Abstract
Evidence indicates that mitogen-activated protein kinase (MAPK) pathways play a crucial role in the neurobiology of the nervous system. In the present study, dopamine receptor-mediated regulation of extracellular signal-regulated kinases (ERKs) was examined in rats in which the nigrostriatal dopaminergic pathway was unilaterally lesioned by 6-hydroxydopamine (6-OHDA). Subcutaneous injections of the D2 receptor agonist quinpirole significantly increased tyrosine-phosphorylated ERK1/2 in lesioned striatum, whereas the D1 receptor agonist SKF38393 failed to activate ERKs. Quinpirole-induced phosphorylation of ERK1/2 was seen as early as 3 min and peaked at 15 min after the challenge. In parallel, striatal ERK kinase activity, measured by the in vitro kinase assay, was increased 2.5-fold on the lesioned side after the administration of quinpirole. Immunohistochemical examination of brain sections after quinpirole administration revealed significant increases in ERK1/2 immunostaining in perinuclear and intranuclear areas of striatal neurons. This increase was much more pronounced on the lesioned than the intact side. Furthermore, quinpirole-induced contralateral rotation was decreased by 48.7 and 50.7%, respectively, when the striatal ERK pathway was selectively inhibited by a single intrastriatal injection of the MAPK/ERK kinase inhibitor PD098059 or after a continuous 7 d intrastriatal infusion of ERK1/2 antisense oligodeoxynucleotide. The results demonstrate, for the first time, that the ERK signaling pathway is activated in denervated striatum in response to stimulation of D2 dopamine receptors and that the resulting imbalance in striatal ERK activity contributes, at least in part, to neuronal plasticity that underlies D2 dopamine receptor-mediated contralateral rotation in unilateral 6-OHDA denervated rats.
Keywords: Dopamine receptor, supersensitivity, ERK pathway, locomotion, phosphorylation, striatum
Mitogen-activated protein kinases (MAPKs) are a group of intracellular protein kinases, including extracellular signal-regulated kinase (ERK), p38 MAPK and c-Jun amino-terminal kinase/stress-activated protein kinase (JNK/SAPK). The first and best characterized MAPK cascade consists of Ras, Raf, MEK1/2, and ERK1/2 and has been demonstrated to be involved in regulation of cell proliferation and differentiation (Boulton et al., 1990, 1991;Blumer and Johnson, 1994; Sale et al., 1995; Robinson and Cobb, 1997). Growth factors, by acting on intrinsic receptor tyrosine kinases (RTK), are primary activators of the MAPK pathway (Schlessinger and Ullrich, 1992; Seger and Kerbs, 1995). Accumulated evidence has also demonstrated that stimulation of G-protein-coupled receptors (GPCRs) activates the MAPK pathways via ras-dependent (Crespo et al., 1994; van Biesen et al., 1995; Touhara et al., 1995; Wan et al., 1996; Della Rocca et al., 1997; Luttrell et al., 1997) orras-independent mechanisms (Pace et al., 1995; Takahashi et al., 1997). Similar to growth factors, GPCR-mediated activation of the MAPK pathway has also been linked to cell proliferation and tissue hypertrophy. For example, activation of MAPK by α1 adrenoceptors is implicated in vascular smooth muscle and cardiac hypertrophy (Bogoyevitch et al., 1996;Glennon et al., 1996; Hu et al., 1996; Ramirez et al., 1997). However, the abundant expression of MAPKs in postmitotic neuronal tissue implies that these pathways mediate functions other than those involved in regulating cell growth (Boulton et al., 1991; Fiore et al., 1993). Indeed, some studies have shown that the MAPK signal pathway is involved in regulating expression of tyrosine hydroxylase, the rate-limited enzyme in the biosynthesis of catecholamines (Gizang-Ginsberg and Ziff, 1990; Haycock et al., 1992; Lewis et al., 1994; Rabinovsky et al., 1995) and may contribute to the increase in tyrosine hydroxylase that develops during chronic treatment with cocaine or morphine (Berhow et al., 1996). Activation of this pathway has recently been found to be related to long-term potentiation (English and Sweatt, 1996, 1997; Impey et al., 1998), long-term facilitation (Martin et al., 1997), and classical conditioning (Crow et al., 1998), and is an essential step for memory formation (Skoulakis and Davis, 1996; Brambilla et al., 1997; Silva et al., 1997; Atkins et al., 1998). In the present study, we examined,in vivo, striatal D2 dopamine receptor-mediated regulation of ERK signaling in 6-hydroxydopamine (6-OHDA)-lesioned rats in which the striatal D2dopamine receptors are upregulated and sensitized. Our results demonstrate that in vivo stimulation of D2 dopamine receptors activates the ERK cascade in the denervated striatum and that this signaling pathway plays an important role in mediating the hypersensitive locomotor response initiated by D2 dopamine receptor stimulation.
MATERIALS AND METHODS
Animal surgery and behavioral assessment. Male Sprague Dawley rats, 220–250 gm, were purchased from Harlan (Indianapolis, IN). Animals were anesthetized with intraperitoneal injections of 50 mg/kg sodium pentobarbital and received a single stereotactic injection of 8 μg of 6-OHDA hydrochloride in 4 μl of artificial CSF with 0.05% ascorbic acid into the medial forebrain bundle using the following coordinates: anteroposterior (AP), −2.5 mm; lateral (L), +2.0 mm; and dorsoventral (DV), −8.5 mm using bregma as the starting point. To limit damage to adrenergic neurons, 25 mg/kg desipramine hydrochloride was administered intraperitoneally 30 min before 6-OHDA. The success of the lesion was assessed by monitoring contralateral rotations in response to a single 0.2 mg/kg apomorphine hydrochloride challenge dose administrated subcutaneously 3 weeks after surgery. For assessing rotational behavior, lesioned rats were placed in 50-cm-diameter bowls and allowed to acclimate to the environment for 30 min before the injection of apomorphine. Animals demonstrating fewer than 20 rotations per 5 min were excluded from further experiments. The selected animals exhibited >90% depletion of striatal dopamine levels on the lesioned side as measured by HPLC. To assess responses of dopamine receptors, the specific D1 receptor agonist SKF38393 (5 mg/kg, s.c.) or the D2receptor agonist quinpirole (1 mg/kg, s.c.) were used.
Antisense oligodeoxynucleotide treatment. Antisense oligodeoxynucleotide (ODN) (5′-GCCGCCGCCGCCGCCAT-3′) and sense control ODN (5′-ATGGCGGCGGCGGCGGC-3′) directed against the initiation translation site of rat ERK1/2 (Sale et al., 1995) and phosphorothioated at the 5′- and 3′-ends were synthesized by the Midland Certified Reagent Company (Midland, TX). The ODNs were dissolved in artificial CSF and delivered via osmotic minipumps connected to Alzet (Palo Alto, CA) brain infusion cannulas, and directed into the lateral dorsal striatum on the lesioned side using the following coordinates: AP, −0.5 mm; L, +5 mm; and DV, −5 mm. The osmotic pumps were placed beneath the skin of the dorsal neck, and the ODNs were continuously infused at a rate of 1 μl/hr (10 ng/d). Contralateral rotations in response to a subcutaneous injection of 1 mg/kg quinpirole was assessed after 7 d of continuous ODN infusion.
