Abstract
It is well established that individual rats exhibit marked differences in behavioral responses to a novel environment. Rats that exhibit high rates of locomotor activity and sustained exploration in such an environment also exhibit high concentrations of stress-induced plasma corticosterone, linking this behavior to the stress system. Furthermore, these high-responding (HR) rats, in contrast to their low-responding (LR) counterparts, have a greater propensity to self-administer drugs. Thus, HR rats have been described as “novelty” seeking in that they are more active and explore novel stimuli more vigorously, despite the fact that this elicits in them high stress responses. In this study, we have further characterized the behavior of HR and LR rats in tests of anxiety and characterized their stress responses to either experimenter- or self-imposed stressors. We then investigated the physiological basis of these individual differences, focusing on stress-related molecules, including the glucocorticoid receptor (GR), the mineralocorticoid receptor (MR), corticotropin-releasing hormone (CRH) and pro-opiomelanocortin (POMC) in the context of the limbic–hypothalamo–pituitary adrenal axis. We have found that HR rats did not differ from LR in their basal expression of POMC in the pituitary. However, HR rats exhibited higher levels of CRH mRNA in the hypothalamic paraventricular nucleus but lower basal levels in the central nucleus of the amygdala. The basal expression of hippocampal MR is not different between HR and LR rats. Interestingly, the basal expression of hippocampal GR mRNA is significantly lower in HR than in LR rats. This low level of hippocampal GR expression in HR rats appears to be responsible, at least in part, for their decreased anxiety in exploring novelty. Indeed, the anxiety level of LR rats becomes similar to HR rats after the administration into the hippocampus of a GR antagonist, RU38486. These data indicate that basal differences in gene expression of key stress-related molecules may play an important role in determining individual differences in responsiveness to stress and novelty. They point to a new role of hippocampal GR, strongly implicating this receptor in determining individual differences in anxiety and novelty-seeking behavior.
Keywords: anxiety, drug addiction, reactivity to novelty, novelty seeking, stress, individual differences
Individual differences in neural and hormonal responses to stress may contribute to observable individual differences in human behavior and vulnerability to psychopathology (Zuckerman, 1990; Anisman and Zacharko, 1992; Holsboer et al., 1995). Humans exhibit individual differences in the degree to which they participate in novelty-seeking or “sensation-seeking” behaviors (Zuckerman, 1984). These behaviors consist of voluntary participation in activities that are associated with personal risk. These activities generally initiate stress and anxiety responses yet provide the participant with a “thrill,” and self-reported measures of sensation seeking are associated with a variety of psychiatric disorders, such as alcoholism and drug addiction (Zuckerman and Neeb, 1979). Although the biological determinants of individual differences in sensation seeking remain unclear, we hypothesized that distinct patterns of gene expression in neuronal circuits that modulate stress responsiveness may underlie these functional tendencies.
An interesting animal model of individual differences in stress responsiveness and sensation seeking has been reported. When experimentally naive rats are exposed to the mild stress of a novel environment, some rats [high-responding (HR)] exhibit high rates of exploratory locomotion, whereas others [low-responding (LR)] exhibit low rates of locomotor activity. The rate of stress-induced locomotion in a novel environment predicts subsequent behavioral responses to drugs such as amphetamine and cocaine. HR rats exhibit higher rates of amphetamine- and cocaine-induced locomotor activity and self-administer these drugs at lower doses than will LR rats (Piazza et al., 1989;Hooks et al., 1991). HR rats also exhibit greater cocaine-induced elevations in extracellular concentrations of dopamine in the nucleus accumbens than LR rats (Hooks et al., 1991). Furthermore, HR rats show a lower density of dopaminergic D2 receptors in the nucleus accumbens (Hooks et al., 1994). In addition, HR rats will seek out novel and varied environments when given a free choice between these environments and environments to which the rats have become habituated (Dellu et al., 1996). HR rats' hyperactivity is associated exclusively with novelty and is not evident in familiar environment (Dellu et al., 1996). These HR rats, which represent an animal model of novelty-seeking behavior, exhibit a prolonged corticosterone response after exposure to the mild stress of a novel environment and exhibit greater stress-induced elevations of mesolimbic dopamine neurotransmission relative to their LR counterparts (Piazza et al., 1989; Dellu et al., 1996). Taken together, these data indicate that individual differences in locomotor response to a mild stress are positively associated with novelty-seeking behavior and drug self-administration and implicate a role of dopamine and corticosterone in these effects. In this series of studies, we undertook contrasting the stress and anxiety behaviors of the HR and LR animals and describing unique patterns of stress-related gene expression that characterize them. We then focused on one particular molecule that exhibited a marked difference in gene expression between the groups and demonstrated that this difference plays an important role in mediating the differences in novelty seeking behavior.
MATERIALS AND METHODS
Male Sprague Dawley rats from Charles River (Wilmington, MA), weighing 250–300 gm, were used in this study. They were housed in pairs (or isolated in experiment 4) in 43 × 21.5 × 25.5 cm Plexiglas cages and kept on a 12 hr light/dark cycle (lights on at 7 A.M.). Food and water were available ab libitum.
After 7 d of habituation to the housing conditions, the rats' locomotor reactivity was monitored during 120 min exposure to the mild stress of a novel environment. The rats were classified as HR (rats that exhibited locomotor counts in the highest third of the sample) or LR (rats that exhibited locomotor counts in the lowest third of the sample).
All the behavioral and anatomical studies were performed in a blind manner.
Experiment 1. Five days after locomotor testing, 40 rats (20 HR and 20 LR) were exposed for 5 min to a light/dark anxiety test. At the end of anxiety testing, the rats were transferred back to their home cages. Independent groups of rats were killed 15, 30, 60, and 90 min after the light/dark anxiety test (groups t= 15, t = 30, t = 60, andt = 90 min). The control rats were quickly removed from their cages and killed by decapitation (groupt = 0) without exposure to the light/dark anxiety testing.
