Abstract
Hyperthermia exacerbates and hypothermia attenuates methamphetamine (METH)-induced dopamine (DA) neurotoxicity. The mechanisms underlying these temperature effects are unknown. Given the essential role of the DA transporter (DAT) in the expression of METH-induced DA neurotoxicity, we hypothesized that the effect of temperature on METH-induced DA neurotoxicity is mediated, at least in part, at the level of the DAT. To test this hypothesis, the effects of small, physiologically relevant temperature changes on DAT function were evaluated in two types of cultured neuronal cells: (1) a neuroblastoma cell line stably transfected with human DAT cDNA and (2) rat embryonic mesencephalic primary cells that naturally express the DAT. Temperatures for studies of DAT function were selected based on core temperature measurements in animals exposed to METH under usual ambient (22°C) and hypothermic (6°C) temperature conditions, where METH neurotoxicity was fully expressed and blocked, respectively. DAT function, determined by measuring accumulation of radiolabeled DA and 1-methyl-4-phenylpyridinium (MPP+), was found to directly correlate with temperature, with higher levels of substrate uptake at 40°C, intermediate levels at 37°C, and lower levels at 34°C. DAT-mediated accumulation of METH also directly correlated with temperature, with greater accumulation at higher temperatures. These findings indicate that relatively small, physiologically relevant changes in temperature significantly alter DAT function and intracellular METH accumulation, and suggest that the effect of temperature on METH-induced DA neurotoxicity is mediated, at least in part, at the level of the DAT.
Keywords: dopamine, dopamine transporter, temperature, methamphetamine, MPP+, neurotoxicity
The psychostimulant methamphetamine (METH) is a potent dopamine (DA) neurotoxin in rodents, nonhuman primates (Gibb et al., 1994; Lew et al., 1998) and, possibly, humans (McCann et al., 1998; Volkow et al., 1999). METH administration leads to long-lasting reductions in a number of DA axonal markers including DA, dihydroxyphenylacetic acid (DOPAC), homovanillic acid, tyrosine hydroxylase activity, and the DA transporter (DAT). Recent studies indicate that the striatal vesicular monoamine transporter is also reduced on a long-term basis after METH (Frey et al., 1997; Villemagne et al., 1998). Morphological studies indicate that loss of these presynaptic DA axonal markers is related to destruction of DA axons and axon terminals (Ellison et al., 1978; Lorez, 1981; Ricaurte et al., 1982, 1984; Bowyer et al., 1994; Broening et al., 1997; Fukumura et al., 1998), generally with sparing of DA nerve cell bodies (Ricaurte et al., 1982; Woolverton et al., 1989; but see Sonsalla et al., 1996;Hirata and Cadet, 1997).
The mechanism by which METH induces dopaminergic neurotoxicity is not known. However, there is compelling evidence that the DAT plays an essential role (Marek et al., 1990; Pu et al., 1994; Fumagalli et al., 1998). The DAT is an integral membrane protein of DA neurons that not only serves to inactivate synaptic dopamine by reuptake into presynaptic dopaminergic neurons, but also is known to mediate the pharmacological and reinforcing properties of a number of psychostimulant drugs (Ritz et al., 1987; Koob and Bloom, 1988; Miller et al., 1999). Moreover, intact function of the DAT is essential for the expression of METH-induced DA neurotoxicity. This is evidenced by the fact that DAT inhibitors prevent METH-induced neurotoxicityin vivo (Marek et al., 1990; Pu et al., 1994) and by the observation that homozygotic (−/−) DAT knock-out mice are resistant to METH-induced DA neurotoxicity, wild-types (+/+) are fully susceptible, and heterozygotes (+/−) develop partial dopaminergic lesions after METH administration (Fumagalli et al., 1998).
Temperature has been found to markedly influence METH-induced DA neurotoxicity. In particular, hyperthermia consistently exacerbates METH-induced DA neurotoxicity, whereas hypothermia is neuroprotective (Bowyer et al., 1992, 1994; Ali et al., 1994; Albers and Sonsalla, 1995; Cappon et al., 1997; Callahan and Ricaurte 1998; Clausing and Bowyer, 1999). Furthermore, a broad range of pharmacological agents that protect against METH-induced DA neurotoxicity appears to do so by producing hypothermia (Bowyer et al., 1992, 1994; Ali et al., 1994;Miller and O'Callaghan, 1994; Albers and Sonsalla, 1995; Callahan and Ricaurte, 1998). The mechanisms underlying these temperature effects are unknown.
We hypothesized that the prominent effect of temperature on METH-induced DA neurotoxicity involved the DAT, possibly by altering its function. To test this hypothesis, we first ascertained core temperatures of mice treated with METH at usual room temperature (22°C, a temperature in which METH-induced neurotoxicity is fully expressed), as well as at a lower ambient temperature (6°C, known to protect from METH-induced DA neurotoxicity). Using these core temperatures as a guide for in vitro studies, the effects of temperature on DAT function were examined in two separate neuronal culture systems containing the DAT.
MATERIALS AND METHODS
Drugs and chemicals.[3H]DA and [3H]MPP+, as hydrochloride salts, were purchased from New England Nuclear (Boston, MA). [3H]Methamphetamine hydrochloride and cocaine hydrochloride were obtained from the National Institute on Drug Abuse (Bethesda, MD). Dopamine hydrochloride and polyornithine were purchased from Sigma (St. Louis, MO), and MPP+ was obtained from Aldrich (Milwaukee, WI). All the other cell culture media and chemicals were purchased from Life Technologies (Grand Island, NY).
Animals. Male albino Swiss-Webster mice weighing 20–25 gm and pregnant Sprague Dawley rats (day 14 of gestation) weighing 250–300 gm were purchased from Taconic (Germantown, NY). Animals were housed individually in clear acrylic cages in a temperature-controlled room before use (22 ± 1°C). Experimental protocols were approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions. The facility for housing and care of the animals is accredited by the American Association for the Assessment and Accreditation of Laboratory Animal Care.
Temperature studies. Two groups (n = 6 per group) of Swiss-Webster mice were treated with either saline or METH (45 mg/kg, s.c.) at room temperature (22 ± 1°C). Another two groups of mice were put in a cold room (6 ± 1°C) 30 min before receiving the same METH regimen, and they were maintained in the cold room for an additional 6 hr. These temperatures were chosen because at 22°C, METH-induced neurotoxicity is fully expressed, whereas at 6°C, there is significant protection from METH-induced DA neurotoxicity (Bowyer et al., 1992; Ali et al., 1994; see below). Rectal temperatures were measured every hour for 6 hr using a BAT-12 thermometer coupled to a RET-3 mouse rectal probe with the resolution of 0.1°C (Physitemp Instruments, Clifton, NJ). All mice were killed 1 week after treatment for measurement of brain biogenic amines.
Determination of brain biogenic amine concentrations. Levels of DA and DOPAC were determined by means of HPLC coupled with electrochemical detection, as described previously (Ricaurte et al., 1992).
