Abstract
Associational connections of pyramidal cells in rat posterior piriform cortex were studied by direct visualization of axons stained by intracellular injection in vivo. The results revealed that individual cells have widespread axonal arbors that extend over nearly the full length of the cerebral hemisphere. Within piriform cortex these arbors are highly distributed with no regularly arranged patchy concentrations like those associated with the columnar organization in other primary sensory areas (i.e., where periodically arranged sets of cells have common response properties, inputs, and outputs). A lack of columnar organization was also indicated by a marked disparity in the intrinsic projection patterns of neighboring injected cells. Analysis of axonal branching patterns, bouton distributions, and dendritic arbors suggested that each pyramidal cell makes a small number of synaptic contacts on a large number (>1000) of other cells in piriform cortex at disparate locations. Axons from individual pyramidal cells also arborize extensively within many neighboring cortical areas, most of which send strong projections back to piriform cortex. These include areas involved in high-order functions in prefrontal, amygdaloid, entorhinal, and perirhinal cortex, to which there are few projections from other primary sensory areas. Our results suggest that piriform cortex performs correlative functions analogous to those in association areas of neocortex rather than those typical of primary sensory areas with which it has been traditionally classed. Findings from other studies suggest that the olfactory bulb subserves functions performed by primary areas in other sensory systems.
Keywords: piriform cortex, olfactory cortex, cortico-cortical, olfaction, association cortex, neural networks
Piriform cortex has long been considered as “primary” olfactory cortex because it is the largest area that receives direct input from the olfactory bulb (OB), the structure that monosynaptically relays input from olfactory receptor neurons. However, physiological and anatomical studies suggest that this cortical area is organized in a fundamentally different way than the primary cortical areas for nonchemical senses (Haberly, 1998). Physiological studies have shown that neurons in piriform cortex typically respond in varying degree to odorant molecules with a broad range of structure (Tanabe et al., 1975; Schoenbaum and Eichenbaum, 1995), in contrast to the exquisite selectivity exhibited by cells in primary sensory areas in neocortex. Studies with extracellularly injected axon tracers have shown that associational (cortico-cortical) projections from restricted regions of piriform cortex are highly distributed spatially, both within piriform cortex and in other olfactory cortical areas (Haberly and Price, 1978; Luskin and Price, 1983). This contrasts with the topographically ordered, columnar architecture of the other primary sensory areas in rat and higher mammals (Chapin et al., 1987; Malach, 1989; Ojima et al., 1991), and is reminiscent of so-called “higher order” or “association” areas, which lack the systematic point to point functional mapping observed in the primary and secondary areas from which they receive input (Kaas, 1993; Zeki, 1993; Pandya, 1995). Previous morphological studies have also shown that piriform cortex projects to areas that are thought to play a role in mediating complex functions related to integrating sensory cues with behavior (prefrontal cortex), assessing the emotional or motivational significance of sensory cues (amygdala), and multisensory association and memory (entorhinal and perirhinal cortex) (Luskin and Price, 1983). This contrasts with other senses in which secondary and higher order areas are interposed between the primary areas that receive ascending sensory input and function-related areas (Pritzel and Markowitsch, 1981; Ottersen, 1982; Burwell and Amaral, 1998). These characteristics of piriform cortex suggest that it is functionally analogous to association areas for other sensory modalities rather than to the primary receiving areas. In other words, rather than being primarily concerned with extracting and refining specific stimulus features like primary neocortical areas, piriform cortex plays a role in linking the combinatorial representations of odorant structure that constitute the olfactory code, and in associating olfactory and other forms of information. This study examines features of cellular-level intrinsic and extrinsic connections of pyramidal cells that provide a basis for evaluating the validity of this conceptual framework for the posterior subdivision of piriform cortex.
MATERIALS AND METHODS
Experiments were performed on adult male Long–Evans rats weighing 160–200 gm. Surgery was performed under chloral hydrate anesthesia (initial dosage, 420 mg/kg). Research protocols were approved by the University of Wisconsin Animal Care and Use Committee and conform to National Institutes of Health animal use guidelines.
Surgery consisted of exposing the surface of piriform cortex by excising and retracting the mandibular musculature, making a small opening in the skull with a dental burr, and incising the dura. The brain was stabilized with 4% agar or 3% agarose in artificial CSF containing (in mm): 147 Na+, 3.5 K+, 1.5 Ca2+, and 0.7 Mg2+.
Intracellular injections were made with micropipettes with tip resistances of 90–120 MΩ when filled with 4% biotinylated dextran amine (BDA; 3000 MW; Molecular Probes, Eugene, OR) in 0.1m K acetate, pH 7.4. Micropipettes were advanced in 2–12 μm increments with an Inchworm piezoelectric drive (Burleigh) while monitoring laminar position by examining the field potential evoked by current pulse stimulation of the OB or lateral olfactory tract through a tungsten microelectrode. After the pipette entered layer II, the compact cell body layer, brief “entry pulses” were applied through a circuit built into the Axoclamp-2A recording amplifier whenever an increase in tip resistance indicated possible contact with neuronal membrane. When the initial membrane potential after impalement was more depolarized than −50 mV, steady hyperpolarizing current was applied through a bridge circuit to bring it into the normal range (−65 to −75 mV). This current was steadily decreased during recovery from impalement damage until the membrane potential and time constant stabilized in the normal range. Because responses of superficial pyramidal (SP) cells to current pulse stimulation are rather uniform across cells and have been previously described in vivo and in slices, they are not illustrated in this report. Injection of BDA was by iontophoresis with +0.4–1 nA, 100 msec square pulses for 10–20 min; survival time after injection was 2–7 d to maximize the extent of axonal labeling. In general, unless cells displayed near-normal membrane potentials and time constants at the termination of injections, they were found to be unsuitable for morphological analysis at the end of the long survival period. Brain fixation was by perfusion through the aorta with 3% fresh formaldehyde and 0.5% glutaraldehyde in 0.1 mPO4. Frozen sections were cut at 60 or 80 μm, usually at 45° to the sagittal plane to reduce their numbers.
Sections were reacted overnight at 4°C in Vector Elite ABC reagent (Vector Laboratories, Burlingame, CA) in 0.05% Triton X-100 and 0.1% crystalline BSA in 0.1 m PBS, pH 7.4, reacted for 10 min in 0.04% diaminobenzidine (DAB) and 0.01% H2O2 in 0.1 mPO4, and dried onto subbed slides. To intensify the DAB reaction product, mounted sections were dehydrated in an alcohol series, defatted overnight in xylene, rehydrated, incubated in 1.4% AgNO3 in dH2O for 1 hr at 56°C, washed three times in dH2O, toned in 0.2% HAuCl4 in dH2O for 10 min at room temperature, washed three times in dH2O, fixed in 5% sodium thiosulfate in dH2O for 10 min, washed three times in dH2O, dehydrated in alcohol, cleared in xylene, and coverslipped with Eukitt. This silver–gold intensification procedure allowed visualization of the finest caliber unmyelinated axons at medium power under bright-field illumination. Cortical lamination was visualized with dark-field illumination.