Experiment 2. Five days after locomotor testing, 14 rats (7 HR and 7 LR) were exposed for 5 min to the elevated plus maze test.
Experiment 3. Five days after locomotor testing, 32 rats (16 HR and 16 LR) were exposed to restraint stress for 30 min. Independent groups of rats were killed 30, 90, and 120 min after the beginning of restraint stress. The control rats were quickly removed from their cages and decapitated (group t = 0).
Experiment 4. Five days after locomotor testing, 24 rats (12 HR and 12 LR) were either group housed or isolated. One week later the rats' anxiety responses were screened in the light/dark boxes.
Experiment 5. Three days after locomotor testing, 36 rats (18 HR and 18 LR) were implanted bilaterally with a cannula aimed at the CA1 field of the dorsal hippocampus. After 5 d of recovery from surgery, rats were injected bilaterally in the hippocampus with either vehicle or the glucocorticoid receptor (GR) antagonist RU38486. One hour after the injection, the rats were screened for their level of anxiety and locomotor activity in the light/dark boxes. The rats were killed after the experiment, and the cannula placements were verified. All the rats had good hippocampal (CA1) placement of the cannulas.
Guide cannula implantation. Rats were anesthetized with sodium pentobarbital (48 mg/kg, i.p.) and placed in a stereotaxic apparatus with the incisor bar 5 mm above the interaural line. All of the rats were implanted bilaterally with a cannula aimed at the CA1 field of the dorsal hippocampus (3.14 mm posterior to bregma, ±2.0 mm from the midsagittal suture, and 3.2 mm ventral from the surface of the skull).
Microinjection. Rats were injected bilaterally in the hippocampus either with vehicle (0.5 μl of artificial CSF) or with the RU38486 (50 or 100 ng/0.5 μl per side). The solutions were injected slowly (over 1 min), and the cannulas were left in place for 2 min to allow for drug diffusion with minimal withdrawal along the cannula paths.
Drug. The RU38486 was purchased from Sigma (St. Louis). It was dissolved in a mixture of artificial CSF and ethanol (2%).
All the experiments started at 8 A.M. At the completion of the studies, trunk blood was collected in polyethylene tubes containing EDTA (20 mg/ml), and the brains were immediately removed and frozen in isopentane cooled to −80°C. The brains were then sectioned on a Bright–Hacker cryostat, and 10-μm-thick coronal sections were mounted on poly-l-lysine-subbed slides. These slides were kept at −80°C until processed for in situhybridization.
In situ hybridization histochemistry. Four brains from HR rats and four brains from LR rats were used for in situhybridization. Each brain was sectioned on a cryostat at 10 μm, and a series of sections were mounted on poly-l-lysine coated slides. Sections were taken at 100 μm intervals, except at the level of the hippocampus, in which sections were collected at 200 μm. The sections were fixed in 4% paraformaldehyde for 1 hr, followed by three washes in 2× SSC (1× SSC is 150 mm sodium chloride and 15 mm sodium citrate). The sections were then placed in a solution containing acetic anhydride (0.25%) in triethanolamine (0.1 m), pH 8, for 10 min at room temperature, rinsed in distilled water, and dehydrated through graded alcohols (50, 75, 85, 95, and 100%). After air drying, the sections were hybridized with a 35S-labeled cRNA probe. The mineralocorticoid receptor (MR) probe was a 400 nucleotide fragment directed against the 3′ untranslated region of MR mRNA. The GR probe was a 451 nucleotide fragment directed against the rat GR mRNA coding region (nucleotides 2364–2815). The rat corticotropin-releasing hormone (CRH) and pro-opiomelanocortin (POMC) probes were 770 and 900 nucleotides, respectively. All these probes were cloned in our laboratory. The probes were labeled in a reaction mixture consisting of 1 μg of linearized plasmid, 1× transcription buffer (Epicenter Technologies, Madison, WI), 125 μCi of [35S]UTP, 125 μCi of [35S]CTP, 150 μmATP and GTP, 12.5 mm dithiothreitol, 20 U of RNase inhibitor, and 6 U of polymerase. The reactions were incubated for 90–120 min at 37°C. Then the probes were separated from unincorporated nucleotides over a Sephadex G50-50 column. The probes were diluted in hybridization buffer (containing 50% formamide, 10% dextran sulfate, 3× SSC, 50 mm sodium phosphate buffer, pH 7.4, 1× Denhardt's solution, 0.1 mg/ml yeast tRNA, and 10 mm dithiothreitol) to yield 106 dpm/70 μl. The sections were coverslipped and placed inside a humidified box overnight at 55°C. After hybridization, the coverslips were removed, and the sections were rinsed and washed twice in 2× SSC for 5 min each and then incubated for 1 hr in RNase (200 μg/ml in Tris buffer containing 0.5m NaCl, pH 8) at 37°C. The sections were washed in increasingly stringent solutions of SSC, 2×, 1×, and 0.5×, for 5 min each, followed by incubation for 1 hr in 0.1× SSC at 65°C. After rinsing in distilled water, the sections were dehydrated through graded alcohols, air-dried, and exposed to Kodak XAR film (Eastman Kodak, Rochester, NY) for 7 d for GR, MR, and CRH probes. The POMC probe was exposed for only 2 hr.
During hybridization, several sections were pretreated for 1 hr with RNase (200 μg/ml) or treated with sense riboprobes from the same plasmid insert as controls.
