Abstract
The direct effects of pituitary adenylate cyclase-activating polypeptides (PACAP) on sympathetic neurons were investigated using rat superior cervical ganglion neurons. Electrophysiological and pharmacological analyses were used to evaluate PACAP modulation of sympathetic neuron membrane potentials and to investigate potential ionic and intracellular signaling mechanisms mediating the responses. More than 90% of the sympathetic neurons were depolarized by the PACAP peptides even when stimulated release was blocked, indicating that the PACAP peptides elicited primary responses in the postganglionic neurons. The response profile was consistent for activation of PACAP-selective PAC1 receptors: nanomolar concentrations of PACAP27 and PACAP38 were required to stimulate depolarization, whereas vasoactive intestinal peptide failed to evoke any response. Furthermore, depolarizations elicited by PACAP27 were reduced by the PAC1 receptor antagonist PACAP(6–38). Both sodium influx and inhibition of a potassium current contributed to the peptide-induced depolarizations. Activation of neither pertussis toxin- nor cholera toxin-sensitive G-proteins was required for generation of the depolarizations. cAMP and diacylglycerol production and activation of protein kinase A or protein kinase C also were not requisite for the responses. By contrast, phospholipase C (PLC)-dependent inositol 1,4,5-triphosphate (IP3) synthesis was crucial to the PACAP-mediated depolarizations. Although calcium release from IP3-sensitive stores was not required for the PACAP-induced responses, inhibition of IP3receptors reduced the depolarizations. Thus, among the many signal transduction pathways coupled to the PAC1 receptor, the PACAP-induced depolarization of sympathetic neurons appears to require activation of PLC and subsequent generation of IP3.
Keywords: sympathetic, superior cervical ganglion, autonomic, pituitary adenylate cyclase-activating polypeptide, PACAP, Trp channel, IP3
The primary preganglionic sympathetic neurotransmitter is acetylcholine, and a major noncholinergic stimulatory factor of sympathetic neurons has been hypothesized to belong to the vasoactive intestinal peptide (VIP) family of related peptides (Ip et al., 1982, 1983, 1985; Kawatani et al., 1985; Schwarzschild et al., 1989). Recently, pituitary adenylate cyclase-activating polypeptides (PACAP) have been suggested to be physiologically relevant regulators of sympathetic physiology. The PACAP precursor molecule is posttranslationally processed to produce either the α-amidated 38 or 27 amino acid peptides PACAP38 or PACAP27 (Arimura, 1998). PACAP peptides not only demonstrate sequence homology with the VIP family of peptides, they also share receptors with VIP. The actions of VIP and PACAP on target tissues are mediated by at least three putative seven-transmembrane G-protein-coupled receptor subtypes identified to date (Spengler et al., 1993; Svoboda et al., 1993; Harmar and Lutz, 1994; Journot et al., 1995; Rawlings and Hezareh, 1996). PACAP peptides are more potent than VIP in binding and stimulating multiple intracellular second messenger pathways at the PACAP-selective PAC1 receptor; in contrast, the VPAC1 and VPAC2receptors exhibit approximate equal high affinity for PACAP27, PACAP38, and VIP, and may represent the prototypic VIP receptors solely coupled to adenylyl cyclase (Christophe, 1993; Harmar and Lutz, 1994; Arimura and Shioda, 1995; Journot et al., 1995).
Previously, we demonstrated potent and efficacious PACAP regulation of superior cervical ganglion (SCG) sympathetic neurotransmitter/neuropeptide expression (May and Braas, 1995; Braas and May, 1996, 1999). In accord with the predicted pharmacological response profile for the PAC1receptor, PACAP27 and PACAP38 are more potent than VIP in stimulating SCG neuron neuropeptide Y and catecholamine release, production, and mRNA expression. Molecular characterization demonstrated SCG PAC1 receptor expression, and morphological studies suggested that nearly all of the principal sympathetic neurons expressed the PAC1 receptor but expressed neither of the VPAC receptors (May and Braas, 1995; Moller et al., 1997a,b;Nogi et al., 1997; Beaudet et al., 1998; May et al., 1998; Braas and May, 1999). Moreover, we demonstrated PACAP expression in a subpopulation of preganglionic sympathetic neurons projecting to the SCG (Beaudet et al., 1998). These results are consistent with the potent neurotrophic activities of PACAP peptides in promoting sympathoneuroblast survival and mitosis (DiCicco-Bloom and Deutsch, 1992; DiCicco-Bloom, 1996; Tanaka et al., 1996; Waschek, 1996; Lu et al., 1998). Together, these results provided substantial evidence establishing central roles for PACAP peptides in sympathetic neuron function and development.
PACAP peptides have been shown to depolarize several neuronal cell types (Lai et al., 1997; Braas et al., 1998; Shibuya et al., 1998). Thus, to gain a better understanding of the diverse roles of PACAP peptides in sympathetic neuron physiology, we have initiated electrophysiological and pharmacological studies to assess both the ionic mechanisms and potential second messenger pathways generating PACAP-induced membrane depolarizations. Both influx of sodium and reduced potassium currents contributed to the PACAP-induced depolarizations. Among the many signal transduction pathways coupled to the PAC1 receptor, the PACAP-induced depolarization of sympathetic postganglionic neurons appeared to require activation of phospholipase C (PLC) and subsequent generation of inositol 1,4,5-triphosphate (IP3).
MATERIALS AND METHODS
Cell culture. Primary SCG neuron cultures were prepared as described previously (May and Braas, 1995; May et al., 1995). All methods involving animals were approved by the University of Vermont Institutional Animal Care and Use Committee. Untimed pregnant Sprague Dawley rats (Charles River, Quebec, Canada) were given commercial rat chow and tap water ad libitum and maintained on a 12 hr light/dark cycle. Donor neonatal rats were rapidly decapitated, and the SCGs from four to five litters (typically 40–60 animals; 80–120 ganglia) were dissociated enzymatically to produce a pooled population of cells. Cells were plated at an initial density of 3 × 103neurons/cm2 onto rat tail collagen-coated Aclar mini-plates (5 cm2 dishes), treated with 10 μm cytosine β-d-arabinofuranoside (Sigma, St. Louis, MO) to eliminate non-neuronal cells, and maintained in defined complete serum-free medium containing 50 ng/ml nerve growth factor (Becton Dickinson Labware, Bedford, MA) (May et al., 1995).
Bathing solutions and electrophysiological recordings. The Aclar miniplate neuron cultures were secured to a Sylgard-lined recording chamber and superfused continuously (6 ml/min) with either oxygenated Krebs' buffer containing (in mm): 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, and 8 glucose, pH 7.3, or a HEPES-buffered physiological solution containing (in mm): 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 8 glucose, and either 26 sodium HEPES, or 26 potassium HEPES, depending on the need to eliminate sodium ions, pH 7.35, at 35–37°C. The compositions of the bathing solutions were modified to investigate the nature of the ionic conductance underlying the PACAP-induced depolarization. A sodium-deficient solution was prepared by substituting 121 mmN-methyl-d-glucamine chloride (NMG; Sigma) for NaCl in the potassium HEPES-buffered solution. To prepare a high potassium solution, the KCl concentration in the HEPES-buffered solution was increased to 20 mm; because the sodium concentration was not modified, this resulted in a small increase in solution osmolarity (∼9%). The osmolarity of the sodium-substituted HEPES-buffered solution was 13% less than the control Krebs'-buffered solution. This slight increase or decrease in osmolarity did not alter the morphological characteristics of the sympathetic neurons. For some experiments, tetrodotoxin (TTX; 300 nm; Sigma) or cadmium (200 μm) was added to either the Krebs'- or HEPES-buffered physiological solutions.
SCG neurons were impaled with potassium citrate (2m)-filled borosilicate microelectrodes (60–80mΩ), and the responses were recorded using an Axoclamp 2A amplifier (Axon Instruments, Foster City, CA). Recordings were made from single isolated neurons or neurons within small clusters (three to five neurons). Neurons were selected based on plasma membrane integrity and neuronal size; impalements were more stable from larger neurons (30–50 μm diameter). Membrane currents were measured in discontinuous single-electrode voltage-clamp mode with a sampling frequency of 8–10 kHz and a 30% current/70% voltage duty cycle. To analyze the voltage dependence of the peptide-induced membrane currents, current–voltage (I–V) relationships were established by recording membrane currents elicited by computer-generated voltage ramps from −120 to −30 mV (pClamp Software, Version 6.0; Axon Instruments) at 25 mV/sec and a sampling rate of 500 Hz. Data were collected on a Gould Brush 2400 chart recorder (Gould Instrument Systems, East Rutherford, NJ) and stored on a PCM recorder (A. R. Vetter Company, Rebersburg, PA); data were acquired digitally for subsequent analysis using the Clampfit program (pClamp 6.03, Axon Instruments).
