Abstract
We have used rapid confocal microscopy to investigate the mechanism of Ca2+ signals in individual dendritic spines of hippocampal CA1 pyramidal cells. The experiments focused on the signals that occur during single weak synaptic responses that were subthreshold for triggering postsynaptic action potentials. These Ca2+ signals were not strongly affected by blocking the EPSPs with the AMPA receptor antagonist CNQX. The signals were also not strongly reduced by blocking T-type voltage-gated Ca2+ channels (VGCCs) with Ni2+or by blocking a broad range of VGCCs with intracellular D890. The spine Ca2+ signals were blocked by NMDA receptor channel (NMDAR) antagonist and had the voltage dependence characteristic of these channels. Neither ryanodine nor cyclopiazonic acid (CPA), substances known to deplete intracellular Ca2+ stores, substantially reduced the amplitude of synaptically evoked Ca2+ signals. CPA slowed the recovery phase of Ca2+ signals in spines produced by synaptic stimulation or by backpropagating action potentials, suggesting a role of intracellular stores in Ca2+reuptake. Thus, we find that Ca2+ release from intracellular stores is not required to produce spine Ca2+ signals. We conclude that synaptic Ca2+ signals in spines are primarily caused by Ca2+ entry through NMDARs. Although these channels are largely blocked by Mg2+ at voltages near the resting potential, they can nevertheless produce significant Ca2+ elevation. The resulting Ca2+ signals are an integral component of individual evoked or spontaneous synaptic events and may be important in the maintenance of synaptic function.
Keywords: dendritic spines, NMDA, Ca2+ channels, Ca2+ stores, subthreshold Ca2+signals, hippocampus, ryanodine, CPA, confocal microscopy
Dendritic spines are the sites of excitatory synaptic input into many types of neurons of the mammalian CNS (Harris and Kater, 1994). Recent advances in Ca2+ imaging have made it possible to detect Ca2+ concentration changes in individual spines during single action potentials or synaptic responses (Yuste and Denk, 1995; Eilers and Konnerth, 1997; Köster and Sakmann, 1998). Depending on how many synaptic inputs into the cell are activated, the EPSP may be either subthreshold or suprathreshold for action potentials. Work on hippocampal neurons has shown that the large depolarization that occurs during suprathreshold stimulation leads to activation of NMDA receptor channels (NMDARs) and that the resulting Ca2+ signals depend on this activation (Regehr and Tank, 1990; Müller and Connor, 1991; Alford et al., 1993; Malinow et al., 1994). These signals are important in triggering the long-term synaptic potentiation (LTP) produced by strong afferent stimulation (Bliss and Collingridge, 1993).
If fewer synapses are activated, action potentials do not occur, but smaller Ca2+ signals in active spines can still be detected (Denk et al., 1995; Eilers et al., 1995; Yuste and Denk, 1995; Finch and Augustine, 1998; Köster and Sakmann, 1998;Takechi et al., 1998; Mainen et al., 1999). These are termed subthreshold signals and have been observed in a variety of cells. In cortical pyramidal cells there have been two very different proposals about their mechanism. According to one proposal, the dominant source of Ca2+ is caused by voltage-gated calcium channels (VGCCs) activated by the depolarization caused by NMDA channels (Schiller et al., 1998). According to the other proposal, the dominant source is Ca2+ entry through the NMDA channel itself (Köster and Sakmann, 1998), an entry that can occurs at resting potential, even when the large AMPA receptor-mediated component of EPSP is blocked. Recent high-resolution imaging work on hippocampal spines has also led to conflicting views. One group has argued that most of the Ca2+ elevation is attributable to Ca2+ entry through NMDARs, but that this entry requires the EPSP to open the NMDA channels (Yuste et al., 1999). Another group has argued that the Ca2+ that enters through NMDARs is very small and must be greatly amplified by intracellular Ca2+ release to be detected (Emptage et al., 1999). Yet another group, using imaging methods with lower spatial resolution, has argued that the subthreshold Ca2+ signal in dendrites is attributable to Ca2+ entry through VGCC, but not through the NMDA channels (Magee et al., 1995).
These apparent disagreements may arise in part because different groups have used somewhat different preparations, different approaches, and have focused on particular mechanisms. There is therefore a need for a systematic examination of all possibilities in the same preparation. It has previously been difficult to identify the component of Ca2+ entry that is directly through NMDARs because blocking these channels blocks both the Ca2+ entry and the depolarization caused by these channels. This depolarization may normally activate VGCC. Here we introduce a method for blocking VGCC from the cytoplasmic side, a method that greatly simplifies the dissection of the signals.
