Abstract
Multi- and single-unit recording was performed in the gracile nucleus in urethane-anesthetized rats to examine estrous variations in responses of its neurons to brushing the hindquarters and mechanical stimulation of the uterus, vaginal canal, cervix, and colon. Six rats each were studied in each of the four estrous stages: proestrus (P), estrus (E), metestrus (M), and diestrus (D). The magnitude of multi-unit responses to gentle brushing of the perineum, hip, and tail, but not the foot and leg, was significantly greater during proestrus than during other stages. Of 70 single units responsive to brush, 56 (80%) responded to stimulation of at least one viscus. Although this percentage did not change with estrous stage, the direction and latency of some responses did. Pressure on the cervix evoked significantly more inhibitory (vs excitatory) responses in P than in E and M, and the response latency was significantly longer in D and P than in E and M. The direction of response to vaginal distention did not change with estrous stage, but response latency was significantly longer in D than in P and E. Uterine distention evoked significantly more inhibitory responses in D than in P, with no estrous changes in latency. Responses to colon distention did not change. These variations in both magnitude of response to tactile stimulation and characteristics of response to stimulation of reproductive organs, but not the colon, correlate with changes in mating behaviors of the female rat, suggesting that the gracile nucleus is a component of neural systems that control reproductive behaviors.
Keywords: female, dorsal column, reproduction, plasticity, somatosensory, pain
It is well known that neurons in the gracile nucleus (NG) receive input from low-threshold cutaneous primary afferent fibers, respond vigorously to gentle tactile stimulation of small areas of the hindquarters, and convey that information to the thalamus and somatosensory regions of the cerebral cortex. It has been found recently, however, that NG neurons in the rat also respond to innocuous and noxious stimulation of female pelvic organs, such as the cervix, uterus, vagina, and colon (Hubscher, 1994; Berkley and Hubscher, 1995).
Such convergent responsiveness suggests that NG may be a component of neural systems that coordinate reproductive behaviors. These behaviors in the female rat include a group of complex pacing and darting movements collectively called “proceptive behaviors,” which are important for successful mating (Erskine, 1989), as well as “lordosis,” which is a mating posture important for copulation and successful fertilization (Schwartz-Giblin et al., 1989; Pfaff, 1997). Both behaviors vary with the rat's estrous cycle in a similar way. Proceptive behaviors occur during the female rat's fertile period when she is sexually receptive, that is, during the afternoon and evening of proestrus and during the early morning of estrus (Erskine, 1989). This period is when estrogen and progesterone rise and fall in conjunction with ovulation (Freeman, 1994). Lordosis is induced by pressure on the rat's cervix and tactile stimulation of the perineum, saddle, base of tail, and proximal legs (Kow et al., 1979). The effectiveness of these stimuli for evoking lordosis is greatest during the rat's fertile period (Komisaruk and Diakow, 1973).
These results suggest that, if NG is involved in reproductive behaviors, then the activity and responsiveness of NG neurons should exhibit estrous variation with the greatest effects occurring during the time of sexual receptivity. Accordingly, the present study tested this prediction. Support for this possibility comes from two sets of studies. First, NG receives input from the pudendal nerve (Ueyama et al., 1987), whose fibers supply the perineum and whose receptive field territory becomes enlarged during the rat's fertile period (Adler et al., 1977). Second, NG neurons project to the inferior olive and cerebellum (Berkley et al., 1986), whose neurons exhibit an increase in the rhythmicity and coordination of their activity during the time of sexual receptivity (Smith, 1995, 1998).
MATERIALS AND METHODS
Subjects
The study was performed on 24 female Sprague Dawley rats (250–325 gm; 90–150 d of age) housed singly in hanging cages and maintained on a 12 hr light/dark cycle. Each rat's estrous stage was evaluated by daily vaginal smears. Only rats that had had at least three regular 4 d cycles before the day of the experiment were used (Long and Evans, 1922). Six rats were studied in each estrous stage, i.e., diestrus (D), proestrus (P), estrus (E), or metestrus (M), and testing was performed at the same time of day for all subjects (7–9 hr after lights on). Experimenters were blind to the rat's estrous stage until after all data had been quantified.
Surgical procedures
Each rat was anesthetized with urethane (1.6 gm/kg, i.p.). The common carotid artery was cannulated to monitor heart rate and blood pressure. The trachea was cannulated to monitor pCO2 and to allow artificial respiration, and the jugular vein was cannulated to administer paralytic agents. Landmarks on the rat's skin were marked with black ink to demarcate standardized numbered cutaneous regions (Fig. 1). After a midline abdominal incision to expose the caudal reproductive tract, each uterine horn was implanted with a water-filled uninflated balloon (latex, 5 mm long) attached to a catheter and brought through the abdominal incision, which was then sutured closed. The rat's head was secured in a clamp that flexed its head ∼30° ventrally for access to the caudal medulla. The body was suspended by hip clamps from a frame for access to the ventral body surface, and the tail was raised and taped to a support pole for access to the perineum, vaginal canal, and colon. The caudal medulla was exposed by a dorsal midline incision and removal of overlying occipital bone and membranes and covered with warm mineral oil. The rat was then paralyzed with pancuronium bromide (0.6 mg in 0.3 ml, i.v.) and artificially respirated to maintain CO2 levels of ∼4%. Body temperature was monitored via an intrascapular thermometer and maintained at ∼37.5°C. Data were used only if mean arterial pressures were >80 mmHg.
Fig. 1.