HDAT-SK-N-MC cell culture. The hDAT-SK-N-MC, a neuroblastoma cell line (SK-N-MC) stably transfected with the human DAT cDNA, constitutively expresses the DAT (Pifl et al., 1993). These cells were used for the present studies because of their established validity for studying the effects of DA neurotoxins (Pifl et al., 1993). Cells were grown in minimum essential medium Eagle containing 1.5 gm/l sodium bicarbonate, 2 mml-glutamine, 0.1 mm nonessential amino acids, 1.0 mm sodium pyruvate, 10% heat-inactivated fetal bovine serum, 100 U/ml of penicillin, and 100 μg/ml of streptomycin at 37°C in humidified air of 5% CO2. For assays, cells were plated into 24-well plates in a final volume of 400 μl and nearly confluent 2 d later, before experimental use.
Rat embryonic mesencephalic cell culture. Rat embryonic mesencephalic tissues were obtained from pregnant Sprague Dawley rats on day 14 of gestation. Briefly, as described by Shimoda et al. (1992), the ventral mesencephalon was dissected free without the membrane covering, and collected in Ca2+ and Mg2+-free Dulbecco's PBS at 4°C. The tissue was minced and dissociated into single cells by mild trituration with a small-bore Pasteur pipette. The cell suspension was plated in polyornithine-coated (0.1 mg/ml) 48-well Falcon plates at a density of 0.65 × 106 cells per cm2. Cultures were maintained in a medium consisting of DMEM/F-12 medium (1:1) supplemented with 6 mg/ml glucose, 15% horse serum, and 2 mm glutamine. The cultures were incubated at 37°C in humidified air of 5% CO2. On day 5 in vitro, the cultures were treated for 24 hr with fluorodeoxyuridine (13 μg/ml) and uridine (33 μg/ml) to prevent excessive proliferation of non-neuronal cells. The cells were cultured for 14 d before experimental use.
[3H]DA uptake. First, the culture medium was completely removed from the plate well. Krebs'-Ringer's-Phosphate (KRP) buffer (pH 7.4, containing 136 mm NaCl, 4.8 mm KCl, 1.2 mmMgSO4, 1.4 mmCaCl2, 10 mm glucose, 1 mm ascorbate, 140 μm EDTA and 120 μm pargyline) was then added to each well (400 μl for hDAT-SK-N-MC cells in 24-well plate and 200 μl for rat embryonic mesencephalic cells in 48-well plate). Cells were preincubated at various predetermined temperatures (34, 37, or 40°C) for 5 min, in the presence or absence of 200 μm cocaine. DA uptake was initiated by the addition of 1/10 total volume of a 10× solution of [3H]DA resulting in a final concentration of 24 nm. For kinetic studies of [3H]DA uptake by hDAT-SK-N-MC cells, 1/10 total volume of 240 nm[3H]DA was added to a range of unlabeled DA (0, 0.1, 0.3, 1, 3, 10, and 30 μm) in KRP. In rat embryonic mesencephalic cells, 24 nm of [3H]DA alone was used to confirm and extend data obtained from hDAT-SK-N-MC cells. Uptake was allowed to proceed for 6 min and stopped by rapid removal of KRP. The cells were rapidly washed twice with same volume of cold KRP, and then solubilized in 1% SDS at room temperature for 2 hr. Cell lysates were placed into scintillation vials containing 5 ml of scintillation fluid and vortexed for 15 sec. Radioactivity was counted at ∼48% efficiency on a Packard 1500 scintillation counter with on board quench correction. The difference between total uptake (in the absence of cocaine) and nonspecific uptake (in the presence of 200 μm cocaine) was defined as specific DAT-mediated uptake.
[3H]MPP+ uptake. The final concentration of 4 nm of [3H]MPP+was mixed with a range of unlabeled MPP+(1, 10, and 100 μm) in hDAT-SK-N-MC cells and final concentration of 13 nm of [3H]MPP+was mixed with a range of unlabeled MPP+(1, 10, and 100 μm) in the embryonic rat mesencephalic cells. The rest of the assay conditions were similar to those used to measure [3H]DA uptake.
[3H]METH accumulation. Time course and dose-effect (concentration-uptake) studies were first performed to determine the best conditions to study the effects of temperature DAT-mediated METH accumulation, because the effects of temperature on cell viability were unknown, and there were previous reports of two concentration-dependent mechanisms for amphetamine to enter cells (Liang and Rutledge, 1982; Zaczek et al., 1991a,b). In the time course study, final concentrations of 20nm[3H]METH and 1 μmunlabeled METH were incubated at 37°C in the presence or absence of 200 μm cocaine for different time periods (0, 3, 5, 7, 9, 15, 20, and 25 min) in hDAT-SK-N-MC cells. In the dose-effect study, a final concentration of 20 nm of [3H]METH was incubated at 37°C for 6 min (the best time point derived from the above time course study) with a range of unlabeled METH concentrations (0, 0.1, 1, 10, 100, and 1000 μm) in the presence or absence of 200 μmcocaine in hDAT-SK-N-MC cells and rat embryonic mesencephalon cells. Based on the above data, the effect of temperature (34, 37, and 40°C) on the DAT-mediated intracellular METH accumulation was tested in hDAT-SK-N-MC cells and rat embryonic mesencephalic cells using a range of unlabeled METH (0.1, 1, and 10 μm) and 20 nm [3H]METH with the incubation time of 6 min. The rest of the assay conditions were similar to those used to measure [3H]DA uptake.
Data analysis. Substrate uptake by the DAT was analyzed using old saturation method of the iterative nonlinear computer fitting program (Kell-Radlig) to estimate Vmax(maximum DA uptake rate of the DAT) andKm (inverse of dopamine affinity for DAT) values, or using the absolute value or the percentage. Data were analyzed by one-way ANOVA, followed by Duncan's multiple rangepost hoc comparisons, where appropriate. Results were considered significant if the p value was <0.05, using a two-tailed test. Data analysis was performed using the Statistical Program for the Social Sciences (SPSS for Windows, Release 6).
RESULTS
Determination of temperatures needed for in vitro studies
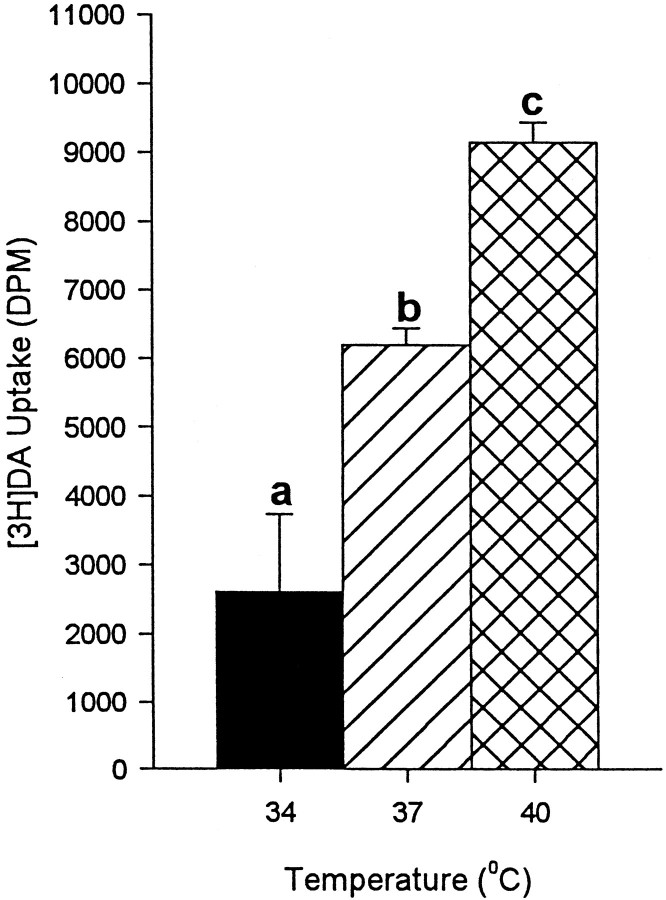
Mice treated with METH at an ambient temperature of 22°C had increases in core temperature up to 39.5°C (Fig.1A). As expected, these animals showed an approximate 70% depletion of DA axonal markers 1 week later (Fig. 1B,C). By contrast, administration of METH at 6°C was associated with decreases in core temperatures as low as 35.5°C (Fig. 1A), and no evidence of DA neurotoxicity (Fig. 1B,C). In addition to confirming the marked influence that temperature can have on METH neurotoxicity (see introductory remarks), these results indicated that a temperature range from 34 to 40°C would be most appropriate for in vitro studies on the effect of temperature on DAT function in the context of METH-induced DA neurotoxicity.