Peptide and drug application. PACAP peptides and VIP were delivered by either chamber superfusion or local pressure application. In the superfusion studies, peptides were diluted from 100 μm stocks to the final concentrations in bathing medium containing 1 mg/ml bovine serum albumin to prevent peptide adsorbance to the perfusion tubing. For local pressure application (Picospritzer, General Valve, Fairfield, NJ), 50 μm peptide in bath solution was released from small-diameter pipettes (5–10 μm) positioned at a distance of 50–100 μm from the cell.N-ethylmaleimide (NEM; 50 μm; Sigma), pertussis toxin (PTX; 500 ng/ml; List Biological Laboratories, Campbell, CA), and cholera toxin (CTX; 500 ng/ml; List Biological Laboratories) were prepared from 1000-fold aqueous stocks. Final concentrations of N6,O2′-dibutyryl cAMP (dBcAMP; 1 mm) and 8-bromo cAMP (8-Br-cAMP; 1 mm; Boehringer Mannheim, Indianapolis, IN) were prepared directly in Krebs' buffer. Forskolin (10 μm), H-89 (25 μm),U73122 (5.6 μm), BimI (2.5 μm), phorbol myristate acetate (PMA; 500 nm), 1-oleoyl-2-acetyl-sn-glycerol (OAG; 200 μm), xestospongin C and xestospongin D (XeC and XeD; 10 and 20 μm, respectively) (all from Calbiochem–Novabiochem, La Jolla, CA), thapsigargin (100 nm; RBI, Natick, MA), and cyclopiazonic acid (CPA; 10 μm; Alexis Corp., San Diego, CA) were prepared from 1000-fold DMSO stocks. For XeC and XeD, bovine serum albumin was deleted from the serum-free defined medium to obviate potential extraneous protein binding (Gafni et al., 1997). Sympathetic neurons were treated directly in the recording bath with dBcAMP or forskolin before pressure application (1 sec) of PACAP27. SCG neurons were preincubated in defined medium containing H-89, U73122, BimI, XeD, XeC, thapsigargin, or CPA for 20–30 min (acute treatments); additional cells were incubated in defined medium containing PTX, CTX, or PMA for 12–15 hr (chronic treatments). After acute or chronic drug treatments, the cells were transferred to the recording chamber for peptide application. Cells were loaded with BAPTA using the membrane-permeant form of the chelator, BAPTA tetra(acetoxymethyl) ester (BAPTA/AM; 10 μm for 15 min at 37°C; Calbiochem–Novabiochem Corp.), and then maintained in defined medium without BAPTA/AM for 20–30 min at 37°C to allow cytoplasmic esterases to de-esterify the molecule. Inhibitors and activators were used at previously established concentrations shown to be effective in our SCG neuron in vitro preparations (May et al., 1995;Braas and May, 1999).
Messenger RNA analysis. Total RNA from brain, SCG, and SCG neuron cultures was prepared using RNA STAT-60 total RNA/mRNA isolation reagent (Tel-Test “B”, Friendswood, TX) as described previously (May and Braas, 1995; Beaudet et al., 1998; Braas et al., 1998). The RNA from brain (2 μg), individual SCG ganglion, or single culture well (3 × 104 neurons) was used to synthesize first-strand cDNA using SuperScript II reverse transcriptase and oligo dT primers with the SuperScript Preamplification System (Life Technologies, Gaithersburg, MD) in a 22 μl final reaction volume. The cDNA was diluted 1:10, and 0.5 μl of the template was used for amplification; amplification of the cDNA templates was performed in a 13 μl reaction volume consisting of 12.5 mm Tris-HCl, pH 8.3, containing 62.5 mm KCl, 2.5 mm MgCl2, 200 μmdeoxynucleotide triphosphates, 0.5 μm primers, 0.5 μl of cDNA template, and 0.3 U AmpliTaq Gold DNA polymerase (PE Applied Biosystems, Norwalk, CT) (May and Braas, 1995) with the cycling parameters as follows: (1) initial denaturation/enzyme activation, 95°C, 10 min; (2) denaturation/enzyme activation, 94°C, 45 sec; annealing, transcript-specific temperature, 30 sec; 72°C, 45 sec (35 cycles); (3) final extension, 72°C, 5 min. Amplification was conducted using oligonucleotide primers specific for the identification of transient receptor potential (Trp) channel mRNA (Table1); for each sample, the same cDNA template was used for amplification of the different Trp mRNA forms. The amplified products were resolved on 2% agarose–GelTwin II (J. T. Baker, Phillipsburg, NJ) gels and visualized by ethidium bromide staining under UV illumination. Complementary DNA synthesis in the absence of either RNA or reverse transcriptase, or amplification without template, primers, or DNA polymerase failed to yield products.
Table 1.
RT-PCR gene-specific primers
| Oligo | Specificity | Sequence | Position | Ta(°C) | Predicted product size (bp) |
|---|---|---|---|---|---|
| Trp1-1 | Rat Trp1 | 5′-CGTAAGCCCACCTGTAAGAAGATAA-3′ | 1003–10271-a | 53.5 | 373 |
| Trp1-2 | Rat Trp1 | 5′-CCAAGTAAAGGGAATTCATAACAAAG-3′ | 1350–13751-a | ||
| Trp2-1 | Rat Trp2 | 5′-AGCCAGCGGTAGTGCGTCGTCT-3′ | 369–3901-b | 60.8 | 413 |
| Trp2-2 | Rat Trp2 | 5′-CTTAAACTCAGGCTCCTTCCGTGCA-3′ | 757–7811-b | ||
| Trp3-1 | Rat Trp3 | 5′-TGTAACTATGGTGGTCGTTCTGCTCA-3′ | 45–701-c | 55.2 | 363 |
| Trp3-2 | Mouse Trp3 | 5′-TGATATCGTGTTGGCTGATTGAGAAT-3′ | 2266–22911-d | ||
| Trp4-1 | Rat Trp4 | 5′-CCTACTTGAATGCCGTGGAAAAG-3′ | 220–2421-e | 56.3 | 343 |
| Trp4-2 | Rat Trp4 | 5′-AAGATGATAGGCGTGATGTCTGG-3′ | 540–5621-e | ||
| Trp5-1 | Rat Trp5 | 5′-AGTTTGTGGGAGCTACTATGTTTGG-3′ | 23–471-f | 54.9 | 431 |
| Trp5-2 | Mouse Trp5 | 5′-TTCTTCTGTTAGCCCCTCATTTGTT-3′ | 2601–26251-g | ||
| Trp6-1 | Rat Trp6 | 5′-TCTTTCTGAAGTGAAGTCGGTGGTCA-3′ | 33–581-h | 55.1 | 465 |
| Trp6-2 | Mouse Trp6 | 5′-CTCCTTGTACTTGATTGTTTGTTGTGTGC-3′ | 2344–23721-i | ||
| Trp7-1 | Mouse Trp7 | 5′-GCAAAGTACAACCCAGCGTTTACCAC-3′ | 1913–19381-j | 57.2 | 558 |
| Trp7-2 | Mouse Trp7 | 5′-CACGTATCTCTTTATGAGCCGCTTCA-3′ | 2445–24701-j |
a–jPrimer positions are given as the nucleotide sequences for the available rat or mouse transient receptor potential protein (Trp) cDNA with the following GenBank accession numbers:
AF061266,rattus norvegicus Trp1 mRNA;
AF136401, rattus norvegicus Trp2 mRNA;
AF061875 rattus norvegicusTrp3 mRNA;
AF190645 mus musculusTrp3 mRNA;
AB008889, rattus norvegicus Trp4 mRNA;
AF061876,rattus norvegicus Trp5 mRNA;
AF060107, mus musculus Trp5 mRNA;
AF061877, rattus norvegicus Trp6 mRNA;
AF057748m mus musculus Trp6;
AF139923, mus musculus Trp7 mRNA.
Curve fitting and statistical methods. All data represent mean neuron responses ± SEM. After pressure application of peptide, the PACAP-induced depolarization recovered slowly such that after 3 and 10 min of wash, the current amplitude was 26 ± 6% and 33 ± 6% of the initial response, respectively. Thus, only one PACAP-induced response was determined per neuron to avoid changes in peptide sensitivity noted with multiple PACAP applications. PACAP concentration-dependence curves were generated assuming a single-site ligand binding isotherm using least squares regression analyses (SigmaPlot 4.0; SPSS Inc., Chicago, IL). Student's t test or one-way ANOVA was used to determine differences among treatments; Newman–Keuls test was used in post hoc analysis to identify which treatments differed from the others. p < 0.05 was considered significant.
RESULTS
PACAP peptides depolarize SCG neurons
Intracellular recordings were compiled from >175 cells from at least 25 different dissociated SCG neuron preparations. The average membrane resting potential recorded from the neonatal SCG neuronsin vitro was −55 ± 0.7 mV. The mean membrane input resistance, determined from the application of 500 msec hyperpolarizing −0.1 nA current pulses, was 153 ± 24 mΩ (n = 17); the majority of SCG neurons generated overshooting action potentials that were followed by membrane afterhyperpolarizations. Most of this action potential activity under resting conditions was eliminated during treatment with 200 μm cadmium, indicating that it resulted primarily from synaptic activation.
