Abstract
Using a newly developed dissociation procedure, we isolated the specialized rhabdomeral membranes from Drosophilaretinal photoreceptors. From these membranes, we have recorded spontaneous active currents in excised patch, voltage-clamp recordings. We observed rapid opening events that closely resembled those ascribed to one class of light-activated channels, TRP. All activity exhibited Ba2+ permeability, little voltage dependence, and sensitivity to La3+ block. Mutational analysis indicated that the spontaneous activity present in these membranes was TRP-dependent. Excised patches from wild-type rhabdomeral membranes exhibited a wide range of conductance amplitudes. In addition, large conductance events exhibited many conductance levels in the open state. Block of activity by La3+ both developed and recovered in a stepwise manner. Our results indicate that TRP-dependent channels have a small unitary conductance and that many channels can be gated coordinately.
Keywords: Drosophila, TRP, phototransduction, channel, barium, rhabdomere
Phototransduction inDrosophila retinal photoreceptor cells involves G-protein-mediated biochemical cascades initiated by the activation of the receptor rhodopsin by light and culminating in an electrical response generated by the opening of many TRP and TRP-related plasma membrane ion channels (for review, see Montell, 1999). Many channels open in response to the activation of a single receptor protein, indicating that amplification of the signal occurs at some step within the biochemical cascades. Amplification and its modulation are essential features of signaling systems, but their underlying mechanisms are unknown in Drosophila phototransduction.
Recent studies indicate that there is little or no amplification within the first three enzymatic steps of the phototransduction pathway (Scott and Zuker, 1998). One light-activated rhodopsin molecule activates a single heterotrimeric G-protein (DGq), which in turn activates a single phospholipase C molecule (PLC). Activation of PLC is thought to lead to the gating of plasma membrane channels. Three channel polypeptides have been identified and implicated inDrosophila phototransduction, TRP, TRPL, and TRP-γ (Phillips et al., 1992; Niemeyer et al., 1996; Reuss et al., 1997; Xu et al., 1997, 2000). However, the assembly of these subunits in vivo and the gating mechanisms of the channels are not well understood.
TRP or TRP-related channel proteins are found in a wide variety of organisms and tissue types (for review, see Putney, 1999; Harteneck et al., 2000). They are generally thought to be store-operated channels, important in Ca2+ regulation and gated through a poorly understood mechanism linked to the depletion of internal Ca2+ stores. Evidence from the heterologous expression of both the Drosophila TRP and TRPL proteins has suggested that the gating of these channels is likewise linked to internal store depletion (Vaca et al., 1994; Dong et al., 1995; Petersen et al., 1995; Yagodin et al., 1998). Recent evidence, however, suggests that they may be gated directly by PLC-generated diacylglycerol (DAG) or by a metabolic byproduct of DAG (Chyb et al., 1999).
Ideally, one would like to study these channels in cell-attached patches, in intact phototransducing cells, but the complicated cellular morphology has precluded this type of recording. To approach these channels directly, we have developed a novel preparation with which to isolate the rhabdomeral membranes, in which TRP- and TRPL-dependent channels are localized. Here we report the first patch-clamp recordings from TRP-dependent channels in their native membranes. We find that groups of channels open and close in a concerted manner. This coordinated gating may contribute to the generation of amplification within the phototransduction cascade.
MATERIALS AND METHODS
Solutions. Bath solution contained (in mm): 120 NMDG, 15 tetraethylammonium chloride, 10N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid buffer, 25 l-proline, and 10 barium chloride (Sigma, St. Louis, MO), pH 7.2. Pipette solution was identical to bath solution, except that the concentration of BaCl2 was only 1 mm.
Membrane preparation. Corneal tissue from the eyes of five adult flies (red- or white-eyed Oregon R, mutantstrpp343;trplp302,trpp343, andtrplp302, courtesy of Dr. Charles Zuker, University of California, San Diego CA; andtrpCM, courtesy of Dr. William Pak, Purdue University, Lafayette, IN) was dissected into an ∼50 μl bubble of bath solution on a dissecting dish. This solution was transferred by pipette into a small-volume Eppendorf tube containing ∼0.5 ml of bath solution and a small amount of 0.1-mm-diameter zirconia-silicone beads (BioSpec Products, Bartlesville, OK). The tube was shaken for 20 sec at 2500 rpm using a Mini-Bead Beater (BioSpec Products) such that the beads sheared the plasma membrane and cytosol from the rhabdomeral membranes. The solution was transferred to the recording chamber by pipette, leaving the beads settled at the bottom of the tube. The cell fragments were allowed to settle to the bottom of the dish mounted to the microscope stage for several minutes before we began recording.
Recording. Recording pipettes were pulled from borosilicate glass using a laser puller (Sutter Instruments, Novato, CA). All recording electrodes had a tip resistance of 25–40 MΩ, measured in bath solution. Viewing and recording conditions were the same as those described previously (Bacigalupo et al., 1995). Suitable rhabdomeral membranes exhibited a smooth surface and were free of attached plasma membrane fragments. Membranes were selected by eye and sampled using a patch recording pipette. Patches were excised by pulling the pipette away from the rhabdomeral fragment, which was stuck to the bottom of the recording chamber. All data were acquired in excised patch voltage-clamp recordings using Axopatch 200A patch clamp (Axon Instruments, Foster City, CA). Currents were filtered at 2 kHz (eight-pole Bessel filter), acquired digitally (DigiData 1200 interface; Axon Instruments), and stored on computer disk. Applications of La3+ were made using a four-bore glass panpipe spritzer (50–80 μm) and a microperfusion system (Goodman and Art, 1996).
