Abstract
In the chick embryo, facial motor neurons comprise branchiomotor and visceral motor subpopulations, which innervate branchial muscles and parasympathetic ganglia, respectively. Although facial motor neurons are known to develop within hindbrain rhombomere 4 (r4) and r5, the precise origins of branchiomotor and visceral motor neuron subpopulations are unclear. We investigated the organization and axon pathfinding of these motor neurons using axonal tracing and rhombomere transplantation in quail-chick chimeras. Our results show that a large majority of branchiomotor neurons originate in r4 but that a cohort of these neurons undergoes a caudal migration from r4 into r5. By contrast, visceral motor neurons develop exclusively in r5. We found that a striking property of facial visceral motor neurons is the ability of their axons to navigate back to appropriate ganglionic targets in the periphery after heterotopic transplantation. These results complement previous studies in which heterotopic facial branchiomotor neurons sent axons to their correct, branchial arch, target. By contrast, when trigeminal branchiomotor neurons were transplanted heterotopically, we found that they were unable to pathfind correctly, and instead projected to an inappropriate target region. Thus, facial and trigeminal motor neuron populations have different axon pathfinding characteristics.
Keywords: facial nerve, branchiomotor neuron, visceral motor neuron, rhombomere, hindbrain, axon pathfinding
Cranial motor neurons innervate a variety of muscles and ganglia in the vertebrate head. Within the embryonic chick hindbrain, trigeminal motor neurons occupy rhombomere 2 (r2) and r3, whereas facial motor neurons occupy r4 and r5 (Lumsden and Keynes, 1989; Lumsden 1990). Trigeminal neurons are of branchiomotor (BM) type and project via a dorsal exit point in r2 to first branchial arch muscles. Facial motor neurons comprise branchiomotor and visceral motor (VM) neuronal subpopulations, which project via a dorsal r4 exit point to second branchial arch muscles and parasympathetic ganglia, respectively (Fig. 1A). The question thus arises of whether facial BM and VM neuronal subpopulations are distributed throughout r4 and r5 or are contained within single rhombomeres.
Fig. 1.
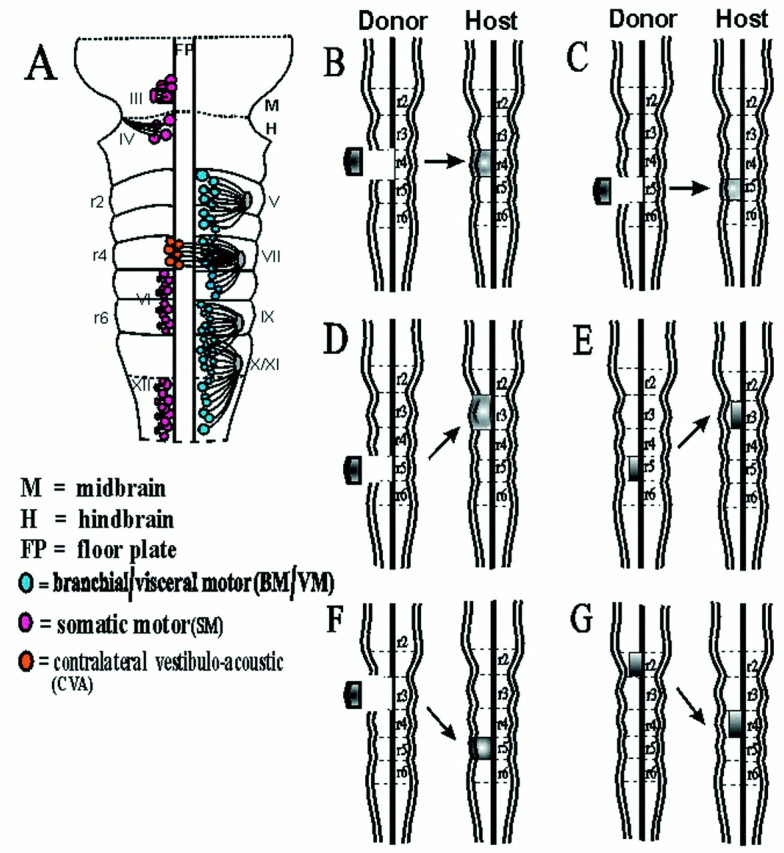
Distribution of motor neurons in the hindbrain and summary of rhombomere transplants. A, Diagram of the ventral aspect of a stage 21 chick embryo showing the motor nuclei of the branchial nerves (V, trigeminal; VII,facial; IX, glossopharyngeal; and X/XI,vagus/cranial accessory) and the somatic motor nuclei (III, oculomotor; IV, trochlear;VI, abducens; and XII, hypoglossal) based on axon tracing (modified after Lumsden, 1990). B–G,Diagrams of orthotopic and heterotopic transplants from donor quail embryos to host chick embryos: r4 orthotopic (B), r5 orthotopic (C), r5 to r3 (D), r5 basal plate to r3 (E), r3 to r5 (F), and r2 basal plate to r4 (G).
In the rodent embryo, BM and VM neurons occupy r4 and r5, respectively (Fritzsch and Nichols, 1993; Auclair et al., 1996; Studer et al., 1996;McKay et al., 1997). The majority of r4 BM neurons migrate caudally, giving rise to the genu of the facial nerve, whereas VM neurons migrate laterally within r5 (Auclair et al., 1996; McKay et al., 1997). In the chick embryo the facial nerve lacks a distinctive genu, and facial motor neurons translocate their cell bodies laterally (Simon et al., 1994). Nevertheless, this does not eliminate the possibility that some BM neurons might also migrate caudally.
BM and VM axons from particular axial levels actively pathfind to target regions that are populated by neural crest cells derived from the same axial level (Lumsden et al., 1991). This topographic correspondence might depend on a system of receptors and ligands on motor axons and branchial arch mesenchyme cells that is governed by the matched expression of repertoires of Hox genes (Lumsden and Keynes, 1989; Hunt et al., 1991). If such a system operates, then motor neurons transplanted to ectopic positions might be able to pathfind back to appropriate targets. However, studies in which the rostrocaudal polarity of r3 was reversed showed that a proportion of trigeminal motor neurons projected via the inappropriate exit point in r4 into the second branchial arch, an inappropriate target (Warrilow and Guthrie, 1999). In contrast, r4 facial motor neurons transplanted to r2 position rerouted their axons to navigate back to the second branchial arch (Bell et al., 1999). It is not clear whether these data highlight a general difference in the behavior of trigeminal and facial motor neurons or in the behavior of motor neurons that reside in odd- and even-numbered rhombomeres.
We have investigated the distribution of facial BM and VM neurons in chick embryos, by axon tracing, and by testing the repertoire of axon projections derived from r4 and r5 when these were transplanted orthopically in quail-chick chimeras. Orthotopic r4 transplants were also used to examine whether BM neurons migrate caudally from r4 into r5. Heterotopic rhombomere transplants were then used to discover whether graft-derived BM or VM axons navigated to appropriate or inappropriate targets in the periphery.