Quantification of the radioactive signal. As a way to standardize optical density measurements, an outline was developed for each brain region based on the shape and size of the region. Using those outlines, optical density measurements were taken for each brain region from the left and right sides of the brain or from rostral-caudal sections spaced by 100 or 200 μm. GR mRNA was quantified in the following areas: cingulate cortex, thalamus, paraventricular nucleus (PVN), arcuate nucleus, central and lateral amygdala, hippocampus, and ventral tegmental area. CRH mRNA was quantified in the PVN, the central amygdala, and Barrington's nucleus. POMC mRNA was quantified in the pituitary. For all the probes, eight sections per region per rat were used. Optical density values were corrected for background, multiplied by the area sampled to produce an integrated density measurement, and then averaged to produce one data point for each brain region for each animal. These data points were averaged per group and compared statistically. Optical density measurements were quantified from x-ray film using NIH Image software.
Corticosterone assay. Corticosterone was assayed using a highly specific antibody developed in our laboratory and characterized by Dr. D. L. Helmreich (Mental Health Research Institute, University of Michigan). Cross-reactivities to related compounds (e.g., cortisol) were <3%. Intra-assay and inter-assay variations were <10% (data not shown).
Horizontal locomotion and rearing behaviors. These behaviors were tested in 43 × 21.5 × 24.5 cm (high) clear acrylic cages with stainless steel grid flooring. Activity was monitored by means of two banks of photocells connected to a microprocessor. Two photocells were located 2.5 cm above the grid floor. Each of these photocells was located 14.3 cm from the end of the cage. Horizontal locomotion was monitored by this lower bank of photocells. Each time a locomotor response was recorded on one of these lower photocells, that photocell was inactive until a response was recorded on the other lower photocell. Thus, each locomotor count recorded a minimum 14.3 cm traversing of the cage. Two additional photocells were located 11.5 cm above the grid floor, 6.5 cm from the end of the cage. Rearing was monitored by this upper bank of photocells. Each time a rearing response was recorded on one of these upper photocells, the response was recorded regardless of activity recorded by the other photocells. Thus, every rearing response was recorded.
Light/dark anxiety test. Anxiety tests were conducted in 30 × 60 × 30 cm Plexiglas shuttlexboxes with translucent covers. Each box had a floor composed of stainless steel bars suspended above corncob bedding, and each box was divided into two equal-sized compartments by a wall with a 12-cm-wide open door. One compartment was painted white and brightly illuminated, and the other compartment was painted black with very dim light. The rats' locomotor activity and time spent in each compartment were monitored by rows of five photocells located 2.5 cm above the grid floor of each compartment. The number of photocell beams interrupted per unit time and times of entry into each compartment were recorded with a microprocessor.
Elevated plus maze test. The apparatus is constructed of black-painted Plexiglas, with four elevated arms (70 cm from the floor, 45 cm long, and 12 cm wide). The arms were arranged in a cross, with two opposite arms being enclosed by 45-cm-high walls. The two other arms were open, having at their intersection a central 12 × 12 cm square platform giving access to all arms. The illumination above the central platform was 85 lux. Each rat was placed in the central square facing an open arm, and the time spent (with the four paws) in every arm was recorded. Horizontal locomotor activity was also quantified.
Restraint stress. This stress was performed by wrapping the rats in flexible Teflon, which was secured with Velcro closures so that the movement was limited.
Statistics. Two-way ANOVAs (group × time) were conducted on measures of stress-induced locomotor activity and on behaviors in the light/dark anxiety test and the elevated plus maze. Additional two-way ANOVAs were conducted on measures of plasma hormone concentrations and on optical densities of radioactive signal in the various in situhybridizations. Fisher's post hoc comparisons followed these ANOVAs. Because of the high number of analyses on the GR probe, a Bonferonni's adjustment was effectuated.
RESULTS
HR rats differed from LR rats in behavioral tests of stress-induced locomotor activation and anxiety, as well as in plasma concentrations of corticosterone after the light/dark stress. These individual differences were also associated with differences in markers of amygdaloid and hypothalamic CRH expression and in markers of hippocampal GR synthesis. We have directly implicated the GR differences between HR and LR rats in the difference in their phenotype. Thus, the blockade of these receptors with RU38486 was able to turn LR rats into HR rats, in terms of their anxiety level and their locomotor response to novelty.
Behavioral studies
In the five following experiments, the rats were first tested for their locomotor activity in a novel environment for 120 min. This allowed the HR or LR classification. The HR rats (the most active one-third of the sample) always exhibited significantly higher locomotor counts than did the LR rats (locomotor counts in the lowest one-third of the sample). There was no significant difference in locomotor counts between experiments.
Experiment 1
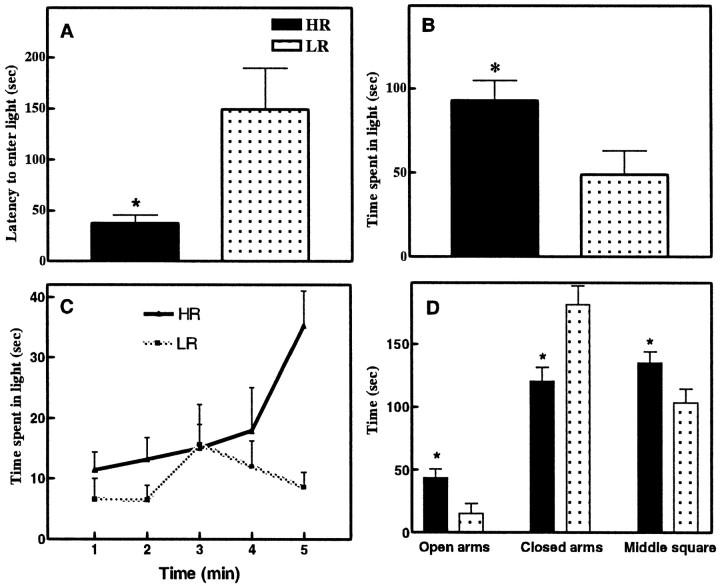
Compared with LR rats (125 ± 32 counts), HR rats (532 ± 87 counts) exhibited a shorter latency to emerge from the dark to the light compartment during the 5 min anxiety test [F(1,26) = 12.69; p< 0.001] (Fig. 1A), and these HR rats spent more time in the light compartment than the LR rats [F(1,26) = 11.67;p < 0.05] (Fig. 1B). Minute-by-minute analysis of the time spent in the light box showed that HR and LR rats had different patterns of exploration [F(4,48) = 3.17; p < 0.05] (Fig. 1C). Compared with LR rats, HR rats had higher locomotor activity in the light box [F(1,26) =16.03; p < 0.001]. However, HR and LR rats did not differ in their locomotor activity in the dark box [F(1,26) = 3.12; p = 0.10].