Analysis. Offline analysis was performed using pClamp 6.0 (Axon Instruments). Gaussian fits to all-points histograms were performed using Pstat (Axon Instruments) and either the Simplex least squares or the Marquardt method. Best fits were chosen by comparing the residuals for each. Calculations of open probability (P(open)) were made with the assumption that opening events could be analyzed as unitary events.P(open) values were calculated using Fetchan and Pstat (Axon Instruments). Records were initially examined in Fetchan. Opening and closing events were counted using the half-maximal conductance of the smallest discernable event as the cutoff criteria. For records in which the events were very small, we altered the cutoff criteria to three-fourths maximal conductance of the smallest events, to reduce the possibility of counting closed state noise as open events. The results of these counts were analyzed in pStat to determine the mean open probability for each entire trace.
Electron microscopy. For transmission electron microscopic images, two preparations of 100 eyes each were dissected from adult Oregon red-eyed flies into bath solution. One preparation was left intact, whereas the second was subjected to dissociation by shaking. Dissociated cells were centrifuged at 13,000 × g for 20 min at 4°C and fixed in 3% glutaraldehyde in 0.1m phosphate buffer, pH 7.0, for 30 min. Samples were suspended in 2% agar in an Eppendorf tube, and post-fixation was done in 1% osmium tetroxide for 1 hr. Inclusion into Epon 812 was done by following a previously described technique (Suzuki et al., 1993), and the samples were observed in a Zeiss (Thornwood, NY) EM 109 transmission electron microscope at the University of Chile School of Medicine (Santiago, Chile).
Western blot analysis. Samples for Western blots were prepared by homogenizing the tissue collected from four dissected eyes and denaturing proteins by boiling for 1 min. Samples were run on SDS-polyacrylamide gels, transferred to nitrocellulose, and blotted according to standard techniques (Sambrook et al., 1989), using anti-TRP antibodies generously provided by Drs. Craig Montell (Johns Hopkins University, Baltimore, MD) and Charles Zuker (University of California, San Diego, CA).
RESULTS
In Drosophila retinal photoreceptors, the light-activated channels reside in a specialized region of tightly packed microvilli known as the rhabdomere, which runs the length of each photoreceptor cell. All of the known enzymes involved in phototransduction are localized to this region. In theDrosophila retina, photoreceptor cells are bound together in an eight-cell bundle, called an ommatidium, with the rhabdomere of each cell oriented toward the center of the bundle (for review, see Ready, 1989; Fig. 1A,C). Thus, in an intact ommatidium normally used for whole-cell patch-clamp recording, the rhabdomeres are inaccessible to the recording pipette.
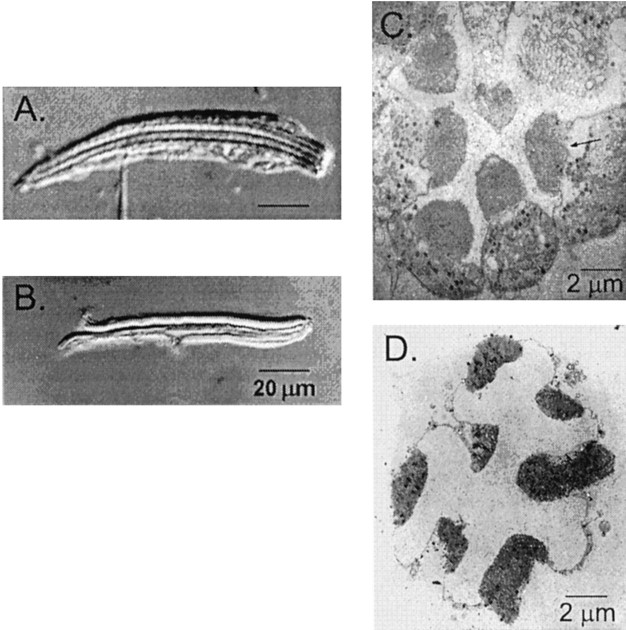
Fig. 1.
A, B, Light micrographs with Nomarski differential interference optics show an intact ommatidium (A) and an isolated rhabdomeral membrane (B) under standard recording conditions. A recording pipette is visible at the bottom ofA, attached to the plasma membrane of a single cell within the intact ommatidium. In the isolated rhabdomeral membranes shown in B, the plasma membranes normally present in intact cells have been stripped away. C, D, Transmitting electron micrographs show cross sections of an intact ommatidium (C) and an isolated rhabdomeral membrane (D). The electron-dense regions in the center of the intact ommatidium are the rhabdomeral membranes.
