Abstract
The G-protein-coupled metabotropic glutamate receptor subtype 7a (mGluR7a) is a member of group III metabotropic glutamate receptors that plays an important role as a presynaptic receptor in regulating transmitter release at glutamatergic synapses. Here we report that the protein interacting with C-kinase (PICK1) binds to the C terminus (ct) of mGluR7a. In the yeast two-hybrid system, the extreme ct of mGluR7a was shown to interact with the PSD-95/Discs large/ZO-1 (PDZ) domain of PICK1. Pull-down assays indicated that PICK1 was retained by a glutathione S-transferase fusion of ct-mGluR7a. Furthermore, recombinant and native PICK1/mGluR7a complexes were coimmunoprecipitated from COS-7 cells and rat brain tissue, respectively. Confocal microscopy showed that both PICK1 and mGluR7a displayed synaptic colocalization in cultured hippocampal neurons. PICK1 has previously been shown to bind protein kinase C α-subunit (PKCα), and mGluR7a is known to be phosphorylated by PKC. We show a relationship between these three proteins using recombinant PICK1, mGluR7, and PKCα, where they were co-immunoprecipitated as a complex from COS-7 cells. In addition, PICK1 caused a reduction in PKCα-evoked phosphorylation of mGluR7a inin vitro phosphorylation assays. These results suggest a role for PICK1 in modulating PKCα-evoked phosphorylation of mGluR7a.
Keywords: metabotropic glutamate receptor subtype 7, protein interacting with C-kinase (PICK1), protein kinase C, protein–protein interactions, PDZ domain, yeast two-hybrid system, protein phosphorylation
Metabotropic glutamate receptors (mGluRs) are proteins that contain seven transmembrane domains, are widely distributed throughout the CNS, and play a crucial role in glutamate-mediated neurotransmission and synaptic plasticity events (for review, see Schoepp et al., 1999). On the basis of molecular, signal transduction, and pharmacological similarities, mGluR1–mGluR8 have been divided into three groups (I–III). The group III receptor member, mGluR7, has two alternative splice isoforms (a and b). The mGluR7a isoform consists of 915 residues with a molecular weight of ∼100 kDa (Okamoto et al., 1994; Saugstad et al., 1994; Flor et al., 1997). Expression of mGluR7 occurs widely throughout the rat brain with its pattern remaining distinct from other group III mGluRs (Okamoto et al., 1994; Saugstad et al., 1994; Ohishi et al., 1995; Kinoshita et al., 1998). On activation, mGluR7 inhibits cAMP formation and is thought to play a presynaptic autoregulatory role, leading to inhibition of transmitter release at glutamatergic synapses (Okamoto et al., 1994; Saugstad et al., 1994). In electrophysiological studies, mGluR7 knockout mice show deficits in synaptic transmission (Bushell et al., 1996). These animals can suffer epileptic seizures, and a recent behavioral study has shown that mGluR7 plays a role in fear response and conditioned taste aversion (Masugi et al., 1999).
PSD-95/Discs large/ZO-1 (PDZ) domain-containing proteins are known to interact with the carboxy terminus (ct) motifs on target proteins (Kornau et al., 1999). These interactions may provide an important mechanism for clustering ion channels and receptors at the plasma membrane, receptor cross-talk, and directing kinases and phosphatases toward their substrates (Kornau et al., 1999). Several examples of glutamate receptor-interacting proteins that contain PDZ domains have been reported. For example, the 95 kDa postsynaptic density protein (PSD 95), which anchors Shaker-type potassium channels (Kim et al., 1995), also binds NMDA receptor subunits (Kornau et al., 1995). For AMPA receptors, molecules that contain PDZ domains such as glutamate receptor-interacting protein (Dong et al., 1997) and AMPA receptor binding protein (Srivastava et al., 1998) and those without PDZ domains such asN-ethylmaleimide-sensitive fusion protein (Nishimune et al., 1998) have been reported as interacting proteins. Proteins interacting with mGluRs include a family of dendritic proteins named Homers (Brakeman et al., 1997), which are known to interact with mGluR1 and mGluR5, and very recently Ca2+/calmodulin (CAM) has been shown to interact with mGluR7a (Nakajima et al., 1999; O'Connor et al., 1999).
Here we show that the PKCα substrate and binding protein, PICK1 (Staudinger et al., 1995, 1997), which contains a single PDZ domain and is known to interact with AMPA receptor subunits (Dev et al., 1999; Xia et al., 1999), ephrin ligands and Eph receptors (Torres et al., 1998), and class I ADP-ribosylation factors (Takeya et al., 2000), interacts with the metabotropic glutamate receptor mGluR7a. Together, these results suggest a possible role for PICK1 in the regulation of synaptic expression and function of various receptor types and raise the possibility that PICK1 is the mediator of a highly coordinated process involved in synaptic transmission and development.
MATERIALS AND METHODS
Yeast two-hybrid system. The ct domains of cDNA mGluR1-8 were amplified from full-length clones by PCR or from rat brain total RNA by RT-PCR, and fragments were subcloned in frame with LexA into one of the pBTM vectors. Overlapping deletion mutants or point mutations of ct-mGluR7 were constructed by PCR (Stemmer and Morris, 1992) and subcloned into pBTM116ADE2. The integrity of the inserts was verified by DNA sequencing. All other information for cDNA constructs is indicated in the Figures or legends or has been described previously (Nishimune et al., 1998; Dev et al., 1999; Nakajima et al., 1999). Briefly, PICK1 subcloned into pGAD10 (Clontech, Palo Alto, CA) was cotransformed with bait plasmids, containing the ct domains of a series of glutamate receptor subunits, into Saccharomyces cerevisiae L-40 reporter strain. Candidates encoding PICK1-interacting glutamate receptor proteins were isolated by colony selection on Trp,His,Ura,Leu,Lys-dropout plates and tested for the activation of β-galactosidase reporter gene by filter β-galactosidase assays.