Fig. 1.
A, Anxiety-like behavior in HR and LR rats in the light/dark boxes. Compared with LR rats, the HR rats exhibit a shorter latency to emerge from the dark to the light compartment. B, These HR rats spend more time in the light compartment. C, Minute-by-minute anxiety-like behavior in HR and LR rats in the light/dark boxes D, Anxiety-like behavior in HR and LR rats in the elevated plus maze. Compared with LR rats, HR rats spent more time in the open arms and the middle portion of the maze. HR rats spent less time than LR rats in the closed arms. Data are expressed as mean ± SEM. *p < 0.05.
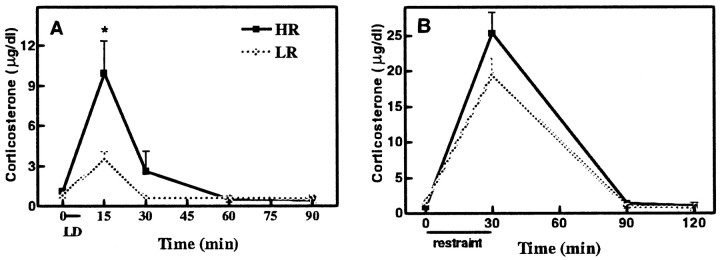
At the onset of the light/dark test, HR and LR rats did not differ in basal plasma corticosterone concentrations. However the HR rats exhibited higher corticosterone secretion 15 and 30 min after exposure to the light/dark anxiety test [F(4,30) = 4.46; p < 0.01] (Fig. 2A).
Fig. 2.
A, Light/dark stress-induced plasma corticosterone values (micrograms per deciliter) for LR and HR rats at various times (15, 30, 60, and 90 min) after the termination of 5 min light/dark stress and in control rats that were not exposed to the light/dark anxiety test (i.e., t = 0). Basal plasma corticosterone concentrations did not differ between the HR and LR rats. However, the HR rats exhibited greater stress-induced secretion of corticosterone measured 15 and 30 min after termination of the anxiety test. B, Restraint stress-induced plasma corticosterone values (micrograms per deciliter) for LR and HR rats at various times (30, 90, and 120 min) after the termination of 30 min restraint stress and in control rats that were not exposed to the restraint stress (i.e., t = 0). Neither the basal (t = 0) nor the stress (t = 30, 90, and 120 min) level of corticosterone was different between HR and LR rats. Data are expressed as mean ± SEM. *p< 0.05.
Experiment 2
After being screened for their locomotor response in a novel environment, the anxiety response of HR (n = 7; 499 ± 88 counts) and LR (n = 7; 144 ± 52 counts) rats was tested in an elevated plus maze. HR rats spent more time in the open arms [F(1,12) = 6.15; p < 0.05] and the middle square [F(1,12) = 4.39; p < 0.05] than LR rats. HR rats spent less time in the closed arms when compared with LR rats [F(1,12) = 9.17; p < 0.01] (Fig. 1D). Compared with LR rats, HR rats had higher locomotor activity in the open arms [F(1,12) = 16.84; p< 0.01]. HR and LR rats had the same locomotor activity in the closed arms [F(1,12) = 1.58;p = 0.23].
Experiment 3
After being screened for their locomotor response in a novel environment, 16 HRs (599 ± 98 counts) and 16 LRs (139 ± 42 counts) were exposed for 30 min to restraint stress. HR and LR rats did not show a difference either in basal corticosterone [F(7,24) = 50.56; p> 0.99] or in corticosterone secretion after 30 min [F(7,24) = 50.56; p = 0.31], 90 min [F(7,24) = 50.56;p > 0.99], or 120 min [F(7,24) = 50.56; p> 0.99] of restraint stress (Fig. 2B).
Experiment 4
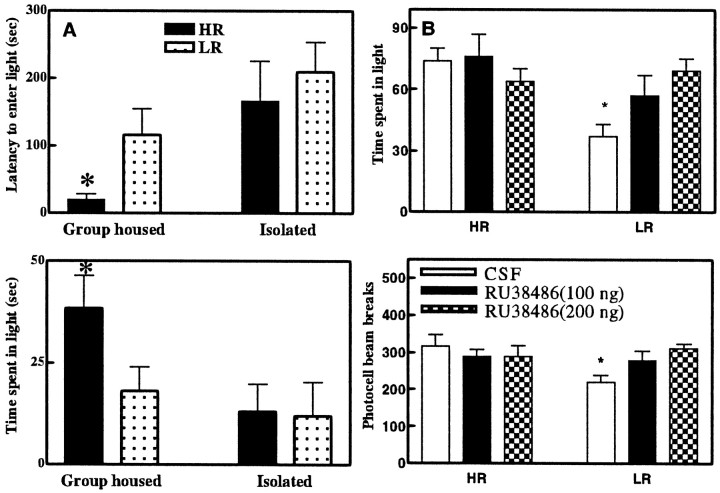
After locomotor screening, 12 HRs (609 ± 86 counts) and 12 LRs (166 ± 44 counts) were either group-housed or isolated. The group-housed HR rats exhibited a shorter latency to enter the light compartment [F(3,19) = 1.75; p < 0.05] and spent more time in the light compartment than any of the other three groups of rats [F(3,19) = 2.15; p < 0.05] (Fig. 3A). Thus, social isolation eliminated the HR–LR difference in the light/dark test.
