Abstract
The role of the cytoskeleton in the activity of GABAAreceptors was investigated in cultured hippocampal neurons. Receptor currents were measured with the whole-cell patch-clamp technique during repetitive stimulation with 1 μm muscimol. After destruction of the microtubular system with nocodazol, muscimol-induced currents showed a rundown by 78%. A similar rundown was observed when actin fibers were destroyed with latrunculin B or C2 toxin of Clostridium botulinum. Because the small GTPases of the Rho family RhoA, Rac1, and Cdc42 are known to control the organization of actin fibers, we investigated their possible involvement. Inactivation of the GTPases with clostridial toxins, as well as intracellular application of recombinant Rho GTPases, indicated that active Rac1 was necessary for full GABAA receptor activity. Immunocytochemical labeling of the receptors showed that the disappearance of receptor clusters in the somatic membrane as induced by muscimol stimulation was enhanced by Rac1 inactivation. It is suggested that Rac1 participates in the regulation of GABAAreceptor clustering and/or recycling.
Keywords: GABAA receptor rundown, actin cytoskeleton, microtubules, hippocampal neurons, Rac1 GTPase, receptor clusters
The open probability and open times of ligand-gated ion channels are regulated by agonists and modulators. In addition, the cytoskeleton can regulate ligand-gated ion channels by mediating their anchorage and organization within the cell membrane. Both tubulin and actin structures seem to be involved. Thus, glutamate receptors of the NMDA type are anchored in the membrane via actin fibers and the linker protein α-actinin-2 (Rosenmund and Westbrook, 1993; Ehlers et al., 1996; Wyszynski et al., 1997; Allison et al., 1998). Depolymerization of the actin fibers by cytochalasin D or the C2 toxin of Clostridium botulinum causes the rapid rundown of the NMDA receptor-mediated currents (Rosenmund and Westbrook, 1993).
The involvement of actin fibers indicates a role of the small GTPases of the Rho family RhoA, Rac1, and Cdc42 in the control of NMDA receptor activity. They act as molecular switches and organize the actin cytoskeleton (Hall, 1994, 1998; Mackay et al., 1995; Nobes and Hall, 1995). Thus, RhoA controls the formation of cytosolic actin fibers, whereas Rac1 organizes the formation of the cortical actin. Indeed, NMDA receptors in cultured hippocampal neurons showed a rapid rundown after the specific inactivation of RhoA by the C3 toxin of C. botulinum (Aktories et al., 1989; Nörenberg et al., 1999).
Glycine-gated anion channels are anchored in the membrane via microtubules and the linker protein gephyrin (Prior et al., 1992;Kirsch and Betz, 1995), whereas actin fibers organize the density of the receptor clusters (Kirsch and Betz, 1995). Apparently, type A receptors for GABA also bind to gephyrin. Thus, the protein has been found at GABAergic postsynaptic membranes (Sassoe-Pognetto et al., 1995; Todd et al., 1995; Craig et al., 1996). Moreover, GABAA receptor subtypes that contain the γ2 subunit form clusters with the help of gephyrin (Essrich et al., 1998). In addition, the protein GABARAP, which is similar or even identical to microtubule-associated protein 1B, seems to connect GABAA and GABAC receptors to microtubules (Hanley et al., 1999; Wang et al., 1999). It is unclear, however, whether microtubules affect the activity of GABAA receptors.
The association of GABAA receptors with actin structures is still controversial. Although actin has been found to coprecipitate with α1 subunits of the GABAAreceptors extracted from bovine brain (Kannenberg et al., 1997), the destruction of actin fibers does not affect the clustering of the receptors (Allison et al., 1998). Although there is yet no evidence that actin fibers influence the activity of GABAAreceptors, their destruction induced the rundown of GABAC receptor-mediated currents (Filippova et al., 1999).
Here, we have investigated whether microtubules and actin fibers regulate the activity of somatodendritic GABAAreceptors in whole-cell voltage-clamped hippocampal neurons in primary culture. The destruction of microtubules, as well as of actin fibers, caused a rundown in GABAA receptor currents. Further studies with use of clostridial cytotoxins to inactivate the GTPases of the Rho family, as well as with recombinant GTPases, showed that Rac1 organized the actin fibers that are functionally connected with GABAA receptor.
MATERIALS AND METHODS
Cultures of hippocampal neurons. Primary cultures of neurons were prepared from hippocampi of newborn Wistar rats (postnatal day 0) as described previously (Benz et al., 1998). The dissociated neuronal and astroglial cells were seeded on laminin-coated coverslips and cultured with DMEM–F12 medium that contained insulin, selenite, and transferrin, as well as B27 supplement. Cultures were incubated at 37°C in an humidified atmosphere of 95% air and 5% CO2 for 8–10 d.
Electrophysiology. Experiments were performed on hippocampal neurons with pyramidal cell-like morphology. Membrane currents through GABAA receptors were recorded in the whole-cell configuration. In some experiments, amphotericin-perforated patch-clamp recordings were made (Rae et al., 1991). The external (bath) solution contained (in mm): NaCl 162, KCl 2.4, CaCl2 1.2, MgCl2 1, HEPES 10, and glucose 11 (∼320 mOsm, pH 7.3, with NaOH). Tetrodotoxin (0.5 μm) was added to inhibit action potentials. The pipette (internal) solution contained (in mm): CsCl 140, CaCl2 1, MgCl2 2, HEPES 10, and EGTA 11 (pH 7.2, ∼300 mOsm). The calculated free Ca2+ concentration was 11 nm (Program WinMAXC; C. Patton, Stanford University, Pacific Grove, CA). All solutions were used at room temperature. All membrane potential values were corrected for the liquid junction potential (5 mV) (Barry, 1994). For amphotericin-perforated patches, patch pipettes were front-filled by dipping for ∼1 sec in filtered pipette solution. Thereafter, they were back-filled with the pipette solution that contained 240 μg/ml amphotericin B. The borosilicate patch pipettes (GB 150–8P; Science Products, Hofheim, Germany) had a resistance of 1.5–3.5 MΩ, when filled with pipette solution.
Whole-cell currents were recorded, and cell capacitance (Cm) and series resistance (Rs) were partially compensated (60–80%) with an EPC-7 amplifier (List, Darmstadt, Germany). At the beginning of the recording period, i.e., 20 min after gaining whole-cell access, control settings were 29.0 ± 1.0 pF forCm and 12.4 ± 0.4 MΩ forRs in conventional whole-cell measurements (n = 161). At the end of the experiment, i.e., 25 min later, these values had not changed significantly, indicating that the recording conditions had remained stable. In amphotericin-perforated patches, stableRs values were consistently achieved within 20 min after seal formation. At that time,Cm andRs values found in perforated patches were similar to those in whole-cell recordings (27.2 ± 1.9 pF and 12.2 ± 0.8 MΩ; n = 24). Again, recording conditions remained stable over time. Current records were filtered at 3 kHz, digitized at 1 kHz (CED 1401; Cambridge Electronic Devices, Cambridge, UK), and analyzed with a laboratory computer using software from Cambridge Electronic Devices.
Unless stated otherwise, experimental agents were introduced into the cells by diffusion from the patch pipette. Therefore, the system was allowed to equilibrate for at least 20 min after whole-cell access had been achieved. To investigate GABAA receptor currents, the agonist muscimol (0.03–100 μm) was applied by means of a fast-flow pressurized superfusion system (DAD-12; Adams and List, New York, NY), which completely exchanges the bath medium in the vicinity of the investigated cells within <200 msec (Kügelgen et al., 1997).
Preparation of clostridial cytotoxins and recombinant proteins. Toxin B and lethal toxin from C. difficileand C. sordellii, respectively, were prepared as described previously (Hofmann et al., 1997). C2I protein, the enzymatic component of C2 toxin from C. botulinum, and C3 toxin from C. limosum were purified as described previously (Böhmer et al., 1996; Barth et al., 1998a,b). If not mentioned otherwise, the toxins were applied intracellularly via the patch pipette.
Full-length cDNAs coding for wild-type Rac1, a dominant negative form of Rac1 with Thr at position 17 mutated to Asn (Rac1N17), and wild-type Cdc42 (Cdc42) were subcloned into the pGEX-2T vector (Amersham Pharmacia Biotech, Uppsala, Sweden). The resulting pGEX-Rac1 and Cdc42 plasmids were used to obtain the glutathione S-transferase (GST) fusion proteins by expression into Escherichia coliBL21 cells. After induction of the expression with 100 μmisopropyl-β-d-thiogalactopyranoside (Sigma, Deisenhofen, Germany) for 20 hr at 29°C, cells were resuspended in PBS containing 0.1% Triton X-100 and then lysed by sonication. Lysates were centrifuged for 10 min at 12,000 ×g, and GST fusion proteins were purified from the supernatants on glutathione Sepharose 4B (Amersham Pharmacia Biotech) according to the instructions of the manufacturer. Cleavage of the desired proteins from the immobilized GST was achieved by incubating the sample with the protease thrombin (Sigma). Thereafter, thrombin was removed from the eluted protein by absorption ontop-aminobenzamidine-agarose. Finally the purified proteins were checked by SDS-PAGE.