Western blotting and antibodies. For Western blots, denatured samples (10–20 μg total protein, as determined by the Bio-Rad protein assay kit) were separated by electrophoresis on 9% SDS-polyacrylamide gels (SDS-PAGE) and, Western blotting was performed as described (Dev et al., 1999). Primary anti-peptide antibodies (Abs) were as follows: affinity-purified anti-PICK-rabbit Ab (rab Ab) and anti-PICK-guinea pig Ab (gpig Ab; dilution 1:200) polyclonal IgGs raised against peptides containing the residues 2-31 and 391-414 of rat PICK1, respectively. Affinity-purified anti-mGluR7a-rab Ab and anti-mGluR7a-gpig Ab polyclonal IgGs were against bacterial fusion proteins containing residues 874-915 of rat mGluR7a (Shigemoto et al., 1997). Polyclonal rabbit Abs were anti-green fluorescent protein (GFP) Ab (Clontech) and anti-hemagglutinin (HA) Ab (Santa Cruz Biotechnology, Santa Cruz, CA). Monoclonal mouse Abs (mAb) were anti-synaptophysin mAb (Roche Diagnostics, Mannhein, Germany), anti-flag M2 mAb (Sigma, St. Louis, MO), and anti-HA mAb (Santa Cruz).
The secondary Abs used in Western blotting were alkaline phosphatase-conjugated and were as follows: goat anti-rabbit IgG (Promega, Madison, WI), goat anti-mouse IgG (Promega), and goat anti-guinea pig IgG (Sigma). The secondary Abs used in immunocytochemistry were as follows: Texas Red-X goat anti-rabbit or anti-mouse IgG (Molecular Probes, Eugene, OR), Oregon green-conjugated donkey anti-rabbit, anti-mouse, or anti-guinea pig IgG (Chemicon, Temecula, CA). Antibodies were used at dilutions recommended by the manufacture or at a dilution of 1:1000 unless indicated otherwise.
Membrane preparation, glutathione S-transferase pull-down and immunoprecipitation. GlutathioneS-transferase (GST) was fused to ct-mGluR7a by subcloning into pGEX-4T-1 (Pharmacia, Uppsala, Sweden). GFP was fused to the N terminus of PICK1 by subcloning it into pEGFP-C2 (Clontech). A flag-tagged mGluR7a was prepared using PCR, with the tag introduced after the signal peptide just after Gly47 and subcloned into pCIneo (Promega). COS-7 cells were transfected with cDNA using LipofectAMINE/PLUS (Life Technologies, Gaithersburg, MD) and used 48 hr after transfection. Sonicates of Escherichia colistrain BL21 expressing GST or GST-fusion proteins and lysates of COS-7 cells transiently expressing the required protein(s) were prepared as described previously (Dev et al., 1999). Rat brain homogenates were prepared in homogenization buffer (HB) containing 0.32m sucrose, 4 mm HEPES, 1 mm EDTA, 1 mm EGTA, pH 7.4, using a glass/Teflon homogenizer (10 passes). Homogenates were centrifuged at 1000 × g for 10 min, and the supernatant (S1) was centrifuged at 48,000 ×g for 30 min to obtain the pellet (P2) fraction. The P2 fraction was resuspended in PtxE containing PBS, 1% Triton X-100, 0.1 mm EDTA, pH 7.4, sonicated, and solubilized. After centrifugation at 100,000 × g for 1 hr, the sonicate was precleared with the appropriate beads. GST pull-down assays were performed as described previously (Dev et al., 1999) using either flag-PICK1 (∼0.2 mg total protein cell sonicate) or native PICK1 (∼2.5 mg total protein rat brain sonicate). For immunoprecipitation, cell sonicates or rat brain sonicates were immunoprecipitated with or without either 20 μl anti-flag M2-agarose affinity beads (Sigma) or with 20 μl protein-G Sepharose beads (Pharmacia) that were previously coupled to 5 μg of either anti-mGluR7a-rab Ab or anti-PICK-rab Ab or anti-HA Ab. All samples were washed before being processed for Western blotting (Dev et al., 1999).
Immunocytochemistry. Low-density hippocampal cultures were prepared as described previously (Malgaroli and Tsien, 1992; Noel et al., 1999) and used after 10–14 d in culture. For immunocytochemistry, hippocampal cultures, grown on coverslips, were washed in HEPES-buffered saline and fixed in 100% ice-cold methanol. After blocking nonspecific binding, the cells were incubated with primary and then secondary Abs and visualized under a confocal Olympus microscope (Tokyo, Japan).
Phosphorylation studies. Maltose binding protein (MAL) was fused to full-length PICK1 by subcloning into pMALc2X (New England Biolabs, Beverly, MA). Purified PKCα was obtained from Calbiochem (Cambridge, MA). MAL and GST proteins were prepared as described by manufacturer's protocol, and GST-ct-mGluR7a was further purified by a cation exchange chromatography. Procedures are essentially the same as those described previously (Nakajima et al., 1999). Briefly, phosphorylation reactions were performed at 30°C in buffer A (40 mm Tris-HCl, pH 7.5, 20 mmMgCl2, 200 μg/mll-α-phosphatidyl-l-serine, 40 μg/ml 1,2-dioleoyl-sn-glycerol) supplemented with reagents and for the times indicated in figure legends. Reactions were stopped by boiling in SDS-PAGE sample buffer, and the samples were run on SDS-PAGE, fixed, and then dried. Finally, the BAS2000 densitometry system (Fuji Film, Tokyo, Japan) was used to determine the amount of [γ-32P]ATP incorporated using a standard curve made from known amounts of 32P.
RESULTS
mGluR/PICK1 interaction in yeast
The specificity of interaction between PICK1 and mGluRs was determined by cotransforming the full-length PICK1 with individual plasmids that contained the complete ct domains of mGluR2, mGluR3, mGluR4, mGluR6, mGluR7a, or mGluR8, or the partial ct products of mGluR1 (A840–P932) or mGluR5 (A826–P918). Yeast cotransformed with PICK1 and ct-mGluR7a gave strong β-galactosidase reporter expression, but no interaction was observed with any of the other mGluR subtypes tested (data not shown).
A series of overlapping deletion constructs of ct-mGluR7a were prepared to determine the PICK1 binding site on ct-mGluR7a. As shown in Figure1A, the last 15 ct-located residues interacted robustly with PICK1, whereas the last seven ct-located residues of mGluR7a were not sufficient for interaction. To further test whether the last 15 residues of ct-mGluR7a contained a PDZ binding motif, we sequentially mutated residue positions 0, −1, and −2. Deletion of the last residue of ct-mGluR7a abolished interaction. Additionally, a mutation of any one of the last three residues of ct-mGluR7a resulted in the loss of interaction with PICK1 (Fig. 1A). Analysis of the ct-mGluR7a sequence reveals a type II PDZ binding motif (-LVI) for the protein (Songyang et al., 1997).
Fig. 1.