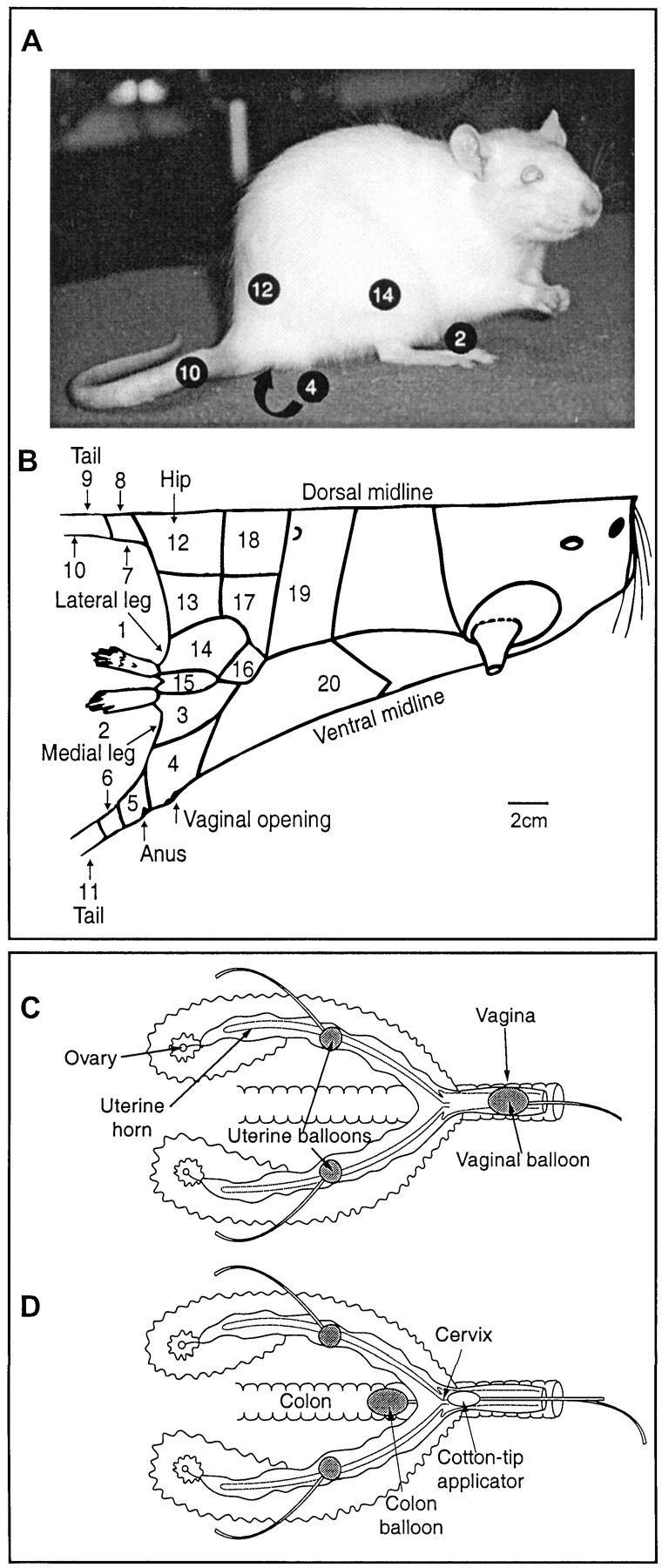
Stimulation protocol. B is a diagram of the rat's body traced directly from one half of a rat's pelt. Areas stimulated during the experiment are demarcated and numbered. Some of these are shown on the picture of the rat inA. C and D are diagrams of the female rat's reproductive tract. C shows the positions of the stimulating balloons implanted in the uterine horns and temporarily placed in the vaginal canal. D shows the position of the stimulating balloon temporarily inserted in the colon and the lubricated cotton swab applicator temporarily positioned next to the cervix.
Recording and experimental protocol
Extracellular recordings were made with glass-coated, platinum-plated tungsten microelectrodes with a 20 μm exposed tip and an average impedance of 2.2 MΩ (Ainsworth et al., 1977) connected to conventional amplification and recording equipment and stored on videotape and computer. The recording electrode was positioned at 200 μm lateral to the obex. To ensure consistency in electrode localization across subjects, the electrode was advanced dorsoventrally through NG, and the vertical extent of NG calculated by measuring the depth of the electrode tip from the surface of the medulla to the level at which brush stimulation of the entire hindquarters no longer evoked any multi-unit activity. Most atlases show that the depth of NG at 200 μm lateral to obex is ∼450 μm. All tracts here had a depth of 450 ± 25 μm. The position at which all recordings were made was 200 μm below the surface (Fig.2A). This location was chosen because Hubscher (1994) found that the highest proportion of viscerally responsive neurons in NG was in the vicinity of this region and because it receives primary afferents from the pudendal nerve (Ueyama et al., 1987).
Fig. 2.
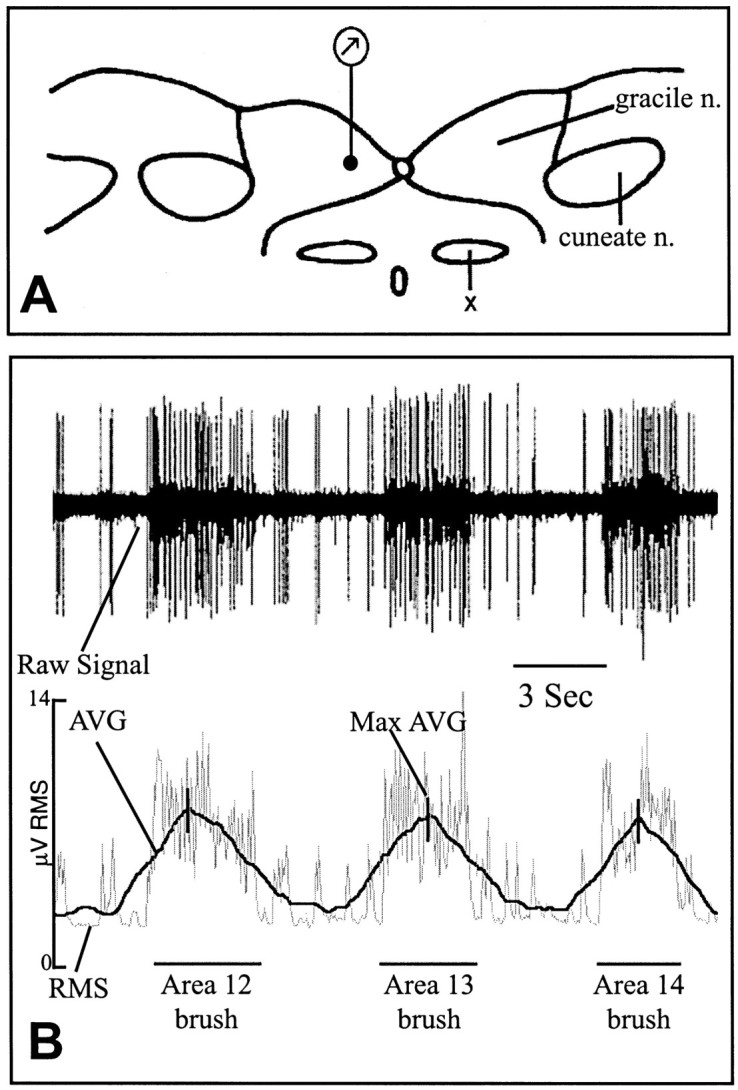
Recording site and data analysis.A, Diagram of a transverse section through the rat brainstem at the level of obex indicating the single recording site (dot at bottom of recording symbol), which was 200 μm lateral to the midline and 200 μm ventral from the surface. X, Nucleus of the solitary tract. B, RMS calculation from the raw multi-unit signal. The top line is a raw multi-unit recording from one rat during skin stimulation of regions 12–14 (see Fig. 1A,B). Thebottom line illustrates and labels the components of the analysis program (see Materials and Methods). The Max AVG generated during each stimulus is demarcated by a vertical slash mark.
