Abstract
The nucleus accumbens, a brain structure ideally situated to act as an interface between corticolimbic information-processing regions and motor output systems, is well known to subserve behaviors governed by natural reinforcers. In the accumbens core, glutamatergic input from its corticolimbic afferents and dopaminergic input from the ventral tegmental area converge onto common dendrites of the medium spiny neurons that populate the accumbens. We have previously found that blockade of NMDA receptors in the core with the antagonist 2-amino-5-phosphonopentanoic acid (AP-5; 5 nmol) abolishes acquisition but not performance of an appetitive instrumental learning task (Kelley et al., 1997). Because it is currently hypothesized that concurrent dopamine D1 and glutamate receptor activation is required for long-term changes associated with plasticity, we wished to examine whether the dopamine system in the accumbens core modulates learning via NMDA receptors. Co-infusion of low doses of the D1receptor antagonist SCH-23390 (0.3 nmol) and AP-5 (0.5 nmol) into the accumbens core strongly impaired acquisition of instrumental learning (lever pressing for food), whereas when infused separately, these low doses had no effect. Infusion of the combined low doses had no effect on indices of feeding and motor activity, suggesting a specific effect on learning. We hypothesize that co-activation of NMDA and D1 receptors in the nucleus accumbens core is a key process for acquisition of appetitive instrumental learning. Such an interaction is likely to promote intracellular events and gene regulation necessary for synaptic plasticity and is supported by a number of cellular models.
Keywords: glutamate, plasticity, striatum, intracellular signals, rat, reinforcement, reward
The nucleus accumbens, a forebrain structure known to subserve behaviors governed by natural reinforcers, receives excitatory glutamatergic input from prefrontal cortex, hippocampus, thalamus, and amygdala (McGeer et al., 1977; Walaas and Fonnum, 1979; Young and Bradford, 1986; Fuller et al., 1987; Robinson and Beart, 1988), as well as a major dopaminergic innervation from the ventral tegmental area (Lindvall and Bjorklund, 1978). These innervations converge on the dendritic spines of the medium spiny neurons that populate the nucleus accumbens (Totterdell and Smith, 1989; Sesack and Pickel, 1990; Smith and Bolam, 1990). Therefore, these neurons are in a unique position to recognize context-driven patterns of activation and to transfer this information to planning and motor regions for appropriate behavioral responses (Houk et al., 1995). Recently, there has been much interest in the neuromodulatory effects of dopamine (DA) receptor activation on NMDA receptor state, as well as the intracellular mechanisms that may govern their interaction. For example, DA D1 receptor activation in striatal slices potentiates responses mediated by NMDA receptor activation (an effect that is blocked by the D1receptor antagonist SCH-23390), whereas dopamine D2 receptors have an attenuating effect (Cepeda et al., 1993; Cepeda and Levine, 1998). Moreover, DA receptor modulation of NMDA receptor-mediated responses is blunted in D1A-deficient mutant mice (Levine et al., 1996). When corticostriatal excitation and dopaminergic activation are temporally coordinated, there is a long-lasting enhancement of synaptic strength in medium spiny neurons (Wickens et al., 1996). In a behavioral study, it was reported that impairment of learning of a one-trial inhibitory avoidance task attributable to post-trial systemic administration of NMDA antagonists is attenuated by systemic administration of low doses of dopamine agonists (Adriani et al., 1998). Taken together, these emerging findings suggest that co-activation of dopamine D1 and glutamate NMDA receptors is required for long-term changes associated with plasticity and perhaps certain forms of learning.
We have previously found that blockade of NMDA receptors in the accumbens core with the antagonist 2-amino-5-phosphonopentanoic acid (AP-5) completely abolishes acquisition but not performance of an appetitive instrumental learning task (acquisition of lever pressing for food; Kelley et al., 1997) and also disrupts spatial learning in the radial arm maze (Smith-Roe et al., 1999). Considering the substantial evidence for DA–NMDA receptor interactions at the physiological and molecular level, we hypothesized that such interactions may play a key role in learning subserved by the nucleus accumbens. Our first objective was to assess the effects of intra-accumbens core infusion of the D1 dopamine receptor antagonist SCH-23390 in an appetitive instrumental learning task. However, a major obstacle to investigating the role of DA receptors in learning and to interpreting effects on behavior is the considerable motor impairment that often results with DA receptor antagonist treatment. Because we indeed found evidence of a motor impairment, we also examined the effects of very low doses of the D1 antagonist as well as combinations of low doses of AP-5 and SCH-23390. We report here that co-activation of D1 and NMDA receptors is necessary for appetitive instrumental learning.
