Abstract
Protein MAP1B was recently reported to link GABACreceptors to the cytoskeleton at neuronal synapses. This interaction was demonstrated in the mammalian retina, where GABACreceptors were thought to be exclusively expressed in bipolar cells. Our previous studies on cultured photoreceptors suggested however the presence of GABAC receptors in cones. To further investigate GABAC receptor expression in cones, we measured GABA responses in mammalian photoreceptors in situ, and we examined the distribution of the receptor and that of protein MAP1B in the mammalian outer retina. Photoreceptors were recorded from flat-mounted retinas of retinal degeneration mice at an age when the retina becomes cone-dominated after rod cell death. GABAAand GABAC-gated currents were produced only in cones but not rods. Recording freshly dissociated retinal cells from wild-type C57 mice confirmed the presence of GABAA and GABAC receptors in cones. Immunohistochemical labeling of mouse and rat retinal sections localized GABAC receptors to cone terminals that were identified by peanut agglutinin lectin staining. As expected from previous studies on bipolar cells, the punctate immunostaining was not restricted to cone terminals in the outer plexiform layer. MAP1B immunolabeling was obtained in rat and pig retinas and was similarly found in cone terminals identified by the peanut agglutinin lectin staining. These results provide physiological and histological evidence that cones receive a GABA feedback in the mammalian retina and are consistent with the notion that protein MAP1B links GABAC receptors to the cytoskeleton at postsynaptic sites.
Keywords: GABA, GABAA receptor, GABACreceptor, MAP1B, retina, photoreceptor, cone
GABACreceptors were recently classified in the GABAAreceptor family (Barnard et al., 1998), although this concept is not widely accepted (Bormann, 2000). GABAC receptors gate a Cl− channel similar to GABAA receptors but exhibit a different pharmacological profile (Bormann, 2000). The main difference with conventional GABAA receptors is their bicuculline and barbiturate resistances; specific antagonists to GABAC receptors are also available (Ragozzino et al., 1996). Furthermore, GABAC receptors, in contrast to GABAA receptors, do not desensitize, rendering them particularly well suited for processing graded potentials.
GABAC receptor ρ subunits share only 30% homology with GABAA receptor subunits. All ρ subunits, except rat ρ2 subunits, can form homomeric GABA-gated Cl− channels, most of which are sensitive to picrotoxin. Only rat and mouse ρ2 subunits are insensitive to picrotoxin and can confer this picrotoxin insensitivity to heteromeric channels (Cutting et al., 1991; Wang et al., 1994; Zhang et al., 1995; Shingai et al., 1996; Enz and Cutting, 1998; Greka et al., 1998). Recently, the microtubule-associated protein MAP1B that was found to specifically interact with GABACreceptor ρ1 subunits, was proposed to link the receptor to the cytoskeleton (Hanley et al., 1999). The MAP proteins are known to stabilize microtubules, the most abundant members of the family being the proteins tau and MAP2, which are located predominantly in axons and dendrites, respectively. By contrast, GABAAreceptors were found to be linked to the cytoskeleton via gephyrin (Kneussel et al., 1999) or GABA receptor-associated protein (GABARAP) (Wang et al., 1999).
In the retina, GABAC receptors have been recorded in bipolar cells (Feigenspan et al., 1993; Lukasiewicz et al., 1994), horizontal cells (Qian and Dowling, 1993; Dong et al., 1994), and ganglion cells (Zhang and Slaughter, 1995). In the mammalian retina, GABAC receptor currents were first reported in bipolar cells (Feigenspan et al., 1993; Euler and Wässle, 1998). This bipolar cell localization was confirmed by immunocytochemistry (Enz et al., 1996; Koulen et al., 1998) andin situ hybridization (Enz et al., 1995). Consistent with its proposed linkage to GABAC receptors, MAP1B was distributed in the inner plexiform layer and more specifically at bipolar cell terminals (Hanley et al., 1999). The ρ3 subunits were localized by in situ hybridization to cells in the ganglion cell layer (Ogurusu et al., 1997). The presence of GABAA and GABAC receptors in mammalian cones was recently suspected after recording porcine photoreceptors using a pure photoreceptor cell culture (Picaud et al., 1998). However, this physiological evidence of GABAC receptors in cones was neither supported by previous immunocytochemical nor in situ hybridization studies (Enz et al., 1995, 1996; Koulen et al., 1998).
To determine whether cones do normally express GABAC receptors, their responses to GABA were recorded in flat-mounted mouse retina. In parallel, retinal sections were immunolabeled with antibodies directed against either MAP1B or the ρ subunits. This study further suggests the implication of GABAC receptors in cone physiology and is in agreement with GABAC receptor interaction with MAP1B.
MATERIALS AND METHODS
Electrophysiological recording. C3H/He/J mice homozygous for the retinal degeneration (rd1) gene aged 20–30 d were procured from Janvier Animal Suppliers (Le Genest St. Isle, France) and maintained in standard laboratory conditions. After killing, the eyes were removed and placed in Ringer's saline. The lens and vitreous were removed, and the retina was separated from the posterior eyecup and placed flat with the photoreceptor surface up in a perfusion chamber. The retina was maintained flat by overlaying with a nylon mesh. As previously described for preparing cultures, retinal cells were enzymatically dissociated in 0.2% papain for 30 min (Picaud et al., 1998). Conventional whole-cell patch-clamp recordings were performed on photoreceptor cells at room temperature using bath solutions containing (in mm): 135 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, and 5 HEPES (pH-adjusted to 7.75 using NaOH). The patch pipettes were pulled from thin-walled borosilicate glass (TW 150F; World Precision Instruments) using a Brown and Flaming type puller (P-87; Sutter Instruments, Novato, CA). They were filled with the recording solutions containing (in mm): 140 KCl, 1 MgCl2, 0.5 EGTA, 5 ATP (disodium), and 4 HEPES (pH-adjusted to 7.4 with KOH). When using the equilibrium potential (ECl) at −30 mV, 98 mm K-gluconate was substituted for KCl. The membrane currents were measured using an RK-400 patch amplifier (Biological, Grenoble, France). Liquid junction potentials were corrected by using standard procedure. Data acquisition and analysis were performed using Patchit and Tack software packages, respectively (Grant and Werblin, 1994). Data were filtered at 3 kHz and digitized at either 10 kHz during voltage steps or 1 Hz during neurotransmitter applications using a data acquisition labmaster board (Scientific Solution, Solon, OH) mounted in an IBM-compatible personal computer (PC).