MATERIALS AND METHODS
Retrograde labeling. Rhode Island Red hens' eggs were incubated at 38°C to stage 20–28 (Hamburger and Hamilton, 1951). After removal of membranes, embryos were pinned ventral side up in a Sylgard-coated dish containing Ringer's solution. Surrounding mesenchyme was removed from the facial nerve and its branches, nerves were transected, and fluorescein and/or rhodamine dextrans (Molecular Probes, Eugene, OR) were applied for retrograde axonal tracing as previously described (Varela-Echavarría et al., 1996). After overnight fixation in 3.5% paraformaldehyde (PFA), hindbrains were flat-mounted and viewed under a confocal microscope (Bio-Rad, Hercules, CA; MRC-600).
Retrograde labeling of the hyoid nerve in stage 25 embryos with an r4 orthotopic graft was performed in a few cases, to track the migration pattern of BM neurons in this rhombomere. These embryos were fixed in Dent's fixative (1:4 DMSO–methanol) before immunofluorescence using QCPN antibody (which recognizes a quail-specific perinuclear antigen; Developmental Hybridoma Bank, Iowa City, IA).
Microsurgery. Stage 10–12 embryos were used in stage-matched quail-to-chick orthotopic and heterotopic grafting experiments. Operations were performed as previously described (Guthrie and Lumsden, 1992). Briefly, pieces of hindbrain neuroepithelium corresponding to whole rhombomeres or basal plate portions of r2, r3, r4, or r5 were excised from host embryos, using tungsten needles (Fig.1B–G). Host neuroepithelium was replaced by orthotopic or heterotopic quail rhombomere grafts as shown (Fig.1B–G). After surgery, eggs were sealed with tape and returned to the incubator. Surviving chimeras were harvested at stage 25–30, and the heads were removed and fixed overnight in either 3.5% PFA, before vibratome-sectioning and immunohistochemistry, or in Dent's fixative for whole-mount immunohistochemistry.
Immunohistochemistry. Fixed embryonic tissues for whole-mount immunostaining were washed extensively in 1% Triton X-100 (Tx; Sigma, Poole, UK) in PBS, and endogenous peroxidases were inactivated using 0.1% hydrogen peroxide solution, before a blocking step in 10% sheep serum and 0.1% Tx–PBS for 2 d at 4°C; all subsequent steps were done at this temperature. Embryos were then incubated in a 1:4 dilution of both the quail-specific antibodies QCPN and QN (which recognizes a quail axon antigen; a generous gift of Dr. H. Tanaka; Tanaka et al., 1990) in 1% sheep serum and 0.1% Tx–PBS for 3 d. After further extensive washing in 0.1% Tx–PBS, embryos were incubated in a peroxidase-conjugated secondary antibody (goat anti-mouse; Jackson ImmunoResearch, West Grove, PA) diluted at 1:100 in the same solution for 3 d. Embryos were washed extensively in PBS, followed by 0.1m Tris buffer, pH 7.2, and the reaction product was developed using diaminobenzidine (DAB; Sigma) at 0.5 mg/ml in the same buffer. Before photography, embryos were cleared in benzylalcohol–benzylbenzoate (50:50) as previously described (Maina et al., 1997). Other embryos were infused with gelatin, vibratome-sectioned, and processed for QCPN–QN immunohistochemistry as described previously (Warrilow and Guthrie, 1999). A few embryos with an r4 orthotopic graft had been retrogradely labeled before immunohistochemistry. The fixed hindbrains of these embryos were immunostained as whole-mounts using QCPN antibodies, and staining was visualized using fluorescently conjugated secondary antibodies (Warrilow and Guthrie, 1999). The whole-mount immunostaining procedure described above was followed, except that the hydrogen peroxide blocking and DAB development steps were omitted, and a Cy3-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch) was used in place of the peroxidase-conjugated secondary antibody. These hindbrains were vibratome-sectioned in the parasagittal plane, mounted in 90% glycerol-PBS, and viewed under a confocal microscope.
RESULTS
Anatomy of the facial nerve in the chick
In the chick, motor neurons that project into the facial nerve develop in r4 and r5 (Fig. 1A) and comprise BM and VM classes (Lumsden and Keynes, 1989). Axons of r5 motor neurons project dorsally and rostrally to the exit point in r4 where they are joined by axons of r4 motor neurons. On leaving the hindbrain, axons of both groups project along the same pathway to their intermediate target, the geniculate ganglion, where the BM and VM components segregate into two major pathways. BM axons turn caudally to form the hyoid nerve, which innervates the second branchial arch musculature, whereas VM axons turn rostrally within the palatine nerve, which also contains sensory axons originating from the geniculate ganglion. The palatine nerve supplies parasympathetic preganglionic innervation to the sphenopalatine and ethmoidal ganglia, which are positioned adjacent to the maxillary division of the trigeminal nerve and retro-orbitally, respectively. Some VM axons also grow out via the smaller, chorda tympani branch of the facial nerve (see Fig. 3A).
Fig. 3.
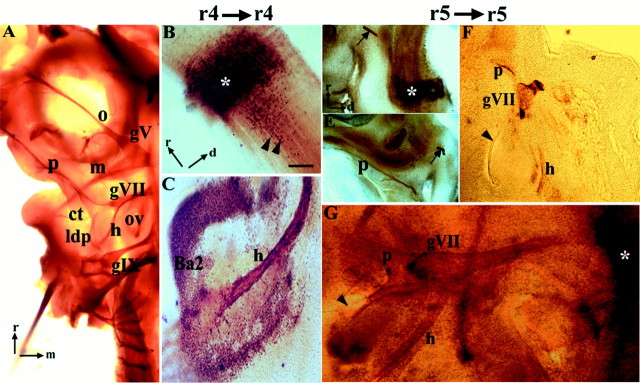
Quail-to-chick orthotopic grafts of r4 and r5.A–G, Anti-neurofilament immunohistochemistry of control (A) and QCPN–QN immunohistochemistry of chimeric (B–G) embryos analyzed at st. 25–30.A, Ventral view of flat-mounted control chick embryo showing the pathways of the branchial nerves V,trigeminal; VII, facial; and IX,glossopharyngeal. Rostral (r) is to thetop, and medial (m) is to theright. B, C, Parasagittal vibratome sections of a chimera with an orthotopic graft of r4. B,Hindbrain with grafted rhombomere (asterisk). Note that many quail cells from the graft have migrated posteriorly (black arrowheads). Rostral and dorsal (d) are indicated by orthogonal arrows. C, Quail axons from motor neurons in r4 innervate the muscle plate of the second branchial arch, which contains quail neural crest cells in the peripheral region. D–G, Parasagittal vibratome sections (D–F) and flat-mount (G) of chimeras with an orthotopic graft of r5. D, Quail graft is visible (asterisk) along with QN+ axons of the ventrally projecting abducens nerve (arrow). Orientation is the same in D–F.E–G, Three examples of the axonal projection patterns of motor neurons in r5. E, In this embryo, motor axons project along the palatine pathway of the facial nerve. An abducens nerve is also visible (arrow). F, This embryo has a palatine nerve and a hyoid projection that terminates abruptly at the level of the first branchial cleft (arrowhead), between the first and second branchial arches. QN+ axons do not reach the core of the second branchial arch (data not shown). G, Ventral view of a st. 25 embryo showing a palatine nerve and a hyoid nerve that terminates proximal to the second branchial arch and caudal to the first branchial cleft (arrowhead). The graft is marked with an asterisk. Scale bar: A, 400 μm;B, C, 100 μm; and D–G, 250 μm. gV, Trigeminal ganglion; gIX,glossopharyngeal ganglion; o, m,ophthalmic and mandibular divisions of trigeminal nerve;ldp, lesser deep petrosal branch of glossopharyngeal nerve.