Defining the mGluR7a/PICK1 interaction in the yeast two-hybrid system. A, Site of interaction on ct-mGluR7a. Overlapping deletion mutants were constructed to determine the site of interaction on ct-mGluR7a for PICK1. The importance of the last three amino acids was determined using a series of single point mutations at the residues 0, −1, and −2. Asteriskindicates a termination site; triangles show the last three residues of ct-mGluR7a and indicate the PDZ binding motif important for PICK1 interaction; positive interactions, as defined by filter β-galactosidase assays, are indicated as + and negative as −.B, Site of interaction on PICK1. Full-length PICK1 (residues 1-416) and a long version fragment of PICK1 (13-358) containing the PDZ domain gave a positive interaction with ct-mGluR7a. A shorter PICK1 fragment (1-305) still containing the PDZ domain gave negative results. PICK1 constructs (305-416 and 305-358) lacking the PDZ domain failed to interact with ct-mGluR7a. The three point mutations, K27E, K27A, and KD27/28AA, abolished the PICK1/ct-mGluR7a association. Boxshows the PDZ domain of PICK1 that is important for ct-mGluR7a interaction; a carboxylate-binding domain (cbd) of eight amino acids is located at the N terminus of the PDZ; positive interactions, as defined by filter β-galactosidase assays, are indicated as + and negative as −.
To define the sites of interaction on PICK1, truncated constructs were tested for their ability to interact with ct-mGluR7a in yeast (Fig.1B). Two fragments were used that contained the PDZ domain of PICK1, and although the residues 13-358 were sufficient for interaction with ct-mGluR7a, the residues 1-305 showed no interaction. To determine whether the binding site of mGluR7a resides outside the PDZ domain, two PICK1 fragments comprising residues 305-358 or 305-416 were tested. Neither fragment showed any interaction with mGluR7a. Three separate point mutations, K27E, K27A, and KD27/28AA, which were previously shown to abolish interaction with PKCα and the AMPA receptor GluR2 subunit but not PICK1 dimerization (Staudinger et al., 1995, 1997; Dev et al., 1999; Xia et al., 1999), were generated in the carboxylate binding motif of the PDZ domain of PICK1. None of these PICK1 mutants interacted with ct-mGluR7a, suggesting that similar to PKCα and GluR2, the PDZ domain of PICK1 is the critical site of interaction and that a large part of the protein is necessary probably to conform a correct protein structure.
GST pull-down assays
To biochemically confirm the interaction between mGluR7a and PICK1, we performed pull-down assays using GST-ct-mGluR7a coupled to glutathione Sepharose 4B beads and then exposed to PICK1. As reported previously, GST-ct-GluR2 retained flag-PICK1 expressed in COS-7 cells, whereas no flag-PICK1 was retained by GST alone (Dev et al., 1999). GST-ct-mGluR7a also bound flag-PICK1 in amounts similar to that observed for GST-ct-GluR2 (Fig.2). The mGluR7a/PICK1 and GluR2/PICK1 interactions were abolished by a single point mutation within the PDZ domain of flag-PICK1(K27E) (Fig. 2). Similar results were obtained using native rat brain PICK1 from P2 sonicates. In these experiments, GST-ct-mGluR7a retained native PICK1 to a similar degree as GST-ct-GluR2 (Fig. 2).
Fig. 2.
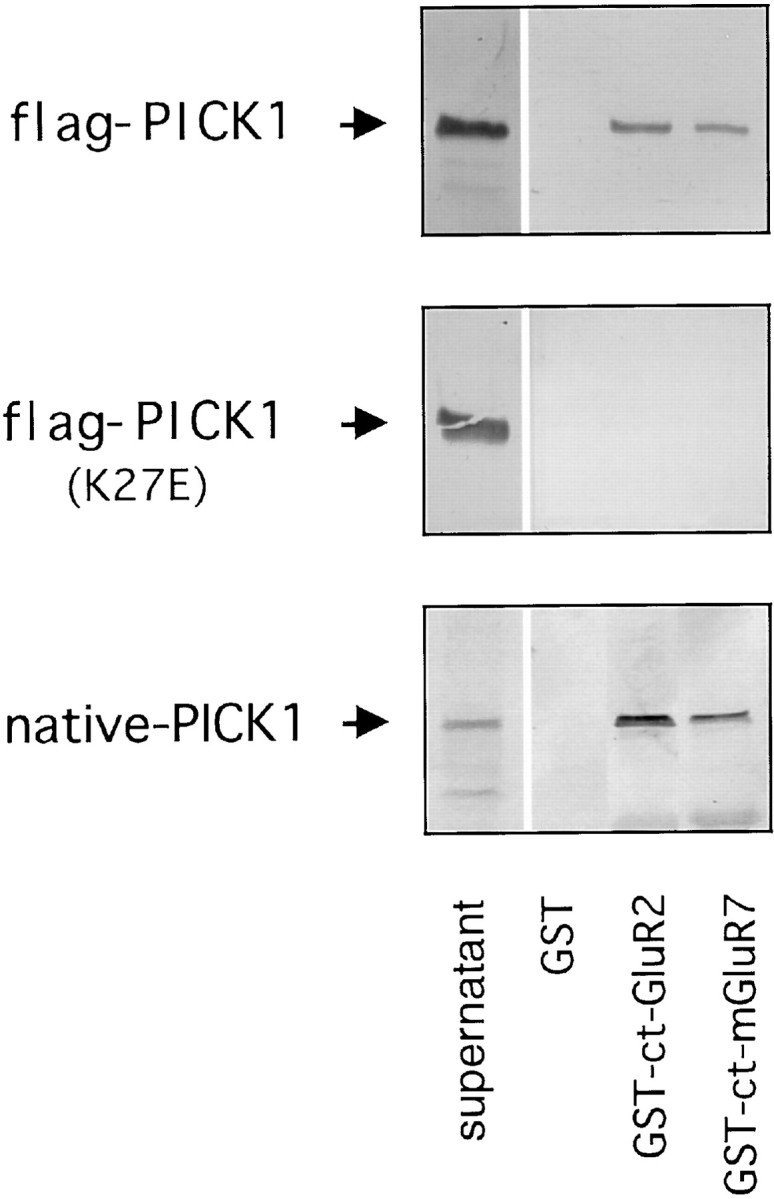
In vitro binding of ct-mGluR7a/PICK1 interaction. Shown are GST-ct-mGluR7a pull-down assays. Western blot using anti-flag M2 mAb showing the levels of COS-7 cell expressed flag-PICK1 (top panel) and point mutated flag-PICK1 (K27E) (center panel) retained by GST alone, GST-ct-GluR2, GST-ct-mGluR7. Western blot using anti-PICK1-rab Ab showing the amount of rat brain lysate-derived native PICK1 (bottom panel) retained by GST alone, GST-ct-GluR2, GST-ct-mGluR7. Supernatant indicates the level of PICK1 expression in the input.