MATERIALS AND METHODS
Experiments were performed on CA1 pyramidal neurons from 300-μm-thick hippocampal slices from 10- to 21-d-old [postnatal day 10 (P10)–P21] Wistar rats (Edwards et al., 1989). Slices were incubated at 33°C in oxygenated standard solution (see below) for at least 40–60 min before transferring them into the recording chamber. The standard solution contained (in mm): 125 NaCl, 2.5 or 3.5 KCl, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 20 glucose, and 0.01 bicuculline, bubbled with 95% O2 and 5% CO2. In some experiments 50 μmdl-2-amino-5-phosphopentanoic acid (dl-APV), 5 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 40–50 μm Ni2+, 20–25 μm ryanodine (Calbiochem, La Jolla, CA), or 30 μm cyclopiazonic acid (CPA) were added to the extracellular solution. Combined electrophysiological recordings and confocal Ca2+ imaging were performed with, respectively, an EPC9 patch-clamp amplifier (Heka, Lambrecht, Germany) and a confocal laser-scanning system (Noran Oz or Noran Odyssey, on Olympus BX50WI microscope, 60× water immersion objective, numerical aperture 0.9). The pipette solution contained (in mm): 140 KCl or K-gluconate, 10 NaCl, 4 Mg-ATP, 0.4 Na-GTP, 10 K-HEPES, 0.10–0.25 Oregon Green 488 BAPTA-1 (Oregon Green;Kd, ∼200 nm;Molecular Probes, Eugene, OR). The pH was adjusted to 7.3 with KOH. In some recordings, the low-affinity calcium indicator dye Magnesium Green (0.3 mm;Kd, ∼6 μm, Molecular Probes) was used. Mg-ATP was replaced by Na2-ATP when Magnesium Green was used. In some experiments 1–2 mm D890 (Knoll, Ludwigshafen, Germany) was added to the pipette solution. At this concentration, in addition to blocking completely currents through voltage-gated Ca2+ channels (Hescheler et al., 1982), D890 also partially blocks voltage-gated Na+ and K+channels, thus allowing a voltage control that is comparable to that obtained when using intracellular Cs+ (O. Garaschuk and Y. Kovalchuk, unpublished observations). The pipette resistance ranged from 2.5 to 3.5 MΩ and the series resistance from 12 to 25 MΩ. No series resistance compensation was applied. Whole-cell recordings were performed at room temperature (21–22°C, if not otherwise indicated) or at 30–32°C (see figure legends). For synaptic stimulation of afferent fibers, voltage pulses (5–20 V, 100 μsec duration) were delivered through a glass pipette that was positioned extracellularly under visual control close to the dendrites under study. The stimulation strength selected was weak and produced Ca2+ signals that were detectable just in a small number of spines (Malinow et al., 1994). Active dendritic spines were identified by imaging the fluorescence increase in response to a burst of three stimuli given at 50 Hz. Generally, the synaptic Ca2+ signals were evoked repeatedly every 2–4 min, and the laser intensity was set to <2–8 μW (measured under the objective). This allowed us to obtain stable Ca2+ recordings over a period of at least 1 hr. In addition, the viability of the dendritic segments under study was tested throughout the experiment by monitoring AP-evoked Ca2+ transients (evoked by short depolarization through the somatic patch pipette). Caffeine (20 or 40 mm) was puffed locally to dendrites from fine-tipped pipettes (Garaschuk et al., 1997). Unless otherwise indicated, chemicals were purchased from Sigma (St. Louis, MO). Fluorescence data are expressed as background-corrected changes in Ca2+-dependent fluorescence divided by the prestimulus fluorescence, ΔF/F. Computer-based data analysis was performed by using Image 1 (Universal Image, West Chester, PA), Igor Pro (Wavemetrics, Lake Oswego, OR), and SigmaStat 2.0 (Jandel Scientific, San Rafael, CA) software.
RESULTS
Experiments were performed on CA1 pyramidal cells in acute rat hippocampal slices. Somatic whole-cell recordings were obtained from visually identified cells near the top surface of the slice. A fluorescent Ca2+ indicator dye (see Materials and Methods) was introduced into the cytosol by diffusion from the patch pipette (concentration range, 100–250 μm). After a period of 30 min, dendrites became highly fluorescent, and single spines on apical dendrites could be easily resolved by confocal imaging (Fig.1A,B). To evoke synaptic responses by focal stimulation, a fine-tipped stimulation pipette was introduced into the stratum radiatum near the dendrite of choice. By adjusting the pipette position, it was possible to find a position at which a brief current pulse evoked a Ca2+ transient in one or more spines. Under these conditions, the EPSP is generally caused both by transmission at the spine in which Ca2+transients were observed and to other synapses not in the field of view. The size of the EPSP was in the range of 1–12 mV and was always below the amplitude required to fire an action potential (AP). We therefore term the Ca2+ signals “subthreshold Ca2+ signals” to distinguish them from the more complex Ca2+ signals that occur when action potentials are involved.
