Abstract
We examined the effects of G-protein β and γ subunit heterodimers on human α1B (N-type) Ca channels expressed in HEK293 cells. All of the known β subunits (β1–β5) produced voltage-dependent inhibition of α1B Ca channels, depending on the γ subunit found in the heterodimer. β1–β4 subunits inhibited Ca channels when paired with γ1–γ3. However, β5 subunits only produced inhibition when paired with γ2. In contrast, heterodimers between β5 subunits and RGS (regulators of G-protein signaling) proteins containing GGL domains did not produce inhibition of Ca channels. However, GGL domain-containing RGS proteins (e.g., RGS6 and RGS11) did block the ability of Gβ5/γ2 heterodimers to inhibit Ca channels. Because all of the G-protein β subunits are found in the nervous system, we conclude that they may all potentially participate in Ca channel inhibition. The interaction of GGL-containing RGS proteins with Gβ5γ2 suggests a novel way in which Ca channels can be regulated.
Keywords: heterotrimeric G-proteins, calcium channel, ion–channel modulation, RGS proteins, presynaptic inhibition, synaptic transmission
Activation of G-protein-coupled receptors (GPCRs) by neurotransmitters has been shown to induce the inhibition of several types of voltage-sensitive Ca channels, including α1B (N-type), α1A(P/Q-type), and α1E (R-type) (Miller, 1998;Simen and Miller, 1998, 2000). The resulting reduction in Ca influx may be important for GPCR-mediated inhibition of neurotransmitter release (Miller, 1998). Investigations of the mechanisms underlying GPCR-mediated Ca channel inhibition have shown that different processes can occur. The best studied of these is rapid and is characterized by its voltage dependence (Hille, 1994; Miller, 1998). The view is widely held that Ca channel inhibition of this type is mediated by the direct binding of G-protein β/γ subunits to one or more sites on the Ca channel α1 subunit (Herlitze et al., 1996;Ikeda, 1996; J. F. Zhang et al., 1996; Qin et al., 1997; Simen and Miller, 1998, 2000; Canti et al., 1999). According to this model, activation of any GPCR should produce inhibition of Ca channels, because β/γ subunits are always released. However, this is clearly not the case, and there are many examples of GPCR activation that does not produce voltage-dependent inhibition of Ca channels (Bernheim et al., 1991; Taussig et al., 1992; Shapiro and Hille, 1993; Hille, 1994;Shapiro et al., 1994; Liu et al., 1995; Margeta-Mitrovic et al., 1997). In many cases these GPCRs are linked to G-proteins of the αq/α11 family. However, the reasons for this selectivity are not clear. One possibility is that not all combinations of β/γ subunits are equally effective in inhibiting Ca channels. There are at least 5 types of β subunits, all of which are found in the nervous system (Betty et al., 1998), and at least 11 types of γ subunits; therefore, many β/γ combinations are potentially possible (Morris and Malbon, 1999). Garcia et al. (1998) examined this question by analyzing the effects of expressing different β subunits in cultured rat superior cervical ganglion (SCG) neurons. They observed that only β1 and β2 produced strong voltage-dependent Ca channel inhibition, whereas β5 and, particularly, β3 and β4 were weak in this regard. Because Gαq is often thought to associate with β5 (Fletcher et al., 1998), it could be argued that lack of effect of β5 was responsible for the modest Ca channel inhibition observed with GPCRs of this type. Recently, however, Ruiz-Velasco and Ikeda (2000)reexamined this question and observed that all β subunits could produce inhibition of Ca channels in rat sympathetic neurons under the appropriate conditions.
In the present series of experiments we examined this question further using cloned human α1B (N-type) Ca channels and different combinations of β/γ subunits. Our data indicates that all of the β subunits can inhibit Ca channels, but that this is dependent on the nature of the γ subunit present in the β/γ heterodimer. Moreover, the effects of some β/γ combinations are influenced by RGS (regulators of G-protein signaling) proteins, suggesting a novel mechanism through which these proteins may regulate neuronal Ca channels.
MATERIALS AND METHODS
Expression constructs. Mouse Gβ4 (GenBank accession number M87286) was prepared from adult male CD4 mouse brain mRNA. Human Gβ1 (X04526), Gβ2 (NM_005273), Gβ3 (NM_002075), and Gβ5 (AF017656) were obtained from human embryonic kidney (HEK) cell line mRNA (Quick Prep Micro mRNA purification kit; Amersham Pharmacia Biotech, Piscataway, NJ). β subunit cDNAs were amplified from the isolated mRNA by reverse transcription-PCR (thermal cycling: 98°C, 20 min; 56°C, 1 min; 72°C, 1.5 min; 35 cycles, preceded by a 3 min 98°C denaturing) with SuperScript II reverse transcriptase (Life Technologies, Rockville, MD) and oligo-dT oligonucleotides and then with specific primers and TakaRa LA Taq DNA polymerase (PanVera, Madison, WI). Subunit specific primers used here were as follows: β1 forward (β1F), GCCGCCACCATGAGTGAGCTTGACCAGTTACGGCAGGAG; β1 reverse (β1R), CTTAGTTCCAGATCTTGAGGAAGCTATCCCA; β2F, GCCGCCACCA-TGAGTGAGCTGGAGCAACTGAG; β2R, CCATTAGTTCCAGATC-TTGAGGAAGG; β3F, GCCACCATGGAGCAACTGCGTCAGGAA-GC; β3R, CCACTTCCCTTTCTCCAGCCTCC; β4F, GCCACCATGA-GCGAGCTGGAGCAGCTGA; β4R, CTCCATGTATCATTGGAGA-ACAG; β5F, GCCGCCACCATGGCAACCGAGGGGCTGC; and β5R, GATGATTAGGCCCAGACTCTGAG.
All constructs contained an expression-optimized Kozak sequence (GCCACC) before the 5′-ATG start codon. The resulting amplification products were first cloned into pCRII/TOPO vector (Invitrogen, San Diego, CA) for verification. Verified constructs were directionally cloned into a mammalian expression vector driven by the cytomegalovirus (CMV) promoter (Gβ1 was cloned in pCMV6C; Gβ2, Gβ3, and Gβ5 were cloned in pcDNA3.1; and Gβ4 was cloned in pCR3.1). The resulting clones were verified by restriction enzyme digestion and automated DNA sequencing (ABI 377 sequencer; Perkin-Elmer, Oak Brook, IL). Gγ1 was subcloned in pCDNA1, Gγ2 in pCDM8.1, and Gγ3 in pCDNA1 (gifts from Dr. Katz, Caltech). Green fluorescent protein (GFP) vector is commercially available from Life Technologies.
