Abstract
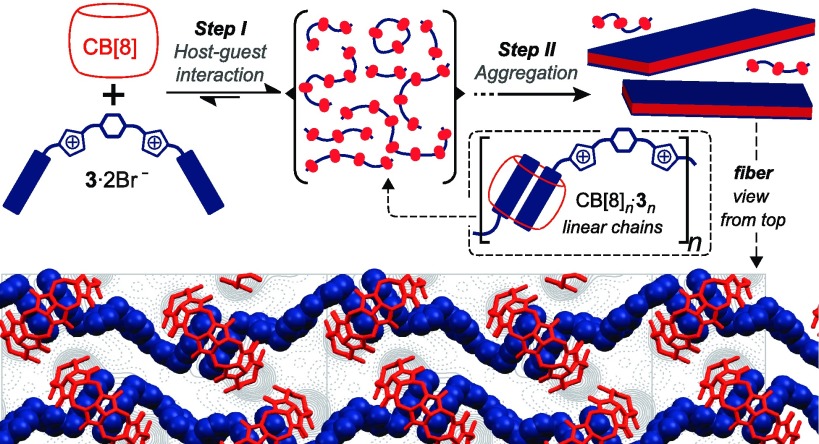
The binding of imidazolium salts to cucurbit[8]uril, CB[8], triggers a stepwise self-assembly process with semiflexible polymer chains and crystalline nanostructures as early- and late-stage species, respectively. In such a process, which involves the crystallization of the host–guest complexes, the guest plays a critical role in directing self-assembly toward desirable morphologies. These include platelet-like aggregates and two-dimensional (2D) fibers, which, moreover, exhibit viscoelastic and lyotropic properties. Our observations provide a deeper understanding of the self-assembly of CB[8] complexes, with fundamental implications in the design of functional 2D systems and crystalline materials.
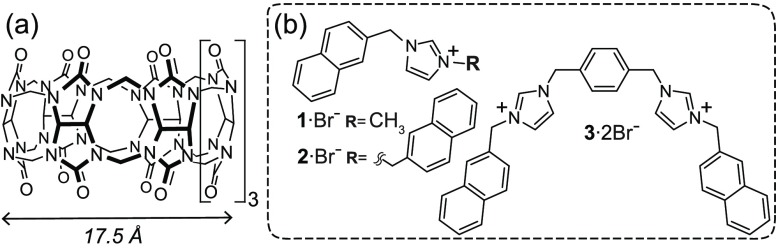
The solution self-assembly of (macro)molecular building blocks provides a convenient pathway to a wide variety of nanoscale structures of broad utility.1 Notable examples include the aggregation of surfactants and amphiphilic block copolymers into micelles of various morphologies,2 as well as the assembly of specific DNA sequences into well-defined nanostructures.3 The architecture and functionality of such assemblies depend on both the molecular design of their individual constituents and the method for nanoscale aggregation. Emergent molecular building blocks can unveil alternative routes for the creation of functional assemblies and strategies to navigate multifaceted equilibria. One such system is the inclusion complexes of imidazolium salts and cucurbit[n]urils, CB[n]s (Chart 1).4 Our group has reported the binding of 1-methyl-3-[(naphthalen-2-yl)methyl]-imidazolium bromide (1 in Chart 1) to CB[8] to form a CB[8]·12 homoternary complex.5a More recently, other groups have reported the preparation of semiflexible CB[8]-based supramolecular polymers by exploiting varied naphthalene-containing guest molecules.5b
Chart 1. Structures of CB[8] and Imidazolium Guests 1–3.
Herein, we report the complexation of naphthalene-derived imidazolium salts 2 and 3 (Chart 1) with CB[8]. These specific host and guest molecules have the ability to engage in strong noncovalent interactions at the mM dilute concentrations yielding, unexpectedly, nanoscopic crystalline structures. These include platelet-like aggregates and two-dimensional (2D) nanofibers, which exhibit viscoelastic and lyotropic behavior.
Compound 2 features two equivalent CB[8]-binding naphthalene residues bridged by one imidazolium group. The CB[8] complexation of 2, similarly to that of 1, is quantitative in the mM concentration range. However, as shown by isothermal titration calorimetry (ITC), CB[8] and 2 interact in a 1:1 ratio (Figure S1). Therefore, a theoretical equilibrium between bimolecular 1:1 and n:n (n ≥ 2) linear and cyclic species may be expected for an equimolar mixture of CB[8] and 2.6 The 1H NMR spectrum of a 1:1 mixture of 2 and CB[8] contained a set of broad and ill-defined signals, which, as with many CB[8] complexes, were difficult to unambiguously assign to the presence of polymeric species.
