Abstract
The interplay between growing axons and the extracellular substrate is pivotal for directing axonal outgrowth during development and regeneration. Here we show an important role for the neuronal cell adhesion molecule α7β1 integrin during peripheral nerve regeneration. Axotomy led to a strong increase of this integrin on regenerating motor and sensory neurons, but not on the normally nonregenerating CNS neurons. α7 and β1 subunits were present on the axons and their growth cones in the regenerating facial nerve. Transgenic deletion of the α7 subunit caused a significant reduction of axonal elongation. The associated delay in the reinnervation of the whiskerpad, a peripheral target of the facial motor neurons, points to an important role for this integrin in the successful execution of axonal regeneration.
Keywords: axonal regeneration, reinnervation, facial nerve, growth cone, motoneuron, integrin, knock-out mice
Changes in adhesion properties of transected axons and their environment are essential for regeneration. In the proximal part, the tips of the transected axons transform into growth cones that home onto the distal part of the nerve and enter the endoneurial tubes on their way toward the denervated tissue (Fawcett, 1992; Bisby, 1995). The distal part of the nerve undergoes Wallerian degeneration, a process involving the removal of the disconnected axons and their myelin sheaths from the associated Schwann cells. The denervated Schwann cells proliferate, attach to each other, and form bands of Büngner, which serve as a permissive substrate for axonal regrowth. These Schwann cells increase synthesis of adhesion molecules, such as L1 and laminin, which are inserted into their cell surface and the surrounding extracellular matrix (ECM) of the endoneurial tubes (Cornbrooks et al., 1983; Salonen et al., 1987;Martini, 1994). The process is mirrored by regenerating axons that upregulate receptors for endoneurial ECM molecules (Lefcort et al., 1992; Kloss et al., 1999).
The integrins are a large family of receptors for ECM molecules (Haas and Plow, 1994; Luckenbill-Edds, 1997) that consists of >20 different heterodimers formed by an α and a β subunit. Although many integrins, particularly β1 family members, are important for neurite outgrowth in vitro (Toyota et al., 1990; Letourneau et al., 1992; Tomaselli et al., 1993; Weaver et al., 1995; Condic and Letourneau, 1997), little is known about their physiological role during axonal regeneration in vivo. In this study we examined the regulation and function of the α7β1 integrin, a receptor for the basement membrane proteins laminins-1, -2, and -4 (Kramer et al., 1991; von der Mark et al., 1991; Yao et al., 1996). This integrin is mainly expressed in skeletal, cardiac, and smooth muscle (Song et al., 1992; Ziober et al., 1993; Martin et al., 1996), but it is also present in the developing brain (Van der Flier et al., 1995; Kil and Bronner-Fraser, 1996; Velling et al., 1996). In the adult nervous system, we now show that α7 is strongly upregulated in axotomized neurons in various injury models during peripheral nerve regeneration, but not after CNS injury. The deletion of the α7 subunit leads to an impairment in axonal outgrowth and a delayed target reinnervation of regenerating facial motoneurons.
MATERIALS AND METHODS
Animals and surgical procedures. Adult homozygous α7−/− and littermate controls (6-month-old) on a 129 Sv background used in this study were obtained from heterozygous crossings (Mayer et al., 1997). Normal adult C57Bl6 mice were obtained from Charles River (Sulzfeld, Germany). The animal experiments and care protocols were approved by the Regierung von Oberbayern (AZ 211-2531-10/93 and AZ 211-2531-37/97); all surgical procedures were performed under anesthesia with intraperitoneal injection of 150 μl of 2.5% avertin/10 gm of body weight. The facial nerve was cut at the foramen stylomastoideum, the hypoglossal nerve just before bifurcation, the vagal nerve at the midcervical level, and the sciatic nerve at the sciatic notch. For optic nerve crush, the eyeball was gently pushed forward, and the optic nerve was crushed repeatedly with fine tweezers for 10 sec. For a direct cerebral trauma, a 2.5-mm-deep, 2.5-mm-long, parasagittal cortical incision was performed on the dorsal forebrain (1.0 mm lateral of the midline, beginning 1.0 mm posterior of bregma, right side), which transected the cerebral cortex, the corpus callosum, and the fimbria fornix. The regeneration and reinnervation studies were performed after a facial nerve crush at the stylomastoid foramen.
Light microscopic immunohistochemistry. The animals were killed in ether, perfusion-fixed in 4% formaldehyde (FA) in PBS (4% FA–PBS), the tissue was removed, post-fixed in 1% FA–PBS for 2 hr, and cryoprotected with 30% sucrose overnight and frozen on dry ice, as previously described (Raivich et al., 1998a). Briefly, 20-μm-thick sections from brainstem, spinal cord, dorsal root ganglia, retina, septum, and cerebral cortex and 10 μm longitudinal sections of the facial and optic nerve were cut at −15°C, collected on gelatin-coated slides, spread in distilled water, fixed in formalin, defatted in acetone, and pretreated with 5% goat serum (Vector, Wiesbaden, Germany) in phosphate buffer (PB). The sections were incubated with primary polyclonal rabbit antibodies against α7 (1:10,000 dilution), galanin (1:400; Penninsula), calcitonin gene-related peptide (CGRP; 1:1000; Penninsula) or glial fibrillary acidic protein (GFAP; 1:5000; Dako, Hamburg, Germany), monoclonal rat antibodies against β1 (1:6000; Chemicon, Palo Alto, CA), αM (1:6000; Serotec, Oxford, UK), and MHC-1 (1:100; Dianova, Hamburg, Germany), or with a monoclonal Syrian hamster antibody against CD3 (1:3000; PharMingen, Hamburg, Germany). Then the sections were incubated with a biotinylated goat anti-rabbit, anti-rat (1:100; Vector) or anti-hamster (1:100; Dianova) secondary antibody, followed by incubation with the ABC reagent (Vector), visualization with diaminobenzidine/H2O2 (DAB; Sigma, Deisenhofen, Germany), dehydration in alcohol and xylene, and mounted with Depex (BDH Chemicals, Poole, UK). Double immunofluorescence was performed with a combination of the primary antibodies against α7 and β1, then biotinylated donkey anti-rabbit and FITC-conjugated goat anti-rat secondary antibodies (1:100; Dianova), followed by Texas Red–Avidin (1:100; Vector) and a FITC-donkey anti-goat tertiary antibody (Dianova), respectively, and scanned in a Leica (Nussloch, Germany) TCS 4D confocal laser microscope with a 10 and 100× objective (pinhole 30/100).
Electron microscopic immunohistochemistry. Perfusion with 40 ml of PBS and 10 mm MgCl2 (Mg–PBS) was followed by 100 ml of 0.5% glutaraldehyde and 4% FA in Mg–PBS and by 100 ml of 4% FA and Mg–PBS, nerve dissection, and a 2 hr immersion in 1% FA–PBS. Vibratome cross sections of 80 μm were obtained at the level of the growth front (5–6 mm distal to the crush), followed by pre-embedding immunohistochemistry as described (Möller et al., 1996). Briefly, free-floating sections were preincubated with goat serum for 4 hr, followed by incubation with the primary antibodies against α7- or β1-subunit. The biotinylated, secondary antibody was applied for 8 hr, followed by ABC reagent overnight and DAB staining, intensified with cobalt and nickel sulfates. After immunostaining, sections were fixed with glutaraldehyde, osmicated, embedded in Araldite (Fluka, Basel, Switzerland), cut (100 nm), counterstained with uranyl acetate and lead citrate, and examined in a Zeiss EM 10 electron microscope.
Quantification of light microscopic immunohistochemistry.Digital images from the stained sections were obtained using a Sony 3 CCD video camera (AVT-Horn, Aachen, Germany) and analyzed with the OPTIMAS 6.2 imaging system (Bethell). Luminosity values for the antibody staining intensity (SI) for each individual facial nucleus were determined using the Mean-SD algorithm as previously described (Kloss et al., 1999) and subsequent subtraction of the SI of the adjacent midline (n = 4 animals per time point).
