Abstract
We studied the expression and distribution of Na/HCO3cotransporters in rat brain using polynucleotide probes and polyclonal antibodies derived from the electrogenic rat kidney Na/HCO3cotransporter (rkNBC). In whole brain, we observed a single mRNA (∼7.5 kb) by Northern hybridization and a major ∼130 kDa protein by immunoblotting with a polyclonal antiserum directed against the C terminus of rkNBC. NBC mRNA and protein were present in cortex, brainstem–diencephalon, and cerebellum. In situhybridization revealed NBC mRNA expression throughout the CNS, with particularly high levels in olfactory bulb, hippocampal dentate gyrus, and cerebellum. NBC mRNA was present in glial cells (e.g., Bergmann glia of cerebellum and hippocampal astrocytes) and neurons (e.g., granule cells of dentate gyrus and neurons of cortex or striatum). Double hybridization of mRNA encoding NBC and glutamate transporter 1 (glial marker) confirmed that both glia and neurons express NBC. Indirect immunofluorescence microscopy demonstrated NBC protein throughout the CNS, particularly in hippocampus and cerebellum. Although NBC mRNA was restricted to cell bodies, NBC protein was distributed diffusely, compatible with a localization in cell processes and perhaps cell bodies. Double labeling with glial fibrillary acidic protein (astrocytic marker), microtubule-associated protein 2 (neuronal marker), or 2′,3′-cyclic mononucleotide 3′-phosphodiesterase (oligodendrocytic marker) demonstrated expression of NBC protein in specific subpopulations of both glia and neurons. Moreover, NBC protein was present in both cultured hippocampal astrocytes and cortical neurons. NBC mRNA and protein were also present in epithelial cells of choroid plexus, ependyma, and meninges. Our results are thus consistent with multiple novel roles for Na/HCO3 cotransport in CNS physiology.
Keywords: NBC, astrocytes, Northern hybridization, in situ hybridization, immunoblotting, indirect immunofluorescence microscopy
Na/HCO3cotransporters (NBCs) move Na+ and HCO3− ions together across plasma membranes, depending on the isoform either as electrogenic cotransporters with Na+:HCO3− stoichiometries of 1:3 and 1:2 or as electroneutral cotransporters with a stoichiometry of 1:1 (Boron et al., 1997). Na/HCO3 cotransport occurs in a wide range of organisms and tissues (Boron and Boulpaep, 1989), including the CNS (Chesler, 1990).
In the CNS, Na/HCO3 cotransporters probably contribute to cellular acid–base homeostasis, and they are candidate players in any one of the numerous physiological and pathological processes with a known acid–base component. For example, acid–base transporters, possibly NBCs, control extracellular pH, contribute to the production of CSF, and participate in the regulation of cell volume and intracranial pressure. Acid–base transporters are also involved in the response to systemic acid–base disturbances (Rabary et al., 1994;Hoffman et al., 1995), as well as in apoptosis (Xu et al., 1998), ischemia, hypoxia, cell swelling, brain edema (Kempski et al., 1990,1991; Siesjö et al., 1990; Staub et al., 1994, 1996), and neoplasia (Okada et al., 1992). Furthermore, Na/HCO3 cotransport in the CNS may play a role in the feedback modulation of neuronal activity, long-term potentiation, and putative “acid signaling” (Chesler and Kaila, 1992; Ransom, 1992; Deitmer and Rose, 1996).
The consequences of Na/HCO3 cotransport depend on stoichiometry and the direction of Na+ and HCO3− transport but also strongly on the cellular and subcellular localization of the transporter. On the basis of functional data, the present consensus is that Na/HCO3cotransporters are exclusively glial transporters (Chesler, 1990;Bevensee and Boron, 1998). Because of the complexity of the CNS and the intrinsic difficulties of physiological studies of Na/HCO3 cotransporters, a comprehensive overview of the cellular and subcellular distribution of Na/HCO3 cotransporters in the CNS has been lacking. For example, the key parameters (e.g., intracellular and extracellular pH and membrane potential) not only change over time but also exhibit steep spatial gradients on a microscopic scale (Chesler and Kaila, 1992). Moreover, it is often difficult to distinguish experimentally between Na/HCO3 cotransporters and other Na+-coupled HCO3−transporters (e.g., Na+-driven Cl-HCO3 exchangers). The direct detection of Na/HCO3 cotransporter mRNA or protein, on the other hand, had been obviated by the lack of molecular probes.
In this study, we used polynucleotide probes and antibodies (Schmitt et al., 1999) derived from rat kidney NBC (Romero et al., 1998) to examine the distribution of Na/HCO3 cotransporter mRNA and protein in the CNS of adult rats by Northern blotting, in situ hybridization, immunoblotting, and immunofluorescence microscopy. We found that NBC is expressed widely throughout the CNS. NBC mRNA and protein are present in various types of glia. Interestingly, we also detected significant levels of NBC mRNA and protein in various types of neurons, as well as in the epithelial cells of the choroid plexus, ependyma, and meninges. Our findings suggest that Na/HCO3 cotransporters in these specialized cells have diverse, complex, and currently unknown roles in CNS physiology.
MATERIALS AND METHODS
Animals
All experiments were conducted on Sprague Dawley rats (Charles River, Wilmington, DE), either ∼33 d old (which are already fairly mature) or ∼105 d old. The rats were fed standard rat chow withad libitum access to tap water. The study was approved by the Yale Animal Care and Use Committee. To harvest brain tissues, rats were killed with pentobarbital or methoxyfluorane (Metofane; Pitman-Moore, Mundelein, IL). Subsequently, the skull was opened, and the whole brain was removed. For immunoblotting and Northern-blotting experiments, brains were placed in ice-cold homogenization buffer (in mm: 200 mannitol, 80 HEPES, 41 KOH, 0.1 pepstatin A, 0.001 leupeptin, 0.23 phenylmethylsulfonyl fluoride, and 1 Na-EDTA, pH 7.5) and dissected into cerebral cortex, brainstem–diencephalon, and cerebellum. The tissue of corresponding regions from three or four animals was pooled, weighed, and transferred into homogenization buffer (4 ml/gm of tissue) for preparation of microsome fractions, or into Trizol reagent for preparation of RNA. For immunofluorescence experiments, rats were anesthetized with methoxyfluorane and perfused intracardially with 0.1 m PBS, pH 7.4, followed by fixative (4% paraformaldehyde in 0.1 m sodium phosphate, pH 7.4). The brains were immediately removed from the cranium, immersion-fixed overnight at 4°C in the same fixative, and cryoprotected by incubation in 30% sucrose in PBS for 24–48 hr. Sections, either 5 or 30 μm thick, were cut on a Reichert cryostat, placed on slides coated with gelatin alum, allowed to dry, and stored at −20°C until used.