Drugs were added to the bath solutions and were applied by a local gravity-driven perfusion system controlled by a manual solution changer. A 1 mm concentration of GABA was puffed on the proximity of the recorded cell through a pulled glass capillary (TW 100F; World precision Instruments) that was controlled (duration as indicated in the figure legends; 20 psi) by a picospritzer (Picospritzer II General Valve Corporation, Fairfield, NJ), monitored through the PC. All experiments were performed on at least three cells, and the results are represented as mean ± SEM.
Immunohistochemistry and cell identification. Recorded cells were filled during the experiments by including Sulforhodamine-101 in the pipette recording solution. Retinas were fixed for 1 hr at 4°C with 4% paraformaldehyde in 0.1 m PBS. Rods were labeled with the rhodopsin antibody rho-4D2 (Hicks and Molday, 1986; kindly provided by D. Hicks) with a similar protocol as the one described below on sections. Immunolabeling for MAP1B (Clone AA6; Sigma, St. Louis, MO) and GABAC receptor (gift of Dr. R. Enz and Prof. H. Wässle, Max Planck Institute, Frankfurt, Germany), as well as staining with peanut agglutinin lectin (Sigma), were performed on frozen retinal sections (8 μm thickness). For the GABAC receptor antibody, the anterior segment was removed, and the whole eyecup was fixed in 4% paraformaldehyde in 0.1 m PBS for 5 min. For MAP1B immunolabeling, fixation was performed directly on sections. Sections were washed in PBS, permeabilized in PBS containing 0.1% Triton X-100 for 5 min, then bathed in PBS containing 1% bovine serum albumin for 30 min at 37°C and incubated in the same solution with the primary antibody for 2 hr at room temperature. The sections were washed three times and incubated with the secondary antibody [rabbit anti-mouse conjugated to Texas Red or fluorescein isothiocyanate (Molecular Probes, Eugene, OR) diluted 1:200] for 1 hr at room temperature. The lectin staining was obtained by bathing the sections with peanut agglutinin lectin coupled to Texas Red (50 μg/ml final concentration) for 1 hr at room temperature. Diamidino-phenylindole (DAPI) nuclear labeling was finally performed in PBS during 2 min.
Western blot of retinal homogenates. Homogenates from entire porcine retinas were centrifuged (65 × g) for 5 min at 4°C in Tris buffer (10 mm, pH 7.4). Pellets were suspended in the Tris buffer containing 1 mmEDTA, a mixture of protease inhibitors (protease inhibitor cocktail tablets; Boehringer Mannheim, Mannheim, Germany), 100 μm phenylmethylsulfonylfluoride (PMSF), and centrifuged (11,000 × g) for 10 min at 4°C. Cytoplasmic proteins in the supernatant were denatured in SDS sample buffer containing 100 mm dithiothreitol; their concentration was measured using bovine serum albumin as a standard. Protein samples of 20 μg were then electrophoresed on a 8% SDS-PAGE and transferred onto a nitrocellulose membrane (Schleicher and Schuell, Dassel, Germany). The membrane was incubated in 1% bovine serum albumin, 0.1 m PBS, pH 7.4, at 37°C for 1 hr, and then with MAP1B monoclonal antibody (1:500 in 1% bovine serum albumin and 0.1% Tween 20, 0.1m PBS, pH 7.4) for 2 hr at room temperature. After a 15 min wash, the nitrocellulose membrane was incubated with the secondary antibody, and rabbit anti-mouse IgGs were conjugated to the alkaline phosphatase (1:5000) for 2 hr at room temperature. The enzyme was visualized with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate in 0.1 m Tris-acetate, pH 9.5.
Chemicals and proteins. All chemicals, lectin, and antibodies for which the source is not mentioned were provided from Sigma. The GABAA antagonist 2-(carboxy propyl-3-amino-6,4-methoxy phenyl) pyradazynium bromide (SR95531) was purchased from Research Biochemicals (Natick, MA), and GABAC antagonist (1,2,5,6-tetrahydro-pyridine-4-yl) methyl phosphonic acid (TPMPA) was obtained from Tocris (Bristol, UK).
RESULTS
Rod and cone identification in flat-mounted retinas
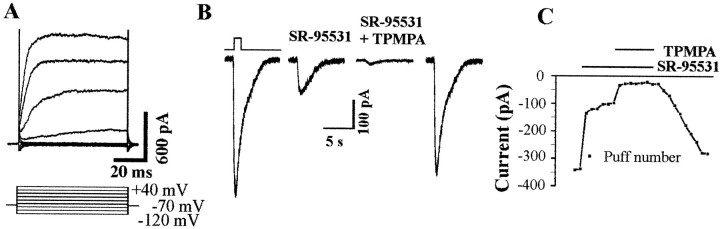
Cone photoreceptors were recorded from 20- to 30-d-oldrd mice, in which a mutation in the rod cGMP-dependent phosphodiesterase induces early onset of rod cell degeneration, leaving cones easily accessible to the patch-clamp recording pipette. Figure1 illustrates a recorded cell with neighboring photoreceptors. Only very few remaining rods can be observed after immunolabeling with an anti-rhodopsin antibody (rho-4D2) among photoreceptors stained in the outer nuclear layer with the nuclear dye DAPI (Fig. 1B,C). Cells were selected for recording in the outer nuclear layer under Nomarski optics. Recorded cells were classified in two groups according to their characteristics (Fig. 2). Based on the rod to cone ratio, these groups were easily assigned to either cones or rods. Figure 2,A and B, illustrates the two representative types of responses to voltage steps that were recorded in rods and cones, respectively. These responses were similar to those obtained from cultured rods and cones from the pig retina (data not shown). To verify cellular identity, recorded cells were filled with sulforhodamine-101 (SR101), and the retina was subsequently labeled with rho-4D2. In Figure 1, the recorded cell is brightly stained with SR101 (Fig. 1A) but is immunonegative for rhodopsin (Fig. 1B) (only the nuclear staining with SR101 leaks through the filter). The outer nuclear layer location established photoreceptor identity, and the opsin immunonegativity indicated it was not a rod but a cone. All cones identified this way had a response to voltage steps similar to that presented in Figure 2,B and C. The other type of response to voltage steps (Fig. 2A,C) was attributed to rods because it was identical to those recorded from cultured pig rods. No dye coupling was observed between photoreceptor cells. These results indicated that both rods and cones can be recorded from the flat-mounted rdmouse.