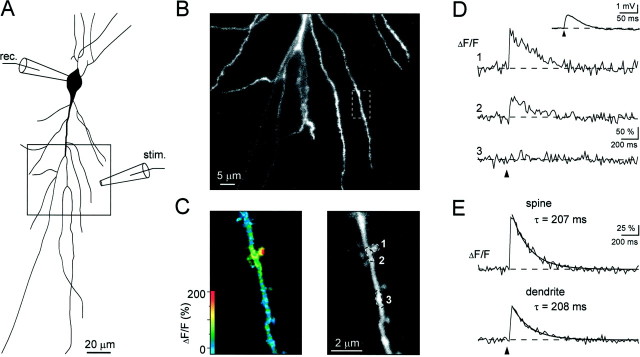
Fig. 1.
Subthreshold synaptic Ca2+signals in individual dendritic spines of CA1 hippocampal pyramidal neurons. A, Camera lucida drawing of a CA1 pyramidal cell (P20), injected with the calcium indicator dye Oregon Green (100 μm) through the recording patch pipette. Afferent fibers were stimulated with an extracellular stimulation electrode located in the stratum radiatum, as shown schematically. B,Confocal image of the boxed dendritic region shown inA. The dashed box indicates the dendritic region that was active during synaptic stimulation, see C–E. C,Left, pseudocolor image of the fluorescence change (ΔF/F) that occurred during the first 150 msec after a single shock synaptic stimulation. Right, Grayscale image of the active dendrite with indicated regions of interest that were analyzed in D. D, waveform of the Ca2+-dependent fluorescence change (ΔF/F) from a spine (trace 1) and two dendritic regions (traces 2 and 3) during single-shock stimulation. The associated EPSP is shown in theinset. Same recording as in C. E,averaged fluorescence transients (n = 6) recorded in the spine and the parent dendrite (region 1 and 2 inC, respectively). Both traces have been fitted with monoexponential functions that had almost identical decay time constants but clearly different amplitudes. The holding potential was −65 mV; recordings were made at 30°C. In this and the following figures arrowheads mark the time points of synaptic stimulation.
The signals evoked by single shocks were highly localized. In the experiment illustrated in Figure 1, the largest signals were observed in the spine at position 1, but there was also a smaller signal in the parent dendrite (position 2). At a distance of ∼4 μm along the dendrite (position 3), there was no detectable signal (Fig.1C,D). With the relatively low indicator concentrations used in this experiment (100 μm Oregon Green), the decay time constant of the signals was ∼200 msec in both the active spine and nearby regions of the parent dendrite (Fig.1E). In other cells, when using higher indicator concentrations (up to 250 μm), decay times of up to 750 msec were encountered (480 ± 260 msec;n = 18; mean ± SD). This signal is similar to that reported by Yuste and Denk (1995) using two-photon microscopy in the line-scanning mode. The rapid confocal imaging (60–120 frames/sec) used here provides information not only about the spine head, but also about nearby regions of the dendrite and reveals a dendritic signal, linked to the spine signal. The peak amplitude of the dendritic signal was ∼20–70% of that recorded in the spine. The amplitude was largest in the immediate neighborhood of the spine and gradually decreased up and down the dendrite. In general, with single-shock stimulation, no Ca2+ signal was detected at distances >4–5 μm from the spine.
To study the mechanism of subthreshold Ca2+ signals, we first investigated the role of the AMPA and NMDA receptor type of glutamate channels. It was useful in this experiment, performed at room temperature, to use a burst of stimulation as the standard stimulus (typically two or three stimuli at 33 or 50 Hz; repetition time, 2 min). This reduced the variability of the response, because we and others have found that at room temperature, the responses to individual stimuli have a significant fraction of failures, presumably because of failure of vesicle release (Hessler et al., 1993; Allen and Stevens, 1994). Figure2 shows that CNQX, an antagonist of AMPA receptor channels, produced at resting membrane potential (−65 mV) only a small (∼30%) reduction in the average spine signal even though the EPSP was almost totally abolished. When the NMDAR antagonist APV was added in addition, the average spine Ca2+ signal was almost totally abolished. Removal of both antagonists restored both the EPSP and the spine signal. Figure 2C summarizes similar results from six experiments. In a separate series of experiments, we found that addition of APV alone greatly reduced the spine signals (by 89 ± 10%; n = 6), but a small residual signal remained.