Northern blotting
Rat brain total RNA was prepared from either whole brain or from dissected cortex, brainstem–diencephalon, or cerebellum using the Trizol reagent (Life Technologies, Gaithersburg, MD) according to the manufacturer's protocol. From total RNA, we further purified poly(A+) RNA on an oligo-dT affinity matrix (Oligotex mRNA mini kit; Qiagen, Valencia, CA). Poly(A+) RNA (5 μg/lane) was separated electrophoretically on a formaldehyde–agarose gel, transferred onto a nylon membrane (Hybond N+; Amersham Pharmacia Biotech, Piscataway, NJ) by downward capillary blotting with 5× SSC, pH 8.8 (technical bulletin 169; Ambion, Austin, TX), cross-linked by UV light, stained (0.3% methylene blue in 300 mm sodium acetate, pH 5.2), and recorded as a photocopy. After destaining in distilled water, the membrane was prehybridized for 1 hr at 70°C (DIG Easy-Hyb; Boehringer Mannheim, Indianapolis, IN), followed by overnight incubation at 70°C with digoxigenin-labeled antisense RNA probe (∼40 ng/ml in DIG Easy-Hyb). The probe corresponded to the entire open reading frame of rat kidney NBC (rkNBC) (Romero et al., 1998) and was synthesized by in vitrotranscription from a pSV-SPORT1 vector using SP6 RNA polymerase (MAXIscript kit; Ambion). Subsequently, the membrane was washed at 70°C (twice for 15 min in 2× SSC and 1% SDS, twice for 15 min in 0.5× SSC and 0.1% SDS, and once for 30 min in 0.2× SSC and 0.1% SDS). This was followed by the immunodetection of the digoxigenin label using an anti-digoxigenin antibody conjugated to alkaline phosphatase (Boehringer Mannheim) and the chemiluminescent substrate CDP-Star (Boehringer Mannheim); chemiluminescence was recorded on x-ray film (X-OMAT AR; Eastman Kodak, Rochester, NY) for 1 hr.
RT-PCR
To obtain templates for RT-PCR analysis of the various brain regions, we synthesized first-strand cDNA from poly(A+) RNA, using oligo-dT primers and a commercial kit (SuperScript II; Life Technologies). The primer pair used for the PCR was originally designed to amplify the 3′-end of rkNBC [nucleotides (nt) 2784–3105]) to generate the immunogen for the anti-[maltose-binding protein (MBP)-NBC-5] sera (Schmitt et al., 1999). These primers also bind to the 3′ end of another NBC isoform that is present in rat brain (Bevensee et al., 2000) but yield a smaller PCR product because of a deletion of 97 bases after nt 2967. PCR products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining and UV transillumination.
Immunoblotting
Primary culture of neurons and astrocytes. Cortical neurons (Brewer et al., 1993) and hippocampal astrocytes (Bevensee et al., 1997a,b) were isolated from embryonic and neonatal rat brain, respectively, and cultured as described previously. Microsomes were prepared as described below after washing cells with homogenization buffer and harvesting them with a rubber policeman in 2 ml of homogenization buffer.
Membrane preparation. Crude microsomes were prepared from each of the three CNS regions or from primary cultures of cortical neurons and hippocampal astrocytes according to the method of Grassl and Aronson (1986). Briefly, tissues were homogenized by 10–20 strokes at 2000 rpm with a Teflon–glass homogenizer (Thomas Scientific, Swedesboro, NJ). The homogenate was then centrifuged at 1000 ×g for 10 min to remove cellular debris. The supernatant was recentrifuged at 100,000 × g in a Beckman (Palo Alto, CA) SW-40T rotor for 1 hr. The resulting microsomal pellet was resuspended in 200–1000 μl of homogenization buffer and stored at −80°C until used.
SDS-PAGE and immunoblotting. Protein concentrations of pooled microsome fractions were determined (DC protein assay; Bio-Rad, Hercules, CA), after which 40 μg of protein of each region was separated on 7.5–8% SDS-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Immobilon-P; Millipore, Bedford, MA). Membranes were blocked for 1–2 hr in BLOTTO, which consists of 5% (w/v) Carnation nonfat dry milk (Nestlé Food Company, Glendale, CA), and 0.1% Tween 20 in PBS (in gm/l: 8 NaCl, 1.44 Na2HPO4, 0.24 KH2PO4, and 0.2 KCl, pH 7.4). Membranes were then incubated overnight at 4°C with the primary antibody, rabbit polyclonal anti-(MBP-NBC-5) serum, diluted 1:400 in BLOTTO. Subsequently, the membranes were rinsed in BLOTTO (twice for 3 min, once for 15 min, and twice for 5 min) and incubated with secondary antibody (anti-rabbit IgG, whole molecule, conjugated to horseradish peroxidase; Zymed, South San Francisco, CA) for 1 hr at room temperature. Membranes were rinsed again in BLOTTO (twice for 3 min, once for 15 min, and twice for 5 min), and bound secondary antibody was visualized by chemiluminescence (ECL; Amersham Pharmacia Biotech). Generation and characterization of the polyclonal NBC antisera used in this study for immunoblotting and immunohistochemistry have been described previously (Schmitt et al., 1999).
In situ hybridization
NBC probes. Digoxigenin-labeled antisense and sense cRNA was synthesized using a Genius kit (Boehringer Mannheim) from PCR fragments that were flanked by promoter sites for SP6 and T7 polymerase. One probe comprised nucleotides 143–2113 of rkNBC, and a second probe comprised nucleotides 2234–3495. The long transcripts were alkali-hydrolyzed to an average length of 200–400 nucleotides. To confirm the specificity of these NBC probes, which were derived from long transcripts that contain stretches with sequence similarity to the anion exchangers (AEs), we also used as probes two shorter transcripts, 180 and 330 nucleotides in length. These two shorter probes correspond exactly to the nucleotides encoding the portions of rkNBC contained in the fusion proteins used to generate the polyclonal NBC antisera (Schmitt et al., 1999); they have no sequence similarity to any of the AEs.
Hybridization. In situ hybridization was performed on 10-μm-thick cryosections of fresh-frozen tissue as described (Schaeren-Wiemers and Gerfin-Moser, 1993). Briefly, sections were hybridized at 68°C for 18 hr in slide mailers containing cRNA probes diluted to ∼200 ng/ml in hybridization buffer [50% formamide, 5× SSC, 2% blocking reagent (Boehringer Mannheim), 0.02% SDS, and 0.1% N-laurylsarcosine]. Sections were then washed three times at 68°C in 2× SSC and twice for 30 min in 0.2× SSC. Hybridized probes were detected with anti-digoxigenin Fab fragments (Boehringer Mannheim) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP)-nitroblue tetrazolium (NBT) substrate (Schaeren-Wiemers and Gerfin-Moser, 1993) for 20 hr, rinsed (10 mm Tris and 1 mm EDTA, pH 8.0), and covered with Vectashield (Vector Laboratories, Burlingame, CA).
Co-localization of GLT-1 mRNA. For the co-localization studies, sections were, after completion of the NBC mRNA detection protocol, co-hybridized with an FITC-labeled RNA probe for glial glutamate transporter 1 (GLT-1). The GLT-1 probe was subsequently detected using (in sequence) mouse anti-FITC antibodies, biotinylated anti-mouse IgG, streptavidin-HRP, biotinylated tyramide signal amplification (New England Nuclear, Boston, MA), and streptavidin-CY3, as described previously (Berger and Hediger, 1998).
Indirect immunofluorescence microscopy
The localization of the NBC protein within the CNS was assessed by immunofluorescence staining. We found that pretreating the tissue sections with SDS (Brown et al., 1996) greatly enhanced the detection of NBC in the CNS, especially in the cerebellum. In short, after rehydrating the tissue sections in Tris-buffered saline (TBS) for 5 min, we incubated the sections with 1% SDS in TBS for 5 min and then washed them thrre to five times for 5 min in TBS. Nonspecific binding sites were blocked for 30 min in blocking buffer, which consisted of 2% bovine serum albumin, 10% normal goat serum, and 0.3% Triton X-100 (TX100) in TBS. Subsequently we incubated the sections overnight at 4°C with rabbit anti-(MBP-NBC-5) serum, diluted 1:25 in blocking buffer. After rinsing three times for 5 min in TBS and 0.3% TX100, we incubated the sections for 1 hr at room temperature with a 1:100 dilution of anti-rabbit IgG (whole molecule, conjugated to TRITC; Vector Laboratories).