PD098059 treatment. PD098059 (2′-amino-3′-methoxyflavone;Biomol, Plymouth Meeting, PA) was dissolved in dimethylsulfoxide (Me2SO) and diluted with PBS to give the desired drug concentration in 0.1% Me2SO. Rats were anesthetized with inhaled halothane, and single injections of 0.4–1.6 μg PD098059 or vehicle were directed into the lateral dorsal striatum ipsilateral to the 6-OHDA lesion at the coordinates: AP, −0.5 mm; L, +5 mm; and DV, −5 mm. The number of rotations in response to a subcutaneous injection of 1 mg/kg quinpirole, administered 2 hr after the intrastriatal injection of PD098059, was counted for 5 min.
Lysate preparation. Striata obtained from both sides of the brain were sonicated in 2 ml of ice-cold lysis buffer containing (in mm): 50 Tris-HCl, pH 7.4, 150 NaCl, 1 EGTA, 10 NaF, 1 Na3VO4, 40 β-glycerophosphate, 1 sodium pyrophosphate, 1 phenylmethylsulfonyl fluoride (PMSF), 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1% Nonidet P-40. The homogenates were allowed to stand on ice for 30 min and centrifuged at 12,000 × g for 15 min at 4°C. The protein content in the supernatants was determined by the Bradford assay using bovine serum albumin as standard. The lysates were stored at −80°C until use.
Immunoprecipitation and immunoblotting. One milligram of striatal lysates were incubated overnight at 4°C with 10 μl agarose-conjugated anti-phosphotyrosine monoclonal antibody (4G10; Upstate Biotechnology, Lake Placid, NY). Immunoprecipitates were washed three times with lysis buffer and resuspended in 40 μl of sample buffer containing 62.5 mm Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 5% 2-mercaptoethanol, and 0.1% bromophenol blue. Striatal lysate supernatant proteins or the immunoprecipitates of phosphotyrosine-containing proteins were size-separated on 12% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated with 1:1000 dilutions of anti-pan ERK antibody (Transduction Laboratories, Lexington, KY), or specific anti-ERK1/2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 hr followed by 1 hr incubation with 1:10,000 dilution of anti-rabbit secondary antibody. The signals were visualized with enhanced chemiluminescence (ECL) Supersignal Western Blot Detection System (Pierce, Rockford, IL) and exposed to x-ray film. The specific bands were quantified by soft-laser densitometry (Biomed Instruments, Fullerton, CA).
In vitro ERK kinase assay. The assay for ERK phosphotransferase activity was performed as described previously (Zhen et al., 1998a) using myelin basic protein (MBP; 0.25 mg/ml) as substrate. The immunoprecipitates obtained with anti-ERK1/2 antibody were washed and suspended in buffer containing (in mm): 25HEPES, pH 7.5, 10 MgCl2, 1 DTT, and 0.2 Na3VO4. The suspended immunoprecipitates were incubated with 10 μm[γ-32P] ATP (3000 Ci/mmol; DuPont NEN, Boston, MA) at 30°C for 20 min. The total reaction volume was 40 μl. The reactions were stopped with 40 μl of a twofold concentrated sample buffer. Twenty microliters of each reaction mixture were subjected to 12% SDS-PAGE. The gels were dried and the radioactivity incorporated into MBP was detected by autoradiography or by scintillation counting.
Immunohistochemical localization of ERK. Whole rat brains were rapidly removed and frozen in dry ice powder. Cryosections of 10 μm thickness were cut and stored at −70°C until use. The sections were returned to room temperature with the aid of cool air generated by a hair dryer. The sections were immersed into ice-cold fixative solution containing 1% paraformaldehyde and 0.2 m lysine, pH 7.4, for 20 min and briefly rinsed with PBS and incubated for 1 hr at room temperature in PBS containing 4% BSA and 0.1% Triton X-100. The sections were incubated overnight with a 1:250 dilution of anti-ERK1/2 polyclonal antibody (R2; Upstate Biotechnology, Lake Placid, NY) and with a 1:50 dilution of anti-MAP-2 monoclonal antibody (a gift of Dr. I. Fisher, MCP Hahnemann University). Sections were rinsed in PBS with 0.1% Triton X-100 and incubated for 1 hr with purified fluorescein-conjugated goat anti-rabbit IgG and purified Texas Red-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) in PBS with Triton X-100. The sections were then rinsed gently in PBS with Triton X-100, mounted onto slides with aqueous mounting media (Fisher Scientific, Pittsburgh, PA), and examined on a Leica (Nussloch, Germany) microscope with a CCD camera connected to a computer, using ultraviolet with 568 or 488 nm excitation frequency filters. Adjacent sections from each animal brain were processed without primary or secondary antibody and used as negative control in each experiment.
Radioligand binding studies. D2dopamine receptor binding was assessed with the selective D2 dopamine receptor antagonist [3H]raclopride. Briefly, saturation binding was performed at 37°C for 30 min in reaction mixture containing (in mm): 50 Tris-HCl buffer, pH 7.4, 120 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, and 0.125–16 nm [3H]raclopride, with 100 μg of striatal membranes. The reaction was terminated by vacuum filtration through Whatsman GF/B filter followed by washing with cold 50 mm Tris-HCl buffer, pH 7.4. Nonspecific binding was defined by binding in the presence of 10 μm spiperone. The radioactivity in the filters was measured by liquid scintillation spectrometry. Receptor density and affinity were determined by Scatchard analysis.
RESULTS
The expression and localization of ERKs in striatal neurons and the effect of dopamine receptor stimulation
Striatal ERK expression was measured in tissue lysates by immunoblot analysis using the anti-pan ERK antibody. As shown in Figure1, two isoforms of ERK, ERK1 (44 kDa) and ERK2 (42 kDa), were detected. ERK2 was the dominant form found in striatum of rat brain. An additional 54 kDa band was also found in the immunoblot. Similar expression patterns and levels of ERKs were found in the control and denervated striata. Stimulation of D2 or D1 dopamine receptors by injections of the respective selective dopamine receptor agonists quinpirole or SKF38393 did not alter the expression levels of striatal ERK proteins (Fig. 1).
Fig. 1.
Effects of 6-OHDA lesion and dopamine receptor stimulation on striatal ERK expression. Unilateral 6-OHDA-lesioned rats were injected subcutaneously with saline (10 min), apomorphine (0.2 mg/kg, 10 min), quinpirole (1 mg/kg, 10 min), or SKF38393 (5 mg/kg, 15 min). Striata from both hemispheres were removed and lysed, and 20 μg of lysates were subjected to SDS-PAGE followed by immunoblotting. The blots were incubated with a 1:1000 dilution of pan-ERK antibody. Immunoreactivity was detected by ECL. A typical blot and summary data (mean ± SEM) obtained from densitometric scans of blots of four independent experiments in relative density units are shown.