Fig. 3.
A, Effect of 1 week isolation stress on the anxiety-like behavior of HR and LR rats. Group-housed HR rats are quicker to first enter the light compartment (top graph), and they spend more time in this light (bottom graph). However, after 1 week of isolation, HR rats exhibit prolonged latencies to enter the light compartment and they spend less time in the light compartment. The behavior of the HR rats after 1 week of isolation stress does not differ from the behavior of the LR rats. Data are expressed as mean ± SEM. *p < 0.05 for comparisons with the group-housed HR rats. B, Anxiety-like behavior in HR and LR rats in the light/dark boxes after microinjection of CSF or RU38486 in the hippocampus. Compared with CSF-treated LR rats, CSF-treated HR rats spent more time in the light box (top graph). The difference between LR and HR rats in the time spent in the light box disappeared with the microinjection of RU38486 at both doses (100 and 200 ng). Bottom graph, Locomotor response to the novel light/dark box environment after microinjection of CSF or RU38486 in the hippocampus. Compared with CSF-treated LR rats, CSF-treated HR rats are more active in the novel environment. The difference in locomotor activity between LR and HR rats disappeared after microinjection of RU38486 at both doses (100 and 200 ng). Data are expressed as mean ± SEM. *p< 0.05. Comparisons are made between HR and LR CSF- or RU 38486-treated rats.
Experiment 5
This experiment was conducted after the anatomical results (see below) indicated that HR animals had significantly lower hippocampal GR than the LR animals. We asked whether lowering the effective activity of the hippocampal glucocorticoid receptors would alter the pattern of individual differences in either group of animals. Thus, 18 HRs (587 ± 92 counts) and 18 LRs (175 ± 43 counts) received microinjections of either CSF or RU38486 (100 or 200 ng) into the hippocampus. One hour later, their anxiety-like behavior and locomotor activity were explored in the light/dark boxes. CSF-treated HR rats spent more time in the light compartment compared with CSF-treated LR rats [F(5,26) = 2.54;p < 0.01]. The difference between HR and LR rats in the time spent in the light compartment disappeared with the injection of either 100 ng [F(5,26) = 2.54;p = 0.13] or 200 ng [F(5,26) = 2.54; p = 0.77] of RU38486 (Fig. 3B). This equalization of the two groups was attributable to the LR rats behaving like HR animals after treatment with the GR antagonist.
CSF-treated HR rats, when compared with LR rats, also exhibited a shorter latency to enter the light compartment [F(5,26) = 6.61; p < 0.01]. The difference between HR and LR rats in the first emergence disappeared with the injection of RU38486 at the higher dose only [F(5,26) = 6.61; p = 0.45] (data not shown).
CSF-treated HR rats exhibited higher locomotor reactivity in the light/dark boxes when compared with CSF-treated LR rats [F(5,26) = 1.77; p < 0.01]. Here again, the difference between HR and LR rats in terms of locomotor reactivity disappeared with the injection of RU38486 both at 100 ng [F(5,26) = 1.77;p = 0.78] and at 200 ng [F(5,26) = 1.77; p = 0.56] (Fig. 3B). As is the case with the other measures, the equalization of the two groups was attributable to the LR rats behaving like HR animals after treatment with the GR antagonist.
Anatomical studies
The animals used in the anatomical study are the control rats from experiment 1 (group t = 0).
HR and LR rats exhibited no significant differences in basal pituitary (anterior and intermediary) POMC mRNA expression (Fig.4C, Table1).
Fig. 4.
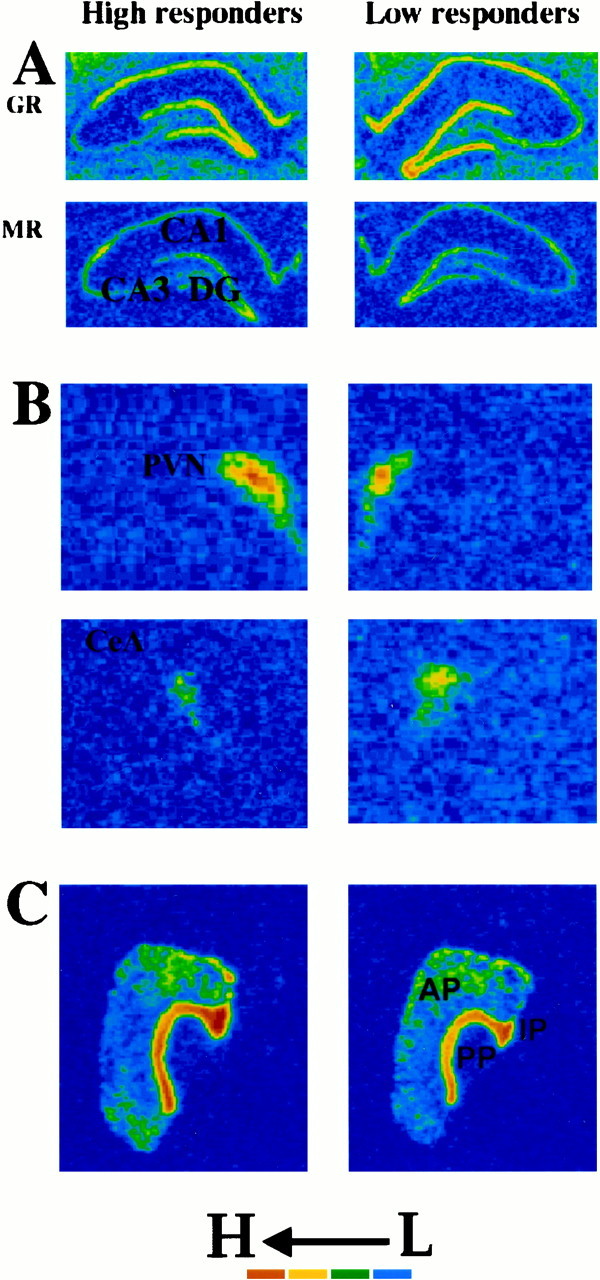
Color-enhanced photomicrographs from x-ray films exposed for 7 d after in situ hybridization with antisense cRNA probes against rat GR and MR mRNAs. Compared with LR rats, the HR rats expressed low basal levels of GR mRNA in the hippocampal CA1 field and dentate gyrus (A, top). There were no differences in MR mRNA expression between HR and LR rats in any hippocampal field (CA1, CA2, CA3, and dentate gyrus) (A, bottom). In the PVN, HR rats expressed higher basal levels of CRH mRNA than LR rats did (b, top). In contrast, HR rats expressed lower basal CRH levels in the CeA than LR rats (B, bottom). HR and LR rats did not differ in POMC expression in the anterior and intermediate pituitary (C).