Stimulus protocol
Spontaneous background activity was recorded for 10–15 min before any stimulation to establish baseline activity levels. Then, each of the 20 skin regions shown in Figure 1B was gently brushed for 3–6 sec, allowing 5–10 sec between stimuli. Next, after ∼5 min without stimulation, a series of five visceral stimuli were delivered ∼5 min apart (Fig. 1C,D). First, the ipsilateral uterine horn was distended (0.1 ml for 30 sec), then the contralateral uterine horn (0.1 ml for 30 sec), followed by distention of the vaginal canal (1 ml for 30 sec) and then the colon (1.5 ml for 30 sec). Finally, a lubricated cotton-tip applicator was inserted deep into the vaginal canal, and firm pressure was applied against the cervix for 30 sec. After another ∼5 min period without stimulation, each of the 20 skin regions was restimulated.
These stimuli can be classified as either noxious or innocuous, as follows. The gentle brush stimulus to the skin was innocuous. The colon stimulus was approximately the size of a large fecal bolus and therefore innocuous. The uterine stimulus evokes escape responses in the unanesthetized rat during M and D but not P and E (Bradshaw et al., 1999). Therefore, this stimulus is noxious during M and D but innocuous during P and E. The vaginal stimulus evokes escape responses in all estrous stages (Bradshaw et al., 1999); therefore, it is always noxious. Cervix stimulation was delivered at an intensity that would evoke lordosis in an awake rat when she is in proestrus but not in the other stages. This single stimulus would not be considered aversive, although the stimulus a female rat receives during multiple intromissions and ejaculation sometimes is (Komisaruk, 1978).
Data analysis
Responses to skin stimulation. Using locally developed hardware and software, the amplitude of the multi-unit signal was quantified using root mean square (RMS) (Counts, 1976) as a measure of total activity. The RMS detector had a bandpass from 1.0 to 170 kHz and a time constant of 50 msec. The analog RMS value was sampled 120 times per second with a 12-bit analog-to-digital converter. Groups of six samples were summed into 50 msec bins, thereby providing 20 data points each second for storage on disk. For data analysis, a 3 sec running average (AVG) was computed for each 50 msec of data. A maximum average (Max AVG) was then calculated as the AVG of the 3 sec period (60 bins) that had the greatest total activity in any given time segment. To provide a measure of the response to brush, the Max AVG during a segment of baseline was subtracted from the Max AVG during a 5 sec segment of brush stimulation for each of the 20 cutaneous regions. Figure 2B illustrates this analysis protocol.
The response values for each stimulus area from the six rats in each estrous stage were averaged and compared across estrous stages. The data were analyzed using repeated measures ANOVA and post hoc Fisher's least significant difference comparisons, with significance set at p ≤ 0.05.
Responses to visceral stimulation. The Spike2 analysis system by Cambridge Electronics Design (Cambridge, UK) was used to isolate single units and to measure their responses. Single units were identified from the multi-unit activity when a rigorous template of the waveform for each unit could be followed throughout an entire experiment; a waveform was required to be within 65–70% of a specific shape and 10–15% of a specific amplitude. All single units that fulfilled these criteria were used in the analysis (two to four per rat). A response was defined as a ≥40% change in frequency in either direction from the average baseline frequency 3–5 min before stimulus onset. The latency to this response was also measured. Examples of some of these responses are shown in Figure 4.
Fig. 4.
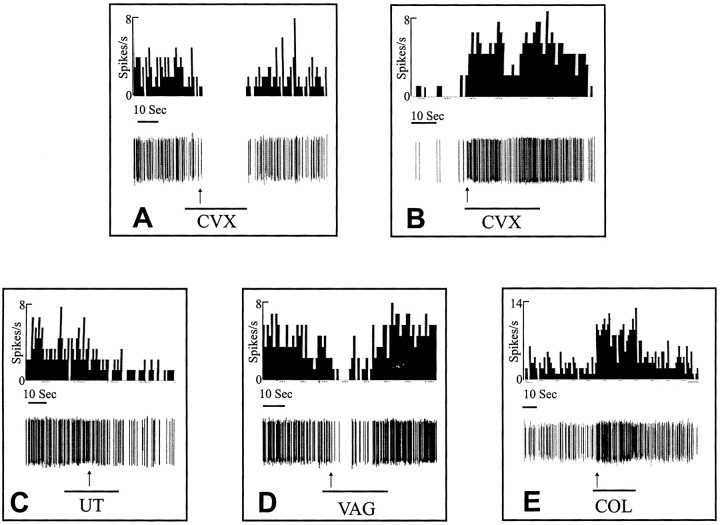
Examples of single-unit responses from five different experiments. The unit in A was from a rat in proestrus and responded by inhibition to cervix pressure (CVX). The unit in B was from a rat in estrus and responded by excitation to cervical pressure. The unit in C was from a rat in diestrus and responded to uterine distention (UT) by inhibition. The unit in D was from a rat in proestrus and responded to vaginal distention (VAG) by inhibition. The unit inE was from a rat in metestrus and responded to colon distention (COL) by excitation. The top lines are frequency histograms for each unit. The middle lines are the discriminated representation of the single unit, and the bottom lines indicate the stimulus. Thearrows indicate latency to response, which was 11 sec for A, 1 sec for B, 13 sec forC, 6 sec for D, and 2 sec forE.