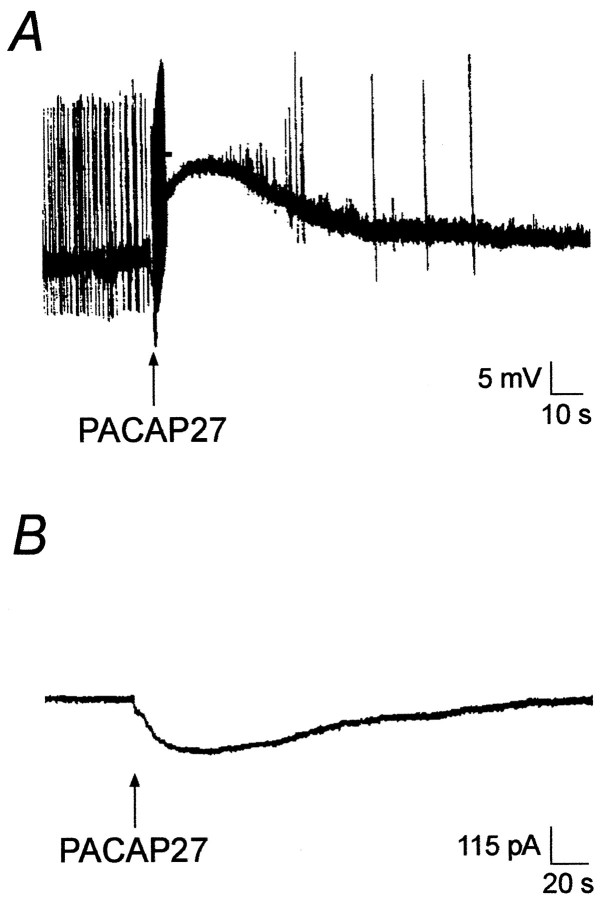
Exposure of SCG neurons to 100 nm PACAP27 elicited membrane depolarizations in >90% of the neurons examined, and the efficacy of PACAP27, applied by either pressure ejection or superfusion, on neuron depolarization was comparable. Pressure application of 50 μm PACAP27 depolarized sympathetic neurons 12.6 ± 0.7 mV (n = 35) (Fig.1A); superfusion of 100 nm PACAP27 for 30 sec depolarized SCG membranes 10.0 ± 0.5 mV (n = 33). An increase in action potential activity commonly accompanied the initial phase of the PACAP-induced depolarization. A similar increase in firing rate occurred with comparable electrotonic depolarization, suggesting that the change in activity resulted, at least in part, from membrane depolarization (data not shown).
Fig. 1.
PACAP27 induces membrane depolarizations and inward currents in sympathetic neurons. A, PACAP-induced depolarization of a sympathetic neuron in response to 1 sec pressure ejection of 50 μm PACAP27 (arrow).B, In the presence of 300 nm TTX, pressure application of 50 μm PACAP27 to sympathetic neurons voltage-clamped to −50 mV revealed the current underlying the PACAP-induced depolarizations.
To ensure that the PACAP-induced membrane depolarizations represented direct neuron responses to PACAP peptides and were not secondary to PACAP-induced release of other neuroregulators, the neurons were treated with either TTX or cadmium before peptide application to block stimulated neurotransmitter or neuromodulator secretion from adjacent terminals. In these experiments, superfusion of 100 nmPACAP27 in the presence of 300 nm TTX or 200 μm cadmium depolarized SCG neurons 12.9 ± 0.9 mV (n = 14) and 9.0 ± 0.8 mV (n = 8), respectively, values that were similar to PACAP-induced depolarizations in the absence of TTX or cadmium. With 300 nm TTX added to the Krebs' solution to inhibit voltage-gated sodium channels and thus eliminate action potential generation, pressure application of 50 μmPACAP27 onto 49 sympathetic neurons, voltage-clamped to −50 mV, produced a mean inward current of −146 ± 9 pA (Fig.1B). These results provided direct evidence that PACAP peptides elicit primary responses in sympathetic postganglionic neurons.
To test whether the PACAP-induced depolarizations were associated with changes in the membrane resistance, 200 msec constant current pulses that elicited 10–15 mV hyperpolarizations were applied before and during PACAP application. Changes in the sizes of the transient hyperpolarizations were used to assess PACAP-induced changes in input resistance. The measurements of input resistance in the presence of PACAP were obtained after electrotonically nulling the PACAP-induced depolarization. The average change in membrane resistance was not different before (185 ± 35 mΩ) or at the peak of the depolarization (185 ± 30 mΩ). However, the PACAP-induced change in membrane resistance was variable: the membrane resistance decreased in four cells, increased in six cells, and was unchanged in one neuron, suggesting that a change in more than one ionic conductance very likely was involved in the generation of the PACAP-induced depolarization.
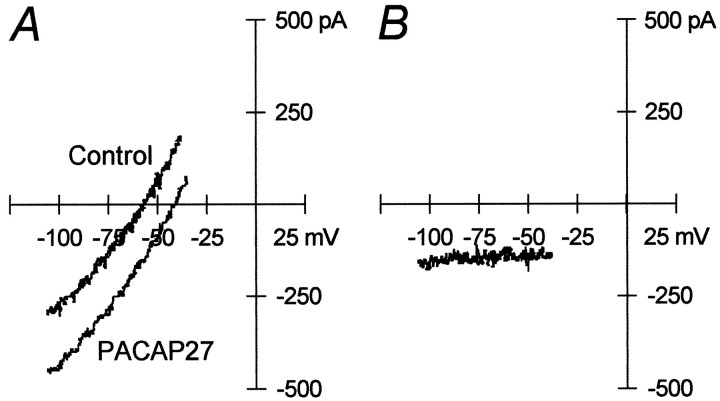
Results of voltage ramp studies supported the conclusion that the PACAP-induced depolarization resulted from modulation of more than one ionic conductance. Neurons were voltage-clamped to −50 mV, and a slow voltage ramp (25 mV/sec) was applied over the voltage range of −120 to −30 mV before and at the peak of the inward current produced by a 1 sec pressure application of PACAP27 (Fig.2A). The PACAP-induced current was determined as the difference in the current recorded before PACAP application from the total current recorded in the presence of PACAP. The results demonstrated the presence of a PACAP-induced inward current over the entire voltage range, which could not be extrapolated to an apparent reversal potential (Fig. 2B) (n = 7). This observation was consistent with both activation of an inward current and inhibition of an outward current contributing to the PACAP-induced current.
Fig. 2.
Current–voltage studies support PACAP modulation of multiple conductances. A,I–V curves generated by voltage ramps from −120 to −30 mV at 25 mV/sec were measured before (Control) and during the peak (PACAP27) of PACAP-induced inward currents.B, Data denote differences in ramp currents before and after PACAP application. Representative data from seven separate experimental recordings.
PACAP-induced depolarizations are mediated by PAC1 receptors
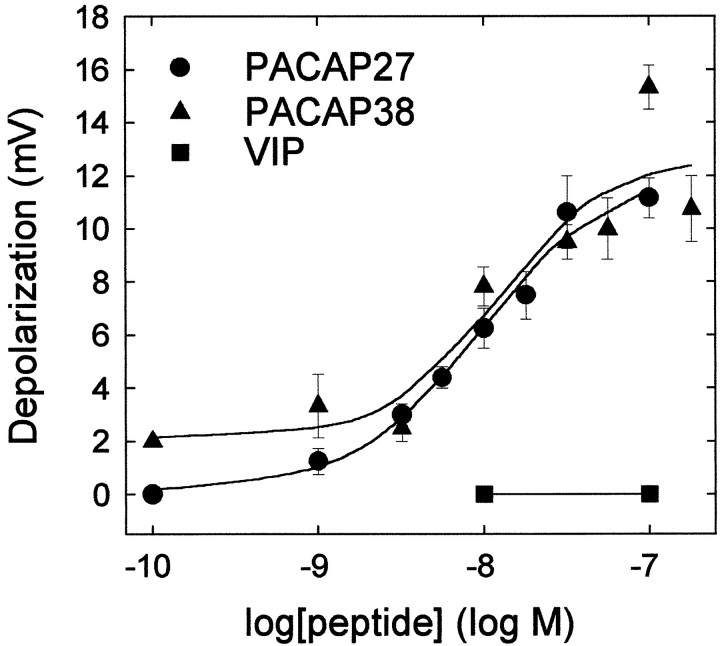
The concentration dependence of PACAP and VIP peptides in eliciting sympathetic neuron depolarizations was examined to establish whether the response profile was consistent for PAC1 receptor activation. Superfusion of varying concentrations of PACAP27 or PACAP38 onto sympathetic neurons current-clamped to −50 mV produced nearly identical concentration–response profiles (Fig.3). Maximal depolarizations of 10–12 mV were obtained with 32–100 nm PACAP27 or PACAP38; half-maximal responses were observed with <10 nm peptide. By contrast, VIP, which shares >68% amino acid sequence identity with PACAP27, failed to elicit any depolarization at the concentrations tested (Fig. 3). This concentration-dependence profile for PACAP and VIP was indicative of PACAP-selective PAC1receptor activation as a primary mechanism mediating the SCG depolarization.
Fig. 3.