Immunohistochemical staining with anti-ERK1/2 antibody revealed ERK immunoreactivity in striatal midsize neurons identified by staining with an MAP-2 antibody, as well as in glial cells (Fig.2a). Neuronal ERK1/2 was readily detectable on the cell surface as well as in dendritic processes, whereas glial ERK was widely distributed throughout the cell cytoplasm. Subcellular localization of neuronal ERK did not exhibit a great deal of colocalization with the MAP-2 signal (Fig.2b,c,e). Few striatal MAP-2-positive cells exhibited ERK immunostaining perinuclearly.
Fig. 2.

ERK and MAP-2 double-immunohistochemistry of striatal neurons of rats injected unilaterally with 6-OHDA.a, Low-power image of ERK immunostaining (fluorescine,green) in control striatum. b, c,High-power images of ERK (green) and MAP-2 (Texas Red, red) immunostaining in control striatum.d, Control immunostaining using peptide preadsorbed ERK1/2 antibody. e–j, Double immunostaining of ERK and MAP-2 in control (e, g, i) and lesioned (f, h, j) striata obtained from rats injected subcutaneously with saline (e, f), SKF38393 (g, h), or quinpirole (i, j).Yellow indicates colocalization of ERK and MAP-2.Arrows indicate neuronal cell bodies.
Selective activation of striatal ERK pathway by D2dopamine receptor stimulation
Activation of the ERK pathway yielded increases in tyrosine-phosphorylated ERKs. The phosphorylated ERKs were determined by immunoblotting with anti-phospho-ERK1/2 antibody or by first immunoprecipitating phosphotyrosine-containing proteins using an anti-phosphotyrosine antibody followed by immunoblotting with anti-ERK1/2 antibody (ERK2). As shown in Figure3A, no difference in phosphorylated ERK1/2 was found between striata obtained from control and lesioned sides of unilaterally 6-OHDA-lesioned rats. However, an increase in phosphorylated ERK1/2 was found on the lesioned side 10 min after a subcutaneous administration of the dopamine receptor agonist apomorphine (0.2 mg/kg) or the specific D2dopamine receptor agonist quinpirole (1 mg/kg). In contrast, the D1 dopamine receptor agonist SKF38393 (5 mg/kg) did not alter the level of phosphorylated ERK1/2. The specificity of the response was further tested with dopamine receptor antagonists. As shown in Figure 4, pretreatment with the selective D2 dopamine receptor antagonist spiperone at a dose (2 mg/kg, i.p.) that totally blocked quinpirole-mediated contralateral rotation, abolished quinpirole-induced increases in phosphorylated ERK in the denervated striatum. However, the effects of quinpirole were not affected by pretreatment with the selective D1 dopamine receptor antagonist SCH23390 (0.1 mg/kg, i.p.).
Fig. 3.

Phosphorylation and activity of ERK1/2 in striatal membranes. Unilateral 6-OHDA-lesioned rats were injected subcutaneously with saline (10 min), quinpirole (1 mg/kg, 10 min), apomorphine (0.2 mg/kg, 10 min), or SKF38393 (5 mg/kg, 15 min). Striata from control (C) and lesioned (L) hemispheres were removed and lysed. For assessing ERK phosphorylation, 20 μg of lysates were subjected to SDS-PAGE followed by immunoblotting. The blots were incubated overnight with a 1:1000 dilution of anti-phospho-MAPK (ERK1/2) antibody (New England Biolabs, Beverly, MA), and immunoreactivity was detected by ECL. A typical blot and summary of the results, in relative density units, obtained from densitometric scans of blots of five independent experiments are shown (A). For measuring ERK activity, 400 μg of lysates were immunoprecipitated with anti-ERK1/2 antibody, and activity was determined in the immunoprecipitates by measuring the phosphorylation of MBP in the presence of [γ-32P] ATP. The data obtained from four independent experiments are presented as mean ± SEM (B). *p < 0.05 and *p < 0.01 compared to intact control side by the paired t test.
Fig. 4.
Effect of dopaminergic antagonists on quinpirole-induced contralateral rotation and increases in striatal phosphorylated ERK1/2. Unilateral 6-OHDA-lesioned rats were injected intraperitoneally with vehicle, 2 mg/kg spiperone, or 0.1 mg/kg SCH23390, 30 min before a subcutaneous administration of 1 mg/kg quinpirole. The rotational behavior in response to quinpirole was assessed for a 5 min period, 5–10 min after the quinpirole injection. Responses to quinpirole administration after antagonist were compared to responses to quinpirole obtained before treatment with the dopaminergic antagonists. The results are presented as percent ± SD of rotations obtained after dopaminergic antagonist treatment to that noted before the treatment in three animals per group (A). **p < 0.01 compared to vehicle-treated group (ANOVA followed by Newman–Keuls test). Immediately after the behavioral test, striata from control (C) and lesioned (L) sides were removed, and 20 μg of lysates were size-fractionated on SDS-PAGE followed by immunoblotting. The blots were incubated with a 1:1000 dilution of anti-phospho-MAPK (ERK1/2) antibody, and immunoreactivity was detected by ECL. A representative blot is shown (B). The experiment was repeated three times, and similar results were obtained.
The time course for D2 dopamine receptor-mediated increases in phosphorylated ERKs was assessed in striata obtained from rats 3, 8, 15, or 30 min after a subcutaneous challenge dose of 1 mg/kg quinpirole. Phosphorylated ERK2 was increased on the lesioned side 3 min after the injection of quinpirole. The increase in phosphorylated ERK reached maximum at 15 min and returned to control levels 30 min after quinpirole administration (Fig. 5). On the control side, no significant change in striatal phosphorylated ERK was observed after the quinpirole challenge.
Fig. 5.
Time-dependent effect of quinpirole on striatal ERK2 phosphorylation. Unilateral 6-OHDA-lesioned rats received subcutaneous injections of saline or 1 mg/kg quinpirole and striata from control (C), and lesioned (L) hemispheres were removed after 3, 8, 15, or 30 min. The tissues were lysed, and 400 μg of lysate proteins were used to immunoprecipitate phosphotyrosine-containing proteins with anti-phosphotyrosine antibody. The immunoprecipitated proteins were separated on SDS-PAGE, proteins were transferred to nitrocellulose membranes, and the blots were incubated with anti-ERK1/2 antibody. Immunoreactivity was detected by ECL. A typical blot and summary data (mean ± SEM) obtained from densitometric scans of blots of four independent experiments in relative density units are shown. *p < 0.05 and **p < 0.01 compared to intact control side by the paired ttest.
To test whether the quinpirole-induced increases in phosphorylated ERK levels are accompanied by a change in ERK activity, striatal ERK activity was measured in tissues obtained from quinpirole-treated (1 mg/kg, s.c.) animals. Whereas comparable basal ERK activities were found in control and lesioned striata, quinpirole challenge increased ERK activity 2.5-fold on the lesioned but not on the control side of unilateral 6-OHDA-lesioned rats (Fig. 3B).