Table 1.
Integrated density of radioactive signal for GR, MR, CRH, and POMC mRNAs in specific regions of rat brain from HR and LR rats
| Brain regions | High responders | Low responders |
|---|---|---|
| GR in CA1 | 1,489 ± 261 | 4,361 ± 578* |
| GR in DG | 1,856 ± 303 | 3,975 ± 328* |
| MR in CA1 | 10,509 ± 1,073 | 9,434 ± 1,029 |
| MR in DG | 6,534 ± 700 | 5,810 ± 469 |
| MR in CA3 | 4,978 ± 1,654 | 5,295 ± 627 |
| CRF in PVN | 2,847 ± 66 | 2,039 ± 741-160 |
| CRF in CeA | 252 ± 36 | 472 ± 1081-160 |
| POMC in AP | 8,780 ± 829 | 10,017 ± 789 |
| POMC in IP | 32,180 ± 2,062 | 34,322 ± 2,363 |
The integrated density represents the optical density (intensity of the signal) multiplied by the area studied. Results are expressed as mean integrated density ± SEM. AP, Anterior pituitary; IP, intermediate pituitary.
p < 0.001.
F1-160: p < 0.05.
Comparison of CRH mRNA expression between HR and LR rats reveals a regionally specific pattern of differences between these two groups of rats. The HR rats expressed a lower level of basal CRH mRNA in the central nucleus of the amygdala (CeA) than the LR rats [F(1,6) = 7.01; p < 0.05] and a higher level in the PVN [F(1,6) = 6.01; p < 0.05] (Fig. 4B, Table 1).
HR and LR rats did not differ in the amount of basal hippocampal MR mRNA expression in any hippocampal field (i.e., CA1, CA3, and dentate gyrus; Fig. 4A, Table 1).
The HR rats exhibited a significantly lower level of basal GR mRNA in the hippocampus. Compared with the LR rats, the HR rats exhibited lower levels of GR mRNA expression in the hippocampal CA1 field [F(1,6) = 67.40; p < 0.006] and in the dentate gyrus [F(1,6) = 24.14; p < 0.006] (Fig. 4A, Table 1). No other differences in GR expression were observed in other brain regions. As stated above, a Bonferonni's adjustment was made to correct for the large number of comparisons made for the GR probe.
DISCUSSION
This study shows (1) that HR rats explore anxiogenic environments more than LR rats, even though this exploration induces in them a high level of corticosterone; (2) that their behavioral phenotype is accompanied by a unique neuronal phenotype, wherein a number of stress-related genes are differentially expressed in brain regions critical for the control of stress responsiveness (particularly, CRH mRNA is decreased in the CeA, and GR mRNA is decreased in the hippocampus of HR rats); and (3) that the decrease in hippocampal GR may contribute to increased novelty seeking, because the infusion of a GR antagonist directly into the hippocampus leads to increased activity and novelty seeking in the previously timid LR rats.
HR rats actively explore an environment that is typically considered anxiogenic or stressful. Such exploration is accompanied by significantly enhanced stress-induced secretion of plasma glucocorticoids in these rats. Thus, HR rats engage in these behaviors not because they find them less stressful, from the neuroendocrine standpoint, but either despite or because of their ability to activate the stress axis.
The high stress response in HR animals is not indicative of a generalized increase in stress responsiveness. In fact, when the stress is experimenter-imposed (restraint), HR and LR animals exhibit identical responses to the stressor. The differential effect of the restraint stress versus the light/dark stress may be attributable to differences in controllability between the two conditions. Indeed, HR animals, when given a choice, repeatedly select the novel and more stressful condition, as indicated by the results of the minute-by-minute monitoring of their behavior, suggesting that the HR rats continue to actively select the bright environment. However, other variables, beyond the issue of choice, might account for the differences between the two stressors. One variable may be the magnitude of the stress response, because a higher level of glucocorticoids is achieved during restraint compared with that in the light/dark box. It is conceivable that the HR–LR difference is most evident at intermediate levels of stress but tends to disappear when the stressor is strong enough that it becomes a primary determinant of the behavior. It is also of interest to note that the differential behavior of HR and LR animals is highly sensitive to environmental conditions. Thus, 1 week of social isolation abolishes the behavioral differences between HR and LR animals by making the HR animals behave more timidly.
Given this behavioral profile, we asked whether HR animals exhibit, at a neuronal level, a different stress system even before any anxiety tests. Our results revealed a unique pattern of differences. Normal expression of POMC in the pituitary and normal levels of MR in the hippocampus but elevated levels of CRH in the PVN decreased levels of CRH in the amygdala and decreased levels of GR in the hippocampus.