Fig. 1.
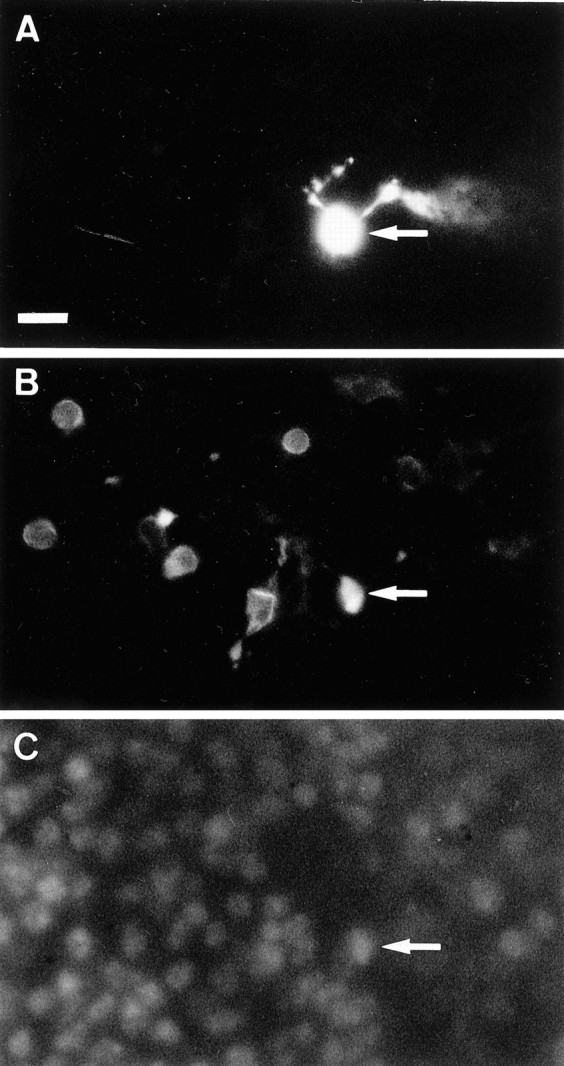
Cone morphology in flat-mounted rdmouse retina. The recorded cell (arrow) was stained with the dye sulforhodamine 101, which was included in the recording pipette solution and passively diffused into the cell during the recording (A). After fixation, rods were immunolabeled with the rhodopsin antibody (rho-4D2) and a secondary antibody coupled to fluorescein (B). The recorded cell is not immunolabeled itself, its nuclei was fluorescent under blue illumination because the red fluorescence of sulforhodamine 101 leaked through the filter. Photoreceptor cell nuclei were visualized with the nuclear dye DAPI (C). Note that the recorded cell was in the same focal plane as immunolabeled rods.
Fig. 2.
Electrophysiological identification of rod and cone photoreceptors in flat-mounted rd mouse retina. Current responses to voltage steps in a representative rod (A) and cone (B). The potential was stepped from a holding potential of −70 mV to potentials ranging from −120 to +40 mV in 20 mV increments. C,Averaged current–voltage curves of rod and cone response to voltage steps (cones, n = 20; rods, n = 5; ±SEM). Cones typically exhibited a large outward current at potential superior to −30 mV, whereas rods showed an inward current activated at the most negative potentials. D, Rod and cone photoreceptor responses to a GABA puff application (1 mm, 1 sec). GABA elicited a large current in cones but not in rods.
Responses to GABA application
When GABA (1 mm) was puff-applied onto recorded mouse photoreceptors held at a potential of −70 mV, rods did not show any response (Fig. 2D). By contrast, cones generated a large current ranging from 150 to 375 pA (Fig. 2D). The reversal potential of GABA-elicited currents was estimated from voltage ramp measurements. Whole-cell currents were recorded in the absence of GABA and during a long GABA puff (Fig.3A). The GABA-elicited current was then calculated by subtracting the measurement in the absence of GABA from that in the presence of GABA. In experiments where the Cl− concentration was almost symmetrical (Fig. 3B), the reversal potential of GABA-elicited currents were close to 0 mV (−4.16 ± 3.8 mV; n = 4). When gluconate was substituted for Cl− in the recording pipette solution (Fig. 3B), the reversal potential approximately followed the Cl−equilibrium potential (ECl = −30 mV;Erev = −33.96 ± 1.27 mV;n = 3). These results indicated that GABA was activating Cl− channels in mouse cone photoreceptors.
Fig. 3.
GABA generated a Cl−conductance in cone photoreceptors. A,Whole-cell currents were recorded from cone photoreceptors in the presence or absence of a GABA (1 mm) puff application, whereas the membrane potential was ramped from −100 to 60 mV at 0.72 mV/msec. Between recordings, cells were held at 0 mV to suppress K+ currents. B, The GABA-elicited current was calculated by subtracting the whole-cell current measured in the absence of GABA from that obtained in the presence of GABA. This measurement is illustrated for two cells in which the equilibrium potentials for Cl−(ECl) were set at either −30 mV (as in A) or 0 mV by using recording pipette solutions with different Cl− concentrations (see Materials and Methods). Under these conditions, the reversal potential of the GABA-elicited current approximately follows the equilibrium potential for Cl−.
Pharmacology of GABA responses
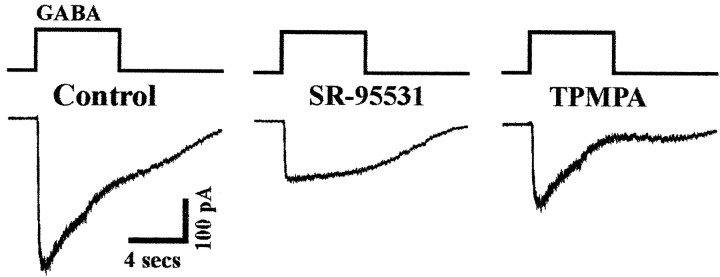
Figure 4 shows the pharmacological profile of the GABA-evoked current recorded from cone photoreceptors voltage-clamped to a holding potential of −70 mV. When the GABAA receptor blocker SR95531 (100 μm) was bath-applied (Fig. 4A), GABA responses were reduced by 74.6% (±1.2%; n = 6), and responses were recovered after washing. Responses were further suppressed by 93.8% (±1.5%) in the same cells when the GABAC receptor antagonist TPMPA (50 μm;Ragozzino et al., 1996), was coapplied with SR95531 to the retina (Fig. 4A). Similarly, TPMPA alone decreased the GABA response by 30.8% (±4.0%; n= 5) (Fig. 4A). Bicuculline (100 μm), another known GABAAreceptor antagonist, also partially blocked the GABA response by 47.81% (±6.6%; n = 3). To measure the effect of picrotoxin (100 μm) on the GABAC receptor, it was applied together with bicuculline. Under these conditions, picrotoxin suppressed 91.89% (±1.3%; n = 3) of the GABACreceptor current (Fig. 4B). In the latter experiment, photoreceptors were pharmacologically isolated from the retinal network with a solution containing CNQX (50 μm), strychnine (30 μm), and AP-4 (100 μm). The observation of both a GABAA and a GABAC receptor component under these conditions (Fig. 4B) confirmed that GABA acted directly on the recorded cells. These pharmacological data strongly suggest that both GABAA and GABAC receptors are present in cone photoreceptors. Note that the decay of GABAAreceptor-mediated current was much faster compared to those of GABAC receptors in the same cell (Fig.4C). Variations in the onset of the responses were also observed from cells to cells and were attributed to the different accessibility to the synaptic cleft in this retinal whole-mount preparation. In experiments described below, the GABAC receptor current was isolated by bath applying SR95531, whereas the GABAA receptor component was obtained by using TPMPA in the perfusion solution.