To study the light-activated channels directly, we developed an isolation procedure that exposes the rhabdomeral membranes in which they reside. Using a mechanical dissociation technique, we stripped away the exposed plasma membrane from the ommatidia, leaving the rhabdomeral membranes exposed (Fig. 1B,D). We examined membrane fragments using light microscopy and selected rhabdomeres with smooth membrane surfaces without apparent plasma membrane for patch recording.
We used a simplified recording solution to maximize the likelihood of observing light-activated channels. This solution substituted Ba2+ for Ca2+as the only permeable cation. Ca2+ has been shown to have modulatory effects on transduction (Hardie and Minke, 1994b, 1995; Hardie, 1995; Scott et al., 1997) and commonly affects the open probabilities of some cation-permeable channels (Yue et al., 1990; Marunaka et al., 1992). Barium, in contrast, permeates light-dependent channels (Reuss et al., 1997), but it does not seem to mediate secondary modulatory effects. Monovalent cations were replaced with nonpermeant NMDG (Hardie and Minke, 1992).
More than 95% of the patches from wild-type membranes exhibited spontaneous channel activity within 1–2 min of excision, if the patch was held at voltages between −70 and −100 or +70 and +100 mV. The majority of the excised patches from wild-type membranes exhibited large-conductance, burst-like behavior, often quickly followed by a dramatic decrease in seal resistance. We have not included data from these patches in the analysis presented here. Some patches (n = 12), however, made a transition from this erratic kinetic behavior to more stereotypical single-channel behavior and maintained a high seal resistance (8–20 GΩ). Data from these patch recordings will be discussed in this paper.
We recorded a wide distribution of spontaneously active conductances in excised patches from wild-type rhabdomeral membranes (Fig.2A). All of the conductances that we recorded, regardless of size, were permeable to barium, exhibited little or no voltage dependence, and were sensitive to block by La3+, reminiscent of TRP-dependent light-sensitive channels observed in whole-cell recordings (Hardie and Mojet, 1995).
Fig. 2.
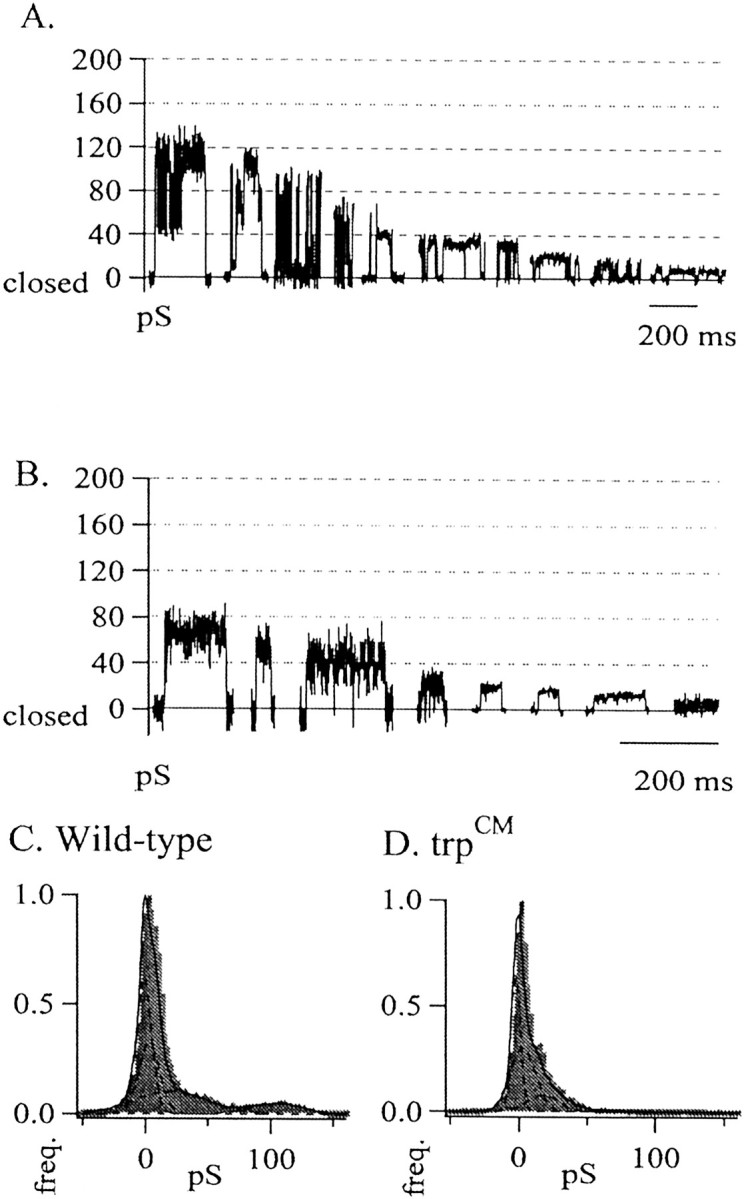
A, Ten individual events selected from 10 different excised patches from wild-type isolated rhabdomeral membranes, plotted in descending order of conductance size. Open state level is upward in each case. Ba2+ in the bath was 10 mm; Ba2+ in the pipette was 1 mm. Conductance values were calculated asg = [I/(Vhold −Erev)], where g is conductance in picosiemens, I is measured current amplitude, and Erev is the reversal potential for each patch, as determined for each individual patch by the slope of the current plotted for multiple holding voltages. All conductances were permeable to barium as determined by the reversal potential of the activity (Erev= 24 ± 6 mV; n = 12). The calculated equilibrium potential for Ba2+, the only permeate ion in our solutions, was +29 mV. B, Eight individual conductance events selected from eight different excised patches taken fromtrpCM isolated rhabdomeral membranes, plotted in descending order of conductance size. C, D, All-points conductance histograms show marked differences in the distribution of conductances observed among all wild-type (C) and trpCM(D) excised patches. Conductance values were determined separately for each patch by dividing the measured current by the driving force. All data from each patch were binned cumulatively, and the resultant histograms were normalized to the zero peak (the largest peak in each histogram) to highlight the relative contribution of each subsequent open state. Wild type,n = 12; trpCM,n = 8.
