the results of cell surface biotinylation, cross-linking, and proteolysis studies in cultured hippocampal neurons (Hall and Soderling, 1997a,b) and in cerebellar granule cells (Huh and Wenthold, 1999).
There is a developmental shift of surface-expressed NMDA and AMPA receptors to synaptophysin-immunopositive sites
Antibodies that recognize extracellular epitopes were used to analyze the surface distribution of AMPA and NMDA receptors in living hippocampal neurons. For the localization of synapses, immunostained cells were first fixed and permeabilized with methanol, followed by a second immunostaining with anti-synaptophysin antibody. Synaptophysin is a widely used marker protein of presynaptic specializations. Cultured neurons were used at 3 time points; 3–5, 7–10, or 14–20 d in culture.
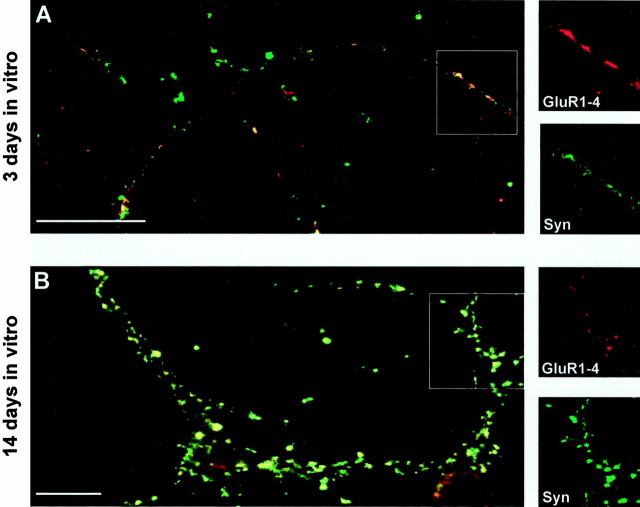
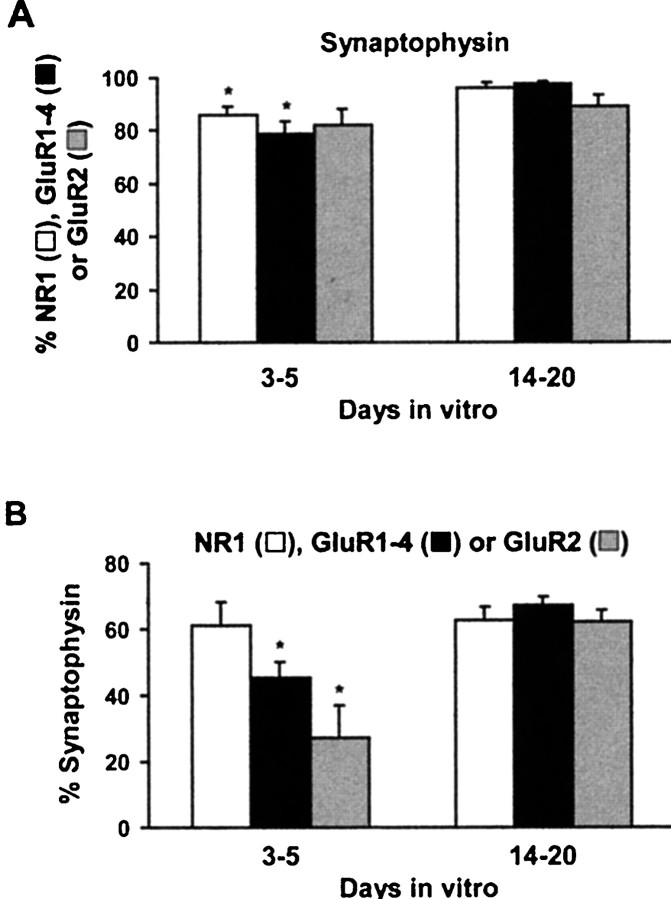
After 3–5 d in culture, the majority of GluR1–4-positive (∼79%; Fig. 5A), NR1-positive (∼85%; Fig. 6A), and GluR2-positive (∼82%; Fig. 6C) clusters colocalized with synaptophysin immunoreactivity, suggesting that most, but not all, NMDA and AMPA clusters are located at synapses (Fig.7A). A relatively small percentage of the total synaptophysin-immunoreactive puncta colocalized with GluR1–4 (∼45%; Fig. 5A) or GluR2 (∼27%; Fig.6C), indicating that the majority of synapses do not contain AMPA receptors at 3–4 d in culture (Fig. 7B).
Fig. 5.