Fig. 1.
Effect of METH on core temperature (A), striatal DA levels (B), and striatal DOPAC levels (C) in mice. Mice were treated with either METH (45 mg/kg, s.c.) or same volume of saline at two different ambient temperatures (22 ± 1 and 6 ± 1°C) and killed 1 week later. Values shown represent the means ± SEM of six mice per group. *Designates significant difference compared to control;p < 0.05, determined by individual comparison after ANOVA showed an F value with p< 0.05.
Effect of temperature on [3H]DA uptake by hDAT-SK-N-MC cells
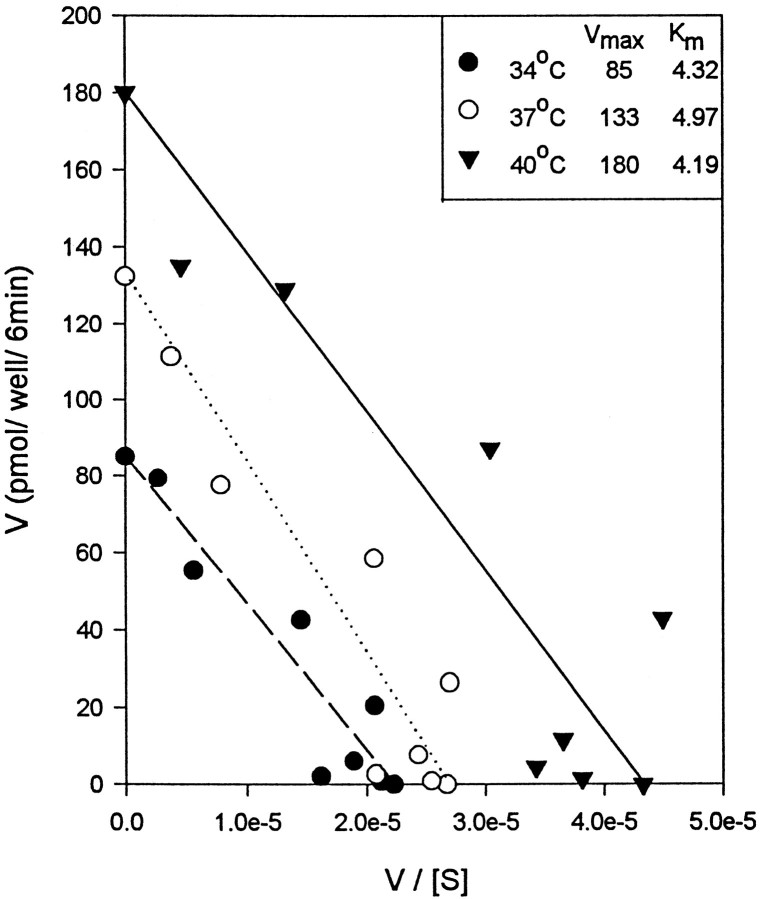
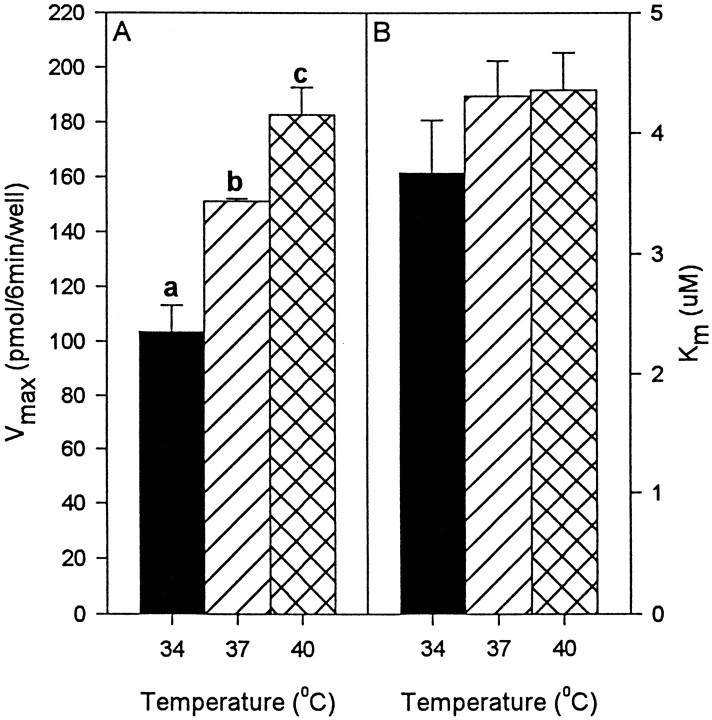
Measurement of DAT function in hDAT-SK-N-MC cells at various temperatures (34, 37, and 40°C; based on experiments described above) showed that the Vmax of DA uptake was significantly greater at higher temperatures, with 103 ± 10 pmol/well/6 min at 34°C, 151 ± 12 pmol/well/6 min at 37°C, and 183 ± 10 pmol/well/6 min at 40°C (Figs.2A,3). TheKm, reflecting the affinity of the DAT for its substrate DA was not significantly different at any of the temperatures tested (Figs. 2B, 3). These data suggest that higher temperatures increases theVmax of DA uptake without changing the affinity of DAT for DA.
Fig. 3.
Representative Eadie-Hofstee plot showing the effect of temperature on the kinetic parameters of [3H]DA uptake by hDAT-SK-N-MC cells. The Eadie-Hofstee plot shows data from one of three experiments, with samples run in triplicate.
Fig. 2.
Effect of temperature on [3H]DA uptake by hDAT-SK-N-MC cells. The experiment was performed as described in Materials and Methods. TheVmax of [3H]DA uptake increased significantly with increased temperature (A). There was no significant change in theKm (B). Values shown in A and B represent the means ± SEM of at least three independent experiments, each performed in triplicate. Results were considered significant ifp < 0.05, one-way ANOVA. aDesignates significant difference from 37 and 40°C. bDesignates significant difference from 34 and 40°C. cDesignates significant difference from 34 and 37°C.