We observed no sustained spontaneous activity in patches excised from the double null mutant trpp343;trplp302 (n = 14) or the single null mutant trpp343(n = 6). Neither did we observe the high-frequency burst-like behavior observed initially in wild-type patches. In some patches (n = 5 of 14,trpp343;trplp302) we did see channel-like events. However, in every case, the duration of this activity was extremely brief, generally a single burst lasting between 5 and 15 sec within 10–15 min of recording time and containing only a few events. The patches otherwise remained silent. The few channel-like events we observed in this mutant always occurred at a single holding potential and totaled less than a dozen events, making it difficult to determine single-channel characteristics. This contrasts sharply with wild-type patches in which we observed prolonged (10–40 min) spontaneous activity in ∼95% of patches.
We also examined patches from the hypomorphic alleletrpCM, in which a small amount of TRP protein is present (as identified by Western blot analysis; data not shown). In patches from this mutant we did observe channel activity but found a distribution of much smaller conductance values (Fig.2B).
For comparison, we normalized all of the data from all holding voltages by converting current to conductance and generated all-points conductance histograms for both wild-type andtrpCM data. (Fig. 2C,D). This allowed us to examine the relative frequency of occurrence of each conductance size. The trpCMhistogram shows primarily small conductance values compared with those of wild type, presumably reflecting the residual TRP channels expressed by this allele.
The lack of channel activity in the trp null mutant and the smaller conductance events observed in the trp hypomorph compared with those observed in wild-type patches suggested that the activity we observed in our patches is TRP-dependent. The prevalence of much smaller conductance values observed in thetrpCM allele further suggests that the unitary conductance of individual TRP channels is small.
A key feature of the activity observed in wild-type patches is the frequent appearance of multiple conductance levels while the channel is open (Fig. 3). If, as our data suggest, all of the activity we observed is TRP-dependent and the unitary conductance of these channels is small, the large conductance events in wild-type patches must be composed of small unitary conductance, TRP-dependent channels.
Fig. 3.
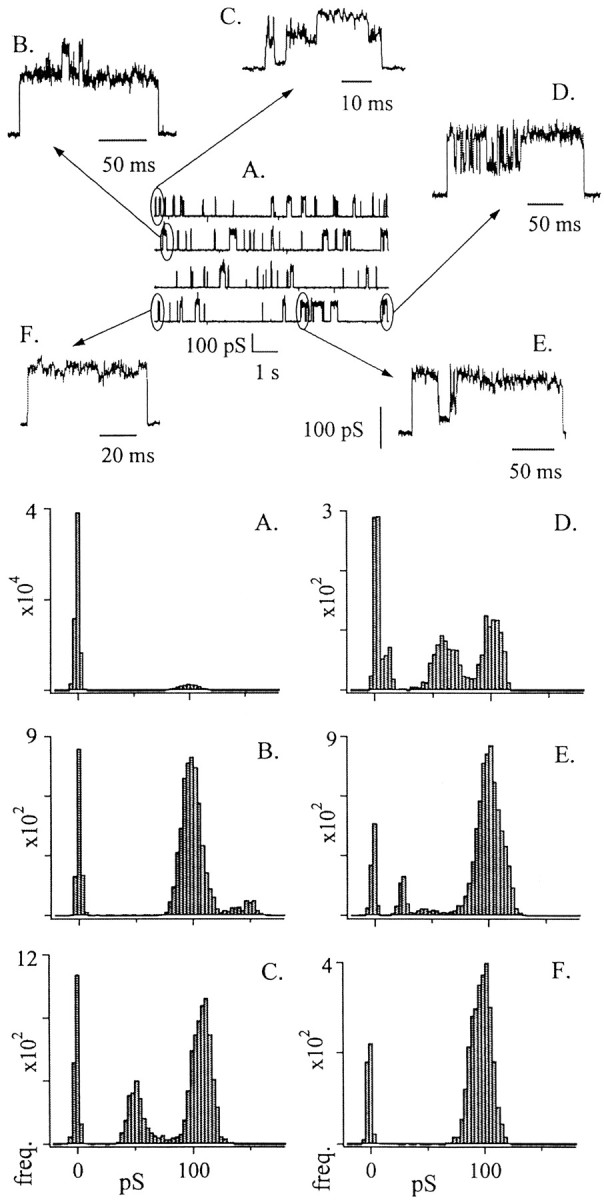
Multiple conductance levels are clearly discernable during large conductance events. Top, A, ∼36 sec of continuously sampled data trace.Vhold = −70 mV. Top, B–F, Examples illustrating multiple conductance levels that occurred within the longer trace. Traces have been enlarged from the indicated regions of A. Bottom, A–F, Histograms corresponding to the data traces in top,A–F. Histogram A was generated from a larger sample of data than that represented in data panelA (top). Histograms B–Fwere generated using only the same-letter data samples. When the data samples include only a single large opening event, the all-points current histograms show distinct variability in amplitudes of opening events (histograms B–F).