Retrograde labeling reveals that BM neurons are present in r4 and r5, whereas VM neurons are restricted to r5
The development of motor neurons in r4 and r5 in the chick was investigated using fluorescein- or rhodamine-dextran as a retrograde tracer (Glover et al., 1986). Dextran was applied either to the hyoid nerve (BM), and/or to the palatine nerve (VM) after nerve transection, to identify BM and VM neuronal groups within the hindbrain (Fig.2). We were able to label neurons via the hyoid nerve (Fig. 2L) from stage 20 onward, consistent with previous studies (Simon et al., 1994). Rhombomere boundaries were clearly recognizable up to stage 25, allowing the unambiguous allocation of BM neurons to either r4 or r5.
Fig. 2.
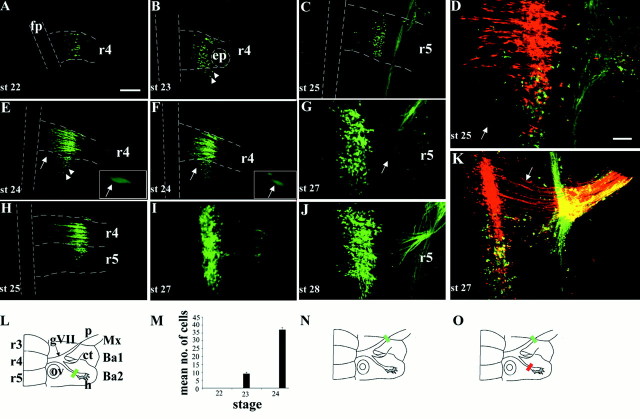
Retrograde labeling of motor neurons in r4 and r5 over a series of developmental stages. A–K, Ventral views of flat-mounted hindbrains showing retrograde labeling of facial BM and/or VM neurons. A, B, E, F, H, I, L, M, Labeling of BM neurons after fluorescein-dextran fills of the hyoid nerve (L, greenbar) at st. 22 (A), st. 23 (B), st. 24 (E, F), st. 25 (H), and st. 27 (I). Between three and five embryos were labeled at each stage. Note the presence of BM neurons in r5 from stage 23 onward. Many of the BM neurons in r5 occupy lateral positions (B, E, arrowheads), whereas others lie closer to the floor plate in a medial position (E, F, arrows). Higher power views of these medially positioned BM neurons are shown in the insets in E andF. C, G, J, Labeling of VM neurons after fluorescein-dextran fills of the palatine nerve (N, green bar) at st. 25 (C), st. 27 (G), and st. 28 (J). Four or five embryos were labeled at each stage. Note the curved trajectory of VM axons (G, arrow). D, K,Double-labeling of BM and VM subpopulations after fluorescein-dextran fills of the palatine nerve (O, green bar) and rhodamine-dextran fills of the hyoid nerve (O, red bar) at st. 25 (D) (n = 3) and st. 27 (K) (n = 5).D, Within r5 a single BM neuron in a medial position has been labeled (arrow), but the majority of facial motor neurons in r5 are located laterally. K, Motor neuron cell bodies at st. 27 occupy the same mediolateral position. The straight or slightly curved axon trajectories of r4 BM neurons are clearly seen (arrow). Presence of apparently double-labeled cells (yellow) in Dand K is an artifact caused by the superposition of labeled neurons at different depths when generating the confocalz-series shown here. Double-labeled cells were never observed in any single focal plane at all depths (data not shown).L, N, O, Schematics of ventral aspect of facial nerve and hindbrain indicating dextran labeling, and quantification of the mean (+SEM) number of BM neurons labeled in r5 at st. 22, 23, and 24 (M). Scale bar (in A):A, B, C, E, F, H, 100 μm. Scale bar (inD): G, I–K, 50 μm. fp,Floor plate; ep, exit point; gVII,geniculate ganglion; ov, otic vesicle; p,palatine nerve; ct, chorda tympani; h,hyoid nerve; Mx, maxilla; Ba1, first branchial arch; Ba2, second branchial arch.Dashed lines in A–C, E, F, andH indicate margins of floor plate and rhombomere boundaries.
From stage 20–22, BM neurons projecting via the hyoid nerve were located exclusively in r4 (Fig. 2A; data not shown), whereas from stage 23 to 27, there was a progressive increase in the number of facial BM neurons located in r5 (Fig.2A,B,E,F,H,I). At stage 23 these neurons appeared confined to rostral r5 (Fig. 2B), but at later stages they were observed throughout the rostrocaudal extent of r5. Quantitation of the number of BM neurons in r5 was possible at stage 23 and 24, owing to the ease of counting individual labeled neurons with minimal overlap of adjacent cells and clear boundary demarcation. An approximately fourfold increase in the number of labeled BM neurons in r5 was observed between stages 23 and 24 (Fig.2M). No BM neurons labeled via the hyoid nerve were present caudal to r5. Most of the labeled BM neurons in r5 were located laterally and did not form a genu. These experiments reveal that facial BM neurons are initially restricted to r4, but are later detected in r5. At earlier stages, BM neuron cell bodies showed considerable mediolateral spread within r4, whereas at later stages they were restricted to more lateral domains (Fig. 2I).
To determine the distribution of facial VM neurons, we retrogradely labeled motor neurons projecting into the palatine nerve, which was possible from stage 25 onward (Fig. 2C,G,J,N). At stage 25, VM neurons were confined to r5 in a lateral position (Fig.2C). This tight clustering of VM cell bodies within r5 was maintained at later stages when rhombomere boundaries had disappeared (Fig. 2J). Thus, from stage 25 onward, r5 contains both facial BM and VM neurons, a spatial overlap that could be demonstrated by double labeling of the hyoid and palatine divisions of the facial nerve at stages 25 and 27 (Fig. 2D,K,O). These results show that from the time when they can first be labeled selectively, BM and VM neurons are confined to r4 and r5, respectively, but that eventually r5 contains both BM and VM subpopulations.
Facial BM neurons develop in r4 and r5, whereas VM neurons develop exclusively in r5
To investigate the rhombomere origin of facial BM and VM neurons, orthotopic transplants of r4 or r5 were performed at stages 10–12 using the quail-chick chimera system (Figs. 1B,C,3). By this developmental stage certain key aspects of rhombomere identity have already been established, including Hox gene expression patterns and some aspects of cranial motor neuron phenotype (Guthrie and Lumsden, 1992; Guthrie et al., 1992; Kuratani and Eichele, 1993; Simon et al., 1995). After such transplants, we used the distinctive peripheral axonal projections of facial BM and VM neurons as phenotypic markers, because there are no distinguishing molecular markers (Varela-Echavarría et al., 1996).
In 100% of chimeras containing an orthotopic r4 graft, we observed the projection of quail-derived axons along exclusively the hyoid pathway (n = 16; Fig. 3B,C; Table1). Quail axons filled the hyoid pathway, which was morphologically indistinguishable from that in control embryos (Fig. 3A), curving caudally from the geniculate ganglion to innervate the second branchial arch (summarized in Fig.5E).
Table 1.