Double labeling with GFAP, CNPase, or MAP2. In addition to NBC, we stained some sections simultaneously with a monoclonal antibody directed against the astrocytic marker glial fibrillary acidic protein (GFAP, monoclonal mouse anti-GFAP; Chemicon, Temecula, CA; diluted 1:500). In other experiments, we double-labeled some sections for the oligodendrocyte and Schwann cell marker protein 2′,3′-cyclic mononucleotide 3′-phosphodiesterase (CNPase, monoclonal anti-CNPase; Sigma, St. Louis, MO; diluted 1:500). Finally, we double-labeled some sections for the neuronal marker microtubule-associated protein (MAP2, monoclonal mouse anti-rat brain-MAP2; Sigma; diluted 1:100). Binding of these mouse monoclonal antibodies was detected with a secondary antibody conjugated to FITC (anti-mouse IgG, heavy and light chain; Vector Laboratories; diluted 1:100).
Detection of fluorescence label. We then rinsed the sections twice for 10 min in TBS and 0.3% TX100, then once for 10 min in TBS alone. Finally, we mounted the sections in an aqueous medium (Vectashield) and assessed the fluorescence labeling on a confocal microscope (600 MRC; Bio-Rad). FITC fluorescence was excited at 488 nm and detected at 522 nm. TRITC fluorescence was excited at 568 nm and detected at 598 nm.
Specificity controls. The following controls were used to assess the specificity of the immunostaining: (1) omitting the primary antibody, (2) blocking the primary antibody by preincubation with the fusion protein that had been used as an immunogen for the generation of the antiserum, and (3) using preimmune serum from the same animal from which we had obtained the primary antibody.
RESULTS
Northern blotting
In Northern blots of mRNA isolated from the whole brain of adult rats (Fig. 1A), NBC-specific polynucleotide probes hybridized to a single mRNA of 7.0–7.5 kb, confirming previously reported results (Romero et al., 1998). In addition, we found this mRNA expressed individually in cortex, brainstem–diencephalon, and cerebellum. The hybridization signal was relatively strong in whole brain, brainstem–diencephalon, and cerebellum but weak in cerebral cortex. Digoxigenin-labeled cRNA probes (Fig. 1A) and a32P-labeled DNA probe corresponding to the entire open reading frame of rkNBC (data not shown) yielded identical results.
Fig. 1.
Expression of NBC in rat brain. A, Northern blot. Poly(A+) RNA from whole brain (WB), cortex (CX), brainstem–diencephalon (BD), and cerebellum (CB) of adult Sprague Dawley rats was hybridized with a digoxigenin-labeled antisense RNA probe corresponding to the entire open reading frame of rkNBC. A 7.5 kb mRNA band could be detected in all lanes. B, RT-PCR. cDNA from whole brain, cortex, cerebellum, and subcortical regions was used as a template in PCR reactions with oligonucleotide primers derived from the 3′ end of rkNBC (sense, nt 2784–3009; antisense, nt 3083–3105). The expected ∼340-bp product was detected in all areas. The ∼250 bp product is attributable to the presence of a brain-specific NBC splice variant with a 97 bp deletion (Bevensee et al., 2000). C, Immunoblot. Proteins from rat brain cortex, subcortical regions, and cerebellum were immunoblotted with a polyclonal antiserum raised in rabbits against the last 108 amino acids of rkNBC fused with MBP. A ∼130 kDa band was detected in all brain regions. The specificity of this labeling was confirmed by preabsorption experiments using MBP fusion proteins with and without the NBC portions present in the fusion protein used for immunization (data not shown).
RT-PCR
RT-PCR with a pair of primers targeting the 3′ end of the rkNBC open-reading frame yielded the expected ∼340 bp product in whole brain as well as in cortex, brainstem, diencephalon, and cerebellum (Fig. 1B). In all preparations, we also saw a ∼250 bp product characteristic of a brain-specific NBC isoform with a 97 bp deletion inside the region spanned by these primers (Bevensee et al., 2000).
Immunoblotting
General
Rabbit anti-(MBP-NBC-5) serum strongly labeled a ∼130 kDa protein from whole rat brain microsomes (data not shown) as well as cortex, brainstem–diencephalon, and cerebellum (Fig. 1C). This band was indistinguishable from the major band detected in kidney microsomes run in parallel on the same gels (data not shown). Scanning densitometry of the 130 kDa bands on the chemiluminograms showed that expression of this protein was highest in the cerebellum; the expression level in the cerebellum was approximately twofold greater than in brainstem–diencephalon and approximately eightfold greater than in the cortex. In addition to the ∼130 kDa band, we observed a smaller band at ∼90 kDa. This observation contrasts with the immunoblot profile of the NBC protein in the rat kidney (Schmitt et al., 1999): there, the major band of ∼130 kDa is accompanied by two weaker bands of ∼100 and ∼85 kDa. On the other hand, the 90 kDa band that we consistently detected in all rat brain microsome preparations was absent from kidney microsomes. It is not clear whether these smaller bands in CNS and kidney correspond to NBC isoforms or proteolytic fragments.
Specificity controls
Two types of control experiment confirmed that the labeling of both the 130 and 90 kDa bands was specific: (1) both bands were absent when we probed with preimmune serum or when we omitted the primary antibody altogether; and (2) both bands were absent when we probed with anti-(MBP-NBC-5) serum that was preabsorbed with the MBP-NBC-5 fusion protein (i.e., with the immunogen used to generate these antibodies). Preabsorption with an MBP fusion protein containing a different part of the rkNBC protein (i.e., MBP-NBC-3; Schmitt et al., 1999) had no effect on the labeling observed with anti-(MBP-NBC-5) serum.
Cultured rat neurons and astrocytes
Immunoblotting with rabbit anti-(MBP-NBC-5) serum showed that both primary cultured cortical neurons and primary cultured hippocampal astrocytes express a ∼130 kDa protein (Fig.2), similar to the one observed in cortex, brainstem–diencephalon, and cerebellum. This protein appeared to be equally abundant in both cell types.
Fig. 2.
Expression of NBC protein in primary cultured neurons and astrocytes. Immunoblots with anti-(MBP-NBC-5) serum on microsomal proteins from rat cortical neurons (N) and hippocampal astrocytes (A) harvested after 6 d of primary culture are shown. A major ∼130 kDa protein is present in both cell types at comparable abundance. Cross-contamination of neurons with glial cells (and vice versa) is typically <3% (data not shown).
In situ hybridization
General
In situ hybridization with two different RNA probes derived from long transcripts (bp 143–2113 and bp 2234–3495) consistently demonstrated significant levels of NBC mRNA throughout the CNS (Figs. 3-5), with certain cell groups exhibiting particularly strong signals. Corresponding sense probes did not yield any labeling (e.g., Fig. 3c). As shown in the low-magnification view of a parasagittal section through rat brain (Fig. 3a), the NBC mRNA labeling was distributed very heterogeneously within the brain in a unique pattern that has not been observed previously with other transporters (Berger and Hediger, 1998;Berger et al., 1998). Both the overview in Figure 3a and individual high-magnification views show strong labeling in, among others areas, the dentate gyrus of the hippocampus (Fig.3b), olfactory bulb (Fig. 3d), piriform cortex (Fig. 3e), striatum (Fig. 3f), superior colliculus (Fig. 3g), and cerebellum (Fig.4c,d). Relatively weak labeling was present in the cerebral cortex (Fig. 4a) and spinal cord (Fig. 4b). Labeling of intermediate intensity was found in midbrain and brainstem regions.