Staining of microtubules. Immunocytochemistry for β-tubulin III was used to analyze microtubules. After fixation with paraformaldehyde (4%), cells were washed and incubated with a monoclonal mouse anti-β-tubulin III antibody (Sigma). The resulting immune complex was visualized with CyTM3-conjugated F(ab′)2 fragment goat anti-mouse IgG (Dianova, Hamburg, Germany).
GABAA receptor immunofluorescence.Immunocytochemistry for the α2 subunit of GABAAreceptors was used to localize GABAA receptors in the neuronal membrane. After fixation with precooled methanol for 10 min at −20°C, cells were washed with PBS, blocked with 4% normal serum, and incubated with a polyclonal guinea pig anti-α2 subunit antiserum (Dr. M. Fritschy, Department of Pharmacology, University of Zurich, Zurich, Switzerland). The resulting immune complex was visualized with CyTM3-conjugated F(ab′)2 fragment goat anti-guinea pig IgG (Dianova, Hamburg, Germany).
Confocal microscopy. Neurons were imaged using a Bio-Rad (Hercules, CA) MRC 1024 (version 3.2) confocal system with a krypton–argon laser and a Zeiss (Oberkochen, Germany) Axiovert 135TV microscope. CyTM3 fluorescence was measured using an excitation wavelength of 554 nm and an emission filter set at 576 nm. A 63× water objective lens was used, and the laser intensity, photomultiplier gain, and pinhole aperture were kept constant for all experiments. Images were obtained using Laser Sharp 2.1T software and processed using Corel Photopaint.
Data evaluation. Current amplitudes were measured at the peak response (average of 10 data points). The current amplitudes at 10 and 25 min were expressed as percent of controls, i.e., the response at 0 min (mean ± SEM of n trials). Differences between means were tested for significance by the Kruskall–Wallis test, followed by Mann–Whitney U test. In some cases, a modifiedt test (Bonferroni–Dunn) for multiple comparisons was used.p < 0.05 was the accepted level of significance.
Materials. Muscimol was from Tocris Cookson (Bristol, UK). If not mentioned otherwise, all drugs and agents used were from Sigma.
RESULTS
Neurons with pyramidal morphology were used for the experiments (see Fig. 2B). In all hippocampal neurons tested (n = 185), a concentration of muscimol >0.03 μm elicited inward currents at a holding potential of −70 mV. This holding potential was used in all experiments. The peak response to the first challenge with 1 μm muscimol ranged from −587 to −3379 pA in cells from different preparations but rarely differed in neurons from the same batch.
Fig. 2.
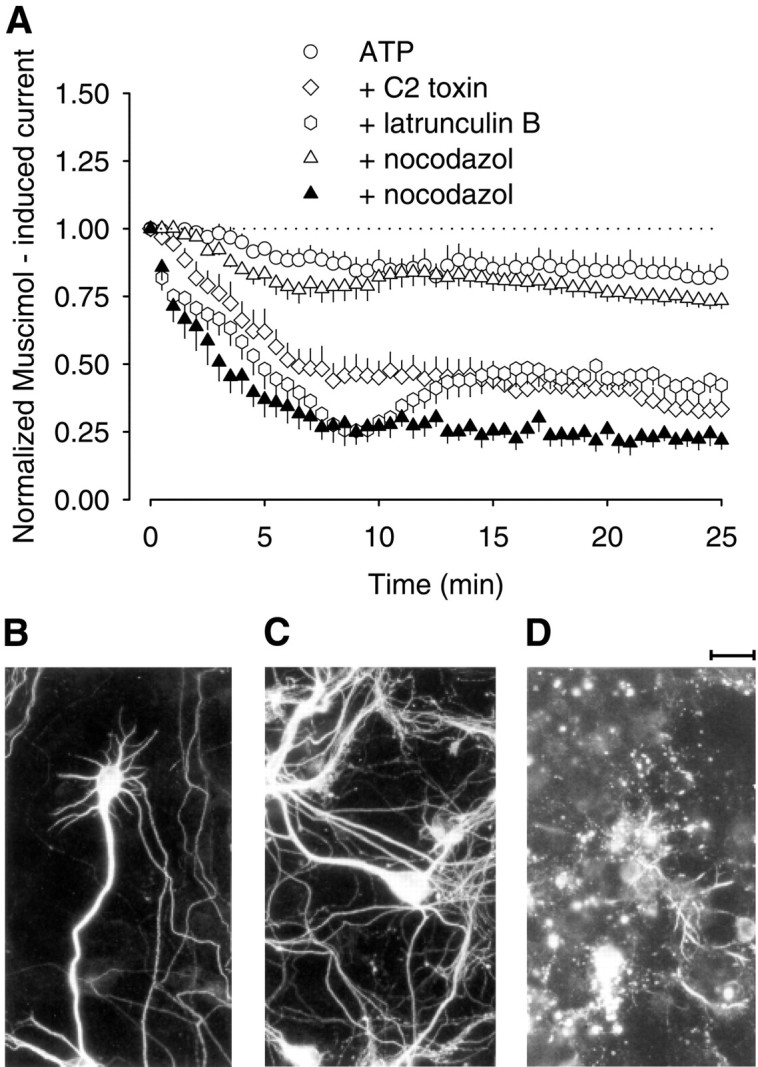
Microtubules, as well as actin filaments, are involved in the maintenance of GABAA receptor activity in rat hippocampal neurons. A, Time course of GABAA receptor current rundown after repetitive stimulation with muscimol. Pipette solutions contained Mg-ATP (4 mm) alone, Mg-ATP plus C2 toxin (10 ng/ml), Mg-ATP plus latrunculin B (2 μm), and Mg-ATP plus nocodazol (2 μm,open triangles; 10 μm, filled triangles). Parameters for repetitive stimulation with 1 μm muscimol correspond to those of Figure 1. In the experiment with 10 μm nocodazol, the culture was also preincubated with 10 μm nocodazol for at least 4 hr. Currents were expressed as percent of the first muscimol application atT0 (n = 5–6 each; means ± SEM). Immunohistochemistry for β-tubulin III in hippocampal neurons in controls (B), after incubation with nocodazol (10 μm) for 0.5 hr (C) and for 4 hr (D).
As was to be expected for GABA-gated Cl−channels, the current reversal potential (Vrev) was −4.2 ± 2.1 mV (n = 5) and thus close to chloride equilibrium potential (−3.7 mV). Furthermore,Vrev responded to changes in the external Cl− concentration like a Nernstian Cl− electrode (data not shown).
The inward currents induced by muscimol were concentration-dependent and reached a plateau at ∼10 μm. The Hill slope was 1.2, and the EC50 was 0.8 μm(n = 9). The GABAAreceptor-selective antagonist bicuculline (10 μm) (Quian and Dowling, 1994; Bormann and Feigenspan, 1995) reduced the currents induced by 1 μm muscimol by 97.0 ± 1.4% (n = 5). Therefore, muscimol apparently activated GABAA and not GABACreceptors in the hippocampal neurons.
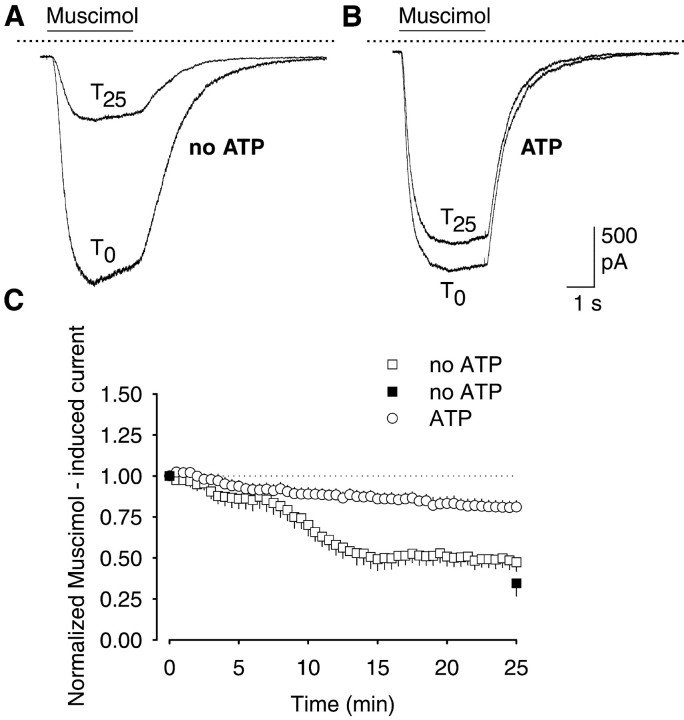
To test the stability of the GABAA receptor currents, the cultured hippocampal neurons were repetitively stimulated with 1 μm muscimol for 25 min, i.e., twice per minute for 3 sec. In the absence of ATP in the patch pipette, there was a time-dependent decrease in GABAA receptor currents (Fig.1A,C). The current decreased to 47.4 ± 5.5% (n = 8) of the first response after 25 min of stimulation. A similar decline was found when cells were only stimulated twice, i.e., at the beginning and at the end of the 25 min period (34.5 ± 7.7%; n= 7) (Fig. 1C). Mg-ATP (4 mm) in the pipette solution largely prevented the time-dependent decrease (Fig.1B,C). The current amplitudes were now 81.0 ± 4.0% of controls after 25 min of repetitive stimulation (n = 7; p > 0.05). All further experiments were performed with 4 mmMg-ATP in the patch pipette.