Analysis was confined to pyramidal cells in the central region of piriform cortex; specifically, the rostral part of the posterior piriform cortex (PPC) (Luskin and Price, 1983). Only cells whose axons appeared to be completely filled were studied in detail. Criteria used to judge the extent of filling included the presence of specializations at axon tips (distinctive hooks or growth cone-like swellings with a terminal spike), and an abrupt rather than gradual decrease in staining intensity that was typically observed after a survival period of 2–7 d. Axons were reconstructed through serial sections with a camera lucida or Neurolucida system (MicroBrightfield) using a 40× oil immersion objective with examination, as needed, at 100×. Reconstructions were rotated and superimposed onto drawings of the brain surface. Shrinkage in depth was corrected based on the original section thickness. Shrinkage in other dimensions was minimal because sections were attached to slides before dehydration, but small corrections were made as needed when rotated arbors were superimposed onto the surface drawings.
Bouton distributions were quantified by stratified random independent sampling of five cells, with sections selected for analysis using a random number table. A grid with 200 μm squares was projected onto sections with the Neurolucida system and a square selected using the random number table. All axon segments within the square were drawn with a 100× oil immersion lens with the Neurolucida system. Boutons were identified using criteria developed in an electron microscopic analysis of intracellularly injected pyramidal cells in opossum piriform cortex (Haberly and Presto, 1986). The close similarities between rat and opossum piriform cortex in terms of the morphology and distribution of pyramidal and nonpyramidal cells, connectivity, and physiology (Haberly, 1998) suggest that such extrapolation to the rat is valid. The Neurolucida system was used to compute distances between boutons in three dimensions, independent of axon course.
RESULTS
Associational (cortico-cortical) connections of SP cells in the central region of piriform cortex were visualized by intracellular injection of the stable intracellular tracer BDA. Previous results obtained with extracellularly injected axon tracers indicate that SP cells are the dominant source of associational axons in the piriform cortex (Haberly and Price, 1978), and morphological and physiological studies (cited below) indicate that they are also the primary target. Cell bodies of these neurons are the primary constituent of layer II, which is the middle, highly compact cellular layer in this phylogenetically old three-layered cortex. Apical dendrites of SP cells extend to the surface through layer I (superficial plexiform layer), and their extensive basal dendrites are concentrated in layer III. Layer III also contains a low density of so-called deep pyramidal cells and nonpyramidal cells that are largely GABAergic (Haberly, 1998).
A total of 17 well stained SP cells were recovered; detailed analysis was performed on five of these cells that appeared to be representative of the full population. Because of the limits on sample size imposed by the difficulties in serially reconstructing the fine-caliber axons that arborized extensively over a distance of ∼1 cm, the analysis focused on the identification of major features of organization common to all injected cells that were not discerned in previous studies with extracellularly injected tracers.
Spatial distribution of intrinsic associational axons
Previous studies with extracellularly injected anterograde and retrograde axonal tracers have revealed that intrinsic associational projections in piriform cortex are highly distributed spatially (Haberly and Price, 1978; Luskin and Price, 1983). However, it cannot be determined from these studies to what extent this feature reflects the connections of individual cells as opposed to populations of neighboring cells, a question of fundamental importance for understanding function as discussed below.
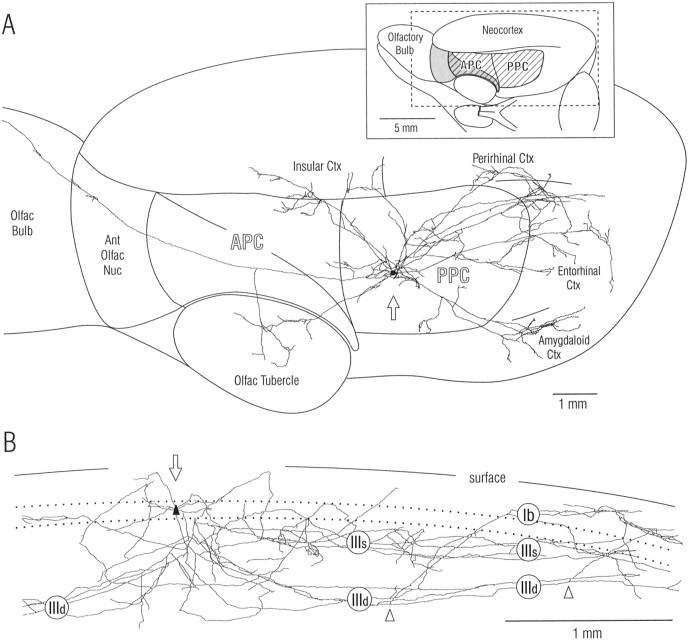
As illustrated in Figures1–3, axon branches from single SP cells in PPC extended over a widespread area that included much of the piriform cortex. Arbors consisted of local collaterals and a widespread system that could extend from the olfactory bulb through the entorhinal cortex (Fig. 1). The widespread system consisted of long association axons that followed relatively straight paths within piriform cortex, giving rise to a small number of shorter branches at irregular intervals. The local system consisted of many short branches in addition to the initial portions of long association fibers.
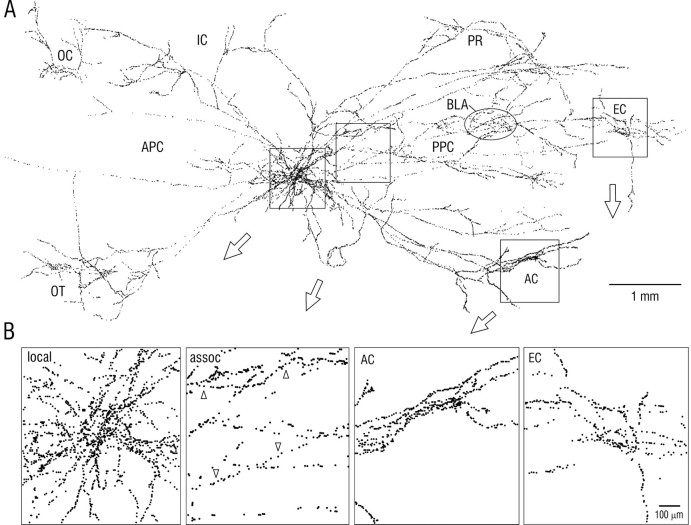
Fig. 1.
Axon from a single pyramidal cell in layer II of rat piriform cortex. Note that axon branches extend over nearly the entire length of the cerebral hemisphere and are widely distributed within piriform cortex and other olfactory and nonolfactory areas. The SP cell in posterior piriform cortex was stained by intracellular injection of biotinylated dextran amine in vivo, and the axon was reconstructed through serial sections with a computer microscope system. A, Spatial distribution of axon branches in surface view. The inset at top right shows the illustrated portion of the rat brain (dashed rectangle) and orientation (45° upward rotation); the hatched area is piriform cortex, and theshaded area is lateral olfactory tract.APC, Anterior piriform cortex; PPC,posterior piriform cortex. B, Depth distribution of same axon within PPC. View is parallel to layers after 90° rotation; rostral is at left as in A; branches outside PPC have been removed; size scale is expanded relative to that in A. Roman numerals indicate layers: Ib, association fiber zone in layer I (molecular layer); IIIs, IIId,superficial and deep portions of layer III; dotted lines, superficial and deep borders of layer II (compact cell body layer). Open arrowheads mark branch points for axon collaterals that ascend to layer I. Open arrows inA and B indicate cell body; dendritic tree is not illustrated (Fig. 3). Ant,Anterior; ctx, cortex; nuc, nucleus;olfac, olfactory.