A subset of the isolated single units responded to stimulation of at least one of the four visceral organs: one or both uterine horns, vaginal canal, colon, or cervix. Percentages and latencies of these responses were quantified and compared across the estrous cycle. The percentage of total units responding in each estrous stage and the percentage of inhibitory verses excitatory responses were analyzed using Kruskal–Wallis H test with post hocMann–Whitney U with p ≤ 0.05. Latencies were analyzed using three-way ANOVA with p ≤ 0.05.
RESULTS
Multi-unit responses to tactile stimulation of the hindquarters
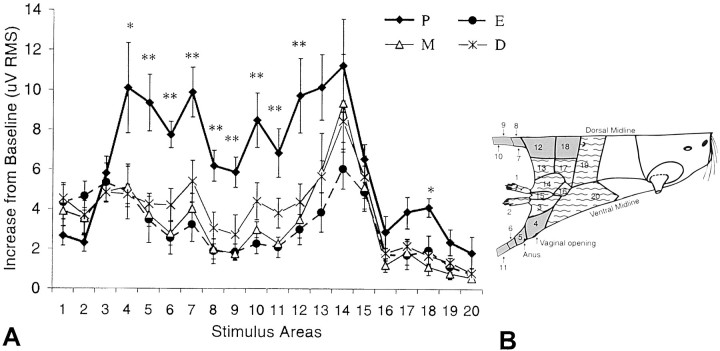
Multi-unit responses to brushing of the hindquarters were always excitatory and stimulus-bound (Fig. 2B). The magnitude of responses to brushing the perineum, hip, and tail (Fig.3B, shaded areas) was significantly greater during the afternoon of proestrus than during all other estrous stages (Fig. 3A). In contrast, the magnitude of responses to brushing the foot, leg, knee, and abdomen (Fig. 3B, dashed areas) did not vary with estrous stage (Fig. 3A). The magnitude of responses to brushing the hindquarters tested before and after visceral stimulation did not differ from each other (data not shown).
Fig. 3.
Multi-unit responses to skin stimulation.A, The Max AVG values of multi-unit responses in each stage of estrous (D, P, E, and M) to stimulation of each of the 20 demarcated skin regions of the hindquarters (see Materials and Methods; Figs. 1B, 2A). *p ≤ 0.05, **p ≤ 0.01. Data are shown as mean ± SEM. B, Shaded areas correspond to those regions whose stimulation produced a significantly greater magnitude of response in P, and the dashed areas correspond to those regions whose stimulation failed to produce estrous changes in response magnitude.
Single-unit responses to pelvic visceral stimulation
Seventy single units were isolated from the multi-unit records of all 24 subjects (two to four units per rat; D, 18; P, 20; E, 15; M, 17). Their mean background activity (in Hertz) did not vary with estrous stage (D, 1.09 ± 0.51; P, 1.22 ± 0.35; E, 1.19 ± 0.53; M, 1.42 ± 0.50). Of the 70 units (all of which responded to tactile stimulation), 56 (80%) also responded to stimulation of at least one viscus (Table 1). This percentage did not vary with estrous stage (D, 83%; P, 85%; E, 73%; M, 76%). Responses to visceral stimulation were either inhibitory or excitatory, sometimes included long-lasting excitatory afterdischarges or long-lasting inhibition, and had wide variations in latency (Fig.4).
Table 1.
Responses of single units to visceral stimulation in different estrous stages
| Estrous stage | # of units responsive to any visceral stimulus | Cervix pressure | Vaginal distention | Uterine distention | Colon distention |
|---|---|---|---|---|---|
| D | 15 | 9 (60%) | 6 (40%) | 12 (80%) | 6 (40%) |
| P | 17 | 14 (82%) | 5 (30%) | 11 (65%) | 6 (35%) |
| E | 11 | 7 (64%) | 7 (64%) | 4 (36%)* | 4 (36%) |
| M | 13 | 7 (54%) | 6 (46%) | 9 (70%) | 4 (31%) |
| Total n(%) | 56 | 37 (66%) | 24 (43%) | 36 (64%) | 20 (36%) |
P < 0.05, significantly less than D.
Cervix
Although the percentage of units responding to pressure on the cervix was the same across estrous stage (Table 1), the characteristics of the responses changed in direction and latency (Fig.5A, Table2). The percentage of units with inhibitory responses was significantly higher in P than in E and M. [Likewise, the percentage of units with excitatory responses was significantly less in P than in E and M (Fig. 5A).] Response latencies were significantly longer in D and P than in E and M (Table 2).
Fig. 5.