In the pituitary, HR and LR rats exhibit equivalent basal expression of POMC mRNA. This is consistent with the finding that HR and LR rats have the same basal level of corticosterone. Surprisingly, HR rats showed an increased basal CRH mRNA in the PVN. One hypothesis is that at the level of HR pituitaries there is a downregulation of CRH receptors, leading to the same POMC synthesis and secretion in stress-free conditions. The basal hyperexpression of PVN CRH mRNA in HR rats may result in exaggerated vesicular stores of CRH in these neurons, allowing for greater stress-induced release of CRH into the hypophyseal portal circulation and greater responsiveness of pituitary corticotrope cells when the system is activated by stress. However, differences between HR and LR rats in their levels of PVN CRH mRNA are likely not implicated in individual differences in anxiety-related behaviors. Indeed, lesions of the CeA, and not the PVN, disrupt CRH-potentiated conditioned fear responses (Liang et al., 1992). Conversely, the decreased CRH expression in the CeA of HR rats is consistent with their phenotype of decreased anxiety behaviors. When injected into the amygdala, CRH antagonists reduce fear-related responses (Koob et al., 1993), and lesions of CeA disrupt CRH-potentiated conditioned fear responses (Liang et al., 1992). Additionally, microinjection of a CRH antagonist into the CeA reverses anxiogenic-like effects of ethanol withdrawal (Rassnick et al., 1993). This evidence strongly supports the idea that CRH in the CeA produces anxiogenic effects. Low levels of CeA CRH in HR rats might represent one of the factors that allow them to engage in novelty-seeking behavior.
HR and LR rats exhibited no differences in hippocampal MR expression. This agrees with the view that MR primarily regulates basal secretion of corticosterone (Dallman et al., 1989; Spencer et al., 1998), because HR and LR exhibit equivalent basal levels of the stress hormone. Thus, MR receptors appear to be uninvolved in individual differences in stress responsiveness and anxiety-related behaviors.
The most novel aspect of this study relates to the implication of glucocorticoid receptors in the hippocampus in novelty-seeking behavior. We found that hippocampal expression of GR mRNA was decreased in HR rats. Low levels of hippocampal GR capacity have also been observed in these rats (Maccari et al., 1991, Kabbaj et al., 1996). Is this decrease in GR expression responsible for the decreased anxiety that is apparent in HR rats when exploring an anxiogenic environment? This was studied in an experiment that revealed that a GR antagonist could lead LR rats to behave indistinguishably from HR rats in terms of their response pattern in an anxiety test and in terms of their locomotor response to novelty. These findings directly implicate the hippocampal GR in individual differences in novelty-seeking behavior. Supporting our results are the findings that transgenic mice that express antisense mRNA against GR exhibit attenuated anxiety responses in the elevated plus maze (Strohle et al., 1998).
It therefore appears that a low level of activity of GR in the hippocampus, whether attributable to reduced gene expression or to a blockade by an antagonist, can promote novelty-seeking behavior. In turn, novelty seeking, as mentioned above, raises the levels of circulating glucocorticoids. The inverse relationship between stress-induced levels of glucocorticoids and GR activity in the hippocampus is well established, because hippocampal GR has been implicated in negative feedback on the stress response (Sapolsky et al., 1984; Herman et al., 1989; Sapolsky et al., 1990). What remains unclear is whether the HR animals seek the anxiogenic environment despite or because of the increased secretion of glucocorticoids. In general, environmental conditions that evoke the release of glucocorticoids are thought to exert negative consequences on the homeostatic functioning of the animal. We generally consider that these environmental challenges should be avoided. However, it should be noted that corticosterone is self-administered by rats (Piazza et al., 1993), and stress actually potentiates self-administration of a variety of abused drugs (Piazza et al., 1990; Goeders and Guerin, 1994; Shaham and Stewart, 1994; Haney et al., 1995; Miczek and Mutschler, 1996). Furthermore, stress will reliably reinstate drug-seeking behavior in rats after extinction of drug-reinforced responding (Piazza et al., 1993; Shaham and Stewart, 1995; Erb et al., 1996; Shaham et al., 1996). These data suggest the existence of common physiological mechanisms underlying responses to stress and to the behaviorally reinforcing actions of abused drugs. In fact, exposure to a stressful situation increases mesolimbic dopamine neurotransmission (Thierry et al., 1976), and activation of these dopaminergic neurons is closely linked to behavioral reinforcement (Wise and Bozarth, 1987; Koob and Bloom, 1988). There is also evidence that glucocorticoid secretion may exert positive hedonic effects. Humans report feelings of euphoria after corticosterone administration (Zuckerman and Neeb, 1979). Thus, although conditions that evoke the release of glucocorticoids are typically considered aversive, this may not always be the case. When animals either self-administer glucocorticoids or select the “stressful” environment, activation of the limbic–hypothalamo–pituitary adrenal axis may indeed be positively reinforcing. In turn, many positively reinforced activities, including feeding and mating, are associated with elevated secretion of glucocorticoids (Dallman et al., 1995; Frye et al., 1996). The decreased expression of hippocampal GR may contribute to the enhanced reinforcing efficacy of stress and drugs of abuse in HR animals by further elevating glucocorticoid levels. These elevated levels of steroid hormones might enhance dopamine release, thereby increasing the behaviorally reinforcing properties of stress and drugs of abuse in HR animals (Rouge-Pont et al., 1998).
Although this combination of findings implicates hippocampal GR expression in individual differences in novelty seeking, it does not imply that GR expression is the only factor that determines these behavioral activities. The combination of decreased hippocampal GR and decreased amygdaloid CRH, as well as altered expression in a number of other genes, may makes these animals particularly willing to explore novel environments.
Are the causes of these individual differences primarily genetic, are they induced by maternal behavior or other environmental and developmental events, and will a certain initial tendency that was either genetic or developmental alter behavior in such a way as to further bias gene expression, thereby exaggerating individual differences? Regardless, such a behavioral and neuronal phenotype may prove interesting as a possible substrate for alterations in tendencies to self-administer drugs and to react to environmental inputs in a highly responsive manner.