Co-immunoprecipitation of mGluR7a/PICK1 complex
COS-7 cells were transiently transfected with GFP-PICK1 and the full-length flag-mGluR7a. As expected, anti-flag M2-agarose affinity beads efficiently precipitated flag-mGluR7a. More importantly, GFP-PICK1 was co-immunoprecipitated with flag-mGluR7a (Fig.3A). These results indicate the specific co-immunoprecipitation of GFP-PICK1 with flag-mGluR7a and show the presence of a GFP-PICK1/flag-mGluR7a complex within COS-7 cells.
Fig. 3.
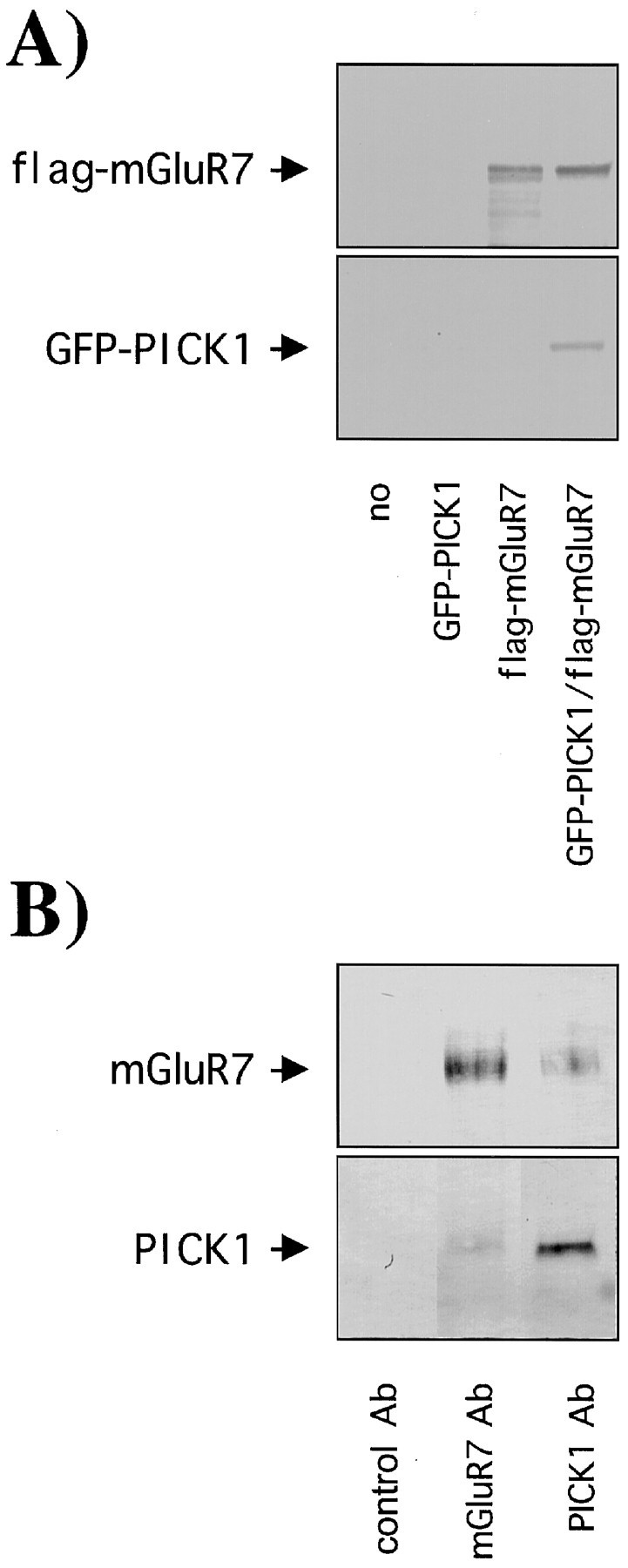
Co-immunoprecipitation of a ct-mGluR7a/PICK1 complex. A, Co-immunoprecipitation studies in COS-7 cells. COS-7 cells transfected with no cDNA, GFP-PICK1, flag-mGluR7a, GFP-PICK1, and flag-mGluR7a. A GFP-PICK1/flag-mGluR7a complex was co-immunoprecipitated using anti-flag M2-agarose affinity beads. The amount of flag-mGluR7a and GFP-PICK1 retained by the beads was detected by Western blotting using anti-flag M2 mAb (top blot) and anti-GFP Ab (bottom blot), respectively. B, Co-immunoprecipitation from rat brain. Rat brain tissue expressing native PICK1 and mGluR7a was precipitated using protein-G Sepharose beads that had been previously coupled to control rabbit IgG, anti-mGluR7a-rab Ab, anti-PICK-rab Ab. The amount of mGluR7a and PICK1 retained by the beads was detected by Western blotting using anti-mGluR7-gpig Ab (top blot) or anti-PICK-gpig Ab (bottom blot), respectively.
We next demonstrated that the interaction occurs in brain by using anti-PICK-rab Ab and anti-mGluR7a-rab Ab to co-immunoprecipitate from solubilized rat brain homogenate (Fig. 3B). Using anti-mGluR7a-gpig Ab and anti-PICK-gpig Ab for Western blotting, the results show that both anti-mGluR7a-rab Ab and anti-PICK-rab Ab co-immunoprecipitated a PICK1/mGluR7a complex from rat brain, whereas no proteins were precipitated with rabbit IgG alone.