Effect of temperature on [3H]DA uptake by embryonic mesencephalic cells
Using similar conditions as those used in hDAT-SK-N-MC cells, we found that DAT function, as reflected by [3H]DA uptake, was significantly greater at higher temperatures in embryonic mesencephalic cells that naturally express the DAT (Fig. 4), suggesting that the function of the DAT is greater at higher temperature regardless of cell model used.
Fig. 4.
Effect of temperature on [3H]DA uptake in embryonic mesencephalic cells. The experiment was performed as described in Materials and Methods. [3H]DA uptake increased significantly with increased temperature. Values shown represent the means ± SEM of at least three independent experiments, each performed in triplicate. Results were considered significant if p < 0.05, one-way ANOVA. aDesignates significant difference from 37 and 40°C. bDesignates significant difference from 34 and 40°C. cDesignates significant difference from 34 and 37°C.
Effect of temperature on [3H]MPP+ uptake by hDAT-SK-N-MC cells and embryonic mesencephalic cells
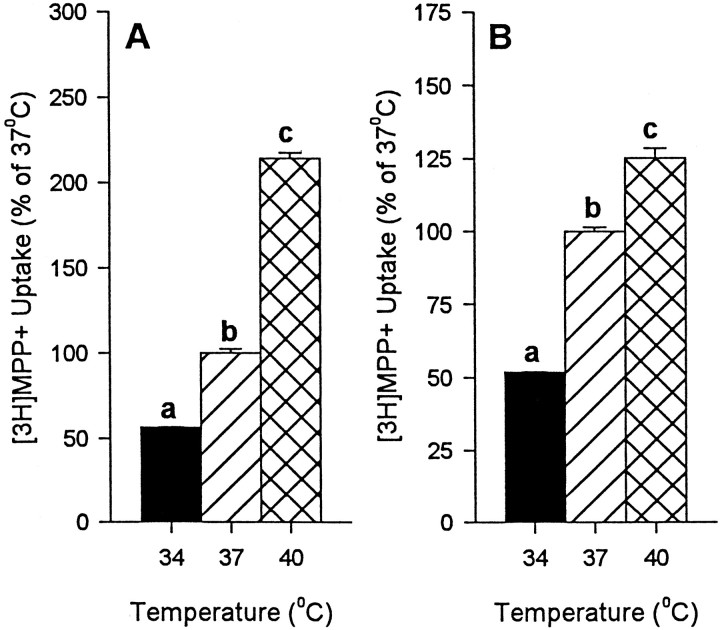
To determine whether findings with [3H]DA generalized to another DAT substrate, additional studies were conducted with the known DA neurotoxin, MPP+. As with [3H]DA, we found significantly greater [3H]MPP+uptake at higher temperatures (Fig. 5). This was the case in both cell culture models used (hDAT-SK-N-MC cells, Fig. 5A; embryonic mesencephalic cells, Fig.5B), suggesting the function of the DAT is greater at higher temperature regardless of cell model or DAT substrate used.
Fig. 5.
Effect of temperature on [3H]MPP+ uptake in hDAT-SK-N-MC cells (A) and embryonic mesencephalic cells (B). Significant increases in [3H]MPP+ uptake were observed at higher temperatures at all concentrations tested, ranging from 1 to 100 μm of cold MPP+ in the presence of 4 nm [3H]MPP+ or 13 nm [3H]MPP+, as described in Materials and Methods. The figure depicts the results of a representative experiment using 10 μm of cold MPP+ in the presence of 4 nm[3H]MPP+(A) or 13 nm[3H]MPP+(B). Values shown represent the mean ± SEM of at least three independent experiments, each performed in triplicate. Results were considered significant ifp < 0.05, one-way ANOVA. aDesignates significant difference from 37 and 40°C. bDesignates significant difference from 34 and 40°C. cDesignates significant difference from 34 and 37°C.
Effect of temperature on [3H]METH accumulation by hDAT-SK-N-MC cells and embryonic mesencephalic cells
Having shown that DAT function was significantly higher at 40°C, intermediate at 37°C, and lower at 34°C, we needed to further determine whether DAT-mediated METH accumulation was similarly influenced by temperature, because this would help link the findings to METH-induced DA neurotoxicity. As noted in Materials and Methods, time course and dose-effect studies were first performed to determine the best conditions in which to study the effect of temperature on DAT-mediated METH accumulation. In a time course study, a positive curvilinear relationship between specific METH accumulation and incubation time was found in the first 9 min, reaching a plateau afterward (Fig. 6). Based on these observations, a 6 min incubation was chosen for subsequent studies because a 6 min incubation had also been used for the studies of DA and MPP+ uptake. In the dose-effect study, DAT-mediated METH accumulation was found only at concentrations of METH that did not exceed 10 μm (Fig.7). This was the case in both hDAT-SK-N-MC cells (Fig. 7A) and embryonic mesencephalic cells (Fig. 7B). At higher concentrations (100 and 1000 μm), METH accumulation largely could not be blocked by cocaine, suggesting that it was largely by passive diffusion (Fig. 7A,B). Because these results were in agreement with those of previous studies examining [3H] amphetamine accumulation by rat striatal synaptosomes (Liang and Rutledge, 1982; Zaczek et al., 1991a,b), METH concentrations not exceeding 10 μm were used in subsequent studies designed to test the effect of temperature on DAT-mediated intracellular METH accumulation.
Fig. 6.
Time course study of [3H]METH accumulation by hDAT-SK-N-MC cells. The experiment was performed three times, as described in Materials and Methods. Values shown represent the mean ± SEM, with each time point run in triplicate.
Fig. 7.
Relationship between METH concentration and DAT-mediated METH accumulation in hDAT-SK-N-MC cells (A) and embryonic mesencephalic cells (B). The experiment was performed as described in Materials and Methods. Accumulation of 20 nm[3H]METH was measured in the presence of increasing concentrations of cold METH and in the presence and absence of cocaine (200 μm). Note that as the concentration of cold METH increases, the amount of DAT-mediated METH accumulation (the area between the lines for absence and presence of cocaine) decreases in both cell models. The non-DAT-mediated METH accumulation (the area below the line for the presence of cocaine) is virtually the same in both cell models. Values shown represent the means ± SEM of at least three independent experiments, each performed in triplicate. The error bars are too small to be seen. Results were considered significant if p < 0.05, one-way ANOVA. *Designates significant DAT-mediated METH accumulation compared with the value at the METH concentration of 100 μm.