Fig. 4.
Pharmacology of cone GABA currents in flat-mountedrd retina. A, GABA puffs (1 mm, 1 sec) were successively applied, as indicated in the presence of either GABAA or GABAC receptor antagonists SR95531 (100 μm) and TPMPA (50 μm), respectively, or both. B, GABA puffs (1 mm, 50 msec) were applied in presence of bicuculline (100 μm) alone and together with picrotoxin (100 μm). These effects of bicuculline and picrotoxin were measured in a bathing solution containing CNQX (50 μm), strychnine (30 μm), and AP-4 (100 μm) to pharmacologically isolate cones. C,The kinetics of the SR95531- and TPMPA-resistant currents are compared after normalizing the recording shown in A.
Modulation of GABA receptors
A major difference between GABAA and GABAC receptors is their respective sensitivity to benzodiazepines, GABAA receptor currents being greatly increased, whereas GABAC currents are not affected (Shimada et al., 1992). To verify the presence of these two types of currents, the effect of pentobarbital (100 μm) was tested on the respective components pharmacologically isolated as described above. Figure 5 illustrates the pentobarbital-induced increase in the TPMPA-resistant GABAA receptor current in a cone while the SR95531-resistant GABAC receptor component was barely affected in the same cell. The GABAAreceptor current increased by 85± 4.6% (n = 3) in amplitude, whereas the GABAC receptor current showed a slight increase (20 ± 0.35%) in the same three cells. These effects are consistent with the presence of both GABAA and GABAC receptor components in cone photoreceptor GABA response.
Fig. 5.
Pentobarbital (PBT) modulation on GABAA and GABAC receptor currents in flat-mounted rd mouse retina. Pentobarbital (100 μm) was bath-applied together with either SR95531 or TPMPA, which isolated the GABAC and GABAAreceptor currents, respectively. Pentobarbital had no significant effect on the SR95531-resistant or GABAC receptor current, whereas it increased the amplitude and slowed down the decay of the TPMPA-resistant or GABAA receptor current (GABA puff, 1 mm, 1 sec).
Kinetics of the GABA response
To further assess the kinetics of the GABA response, GABA was applied for long duration (6 sec). When the GABACreceptor component was suppressed by TPMPA application (50 μm; Fig. 6), the remaining GABAA response desensitized during the 6 sec application by 84% of its initial amplitude. By contrast, the GABAC receptor component isolated in the presence of SR95531 (100 μm) was only reduced by 24% during the 6 sec application (Fig. 6). These different kinetics are consistent with the expression of desensitizing GABAA receptors and nondesensitizing GABAC receptors in cone photoreceptors.
Fig. 6.
Kinetics of the GABAA and GABAC receptor currents. Long GABA puff applications (1 mm, 6 sec) were delivered in the absence or presence of SR95531 (100 μm) and TPMPA (50 μm). The SR95531-resistant or GABAC receptor current remained sustained during the time of application, whereas the TPMPA-resistant or GABAA receptor current rapidly decreased in amplitude during the puff.
Recordings of freshly dissociated photoreceptors
To test whether cones in wild-type mice would similarly generate GABAA and GABAC receptor currents, retinal neurons from C57 mouse retina were dissociated and immediately recorded. Because cones represent only 1% of all photoreceptors in the mouse retina, many experiments were required to identify and record only four cones. These cones had similar responses to voltage steps (Fig. 7A) as those recorded from cones in the rd mouse (Fig.2B). GABA application elicited responses that were blocked partially (70.6 ± 2.56%; n = 4) by SR95531 (100 μm) and completely (97.62 ± 0.27%) by coapplication of SR95531 together with TPMPA (50 μm) (Fig. 7B,C). This observation confirmed our results on the rd mouse, demonstrating the presence of GABAA and GABACreceptors in mammalian cone photoreceptors in situ.
Fig. 7.
GABAA and GABAC receptors in freshly dissociated C57 mouse cones. A, Current responses to voltage steps in an isolated cone voltage-clamped at −70 mV and stepped in 20 mV increments from −120 to +40 mV.B, GABA-elicited response in the same freshly dissociated cone in the presence of GABAA receptor antagonist SR95531 alone and with TPMPA, respectively (GABA puff 1 mm, 1 sec). C, Maximum amplitude of the GABA responses in the absence or presence of SR95531 (100 μm) and TPMPA (50 μm).
GABAC receptor and MAP1B localizations in the outer retina
To determine whether GABAC receptors are expressed in mammalian cone photoreceptors, we investigated the distribution of the receptors and that of protein MAP1B in the outer retina. Because the MAP1B antibody did not cross-react with mouse tissue, we examined MAP1B localization in the rat retina (Fig.8). Photoreceptor outer segments, fibers in the outer nuclear layer, and structures in the outer plexiform layer were MAP1B-immunopositive (Fig. 8A). To verify that these structures represented cone terminals, retinal sections were labeled with peanut agglutinin lectin that binds selectively to cone extracellular matrix. A few cones were stained in each microscopic field at the level of their outer segments (Fig.8B–D). Similar to the MAP1B antibody, structures with the same shape were also heavily stained at the same position and spacing in the outer plexiform layer, suggesting that cone terminals were MAP1B-immunopositive. In addition to the inner plexiform labeling previously described (Hanley et al., 1999), rare cell bodies were also brightly stained in the proximal part of the inner nuclear layer with processes extending in the inner plexiform layer (data not shown).