Cell counts. To quantify microglial proliferation, the animals were injected with 200 μCi of [3H]thymidine (Amersham, Braunschweig, Germany) 3 d after facial nerve axotomy and 2 hr before killing by perfusion. Fixed brainstem sections were obtained as described, autoradiographed (Raivich et al., 1994), and labeled cells were counted for the whole facial motor nucleus (six sections per animal). GFAP-positive stellate astrocytes and CD3-positive lymphocytes were counted in the facial motor nuclei of two sections per animal.
Detection of α7β1 integrin mRNA. To study the mRNA, the facial motor nuclei, the gastrocnemius, and the heart muscle were excised immediately after killing and frozen on dry ice. Individual tissue samples were homogenized and processed using Tristar (Angewandte Gentechnologie Systeme AGS, Heidelberg, Germany) according to the manufacturer's protocol. RNA extracts (1 μg of total RNA) were reverse-transcribed with random hexamer primers using superscript II Moloney murine leukemia virus reverse transcriptase (Life Technologies, Eggenstein, Germany). Thirty-five cycle thermoenzymatic amplification of integrin mRNA was performed in a MJ Research DNA Engine (Peltier Thermocycler, Biometra, Göttingen, Germany), using the following primers: α7-sense 5′-tgctcagagatgcatcc-3′, α7-antisense 5′-caccggatgctcatcaggac-3′ and β1-sense 5′-ggcaacaatgaagctatcgt-3′, β1-antisense 5′-ccctcaacttcggattgac-3′. Amplification products were analyzed with gel electrophoresis.
Regeneration rate in the facial nerve. Four days after facial nerve crush, the animals were killed, followed by a brief, 5 min perfusion-fixation with 4% FA–PBS and then by a slow, 60 min perfusion with 1% FA–PBS, followed by immediate dissection and freezing on dry ice. Nerves were cut longitudinally, and the regenerating axons were visualized by immunostaining for galanin or CGRP. Every fifth section was used per antibody, with an interval of 50 μm, and the distance between the most distal-labeled growth cone and the crush site measured using light microscopic grid scaling. The average distance for each animal was calculated from four or five tissue sections.
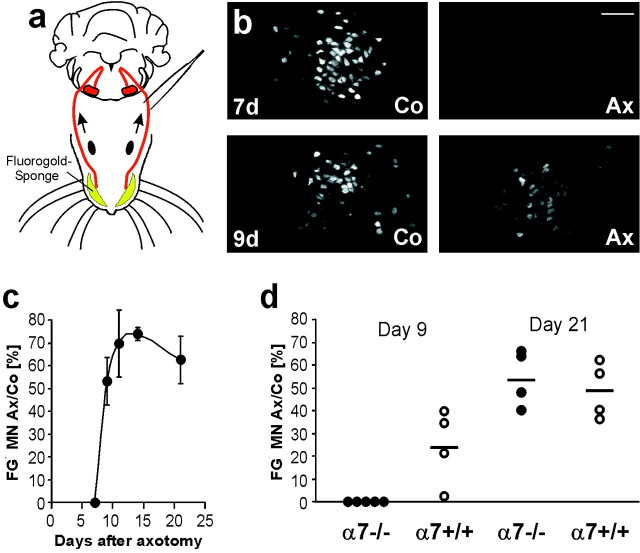
Reinnervation of the whiskerpad. Under avertin anesthesia, a flat cut was performed under the skin of the right and left whiskerpad, a gelatin sponge (whiskerpad size, 1-mm-thick) filled with 15 μl of 4% FluoroGold (FG; Fluorochrome, Denver, CO) in H2O was inserted under the pad, removed after 20 min, and the wound tissue was rinsed with PBS before suture. The animals were perfused 48 hr after instillation of the retrograde tracer, and the brainstem sections were spread and dried for 10 min. The retrograde-labeled motoneurons within the facial motor nucleus (six sections per animal) were immediately counted under a fluorescence microscope with UV-light illumination. For illustrations (see Fig.5B) the sections were covered with Vectorshield (Vector) and scanned with a Leica TCS 4D confocal laser microscope (488 nm excitation, 590 nm longpass filter).
Fig. 5.
Lack of α7 integrin subunit causes a delay of target reinnervation in the axotomized facial nerve.a–c, Experimental model. a, Fluorogold was applied into both whiskerpads 2 d before killing, retrogradely transported by normally connected (control side, Co) and reinnervating motoneurons (axotomized side, Ax), and detected in the brainstem (b) at different time points after facial nerve crush. The onset of whiskerpad reinnervation is observed between day 7 and day 9. c, Time course of whiskerpad reinnervation, with total number of FG-positive motoneurons (FG + MN) on the axotomized side, expressed as percentage of the number on the contralateral side; six brainstem sections were counted per animal. Scale bar, 100 μm. d, Effect of α7 deficiency on whiskerpad reinnervation 9 and 21 d after facial nerve crush. Unlike the α7+/+ mice (n = 4), no FG-positive motoneurons were found in the axotomized, facial motor nuclei of the α7−/− animals (n = 5) at day 9 (p < 0.05%, Wilcoxon test). Both groups of animals (n = 4) showed a similar reinnervation index at day 21. The horizontal bar shows the mean percentage per group.
RESULTS
Regulation of α7 integrin subunit in the regenerating facial motor nucleus
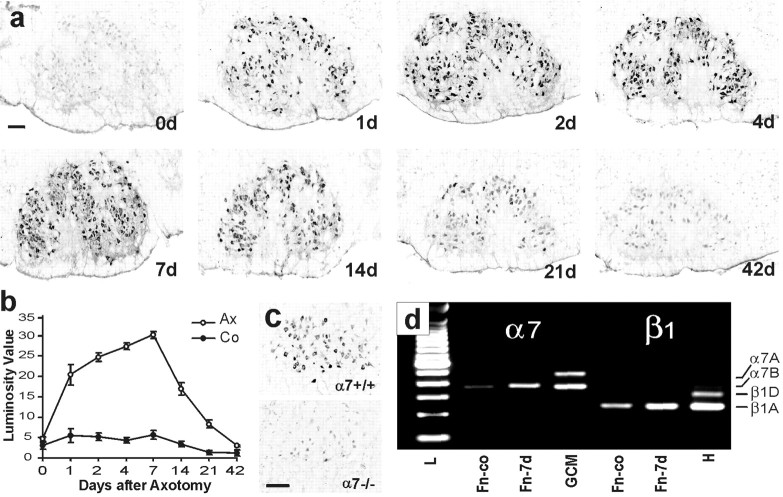
Low levels of α7 were already observed in the normal brainstem (Fig. 1a,b). Transection of the facial nerve led to a massive increase of α7 immunoreactivity on the axotomized facial motoneurons (Fig. 1a–c). The increase of α7 was already apparent 1 d after axotomy, reached a maximum at day 7, and was followed by a decrease at the start of reinnervation at day 14. A very similar time course was previously shown for neuronal β1 immunoreactivity in the axotomized facial motor nucleus (Kloss et al., 1999). The specificity of the polyclonal rabbit anti-α7 antibody is shown by the disappearance of the α7 immunoreactivity on axotomized motoneurons in the α7-deficient mice (Fig. 1c). The mRNA for the α7 integrin subunits is known to undergo alternative splicing resulting in α7A and α7B isoforms; both isoforms associate with β1A and β1D integrin variants (Collo et al., 1993; Ziober et al., 1993; Van der Flier et al., 1995; Velling et al., 1996). However, only the mRNA for the α7B and β1A variants was detected both in the normal and axotomized facial motor nuclei by RT-PCR (Fig.1d).
Fig. 1.
α7 integrin subunit in the facial motor nucleus after peripheral nerve transection. a, α7 immunoreactivity is weak in the normal brainstem (0 d), but rapidly increases after transection of the facial nerve (1–42 d).b, Quantification of α7 staining intensity in the normal (Co) and axotomized facial motor nucleus (Ax) shows a strong increase at day 1 and is maximum at day 7 (n = 4 animals; mean values; error bars indicate SEM). c, Specificity of the polyclonal rabbit anti-α7 antibody is demonstrated by the disappearance of motoneuronal staining (at day 3) in the α7−/− mouse, compared to the wild-type animal (α7+/+). Scale bars, 125 μm. d, Analysis of the mRNA isoforms of α7 and β1 integrin subunits by RT-PCR. The normal and axotomized facial nucleus (Fn-co, Fn-7d) only contains α7B and β1A mRNA splice isoforms. Gastrocnemius muscle (GCM) and heart were used as positive controls; the GCM contains both α7A and α7B, and the heart contains both β1A and β1D isoforms. L, 100 bp ladder.