PACAP-induced SCG sympathetic neuron depolarizations are concentration-dependent. SCG neurons were superfused (30 sec) with the indicated concentrations of PACAP27 (●), PACAP38 (▴), or VIP (▪), and neuronal depolarization was measured by intracellular recording. Maximal depolarizations of ∼10 mV were attained with 32–100 nm PACAP peptides; PACAP27 and PACAP38 demonstrated identical half-maximal responses at 5 nm peptide. The data represent the mean depolarization (mV) ± SEM (n = 3–7 neurons per concentration).
To assess further the specificity of the PACAP-induced responses, sympathetic neurons were pretreated with 100 nmPACAP(6–38) for 15 min before 100 nm PACAP27 or PACAP38 superfusion. PACAP(6–38) reduced PACAP27-induced depolarizations 88% (1.5 ± 2 mV; n = 4; p < 0.001). In contrast, PACAP(6–38) did not reduce PACAP38-elicited responses (13.3 ± 2 mV; n = 3; p = 0.069). The pharmacological profile for PACAP and VIP in the concentration-dependence studies and the peptide antagonist effects were consistent with PACAP-selective PAC1receptor activation as a primary mechanism mediating the SCG response.
PACAP-induced depolarizations result from mechanisms requiring extracellular sodium and a reduction of a potassium current
Experiments were undertaken to examine the ionic mechanisms underlying the observed PACAP-induced depolarizations. For sympathetic neurons, held at −50 mV and maintained in the HEPES-buffered physiological solution containing 300 nm TTX to eliminate action potential generation, a 1 sec pressure application of 50 μm PACAP27 elicited inward currents of −142 ± 16 pA (n = 6). To investigate whether extracellular sodium contributed to the PACAP-induced currents recorded in these neurons voltage-clamped at −50 mV, the sodium ion concentration in the HEPES-buffered bathing solution was decreased by replacing sodium ions with the membrane-impermeant ion NMG (sodium-deficient solution). In the sodium-deficient solution, the PACAP-induced currents were reduced ∼70% from a control value of −142 ± 16 pA to −40 ± 10 pA (n = 8; p < 0.001) (Table2). Therefore, sodium influx appeared to be a critical factor contributing to the PACAP-induced currents.
Table 2.
Electrophysiological recording solutions used to assess ionic mechanisms underlying the PACAP-induced current
| Solution | Current amplitude (pA) |
|---|---|
| HEPES buffer | −142 ± 16 (6) |
| HEPES buffer + 20 mm KCl | −92 ± 11 (8)* |
| HEPES buffer + NMG | −40 ± 10 (8)* |
| HEPES buffer + 20 mm KCl + NMG | −12 ± 3 (10)* |
Sympathetic neurons were voltage-clamped at −50 mV, and the current amplitude measured after PACAP27 (1 sec) pressure application. The data represent the mean current amplitude ± SEM. The numbers of cells tested are shown in parentheses.
Significantly different from control (p < 0.05).
Subsequent studies tested whether a component of the PACAP-induced currents could be attributed to inhibition of a potassium conductance. Initially, PACAP-induced currents were compared in cells maintained in the sodium-deficient NMG solution with control and elevated potassium concentrations. Elevation of the extracellular potassium concentrations from control levels of 5.9 mm to 20 mm was expected to shift the potassium equilibrium from approximately −90 mV to approximately −50 mV (Schofield and Ikeda, 1989) and thus should eliminate the contribution of any potassium current component in cells voltage-clamped at −50 mV. The PACAP-induced current recorded from cells maintained in the HEPES-buffered sodium-deficient solution containing elevated potassium was −12 ± 3 pA (n= 10) (Table 2), a value significantly less than that obtained in the sodium-deficient solution containing 5.9 mmpotassium (−40 ± 10 pA; p = 0.043).
To further appraise the contribution of the potassium conductance to the PACAP-induced current, the effects of elevating the external potassium concentration in the presence of sodium ions were also evaluated in complementary experiments. Increasing the external potassium concentration to 20 mm in the HEPES-buffered medium decreased the PACAP-induced inward currents by ∼35% (−92 ± 11 pA; n = 8) (Table 2), compared with currents in the same solution containing 5.9 mmpotassium (−142 ± 16 pA; n = 6;p = 0.002). Thus, in neurons voltage-clamped at −50 mV, both sodium influx and inhibition of a potassium conductance contributed to the generation of the PACAP-induced currents.
To test whether calcium influx contributed to the residual PACAP-induced current present in the sodium-deficient solution, PACAP-induced currents were measured at −50 mV in neurons maintained in a HEPES-buffered, NMG-substituted 20 mm KCl solution with either no added calcium (nominally calcium deficient) or elevated calcium (5 mm). In sodium- and calcium-deficient solution, the PACAP-induced current was −20 ± 2.2 pA (n = 5); although larger, the current was not statistically different from that recorded when external calcium was 2.5 mm(−12 ± 3.1 pA, n = 10). When external calcium in the sodium-deficient solution was raised to 5 mm, there was no measurable PACAP-induced current (n = 5); thus elevation of external calcium inhibited the residual inward current.
Experiments also were completed to establish whether elevation of external calcium levels to 5 mm inhibited PACAP-induced current in the presence of sodium. In preparations maintained in HEPES-buffered sodium solution with 20 mm KCl and 5 mm calcium, the PACAP-induced current was −34 ± 10 pA (n = 5), an averaged current value significantly different from currents recorded in neurons maintained in comparable solution containing 2.5 mm calcium (−92 ± 11 pA, n = 8; p < 0.01).
Neither PTX- nor CTX- sensitive G-proteins are involved in the PACAP-induced depolarizations
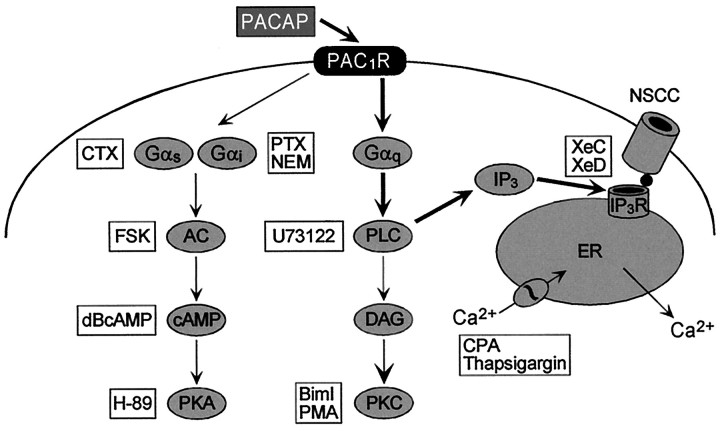
The PAC1 receptor belongs to group III of G-protein-coupled receptors and can initiate several transduction cascades, including the adenylyl cyclase and PLC signaling pathways (Fig. 4) (Absood et al., 1992; Deutsch and Sun, 1992; Hashimoto et al., 1993; Spengler et al., 1993; Journot et al., 1995; Braas and May, 1996, 1999; Lu et al., 1998). To investigate the potential roles of Gαs, SCG neurons were treated with 500 ng/ml CTX for 12–15 hr to downregulate Gαs subunits (Murayama and Ui, 1984; Kaziro et al., 1991); chronic CTX treatment did not inhibit PACAP-induced depolarization in any of the cells examined (Table3). In evaluating the potential roles of Gαi/o, superfusion of cultured sympathetic neurons with the sulfhydryl alkylating agent NEM (50 μm), shown previously to selectively target Gαi/o(Shapiro et al., 1994), had no effect on membrane potential and did not occlude the PACAP-initiated depolarizations in SCG neurons (Table 3). Additionally, selective ADP-ribosylation of cellular Gαi/o subunits by treatment with 500 ng/ml PTX for 12–15 hr also failed to attenuate the peptide-induced depolarizations (Table 3). Hence it appeared that neither Gαs nor Gαi/o mediated the SCG PAC1 receptor-induced depolarizations.
Fig. 4.
Schematic representation of PAC1receptor intracellular signaling. PAC1 receptors are coupled to SCG sympathetic neurons adenylyl cyclase and PLC, resulting in diverse intracellular signaling responses. Second messenger pathway activators and inhibitors used to elucidate the signaling mechanisms underlying the PACAP-induced depolarization are indicated inboxes. The data suggested that PACAP-induced stimulation of IP3 production results in IP3 receptor activation; activated IP3 receptors may directly gate a nonselective cationic conductance, postulated to be a mammalian Trp family channel. PAC1R, PACAP-selective, PAC1 receptor; CTX, cholera toxin; FSK, forskolin; dBcAMP, dibutyryl cAMP; PTX, pertussis toxin; NEM,N-ethylmaleimide; BimI, bisindolylmaleimide I; PMA, phorbol myristate acetate;CPA, cyclopiazonic acid; XeD, xestospongin D; XeC, xestospongin C;IP3R, IP3 receptor;NSCC, nonselective cationic conductance;AC, adenylyl cyclase; PKA, protein kinase A; PLC, phospholipase C; DAG, diacylglycerol; PKC, protein kinase C.
Table 3.