Colocalization of GluR1–4 and synaptophysin in hippocampal neurons in culture for 3 and 14 d. Colocalization (yellow) of GluR1–4 (red) and synaptophysin (Syn, green) on hippocampal neurons in culture for 3 (A) and 14 (B) d. The individual immunoreactivity for GluR1–4 and synaptophysin (Syn) in regions highlighted in the boxes are shown in the side panels. At 3–5 d in culture (A), 79 ± 5% of surface-expressed GluR1–4 puncta (4 fields, 950 puncta) contained synaptophysin-immunoreactive puncta. At 14–20 d in culture, 98 ± 1% of surface-expressed GluR1–4 puncta (4 fields, 375 puncta) contained synaptophysin-immunoreactive puncta. At 3–5 d in vitro 45 ± 5% of total synaptophysin puncta (4 fields, 950 puncta) contained GluR1–4 immunoreactivity, which increased to 67 ± 2% (4 fields, 375 puncta) at 14–20 d in vitro. Scale bars, 10 μm.
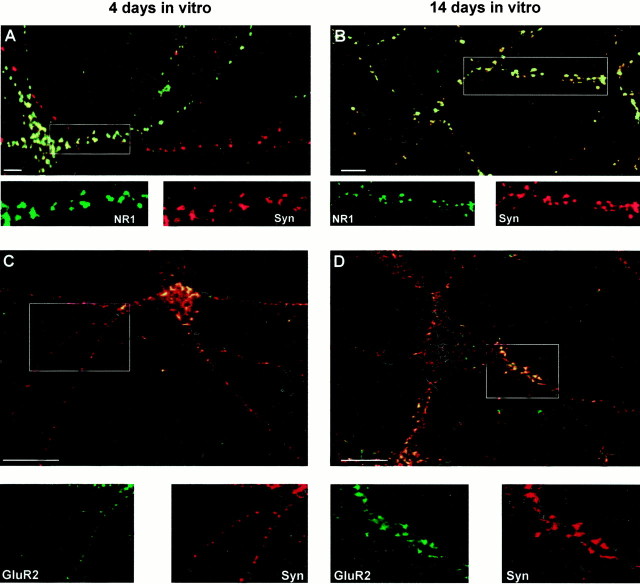
Fig. 6.
Colocalization of NR1 and GluR2 with synaptophysin in hippocampal neurons in culture for 4 and 14 d. Colocalization (yellow) of NR1 (green; A,B) or GluR2 (green; C,D) and synaptophysin (Syn, red) on hippocampal neurons in culture for 4 (A, C) and 14 (B, D) d. The individual immunoreactivity for NR1, GluR2, and synaptophysin (Syn) in regions highlighted in the boxesare shown in the bottom panels. At 3–5 d in culture (A), 85 ± 3% of surface-expressed NR1 puncta (6 fields, 748 puncta) contained synaptophysin-immunoreactive puncta. At this age 61 ± 7% of the total synaptophysin puncta (6 fields, 748 puncta) contained NR1. At 14–20 d in culture (B), 96 ± 2% of surface-expressed NR1 puncta (4 fields, 413 puncta) contained synaptophysin-immunoreactive puncta, whereas 63 ± 4% of total synaptophysin puncta (4 fields, 413 puncta) colocalized with NR1. At 3–5 d in culture (C), 82 ± 6% of surface-expressed GluR2 puncta (3 fields, 381 puncta) contained synaptophysin-immunoreactive puncta, whereas 27 ± 10% of the total synaptophysin (3 fields, 418 puncta) contained GluR2. At 14–20 d in culture (D) 89 ± 4% of surface-expressed GluR2 (4 fields, 366 puncta) contained synaptophysin-immunoreactive puncta, and 62 ± 3% of the total synaptophysin (4 fields, 366 puncta) contained GluR2. Scale bars, 5 μm.
Fig. 7.
Quantification of developmental changes in the colocalization of AMPA and NMDA receptor subunit proteins and synaptophysin. A, The majority of surface NR1 (■), GluR1–4 (▪), and GluR2 (░) clusters colocalized with the presynaptic marker protein synaptophysin. B, The percentage of the total synaptophysin-immunoreactive puncta containing NR1 (■) remained the same, whereas GluR1–4 (▪) and GluR2 (░) increased during development. Mean and SE was calculated based on at least three independent determinations; *p < 0.001 compared with samples in the 14- to 20-d-old groups.
After 14–20 d in culture almost all the surface GluR1–4 (∼98%; Fig. 5B), NR1 receptors (∼96%; Fig.6B), and a majority of the surface GluR2-containing clusters (∼89%; Fig. 6D) were colocalized with synaptophysin. At this age there was also an increase in the percentage of the total synaptophysin-immunoreactive puncta containing GluR1–4 (∼67%; Fig. 5B) or GluR2 (∼62%; see Fig.6D). The percentage of the total synaptophysin-positive puncta containing NR1 (∼60%) remained essentially the same during development (Fig. 7B).
The developmental increase in the percentage of synaptophysin-positive puncta expressing AMPA receptors could be attributable to the appearance of AMPA receptors at synapses that (1) previously only expressed NMDA receptors or (2) did not express either class of iGluR. To distinguish between these possibilities we have performed colocalization studies of AMPA and NMDA receptors.