Fig. 1.
Intracellular ATP prevents rundown of GABAA receptor currents in rat hippocampal neurons during repetitive stimulation. Inward currents were evoked by 3 sec pressure application of 1 μm muscimol at 30 sec intervals over 25 min. A, B, Superimposed inward currents after the first (T0) and last (T25) application of muscimol. Currents were recorded at a holding potential of −70 mV with pipette solutions without (A) or with (B) 4 mm Mg-ATP. Thesolid, horizontal linesabove the currenttracesindicate the periods of muscimol pressure application; thedotted lines indicate the zero current level.C, Grouped mean data showing the time course of GABAA receptor current rundown. Currents were expressed as percent of the first muscimol application atT0 (n = 7–8; means ± SEM). The filled squares show the effects of muscimol (1 μm) when applied only twice, i.e., atT0 and T25.
Although these findings confirmed that intracellular ATP is necessary for maintaining GABAA receptor function (Gyenes et al., 1988, 1994; Stelzer et al., 1988; Sweetnam et al., 1988; Chen et al., 1990), they also emphasized the difference between GABAA and GABAC receptors, because the latter show an ATP-induced decrement in current amplitude (Filippova et al., 1999). GABAA receptor channels can undergo desensitization, which has been defined on the macroscopic level as current decay in the presence of an agonist (Jones and Westbrook, 1995; Berger et al., 1998). We also considered the possibility that the decrease in GABAA receptor currents observed during repetitive stimulation was attributable to cumulative desensitization, which trapped a progressively increasing fraction of GABAA receptor channels in the nonconductive state. In our experiments, currents desensitized by ∼10% at the beginning of repetitive stimulation and after 25 min (Table 1). Moreover, the extent of desensitization was independent of the absence or presence of intracellular Mg-ATP, which strongly affected rundown. Also neurons stimulated either 51 times or only twice showed the same extent of desensitization (Table 1, Fig. 1C). Hence, current desensitization and current rundown seemed to be independent of each other.
Table 1.
Effects of ATP and lethal toxin on desensitization of GABAA receptor channels in rat hippocampal neurons
| Pipette | Desensitization (%) | |||
|---|---|---|---|---|
| T0 | T10 | T20 | T25 | |
| No ATP | 10.0 ± 2.0 | 10.2 ± 1.7 | 10.0 ± 1.1 | 10.2 ± 1.6 |
| No ATP | 11.0 ± 2.0 | 11.3 ± 2.2 | ||
| ATP | 9.5 ± 2.2 | 10.2 ± 2.3 | 10.6 ± 2.4 | 10.6 ± 2.0 |
| ATP + lethal toxin | 9.4 ± 3.6 | 11.6 ± 2.7 | 11.5 ± 2.2 | 12.5 ± 3.1 |
Muscimol (1 μm) was pressure applied for 3 sec to cultured rat hippocampal neurons, and current amplitudes were measured at the peak response, as well as at the end of the 3 sec application period by means of the whole-cell patch clamp technique. Starting 20 min after gaining whole-cell access (T0), muscimol was applied either regularly at every 30 sec over 25 min or twice, once at the beginning (T0) and once at the end (T25) of the experiment. The extent of current desensitization is shown at the start of the experiment (T0) and 10 (T10), 20 (T20), and 25 (T25) min after the first agonist application. It is expressed as the percentage by which current amplitudes decayed within the 3 sec of muscimol application. Data were obtained from the experiments shown in Figures 1and 3. Mean ± SEM are shown. The desensitization values did not differ.
Microtubules are involved in the maintenance of GABAAreceptor activity
First, we tested the possible role of tubulin structures in GABAA receptor activity. Because colchicine can act as a competitive antagonist at GABAAreceptors, we used nocodazol to destroy the microtubules (Samson et al., 1979; Weiner et al., 1998). Nocodazol (2 μm) had no effect when applied into neurons via the patch pipette for 20 min before and during the experiment. After 25 min of repetitive stimulation with muscimol, the current peak amplitudes were 73.5 ± 2.9% of the control value measured at 0 min (n = 6) (Fig. 2A). To evaluate the destructive effect of nocodazol on the microtubules, we used immunocytochemistry for β-tubulin III. When neurons were treated with 10 μm nocodazol for 30 min, no change of the tubular cytoskeleton was observed (Fig. 2C). When cells were preincubated with 10 μm nocodazol for 4 hr, however, the microtubular network was completely destroyed (Fig.2D). Under these conditions, the muscimol-induced currents showed a pronounced rundown (21.9 ± 3.4% of control;n = 6) (Fig. 2A). These findings indicated that intact microtubules were essential for the activity of GABAA receptors.
Actin filaments are also necessary for GABAAreceptor activity
Next, we tested whether actin fibers affected the activity of GABAA receptors. The C2 toxin of C. botulinum selectively ADP-ribosylates actin monomers and thereby prevents their polymerization (Aktories et al., 1986;Vandekerckhove et al., 1988; Aktories and Wegner, 1992). Because actin filaments are continuously rebuilt, C2 toxin ultimately causes their breakdown. When applied via the patch pipette, C2 toxin (10 ng/ml) reduced the current amplitudes induced by repetitive stimulation with muscimol. After 10 min of stimulation, the current was reduced to 45.2 ± 5.9% of the initial value. After 25 min, it declined further to 33.4 ± 3.9% (n = 6) (Fig.2A). In contrast, in controls, the current was only reduced to 83.7 ± 5.1% of the initial value after 25 min of stimulation (n = 5) (Fig. 2A). Next, we used latrunculin B, which also prevents actin polymerization. Within 25 min of repetitive stimulation with muscimol, latrunculin B (2 μm) reduced the current to 42.3 ± 5.4% of the initial value (n = 6) (Fig.2A). Together, these findings indicated that actin filaments were essential for the activity of the GABAA receptors.
The GTPase Rac1 maintains the GABAAreceptor activity
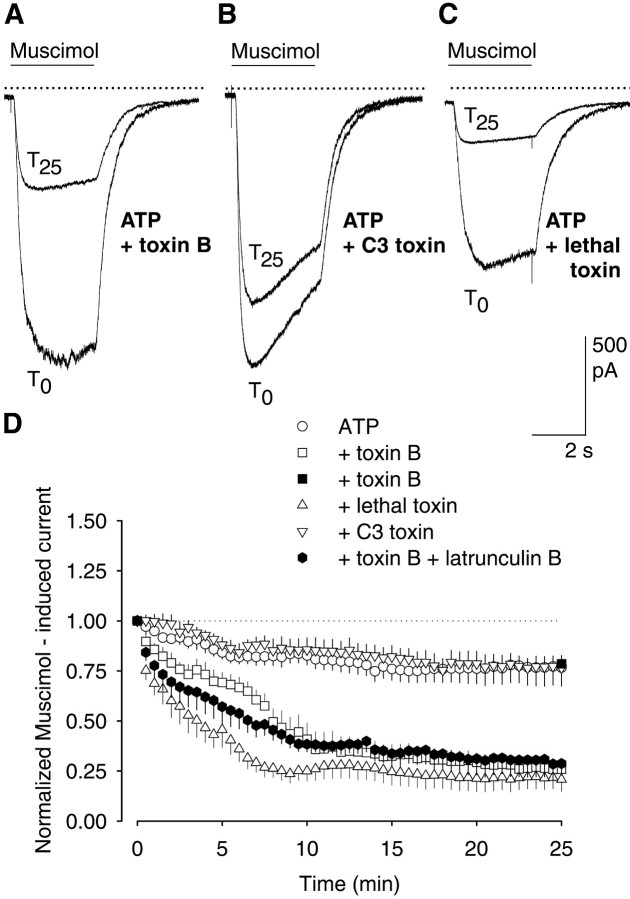
GTPases of the Rho family control the polymerization of actin but do not affect tubulin fibers (Hall, 1994, 1998; Mackay et al., 1995;Nobes and Hall, 1995; Best et al., 1996). To test whether Rho GTPases regulate the function of GABAA receptors, we used toxin B from C. difficile, which inactivates Rho, Rac1, and Cdc42 (Just et al., 1995). When applied via the patch pipette, toxin B reduced within 25 min the muscimol-induced current amplitudes to 25.9 ± 4.2% (n = 7) (Fig.3A,D), whereas in controls, the currents were reduced to only 85.7 ± 2.1% (n = 6) (Fig. 3D). Not only was the extent of the toxin B-induced reduction similar to the effects of C2 toxin and latrunculin B, but so was the time course (compare with Fig.2). Apparently, Rho GTPases were indeed involved in maintaining the activity of GABAA receptors.
Fig. 3.
Inactivation of Rho GTPases by clostridial toxins induces a use-dependent GABAA receptor rundown in rat hippocampal neurons. A–C, Superimposed inward currents evoked at T0 andT25. Toxins were added to pipette solutions containing 4 mm Mg-ATP. A, Toxin B (5 ng/ml); B, C3 toxin (6 μg/ml); C, lethal toxin (50 ng/ml). Parameters for repetitive stimulation with 1 μm muscimol correspond to those of Figure 1. Thesolid, horizontal linesabove the currenttracesindicate the periods of muscimol pressure application; thedotted lines indicate the zero current level.D, Grouped mean data showing the time course of GABAA receptor current rundown. Filled squares show effects of muscimol (1 μm) applied only twice, i.e., at T0 andT25, in the presence of intracellular toxin B. Currents were expressed as percent of the first muscimol application at T0 (n = 6–7; means ± SEM).