Effects of second messenger activators or inhibitors on PACAP-induced neuron depolarizations
| Treatment | Membrane potential (mV) | PACAP-induced depolarizations (mV) |
|---|---|---|
| Vehicle, water | −56 ± 2 | 12.9 ± 0.9 (14) |
| NEM (50 μm) | −56 ± 3 | 12.5 ± 0.7 (8) |
| PTX (500 ng/ml) | −59 ± 3 | 14.6 ± 1.2 (10) |
| CTX (500 ng/ml) | −64 ± 4 | 13.5 ± 1.4 (6) |
| dBcAMP (1 mm) | −54 ± 1 | 11.5 ± 0.5 (4) |
| Vehicle, DMSO | −54 ± 10 | 15.6 ± 1.3 (10) |
| Forskolin (10 μm) | −59 ± 3 | 11.5 ± 1.2 (4) |
| H-89 (25 μm) | −56 ± 5 | 12.4 ± 1.9 (5) |
| U73122 (5.6 μm) | −46 ± 1 | 1.1 ± 0.5 (7)3-150 |
| Biml (2.5 μm) | −58 ± 3 | 13.3 ± 1.5 (6) |
| PMA (500 nm) | −52 ± 8 | 11.0 ± 0.6 (3) |
| XeD (20 μm) | −52 ± 5 | 7.6 ± 1.2 (10)3-150 |
| XeC (10 μm) | −47 ± 3 | 7.7 ± 1.8 (3)3-150 |
SCG neurons in vitro were incubated in defined medium containing the indicated drugs as described in Materials and Methods. The effects of these agents on PACAP27-induced depolarization (pressure application) were examined in the presence of 300 nm TTX. The data represent the mean membrane potential or depolarization ± SEM, and the numbers of cells tested under each paradigm are shown in parentheses.
F3-150: Significantly different from vehicle control (p < 0.05). The resting membrane potential of neurons in Krebs' solution in the absence of TTX was −55 ± 1 mV (n = 35); PACAP27-induced depolarization in these cells was 12.6 ± 0.7 mV.
PACAP-induced SCG neuron depolarizations are not mediated by activation of protein kinase A or protein kinase C
Subsequent studies tested the potential involvement of the cAMP/protein kinase A (PKA) pathway in generating the PACAP-induced depolarizations using activators or inhibitors of this signaling cascade. Inhibition of SCG neuron PKA with the selective kinase inhibitor H-89 (25 μm) had no apparent effects on the depolarizations (Table 3). In addition, neither direct activation of adenylyl cyclase by forskolin (10 μm) nor application of the cAMP analogs, dBcAMP (1 mm) or 8-Br-cAMP (1 mm), simulated the PACAP-induced depolarizations (data not shown). Furthermore, pretreatment of sympathetic neurons with either forskolin or dBcAMP did not affect the magnitude of the PACAP-mediated depolarizations (Table 3). These observations indicated that the depolarizations elicited by PACAP were not mediated by the generation of cAMP or subsequent activation of PKA.
To investigate the potential roles of PLC activation by PACAP in eliciting sympathetic neuron depolarizations, cells were pretreated with the PLC inhibitor U73122. Pretreatment of cells with 5.6 μmU73122 for 20 min reduced the PACAP-induced depolarizations >90% (1.1 ± 0.5 mV; n = 7;p < 0.001) (Table 3). These results suggested that PLC activation was critical for the PACAP-induced depolarizations. Additional experiments were conducted to test whether the PACAP-mediated depolarizations were secondary to either diacylglycerol (DAG) production or protein kinase C (PKC) activation, or both (Chyb et al., 1999). The effects of the membrane-permeable DAG analog OAG was examined to determine whether the PACAP-induced production of DAG was potentially involved in the generation of the sympathetic neuron depolarizations. Superfusion of 100 or 200 μm OAG did not elicit depolarization in any cells tested (n = 5); additionally, pretreatment with OAG did not occlude the depolarizations elicited by PACAP (data not shown). The results suggested that PACAP-stimulated generation of DAG does not directly mediate the depolarizations. Subsequently, SCG neurons were treated with PMA acutely to activate PKC; alternatively, sympathetic neurons were chronically treated with PMA to downregulate PKC, or cells were exposed to the PKC inhibitor BimI (2.5 μm) before PACAP application. Acute treatment of cells with 500 nm PMA did not mimic the PACAP-induced depolarizations (data not shown); similarly, neither pretreatment of cells with PMA for 12–16 hr to downregulate PKC protein levels nor incubation with the PKC inhibitor BimI had an effect on the depolarizations (Table 3).
A component of the SCG PACAP-induced depolarizations is mediated by the IP3 receptor
The preceding results indicated that neither PKC activation nor DAG generation were involved in the PACAP-induced depolarization. Additional experiments tested whether PACAP-stimulated IP3 production might be involved (Fig. 4). Recently, several cell-permeant xestospongin compounds have been described that inhibit IP3 receptors (Gafni et al., 1997). Among these reagents, XeD and XeC were pharmacologically active at micromolar concentrations, with XeC demonstrating greater potency between the two compounds. These compounds were used to investigate potential roles of IP3 in the generation of the depolarizations induced by PACAP. Pretreatment of SCG neurons with XeD (20 μm) or XeC (10 μm) for 20 min decreased the PACAP-elicited depolarizations 51% (p < 0.001; p = 0.012, respectively) (Table 3).
To investigate whether calcium ions released from intracellular stores in response to PACAP-stimulated IP3 production and subsequent IP3 receptor activation elicited the peptide-mediated depolarizations, SCG neurons were pretreated with 10 μm CPA or 100 nm thapsigargin to inhibit the endoplasmic reticulum calcium ATPase, thus allowing internal cellular calcium stores to deplete progressively. CPA or thapsigargin alone did not alter the resting potential of the sympathetic neurons; in addition, neither CPA nor thapsigargin pretreatment diminished the PACAP-induced membrane depolarizations (Table4). PACAP-stimulated depolarizations were also examined in cells loaded with the calcium chelator BAPTA, which efficiently buffers transient elevations of intracellular calcium (Neher, 1998). Pretreatment of the sympathetic neurons with BAPTA/AM (10 μm), the membrane-permeable form of the calcium chelator, had no effect on the magnitude of the PACAP-induced depolarizations (Table 4). On the basis of these observations, we conclude that release of calcium from IP3-sensitive stores was not involved in the generation of the PACAP-induced depolarization. Thus, we hypothesized that the IP3-induced component of the PACAP-induced depolarizations resulted from direct coupling of IP3 receptor activation to an ionic conductance in the plasma membrane.
Table 4.
Effects of modulation of intracellular calcium stores on PACAP-induced neuron depolarization
| Treatment | Membrane potential (mV) | PACAP-induced depolarization (mV) |
|---|---|---|
| Vehicle | −54 ± 10 | 15.6 ± 1.3 (10) |
| Thapsigargin (100 nm) | −56 ± 6 | 16.3 ± 1.3 (4) |
| CPA (10 μm) | −56 ± 3 | 13.3 ± 1.4 (7) |
| BAPTA (10 μm) | −51 ± 3 | 13.0 ± 1.1 (4) |
Sympathetic neurons were incubated in defined medium containing 100 nm thapsigargin, 10 μm CPA, or 10 μm BAPTA for 30 min as described in Materials and Methods. The effects of these agents on PACAP27-induced depolarization (pressure application) was examined in the presence of 300 nm TTX; for all of the treatments, the drug vehicle was DMSO. The data represent the mean membrane potential or depolarization ± SEM. The numbers of neurons tested under each paradigm are shown in parentheses.
SCG neuronal expression of specific Trp channels may mediate PACAP-induced currents
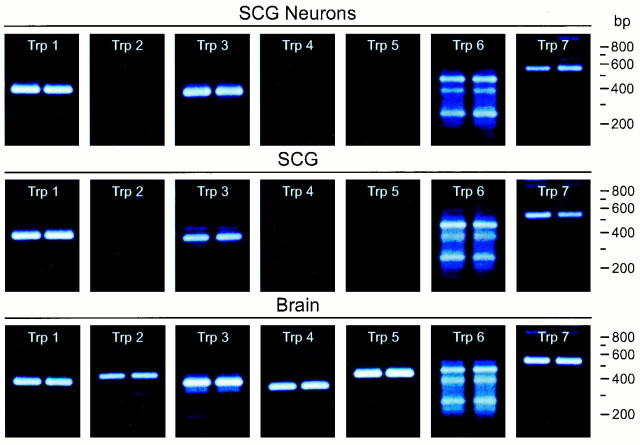
Recent studies suggest that IP3 can engage a subfamily of mammalian Trp channels, thus activating a nonselective cationic conductance (Hu et al., 1994; Dong et al., 1995; Kiselyov et al., 1998). For some Trp subtypes, IP3 receptor occupancy and interactions with Trp to maintain channels in the active state could be attenuated markedly after xestospongin treatment (Kiselyov et al., 1998). At least one Trp isoform has been described to be activated directly by IP3 (Hu et al., 1994;Dong et al., 1995). To evaluate whether Trp-related molecules exist in the SCG and, importantly, whether specific Trp channels shown to be store independent are found in sympathetic neurons to potentially represent PACAP-activated cationic conductances, RT-PCR experiments were undertaken. Using oligonucleotide primers directed against the seven mammalian Trp cDNAs identified to date, all Trp1–Trp7 channels were identified in brain (Fig. 5). By contrast, rat sympathetic neurons demonstrated a different pattern of Trp channel expression. Using our cultures enriched in postganglionic neurons, Trp1, Trp3, Trp6, and Trp7 were well expressed; Trp2, Trp4, and Trp5 mRNA expression was either low or undetectable. These observations indicated expression of mammalian Trp channels in the SCG; the expression of Trp3, Trp6, and Trp7 in sympathetic neurons is significant because the channels belong to a subfamily characterized by store-independent activation and low cationic specificity.