Expression of α7β1 integrin in different central and peripheral injury models
The increase of α7 immunoreactivity on axotomized neurons was consistently present in different models of peripheral regeneration, but not after CNS injury. Four days after peripheral nerve injury, strong α7 staining was found on the axotomized motoneurons of the vagal and hypoglossal nuclei (Fig.2a,b). In the spinal cord, transection of the sciatic nerve caused an increase of α7 immunoreactivity on the spinal motoneurons and in the substantia gelatinosa (Fig. 2c). Dorsal rhizotomy abolished the staining in the dorsal but not the ventral horn, suggesting the localization of α7 on the primary sensory afferents in the substantia gelatinosa reacting to sciatic injury (data not shown). Axotomized dorsal root ganglia (DRG) showed a redistribution of α7 immunoreactivity (Fig. 2d). As in previous studies (Velling et al., 1996), normal, uninjured DRG showed strong α7 immunostaining on perineuronal satellite cells. This α7 immunostaining disappeared from the satellite cells after axotomy, but increased on the injured, small sensory neurons. Interestingly, small caliber sensory neurons show a particularly robust and rapid neurite outgrowth after axotomy (Brown et al., 1992). In the normal sciatic nerve, weak α7 was only detected in the perineurium. It increased strongly on the regenerating axons after crush (Fig. 2e).
Fig. 2.
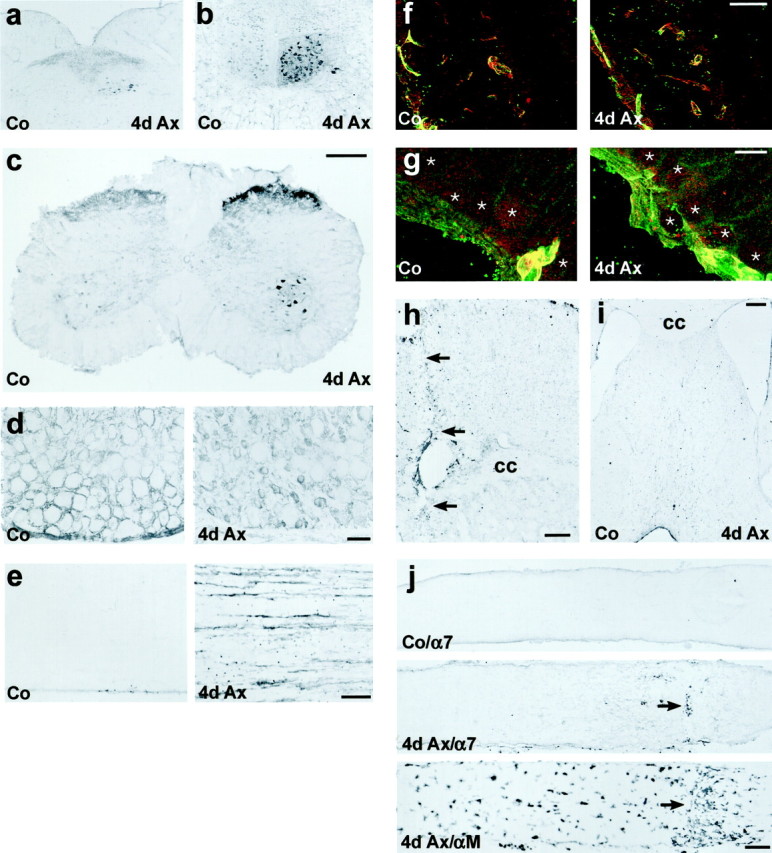
Enhanced α7 and β1 integrin immunoreactivity (IR) in different models of peripheral but not of central axotomy.a–e, Peripheral nerve transection. a, b,Axotomy of the vagal (a) or hypoglossal nerve (b) leads to a strong increase of α7-IR in the corresponding nucleus (4d Ax). c–e,Transection of the sciatic nerve also leads to strong α7-IR on the axotomized motoneurons and in the substantia gelatinosa in the lumbar spinal cord (4d Ax). d, In the normal DRG (Co), α7 is localized to satellite cells surrounding the sensory neurons. Sciatic axotomy leads to a disappearance of this staining and a strong increase of α7-IR on the small sensory neurons (4d Ax). e, α7 is also upregulated on the regenerating axons in the sciatic nerve (4d Ax).f–j, Transection of CNS axons. f, g,Immunofluorescence double labeling in the axotomized retina revealed a colocalization of α7 (red) and β1 (green) on blood vessels (yellow profiles), but no staining of both subunits on normal (Co) and axotomized retinal ganglion cells (4d Ax, asterisks). h, i, Cortical incision with resulting transection of the underlying corpus callosum (h; cc, arrows) and the septohippocampal tract (i) induced α7-IR on peritraumatic blood vessels but not on the axotomized pyramidal cells and septal neurons.j, α7 is also absent from the axons of normal (Co/α7) and axotomized (4d Ax/α7) RGCs. The arrow points to the transversal accumulation of peroxidase-positive leukocytes at the lesion site (4d Ax/α7). The optic nerve crush is also demarcated by the accumulation of αMβ2-positive macrophages in an adjacent section (4d Ax/αM, arrow). Scale bars:c (also applicable to a, b), h, j, 250 μm; d, e, i, 100 μm;f, 50 μm; g, 10 μm.
In addition to the upregulation of the α7 subunit during regeneration, there was a parallel increase in immunostaining of the β1 subunit in the axotomized facial nucleus (see Fig. 7; Kloss et al., 1999). A similar increase was also present in regenerating hypoglossal, vagal, and spinal motoneurons and in the DRGs (data not shown).
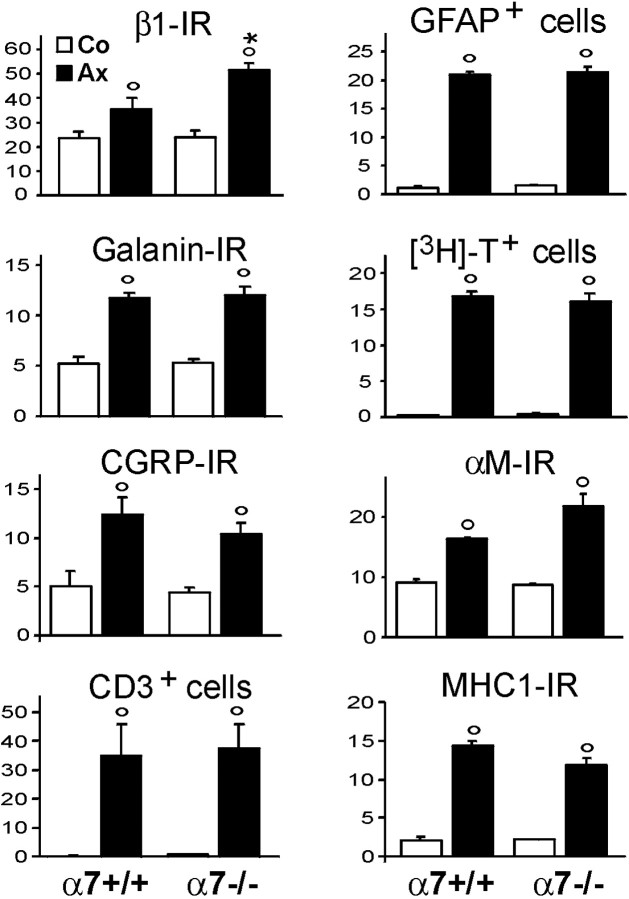
Fig. 7.