To test whether the toxin B-induced current reduction depended on the repetitive stimulation of the GABAA receptors, we applied muscimol only twice, i.e., at the beginning and at the end of the 25 min period. Under these conditions, the second current amplitude was 78.5 ± 4.1% (n = 6) of the initial value, despite the presence of toxin B in the cytosol (Fig. 3D). Thus, toxin B only decreased the currents if the GABAA receptors were repetitively stimulated.
Next, we applied toxin B (5 ng/ml) together with latrunculin B (2 μm), to test whether toxin B had effects that were independent of the actin fibers. The combined application reduced the muscimol currents within 25 min to 24.7 ± 5.6% of the initial value (n = 6) (Fig. 3D). This decrease was not significantly different from the reduction observed with toxin B alone (Fig. 3).
Because toxin B inactivates Rho, Rac1, and Cdc42, we next tried to identify the GTPase involved. The possible role of Rho A was tested with C3 toxin from C. botulinum, which selectively inactivates this GTPase (Aktories et al., 1989). However, C3 toxin did not reduce the muscimol-induced currents (76.5 ± 4.6% after 25 min of repetitive stimulation; p > 0.05;n = 7) (Fig. 3B,D). Because Rac1 or Cdc42 seemed to be involved, we next applied the lethal toxin of C. sordellii, which is known to inactivate these two GTPases (Just et al., 1996). Indeed, in the presence of lethal toxin, the GABAA receptor-mediated current declined during repetitive stimulation with muscimol (Fig.3C,D). It was 20.8 ± 5.8% of the initial value at the end of the 25 min period (Fig. 3D), whereas the maximal reduction was observed after 15 min (n = 7) (Fig. 3D).
In contrast to its effects on current peak amplitudes, lethal toxin had no influence on the current decay during repetitive muscimol applications. Hence, inactivation of Rac1 and/or Cdc42 did not seem to interfere with GABAA receptor desensitization (Table 1).
Finally, we tested whether the lethal toxin (50 ng/ml) from C. sordellii affected the activity of the GABAAreceptors during the usual waiting period before the stimulation. For this purpose, neurons from the same preparation were dialyzed with normal or lethal toxin containing pipette solution for 20 min in the whole-cell configuration. Thereafter, the cells were stimulated once with 1 μm muscimol. The current responses under these conditions were −1426 ± 320 and −1309 ± 252 pA, respectively (n = 6 each; p > 0.05). Together, these experiments showed that inactivation of the GTPases Rac1 and/or Cdc42 by clostridial toxins reduced GABAA receptor activity during the period of repetitive stimulation.
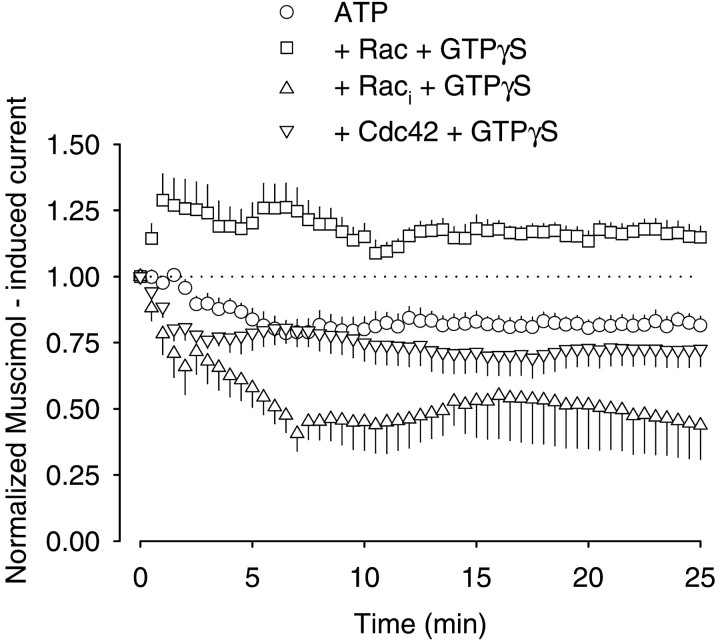
Because there are no toxins available that selectively inactivate Rac1 or Cdc42, a different approach was used to determine which of the two GTPases regulated the GABAA receptors. We applied recombinant GTPase proteins via the patch pipette together with 0.3 mm GTPγS, which is necessary for their activation. After 25 min of stimulation with muscimol, currents were 81.5 ± 3.5% (n = 5) of the initial value in the presence of intracellular Mg-ATP alone. When GTPγS (0.3 mm) was added, they declined to 78.4 ± 5.4% (n = 6;p > 0.05). Recombinant Cdc42 (100 ng/ml) also had no effect on the muscimol-induced currents (72.5 ± 6.5% after 25 min of stimulation; n = 6) (Fig.4). However, 100 ng/ml recombinant Rac1 significantly elevated the currents as a result of repetitive stimulation. An increase over the initial value was observed within 5 min of stimulation (Fig. 4). After the 25 min stimulation period, the current was still 114.8 ± 4.2% of the initial value [compared with 81.5 ± 3.5% in controls (p < 0.05;n = 7)] (Fig. 4). In contrast, application of a dominant inactive form of recombinant Rac1 reduced the current to 43.8 ± 12.9% (p < 0.05) of the initial value (n = 6) (Fig. 4). Together, these findings indicated that Rac1 was involved in the control of GABAAreceptor activity.
Fig. 4.
Effects of recombinant Rac1 on GABAAreceptor currents in rat hippocampal neurons. Pipette solutions contained Mg-ATP (4 mm) or Mg-ATP plus the recombinant GTPases Rac, its constitutively inactive form Raci together with GTPγS, and Cdc42 together with GTPγS (0.3 mm). Parameters for repetitive stimulation with 1 μm muscimol corresponded to those in Figure 1. Currents were normalized by comparison to the first muscimol application atT0 (n = 5–7; means ± SEM).
Inactivation of Rac does not affect GABAA receptor affinity for muscimol
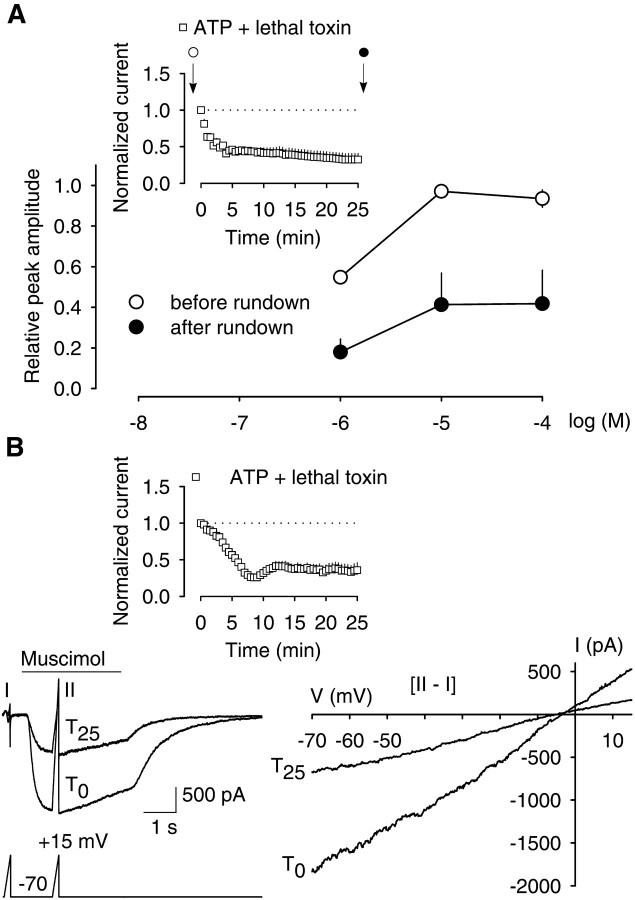
The inactivation of Rac1 by clostridial toxins may diminish GABAA receptor activity by disturbing ligand–receptor interactions or the resulting downstream events. Next, we tested whether inactivation of Rac1 by lethal toxin affected the ligand–receptor interaction. For this purpose, two concentration–response curves with muscimol were made in the presence of intracellular lethal toxin (50 ng/ml). The first curve was done at the beginning of the stimulation period when the effect of the lethal toxin was not yet observed. The second curve was made at the end of the usual 25 min period of repetitive stimulation, when the effect of the lethal toxin was pronounced (rundown to 32.4 ± 8.4% after 25 min) (Fig. 5A,inset). Muscimol concentrations of 1, 10, and 100 μm were chosen because they represented the EC50 (1 μm), as well as maximal and supramaximal concentrations. Before and after repetitive stimulation, muscimol concentrations of 10 and 100 μm produced maximal responses, the absolute values of which differed. In both curves, 1 μmmuscimol caused a half-maximal response, i.e., the EC50 was independent of treatment with lethal toxin. However, lethal toxin plus repetitive stimulation lowered the current amplitudes induced by 10 or 100 μmmuscimol to ∼40% of the respective pretreatment values (n = 5) (Fig. 5A). Thus, a reduction in GABAA receptor affinity was not the reason for the observed rundown.