Fig. 3.
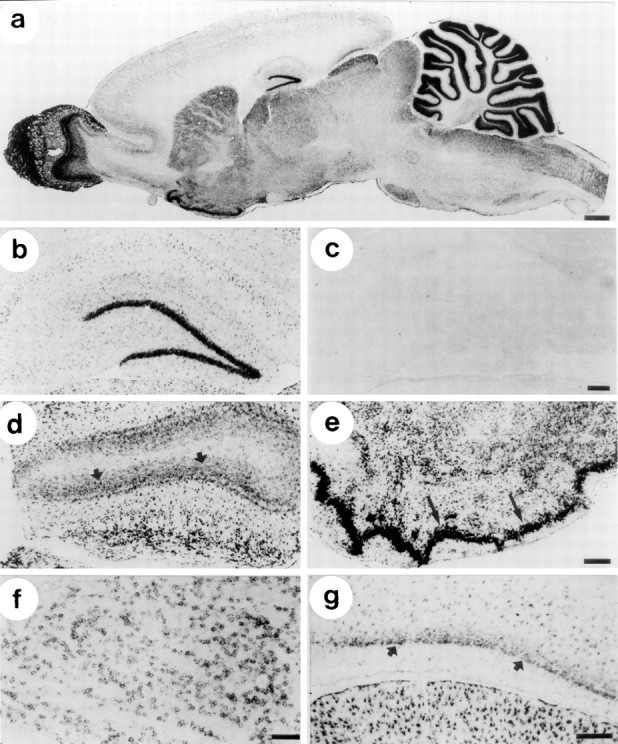
Localization of NBC mRNA in rat brain I: in situ hybridization of cryosections with digoxigenin-labeled NBC antisense RNA and colorimetric detection with BCIP-NBT.a, Parasagittal section, overview. The signals show a unique distribution of NBC mRNA with high levels in olfactory bulb, striatum and piriform cortex, and cerebellum. b, Hippocampus. NBC mRNA is present in dentate gyrus granule cells and in astrocytes. c, Hippocampus, sense probe. No labeling is visible. d, Olfactory bulb. NBC mRNA is present in astrocytes and also in neurons in the granule cell layer (arrows). e, Piriform cortex. NBC mRNA is strongly expressed in pyramidal cells (arrows). The other labeled cells in this region are astrocytes. f, Striatum. NBC mRNA is expressed in neurons. g, Entorrhinal cortex. NBC mRNA is expressed by neurons in layer II (arrows) and by astrocytes throughout all layers. In the superior colliculus (bottom), NBC mRNA is strongly expressed by astrocytes, Scale bars: a–c, 250 μm;d, e, 300 μm; f, g, 100 μm.
Fig. 4.

Localization of NBC mRNA in rat brain II:in situ hybridization of cryosections with digoxigenin-labeled antisense RNA and colorimetric detection with BCIP-NBT. a, Cerebral cortex. NBC mRNA is relatively weakly expressed in cells scattered throughout all layers; most of these cells appear as astrocytes. b, Spinal cord. NBC mRNA is relatively strongly expressed by astrocytes in both gray and white matter. c, d, Cerebellum. NBC is strongly expressed by the Bergmann glia (d, arrows) and more moderately in astrocytes within the granule cell layer. Relatively weak labeling is present in the granule cells (c, asterisks), suggesting NBC RNA expression in these cerebellar neurons.e, Choroid plexus. NBC mRNA is expressed moderately in epithelial cells (arrow). f, Meninges. NBC mRNA is detectable in the outermost meningeal layer (arrow). Scale bars: a, b, 200 μm;c, 300 μm; d–f, 100 μm.
Fig. 5.

Localization of NBC mRNA in rat brain III: co-localization of mRNA for NBC and GLT-1, an astrocytic marker protein. a, d, g, j, NBC mRNA, colorimetric detection, transmitted light. b, e, h, k, Co-localization of NBC mRNA and GLT-1 mRNA. The color label of NBC mRNA appearsblack (transmitted light), and GLT1 mRNA fluorescence appears white (epifluorescence). c, f, i, l, GLT1 mRNA, fluorescence detection. Sets of twoarrows point to identical cells in each of the three rows. The mRNA for GLT-1, a glutamate uptake system, is used here to label astrocytes. a–c, Layer II of cerebral cortex. NBC mRNA is weakly expressed by cells that are also positive for GLT1 mRNA (large arrows), indicating their astrocytic nature. Ina, three neurons are indicated that express NBC mRNA (small arrows). d–f, Brainstem. NBC mRNA-positive cells express higher levels of NBC mRNA than in cortex (a), and they all also express GLT1, indicating their astrocytic nature. g–i, Striatum. NBC is relatively strongly expressed by neurons (small arrows). Astrocytes in this region express very little NBC mRNA (large arrows). j–l, Dentate gyrus. NBC is strongly expressed by granule cells (i.e., neurons on left) and also by weakly stained cells in the molecular layer. These latter cells are astrocytes, because they also express GLT1 mRNA. Scale bar (shown in a), 40 μm.
Two additional, highly specific short probes, which corresponded exactly to the amino acid sequences of rkNBC used to raise the antibodies used in this study, yielded a hybridization pattern identical to the one observed with the longer probes, albeit with a weaker signal (data not shown). The similarity of the staining patterns with long and two short probes shows that the observed labeling specifically corresponds to NBC mRNA and rules out cross-hybridization of the longer probes with mRNAs of the AE anion exchanger family. In addition, the hybridization with the short probe corresponding to amino acids 928–1035 of rkNBC, the C terminus that is present only in NBC isoforms that are electrogenic, confirms the results of the RT-PCR experiments (Fig. 1B). Thus, we consistently detected these epitopes on the mRNA level by RT-PCR and in situhybridization as well as on the protein level by immunoblotting using the anti-(MBP-NBC-5) serum (Fig. 1C).
Assignment to neurons and astrocytes
At higher magnification it became apparent that astrocytes as well as neurons express NBC mRNA. Assignment of NBC labeling to glial cells was possible in some cases because of the location of the positive cells (e.g., optic nerve or white matter of spinal cord) or some characteristic morphological feature (e.g., the typical star-shaped astrocytes in ventral forebrain and brainstem; Berger and Hediger, 1998). In other cases, we identified astrocytes by co-localizing the mRNA for the astrocytic glutamate transporter GLT-1 simultaneously with the NBC mRNA, as demonstrated for the cerebral cortex (Fig.5a–c), brainstem (Fig.5d–f), and dentate gyrus of the hippocampus (Fig.5j–l). Neurons, on the other hand, we recognized by their unique distribution within a particular region (e.g., in the granular layer of the dentate gyrus; Fig. 3b) or by the absence of GLT-1 mRNA staining, as for cerebral cortex (Fig.5a–c), striatum (Fig. 5g–i), and dentate gyrus (Fig. 5j–l).