During development, NMDA receptors are expressed at the synapse before AMPA receptors
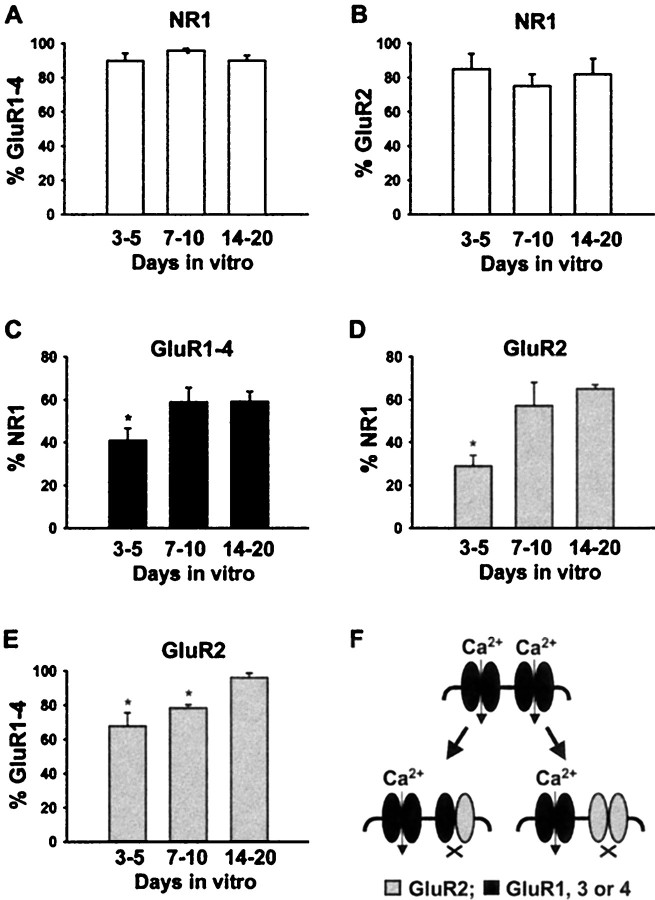
After 3–5 d in culture, numerous NR1 clusters were detected on the surface of pyramidal-shaped neurons (Fig.8A). At this age, only ∼41 and ∼29% of the NR1 clusters colocalized with GluR1–4 or GluR2, respectively (Figs. 8A,9A).
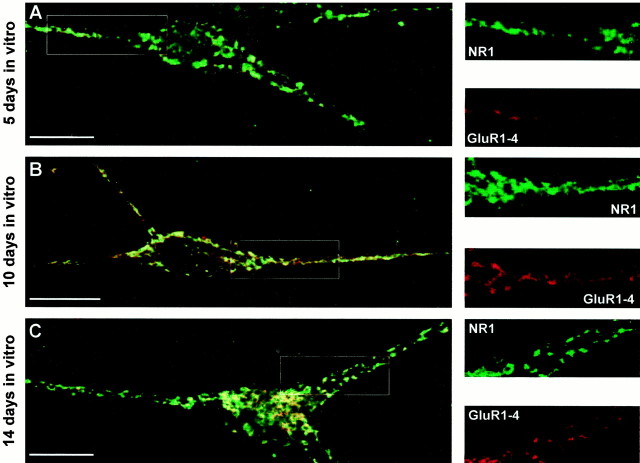
Fig. 8.
Surface distribution of GluR1–4 and NR1 immunoreactivity during development of living hippocampal neurons in culture. Surface distribution of GluR1–4 (red) and NR1 (green) immunoreactivity on living hippocampal neurons in culture for 5 (A), 10 (B), and 14 (C) d. Areas of colocalization are shown in yellow on the left panels. The individual immunoreactivities for GluR1–4 and NR1 in region highlighted in the box are shown in theright panels. In 3- to 5-d-old living neurons (A) 41 ± 6% of surface-expressed NR1 puncta (5 fields, 610 puncta) contained GluR1–4 puncta. This increased to 59 ± 7% (8 field, 1255 puncta) and 59 ± 5% (6 field, 875 puncta) after 7–10 (B) and 14–20 d (C) in culture, respectively. Scale bars, 10 μm.
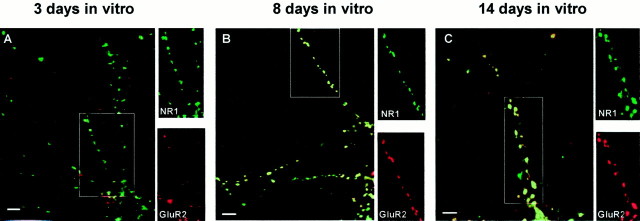
After 7–10 d in culture, the percentage of NR1 clusters that colocalized with GluR1–4 and GluR2 increased by ∼1.5- and 2-fold, respectively, to ∼60% (Figs. 8B, 9B), decreasing the number of NR1 only synapses to ∼40%.
Fig. 9.
Surface distribution of GluR2 and NR1 immunoreactivity during development of living hippocampal neurons in culture. Surface distribution of GluR2 and NR1 immunoreactivity on living hippocampal neurons in culture for 3 (A), 8 (B), and 14 (C) d. Colocalization (yellow) of GluR2 (red) and NR1 (green) immunoreactivity on living hippocampal neurons. The individual immunoreactivities for GluR2 and NR1 in region highlighted in thebox are shown in the side panels. In 3- to 5-d-old neurons (A) 29 ± 5% of surface-expressed NR1 puncta (3 fields, 240 puncta) contained GluR2 puncta. This increased to 57 ± 11% (3 fields, 124 puncta) and 67 ± 2% (3 fields, 173 puncta) after 7–10 (B) and 14–20 d (C) in culture, respectively. Scale bars, 5 μm.
After 14–20 d in culture, the percentage of NR1 clusters colocalized with GluR1–4 remained the same (∼60%), whereas colocalization with GluR2 moderately increased (Figs. 8C, 9C).
These results suggest a developmental rearrangement in the distribution of AMPA receptors within neurons, such that there is an increase in the targeting of AMPA receptors to NMDA receptor containing synapses between days 3 and 20 in culture (Fig.10A–D).
Fig. 10.
Quantification of developmental changes in the colocalization of AMPA and NMDA receptor subunit proteins. The percentage of GluR1–4 (A) and GluR2 (B) clusters that contain NR1 on the surface of living hippocampal neurons at different time points. The number of NR1 clusters containing GluR1–4 (C) or GluR2 (D) increased on the surface of living hippocampal neurons during development. E, The number of GluR2-containing GluR1–4 clusters increased between day 3 and 20 in culture. F, Schematic diagram of two potential molecular mechanisms by which the relative increase in synaptic GluR2 can decrease Ca2+ influx at synapses by forming Ca2+-impermeable AMPA receptors. Mean and SE was calculated based on at least three independent determinations; *p < 0.001 compared with samples in the 14- to 20-d-old groups.
These experiments also revealed an unexpected difference between the colocalization of NR1/GluR1–4 versus NR1/GluR2 at 3–5 d in culture and raised the possibility of developmental changes in surface expressed AMPA receptor subunit composition. We therefore compared the distribution of GluR1–4 and GluR2 subunits directly.
The number of AMPA receptor clusters containing GluR2 subunits increased during development
At 3–5 d in culture ∼67% of GluR1–4-positive puncta contained GluR2 immunoreactivity. This ratio increased to ∼78% at 7–10 d in vitro. After 14 d, colocalization was almost complete (∼96%; Fig. 10E). These data are consistent with the increase in GluR2/NR1 (Fig. 10D) and GluR2/synaptophysin (Fig. 7B) colocalization during development, when compared to changes in GluR1–4/NR1 (Fig.10C) and GluR1–4/synaptophysin (Fig. 7B) colocalization ratios. These results indicate an increase in GluR2 subunit-containing AMPA receptors during development (Fig.10E).
DISCUSSION
Antibodies that recognize all subunit combinations of surface expressed AMPA and NMDA receptors
To allow us to perform studies on live, developing neurons we used site-directed antibodies against extracellular epitopes that should recognize all NMDA and AMPA receptors. AMPA receptors were identified with antibodies raised against fusion proteins derived from the TM3-TM4 linker region of the GluR1flop subunit. The use of these GluR1–4-specific antibodies eliminated the possibility that the observed changes are attributable to changes in subunit composition of AMPA receptors during development or differential targeting of individual subunits in neurons. NMDA receptors were identified by antibodies against N-terminal residues 436–450 of the NR1 subunit. NR1 is part of every NMDA receptor expressed at the cell surface, and the selected sequence is identical in all splice variants of this protein, but different in other iGluR subunits.
The regional distribution of the GluR1–4 and NR1 receptor subunit immunoreactivity was analyzed in the adult rat brain using a histoblot procedure (Tönnes et al., 1999). The direct transfer of native proteins from unfixed frozen tissue sections to an immobilizing matrix offers improved accessibility of the transferred proteins for immunochemical analysis. The histoblot patterns obtained agree well with previous autoradiographic studies using AMPA and NMDA receptor-selective radioligands (Monaghan et al., 1984; Bowery et al., 1988; Insel et al., 1990) and are consistent with the cellular distribution of different AMPA and NMDA receptor subunit mRNAs (Pellegrini-Giampietro et al., 1991; Monyer et al., 1994; Standley et al., 1995). However, some of the previously published immunocytochemical studies performed on fixed tissue reported more uniform distribution of labeling for AMPA receptor subunits in different layers of the cerebral cortex (Petralia and Wenthold, 1992). This difference could be attributable to the fact that synaptic receptors may be relatively inaccessible in the fixed tissue, or there are regional differences in mechanical properties, tissue permeability, or myelin content. The expression of both GluR1–4 and NR1 proteins is strongest in the hippocampus, therefore cultured hippocampal neurons provide a good model system to study the targeting of native AMPA and NMDA receptors.
Both AMPA and NMDA receptors concentrate at synaptic sites on the cell surface during development
In our study, most NMDA receptor clusters colocalized with the presynaptic marker synaptophysin. However, despite its extensive use as a synaptic marker, we cannot formally exclude the possibility that synaptophysin might sometimes occur at nonsynaptic loci or that some synapses might lack synaptophysin. Our results are consistent with electrophysiological data showing that the distribution of the presynaptic and postsynaptic terminals are rapidly synchronized, and most synapses have NMDA receptors throughout development (Tovar and Westbrook, 1999). As neurons develop, synapses acquire AMPA receptors. The ∼1.5 fold increase in the relative number of GluR1–4-positive synaptic puncta correlates only partially with the results of the cell-ELISA experiments, which indicate an approximately twofold increase in total surface expression of GluR1–4 subunits over a similar period of time. This suggests an increase in both the number of AMPA receptor-containing synapses and the density of AMPA receptors at synapses. This result agrees well with electrophysiological data showing that the developmental increase in AMPA receptor EPSCs is attributable to both an increase in synapse number and an increase in quantal size (Gomperts et al., 2000).
In the present study we have detected a small but significant proportion of extrasynaptic clusters of both AMPA and NMDA receptors. This observation is consistent with previous studies on AMPA (Richmond et al., 1996; Nusser et al., 1998) and NMDA receptors (Aoki et al., 1994; Liao et al., 1999) using cultured neurons and immunocytochemical methods. Here we have found a developmental decrease in the proportion of extrasynaptic clusters of both NMDA and AMPA receptors. This may be induced by the activity of newly formed synapses (Rao and Craig, 1997).
NMDA receptor containing synapses progressively acquire AMPA receptors in developing hippocampal neurons
Recent studies have indicated that AMPA and NMDA receptor accumulation at excitatory synapses is independently regulated (Gomperts et al., 1998; Archibald et al., 1999; Liao et al., 1999;Lissin et al., 1999; Lüscher et al., 1999; Noel et al., 1999;Petralia et al., 1999; Shi et al., 1999; Takumi et al., 1999; Gomperts et al., 2000). Early in postnatal development most excitatory hippocampal synapses contain functional NMDA receptors without detectable AMPA receptor responses (Isaac et al., 1995; Liao et al., 1995; Durand et al., 1996). During the first two postnatal weeks, synapses acquire functional AMPA receptors (Durand et al., 1996). Our results are consistent with the evidence that expression and synaptic delivery of AMPA and NMDA receptor subunits are different and that these processes are developmentally regulated in hippocampal neurons. The molecular mechanisms regulating this differential targeting remain largely unknown, but proteins that interact directly with AMPA and NMDA receptor subunits are likely to play central roles in this process (Dong et al., 1997; Kornau et al., 1997; Nishimune et al., 1998;O'Brien et al., 1998a; Osten et al., 1998; Song et al., 1998;Dev et al., 1999; Lüscher et al., 1999; Lüthi et al., 1999;Noel et al., 1999; Wyszynski et al., 1999; Xia et al., 1999; Man et al., 2000).
Other studies have also recently addressed developmental differences in the colocalization of AMPA and NMDA receptors. In an immunogold-labeling study, the amount of immunolabeling per synapse was initially high and remained constant for NMDA receptors. In contrast, gold labeling was initially low and increased during development for AMPA receptors (Petralia et al., 1999). In the most comparable study, the distribution of AMPA and NMDA receptors was analyzed in fixed hippocampal neurons during development in culture (Liao et al., 1999). Our results are in general agreement with the view that NMDA receptor only synapses are replaced by NMDA and AMPA receptor synapses during development, but there are significant quantitative differences between the two studies. For example, at 1 week in vitro >90% of synapses are silent in the study of Liao et al. (1999), whereas we find <50% silent synapses at this time. There are several possible explanations for this difference: for example, (1) our experiments were performed on postnatal cultures compared with embryonic cultures. (2) We labeled AMPA receptors with an antibody that recognizes all GluR1–4 subunits, whereas Liao et al. (1999) used GluR1 and GluR2/3 subunit-specific antibodies. (3) We labeled AMPA and NMDA receptors on the surface of neurons while they were still alive, whereas Liao et al. (1999) identified the total NMDA and AMPA receptor population after fixation and permeabilization.
The use of living neurons offers distinct advantages over fixed or fixed and permeabilized cells, e.g., fixation can hinder access to epitopes by covalent cross-linking, and permeabilization provides access to intracellular pools of receptors that clouds the interpretation of the synaptic distribution of receptors. Finally, antibodies that label living neurons can be used to study AMPA and NMDA receptor dynamics in physiological experiments.
A pertinent issue, in immunocytochemical experiments on both living and fixed cells, is the possibility that low levels of AMPA receptor protein could be misclassified as no AMPA receptor expression because of a lack of sensitivity. This is analogous to the misclassification of failures in electrophysiological experiments. However, we do not consider this to be a major source of error in our study because the mean intensity of the puncta were ∼10-fold greater than the background.
The subunit composition of surface-expressed AMPA receptors changes during development
The GluR2 subunit in the edited form is responsible for the calcium impermeability of AMPA receptors. Our data indicate that early in development a high proportion of AMPA receptors are present at synapses that do not possess GluR2 immunoreactivity. However, after 2 weeks in culture the GluR2 immunoreactivity was present in nearly every AMPA receptor-positive synapse. The relative increase in synaptic GluR2 can reduce Ca2+ influx by forming Ca2+-impermeable AMPA receptors on their own or in combination with other subunits (Fig.10F). It is more likely that in hippocampal neurons GluR2 forms heteromeric AMPA receptor complexes with GluR1 or GluR3 subunits (Wenthold et al., 1996). It is interesting to note that the relative increase in synaptic GluR2 continues during the second week, when there are only moderate changes in the surface expression and total amount of AMPA receptors, suggesting that this process is independently regulated. These results demonstrate that not only the expression and synaptic targeting of AMPA receptors is regulated, but that the subunit composition is also under developmental control.
Since the submission of our manuscript, electrophysiological studies have provided additional support for our results. A rapid and long-lasting change in the subunit composition and Ca2+ permeability of AMPA receptors has been identified at cerebellar stellate cell synapses after synaptic activity (Liu and Cull-Candy, 2000). Additionally, a new AMPA receptor-trafficking model for synaptic plasticity has been proposed, which is based on the differential targeting of GluR2 subunit-containing AMPA receptors (Malinow et al., 2000).
Footnotes
We are grateful to the Medical Research Council, the Royal Society, and the Wellcome Trust for financial support. The mammalian expression vector containing the genes coding for various ionotropic glutamate receptor subunits was the generous gift of Dr. H. Monyer (University of Heidelberg, Heidelberg, Germany).
L.P. and J.N. contributed equally to this work.
Correspondence should be addressed to Dr. Elek Molnar, Medical Research Council Centre for Synaptic Plasticity, Department of Anatomy, University of Bristol, School of Medical Sciences, University Walk, Bristol BS8 1TD, UK. E-mail: Elek.Molnar@bristol.ac.uk.
Dr. Noël's present address: Universite de Nice-Sophia Antipolis, Biologie cellulaire des compartiments calciques, Groupe de neurobiologie fondamentale et clinique, EA 2674 Parc Valrose Faculte des sciences, 06108 Nice Cedex 2, France.
REFERENCES
- 1.Aoki C, Venkatesan C, Go C-G, Mong JA, Dawson TM. Cellular and subcellular localization of NMDA-R1 subunit immunoreactivity in the visual cortex of adult and neonatal rats. J Neurosci. 1994;14:5202–5222. doi: 10.1523/JNEUROSCI.14-09-05202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald K, Molnar E, Henley JM. Differential changes in the subcellular distribution of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate and N-methyl-d-aspartate receptors in neonate and adult rat cortex. Neurosci Lett. 1999;270:49–52. doi: 10.1016/s0304-3940(99)00466-8. [DOI] [PubMed] [Google Scholar]
- 3.Bear MF, Abraham WC. Long-term depression in the hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- 4.Bennett JA, Dingledine R. Topology profile for a glutamate receptor: three transmembrane domains and a channel-lining reentrant membrane loop. Neuron. 1995;14:373–384. doi: 10.1016/0896-6273(95)90293-7. [DOI] [PubMed] [Google Scholar]
- 5.Benke TA, Jones OT, Collingridge GL, Angelides KJ. N-methyl-d-aspartate receptors are clustered and immobilized on dendrites of living cortical-neurons. Proc Natl Acad Sci USA. 1993;90:7819–7823. doi: 10.1073/pnas.90.16.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benke D, Wenzel A, Scheurer L, Fritschy JM, Möhler H. Immunobiochemical characterization of the NMDA-receptor subunit NR1 in the developing and adult rat brain. J Recept Signal Transduct Res. 1995;15:393–411. doi: 10.3109/10799899509045229. [DOI] [PubMed] [Google Scholar]
- 7.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 8.Bowery NG, Wong EHF, Hudson AL. Quantitative autoradiography of [3H]MK-801 binding sites in mammalian brain. Br J Pharmacol. 1988;93:944–954. doi: 10.1111/j.1476-5381.1988.tb11484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll RC, Beattie EC, Xia H, Lüscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci USA. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cockcroft VB, Ortells MO, Thomas P, Lunt GG. Homologies and disparities of glutamate receptors: a critical analysis. Neurochem Int. 1993;23:583–594. doi: 10.1016/0197-0186(93)90107-g. [DOI] [PubMed] [Google Scholar]
- 11.Dev KK, Nishimune A, Henley JM, Nakanishi S. The protein kinase Cα binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology. 1999;38:635–644. doi: 10.1016/s0028-3908(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 12.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 13.Dong HL, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 14.Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 15.Durand J, Kojic L, Wang Y, Lee P, Cynader MS, Gu Q. Confocal imaging of N-methyl-d-aspartate receptors in living cortical neurons. Neuroscience. 2000;97:11–23. doi: 10.1016/s0306-4522(99)00595-3. [DOI] [PubMed] [Google Scholar]
- 16.Gomperts SN, Rao A, Craig AM, Malenka RC, Nicoll RA. Postsynaptically silent synapses in single neuron cultures. Neuron. 1998;21:1443–1451. doi: 10.1016/s0896-6273(00)80662-5. [DOI] [PubMed] [Google Scholar]
- 17.Gomperts SN, Carroll R, Malenka RC, Nicoll RA. Distinct roles for ionotropic and metabotropic glutamate receptors in the maturation of excitatory synapses. J Neurosci. 2000;20:2229–2237. doi: 10.1523/JNEUROSCI.20-06-02229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall RA, Soderling TR. Quantitation of AMPA receptor surface expression in cultured hippocampal neurons. Neuroscience. 1997a;78:361–371. doi: 10.1016/s0306-4522(96)00525-8. [DOI] [PubMed] [Google Scholar]
- 19.Hall RA, Soderling TR. Differential surface expression and phosphorylation of the N-methyl-d-aspartate receptor subunits NR1 and NR2 in cultured hippocampal neurons. J Biol Chem. 1997b;272:4135–4140. doi: 10.1074/jbc.272.7.4135. [DOI] [PubMed] [Google Scholar]
- 20.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 21.Hollmann M, O'Shea-Greenfield A, Rogers SW, Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989;342:643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- 22.Hollmann M, Maron C, Heinemann S. N-glycosilation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1. Neuron. 1994;13:1331–1343. doi: 10.1016/0896-6273(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 23.Huh K-H, Wenthold RJ. Turnover analysis of glutamate receptors identifies a rapidly degraded pool of the N-methyl-d-aspartate receptor subunit, NR1, in cultured cerebellar granule cells. J Biol Chem. 1999;274:151–157. doi: 10.1074/jbc.274.1.151. [DOI] [PubMed] [Google Scholar]
- 24.Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain I. N-methyl-d-aspartate and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- 25.Isaac JTR, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical input. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- 26.Isaac JTR, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishi S. Molecular characterization of the family of the N-methyl-d-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- 28.Klotz IM, Heiney RE. Introduction of sulfhydryl groups into proteins using acetylmercaptosuccinil anhydride. Arch Biochem Biophys. 1962;96:605–612. doi: 10.1016/0003-9861(62)90345-4. [DOI] [PubMed] [Google Scholar]
- 29.Kornau HC, Seeburg PH, Kennedy MB. Interaction of ion channels and receptors with PDZ domain proteins. Curr Opin Neurobiol. 1997;7:368–373. doi: 10.1016/s0959-4388(97)80064-5. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature. 1998;393:695–698. doi: 10.1038/31496. [DOI] [PubMed] [Google Scholar]
- 32.Liao DZ, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 33.Liao D, Zhang X, O'Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci. 1999;2:37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- 34.Lissin DV, Carroll RC, Nicoll RA, Malenka RC, von Zastrow M. Rapid, activity-induced redistribution of ionotropic glutamate receptors in cultured hippocampal neurons. J Neurosci. 1999;19:1263–1272. doi: 10.1523/JNEUROSCI.19-04-01263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S-QJ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- 36.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement by the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 37.Lüscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 38.Lüthi A, Chittajallu R, Duprat F, Palmer MJ, Benke TA, Kidd FL, Henley JM, Isaac JTR, Collingridge GL. Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron. 1999;24:389–399. doi: 10.1016/s0896-6273(00)80852-1. [DOI] [PubMed] [Google Scholar]
- 39.Malinow R, Mainen ZF, Hayashi Y. LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 40.Mammen AL, Huganir RL, O'Brien RJ. Redistribution and stabilization of cell surface glutamate receptors during synapse formation. J Neurosci. 1997;17:7351–7358. doi: 10.1523/JNEUROSCI.17-19-07351.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Man H-Y, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalisation. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 42.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurons. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 43.McIlhinney RAJ, Molnar E. Characterization, cell-surface expression and ligand-binding properties of different truncated N-terminal extracellular domains of the ionotropic glutamate receptor subunit GluR1. Biochem J. 1996;315:217–225. doi: 10.1042/bj3150217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McIlhinney RAJ, Molnar E, Atack JR, Whiting PJ. Cell surface expression of the human N-ethyl-d-aspartate receptor subunit 1a requires the co-expression of the NR2A subunit in transfected cells. Neuroscience. 1996;70:989–997. doi: 10.1016/0306-4522(95)00419-x. [DOI] [PubMed] [Google Scholar]
- 45.McIlhinney RAJ, Le Bourdellès B, Molnar E, Tricaud N, Streit P, Whiting PJ. Assembly intracellular targeting and cell surface expression of the human N-methyl-d-aspartate receptor subunits NR1a and NR2A in transfected cells. Neuropharmacology. 1998;37:1355–1367. doi: 10.1016/s0028-3908(98)00121-x. [DOI] [PubMed] [Google Scholar]
- 46.Molnar E, Baude A, Richmond SA, Patel PB, Somogyi P, McIlhinney RAJ. Biochemical and immunocytochemical characterization of antipeptide antibodies to a cloned GluR1 glutamate receptor subunit: Cellular and subcellular distribution in the rat forebrain. Neuroscience. 1993;53:307–326. doi: 10.1016/0306-4522(93)90198-o. [DOI] [PubMed] [Google Scholar]
- 47.Monaghan DT, Yao D, Cotman CW. Distribution of [3H]AMPA binding sites in rat brain as determined by quantitative autoradiography. Brain Res. 1984;324:160–164. doi: 10.1016/0006-8993(84)90636-x. [DOI] [PubMed] [Google Scholar]
- 48.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 49.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 50.Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- 51.Nishimune A, Isaac JTR, Molnar E, Noel J, Nash R, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- 52.Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, Collingridge GL, Henley JM. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron. 1999;23:365–376. doi: 10.1016/s0896-6273(00)80786-2. [DOI] [PubMed] [Google Scholar]
- 53.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurons. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 54.Nusser Z, Lujan R, Laube G, Roberts JDB, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- 55.O'Brien RJ, Lau L-F, Huganir RL. Molecular mechanism of glutamate receptor clustering at excitatory synapses. Curr Opin Neurobiol. 1998a;8:364–369. doi: 10.1016/s0959-4388(98)80062-7. [DOI] [PubMed] [Google Scholar]
- 56.O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998b;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- 57.Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, Ziff EB. The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron. 1998;21:99–110. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- 58.Pellegrini-Giampietro DE, Bennett MVL, Zukin RS. Differential expression of 3 glutamate receptor genes in developing rat brain –an in situ hybridisation study. Proc Natl Acad Sci USA. 1991;88:4157–4161. doi: 10.1073/pnas.88.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- 60.Petralia RS, Esteban JA, Wang Y-X, Partridge JG, Zhao H-M, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- 61.Pow DV, Crook DK. Extremely high titre polyclonal antisera against small neurotransmitter molecules: rapid production, characterisation and use in light- and electron-microscopic immunochemistry. J Neurosci Methods. 1993;48:51–63. doi: 10.1016/s0165-0270(05)80007-x. [DOI] [PubMed] [Google Scholar]
- 62.Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- 63.Richmond SA, Irving AJ, Molnar E, McIlhinney RAJ, Michelangeli F, Henley JM, Collingridge GL. Localization of the glutamate receptor subunit GluR1 on the surface of living and within cultured hippocampal neurons. Neuroscience. 1996;75:69–82. doi: 10.1016/0306-4522(96)00217-5. [DOI] [PubMed] [Google Scholar]
- 64.Shi S-H, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 65.Sommer B, Keinänen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Köhler M, Takagi T, Sakmann B, Seeburg PH. Flip and Flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- 66.Song I, Kamboj S, Xia J, Dong HL, Liao DZ, Huganir RL. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- 67.Standley S, Tocco G, Tourigny M-F, Massicotte G, Thompson RF, Baudry M. Developmental changes in AMPA receptor proteins and expression in the rat hippocampal formation. Neuroscience. 1995;67:881–892. doi: 10.1016/0306-4522(95)00075-t. [DOI] [PubMed] [Google Scholar]
- 68.Takumi Y, Ramírez-León V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- 69.Tönnes J, Stierli B, Cerletti C, Behrmann JT, Molnar E, Streit P. Regional distribution and developmental changes of GluR1-flop protein revealed by monoclonal antibody in rat brain. J Neurochem. 1999;73:2195–2205. [PubMed] [Google Scholar]
- 70.Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vissavajjhala P, Janssen WGM, Hu Y, Gazzaley AH, Moran T, Hof PR, Morrison JH. Synaptic distribution of the AMPA-GluR2 subunit and its colocalization with calcium-binding proteins in rat cerebral cortex: an immunohistochemical study using a GluR2-specific monoclonal antibody. Exp Neurol. 1996;142:296–312. doi: 10.1006/exnr.1996.0199. [DOI] [PubMed] [Google Scholar]
- 72.Wenthold RJ, Petralia RS, Blahos J, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wenzel A, Fritschy JM, Möhler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 1997;68:469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- 74.Wu GY, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- 75.Wyszynski M, Valtschanoff JG, Naisbitt S, Dunah AW, Kim E, Standaert DG, Weinberg R, Sheng M. Association of AMPA receptors with a subset of glutamate receptor-interacting protein in vivo. J Neurosci. 1999;19:6528–6537. doi: 10.1523/JNEUROSCI.19-15-06528.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]