Fig. 8.

GABAC receptor and MAP1B immunolocalizations in the rat retina. A–D, Rat retinal sections were labeled with a MAP1B antibody (A), peanut agglutinin lectin (B), and nuclear stain DAPI (C) or visualized with Nomarski image (D). Photographs (B–D) were taken from the same section, only the right part ofB is shown in C and D. MAP1B immunolabeling (A) is observed in photoreceptor outer segments, processes crossing the outer nuclear layer and structures in the outer plexiform layer (A, small arrow). Similar structures are also observed in sections stained with peanut agglutinin lectin that selectively labels cones (B, large arrow) identifying these structures as cone photoreceptor terminals (B, small arrow). E, Western blot of MAP1B from a retinal homogenate showing that the antibody identified a retinal protein at the expected molecular mass (∼300 kDa; Edelmann et al., 1996) of protein MAP1B. F–H, Rat retinal sections were labeled for the GABAC receptor (F, G) and peanut lectin agglutinin (H). A punctate immunolabeling is observed in the outer plexiform layer where some immunopositive structures (G, arrows) are also stained with the cone peanut agglutinin lectin (H, arrow). Scale bar: A–D, 10 μm; F, 16 μm;G, H, 7.3 μm.
When rat retinas were stained with GABAC receptor antibodies, punctate immunolabeling was observed in the outer plexiform layer (Fig. 8F). By contrast to the porcine retina (Picaud et al., 1998), no staining was found in the outer nuclear layer. Double staining of the sections with the peanut agglutinin lectin identified some of the immunopositive structures as cone terminals (Fig. 8G,H). A similar GABAC receptor staining was also observed in the mouse outer retina (Fig. 9A), where immunopositive structures appeared to colocalize with the peanut agglutinin lectin staining of cone terminals (Fig. 9B–D). Although these stainings clearly demonstrated that GABAC receptors were not restricted to cone terminals in the outer plexiform layer, they indicated that MAP1B and GABAC receptors were both localized at mammalian cone terminals.
Fig. 9.
GABAC receptor immunostaining in the mouse retina. Mouse retinal sections were labeled with the GABAC receptor antibodies (A, C) and peanut lectin agglutinin (B). A punctate immunolabeling is observed in the outer plexiform layer where some immunopositive structures (C, arrows) appear also stained with the cone peanut agglutinin lectin (B, arrows). An image taken with the two stainings superimposed is presented to ease the localization of the cone terminals labeled with the peanut agglutinin lectin (D, arrows). Scale bar:A, 30 μm; B–D, 9 μm.
The distribution of MAP1B was also investigated in the pig retina because cones are more numerous than in rats and are more easily identifiable as a single row of nuclei at the distal edge of the outer nuclear layer (Fig. 10). Furthermore, the presence of GABAC receptors in porcine cones was suggested by our previous study on cultured photoreceptors (Picaud et al., 1998). MAP1B immunolabeling was mostly restricted to the outer retina (Fig. 10A,B), but weak discrete staining was also observed in the inner plexiform layer and in ganglion cell axons. At higher magnification, photoreceptor outer segments, a row of nuclei at the distal border of the outer nuclear layer, their cell processes, and terminals in the outer plexiform layer were all intensely labeled (Fig. 10D). To confirm that immunopositive terminals in the outer plexiform layer belonged to cones, retinal sections were double-stained with peanut agglutinin lectin (Fig.10F) and MAP1B antibody (Fig. 10G). Cone terminals that were stained with the lectin were clearly immunolabeled with the MAP1B antibody (Fig. 10F,G, arrows). The recording of GABAC receptors in cones and the MAP1B immunostaining of these neurons are consistent with the notion that MAP1B may link GABAC receptors to the cytoskeleton in mammalian cone photoreceptors.
Fig. 10.

MAP1B distribution in porcine retinal sections. Porcine retinal sections were examined after staining with MAP1B antibody (A, D, F), DAPI (B, E) and peanut agglutinin lectin (G) or visualized under Nomarski image (C). An intense MAP1B immunolabeling is observed in the outer retina contrasting with the weak or no labeling in the inner retina (A, B). At higher magnification (D), this labeling is found in photoreceptor outer segments, a row of nuclei at the distal border of the outer nuclear layer where cone cell bodies are localized, in the axons of these cell bodies, and finally in their terminals located at the distal border of the outer plexiform layer. When retinal sections were double-labeled with fluorescein for MAP1B (F) and Texas Red for peanut agglutinin lectin (G), lectin-stained terminals (G, arrows) were also immunopositive for MAP1B (F, arrows), identifying these labeled cells as cone photoreceptors. Scale bar: A, B, 20 μm;C–G, 10 μm.
DISCUSSION
In the present study, mouse rods and cones were electrophysiologically recorded in situ. Both GABAA and GABAC receptor activation was detected in cone photoreceptors, whereas rods did not respond to GABA application. MAP1B that is known to link GABAC receptors to the cytoskeleton was localized throughout mammalian cone photoreceptors up to their terminals. This localization of MAP1B in cone terminals is in agreement with the idea that GABAC receptors are localized at cone photoreceptor terminals. This was confirmed by GABAC receptor immunolabeling of mouse and rat cone terminals. This distribution of GABA receptors would be consistent with cone photoreceptors receiving a GABA feedback at their terminals.
GABAA and GABAC receptors in cones
In the outer retina of nonmammalian vertebrates, the functional role of GABA feedback is still a matter of controversy (Werblin, 1991;Piccolino, 1995; Kamermans and Spekreijse, 1999). Classically, horizontal cells are considered to release GABA by a carrier-mediated mechanism (Schwartz, 1982, 1987; Yazulla and Kleinschmidt, 1983), the cone photoreceptors responding to transmitter at GABAA receptors as been demonstrated in turtle cones (Kaneko and Tachibana, 1986). This feedback could mediate the complex surround response in cone photoreceptors and thus contribute to enhancing contrast at the border of objects (Piccolino, 1995). However, producing the surround response has also been attributed to Ca2+ channel modulation, the GABA feedback would then adjust cone photoreceptor light sensitivity and responsiveness (Kamermans and Spekreijse, 1999).