Expression of NBC mRNA in astrocytes
We found astrocytes expressing NBC mRNA in all areas of the rat brain that we examined. Astrocytes in cerebral cortex (Figs.4a, 5a–c) and hippocampus were stained less intensely than astrocytes in the ventral forebrain, midbrain (e.g., superior colliculus; Fig. 3a), brainstem (Fig.5d–f), or spinal cord (Fig. 4b). The olfactory bulb (Fig. 3a) also showed a higher intensity of labeling of astrocytes than cerebral cortex. The strongest labeling for NBC mRNA was seen in the Bergmann glial cells of the cerebellum, which were recognizable by the characteristic pattern of their processes radiating into the molecular layer (Figs. 3a,4c,d). In general, astrocytes in gray matter areas were more intensely labeled than astrocytes in white matter.
Expression of NBC mRNA in neurons
Several populations of neurons expressed NBC mRNA, including the granule cells in the dentate gyrus (Fig. 3b,c), the granule cells in olfactory bulb (Fig. 3d), the pyramidal cells in piriform cortex (Fig. 3e), striatal neurons (Fig.3f), and layer II/III neurons in the entorrhinal cortex (Fig. 3g). The granule cells in the cerebellum also appeared to stain for NBC mRNA (Fig. 4c,d), but background labeling in that area was high.
Double in situ hybridization of sections from the cerebral cortex with NBC and GLT-1 probes (Fig. 5a–c) revealed scattered neurons as well as numerous astrocytes that stained positively for NBC mRNA. Similarly, expression of NBC in the striatum (Fig. 3f) was detectable in both neurons and, more weakly, in astrocytes (Fig. 5g–i). Several neuron populations did not express detectable amounts of NBC mRNA, including neurons in the thalamus, hypothalamus, midbrain, brainstem, and spinal cord (data not shown).
Other cell types
Apart from neurons and astrocytes, we also detected moderate levels of NBC mRNA in the epithelial cells of the choroid plexus (Fig.4e) and relatively high levels in cells of the meningeal layers surrounding the brain (Fig. 4f). Except for moderate levels of NBC mRNA in the cells lining the third ventricle, ependymal cells generally showed little labeling. Similarly, NBC mRNA expression was below the detection limit in subependymal cells of the rostral forebrain or in other glial cells, such as oligodendrocytes or microglia.
Indirect immunofluorescence microscopy
Overview
Confocal microscopy on coronal cryostat sections, 5–20 μm thick, stained with the anti-(MBP-NBC-5) serum consistently revealed a diffuse, heterogeneous staining pattern throughout the neuraxis (data not shown). The most intense staining was apparent in the olfactory bulb (data not shown) and the cerebellum (Figs.6A,7A). Other regions demonstrated a light and diffuse immunostaining for NBC, including the thalamus, hypothalamus and basal ganglia, brainstem, and spinal cord. We also observed light staining for NBC in the cerebral cortex, mainly in the medial and basolateral cortex. Because of the robust NBCin situ hybridization signal noted in the cerebellum and hippocampus, we focused on these two structures in our subsequent double-labeling experiments.
Fig. 6.

Co-localization of NBC and GFAP in hippocampus and cerebellum. Indirect double-label immunofluorescence microscopy was performed on 5 μm cryosections of paraformaldehyde-fixed adult rat brain; confocal image. A–C, Cerebellum;D–F, hippocampus. NBC labeling was visualized by TRITC-conjugated secondary antibody (red fluorescence inA, C, D, F); GFAP labeling was visualized by FITC-conjugated secondary antibody (greenfluorescence in B, C, E, F); and overlay of NBC and GFAP signals is shown in C and F. NBC labeling is distributed diffusely in cerebellum (A) and hippocampus (D). Cell bodies of pyramidal neurons do not show NBC labeling (D). GFAP labeling reveals characteristically star-shaped astrocytes scattered throughout the cerebellum (B), including the fiber tracts (f), granule cell layer (g), and molecular layer (m), and within the hippocampus (E). Co-localization of NBC and GFAP immunoreactivity (yellow), indicative of co-expression in astrocytes, is observed within the cerebellum (C) in the fiber tract but also in the granule cell layer. In the hippocampus (F), only little of the NBC label co-localizes with GFAP, suggesting that NBC is expressed mainly in nonastrocytic cells, presumably neurons (see Fig.7).
Cerebellum and hippocampus
Indirect immunofluorescence microscopy performed with the anti-(MBP-NBC-5) serum on coronal sections of 5–12 μm thickness consistently yielded a diffuse distribution of the label throughout the cerebellum and hippocampus. Preabsorbing the antiserum with the MBP-NBC-5 fusion protein reduced this labeling to background levels, indicating the specificity of the diffuse signal (data not shown). The intensity of this labeling was highest in the cerebellum (Figs.6A, 7A). Here, the NBC staining was diffuse throughout the molecular layer, fibrous or membranal in the granule cell layer, and relatively light in the white matter tracts of the cerebellum. We also observed NBC staining throughout the hippocampal formation (Figs. 6D,7D). Both in cerebellum and hippocampus, some large cells were conspicuous by the absence of NBC labeling from the soma, including Purkinje cells of the cerebellum (Figs. 6A,7A), cells within the dentate gyrus of the hippocampus (Figs. 6D, 7D), and pyramidal neurons of the hippocampus.
Fig. 7.

Co-localization of NBC and MAP2 in hippocampus and cerebellum. Indirect double-label immunofluorescence microscopy is shown as in Figure 6. A–C, Cerebellum;D–F, hippocampus. NBC labeling was visualized by TRITC-conjugated secondary antibody (red fluorescence inA, C, D, F); MAP2 labeling was visualized by FITC-conjugated secondary antibody (greenfluorescence in B, C, E, F); and overlay of NBC and MAP2 signals is shown in C and F. Diffuse distribution of NBC label is seen in cerebellum (A) and hippocampus (D), similar to Figure 6. Diffuse labeling for MAP2, a marker for dendritic processes of neurons, is apparent in all layers of the cerebellum (B) except for the fiber tract and throughout the hippocampus (E). Overlay of NBC and MAP2 immunofluorescence (C, F) suggests the presence of NBC in neuronal processes within the molecular and granule cell layers of the cerebellum (C) and extensive neuronal expression of NBC throughout the hippocampus.
To determine whether the diffuse staining for NBC in the CNS corresponds to expression of NBC protein in glia and/or neurons, we performed double-labeling experiments of NBC together with marker proteins for astrocytes, oligodendrocytes, or neurons.
Double labeling of NBC and GFAP (Fig. 6)
GFAP staining revealed cells with the characteristic star shape of astrocytes scattered sparsely throughout the cerebrum (data not shown). Within the cerebellum (Fig. 6B), we observed GFAP-positive cells in the molecular and granular layers, as well as in the white matter tracts. Astrocytic processes extended upward from the Purkinje cell layer, interdigitating within the molecular layer (Fig.6B). An overlay of NBC and GFAP fluorescence (Fig.6C) shows co-localization of NBC and GFAP (yellow) mostly in the fiber tract but also within the granule cell layer and in processes radiating into the molecular layer. The somata of the Purkinje cells exhibited neither NBC nor GFAP labeling.
In the hippocampus (Fig. 6E), astrocytes were scattered throughout the molecular layer. An overlay of NBC and GFAP fluorescence (Fig. 6F) suggests co-localization of NBC and GFAP in some astrocytes throughout the hippocampal formation, within the fibrous glial processes at the boundary between hippocampus and thalamus, and in some astrocytes close to the pyramidal cell layer. However, the major part of the NBC labeling was present in GFAP-negative cells, probably neurons.
Together, these findings from cerebellum and hippocampus demonstrate that the NBC protein is expressed in several populations of glial cells, in keeping with the findings from our immunoblotting andin situ hybridization experiments (Figs. 4, 5). The diffuse appearance of the immunolabeling at the light microscopic level suggests that the NBC protein is mainly localized on the cell processes rather than in the cell bodies.