The RGS11n construct encoding the N-terminal half of the RGS11 protein contains DEP and GGL domains, and the RGS11c construct encoding the C-terminal half of the RGS11 protein contains an RGS domain. They were generated by PCR (T7 primer with R11R, CTTCGTGGGGGCAGCCTCGAGGGGGGCATTCATGAC; and pcDNA3.1R primer with R11F, GTGGTGGAATTCGACCATGCCGCATCTGAG-GAAGATGGAGCGGGTGGTCGTGAGCATGCAGGACGTCATGAATGCCCCCACGGTGGCT) using pfu DNA polymerase (Stratagene, La Jolla, CA) and were subcloned into a pcDNA3.1 vector. The two constructs were designed to have the same sequence around the start codon as in full-length RGS11 to achieve similar expression levels. Full-length RGS11, RGS11ΔD, and RGS11*(PW274AS), full-length RGS6, RGS6ΔD, and RGS6*(D297A) constructs were tagged with an N-terminal hemagglutinin epitope and subcloned into the pcDNA3.1 vector as described (Snow et al., 1998,1999). RGS2, RGS4, and untagged RGS11 constructs, cloned into the mammalian expression vector pcDNA3.1, were gifts from Drs. A. Gilman and A. Krumins (University of Texas Southwestern Medical Center).
Cell culture and transient transfection. The C2D7 cell line, derived from HEK293 cells stably expressing the N-type Ca channel α1B, α2δ, and β1–3 subunits (Simen and Miller, 1998, 2000), was kindly provided by SIBIA Neurosciences and kept in medium (11995DMEM, 1% penicillin/streptomycin, 5% bovine calf serum, 0.5 g/l geneticin, and 70 μl/500 ml hygromicin). The cells were transfected with 5 μg of G-protein β subunit, 5 μg of G-protein γ subunit, and 1 μg of GFP DNA, using the polyethylenimine method as described by Boussif et al. (1995). In RGS cotransfection experiments, different amounts of Gβ, Gγ, and RGS DNA were used as indicated in the text. Successfully transfected cells were identified by GFP fluorescence.
Electrophysiological recording. At 40–72 hr after transfection, total Ba2+ currents were measured using the whole-cell patch-clamp technique. The coverslips were mounted in a perfusion chamber and constantly perfused by a gravity feed system with a modified HEPES-balanced external solution (151 mm tetraethylammonium chloride, 10 mmHEPES, 5 mm BaCl2, 1 mmMgCl2, and 10 mm glucose, pH adjusted to 7.4 and osmolarity to 310 mOsm) to isolate theIBa. Pipettes of 3–5 MΩ were pulled from microhematocrit capillary tubes (VWR Scientific, West Chester, PA) with a Flaming–Brown P-97 micropipette puller (Sutter Instrument Co., Novato, CA). The pipette solution contained 100 mm CsCl, 1 mmMgCl2, 10 mm HEPES, 10 mm BAPTA, 3.6 mm MgATP, 14 mm phosphocreatine (CrP), 0.1 mm LiGTP, and 50 U/ml creatine phosphokinase (CrPK); pH was adjusted to 7.2 with Cs(OH), and osmolarity was near 290mOsm. The tip solution was similar to the pipette solution without MgATP, CrP, LiGTP, or CrPK.
IBa was measured and recorded with an Axopatch 200B (Axon Instruments, Foster City, CA) using the Clampex program (pClamp 6 software suite; Axon Instruments). Data were digitized at 10 kHz and filtered at 5 kHz. Series resistance was compensated to 70%, and currents were leak-corrected on-line using a P4 protocol. Prepulse experiments were performed using a prepulse protocol consisting of a 50 msec +10 mV depolarization test pulse from −80 mV holding potential with (test pulse 2) or without (test pulses 1) a 50 msec +80 mV prepulse (Fig. 1A). There was a 5 msec −80 mV interval between the prepulse and test pulse. Currents were analyzed off-line using the Clampfit program. Facilitation was indicated by calculating the “facilitation ratio” (P2/P1), which we defined as the peak current of test pulse 2 divided by the current of test pulse 1 at the same time point (See Simen and Miller, 1998). This time point was normally between 6 and 15 msec after the start of the depolarizing test pulse. All experiments and solutions were used at room temperature.
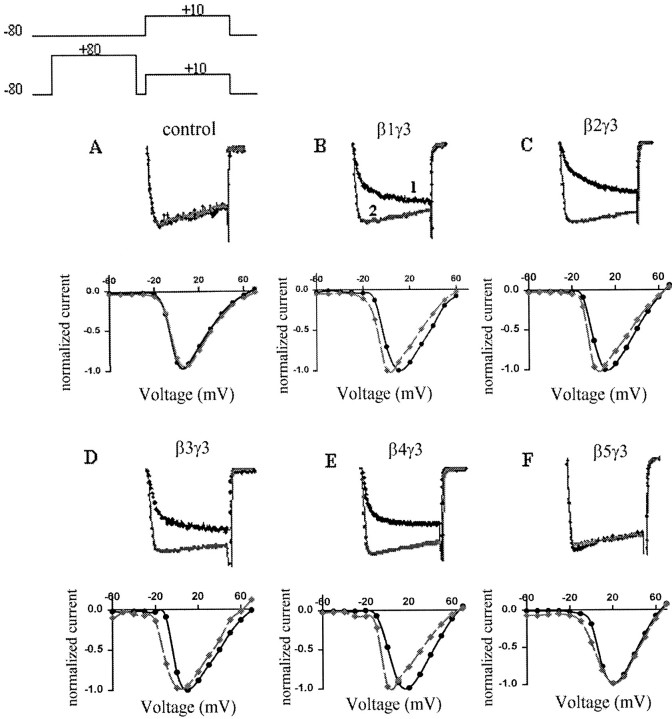
Fig. 1.