Fig. 5.
A, Lethal toxin of C. sordellii does not affect the affinity of GABAAreceptors to muscimol. Half-maximal (1 μm), maximal (10 μm), and supramaximal (100 μm) muscimol concentrations were tested in neurons immediately before and after the rundown of GABAA receptor currents (seearrows in inset), which was induced by intracellular lethal toxin (50 ng/ml) plus repetitive stimulation with 1 μm muscimol. Currents were reduced to 32.4 ± 8.5% after 25 min of stimulation. The resulting current amplitudes were normalized by comparison to the maximal response obtained with 100 μm muscimol in the respective individual experiments before induction of rundown. Shown are means ± SEM (n = 5). B, Lethal toxin ofC. sordellii does not affect the reversal potential (Vrev) of GABAAreceptor-mediated currents. In this experiment, rundown of GABAA receptor currents was also induced by intracellular lethal toxin (50 ng/ml) plus repetitive stimulation with 1 μm muscimol. A representative recording of five experiments shows how Vrev of GABAA receptor-mediated currents was assessed (left panel). Fast voltage ramps, 200 msec from the holding potential of −70 to +15 mV, were applied immediately before every application of muscimol (T0 toT25), as well as at approximately each peak response to the agonist (T0 toT25; bottom trace). Also shown are superimposed current traces obtained in response to the voltage-ramps imposed before (I) and during (II) the application of muscimol at the first (T0) and last (T25) challenge with muscimol (left panel, top trace). Under these conditions, the current was decreased to 35.9 ± 10.0% (inset; n = 5). The respective current–voltage (I–V) relationships are shown in the right panel. I–V curves obtained in response to the first (T0) and last (T25) challenge were superimposed. Curves were obtained by subtracting current responses to the voltage ramp in the absence of muscimol from those in its presence (II–I).
Next, we tested whether the inactivation of Rac1 by lethal toxin affected the driving force through open GABAAchannels, which is one determinant of the GABAAreceptor-mediated peak current. This parameter is determined by the difference between membrane potential V (voltage-clamped at −70 mV) minus the reversal potential for the current,Vrev. When fast voltage ramps were used to measure the Vrev of muscimol-induced currents during the repetitive stimulation protocol in the presence of intracellular lethal toxin (50 ng/ml) (Fig.5B), Vrev did not change; at the start it was −6.2 ± 3.4, and at the end of the repetitive stimulation it was −5.6 ± 2.5 mV. Under these conditions, the muscimol-induced currents showed the usual rundown (to 35.9 ± 10.0%;n = 5) (Fig. 5B, inset). Thus, inhibition of Rac1 did not alter the driving force through open GABAA receptor channels, suggesting that the electrochemical Cl− gradient over the cell membrane, as well as the selective Cl− permeability of the channel pore, remained unchanged.
GABAA receptor clusters in the neuronal membrane depend on the cytoskeleton
Continuous stimulation with GABAA receptor agonists has been shown to reduce the current amplitude and, in addition, receptor density in the plasma membrane (Tehrani and Barnes, 1991). Therefore, we investigated with use of GABAA receptor immunocytochemistry whether the nocodazol and lethal toxin affected the organization of GABAA receptors in the plasma membrane. Because hippocampal neurons are known to express GABAAreceptors, which contain the α2 subunit (Essrich et al., 1998;Kannenberg et al., 1999), we used a respective antiserum. Untreated hippocampal neurons showed granular immunoreactivity in dendritic and somatic membranes (Fig.6A). When the neurons were treated for 25 min with 1 μm muscimol, the number of these granules appeared to be reduced (Fig.6B). In contrast, treatment of the neurons with lethal toxin for 1 hr did not reduce the number of GABAA receptor clusters (Fig.6D). After pretreatment with lethal toxin and subsequent stimulation with muscimol, however, hardly any clusters were observed in the somatic membrane (Fig. 6F). To test the involvement of microtubules in GABAA receptor organization, a respective experiment was performed with nocodazol. Treatment of the neurons with nocodazol for 4 hr did not affect the clusters (Fig. 6C), whereas the subsequent treatment with muscimol strongly reduced the number of receptor clusters in the somatic membrane (Fig. 6E). Together, these data showed that destruction of the actin, as well as of the microtubular cytoskeleton alone, did not affect the number of receptor clusters in the somatic membrane, although it facilitated the muscimol-induced reduction.
Fig. 6.
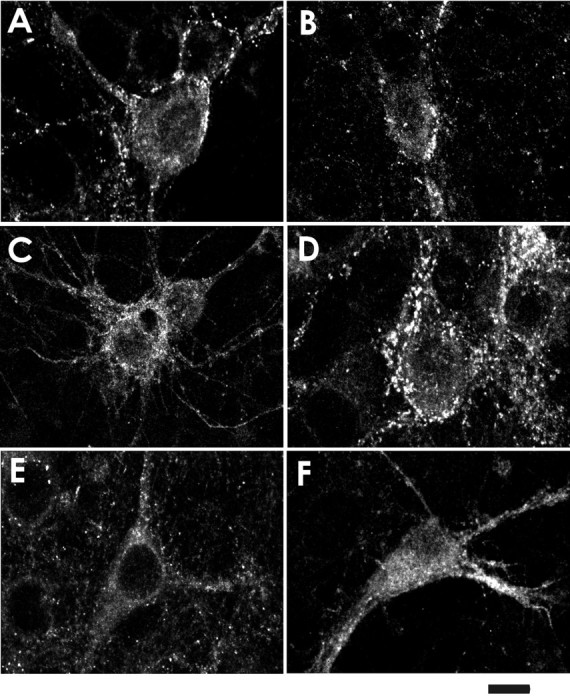
Nocodazol and lethal toxin of C. sordellii enhance the reduction in GABAA receptor clusters induced by repetitive stimulation with muscimol. Immunohistochemistry for α2 subunit of GABAA receptors after destruction of microtubular or actin cytoskeleton with or without subsequent muscimol application. A, Controls; B, muscimol (1 μm for 25 min);C, nocodazol (10 μm for 4 hr);D, lethal toxin (50 ng/ml for 1 hr); E, nocodazol (10 μm for 4 hr) plus subsequent muscimol (1 μm for 25 min); F, lethal toxin (50 ng/ml for 1 hr) plus subsequent muscimol (1 μm for 25 min). Scale bar, 25 μm.
Lethal toxin of C. sordellii also affects GABAA receptors in perforated patches
A relatively high negative pressure is necessary to disrupt the cell membrane to gain whole-cell access. Consequently, cytoskeletal structures may be disturbed (Rosenmund and Westbrook, 1993), which may affect the activity of GABAA receptors in an unspecific manner. In addition, cytosolic components may be lost by diffusion, when the whole-cell patch mode is applied. Such a loss of ATP may have contributed to the decreased response to muscimol observed in Figure 1. Because the cytoskeleton is much less disturbed in the perforated patch configuration, we used this procedure for further analysis. Amphotericin was applied to form membrane pores that allow the diffusion of small ions but not of larger molecules (Cass et al., 1970; Holz and Finkelstein, 1970). In such hippocampal neurons, there was no decrease in muscimol-induced current when cells were stimulated only twice (96.3 ± 9.6%; n = 7) (Fig.7D). Repetitive stimulation with 1 μm muscimol, however, reduced the current within 25 min to 43.4 ± 3.2% of the initial value (n = 5) (Fig.7A,D).
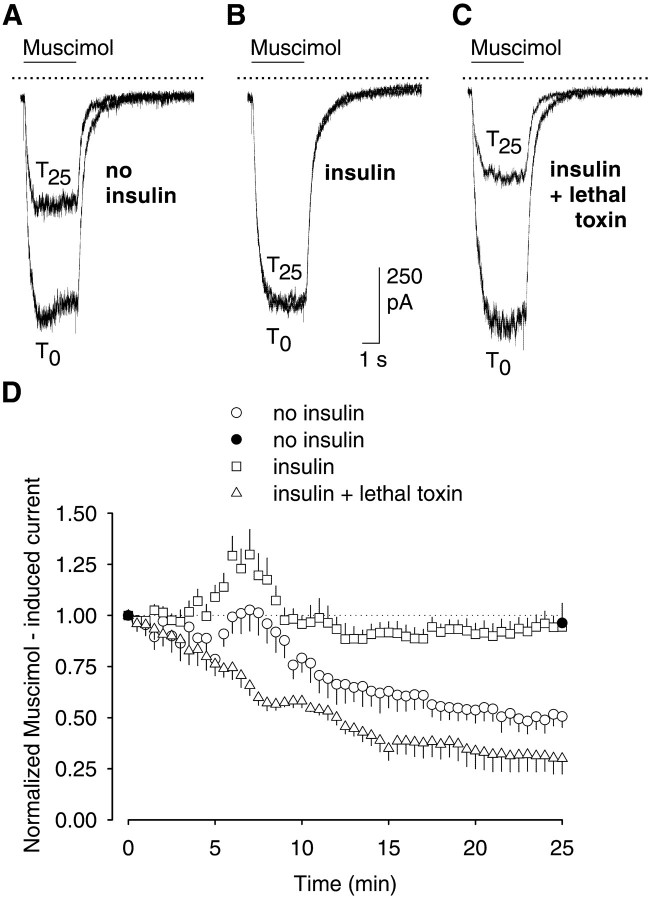
Fig. 7.