Frequency with which quail axons were observed in various axon pathways after orthotopic and heterotopic quail to chick rhombomere transplants
| Transplant | Exit via r2 (ectopic nerve) | Exit via r4 | Hyoid | Palatine | Mandibular | N | |||
|---|---|---|---|---|---|---|---|---|---|
| + | ++ | + | ++ | + | ++ | ||||
| r4 → r4 | 16 | 16 | |||||||
| r5 → r5 | 12 | 5 | 1 | 20 | 22 | ||||
| r5 → r31-a | 9 | 9 | 3 | 4 | 11 | 3 | 1 | 14 | |
| r3 → r5 | 9 | 9 | |||||||
| r2 → r41-b | 5 | 5 | |||||||
Pooled data from whole r5 grafts and r5 basal plate alone.
Only r2 basal plate was transplanted to r4.
Scoring for the palatine pathway: + indicates the nerve has not extended to the level of the maxilla; ++ indicates the nerve has extended to at least the axial level of the maxilla.
Scoring for the hyoid pathway: + indicates the nerve has not extended distal to the first branchial cleft; ++ indicates the nerve has grown beyond the first branchial cleft into the second branchial arch.
Scoring for the mandibular pathway: + indicates sparse projections into the first branchial arch, at the limit of detectability; ++ indicates prominent projection to the first branchial arch.
Fig. 5.
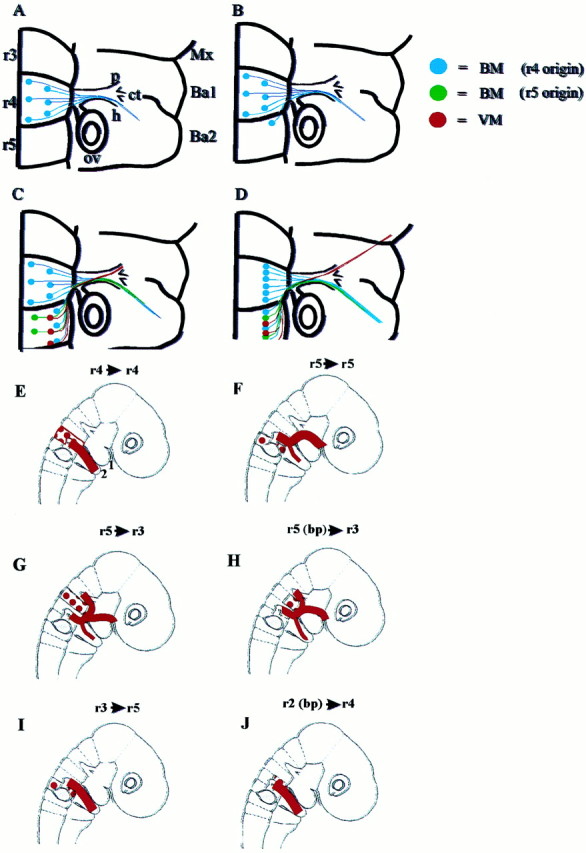
Summary diagram of the temporal order of development of motor pathways of the facial nerve in the chick, based on retrograde axon tracing and orthotopic grafts. A, St. 20–22: all BM neurons are confined to r4 and send their axons into the hyoid nerve. B, St. 23: BM neurons are also identified in r5. C, St. 25: BM neurons born in r5 extend axons along the hyoid pathway, and VM neurons in r5 project axons to form the anlage of the palatine nerve. D, St. 27: Cell bodies of BM and VM neurons occupy the same mediolateral position. The palatine nerve has extended to the maxilla to innervate the target ganglia. For abbreviations, see Figure 2 legend. E–J, Motor pathways that result from orthotopic (E, F) and heterotopic (G–J) quail to chick grafts.E, r4 orthotopic grafts: quail axons grow into the second branchial arch. 1 and 2 point out first and second branchial arches. F, r5 orthotopic grafts: quail axons grow into the palatine branch and into the second branchial arch. G, H, r5 to r3 grafts (G,whole rhombomere; H, basal plate graft only): quail axons that exit via the r2 dorsal exit point reroute to join the facial nerve pathway at the geniculate ganglion. Other quail axons exit the hindbrain via the exit point in r4. Axons are distributed in the palatine and hyoid nerves en route to the correct targets. For clarity, axons that traverse the trigeminal nerve in a small minority of chimeras have been omitted. I, r3 to r5 grafts: axons project to an incorrect target, the second branchial arch.J, r2 (basal plate) to r4 grafts: axons project to an incorrect target, the second branchial arch. For abbreviations, see Figure 2 legend.
For orthotopic r5 transplants, analysis was initially done at stage 23–24 (n = 6; data not shown), but no quail-derived axons were observed in the periphery. This is likely to be attributable to the late formation of facial VM projections along the palatine nerve relative to BM projections (see above). We therefore confined all subsequent analyses to chimeras of stage 25 or older. In all operated embryos at stage 25–30, a quail-derived abducens nerve was detected (22 of 22 of r5 grafts; Table 1). The abducens nucleus lies within r5 and 6 in the chick embryo and is formed by somatic motor (SM) neurons that project ventrally (Fig. 1A; Lumsden and Keynes, 1989). In our experiments, the abducens served as a robust marker of the r5 phenotype, independent of the presence of additional dorsal axon pathways of the facial nerve.
After orthotopic transplantation of r5, 95% of embryos contained quail axons within the palatine nerve (n = 21 of 22; Fig.3D–G; Table 1). In 4 of 22 chimeras, only a quail-derived palatine nerve was present, whereas in a further 17 of 22 cases, both a palatine and a hyoid projection were present. In just one chimera, dorsal projections of quail-derived axons grew via the hyoid nerve exclusively. The pattern of quail palatine projections was normal, extending rostrally past the first branchial arch and then over the palate to its target, the sphenopalatine ganglion. By contrast, in these embryos, r5 quail neurons formed only sparse projections within the hyoid nerve, and often failed to reach the branchial arch muscle plate (Fig. 3F, Table 1). The motor axon pathways resulting from these orthotopic grafts are summarized in Figure 5, Eand F.
Although we could not precisely quantify the number of QN+ axons in the different pathways, these observations imply that the majority of dorsally projecting motor neurons in r5 follow VM rather than BM axon pathways. Taken together, the results of these orthotopic grafts show that r4 and r5 both generate facial BM neurons, but that the majority originate in r4. By contrast, facial VM neurons develop exclusively in r5.
A subpopulation of facial BM neurons that originate in r4 migrates into r5
Between stages 23 and 25, our retrograde labeling studies show that there is a dramatic increase in the number of facial BM neurons within r5. This period corresponds to the onset of rhombomere boundary disappearance, and is in line with previous observations that inter-rhombomeric cell mixing occurs among differentiated neurons of the mantle zone at these stages (Hemond and Glover, 1993; Wingate and Lumsden, 1996). Therefore, in addition to the intrinsic generation of BM neurons within r5, a caudal migration of BM neurons from r4 might account for the presence of BM neurons in r5 from stage 23 onward (see above).