Dopamine receptor-mediated regulation of striatal ERKs was further examined immunohistologically in animals treated with dopamine receptor agonists. No noticeable differences in cellular and subcellular ERK distributions were found between striata taken from control and lesioned sides after injections of saline (Fig. 2e,f) or 5 mg/kg SKF38393 (Fig. 2g,h). In contrast, 1 mg/kg quinpirole induced small increases in cytosol and cell surface ERK immunostaining in striatal MAP-2-positive cells on the intact side (Fig. 2i). In denervated striatal neurons, quinpirole dramatically increased ERK immunostaining in perinuclear and nuclear regions of neurons. This subcellular ERK redistribution resulted in reduced overlap between ERK and MAP-2 staining (Fig. 2j). The observations demonstrate that D2 dopamine receptor stimulation elicits a redistribution of ERK from cell surface regions to perinuclear and nuclear regions of neurons and that this effect is more pronounced in striata on the lesioned side of unilateral 6-OHDA-injected rats.
Dependence of D2 dopamine receptor-mediated rotation in unilateral 6-OHDA-lesioned rats on activation of striatal ERK pathway
Dopamine receptor agonist-mediated contralateral rotation in unilateral 6-OHDA-lesioned rats has been previously demonstrated in numerous laboratories to be a function of striatal dopamine receptor supersensitivity that develops after denervation of dopaminergic input to the striatum on the lesioned side. To test whether the ERK pathway plays a role in mediating dopamine receptor-stimulated rotational behavior, quinpirole-induced contralateral rotation was determined after inhibition of the striatal ERK1/2 signaling cascade, ipsilateral to the lesion, with a direct intrastriatal injection of the MAPK/ERK kinase (MEK) inhibitor PD098059 (Alessi et al., 1995). The injection of PD098059 dose-dependently inhibited the increase in striatal ERK activity that was produced by injections of quinpirole. ERK activation was inhibited by >80% at a dose of 1.6 μg PD098059 (Fig.6A). Correspondingly, quinpirole-induced rotational behavior, tested 2 hr after the administration of PD098059, was dose-dependently inhibited by the inhibitor. Inhibition of 48.7% of quinpirole-induced rotations was achieved after the injection of 1.6 μg PD098059 (Fig.6B). In contrast, the contralateral rotation that was produced by the D1 dopamine receptor agonist SKF38393 (5 mg/kg) was not altered by the intrastriatal injection of 1.6 μg PD098059 (63 ± 9 rotations/5 min vs 69 ± 7 rotations/5 min; n = 4).
Fig. 6.
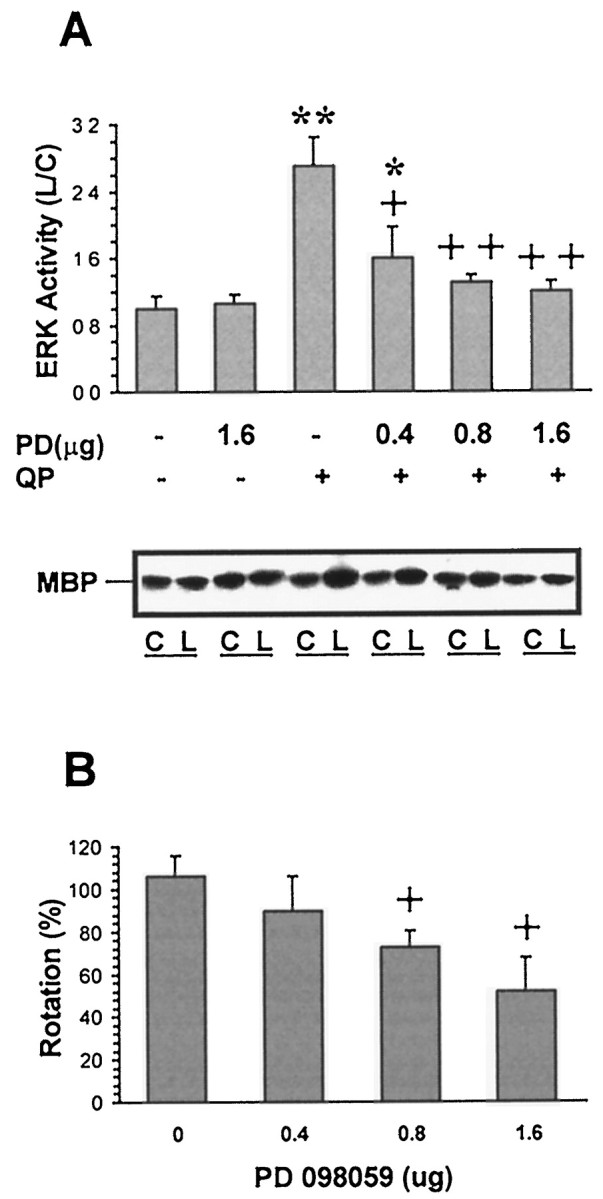
Effects of PD098059 on quinpirole-stimulated striatal ERK activity and rotational behavior. Under halothane anesthesia, unilateral 6-OHDA-lesioned rats received an intrastriatal injection of vehicle or PD098059 (PD) (0.4, 0.8, or 1.6 μg) ipsilateral to the 6-OHDA lesion. Rats were injected subcutaneously with 1 mg/kg quinpirole (QP) 2 hr after PD injection, and rotational behavior was assessed for a 5 min period, 5–10 min after QP. Striata from control (C) and lesioned (L) hemispheres were removed after 10 min, lysed, and 400 μg of lysates were immunoprecipitated with ERK1/2 antibody. ERK activity was determined by measuring the phosphorylation of MBP in the presence of [γ-32P]ATP, and proteins were separated on SDS-PAGE. The gels were dried, and the radioactivity incorporated into MBP was assessed by autoradiography. The ratios of radioactivity on the lesioned over the control sides were calculated from the densitometric assessment of the autoradiograms. A representative autoradiogram and summary data from three to four independent experiments are shown (A). The behavioral response to QP after administration of PD was compared to the number of rotations elicited by QP in a test performed 4 d previously. The results obtained from three to four individual experiments are presented as percent ± SEM of change in response to PD treatment (B). *p < 0.05 and **p < 0.01 compared to the vehicle-treated group in the absence of quinpirole. +p < 0.05 and ++p < 0.01 compared to vehicle-treated group in the presence of quinpirole (ANOVA followed by Newman–Keuls test).
In an attempt to further test the role of ERK in the expression of D2 dopamine receptor supersensitivity that follows denervation of dopaminergic neuronal input to striatum, an antisense approach was used to specifically target the ERK1/2 isoforms in striatum. After a 7 d intrastriatal infusion of 10 ng/d of ERK1/2 antisense ODN ipsilateral to the 6-OHDA injection, the level of phosphorylated ERK that was induced by quinpirole was reduced by >70% (Fig. 7A); the same antisense ODN treatment resulted in a 22% decrease in expression of striatal ERKs on the denervated side when compared to the control side (Fig.7B). Neither ERK expression level nor quinpirole-mediated elevation in phosphorylated ERK were altered by intrastriatal treatment with the control sense ODN (Fig. 7A,B). Correspondingly, quinpirole-induced contralateral rotation was inhibited by 50.7% after intrastriatal infusion with the ERK1/2 antisense ODN. Intrastriatal infusion of the sense ODN did not affect quinpirole-induced rotation (Fig. 7C).