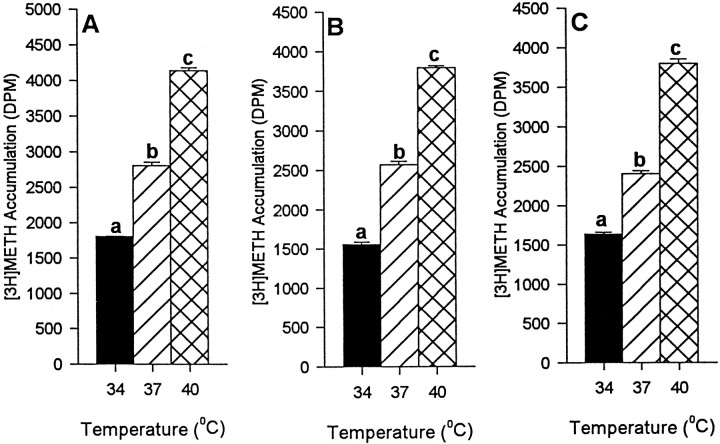
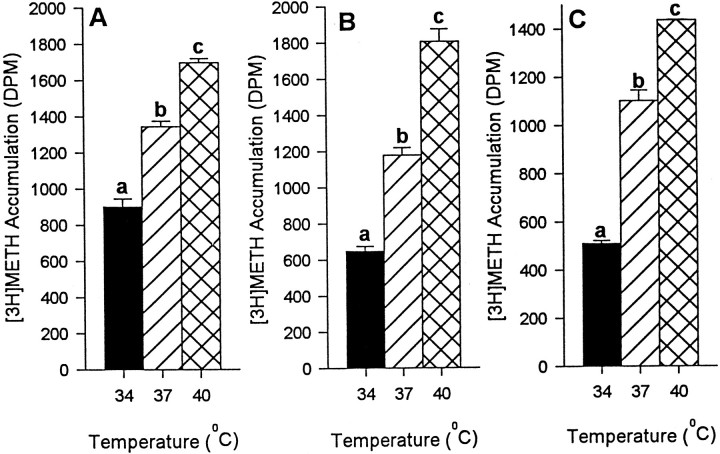
Increased temperature (34, 37, and 40°C) was associated with greater [3H]METH accumulation at all concentrations of METH tested (final concentration of 0.1, 1, and 10 μm cold METH mixed with 20 nm[3H]METH). This was the case in both hDAT-SK-N-MC cells (Fig.8A–C, respectively) and embryonic mesencephalic cells (Fig.9A–C, respectively), indicating that DAT-mediated METH accumulation is also directly correlated with temperature, with significantly higher accumulation at 40°C, intermediate accumulation at 37°C, and lowest accumulation at 34°C.
Fig. 8.
Effect of temperature on DAT-mediated [3H]METH accumulation in hDAT-SK-N-MC cells. The experiment was performed as described in Materials and Methods. Significantly greater METH accumulation was observed at higher temperatures at all concentrations of METH (0.1, 1, and 10 μm; A–C, respectively) tested in the presence of 20 nm [3H]METH. Values shown represent the mean ± SEM of at least three independent experiments, each performed in triplicate. Results were considered significant if p < 0.05, one-way ANOVA.aDesignates significant difference from 37 and 40°C.bDesignates significant difference from 34 and 40°C.cDesignates significant difference from 34 and 37°C.
Fig. 9.
Effect of temperature on DAT-mediated [3H]METH accumulation in embryonic mesencephalic cells. The experiment was performed as described in Materials and Methods. Significantly greater METH accumulation was observed at higher temperatures at all concentrations of METH (0.1, 1, and 10 μM; A–C, respectively) tested in the presence of 20 nm [3H]METH. Values shown represent the mean ± SEM of at least three independent experiments, each performed in triplicate. Results were considered significant if p < 0.05, one-way ANOVA.aDesignates significant difference from 37 and 40°C.bDesignates significant difference from 34 and 40°C.cDesignates significant difference from 34 and 37°C.
DISCUSSION
The present results indicate that small, stepwise increases in temperature (ranging from 34 to 40°C) are directly correlated with significant increases in DAT function and DAT-mediated METH cellular accumulation. The fact that similar observations were made using three different DAT substrates (DA, MPP+, and METH) in two distinct neuronal cell culture models suggests that the findings are not related to peculiarities of an individual substrate or cell culture model and strongly support the validity of the observations. Because changes in temperature can profoundly influence METH neurotoxicity (Bowyer et al., 1992, 1994; Ali et al., 1994; Albers and Sonsalla, 1995; Cappon et al., 1997; Callahan and Ricaurte 1998;Clausing and Bowyer, 1999) and because the DAT plays an essential role in METH-induced DA neurotoxicity (Marek et al., 1990; Pu et al., 1994;Fumagalli et al., 1998), the effect of temperature on METH-induced DA neurotoxicity is likely to be mediated, at least in part, at the level of the DAT.
Whereas previous studies have demonstrated that DAT function is decreased at extremely low temperatures (4°C) (Shimada et al., 1991), this is the first study to demonstrate that small changes in temperature that are in close proximity to normal core temperature (37°C) significantly influence DAT function. The importance of this finding is underscored by the fact that the temperature range used in the present studies was selected based on core temperature measurements from METH-treated animals. Thus, the “high” temperature (40°C) mimicked the core temperature of animals in which METH toxicity was fully expressed, whereas the “low” temperature (34°C) mimicked the core temperature of animals that were fully protected by reducing the ambient temperature (Fig. 1). Notably, these temperatures also reflect brain temperature, which closely parallels core temperature (Clausing and Bowyer, 1999).
The present results also indicate that increased temperature is associated with greater DAT-mediated METH accumulation (Figs. 8, 9). This observation is consistent with the hypothesis that temperature influences METH neurotoxicity, at least in part, by altering DAT function, possibly by increasing intraneuronal METH concentrations. It is well established that METH-induced DA neurotoxicity is dose-dependent and that increased striatal levels of amphetamines are associated with increased neurotoxicity (Ricaurte et al., 1983; seeSeiden and Ricaurte, 1987). Furthermore, it is known that the DAT plays an essential role in METH-induced dopaminergic neurotoxicity (Marek et al., 1990; Pu et al., 1994; Fumagalli et al., 1998). Collectively, these observations suggest that transport of METH into cells via the DAT or an interaction between METH and the DAT is necessary (although perhaps not sufficient) for the expression of METH neurotoxicity. The fact that there are brain regions in the hyperthermic animal that contain the DAT yet do not sustain the same degree of neural injury as the striatum (e.g., nucleus accumbens, hypothalamus, and substantia nigra) (Broening et al., 1997) suggests that regional differences in DAT function or factors beyond the DAT (e.g., age, species or metabolic differences) may also modify the neurotoxicity of METH and related drugs (Broening et al., 1995; Cappon et al., 1997).
Consistent with previous reports using amphetamine as a DAT substrate (Liang and Rutledge, 1982; Zaczek et al., 1991a,b), the present results indicate that the nature of the interaction between METH and DA neurons is dependent on the concentration of METH tested. Specifically, at lower concentrations (20 nm to 10 μm), the bulk of METH appears to enter DA cells via the DAT, and is thus sensitive to cocaine inhibition. In contrast, at higher concentrations (100–1000 μm), the bulk of METH appears to enter DA cells by passive diffusion, because most of it can no longer be blocked by cocaine. The finding that DAT-mediated intracellular METH accumulation was strongly influenced by temperature provides additional indication that transport of METH into cells through the DAT or an interaction between METH and the DAT plays a role in METH neurotoxicity. Notably, the concentrations of METH used for DAT-mediated uptake in the present study (0.1–10 μM) are in the range of those found in the setting of METH neurotoxicity in vivo (Clausing and Bowyer, 1999).