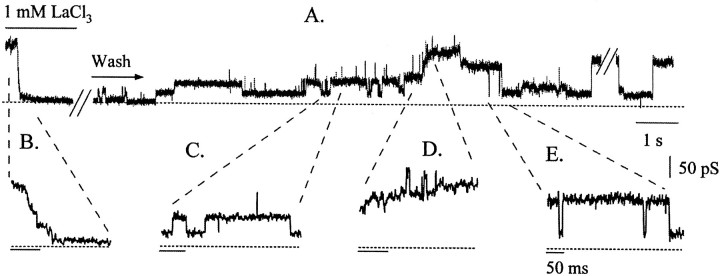
To examine this possibility, we tested the hypothesis that pharmacological inhibition of wild-type channel activity would reduce the number of active channels and reveal smaller-amplitude events, which would be expected if many channels contribute to each large event. One micromolar La3+ quickly and completely blocked all activity, and this block was at least partially reversible (Fig. 4A). The block developed in a stepwise manner (Fig. 4B). The recovery of the activity occurred in similar stepwise opening events that initially flickered open and closed and then remained open (Fig. 4C,D). These steps occurred primarily sequentially and were not uniform in amplitude, nor did they correspond to the amplitude of the steps observed in the initial block. After several such stepwise events, the now large current closed nearly to zero in a single fast transition (Fig. 4E). A large conductance event dominated the record thereafter (Fig. 4A,E, latter part of trace). These results indicate that large events are not unitary channel openings but are made up of smaller conductance channels. The rapidity of transitions exhibited by the conductance in the latter part of Figure 4A suggests that the smaller channels open and close in a coordinated manner.
Fig. 4.
La3+ block and recovery occurred in a stepwise manner. A large conductance event with very long open times was blocked completely by 1 mmLaCl3. This block occurred in a stepwise manner (B). After several minutes of continuous wash, the activity began to reappear, occurring again in small stepwise events that were quite variable in amplitude (C, D). After many such small steps open, the events appear to become concerted, opening and closing as a single unit (E). The dashed line at thebottom of each trace represents the closed level. Two stretches of the data have been removed for clarity (at slash marks) because of the long periods of inactivity after the application of the blocker and while it remained open near the end of the top trace. The current trace begins slightly before the application of LaCl3 (bar). Subsequent wash of the patch with bath solution continued for the duration of the trace. Vhold = −40 mV.
When we examined the action 10 μmLa3+, which blocks activity much more slowly than 1 mm La3+, we found a similar phenomenon (Fig. 5). Ten micromolar La3+ decreased the amplitudes of the conductance events without significantly changing their calculated P(open) values (Fig.5A,B). After initiating the wash-off of La3+, we recorded conductance events of smaller amplitudes than those of the preblock events. Nevertheless,P(open) recovered almost completely. This suggests that La3+ block did not occur through the obstruction of a single pore, but instead it may block many individual channels that underlie the large conductance.
Fig. 5.
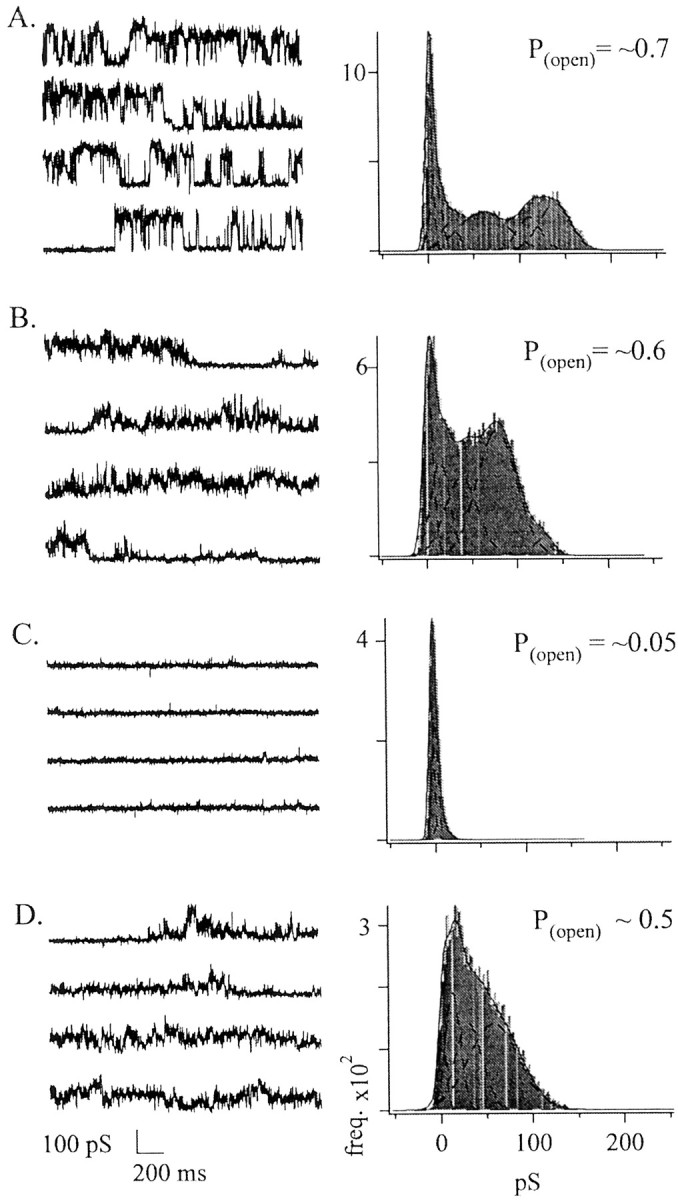
La3+ reversibly blocked channel activity. We found that 1 mm La3+blocked all conductances completely within seconds. Block by micromolar concentrations of La3+ was much slower.A, Current activity is shown before the application of the blocker. The all-points current histogram (right) was generated from the sample data (left). TheP(open) shown in the top right corner of the histogram plot is the averageP(open) calculated from the data shown on the left. Calculations ofP(open) were made as described in Materials and Methods. B, Example of the activity seen during the application of 10 μm LaCl3. The application of LaCl3 occurred at the beginning of the trace and continued through the entire trace. C, Data are shown for a patch exposed to 1 mm LaCl3, after which activity was almost completely blocked. D,Activity that began to recover several seconds after we removed LaCl3. Vhold = −20 mV.
DISCUSSION