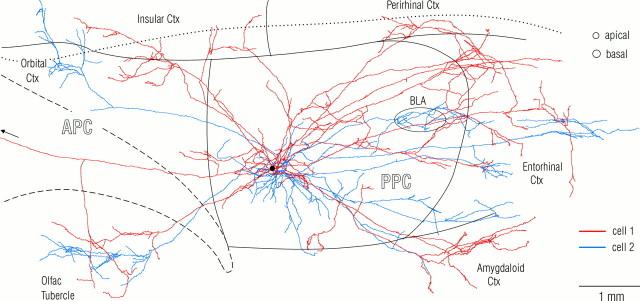
Comparison of the arborizations of SP cell axons within piriform cortex with their arbors in other cortical areas revealed a marked difference. Within piriform cortex, associational axons displayed little tendency for a clustering of branches outside the local collateral region. In contrast, as best seen in Figure 2, portions of the same axons in other cortical areas displayed a clear tendency for restricted “patchy” concentrations (<500 μm diameter) within otherwise spatially distributed arbors.
Fig. 2.
Association (cortico-cortical) axons from a pair of neighboring superficial pyramidal cells in posterior piriform cortex. Note the minimal overlap of the two axonal arbors outside the ∼1 mm diameter local collateral region that surrounds SP somata. The axon drawn in red (Fig. 3, cell 1) is the same as in Figure 1. The arborizations from the second cell (blue) in the orbital cortex (top left) and basolateral amygdala (BLA, oval) are deep to piriform cortex. The black spot indicates the position of the cell bodies. The circles at top right denote typical diameters of pyramidal cell dendritic trees at the depths where they are contacted by association fibers (proximal apical dendrites in layer Ib and basal dendrites in layer III). The borders of piriform cortex and the insular-perirhinal border are indicated by solid lines; the dashed line outlines the lateral olfactory tract; the dotted line is the rhinal sulcus.
Depth distribution of intrinsic associational axons
Analysis of the depth distribution of axonal arbors in piriform cortex can provide insight into postsynaptic targets as a consequence of the laminar segregation of different neuronal elements. Although depth distributions of intrinsic projections have been studied in piriform cortex with extracellular tracers (Luskin and Price, 1983), this analysis was repeated for the arbors of intracellularly injected cells because contributions from nonpyramidal cells cannot be distinguished in the previous data.
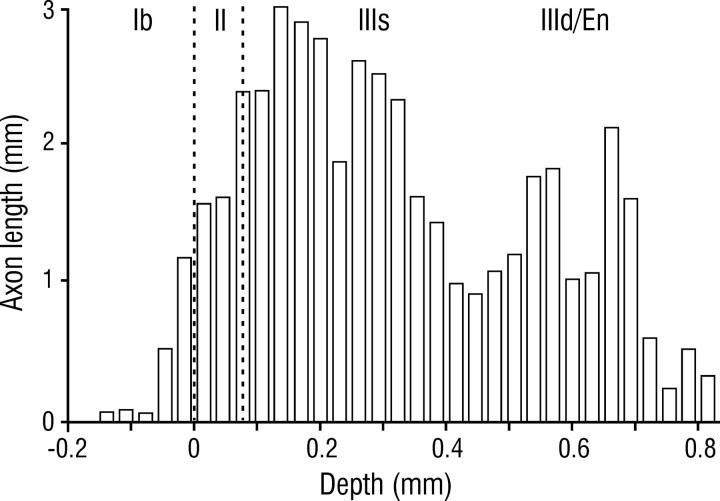
Within piriform cortex, associational projections from SP cells were concentrated in layer III, with relatively sparse input to layer Ib, and an intermediate density in layer II (Figs. 1B,4). Within layer III, axons were most numerous in its superficial part (layer IIIs) at the depth of basal dendrites of SP cells, but were also present in its deep part (layer IIId) and the subjacent endopiriform nucleus where dendrites from deep pyramidal and nonpyramidal cells predominate (Haberly, 1998). In layer IIId, long associational axons gave rise to collaterals that ascended to more superficial layers at varying distances from the cell body (Fig. 1B, arrowheads). Within layer Ib (the deep portion of layer I from which afferent input is excluded), axons were concentrated at its deep border at the depth of proximal apical dendrites. Potential targets within layer II include basal dendrites (Fig. 5A, arrowheads) and smaller numbers of apical dendritic processes (associational axons do not synapse on pyramidal somata; Haberly and Behan, 1983; Haberly and Presto, 1986).
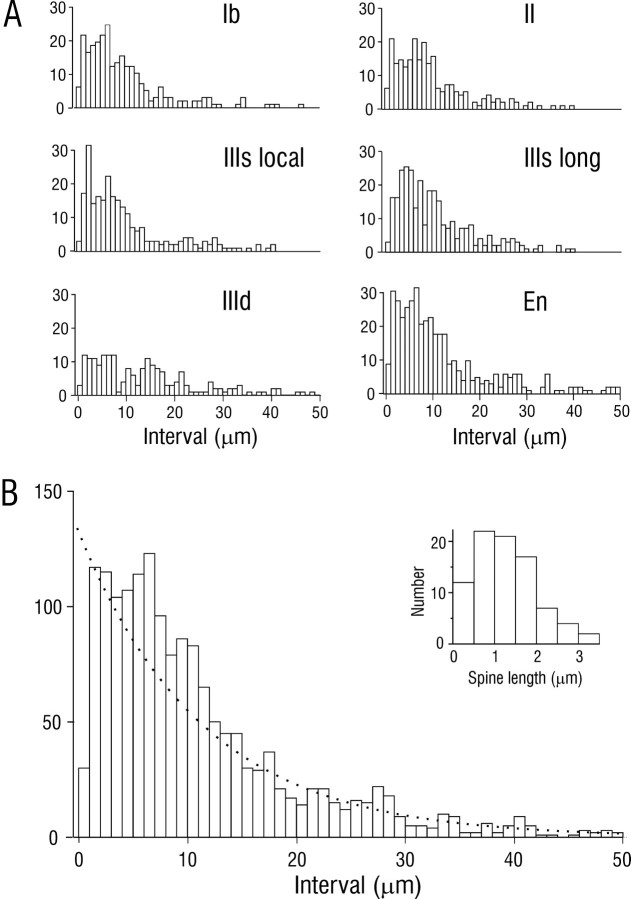
Fig. 4.
Depth distribution of superficial pyramidal cell axons in piriform cortex. Histogram shows axon length as a function of depth for five intracellularly injected cells. En, Endopiriform nucleus.