To determine whether facial BM neurons migrate from r4 to r5, we examined some chimeras containing orthotopic r4 grafts for evidence of neuronal migration from r4 to r5 (n = 3). These embryos were analyzed at stage 26–27 by dextran labeling the hyoid nerve, and the hindbrains were immunostained using QCPN antibodies to detect quail cells, before vibratome sectioning (Fig.4). Quail-derived, dextran-labeled facial BM neurons (arrowheads) were observed in r5 as well as in r4, suggesting that facial BM neurons in r4 indeed migrate caudally into r5 (Fig. 4B,C). Some dextran-labeled BM neurons in r5 were not QCPN-positive, implying that they were derived from the chick host, and consistent with the generation of BM neurons in r5 demonstrated earlier. Some QCPN-positive cells in r5 were not labeled with dextrans, and probably represent other cell or neuronal types with a similar migration pattern. Thus, avian embryos display cell migrations from r4 into r5, and a proportion of these cells are facial BM neurons.
Fig. 4.
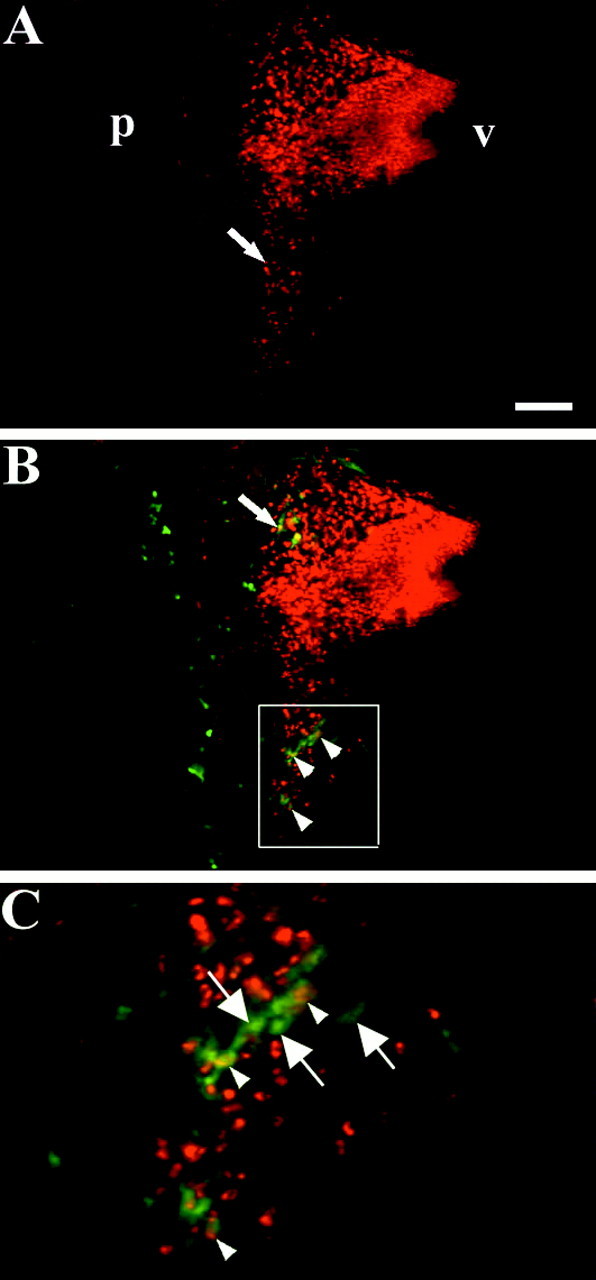
Retrograde labeling of the hyoid nerve in a chimera with an orthotopic graft of r4. A–C,Parasagittal vibratome sections through the hindbrain of a st. 26 embryo with an orthotopic r4 graft, after a fluorescein-dextran (green) fill of the hyoid nerve and whole-mount QCPN immunohistochemistry (red). A, Quail cells (red) that have migrated posteriorly from r4 into r5 are clearly seen near the pial surface (arrow).B, QCPN+–dextran-labeled cells are quail BM neurons that are present in r4 (arrow) and r5 (arrowheads). C, Higher power view of theboxed area in B to show quail BM neurons (yellow or redcenter and green surround) in r5 that have migrated posteriorly from r4 (arrowheads). Also visible are QCPN−–dextran-labeled endogenous chick BM neurons (arrows) that originate in r5.p, Pial surface; v, ventricular surface. Scale bar: A, B, 100 μm;C, 25 μm.
Temporal differences in the formation of BM and VM pathways
Our retrograde labeling and grafting studies also gave information about the timing of BM and VM peripheral axon outgrowth. Orthotopic r4 transplants revealed that axonal projections to the second branchial arch are well established by stage 25 (n = 4 chimeras; Fig. 3C), the earliest stage examined. These observations are in keeping with earlier reports that showed the formation of hyoid projections to the second branchial arch as early as stage 21 (Simon et al., 1994). By contrast, outgrowth of r5-derived BM neurons was relatively delayed, so that only partial outgrowth along the hyoid nerve was observed at the equivalent stage (n = 2 chimeras at stage 25; Fig. 3G). Analysis of the two r5 chimeras fixed at stage 25 shed light on the timing of outgrowth of BM and VM pathways from r5. In one of these chimeras only an incompletely developed hyoid pathway was present. In the other, a more extensive hyoid projection was present, and in addition, a contingent of QN-positive axons projected only a short distance along the palatine nerve, just beyond its origin from the geniculate ganglion (Fig.3G). Therefore, projection of VM fibers beyond the geniculate ganglion occurs within the narrow time window of a single developmental stage, consistent with our retrograde labeling study (see above). Embryos analyzed at stage 26–31 all contained a quail palatine nerve. These findings suggest that our failure to observe a palatine pathway in one (stage 25) chimera is attributable to the fact that this nerve had not yet formed at the stage of analysis. Therefore, r4 BM neurons constitute the first subpopulation of facial motor neurons to project to their peripheral target. Next, the smaller r5-derived contingent of BM neurons sends its axons along the same pathway. Finally, VM neurons project rostrally to form the palatine nerve (summarized in Fig. 5).
Ectopic motor neurons with distinct segmental origins display different specificity in their axon pathfinding
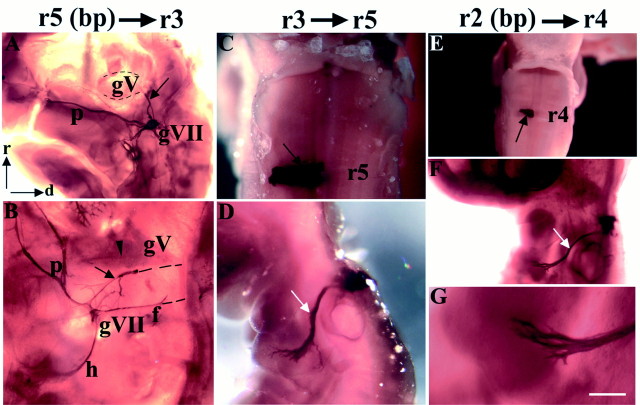
To test the specificity of axon pathfinding of motor neurons with distinct segmental origins, heterotopic rhombomere transpositions were performed, and chimeras were analyzed as before, at stages 25–30 (Figs. 1D–G, 6, Table1). When r5 was transposed to r3 position, quail-derived axons projected along several distinct pathways (n = 6; Figs.1D, 5G, Table 1). The most striking observation was that in some cases an ectopic longitudinal QN-positive nerve bundle exited the hindbrain via the r2 exit point before turning caudally and projecting to the geniculate ganglion. This tract is likely to be composed of facial motor axons, which had navigated back to their intermediate target. Such a tract is unlikely to contain neural crest-derived neurons because r5 gives rise to few neural crest cells (Lumsden et al., 1991).