Ideally, to directly test the hypothesis that increased uptake of METH at higher temperatures is responsible for increased DA neurotoxicity observed at higher ambient temperatures, in vivo and/or DA cell culture studies should be performed to demonstrate that increased concentrations of METH within DA terminals are associated with increased METH neurotoxicity. However, because only a very small fraction of nerve terminals in the striatum are dopaminergic, in vivo studies using the entire striatum of intact animals stand a high chance of yielding false negative results. Moreover, they would not permit conclusions regarding changes in METH concentrations within DA nerve terminals. Likewise, neurotoxicity studies using DA cells in culture would be inconclusive, because efforts to protect DA cells in culture from METH-induced neurotoxicity with DAT blockers have thus far been unsuccessful (Callahan et al., 2000). In addition, these studies would require prolonged incubations (3–5 d) (Bennett et al., 1993,1998; Cubells et al., 1994) at nonphysiological temperatures, a process that would be associated with exceedingly high rates of cell death unrelated to METH. In this regard, however, it is noteworthy that in a recently developed in vitro model, small increments in temperature identical to those used in the present study have been linked to increases in METH-induced DA neurotoxicity, as indexed in that model system (Kim et al., 2000).
The observation that increased temperature leads to increased DAT function does not preclude the possibility that other mechanisms potentially involved in METH-induced DA neurotoxicity are also influenced by temperature. For example, increased temperature may be associated with increased METH-induced DA release or redistribution, possibly leading to increased formation of reactive oxidative species (Cubells et al., 1994; Hirata et al., 1996; Huang et al., 1997;Fumagalli et al., 1999). Increased temperature is also likely to be associated with increased metabolic demand, and this could also influence METH neurotoxicity (Chan et al., 1994; Albers et al., 1996;Bowyer et al., 1996; Huang et al., 1997; Stephans et al., 1998; Burrows et al., 2000), although results of studies evaluating the role of energy consumption in METH neurotoxicity have not always been consistent (Chan et al., 1994; Callahan and Ricaurte, 1998). Alternatively, higher temperatures may increase the release of excitatory amino acids (EAAs) potentially involved in METH-induced DA injury (Sonsalla et al., 1989, 1991; Stephans and Yamamoto, 1994). However, because the ability of EAA antagonists to protect from METH neurotoxicity appears to be largely dependent on their hypothermic effects (Bowyer et al., 1994; Miller and O'Callaghan, 1994), the role of EAAs in METH neurotoxicity is uncertain. Nonetheless, these or other as yet unidentified mechanisms or processes underlying METH neurotoxicity could all theoretically be influenced by temperature in a manner that would lead to an exacerbation of METH-induced neurotoxic injury.
Findings from the present study also shed light on neurotoxic processes induced by the dopaminergic neurotoxin MPP+ (Langston et al., 1983, 1984;Heikkila et al., 1984). In particular, a key event in the expression of the neurotoxicity of MPTP is the active uptake of MPP+ into dopaminergic neurons (Javitch et al., 1985; Melamed et al., 1985; Chiba et al., 1985). Like METH, a positive relationship between DAT function and MPTP-induced neurotoxicity has been revealed (Ricaurte et al., 1985; Gainetdinov et al., 1997; Bezard et al., 1999; Donovan et al., 1999). However, in contrast to what is observed with METH, hypothermia enhances MPTP-induced neurotoxicity in mice, via an unknown mechanism (Freyaldenhoven et al., 1995; Moy et al., 1998). Results from the present study indicate that the ability of hypothermia to exacerbate MPTP-induced DA neurotoxicity is not caused by the increase in the transmembrane MPP+ incorporation. Other factors capable of increasing the bioavailability of MPTP in brain under conditions of hypothermia are likely to be responsible for this apparent paradox. Nevertheless, the fact that with relatively small increases in temperature, there are significant increases in the accumulation of a potent DA neurotoxin such as MPP+ suggests that the present findings may have relevance to idiopathic disease processes involving brain DA neurons (e.g., Parkinson's disease).
In summary, data from the current study indicate that small, physiologically relevant changes in temperature designed to parallel those in METH-treated animals can significantly influence DAT function and DAT-mediated METH cellular accumulation measured in isolated cell systems. Given the central role of the DAT in METH-induced DA neurotoxicity, it seems likely that temperature exerts its effect on METH-induced DA neurotoxicity, at least in part, at the level of the DAT. The fact that temperature also influences MPP+ accumulation raises the possibility that the present results may be of relevance to pathophysiological insults related to DAT-mediated entry of toxic species. Finally, it seems likely that the present studies have implications for other toxic amphetamine derivatives (e.g., MDMA), because the neurotoxic effects of these drugs are also highly dependent on intact neurotransporter function (Rudnick and Wall, 1992; Shankaran et al., 1999) and influenced by temperature (Malberg and Seiden, 1998).
Footnotes
This work was supported by National Institute on Drug Abuse/National Institutes of Health Grants DA09487, DA05707, DA05938, and DA10217 (G.A.R.). The SK-N-MC cell line was kindly provided by Prof. Marc G. Caron at Duke University.
Correspondence should be addressed to Dr. George A. Ricaurte, Department of Neurology, Johns Hopkins Medical Institutions, 5501 Hopkins Bayview Circle, Room 5B.71E, Baltimore, MD 21224. E-mail:Ricaurte@jhmi.edu.
REFERENCES
- 1.Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. [PubMed] [Google Scholar]
- 2.Albers DS, Zeevalk GD, Sonsalla PK. Damage to dopaminergic nerve terminals in mice by combined treatment of intrastriatal malonate with systemic methamphetamine or MPTP. Brain Res. 1996;718:217–220. doi: 10.1016/0006-8993(96)00135-7. [DOI] [PubMed] [Google Scholar]
- 3.Ali SF, Newport GD, Holson RR, Slikker W, Jr, Bowyer JF. Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res. 1994;658:33–38. doi: 10.1016/s0006-8993(09)90007-5. [DOI] [PubMed] [Google Scholar]
- 4.Bennett BA, Hyde CE, Pecora JR, Clodfelter JE. Differing neurotoxic potencies of methamphetamine, mazindol, and cocaine in mesencephalic cultures. J Neurochem. 1993;60:1444–1452. doi: 10.1111/j.1471-4159.1993.tb03307.x. [DOI] [PubMed] [Google Scholar]
- 5.Bennett BA, Hollingsworth CK, Martin RS, Harp JJ. Methamphetamine-induced alterations in dopamine transporter function. Brain Res. 1998;782:219–227. doi: 10.1016/s0006-8993(97)01281-x. [DOI] [PubMed] [Google Scholar]
- 6.Bezard E, Gross CE, Fournier MC, Dovero S, Bloch B, Jaber M. Absence of MPTP-induced neuronal death in mice lacking the dopamine transporter. Exp Neurol. 1999;155:268–273. doi: 10.1006/exnr.1998.6995. [DOI] [PubMed] [Google Scholar]
- 7.Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in striatum. J Pharmacol Exp Ther. 1992;260:817–824. [PubMed] [Google Scholar]
- 8.Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- 9.Bowyer JF, Clausing P, Schmued L, Davies DL, Binienda Z, Newport GD, Scallet AC, Slikker W., Jr Parenterally administered 3-nitropropionic acid and amphetamine can combine to produce damage to terminals and cell bodies in the striatum. Brain Res. 1996;712:221–229. doi: 10.1016/0006-8993(95)01417-9. [DOI] [PubMed] [Google Scholar]
- 10.Broening HW, Bowyer JF, Slikker W., Jr Age-dependent sensitivity of rats to the long-term effects of the serotonergic neurotoxicant (+/−)-3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J Pharmacol Exp Ther. 1995;275:325–333. [PubMed] [Google Scholar]
- 11.Broening HW, Pu C, Vorhees CV. Methamphetamine selectively damages dopaminergic innervation to the nucleus accumbens core while sparing the shell. Synapse. 1997;27:153–160. doi: 10.1002/(SICI)1098-2396(199710)27:2<153::AID-SYN6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Burrows KB, Nixdorf WL, Yamamoto BK. Central administration of methamphetamine synergizes with metabolic inhibition to deplete striatal monoamines. J Pharmacol Exp Ther. 2000;292:853–860. [PubMed] [Google Scholar]
- 13.Callahan BT, Ricaurte GA. Effect of 7-nitroindazole on body temperature and methamphetamine-induced dopamine toxicity. NeuroReport. 1998;9:2691–2695. doi: 10.1097/00001756-199808240-00001. [DOI] [PubMed] [Google Scholar]
- 14.Callahan BT, Kim S, Yuan J, Ricaurte G (2000) Effect of dopamine uptake blockers on methamphetamine toxicity in primary mesencephalic cultures. Soc Neurosci Abstr, in press.
- 15.Cappon GD, Morford LL, Vorhees CV. Ontogeny of methamphet-amine-induced neurotoxicity and associated hyperthermic response. Brain Res Dev Brain Res. 1997;103:155–162. doi: 10.1016/s0165-3806(97)81791-9. [DOI] [PubMed] [Google Scholar]
- 16.Chan P, Di Monte DA, Luo J-J, Delanney LE, Irwin I, Langston JW. Rapid ATP loss caused by methamphetamine in the mouse striatum: relationship between energy impairment and dopaminergic neurotoxicity. J Neurochem. 1994;62:2484–2487. doi: 10.1046/j.1471-4159.1994.62062484.x. [DOI] [PubMed] [Google Scholar]
- 17.Chiba K, Trevor AJ, Castagnoli N., Jr Active uptake of MPP+, a metabolite of MPP+, by brain synaptosomes. Biochem Biophys Res Commun. 1985;128:1228–1232. doi: 10.1016/0006-291x(85)91071-x. [DOI] [PubMed] [Google Scholar]
- 18.Clausing P, Bowyer JF. Time course of brain temperature and caudate/putamen microdialysate levels of amphetamine and dopamine in rats after multiple doses of d-amphetamine. Ann NY Acad Sci. 1999;890:495–504. doi: 10.1111/j.1749-6632.1999.tb08031.x. [DOI] [PubMed] [Google Scholar]
- 19.Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donovan DM, Miner LL, Perry MP, Revay RS, Sharpe LG, Przedborski S, Kostic V, Philpot RM, Kirstein CL, Rothman RB, Schindler CW, Uhl GR. Cocaine reward and MPTP toxicity: alteration by regional variant dopamine transporter overexpression. Mol Brain Res. 1999;73:37–49. doi: 10.1016/s0169-328x(99)00235-1. [DOI] [PubMed] [Google Scholar]
- 21.Ellison G, Eison MS, Huberman HS, Daniel F. Long-term changes in dopaminergic innervation of caudate nucleus after continuous amphetamine administration. Science. 1978;201:276–278. doi: 10.1126/science.26975. [DOI] [PubMed] [Google Scholar]
- 22.Frey K, Kilbourn M, Robinson T. Reduced striatal vesicular monoamine transporters after neurotoxic but not after behaviorally-sensitizing doses of methamphetamine. Eur J Pharmacol. 1997;334:273–279. doi: 10.1016/s0014-2999(97)01152-7. [DOI] [PubMed] [Google Scholar]
- 23.Freyaldenhoven TE, Ali SF, Hart RW. MPTP and MPP+-induced effects on body temperature exhibit age- and strain-dependence in mice. Brain Res. 1995;688:161–170. doi: 10.1016/0006-8993(95)00529-y. [DOI] [PubMed] [Google Scholar]
- 24.Fukumura M, Cappon GD, Pu CF, Broening HW, Vorhees CV. A single dose model of methamphetamine-induced neurotoxicity in rats: effects on neostriatal monoamines and glial fibrillary acidic protein. Brain Res. 1998;806:1–7. doi: 10.1016/s0006-8993(98)00656-8. [DOI] [PubMed] [Google Scholar]
- 25.Fumagalli F, Gainetdinov RR, Valenzano KJ, Caron MG. Role of dopamine transporter in methamphetamine-induced neurotoxicity: evidence from mice lacking the transporter. J Neurosci. 1998;18:4861–4869. doi: 10.1523/JNEUROSCI.18-13-04861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci. 1999;19:2424–2431. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochem. 1997;69:1322–1325. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- 28.Gibb JW, Hanson GR, Johnson M. Neurochemical mechanisms of toxicity. In: Cho AK, Segal DS, editors. Amphetamine and its analogs. Academic; Los Angeles: 1994. pp. 269–295. [Google Scholar]
- 29.Heikkila RE, Manzino L, Cabbat FS, Duvoisin RC. Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature. 1984;311:467–469. doi: 10.1038/311467a0. [DOI] [PubMed] [Google Scholar]
- 30.Hirata H, Cadet JL. p53-knockout mice are protected against the long-term effects of methamphetamine on dopaminergic terminals and cell bodies. J Neurochem. 1997;69:780–790. doi: 10.1046/j.1471-4159.1997.69020780.x. [DOI] [PubMed] [Google Scholar]
- 31.Hirata H, Ladenheim B, Carlson E, Epstein C, Cadet JL. Autoradiographic evidence for methamphetamine-induced striatal dopaminergic loss in mouse brain: attenuation in CuZn-superoxide dismutase transgenic mice. Brain Res. 1996;714:95–103. doi: 10.1016/0006-8993(95)01502-7. [DOI] [PubMed] [Google Scholar]
- 32.Huang NK, Wan FJ, Tseng CJ, Tung CS. Nicotinamide attenuates methamphetamine-induced striatal dopamine depletion in rats. NeuroReport. 1997;8:1883–1885. doi: 10.1097/00001756-199705260-00018. [DOI] [PubMed] [Google Scholar]
- 33.Javitch JA, D'Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci USA. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S, Westphalen R, Callahan B, Hatzidimitriou G, Yuan J, Ricaurte GA. Toward development of an in vitro model of methamphetamine-induced dopamine nerve terminal toxicity. J Pharmacol Exp Ther. 2000;293:625–633. [PubMed] [Google Scholar]
- 35.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 36.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 37.Langston JW, Forno LS, Rebert CS, Irwin I. Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyrine (MPTP) in the squirrel monkey. Brain Res. 1984;292:390–394. doi: 10.1016/0006-8993(84)90777-7. [DOI] [PubMed] [Google Scholar]
- 38.Lew R, Malgrange B, Ricaurte GA, Seiden LS. Evidence for mechanism of action of neurotoxicity of amphetamine related compounds. In: Kostrzewa RM, editor. Highly selective neurotoxins: basic and clinical applications. Humana; Totowa, NJ: 1998. pp. 235–268. [Google Scholar]
- 39.Liang NY, Rutledge CO. Comparison of the release of [3H]dopamine from isolated corpus striatum by amphetamine, fenfluramine and unlabelled dopamine. Biochem Pharmacol. 1982;31:983–992. doi: 10.1016/0006-2952(82)90332-x. [DOI] [PubMed] [Google Scholar]
- 40.Lorez H. Fluorescence histochemistry indicates damage of striatal dopamine nerve terminals in rats after multiple doses of methamphetamine. Life Sci. 1981;28:911–916. doi: 10.1016/0024-3205(81)90053-9. [DOI] [PubMed] [Google Scholar]
- 41.Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marek GJ, Vosmer G, Seiden LS. Dopamine uptake inhibitors block long-term neurotoxic effects of methamphetamine upon dopaminergic neurons. Brain Res. 1990;513:274–279. doi: 10.1016/0006-8993(90)90467-p. [DOI] [PubMed] [Google Scholar]