Distribution of PICK1 and mGluR7a in hippocampal neurons
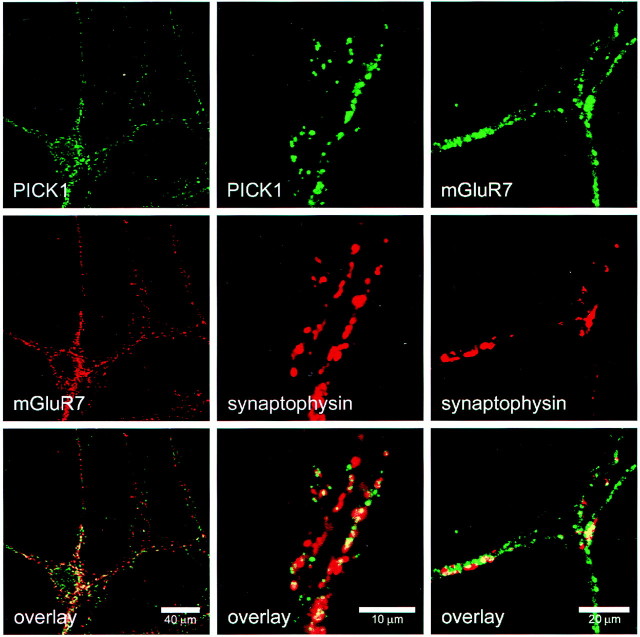
A number of reports have shown previously the presynaptic localization of mGluR7 (Okamoto et al., 1994;Saugstad et al., 1994; Ohishi et al., 1995; Kinoshita et al., 1998). A ubiquitous localization of PICK1 has been reported, and recently, subcellular fractionation experiments have shown PICK1 in multiple locations (Staudinger et al., 1995, 1997; Torres et al., 1998; Xia et al., 1999). More specifically, PICK1 has been found to be enriched in synaptic membrane and synaptic vesicle fractions, suggesting both presynaptic and postsynaptic localizations (Torres et al., 1998). Here we show that double labeling of cultured hippocampal neurons for PICK1 and mGluR7a indicated some, but not an exclusive, overlap of PICK1 and mGluR7a puncta. In agreement with previous reports (Stowell and Craig, 1999; Xia et al., 1999), both PICK1 and mGluR7a showed a punctate distribution and an overlap with synaptophysin, suggesting their enrichment at synapses (Fig.4). Some synaptophysin-positive, PICK1- or mGluR7a-negative puncta were also observed. Conversely, a minor portion of puncta was PICK1 positive but synaptophysin negative.
Fig. 4.
PICK1 and mGluR7a distribution in rat hippocampal neurons. Co-staining of cultured hippocampal neurons for PICK1 and mGluR7a, PICK1 and synaptophysin, mGluR7a and synaptophysin. Top panel, Green channel; center panel, red channel; bottom panel, overlay seen inyellow. Immunoreactivity for mGluR7a shows a punctate distribution that is colocalized with immunoreactivity for PICK1 and synaptophysin. A portion of PICK1 immunoreactivity is also found not colocalized with mGluR7a or with synaptophysin. Scale bar is indicated in white.
Co-immunoprecipitation of a PKCα/PICK1/mGluR7a complex from COS-7 cells
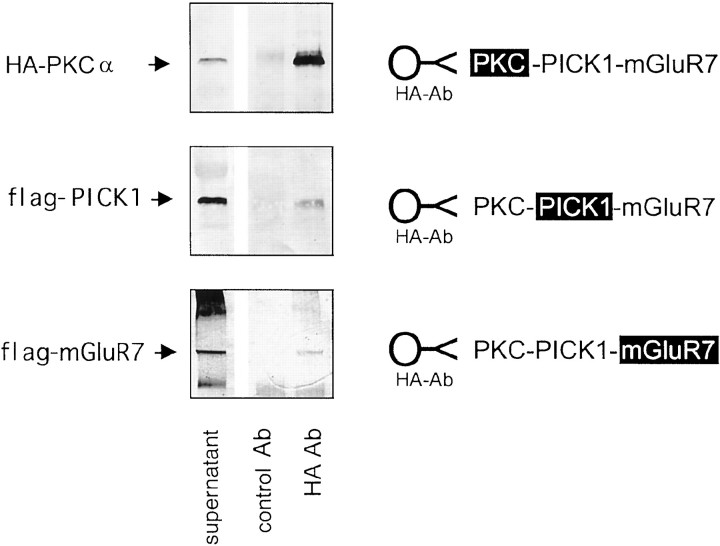
Previous studies have shown that mGluR7a serves as a phosphorylation substrate for PKC (Nakajima et al., 1999). Furthermore, in addition to interacting with PKCα, PICK1 is also phosphorylated by PKC (Staudinger et al., 1995,1997). To establish a possible link between these three proteins, we determined the existence of a PKCα/PICK1/mGluR7a complex within COS-7 cells. After transiently transfecting COS-7 cells with the full-length clones of flag-PICK1, flag-mGluR7a, and HA-PKCα, the cell sonicates were incubated with anti-HA Ab that had been coupled to protein-G Sepharose beads. The amount of HA-PKCα, flag-PICK1, and flag-mGluR7a was assessed by Western blotting using anti-HA mAb, anti-PICK-gpig Ab, and anti-flag M2 mAb, respectively. The blots show that anti-HA Ab efficiently immunoprecipitated HA-PKCα. Co-immunoprecipitation of PICK1 and mGluR7a also occurred, indicating a complex formation of PKCα/PICK1/mGluR7a in COS-7 cells (Fig.5).
Fig. 5.
PKCα/PICK1/mGluR7a form a complex in COS-7 cells. Shown are immunoprecipitation studies. Lysates of COS-7 cells co-transfected with HA-PKCα, flag-PICK1, and flag-mGluR7a were precipitated using protein-G Sepharose beads that had been previously coupled to either control rabbit IgG (control Ab) or anti-HA Ab (HA Ab). The amount of HA-PKCα, flag-PICK1, and flag-mGluR7a retained by the beads was detected by Western blotting using anti-HA mAb (top blot), anti-PICK1-gpig Ab (middle blot), or anti-flag M2 mAb (bottom blot), respectively. The schematics next to each blot indicate the number of protein/protein interactions between the anti-HA Ab and the precipitated protein.
Inhibition of PKCα-evoked phosphorylation of mGluR7a by PICK1
To investigate what role PICK1 plays in a PKCα/PICK1/mGluR7a complex, we investigated the effects of PICK1 on PKCα-evoked phosphorylation of mGluR7a. The effects of PICK1 binding on PKCα phosphorylation were examined by incubating a fixed amount of GST-ct-mGluR7a with fixed amounts of MAL (control) or MAL-PICK1 and [γ-32P]ATP in the absence or presence of increasing concentrations of PKCα. When compared with MAL (control), MAL-PICK1 was shown to have an inhibitory action on PKCα-evoked phosphorylation of mGluR7a throughout the range of PKCα concentrations examined (Fig.6A). Although we have not formally excluded the possibility that MAL, while tethered to PICK1, could hinder PKCα phosphorylation, we believe this to be unlikely. Indeed, to further investigate the mechanism of PICK1 inhibition of PKCα phosphorylation, we determined its effects on a GST-ct-mGluR7a mutant, which contains the PKCα phosphorylation sites but not the region that interacts with PICK1. Figure6B shows that MAL-PICK1 has no effect on the rate of PKCα-evoked phosphorylation of the GST-ct-mGluR7a mutant as compared with PKCα-evoked phosphorylation of the mutant protein without addition of MAL-PICK1. These data suggest that the binding of PICK1 to mGluR7a is required for inhibition of PKCα phosphorylation of mGluR7a.