Quantitative effects of α7 deficiency on a panel of neuroglial activation markers. There is a significant increase in the immunoreactivity for the neuronal (β1, galanin, CGRP) and microglial (αM) markers, the number of GFAP-positive, stellate astrocytes, and the number of [3H]thymidine-labeled, proliferating cells in the axotomized facial motor nucleus 3 d after nerve transection compared to contralateral side. There is also a strong increase in microglial MHC1 immunoreactivity and the number of CD3-positive lymphocytes at day 14. Only the immunoreactivity for the β1 shows a statistically significant change in the axotomized nucleus between the two groups of animals, with an increase of 70% in the α7−/− mice. Open circle, Significant increase for the axotomized versus the contralateral side (p < 0.03, paired t test).Asterisk, Significant increase for the α7−/− versus the α7+/+ animals (p < 0.02, unpairedt test).
Central axotomy did not induce the expression of the α7β1 integrin (Fig. 2f–j). In the retina, α7 (red) and β1 (green) colocalized on the retinal blood vessels (Fig. 2f,g, yellow profiles), similar to the presence of α7β1 on the cerebral blood vessels (Velling et al., 1996). However, both integrin subunits were absent from the retinal ganglion cells in the normal retina and 4 d after optic nerve crush (Fig. 2g, asterisks). Similar lack of α7 immunoreactivity was also observed in the crushed optic nerve (Fig. 2j). The local accumulation of αMβ2-positive macrophages was used to demarcate the lesion site (Fig. 2j, arrows) and served as a positive control.
Cortical incision with ensuing transection of the cerebral cortex and the underlying corpus callosum and fimbria fornix caused moderate α7 labeling on blood vessels around the wound (Fig. 2h, arrows) but not on the adjacent cortical neurons, including the widely projecting pyramidal cells in the third cortical layer. No α7 increase was observed on the septal neurons affected by the transection of the fimbria fornix (Fig. 2i).
In the distal part of the crushed facial nerve, the specific immunoreactivity to the cytoplasmic part of the α7 subunit was restricted to regenerating axons, with moderate submembranous staining of growth cones and strong immunoreactivity in fine axonal sprouts (Fig. 3a–c). This immunoreactivity was not detected on the associated Schwann cells (s), irrespective of the stage of axon and myelin (m) detachment. Immunoreactivity against the extracellular part of the β1 subunit (Fig. 3d) showed a cell surface staining of axons (n) and the Schwann cells (s). Specific β1 staining was most pronounced at the axon–axon (arrow) and axon–Schwann cell contacts (arrowhead).
Fig. 3.
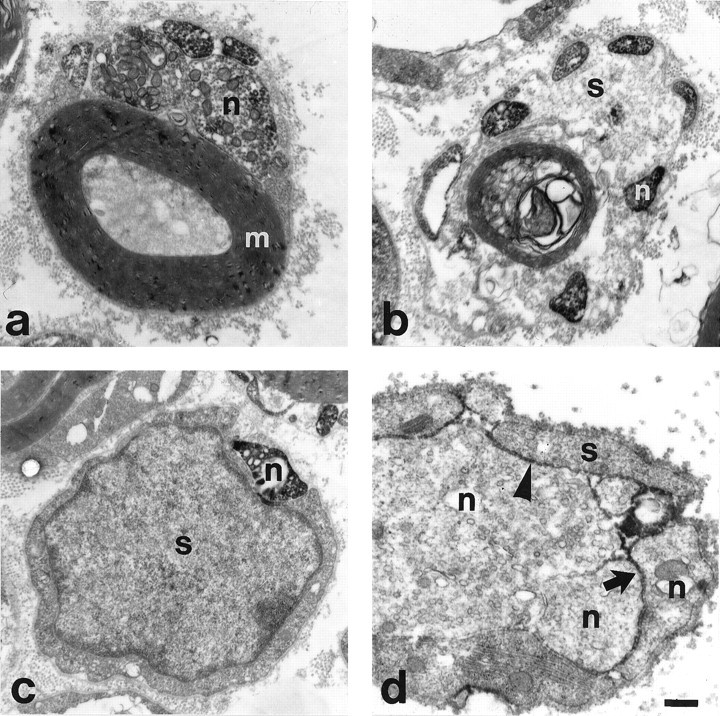
Regenerating facial neurites contain α7- and β1-integrin subunits at the ultrastructural level.a–c, α7- in regenerating motoneurites (n) 4 d after nerve crush in association with intact myelin (m) (a), with a partially demyelinated (b), and a completely demyelinated (c), α7-negative Schwann cell (s). The submembranous and cytoplasmic staining is attributable to immunoreactivity against the cytoplasmic part of the α7-subunit. d, The immune staining against the extracellular part of the β1-subunit is localized on the cell surface of regenerating neurites (n) and Schwann cells (s) at day 7. Pronounced staining at sites of axon–axon and axon–Schwann cell contacts (arrowhead). Scale bar, 0.45 μm.
Effects of α7 deficiency on axonal outgrowth and target reinnervation
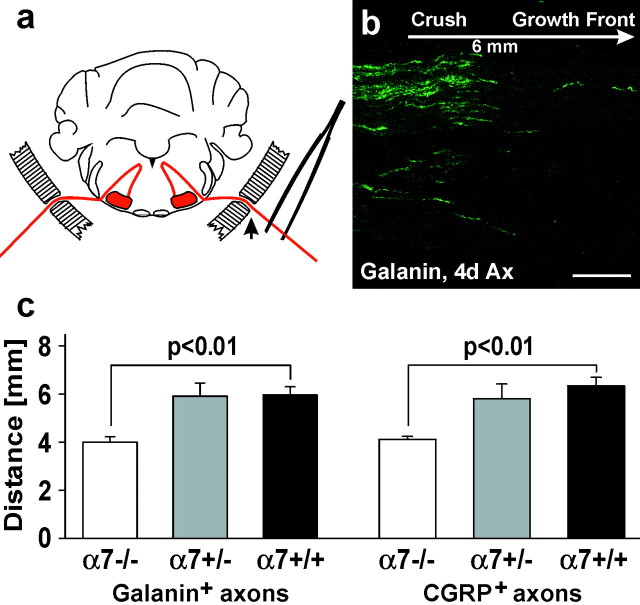
To examine the functional role of α7 integrin during nerve regeneration, we compared the regeneration rate in α7-deficient mice and wild-type controls obtained from heterozygous crossings (Mayer et al., 1997). The facial nerve was crushed near the stylomastoid foramen (Fig. 4a, arrow), allowed to regenerate for 4 d, fixed, and sectioned longitudinally. Nerve sections were stained for the neuropeptides CGRP or galanin (Fig.4b), and the distance between the lesion site and the axonal growth front was measured with a light microscopic grid scaling. At day 4, the regeneration distance for the wild-type animals was 6.01 ± 0.35 mm for galanin- and 6.38 ± 0.29 for CGRP-immunoreactive axons (Fig. 4c; mean ± SE,n = 4 animals). A similar distance was also determined for heterozygous mice (5.96 ± 0.55 for galanin and 5.84 ± 0.55 mm for CGRP-positive axons, n = 2). The homozygous α7-deficient mice (n = 4) showed a significant, 33–35% reduction to 4.04 ± 0.24 mm for galanin- and 4.15 ± 0.48 mm and for CGRP-immunoreactive axons (p< 0.01 for each axonal marker; unpaired t test).
Fig. 4.
α7 deficiency leads to reduced axonal outgrowth of axotomized facial motorneurons. a, The facial nerve was crushed near the foramen stylomastoideum (arrow).b, After 4 d, the nerve was cut longitudinally and stained for galanin or CGRP, which accumulate in the terminals of the elongating neurites. The average distance between the crush site and the growth front was determined for each axonal marker in five tissue sections per animal. The micrograph shows axonal regneration in a wild-type animal. c, Both the CGRP- and galanin-positive axonal populations show a regeneration distance of ∼6 mm at day 4 in the α7+/+ and α7+/−. This regeneration distance was reduced by 33% (galanin) and 35% (CGRP) in the α7−/− mice (α7−/−, α7+/+, n = 4 mice; α7+/−,n = 2 mice; mean values; error bar indicates SE). Scale bar, 200 μm.