Effects of different G β subunits on barium current facilitation. Top panel, SuperimposedIBa during a 50 msec depolarization to +10 mV from −80 mV holding potential, without (trace 1) and with (trace 2) a 50 msec +80 mV prepulse. Bottom panels, Leftward shift of the I–V curve after the prepulse (dashed gray line). Currents were recorded 40–72 hr after transfection of C2D7 cells with GFP alone (A) or with G-protein β1 (B), β2 (C), β3 (D), β4 (E), or β5 (F), expressed together with Gγ3.
Statistical analysis for multiple comparisons was performed using one-way ANOVA followed by a nonparametric Kolmogorov–Smirnov test. The unpaired t test was used for two-group comparison.p < 0.01 was considered statistically significant.
Northern blots. Total RNA was isolated from transfected (48 hr) C2D7 cells using the guanidine thiocyanate-phenol method (Trizol reagent; Life Technologies). Total RNA from untransfected cells was used as a control. Twenty micrograms of RNA for each sample were separated on formaldehyde-agarose gels and transferred to Hybond-N+ nylon membranes (Amersham Pharmacia Biotech). Each β subunit was probed with a subunit-specific oligonucleotide (β1, TTCCCACTGGGTCGATGTTGTT-TGTG; β2, GGAGCAGATGTTGTCCAACC; β3, AATGAAGAGATTG-AAGTCAGGAGACAC; β4, TCCCGAACTTGTAATGATTT GTCCA; β5: CGTGCAGGGCATGGTGACCGCGTGCT). The probe was labeled with T4 polynucleotide kinase (New England Biolabs, Beverly, MA) and [γ32P]ATP (6000Ci/mmol; Amersham Pharmacia Biotech). Membranes were prehybridized for 2 hr at 42°C (6× SSPE, 5× Denhardt's solution, 0.1% SDS, and 100 μg/ml boiled salmon sperm DNA) and hybridized at 42°C overnight (prehybridization solution plus labeled probes). The blots were washed in 5× SSC and 0.5% SDS at room temperature for 10 min and in 0.5× SSC and 0.1% SDS at 42°C for 10–60 min.
RESULTS
We subcloned human G-protein β1, β2, β3, and β5 and mouse (m) β4 into CMV vectors. All β subunits were sequenced and matched sequences formerly reported, with the exception that the mβ4 sequence showed four differences from that reported in the database (M87286). As previously observed (Snow et al., 1999), there were two silent mutations at positions 433 (A→G) and 634 (G→C), and differences at positions 434 (G→A) and 458 (G→C) corresponded to Asp→Asn and Ala→Pro substitutions. Three independent clones were sequenced, and all showed the same changes.
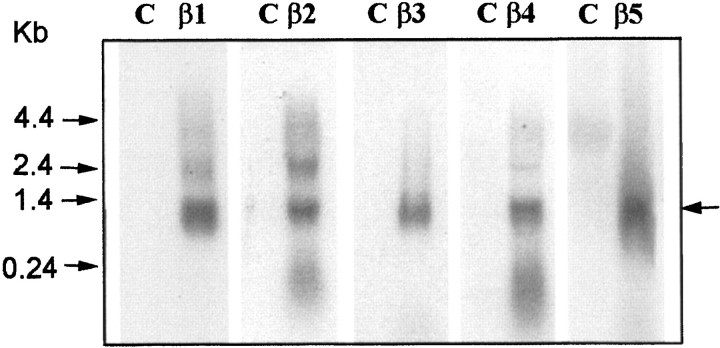
We examined the effects of overexpressing different G-protein β subunits on cloned human α1B (N-type) Ca channels (α1B-1, α2δ, and β1–3) stably expressed in HEK293 cells (C2D7 cells). In control cells, a 50 msec depolarizing test pulse to +10 mV elicited a rapidly activating and slowly inactivatingIBa, which was not significantly altered by a 50 msec prepulse to +80 mV (Fig.1A). To study the ability of different G protein β/γ dimers to inhibit theIBa, we transiently transfected C2D7 cells with different G-protein β subunits, together with γ3 subunits and GFP DNA. Ba currents were only recorded from GFP fluorescent cells. Expression of β1γ3 subunits in C2D7 cells reduced the IBa amplitude and slowed its activation rate (Fig. 1B, trace 1). The inhibition was “relieved” by a depolarizing prepulse (Fig.1B, trace 2). The resulting “facilitation ratio” (see Materials and Methods) has frequently been used as an index of voltage-dependent, membrane-delimited inhibition of Ca channels by G protein β/γ subunits (Simen and Miller, 1998, 2000). In addition, the I–V curve for the IBawas shifted to the left by ∼10–15 mV after the prepulse (Fig.1B, bottom traces). As did Garcia et al. (1998), we observed that both β1γ3 and β2γ3 inhibited N-type currents in a voltage dependent manner, with facilitation ratios of 1.76 ± 0.16 and 1.87 ± 0.21, respectively (Fig. 1B,C). In contrast to Garcia et al. (1998), we found that β4γ3 and β3γ3 also significantly inhibited the IBa(Fig. 1D,E). β4γ3 and β3γ3 produced facilitation ratios of 2.01 ± 0.18 and 1.50 ± 0.12, respectively. However, no effects were observed in cells transfected with Gβ5γ3 DNA (Fig. 1F). Moreover, overexpression of any β subunits alone failed to produce inhibition of the IBa (data not shown). To exclude the possibility that the inability of β5γ3 to inhibit theIBa was attributable to a failure to express the β5 subunit, total RNA was isolated from transfected cells and probed with β subunit-specific oligonucleotides. Each β subunit was expressed as a transcript of ∼1.2 kb (Fig.2).
Fig. 2.
Northern blot of G protein β subunit expression. Total mRNA (20 μg) isolated from untransfected C2D7 cells (as control, C) and cells transfected with different G protein β subunits (as marked) were hybridized with subunit-specific oligonucleotide probes (see Materials and Methods). Positions of the RNA size standards (in kilobases) are shown on the left. Heterologously expressed Gβ subunits are marked with an arrow on theright. Endogenous Gβ subunits were expected to have similar sizes. However, because they were expressed in much lower amounts, they would not be detected under these experimental conditions.