Fig. 6.
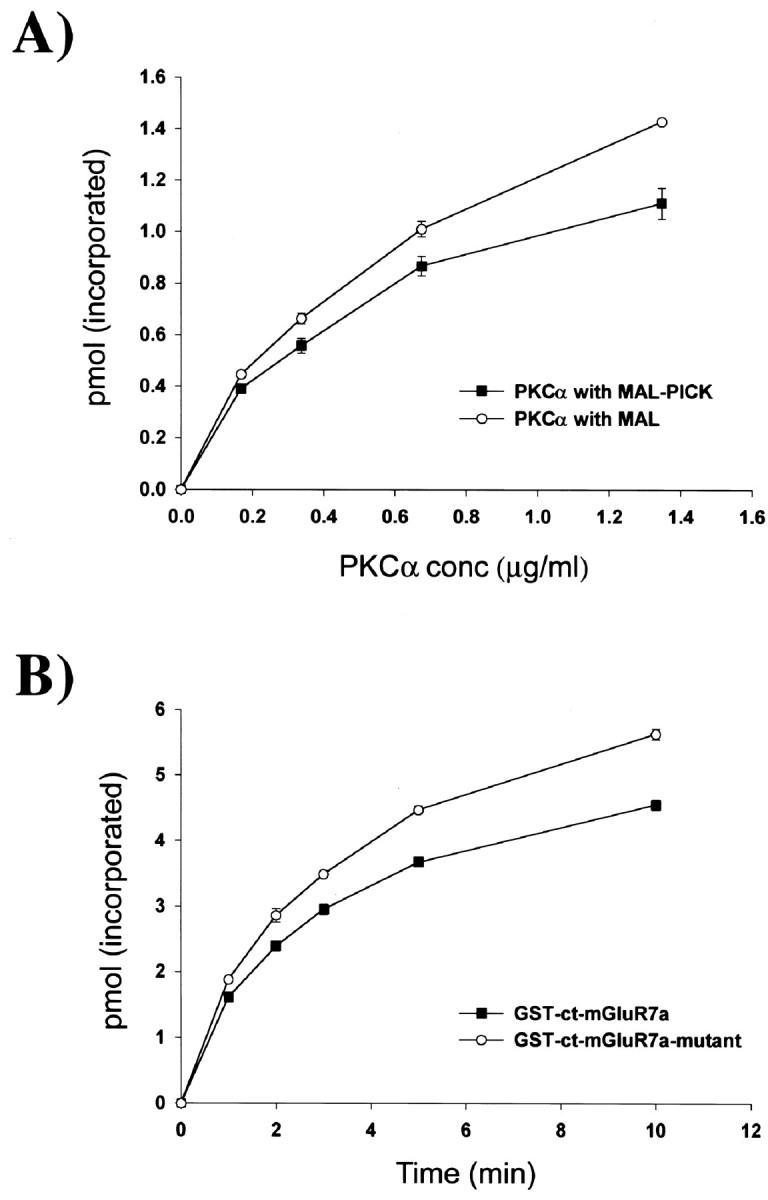
PICK1 inhibits PKCα phosphorylation of mGluR7a.A, Inhibition of PKCα phosphorylation by PICK1. GST-ct-mGluR7a (15 pmol) was preincubated with 15 pmol MAL-PICK1 or MAL (control) in 10 μl buffer B (20 mm HEPES, pH 7.4, 120 mm NaCl, 1 mm CaCl2) for 3 hr at 4°C. The phosphorylation reaction was started by adding varying concentrations of PKCα in buffer A (supplemented with 200 μm [γ-32P]ATP, 50 mCi/mmol) and allowed to proceed for a total of 3 min. When compared with MAL alone, MAL-PICK1 inhibited PKCα-evoked phosphorylation of GST-ct-mGluR7a throughout the range of PKCα concentrations examined. B, Inhibition of PKCα phosphorylation requires PICK1/mGluR7a interaction. GST-ct-mGluR7a (15 pmol) or GST-ct-mGluR7a-mutant (H851–L892, a construct that contains the PKCα phosphorylation sites but not the interaction site for PICK1) was preincubated with 15 pmol MAL-PICK1 and 1.35 μg/ml PKCα in 10 μl buffer A (supplemented with 1 mmCaCl2) for 3 hr at 4°C. The phosphorylation reaction was started by addition of 10 μl buffer C (40 mmTris-HCl, pH 7.5, 200 μm [γ-32P]ATP, 50 mCi/mmol) and allowed to proceed for times indicated. The rate of PKC-evoked phosphorylation of GST-ct-mGluR7a was reduced as compared with the GST-ct-mGluR7a-mutant. The mean percentage inhibitions of PKCα phosphorylation of GST-ct-mGluR7a as compared with GST-ct-mGluR7a-mutant were 14.4, 16.4, 15.5, 17.9, and 19.2% at the time points 1, 2, 3, 5, and 10 min, respectively.
DISCUSSION
Our data show that PICK1 associates with mGluR7a via a PDZ domain interaction. Deletion and mutant constructs of ct-mGluR7a indicate a vital role for the last three residues of ct-mGluR7a for interaction with PICK1 and suggest an additional importance for the last 15 residues. Point mutations made within the carboxylate binding domain of PICK1 show the involvement of its PDZ domain, and use of truncated fragments implies that nearly the full length of PICK1 is required for interaction, probably because of the need for PICK1 to be in a correct conformation. Biochemical experiments verified the interaction showing that recombinant and native PICK1 were retained by GST-ct-mGluR7a. After expression in COS-7 cells, GFP-PICK1 was co-immunoprecipitated with flag-mGluR7a, indicating the formation of a PICK1/mGluR7a complex within these cells. Importantly, a native rat brain PICK1/mGluR7a complex was immunoprecipitated with antibodies directed against PICK1 or mGluR7a. In hippocampal neurons, both PICK1 and mGluR7a showed a partial overlapping distribution with synaptophysin, indicating their presence at synapses. Finally, PKCα was shown to form a complex with mGluR7a and PICK1 where PICK1 evoked an inhibitory effect on the phosphorylation of mGluR7a.