Reduced axonal regeneration could lead to a delay in the reinnervation of the peripheral target. We first examined the time course of reinnervation of the whiskerpad in normal, C57Bl6 mice (Fig.5a–c). FG was applied into the whiskerpads on the operated and unoperated side at different time points after facial nerve crush. The number of retrogradely labeled facial motoneurons was counted 2 d after application of the retrograde tracer (Fig. 5a,b) and compared to the unoperated side. No FG-labeled motoneurons were observed in the operated facial motor nucleus 7 d after the initial facial nerve crush. At day 9, there was a steep increase to 53 ± 10% of the contralaterally labeled neurons, which reached a maximum at day 14 (Fig.5c).
To determine the effects of α7 deficiency, the number of FG-labeled motoneurons in α7−/− and α7+/+ mice were compared 9 and 21 d after facial nerve crush (Fig. 5d). At day 9, wild-type animals showed a definite onset of reinnervation, with 25 ± 9% of the motoneurons labeled compared to that on the contralateral side. At the same time point, there were no FG-positive motoneurons in the operated facial motor nucleus of the α7−/− mice (p < 0.05 compared to wild-type animals, Wilcoxon test). This effect disappeared at day 21, with both animal groups showing a similar level of whiskerpad reinnervation.
Neuroglial response in the facial motor nucleus of normal and α7−/− mice
To determine if α7 deficiency also causes central changes in the injured facial motor nucleus that could impair regeneration, we examined characteristic markers of the neuroglial response using established histology and immunohistochemistry protocols (Raivich et al., 1994; Möller et al., 1996), shown in Figure6. The neuronal response was assessed with immunohistochemistry for the β1 integrin subunit, CGRP and galanin, neuropeptides in axotomized motoneurons (Möller et al., 1996; Klein et al., 1997), and the astrocyte response with the number of GFAP-immunoreactive, stellate figures. The early microglial activation was examined using autoradiography for [3H]thymidine-labeled, proliferating cells and immunoreactivity for the αMβ2-integrin. For the late response at day 14 we stained against MHC-1 on the microglia and counted the number of CD3-positive, infiltrating lymphocytes. As shown in Figures 6 and 7, there was no apparent difference for the neuronal peptides, early and late glial activation markers, and the recruitment of lymphocytes between the wild-type and the α7-deficient mice, either with light microscopy (Fig. 6) or at the quantitative level (Fig. 7). However, there was a clear and statistically significant increase in the neuronal staining for the β1 integrin subunit on the regenerating motoneurons in the α7−/− mice (Figs. 6, 7).
Fig. 6.
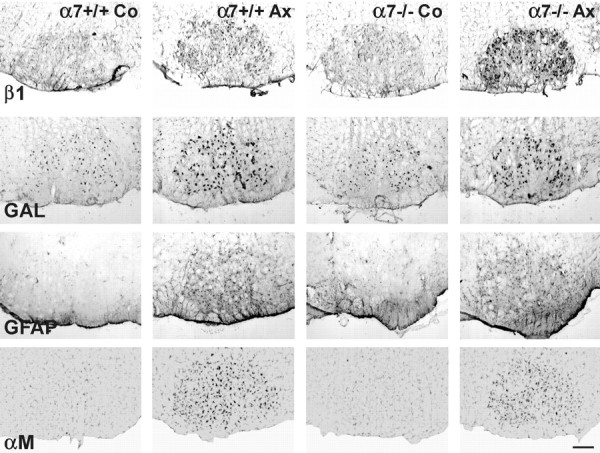
Effect of α7 deficiency on the neuroglial response. All activation markers increase 3 d after facial nerve transection in the regenerating facial motor nucleus (Ax), compared to the contralateral, unoperated side (Co). Note the stronger increase in the neuronal β1 integrin immunoreactivity after axotomy in the α7−/− mice. No apparent difference in the immunostaining for neuronal galanin (GAL), the GFAP-positive astrocytes or the αMβ2-positive microglia (αM) between the α7+/+ and α7−/− animals. Scale bar, 200 μm.
DISCUSSION
Expression of α7 and β1 integrin subunits is linked to peripheral nerve regeneration
The current study shows a highly consistent increase in α7β1 integrin immunoreactivity on axotomized motor and sensory neurons in different models of successful, peripheral regeneration. In contrast, this immunoreactivity for α7 and β1 subunits was not detected on intact and axotomized adult retinal ganglion cells, which normally do not regenerate. Similar lack of immunoreactivity was also observed for axotomized pyramidal cells and the septal neurons. Both the β1 integrin and different associated α-subunits are strongly expressed during brain and retinal development (Neugebauer and Reichardt, 1991;Tomaselli et al., 1993; Weaver et al., 1995; Kil and Bronner-Fraser, 1996; Velling et al., 1996). Previous studies also show an important role for β1 integrin family members in the neurite outgrowth of embryonic retinal ganglion cells (Neugebauer and Reichardt, 1991; Stone and Sakaguchi, 1996; Ivins et al., 1998; Treubert and Brummendorf, 1998). However, blocking antibodies against the β1 integrin subunit did not affect axonal regeneration in the adult retinal explants (Bates and Meyer, 1997). In view of the current findings, high levels of α7 and β1 integrin expression appear not to be linked to axotomy per se but to a successful type of axonal regeneration in vivo.
Quantitative methods to measure axonal regeneration
In the current study we used two separate methods to compare the speed of nerve regeneration in normal and α7 knock-out mice: at the early phase of axonal outgrowth and at the onset of target reinnervation. In the first method, we immunostained longitudinal nerve sections against the axon-specific neuropeptides CGRP and galanin (Gray et al., 1992). These peptides are synthesized in two apparently nonoverlapping motoneuron populations (Moore, 1989) and are transported anterogradely into the axonal growth cones. Because the facial nerve is almost purely motoric, the staining of the growth front is specific to motor axons. Four days after injury, the growth distance in normal mice was 6.01 ± 0.35 mm for the galanin- and 6.38 ± 0.29 mm for the CGRP-positive motor axons. Previous studies using electrophysiological and autoradiographic techniques revealed a regeneration rate of motor and sensory neurons of ∼4 mm/d (Forman and Berenberg, 1978; McQuarrie et al., 1978; Bisby and Keen, 1985; Chen and Bisby, 1993). However, they also showed an initial delay of ∼2 d (Forman and Berenberg, 1978). If this delaying effect was included, the resulting regeneration rate in the current study would be ∼3 mm/d.
The second method addressed the effects on the late stage of axonal regeneration, at the onset of target reinnervation. Here, we applied FG to the whiskerpads at different time points after crush (Hirota et al., 1996). The observed onset of FG uptake by reinnervating motor axons after day 7 corresponds well with the beginning of whiskerpad movements at day 9 (Chen and Bisby, 1993). Because the total distance between crush site and whiskerpad is ∼18 mm, the axons appear to grow at a rate of 3.6 mm/d, again assuming an initial delay of 48 hr. The slightly lower regeneration rate at the early phase of axonal outgrowth might be attributable to the previously reported suboptimal growth speed during the first days after injury (Forman and Berenberg, 1978). Overall, the current data on the speed of axonal outgrowth are in agreement with previous studies. Importantly, both methods used produce similar results and show little intragroup variation, allowing us to observe statistically significant changes in relatively small groups of animals.
α7 integrin subunit plays an important role in the regenerating facial nerve
Both α7 and β1 integrin subunits were present on growing axons in the distal part of the crushed facial nerve and participated in contacts among axons and between axons and Schwann cells. The importance of the α7β1 integrin is also underlined by transgenic experiments. α7 null mice show a reduced rate of axonal outgrowth and a delay in the reinnervation of the whiskerpad, a peripheral target of facial motoneurons. This effect of α7 deficiency was present in two nonoverlapping populations of facial motoneurons that express galanin or CGRP (Moore, 1989), pointing to a rather general role of this adhesion molecule in promoting regeneration.