Footnotes
This work was supported by National Institute on Drug Abuse Grant DA02265, National Institute of Mental Health Grant MH42251, a Bristol-Myers Squibb research award, and Nancy Friend Pritzker Network Grant 961629 to H.A.
Correspondence should be addressed to Mohamed Kabbaj, Mental Health Research Institute, University of Michigan, 205 Zina Pitcher Place, Ann Arbor, MI 48109-0720. E-mail: kabbaj@umich.edu.
REFERENCES
- 1.Anisman H, Zacharko RM. Depression as a consequence of inadequate neurochemical adaptation in response to stressors. Br J Psychiatry [Suppl] 1992;15:36–43. [PubMed] [Google Scholar]
- 2.Dallman MF, Levin N, Cascio CS, Akana SF, Jacobson L, Kuhn RW. Pharmacological evidence that the inhibition of diurnal adrenocorticotropin secretion by corticosteroids is mediated via type I corticosterone-preferring receptors. Endocrinology. 1989;124:2844–2850. doi: 10.1210/endo-124-6-2844. [DOI] [PubMed] [Google Scholar]
- 3.Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Ann NY Acad Sci. 1995;771:730–742. doi: 10.1111/j.1749-6632.1995.tb44724.x. [DOI] [PubMed] [Google Scholar]
- 4.Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats—biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- 5.Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- 6.Frye CA, McCormick CM, Coopersmith C, Erskine MS. Effects of paced and non-paced mating stimulation on plasma progesterone, 3 alpha-diol and corticosterone. Psychoneuroendocrinology. 1996;21:431–439. doi: 10.1016/0306-4530(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 7.Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology. 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- 8.Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- 9.Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, Watson SJ. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holsboer F, Lauer CJ, Schreiber W, Krieg JC. Altered hypothalamic-pituitary-adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology. 1995;62:340–347. doi: 10.1159/000127023. [DOI] [PubMed] [Google Scholar]
- 11.Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB. Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991;9:121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- 12.Hooks MS, Juncos JL, Justice JB, Jr, Meiergerd SM, Povlock SL, Schenk JO, Kalivas PW. Individual locomotor response to novelty predicts selective alterations in D1 and D2 receptors and mRNAs. J Neurosci. 1994;14:6144–6152. doi: 10.1523/JNEUROSCI.14-10-06144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kabbaj M, Piazza P, Le Moal M, Maccari S. Individual differences in the noradrenergic regulation of hippocampal corticosteroid receptors. Soc Neurosci Abstr. 1996;22:18. [Google Scholar]
- 14.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 15.Koob GF, Heinrichs SC, Pich EM, Menzaghi F, Baldwin H, Miczek K, Britton KT. The role of corticotropin-releasing factor in behavioural responses to stress. Ciba Found Symp. 1993;172:277–289. doi: 10.1002/9780470514368.ch14. [DOI] [PubMed] [Google Scholar]
- 16.Liang KC, Melia KR, Campeau S, Falls WA, Miserendino MJ, Davis M. Lesions of the central nucleus of the amygdala, but not the paraventricular nucleus of the hypothalamus, block the excitatory effects of corticotropin-releasing factor on the acoustic startle reflex. J Neurosci. 1992;12:2313–2320. doi: 10.1523/JNEUROSCI.12-06-02313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maccari S, Piazza PV, Deminiere JM, Angelucci L, Simon H, Le Moal M. Hippocampal type I and type II corticosteroid receptor affinities are reduced in rats predisposed to develop amphetamine self-administration. Brain Res. 1991;548:305–309. doi: 10.1016/0006-8993(91)91137-p. [DOI] [PubMed] [Google Scholar]
- 18.Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology. 1996;128:256–264. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- 19.Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 20.Piazza PV, Deminiere JM, Le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self- administration. Brain Res. 1990;514:22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- 21.Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci USA. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- 23.Rouge-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci. 1998;10:3903–3907. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 24.Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci USA. 1984;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sapolsky RM, Armanini MP, Packan DR, Sutton SW, Plotsky PM. Glucocorticoid feedback inhibition of adrenocorticotropic hormone secretagogue release. Relationship to corticosteroid receptor occupancy in various limbic sites. Neuroendocrinology. 1990;51:328–336. doi: 10.1159/000125357. [DOI] [PubMed] [Google Scholar]
- 26.Shaham Y, Stewart J. Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology. 1994;114:523–527. doi: 10.1007/BF02249346. [DOI] [PubMed] [Google Scholar]
- 27.Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- 28.Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking in rats under opioid maintenance: the effects of stress, heroin priming, and withdrawal. J Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer RL, Kim PJ, Kalman BA, Cole MA. Evidence for mineralocorticoid receptor facilitation of glucocorticoid receptor-dependent regulation of hypothalamic-pituitary-adrenal axis activity. Endocrinology. 1998;139:2718–2726. doi: 10.1210/endo.139.6.6029. [DOI] [PubMed] [Google Scholar]
- 30.Strohle A, Poettig M, Barden N, Holsboer F, Montkowski A. Age- and stimulus-dependent changes in anxiety-related behaviour of transgenic mice with GR dysfunction. NeuroReport. 1998;9:2099–2102. doi: 10.1097/00001756-199806220-00035. [DOI] [PubMed] [Google Scholar]
- 31.Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- 32.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 33.Zuckerman M. Sensation-seeking: a comparative approach to a human trait. Behav Brain Sci. 1984;7:413–471. [Google Scholar]
- 34.Zuckerman M. The psychophysiology of sensation seeking. J Pers. 1990;58:313–345. doi: 10.1111/j.1467-6494.1990.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 35.Zuckerman M, Neeb M. Sensation seeking and psychopathology. Psychiatry Res. 1979;1:255–264. doi: 10.1016/0165-1781(79)90007-6. [DOI] [PubMed] [Google Scholar]