To further assess the potential role of G-protein γ subunits, we investigated the ability of other combinations between β1–β4 and γ1–γ3 to inhibit the IBa. These results are summarized in Figure 3. For βγ1 combinations, β1γ1 exhibited the largest facilitation ratio (1.72 ± 0.14). β2γ1, β3γ1,and β4γ1 exhibited smaller effects, although they were still significantly different from control (one-way ANOVA, p < 0.01). As with β5γ3, β5γ1 failed to inhibit the IBa. βγ2 combinations showed similar facilitation ratios for β1–β4 as did βγ3 and βγ1 combinations. A striking difference, however, was that β5γ2 strongly inhibited theIBa, exhibiting a facilitation ratio of 1.76 ± 0.07.
Fig. 3.
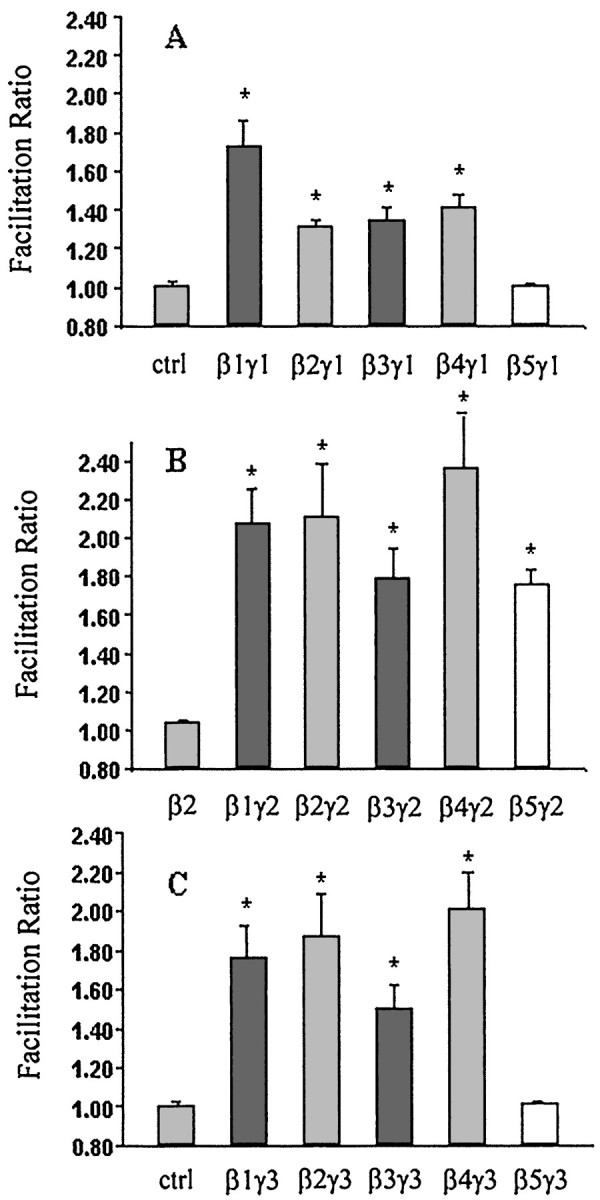
Summary of facilitation ratios for barium current inhibition after transfection of different Gβγ combinations. G-protein β subunits were coexpressed with Gγ1 (A), Gγ2 (B), and Gγ3 (C). Gβ expressed alone did not produce facilitation of IBa, as represented by Gβ2 in B. Data are plotted as mean ± SEM; *p < 0.01, one-way ANOVA analysis followed by nonparametric Kolmogorov–Smirnov test (n = 7∼15).
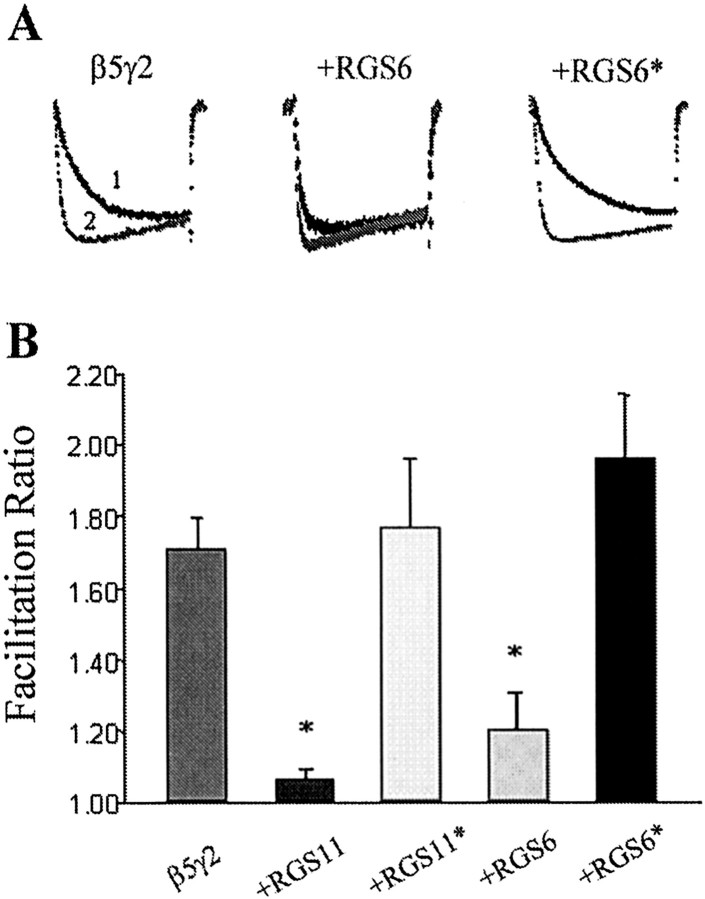
It is interesting to note that G protein γ subunits are not the only proteins that can potentially form heterodimers with β-subunits. RGS6, 7, 9,and 11 belong to a subfamily of G protein α-subunit GTPase-activating proteins (GAPs) that contain a γ-subunit-like (GGL) domain (Hepler, 1999; Siderovski et al., 1999). It has been shown that GGL domain-containing RGS proteins form heterodimers with Gβ5 (Snow et al., 1998, 1999; Posner et al., 1999), raising the possibility that β5/RGS heterodimers could effectively inhibit Ca channels or that RGS proteins could act as “antagonists” by inhibiting the interaction between γ2 and β5. The next series of experiments were designed to investigate these possibilities. Coexpression of the GGL domain containing RGS11 with Gβ5 did not result in Ca channel inhibition (data not shown). Furthermore, neither RGS11 nor Gβ5 expressed alone produced any effect on the IBa (Fig.4A; data not shown for Gβ5 alone). Interestingly, however, we observed that coexpression of RGS6 or RGS11 with β5γ2 was able to antagonize the effects of the β/γ heterodimers (Fig. 5). Thus, increasing the ratio of RGS11 to γ2 in the transfection produced a dose-dependent reduction in the facilitation ratio observed (Fig.4A). This inhibition was selective for the β5γ2 combination; when RGS11 was coexpressed at an 8:1 ratio to γ2, together with β2, no reduction in the effects of the β2γ2 subunits was observed (Fig. 4B). It has been shown that point mutations in the GGL domain (D297A in RGS6 and PW274AS in RGS11) abolish the interaction between GGL-containing RGS proteins and Gβ5 (Snow et al., 1999). Expression of full-length RGS6/11 cDNA constructs with these mutations failed to block the effects of Gβ5γ2 (Fig. 5), thus supporting the role of the GGL domain in the interaction between RGS11 and β5γ2.