Fig. 5.
SCG neurons express multiple Trp channel transcripts. Total RNA from rat brain and SCG and primary rat sympathetic neurons in vitro was reverse-transcribed, and the cDNA was amplified using each of the seven oligonucleotide primer sets specific for the Trp channel transcripts (Table 1). The amplified products were resolved on 2% agarose–GelTwin II gels, stained with ethidium bromide, and visualized by UV illumination. The predicted sizes of the seven products are as follows:Trp1, 373 bp; Trp2, 413 bp;Trp3, 363 bp; Trp4, 343 bp;Trp5, 431 bp; Trp6, 465 bp; andTrp7, 558 bp. Trp6 may exhibit multiple transcript isoforms. Among Trp channels, Trp3 Xe sensitivity and store independence have been best studied to date.
DISCUSSION
Studies have suggested that VIP or VIP-related molecules may be noncholinergic modulators of SCG function (Ip et al., 1982, 1983, 1985;Schwarzschild et al., 1989; Zigmond et al., 1989). Recent studies have shown that many of the sympathetic neuron responses described for VIP appear to be mediated largely by PACAP peptides. Both PACAP27 and PACAP38 stimulated with high potency and efficacy SCG neuron transmitter production and secretion, second messenger production, and differentiation (May and Braas, 1995; Braas and May, 1996; Lu et al., 1998, 1999). Considerably higher concentrations of VIP were necessary to elicit sympathetic neurosecretion, which appeared consistent with the preferential expression of PACAP-selective PAC1 receptor expression by sympathetic neurons. Sympathetic preganglionic neurons in the intermediolateral cell column of the spinal cord projecting to the SCG express PACAP mRNA (Beaudet et al., 1998), and transection of the cervical sympathetic trunk diminished PACAP-immunoreactive fibers in the SCG (Sundler et al., 1996). In sum, these results presented strong anatomical and physiological evidence implicating PACAP peptides as potential noncholinergic regulators of sympathetic function. The present studies analyzed the direct effects of PACAP peptides on SCG neurons. Using electrophysiological and pharmacological approaches, the studies evaluated PACAP modulation of sympathetic neuron membrane potentials and investigated potential ionic mechanisms and intracellular signaling mechanisms mediating the PACAP-induced depolarizations.
Nearly all of the sympathetic neurons examined exhibited PACAP27- or PACAP38-elicited depolarizations when we used the same SCG neuron system as in our previous regulatory studies (May et al., 1995; May and Braas, 1995; Braas and May, 1996, 1999). The PACAP-induced depolarizations remained when stimulated release was blocked with either TTX or cadmium, demonstrating that the depolarizations represented direct peptidergic effects on sympathetic neurons and were not mediated by interneuronal signaling molecules within the in vitro preparation. The depolarizations mediated by PACAP27 were blunted by the peptide antagonist PACAP(6–38), demonstrating the specificity of the peptide responses; PACAP(6–38) did not inhibit the PACAP38-induced depolarizations, a difference that may be related to sympathetic neuron expression of specific PAC1receptor isoforms. Similarly, PACAP(6–38) was unable to inhibit depolarization of rat sympathetic preganglionic neurons induced by PACAP38 (Lai et al., 1997). PACAP27 and PACAP38 exhibited equal high potency in sympathetic neuron depolarization, whereas VIP had no apparent effects at the concentrations tested. These results were in agreement with investigations establishing the preferential expression of the PACAP-selective PAC1(short)HOP1 receptor splice variant in rat SCG postganglionic neurons (Lu et al., 1998; Braas and May, 1999). PAC1 receptor mRNA and protein were identified in >90% of sympathetic neurons byin situ hybridization histochemistry and immunocytochemistry, respectively (Moller et al., 1997a,b; Nogi et al., 1997; Braas and May, 1999). The potencies of PACAP27 and PACAP38 in eliciting depolarizations were similar to those required for sympathetic neuron second messenger production and neurotransmitter/neuropeptide secretion (May and Braas, 1995; Braas and May, 1999).
The present results indicated that the PACAP-induced inward currents and resultant depolarizations in sympathetic postganglionic neurons required extracellular sodium, demonstrating that the influx of sodium ions is a key component of the currents. Similar to many other neuropeptide-induced depolarizations, sodium influx may be mediated by nonselective cationic channels. PACAP-induced currents also were reduced after elevation of extracellular potassium levels, a result that is consistent with inhibition of a potassium current activated at −50 mV contributing to the PACAP-induced depolarization. Therefore, at −50 mV the PACAP-induced inward currents are mediated by a combination of inhibition of a potassium conductance and activation of a sodium-permeable channel.
We have not identified the potassium conductance contributing to the PACAP-induced depolarizations; however, one potassium conductance commonly inhibited by neuropeptides and other transmitters in rat SCG is the voltage-dependent, noninactivating conductanceIM (Brown, 1988). Cruzblanca et al. (1998) demonstrated that bradykinin-induced inhibition ofIM required PLC activation and calcium release from IP3-sensitive stores in acutely dissociated adult SCG neurons. Thus, a component of the PACAP-induced currents was anticipated to be caused by calcium-dependent inhibition of IM. However, the lack of thapsigargin, CPA, or BAPTA effects on the PACAP-induced depolarizations strongly indicated that release of calcium from intracellular stores was not required for initiating the PACAP-induced depolarizations. Although a membrane-delimited inhibition ofIM could have been involved in the generation of the depolarizations, preliminary results indicated that this conductance did not represent a significant component of the PACAP-induced currents. Pretreatment of sympathetic neurons voltage-clamped to −50 mV with 1 mm barium, a concentration that effectively inhibitsIM (Constanti and Brown, 1981), did not significantly reduce the PACAP-mediated inward currents (<5%) (M. M. Beaudet, unpublished observation). Given the voltage dependence of IM, i.e., that only a small portion of IM conductance would be activated at −50 mV, inhibition ofIM under these recording conditions would not be expected to be a prominent contributor to the PACAP-induced currents.
SCG neurons express preferentially PACAP-selective PAC1(short) HOP1 receptor variants that demonstrate potent and efficacious stimulation of cAMP and inositol phosphate production by PACAP27 and PACAP38 (Lu et al., 1998;Braas and May, 1999). Studies using bacterial toxins, and intracellular second messenger activators or inhibitors indicated that the cAMP/PKA pathway did not participate in the generation of the PACAP-induced currents or depolarizations. Because inhibition of PLC attenuated markedly the PACAP-induced depolarizations, PLC-dependent IP3 or DAG production appeared to be important for the generation of the peptide-induced depolarizations. However, application of OAG did not elicit membrane depolarizations, and inhibition of PKC did not affect the PACAP-induced depolarizations, suggesting that synthesis of DAG did not contribute to the responses.
By contrast, inhibition of IP3 receptors suggested that IP3 production was crucial to the peptide-induced depolarizations. However, neither depletion of calcium stores nor intracellular calcium chelation affected the PACAP-induced membrane depolarizations, suggesting that calcium release from IP3-sensitive stores was not required for the responses. Rather, the depolarizations appeared to depend on direct IP3-mediated modulation of an ionic conductance in the plasma membrane. Direct IP3 activation of nonselective cationic conductances has been reported for a number of cell types, including lymphocytes, cerebellar Purkinje cells, and vascular endothelial cells (Kuno and Gardner, 1987; Mozhayeva et al., 1990; Brent et al., 1993; McDonald et al., 1993; Kuno et al., 1994;Vaca and Kunze, 1995), and therefore could be involved in the generation of the PACAP-induced depolarizations of SCG neurons.