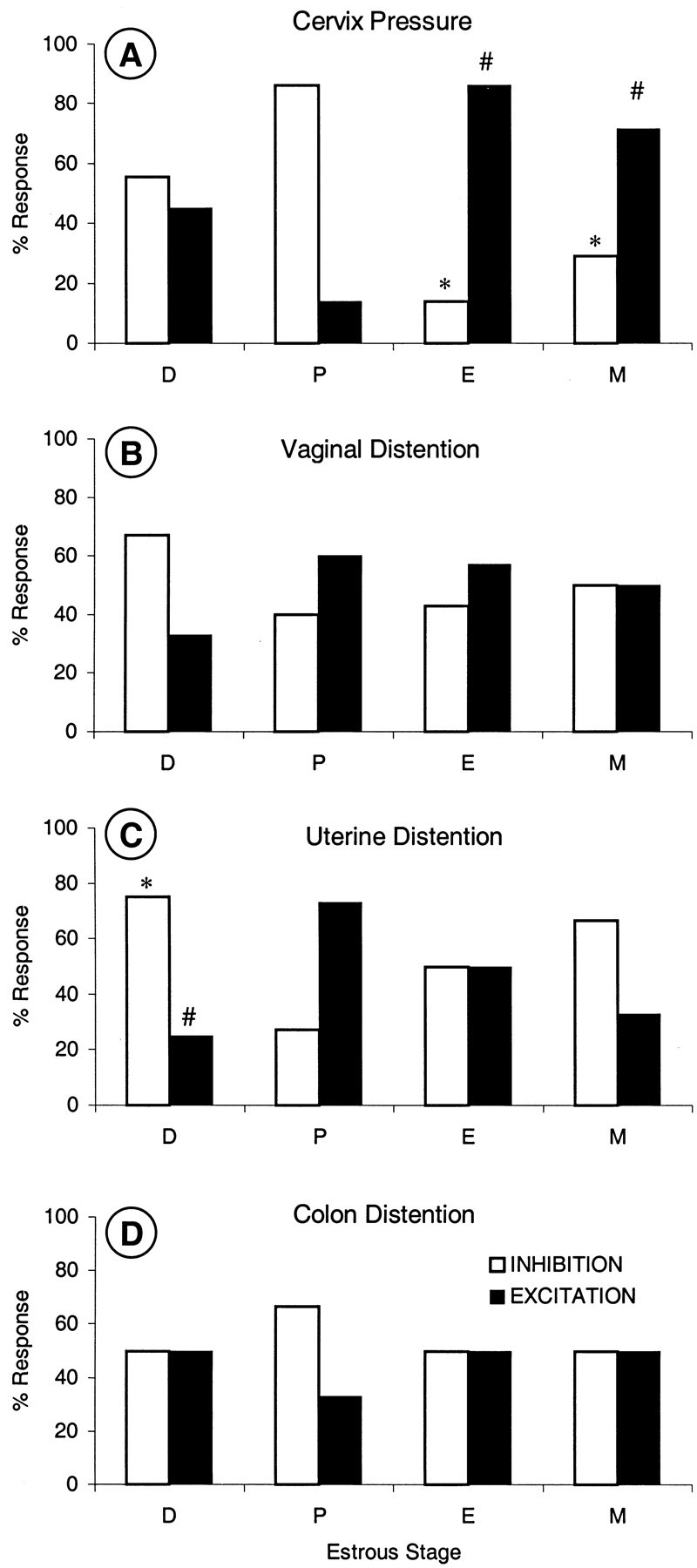
Single-unit responses to visceral stimulation. Percent inhibitory and excitatory responses to stimulation of cervix (A), vaginal canal (B), uterine horn (C), and colon (D) in each stage of estrous (D, P, E, and M). * and #p ≤ 0.05, significantly different from P.
Table 2.
Latency of response (in seconds) to each visceral stimulus in each estrous stage
| Estrous stage | Cervix pressure | Vaginal distention | Uterine distention | Colon distention |
|---|---|---|---|---|
| D | 16.7 ± 3.1 | 13.1 ± 4.1* | 13.1 ± 2.5 | 9.0 ± 3.7 |
| P | 14.0 ± 3.2 | 3.2 ± 1.7 | 7.8 ± 2.6 | 6.1 ± 1.8 |
| E | 3.5 ± 2.3* | 3.7 ± 1.6 | 16.5 ± 6.0 | 12.2 ± 6.6 |
| M | 3.4 ± 1.9* | 7.1 ± 3.7 | 9.8 ± 3.1 | 3.2 ± 1.4 |
p ≤ 0.05, significantly different from P. Data are represented as mean ± SEM.
Vaginal distention
The percentage of units responding to vaginal distention was the same across estrous stage (Table 1), as were the inhibitory or excitatory characteristics of those responses (Fig. 5B). However, response latencies were significantly longer in D than in P and E (Table 2).
Uterine distention
The percentage of units responding to uterine distention in D (80%) was significantly greater than in E (36%) (Table 1), whereas the direction of the responses shifted between D and P (Fig.5C). The percentage of units with inhibitory responses was significantly greater in D than in P. [Likewise, the percentage of units with excitatory responses was significantly less in D than in P (Fig. 5C).] Response latencies did not change (Table2).
Colon distention
The percentage of neurons responding to distention of the colon was the same across estrous stage (Table 1), as were the percentages of inhibitory and excitatory responses (Fig. 5D) and latencies (Table 2).
The changes in magnitude of response, direction, and latency described above are summarized in Table 3, which shows that changes occurred to stimulation of perineum, hip, tail and reproductive structures, but not the feet, leg, abdomen, and colon, between D and P or between P and E. In other words, all changes occurred in association with P.
Table 3.
Summary of estrous changes in responses of NG neurons to hindquarter skin and pelvic visceral stimulation
| Cervix pressure | Vaginal distention | Uterus distention | Colon distention | Brush of perineum, hip, tail | Brush of foot, leg, abdomen | |
|---|---|---|---|---|---|---|
| Magnitude of response | — | — | — | — | greatest in P | ∅ |
| Response shifts from inhibition to excitation | P → E | ∅ | D → P | ∅ | — | — |
| Latency shifts from long to short | P → E | D → P | ∅ | ∅ | — | — |
—, Not tested; ∅, no change.
DISCUSSION
The present results showed that there are significant estrous variations in responses of NG neurons to both skin and pelvic visceral stimulation. Observations of plasticity in NG are not new. Others have found that receptive fields, response properties, c-Fos expression, or levels of neuropeptides in NG change after peripheral nerve and spinal cord transections, colon inflammation, or administration of GABA antagonists (Millar et al., 1976; Dostrovsky and Millar, 1977; MacMahon and Wall, 1983; Pettit and Schwark, 1993;Berkley and Hubscher, 1995; Al-Chaer et al., 1996; Schwark et al., 1998; Ma and Bisby, 1999).