Nevertheless, to confirm that this ectopic fascicle was a motor rather than a sensory pathway we also grafted r5 basal plate alone in place of r3 (n = 8; Fig. 1E), thus eliminating possible contamination by neural crest cells. These chimeras showed the same ectopic nerve bundle directed from the r2 trigeminal nerve exit point to the geniculate ganglion in 9 of 14 cases, and data from both types of operation were subsequently pooled because the findings were identical (Figs. 5H, 6A,B, Table 1). Ectopic r5-derived motor axons also followed a second axon pathway, which turned caudally within the neuroepithelium, exited the hindbrain via the r4 facial exit point, and projected via the facial nerve to the geniculate ganglion (n = 9 of 14). Thus, r5-derived motor neurons were able to project back to their intermediate target either via the inappropriate (r2) exit point or by projecting aberrantly in a caudal direction toward the appropriate (r4) exit point. Overall, motor axons from r5 projected along novel pathways to the facial nerve or geniculate ganglion in 93% of cases.
Fig. 6.
BM and VM axon pathfinding after heterotopic quail-to-chick transplants. A–G, QCPN and QN immunohistochemistry in embryos at st. 25–30 after heterotopic grafts of r5 basal plate to r3 (A, B), r3 to r5 (C, D), and r2 basal plate to r4 (E–G). A, B, Ventral views of two flat-mounted embryos showing ectopic QN+ axon tract (A, B, black arrows) projecting from the trigeminal nerve exit point in r2 to the geniculate ganglion. InB, QN+ axons also exit the hindbrain via the exit point in r4 and project to the geniculate ganglion in the facial nerve (f). A palatine nerve is recognizable in both cases and has bifurcated in B to supply both the sphenopalatine and ethmoidal ganglia (data not shown). Two other motor pathways additionally present in B are a hyoid nerve and mandibular projection (arrowhead), which is at the limit of detectability. In B, the connection of the ectopic bundle and facial nerve to the hindbrain was severed during flat-mounting, as indicated by black dashed lines. The grafted rhombomeres (data not shown) resembled the basal plate graft shown in E. C, D, Dorsal (C) and lateral (D) views of whole-mount embryo showing, respectively, the graft (C, black arrow) and axonal projection of trigeminal BM neurons to the second branchial arch (D, white arrow).E–G, Dorsal (E) and lateral (F, G) views of whole-mount embryo showing the grafted basal plate (E, black arrow), the projection of trigeminal BM axons to the second branchial arch (F, white arrow), and, at higher power, the second branchial arch, which is devoid of quail neural crest cells (G). Scale bar (in G): C, G, 200 μm; A, E, 300 μm; B, 500 μm; F, 1200 μm; and D, 1600 μm.
Projections along the distal, motor pathways of the facial and trigeminal nerves were also assessed; QN-positive projections were observed in the palatine (n = 11 of 14) and hyoid (n = 7 of 14) divisions of the facial nerve and along the mandibular branch of the trigeminal nerve (n = 4 of 14). A ventrally projecting abducens nerve was observed in all (14 of 14) chimeras, supporting the view that r5 identity is not altered when it is transposed rostrally, because SM abducens neurons are generated within it. The broad conclusion of this experiment is therefore that many ectopic VM axons were able to pathfind to their eventual target, by following novel pathways incorporating one or both of their bona fide intermediate targets, the r4 exit point and/or the geniculate ganglion.
After transposition of r3 to r5 position, in 100% of cases quail BM neurons extended their axons rostrally, exited the hindbrain via r4, and grew along the hyoid pathway to the second branchial arch (n = 9; Figs. 1F, 6C,D, Table 1). No quail-derived neural crest cells were identified in the periphery in these transplants, in accordance with observations that r3 is neural crest-depleted (Lumsden et al., 1991). In addition, we transposed r2 to r4 (Fig. 1G) to determine whether r2 trigeminal motor neurons would show the same lack of specificity in axon pathfinding as those in r3. Only the basal plate of r2 was transplanted, because previous studies have shown that r2 produces many neural crest cells, which might confer on the second branchial arch first branchial arch identity, and thus confound analysis of axon pathways (Noden, 1983; Lumsden et al., 1991). The results of r2 to r4 transposition experiments showed that motor neurons in the transposed rhombomere extended axons along the hyoid pathway to the second branchial arch in all cases (n = 5; Fig.6E–G, Table 1), forming a nerve that was morphologically indistinguishable from the normal hyoid projection (compare Figs. 3A and 6F). These results are closely similar to those derived from r3 to r5 transpositions. Thus, ectopic trigeminal motor neurons of r2 or r3 origin are capable of extending axons along incorrect, facial nerve pathways to the second branchial arch and do not reroute to their correct, first branchial arch targets. These results contrast with those of r5 transpositions, in which r5-derived neurons were able to navigate back to their targets (for comparison, see Fig. 5E–J).
DISCUSSION
Our study has revealed that facial branchiomotor and visceral motor neurons have different segmental origins in the chick embryo hindbrain. Visceral motor neurons are generated exclusively in rhombomere 5, whereas the majority of branchiomotor neurons originate in rhombomere 4. This early segmental ground plan is modified by the migration of a cohort of BM neurons from r4 into r5 and the generation of a smaller population of BM neurons within r5. When transplanted rostrally, r5 facial VM neurons displayed the ability to pathfind back to their correct targets. By contrast, r2 and r3 trigeminal BM neurons transplanted caudally did not pathfind back to the first branchial arch, but grew into the second branchial arch, an incorrect target region. Our findings thus suggest that facial motor axons are capable of rerouting to their targets, whereas trigeminal motor axons are not, consistent with previous experiments showing that ectopic r4 facial BM neurons could navigate back to the second branchial arch (Bell et al., 1999).
Time course of the development of facial motor neurons in the chick
The sequence of motor neuron development in r4 and r5 can be inferred from the expression of markers, axonal tracing, and grafting experiments, derived from this and previous studies. Postmitotic facial motor neurons are first recognized in r4 and r5 by Islet-1 and SC1 expression at stage 14–15 (Simon et al., 1994;Varela-Echavarría et al., 1996). At stage 16, axons of r4 vestibuloacoustic neurons exit the hindbrain (Simon and Lumsden, 1993), followed at stage 19–20 by those of BM and VM neurons in r4 and r5 (Simon and Lumsden, 1993; Simon et al., 1994). The first hyoid-projecting axons have reached the second branchial arch by stage 21–22, based on anterograde and retrograde axon tracing.
Based on axon tracing, r5 BM axons have reached the branchial arch by stage 23, even though the results of orthotopic r5 transplantation experiments show that projections from r5 BM neurons cannot be detected in the hyoid nerve until stage 25. Taken together, these results imply that the projections formed by BM neurons resident in r5 at stage 23 must originate from r4-derived BM neurons that have migrated caudally into r5. At approximately stage 25, BM neurons born in r5 then send projections to the branchial arch, and r5 VM neurons project into the palatine nerve. By stage 27, the cell bodies of BM and VM neurons have migrated laterally, and VM axons have reached their target parasympathetic ganglia (Fig. 5A–D).