The spontaneous activity recorded from excised wild-type patches exhibited strikingly similar single-channel characteristics, despite the range of amplitudes observed from patch to patch. All were permeable to the divalent cation Ba2+, were sensitive to La3+ block, and exhibited little or no voltage dependence. The lack of activity in rhabdomeral membranes from both trp null alleles andtrp−;trpl− double null alleles and the reduced activity observed in thetrpCM hypomorph suggest that the spontaneous activity we observe in wild-type andtrpCM rhabdomeres is composed of TRP-dependent channels. Taken together, these data indicate that the spontaneous activity we observed in excised patches from isolated rhabdomeral membranes was dependent on the presence of the TRP protein.
Furthermore, these data suggest that little if any spontaneous TRPL-dependent current was present in these patches under our recording conditions. Excised patch recordings from the null mutanttrplp302 were nearly indistinguishable from those from wild type (data not shown). It may be that TRPL-dependent channels are present in these membranes but simply do not activate spontaneously. Alternatively, these data do not address the question of whether TRPL polypeptides can be components of TRP-dependent channels through heterologous assembly.
The spontaneous activity we observed in these patches is similar to a phenomenon that develops in intact, functioning photoreceptor cells under whole-cell recording conditions (Hardie and Minke, 1994a). The rundown current (RDC) is a sustained inward current that develops minutes after the whole-cell configuration is achieved and is accompanied by a dramatic loss in light sensitivity. Although its origin is not understood, the RDC is generally thought to be caused by the dissociation of the light-activated TRP-dependent channels from the rest of the phototransduction machinery, because it does not occur intrp− flies (Hardie and Minke, 1994a). Spontaneous activation of TRP channels from human has been observed in heterologous expression systems (Yamada et al., 2000).
The preponderance of small amplitude events observed in the hypomorphictrpCM allele suggests that the unitary conductance of individual channels is small (Fig. 2). We conclude that the large conductance events (as large as 144 pS) arise from the coordinated gating of many of these small conductance channels. There are two lines of evidence to support this idea. First, large conductance events consistently exhibit many open-state conductance transitions that are quite variable in amplitude (Fig. 3). Second, the amplitudes of large conductance events can be broken down into smaller events pharmacologically.
Determining the number of channels that open coordinately in an event requires an estimate of the single-channel conductance. Although they are rare, we have observed clear individual events of 4 pS as transitions from the closed state. Transitions of 8 pS can frequently be discerned within the open state, which contains higher-frequency noise than that of the closed state. If the unitary channel conductance is 4 pS, this would mean that 144 pS, approximately the largest wild-type conductance we measure, would correspond to 36 channels opening coordinately. This is close to the number of channels estimated to be open at the peak of a quantum bump, a single photon response (Henderson et al., 2000). Wild-type flies exhibit average bump peak amplitudes of ∼12 pA when voltage-clamped to −80 mV (Cook et al., 2000). Assuming the reversal potential for each patch (Erev) = 9 mV (Reuss et al., 1997), this translates to ∼35 channels of 4 pS unitary conductance open at the quantum bump peak. Thus, it is possible that coordinated gating of TRP-dependent channels contributes to quantum bump formation.