Insulin prevents the ATP-independent rundown of GABAA receptor currents in rat hippocampal neurons. Inward currents were evoked by 3 sec pressure application of 1 μm muscimol at 30 sec intervals over 25 min. Representative inward currents evoked at the holding potential of −70 mV in amphotericin B perforated patch recording configuration after first (T0) and last (T25) application of muscimol were superimposed and are shown in A–C. A, Control; B, insulin (10 μg/ml) was present in the incubation medium during a 12 hr preincubation period, as well as during the actual experiment; C, protocol (B) was used, and in addition, lethal toxin (50 ng/ml) was added 1 hr before the start of the experiments. The solid, horizontal lines above thecurrent traces indicate the period of muscimol pressure application; the dotted lines indicate the zero current level. The respective time courses of GABAA receptor currents are shown in D. In addition, the effects of muscimol (1 μm) are shown when it was applied only twice, i.e., at T0 andT25, in the absence of extracellular insulin (filled circles). Currents were normalized by comparison to the first muscimol application atT0 (n = 7 each; means ± SEM).
To also test the possible involvement of Rac1 in this model, we used insulin, which has been reported to activate Rac1 (Nishiyama et al., 1994). Incubation of the cells with insulin (10 μg/ml) for 12 hr before and during the actual experiment prevented the use-dependent reduction in muscimol-induced current (n = 5) (Fig.7B,D). This effect of insulin was blocked when the cells were additionally incubated with lethal toxin (50 ng/ml) for 60 min before the experiment (Fig.7C,D). Under these conditions, the muscimol-induced current was 30.0 ± 7.6% of the initial value (n = 7). However, when insulin was added to the bath medium after 12.5 min of repetitive stimulation, i.e., when the rundown was already established, it did not reverse the rundown (data not shown).
These results indicated that Rac1 also regulated GABAA receptor activity in cells in which the intracellular milieu, as well as the cytoskeleton, remained relatively undisturbed.
DISCUSSION
The present study tested the hypothesis that microtubules and microfilaments are relevant for the function of GABAA receptors. Hippocampal neurons were treated with nocodazol, which breaks down microtubules. Latrunculin B and the C2 toxin of C. botulinum were used to depolymerize F-actin and thus break down cytoskeletal actin fibers (Spector et al., 1983, 1989; Aktories et al., 1986; Allison et al., 1998). After these treatments, muscimol-induced currents showed a rundown of 60–82% during 25 min of repetitive stimulation. Together, these data show that the function of GABAAreceptors is connected to microtubules, as well as microfilaments. These actin fibers seem to be under the control of the small GTPase Rac1, inactivation of which also facilitated the muscimol-induced rundown of GABAA receptor activity. In view of our previous finding that glutamate receptors of the NMDA type are functionally coupled to RhoA-dependent actin fibers (Nörenberg et al., 1999), we present the first evidence that different GTPases of the Rho family may regulate different types of ligand-gated ion channels.
Until now, there was only indirect evidence that the activity of GABAA receptors is affected by the cytoskeleton. The lack of direct evidence is surprising because microtubular depolymerization has been shown previously to inhibit the muscimol-induced uptake of Cl− into cerebral cortical microsacs (Whatley et al., 1994). Moreover, biochemical data suggest a connection between GABAA receptors and tubulin via the linker proteins gephyrin and GABARAP (Item and Sieghart, 1994; Essrich et al., 1998; Wang et al., 1999). The previous lack of electrophysiological data on receptor activity may be attributable to the kinetics of tubular reorganization in neurons. Thus, destruction of the microtubules could not be induced by a 30 min but only by a 4 hr pretreatment with nocodazol, which indeed facilitated the muscimol-induced rundown.
Data on the connections of actin filaments with GABAA receptors are also sparse. Here, biochemical findings show that actin coprecipitates with α1 subunits of GABAA receptors extracted from bovine brain (Kannenberg et al., 1997). It is unknown, however, whether other α subunit isoforms, such as the α2 subunit found in rat hippocampal neurons (Essrich et al., 1998), are also coupled to actin filaments. Thus, to our knowledge, the present data are the first evidence that GABAA receptor activity is coupled to actin fibers.
The GTPases of the Rho family RhoA, Rac1, and Cdc42 regulate the organization of actin fibers in cells. Toxin B from C. difficile and lethal toxin from C. sordellii inactivate the GTPases via glucosylation and galactosylation, respectively (Just et al., 1995, 1996). Toxin B inhibits all GTPases of the Rho family, whereas lethal toxin blocks Rac1 and Cdc42. Both toxins facilitated the rundown of GABAA receptor activity. When toxin B was applied together with latrunculin B, there was no synergistic effect. Therefore, we conclude that inactivation of the GTPase, which was subsequently identified as Rac1, induced the rundown of GABAA receptor activity via degradation of microfilaments. This identification was performed with recombinant Rho proteins. Upon intracellular application via the patch pipette, the wild-type form of Cdc42 proved to be inactive, whereas that of Rac1 enhanced the current induced by muscimol. Some Rho GTPase effector molecules, such as proteins of the PAK family or p70S6K, seem to be shared by Rac1 and Cdc42 (for review, see Ridley, 1996). The selective effect of Rac1 indicated, however, that these effectors were not involved in the control of GABAA receptor activity. The additional finding that the constitutively inactive form of Rac1 also decreased GABAA receptor activity corroborated the involvement of this GPTase, because inactive forms of the GTPases can block the respective endogenous proteins by binding and thereby trapping GDP/GTP exchange factors, which are necessary for activation (Seasholtz et al., 1999).
Several mechanisms were excluded by which Rac1 and the dependent actin fibers may have reduced GABAA receptor activity. Thus, inactivation of Rac1 by lethal toxin decreased neither the affinity of the GABAA receptors to muscimol nor the Cl− driving force through open GABAA receptor channels. It also seems unlikely that inactivation of Rac1 with lethal toxin induced a state of cumulative receptor desensitization, which then caused the rundown, because desensitization was not affected by the toxin. It remains possible, however, that inactivation of Rac1 may have reduced the unitary conductance of the channel and/or the channel open probability. The latter mechanism is of particular interest in view of reports that cytoskeletal elements and Rho GTPases can regulate the open probability of some K+ channels (Benz et al., 1998;Cachero et al., 1998), as well as of NMDA receptor channels (Ehlers et al., 1996).
Inhibition of Rac1 with lethal toxin or destruction of actin or tubulin fibers did not affect the GABAA currents induced by the initial pulses of muscimol but enhanced the rundown caused by repetitive stimulation with the agonist. Similarly, treatment of hippocampal neurons with lethal toxin or nocodazol did not reduce the density of GABAA receptor clusters in the somatic membrane as shown by immunocytochemistry (Allison et al., 1998) but facilitated the disappearance of receptor clusters caused by continuous receptor stimulation with muscimol. Thus, the disappearance of GABAA receptor clusters was related to the measured loss of activity.
It is presently unknown whether disassembly of the clusters into single receptors already caused their loss of activity. There is some indirect evidence in favor of this speculation. Thus, GABAA receptors that lack the γ2 subunit do not cluster and show a reduced conductance (Günther et al., 1995;Essrich et al., 1998). Moreover, cluster formation depends on the presence of gephyrin in the membrane, indicating that the cytoskeleton is involved (Essrich et al., 1998). However, it cannot be excluded that receptor internalization caused the disappearance of the clusters and the reduction of receptor activity.
There is growing evidence that Rac1 and actin fibers are involved in membrane organization and recycling (Bretscher, 1996; Radhakrishna et al., 1999). The process of receptor recycling seems to consist of two steps that are regulated separately, i.e., endocytosis of receptors and their subsequent reinsertion into the membrane (exocytosis). Endocytosis of GABAA receptors via clathrin-coated vesicles is induced by repetitive stimulation with an agonist and can result in a significant reduction in membrane-located GABAA receptors, i.e., downregulation (Tehrani and Barnes, 1991; Tehrani et al., 1997; Connolly et al., 1999). The present data are in accord with the hypothesis that destruction of the actin or tubulin cytoskeleton facilitated the muscimol-induced receptor endocytosis, resulting in the reduction of muscimol-induced currents. This hypothesis would imply that an intact cytoskeleton reduces receptor endocytosis.
Recently, it has been shown that GABAA receptors are not only internalized but also recycled (Filippova et al., 2000). Activation of protein kinase C facilitates rundown of GABAA receptor currents and receptor downregulation after repetitive stimulation by inhibiting receptor exocytosis (Chapell et al., 1998; Connolly et al., 1999; Filippova et al., 2000). It is doubtful that destruction of the cytoskeleton diminished receptor exocytosis in our experiments, because inhibition of constitutive exocytosis should have resulted in reduced receptor clusters already in the absence of muscimol.