Double labeling of NBC and CNPase (data not shown)
Labeling with CNPase (an oligodendrocytic marker) was abundantly present in the fiber tracts systems of the CNS, particularly in the corpus callosum and the cerebellum. A fibrous or membranal staining pattern for CNPase was visible in the granule cell layer of the cerebellum and throughout the cerebral cortex, hippocampus, and subcortical structures, including the thalamus and basal ganglia. Overlay of NBC and CNPase staining did not reveal any co-localization of CNPase and NBC in the structures studied.
Double labeling of NBC and MAP2 (Fig. 7)
In the cerebellum, we observed MAP2 staining in the molecular and granule cell layers but, as expected, not in the fiber tract. An overlay of NBC and MAP2 fluorescence shows that some, but not all, NBC labeling in the molecular and granular layers co-localizes with the neuronal marker MAP2 (Fig. 7C).
In the hippocampus, we found MAP2 staining throughout the entire formation, including the molecular and pyramidal cell layers, the stratum oriens, and the dentate gyrus (Fig. 7E). An overlay of NBC and MAP2 fluorescence (Fig. 7F) reveals extensive co-localization of NBC and MAP2. This observation suggests widespread expression of NBC by hippocampal neurons, in keeping with our previous finding that most of the NBC protein in the hippocampus is localized in GFAP-negative cells (Fig. 6F).
DISCUSSION
The present study is the first systematic survey of the expression and localization of Na/HCO3 cotransporters in the CNS. Our findings show that some glial cells express NBC, in keeping with previous functional studies. Surprisingly, we also observed NBC expression in many types of neurons, including granule cells of hippocampus and piriform cortex, and neurons within the olfactory bulb. Double labeling of NBC together with neuronal and glial markers, as well as immunoblot detection of NBC in cultured neurons and astrocytes, confirmed that neurons as well as glial cells express NBC. The mRNA localizes to cell bodies, reflecting the site of mRNA and protein synthesis. The NBC protein, on the other hand, appears to be present mainly on processes of both glia and neurons, as evidenced by the absence of NBC staining in cell bodies and by the diffuse distribution of the NBC immunoreactivity throughout the CNS. In addition to glia and neurons, NBC mRNA is also present in the epithelial cells of the choroid plexus, ependyma, and meninges.
Specificity of polynucleotide probes and antibodies
Nucleotide probes, PCR primers, and the anti-(MBP-NBC-5) serum used in this study were based on cDNA sequence of the rat kidney Na/HCO3 cotransporter (Romero et al., 1998). We previously demonstrated the epitope specificity of the antiserum used in this study, ruling out cross-reactivity with known related proteins, such as members of the AE family (Schmitt et al., 1999). The mammalian Na+-driven Cl-HCO3exchanger, which is present in hippocampal pyramidal cells (Schwiening and Boron, 1994), has yet to be cloned. However, anti-(MBP-NBC-5) does not appear to recognize the Na+-driven Cl-HCO3 exchanger in renal mesangial cells (Boyarsky et al., 1988), either by immunocytochemistry (Schmitt et al., 1999) or by immunoblot of proteins isolated from cultured rat mesangial cells (B. M. Schmitt and B. A. Davis, unpublished results). Furthermore, the C terminus of the recently clonedDrosophila Na+-driven Cl-anion exchanger (Romero et al., 2000) is so distantly related to NBC that we would not expect immunocrossreactivity.
For our in situ hybridization experiments, we established the specificity of our cRNA probes with sense controls and very specific short probes. Furthermore, we found in our Northern and immunoblotting experiments that the apparent molecular masses for the rat brain NBC mRNA and protein are identical to those of the rat kidney NBC mRNA and protein, respectively.
These types of specificity control experiments make the contribution of other known proteins unlikely, suggesting that the signals represent true NBC reactivity. We are aware by now, however, of multiple NBC-related clones. Some of these clones are so closely related to rkNBC that they react equally well with our probes and also cannot be distinguished, within a given species, by the size of their mRNAs or proteins (Burnham et al., 1997; Abuladze et al., 1998; Romero et al., 1998; Choi et al., 1999; Bevensee et al., 2000). In this study, our antisera readily reacted with the astrocytic Na/HCO3 cotransporter, which is electrogenic and probably has a stoichiometry of 1:2 (Bevensee et al., 1997b).
Although our probes thus recognize rkNBC and the other known electrogenic Na/HCO3 cotransporter isoforms, we would not expect them to react with the electroneutral Na/HCO3 cotransporter recently cloned from rat aorta (Choi et al., 2000). The probes do not react either with another NBC isoform of unknown function that has been cloned from NT2 cells and is highly expressed in the retina (Ishibashi et al., 1998; B. M. Schmitt, unpublished observation). It appears reasonable to regard our cRNAs and antisera as specific probes for electrogenic Na/HCO3 cotransporters.
NBC in glia
Investigators working on invertebrates and amphibians were the first to obtain functional evidence for electrogenic Na/HCO3 cotransport in glia (Astion and Orkand, 1988; Deitmer and Schlue, 1989; Newman and Astion, 1991). Subsequently, others detected Na/HCO3 cotransport in the mammalian CNS, including rat forebrain astrocytes (Boyarsky et al., 1993), cerebellar astrocytes (Brune et al., 1994), and hippocampal astrocytes (Bevensee et al., 1997a,b). In cerebellar oligodendrocytes, two groups have detected Na/HCO3 cotransport, reported to be electroneutral in mouse (Kettenmann and Schlue, 1988) but electrogenic in rat (Boussouf et al., 1997). The expression of an NBC in various glial cell populations was recently confirmed on the mRNA level by Giffard et al. (2000).
In the present study, we detected NBC expression in astrocytes from all regions of the CNS. Some astrocytes, however, did not express detectable NBC mRNA. Moreover, gray matter astrocytes seemed to express higher levels of NBC mRNA than did white matter astrocytes. This heterogeneity may be associated with different functional specialization of these subpopulations and may coincide with known distinction into fibrous and protoplasmic astrocytes. We saw very high expression of NBC in the Bergmann glia of the cerebellum, which is located in close proximity to neurons of the Purkinje cell layer.
Double labeling for NBC and the oligodendrocytic marker protein CNP provided no evidence for significant NBC expression in cerebellar oligodendrocytes, in contrast to the two aforementioned functional studies on mouse and rat cerebellar oligodendrocytes (Kettenmann and Schlue, 1988; Boussouf et al., 1997). Determining whether NBC is present in microglia would require a technical approach different from the one used in the present study (e.g., immunoelectron microscopy).
NBC in neurons
Functional studies on neurons have demonstrated the presence of several acid–base transporters, including Na-H exchangers, H+ pumps, Na+-driven Cl-HCO3exchangers, Na+-independent Cl-HCO3 exchangers and Ca-H pumps (for overview, see Bevensee and Boron, 1998). However, there is no functional evidence for a neuronal Na/HCO3 cotransporter.