Many molecular, biochemical, and electrophysiological studies have suggested that Trp, Trpl, and other Trp-related molecules, first identified by Drosophila genetics, function as membrane ion channels (Birnbaumer et al., 1996; Zhu et al., 1996; Montell, 1997;Philipp et al., 1998; Okada et al., 1998, 1999). Congruous with the presented data, activation of a nonselective cationic conductance of the Trp family could be a component of the depolarization of SCG neurons elicited by PACAP. Several Trp channel members demonstrate characteristics consistent with our observations. Among the seven mammalian Trp channels identified to date, Trp3, Trp6, and Trp7 from recombinant expression demonstrate relatively low selectivity for divalent and monovalent cations. Contrary to early suggestions, not all members of the Trp family of channels are activated simply after calcium store depletion, and the Trp3, Trp6, and Trp7 subfamily has been shown to be store independent (Okada et al., 1999). The mechanisms of Trp channel activation have not been completely elucidated, but recent studies have supported the IP3 receptor conformational coupling hypothesis with Trp for sustained channel activation (Kiselyov et al., 1998; Boulay et al., 1999; Zubov et al., 1999). IP3 receptor-mediated activation of the human Trp homolog HTrp3 for example, can be blocked by heparin and xestospongin IP3 receptor inhibition (Kiselyov et al., 1998), and IP3 receptors can be coimmunoprecipitated with HTrp3 channels. These properties are similar to our experimental results. PACAP activation of sympathetic neuron PAC1 receptors potently stimulates PLC activity and IP3 production, and inhibition of PLC or IP3 receptors attenuated the depolarizations, supporting the contention that IP3-mediated activation of a nonselective cationic conductance contributes to the responses. Furthermore, depolarization occurred in the absence of intracellular calcium release, and sympathetic postganglionic neuron expression of Trp channel transcripts is consistent with direct IP3/IP3 receptor interaction with these channel proteins. Although several members of the Trp3 channel family are activated directly by DAG (Hofmann et al., 1999; Okada et al., 1999), OAG did not depolarize these sympathetic neurons, results inconsistent with DAG-operated channel involvement in the PACAP-generated depolarization. However, our present results do not rule out a DAG metabolite in the generation of a component of the PACAP-induced depolarizations (Chyb et al., 1999).
We considered that the residual inward current recorded with elevated potassium and sodium-deficient NMG-substituted solution might have been produced by calcium influx. However, in a calcium-deficient solution, the residual current amplitude was not diminished but enhanced nominally. From these observations, calcium influx did not appear to contribute significantly to the PACAP-induced currents under these experimental conditions. Also, raising extracellular calcium inhibited the PACAP-induced current. The current amplitude was decreased when the external calcium concentration was raised twofold; similarly, increasing external calcium levels twofold decreased the inward sodium current recorded at −50 mV in neurons maintained in HEPES-buffered sodium solution with elevated KCl. These results were consistent with the previously described calcium-mediated inactivation of some Trp channels in vitro (Montell, 1997; Okada et al., 1999).
In summary, the present electrophysiological studies demonstrated that >90% SCG sympathetic neurons are depolarized after PACAP activation of PAC1 receptors. The PACAP-induced depolarizations were mediated by concomitant activation of sodium influx and inhibition of a potassium current. The depolarizations required PLC activation and subsequent IP3production. Given the selective expression of Trp3, Trp6, and Trp7 family members in sympathetic neurons, these studies support PAC1 receptor-mediated IP3receptor activation of a nonselective cationic conduction potentially related to a mammalian Trp.
Footnotes
This work was supported by National Institutes of Health Grants HD-27468 and NS-01636 (V.M.) and NS-23978 (R.L.P.), and American Hearth Association Grants 975043N (R.L.P.) and 94015540 (K.M.B.). We thank Dr. Jean Hardwick for technical advice during early phases of this work, Lei Zhang for design of some of the oligonucleotide primers, and Thomm Buttolph for technical support with the initial mouse Trp channel reverse transcription PCR. We also thank Drs. Mark Nelson and David Saffen for enlightening scientific discussions and guidance.
Correspondence should be addressed to Dr. Victor May, Department of Anatomy and Neurobiology, University of Vermont College of Medicine, Given Health Science Building, Burlington, VT 05405. E-mail:vmay@zoo.uvm.edu.
REFERENCES
- 1.Absood A, Chen D, Hakanson R. Neuropeptides of the vasoactive intestinal peptide/helodermin/pituitary adenylate cyclase activating peptide family elevate plasma cAMP in mice: comparison with a range of other regulatory peptides. Regul Pept. 1992;40:311–322. doi: 10.1016/0167-0115(92)90518-y. [DOI] [PubMed] [Google Scholar]
- 2.Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- 3.Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol. 1995;16:53–88. doi: 10.1006/frne.1995.1003. [DOI] [PubMed] [Google Scholar]
- 4.Beaudet MM, Braas KM, May V. Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J Neurobiol. 1998;36:325–336. [PubMed] [Google Scholar]
- 5.Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proc Natl Acad Sci USA. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Modulation of Ca2+ entry by polypeptides of the inositol 1,4,5-trisphophate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proc Natl Acad Sci USA. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides, PACAP-38 and PACAP-27, regulation of sympathetic neuron catecholamine, and neuropeptide Y expression through activation of type I PACAP/VIP receptor isoforms. Ann NY Acad Sci. 1996;805:204–216. doi: 10.1111/j.1749-6632.1996.tb17484.x. [DOI] [PubMed] [Google Scholar]
- 8.Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC1 receptor isoform activation of specific intracellular signaling pathways. J Biol Chem. 1999;274:27702–27710. doi: 10.1074/jbc.274.39.27702. [DOI] [PubMed] [Google Scholar]
- 9.Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci. 1998;18:9766–9779. doi: 10.1523/JNEUROSCI.18-23-09766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brent LH, Gong Q, Ross JM, Wieland SJ. Mitogen-activated Ca++ channels in human B lymphocytes. J Cell Physiol. 1993;155:520–529. doi: 10.1002/jcp.1041550310. [DOI] [PubMed] [Google Scholar]
- 11.Brown D. M-currents: an update. Trends Neurosci. 1988;11:294–299. doi: 10.1016/0166-2236(88)90089-6. [DOI] [PubMed] [Google Scholar]
- 12.Christophe J. Type I receptors for PACAP (a neuropeptide even more important than VIP?). Biochim Biophys Acta. 1993;1154:183–199. doi: 10.1016/0304-4157(93)90011-c. [DOI] [PubMed] [Google Scholar]
- 13.Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- 14.Constanti A, Brown DA. M-currents in voltage-clamped mammalian sympathetic neurones. Neurosci Lett. 1981;24:289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- 15.Cruzblanca H, Koh DS, Hille B. Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3-sensitive Ca2+ stores in rat sympathetic neurons. Proc Natl Acad Sci USA. 1998;95:7151–7156. doi: 10.1073/pnas.95.12.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deutsch PJ, Sun Y. The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J Biol Chem. 1992;267:5108–5113. [PubMed] [Google Scholar]
- 17.DiCicco-Bloom E. Region-specific regulation of neurogenesis by VIP and PACAP: direct and indirect modes of action. Ann NY Acad Sci. 1996;805:244–256. doi: 10.1111/j.1749-6632.1996.tb17487.x. [DOI] [PubMed] [Google Scholar]
- 18.DiCicco-Bloom E, Deutsch P. Pituitary adenylate cyclase activating polypeptide (PACAP) potently stimulates mitosis, neuritogenesis and survival in cultured rat sympathetic neuroblasts. Regul Pept. 1992;37:319. [Google Scholar]
- 19.Dong Y, Kunze DL, Vaca L, Schilling WP. Ins(1,4,5)P3 activates Drosophila cation channel Trpl in recombinant baculovirus-infected Sf9 insect cells. Am J Physiol. 1995;269:C1332–1339. doi: 10.1152/ajpcell.1995.269.5.C1332. [DOI] [PubMed] [Google Scholar]
- 20.Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 21.Harmar T, Lutz E. Multiple receptors for PACAP and VIP. Trends Pharmacol Sci. 1994;15:97–99. doi: 10.1016/0165-6147(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto H, Ishihara T, Shigemoto R, Mori K, Nagata S. Molecular cloning and tissue distribution of a receptor for pituitary adenylate cyclase-activating polypeptide. Neuron. 1993;11:333–342. doi: 10.1016/0896-6273(93)90188-w. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y, Vaca L, Zhu X, Birnbaumer L, Kunze DL, Schilling WP. Appearance of a novel Ca2+ influx pathway in Sf9 insect cells following expression of the transient receptor potential-like (trpl) protein of Drosophila. Biochem Biophys Res Commun. 1994;201:1050–1056. doi: 10.1006/bbrc.1994.1808. [DOI] [PubMed] [Google Scholar]
- 25.Ip NY, Ho CK, Zigmond RE. Secretin and vasoactive intestinal peptide acutely increase tyrosine 3-monooxygenase in the rat superior cervical ganglion. Proc Natl Acad Sci USA. 1982;79:7566–7569. doi: 10.1073/pnas.79.23.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ip NY, Perlman RL, Zigmond RE. Acute transsynaptic regulation of tyrosine 3-monooxygenase activity in the rat superior cervical ganglion: evidence for both cholinergic and noncholinergic mechanisms. Proc Natl Acad Sci USA. 1983;80:2081–2085. doi: 10.1073/pnas.80.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ip NY, Baldwin C, Zigmond RE. Regulation of the concentration of adenosine 3′,5′-cyclic monophosphate and the activity of tyrosine hydroxylase in the rat superior cervical ganglion by three neuropeptides of the secretin family. J Neurosci. 1985;5:1947–1954. doi: 10.1523/JNEUROSCI.05-07-01947.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Journot L, Waeber C, Pantaloni C, Holsboer F, Seeburg PH, Bockaert J, Spengler D. Differential signal transduction by six splice variants of the pituitary adenylate cyclase-activating peptide (PACAP) receptor. Biochem Soc Trans. 1995;23:133–137. doi: 10.1042/bst0230133. [DOI] [PubMed] [Google Scholar]
- 29.Kawatani M, Rutigliano M, de Groat WC. Depolarization and muscarinic excitation induced in a sympathetic ganglion by vasoactive intestinal polypeptide. Science. 1985;229:879–881. doi: 10.1126/science.3895438. [DOI] [PubMed] [Google Scholar]
- 30.Kaziro Y, Itoh H, Kozasa T, Nakafuku M, Satoh T. Structure and function of signal-transducing GTP-binding proteins. Annu Rev Biochem. 1991;60:349–400. doi: 10.1146/annurev.bi.60.070191.002025. [DOI] [PubMed] [Google Scholar]
- 31.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 32.Kuno M, Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987;326:301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- 33.Kuno M, Maeda N, Mikoshiba K. IP3-activated calcium-permeable channels in the inside-out patches of cultured cerebellar Purkinje cells. Biochem Biophys Res Commun. 1994;199:1128–1135. doi: 10.1006/bbrc.1994.1348. [DOI] [PubMed] [Google Scholar]
- 34.Lai CC, Wu SY, Lin HH, Dun NJ. Excitatory action of pituitary adenylate cyclase activating polypeptide on rat sympathetic preganglionic neurons in vivo and in vitro. Brain Res. 1997;748:189–194. doi: 10.1016/s0006-8993(96)01297-8. [DOI] [PubMed] [Google Scholar]
- 35.Lu N, Zhou R, DiCicco-Bloom E. Opposing mitogenic regulation by PACAP in sympathetic and cerebral cortical precursors correlates with differential expression of PACAP receptor (PAC1-R) isoforms. J Neurosci Res. 1998;53:651–662. doi: 10.1002/(SICI)1097-4547(19980915)53:6<651::AID-JNR3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.May V, Braas KM. Pituitary adenylate cyclase-activating polypeptide (PACAP) regulation of sympathetic neuron neuropeptide Y and catecholamine expression. J Neurochem. 1995;65:978–987. doi: 10.1046/j.1471-4159.1995.65030978.x. [DOI] [PubMed] [Google Scholar]
- 37.May V, Brandenburg CA, Braas KM. Differential regulation of sympathetic neuron neuropeptide Y and catecholamine content and secretion. J Neurosci. 1995;15:4580–4591. doi: 10.1523/JNEUROSCI.15-06-04580.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.May V, Beaudet MM, Parsons RL, Hardwick JC, Gauthier EA, Durda JP, Braas KM. Mechanisms of pituitary adenylate cyclase activating polypeptide (PACAP)-induced depolarization of sympathetic superior cervical ganglion (SCG) neurons. Ann NY Acad Sci. 1998;865:164–175. doi: 10.1111/j.1749-6632.1998.tb11175.x. [DOI] [PubMed] [Google Scholar]
- 39.McDonald TV, Premack BA, Gardner P. Flash photolysis of caged inositol 1,4,5-trisphosphate activates plasma membrane calcium current in human T cells. J Biol Chem. 1993;268:3889–3896. [PubMed] [Google Scholar]
- 40.Moller K, Reimer M, Hannibal J, Fahrenkrug J, Sundler F, Kanje M. Pituitary adenylate cyclase-activating peptide (PACAP) and PACAP type 1 receptor expression in regenerating adult mouse and rat superior cervical ganglia in vitro. Brain Res. 1997a;775:156–165. doi: 10.1016/s0006-8993(97)00937-2. [DOI] [PubMed] [Google Scholar]
- 41.Moller K, Reimer M, Ekblad E, Hannibal J, Fahrenkrug J, Kanje M, Sundler F. The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion. Brain Res. 1997b;775:166–182. doi: 10.1016/s0006-8993(97)00923-2. [DOI] [PubMed] [Google Scholar]
- 42.Montell C. New light on TRP and TRPL. Mol Pharmacol. 1997;52:755–763. doi: 10.1124/mol.52.5.755. [DOI] [PubMed] [Google Scholar]
- 43.Mozhayeva GN, Naumov AP, Kuryshev YA. Inositol 1,4,5-trisphosphate activates two types of Ca2(+)-permeable channels in human carcinoma cells. FEBS Lett. 1990;277:233–234. doi: 10.1016/0014-5793(90)80853-b. [DOI] [PubMed] [Google Scholar]
- 44.Murayama T, Ui M. [3H]GDP release from rat and hamster adipocyte membranes independently linked to receptors involved in activation or inhibition of adenylate cyclase. Differential susceptibility to two bacterial toxins. J Biol Chem. 1984;259:761–769. [PubMed] [Google Scholar]
- 45.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 46.Nogi H, Hashimoto H, Hagihara N, Shimada S, Yamamoto K, Matsuda T, Tohyama M, Baba A. Distribution of mRNAs for pituitary adenylate cyclase-activating polypeptide (PACAP), PACAP receptor, vasoactive intestinal polypeptide (VIP), and VIP receptors in the rat superior cervical ganglion. Neurosci Lett. 1997;227:37–40. doi: 10.1016/s0304-3940(97)00295-4. [DOI] [PubMed] [Google Scholar]
- 47.Okada T, Shimizu S, Wakamori M, Maeda A, Kurosaki T, Takada N, Imoto K, Mori Y. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J Biol Chem. 1998;273:10279–10287. doi: 10.1074/jbc.273.17.10279. [DOI] [PubMed] [Google Scholar]
- 48.Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. J Biol Chem. 1999;274:27359–27379. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- 49.Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, Cavalie A, Flockerzi V. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 1998;17:4274–4282. doi: 10.1093/emboj/17.15.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rawlings SR, Hezareh M. Pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP/vasoactive intestinal polypeptide receptors: actions on the anterior pituitary gland. Endocr Rev. 1996;17:4–29. doi: 10.1210/edrv-17-1-4. [DOI] [PubMed] [Google Scholar]
- 51.Schofield GG, Ikeda SR. Potassium currents of acutely isolated adult rat superior cervical ganglion neurons. Brain Res. 1989;485:205–214. doi: 10.1016/0006-8993(89)90563-5. [DOI] [PubMed] [Google Scholar]
- 52.Schwarzschild MA, Vale W, Corigliano-Murphy AC, Pisano JJ, Ip NY, Zigmond RE. Activation of ganglionic tyrosine hydroxylase by peptides of the secretin-glucagon family: structure-function studies. Neuroscience. 1989;31:159–167. doi: 10.1016/0306-4522(89)90037-7. [DOI] [PubMed] [Google Scholar]
- 53.Shapiro MS, Wollmuth LP, Hille B. Modulation of Ca2+ channels by PTX-sensitive G-proteins is blocked by N-ethylmaleimide in rat sympathetic neurons. J Neurosci. 1994;14:7109–7116. doi: 10.1523/JNEUROSCI.14-11-07109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shibuya I, Kabashima N, Tanaka K, Setiadji VS, Noguchi J, Harayama N, Ueta Y, Yamashita H. Patch-clamp analysis of the mechanism of PACAP-induced excitation in rat supraoptic neurones. J Neuroendocrinol. 1998;10:759–768. doi: 10.1046/j.1365-2826.1998.00260.x. [DOI] [PubMed] [Google Scholar]
- 55.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 56.Sundler F, Ekblad E, Hannibal J, Moller K, Zhang YZ, Mulder H, Elsas T, Grunditz T, Danielsen N, Fahrenkrug J, Uddman R. Pituitary adenylate cyclase-activating peptide in sensory and autonomic ganglia: localization and regulation. Ann NY Acad Sci. 1996;805:410–426. doi: 10.1111/j.1749-6632.1996.tb17501.x. [DOI] [PubMed] [Google Scholar]
- 57.Svoboda M, Tastenoy M, Ciccarelli E, Stievenart M, Christophe J. Cloning of a splice variant of the pituitary adenylate cyclase-activating polypeptide (PACAP) type I receptor. Biochem Biophys Res Commun. 1993;195:881–888. doi: 10.1006/bbrc.1993.2127. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka J, Koshimura K, Murakami Y, Kato Y. Stimulatory effect of PACAP on neuronal cell survival. Ann NY Acad Sci. 1996;805:473–475. doi: 10.1111/j.1749-6632.1996.tb17506.x. [DOI] [PubMed] [Google Scholar]
- 59.Vaca L, Kunze DL. IP3-activated Ca2+ channels in the plasma membrane of cultured vascular endothelial cells. Am J Physiol. 1995;269:C733–C738. doi: 10.1152/ajpcell.1995.269.3.C733. [DOI] [PubMed] [Google Scholar]
- 60.Waschek JA. VIP and PACAP receptor-mediated actions on cell proliferation and survival. Ann NY Acad Sci. 1996;805:290–300. doi: 10.1111/j.1749-6632.1996.tb17491.x. [DOI] [PubMed] [Google Scholar]
- 61.Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- 62.Zigmond RE, Schwarzschild MA, Rittenhouse AR. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]
- 63.Zubov AI, Kaznacheeva EV, Nikolaev AV, Alexeenko VA, Kiselyov K, Muallem S, Mozhayeva GN. Regulation of the miniature plasma membrane Ca2+ channel Imin by inositol 1,4,5-trisphosphate receptors. J Biol Chem. 1999;274:25983–25985. doi: 10.1074/jbc.274.37.25983. [DOI] [PubMed] [Google Scholar]