Endocytosis and exocytosis of membrane proteins, such as the Na+/H+exchanger and/or the transferrin receptor, are under the control of phosphatidylinositol 3-kinase (PI3-K) (Martys et al., 1996; Kurashima et al., 1998). This has been also shown for GABAAreceptors (Connolly et al., 1999). Because PI3-K is an effector of Rac1 (Hartwig et al., 1995; Tolias et al., 1995), these findings present indirect evidence for a control of the GABAAreceptor recycling by the GTPase. There is further evidence for such a role of Rac1. Insulin, which activates Rac1 (Nishiyama et al., 1994), has been shown to recruit functional GABAAreceptors into the membrane within 10 min of application (Wan et al., 1997). In our perforated patch experiments, pretreatment with insulin prevented the use-dependent reduction in current. This effect was abolished by lethal toxin. However, insulin did not reverse the use-dependent reduction once it was established, indicating that receptor exocytosis did not occur within the observation period.
In contrast to GABAA receptors, NMDA receptors do not seem to be cycled into and out of the synaptic membrane (Carroll et al., 1999). This observation may explain our previous finding that NMDA receptor activity is independent of Rac1 activity. Instead, it is functionally coupled to RhoA-dependent actin fibers (Nörenberg et al., 1999), which may provide anchorage within the membrane (Rosenmund and Westbrook, 1993).
Our previous finding that Rac1 is strongly expressed in pyramidal neurons of the hippocampus of the adult rat (Olenik et al., 1997) indicates that the GTPase may have important functions in differentiated neurons. The present findings suggest that Rac1 is involved in the control of GABAA receptor density and function. If the GTPase indeed regulates GABAA receptor clustering and/or endocytosis, other receptors that undergo recycling could also be under the control of Rac1. Such a role requires that the GTPase itself is regulated in its activity. Among several candidates, insulin deserves some attention because it is found in relevant concentrations in the brain (Havrankova et al., 1978, 1979).
Footnotes
The study was funded by Deutsche Forschungsgemeinschaft Grant SFB 505/B6. We thank Drs. P. Jonas and M. Kohlhardt for critical reading of this manuscript. The help of Dr. S. Cox in editing this manuscript is appreciated.
Correspondence should be addressed to Dr. D. K. Meyer, Department of Pharmacology, Albert-Ludwigs-University, Hermann-Herder-Straße 5, D-79104 Freiburg, Germany. E-mail meyerdk@uni-freiburg.de.
REFERENCES
- 1.Aktories K, Wegner A. Mechanisms of the cytopathic action of actin-ADP-ribosylating toxins. Mol Microbiol. 1992;6:2905–2908. doi: 10.1111/j.1365-2958.1992.tb01749.x. [DOI] [PubMed] [Google Scholar]
- 2.Aktories K, Barmann M, Ohishi I, Tsuyama S, Jakobs KH, Habermann E. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986;322:390–392. doi: 10.1038/322390a0. [DOI] [PubMed] [Google Scholar]
- 3.Aktories K, Braun U, Rosener S, Just I, Hall A. The rho gene product expressed in E. coli is a substrate of botulinum ADP-ribosyltransferase C3. Biochem Biophys Res Commun. 1989;158:209–213. doi: 10.1016/s0006-291x(89)80199-8. [DOI] [PubMed] [Google Scholar]
- 4.Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry P. JPCalc, a software package for calculating liquid junction potential in patch clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 6.Barth H, Preiss JC, Hofmann F, Aktories K. Characterization of the catalytic site of the ADP-ribosyltransferase Clostridium botulinum C2 toxin by site-directed mutagenesis. J Biol Chem. 1998a;273:29506–29511. doi: 10.1074/jbc.273.45.29506. [DOI] [PubMed] [Google Scholar]
- 7.Barth H, Hofmann F, Olenik C, Just I, Aktories K. The N-terminal part of the enzyme component (C2I) of the binary Clostridium botulinum C2 toxin interacts with the binding component C2II and functions as a carrier system for a Rho ADP-ribosylating C3-like fusion toxin. Infect Immun. 1998b;66:1364–1369. doi: 10.1128/iai.66.4.1364-1369.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benz I, Meyer DK, Kohlhardt M. Properties and the cytoskeletal control of Ca++-independent large conductance K+ channels in neonatal rat hippocampal neurons. J Membr Biol. 1998;161:275–286. doi: 10.1007/s002329900334. [DOI] [PubMed] [Google Scholar]
- 9.Berger T, Schwarz C, Kraushaar U, Monyer H. Dentate gyrus basket cell GABAA receptors are blocked by Zn2+ via changes of their desensitization kinetics: an in situ patch-clamp and single-cell PCR study. J Neurosci. 1998;18:2437–2448. doi: 10.1523/JNEUROSCI.18-07-02437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Best A, Ahmed S, Kozma R, Lim L. The Ras-related GTPase Rac1 binds tubulin. J Biol Chem. 1996;271:3756–3762. doi: 10.1074/jbc.271.7.3756. [DOI] [PubMed] [Google Scholar]
- 11.Böhmer J, Jung M, Sehr P, Fritz G, Popoff M, Just I, Aktories K. Active site mutation of the C3-like ADP-ribosyltransferase from Clostridium limosum: analysis of glutamic acid 174. Biochemistry. 1996;35:282–289. doi: 10.1021/bi951784+. [DOI] [PubMed] [Google Scholar]
- 12.Bormann J, Feigenspan A. GABAC receptors. Trends Neurosci. 1995;18:515–518. doi: 10.1016/0166-2236(95)98370-e. [DOI] [PubMed] [Google Scholar]
- 13.Bretscher MS. Getting membrane flow and the cytoskeleton to cooperate in moving cells. Cell. 1996;87:601–606. doi: 10.1016/s0092-8674(00)81380-x. [DOI] [PubMed] [Google Scholar]
- 14.Cachero TG, Morielli AD, Peralta EG. The small GTP-binding protein RhoA regulates a delayed rectifier potassium channel. Cell. 1998;93:1077–1085. doi: 10.1016/s0092-8674(00)81212-x. [DOI] [PubMed] [Google Scholar]
- 15.Carroll RC, Beattie EC, Xia H, Lüscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci USA. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cass A, Finkelstein A, Krespi V. The ion permeability induced in lipid membranes by the polyene antibiotics nystatin and amphotericin B. J Gen Physiol. 1970;56:100–124. doi: 10.1085/jgp.56.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapell R, Bueno OF, Alvarez-Hernandez X, Robinson LC, Leidenheimer NJ. Activation of protein kinase C induces gamma-aminobutyric acid type A receptor internalization in Xenopus oocytes. J Biol Chem. 1998;273:32595–32601. doi: 10.1074/jbc.273.49.32595. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Stelzer A, Kay A, Wong R. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol (Lond) 1990;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connolly CN, Kittler JT, Thomas P, Uren JM, Brandon NJ, Smart TG, Moss SJ. Cell surface stability of gamma-aminobutyric acid type A receptors. Dependence on protein kinase C activity and subunit composition. J Biol Chem. 1999;274:36565–36572. doi: 10.1074/jbc.274.51.36565. [DOI] [PubMed] [Google Scholar]
- 20.Craig AM, Banker G, Chang W, McGrath ME, Serpinskaya AS. Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons. J Neurosci. 1996;16:3166–3177. doi: 10.1523/JNEUROSCI.16-10-03166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehlers MD, Zhang S, Bernhadt JP, Huganir RL. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell. 1996;84:745–755. doi: 10.1016/s0092-8674(00)81052-1. [DOI] [PubMed] [Google Scholar]
- 22.Essrich C, Lorez M, Benson JA, Fritschy JM, Lüscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 23.Filippova N, Dudley R, Weiss DS. Evidence for phosphorylation-dependent internalization of recombinant human rho1 GABAC receptors. J Physiol (Lond) 1999;518:385–399. doi: 10.1111/j.1469-7793.1999.0385p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filippova N, Sedelnikova A, Zong Y, Fortinberry H, Weiss DS. Regulation of recombinant gamma-aminobutyric acid (GABA)(A) and GABA(C) receptors by protein kinase C. Mol Pharmacol. 2000;57:847–856. [PubMed] [Google Scholar]
- 25.Günther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, Lang Y, Bluethmann H, Möhler H, Lüscher B. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gyenes M, Farrant M, Farb D. “Run-down” of γ-aminobutyric acidA receptor function during whole-cell recording: a possible role for phosphorylation. Mol Pharmacol. 1988;34:719–723. [PubMed] [Google Scholar]
- 27.Gyenes M, Wang Q, Gibbs T, Farb D. Phosphorylation factors control neurotransmitter and neuromodulator actions at the γ-aminobutyric acid type A receptor. Mol Pharmacol. 1994;46:542–549. [PubMed] [Google Scholar]
- 28.Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Ann Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- 29.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 30.Hanley JG, Koulen P, Bedford F, Gordon-Weeks PR, Moss SJ. The protein MAP-1B links GABA(C) receptors to the cytoskeleton at retinal synapses. Nature. 1999;397:66–69. doi: 10.1038/16258. [DOI] [PubMed] [Google Scholar]