Subpopulations of BM neurons in r5
We have demonstrated that, in addition to VM neurons, r5 contains two distinct subpopulations of BM neurons, one group that migrates caudally from r4, and a second that is intrinsic to r5. The caudal migration of a subset of facial BM neurons in the chick hindbrain appears highly directed, as in the rodent (Auclair et al., 1996; McKay et al., 1997). In the mouse and rat, however, facial BM neurons migrate as far as r6, and their characteristic migration route close to the midline forms a loop round the abducens nucleus, giving rise to the genu of the facial nerve. By contrast, in the chick, BM neurons migrate only as far as r5, and their position remains more lateral, so that their migration path does not form a genu.
Two types of evidence support the existence of a subpopulation of BM neurons that is born in r5. First, retrograde axon tracing of the hyoid (BM) nerve in chimeras with an r4 orthotopic graft labels BM neurons that have originated in r5, in addition to those that migrate from r4. Second, in the great majority of embryos with an r5 orthotopic graft, dual palatine (VM) and hyoid (BM) pathways are detected. The latter pathway was observed less frequently, contained fewer axons, and sometimes could not be traced all the way to the second branchial arch. One explanation for this finding is that the later development of r5 BM neurons relative to r4 BM neurons could deprive them of limiting amounts of trophic support from the branchial arch (Oppenheim et al., 1988), leading to programmed cell death (Hamburger, 1975). A small population of r5-derived neurons that forms part of the facial motor nucleus has previously been reported in a fate-mapping study of hindbrain nuclei using the quail-chick chimera system, but axon projections were not assessed (Marin and Puelles, 1995)
Axon pathfinding of motor neurons after heterotopic grafts
When r5 was transplanted ectopically, we found that in the majority of cases, motor axons were able to reroute back to their intermediate target, the geniculate ganglion, and formed a palatine nerve en route to the sphenopalatine ganglion. In some cases axons formed an ectopic fascicle extending longitudinally from r2 exit point to the geniculate ganglion. This feature is reminiscent of the longitudinal fascicle observed after transplantation of r4 to r2 position, which resulted from the projection of r4 facial motor axons back to the second branchial arch (Bell et al., 1999). Therefore, axonal rerouting toward the appropriate target appears to be a characteristic of facial motor neurons in r4 and r5. The ability of r5 motor axons to deviate from the r2 exit point toward the geniculate ganglion suggests that guidance cues may emanate from this structure. Ganglionic intermediate targets have previously been suggested to provide guidance cues for cranial motor axons, because the latter exhibited increased outgrowth and chemoattraction when cocultured in the presence of trigeminal ganglia (Tucker et al., 1996; Caton et al., 2000). Other studies have also shown that when an impermeable filter blocked the growth of trigeminal motor axons, many axons followed aberrant pathways around the barrier to reach the trigeminal ganglion (Moody and Heaton, 1983).
The axon pathfinding of r5 VM neurons contrasts with the lack of pathfinding selectivity of heterotopic trigeminal motor axons, which projected to the inappropriate ganglion and branchial arch. This behavior is unlikely to be explained by a change in the phenotype of the transposed segment, because previous studies using molecular markers have shown that neither rostral nor caudal transposition within the pre-otic region is associated with the respecification of rhombomere identity (Guthrie et al., 1992; Graham and Lumsden, 1996). A lack of specificity of early trigeminal projections was also shown in r3 reversal experiments, which resulted in the projection of a large contingent of trigeminal motor neurons via the r4 exit point to the second branchial arch, although these projections were eliminated later in development (Warrilow and Guthrie, 1999).
Guidance cues for BM and VM axon pathfinding in the head
The normal topographic projection of motor neurons to the branchial arches may depend on the corresponding expression on motor axons and peripheral mesenchyme of molecules whose expression is governed by the same repertoire of Hox genes (Lumsden and Keynes, 1989; Hunt et al., 1991). For example, Hoxb1 is expressed within BM neurons of rhombomere 4 and within the mesenchyme of the second branchial arch, but not at more rostral axial levels. A role for Hoxb1 in axon guidance has been shown in experiments in which Hoxb1 was ectopically expressed in both r2 motor neurons and the first branchial arch mesenchyme, leading to innervation by ectopic Hoxb-1-expressing motor axons of this territory (Bell et al., 1999). Corresponding experiments showed that when Hoxb1 was expressed in r2 but not in the periphery,Hoxb1-expressing neurons rerouted their axons to theHoxb1-expressing environment of the second branchial arch (Bell et al., 1999). Our study extends the notion that motor axons innervate regions populated by neural crest cells from the same axial level to include r5 VM neurons, because the sphenopalatine and ethmoidal ganglia are derived from neural crest cells at r4 and r5 levels (D'Amico-Martel and Norden, 1983).
The guidance molecules that ensure selective pathfinding of VM and BM neurons at individual axial levels remain largely unidentified. The branchial arches have been shown to exert a growth-promoting and chemoattractive influence on cranial motor axons, mediated partly by the production of hepatocyte growth factor (HGF) (Caton et al., 2000). Motor neurons from various axial levels display similar responses to branchial arch explants, and to HGF, implying that arch-derived factors are general rather than axial level-specific cues (Caton et al., 2000). However, because VM neurons do not innervate the branchial arches, they might be less responsive to branchial arch-derived cues, a possibility that we could not examine because of the lack of VM markers. VM neurons might gain guidance information from their intermediate and final target ganglia, as shown by the rerouting of VM axons to the geniculate and the sphenopalatine ganglion. This complements our recent studies on mouse embryos, in which the absence of the sphenopalatine ganglion target leads to a disruption of facial VM neuron pathfinding, implying that this structure is a source of guidance cues for VM axons (Jacob et al., 2000). Thus, the generation of facial BM and VM pathways might involve a common pathway to the geniculate ganglion, followed by selective responses of BM and VM neurons to the branchial arch and the sphenopalatine ganglion, respectively.
Footnotes
This work was supported by grants from the Special Trustees of Guy's Hospital and the Wellcome Trust. J.J. is a Wellcome Clinical Training Fellow. We thank F. Prin for assistance with figures, C. Barrett for assistance with immunohistochemistry, and N. Pringle for antibodies. We are grateful to A. Lumsden, R. Wingate, and a helpful reviewer for insightful comments on this manuscript.
Correspondence should be addressed to Sarah Guthrie, Medical Research Council Centre for Developmental Neurobiology, Fourth Floor, New Hunt's House, King's College, Guy's Campus, London SE1 1UL, UK. E-mail: sarah.guthrie@kcl.ac.uk.