Fig. 7.
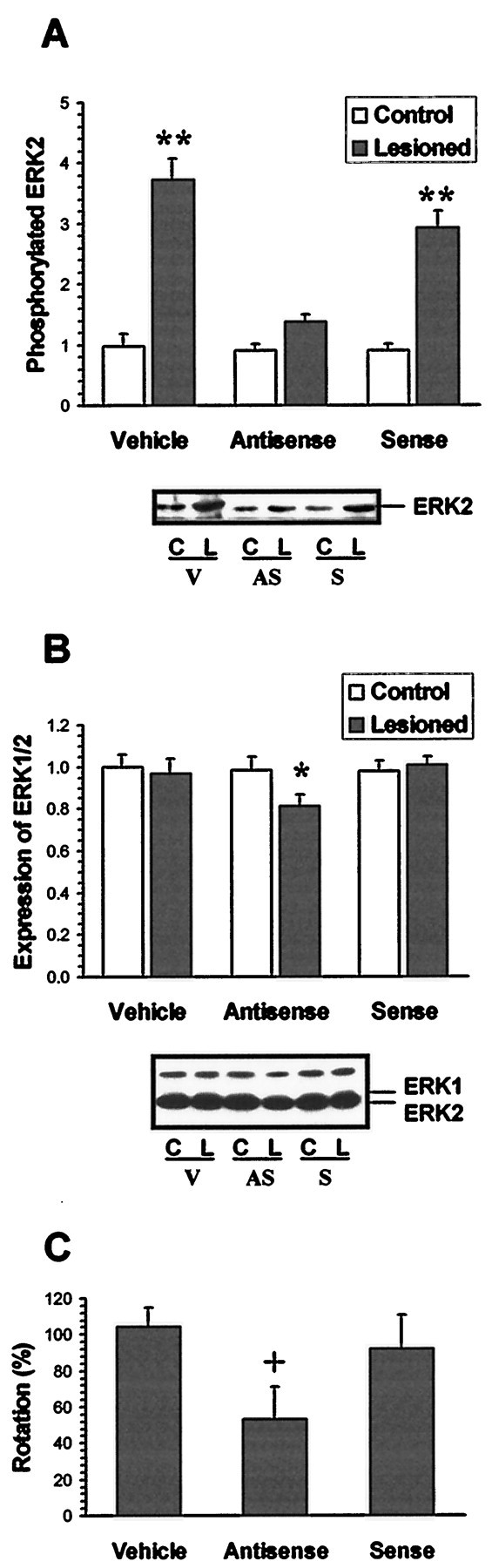
Effects of ERK1/2 antisense ODN treatment on quinpirole-induced striatal ERK phosphorylation, expression, and rotational behavior. Under sodium pentobarbital anesthesia, a cannula connected to an Alzet osmotic minipump was stereotactically placed into the dorsal lateral striatum on the 6-OHDA-lesioned side. Vehicle (V), sense ODN (S), or antisense (AS) ODNs were continuously delivered at 1 μl/hr (10 ng/d for the ODNs) for 7 d. Rats were then challenged with a subcutaneous injection of 1 mg/kg quinpirole, and the rotational response was assessed for a 5 min period, 5–10 min after the quinpirole injection. Striata from control (C) and lesioned (L) sides were removed 10 min after quinpirole challenge. To assess ERK phopshorylation, 400 μg of striatal lysates were immunoprecipitated with anti-phosphotyrosine antibody. The immuno- precipitates were subjected to SDS-PAGE, blotted with ERK1/2 antibody, and ERK immunoreactivity was detected by ECL. A typical blot and summary data (mean ± SEM) obtained from the densitometric scans of four to six independent experiments in relative density units are shown (A). To assess ERK expression, 20 μg of striatal lysates were subjected to SDS-PAGE followed by immunoblotting with the pan-ERK antibody, and immunoreactivity was detected by ECL. A typical blot and summary data (mean ± SEM) obtained from densitometric scans of four to six independent experiments in relative density units are shown (B). The behavioral response to quinpirole after administration of ODNs were compared to the responses to quinpirole obtained before treatment with the respective ODNs. The results are presented as the percent ± SEM of rotations obtained after ODN treatment to that noted before the treatment in six to eight individual experiments (C). *p < 0.05 and **p < 0.01 compared to intact control side by the paired t test. +p < 0.05 compared to vehicle-treated group (ANOVA followed by Newman–Keuls test).
The above treatments with PD098059 or ERK antisense ODN did not affect D2 dopamine receptor binding density (Bmax) (vehicle, 176 ± 11 fmol/mg; PD098059, 173 ± 4 fmol/mg; ERK antisense, 170 ± 20 fmol/mg) or binding affinity (Kd) (6.1 ± 0.6 nm; 6.0 ± 0.5 nm; 5.8 ± 0.5 nm), as determined by the assessment of specific D2dopamine receptor binding using the selective D2dopamine receptor antagonist [3H]raclopride.
DISCUSSION
The present data demonstrate that in vivostimulation of D2 dopamine receptors activates the ERK signaling pathway in striata in which the dopaminergic input has been denervated via a unilateral injection of 6-OHDA. Moreover, activation of the ERK cascade in the striatum is necessary for the expression of locomotor hyperactivity that underlies D2 dopamine receptor-mediated rotational behavior in unilateral 6-OHDA denervated rats.
Activation of striatal ERK signaling by D2 dopamine receptors was evident by the fact that acute challenge with the selective D2dopamine receptor agonist quinpirole increased tyrosine-phosphorylated ERK1/2 and ERK activity in denervated striata. The increases in phosphorylated ERK1/2 and in ERK activity were not accompanied by an apparent change in ERK protein expression, implying that D2 dopamine receptor stimulation activates this MAPK pathway. Furthermore, immunohistochemical analysis revealed that stimulation of D2 dopamine receptors increased ERK immunoreactivity in nuclear and perinuclear areas of striatal medium-size neurons on the denervated side. The results also indicate that stimulation of D2 dopamine receptor results in a translocation of cellular ERK1/2 into nuclei of striatal neurons. These results are the first to demonstrate that stimulation of D2 dopamine receptors leads to the activation of the ERK pathway in brain neurons and are consistent with previous studies obtained in cultured cells overexpressing D2 dopamine receptors (Lajiness et al., 1993; Yan et al., 1997). This action appears to be specific for the D2 receptor because it is selectively inhibited by a D2 but not by a D1dopamine receptor antagonist, and stimulation of D1 dopamine receptors did not influence the ERK signaling pathway assessed either by tyrosine-phosphorylated ERK levels or intracellular redistribution of ERK1/2 in striatal neurons. Nevertheless, stimulation of D1 dopamine receptors were previously shown to inhibit PDGF-stimulated MAPK activity in vascular smooth muscle cells (Yasunari et al., 1997), and recent studies in our laboratory have shown that stimulation of D1 dopamine receptors activate the p38 MAPK and JNK pathways via a PKA-dependent mechanism in SK-N-MC human neuroblastoma cells (Zhen et al., 1998a).