The present experiments clearly suggest a peripheral site of α7β1 action. The α7β1 integrin is a specific receptor for laminin-1 (α1β1γ1), laminin-2 (α2β1γ1), and laminin-4 (α2β2γ1) (Kramer et al., 1991; von der Mark et al., 1991; Yao et al., 1996). The laminin-2 and laminin-4 isoforms are present in the normal peripheral nerve (Kuecherer-Ehret et al., 1990; Hsiao et al., 1993), and their synthesis is increased after injury (Kuecherer-Ehret et al., 1990; Doyu et al., 1993; LeBeau et al., 1994). At the ultrastructural level, laminin immunoreactivity is present on the Schwann cell surface and the basal membranes both facing the regenerating axons (Kuecherer-Ehret et al., 1990; Wang et al., 1992; Ide, 1996). Immune neutralization of laminin inhibits axonal growth inside the basal lamina scaffolds in peripheral nerves (Ide, 1996); a similar effect was observed using antibodies specific for the α2-laminin chain (Agius and Cochard, 1998). Thus, the α2β1γ1 and α2β2γ1 laminin isoforms are most likely, functionally active targets for the axonal α7β1 integrin in the regenerating peripheral nerve. In contrast to the periphery, antibodies against laminin failed to inhibit neurite outgrowth on the immature spinal cord substrate (Agius et al., 1996).
Absence of the α7 integrin subunit causes only a partial reduction in the speed of nerve fiber regeneration. This suggests the presence of additional axonal molecules promoting axon outgrowth, leading to a partial functional compensation for the α7 deficiency. The particularly strong increase of the β1 subunit after axotomy in the α7−/− mice clearly supports such a compensatory mechanism via other associated α-subunits (Toyota et al., 1990; Condic and Letourneau, 1997). Other potential groups include cadherins (Seilheimer and Schachner, 1988; Doherty et al., 1990) and the Ig superfamily of cell adhesion molecules (Seilheimer and Schachner, 1988; Doherty et al., 1990; Martini, 1994). Here, a conditional gene-targeting approach to these molecules in axotomized neurons will shed more light into the mechanisms regulating cell adhesion and the overall process of successful axonal regeneration.
α7 integrin deficiency does not affect the central neuroglial response
In contrast to axonal regeneration, the absence of the α7 integrin subunit did not lead to a change of the cellular response in the axotomized facial nucleus, the affected part of the CNS. Nerve transection is known to cause a pronounced cellular response at two different sites: distal to the site of axotomy, but also in and around the cell body of the affected neurons. Distal to the lesion, the nerve undergoes Wallerian degeneration, followed by the outgrowth of the sprouting axons into the distal nerve toward the peripheral tissues (Fawcett, 1992; Bisby, 1995).
At the level of the neuronal cell body, injured motoneurons increase protein synthesis, including adhesion molecules (Martini and Schachner, 1988; Möller et al., 1996; Jones et al., 1997) and neuropeptides (Raivich et al., 1995). Reactive astrocytes upregulate cytoskeletal proteins like GFAP and convert to a stellate type (Graeber and Kreutzberg, 1986; Raivich et al., 1999). Activated microglial cells proliferate, upregulate activation markers such as αMβ2 integrin (Raivich et al., 1994), and transform after neuronal cell death into phagocytes that degrade neuronal debris (Torvik and Skjorten, 1971;Kreutzberg, 1996; Klein et al., 1997). The upregulation of antigen presenting molecule MHC1 and costimulatory factors such as B7.2 and ICAM-1 (Raivich et al., 1998a; Werner et al., 1998; Bohatschek et al., 1999) on these phagocytotic cells coincides with the recruitment of lymphocytes (Raivich et al., 1998a).
Although these different aspects of glial activation have been thought to support nerve regeneration (Streit, 1993), recent studies have put some doubt on this notion. Thus, the cytostatic ablation of proliferating microglia with cytosine-arabinoside does not affect the speed of axonal regeneration in the hypoglossal nerve (Svensson and Aldskogius, 1993). A similar absence of effect on the regenerating facial nerve is also observed in mice that are deficient for the macrophage-colony stimulating factor with a severe reduction of microglial activation and proliferation (A. Werner and G. Raivich, unpublished observations). An alternative hypothesis is that neuronal injury leads to two separate sets of effects: it induces a neuronal regeneration program and at the same time, a central glial activation, with little effect of the latter on the former. This glial response was recently suggested to be part of the anti-infectious repertoire of the injured CNS, which could help to protect the damaged neurons and the surrounding environment from possible infection (Raivich et al., 1999). Interestingly, the data provided by the current study support the notion of two separate, relatively independent sets of effects. In addition to the reduction in axonal regeneration, the lack of the α7 subunit also causes a strong increase in the level of the associated neuronal β1 integrin. This upregulation of β1 may be part of a compensatory mechanism, to ensure successful regeneration in the injured peripheral nerve. Surprisingly, the contralateral, upoperated facial nucleus shows normal levels of β1 immunoreactivity both in the wild-type and the α7−/− mice, suggesting that the effect of α7 deficiency on the expression of β1 is triggered by the neuronal regeneration program.
In contrast, the absence of α7 did not appear to affect the response to injury by glia or lymphocytes, suggesting that the immune surveillance of the injured CNS is unaffected by the absence of the α7 subunit. At present, little is known about the neuronal trigger that initiates these central reactions. However, the absence of the α7 integrin subunit did not affect the expression of neuronal peptides like CGRP or galanin. In vitro, these neuropeptides play an important role in the activation of astrocytes and microglia (Lazar et al., 1991; Priller et al., 1998) and might be central mediators of the glial response.
In summary, the current study suggests that the α7β1 integrin is an important mediator of axonal regeneration. Axotomy leads to a highly consistent increase of α7β1 integrin immunoreactivity on axotomized motor and sensory neurons in different models of successful regeneration. Both the α7 and β1 subunit are concentrated on growth cones in the regenerating nerve, and the transgenic deletion of the α7 subunit led to a reduced rate of axonal elongation in the axotomized nerve and a delayed target reinnervation. Moreover, the lack of changes in the central neuroglial response to injury clearly indicates a peripheral site of action for this cell adhesion molecule in the interaction with the extracellular matrix in the injured peripheral nerve.
Footnotes
This work was supported by grants 01KO9401/3 and 01KO9703/3 of the Bundesministerium für Bildung und Forschung. We thank Andrea Koppius, Dietmute Büringer, and Karin Brückner for their expert technical assistance and Dr. Jim Chalcroft for his help with digital photography.
Correspondence should be addressed to Dr. Gennadij Raivich, Department of Neuromorphology, Max-Planck-Institute of Neurobiology, Am Klopferspitz 18a 82152 Martinsried, Germany. E-mail:Raivich@neuro.mpg.de.