What is important here, however, is that the response variations occurred in association with natural cyclical events rather than a deliberate experimental manipulation. A specific feature of the variations was that the changes occurred across the period of diestrus through estrus (i.e., proestrus, Table 3). As described in the introductory remarks, this interval spans ovulation, during which the female rat also exhibits substantive changes in her social and reproductive behaviors.
Sources and mechanisms of estrous variation in responses
Responses to skin stimulation
One possible source of the increase in magnitude of responses to stimulation of the skin of the perineum, hip, and tail during proestrus are changes in the sensitivity of afferent fibers in the pudendal nerve. These afferents innervate the skin of the perineum and tail (Peters et al., 1987) and project directly to the NG area studied here (Ueyama et al., 1987), and their receptive fields increase during the afternoon of proestrus (Adler et al., 1977). Of relevance is that a similar increase in receptive field size of pudendal afferents occurs when ovariectomized rats are given estrogen replacement (Komisaruk et al., 1972; Kow and Pfaff, 1973). This finding suggests that part of the basis for the increased responses of NG neurons during the afternoon of proestrus are the increases in estrogen levels that occur at that time (Freeman, 1994), possibly acting on dorsal root ganglion cells, whose estrogen receptors also increase with estrogen replacement (Taleghany et al., 1999).
Responses to visceral stimulation
Mechanisms that might underlie estrous changes in the responses of NG neurons to pelvic visceral stimulation are less evident. Although sensory neurons that innervate the pelvic viscera express estrogen receptors (Papka et al., 1997) and show estrous variations in responses to distention of the vaginal canal and uterus (Robbins et al., 1992), it seems unlikely that these afferents are a major source of visceral response variations in NG. Part of the reason for this conclusion is that the pattern of estrous changes in the afferent fibers differs from those found here in NG. For example, whereas the percentage of neurons in NG responding to vaginal distention did not vary with estrous stage and their response latency was longer in D than in P and E, the response threshold of fibers in the pelvic nerve to distention of the vaginal canal was lower in P than in the other three stages (Robbins et al., 1992). The main reason, however, is that the overall percentages of responses of NG neurons to visceral stimulation did not change with estrous stage. Instead, the character of the responses to stimulation of reproductive organs, but not the colon, changed, i.e., inhibition versus excitation and latency. Such changes suggest that the main sources of the estrous variation are likely within the CNS.
Because α or β estrogen receptors have not been found in NG (Shughrue et al., 1997), it seems unlikely the changes are attributable to the actions of estrogen within NG itself. On the other hand, estrogen receptors in the lumbrosacral region of the dorsal horn increase in density during proestrus (Amandusson et al., 1995;Williams et al., 1997). Thus, one source of the changes in NG might be second-order neurons from the spinal cord conveying visceral information either directly or indirectly via other areas in the brain to NG (Berkley and Hubscher, 1995; Al-Chaer et al., 1996), assuming these second-order neurons express estrogen receptors. What is most difficult to explain, however, are potential mechanisms that would produce different changes in both direction and latency of responses to reproductive stimulation of different reproductive organs but not the colon. Given the long latencies of response of NG neurons and that the activity or neurochemistry of many regions in the brain and spinal cord varies with reproductive status (McEwen and Alves, 1999), it would appear that the complex changes in NG are likely the end result of multiple influences from different sources eventually acting on NG neurons.
Functional significance
Tactile stimulation of the female rat's perineum, legs, and tail, but not the rest of the hindquarters, or mechanical stimulation of the vagina and cervix evoke an important mating posture called lordosis primarily when the rat is fertile (i.e., during proestrus), with the combination of both types of stimuli being most effective (Komisaruk and Diakow, 1973; Kow et al., 1979). The similarity of estrous changes in the ability to evoke this posture to estrous changes in responses of NG neurons to tactile stimulation of perineum, hip, and tail, but not the rest of the hindquarters, and to stimulation of reproductive organs, but not the colon, support the hypothesis discussed in the introductory remarks that NG is part of the neural circuitry for lordosis (Schwartz-Giblin et al., 1989). The facts that NG neurons project to the inferior olive and cerebellum (Berkley et al., 1986) and that neurons in both regions become more excited, rhythmic, and coordinated during the time of sexual receptivity (Smith, 1995, 1998;Smith and Chapin, 1996a,b) further suggest that NG is an important component of the sensorimotor neural circuitry that modulates sexual behaviors in general.
Although the involvement of NG in sensorimotor integration is not a new concept (Wall and Dubner, 1972), its involvement in pain has recently come under discussion, as a component of either an ensemble of systems that mutually and dynamically contribute to various somatovisceral experiences, including pain (Berkley and Hubscher, 1995; Berkley, 1997,1998), or a pelvic “visceral pain pathway” (Willis et al., 1999).
It is therefore relevant to compare the results obtained here with results from previous studies in which estrous changes in behavioral nociceptive escape responses to some of the same stimuli were measured (Bradshaw et al., 1999). For vaginal stimulation, the behavioral studies showed that high pressures of vaginal distention in the unanesthetized rat give rise to significantly more escape responses during diestrus than during proestrus and estrus. These behavioral results correlate to some extent with responses of NG neurons in that response latencies of the neurons to vaginal distention were significantly shorter during proestrus and estrus than during diestrus (Table 2). For uterine stimulation (0.l ml distention), the behavioral studies showed that this same stimulus gives rise to escape responses during metestrus and diestrus but not proestrus and estrus. This pattern did not occur for responses of NG neurons (i.e., M and D different from P and E). Instead, uterine distention was more likely to evoke neuronal responses in diestrus than in estrus (Table 1), with no differences between proestrus, estrus, and metestrus, and the responses were more likely to be inhibitory in diestrus than in proestrus (Fig.5C, Table 3). Thus, although some of the estrous variations in behavioral escape and neuronal responses appear correlated (responses to vaginal stimulation), others do not (responses to uterine stimulation). Such findings indicate that the contribution of NG to visceral nociception changes under different physiological conditions.