The signal transduction pathway for GPCR-mediated activation of MAPKs is less well defined than that for the growth factor receptors. It appears that GPCRs may be linked to MAPKs via different mechanisms, depending on the specific G-protein subtype with which the receptor interacts (Faure et al., 1994; Crespo et al., 1995; Hanford and Glembotski, 1996; Xing and Insel, 1996; Yu et al., 1996; Pende et al., 1997). The linkage of Gi-protein-coupled receptors to MAPKs is mediated via the βγ dimer of G-proteins that is released by the dissociation of the trimeric G-protein after receptor stimulation (Crespo et al., 1994; Koch et al., 1994; van Biesen et al., 1995; Luttrell et al., 1997). This signaling pathway has been demonstrated for many Gi-coupled receptors, including α2 adrenoceptor and 5-HT1A serotonin receptor (Flordellis et al., 1995; Cowen et al., 1996; Garnovskaya et al., 1996). D2 dopamine receptors belong to the Gi-protein-coupled receptor family and have been shown to inhibit adenylyl cyclase activity (Sokoloff and Schwartz, 1995). The molecular signaling components that link the D2 dopamine receptor to ERKs are yet to be determined. Recent studies performed in SK-N-MC human neuroblastoma cells and in MN9D cells that express D4 dopamine receptors have demonstrated that the D4 dopamine receptor-mediated activation of ERK signaling requires Src and SHC-Grb2 for interaction with a pertussis toxin-sensitive G-protein (Zhen et al., 1998b). It remains to be demonstrated whether D2 dopamine receptors share the same pathway as D4 dopamine receptors, both of which belong to the Gi-linked D2-like dopamine receptor family.
The nigrostriatal dopaminergic pathway is a major brain dopamine-containing neuronal projection. Chronic interruption of this pathway or depletion of dopamine mimics the pathogenesis of Parkinson's disease and results in sensitization of locomotor responses to striatal D1 and D2 dopamine receptors in rodents (Arnt and Hyttel, 1984; Hu et al., 1990). Increased D1dopamine receptor–G-protein coupling rather than altered D1 receptor expression appears to underlie the sensitized response to D1 dopamine receptor stimulation (Butkerait et al., 1994; Cai et al., 1998). D1 dopamine receptor-mediated cAMP-dependent activation of protein kinase A has been suggested to mediate the development of altered motor responses during chronic levodopa treatment (Oh et al., 1997). On the other hand, the mechanism that leads to the development of supersensitivity of D2 dopamine receptor-mediated responses is thought to be related to an increase in expression of D2 dopamine receptors (Creese et al., 1977;Norman et al., 1987; Savasta et al., 1987; Qin et al., 1994) as well as enhanced D2receptor–Gi-protein coupling (Rubinstein et al., 1990; Butkerait et al., 1994; Marcotte et al., 1994; Cai et al., 1998). However, the intracellular signaling pathway that underlies hypersensitization of D2 dopamine receptor-mediated locomotion was not previously determined. The present data demonstrate high ERK expression levels in striatal medium-size neurons that are involved in initiation and modulation of locomotor behavior (Kitai, 1981; Gerfen, 1995). In vivo stimulation of D2 dopamine receptors elicited contralateral rotation as well as activation of striatal ERK pathway in unilateral 6-OHDA-lesioned rats. More interestingly, D2dopamine receptor-mediated activation of ERKs was predominantly noted in striata ipsilateral to the 6-OHDA lesion, thus resulting in an imbalance in ERK signaling between the two sides of the brain. The predominant receptor-mediated activation of the ERK pathway in the denervated striatum appears to be a consequence of D2 dopamine receptor upregulation and enhanced D2 dopamine receptor-G-protein coupling that result in increased transmembrane signaling and ultimately enhanced intracellular signaling. Alternatively, this asymmetry in the activation of ERK may be mediated by the recruitment of the ERK signaling pathway during the development of dopaminergic supersensitivity. Furthermore, the phosphorylation and activation of striatal ERKs preceded the initiation of rotational behavior, and interference with this cascade either via striatal MEK inhibition or with ERK antisense ODN markedly inhibited quinpirole-elicited contralateral rotation. These data, therefore, indicate that enhanced striatal D2 dopamine receptor-activated ERK signaling underlies the supersensitivity that ultimately results in enhanced locomotor activity. This is the first report documenting the involvement of brain ERK pathway in mediating an acute behavioral activity as opposed to the role of this cascade in long-term changes in behavior or in neuronal plasticity. Nevertheless, the role of striatal ERK in the present context reflects alterations in intracellular signaling that arise during the development of dopaminergic supersensitivity.
The present study provides evidence that activation of striatal ERK signaling is required for mediating the contralateral rotational behavior that is elicited in response to D2dopamine receptor stimulation in unilateral 6-OHDA-lesioned rats. The evidence indicates that striatal neuronal MAPK pathway is essential in D2 dopamine receptor-mediated regulation of locomotor activity particularly under the condition of dopamine receptor supersensitivity, thus supporting a role for ERK signaling in neuronal plasticity.
Footnotes
This work was supported by United States Public Health Service Grants NS29514 and DA11029.
G.C. and X.Z. contributed equally to this work.
Correspondence should be addressed to Dr. Eitan Friedman, Department of Pharmacology and Physiology, MCP Hahnemann School of Medicine, 3200 Henry Avenue, Philadelphia, PA 19129. E-mail:eitan.friedman@drexel.edu.
REFERENCES
- 1.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltie AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Arnt J, Hyttel J. Differential inhibition by dopamine D-1 and D-2 antagonists of circling behavior induced by dopamine agonists in rats with unilateral 6-hydroxydopamine lesions. Eur J Pharmacol. 1984;102:349–354. doi: 10.1016/0014-2999(84)90267-x. [DOI] [PubMed] [Google Scholar]
- 3.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 4.Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci. 1996;16:4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumer KJ, Johnson GL. Diversity in function and regulation of MAP kinase pathways. Trends Biol Sci. 1994;19:236–240. doi: 10.1016/0968-0004(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 6.Bogoyevitch MA, Andersson MB, Gillespie-Brown J, Clerk A, Glennon PE, Fuller SJ, Sugden PH. Adrenergic receptor stimulation of the mitogen-activated protein kinase cascade and cardiac hypertrophy. Biochem J. 1996;314:115–121. doi: 10.1042/bj3140115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulton TG, Yancopoulos GD, Gergory JS, Slavughter C, Moomaw C, Hsu J, Cobb MH. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990;249:64–67. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- 8.Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 9.Brambilla R, Gnesutta N, Minichiello L, White G, Roylance AJ, Herron CE, Ramsey M, Wolfer DP, Cestari V, Rossi-Arnaud C, Grant SG, Chapman PF, Sturani E, Klein R. A role for the Ras signaling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- 10.Butkerait P, Wang HY, Friedman E. Increase in guanine nucleotide binding to striatal G proteins is associated with dopamine receptor supersensitivity. J Pharmacol Exp Ther. 1994;271:422–428. [PubMed] [Google Scholar]
- 11.Cai G, Wang HY, Bhamre S, Friedman E. Enhanced D1 dopamine receptor/G protein coupling in unilateral 6-hydroxydopamine-lesioned rats. Soc Neurosci Abstr. 1998;24:859. [Google Scholar]
- 12.Cowen DS, Sowers RS, Manning DR. Activation of a mitogen-activated protein kinase (ERK2) by the 5-hydroxytryptamine1A receptor is sensitive not only to inhibitors of phosphatidylinositol 3-kinase, but to an inhibitor of phosphatidylinositol hydrolysis. J Biol Chem. 1996;271:22297–22300. doi: 10.1074/jbc.271.37.22297. [DOI] [PubMed] [Google Scholar]
- 13.Creese I, Burt DR, Snyder SH. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science. 1977;197:596–598. doi: 10.1126/science.877576. [DOI] [PubMed] [Google Scholar]
- 14.Crespo P, Xu N, Simonds WF, Gutkind JS. Ras-dependent activation of MAP kinase pathway mediated by G-protein βγ subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 15.Crespo P, Cachero TG, Xu N, Gutkind JS. Dual effect of β-adrenergic receptors on mitogen-activated protein kinase. Evidence for a βγ-dependent activation and a Gαs-cAMP-mediated inhibition. J Biol Chem. 1995;270:25259–25265. doi: 10.1074/jbc.270.42.25259. [DOI] [PubMed] [Google Scholar]
- 16.Crow T, Xue-Bian JJ, Siddiqi V, Kang Y, Neary JT. Phosphorylation of mitogen-activated protein kinase by one-trial and multi-trial classical conditioning. J Neurosci. 1998;18:3480–3487. doi: 10.1523/JNEUROSCI.18-09-03480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Della Rocca GJ, van Biesen T, Daaka Y, Luttrell DK, Luttrell LM, Lefkowitz RJ. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2 and Src Kinase. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 18.English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- 19.English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- 20.Faure M, Voyno-Yasenstskaya TA, Bourne HR. cAMP and βγ subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J Biol Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- 21.Fiore RS, Bayer VE, Pelech SL, Posada J, Cooper JA, Baraban JM. p42 mitogen-activated protein kinase in brain: prominent localization in neuronal cell bodies and dendrites. Neuroscience. 1993;55:463–472. doi: 10.1016/0306-4522(93)90516-i. [DOI] [PubMed] [Google Scholar]
- 22.Flordellis CS, Bergurand M, Gouache P, Barbu V, Gavras H, Handy DE, Bereziat G, Masliah J. α2 adrenergic receptor subtypes expressed in Chinese hamster ovary cells activate differentially mitogen -activated protein kinase by a p21ras independent pathway. J Biol Chem. 1995;270:3491–3494. doi: 10.1074/jbc.270.8.3491. [DOI] [PubMed] [Google Scholar]
- 23.Garnovskaya MN, van Biesen T, Hawes B, Ramos SC, Lefkowitz RJ, Raymond JR. Ras-dependent activation of fibroblast mitogen-activated protein kinase by 5-HT1A receptor via a G protein βγ-subunit-initiated pathway. Biochemistry. 1996;35:13716–13722. doi: 10.1021/bi961764n. [DOI] [PubMed] [Google Scholar]
- 24.Gerfen CR. Dopamine receptor function in the basal ganglia. Clin Neuropharmacol. 1995;18:S162–S177. [Google Scholar]
- 25.Gizang-Ginsberg E, Ziff EB. Nerve growth factor regulates tyrosine hydroxylase gene transcription through a nucleoprotein complex that contains c-Fos. Genes Dev. 1990;4:477–491. doi: 10.1101/gad.4.4.477. [DOI] [PubMed] [Google Scholar]
- 26.Glennon PE, Kaddoura S, Sale EM, Sale GJ, Fuller SJ, Sugden PH. Depletion of mitogen-activated protein kinase using an antisense oligodeoxynucleotide approach downregulates the phenylephrine-induced hypertrophic response in rat cardiac myocytes. Circ Res. 1996;78:954–961. doi: 10.1161/01.res.78.6.954. [DOI] [PubMed] [Google Scholar]
- 27.Hanford DS, Glembotski CC. Stabilization of B-type natriuretic peptide mRNA in cardiac myocytes by alpha-adrenergic receptor activation: potential roles for protein kinase C and mitogen-activated protein kinase. Mol Endocrinol. 1996;10:1719–1727. doi: 10.1210/mend.10.12.8961280. [DOI] [PubMed] [Google Scholar]
- 28.Haycock JW, Ahn NG, Cobb MH, Krebs EG. ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc Natl Acad Sci USA. 1992;89:2365–2369. doi: 10.1073/pnas.89.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu XT, Wachtel SR, Galloway MP, White FJ. Lesions of the nigrostriatal dopamine projection increase the inhibitory effects of D1 and D2 dopamine agonists on caudate-putamen neurons and relieve D2 receptors from the necessity of D1 receptor stimulation. J Neurosci. 1990;10:2318–2329. doi: 10.1523/JNEUROSCI.10-07-02318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu ZW, Shi XY, Lin RZ, Hoffman BB. α1 adrenergic receptors activate phosphatidylinositol 3-kinase in human vascular smooth muscle cells. J Biol Chem. 1996;271:8977–8982. doi: 10.1074/jbc.271.15.8977. [DOI] [PubMed] [Google Scholar]
- 31.Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 32.Kitai ST. Anatomy and physiology of the neostriatum. Adv Biochem Psycopharmacol. 1981;30:1–21. [PubMed] [Google Scholar]
- 33.Koch WJ, Hawes BE, Allen LF, Lefkowitz RJ. Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by G beta gamma activation of p21ras. Proc Natl Acad Sci USA. 1994;91:12706–12710. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lajiness ME, Chio CL, Huff R. D2 dopamine receptor stimulation of mitogenesis in transfected Chinese hamster ovary cells: relationship to dopamine stimulation of tyrosine phosphorylations. J Pharmacol Exp Ther. 1993;267:1573–1581. [PubMed] [Google Scholar]