In the mammalian retina, recording of GABAA and GABAC receptors in cultured porcine cone photoreceptors suggested the existence of a GABA feedback to cone photoreceptors (Picaud et al., 1998). The presence of GABAA receptors in mammalian cones was supported by immunocytochemical staining of cat retina (Hughes et al., 1991). In the case of GABAC receptors, their expression in mammalian cones was not consistent with previous in situ hybridization studies (Enz et al., 1996; Ogurusu et al., 1997) but could explain the immunostaining observed in the outer plexiform layer that was attributed to bipolar cells (Enz et al., 1995;Koulen et al., 1998). Our immunolabeling of rat retinal sections is in agreement with GABAC receptor localization in the cone terminals. Because the labeling was not restricted to cone terminals, the receptor might also be expressed in bipolar cell dendrites as previously proposed.
The picrotoxin sensitivity of cone GABAC receptor currents indicates these receptors may not contain ρ2 subunits that are picrotoxin-insensitive and can confer this picrotoxin resistance to the heteromeric channels in the mouse retina (Greka et al., 1998). The presence of MAP1B that specifically interacts with the ρ1 subunits (Hanley et al., 1999) suggests that ρ1 subunits are also present in cone GABAC receptors. These reported interactions does not necessarily mean, however, that MAP1B presence is required and sufficient for ρ1 subunit expression. The glycine transporter GLYT-1 was indeed found to similarly interact with ρ1 subunits (Hanley et al., 2000), although it does not colocalize with GABAC receptors in the retina (Pow and Hendrickson, 1999). The 20% pentobarbital-elicited increase in the GABAC receptor current may indicate that ρ1 subunits can form heteromeric channels with GABAAreceptor subunits, as reported by Qian and Ripps (1999). These results suggest that ρ1 subunits and not ρ2 subunits are included in the composition of cone GABAC receptors.
The localization of GABA receptors in mammalian cone photoreceptorsin situ is consistent with the notion that mammalian cone photoreceptors receive a GABA feedback. In contrast to nonmammalian vertebrate animals (Kaneko and Tachibana, 1986), this GABA feedback to cones may not be solely mediated by GABAAreceptors but by both GABAA and GABAC receptors. This combination of receptors could extend the range of sensitivity and allow differential modulation (Bormann, 2000). Furthermore, nondesensitizing GABAC receptors might be more adapted to process the graded signals generated in the outer retina. The sign of this feedback signal that depends on the equilibrium potential for Cl−(ECl) and its origin are still unclear. GABA could indeed be released by either horizontal cells that can be immunolabeled for GABA and glutamic acid decarboxylase (GAD) (Nishimura et al., 1985) or by interplexiform cells that can also be immunolabeled with GABA (Ryan and Hendrickson, 1987) or can take up radioactive GABA (Nakamura et al., 1980). Further studies will address this question on the respective functional contribution of GABAA and GABAC receptors to cone light responses.
Protein MAP1B and GABAC receptors
Protein MAP1B mutant homozygous mice were found to diein utero, whereas the heterozygous mice survived but with major loss in visual acuity caused by eye malformation. Histological examination of the retina revealed a disorganization of the inner and outer nuclear layers with a loss of demarcation at the external plexiform layer (Edelmann et al., 1996). Such an alteration of the outer nuclear and plexiform layers is consistent with our localization of MAP1B in photoreceptors and especially at cone terminals in the outer plexiform layer.
MAP1B is found in embryonic nervous tissue within developing axonal processes (Schoenfeld et al., 1989) where phosphorylated MAP1B seems to play an important role in neurite elongation by interfering with microtubule extension (Goold et al., 1999). In the adult tissue, although its expression normally regresses, it was recently proposed to link the ρ1 subunits of GABAC receptors to the cytoskeleton, as exemplified on rat retinal bipolar cells (Hanley et al., 1999). In the present study we localized MAP1B to cone photoreceptor terminals by double staining with a cone-specific lectin. The recording of GABAC receptors in cones and the immunolocalization of the receptors to cone terminals are in agreement with the notion that MAP1B links GABAC receptors to the cytoskeleton. This property would provide another fundamental distinction from GABAA receptors that were found to be linked to the cytoskeleton via gephyrin (Kneussel et al., 1999) or GABARAP (Wang et al., 1999).
Protein MAP1B was not restricted to cone terminals but was also detected in photoreceptor cell bodies and outer segments (Figs. 8, 10). Cone cell bodies were similarly labeled with the ρ subunit antibody in the porcine retina (Picaud et al., 1998) but not in the rat retina (Fig. 8F; Enz et al., 1996). This expression of MAP1B in photoreceptors is consistent with its isolation by a cloning strategy from a photoreceptor cDNA bank (D. Farber, personal communication). In rods, it seems unlikely that MAP1B links GABAC receptors because these receptors were not recorded from these cells, either in cultured porcine photoreceptors (Picaud et al., 1998) or in flat-mounted rd mouse retinas (Fig. 2D). Because MAP1B plays a major role in neurite elongation at embryonic stages (Goold et al., 1999), it may also contribute to the continuous elongation of photoreceptor outer segments in the adult. Interestingly, MAP1B was also found in another ciliated sensory neuron, the hair cell (Jaeger et al., 1994). In these cells, the protein was found at the base of stereocilia in the cuticular plate.
In all species studied so far, GABAC receptors have been found in bipolar cell axon terminals. It is therefore unlikely that they are not present in porcine bipolar cells, which appears in contradiction to the weak MAP1B immunolabeling in porcine inner plexiform layer (Fig. 10). MAP1B was however reported to only link to ρ1 subunits, but not to other ρ subunits, which can also form homomeric channels (Cutting et al., 1991; Wang et al., 1994; Zhang et al., 1995; Shingai et al., 1996; Greka et al., 1998). The absence of MAP1B staining in pig bipolar cells may therefore reveal an absence of ρ1 subunits in pig bipolar cells. Otherwise, GABAC receptors were reported to contribute a minor component of GABA responses in cone bipolar cells by contrast to rod bipolar cells (Euler and Wässle, 1998). Because pig vision is more cone-dominated than that of rats, this difference between rod and cone bipolar cells may partly explain the different MAP1B staining in the inner plexiform layer (Hanley et al., 1999). Moreover, porcine rod bipolar cells stained with protein kinase C antibody exhibit very fine axon terminals (data not shown), highly contrasting with large buttons of rat rod bipolar cells (Karschin and Wässle, 1990). Therefore, rod bipolar cell terminals may still be MAP1B-immunopositive in the inner plexiform layer, but their different morphology and lower density may explain the faint MAP1B immunostaining observed in the inner plexiform layer of the pig retina.