MATERIALS AND METHODS
Animals and surgery. A total of 48 male Sprague Dawley rats (Harlan Sprague Dawley, Madison, WI) weighing 275–300 gm were used for these experiments. Care of animals was in accordance with institutional guidelines. Rats were housed two per cage in a temperature-controlled (21°C) and light-controlled (12 hr light/dark cycle) animal colony. For cannula implantation, rats were anesthetized with a ketamine-xylazine mixture (100 and 10 mg/kg, respectively; Research Biochemicals, Natick, MA). Standard stereotaxic procedures were used to implant bilateral 23 gauge stainless steel guide cannulas, with coordinates based on flat-skull stereotaxic orientation. Cannulas were secured with dental acrylic and stainless steel screws, and a wire stylet occluded the guide to maintain patency. For all experiments, rats were implanted with cannulas placed 2.5 mm above the nucleus accumbens core at the following coordinates: anteroposterior, +1.4 mm; lateromedial, ±1.7 mm from midline; and dorsoventral, −5.5 mm from skull. After several days of recovery from surgery, all rats were put on a restricted diet that maintained body weight at 85% of free-feeding weight. Water was available ad libitum at all times in the home cage.
Drugs and microinfusions. The selective, competitive NMDA receptor antagonist AP-5 and the D1 receptor antagonist SCH-23390 HCl were obtained from Research Biochemicals. All drugs were dissolved in isotonic sterile saline and kept at 4°C in 200 μl aliquots. Intracerebral microinfusions were bilateral in a volume of 0.5 μl/side. AP-5 was administered in a dose per side of 0.5 nmol (0.1 μg), and SCH-23390 was administered in two doses: 3 nmol (1 μg) and 0.3 nmol (0.1 μg). Infusions of drug or vehicle were given by lowering 30 gauge injector cannulas to the site of infusion (−8.0 mm from skull). A Harvard Apparatus microdrive pump was used to administer drug infusions with an infusion time of 1 min 33 sec, followed by 1 min of diffusion time. The injectors were then removed, the stylets were replaced, and the rats were placed into the test apparatus immediately. For all experiments, rats were given two preliminary sham injections, in which a dummy injector was lowered through the guide to adapt them to the procedure.
Behavioral training. All rats (except those in the feeding and locomotion study described below) were trained in operant chambers (Coulbourn Instruments, Allentown, PA) equipped with two levers, a house light, and a red signal light. All stimulus events and data acquisition were controlled with a computer (Paul Fray, Cambridge, UK). Before training, rats were adapted to the food pellets (45 mg sucrose pellets). Additionally, the rats had preexposure for two 10 min sessions to the operant test cages with several pellets availablead libitum in the food tray (with no levers present). On the first test day and all days thereafter, rats were placed in the operant chamber for a 15 min session. Responding on one lever resulted in delivery of a food pellet on a variable ratio 2 schedule of reinforcement, rewarding an average of every two responses. The other lever was not distinguishable from the first lever but did not deliver a food pellet or alter house lighting. The correct lever was randomized among animals but was always the same for an individual animal. When a correct response was made, a food pellet was delivered into a food tray located in between the two levers. A photocell located in the food tray recorded nose pokes. Pellet delivery was accompanied by house light offset and illumination of a red stimulus light on the response panel (3 sec), as well as a light in the food tray. Dependent variables recorded included correct responses, incorrect responses, and nose pokes. Animals were tested between 9 A.M. and 2 P.M.
Experimental procedure. Rats were given the appropriate microinfusion immediately before the session for the first 4 test days. They were then tested without any infusion for days 5–9. On day 10, all animals received their initial treatment to test for performance effects once learning had occurred. Five groups of rats were given one of five different treatments: a high dose of SCH-23390 (3 nmol),n = 7; a low dose of SCH-23390 (0.3 nmol),n = 8; a low dose of AP-5 (0.5 nmol), n= 8; a combined infusion of the low doses of AP-5 and SCH-23390,n = 7, and saline vehicle, n = 10. Previous work in our laboratory has demonstrated that microinfusion of a 5 nmol dose of AP-5 completely blocks learning (Kelley et al., 1997), and the high dose of SCH-23390 effectively blocks DA receptors (Delfs and Kelley, 1990).
Feeding and locomotion in food-deprived rats. To examine the possibility of motor and motivational effects of these treatments on behavior, feeding and locomotion were observed in rats with the combined treatment of AP-5 and SCH-23390 and vehicle (n= 8) or with the 3 nmol dose of SCH-23390 and vehicle (n = 8) infused into the nucleus accumbens core. Rats were food-deprived in a manner similar to that described above. After infusion, the animals were placed immediately in a cage similar to their home cage and observed for 15 min using an event recorder. Behaviors recorded were locomotion (crossing center of cage) counts, rearing (counts and duration), feeding (bouts, total duration, and mean duration of a bout), food intake (grams), and latency to eat. A Latin square design was used to randomize drug and vehicle infusions. Rats were given two acclimation sessions of 15 min, during which they were given mock infusions. Test sessions were several days apart.
Histological analysis. At the completion of testing, all rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with 0.9% saline followed by 10% formalin. The brains were stored in a 10% sucrose-formalin mixture for several days before sectioning. Brains were cut into 60 μm sections and stained for Nissl substance with cresyl violet. The sections were examined with light microscopy, and estimated locations of infusion sites were recorded on atlas sections. All infusion sites fell within the boundaries of the accumbens core (histology results are shown in Fig. 4).
Fig. 4.

Representative histology from a rat infused with the co-treatment (SCH-23390, 0.3 nmol; AP-5, 0.5 nmol) as shown inA and from a rat infused with saline, as shown inB.
Statistical analysis. Learning data were analyzed using one-, two-, or three-factor ANOVA, with treatment as the between-subjects factor and days and lever as the within-subjects (repeated measures) factors in the multifactorial analyses. For each experiment, three separate analyses were performed: days 1–4 (acquisition during treatment), days 5–9 (acquisition/performance with no treatment), and days 9–10 (comparison of performance on treatment day 10 with performance on previous day of no treatment). The locomotor and feeding data were analyzed by a one-factor ANOVA.
RESULTS
Infusion of a high dose of SCH-23390 impairs instrumental learning and performance
As shown in Figure1A, the 3 nmol dose of SCH-23390 infused into the nucleus accumbens impaired acquisition of lever pressing. Analysis of data from days 1–4 revealed a significant treatment effect [F(1,15) = 36.12;p < 0.0001] as well as day × treatment [F(3, 45) = 11.25; p< 0.0001], lever × treatment [F(1, 15) = 9.69; p < 0.007], and day × lever × treatment [F(1,3) = 7.99; p < 0.0002] interactions. There were no significant effects on days 5–9. Readministration of this dose strongly impaired performance on day 10, because comparison of days 9 and 10 revealed a treatment effect [F(1,15) = 12.09; p< 0.003] as well as day × treatment [F(1,15) = 25.16; p< 0.0002], lever × treatment [F(1,15) = 13.73; p< 0.002], and day × lever × treatment [F(1,15) = 22.17; p< 0.0003] interactions.
Fig. 1.
Influence of the high dose (3 nmol) of D1 receptor antagonist infusion into the nucleus accumbens core on acquisition of lever pressing for sucrose pellets: correct and incorrect lever responses. Animals received intra-accumbens infusion of SCH-23390 (3 nmol) or vehicle (saline) on the first 4 test days; on the remaining training days, no infusion was given except on day 10, when animals received their initial treatments (underscoreddays indicate infusion days). A, Lever presses. **p < 0.01, treatment effect;††p < 0.01, interactions.B, Nose pokes into the food tray during learning. **p < 0.01, treatment effect;††p < 0.01, interactions. See Materials and Methods for statistical details.
Rats treated with the high dose of SCH-23390 demonstrated a nose poking profile quite different from that of vehicle-treated rats (Fig.1B). In vehicle-treated rats, this unconditioned behavior parallels the lever-pressing curve, increasing markedly as animals begin to learn and gradually leveling off. Analysis of days 1–4 showed a treatment effect [F(1,15) = 23.16; p< 0.0002] and day × treatment interaction [F(1,3) = 9.46; p < 0.0001]. The day × treatment interaction [F(1,3) = 3.82; p < 0.008] persisted for days 5–9. A dramatic decrease in nose poking was observed in SCH-23390-treated rats between days 9 and 10, revealing a treatment effect [F(1,15) = 6.14;p < 0.03] and a day × treatment interaction [F(1,1) = 12.16; p < 0.003].
Low doses of SCH-23390 and AP-5 administered separately have no effect on learning
It can be observed from Figure2A that intra-accumbens infusion of either the 0.3 nmol dose of the D1antagonist or the 0.5 nmol dose of the NMDA antagonist, administered separately, had no effect on response learning. The learning curve of drug-treated animals was similar to that of controls. There was also no effect of reinfusion on day 10. A similar profile was noted for nose pokes (Fig. 2B).
Fig. 2.
Influence of D1 receptor antagonist SCH-23390 (0.3 nmol) or NMDA receptor antagonist AP-5 (0.5 nmol) infusion into the nucleus accumbens core on acquisition of lever pressing for sucrose pellets. See legend of Figure 1 for further details. A, Lever presses. B, Nose pokes. See Materials and Methods for statistical details.
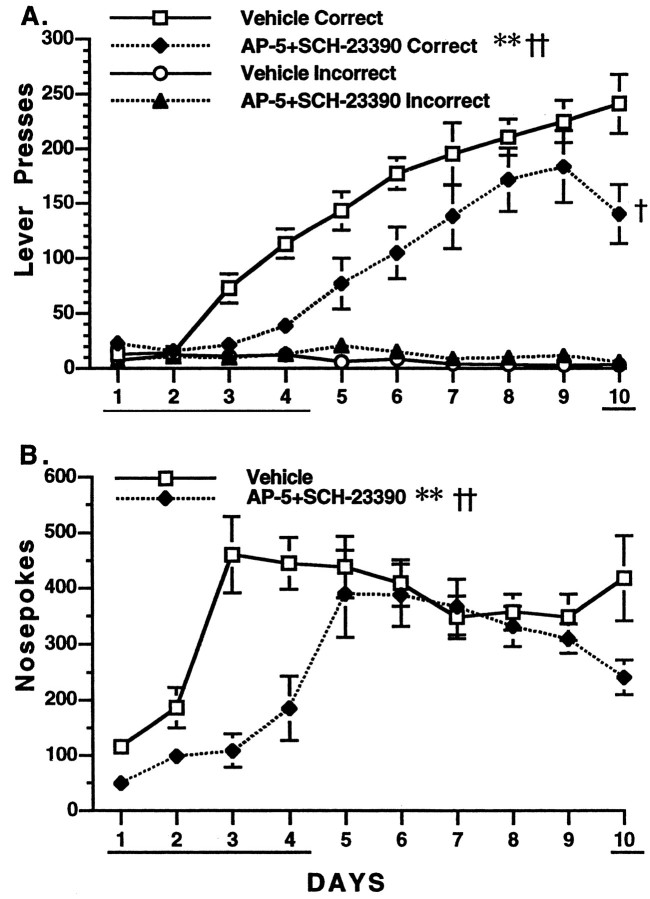
Co-infusion of low doses of SCH-23390 and AP-5 inhibits learning
In contrast to the lack of effect of low doses of SCH-23390 and AP-5 when infused separately into the nucleus accumbens core, co-infusion of these drugs clearly inhibited learning (Fig.3A). Analysis of days 1–4 revealed a significant treatment effect [F(1,15) = 12.5; p < 0.003] as well as day × treatment [F(3,45) = 11.35; p< 0.0001], lever × treatment [F(1,1) = 9.438; p < 0.008], and day × lever × treatment [F(3,45) = 9.21; p < 0.0001] interactions. Drug-treated animals sampled the levers but did not begin to discriminate between them until day 4. The lever × treatment effect [F(1,15) = 5.15;p < 0.04] persisted on days 5–9, indicating a residual effect on learning. Comparison of days 9 and 10 revealed significant day × treatment [F(1,15) = 6.90; p < 0.02], lever × treatment [F(1,15) = 4.90; p < 0.04], and day × lever × treatment [F(1,15) = 5.38; p < 0.03] interactions. It can be observed in Figure 3A that performance declined somewhat after readministration of the combined drug treatment, whereas the performance of vehicle-treated rats continued to increase.
Fig. 3.
Influence of the co-infusion of D1receptor antagonist SCH-23390 (0.3 nmol) and NMDA receptor antagonist AP-5 (0.5 nmol) into the nucleus accumbens core on acquisition of lever pressing for sucrose pellets. See legend of Figure 1 for further details. A, Lever presses. **p < 0.01, treatment effect; ††p < 0.01, interactions. B, Nose pokes. *p < 0.05, treatment effect; ††p < 0.01, interactions. See Materials and Methods for statistical details.
Analysis of days 1–4 revealed a significant treatment effect [F(1,15) = 15.74; p< 0.001] and a day × treatment interaction [F(3,45) = 9.37; p < 0.0001] for nose poking behavior (Fig. 3B). Although a significant reduction in lever pressing occurred on day 10, nose poking in combined dose animals did not change significantly from day 9 to 10.
Combined infusion of AP-5 and SCH-23390 does not affect food intake or motor activity
Combined infusion of AP-5 (0.5 nmol) and SCH-23390 (0.3 nmol) into the nucleus accumbens core had no effect on locomotor or feeding behavior in hungry rats (Table 1). However, animals infused with the higher dose of SCH-23390 (3 nmol) had significantly fewer counts of locomotion [F(1,7) = 18.03; p < 0.004] and engaged in longer bouts of feeding [F(1,7) = 7.56; p < 0.03] yet did not differ in total food intake. Thus, the high dose of SCH-23390 infused into the accumbens core did not alter motivation to feed but did cause motor inhibition and prolonged bout length, eliciting a classic neuroleptic profile.
Table 1.
Feeding and motor behavior after intra-accumbens treatments in food-deprived rats (15 min test)
| Behavioral indices | Treatment | |||
|---|---|---|---|---|
| Experiment 1 | Experiment 2 | |||
| VEH | AP-5/SCH | VEH | SCH | |
| Locomotion | 15 ± 2 | 15 ± 2 | 11 ± 2 | 4 ± 2* |
| Rears | 22 ± 2 | 30 ± 4 | 14 ± 3 | 8 ± 3 |
| Feeding duration (sec) | 530 ± 32 | 485 ± 26 | 618 ± 42 | 660 ± 39 |
| Average bout duration (sec) | 25 ± 2 | 28 ± 3 | 41 ± 5 | 89 ± 181-160 |
| Total intake (gm) | 3.6 ± 0.3 | 3.2 ± 0.2 | 4.3 ± 0.4 | 4.3 ± 0.3 |
Data represent means ± SEM. Locomotion is frequency of cage crossing. AP-5/SCH is the low-dose combination of the two drugs (AP-5, 0.5 nmol; SCH-23390, 0.3 nmol); SCH is the high dose of SCH-23390 (3 nmol). Average bout duration is the total duration per number of bouts.
P < 0.004.
F1-160: P < 0.05.
Figure 4 shows examples of representative histology from both an experimental (AP5- and SCH-23390-infused) rat and a control (saline-infused) rat. Cannula tracks from all rats were well localized in the nucleus accumbens core. Damage from microinjections was generally minimal, and there were no observable differences between drug- and saline-infused brains.
DISCUSSION
The results reported here suggest that coincident activation of dopamine D1 receptors and glutamatergic NMDA receptors within the nucleus accumbens core may be an important mechanism for appetitive response learning. Previous work has shown a nearly complete inhibition of such learning after infusion of an effective dose of the selective NMDA antagonist AP-5 into the accumbens core (Kelley et al., 1997; Baldwin et al., 2000). Given the convincing evidence for DA–NMDA interactions in cellular and molecular models of plasticity, it seemed logical to propose that such an interaction may participate in accumbens-mediated response learning. Although dopamine has been implicated in many theories of learning (Beninger, 1983;Ettenberg, 1989; Schultz et al., 1997; Berridge and Robinson, 1998), to our knowledge there has been no direct test of intra-accumbens selective DA antagonists on acquisition of instrumental learning. Data resulting from such an approach are quite difficult to interpret, because instrumental behavior is often very sensitive to the motor-inhibitory effects of DA antagonists (Fibiger et al., 1976;Salamone, 1987). Indeed, in the paradigm described here, learning appeared to be severely impaired with infusion of the higher dose of SCH-23390. However, once the task was learned, performance was also drastically reduced by the treatment, rendering interpretation of the initial “learning” effect more complex.
To circumvent this problem, we infused a dose of the D1 antagonist one order of magnitude lower than the performance-impairing dose. A 10-fold lower dose of AP-5 than that necessary to inhibit learning was also tested. Neither of these treatments by itself was found to affect acquisition of the lever-pressing task. However, co-infusion of both the NMDA and D1 antagonists together strongly impaired learning, suggesting that a critical level of co-activation of D1 and NMDA receptors is necessary for learning. Because this treatment had no effect in control tests of motor behavior, it is unlikely that motor impairment could account for the learning deficit. Moreover, although the combined treatment lowered performance somewhat once the task was learned, this was a very small effect compared with that of the high dose of SCH-23390. This profile suggests a preferential role for DA–NMDA interaction in the early stages of motor learning, rather than in performance of learned motor behavior. It is important to note further that neither the combined dosage nor even the high dose of SCH-23390 had any effect on food intake, demonstrating that motivation for primary reward is not affected by blockade of DA D1 receptors within the accumbens. This result supports previous work showing that treatment with low doses of SCH-23390 reduces lever pressing for food but actually increases food intake (Cousins et al., 1994).
It is also very interesting that nose poking behavior was impaired by the combined treatment of AP-5 and SCH-23390, an effect that appears to recover more quickly than the impairment of instrumental learning, from close inspection of Figure 3. Thus, although no overt inhibition of locomotor or feeding behavior was found, the combined treatment nevertheless appears to inhibit this form of behavioral activation. Combined activation of NMDA receptors and DA D1receptors may also be necessary for enhancing arousal and promotion of behaviors that would serve to bring the animal in contact with potentially important environmental stimuli.
The present results provide additional evidence for emerging theories of DA D1–NMDA receptor interactions in the control of activity of striatal medium spiny neurons (Cepeda and Levine, 1998). In anatomical terms, these two classes of receptors are localized on the same dendritic spines, providing a locus of close interaction. In rat striatal slices, a number of studies show that dopamine can enhance glutamate- and particularly NMDA receptor-mediated excitation (Cepeda et al., 1993, 1998; Galarraga et al., 1997; Harvey and Lacey, 1997; Hernandez-Lopez et al., 1997; Hu and White, 1997). For example, neuronal excitation evoked by NMDA was markedly potentiated by iontophoretic application of dopamine; the potentiation was mimicked by a D1 agonist and blocked by co-application of SCH-23390 (Cepeda et al., 1993). Although DA has often been found to inhibit postsynaptic currents (Uchimura et al., 1986; Calabresi et al., 1987), in vivo models often report the opposite effect under particular conditions (Pierce and Rebec, 1995; Kiyatkin and Rebec, 1996). For example, Hernandez-Lopez et al. (1997) found that D1 agonists or cAMP analogs enhanced evoked activation in medium spiny neurons when membranes were relatively depolarized, an effect that was dependent on L-type Ca2+ channels.
Molecular approaches also support a general convergence or interdependence between NMDA receptor- and D1-mediated intracellular signal transduction. Studies using primary striatal cell cultures showed that dopamine D1-induced immediate early gene (IEG) expression is dependent on NMDA receptor activation (Konradi et al., 1996). These studies also showed that blockade with NMDA antagonists reduced the ability of dopamine to induce phosphorylation of the cAMP response element-binding protein (CREB), a transcription factor activated in many forms of learning. Similar results were reported by Das et al. (1997), who found that NMDA- induced CREB phosphorylation was dependent on calcium/calmodulin-dependent protein kinase and that D1-induced IEG expression and CREB phosphorylation was dependent on protein kinase A (PKA) activity. Another potential site of interaction is phosphorylation of NMDA receptors, which can occur via both PKA and calcium/calmodulin-dependent protein kinase (Leonard and Hell, 1997;Leonard et al., 1999). Thus, one can imagine a scenario in which temporal coordination of specific glutamate inputs with enhanced DA release would result in molecular integration of postsynaptic signals. Such resultant integration within the dendrites of medium spiny neurons could be a basis for the synaptic modification necessary for motor learning (Kotter, 1994; Kelley, 1999). In support of this hypothesis, we have recently found that PKA inhibitors also selectively impair response learning when infused into the accumbens core (Baldwin et al., 1999).
This hypothesis is further supported by evidence of neuronal plasticity within striatum in physiological models. Both long-term potentiation and long-term depression, phenomena hypothesized to underlie associative processes in learning, have been demonstrated in striatum and nucleus accumbens (Boeijinga et al., 1993; Lovinger et al., 1993;Pennartz et al., 1993; Kombian and Malenka, 1994; Calabresi et al., 1996; Charpier and Deniau, 1997). Most relevant to the present study is the demonstration of long-term enhancement of synaptic strength when corticostriatal excitation and dopaminergic activation are temporally coordinated (Wickens et al., 1996). For appetitive instrumental learning to occur, an animal must make an association between a motor response and the positive outcome of that response. It is possible that glutamate-coded afferent information arising from key corticolimbic structures, such as amygdala and prefrontal and cingulate cortex, may provide the sensorimotor and motivational information to medium spiny dendrites, whereas the primary reward of food (and/or motivational state of hunger) enhances DA release. The probability of temporal and spatial convergence of glutamate- and dopamine-coded signals may increase as the animal begins to gain experience in the chamber. For example, Pavlovian cues (association of the environment with food) would activate the amygdalostriatal pathway, preliminary and initially random contact with the food would activate dopamine, and experience with a positive outcome would promote bias toward correct response selection, which may be the domain of the prefrontostriatal pathway. Thus, a critical level of convergence may be reached to trigger intracellular gene expression that enables motor learning. In support of this hypothesis, parallel work has shown that NMDA receptor-dependent plasticity in amygdala and prefrontal cortex is also necessary for instrumental learning (Baldwin et al., 2000).
In summary, these findings suggest that coincident activation of dopamine D1 and NMDA receptors in the nucleus accumbens core contributes to a process whereby animals acquire a new motor response that results in a positive outcome. Further work is required to know more precisely what intracellular signals and transcriptional alterations mediate the synaptic modifications necessary for such learning.
Footnotes
This research was supported by National Institute on Drug Abuse Grant DA04788 to A.E.K.
Correspondence should be addressed to Dr. Ann E. Kelley, Department of Psychiatry, University of Wisconsin-Madison Medical School, 6001 Research Park Boulevard, Madison, WI 53719. E-mail:aekelley@macc.wisc.edu.
REFERENCES
- 1.Adriani W, Felici A, Sargolini F, Roullet P, Usiello A, Oliverio A, Mele A. N-Methyl-d-aspartate and dopamine receptor involvement in the modulation of locomotor activity and memory processes. Exp Brain Res. 1998;123:52–59. doi: 10.1007/s002210050544. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin AE, Sadeghian K, Holahan MR, Kelley AE. cAMP-dependent protein kinase within the nucleus accumbens core mediate appetitive instrumental learning. Soc Neurosci Abstr. 1999;25:638. doi: 10.1006/nlme.2000.4002. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin AE, Holahan MR, Sadeghian K, Kelley AE. N-Methyl-d-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behav Neurosci. 2000;114:1–15. doi: 10.1037//0735-7044.114.1.84. [DOI] [PubMed] [Google Scholar]
- 4.Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Res Rev. 1983;6:173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- 5.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 6.Boeijinga PH, Mulder AB, Pennartz CM, Manshanden I, Lopes da Silva FH. Responses of the nucleus accumbens following fornix/fimbria stimulation in the rat. Identification and long-term potentiation of mono- and polysynaptic pathways. Neuroscience. 1993;53:1049–1058. doi: 10.1016/0306-4522(93)90488-2. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi P, Mercuri N, Stanzione P, Stefani A, Bernardi G. Intracellular studies on the dopamine-induced firing inhibition of neostriatal neurons in vitro: evidence for D1 receptor involvement. Neuroscience. 1987;20:757–771. doi: 10.1016/0306-4522(87)90239-9. [DOI] [PubMed] [Google Scholar]
- 8.Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 1996;19:19–24. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- 9.Cepeda C, Levine MS. Dopamine and N-methyl-d-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20:1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- 10.Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS. Dopaminergic modulation of NMDA-induced whole cell currents in neostriatal neurons in slices: contribution of calcium conductances. J Neurophysiol. 1998;79:82–94. doi: 10.1152/jn.1998.79.1.82. [DOI] [PubMed] [Google Scholar]
- 12.Charpier S, Deniau JM. In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proc Natl Acad Sci USA. 1997;94:7036–7040. doi: 10.1073/pnas.94.13.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cousins MS, Wei W, Salamone JD. Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs. Psychopharmacology. 1994;116:529–537. doi: 10.1007/BF02247489. [DOI] [PubMed] [Google Scholar]
- 14.Das S, Grunert M, Williams L, Vincent SR. NMDA and D1 receptors regulate the phosphorylation of CREB and the induction of c-fos in striatal neurons in primary culture. Synapse. 1997;25:227–233. doi: 10.1002/(SICI)1098-2396(199703)25:3<227::AID-SYN1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.Delfs JM, Kelley AE. The role of D-1 and D-2 dopamine receptors in oral stereotypy induced by dopaminergic stimulation of the ventrolateral striatum. Neuroscience. 1990;39:59–67. doi: 10.1016/0306-4522(90)90221-o. [DOI] [PubMed] [Google Scholar]
- 16.Ettenberg A. Dopamine, neuroleptics and reinforced behavior. Neurosci Biobehav Rev. 1989;13:105–111. doi: 10.1016/s0149-7634(89)80018-1. [DOI] [PubMed] [Google Scholar]
- 17.Fibiger HC, Carter DA, Phillips AG. Decreased intracranial self-stimulation after neuroleptics or 6-hydroxydopamine:evidence for mediation by motor deficits rather than by reduced reward. Psychopharmacology. 1976;47:21–27. doi: 10.1007/BF00428696. [DOI] [PubMed] [Google Scholar]
- 18.Fuller TA, Russchen FT, Price JL. Sources of presumptive glutamergic/aspartergic afferents to the rat ventral striatopallidal region. J Comp Neurol. 1987;258:317–338. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- 19.Galarraga E, Hernandez-Lopez S, Reyes A, Barral J, Bargas J. Dopamine facilitates striatal EPSPs through an L-type Ca2+ conductance. NeuroReport. 1997;8:2183–2186. doi: 10.1097/00001756-199707070-00019. [DOI] [PubMed] [Google Scholar]
- 20.Harvey J, Lacey MG. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci. 1997;17:5271–5280. doi: 10.1523/JNEUROSCI.17-14-05271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houk JC, Adams JL, Barto AG. A model of how the basal ganglia generate and use neural signals that predict reinforcement. In: Houk JC, Davis JL, Beiser DG, editors. Models of information processing in the basal ganglia. MIT; Cambridge, MA: 1995. pp. 249–270. [Google Scholar]
- 23.Hu X-T, White FJ. Dopamine enhances glutamate-induced excitation of rat striatal neurons by cooperative activation of D1 and D2 class receptors. Neurosci Lett. 1997;224:61–65. doi: 10.1016/s0304-3940(97)13443-7. [DOI] [PubMed] [Google Scholar]
- 24.Kelley AE. Neural integrative activities of nucleus accumbens subregions in relation to motivation and learning. Psychobiology. 1999;27:198–213. [Google Scholar]
- 25.Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on NMDA receptor activation in the nucleus accumbens core. Proc Natl Acad Sci USA. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiyatkin EA, Rebec GV. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol. 1996;75:142–153. doi: 10.1152/jn.1996.75.1.142. [DOI] [PubMed] [Google Scholar]
- 27.Kombian SB, Malenka RC. Simultaneous LTP of non-NMDA and LTD of NMDA-receptor mediated responses in the nucleus accumbens. Nature. 1994;368:242–245. doi: 10.1038/368242a0. [DOI] [PubMed] [Google Scholar]
- 28.Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci. 1996;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotter R. Postsynaptic integration of glutamatergic and dopaminergic signals in the striatum. Prog Neurobiol. 1994;44:163–196. doi: 10.1016/0301-0082(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 30.Leonard AS, Hell JW. Cyclic AMP-dependent protein kinase and protein kinase C phosphorylate N-methyl-d-aspartate receptors at different sites. J Biol Chem. 1997;272:12107–12115. doi: 10.1074/jbc.272.18.12107. [DOI] [PubMed] [Google Scholar]
- 31.Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA. 1999;96:3239–3244. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine MS, Altemus KL, Cepeda C, Cromwell HC, Crawford C, Ariano MA, Drago J, Sibley DR, Westphal H. Modulatory actions of dopamine on NMDA receptor-mediated responses are reduced in D1A-deficient mutant mice. J Neurosci. 1996;16:5870–5882. doi: 10.1523/JNEUROSCI.16-18-05870.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindvall O, Bjorklund A. Anatomy of the dopaminergic neuron systems in the rat brain. Adv Biochem Psychopharmacol. 1978;19:1–23. [PubMed] [Google Scholar]
- 34.Lovinger DM, Tyler EC, Merritt A. Short- and long-term synaptic depression in rat neostriatum. J Neurophysiol. 1993;70:1937–1949. doi: 10.1152/jn.1993.70.5.1937. [DOI] [PubMed] [Google Scholar]
- 35.McGeer PL, McGeer EG, Scherer U, Singh K. A glutamatergic corticostriatal pathway? Brain Res. 1977;128:369–373. doi: 10.1016/0006-8993(77)91003-4. [DOI] [PubMed] [Google Scholar]
- 36.Pennartz CM, Ameerun RF, Groenewegen HJ, Lopes da Silva FH. Synaptic plasticity in an in vitro slice preparation of the rat nucleus accumbens. Eur J Neurosci. 1993;5:107–117. doi: 10.1111/j.1460-9568.1993.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 37.Pierce RC, Rebec GV. Iontophoresis in the neostriatum of awake, unrestrained rats: differential effects of dopamine, glutamate and ascorbate on motor- and nonmotor-related neurons. Neuroscience. 1995;67:313–324. doi: 10.1016/0306-4522(95)00012-8. [DOI] [PubMed] [Google Scholar]
- 38.Robinson TG, Beart PM. Excitant amino acid projections from rat amygdala and thalamus to nucleus accumbens. Brain Res Bull. 1988;20:467–471. doi: 10.1016/0361-9230(88)90136-0. [DOI] [PubMed] [Google Scholar]
- 39.Salamone JD. The actions of neuroleptic drugs on appetitive instrumental behaviors. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of psychopharmacology, Vol 19. Plenum; New York: 1987. pp. 575–608. [Google Scholar]
- 40.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1598. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 41.Sesack SR, Pickel VM. In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res. 1990;527:266–279. doi: 10.1016/0006-8993(90)91146-8. [DOI] [PubMed] [Google Scholar]
- 42.Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- 43.Smith-Roe SL, Sadeghian K, Kelley AE. Spatial learning in the radial arm maze is impaired following NMDA receptor blockade in striatal subregions. Behav Neurosci. 1999;113:703–717. doi: 10.1037//0735-7044.113.4.703. [DOI] [PubMed] [Google Scholar]
- 44.Totterdell S, Smith AS. Convergence of hippocampal and DA-ergic input onto identified neurons in the nucleus accumbens of the rat. J Chem Neuroanat. 1989;2:285–298. [PubMed] [Google Scholar]
- 45.Uchimura N, Higashi H, Nishi S. Hyperpolarizing and depolarizing actions of dopamine via D-1 and D-2 receptors on nucleus accumbens neurons. Brain Res. 1986;375:368–372. doi: 10.1016/0006-8993(86)90760-2. [DOI] [PubMed] [Google Scholar]
- 46.Walaas I, Fonnum F. The effects of surgical and chemical lesions on neurotransmitter candidates on the nucleus accumbens. Neuroscience. 1979;4:209–216. doi: 10.1016/0306-4522(79)90083-6. [DOI] [PubMed] [Google Scholar]
- 47.Wickens JR, Begg AJ, Arbuthnott GW. Dopamine reverses the depression of rat corticostriatal synapses which normally follows high-frequency stimulation of cortex in vitro. Neuroscience. 1996;70:1–5. doi: 10.1016/0306-4522(95)00436-m. [DOI] [PubMed] [Google Scholar]
- 48.Young AM, Bradford HF. Excitatory amino acid neurotransmitters in the corticostriate pathway: studies using intracerebral microdialysis in vivo. J Neurochem. 1986;47:1399–1404. doi: 10.1111/j.1471-4159.1986.tb00771.x. [DOI] [PubMed] [Google Scholar]