- 35.Lewis SE, Rao MS, Symes AJ, Daurer WT, Fink JS, Landis SC, Hyman SE. Coordinated regulation of choline acetyltransferase, tyrosine hydroxylase, and neuropeptide mRNAs by ciliary neurotrophic factor and leukemia inhibitory factor in cultured sympathetic neurons. J Neurochem. 1994;63:429–438. doi: 10.1046/j.1471-4159.1994.63020429.x. [DOI] [PubMed] [Google Scholar]
- 36.Luttrell LM, Della Rocca GJ, van Biesen T, Luttrell DK, Lefkowitz RJ. Gβγ subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor. A scaffold for G protein-coupled receptor-mediated ras activation. J Biol Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- 37.Marcotte ER, Sullivan RM, Mishra RK. Striatal G-proteins: effects of unilateral 6-hydroxydopamine lesions. Neurosci Lett. 1994;169:195–198. doi: 10.1016/0304-3940(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 38.Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocated into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- 39.Norman AB, Battaglia G, Creese I. Differential recovery rates of rat D2 dopamine receptors as a function of aging and chronic reserpine treatment following irreversible modification: a key to receptor regulatory mechanisms. J Neurosci. 1987;7:1484–1491. doi: 10.1523/JNEUROSCI.07-05-01484.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh JD, Dotto PD, Chase TN. Protein kinase A inhibitor attenuates levodopa-induced motor response alterations in hemi-parkinsonian rat. Neurosci Lett. 1997;228:5–8. doi: 10.1016/s0304-3940(97)00355-8. [DOI] [PubMed] [Google Scholar]
- 41.Pace AM, Faure M, Bourne HR. Gi2-mediated activation of the MAP kinase cascade. Mol Biol Cell. 1995;6:1685–1695. doi: 10.1091/mbc.6.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pende M, Fisher TL, Simpson PB, Russell JT, Blenis J, Gallo V. Neurotransmitter- and growth factor-induced cAMP response element binding protein phosphorylation in glial cell progenitors: role of calcium ions, protein kinase C, and mitogen-activated protein kinase/ribosomal S6 kinase pathway. J Neurosci. 1997;17:1291–1301. doi: 10.1523/JNEUROSCI.17-04-01291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin ZH, Chen JF, Weiss B. Lesions of mouse striatum induced by 6-hydroxydopamine differentially alter the density, rate of synthesis, and level of gene expression of D1 and D2 dopamine receptors. J Neurochem. 1994;62:411–420. doi: 10.1046/j.1471-4159.1994.62020411.x. [DOI] [PubMed] [Google Scholar]
- 44.Rabinovsky ED, Ramchatesingh J, McManaman JL. Regulation of tyrosine hydroxylase gene expression in IMR-32 neuroblastoma cells by basic fibroblast growth factor and ciliary neurotrophic factor. J Neurochem. 1995;64:2401–2412. doi: 10.1046/j.1471-4159.1995.64062404.x. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez MT, Sah VP, Zhao XL, Hunter JJ, Chien KR, Brown JH. The MEKK-JNK pathway is stimulated by α1-adrenergic receptor and ras activation and is associated with in vitro and in vivo cardiac hypertrophy. J Biol Chem. 1997;272:14057–14061. doi: 10.1074/jbc.272.22.14057. [DOI] [PubMed] [Google Scholar]
- 46.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 47.Rubinstein M, Muschietti JP, Gershanik O, Flawia MM, Stefano FJE. Adaptive mechanisms of striatal D1 and D2 dopamine receptors in response to a prolonged reserpine treatment in mice. J Pharmacol Exp Ther. 1990;252:810–816. [PubMed] [Google Scholar]
- 48.Sale EM, Atkinson PGP, Sale GJ. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DMA synthesis. EMBO J. 1995;14:674–684. doi: 10.1002/j.1460-2075.1995.tb07046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savasta M, Dubois A, Feuerstein C, Manier M, Scatton B. Denervation supersensitivity of striatal D2 dopamine receptors is restricted to the ventro- and dorsolateral regions of the striatum. Neurosci Lett. 1987;74:180–186. doi: 10.1016/0304-3940(87)90146-7. [DOI] [PubMed] [Google Scholar]
- 50.Schlessinger J, Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992;9:383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- 51.Seger R, Kerbs E. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 52.Silva AJ, Frankland PW, Marowitz Z, Friedman E, Lazlo G, Cioffi D, Jacks T, Bourtchuladze R. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat Genet. 1997;15:281–284. doi: 10.1038/ng0397-281. [DOI] [PubMed] [Google Scholar]
- 53.Skoulakis EM, Davis RL (1996) Olfactory learning deficits in mutants for leonardo, a Drosophila gene. 17:931–944. [DOI] [PubMed]
- 54.Sokoloff P, Schwartz JC. Novel dopamine receptors half a decade later. Trends Pharmacol Sci. 1995;16:270–275. doi: 10.1016/s0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi T, Kawahara Y, Okuda M, Ueno H, Takeshita A, Yokoyama M. Angiotensin II stimulates mitogen-activated protein kinases and protein synthesis by a Ras-independent pathway in vascular smooth muscle cells. J Biol Chem. 1997;272:16018–16022. doi: 10.1074/jbc.272.25.16018. [DOI] [PubMed] [Google Scholar]
- 56.Touhara K, Hawes BE, van Biesen T, Lefkowitz RJ. G protein βγ subunits stimulate phosphorylation of Shc adaptor protein. Proc Natl Acad Sci USA. 1995;92:9284–9287. doi: 10.1073/pnas.92.20.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Biesen T, Hawes BE, Luttrell DK, Krueger KM, Touhara K, Porfiri E, Sakaue M, Luttrell LM, Lefkowitz RJ. Receptor-tyrosine-kinase- and Gβγ-mediated MAP kinase activation by a common signaling pathway. Nature. 1995;376:781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- 58.Wan Y, Kurosaki T, Huang XY. Tyrosine kinases in activation of the MAP kinase cascade by G-protein-coupled receptors. Nature. 1996;380:541–544. doi: 10.1038/380541a0. [DOI] [PubMed] [Google Scholar]
- 59.Xing M, Insel PA. Protein kinase C-dependent activation of cytosolic phospholipase A2 and mitogen-activated protein kinase by alpha1-adrenergic receptors in Madin-Darby canine kidney cells. J Clin Invest. 1996;97:1302–1310. doi: 10.1172/JCI118546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan Y, Chi PP, Bourne HR. RGS4 inhibits Gq-mediated activation of mitogen-activated protein kinase and phosphoinositide synthesis. J Biol Chem. 1997;272:11924–11927. doi: 10.1074/jbc.272.18.11924. [DOI] [PubMed] [Google Scholar]
- 61.Yasunari K, Kohno M, Kano H, Yokokawa K, Minami M, Yoshikawa J. Dopamine D1-like receptor stimulation inhibits hypertrophy induced by platelet-derived growth factor in cultured rat renal vascular smooth muscle cells. Hypertension. 1997;29:350–355. doi: 10.1161/01.hyp.29.1.350. [DOI] [PubMed] [Google Scholar]
- 62.Yu SM, Tsai SY, Guh JH, Ko FN, Teng CM, Ou JT. Mechanism of catecholamine-induced proliferation of vascular smooth muscle cells. Circulation. 1996;94:547–554. doi: 10.1161/01.cir.94.3.547. [DOI] [PubMed] [Google Scholar]
- 63.Zhen X, Uryu K, Wang HY, Friedman E. D1-dopamine receptor agonists mediate activation of p38 MAPK and JNK by a PKA-dependent mechanism in SK-N-MC human neuroblastoma cells. Mol Pharmacol. 1998a;54:453–458. doi: 10.1124/mol.54.3.453. [DOI] [PubMed] [Google Scholar]
- 64.Zhen X, Wang HY, Uryu K, Cai G, Smith C, Friedman E. Activation of extracellular signal-regulated kinase (ERK) by D4 dopamine receptors (DAR) requires Src, SHC-Grb2 via Gi protein. Soc Neurosci Abstr. 1998b;24:859. [Google Scholar]