In Figure 4, during the washout of La3+, small unitary events initially appeared sequentially and then began to gate in a concerted manner. The transitions at the end of this trace are larger than any previous individual opening event (Fig.4A,E, latter part of trace). These large transitions exhibit several conductance amplitudes, suggesting that coordination does not require a fixed number of elementary components. This is also the case in Figure 5. Both in the presence of a lower concentration of La3+ (10 μm) and after wash, the amplitudes of the current were distinct from those before block by La3+.
Coordinated gating may provide a mechanism for the generation or regulation of gain that appears to occur late in the phototransduction cascade. The idea that a variable number of TRP-dependent channels can be gated together to form a single unitary event provides an interesting model for regulating gain. Although the underlying gating mechanisms of these channels remains unclear, the TRP protein has been shown to be part of a supramolecular signaling assembly in the rhabdomeral membrane (Huber et al., 1996; Shieh and Zhu, 1996;Chevesich et al., 1997; Tsunoda et al., 1997). These assemblies might play a role in coordinated gating and possibly induce amplification and its regulation.
Finally, although many single-channel characteristics of TRP and TRPL channels appear to depend heavily on the environment in which they are placed, coordinated gating may explain at least one puzzling phenomenon. Coordinated gating may underlie the large range of unitary conductance values reported for the light-activated conductance (10–20 pS), the light-activated conductance in the absence of TRPL (4 pS), the RDC (1.5–4.5 pS), and TRP channels expressed in heterologous expression systems (Vaca et al., 1994; Hardie and Minke, 1994a; Hardie and Mojet, 1995; Hardie et al., 1997; Reuss et al., 1997). Although isolation procedure and recording conditions certainly do not provide an environment in which the channels normally find themselves, our approach nevertheless opens new technological means by which to gain a more complete understanding of channel function.
Footnotes
This work was supported by the American Heart Association, Oregon Affiliate, and by National Science Foundation Grant INT-9604977 in collaboration with Fundacion Andes/Conicyt Grants Fundecyt-1990938 and 1981053 and Grant P99-031 Program ICM Mideplan. J.E.H. was supported by National Institutes of Health predoctoral training Grant 5-T32-GM07257. We gratefully acknowledge Drs. Judith Eisen, William Roberts, Roger Hardie, Craig Montell, and Enrico Nasi for critical reading of earlier versions of this manuscript, Drs. William Pak and Charles Zuker for mutant fly strains, Drs. Charles Zuker and Craig Montell for anti-TRP antibodies, and Dr. Nancy Olea for electron microscopy sample preparation.
Correspondence should be addressed to Dr. Peter O'Day, Institute of Neuroscience, University of Oregon, Eugene, OR 97403. E-mail:oday@uoneuro.uoregon.edu.
REFERENCES
- 1.Bacigalupo J, Bautista DM, Brink DL, Hetzer JF, O'Day PM. Cyclic-GMP enhances light-induced excitation and induces membrane currents in Drosophila retinal photoreceptors. J Neurosci. 1995;15:7196–7200. doi: 10.1523/JNEUROSCI.15-11-07196.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chevesich J, Kreuz AJ, Montell C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron. 1997;18:95–105. doi: 10.1016/s0896-6273(01)80049-0. [DOI] [PubMed] [Google Scholar]
- 3.Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- 4.Cook B, Bar-Yaacov M, Ben-Ami HC, Goldstein RE, Paroush Z, Selinger Z, Minke B. Phospholipase C and termination of G-protein-mediated signalling in vivo. Nat Cell Biol. 2000;2:296–301. doi: 10.1038/35010571. [DOI] [PubMed] [Google Scholar]
- 5.Dong Y, Kunze DL, Vaca L, Schilling WP. Ins(1,4,5)P3 activates Drosophila cation channel Trpl in recombinant baculovirus-infected Sf9 insect cells. Am J Physiol. 1995;269:C1332–C1339. doi: 10.1152/ajpcell.1995.269.5.C1332. [DOI] [PubMed] [Google Scholar]
- 6.Goodman MB, Art JJ. Positive feedback by a potassium-selective inward rectifier enhances tuning in vertebrate hair cells. Biophys J. 1996;71:430–442. doi: 10.1016/S0006-3495(96)79245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardie RC. Photolysis of caged Ca2+ facilitates and inactivates but does not directly excite light-sensitive channels in Drosophila photoreceptors. J Neurosci. 1995;15:889–902. doi: 10.1523/JNEUROSCI.15-01-00889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 9.Hardie RC, Minke B. Spontaneous activation of light-sensitive channels in Drosophila photoreceptors. J Gen Physiol. 1994a;103:389–407. doi: 10.1085/jgp.103.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardie RC, Minke B. Calcium-dependent inactivation of light-sensitive channels in Drosophila photoreceptors. J Gen Physiol. 1994b;103:409–427. doi: 10.1085/jgp.103.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardie RC, Minke B. Phosphoinositide-mediated phototransduction in Drosophila photoreceptors: the role of Ca2+ and trp. Cell Calcium. 1995;18:256–274. doi: 10.1016/0143-4160(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 12.Hardie RC, Mojet MH. Mg2+ dependent block of the light-activated and trp dependent conductance in Drosophila photoreceptors. J Neurophysiol. 1995;74:2590–2599. doi: 10.1152/jn.1995.74.6.2590. [DOI] [PubMed] [Google Scholar]