REFERENCES
- 1.Agius E, Cochard P. Comparison of neurite outgrowth induced by intact and injured sciatic nerves: a confocal and functional analysis. J Neurosci. 1998;18:328–338. doi: 10.1523/JNEUROSCI.18-01-00328.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agius E, Sagot Y, Duprat AM, Cochard P. Antibodies directed against the beta 1-integrin subunit and peptides containing the IKVAV sequence of laminin perturb neurite outgrowth of peripheral neurons on immature spinal cord substrata. Neuroscience. 1996;71:773–786. doi: 10.1016/0306-4522(95)00447-5. [DOI] [PubMed] [Google Scholar]
- 3.Bates CA, Meyer RL. The neurite-promoting effect of laminin is mediated by different mechanisms in embryonic and adult regenerating mouse optic axons in vitro. Dev Biol. 1997;181:91–101. doi: 10.1006/dbio.1996.8438. [DOI] [PubMed] [Google Scholar]
- 4.Bisby MA. Regeneration of peripheral nervous system axons. In: Waxman SG, Kocsis JD, Stys PK, editors. The axon. Oxford UP; New York: 1995. pp. 553–578. [Google Scholar]
- 5.Bisby MA, Keen P. The effect of a conditioning lesion on the regeneration rate of peripheral nerve axons containing substance P. Brain Res. 1985;336:201–206. doi: 10.1016/0006-8993(85)90646-8. [DOI] [PubMed] [Google Scholar]
- 6.Bohatschek M, Gschwendtner A, von Maltzan X, Kloss CUA, Pfeffer K, Labow M, Bluthmann H, Kreutzberg GW, Raivich G. Cytokine mediated regulation of MHC-1, MHC-2 and B7–2 in the axotomized mouse facial motor nucleus. Soc Neurosci Abstr. 1999;25:1535. [Google Scholar]
- 7.Brown MC, Lunn ER, Perry VH. Consequences of slow Wallerian degeneration for regenerating motor and sensory axons. J Neurobiol. 1992;23:521–536. doi: 10.1002/neu.480230507. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Bisby MA. Impaired motor axon regeneration in the C57BL/Ola mouse. J Comp Neurol. 1993;333:449–454. doi: 10.1002/cne.903330310. [DOI] [PubMed] [Google Scholar]
- 9.Collo G, Starr L, Quaranta V. A new isoform of the laminin receptor integrin alpha 7 beta 1 is developmentally regulated in skeletal muscle. J Biol Chem. 1993;268:19019–19024. [PubMed] [Google Scholar]
- 10.Condic ML, Letourneau PC. Ligand-induced changes in integrin expression regulate neuronal adhesion and neurite outgrowth. Nature. 1997;389:852–856. doi: 10.1038/39878. [DOI] [PubMed] [Google Scholar]
- 11.Cornbrooks CJ, Carey DJ, McDonald JA, Timpl R, Bunge RP. In vivo and in vitro observations on laminin production by Schwann cells. Proc Natl Acad Sci USA. 1983;80:3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty P, Cohen J, Walsh FS. Neurite outgrowth in response to transfected N-CAM changes during development and is modulated by polysialic acid. Neuron. 1990;5:209–219. doi: 10.1016/0896-6273(90)90310-c. [DOI] [PubMed] [Google Scholar]
- 13.Doyu M, Sobue G, Ken E, Kimata K, Shinomura T, Yamada Y, Mitsuma T, Takahashi A. Laminin A, B1, and B2 chain gene expression in transected and regenerating nerves: regulation by axonal signals. J Neurochem. 1993;60:543–551. doi: 10.1111/j.1471-4159.1993.tb03183.x. [DOI] [PubMed] [Google Scholar]
- 14.Fawcett JW. Intrinsic neuronal determinants of regeneration. Trends Neurosci. 1992;15:5–8. doi: 10.1016/0166-2236(92)90338-9. [DOI] [PubMed] [Google Scholar]
- 15.Forman DS, Berenberg RA. Regeneration of motor axons in the rat sciatic nerve studied by labeling with axonally transported radioactive proteins. Brain Res. 1978;156:213–225. doi: 10.1016/0006-8993(78)90504-8. [DOI] [PubMed] [Google Scholar]
- 16.Graeber MB, Kreutzberg GW. Astrocytes increase in glial fibrillary acidic protein during retrograde changes of facial motor neurons. J Neurocytol. 1986;15:363–373. doi: 10.1007/BF01611438. [DOI] [PubMed] [Google Scholar]
- 17.Gray C, Hukkanen M, Konttinen YT, Terenghi G, Arnett TR, Jones SJ, Burnstock G, Polak JM. Rapid neural growth: calcitonin gene-related peptide and substance P-containing nerves attain exceptional growth rates in regenerating deer antler. Neuroscience. 1992;50:953–963. doi: 10.1016/0306-4522(92)90218-q. [DOI] [PubMed] [Google Scholar]
- 18.Haas TA, Plow EF. Integrin-ligand interactions: a year in review. Curr Opin Cell Biol. 1994;6:656–662. doi: 10.1016/0955-0674(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 19.Hirota H, Kiyama H, Kishimoto T, Taga T. Accelerated Nerve Regeneration in Mice by upregulated expression of interleukin (IL) 6 and IL-6 receptor after trauma. J Exp Med. 1996;183:2627–2634. doi: 10.1084/jem.183.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsiao LL, Engvall E, Peltonen J, Uitto J. Expression of laminin isoforms by peripheral nerve-derived connective tissue cells in culture. Comparison with epitope distribution in normal human nerve and neural tumors in vivo. Lab Invest. 1993;68:100–108. [PubMed] [Google Scholar]
- 21.Ide C. Peripheral nerve regeneration. Neurosci Res. 1996;25:101–121. doi: 10.1016/0168-0102(96)01042-5. [DOI] [PubMed] [Google Scholar]
- 22.Ivins JK, Colognato H, Kreidberg JA, Yurchenco PD, Lander AD. Neuronal receptors mediating responses to antibodyactivated laminin-1. J Neurosci. 1998;18:9703–9715. doi: 10.1523/JNEUROSCI.18-23-09703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones LL, Kreutzberg GW, Raivich G. Regulation of CD44 in the regenerating mouse facial motor nucleus. Eur J Neurosci. 1997;9:1854–1863. doi: 10.1111/j.1460-9568.1997.tb00752.x. [DOI] [PubMed] [Google Scholar]
- 24.Kil SH, Bronner-Fraser M. Expression of the avian alpha 7-integrin in developing nervous system and myotome. Int J Dev Neurosci. 1996;14:181–190. doi: 10.1016/0736-5748(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 25.Klein MA, Möller JC, Jones LL, Bluethmann H, Kreutzberg GW, Raivich Impaired neuroglial activation in interleukin-6 deficient mice. Glia. 1997;19:227–233. doi: 10.1002/(sici)1098-1136(199703)19:3<227::aid-glia5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Kloss CUA, Shen YJ, Menuz K, Probst JC, Kreutzberg GW, Raivich G. The integrin family of cell adhesion molecules in the injured brain: regulation and cellular localization in the normal and regenerating mouse facial motor nucleus. J Comp Neurol. 1999;411:162–178. doi: 10.1002/(sici)1096-9861(19990816)411:1<162::aid-cne12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Kramer RH, Vu MP, Cheng YF, Ramos DM, Timpl R, Waleh N. Laminin-binding integrin alpha 7 beta 1: functional characterization and expression in normal and malignant melanocytes. Cell Regul. 1991;2:805–817. doi: 10.1091/mbc.2.10.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 29.Kuecherer-Ehret A, Graeber MB, Edgar D, Thoenen H, Kreutzberg GW. Immunoelectron microscopic localization of laminin in normal and regenerating mouse sciatic nerve. J Neurocytol. 1990;19:101–109. doi: 10.1007/BF01188442. [DOI] [PubMed] [Google Scholar]
- 30.Lazar P, Reddington M, Streit W, Raivich G, Kreutzberg GW. The action of calcitonin gene-related peptide on astrocyte morphology and cyclic AMP accumulation in astrocyte cultures from neonatal rat brain. Neurosci Lett. 1991;130:99–102. doi: 10.1016/0304-3940(91)90237-n. [DOI] [PubMed] [Google Scholar]
- 31.LeBeau JM, Liuzzi FJ, Depto AS, Vinik AI. Differential laminin gene expression in dorsal root ganglion neurons and nonneuronal cells. Exp Neurol. 1994;127:1–8. doi: 10.1006/exnr.1994.1074. [DOI] [PubMed] [Google Scholar]
- 32.Lefcort F, Venstrom K, McDonald JA, Reichardt LF. Regulation of expression of fibronectin and its receptor, alpha 5 beta 1, during development and regeneration of peripheral nerve. Development. 1992;116:767–782. doi: 10.1242/dev.116.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letourneau PC, Condic ML, Snow DM. Extracellular matrix and neurite outgrowth. Curr Opin Genet Dev. 1992;2:625–634. doi: 10.1016/s0959-437x(05)80183-2. [DOI] [PubMed] [Google Scholar]