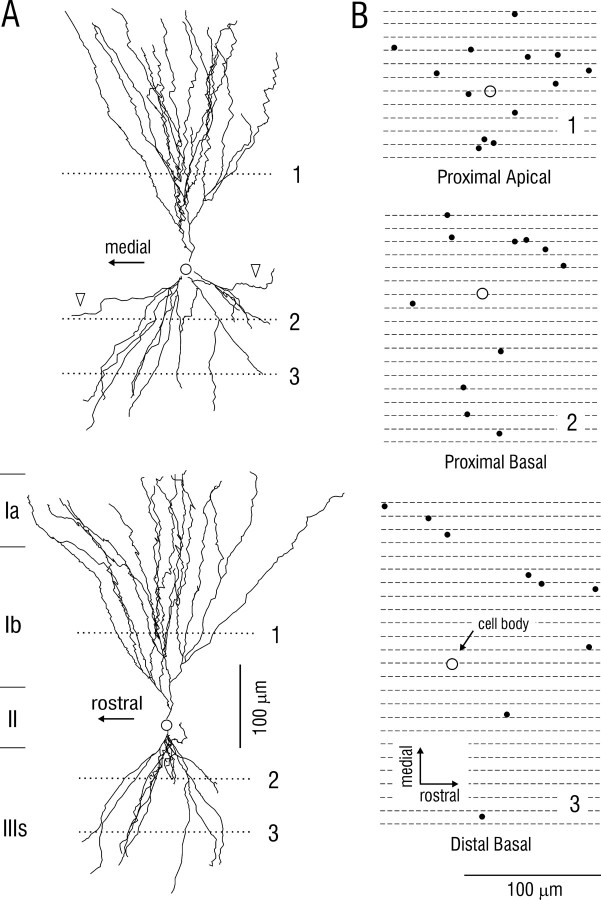
Fig. 5.
Dendritic morphology of superficial pyramidal cell in posterior piriform cortex. A, Top, Reconstruction in frontal plane (perpendicular to predominant course of associational axons); bottom, same cell after 90° rotation. B, Positions of dendrites from same cell at depths indicated by the dotted lines in A. Black spots indicate points of intersection of dendrites with horizontal planes (parallel to surface) at depths of proximal apical, proximal basal, and distal basal segments where SP cells receive association fiber synapses. Circles indicate cell body position, orthogonally projected onto the three horizontal planes. Diameters of black spots in B correspond to area that contains ∼90% of the dendritic spines (sites of association fiber synapses). Comparison of dendritic and axonal morphologies suggests that each SP cell makes a small number of contacts on a large number of other SP cells; see Results.
Spatial distribution of boutons
A previous study showed that ∼80% of the en passantboutons that are visualized with the light microscope, and most or all of the stalked boutons give rise to synaptic contacts as determined by electron microscopy (Haberly and Presto, 1986). As a result, it will be assumed that the distribution of synapses from pyramidal cells in piriform cortex can be approximated by light microscopic analysis. Knowledge of this distribution is necessary for assessing the nature and extent of intrinsic connectivity; for example, despite an absence of locally exuberant axonal branching, a concentration of synaptic input onto individual cells or “columns” of cells could be achieved through appropriate arrangement of boutons on unbranched axons.
Plots of bouton distribution (Fig. 6) revealed a nonuniform arrangement with both laminar and regional differences. Mean interbouton intervals for five cells (Table1) were ∼10 μm in layers Ib, II, and IIIs where pyramidal cell dendrites are the dominant postsynaptic component, and somewhat greater in layer IIId (∼14 μm;p < 0.01 vs layers Ib, II, or IIIs) and the endopiriform nucleus (∼13 μm; p < 0.02 vs layers Ib or II). This relationship between axonal depth and bouton spacing was readily apparent on visual examination of labeled axons (Fig.6B, second panel), although there was substantial variation at all locations and depths. Irregularly arranged bouton clusters of varying dimension were observed on axons in both the piriform cortex and adjoining areas (Fig. 6B). However, there was no hint of any regularity or periodicity in size or location of such clusters in piriform cortex as observed in cortical areas with a columnar organization (see Discussion).
Fig. 6.
A, Surface plot of the locations of putative synaptic boutons for the axons illustrated in Figure 2. Eachdot represents a bouton visualized by light microscopy.B, Enlargements of regions indicated byboxes in A. First panel (left) shows local axon collaterals; second panel shows longer association (assoc) fibers; upward arrowheads indicate an axon in layer IIIs with a relatively high bouton density; downward arrowheads indicate an axon in layer IIId with a lower density. Third andfourth panels show boutons in amygdaloid cortex (AC) and entorhinal cortex (EC).Oval in A indicates a cluster of boutons in the basolateral amygdala (BLA), deep to piriform cortex. IC, Insular cortex; OC, orbital cortex; OT, olfactory tubercle; PR,perirhinal cortex.
Table 1.
Intervals between boutons (mean ± SEM) in different layers of the posterior piriform cortex and subjacent endopiriform nucleus (En) derived by random sampling of sections from five intracellularly injected superficial pyramidal cells
| Layer | n | Mean |
|---|---|---|
| Ib | 255 | 10.06 ± 0.60 |
| II | 228 | 9.86 ± 0.52 |
| IIIs | 294 | 10.92 ± 0.52 |
| IIId | 200 | 14.38 ± 0.891-160 |
| En | 425 | 12.84 ± 0.67* |
F1-160: Significantly greater than for layers Ib, II, and IIIs, p < 0.01 for each;
significantly greater than layers Ib and II, p < 0.02 for each, Tukey's HSD test.
Distributions of interbouton intervals were also analyzed for axons at different depths and locations. Figure 7presents interval histograms from five cells for individual layers of PPC and the subjacent endopiriform nucleus (Fig. 7A), and for pooled data (Fig. 7B). The moderately good fit of the composite histogram by an exponential distribution, which would be expected for binned intervals between independently placed boutons, suggests that there is a substantial random component in the determination of bouton placement. However, there were double peaks at ∼2.5 and 6 μm in several individual histograms (Fig. 7A) and clear differences in the distributions of intervals in different layers (e.g., peak at much larger intervals in layer IIId), suggesting that synaptic targeting may be determined by multiple factors.
Fig. 7.
Distribution of interbouton intervals for association axons in PPC. Data were derived by random sampling of sections from five injected cells. A, Interval histograms from individual layers in PPC and the subjacent endopiriform nucleus (En). IIIs local, Local axon collaterals in the superficial part of layer III; IIIs long, long association axons in superficial III.B, Composite histogram from individual plots.Dotted line is an exponential distribution with identical mean. Inset is a histogram of dendritic spine length for SP cells; mean = 1.21 ± 0.08 μm (SEM);n = 85.
Cell targeting
A important question for understanding the nature of information processing is the extent of interconnectivity between principal cells. Results from previous morphological studies indicate that most (>90%) of the boutons on associational axons, outside the dense local collateral region, synapse on pyramidal cell dendrites (Haberly and Behan, 1983; Luskin and Price, 1983; Haberly and Presto, 1986). Based on their large numbers and findings from physiological studies (Haberly and Bower, 1984; Rodriguez and Haberly, 1989; Tseng and Haberly, 1989;Ketchum and Haberly, 1993), it can be concluded that SP cells are the predominant source of these dendrites. Therefore, to examine the key issue of the number of synaptic contacts that each association axon makes on a given pyramidal cell, morphological features of the dendritic trees of SP cells were examined in detail.
As illustrated in Figure 5A, the apical and basal dendritic trees of SP cells consist of a modest number of dendritic branches that are concentrated in conical volumes. To assess the extent to which single unbranched axons could contact more than one dendrite from a given SP cell by chance alone, the positions of dendrites were plotted in horizontal sections (parallel to the cortical surface) through these apical and basal dendritic cones. As seen in Figure 5B, these plots revealed that if an axon contacts one dendrite, the probability that it will contact a second dendrite from the same cell is low (estimated as <15%).
An increase in the targeting of multiple dendrites of the same cell over that expected by chance could be achieved through abrupt, local changes in axonal or dendritic trajectories. However, dendrites tended to follow relatively linear trajectories as described earlier for axons, rather than erratic paths. An increase in multiple targeting also could be achieved through stalked boutons that might extend to contact additional dendrites. However, counts revealed that stalked boutons were present in relatively small numbers (Table2), and length measurements revealed that few stalks were longer than 4 μm.
Table 2.
Relative numbers of en passant and stalked boutons on association axons from superficial pyramidal cells in piriform cortex and endopiriform nucleus (En)
| Layer | En passant | Stalked | % Stalked |
|---|---|---|---|
| Ib | 212 | 62 | 22.6 |
| II | 209 | 44 | 17.4 |
| IIIs | 264 | 55 | 17.2 |
| IIId | 169 | 55 | 24.6 |
| En | 148 | 45 | 23.3 |
Data were derived by random sampling of the same five cells used for Table 1.
To assess the extent to which axons could make multiple contacts on an individual dendrite by synapsing on more than one dendritic spine, the lengths of spine necks were analyzed. This analysis revealed that, in the frontal plane (perpendicular to the predominant rostrocaudal course of association axons), ∼90% of spines on pyramidal cells are ≤2 μm from the center lines of dendrites (Fig. 7B, inset). Therefore, if there are extensive multiple contacts on single dendritic branches, it would be reflected in an excess of interbouton intervals (4 μm; 2 μm on each side of the shaft center). Inspection of interval plots (Fig. 7) revealed that, although there was a peak in the appropriate range, the proportion of total boutons that could be attributed to this factor was <15%.
Finally, to assess the extent to which more than one branch from the same axon might converge onto a single SP cell, typical diameters of dendritic trees (Fig. 5A) were compared with surface plots of axonal distributions. As seen in Figure 2 (top right, circles), there appeared to be little tendency for neighboring axon collaterals from a given cell to maintain a spacing that would allow extensive multiple contacts on single pyramidal cells in piriform cortex.
For the two fully reconstructed cells in Figure 2, lower limits for the number of pyramidal cells contacted were estimated as follows. Within the PPC where cell bodies were located, cell 1 (Fig. 2, red) gave rise to 4311 bouton-like swellings and cell 2 (Fig. 2,blue), 2513. Assuming that 80% of bouton-like swellings make synaptic contacts (Haberly and Presto, 1986), 90% of which are on pyramidal cells (Haberly and Behan, 1983), with 10% of cells receiving input from more than one collateral from a single cell (Fig. 2), 15% of contacts involving two dendritic branches from the same cell (Fig.5), and 15% involving two spines from the same dendritic branch (Fig.7B, inset), it follows that the axon from cell 1 contacted a minimum of ∼2000 pyramidal cells in PPC and the axon from cell 2, a minimum of ∼1200 cells.
Degree of overlap in axonal arbors from neighboring cells
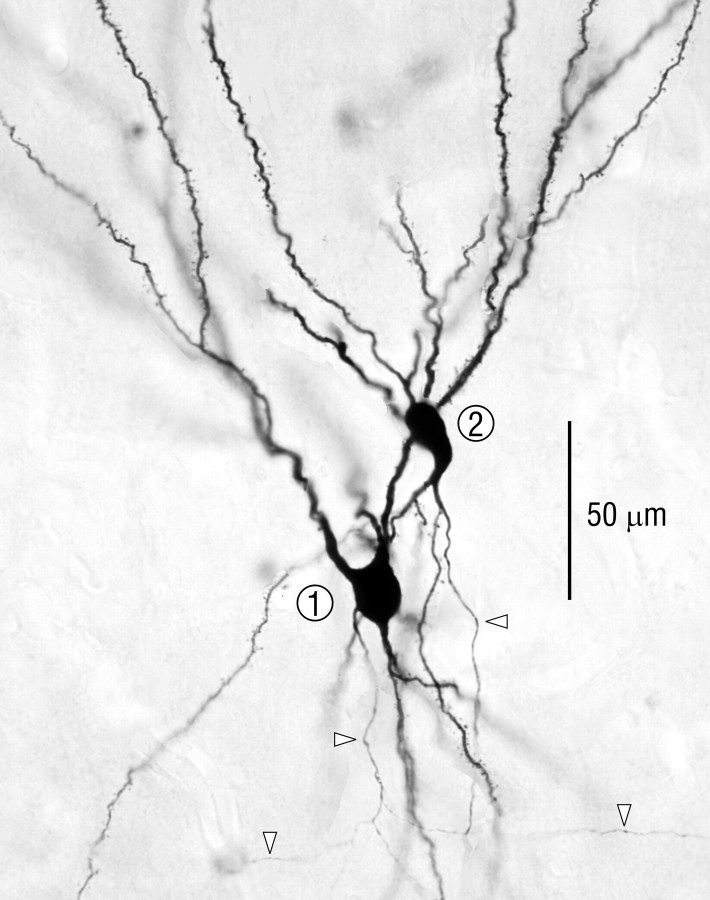
To determine if cells in PPC with similar connections are grouped as observed in cortical areas with a columnar organization, the arborizations of axons from adjacent pyramidal cells were compared. Two pairs were studied; one pair was fully reconstructed in three dimensions with the computer-microscope system (Fig. 2), and selected regions from the second pair were reconstructed. Cell bodies for both pairs were in layer II and separated by <20 μm in the horizontal dimension. Apical and basal dendritic trees of both pairs overlapped extensively (Fig. 3).
Fig. 3.
Photomicrograph of the neighboring pyramidal cells reconstructed in Figure 2. Arrowheads indicate axons.
To interpret these data, the assumption was made that if there are functional “modules” in piriform cortex with dimensions comparable to neocortical columns (200–500 μm), the probability would be high that cells separated horizontally by <20 μm would be in the same rather than adjoining modules. The technical difficulty of staining and reconstructing adjacent cells limited the analysis to two pairs; however, the probability that both pairs would have spanned a boundary between modules, if they exist, would appear to be very low.
Comparison of the axon distribution patterns within piriform cortex for both pairs of pyramidal cells revealed that despite certain parallels in course and branching patterns, there was relatively little overlap in the positions of individual axon branches outside the local collateral region (Fig. 2). As seen by comparing the dimensions of typical pyramidal cell dendritic trees (Fig. 2, top right, circles) with the spaces between axon branches from the neighboring cells in Figure 2, there were few sites in which a single cell in piriform cortex could have received input from both of these cells. Furthermore, within piriform cortex there were no obvious regions of concentrated overlap between the arbors from both cells in a pair with dimensions comparable to columns in neocortex (hundreds of micrometers).
Degree of divergence of output projections
The final question investigated was the degree to which projections from piriform cortex to other cortical areas are derived from different populations of pyramidal cells as opposed to overlapping populations of cells that have branched axons.
Examination of injected cells revealed that axons from all SP cells branched extensively and arborized in multiple cortical areas. The two neighboring cells in Figure 2 together projected to most other olfactory areas (olfactory tubercle, anterior olfactory nucleus, and olfactory bulb), to amygdaloid cortex and nuclei, prefrontal cortex (agranular insula and orbital cortex), entorhinal cortex, and perirhinal cortex. For cell 1 (red), 4670 of the total of 9471 boutons (49%), and for cell 2 (blue), 2318 of 4902 (47%), were in areas outside of piriform cortex.
DISCUSSION
New features of neuronal circuitry
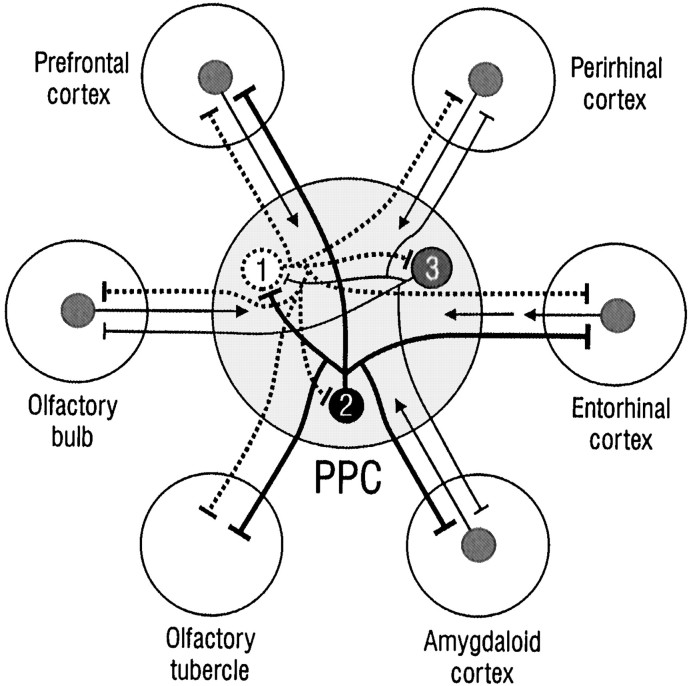
The results have revealed that axons from single SP cells in PPC are highly branched and distributed over an area that can encompass virtually an entire cerebral hemisphere. A particularly intriguing feature is that axons from individual cells are not only widely distributed in piriform cortex, but also arborize extensively in other cortical areas with diverse functional roles including those related to behavior, cognition, emotion, and memory (Fig.8).
Fig. 8.
Summary of intrinsic and extrinsic cortical connections of superficial pyramidal cells in PPC. Each SP cell (numbered circles) has an extensively branching axon that contacts a large number of other SP cells in piriform cortex at disparate locations (flat end bars are synapses). Axons from each cell also arborize in adjoining cortical areas, including those involved in the highest order brain functions. Axonal branches from each cell extend to most, but not all of the target areas (e.g., cell 1 projects to all areas with the exception of amygdaloid cortex; cell 2 does not project to the olfactory bulb or perirhinal cortex). Reciprocal projections from target areas are indicated by arrows. Connections with cortical areas in the olfactory peduncle are not illustrated.
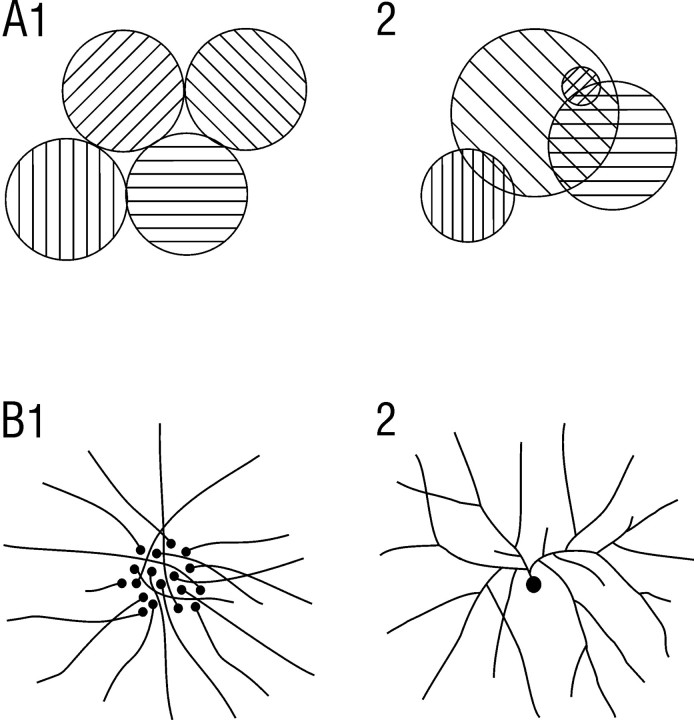
Within piriform cortex, associational axons typically followed rather linear trajectories, and regions of exuberant branching were not observed. Boutons were distributed over the entire extents of axons rather than limited to specialized terminal regions. Although boutons were irregularly arranged with occasional regions of higher concentration, such regions displayed no apparent regularity in size or periodicity in location. Furthermore, projections from neighboring cell pairs did not converge within restricted regions. Although a definitive argument cannot be made on the basis of negative evidence, the present findings suggest that, as illustrated in Figure9A, if there is a modular grouping of cells with similar connections in PPC, it would have to be irregular and overlapping in nature (A2), quite unlike the columnar organization in other primary sensory areas (A1). Finally, comparison of axon branching patterns and bouton distributions relative to dendritic morphologies suggests that each SP cell makes a small number of synapses on a very large number of other pyramidal cells (>1000).
Fig. 9.
Alternative schemes for modular organization (A), and spatially distributed associational connections (B) in PPC. A1,Modular organization as observed in primary neocortical areas where discrete columns of cells defined by response properties and connections are regular in size and arrangement, and do not overlap.A2, Organization in which cells with similar response properties are spatially grouped, but in an irregular overlapping manner. B1, Distributed connectivity in which each cell projects to a specific spot, but neighboring cells project to spatially disparate spots. B2, Distributed connectivity in which individual cells have highly branched, spatially divergent axons.
Arborization patterns of SP axons were clearly different in piriform cortex and in cortical areas outside piriform cortex. Rather than an impression of relative uniformity as in PPC, there was a clear tendency for irregular patchy arborization in prefrontal, amygdaloid, and entorhinal cortex, as well as the olfactory tubercle. Another rather striking feature was that such patches from neighboring pairs of SP cells displayed little tendency for overlap.
Limitations
This study was performed on SP cells because they are the predominant principal cell population in piriform cortex and the primary source of association axons. Although the operation of this system cannot be understood without comparable findings for other cell types, the findings from SP cells alone have revealed new features of organization with potential functional significance.
An important issue is the extent to which the findings from the necessarily small sample of injected SP cells are representative of the full population. A number of factors support a representative nature. First, results of previous studies with a wide variety of morphological and physiological techniques suggest that SP cells are a rather homogeneous population (Haberly, 1998). Second, observations from small extracellular injections of axonal tracers (D. M. G. Johnson, unpublished observations) were consistent with the single cell data. Third, the interpretations discussed below are based on robust features that were apparent in all cells.
A final issue is the extent to which findings for PPC apply to other parts of piriform cortex. Although findings from extracellular injections have revealed general similarities between subdivisions (Luskin and Price, 1983), there could be substantial differences that would not be apparent in such population level studies.
Implications for information processing and olfaction
For understanding functions of piriform cortex it is important to consider how information is represented in the OB. The rat OB contains ∼2000 glomeruli, each one of which receives converging input from receptor neurons that express a single olfactory receptor (Buck, 1999). Because single molecules can activate many different receptors (Malnic et al., 1999), the olfactory code consists of spatially distributed combinations of active glomeruli (Sharp et al., 1975; Shepherd, 1994;Buck, 1999; Rubin and Katz, 1999). It would appear, therefore, that one cortical level function must be a linking of activity in spatially distributed OB neurons with particular odors, as defined by their significance to the animal. Such correlation would require a convergence of information encoded by cells in different parts of the OB, as well as input from areas that represent contextual information.
Studies in behaving rats have shown that individual cells in piriform cortex display “combinatorial” responses to multiple odor qualities, as well as robust responses to contextual stimuli such as the ready light for discrimination trials (Schoenbaum and Eichenbaum, 1995). Such responses are consistent with the observed distributed connections within piriform cortex and between piriform cortex and higher order areas that encode information from other modalities. The presence of extensive return projections from most of the higher order areas to which it projects (Fig. 8) (Haberly, 1998), together with the complex nature of its cellular responses, suggest that rather than unidirectionally supplying these areas with olfactory information, the piriform cortex interacts with them during the mediation of olfactory-guided behavior.
A key issue is the extent to which the complex cellular responses in piriform cortex are established through specific connections as opposed to learning-related adjustments in synaptic strengths. If cells of a given type in PPC are spatially dispersed rather than grouped, it must be asked if it is developmentally feasible for cells, especially those with highly branched axons, to be connected in a specific manner. Our finding of relatively linear axon trajectories and irregular bouton distributions suggests one possibility: rather than being established through selective axon pathfinding as for the projection from receptor neurons to OB glomeruli (Mombaerts et al., 1996), axons in PPC establish synapses with neurons of appropriate type that are encountered throughout their paths. However, the results are also consistent with a role of “parallel-distributed” processes based on adjustments in synaptic strength (Hertz et al., 1991) instead of, or in addition to, specific axonal connections. Thus, the highly branched, spatially distributed axons from individual cells (Fig. 9B) that make a small number of synapses on a large number of neurons are reminiscent of the architectures of artificial “neural networks” that can perform complex pattern analysis.
Comparison to other primary sensory areas
The term “primary sensory” has been applied to piriform cortex and certain areas in neocortex because they are the dominant receiving areas for sensory input. However, despite the parallel in this defining characteristic, the features of intracortical connections visualized in this study have little in common with those in primary sensory areas for nonchemical senses (Gilbert and Wiesel, 1983; Chapin et al., 1987; Zeki and Shipp, 1988;Malach, 1989, 1994; Burkhalter and Charles, 1990; Ojima et al., 1991;Kaas, 1993; Romanski and LeDoux, 1993; Young et al., 1994;Pandya, 1995; Fitzpatrick, 1996; Sonty and Juniano, 1997; Kaas et al., 1999). Primary visual (V1), auditory (A1), and somatosensory (S1) areas in diverse species including the rat have discrete columnar organizations so that intrinsic associational axons arborize in periodic patches that we did not observe in PPC. Second, with the exception of projections from S1 to prefrontal cortex that are thought to be involved in feeding (Price, 1999), these other primary areas lack direct projections to the higher order areas that receive extensive input from PPC (Fig. 8). Third, in contrast to the branching projections from single pyramidal cells in PPC to multiple areas, efferents from V1 to each of its cortical targets arise from independent sets of pyramidal cells that encode different aspects of visual stimuli. This means that information on form, color, motion, and other features is routed to different areas, whereas PPC provides a broad readout from a high proportion of its cells to each downstream area. Limited data for A1 and S1 support a similar principle for these systems.
Comparison to sensory association cortex
In view of their combinatorial responses to odor as well as to task-related stimuli, cells in piriform cortex are reminiscent of those in “sensory association” areas of neocortex (Felleman and Van Essen, 1991). Consequently, one may ask whether there are parallels in connectivity that may contribute to the functional similarities. Data for most higher order sensory areas (Pritzel and Markowitsch, 1981;Burwell and Amaral, 1998; McDonald, 1998) are inadequate for detailed comparison; however, relevant features have been examined in the inferotemporal (IT) cortex where cells encode complex visual form. Three similarities are apparent: (1) cells in IT project to prefrontal, amygdaloid, entorhinal, and perirhinal cortex (Pritzel and Markowitsch, 1981; Ottersen, 1982; Cheng et al., 1997; Burwell and Amaral, 1998); (2) in contrast to primary areas, individual cells in IT can project to more than one area (Cheng et al., 1997); (3) there is no discernable topographical order in anterior IT (area TE) in relationship to other cortical areas (Tanaka, 1997a), as in PPC. Although there is a columnar organization in IT and a corresponding patchiness in connections that clearly differs from PPC (Tanaka, 1997b), optical imaging experiments have revealed a functional overlap between columns and a nonselective response from >90% of the area of TE to all complex visual forms (Wang et al., 1998), suggesting that some form of spatially distributed processing is performed (Higuchi and Miyashita, 1996; Saleem and Tanaka, 1996).
Relationship to findings for OB
If piriform cortex lacks a systematic modular organization and associated spatially ordered connections as in other primary areas, recent findings revealing such features in the OB are of particular interest. In addition to the morphological studies cited earlier, physiological studies have shown that glomeruli and associated neurons in the OB are spatially ordered with respect to stimulus parameters (Mori and Yoshihara, 1995; Johnson et al., 1999) and have provided evidence that response specificities to molecular features are enhanced through interactions between glomeruli (Yokoi et al., 1995). Thus, it can be proposed that the OB performs “feature extraction” operations like those performed by primary areas in other systems, whereas piriform cortex synthesizes these features and links them with other brain functions, analogous to association areas in other systems.
Footnotes
This work was supported by National Institutes of Health Grant DC03271 from the National Institute on Deafness and Other Communication Disorders. We thank Joshua Chover for consultations regarding quantitative interpretations and Sherry Feig, Ray Guillery, and Philip Smith for critical reading.
Correspondence should be addressed to Lewis Haberly, Department of Anatomy, University of Wisconsin, 1300 University Avenue, Madison, WI 53706. E-mail: lhaberly@facstaff.wisc.edu.
REFERENCES
- 1.Buck LB. Information coding in the vertebrate olfactory system. Annu Rev Neurosci. 1999;19:517–544. doi: 10.1146/annurev.ne.19.030196.002505. [DOI] [PubMed] [Google Scholar]
- 2.Burkhalter A, Charles V. Organization of local axon collaterals of efferent projection neurons in rat visual cortex. J Comp Neurol. 1990;302:920–934. doi: 10.1002/cne.903020417. [DOI] [PubMed] [Google Scholar]
- 3.Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Chapin JK, Sadeq M, Guise JL. Corticocortical connections within the primary somatosensory cortex of the rat. J Comp Neurol. 1987;263:326–346. doi: 10.1002/cne.902630303. [DOI] [PubMed] [Google Scholar]
- 5.Cheng K, Saleem KS, Tanaka K. Organization of corticostriatal and corticoamygdalar projections arising from the anterior inferotemporal area TE of the macaque monkey: a Phaseolus vulgaris leucoagglutinin study. J Neurosci. 1997;17:7902–7925. doi: 10.1523/JNEUROSCI.17-20-07902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick D. The functional organization of local circuits in visual cortex: Insights from the study of tree shrew striate cortex. Cereb Cortex. 1996;6:329–341. doi: 10.1093/cercor/6.3.329. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert CD, Wiesel TN. Clustered intrinsic connections in cat visual cortex. J Neurosci. 1983;3:1116–1133. doi: 10.1523/JNEUROSCI.03-05-01116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haberly LB. Olfactory cortex. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford UP; New York: 1998. pp. 377–416. [Google Scholar]
- 10.Haberly LB, Behan M. Structure of opossum piriform cortex. III. Ultrastructural characterization of synaptic terminals of association and olfactory bulb afferent fibers. J Comp Neurol. 1983;219:448–460. doi: 10.1002/cne.902190406. [DOI] [PubMed] [Google Scholar]
- 11.Haberly LB, Bower JM. Analysis of association fiber system in piriform cortex with intracellular recording and staining methods. J Neurophysiol. 1984;51:90–112. doi: 10.1152/jn.1984.51.1.90. [DOI] [PubMed] [Google Scholar]
- 12.Haberly LB, Presto S. Ultrastructural analysis of synaptic relationships of intracellularly stained pyramidal cell axons in piriform cortex. J Comp Neurol. 1986;248:464–474. doi: 10.1002/cne.902480403. [DOI] [PubMed] [Google Scholar]
- 13.Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. I. Systems originating in piriform cortex and adjacent areas. J Comp Neurol. 1978;178:711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- 14.Hertz J, Krogh A, Palmer RG. Introduction to the theory of neural computation. Addison-Wesley; Reading, PA: 1991. [Google Scholar]
- 15.Higuchi S, Miyashita Y. Formation of mnemonic neuronal responses to visual paired associates in inferotemporal cortex is impaired by perirhinal and entorhinal lesions. Proc Natl Acad Sci USA. 1996;93:739–743. doi: 10.1073/pnas.93.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson BA, Woo CC, Hingco EE, Pham KL, Leon M. Multidimensional chemotopic responses to n-aliphatic acid odorants in rat olfactory bulb. J Comp Neurol. 1999;409:529–548. [PubMed] [Google Scholar]
- 17.Kaas JH. The functional organization of somatosensory cortex in primates. Anat Embryol. 1993;175:509–518. doi: 10.1016/s0940-9602(11)80212-8. [DOI] [PubMed] [Google Scholar]
- 18.Kaas JH, Hackett TA, Tramo MJ. Auditory processing in primate cerebral cortex. Curr Opin Neurobiol. 1999;9:164–170. doi: 10.1016/s0959-4388(99)80022-1. [DOI] [PubMed] [Google Scholar]
- 19.Ketchum KL, Haberly LB. Membrane currents evoked by afferent stimulation in rat piriform cortex: II. Analysis with a system model. J Neurophysiol. 1993;69:261–281. doi: 10.1152/jn.1993.69.1.261. [DOI] [PubMed] [Google Scholar]
- 20.Luskin MB, Price JL. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- 21.Malach R. Patterns of connections in rat visual cortex. J Neurosci. 1989;9:3741–3752. doi: 10.1523/JNEUROSCI.09-11-03741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malach R. Cortical columns as devices for maximizing neuronal diversity. Trends Neurosci. 1994;17:101–103. doi: 10.1016/0166-2236(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 23.Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- 24.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 25.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmonson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 26.Mori K, Yoshihara Y. Molecular recognition and olfactory processing in the mammalian olfactory system. Prog Neurobiol. 1995;45:585–619. doi: 10.1016/0301-0082(94)00058-p. [DOI] [PubMed] [Google Scholar]
- 27.Ojima H, Honda CN, Jones EG. Patterns of axon collateralization of identified supragranular pyramidal neurons in cat auditory cortex. Cereb Cortex. 1991;1:80–94. doi: 10.1093/cercor/1.1.80. [DOI] [PubMed] [Google Scholar]
- 28.Ottersen OP. Connections of the amygdala of the rat. IV: Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J Comp Neurol. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- 29.Pandya DN. Anatomy of the auditory cortex. Rev Neurol. 1995;151:486–494. [PubMed] [Google Scholar]
- 30.Price JL. Prefrontal cortical networks related to visceral function and mood. Ann NY Acad Sci. 1999;877:383–96. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- 31.Pritzel M, Markowitsch HJ. Cortico-prefrontal afferents in the guinea pig. Brain Res Bull. 1981;7:427–434. doi: 10.1016/0361-9230(81)90041-1. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez R, Haberly LB. Analysis of synaptic events in opossum piriform cortex with improved current source density techniques. J Neurophysiol. 1989;61:702–718. doi: 10.1152/jn.1989.61.4.702. [DOI] [PubMed] [Google Scholar]
- 33.Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- 34.Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- 35.Saleem KS, Tanaka K. Divergent projections from the anterior inferotemporal area TE to the perirhinal and entorhinal cortices in the macaque monkey. J Neurosci. 1996;16:4757–4775. doi: 10.1523/JNEUROSCI.16-15-04757.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- 37.Sharp FR, Kauer JS, Shepherd GM. Local sites of activity-related glucose metabolism in rat olfactory bulb during olfactory stimulation. Brain Res. 1975;98:596–600. doi: 10.1016/0006-8993(75)90377-7. [DOI] [PubMed] [Google Scholar]
- 38.Shepherd GM. Discrimination of molecular signals by the olfactory receptor neuron. Neuron. 1994;13:771–790. doi: 10.1016/0896-6273(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 39.Sonty RY, Juniano SL. Development of intrinsic connections in cat somatosensory cortex. J Comp Neurol. 1997;384:501–516. [PubMed] [Google Scholar]
- 40.Tanabe T, Iino M, Takagi SF. Discrimination of odors in olfactory bulb, pyriform-amygdaloid areas, and orbitofrontal cortex of the monkey. J Neurophysiol. 1975;38:1284–1296. doi: 10.1152/jn.1975.38.5.1284. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka K. Mechanisms of visual object recognition: monkey and human. Curr Opin Neurobiol. 1997a;7:523–529. doi: 10.1016/s0959-4388(97)80032-3. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka K. Columnar organization in inferotemporal cortex. In: Rockland KS, editor. Cerebral cortex, Vol 11. Plenum; New York: 1997b. pp. 469–498. [Google Scholar]
- 43.Tseng G-F, Haberly LB. Deep neurons in piriform cortex. I. Morphology and synaptically evoked responses including a unique high amplitude paired shock facilitation. J Neurophysiol. 1989;62:369–385. doi: 10.1152/jn.1989.62.2.369. [DOI] [PubMed] [Google Scholar]
- 44. Wang G, Tanifuju M, Tanaka K. Functional architecture in monkey inferotemporal cortex revealed by in vivo optical imaging. Neurosci Res 32 1998. 33:46. [DOI] [PubMed] [Google Scholar]
- 45.Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young MP, Scannell JW, Burns GA, Blakemore C. Analysis of connectivity: neural systems in the cerebral cortex. Rev Neurosci. 1994;5:227–250. doi: 10.1515/revneuro.1994.5.3.227. [DOI] [PubMed] [Google Scholar]
- 47.Zeki S. A vision of the brain, pp 151–157. Blackwell Scientific; Oxford: 1993. [Google Scholar]
- 48.Zeki S, Shipp S. The functional logic of cortical connections. Nature. 1988;335:311–317. doi: 10.1038/335311a0. [DOI] [PubMed] [Google Scholar]