Of further relevance to this discussion are results from an extensive series of studies showing that the same vaginocervical stimulation that evokes lordosis also produces an increase in nociceptive thresholds to noxious cutaneous stimuli, an effect termed vaginocervical- or mating-induced analgesia (Komisaruk and Whipple, 1995). Both lordosis and analgesia are hypothesized to be brought about by a common mechanism involving complex interactions among central neural regions that process information from hindquarter skin, muscles, the vagina, and cervix (Komisaruk, 1978; Komisaruk and Whipple, 1995). The convergence of pelvic somatic and visceral information in NG neurons together with the estrous changes in their responses observed in the present study suggest that NG is a component of this common mechanism.
Conclusions
The functional significance of the recently discovered visceral–somatic and visceral–visceral convergence within NG is not clear. The present findings showing how physiological changes brought about by the ovarian cycle influence the activity of its neurons support the hypothesis of the involvement of NG in the regulation of reproductive behaviors. Comparison of the findings with behavioral data and vaginocervix-induced cutaneous analgesia support its involvement as well in nociceptive modulation, both somatic and visceral. Together, these considerations indicate that NG is a component of an ensemble of systems that mutually and dynamically contribute to a variety of somatovisceral experiences, including pain.
Footnotes
This work was supported by National Institutes of Health Grant RO1-NS11892. We thank Paul Hendricks and Ross Henderson for hardware and software production, John Chalcraft for help with illustration, and Michael Torlone, Elizabeth Wood, and Jennifer Temple for various constructive contributions to this project.
Correspondence should be addressed to Dr. Karen J. Berkley, Program in Neuroscience, Florida State University, Tallahassee, FL 32306-1270. E-mail: kberkley@psy.fsu.edu.
REFERENCES
- 1.Adler NT, Davis PG, Komisaruk BR. Variation in the size and sensitivity of a genital sensory field in relation to the estrous cycle in rats. Horm Behav. 1977;9:334–344. doi: 10.1016/0018-506x(77)90068-x. [DOI] [PubMed] [Google Scholar]
- 2.Ainsworth A, Dostrovsky JO, Merrill EG, Millar J. An improved method for insulating tungsten micro-electrodes with glass. J Physiol (Lond) 1977;269:4P–5P. [PubMed] [Google Scholar]
- 3.Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Pelvic visceral input into the nucleus gracilis is largely mediated by the postsynaptic dorsal column pathway. J Neurophysiol. 1996;76:2675–2690. doi: 10.1152/jn.1996.76.4.2675. [DOI] [PubMed] [Google Scholar]
- 4.Amandusson A, Hermanson O, Blomqvist A. Estrogen receptor-like immunoreactivity in the medullary and spinal dorsal horn of the female rat. Neurosci Lett. 1995;196:25–28. doi: 10.1016/0304-3940(95)11828-k. [DOI] [PubMed] [Google Scholar]
- 5.Berkley KJ. On the dorsal columns: translating basic research hypotheses to the clinic. Pain. 1997;70:103–107. [PubMed] [Google Scholar]
- 6.Berkley KJ. Lesions of the dorsal columns: definitely disturbing. Pain Forum. 1998;7:119–124. [Google Scholar]
- 7.Berkley KJ, Hubscher CH. Are there separate central nervous system pathways for touch and pain? Nat Med. 1995;1:766–773. doi: 10.1038/nm0895-766. [DOI] [PubMed] [Google Scholar]
- 8.Berkley KJ, Budell RJ, Blomqvist A, Bull M. Output systems of the dorsal column nuclei in the cat. Brain Res. 1986;396:199–225. doi: 10.1016/0165-0173(86)90012-3. [DOI] [PubMed] [Google Scholar]
- 9.Bradshaw HB, Temple JL, Wood E, Berkley KJ. Estrous variations in behavioral responses to vaginal and uterine distention in the rat. Pain. 1999;82:187–197. doi: 10.1016/S0304-3959(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 10.Counts L. Root mean square. In: Sheingold DH, editor. Nonlinear circuits handbook: designing with analog function modules and ICs. Analog Devices; Norwood, MA: 1976. pp. 389–416. [Google Scholar]
- 11.Dostrovsky JO, Millar J. Receptive fields of gracile neurons after transection of the dorsal columns. Exp Neurol. 1977;56:610–621. doi: 10.1016/0014-4886(77)90324-7. [DOI] [PubMed] [Google Scholar]
- 12.Erskine MS. Solicitation behavior in the estrous female rat: a review. Horm Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- 13.Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neil JD, editors. The physiology of reproduction, Vol 2, Ed 2. Raven; New York: 1994. pp. 129–148. [Google Scholar]
- 14.Hubscher CH. Sensory input from pelvic reproductive organs to the gracile and solitary nuclei in the female rat. PhD thesis. Florida State University; 1994. Responses of neurons in the gracile nucleus of female rats to stimulation of internal pelvic reproductive organs. [Google Scholar]
- 15.Komisaruk BR. The nature of the neural substrate of female sexual behaviour in mammals and its hormonal sensitivity: review and speculations. In: Hutchison JB, editor. Biological determinants of sexual behavior. Wiley; New York: 1978. pp. 349–393. [Google Scholar]
- 16.Komisaruk BR, Diakow C. Lordosis reflex intensity in rats in relation to the estrous cycle, ovariectomy, estrogen administration and mating behavior. Endocrinology. 1973;93:548–557. doi: 10.1210/endo-93-3-548. [DOI] [PubMed] [Google Scholar]