A number of PDZ-mediated interactions have been reported to occur via the recognition of a short ct peptide motif that usually contains three to seven important residues located at the extreme ct domain (Songyang et al., 1997). Analysis of the ct domains of metabotropic glutamate receptor subtypes shows that the last three residues of group I and II mGluRs contain a common St/sL motif, similar to the type I PDZ binding motift/sXv/i. In contrast, the sequences for group III mGluRs are more divergent; in particular, ct-mGluR7a ends with -LVI residues, which resemble the type II PDZ binding motif (Songyang et al., 1997). To date, PICK1 has been shown to bind to a number of ct-located motifs. PICK1 shows a diverse binding profile with the ability to interact with both type I and type II PDZ binding motifs. Examples include PKCα (type I) (Staudinger et al., 1995, 1997), ephrin ligands and Eph receptors (type II) (Torres et al., 1998), and the short alternative splice variants of AMPA receptor subunits (type II) (Dev et al., 1999; Xia et al., 1999). PICK1 also has the ability to form dimers (Staudinger et al., 1995, 1997), thereby allowing it to have a possible role in cross-linking its interacting proteins. This array of interactions opens the possibility of a wide range of cellular roles for PICK1.
Recently, mGluR7a has been shown to interact with CAM in a manner that is mutually exclusive with the βγ subunits of G-proteins (O'Connor et al., 1999) and is modulated by PKC phosphorylation (Nakajima et al., 1999). However, this interaction does not require the presence of a PDZ-binding motif or of a PDZ domain in the mGluR7a or CAM proteins, respectively, and occurs in the proximal part of ct-mGluR7a (Nakajima et al., 1999). In a similar set of studies, PKC has been suggested to disrupt mGluR7a function by uncoupling the receptor from the GTP-binding protein by direct phosphorylation of the receptor protein (Macek et al., 1999; O'Connor et al., 1999). In addition, agonist-induced receptor phosphorylation of ct-located sequences has been shown to cause internalization of G-protein-coupled receptors (Ferguson et al., 1996;Goodman et al., 1996).
Because of the importance of PKC phosphorylation events in controlling mGluR7a, the possible role of PICK1 in linking PKCα to mGluR7a was investigated. Recent reports investigating the interaction between ct-GluR2 (an AMPA receptor subunit) and the glutamate receptor interacting protein (a PDZ domain containing protein) have suggested that receptor–PDZ interactions prevent PKC-evoked phosphorylation of the receptor (Li et al., 1999; Matsuda et al., 1999). Here we provide a possible molecular mechanism for the regulation of PKCα-evoked phosphorylation of mGluR7a by PICK1. We report the complex formation between PKCα/PICK1/mGluR7a and show that PICK1 plays an inhibitory role in PKCα phosphorylation events of mGluR7a. Furthermore, this inhibition is dependent on the interaction of PICK1 with mGluR7a. One possible mechanism to explain this observation could be that PICK1 masks or allosterically alters the mGluR7a phosphorylation sites, thereby inhibiting PKCα phosphorylation. Because PICK1 is known to form dimers (Staudinger et al., 1995, 1997), another possible inhibitory mechanism could be that these dimers function as an “inhibitory bridge” such that one PICK1 molecule binds to mGluR7a and the other binds to PKCα, causing PKCα to be held at a distance from mGluR7a.
Previous reports have shown a predominantly presynaptic localization of mGluR7 (Kinoshita et al., 1998), whereas PICK1 has been found in separated subcellular fractions that occur presynaptically and postsynaptically (Torres et al., 1998). Interestingly, mGluR7 has been specifically located in the active zone/presynaptic grid of the excitatory synapse (Shigemoto et al., 1996), whereas other mGluRs that do not interact with PICK1 (such as mGluR2) do not show this type of localization. Whether PICK1 plays a role in targeting mGluR7 to this region of the neuron is presently unclear. A recent study has suggested that the latter half of ct-mGluR7a is responsible for axonal targeting (Stowell and Craig, 1999). However, because the target signal was able to function without its placement at the extreme C terminus (thought to be a normal requirement for PDZ type interactions), the mechanism was not believed to involve the PDZ binding motif of ct-mGluR7 or to be dependent on PDZ-based interactions. Nevertheless, PICK1 may serve to regulate mGluR7a trafficking by modulating as yet undiscovered transport proteins for mGluR7a. Indeed, the regulation of PKCα phosphorylation by PICK1 may itself act as a signal for receptor turnover and trafficking events. Because phosphorylation may be important in modulating mGluR7 function or in controlling internalization and turnover events, under certain conditions, PICK1 may regulate glutamate release at the presynaptic terminal. Clearly, evidence is now emerging that the C terminal of mGluR7 is crucial in both the transport and function of this receptor. The processes by which interacting proteins (PICK1 and CAM), regulatory proteins (PKC), and unknown targeting proteins function in combination to control the mGluR7 receptor are sure to play important roles in modulating glutamate-mediated transmission.
Footnotes
This work was supported in part by research grants from the Medical Research Council (UK), the Wellcome Trust (UK), and the Ministry of Education, Science and Culture of Japan. K.K.D. is a Wellcome Trust Research Fellow. We are very grateful to Guido Meyer and Graham L. Collingridge for their helpful discussions, to Lisa Pickard for assistance in hippocampal cell preparations, and to Dai Watanabe for help in the preparation of this manuscript. We also thank Atsushi Nishimune for providing some yeast two-hybrid constructs, Yoshiaki Tagawa for the pBSKII flag-mGluR7a, Naoaki Saito for providing the HA-PKCα construct, and Ryuchi Shigemoto for both mGluR7a antibodies.
Correspondence should be addressed to Shigetada Nakanishi, Department of Biological Sciences, Kyoto University, Faculty of Medicine, Yoshida Sakyo-ku, Kyoto 606-8501, Japan. E-mail:snakanis@phy.med.kyoto-u.ac.jp.