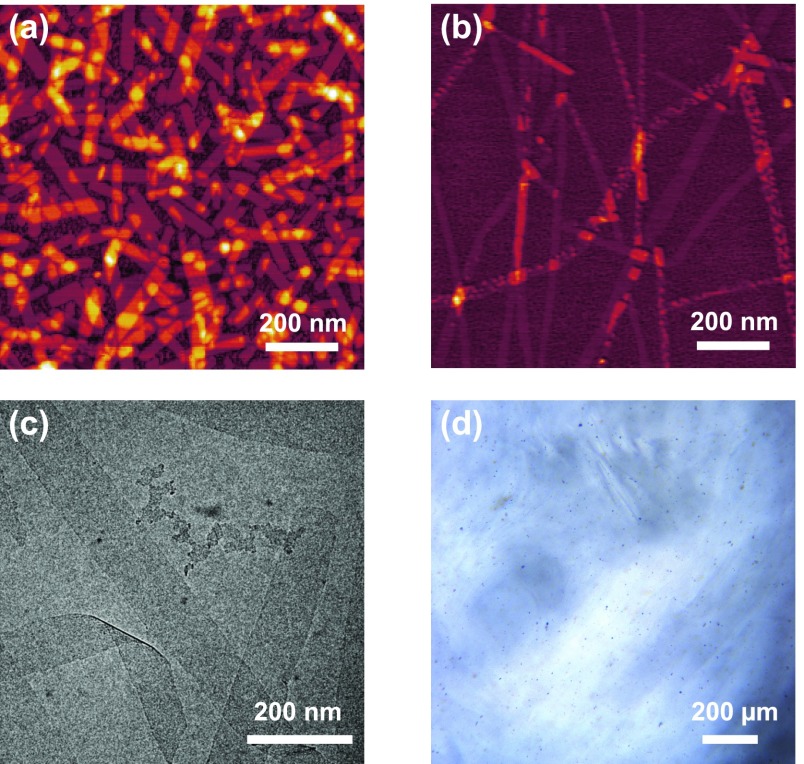
In an attempt to clarify this characteristic, a series of morphological studies were performed on 1:1 mixtures of CB[8] and 2. The combined small-angle X-ray scattering (SAXS) and static light scattering patterns of an equimolar (1.0 mM) mixture of CB[8] and 2 exhibits a clear power-law dependence of q–2 in the high-q region (0.008 Å < q < 0.1 Å), which is associated with the presence of semiflexible polymer chains of CB[8]n·2n (Figures S2 and S3).7 A radius of gyration (Rg) of ca. 30 nm was calculated by fitting the SAXS scattering intensity to the Debye function for Gaussian chains (Figures S2). Remarkably, the additional scattering at low q values (q < 0.005 Å) follows a q–1 power law (Figures S3 and S4), which is a signature of rod-like structures. These results can be rationalized by assuming that the polymer chains of CB[8]n·2n coexist with some sort of larger rod-like structures. Indeed, elongated platelet-like aggregates were observed by atomic force microscopy (AFM images in Figures 1a, S9 and S12) and transmission electron microscopy (TEM images in Figure S22). Longitudinal height profiles showed that the aggregates exhibit a thickness between 2 and 4 nm (Figure S9).
Figure 1.
AFM morphological analysis of incubated samples of CB[8]+2 (a) and CB[8]+3 (b). Cryo-TEM micrograph of a sample of the CB[8]+3 fibers (c). Optical polarized micrograph of a 1.0 wt % aqueous solution of the CB[8]+3 fibers (d).
We hypothesized that 3, which is structural analogue of 2, could also produce polymers and higher-order assemblies in the presence of CB[8]. A series of ITC experiments indicated that 3 and CB[8] preferentially interact in a 1:1 ratio (Figure S1), similar to mixtures of 2 and CB[8]. A combination of small-angle neutron scattering, AFM and TEM measurements showed the coexistence of small semiflexible polymer chains and large nanoscale assemblies in incubated samples of 3 and CB[8] (Figures 1b, 1c and S7), echoing the behavior of mixtures of 2 and CB[8].7 High aspect ratio fibers were clearly observed by AFM and TEM in aqueous equimolar (1.0 mM) mixtures of CB[8] and 3 (Figure 1b, 1c, S10 and S13).
The assembly of such small molecular weight building blocks into highly anisotropic micrometer-sized species is remarkable and prompted us to perform a series of control experiments aimed at shedding light on the fiber formation process. Samples obtained by casting aqueous solutions of 3 (1.0 mM) alone onto mica substrates were completely free from fibers (Figure S14). Fibers were also absent from samples that were prepared immediately after mixing 3 and CB[8] (Figure S15). This suggested that both 3 and CB[8] are indispensable in the formation of fibers, a process which also has an associated incubation time (vide infra). Taking all the previous results into consideration, we propose that 1:1 mixtures of 3 and CB[8] transition through a two-step self-assembly process according to Figure 2a. Initially, the rapid host–guest recognition of 3 by CB[8] yields linear, and possibly also cyclic, polymer chains (Figure 2a). At a later point, aggregation initiates which results in the formation of fibers. Such self-assembly phenomenon also applies to 1:1 mixtures of 2 and CB[8], which generate platelet-like aggregates instead of fibers.
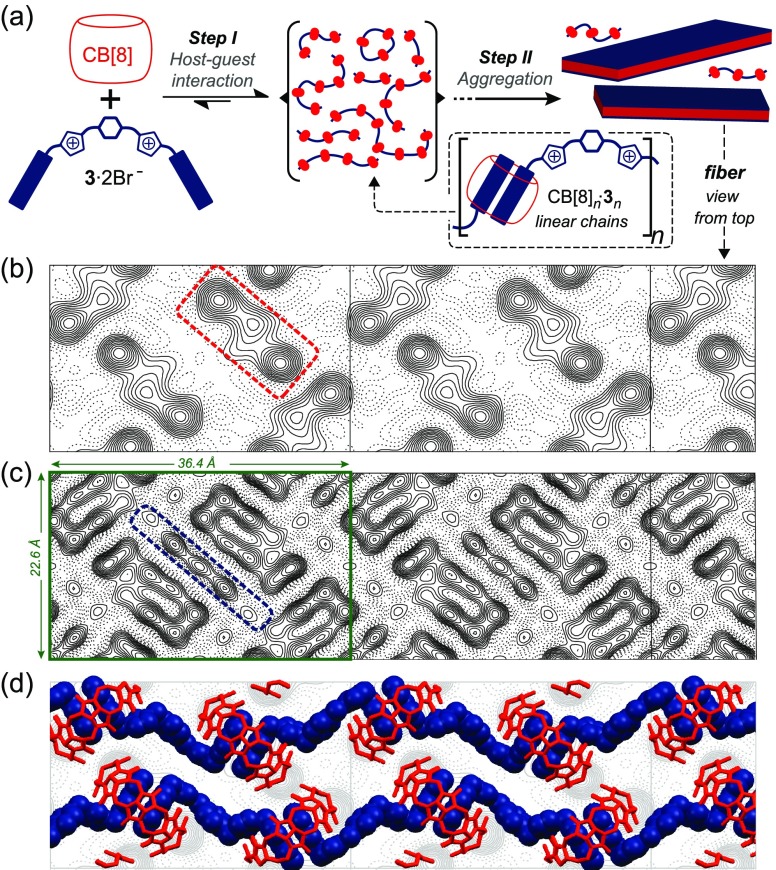
Figure 2.
Step-wise self-assembly scheme (a) of CB[8] (in red) and 3 (in blue), cryo-TEM maps at a resolution of 5 (b) and 4 Å (c), and packing model for the CB[8]+3 fibers (d) superimposed to panel b. Unit cell parameters (in green) are shown in panel c.
It has recently been reported that the binding of specific guest molecules, such as viologen and cyanostilbene derivatives, to CB[8] results in the formation of various higher-order structures including three-dimensional organic frameworks and nanobundles.8a−8e In our case, both 2 and 3, which share many similar structural characteristics (Chart 1), are able to produce large-scale aggregates in the presence of CB[8]. We hypothesize that the aggregation is possibly driven by the combination of several factors including host–guest and hydrophobic interactions, as well as attractive interactions between the CB[8] molecules themselves. Highly elongated fibers grow in 1:1 mixtures of 3 and CB[8], whereas only relatively short platelet-like aggregates are obtained from those of 2 and CB[8]. Such divergent behavior, in analogy to other supramolecular systems,8 could be associated with the hydrophilic–hydrophobic ratio of the host–guest complexes (compound 3 features two positively charged imidazolium groups whereas 2 contains only one),9 and the packing of the host and the guest molecules within the aggregates.
With the idea of investigating such packing, we then imaged the fibers by means of liquid AFM and high-resolution (HR) cryo-TEM. The fibers have a length over several microns and a typical width of ca. 40 nm. According to our liquid AFM analysis, the fiber thickness is 1.8 nm (Figures S10). Coulomb potential distribution maps obtained by HR cryo-TEM showed high density structural features which were attributed to the CB[8] molecules bound to the naphthalene residues of 3 (Figure 2b). Higher resolution maps showed additional features which were associated with the naphthalene-bridging linker of 3 (Figure 2c). A plausible packing model, considering the cryo-TEM maps and the fact that the outer diameter of CB[8] matches the thickness of the fibers,4a consists of a single-layer structure of host–guest complexes. In our model, individual CB[8]n·3n chains are laterally assembled into zigzagged strands, which run along the long fiber axis. The host molecules are organized in a “side to portal” arrangement (herringbone structure) with their equatorial planes orthogonal to the fiber main lattice. As such, adjacent CB[8]s from individual strands are capable of engaging in multiple hydrogen-bonding interactions (between the carbonyl groups of the portals and the methylene and equatorial methine groups),10 a feature which likely contributes to stitching the CB[8]n·3n chains together (Figure S28). Ion-dipole and C—H···O=C hydrogen bonding interactions between the imidazolium groups of 3 and the carbonyl portals of neighboring CB[8]s may also play an important role in reinforcing the lateral assembly of the strands.
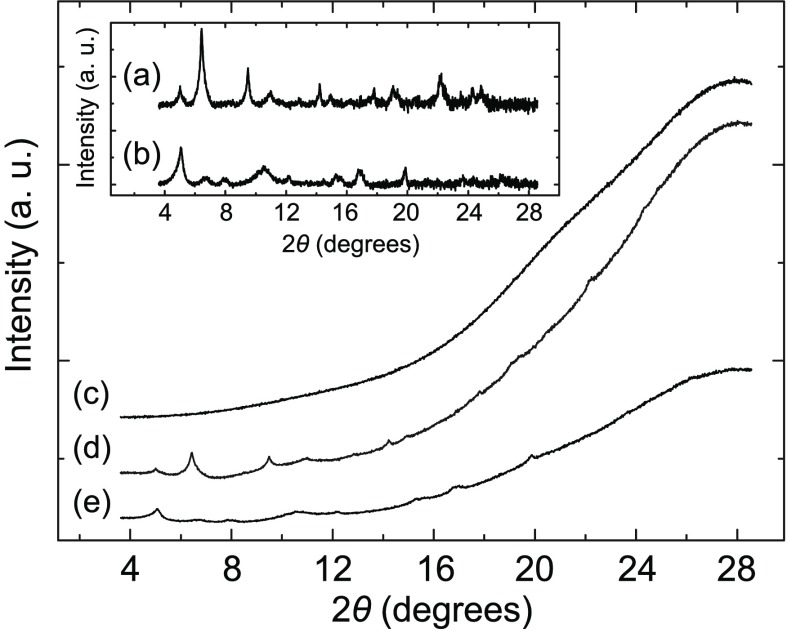
Solution-phase X-ray diffraction (XRD) experiments of samples of the fibers revealed a series of relatively sharp peaks (Figure 3), which is characteristic of crystalline materials.11 The HR cryo-TEM data matches the XRD patterns and indicates that CB[8]n·3n crystallizes in the P22121 plane group symmetry (Figure S18 and Table S1). The platelet-like aggregates are also crystalline in nature (Figure 3 and S22), and exhibit a different microstructure in comparison to the fibers. We have been, however, unable to suggest possible symmetry unit cells and parameters solely on the basis of our TEM and XRD results.
Figure 3.
XRD patterns corresponding to aqueous solutions of the CB[8]+3 fibers (a, d), the CB[8]+2 platelet-like aggregates (b, e), and neat H2O (c). For clarity, data set in the inset (a, b) has been background-subtracted.
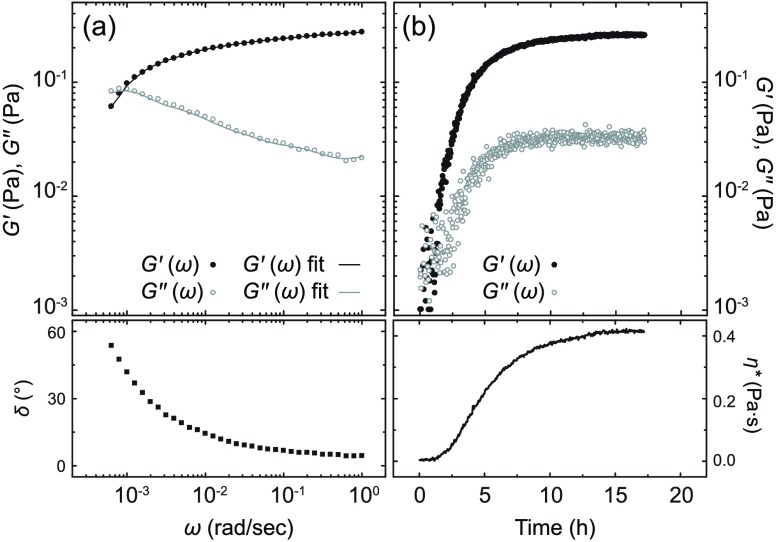
Samples of the fibers are free-flowing fluids but noticeably more viscous than samples of the platelet-like aggregates or 3 alone at analogous molar concentration. In a similar fashion to many suspensions of fibrillar aggregates,12 shear-thinning was observed in samples of the fibers (Figure S26). The linear viscoelasticity of an equimolar mixture of 3 and CB[8] (1.0 mM) after incubation was characterized by monitoring the variation of the storage (G′) and loss moduli (G″), at a constant strain, as a function of the angular frequency (Figure 4a). At 20 °C, G′ dominates over G″ above a critical frequency value ωc; whereas G′ crosses over and drops below G″ at frequency values below ωc. These trends are reminiscent of the Maxwellian behavior found in many viscoelastic worm-like micellar solutions,13 but differs from it at high angular frequency values (ω > 0.002 rad/s). When concentrated up to a dry matter content of ca. 1.0 wt %, free-flowing samples of the fibers turned into gels, which furthermore exhibited lyotropic properties (Figure 1d). Such behavior is also distinctive of many fluids containing rod-like particles.14
Figure 4.
Variation of G′, G″ and phase angle as a function of angular frequency, at 20 °C, for an equimolar (1.0 mM) mixture of CB[8] and 3 after incubation, and fits (solid lines) to the Maxwell model with three elements (a). Variation of G′, G″ η* at 20 °C as a function of time for an equimolar (1.0 mM) mixture of CB[8] and 3 (b). See Figure S25 and Table S2 for the fitting of the rheological data in (a).
The kinetics of fiber formation were also elucidated through a series of rheological measurements. We tracked the evolution of a freshly prepared equimolar mixture of 3 and CB[8] (1.0 mM) by recording the complex viscosity, η*, of the sample over time at a constant frequency and strain amplitude (Figure 4b). After an induction period, values of η* increase with time and then plateau (Figure 4b). As fibers were apparent from the morphological analysis, the initial η* increase is likely associated with the growth of the fibers, provided sufficient 3 and CB[8] are available. After the fibers reach a critical length, η* plateaus. The final values of G′, G″ and η* are stable and match those of samples subjected to an incubation period of ca. 24 h at 20 °C (see Figure 4). The kinetic data corresponding to the initial increase of the viscoelastic moduli was analyzed according to the Avrami theory and a value of the Avrami exponent (n) of approximately one was determined (Figure S27).15 Considering the existence of fibrillar aggregates, such a value for n is consistent with a one-dimensional and interfacial-controlled growth process.16
In summary, we have demonstrated how nanoscale assemblies of different morphologies and macroscopic properties can be prepared in a one-pot fashion from complementary components of subnanometer dimensions. Guest molecules 2 and 3 undergo a stepwise self-assembly process in the presence of CB[8], which yields anisotropic crystalline aggregates. Semiflexible polymer chains have been identified as intermediate species in the assembly mechanism. Micron-sized two-dimensional fibers with a thickness of a single CB[8] macrocycle (1.8 nm) are produced from 1:1 mixtures of CB[8] and 3, which is unprecedented in the field of CB[n]-based molecular recognition. The molecular designs of our individual building blocks (pairs of host and guest molecules), encode structural information which translates to significant differences in aggregate morphology. This, in turn, has important implications in the macroscopic properties of the systems as illustrated by the identification of the viscoelastic and lyotropic properties of the fibers (Figures 1d and 4).
These unique features, which arise from a combination of tight binding and crystallization-assisted self-assembly, will ensure a variety of applications. For example, supramolecular fibers made from low molecular weight components may become ideal materials as drag reducers or viscosity modifiers in applications where stimuli-responsive degradable systems are preferred over traditional high molecular weight polymers.17 Additionally, this fundamental study will enable the development of hierarchical crystalline nanostructures and functional 2D systems.
Acknowledgments
This work was supported by the EPSRC (reference no. EP/G060649/1), an ERC Starting Investigator Grant (project no. 240629, ASPiRe) and a Next Generation Fellowship from the Walters-Kundert Foundation. J.d.B. acknowledges the MINECO, the FSE and the FEDER for funding through projects RYC-2015-18471 (Ramón y Cajal program) and CTQ2017-84087-R. L.D.M. acknowledges support from a Royal Society University Research Fellowship UF160152. R.A.B. acknowledges support from the EPSRC CDT in Nanoscience and Nanotechnology (NanoDTC), grant number EP/L015978/1. The authors thank Dr. Sean Lovett for his help with the fitting of the oscillatory rheology frequency sweep data, Dr. Aurel Radulescu for his assistance with some of the SANS measurements and Dr. Silvia Hernández-Ainsa for helpful discussions.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b07506.
Experimental procedures, synthesis of guest molecules, preparation of complexes and additional characterization details (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Whitesides G. M.; Mathias J. P.; Seto C. T. Molecular Self-Assembly and Nanochemistry: a Chemical Strategy for the Synthesis of Nanostructures. Science 1991, 254, 1312–1319. 10.1126/science.1962191. [DOI] [PubMed] [Google Scholar]; b Aida T.; Meijer E. W.; Stupp S. I. Functional Supramolecular Polymers. Science 2012, 335, 813–817. 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Israelachvili J. N.; Mitchell D. J.; Ninham B. W. Theory of Self-Assembly of Hydrocarbon Amphiphiles into Micelles and Bilayers. J. Chem. Soc., Faraday Trans. 2 1976, 72, 1525–1568. 10.1039/f29767201525. [DOI] [Google Scholar]; b Laughlin R. G.The Aqueous Phase Behavior of Surfactants; Academic Press: New York, 1994. [Google Scholar]; c Jönsson B.; Lindman B.; Holmberg K.; Kronberg B.. Surfactants and Polymers in Aqueous Solution; John Wiley & Sons: West Sussex, 1998. [Google Scholar]; d Antonietti M.; Förster S. Vesicles and Liposomes: a Self-Assembly Principle beyond Lipids. Adv. Mater. 2003, 15, 1323–1333. 10.1002/adma.200300010. [DOI] [Google Scholar]; e Glotzer S. C.; Solomon M. J. Anisotropy of Building Blocks and their Assembly into Complex Structures. Nat. Mater. 2007, 6, 557–562. 10.1038/nmat1949. [DOI] [PubMed] [Google Scholar]; f Mai Y.; Eisenberg A. Self-Assembly of Block Copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. 10.1039/c2cs35115c. [DOI] [PubMed] [Google Scholar]; g Schacher F. H.; Rupar P. A.; Manners I. Functional Block Copolymers: Nanostructured Materials with Emerging Applications. Angew. Chem., Int. Ed. 2012, 51, 7898–7921. 10.1002/anie.201200310. [DOI] [PubMed] [Google Scholar]; h Kang Y.; Liu K.; Zhang X. Supra-Amphiphiles: a New Bridge between Colloidal Science and Supramolecular Chemistry. Langmuir 2014, 30, 5989–6001. 10.1021/la500327s. [DOI] [PubMed] [Google Scholar]; i Yu G.; Jie K.; Huang F. Supramolecular Amphiphiles Based on Host–Guest Molecular Recognition Motifs. Chem. Rev. 2015, 115, 7240–7303. 10.1021/cr5005315. [DOI] [PubMed] [Google Scholar]
- a Seeman N. C. Nucleic Acids Junctions and Lattices. J. Theor. Biol. 1982, 99, 237–247. 10.1016/0022-5193(82)90002-9. [DOI] [PubMed] [Google Scholar]; b Rothemund P. W. K. Folding DNA to Create Nanoscale Shapes and Patterns. Nature 2006, 440, 297–302. 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]; c Aldaye F. A.; Palmer A. L.; Sleiman H. F. Assembling Materials with DNA as the Guide. Science 2008, 321, 1795–1799. 10.1126/science.1154533. [DOI] [PubMed] [Google Scholar]; d Ke Y.; Ong L. L.; Shih W. M.; Yin P. Three-dimensional Structures Self-Assembled from DNA Bricks. Science 2012, 338, 1177–1183. 10.1126/science.1227268. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Hernández-Ainsa S.; Keyser U. F. DNA Origami Nanopores: Developments, Challenges and Perspectives. Nanomedicine 2013, 8, 1551–1554. 10.2217/nnm.13.145. [DOI] [PubMed] [Google Scholar]
- a Kim J.; Jung I.-S.; Kim S.-Y.; Lee E.; Kang J.-K.; Sakamoto S.; Yamaguchi K.; Kim K. New Cucurbituril Homologues: Syntheses, Isolation, Characterization, and X-ray Crystal Structures of Cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 2000, 122, 540–541. 10.1021/ja993376p. [DOI] [Google Scholar]; b Lagona J.; Mukhopadhyay P.; Chakrabarti S.; Isaacs L. The Cucurbit[n]uril Family. Angew. Chem., Int. Ed. 2005, 44, 4844–4870. 10.1002/anie.200460675. [DOI] [PubMed] [Google Scholar]; c Kim K.; Selvapalam N.; Ko Y. H.; Park K. M.; Kim D.; Kim J. Functionalized Cucurbiturils and their Applications. Chem. Soc. Rev. 2007, 36, 267–279. 10.1039/B603088M. [DOI] [PubMed] [Google Scholar]; d Dsouza R. N.; Pischel U.; Nau W. M. Fluorescent Dyes and Their Supramolecular Host-Guest Complexes with Macrocycles in Aqueous Solution. Chem. Rev. 2011, 111, 7941–7980. 10.1021/cr200213s. [DOI] [PubMed] [Google Scholar]; e Barrow S. J.; Kasera S.; Rowland M. J.; del Barrio J.; Scherman O. A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. 10.1021/acs.chemrev.5b00341. [DOI] [PubMed] [Google Scholar]
- a Jiao D.; Biedermann F.; Tian F.; Scherman O. A. A Systems Approach to Controlling Supramolecular Architecture and Emergent Solution Properties via Host-Guest Complexation in Water. J. Am. Chem. Soc. 2010, 132, 15734–15743. 10.1021/ja106716j. [DOI] [PubMed] [Google Scholar]; b Zou H.; Liu J.; Li Y.; Li X.; Wang X. Cucurbit[8]uril-Based Polymers and Polymer Materials. Small 2018, 14, 1802234. 10.1002/smll.201802234. [DOI] [PubMed] [Google Scholar]
- del Barrio J.; Horton P. N.; Lairez D.; Lloyd G. O.; Toprakcioglu C.; Scherman O. A. Photocontrol over Cucurbit[8]uril Complexes: Stoichiometry and Supramolecular Polymers. J. Am. Chem. Soc. 2013, 135, 11760–11763. 10.1021/ja406556h. [DOI] [PubMed] [Google Scholar]
- Aqueous 1:1 mixtures of CB[8] and guest molecule 2 (or 3) were incubated for ca. 24 h at 20 °C prior to use unless otherwise stated.
- a An Q.; Chen Q.; Zhu W.; Li Y.; Tao C.; Yang H.; Li Z.; Wan L.; Tian H.; Li G. A Facile Method for Preparing One-Molecule-Thick Free-Standing Organic Nanosheets with Regular Square Shape. Chem. Commun. 2010, 46, 725–727. 10.1039/B920623J. [DOI] [PubMed] [Google Scholar]; b Lin F.; Zhan T. G.; Zhou T. Y.; Zhang K. D.; Li G. Y.; Wu J.; Zhao X. The Construction of Rigid Supramolecular Polymers in Water Through the Self-Assembly of Rod-Like Monomers and Cucurbit[8]uril. Chem. Commun. 2014, 50, 7982–7985. 10.1039/C4CC02971B. [DOI] [PubMed] [Google Scholar]; c Yu Y.; Li J.; Zhang M.; Cao L.; Isaacs L. Hydrophobic Monofunctionalized Cucurbit[7]uril Undergoes Self-Inclusion Complexation and Forms Vesicle-Type Assemblies. Chem. Commun. 2015, 51, 3762–3765. 10.1039/C5CC00236B. [DOI] [PubMed] [Google Scholar]; d Kim H. J.; Whang D. R.; Gierschner J.; Park S. Y. Highly Enhanced Fluorescence of Supramolecular Polymers Based on a Cyanostilbene Derivative and Cucurbit[8]uril in Aqueous Solution. Angew. Chem., Int. Ed. 2016, 55, 15915–15919. 10.1002/anie.201609699. [DOI] [PubMed] [Google Scholar]; e Tian J.; Chen L.; Zhang D.-W.; Liu Y.; Li Z.-T. Supramolecular Organic Frameworks: Engineering Periodicity in Water through Host-Guest Chemistry. Chem. Commun. 2016, 52, 6351–6362. 10.1039/C6CC02331B. [DOI] [PubMed] [Google Scholar]; f Zhang X.; Wang C. Supramolecular Amphiphiles. Chem. Soc. Rev. 2011, 40, 94–101. 10.1039/B919678C. [DOI] [PubMed] [Google Scholar]; g Yan X.; Zhu P.; Li Self-Assembly and Application of Diphenylalanine-Based Nanostructures. Chem. Soc. Rev. 2010, 39, 1877–1890. 10.1039/b915765b. [DOI] [PubMed] [Google Scholar]
- The fact that relatively high ionic strength did not hinder aggregation (Figure S11) suggests that hydrophobic interactions contribute to some extent to the formation of higher-order structures.
- a Lü J.; Lin J.-X.; Cao M.-N.; Cao R. Cucurbituril: A Promising Organic Building Block for the Design of Coordination Compounds and Beyond. Coord. Chem. Rev. 2013, 257, 1334–1356. 10.1016/j.ccr.2012.12.014. [DOI] [Google Scholar]; b Danylyuk O. Exploring cucurbit[6]uril–peptide interactions in the solid state: crystal structure of cucurbit[6]uril complexes with glycyl-containing dipeptides. CrystEngComm 2017, 19, 3892–3897. 10.1039/C7CE00881C. [DOI] [Google Scholar]
- a Smith B. J.; Overholts A. C.; Hwang N.; Dichtel W. R. Insight into the Crystallization of Amorphous Imine-Linked Polymer Networks to 2D Covalent Organic Frameworks. Chem. Commun. 2016, 52, 3690–3693. 10.1039/C5CC10221A. [DOI] [PubMed] [Google Scholar]; b Tsarfati Y.; Rosenne S.; Weissman H.; Shimon L. J. W.; Gur D.; Palmer B. A.; Rybtchinski B. Crystallization of Organic Molecules: Nonclassical Mechanism Revealed by Direct Imaging. ACS Cent. Sci. 2018, 4, 1031–1036. 10.1021/acscentsci.8b00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Larson R. G.The Structure and Rheology of Complex Fluids; Oxford University Press: New York, 1999. [Google Scholar]; b Tschoegl N. S.The phenomenological theory of linear viscoelastic behavior. An introduction; Springer-Verlag: Berlin, 1989. [Google Scholar]
- Cates M. E. Reptation of Living Polymers: Dynamics of Entangled Polymers in the Presence of Reversible Chain-Scission Reaction. Macromolecules 1987, 20, 2289–2296. 10.1021/ma00175a038. [DOI] [Google Scholar]
- a Onsager L. The Effects of Shape on the Interaction of Colloidal Particles. Ann. N. Y. Acad. Sci. 1949, 51, 627–659. 10.1111/j.1749-6632.1949.tb27296.x. [DOI] [Google Scholar]; b Lyotropic Colloidal and Macromolecular Liquid-CrystalsLekkerkerker H. N. W.; Vroege G. J. Lyotropic colloidal and macromolecular liquid crystals. Philos. Trans. R. Soc. London, Ser. A 1993, 344, 419–440. 10.1098/rsta.1993.0098. [DOI] [Google Scholar]
- a Avrami M. Kinetics of Phase Change. I General Theory. J. Chem. Phys. 1939, 7, 1103–1112. 10.1063/1.1750380. [DOI] [Google Scholar]; b Liu X. Y.; Sawant P. D. Formation Kinetics of Fractal Nanofiber Networks in Organogels. Appl. Phys. Lett. 2001, 79, 3518–3520. 10.1063/1.1415609. [DOI] [Google Scholar]
- Henry M.Nanocrystals from Solutions and Gels. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa H. S., Ed.; American Scientific Publishers: Valencia, CA, 2004; Vol. 6, p 555. [Google Scholar]
- Supramolecular systems which have been evaluated as drag reducers or viscosity modifiers can be found here:; a Sabadini E.; Francisco K. R.; Bouteiller L. Bis-Urea-Based Supramolecular Polymer: The First Self-Assembled Drag Reducer for Hydrocarbon Solvents. Langmuir 2010, 26, 1482–1486. 10.1021/la903683e. [DOI] [PubMed] [Google Scholar]; b Li L.; Guo X.; Fu L.; Prud’homme R. K.; Lincoln S. F. Complexation Behavior of α-, β-, and γ-Cyclodextrin in Modulating and Constructing Polymer Networks. Langmuir 2008, 24, 8290–8296. 10.1021/la800859w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.