Fig. 4.
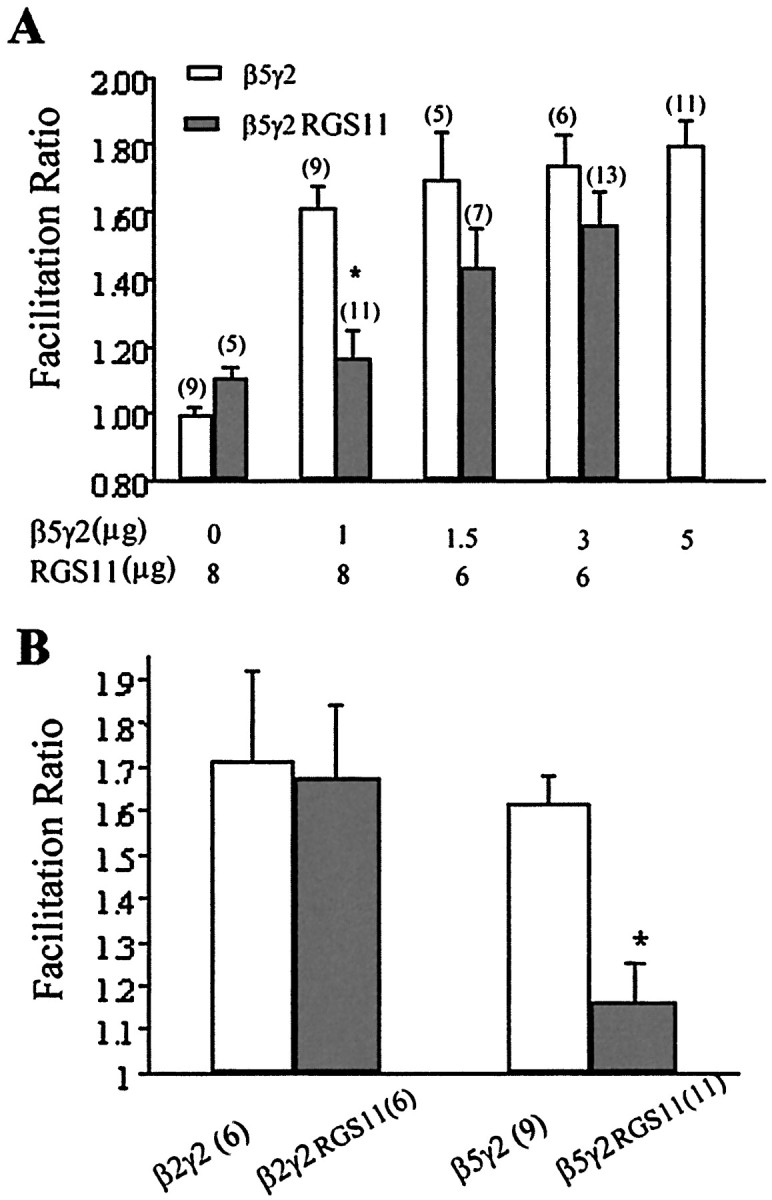
RGS11 antagonized the effects of β5γ2. Increasing the ratio of RGS11 to γ2 reduced theIBa facilitation ratio produced by β5γ2 (A). However, RGS11 did not antagonize the effects of β2γ2 (B). In the second set of experiments (B), 8 μg of RGS11 was cotransfected with 1 μg of β2γ2 or β5γ2.Numbers of experiments are inparentheses. Data are plotted as mean ± SEM; *p < 0.01, unpaired t test between β5γ2 and β5γ2RGS11.
Fig. 5.
RGS6 and RGS11 antagonism of Gβ5γ2 facilitation of Ca currents depends on their GGL domains. RGS6 and RGS11 constructs were cotransfected with Gβ5γ2 at an 8:1 ratio. (RGS6* and RGS11* represent full-length cDNA constructs with mutations in the GGL domain that inhibit binding to β5; see Results and Snow et al., 1999). A, Typical current traces with (trace 2) or without (trace 1) prepulse. B, Summary of facilitation ratios for IBa inhibition after cotransfection with β5γ2 and different RGS constructs. Data are plotted as mean ± SEM; *p < 0.01, one-way ANOVA analysis followed by nonparametric Kolmogorov–Smirnov test (n = 6∼15).