- 17.Komisaruk BR, Whipple B. The suppression of pain by genital stimulation in females. Ann Rev Sex Res. 1995;6:151–186. [Google Scholar]
- 18.Komisaruk BR, Adler NT, Hutchison J. Genital sensory field: enlargement by estrogen treatment in female rats. Science. 1972;178:1295–1298. doi: 10.1126/science.178.4067.1295. [DOI] [PubMed] [Google Scholar]
- 19.Kow LM, Pfaff DW. Effects of estrogen treatment on the size of receptive field and response threshold of pudendal nerve in the female rat. Neuroendocrinology. 1973;13:299–313. doi: 10.1159/000122214. [DOI] [PubMed] [Google Scholar]
- 20.Kow LM, Montgomery MO, Pfaff DW. Triggering of lordosis reflex in female rats with somatosensory stimulation: quantitative determination of stimulus parameters. J Neurophysiol. 1979;42:195–202. doi: 10.1152/jn.1979.42.1.195. [DOI] [PubMed] [Google Scholar]
- 21.Long JA, Evans HM. The oestrous cycle in the rat and its associated phenomena. Mem Univ Calif. 1922;6:1–128. [Google Scholar]
- 22.Ma W, Bisby MA. Ultrastructural localization of increased neuropeptide immunoreactivity in the axons and cells of the gracile nucleus following chronic constriction injury of the sciatic nerve. Neuroscience. 1999;93:335–348. doi: 10.1016/s0306-4522(99)00145-1. [DOI] [PubMed] [Google Scholar]
- 23.MacMahon SB, Wall PD. Plasticity in the nucleus gracilis of the rat. Exp Neurol. 1983;80:195–207. doi: 10.1016/0014-4886(83)90016-x. [DOI] [PubMed] [Google Scholar]
- 24.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 25.Millar J, Basbaum AI, Wall PD. Restructuring of the somatotopic map and appearance of abnormal neuronal activity in the gracile nucleus after partial deafferentation. Exp Neurol. 1976;50:658–672. doi: 10.1016/0014-4886(76)90035-2. [DOI] [PubMed] [Google Scholar]
- 26.Papka RE, Srinivasan B, Miller KE, Hayashi S. Localization of estrogen receptor protein and estrogen receptor messenger RNA in peripheral autonomic and sensory neurons. Neuroscience. 1997;79:1153–1163. doi: 10.1016/s0306-4522(97)00076-6. [DOI] [PubMed] [Google Scholar]
- 27.Peters LC, Kristal MB, Komisaruk BR. Sensory innervation of the external and internal genitalia of the female rat. Brain Res. 1987;408:199–204. doi: 10.1016/0006-8993(87)90372-6. [DOI] [PubMed] [Google Scholar]
- 28.Pettit MJ, Schwark HD. Receptive field reorganization in dorsal column nuclei during temporary denervation. Science. 1993;262:2054–2056. doi: 10.1126/science.8266104. [DOI] [PubMed] [Google Scholar]
- 29.Pfaff DW. Hormones, genes, and behavior. Proc Natl Acad Sci USA. 1997;94:14213–14216. doi: 10.1073/pnas.94.26.14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins A, Berkley KJ, Sato Y. Estrous cycle variation of afferent fibers supplying reproductive organs in the female rat. Brain Res. 1992;596:353–356. doi: 10.1016/0006-8993(92)91572-v. [DOI] [PubMed] [Google Scholar]
- 31.Schwark HD, Petit MJ, Fuchs JL. Distribution of substance P receptor binding in dorsal column nuclei of rat, cat, monkey and human. Brain Res. 1998;786:259–262. doi: 10.1016/s0006-8993(97)01436-4. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz-Giblin S, McEwen BS, Pfaff DW. Mechanisms of Female reproductive behavior. In: Brush FR, Levine S, editors. Psychoendocrinology. Academic; San Diego: 1989. pp. 41–104. [Google Scholar]
- 33.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Smith SS. Sensorimotor-correlated discharge recorded from ensembles of cerebellar Purkinje cells varies across the estrous cycle of the rat. J Neurophysiol. 1995;74:1095–1108. doi: 10.1152/jn.1995.74.3.1095. [DOI] [PubMed] [Google Scholar]
- 35.Smith SS. Estrous hormones enhance coupled, rhythmic olivary discharge in correlation with facilitated limb stepping. Neuroscience. 1998;82:83–95. doi: 10.1016/s0306-4522(97)00211-x. [DOI] [PubMed] [Google Scholar]
- 36.Smith SS, Chapin JK. The estrous cycle and the olivo-cerebellar circuit. I. Contrast enhancement of sensorimotor-correlated cerebellar discharge. Exp Brain Res. 1996a;111:371–384. doi: 10.1007/BF00228726. [DOI] [PubMed] [Google Scholar]
- 37.Smith SS, Chapin JK. The estrous cycle and the olivo-cerebellar circuit. II. Enhanced selective sensory gating of responses from the rostral dorsal accessory olive. Exp Brain Res. 1996b;111:385–392. doi: 10.1007/BF00228727. [DOI] [PubMed] [Google Scholar]
- 38.Taleghany N, Sarajari S, DonCarlos LL, Gollapudi L, Oblinger MM. Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. J Neurosci Res. 1999;57:603–615. [PubMed] [Google Scholar]
- 39.Ueyama T, Arakawa H, Mizuno N. Central distribution of efferent and afferent components of the pudendal nerve in rat. Anat Embryol (Berl) 1987;177:37–49. doi: 10.1007/BF00325288. [DOI] [PubMed] [Google Scholar]
- 40.Wall PD, Dubner R. Somatosensory pathways. Annu Rev Physiol. 1972;34:315–336. doi: 10.1146/annurev.ph.34.030172.001531. [DOI] [PubMed] [Google Scholar]
- 41.Williams SJ, Chung K, Om AS, Papka RE. Cytosolic estrogen receptor concentrations in the lumbosacral spinal cord fluctuate during the estrous cycle. Life Sci. 1997;61:2551–2559. doi: 10.1016/s0024-3205(97)01009-6. [DOI] [PubMed] [Google Scholar]
- 42.Willis WD, Al-Chaer ED, Quast MJ, Westlund KN. A visceral pain pathway in the dorsal column of the spinal cord. Proc Natl Acad Sci USA. 1999;96:7675–7679. doi: 10.1073/pnas.96.14.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]