Against this background, we were surprised to find significant and widespread expression of NBC mRNA and protein in neurons. Four results support this conclusion: (1) strong expression of NBC mRNA is evident in several neuronal populations that are easily identifiable anatomically; these include cortical neurons, granule cells of hippocampal dentate gyrus, and granule cells in the piriform cortex; (2) we frequently find NBC mRNA in cells with a neuronal morphology and that do not label with the mRNA for the astrocytic marker GLT-1; (3) NBC protein co-localizes in some cells with the neuronal marker protein MAP2; and (4) cultured cortical neurons express NBC protein. Because the apparent expression levels were similar in cultured neurons and cultured astrocytes (Fig. 2), this last finding cannot be explained by contamination of the neuronal culture by glial cells. In a recentin situ hybridization study, Giffard et al. (2000) concluded that NBC is expressed primarily in glial cells in the rat CNS. However, the in situ hybridization pattern of Giffard et al. (2000) is in fact very similar to ours (Figs. 3-5). For instance, the intense dentate gyrus hybridization signal observed in both studies is consistent with neuronal NBC expression.
Although our molecular data provide compelling evidence for the presence of NBC mRNA and protein in certain neurons, one might ask why physiological experiments have heretofore not detected NBCs. First, the absence of detectable NBC signals in many brain regions implies that NBCs are not present in all neurons. Second, an Na+-driven Cl-HCO3exchanger could mask the activity of an NBC. Third, NBC protein might be restricted to cell processes, as implied by the diffuse staining in our immunofluorescence studies, making it impossible to detect in fluorescence studies targeting the soma. Finally, neuronal NBCs might become active only under special conditions (e.g., cell shrinkage and depolarization), reflecting the presence of specialized regulatory factors and/or ion gradients.
The functional role of NBC in neurons is unclear. An electroneutral neuronal NBC could simply regulate intracellular (and extracellular) pH as well as cell volume, similar to Na-H exchangers. If the neuronal NBC is electrogenic, as suggested by the reactivity profile of our probes, stoichiometry becomes a crucial issue. In resting neurons, a 1:3 Na/HCO3 cotransporter would likely function as an HCO3− extruder (i.e., acid loader), whereas a 1:2 Na/HCO3 cotransporter would function as an acid extruder. Depolarization would probably reverse a 1:2 cotransporter (turning it into an acid extruder) and accelerate a 1:3 cotransporter. In either case, the HCO3− uptake would counteract activity-dependent acid loading in neurons (Chesler and Kaila, 1992).
Regardless of stoichiometry, a neuronal electrogenic Na/HCO3 cotransporter might contribute to another homeostatic mechanism. Ransom (1992) and Chesler and Kaila (1992)speculated that the increased [K+]o that accompanies neuronal activity would depolarize astrocytes, stimulating their electrogenic Na/HCO3 cotransporters and turning them into an “HCO3− sink.” Because the resulting extracellular acidification would inhibit neuronal activity, astrocytic Na/HCO3 cotransport may be part of a negative feedback mechanism, preventing excess neuronal excitability. Reversing 1:3 or stimulating 1:2 neuronal Na/HCO3cotransport would minimize neuronal acidification but would reinforce extracellular acidification. Whether stimulating neuronal Na/HCO3 cotransport would increase or decrease excitability would depend on the combined effects of intracellular and extracellular pH changes on excitability.
NBC in choroid plexus, ependyma, and meninges
We detected NBC mRNA in choroid plexus epithelial cells. Preliminary immunolocalization experiments suggest that NBC protein is present within the basolateral membrane of these cells (R. M. Douglas and B. M. Schmitt, unpublished observations). We also detected NBC mRNA in ependymal cells and within the pia mater. The presence of NBC in the choroid plexus suggests that Na/HCO3 cotransport participates in the transepithelial transport of NaHCO3 and production of CSF. Although the cellular mechanisms are unclear, CSF formation has an absolute requirement for HCO3−. The basolateral membrane of the choroid plexus epithelial cell expresses a Cl-HCO3 exchanger (Lindsey et al., 1990). If NBC in the choroid plexus epithelial cell is indeed basolateral, inwardly directed Na/HCO3 cotransport cotransport, in parallel with exchange of extracellular Cl− for intracellular HCO3−, would mediate net uptake of NaCl while cycling HCO3− across the basolateral membrane. If NBC in the choroid plexus epithelial cell is apical, it would secrete NaHCO3 into the ventricular lumen.
Conclusion
We have examined the distribution of Na/HCO3cotransporters in the CNS of adult rats. NBC mRNA and protein are found in subpopulations of both neuronal and glial cells and in epithelial cells of choroid plexus, ependyma, and meninges. The widespread and abundant expression throughout the CNS suggests important roles for NBCs in CNS physiology. Given the rather diverse functional specializations of these cells, these roles are probably far more diverse and complex than previously appreciated.
Footnotes
This work was supported by National Institutes of Health Grants P01 HD32573 (G.G.H. and W.F.B.), NS 35918 (G.G.H.), NS18400 (W.F.B.), and NS32001 (M.A.H.). B.M.S. was supported by Deutsche Forschungsgemeinschaft Forschungsstipendium Schm 1297/1-1.
Correspondence should be addressed to Dr. Walter F. Boron, Department of Cellular and Molecular Physiology, Yale University School of Medicine, 333 Cedar Street, New Haven, CT 06520-8026. E-mail:walter.boron@yale.edu.
REFERENCES
- 1.Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998;273:17689–17695. doi: 10.1074/jbc.273.28.17689. [DOI] [PubMed] [Google Scholar]
- 2.Astion ML, Orkand RK. Electrogenic Na+/HCO3− cotransport in neuroglia. Glia. 1988;1:355–357. doi: 10.1002/glia.440010508. [DOI] [PubMed] [Google Scholar]
- 3.Berger UV, Hediger MA. Comparative analysis of glutamate transporter expression in rat brain using differential double in situ hybridization. Anat Embryol. 1998;198:13–30. doi: 10.1007/s004290050161. [DOI] [PubMed] [Google Scholar]
- 4.Berger UV, Tsukaguchi H, Hediger MA. Distribution of mRNA for the facilitated urea transporter UT3 in the rat nervous system. Anat Embryol. 1998;197:405–414. doi: 10.1007/s004290050152. [DOI] [PubMed] [Google Scholar]
- 5.Bevensee MO, Boron WF. pH regulation in mammalian neurons. In: Kaila K, Ransom BR, editors. pH and brain function. Wiley-Liss; New York: 1998. pp. 211–231. [Google Scholar]
- 6.Bevensee MO, Weed RA, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. I. Role of HCO3−. J Gen Physiol. 1997a;110:453–465. doi: 10.1085/jgp.110.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bevensee MO, Apkon M, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. II. Electrogenic Na/HCO3 cotransport. J Gen Physiol. 1997b;110:467–483. doi: 10.1085/jgp.110.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na+-HCO3− cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am J Physiol. 2000;278:C1200–C1211. doi: 10.1152/ajpcell.2000.278.6.C1200. [DOI] [PubMed] [Google Scholar]