REFERENCES
- 1.Auclair F, Valdes N, Marchand R. Rhombomere-specific origin of branchial and visceral motoneurones of the facial nerve in the rat embryo. J Comp Neurol. 1996;369:451–461. doi: 10.1002/(SICI)1096-9861(19960603)369:3<451::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Bell E, Wingate RJ, Lumsden A. Homeotic transformation of rhombomere identity after localized Hoxb1 misexpression. Science. 1999;284:2168–2171. doi: 10.1126/science.284.5423.2168. [DOI] [PubMed] [Google Scholar]
- 3.Caton A, Hacker A, Naeem A, Livet J, Maina F, Klein R, Birchmeier C, Guthrie S. The branchial arches and HGF are growth-promoting and chemoattractant for cranial motor axons. Development. 2000;127:1751–1766. doi: 10.1242/dev.127.8.1751. [DOI] [PubMed] [Google Scholar]
- 4.D'Amico-Martel A, Norden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- 5.Fritzsch B, Nichols DH. DiI reveals a prenatal arrival of efferents at the differentiating otocyst of mice. Hear Res. 1993;65:51–60. doi: 10.1016/0378-5955(93)90200-k. [DOI] [PubMed] [Google Scholar]
- 6.Glover JC, Petursdottir G, Jansen JKS. Fluorescent dextran-amines used as axonal tracers in the nervous system of the chicken embryo. J Neurosci Methods. 1986;18:243–254. doi: 10.1016/0165-0270(86)90011-7. [DOI] [PubMed] [Google Scholar]
- 7.Graham A, Lumsden A. Interactions between rhombomeres modulate Krox-20 and follistatin expression in the chick embryo hindbrain. Development. 1996;122:473–480. doi: 10.1242/dev.122.2.473. [DOI] [PubMed] [Google Scholar]
- 8.Guthrie S, Lumsden A. Motor neuron pathfinding following rhombomere reversals in the chick embryo hindbrain. Development. 1992;114:663–673. doi: 10.1242/dev.114.3.663. [DOI] [PubMed] [Google Scholar]
- 9.Guthrie S, Muchamore I, Kuroiwa A, Marshall H, Krumlauf R, Lumsden A. Neuroectodermal autonomy of Hox-2.9 expression revealed by rhombomere transpositions. Nature. 1992;356:157–159. doi: 10.1038/356157a0. [DOI] [PubMed] [Google Scholar]
- 10.Hamburger V. Cell death in the development of the lateral motor column of the chick embryo. J Comp Neurol. 1975;160:535–546. doi: 10.1002/cne.901600408. [DOI] [PubMed] [Google Scholar]
- 11.Hamburger V, Hamilton HL. A series of normal stages in development of the chick embryo. J Morphol. 1951;88:49–87. [PubMed] [Google Scholar]
- 12.Hemond SG, Glover JC. Clonal patterns of cell proliferation migration, and dispersal in the brainstem of the chicken embryo. J Neurosci. 1993;13:1387–1402. doi: 10.1523/JNEUROSCI.13-04-01387.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt P, Gulisano M, Cook M, Sham MH, Faiella A, Wilkinson D, Boncinelli E, Krumlauf R. A distinct Hox code for the branchial region of the vertebrate head. Nature. 1991;353:861–864. doi: 10.1038/353861a0. [DOI] [PubMed] [Google Scholar]
- 14.Jacob J, Tiveron M-C, Brunet J-F, Guthrie S. Role of the target in the pathfinding of facial visceral motor axons. Mol Cell Neurosci. 2000;16:14–26. doi: 10.1006/mcne.2000.0855. [DOI] [PubMed] [Google Scholar]
- 15.Kuratani SC, Eichele G. Rhombomere transplantation repatterns the segmental organisation of cranial nerves and reveals cell-autonomous expression of a homeodomain protein. Development. 1993;117:105–117. doi: 10.1242/dev.117.1.105. [DOI] [PubMed] [Google Scholar]
- 16.Lumsden A. The cellular basis of segmentation in the developing chick hindbrain. Trends Neurosci. 1990;13:329–335. doi: 10.1016/0166-2236(90)90144-y. [DOI] [PubMed] [Google Scholar]
- 17.Lumsden A, Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989;337:424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- 18.Lumsden A, Sprawson N, Graham A. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development. 1991;113:1281–1291. doi: 10.1242/dev.113.4.1281. [DOI] [PubMed] [Google Scholar]
- 19.Maina F, Hilton MC, Ponzetto C, Davies AM, Klein R. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes Dev. 1997;11:3341–3350. doi: 10.1101/gad.11.24.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin F, Puelles L. Morphological fate of rhombomeres in quail/chick chimaeras: a segmental analysis of hindbrain nuclei. Eur J Neurosci. 1995;7:1714–1738. doi: 10.1111/j.1460-9568.1995.tb00693.x. [DOI] [PubMed] [Google Scholar]
- 21.McKay I, Lewis J, Lumsden A. Organisation and development of facial motor neurons in the kreisler mutant mouse. Eur J Neurosci. 1997;9:1499–1506. doi: 10.1111/j.1460-9568.1997.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 22.Moody SA, Heaton MB. Developmental relationships between trigeminal ganglia and trigeminal motoneurons in chick embryos. III. Ganglion perikarya direct motor axon growth in the periphery. J Comp Neurol. 1983;213:350–364. doi: 10.1002/cne.902130310. [DOI] [PubMed] [Google Scholar]
- 23.Noden DM. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. 1983;96:144–165. doi: 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- 24.Oppenheim RW, Haverkamp LJ, Prevette D, McManaman JL, Appel SH. Reduction of naturally occurring motoneuron death in vivo by a target-derived neurotrophic factor. Science. 1988;240:919–922. doi: 10.1126/science.3363373. [DOI] [PubMed] [Google Scholar]
- 25.Simon H, Lumsden A. Rhombomere-specific origin of the contralateral vestibulo-acoustic efferent neurons and their migration across the embryonic midline. Neuron. 1993;11:209–220. doi: 10.1016/0896-6273(93)90179-u. [DOI] [PubMed] [Google Scholar]
- 26.Simon H, Guthrie S, Lumsden A. Regulation of SC1/DM-GRASP during the migration of motor neurons in the chick embryo brain stem. J Neurobiol. 1994;25:1129–1143. doi: 10.1002/neu.480250908. [DOI] [PubMed] [Google Scholar]
- 27.Simon H, Hornbruch A, Lumsden A. Independent assignment of antero-posterior and dorso-ventral positional values in the developing chick hindbrain. Curr Biol. 1995;5:205–214. doi: 10.1016/s0960-9822(95)00041-8. [DOI] [PubMed] [Google Scholar]
- 28.Studer M, Lumsden A, ArizaMcNaughton L, Bradley A, Krumlauf R. Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature. 1996;384:630–634. doi: 10.1038/384630a0. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka H, Kinutani M, Agata A, Takashima Y, Obata K. Pathfinding during spinal tract formation in the chick-quail chimaera analysed by species-specific monoclonal antibodies. Development. 1990;110:565–571. doi: 10.1242/dev.110.2.565. [DOI] [PubMed] [Google Scholar]
- 30.Tucker A, Lumsden A, Guthrie S. Cranial motor axons respond differentially to the floor plate and sensory ganglia in collagen gel co-cultures. Eur J Neurosci. 1996;8:906–916. doi: 10.1111/j.1460-9568.1996.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 31.Varela-Echavarría A, Pfaff SL, Guthrie S. Differential expression of LIM homeobox genes among motor neuron subpopulations in the developing chick brain stem. Mol Cell Neurosci. 1996;8:242–257. doi: 10.1006/mcne.1996.0061. [DOI] [PubMed] [Google Scholar]
- 32.Warrilow J, Guthrie S. Rhombomere origin plays a role in the specificity of cranial motor axon projections in the chick. Eur J Neurosci. 1999;11:1403–1413. doi: 10.1046/j.1460-9568.1999.00559.x. [DOI] [PubMed] [Google Scholar]
- 33.Wingate RJ, Lumsden A. Persistence of rhombomeric organisation in the postsegmental hindbrain. Development. 1996;122:2143–2152. doi: 10.1242/dev.122.7.2143. [DOI] [PubMed] [Google Scholar]