- 43.McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melamed E, Rosenthal J, Cohen O, Globus M, Uzzan A. Dopamine but not norepinephrine or serotonin uptake inhibitors protect mice against neurotoxicity of MPTP. Eur J Pharmacol. 1985;116:179–181. doi: 10.1016/0014-2999(85)90201-8. [DOI] [PubMed] [Google Scholar]
- 45.Miller DB, O'Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:752–760. [PubMed] [Google Scholar]
- 46.Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999;20:424–429. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- 47.Moy LY, Albers DS, Sonsalla PK. Lowering ambient or core body temperature elevates striatal MPP+ levels and enhances toxicity to dopamine neurons in MPTP-treated mice. Brain Res. 1998;790:264–269. doi: 10.1016/s0006-8993(98)00069-9. [DOI] [PubMed] [Google Scholar]
- 48.Pifl C, Giros B, Caron MG. Dopamine transporter expression confers cytotoxicity to low doses of the Parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium. J Neurosci. 1993;13:4246–4253. doi: 10.1523/JNEUROSCI.13-10-04246.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pu C, Fisher JE, Cappon GD, Vorhees CV. The effects of amfonelic acid, a dopamine uptake inhibitor, on methamphetamine-induced dopaminergic terminal degeneration and astrocytic response in rat striatum. Brain Res. 1994;649:217–224. doi: 10.1016/0006-8993(94)91067-7. [DOI] [PubMed] [Google Scholar]
- 50.Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- 51.Ricaurte GA, Fuller RW, Perry KW, Seiden LS, Schuster CR. Fluoxetine increases long-lasting neostriatal dopamine depletion after administration of d-methamphetamine and d-amphetamine. Neuropharmacology. 1983;22:1165–1169. doi: 10.1016/0028-3908(83)90075-8. [DOI] [PubMed] [Google Scholar]
- 52.Ricaurte GA, Seiden LS, Schuster CR. Further evidence that amphetamines produce long-lasting dopamine neurochemical deficits by destroying dopamine nerve fibers. Brain Res. 1984;303:359–364. doi: 10.1016/0006-8993(84)91221-6. [DOI] [PubMed] [Google Scholar]
- 53.Ricaurte GA, Langston JW, DeLanney LE, Irwin I, Brooks JD. Dopamine uptake blockers protect against the dopamine depleting effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse striatum. Neurosci Lett. 1985;59:259–264. doi: 10.1016/0304-3940(85)90141-7. [DOI] [PubMed] [Google Scholar]
- 54.Ricaurte GA, Martello AL, Katz JL, Martello MB. Lasting effects of (+-)-3,4-methylenedioxymethamphetamine (MDMA) on central serotonergic neurons in nonhuman primates: neurochemical observations. J Pharmacol Exp Ther. 1992;261:616–622. [PubMed] [Google Scholar]
- 55.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 56.Rudnick G, Wall SC. The molecular mechanism of “ecstasy” [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci USA. 1992;89:1817–1821. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seiden LS, Ricaurte GA. Neurotoxicity of methamphetamine and related drugs. In: Meltzer HY, editor. Psychopharmacology: the third generation of progress. Raven; New York: 1987. pp. 359–366. [Google Scholar]
- 58.Shankaran M, Yamamoto BK, Gudelsky GA. Involvement of the serotonin transporter in the formation of hydroxyl radicals induced by 3,4-methylenedioxymethamphetamine. Eur J Pharmacol. 1999;385:103–110. doi: 10.1016/s0014-2999(99)00728-1. [DOI] [PubMed] [Google Scholar]
- 59.Shimada S, Kitayama S, Lin CL, Patel A, Nanthakumar E, Gregor P, Kuhar M, Uhl G. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991;254:576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- 60.Shimoda K, Sauve Y, Marini A, Schwartz JP, Commissiong JW. A high percentage yield of tyrosine hydroxylase-positive cells from rat E14 mesencephalic cell culture. Brain Res. 1992;586:319–331. doi: 10.1016/0006-8993(92)91642-r. [DOI] [PubMed] [Google Scholar]
- 61.Sonsalla PK, Nicklas WJ, Heikkila RE. Role for excitatory amino acids in methamphetamine-induced nigrostriatal dopaminergic toxicity. Science. 1989;243:398–400. doi: 10.1126/science.2563176. [DOI] [PubMed] [Google Scholar]
- 62.Sonsalla PK, Riordan DE, Heikkila RE. Competitive and noncompetitive antagonists at N-methyl-D-aspartate receptors protect against methamphetamine-induced dopaminergic damage in mice. J Pharmacol Exp Ther. 1991;256:506–512. [PubMed] [Google Scholar]
- 63.Sonsalla PK, Jochnowitz ND, Zeevalk GD, Oostveen JA, Hall ED. Treatment of mice with methamphetamine produces cell loss in the substantia nigra. Brain Res. 1996;738:172–175. doi: 10.1016/0006-8993(96)00995-x. [DOI] [PubMed] [Google Scholar]
- 64.Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: roles for glutamate and dopamine efflux. Synapse. 1994;17:203–209. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- 65.Stephans SE, Whittingham TS, Douglas AJ, Lust WD, Yamamoto BK. Substrates of energy metabolism attenuate methamphetamine-induced neurotoxicity in striatum. J Neurochem. 1998;71:613–621. doi: 10.1046/j.1471-4159.1998.71020613.x. [DOI] [PubMed] [Google Scholar]
- 66.Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci. 1998;18:419–427. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Gatley SJ, Wong CT, Hitzemann RJ, Pappas NR. In vivo evidence that methamphetamine abuse produces long-lasting changes in dopamine transporters in human brain. J Nucl Med. 1999;40:110. [Google Scholar]
- 68.Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486:73–78. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]
- 69.Zaczek R, Culp S, De SE. Interactions of [3H]amphetamine with rat brain synaptosomes. II. Active transport. J Pharmacol Exp Ther. 1991a;257:830–835. [PubMed] [Google Scholar]
- 70.Zaczek R, Culp S, Goldberg H, McCann DJ, De SE. Interactions of [3H]amphetamine with rat brain synaptosomes. I. Saturable sequestration. J Pharmacol Exp Ther. 1991b;257:820–829. [PubMed] [Google Scholar]