- 9.Boron WF, Boulpaep EL. The electrogenic Na/HCO3 cotransporter. Kidney Int. 1989;36:392–402. doi: 10.1038/ki.1989.208. [DOI] [PubMed] [Google Scholar]
- 10.Boron WF, Hediger MA, Boulpaep EL, Romero MF. The renal electrogenic Na+:HCO3− cotransporter. J Exp Biol. 1997;200:263–268. doi: 10.1242/jeb.200.2.263. [DOI] [PubMed] [Google Scholar]
- 11.Boussouf A, Lambert RC, Gaillard S. Voltage-dependent Na+-HCO3− cotransporter and Na+/H+ exchanger are involved in intracellular pH regulation of cultured mature rat cerebellar oligodendrocytes. Glia. 1997;19:74–84. doi: 10.1002/(sici)1098-1136(199701)19:1<74::aid-glia8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Boyarsky G, Ganz MB, Sterzel B, Boron WF. pH regulation in single glomerular mesangial cells. II. Na-dependent and -independent Cl-HCO3 exchangers. Am J Physiol. 1988;255:C857–C869. doi: 10.1152/ajpcell.1988.255.6.C857. [DOI] [PubMed] [Google Scholar]
- 13.Boyarsky G, Ransom B, Schlue W-R, Davis MBE, Boron WF. Intracellular pH regulation in single cultured astrocytes from rat forebrain. Glia. 1993;8:241–248. doi: 10.1002/glia.440080404. [DOI] [PubMed] [Google Scholar]
- 14.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 15.Brown D, Lydon J, McLaughlin M, Stuart-Tilley A, Tyszkowski R, Alper S. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS). Histochem Cell Biol. 1996;105:261–267. doi: 10.1007/BF01463929. [DOI] [PubMed] [Google Scholar]
- 16.Brune T, Fetzer S, Backus KH, Deitmer JW. Evidence for electrogenic sodium-bicarbonate cotransport in cultured rat cerebellar astrocytes. Pflügers Arch. 1994;429:64–71. doi: 10.1007/BF02584031. [DOI] [PubMed] [Google Scholar]
- 17.Burnham CE, Amlal H, Wang Z, Shull GE, Soleimani M. Cloning and functional expression of a human kidney Na+:HCO3− cotransporter. J Biol Chem. 1997;272:19111–19114. doi: 10.1074/jbc.272.31.19111. [DOI] [PubMed] [Google Scholar]
- 18.Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34:401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- 19.Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- 20.Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of a human electrogenic Na+-HCO3− cotransporter isoform (hhNBC). Am J Physiol. 1999;276:C576–C584. doi: 10.1152/ajpcell.1999.276.3.C576. [DOI] [PubMed] [Google Scholar]
- 21.Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- 22.Deitmer JW, Rose CR. pH regulation and proton signalling by glial cells. Prog Neurobiol. 1996;48:73–103. doi: 10.1016/0301-0082(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 23.Deitmer JW, Schlue W-R. An inwardly directed electrogenic sodium-bicarbonate cotransport in leech glial cells. J Physiol (Lond) 1989;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giffard RG, Papadopoulos MC, van Hooft JA, Xu L, Giuffrida R, Monyer H. The electrogenic sodium bicarbonate cotransporter: developmental expression in rat brain and possible role in acid vulnerability. J Neurosci. 2000;20:1001–1008. doi: 10.1523/JNEUROSCI.20-03-01001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grassl SM, Aronson PS. Na+/ HCO3− co-transport in basolateral membrane vesicles isolated from rabbit renal cortex. J Biol Chem. 1986;261:8778–8783. [PubMed] [Google Scholar]
- 26.Hoffman WE, Charbel FT, Edelman G, Ausman JI. Brain tissue acid-base response to hypercapnia in neurosurgical patients. Neurol Res. 1995;17:417–420. [PubMed] [Google Scholar]
- 27.Ishibashi K, Sasaki S, Marumo F. Molecular cloning of a new sodium bicarbonate cotransporter cDNA from human retina. Biochem Biophys Res Commun. 1998;246:535–538. doi: 10.1006/bbrc.1998.8658. [DOI] [PubMed] [Google Scholar]
- 28.Kempski O, Staub F, Jansen M, Baethmann A. Molecular mechanisms of glial cell swelling in acidosis. Adv Neurol. 1990;52:39–45. [PubMed] [Google Scholar]
- 29.Kempski O, von Rosen S, Weigt H, Staub F, Peters J, Baethmann A. Glial ion transport and volume control. Ann NY Acad Sci. 1991;633:306–317. doi: 10.1111/j.1749-6632.1991.tb15622.x. [DOI] [PubMed] [Google Scholar]
- 30.Kettenmann H, Schlue W-R. Intracellular pH regulation in cultured mouse oligodendrocytes. J Physiol (Lond) 1988;406:147–162. doi: 10.1113/jphysiol.1988.sp017373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindsey AE, Schneider K, Simmons DM, Baron R, Lee BS, Kopito RR. Functional expression and subcellular localization of an anion exchanger cloned from choroid plexus. Proc Natl Acad Sci USA. 1990;87:5278–5282. doi: 10.1073/pnas.87.14.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman EA, Astion ML. Localization and stoichiometry of electrogenic sodium bicarbonate cotransport in retinal glial cells. Glia. 1991;4:424–428. doi: 10.1002/glia.440040411. [DOI] [PubMed] [Google Scholar]
- 33.Okada Y, Kloiber O, Hossmann KA. Regional metabolism in experimental brain tumors in cats: relationship with acid/base, water, and electrolyte homeostasis. J Neurosurg. 1992;77:917–926. doi: 10.3171/jns.1992.77.6.0917. [DOI] [PubMed] [Google Scholar]
- 34.Rabary O, Boussofara M, Grimaud D. Acid-base equilibrium and the brain. Ann Fr Anesth Reanim. 1994;13:111–122. doi: 10.1016/s0750-7658(94)80194-0. [DOI] [PubMed] [Google Scholar]
- 35.Ransom BR. Glial modulation of neural excitability mediated by extracellular pH: a hypothesis. Prog Brain Res. 1992;94:37–46. doi: 10.1016/s0079-6123(08)61737-9. [DOI] [PubMed] [Google Scholar]
- 36.Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol. 1998;274:F425–F432. doi: 10.1152/ajprenal.1998.274.2.F425. [DOI] [PubMed] [Google Scholar]
- 37.Romero MF, Henry D, Nelson S, Harte PJ, Dillon AK, Sciortino CM. Cloning and characterization of a Na+ driven anion exchanger (NDAE1). A new HCO3− transporter. J Biol Chem. 2000;275:24552–24559. doi: 10.1074/jbc.M003476200. [DOI] [PubMed] [Google Scholar]
- 38.Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- 39.Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF. Immunolocalization of the electogenic Na+/HCO3− cotransporter in mammalian and amphibian kidney. Am J Physiol. 1999;276:F27–F36. doi: 10.1152/ajprenal.1999.276.1.F27. [DOI] [PubMed] [Google Scholar]
- 40.Schwiening CJ, Boron WF. Regulation of intracellular pH in pyramidal neurons from the rat hippocampus by Na+-dependent Cl--HCO3− exchange. J Physiol (Lond) 1994;475:59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siesjö BK, Ekholm A, Katsura K, Theander S. Acid-base changes during complete brain ischemia. Stroke. 1990;21:194–199. [PubMed] [Google Scholar]
- 42.Staub F, Mackert B, Kempski O, Haberstok J, Peters J, Baethmann A. Swelling and damage to nerves and glial cells by acidosis. Anasthesiol Intensivmed Notfallmed Schmerzther. 1994;29:203–209. doi: 10.1055/s-2007-996719. [DOI] [PubMed] [Google Scholar]
- 43.Staub F, Winkler A, Haberstok J, Plesnila N, Peters J, Chang RC, Kempski O, Baethmann A. Swelling, intracellular acidosis, and damage of glial cells. Acta Neurochir Suppl. 1996;66:56–62. doi: 10.1007/978-3-7091-9465-2_10. [DOI] [PubMed] [Google Scholar]
- 44.Xu L, Glassford AJ, Giaccia AJ, Giffard RG. Acidosis reduces neuronal apoptosis. NeuroReport. 1998;9:875–879. doi: 10.1097/00001756-199803300-00021. [DOI] [PubMed] [Google Scholar]