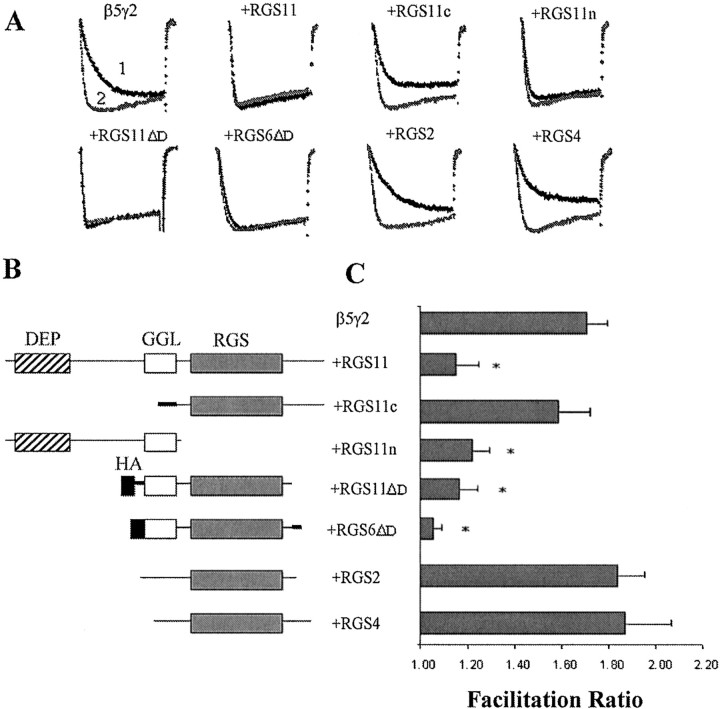
All four of the GGL-containing RGS proteins, RGS6, 7, 9, and 11, contain a DEP (dishevelled/Egl-10/pleckstrin) domain as well as GGL and RGS domains. To further answer the question of which domain(s) is involved in the interaction with Gβ5γ2, we made two constructs consisting of the N-terminal (RGS11n) and C-terminal (RGS11c) halves of the RGS11 protein, with RGS11n containing the DEP and GGL domains and RGS11c containing the RGS domain and a C-terminal tail (Fig.6B). At an 8:1 ratio, cotransfection of the RGS11n construct reduced the facilitation ratio produced by Gβ5γ2 to a degree similar to that observed with the full-length RGS11. However, cotransfection of RGS11c did not reduce the facilitation ratio significantly (Fig. 6C). In accordance with this result, RGS2 and RGS4 proteins, which contain only an RGS domain, failed to reduce the facilitation ratio when coexpressed with Gβ5γ2 (Fig. 6C). To further address the question of whether the DEP domain is a requirement for interaction with Gβ5, the DEP domains of RGS6 and RGS11 were deleted. Both constructs blocked the facilitation by Gβ5γ2 to an extent similar to that of full-length RGS6 and RGS11 (Fig. 6C).
Fig. 6.
RGS protein constructs have different abilities to antagonize IBa inhibition by β5γ2. Various RGS constructs were cotransfected with Gβ5γ2 at an 8:1 ratio. A, Typical current traces with (trace 2) or without (trace 1) prepulse.B, Schematic structures of different RGS protein constructs. Artificial linkers or termini are drawn in thick lines. Hatched boxes, DEP domain; open boxes, GGL domain; filled boxes, hemagglutinin (HA) epitope; gray boxes, RGS domain.C, Summary of facilitation ratios forIBa inhibition after cotransfection with β5γ2 and different RGS protein constructs. Data are plotted as mean ± SEM; *p < 0.01, one-way ANOVA analysis followed by nonparametric Kolmogorov–Smirnov test (n = 8∼15).
DISCUSSION
Voltage-dependent inhibition of Ca channels is thought to be mediated by direct binding of G protein β/γ subunits to one or more sites on the α1 subunit of the Ca channel (Herlitze et al., 1996; Ikeda 1996; Simen and Miller, 1998, 2000). An important question is how selectivity can be imparted to this process. That this is indeed important is indicated by studies showing that the productive activation of some GPCRs, particularly those linked to the αq family of heterotrimeric G proteins, does not produce voltage dependent inhibition of Ca channels, or at least rather little in comparison with other receptors (see introductory remarks). Because the activation of all GPCRs presumably results in the release of β/γ subunits, it is unclear what factors underlie specificity. However, because the precise composition of the heterotrimeric G proteins that interact with each receptor presumably differs, this may dictate specificity in some manner that is not currently understood. One important possibility is that not all combinations of β/γ heterodimers are able to inhibit Ca channels. Considering that there are at least 5 different β subunits and at least 11 types of γ subunits, the number of possible combinations is very large (Morris and Malbon, 1999). Garcia et al. (1998), examined the ability of all of the known β subunits to produce Ca channel inhibition when they overexpressed them in rat SCG neurons. They observed that only β1 and β2 produced strong Ca channel inhibition. Our results complement those of Garcia et al. (1998) in certain important respects. We have shown that all of the β subunits can in fact produce strong Ca channel inhibition, but that this may depend critically on the nature of the γ-subunit involved. For example, although β1–β4 are effective when combined with γ1, 2, or 3, β5 is only effective when expressed with γ2. Similar conclusions can be drawn from the recent work of Ruiz-Velasco and Ikeda (2000). These results indicate that it is possible that in the SCG neurons used by Garcia et al. (1998), γ2 was not highly expressed, or that any other γ subunits that form heterodimers with β5 are also probably ineffective (Watson et al., 1994, 1996). Other explanations are also possible (see below).
The observation that only γ2 was effective in combination with β5 is consistent with several other observations in the literature. It has been shown that β5 will associate with several different γ subunits, including γ2, 4, 5, and 7 (Watson et al., 1994, 1996). Comparison of the functional effects of these heterodimers is limited. However, β5γ2 was shown to be a far more effective activator of phospholipase C (PLC)-β2 than either β5γ4 or β5γ7 (S.Zhang et al., 1996). On the other hand, none of these combinations proved to be effective activators of the MAPK and JNK pathways, although they were all effective in combination with β1 (S. Zhang et al., 1996). Thus, β5-containing heterodimers are not effective in all signaling pathways. Clearly, in the case of N-type Ca channels, the β5/γ2 combination is effective, but β5γ1 and β5γ3 are not. Of particular interest in this regard are observations that β5γ2 heterodimers are selectively found in association with αq or other members of this family of heterotrimeric G proteins (Fletcher et al., 1998). Thus, activation of receptors such as the M1 muscarinic receptor, the ET1 endothelin receptor, or other αq linked receptors can be expected to release β5γ2 subunits (Lindorfer et al., 1998). Because we have now shown that these heterodimers are effective inhibitors of α1B Ca channels, it is unlikely that this explains the ineffectiveness of αq-linked GPCRs in inhibiting Ca channels.