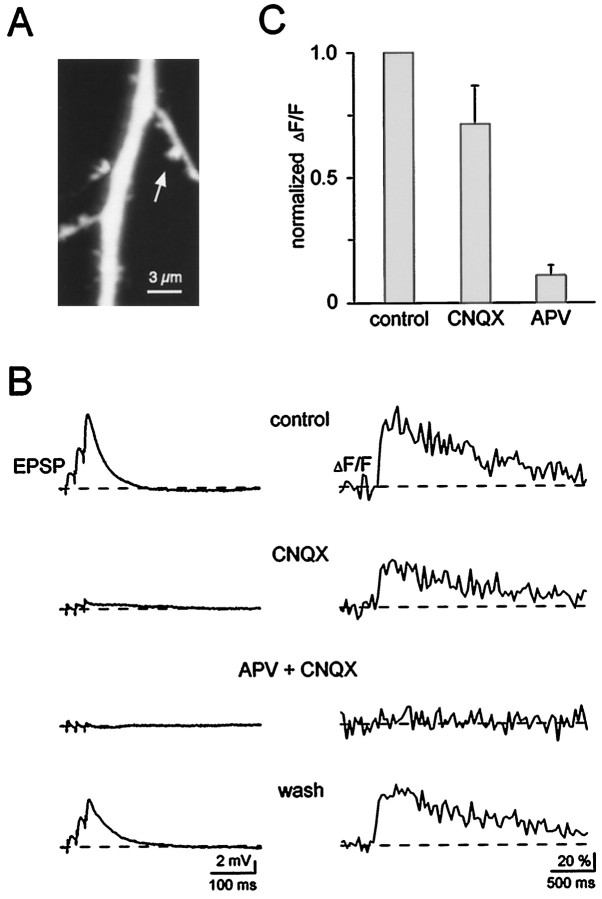
Fig. 2.
Subthreshold Ca2+ signals in spines require activation of NMDARs. A, Confocal image of a dendritic segment containing an active spine (arrow). B, EPSPs (left traces) and associated spine Ca2+ signals (ΔF/F, right traces). Afferent fibers were stimulated with a short burst consisting of three stimuli given at 50 Hz. Bath-applied CNQX (5 μm) blocked the fast component of the EPSPs, whereas it reduced the Ca2+ transient only by ∼30%. Additional application of APV (50 μm) completely and reversibly blocked the EPSP as well as the associated Ca2+ signal. Each trace is an average of three or four consecutive recordings. Membrane potential was −65 mV.C, Bar graph comparing the effects of CNQX (n = 6) and APV (n = 6) on spine Ca2+ signals (triplet stimulation, mean + SD). ΔF/F amplitudes were normalized to control values.
NMDARs are controlled by Mg2+, which produces a voltage-dependent block of these channels (Mayer et al., 1984; Nowak et al., 1984). Consistent with this, we and others find that in the absence of extracellular Mg2+, the spine signals become very large and saturate the high-affinity calcium indicator dye (data not shown). Together these results clearly indicate that the subthreshold spine Ca2+signals require the NMDAR. The fact that CNQX produced only a small reduction in the spine signal, even though it almost completely blocked the EPSP, indicates that depolarization is not critical for triggering Ca2+ entry. The results are difficult to reconcile with the view that the Ca2+ entry is primarily through VGCC because such signals would be greatly reduced when the EPSP amplitude is reduced by CNQX. It is also important to note that in many cases, spine Ca2+ signals can be recorded when the somatically recorded EPSP is so small (1–2 mV, as in Fig. 1; see also Zamanillo et al., 1999) that activation of VGCC should be minimal.
To directly test the role of voltage-gated Ca2+ channels, we attempted to eliminate their contribution. It has been reported that local dendritic signals can be blocked by low concentrations of Ni2+ (Markram and Sakmann, 1994; Magee et al., 1995), and it was therefore of interest to test the effect of this blocker on spine signals. Figure 3 shows that Ni2+ produced only a small reduction in the average synaptically evoked Ca2+signal in the spines (note also a small reduction in the EPSP; Fig.3B, left). A summary of 10 experiments is given in Figure3C. These results indicate that Ca2+ entry through Ni2+-sensitive channels makes at most a small contribution to spine signals. To examine the role of a wider range of voltage-gated Ca2+ channels, we used a patch pipette solution containing D890, a compound that blocks voltage-gated Ca2+ channels from the inside (Hescheler et al., 1982). Intracellular application is vital for the analysis of synaptically induced signals because extracellular application would interfere with transmitter release. