Abstract
Neural correlates of responses to emotionally valenced olfactory, visual, and auditory stimuli were examined using positron emission tomography. Twelve volunteers were scanned using the water bolus method. For each sensory modality, regional cerebral blood flow (rCBF) during presentation of both pleasant and unpleasant stimuli was compared with that measured during presentation of neutral stimuli. During the emotionally valenced conditions, subjects performed forced-choice pleasant and unpleasant judgments. During the neutral conditions, subjects were asked to select at random one of a two key-press buttons. All stimulations were synchronized with inspiration, using an airflow olfactometer, to present the same number of stimuli for each sensory modality. A no-stimulation control condition was also performed in which no stimulus was presented. For all three sensory modalities, emotionally valenced stimuli led to increased rCBF in the orbitofrontal cortex, the temporal pole, and the superior frontal gyrus, in the left hemisphere. Emotionally valenced olfactory and visual but not auditory stimuli produced additional rCBF increases in the hypothalamus and the subcallosal gyrus. Only emotionally valenced olfactory stimuli induced bilateral rCBF increases in the amygdala. These findings suggest that pleasant and unpleasant emotional judgments recruit the same core network in the left hemisphere, regardless of the sensory modality. This core network is activated in addition to a number of circuits that are specific to individual sensory modalities. Finally, the data suggest a superior potency of emotionally valenced olfactory over visual and auditory stimuli in activating the amygdala.
Keywords: emotion, hedonic judgment, odor processing, visual processing, auditory processing, PET
Everyday, we make numerous judgments about the pleasantness or unpleasantness of external sensory stimuli. Exposure to such stimuli can induce subjective emotional experiences such as pleasure or fear and behavioral responses aimed at engaging or avoiding continued exposure. Neurobehavioral studies in animals have historically implicated structures related to the limbic system in these emotional processes, with a particular emphasis on the amygdala and hypothalamus (LeDoux, 1987, 1995; Davis, 1992; Rolls, 1999). Electrophysiological and lesion studies also indicate that the orbitofrontal cortex (OFC) makes a significant contribution to these processes in animals (Zald and Kim, 1996b; Rolls, 1999).
Several recent neuroimaging studies have attempted to delineate the cortical and subcortical regions involved in processing emotionally valenced stimuli in humans. Such studies have examined responses to pleasant and/or unpleasant visual (Cahill et al., 1996; Irwin et al., 1996; Morris et al., 1996; Lane et al., 1997a,b; Taylor et al., 1998;Whalen et al., 1998) auditory (Blood et al., 1999), olfactory (Zald and Pardo, 1997; Fulbright et al., 1998; Zald et al., 1998a; Royet et al., 2000), gustatory (Zald et al., 1998b), and somatosensory (Francis et al., 1999) stimuli. Consistent with the animal literature, the amygdala has been observed to activate during exposure to emotionally valenced stimuli in multiple sensory modalities, particularly during exposure to aversive stimuli. OFC activations have emerged during exposure to pleasant and unpleasant stimuli in multiple sensory modalities. Although, not quite as frequent, activations have also localized to the hippocampal or parahippocampal region in several sensory modalities (Lane et al., 1997a; Zald et al., 1998b; Blood et al., 1999). Several additional brain regions have been observed to activate in these studies, although their consistency across studies or across modalities remains unclear. For instance, Lane et al. (1997b) reported hypothalamic activation during exposure to both pleasant and unpleasant visual stimuli, but similar activations have yet to be confirmed in other studies.
A major difficulty in interpreting the above neuroimaging literature arises because of the variable methods used across studies. The studies differ in the emotional intensity and number of stimuli presented, the tasks performed by the subjects, the subject population, and imaging methods. It thus remains unclear to what extent variable results reflect sensory-specific engagement of different brain regions or methodological discrepancies. Even within areas that have emerged across studies using different sensory modalities, it is not known to what extent variability in the magnitude, laterality, or specific location of responses, reflect sensory specific or methodological factors. For instance, it remains unclear whether stimuli across different sensory modalities engage a common orbitofrontal region or different discrete sensory-specific subregions. To allow straightforward comparison of structures activated by emotionally valenced stimuli in different sensory modalities, we measured the regional cerebral blood flow (rCBF) changes induced by visual, auditory, and olfactory emotional stimuli in the same subjects. Subjects performed the same task of hedonic judgment across sensory modalities, and the methods of stimulus presentation were kept as similar as possible. The study thus provides the first direct comparison of emotional processing in different sensory modalities.
MATERIALS AND METHODS
Subjects. Twelve right-handed male subjects (20–30 years of age) participated in this study. They were selected after a screening of their olfactory detection ability and mean duration of their breath cycle. These subjects achieved at least 80% of correct responses and had a mean duration of breath cycle ranging from 3 to 6 sec. Subjects with asthma or a pronounced tendency for allergies were excluded. In addition, the subject's level of anhedonia, that is, the loss of ability to experience pleasure, was rated with the Physical Anhedonia Scale (Chapman et al., 1976). Only subjects receiving a score <29 were included. Finally, the inclusion of subjects required a medical visit to rule out hereditary genetic diseases or other factors that might increase the risk of radiation exposure. The subjects participating in the study provided informed written consent, and the experiment was approved by the local ethic committee and conducted according to French regulations on biomedical experiments on healthy volunteers.
Twenty-four subjects (12 male and 12 female students) were used in a pilot study to rate the hedonic value of visual and auditory stimuli with a rating scale ranging from 1 (maximum unpleasant) to 10 (maximum pleasantness). For olfactory stimuli, the hedonic value (also rated from 0 to 10) had been determined in a previous study of 71 subjects (Royet et al., 1999).
Odorous stimuli. One hundred twenty stimuli were used. They were distributed into four sets of 24 odorants as a function of hedonicity rating. For emotional scans, each set contained 12 pleasant odorants selected so as to provide the highest scores (mean score of 6.37, range of 5.63–7.24) and 12 unpleasant odorants selected as presenting the lowest scores (mean score of 1.48, range of 0.45–2.42). In each set, the order of presentation of pleasant and unpleasant odors was pseudorandomized. For neutral scans, 24 odors were selected so as to provide mean scores (mean score of 3.97, range of 3.21–4.69). Pleasant odorants included chewing-gum, mint, rose, lilac, lemon, lavender, raspberry, and caramel. Unpleasant odorants included tetrahydrothiophene, butyl sulfide, butyric acid, onion, ethylmercaptan, pentanoic acid, and isovaleric acid. Neutral stimuli included sandalwood, incense, gingerbread, pepper, chamomile, tobacco, cinnamon, and pine.
Odorants were presented in white polyethylene squeeze bottles (100 ml) provided with a dropper (Osi, Elancourt, France). Odorants were diluted in mineral oil so that 5 ml of odorous solution (10%) were prepared and adsorbed by compressed filaments of polypropylene. The concentration of the products with very high potency was limited to 1%.
Auditory stimuli. Ninety-six auditory stimuli were selected based on the pilot study. They consisted of environmental sounds and vocalizations from animals and people. For both emotional scans, 24 pleasant (mean score of 7.65, range of 6.75–8.40) and 24 unpleasant stimuli (mean score of 1.94, range of 0.83–2.62) were selected. For both neutral scans, 24 stimuli rated as neutral (mean score of 4.77, range of 3.10–6.70) by the reference group were chosen. Examples of pleasant stimuli include a baby's laugh, a flowing river, a bird song, a cicada, a tree frog, and a melody. Examples of unpleasant stimuli include an alarm clock, a woman crying, an explosion, a snoring sound, a sawmill, and a breaking of glass. Examples of neutral stimuli include the noise of a train, wind, a plane, and drumming. The volume of stimuli was controlled to avoid presenting stimuli at too high of an intensity.
Visual stimuli. Ninety-six visual stimuli were selected based on the pilot study. They represented complex scenes of landscape, animals, and people. Half of these stimuli were rated as pleasant (mean score of 8.53, range of 8.01–9.12) or unpleasant (mean score of 1.87, range of 0.63–2.56), and the other half were rated as neutral (mean score of 5.15, range of 3.08–7.58). Examples of pleasant stimuli include pictures of a skier, a lake, a picnic, a beach, flowers, and a field of wheat. Unpleasant pictures showed items such as a face with abscesses, a polluted river, a surgical operation, road accident victims, a trapdoor spider, and a butchered woman. Neutral stimuli included images of a bird, a squirrel, a forest, a clown fish, a fire-balloon, and a dancer. Images were presented in a landscape format with a spatial resolution of at least 75 pixels/inch. They were projected on to a screen, producing an image with dimensions of 34 × 26 cm.
Stimulating material. Odors were presented with an airflow olfactometer, which allowed synchronization of stimulation with breathing. The stimulating material was described previously (Royet et al., 1999). Briefly, vector air was pumped with a compressor, treated with a charcoal filter, and then pumped into an air-dilution olfactometer. At the beginning of each inspiration, odor was injected into the olfactometer and was delivered into an anesthesia mask. Breathing was recorded with the aid of a thermic probe located near the right nostril.
Auditory stimuli were presented with high-quality stereo headphones. Visual stimuli were presented on a translucent pearl gray screen (80 × 72 cm; Juillet AudioVisuel, Lyon, France) from a video-projector (Sony, Paris, France). The screen was placed over a polystyrene black support that was fitted into the posterior part of the camera tunnel. Images were reflected on a two-way mirror (15 × 10 cm) positioned in front of the camera above the subject's head.
Experimental procedure. The day before scanning, subjects were trained to breathe regularly without sniffing to detect (odor vs no odor) stimulations during inspiration and to give a manual response with two key-press buttons before the next breathing cycle. On the scanning day, each subject was given a total of 12 positron emission tomography (PET) scans: four scans for each of the three modalities, including two neutral and two emotional scans. The order of presentation of the three sensory modalities differed among subjects to obtain a balanced experimental design (Latin square). Thus, there were six different orders of scans, with each order being administered to two subjects. Eight of 12 subjects were also given at the end of the experiment two control scans during which no stimulus was presented (no-stimulation control task). The scans were performed every 9–10 min.
Visual and auditory stimulations were, like olfactory stimulations, synchronized with the respiratory cycle to obtain the same number of presentations for each sensory modality. For a mean respiratory cycle of ∼4–5 sec, ∼12–16 stimulations were performed during each scan. Auditory and visual stimuli were delivered when pure air was injected with an empty bottle in the airflow olfactometer. Auditory stimuli were presented from 1 to 3 sec, with an average duration of 2.5 sec, so that each stimulus could be identified by subjects. Visual stimuli were presented for 2.5 sec. For no-stimulation control scans, only pure air was delivered in the mask for each breath cycle.
Instructions were provided to the subjects before each scan. For each stimulus (image, sound, or odor) in the neutral condition and for each inspiration (pure air used as stimulus) in the no-stimulation control condition, subjects were asked to select, at random, one of the two key-press buttons (the right button with the middle finger, and the left button with the index finger of the right hand). During each emotional scan, they were asked to select one of the two key-press buttons according to whether the response was “the stimulus is pleasant” or “the stimulus is unpleasant.” Half of the subjects used the index finger for the “pleasant responses” and the middle finger for the “unpleasant responses.” For the other half of the subjects, the meaning of the two key-press buttons was reversed. The pleasant–unpleasant judgments and reaction times were recorded with a Macintosh PowerBook G3 computer (Apple Computers, Cupertino, CA). The experimental design was programmed using PsyScope software (Cohen et al., 1993). Except in the visual conditions, scans were performed while subjects wore a blindfold over their eyes, and room illumination was dimmed.
PET and magnetic resonance imaging scanning. The PET camera was a whole-body tomograph (Siemens EXACT HR+) with 32 contiguous rings of 376 detectors and a transaxial resolution of 4.5 mm [full-width half-maximal (FWHM)]. It provided 63 plans of 2.43 mm, providing a field of axial view of 15.2 mm. The subject's head was immobilized with a thermoplastic facemask (Tru-Scan Imaging Inc., Annapolis, MD) allowing control of patient movement and reproducible positioning. The effects of radiation self-attenuation were corrected by an initial transmission scan of each subject using an external positron-emitting isotope (68Ge). An intravenous bolus injection of 333 MBq H215O was given for each run in the left forearm brachial vein through an indwelling catheter. Scan to record brain activity began when radioactive counts exceeded the background activity by 200% and lasted for 60 sec. The images were attenuation-corrected and reconstructed with filtered back projection using a Hamming filter. PET scans were analyzed using statistical parametric mapping (SPM96; MRC Cyclotron Unit, London, UK) (Friston et al., 1995a,b). Image processing included interscan realignment, spatial normalization to stereotactic space as defined by the ICBM template provided by the Montreal National Institute, and smoothing of the images using a three-dimensional gaussian filter (FWHM, 20 mm) to overcome residual anatomical variability. The localization of activated areas was also examined by reference to a magnetic resonance imaging (MRI) template. Cortical regions are presented using the nomenclature of Duvernoy (1991). Global differences in CBF were covaried out for all voxels, and comparisons across conditions were made using t tests. The significance of rCBF differences was assessed through z-scores in an omnibus sense (Friston et al., 1995b). Threshold for significance was set atp < 0.05 and p < 0.01 (corrected).
Principal component analysis. To assess functional connectivity between the different brain regions, we submitted data related to the three modalities to a principal component analysis (PCA). A singular value decomposition was used to divide the original data set into a series of independent components with decreasing contributions to the variance in the voxel values. For each component, the singular value decomposition provides three parameters: (1) an eigenimage, i.e., a pattern of covariation structures that can be displayed as a brain image; (2) an eigenvalue, which indicates the proportional contribution of that component to the global variance; and (3) a condition-dependent profile, called an eigenvector, which represents its influence on the different conditions of activation (Friston et al., 1993).
Main and simple effects. Simple effects were deduced from comparisons between emotional (E) and neutral (N) conditions (E − N and N − E) in each of the three modalities: auditory (A), olfactory (O), and visual (V). Main effects were determined to evidence brain areas significantly activated in the three modalities by performing an analysis of conjunction according to the formulas [(AE − AN) and (OE − ON) and (VE − VN)].
Regions of interest. The exploratory statistical parametric mapping analyses failed to consistently demonstrate activations in several regions of interest (ROIs) pertaining to the limbic system. Using a priori hypotheses, we performed complementary analyses by restricting the search to four anatomical ROIs (the amygdala, the hypothalamus, and the hippocampus). Right and left ROIs were delineated from the IRM template and the Duvernoy Atlas with the aid of the Analyze software (Mayo Foundation). After outlining of the ROIs, anatomical masks of these regions were computed. Image smoothing was performed with a three-dimensional gaussian filter (FWHM) of 16 mm. A new statistical analysis was performed on the same set of data, masked with the anatomical mask. The magnitude of the rCBF changes were the same as in the exploratory analysis, but the level of uncorrected statistical significance was reduced because of the considerable decrease in the search space. The geometrical characteristics of the search space were computed to determine the probability of exceeding the statistical threshold within a limited gaussian field (Worsley et al., 1996).
RESULTS
Behavioral data
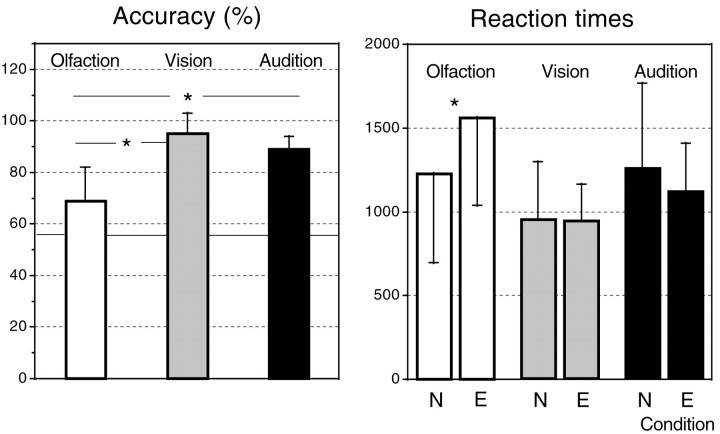
Response accuracy and reaction times are shown in Figure1. For odors, response accuracy was determined as a function of results obtained in our previous study (Royet et al., 1999). For visual and auditory stimuli, accuracy was determined as a function of results obtained in the pilot study. Thus, a binary response (pleasant vs unpleasant) was considered as correct when it was congruent with mean scores found in these earlier studies. A one-way ANOVA with repeated measures (Winer, 1962) on the response accuracy (Fig. 1, left) showed a significant effect of the sensory modality (F(2,22) = 49.03, p < 0.0005). Multiple orthogonal comparisons revealed that response accuracy was significantly higher for visual (96.3%) and auditory (91.5%) stimuli than for olfactory stimuli (71.7%) (F(1,33) = 73.98,p < 0.0005; andF(1,33) = 48.17, p < 0.0005, respectively). We also determined the number of times for which subjects selected the index finger or the middle finger during the neutral scans. The results indicated that they selected one or the other of these fingers at random in the three neutral sensory conditions (i.e., ∼50% of the time).
Fig. 1.
Left, Accuracy (%) of behavioral responses as a function of the three modalities and repetition (sequences 1 and 2). Right, Mean reaction times (in milliseconds) of subjects as a function of modality and emotional and neutral conditions.
A two-way repeated measures ANOVA was performed on the reaction times (Fig. 1, right), taking into account the sensory modality (first factor) and the neutral versus emotional condition (second factor). The ANOVA demonstrated a significant effect of the sensory modality (F(2,33) = 3.89,p < 0.05), no significant effect of the second factor (F(1,33) = 1.59, p = NS), and a significant interaction between both factors (F(2,33) = 8.04, p < 0.005). Multiple orthogonal comparisons showed that subjects required more time to perform the olfactory tasks than the visual tasks (F(1,33) = 7.76, p < 0.01). They also demonstrated that subjects took more time to perform the emotional olfactory task than the neutral olfactory task (F(1,33) = 15.04, p < 0.0005).
PET data
The origin of the variance in the different experimental conditions
The PCA showed that 95.9% of the variance were represented by the first four components of the analysis (Fig.2). The first component (86.5% of the variance) was characterized by the neural structures involved in all three sensory modalities. The positive eigenimage (auditory and olfactory conditions) bilaterally engaged the temporal and orbitofrontal lobes, whereas the negative eigenimage (visual conditions) engaged the occipital region. The second component (4.8% of the variance) mainly discriminated activation patterns related to the auditory (temporal lobes depicted in the negative eigenimage) and olfactory (orbitofrontal lobes shown in the positive eigenimage) conditions. The third component (3.2% of the variance) systematically contrasted the emotional and neutral conditions. The positive eigenimage (neutral condition) mainly engaged the bilateral inferior parietal lobule [Brodmann's area (BA) 40], the middle frontal gyrus (BA 46), and the precuneus (BA 7), whereas the negative eigenimage (emotional condition) mainly engaged the left temporal pole (BA 38), the left orbitofrontal gyrus (BA 11/47), and the left superior frontal gyrus (BA 9). Finally, the fourth component (1.4% of the variance) opposed both repetitions in each condition. The positive eigenimage (first scan) engaged the right precentral gyrus, whereas the negative eigenimage (second scan) engaged the right cerebellum.
Fig. 2.
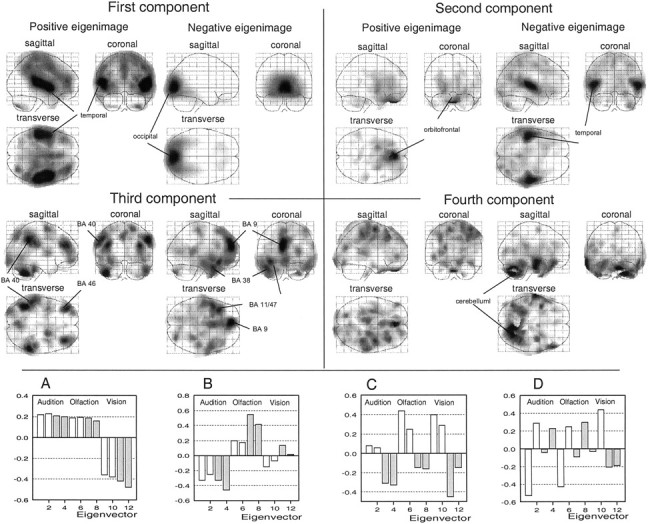
The first four components of the PCA performed on the three activation conditions (modality, emotional, and repetition). Patterns of positive and negative covariance (eigenimages) of the four components that account for 86.5, 4.8, 3.2, and 1.4% of the variance, respectively. Bottom, Condition-dependent profiles (eigenvector) corresponding to positive and negative eigenimages of the first (A), second (B), third (C), and fourth (D) components. The first component discriminated activation patterns related to the auditory and olfactory conditions (positive eigenimage) and to the visual condition (negative eigenimage). The second component discriminated activation patterns related to the auditory (negative eigenimage) and olfactory (positive eigenimage) conditions. The third component discriminated activation patterns related to emotional (negative eigenimage) and neutral (positive eigenimage) conditions. The fourth component discriminated activation patterns related to the first (negative eigenimage) and the second (positive eigenimage) repetitions in each condition.Gray, Emotional condition; white, neutral condition.
Areas activated in the emotional condition for the three sensory modalities
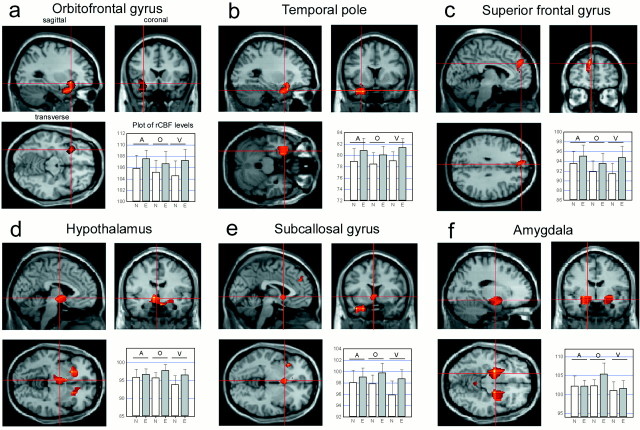
When the rCBF images in the neutral tasks were subtracted from the rCBF images obtained in the emotional tasks, significant rCBF increases were found in the frontal and temporal lobes (Table1, Fig. 3). For the auditory modality, significant rCBF increases were observed in the superior frontal gyrus (BA 8) and the temporal pole (anterior part of the superior temporal gyrus, BA 38). For the olfactory modality, significant rCBF increases were obtained in the left OFC (BA 11/47) and the right superior frontal gyrus (BA 9). A weaker rCBF increase was observed in the right OFC (BA 11/47) and the hypothalamus. A higher resolution analysis (10 mm FWHM) confirmed that the maxima of the hypothalamic focus fell well within the boundaries of the hypothalamus and could not be attributed to movement-related edge artifacts. For the visual modality, rCBF increases were found in the subcallosal gyrus (BA 25), the superior frontal gyrus (BA 9), and the temporal pole (BA 38). An analysis of conjunction performed between these different contrasts revealed significant rCBF increases in the right OFC (BA 11/47) and in three areas in the left hemisphere: an area circumscribed by the border of the OFC (BA 11/47) and anterior agranular insula and extending in to the subcallosal region, the superior frontal gyrus (BA 9), and the temporal pole (BA 38). Thus, although activation in the OFC did not reach statistical significance in the auditory and visual conditions in the exploratory analyses, a significant OFC activation emerged in the three sensory conditions in the conjunction analysis.
Table 1.
Brain regions with significant rCBF increases when comparing the rCBF images obtained in the emotional tasks with those obtained in the neutral and control tasks
| Contrasts | Modality | Brain regions | BA | Size | p (mmax >k) | Z | p (Z > u) | x,y, z MNI |
|---|---|---|---|---|---|---|---|---|
| Emotional–neutral | Audition | Superior frontal gyrus | 8 | 1597 | 0.090 | 4.27 | 0.052 | −16, 44, 58 |
| Temporal pole | 38 | 1658 | 0.118 | 4.17 | 0.076 | −44, 14, −24 | ||
| Olfaction | Orbitofrontal gyrus | 11/47 | 14306 | 0.000 | 4.34 | 0.041 | −24, 30, −8 | |
| Hypothalamus | 4.03 | 0.121 | −6, −2, −6 | |||||
| Orbitofrontal gyrus | 11/25/47 | 3.91 | 0.178 | 28, 26, −14 | ||||
| Superior frontal gyrus | 9 | 886 | 0.085 | 4.29 | 0.048 | 60, 22, 34 | ||
| Vision | Subcallosal gyrus | 25 | 19399 | 0.000 | 5.05 | 0.002 | −4, 10, −4 | |
| Superior frontal gyrus | 9 | 4.90 | 0.004 | −6, 52, 28 | ||||
| Temporal pole | 38 | 4.50 | 0.022 | −34, 18, −24 | ||||
| Conjunction | Temporal pole | 38 | 1031 | 0.002 | 5.88 | 0.000 | −30, 10, −26 | |
| Orbitofrontal gyrus | 11/47 | 5.48 | 0.000 | −30, 18, −12 | ||||
| Superior frontal gyrus | 9 | 450 | 0.006 | 5.35 | 0.000 | −6, 50, 30 | ||
| Orbitofrontal gyrus | 11/47 | 3.95 | 0.108 | 26, 30, −12 | ||||
| Emotional– no-stimul. control | Olfaction | Amygdala | 18584 | 0.000 | 6.47 | 0.000 | −22, 0, −12 | |
| Amygdala/parainsular region | 5.82 | 0.000 | 34, 4, −14 | |||||
| Cerebellum | 4.25 | 0.060 | 4, −46, −14 | |||||
| Neutral–emotional | Audition | Inferior parietal lobule | 40 | 2502 | 0.083 | 4.09 | 0.101 | −50, −34, 28 |
| Olfaction | Inferior parietal lobule | 40 | 2244 | 0.108 | 4.06 | 0.113 | 64, −40, 36 | |
| Inferior parietal lobule | 40 | 4.02 | 0.125 | 60, −44, 48 | ||||
| Vision | Inferior parietal lobule | 40 | 4339 | 0.019 | 4.61 | 0.014 | 52, −46, 40 | |
| Superior frontal gyrus | 6 | 5289 | 0.011 | 4.53 | 0.019 | 22, −4, 64 | ||
| Inferior parietal lobule | 40 | 6761 | 0.005 | 4.38 | 0.035 | −56, −38, 50 | ||
| Inferior parietal lobule | 40 | 4.25 | 0.057 | −64, −28, 32 | ||||
| Superior parietal lobule | 7 | 4.24 | 0.058 | −36, −58, 60 | ||||
| Conjunction | Middle frontal gyrus | 46 | 488 | 0.003 | 5.56 | 0.000 | 34, 34, 22 | |
| Inferior parietal gyrus | 40 | 1827 | 0.001 | 6.07 | 0.000 | −50, −38, 34 | ||
| Inferior parietal lobule | 40 | 1507 | 0.000 | 6.60 | 0.000 | 56, −46, 32 | ||
| Precuneus | 7 | 472 | 0.027 | 4.70 | 0.009 | 4, −66, 46 |
A minus for the x coordinate denotes the left hemisphere. MNI, Montreal Neurological Institute.
Fig. 3.
Sagittal, coronal, and transverse sections through the z maps on an anatomically normalized standard brain with areas activated in the three modalities in the emotional minus neutral conditions: in (a) the left inferior frontal gyrus, (b) the left temporal pole, (c) the left superior frontal gyrus; in olfaction and vision in (d) the hypothalamus and (e) the subcallosal gyrus; and (f) in olfaction in the emotional minus no-stimulation control conditions in both amygdalae. For each area activated, the plots show rCBF levels in the six activation conditions for this coordinate [5.35 ≤ z ≤ 6.27,p = 0.000 (corrected)].
When the rCBF images in the no-stimulation control task were subtracted from those observed in the emotional olfactory task, significant rCBF increases were found in the amygdala bilaterally and in the cerebellum (Table 1). ROI analysis corroborated these findings for the amygdala. However, because in the exploratory analysis the activated area appeared to extend beyond the boundaries of the amygdala, we additionally reanalyzed the data using images with less blurring (FWHM of 10 mm). At this higher resolution, an rCBF increase emerged in the amygdala and the piriform cortex in the left hemisphere and in an area bordering the superior temporal gyrus and the claustrum in the right hemisphere. Contrasts between emotional auditory and visual stimuli with the no-stimulation control condition did not reveal any additional activations beyond those reported in contrast with the neutral condition or those usually involved in auditory and visual processing (temporal and occipital areas, respectively).
Areas activated in the neutral condition
When the rCBF images in the emotional tasks were subtracted from the rCBF images obtained in the neutral tasks, rCBF levels were significantly higher in the neutral than in the emotional conditions in the right and left inferior parietal lobules (BA 40) for the three modalities and in the superior frontal gyrus (BA 6) and the superior parietal lobule (BA 7) for the visual modality (Table 1). The analysis of conjunction performed between these three contrasts confirmed that rCBF levels in the bilateral inferior parietal lobule (BA 40), the right middle frontal gyrus (BA 46), and the right precuneus (BA 7) were higher in the neutral than in the emotional conditions.
Regions of interest
The focused ROI analyses of emotional versus neutral conditions revealed a significant rCBF increase in the hypothalamus for visual stimuli, the right and left hippocampus for visual and olfactory stimuli, and the left hippocampus for auditory stimuli. Because the hippocampal region was found to be activated in the three modalities, recent imaging experiments suggest that such an activation could reflect a gating process through which highly arousing stimuli preferentially gain access to hippocampal mnemonic processes (Zald et al., 1998b; Zald and Pardo, 2000).
DISCUSSION
The present paper examined the neural correlates of responses to emotional olfactory, visual, and auditory stimuli. We observed that making emotional judgments about emotionally valenced stimuli recruits a core network in the left hemisphere, regardless of the sensory modality. This core network was activated in addition to a number of areas that appear specific to individual sensory modalities.
Neural structures activated by emotional stimuli for all three sensory modalities
The first two components of the PCA reveal activation patterns as a function of the sensory modality, thus reflecting areas involved in perceptual processing. The third component takes into account the variance linked to emotional and neutral conditions only. This indicates that a distinct set of structures, separate from those involved in modality-specific sensory processing, show functional connectivity during emotional processing. This component primarily consists of three cortical regions activated in the left hemisphere: the posterior part of the OFC (BA 11/47), the temporal pole (BA 38), and the superior frontal gyrus (BA 9). Although the PCA is primarily descriptive (and represents functional connectivity as opposed to activation), the pattern is striking in its convergence with the conjunction analysis, which revealed significantly increased rCBF in all three areas during the emotional condition for in each sensory modality.
The activation of the OFC in response to emotional stimuli is consistent with current hypotheses of OFC functions that focus on its role in evaluating the appetitive or aversive reinforcement value of sensory stimuli (Zald and Kim, 1996b; Rolls, 1999). Damage to this area has been shown to induce changes in personality and affect (Hecaen and Albert, 1978; Milner, 1982; Damasio et al., 1994; Rolls et al., 1994,Zald and Kim, 1996b). Recent neuroimaging studies show enhanced activity in the OFC in response to different kinds of emotional stimuli, such as sad and angry visual expressions (Morris et al., 1996), film-generated emotions (Reiman et al., 1997), pleasant touch (Francis et al., 1999), pleasant music (Blood et al., 1999), and aversive odorants or tastes (Zald and Pardo, 1997; Zald et al., 1998a,b; Royet et al., 2000). Thus, our data confirm and extend these findings by showing that the OFC activates during visual, olfactory, and auditory hedonic judgments. These results are supported by anatomical data showing that the OFC receives not only olfactory (Carmichael et al., 1994) but also visual (Zald and Kim, 1996a) and auditory projections (Romanski et al., 1999).
Activation in the temporal pole has been found in studies involving visual modality for different types of emotion (Reiman et al., 1997;Phillips et al., 1998; Blair et al., 1999; Dougherty et al., 1999). Our results extend these data to the olfactory and auditory modalities. The OFC and the temporal pole are heavily connected (Dejerine and Dejerine-Klumpke, 1895; Curran, 1909; Barbas et al., 1999).Livingston and Escobar (1971) argued that both areas pertain to the basolateral division of the limbic system, a division that involves brain regions connected with the amygdala.
The activation in the superior frontal gyrus (BA 9) emerged in an area very close to the one found by Reiman et al. (1997) and Lane et al. (1997b) in response to film- and picture-generated emotion. Thus, this cortex is not only involved in emotion generated by visual stimuli but also by auditory and olfactory stimuli. Reiman and colleagues suggested that it could participate in aspects of emotion that are unrelated to the type of emotion, emotional valence, or the nature of the emotional stimulus, but “in the conscious experience of emotion, inhibition of potentially excessive emotion, or the process of monitoring one's own emotional state to make personally relevant decisions.” Our data support its involvement in emotional processing but leave unclear exactly what aspect of emotional processing it serves.
In summary, we demonstrated that emotional processing involves a neural network, including at least three brain areas that appear to perform similar functions across the three investigated sensory modalities. These data indicate that this neural network is not modality-specific, but rather it appears to perform similar functions across multiple sensory modalities.
Emotional activation in modality-specific areas?
Unexpectedly, rCBF increases arose in the amygdala during olfaction but not in vision and audition. A recent study using unpleasant music similarly failed to observe activation of the amygdala (Blood et al., 1999). In contrast, several studies using PET or functional MRI have reported amygdala activation after negative visual emotional stimuli, including when fearful or angry facial expressions were unconsciously perceived (Morris et al., 1996, 1998;Whalen et al., 1998). The lack of activation during audition and vision in the present study is not easily attributable to weak emotional valence. For instance, the visual stimuli contained particularly unpleasant images and induced strong emotional reactions as attested by subjects. In addition, the level of correct responses in hedonic judgment (Fig. 1) was very high in both the visual and auditory conditions (90%), indicating that subjects effectively recognized these stimuli as emotional. Some previous studies have suggested that the amygdala preferentially responds to aversive stimuli (Lane et al., 1997a,b; Zald and Pardo, 1997; Zald et al., 1998b). It may be that the combination of pleasant and unpleasant stimuli within the same scans restricted our ability to observe amygdala activation. However, this does not explain why amygdala activation arose during emotional olfaction but not during emotional vision and audition. Apart from methodological differences, the most obvious possibility could be a stronger ability of odorants to induce emotional states. Indeed, olfactory projections onto the amygdala are bisynaptic and dense (Shipley and Reyes, 1991; Carmichael et al., 1994). Swanson and Petrovich (1998) distinguished 13 structural areas in the rat amygdala, of which 10 constituted the corticomedial group, which are implicated in olfactory functions. In man, five of eight amygdaloid areas pertain to the corticomedial group (Crosby et al., 1962). In addition, human experience of odors is primarily hedonic or esthetic (Herz and Engen, 1996), and the prominent role of olfaction in emotion over vision and audition has been demonstrated in several behavioral studies (Hinton and Henley, 1993; Herz and Cupchik, 1995; Herz, 1996, 1997).
We found an rCBF increase in the hypothalamus in olfaction and vision.Reiman et al. (1997) also reported hypothalamic activation during film-generated emotion. The hypothalamus has long been implicated as a critical output pathway for the limbic system. Electrophysiological studies in animals indicate that it responds to stimuli with emotional characteristics (Davis, 1992; LeDoux, 1995). Indeed, it receives strong input from several of the other areas implicated in this study, including the amygdala and OFC (Swanson and Petrovich, 1998).
Another well established area involved in the emotional circuit is the cingulate cortex. Damage to this cortex affects emotional behavior in animals and humans (LeDoux, 1987). Human brain imaging investigations of anxiety, fear, dysphoria, depression, and pain, and studies involving exposure to pleasant or unpleasant images, music, touch, or taste have implicated the subcallosal anterior cingulate region (BA 25) (George et al., 1995; Rauch et al., 1995; Vogt et al., 1996; Drevets et al., 1997; Lane et al., 1997b; Mayberg et al., 1997; Blood et al., 1999; Francis et al., 1999). Thus, the rCBF increases found in the subcallosal gyrus in response to emotional visual and olfactory stimuli provides a convergent picture. This gyrus represents the most important autonomic region of the frontal lobe, and it provides an astonishing amount of input into the lateral hypothalamus (Öngür et al., 1998).
Lateralization of emotional processing
The current study indicates a strong lateralization of cerebral areas participating in emotion, with the OFC, the temporal pole, and the frontal gyrus clearly presenting an rCBF increase in the left side. The right OFC was also activated but with a weakly significant level. In previous studies, we suggested that this area is mainly related to the familiarity judgment, that is, odor recognition (Royet et al., 1999, 2000).
Previous theories on the lateralization of emotion have labeled the right hemisphere as superior for the control of emotional processing, irrespective of the valence of emotion, or as specifically associated to the processing of negative emotions (Ahren and Schwartz, 1985;Gainotti, 1989; Jones and Fox, 1992; Wittling and Roschmann, 1993;Canli et al., 1998; Angrilli et al., 1999). However, a number of neuroimaging studies agree with the present results in indicating a strong involvement of left hemisphere structures in emotional processing (Drevets et al., 1992, Pardo et al., 1993; Rauch et al., 1994; Ketter et al., 1996; Morris et al., 1996, 1998; Zald and Pardo, 1997; Zald et al., 1998a,b; Dougherty et al., 1999). In particular, activity in the left OFC has been observed in PET studies with aversive odors and tastes (Zald and Pardo, 1997; Zald et al., 1998a,b; Royet et al., 2000), the subjective experience of anger (Dougherty et al., 1999), and negative emotional inductions, such as dysphoria (Pardo et al., 1993) and obsessive–compulsive symptoms (Rauch et al., 1994). Thus, the present results, as well as previous neuroimaging data, indicate that the left hemisphere structures play a more prominent role in emotional processing than is accounted for by traditional accounts of the lateralization of emotions.
Footnotes
This work was supported by research grants from the Rhône-Alpes Region, the Groupement d'Intérêt Scientifique (Sciences de la Cognition), the Centre National de la Recherche Scientifique, and the Université Claude-Bernard de Lyon. The Institut des Sciences Cognitives and the Laboratoire des Neurosciences et Systèmes Sensoriels belong to the Institut Fédératif des Neurosciences de Lyon. We thank the technical and medical staff of Centre d'Exploration et de Recherche Médicale par Emission de Positons for their valuable assistance and our volunteer subjects for their participation and patience. We also thank V. Merienne and D. Sanders for the selection and preparation of visual and auditory stimuli, A. C. Batut and N. Zaafouri for assistance during PET experiments, M. Vigouroux and V. Farget for conceiving the screen support, and M. Meunier for helpful comments on this manuscript. We are grateful to societies of perfume and/or aroma (Givaudan-Roure and the International Flavour and Fragrances) for supplying the odorants used in this study.
Correspondence should be addressed to Dr. J.-P. Royet, Laboratoire de Neurosciences et Systèmes Sensoriels, Centre National de la Recherche Scientifique, Unité Mixte de Recherche 5020, Université Claude-Bernard Lyon 1, 69622 Villeurbanne, France. E-mail: royet@olfac.univ-lyon1.fr.
REFERENCES
- 1.Ahren GL, Schwartz GE. Differential lateralization for positive and negative emotion in the human brain: EEG spectral analysis. Neuropsychologia. 1985;23:745–755. doi: 10.1016/0028-3932(85)90081-8. [DOI] [PubMed] [Google Scholar]
- 2.Angrilli A, Palomba D, Cantagallo A, Maietti A, Stegagno L. Emotional impairment after right orbitofrontal lesion in a patient without cognitive deficits. NeuroReport. 1999;10:1741–1746. doi: 10.1097/00001756-199906030-00021. [DOI] [PubMed] [Google Scholar]
- 3.Barbas H, Ghasghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol. 1999;410:343–367. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- 5.Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 1999;2:382–387. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- 6.Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci USA. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canli T, Desmond JE, Zhao Z, Glover G, Gabrieli JD. Hemispheric asymmetry for emotional stimuli detected with fMRI. NeuroReport. 1998;9:3233–3239. doi: 10.1097/00001756-199810050-00019. [DOI] [PubMed] [Google Scholar]
- 8.Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346:403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- 9.Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- 10.Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: a new graphic interactive environment for designing psychology experiments. Behav Res Methods Instrum Comput. 1993;25:257–271. [Google Scholar]
- 11.Crosby EC, Humphrey T, Lauer EW. Correlative anatomy of the nervous system, Ch 7, Telencephalon, Pt 2, Subcortical telencephalic nuclei, pp 356–393. MacMillan; New York: 1962. [Google Scholar]
- 12.Curran EJ. A new association tract in the cerebrum with remarks on the fiber tract dissection method of studying the brain. J Comp Neurol. 1909;19:645–657. [Google Scholar]
- 13.Damasio H, Grabowski T, Frank R, Galaburda A, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- 14.Davis M. The role of the amygdala in fear and anxiety. Ann Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 15.Dejerine JJ, Dejerine-Klumpke . Anatomie des Centres Nerveux. Rueff; Paris: 1895. [Google Scholar]
- 16.Dougherty DD, Shin LM, Alpert NM, Pitman RK, Orr SP, Lasko M, Macklin ML, Fischman AUJ, Rauch SL. Anger in healthy men: a PET study using script-driven imagery. Biol Psychiat. 1999;46:466–472. doi: 10.1016/s0006-3223(99)00063-3. [DOI] [PubMed] [Google Scholar]
- 17.Drevets WC, Vieen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomy study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 19.Duvernoy HM. The human brain: surface, three dimensional sectional anatomy and MRI. Springer; Wien, Austria: 1991. [Google Scholar]
- 20.Francis S, Rolls ET, Bowtell R, McGlone F, O'Doherty J, Browning A, Clare S, Smith E. The representation of pleasant touch in the brain and its relationship with taste and olfactory area. NeuroReport. 1999;10:453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- 21.Friston KJ, Frith CD, Liddle PF, Frackowiak RSJ. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- 22.Friston KJ, Ashburner J, Frith CD, Poline J-B, Healther JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995a;3:165–189. [Google Scholar]
- 23.Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995b;2:189–210. [Google Scholar]
- 24.Fulbright RK, Skudlarski P, Lacadie CM, Warrenburg S, Bowers AA, Gore JC, Wexler BE. Functional MR imaging of regional brain responses to pleasant and unpleasant odors. Am J Neuroradiol. 1998;19:1721–1726. [PMC free article] [PubMed] [Google Scholar]
- 25.Gainotti G. Disorders of emotions and affect in patients with unilateral brain damage. In: Boller F, Grafman J, editors. Handbook of neurophysiology III. Elsevier; Amsterdam: 1989. pp. 345–361. [Google Scholar]
- 26.George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post M. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry. 1995;152:341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- 27.Hecaen H, Albert ML. Human neuropsychology. Wiley; New York: 1978. [Google Scholar]
- 28.Herz RS. A comparison of olfactory, tactile and visual as associated memory cues. Chem Senses. 1996;21:614–615. [Google Scholar]
- 29.Herz RS. Emotion experienced during encoding enhances odor retrieval cue effectiveness. Am J Psychol. 1997;110:489–505. [PubMed] [Google Scholar]
- 30.Herz RS, Cupchik GC. The emotional distinctiveness of odor-evoked memories. Chem Senses. 1995;20:517–528. doi: 10.1093/chemse/20.5.517. [DOI] [PubMed] [Google Scholar]
- 31.Herz RS, Engen T. Odor memory: review and analysis. Psychon Bull Rev. 1996;3:300–313. doi: 10.3758/BF03210754. [DOI] [PubMed] [Google Scholar]
- 32.Hinton PB, Henley TB. Cognitive and affective components of stimuli presented in three modes. Bull Psychon Soc. 1993;31:595–598. [Google Scholar]
- 33.Irwin W, Davidson RJ, Lowe MJ, Mock BJ, Sorenson JA, Turski PA. Human amygdala activation detected with echo-planar functional magnetic resonance imaging. NeuroReport. 1996;7:1765–1769. doi: 10.1097/00001756-199607290-00014. [DOI] [PubMed] [Google Scholar]
- 34.Jones NA, Fox NA. Electroencephalogram asymmetry during emotionally evocative films and its relation to positive and negative affectivity. Brain Cogn. 1992;20:182–198. doi: 10.1016/0278-2626(92)90021-d. [DOI] [PubMed] [Google Scholar]
- 35.Ketter TA, Andreason PJ, George MS, Lee, Gil DS, Parekh PI, Willis MW, Herscovitch P, Post RM. Anterior paralimbic mediation of procaine-induced emotional and psychosensory experiences. Arch Gen Psychiatry. 1996;53:59–69. doi: 10.1001/archpsyc.1996.01830010061009. [DOI] [PubMed] [Google Scholar]
- 36.Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997a;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- 37.Lane RD, Reiman E, Bradley MM, Lang PJ, Ahern GL, Davidson RJ. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997b;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- 38.LeDoux JE. Emotion. In: Plum F, Mountcastle VB, editors. Handbook of physiology. The nervous system. American Physiological Society; Bethesda, MD: 1987. pp. 419–459. [Google Scholar]
- 39.LeDoux JE. Emotion: clues from the brain. Ann Rev Psychol. 1995;40:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- 40.Livingston KE, Escobar A. Anatomical bias of the limbic system concept. Arch Neurol. 1971;24:17–21. doi: 10.1001/archneur.1971.00480310045003. [DOI] [PubMed] [Google Scholar]
- 41.Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. NeuroReport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 42.Milner B. Some cognitive effects of frontal-lobe lesions in man. Philos Trans R Soc B Biol Sci. 1982;298:211–226. doi: 10.1098/rstb.1982.0083. [DOI] [PubMed] [Google Scholar]
- 43.Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 44.Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- 45.Öngür D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- 46.Pardo JV, Pardo PJ, Raichle ME. Neural correlates of self-induced dysphoria. Am J Psychiatry. 1993;151:784–785. doi: 10.1176/ajp.150.5.713. [DOI] [PubMed] [Google Scholar]
- 47.Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Williams SC, Bullmore ET, Brammer M, Gray JA. Neural responses to facial and vocal expressions of fear and disgust. Proc R Soc Lond B Biol Sci. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, Fischman AJ. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- 49.Rauch SL, Savage CR, Alpert NM, Miguel EC, Baer L, Breiter HC, Fischman AJ, Manzo PA, Moretti C, Jenike MA. A positron emission tomographic study of simple phobic symptom provocation. Arch Gen Psychiatry. 1995;52:20–28. doi: 10.1001/archpsyc.1995.03950130020003. [DOI] [PubMed] [Google Scholar]
- 50.Reiman EM, Richard MD, Lane MD, Geoffrey L, Ahern MD, Schwartz GE, Davidson RJ, Friston KJ, Yun LS, Chen K. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- 51.Rolls ET. The brain and emotion Neuilly-sur-Seine, France: pp 75–145. Oxford UP; Oxford: 1999. [Google Scholar]
- 52.Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romanski LM, Bates JF, Goldman-Rakic PS. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1999;403:141–157. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 54.Royet JP, Koenig O, Gregoire MC, Cinotti L, Lavenne F, Le Bars D, Costes N, Vigouroux M, Farget V, Sicard G, Holley A, Mauguière F, Comar D, Froment JC. Functional anatomy of perceptual and semantic processing for odors. J Cognit Neurosci. 1999;11:94–109. doi: 10.1162/089892999563166. [DOI] [PubMed] [Google Scholar]
- 55. Royet JP, Hudry J, Vigouroux M. Application de l'Imagerie Cérébrale à l'Etude de l'Olfaction. Rencontres IPSEN en ORL Christen Y, Collet L. 2000. Editions Irvinn; Neuilly-sur-Seine, France, in press. [Google Scholar]
- 56.Shipley M, Reyes P. Anatomical of the human olfactory bulb and central olfactory pathways. In: Laing DG, Doty RL, Breipohl W, editors. The human sense of smell. Springer; Berlin: 1991. pp. 29–60. [Google Scholar]
- 57.Swanson LA, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 58.Taylor SF, Liberzon I, Fig LM, Decker L, Minoshima S, Koeppe RA. The effect of emotional content on visual recognition memory: a PET activation study. NeuroImage. 1998;8:188–197. doi: 10.1006/nimg.1998.0356. [DOI] [PubMed] [Google Scholar]
- 59.Vogt BA, Derbyshire S, Jones AKP. Pain processing in four regions of human cingulate cortex localized with co-registered PET and PR imaging. Eur J Neurosci. 1996;8:1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- 60.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenik MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winer BJ. Statistical principles in experimental design. McGraw-Hill; New York: 1962. [Google Scholar]
- 62.Wittling W, Roschmann R. Emotion-related hemisphere asymmetry: subjective emotional responses to laterally presented films. Cortex. 1993;29:431–448. doi: 10.1016/s0010-9452(13)80252-3. [DOI] [PubMed] [Google Scholar]
- 63.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical Approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 64.Zald DH, Kim SW. Anatomy and function of the orbital frontal cortex. I. Anatomy, neurocircuitry, and obsessive-compulsive disorder. J Neuropsychiat. 1996a;8:125–138. doi: 10.1176/jnp.8.2.125. [DOI] [PubMed] [Google Scholar]
- 65.Zald DH, Kim SW. Anatomy and function of the orbital frontal cortex. II. Function and relevance to obsessive-compulsive disorder. J Neuropsychiat. 1996b;8:249–261. doi: 10.1176/jnp.8.3.249. [DOI] [PubMed] [Google Scholar]
- 66.Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zald DH, Pardo JV. Functional neuroimaging of the olfactory system in humans. Int J Psychophysiol. 2000;36:165–181. doi: 10.1016/s0167-8760(99)00110-5. [DOI] [PubMed] [Google Scholar]
- 68.Zald DH, Donndelinger MJ, Pardo JV. Elucidating dynamic brain interactions with across-subjects correlational analyses of positron emission tomographic data: the functional connectivity of the amygdala and orbitofrontal cortex during olfactory tasks. J Cereb Blood Flow Metab. 1998a;18:896–905. doi: 10.1097/00004647-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 69.Zald DH, Lee JT, Fluegel KW, Pardo JV. Aversive gustatory stimulation activates limbic circuits in humans. Brain. 1998b;121:1143–1154. doi: 10.1093/brain/121.6.1143. [DOI] [PubMed] [Google Scholar]