Conclusion
The preparation developed in this study should enable us to characterize further the pharmacology of mammalian photoreceptors. The demonstration of GABAA and GABAC receptors in mammalian cone photoreceptors already provides some new insight into the physiological and pathological functions of the cone pathway. It provides strong evidence that cone photoreceptors receive a GABA feedback in the mammalian retina. Analysis of GABAC receptor knock-out mice (Zheng et al., 1999) should help understand further the contribution of these receptors to cone physiology and, more generally, to their function in neuronal transmission. Our finding of these receptors in another retinal neuron suggest that these nondesensitizing receptors might be particularly adapted to process graded potential neurotransmission.
Footnotes
This work was supported by Institut National de la Santé et de la Recherche Médicale, Retina France, Association pour le Developpement de la Recherche sur la régénération de la rEtine et sa Transplantation (ADRET-Alsace), Fédération des Aveugles de France, Ministère de l'Education Nationale et de la Recherche, and Institut de Recherches Internationales Servier. B.P. received a fellowship from Retina France. We thank Anne Feltz, David Copenhagen, Alvaro Rendon, and David Hicks for critically reading this manuscript and Valérie Forster for expert technical assistance. The ρ subunit antibody was kindly provided by Ralf Enz and Heinz Wässle.
Correspondence should be addressed to Serge Picaud, Institut National de la Santé et de la Recherche Médicale EMI 99–18, Médicale A, BP426, 1 Place de l'Hôpital, 67091 Strasbourg Cedex, France. E-mail: picaud@neurochem.u-strasbg.fr.
REFERENCES
- 1.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 2.Bormann J. The “ABC” of GABA receptors. Trends Pharmacol Sci. 2000;21:16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- 3.Cutting GR, Lu L, O'Hara BF, Kasch LM, Montrose-Rafizadeh C, Donovan DM, Shimada S, Antonarakis SE, Guggino WB, Uhl GR, Kazazian HH. Cloning of the γ-aminobutyric acid (GABA) P1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc Natl Acad Sci USA. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong CJ, Picaud S, Werblin FS. GABA transporters and GABAC-like receptors on catfish cone- but not rod-driven horizontal cells. J Neurosci. 1994;14:2648–2658. doi: 10.1523/JNEUROSCI.14-05-02648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edelmann W, Zervas M, Costello P, Roback L, Fischer I, Hammarback JA, Cowan N, Davies P, Wainer B, Kucherlapati R. Neuronal abnormalities in microtubule-associated protein 1B mutant mice. Proc Natl Acad Sci USA. 1996;93:1270–1275. doi: 10.1073/pnas.93.3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enz R, Cutting GR. Molecular composition of GABAC receptors. Vision Res. 1998;38:1431–1441. doi: 10.1016/s0042-6989(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 7.Enz R, Brandstatter JH, Hartveit E, Wässle H, Bormann J. Expression of GABA receptor r-1 and r-2 subunits in the retina and brain of the rat. Eur J Neurosci. 1995;7:1495–1501. doi: 10.1111/j.1460-9568.1995.tb01144.x. [DOI] [PubMed] [Google Scholar]
- 8.Enz R, Brandstatter JH, Wässle H, Bormann J. Immunocytochemical localization of the GABAC receptor rho subunit in the mammalian retina. J Neurosci. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Euler T, Wässle H. Different contribution of GABAA and GABAC receptors to rod and cone bipolar cells in rat retinal slice preparation. J Neurophysiol. 1998;79:1384–1395. doi: 10.1152/jn.1998.79.3.1384. [DOI] [PubMed] [Google Scholar]
- 10.Feigenspan A, Wässle H, Bormann J. Pharmacology of GABA receptor Cl− channels in rat retinal bipolar cells. Nature. 1993;361:159–161. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- 11.Goold RG, Owen R, Gordon-Weeks PR. Glycogen synthase kinase 3 phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. J Cell Sci. 1999;112:3373–3384. doi: 10.1242/jcs.112.19.3373. [DOI] [PubMed] [Google Scholar]
- 12.Grant GB, Werblin FS. Low-cost data acquisition and analysis programs for electrophysiology. J Neurosci Methods. 1994;55:89–98. doi: 10.1016/0165-0270(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 13.Greka A, Koolen JA, Lipton SA, Zhang D. Cloning and characterization of mouse GABAC receptor subunits. NeuroReport. 1998;9:229–232. doi: 10.1097/00001756-199801260-00010. [DOI] [PubMed] [Google Scholar]
- 14.Hanley JG, Koulen P, Bedford F, Gordon-Weeks PR, Moss SJ. The protein MAP1B links GABAC receptors to the cytoskeleton at retinal synapses. Nature. 1999;397:66–69. doi: 10.1038/16258. [DOI] [PubMed] [Google Scholar]
- 15.Hanley JG, Jones EM, Moss SJ. GABA receptor rho1 subunit interacts with a novel splice variant of the glycine transporter, GLYT-1. J Biol Chem. 2000;275:840–846. doi: 10.1074/jbc.275.2.840. [DOI] [PubMed] [Google Scholar]
- 16.Hicks D, Molday RS. Differential immunogold-dextran labeling of bovine and frog rod and cone cells using monoclonal antibodies against bovine rhodopsin. Exp Eye Res. 1986;42:55–71. doi: 10.1016/0014-4835(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 17.Hughes TE, Grünert U, Karten HJ. GABAA receptors in the retina of the cat: an immunocytochemical study of wholemounts, sections, and dissociated cells. Vis Neurosci. 1991;6:229–238. doi: 10.1017/s0952523800006246. [DOI] [PubMed] [Google Scholar]
- 18.Jaeger RG, Fex J, Kachar B. Structural basis for mechanical transduction in the frog vestibular sensory apparatus: II. The role of microtubules in the organization of the cuticular plate. Hear Res. 1994;77:207–215. doi: 10.1016/0378-5955(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 19.Kamermans M, Spekreijse H. The feedback pathway from horizontal cells to cones. A mini review with a look ahead. Vision Res. 1999;39:2449–2468. doi: 10.1016/s0042-6989(99)00043-7. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko A, Tachibana M. Effects of γ-aminobutyric acid on isolated cone photoreceptors of the turtle retina. J Physiol (Lond) 1986;373:442–461. doi: 10.1113/jphysiol.1986.sp016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karschin A, Wässle H. Voltage and transmitter-gated currents in isolated bipolar cells of the rat retina. J Neurophysiol. 1990;63:860–876. doi: 10.1152/jn.1990.63.4.860. [DOI] [PubMed] [Google Scholar]
- 22.Kneussel M, Hermann A, Kirsch J, Betz H. Hydrophobic interactions mediate binding of the glycine receptor beta-subunit to gephyrin. J Neurochem. 1999;72:1323–1326. doi: 10.1046/j.1471-4159.1999.0721323.x. [DOI] [PubMed] [Google Scholar]
- 23.Koulen P, Brandstatter JH, Enz R, Wässle H. Synaptic clustering of GABAC receptor r-subunits in the rat retina. Eur J Neurosci. 1998;10:115–127. doi: 10.1046/j.1460-9568.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 24.Lukasiewicz PD, Maple BR, Werblin F. A novel GABA receptor on bipolar cell terminals in the tiger salamander retina. J Neurosci. 1994;14:1202–1212. doi: 10.1523/JNEUROSCI.14-03-01202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura Y, McGuire BA, Sterling P. Interplexiform cell in cat retina: identification by uptake of gamma-[3H]aminobutyric acid and serial reconstruction. Proc Natl Acad Sci USA. 1980;77:658–661. doi: 10.1073/pnas.77.1.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura Y, Schwartz ML, Rakic P. Localization of γ-aminobutyric acid and glutamic acid decarboxylase in rhesus monkey retina. Brain Res. 1985;359:351–355. doi: 10.1016/0006-8993(85)91449-0. [DOI] [PubMed] [Google Scholar]
- 27.Ogurusu T, Eguchi, Shinai R. Localization of γ-aminobutyric acid (GABA) receptor ρ3 subunit in rat retina. NeuroReport. 1997;8:925–927. doi: 10.1097/00001756-199703030-00022. [DOI] [PubMed] [Google Scholar]
- 28.Picaud S, Pattnaik B, Hicks D, Forster V, Fontaine V, Sahel J, Dreyfus H. GABAA and GABAC receptors in adult porcine cones: evidence from a photoreceptor/glia co-culture model. J Physiol (Lond) 1998;513:33–42. doi: 10.1111/j.1469-7793.1998.033by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccolino M. Cross-talk between cones and horizontal cells through the feedback circuit. In: Djamgoz MBA, Archer SN, Vallerga S, editors. Neurobiology and clinical aspects of the outer retina. Chapman and Hall; London: 1995. pp. 221–248. [Google Scholar]
- 30.Pow DV, Hendrickson AE. Distribution of the glycine transporter glyt-1 in mammalian and nonmammalian retinae. Vis Neurosci. 1999;16:231–239. doi: 10.1017/s0952523899162047. [DOI] [PubMed] [Google Scholar]
- 31.Qian H, Dowling JE. Novel GABA responses from rod driven retinal horizontal cells. Nature. 1993;361:162–164. doi: 10.1038/361162a0. [DOI] [PubMed] [Google Scholar]
- 32.Qian H, Ripps H. Response kinetics and pharmacological properties of heteromeric receptors formed by coassembly of GABA rho- and gamma 2-subunits. Proc R Soc Lond B Biol Sci. 1999;266:2419–2425. doi: 10.1098/rspb.1999.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragozzino D, Woodward RM, Murata Y, Eusobi F, Dverman LE, Miledi R. Design and in vitro pharmacology of a selective gamma-aminobutyric acid C receptor antagonist. Mol Pharmacol. 1996;50:1024–1030. [PubMed] [Google Scholar]
- 34.Ryan MK, Hendrickson AE. Interplexiform cells in macaque monkey retina. Exp Eye Res. 1987;45:57–66. doi: 10.1016/s0014-4835(87)80078-7. [DOI] [PubMed] [Google Scholar]
- 35.Schoenfeld TA, McKerracher L, Obar R, Vallee RB. MAP1A and MAP1B are structurally related microtubule associated proteins with distinct developmental patterns in the CNS. J Neurosci. 1989;9:1712–1730. doi: 10.1523/JNEUROSCI.09-05-01712.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz EA. Calcium-independent release of GABA from isolated horizontal cells of the toad retina. J Physiol (Lond) 1982;323:211–227. doi: 10.1113/jphysiol.1982.sp014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz EA. Depolarization without calcium can release γ-aminobutyric acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- 38.Shimada S, Cutting G, Uhl GR. Gamma-aminobutyric acid A or C receptor? Gamma-aminobutyric acid rho1 receptor RNA induces bicuculline-, barbiturate-, and benzodiazepine-insensitive gamma-aminobutyric acid response in Xenopus oocytes. Mol Pharmacol. 1992;41:683–687. [PubMed] [Google Scholar]
- 39.Shingai R, Yanagi K, Fukushima T, Sakata K, Ogurusu T. Functional expression of GABA ρ3 receptors in Xenopus oocytes. Neurosci Res. 1996;26:387–390. doi: 10.1016/s0168-0102(96)01114-5. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABAA-receptor-associated protein links GABAA receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- 41.Wang TL, Guggino WB, Cutting GR. A novel γ-aminobutyric acid receptor subunit (ρ2) cloned from human retina forms bicuculline-insensitive homooligomeric receptors in Xenopus oocytes. J Neurosci. 1994;14:6524–6531. doi: 10.1523/JNEUROSCI.14-11-06524.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Werblin FS. Synaptic connections, receptive fields, and patterns of activity in the tiger salamander retina. A simulation of patterns of activity formed at each cellular level from photoreceptors to ganglion cells. Invest Ophthalmol Vis Sci. 1991;32:459–483. [PubMed] [Google Scholar]
- 43.Yazulla S, Kleinschmidt J. Carrier-mediated release of GABA from retinal horizontal cells. Brain Res. 1983;263:63–75. doi: 10.1016/0006-8993(83)91201-5. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Slaughter MM. Preferential suppression of the ON pathway by GABAC receptors in the amphibian retina. J Neurophysiol. 1995;74:1583–1592. doi: 10.1152/jn.1995.74.4.1583. [DOI] [PubMed] [Google Scholar]
- 45.Zheng W, Zhang JH, Xu M, Lu L. Genomic cloning and genetic manipulations of γ-aminobutyric acid type C (GABAC) receptor rho-1 subunit: the molecular construction for rho-1 deficient mice. Invest Ophthalmol Vis Sci. 1999;40:1138. [Google Scholar]