REFERENCES
- 1.Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 2.Bushell TJ, Sansig G, Shigemoto R, Flor P, Khun R, Knoepfel T, Scheroeder M, Collet VL, Collingridge GL, van der Putten H. An impairment of hippocampal synaptic plasticity in mice lacking mGlu7 receptors. Neuropharmacology. 1996;35:A6. [Google Scholar]
- 3.Dev KK, Nishimune A, Henley J, Nakanishi S. The protein kinase Cα binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology. 1999;38:635–644. doi: 10.1016/s0028-3908(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 4.Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson SS, Downey WE, III, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 6.Flor PJ, van der Putten H, Rüegg D, Lukic S, Leonhardt T, Bence M, Sansig G, Knoepfel T, Kuhn R. A novel splice variant of a metabotropic glutamate receptor, human mGluR7b. Neuropharmacology. 1997;36:153–159. doi: 10.1016/s0028-3908(96)00176-1. [DOI] [PubMed] [Google Scholar]
- 7.Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 8.Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita A, Shigemoto R, Ohishi H, van der Putten H, Mizuno N. Immunohistochemical localisation of metabotropic glutamate receptors, mGluR7a and mGluR7b, in the central nervous system of the adult rat and mouse: a light and electron microscopic study. J Comp Neurobiol. 1998;393:332–352. [PubMed] [Google Scholar]
- 10.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 11.Kornau HC, Seeburg PH, Kennedy MB. The synaptic protein network associated with ionotropic glutamate receptors. In: Jonas P, Monyer H, editors. Handbook of experimental pharmacology. Berlin; Springer: 1999. pp. 121–142. [Google Scholar]
- 12.Li P, Kerchner GA, Sala C, Wei F, Huettner JE, Sheng M, Zhuo M. AMPA receptor-PDZ interactions in facilitation of spinal sensory synapses. Nat Neurosci. 1999;2:972–977. doi: 10.1038/14771. [DOI] [PubMed] [Google Scholar]
- 13.Macek TA, Schaffhauser H, Conn PJ. Activation of PKC disrupts presynaptic inhibition by group II and group III metabotropic glutamate receptors and uncouples the receptor from GTP-binding proteins. Ann NY Acad Sci. 1999;868:554–557. doi: 10.1111/j.1749-6632.1999.tb11327.x. [DOI] [PubMed] [Google Scholar]
- 14.Malgaroli A, Tsien RW. Glutamate-induced long-term potentiation of the frequency of miniature synaptic currents in cultured hippocampal neurones. Nature. 1992;375:134–139. doi: 10.1038/357134a0. [DOI] [PubMed] [Google Scholar]
- 15.Masugi M, Yokoi M, Shigemoto R, Mugurama K, Watanabe Y, Sansig G, van der Putten H, Nakanishi S. Metabotropic glutamate receptor subtype 7 ablation causes deficit in fear response and conditioned taste aversion. J. Neurosci. 1999;19:955–963. doi: 10.1523/JNEUROSCI.19-03-00955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda S, Mikawa S, Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem. 1999;73:1765–1768. doi: 10.1046/j.1471-4159.1999.731765.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima Y, Yamamoto T, Nakayama T, Nakanishi S. A relationship between protein kinase C phosphorylation and calmodulin binding to the metabotropic glutamate receptor subtype 7. J Biol Chem. 1999;274:27573–27577. doi: 10.1074/jbc.274.39.27573. [DOI] [PubMed] [Google Scholar]
- 18.Nishimune A, Isaac JTR, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- 19.Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, Collingridge GL, Henley JM. Surface expression of AMPA receptors in hippocampal neurones is regulated by an NSF-dependent mechanism. Neuron. 1999;23:365–376. doi: 10.1016/s0896-6273(00)80786-2. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor V, El Far O, Bofill-Cardona E, Nanoff C, Freissmuth M, Karschin A, Airas JM, Betz H, Boehm S. Calmodulin dependence of presynaptic metabotropic glutamate receptor signalling. Science. 1999;286:1180–1184. doi: 10.1126/science.286.5442.1180. [DOI] [PubMed] [Google Scholar]
- 21.Ohishi H, Nomura S, Ding Y.-Q, Shigemoto R, Wada E, Kinoshita A, Li J-L, Neki A, Nakanishi S, Mizuno N. Presynaptic localisation of a metabotropic glutamate receptor, mGluR7, in the primary afferent neurones: an immunohistochemical study in the rat. Neurosci Lett. 1995;202:85–88. doi: 10.1016/0304-3940(95)12207-9. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto N, Hori S, Akazawa C, Hayashi Y, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterisation of a new metabotropic glutamate receptor mGluR7 coupled to inhibitory cyclic AMP signal transduction. J Biol Chem. 1994;269:1231–1236. [PubMed] [Google Scholar]
- 23.Saugstad JA, Kinzie JM, Mulvihill ER, Segerson TP, Westbrook GL. Cloning and expression of a new member of the l-2-amino-4-phosphonobutyric acid-sensitive class of metabotropic glutamate receptors. Mol Pharmacol. 1994;45:367–372. [PubMed] [Google Scholar]
- 24.Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- 25.Shigemoto R, Kulik A, Roberts JD, Ohishi H, Nusser Z, Kaneko T, Somogyi P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature. 1996;381:523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- 26.Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, Weinberg RJ, Ziff EB. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- 29.Staudinger J, Zhou J, Burgess R, Elledge SJ, Olson EN. PICK1: a perinuclear binding protein and substrate for protein kinase C isolated by the yeast two-hybrid system. J Cell Biol. 1995;128:263–271. doi: 10.1083/jcb.128.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staudinger J, Lu J, Olson EN. Specific interaction of the PDZ domain protein PICK1 with the COOH terminus of protein kinase C-α. J Biol Chem. 1997;272:32019–32024. doi: 10.1074/jbc.272.51.32019. [DOI] [PubMed] [Google Scholar]
- 31.Stemmer WP, Morris SK. Enzyme Inverse PCR: a restriction site independent, single-fragment method for high efficiency, site directed mutagenesis. Biotechniques. 1992;13:214–220. [PubMed] [Google Scholar]
- 32.Stowell JN, Craig AM. Axon/dendrite targeting of metabotropic glutamate receptors by their cytoplasmic carboxy-terminal domains. Neuron. 1999;22:525–536. doi: 10.1016/s0896-6273(00)80707-2. [DOI] [PubMed] [Google Scholar]
- 33.Takeya R, Takeshige K, Sumimoto H. Interaction of the PDZ domain of human PICK1 with class I ADP-ribosylation factors. Biochem Biophys Res Commun. 2000;267:149–155. doi: 10.1006/bbrc.1999.1932. [DOI] [PubMed] [Google Scholar]
- 34.Torres R, Firestein BL, Dong H, Staudinger J, Olson EN, Huganir RL, Bredt DS, Gale NW, Yancopoulos GD. PDZ proteins bind, cluster and synaptically colocalise with Eph receptors and their ephrin ligands. Neuron. 1998;21:1453–1463. doi: 10.1016/s0896-6273(00)80663-7. [DOI] [PubMed] [Google Scholar]
- 35.Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]