- 31.Hartwig JH, Bokoch GM, Carpenter CL, Janmey PA, Taylor LA, Toker A, Stossel TP. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 32.Havrankova J, Schmechel D, Roth J, Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci USA. 1978;75:5737–5741. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Havrankova J, Roth J, Brownstein MJ. Concentrations of insulin and insulin receptors in the brain are independent of peripheral insulin levels. Studies of obese and streptozotocin-treated rodents. J Clin Invest. 1979;64:636–642. doi: 10.1172/JCI109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofmann F, Busch C, Prepens U, Just I, Aktories K. Localization of the glucosyltransferase activity of Clostridium difficile toxin B to the N-terminal part of the holotoxin. J Biol Chem. 1997;272:11074–11078. doi: 10.1074/jbc.272.17.11074. [DOI] [PubMed] [Google Scholar]
- 35.Holz R, Finkelstein A. The water and non-electrolyte permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J Gen Physiol. 1970;56:125–145. doi: 10.1085/jgp.56.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Item C, Sieghart W. Binding of gamma-aminobutyric acidA receptors to tubulin. J Neurochem. 1994;63:1119–1125. doi: 10.1046/j.1471-4159.1994.63031119.x. [DOI] [PubMed] [Google Scholar]
- 37.Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 38.Just I, Selzer J, Wilm M, Eichel-Streiber CV, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 39.Just I, Selzer J, Hofmann F, Green GA, Aktories K. Inactivation of Ras by Clostridium sordellii lethal toxin-catalyzed glucosylation. J Biol Chem. 1996;271:10149–10153. doi: 10.1074/jbc.271.17.10149. [DOI] [PubMed] [Google Scholar]
- 40.Kannenberg K, Baur R, Sigel E. Proteins associated with alpha 1-subunit-containing GABAA receptors from bovine brain. J Neurochem. 1997;68:1352–1360. doi: 10.1046/j.1471-4159.1997.68041352.x. [DOI] [PubMed] [Google Scholar]
- 41.Kannenberg K, Sieghart W, Reuter H. Clusters of GABAA receptors on cultured hippocampal cells correlate only partially with functional synapses. Eur J Neurosci. 1999;11:1256–1264. doi: 10.1046/j.1460-9568.1999.00533.x. [DOI] [PubMed] [Google Scholar]
- 42.Kirsch J, Betz H. The postsynaptic localization of the glycine receptor-associated protein gephyrin is regulated by the cytoskeleton. J Neurosci. 1995;15:4148–4156. doi: 10.1523/JNEUROSCI.15-06-04148.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kügelgen Nörenberg W, IV, Illes P, Schobert A, Starke K. Differences in the mode of stimulation of cultured rat sympathetic neurones between ATP and UDP. Neuroscience. 1997;78:935–941. doi: 10.1016/s0306-4522(96)00691-4. [DOI] [PubMed] [Google Scholar]
- 44.Kurashima K, Szabo EZ, Lukacs G, Orlowski J, Grinstein S. Endosomal recycling of the Na+/H+ exchanger NHE3 isoform is regulated by the phosphatidylinositol 3-kinase pathway. J Biol Chem. 1998;273:20828–20836. doi: 10.1074/jbc.273.33.20828. [DOI] [PubMed] [Google Scholar]
- 45.Mackay DJG, Nobes CD, Hall A. The Rho's progress: a potential role during neuritogenesis for the Rho family of GTPases. Trends Neurosci. 1995;18:496–501. doi: 10.1016/0166-2236(95)92773-j. [DOI] [PubMed] [Google Scholar]
- 46.Martys JL, Wjasow C, Gangi DM, Kielian MC, McGraw TE, Backer JM. Wortmannin-sensitive trafficking pathways in Chinese hamster ovary cells. Differential effects on endocytosis and lysosomal sorting. J Biol Chem. 1996;271:10953–10962. doi: 10.1074/jbc.271.18.10953. [DOI] [PubMed] [Google Scholar]
- 47.Nishiyama T, Sasaki T, Takaishi K, Kato M, Yaku H, Araki K, Matsuura Y, Takai Y. rac p21 is involved in insulin-induced membrane ruffling and rho p21 is involved in hepatocyte growth factor- and 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced membrane ruffling in KB cells. Mol Cell Biol. 1994;14:2447–2456. doi: 10.1128/mcb.14.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 49.Nörenberg W, Hofmann F, Illes P, Aktories K, Meyer DK. Rundown of somatodendritic N-methyl-d-aspartate (NMDA) receptor channels in rat hippocampal neurones: evidence for a role of the small GTPase RhoA. Br J Pharmacol. 1999;127:1060–1063. doi: 10.1038/sj.bjp.0702643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olenik C, Barth H, Just I, Aktories K, Meyer DK. Gene expression of the small GTP binding proteins RhoA, RhoB, Rac1, and Cdc42 in adult brain. Mol Brain Res. 1997;52:263–269. doi: 10.1016/s0169-328x(97)00270-2. [DOI] [PubMed] [Google Scholar]
- 51.Prior P, Schmitt B, Grenningloh G, Pribilla I, Multhaup G, Beyreuther K, Maulet Y, Werner P, Langosch D, Kirsch J. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron. 1992;8:1161–1170. doi: 10.1016/0896-6273(92)90136-2. [DOI] [PubMed] [Google Scholar]
- 52.Quian H, Dowling J. Pharmacology of novel GABA receptors found on rod horizontal cells of the white perch retina. J Neurosci. 1994;14:4299–4307. doi: 10.1523/JNEUROSCI.14-07-04299.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson JG. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci. 1999;112:855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- 54.Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- 55.Ridley AJ. Rho: theme and variations. Curr Biol. 1996;6:1256–1264. doi: 10.1016/s0960-9822(02)70711-2. [DOI] [PubMed] [Google Scholar]
- 56.Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- 57.Samson F, Donoso JA, Heller-Bettinger I, Watson D, Himes RH. Nocodazol action on tubulin assembly, axonal ultrastructure and fast axoplasmic transport. J Pharmacol Exp Ther. 1979;208:411–417. [PubMed] [Google Scholar]
- 58.Sassoe-Pognetto M, Kirsch J, Grunert U, Greferath U, Fritschy JM, Mohler H, Betz H, Wassle H. Colocalization of gephyrin and GABAA-receptor subunits in the rat retina. J Comp Neurol. 1995;357:1–14. doi: 10.1002/cne.903570102. [DOI] [PubMed] [Google Scholar]
- 59.Seasholtz TM, Majumdar M, Brown JH. Rho as a mediator of G protein-coupled receptor signaling. Mol Pharmacol. 1999;55:949–956. doi: 10.1124/mol.55.6.949. [DOI] [PubMed] [Google Scholar]
- 60.Spector I, Shochet N, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- 61.Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins—novel marine macrolides that disrupt microfilament organization and affect cell growth. I. Comparison with cytochalasin D. Cell Motil Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- 62.Stelzer A, Kay A, Wong R. GABAA receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988;241:339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- 63.Sweetnam P, Lloyd J, Gallombardo P, Malison R, Gallager D, Tallman J, Nestler E. Phosphorylation of the GABAa/Benzodiazepine receptor a subunit by a receptor associated protein kinase. J Neurochem. 1988;51:1274–1284. doi: 10.1111/j.1471-4159.1988.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 64.Tehrani MH, Barnes EM., Jr Agonist-dependent internalization of gamma-aminobutyric acidA/benzodiazepine receptors in chick cortical neurons. J Neurochem. 1991;57:1307–1312. doi: 10.1111/j.1471-4159.1991.tb08295.x. [DOI] [PubMed] [Google Scholar]
- 65.Tehrani MH, Baumgartner BJ, Barnes EM., Jr Clathrin-coated vesicles from bovine brain contain uncoupled GABAA receptors. Brain Res. 1997;776:195–203. doi: 10.1016/s0006-8993(97)01037-8. [DOI] [PubMed] [Google Scholar]
- 66.Todd AJ, Spike RC, Chong D, Neilson M. The relationship between glycine and gephyrin in synapses of the rat spinal cord. Eur J Neurosci. 1995;7:1–11. doi: 10.1111/j.1460-9568.1995.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 67.Tolias KF, Cantley LC, Carpenter CL. Rho family GTPases bind to phosphoinositide kinases. J Biol Chem. 1995;270:17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- 68.Vandekerckhove J, Schering B, Barmann M, Aktories K. Botulinum C2 toxin ADP-ribosylates cytoplasmic beta/gamma-actin in arginine 177. J Biol Chem. 1988;263:696–700. [PubMed] [Google Scholar]
- 69.Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- 70.Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- 71.Weiner JL, Buhler AV, Whatley VJ, Harris RA, Dunwiddie TV. Colchicine is a competitive antagonist at human recombinant gamma-aminobutyric acidA receptors. J Pharmacol Exp Ther. 1998;284:95–102. [PubMed] [Google Scholar]
- 72.Whatley VJ, Mihic SJ, Allan AM, McQuilkin SJ, Harris RA. Gamma-aminobutyric acidA receptor function is inhibited by microtubule depolymerization. J Biol Chem. 1994;269:19546–19552. [PubMed] [Google Scholar]
- 73.Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M. Competitive binding of alpha-actinin and calmodulin to the NMDA receptor. Nature. 1997;385:439–442. doi: 10.1038/385439a0. [DOI] [PubMed] [Google Scholar]