The structure of the β5 subunit is the most unusual of all of the β subunits, being only ∼50% identical to β1–β4, all of which are very similar to one another (Morris and Malbon, 1999). It has recently been shown that the β5 subunit can associate not only with γ subunits but also with proteins of the RGS family that possess γ subunit-like GGL domains (Hepler, 1999; Siderovski et al., 1999). The question arises of the biological significance of these β5/RGS heterodimers. One possibility is that they might support the same effector signaling events as β5/γ2 heterodimers. However, as we demonstrate here, this does not necessarily appear to be the case. Coexpression of β5 with RGS11 did not produce Ca channel inhibition. It has been shown biochemically that overexpression of β5 (but not β1–β4) with GGL-containing RGS proteins does actually lead to the formation of tight complexes in the cytosol of cells (Snow et al., 1998, 1999; Posner et al., 1999; Liang et al., 2000). Thus, it is unlikely that the lack of effect of the RGS11 seen in our experiments is attributable to the lack of formation of heterodimers. Recently,Posner et al. (1999) and Snow et al. (1998) demonstrated that although heterodimers formed between RGS6, 7, or 11 and β5 possessed appreciable GAP activity, they were ineffective in activating or inhibiting adenylate cyclases I and II and were also unable to antagonize the ability of β1γ2 to activate cyclase. In addition, neither β5/RGS complex was able to activate PLC-β2, although high concentrations of the heterodimer had a small inhibitory effect on the enzyme activated by β1γ2 (Posner et al., 1999). Thus, the lack of effect of β5/RGS11 complexes on Ca channels mirrors other data indicating that these complexes are generally inactive in traditional assays of β/γ-mediated signal transduction.
On the other hand, it is also possible that GGL-containing RGS proteins might compete with γ2 for β5 subunits and therefore antagonize the effects of the β5γ2 heterodimer. Indeed, it has been proposed that GGL-containing RGS proteins have a higher affinity for Gβ5 than Gγ2 does, because conversion from phenylalanine (Phe-61) within Gγ2 to tryptophan (Trp-274) at the analogous position found within GGL domains of RGS proteins increases Gβ5/γ2 binding under low-detergent conditions (Snow et al., 1999). We have now obtained evidence for this competing interaction, although the relative affinities of the binding partners cannot be accurately assessed from our experiments. Thus, coexpression of both RGS6 and RGS11 blocked the inhibitory effects of β5γ2 on Ca channels, although they were unable to block the effects of β2γ2. The role of the GGL domains in these effects is clear from the fact that mutations that prevent binding of the GGL domain to β5 also prevented the effect of RGS6 and 11. Furthermore, when we cut the RGS11 proteins into two sections, the portion containing the GGL and DEP domains inhibited the effect of β5γ2, whereas the portion containing the RGS domain did not. The function of the DEP domain is unclear. It has been suggested that DEP domains may play a role in membrane targeting of proteins (Axelrod et al., 1998). However, in the present case, deletion of the DEP domains from RGS6 or 11 did not abolish their effects on β5γ2. Thus, our data are consistent with the possibility that GGL-containing RGS proteins may regulate β5γ2-dependent inhibition of Ca channels through interaction of their GGL domains with β5. In support of this possibility, β5/RGS6 and β5/RGS7 complexes have been isolated from the brain (Liang et al. 2000, Zhang and Simonds, 2000). It is also possible that the decreased effectiveness observed by Garcia et al. (1998) and Ruiz-Velasco and Ikeda (2000) when β5 was expressed in SCG neurons reflects the expression of GGL-containing RGS proteins in these neurons.
In summary, our results indicate that all of the known G-protein β subunits are capable of producing rapid, voltage-dependent inhibition of Ca channels, although this ability may depend on the type of γ -subunit found in the heterodimer. Because all of the β subunits are highly expressed in the nervous system (Betty et al., 1998), it is likely that they may all participate in the receptor-mediated regulation of Ca channels. Thus, the reasons why activation of some GPCRs does not produce strong Ca channel inhibition may not simply depend on the selectivity of β/γ heterodimers for Ca channel inhibition (Garcia et al., 1998). Our data also suggest that GGL-containing RGS proteins may act as antagonists of heterotrimeric G proteins in two ways. First, they can act as GAP proteins for G-protein α subunits, and second, as we show here, they may act as antagonists of β5γ2 heterodimers through their interactions with β5 subunits.
Footnotes
This study was supported by US Public Health Service Grants DA-02121, DA-13141, DA-44840, MH-40165, NS-33826, and NS-21442. We thank Dr. C. Lee for helpful suggestions and comments throughout the project and D. Ren for excellent technical assistance. We are grateful to Drs. A. Gilman and A. Krumins for kindly providing cDNA for RGS proteins, to Dr. Katz for Gγ subunit cDNA, and to SIBIA Neuroscience for cell lines expressing α1B Ca channels.
Correspondence should be addressed to Dr. Richard J. Miller, Department of Neurobiology, Pharmacology, and Physiology, University of Chicago, 947 East 58th Street (MC 0926), Chicago, IL 60637. E-mail:rjmx@midway.uchicago.edu.