- 13.Hardie RC, Reuss H, Lansdell SJ, Millar NS. Functional equivalence of native light-sensitive channels in the Drosophila trp301 mutant and TRPL cation channels expressed in a stably transfected Drosophila cell line. Cell Calcium. 1997;21:431–440. doi: 10.1016/s0143-4160(97)90054-3. [DOI] [PubMed] [Google Scholar]
- 14.Harteneck C, Plant T, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- 15.Henderson SR, Reuss H, Hardie RC. Single photon responses in Drosophila photoreceptors and their regulation by Ca2+. J Physiol (Lond) 2000;524:179–194. doi: 10.1111/j.1469-7793.2000.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huber A, Sander P, Gobert A, Bahner M, Hermann R, Paulsen R. The transient receptor potential protein (Trp), a putative store-operated Ca channel essential for phosphoinositide-mediated photoreception, forms a signaling complex with NorpA, InaC and InaD. EMBO J. 1996;15:7036–7045. [PMC free article] [PubMed] [Google Scholar]
- 17.Marunaka Y, Tohda H, Hagiwara N, O'Brodovich H. Cytosolic Ca(2+)-induced modulation of ion selectivity and amiloride sensitivity of a cation channel and beta agonist action in fetal lung epithelium. Biochem Biophys Res Commun. 1992;187:648–656. doi: 10.1016/0006-291x(92)91244-k. [DOI] [PubMed] [Google Scholar]
- 18.Montell C (1999) Visual transduction inDrosophila. Annu Rev Cell Dev Biol 231–268. [DOI] [PubMed]
- 19.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- 20.Petersen CC, Berridge MJ, Borgese MF, Bennett DL. Putative capacitative calcium entry channels: expression of Drosophila trp and evidence for the existence of vertebrate homologues. Biochem J. 1995;311:41–44. doi: 10.1042/bj3110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- 22.Putney JW., Jr TRP, inositol 1,4,5-trisphosphate receptors, and capacitative calcium entry (comment). Proc Natl Acad Sci USA. 1999;96:14669–14671. doi: 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ready DF. A multifaceted approach to neural development. Trends Neurosci. 1989;12:102–110. doi: 10.1016/0166-2236(89)90166-5. [DOI] [PubMed] [Google Scholar]
- 24.Reuss H, Mojet MH, Chyb S, Hardie RC. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron. 1997;19:1249–1259. doi: 10.1016/s0896-6273(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 26.Scott K, Zuker CS. Assembly of the Drosophila phototransduction cascade into a signalling complex shapes elementary responses. Nature. 1998;395:805–808. doi: 10.1038/27448. [DOI] [PubMed] [Google Scholar]
- 27.Scott K, Sun Y, Beckingham K, Zuker CS. Calmodulin regulation of Drosophila light-activated channels and receptor function mediates termination of the light response in vivo. Cell. 1997;91:375–383. doi: 10.1016/s0092-8674(00)80421-3. [DOI] [PubMed] [Google Scholar]
- 28.Shieh BH, Zhu MY. Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron. 1996;16:991–998. doi: 10.1016/s0896-6273(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki E, Katayama E, Hirosawa K. Structure of photoreceptive membranes of Drosophila compound eyes as studied by quick-freezing electron microscopy. J Electron Microsc. 1993;42:178–184. [PubMed] [Google Scholar]
- 30.Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, Becker A, Socolich M, Zuker CS. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–249. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- 31.Vaca L, Sinkins WG, Hu Y, Kunze DL, Schilling WP. Activation of recombinant trp by thapsigargin in Sf9 insect cells. Am J Physiol. 1994;267:C1501–C1505. doi: 10.1152/ajpcell.1994.267.5.C1501. [DOI] [PubMed] [Google Scholar]
- 32.Xu XS, Li H, Guggino WB, Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- 33.Xu X-ZS, Chien F, Butler A, Salkoff L, Montell C. TRPγ, a new Drosophila TRP family member, forms a regulated cation channel with TRPL. Neuron. 2000;26:647–657. doi: 10.1016/s0896-6273(00)81201-5. [DOI] [PubMed] [Google Scholar]
- 34.Yagodin S, Hardie RC, Lansdell SJ, Millar NS, Mason WT, Sattelle DB. Thapsigargin and receptor-mediated activation of Drosophila TRPL channels stably expressed in a Drosophila S2 cell line. Cell Calcium. 1998;23:219–228. doi: 10.1016/s0143-4160(98)90120-8. [DOI] [PubMed] [Google Scholar]
- 35.Yamada H, Wakamori M, Hara Y, Takahashi Y, Konishi K, Mori Y. Spontaneous single-channel activity of neuronal TRP5 channels recombinantly expressed in HEK293 cells. Neurosci Lett. 2000;12:111–114. doi: 10.1016/s0304-3940(00)01033-8. [DOI] [PubMed] [Google Scholar]
- 36.Yue DT, Backx PH, Imredy JP. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 1990;250:1735–1738. doi: 10.1126/science.2176745. [DOI] [PubMed] [Google Scholar]