- 34.Luckenbill-Edds L. Laminin and the mechanism of neuronal outgrowth. Brain Res Brain Res Rev. 1997;23:1–27. doi: 10.1016/s0165-0173(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 35.Martin PT, Kaufman SJ, Kramer RH, Sanes JR. Synaptic integrins in developing, adult, and mutant muscle: selective association of alpha1, alpha7A, and alpha7B integrins with the neuromuscular junction. Dev Biol. 1996;174:125–139. doi: 10.1006/dbio.1996.0057. [DOI] [PubMed] [Google Scholar]
- 36.Martini R. Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. J Neurocytol. 1994;23:1–28. doi: 10.1007/BF01189813. [DOI] [PubMed] [Google Scholar]
- 37.Martini R, Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and myelin-associated glycoprotein) in regenerating adult mouse sciatic nerve. J Cell Biol. 1988;106:1735–1746. doi: 10.1083/jcb.106.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer U, Saher G, Fassler R, Bornemann A, Echtermeyer F, von der M, Miosge N, Poschl E. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- 39.McQuarrie IG, Grafstein B, Dreyfus CF, Gershon MD. Regeneration of adrenergic axons in rat sciatic nerve: effect of a conditioning lesion. Brain Res. 1978;141:21–34. doi: 10.1016/0006-8993(78)90614-5. [DOI] [PubMed] [Google Scholar]
- 40.Möller JC, Klein MA, Haas S, Jones LL, Kreutzberg GW, Raivich G. Regulation of thrombospondin in the regenerating mouse facial motor nucleus. Glia. 1996;17:121–132. doi: 10.1002/(SICI)1098-1136(199606)17:2<121::AID-GLIA4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Moore RY. Cranial motor neurons contain either galanin- or calcitonin gene-related peptidelike immunoreactivity. J Comp Neurol. 1989;282:512–522. doi: 10.1002/cne.902820404. [DOI] [PubMed] [Google Scholar]
- 42.Neugebauer KM, Reichardt LF. Cell-surface regulation of beta 1-integrin activity on developing retinal neurons. Nature. 1991;350:68–71. doi: 10.1038/350068a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Priller J, Haas CA, Reddington M, Kreutzberg GW. Cultured astrocytes express functional receptors for galanin. Glia. 1998;24:323–328. doi: 10.1002/(sici)1098-1136(199811)24:3<323::aid-glia6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 44.Raivich G, Moreno-Flores MT, Möller JC, Kreutzberg GW. Inhibition of posttraumatic microglial proliferation in a genetic model of macrophage colony-stimulating factor deficiency in the mouse. Eur J Neurosci. 1994;6:1615–1618. doi: 10.1111/j.1460-9568.1994.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 45.Raivich G, Reddington M, Haas CA, Kreutzberg GW. Peptides in motoneurons. Prog Brain Res. 1995;104:3–20. [PubMed] [Google Scholar]
- 46.Raivich G, Haas S, Werner A, Klein MA, Kloss C, Kreutzberg GW. Regulation of MCSF receptors on microglia in the normal and injured mouse central nervous system: a quantitative immunofluorescence study using confocal laser microscopy. J Comp Neurol. 1998a;395:342–358. doi: 10.1002/(sici)1096-9861(19980808)395:3<342::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 47.Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998b;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raivich G, Bohatschek M, Kloss CUA, Werner A, Jones LL, Kreutzberg GW. The neuroglial activation repertoire in the injured brain: molecular mechanisms and cues to physiological function. Brain Res Rev. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 49.Salonen V, Peltonen J, Roytta M, Virtanen I. Laminin in traumatized peripheral nerve: basement membrane changes during degeneration and regeneration. J Neurocytol. 1987;16:713–720. doi: 10.1007/BF01637662. [DOI] [PubMed] [Google Scholar]
- 50.Seilheimer B, Schachner M. Studies of adhesion molecules mediating interactions between cells of peripheral nervous system indicate a major role for L1 in mediating sensory neuron growth on Schwann cells in culture. J Cell Biol. 1988;107:341–351. doi: 10.1083/jcb.107.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song WK, Wang W, Foster RF, Bielser DA, Kaufman SJ. H36-alpha 7 is a novel integrin alpha chain that is developmentally regulated during skeletal myogenesis. J Cell Biol. 1992;117:643–657. doi: 10.1083/jcb.117.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stone KE, Sakaguchi DS. Perturbation of the developing Xenopus retinotectal projection following injections of antibodies against beta1 integrin receptors and N-cadherin. Dev Biol. 1996;180:297–310. doi: 10.1006/dbio.1996.0302. [DOI] [PubMed] [Google Scholar]
- 53.Streit WJ. Microglial-neuronal interactions. J Chem Neuroanat. 1993;6:261–266. doi: 10.1016/0891-0618(93)90047-8. [DOI] [PubMed] [Google Scholar]
- 54.Svensson M, Aldskogius H. Regeneration of hypoglossal nerve axons following blockade of the axotomy-induced microglial cell reaction in the rat. Eur J Neurosci. 1993;5:85–94. doi: 10.1111/j.1460-9568.1993.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 55.Tomaselli KJ, Doherty P, Emmett CJ, Damsky CH, Walsh FS, Reichardt LF. Expression of beta 1 integrins in sensory neurons of the dorsal root ganglion and their functions in neurite outgrowth on two laminin isoforms. J Neurosci. 1993;13:4880–4888. doi: 10.1523/JNEUROSCI.13-11-04880.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torvik A, Skjorten F. Electron microscopic observations on nerve cell regeneration and degeneration after axon lesions. I. Changes in the nerve cell cytoplasm. Acta Neuropathol. 1971;17:248–264. doi: 10.1007/BF00685058. [DOI] [PubMed] [Google Scholar]
- 57.Toyota B, Carbonetto S, David S. A dual laminin/collagen receptor acts in peripheral nerve regeneration. Proc Natl Acad Sci USA. 1990;87:1319–1322. doi: 10.1073/pnas.87.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Treubert U, Brummendorf T. Functional cooperation of beta1-integrins and members of the Ig superfamily in neurite outgrowth induction. J Neurosci. 1998;18:1795–1805. doi: 10.1523/JNEUROSCI.18-05-01795.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van der Flier A, Kuikman I, Baudoin C, van der Neut R, Sonnenberg A. A novel beta 1 integrin isoform produced by alternative splicing: unique expression in cardiac and skeletal muscle. FEBS Lett. 1995;369:340–344. doi: 10.1016/0014-5793(95)00814-p. [DOI] [PubMed] [Google Scholar]
- 60.Velling T, Collo G, Sorokin L, Durbeej M, Zhang H, Gullberg D. Distinct alpha 7A beta 1 and alpha 7B beta 1 integrin expression patterns during mouse development: alpha 7A is restricted to skeletal muscle but alpha 7B is expressed in striated muscle, vasculature, and nervous system. Dev Dyn. 1996;207:355–371. doi: 10.1002/(SICI)1097-0177(199612)207:4<355::AID-AJA1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 61.von der Mark H, Durr J, Sonnenberg A, Deutzmann R, Goodman SL. Skeletal myoblasts utilize a novel beta 1-series integrin and not alpha 6 beta 1 for binding to the E8 and T8 fragments of laminin. J Biol Chem. 1991;266:23593–23601. [PubMed] [Google Scholar]
- 62.Wang GY, Hirai K, Shimada H, Taji S, Zhong SZ. Behavior of axons, Schwann cells and perineurial cells in nerve regeneration within transplanted nerve grafts: effects of anti-laminin and anti-fibronectin antisera. Brain Res. 1992;583:216–226. doi: 10.1016/s0006-8993(10)80027-7. [DOI] [PubMed] [Google Scholar]
- 63.Weaver CD, Yoshida CK, de Curtis I, Reichardt LF. Expression and in vitro function of beta 1-integrin laminin receptors in the developing avian ciliary ganglion. J Neurosci. 1995;15:5275–5285. doi: 10.1523/JNEUROSCI.15-07-05275.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Werner A, Kloss CUA, Walter J, Kreutzberg GW, Raivich G. Intercellular adhesion molecule-1 (ICAM1) in the regenerating mouse facial motor nucleus. J Neurocytol. 1998;27:219–232. doi: 10.1023/a:1006928830251. [DOI] [PubMed] [Google Scholar]
- 65.Yao CC, Ziober BL, Squillace RM, Kramer RH. Alpha7 integrin mediates cell adhesion and migration on specific laminin isoforms. J Biol Chem. 1996;271:25598–25603. doi: 10.1074/jbc.271.41.25598. [DOI] [PubMed] [Google Scholar]
- 66.Ziober BL, Vu MP, Waleh N, Crawford J, Lin CS, Kramer RH. Alternative extracellular and cytoplasmic domains of the integrin alpha 7 subunit are differentially expressed during development. J Biol Chem. 1993;268:26773–26783. [PubMed] [Google Scholar]