REFERENCES
- 1.Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernheim L, Beech DJ, Hille B. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 1991;6:859–867. doi: 10.1016/0896-6273(91)90226-p. [DOI] [PubMed] [Google Scholar]
- 3.Betty M, Harnish SW, Rhodes KJ, Cockett MI. Distribution of heterotrimeric G-protein β and γ subunits in the rat brain. Neurosci. 1998;85:475–486. doi: 10.1016/s0306-4522(97)00623-4. [DOI] [PubMed] [Google Scholar]
- 4.Boussif O, Lezoualch F, Zania MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canti C, Page KM, Stephens GJ, Dolphin AC. Identification of residues in the N terminus of α1B critical for inhibition of the voltage-dependent calcium channel by Gβγ. J Neurosci. 1999;19:6855–6864. doi: 10.1523/JNEUROSCI.19-16-06855.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher JE, Lindorfer MA, De Filippo JM, Yasuda H, Guilmard M, Garrison JC. The G-protein β5 subunit interacts selectively with the Gq α subunit. J Biol Chem. 1998;273:636–644. doi: 10.1074/jbc.273.1.636. [DOI] [PubMed] [Google Scholar]
- 7.Garcia DE, Li B, Garcia-Ferreiro RE, Hernandez-Ochoa EO, Yan K, Gautam N, Catterall WA, Mackie K, Hille B. G-protein β-subunit specificity in the fast membrane delimited inhibition of Ca channels. J Neurosci. 1998;18:9163–9170. doi: 10.1523/JNEUROSCI.18-22-09163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hepler JR. Emerging roles for RGS proteins in cell signaling. Trends Pharmacol Sci. 1999;20:376–382. doi: 10.1016/s0165-6147(99)01369-3. [DOI] [PubMed] [Google Scholar]
- 9.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca channels by G-protein β/γ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 10.Hille B. Modulation of ion channel function by G-protein coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda SR. Voltage dependent modulation of N-type calcium channels by G-protein β/γ subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 12.Liang JJ, Chen HH, Jones PG, Khawaja XZ. RGS7 complex formation and colocalization with the Gβ5 subunit in the adult rat brain and influence on Gβ5γ2-mediated PLCβ signaling. J Neurosci Res. 2000;60:58–64. doi: 10.1002/(SICI)1097-4547(20000401)60:1<58::AID-JNR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Lindorfer MA, Myung CS, Savino Y, Yasuda H, Khazan R, Garrison JC. Differential activity of the G-protein β5γ2 subunit at receptors and effectors. J Biol Chem. 1998;273:34429–34436. doi: 10.1074/jbc.273.51.34429. [DOI] [PubMed] [Google Scholar]
- 14.Liu NJ, Xu T, Xu C, Li CQ, Yu YX, Kang HG, Han JS. Cholecystokinin octapeptide reverses μ-opioid receptor mediated inhibition of calcium current in rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 1995;275:1293–1299. [PubMed] [Google Scholar]
- 15.Margeta-Mitrovic M, Grigg JJ, Koyano K, Nakajima Y, Nakajima S. Neurotensin and substance P inhibit low and high voltage activated Ca channels in cultured newborn rat nucleus basalis neurons. J Neurophysiol. 1997;78:1341–1352. doi: 10.1152/jn.1997.78.3.1341. [DOI] [PubMed] [Google Scholar]
- 16.Miller RJ. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- 17.Morris AJ, Malbon CC. Physiological regulation of G-protein linked signalling. Physiol Rev. 1999;79:1373–1430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- 18.Posner BA, Gilman AG, Harris BA. Regulators of G-protein signalling 6 and 7. J Biol Chem. 1999;274:31087–31093. doi: 10.1074/jbc.274.43.31087. [DOI] [PubMed] [Google Scholar]
- 19.Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Direct interaction of Gβγ with a C-terminal Gβγ-binding domain of the Ca2+ channel α1 subunit is responsible for channel inhibition by G protein-coupled receptors. Proc Natl Acad Sci USA. 1997;4:8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz-Velasco V, Ikeda SR. Multiple G-protein βγ combinations produce voltage-dependent inhibition of N-type calcium channels in rat superior cervical ganglion neurons. J Neurosci. 2000;20:2183–2191. doi: 10.1523/JNEUROSCI.20-06-02183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro MS, Hille B. Substance P and somatostatin inhibit calcium channels in rat sympathetic neurons via different G-protein pathways. Neuron. 1993;10:11–20. doi: 10.1016/0896-6273(93)90237-l. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro MS, Wollmuth LP, Hille B. Angiotensin 11 inhibits calcium and M current channels in rat sympathetic neurons via G-proteins. Neuron. 1994;12:1319–1329. doi: 10.1016/0896-6273(94)90447-2. [DOI] [PubMed] [Google Scholar]
- 23.Siderovski DP, Strockbine B, Behe CI. Whither goest the RGS proteins? Crit Rev Biochem Mol Biol. 1999;34:215–251. doi: 10.1080/10409239991209273. [DOI] [PubMed] [Google Scholar]
- 24.Simen AA, Miller RJ. Structural features determining differential receptor regulation of neuronal Ca channels. J Neurosci. 1998;18:3689–3698. doi: 10.1523/JNEUROSCI.18-10-03689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simen AA, Miller RJ. Involvement of regions in domain I in the opioid receptor sensitivity of alpha1B Ca(2+) Channels. Mol Pharmacol. 2000;57:1064–1074. [PubMed] [Google Scholar]
- 26.Snow BE, Krumins AM, Brothers GM, Lee S, Wall MA, Chung S, Mangion J, Arya S, Gilman AG, Siderovske DP. A G protein γ subunit-like domain shared between RGS11 and other RGS proteins specifies binding to Gβ5 subunits. Proc Natl Acad Sci USA. 1998;95:13307–13312. doi: 10.1073/pnas.95.22.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snow BE, Betts L, Mangion J, Sondek J, Siderovski DP. Fidelity of G protein β-subunit association by the G protein γ-subunit-like domains of RGS6, RGS7, and RGS11. Proc Natl Acad Sci USA. 1999;96:6489–6494. doi: 10.1073/pnas.96.11.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taussig R, Sanchez R, Rifo M, Gilman AG, Belardetti F. Inhibition of the ω-conotoxin sensitive calcium current by distinct G-proteins. Neuron. 1992;8:799–809. doi: 10.1016/0896-6273(92)90100-r. [DOI] [PubMed] [Google Scholar]
- 29.Watson AJ, Katz A, Simon MI. A fifth member of the mammalian G-protein β-subunit family. J Biol Chem. 1994;269:22150–28160. [PubMed] [Google Scholar]
- 30.Watson AJ, Aragay AM, Slepak VZ, Simon MI. A novel form of the G-protein β subunit Gβ5 is specifically expressed in the vertebrate retina J. Biol Chem. 1996;271:28154–28160. doi: 10.1074/jbc.271.45.28154. [DOI] [PubMed] [Google Scholar]
- 31.Zhang JF, Ellinor PT, Aldrich RW, Tsien RW. Multiple structural elements in voltage-dependent Ca2+ channels support their inhibition by G proteins. Neuron. 1996;17:991–1003. doi: 10.1016/s0896-6273(00)80229-9. [DOI] [PubMed] [Google Scholar]
- 32.Zhang JH, Simonds WF. Copurification of brain G-protein beta5 with RGS6 and RGS7. J Neurosci. 2000;20(RC59):1–5. doi: 10.1523/JNEUROSCI.20-03-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang S, Coso OA, Lee C, Gutkind JS, Simmonds WF. Selective activation of effector pathways by brain specific Gprotein β5. J Biol Chem. 1996;271:33575–33579. doi: 10.1074/jbc.271.52.33575. [DOI] [PubMed] [Google Scholar]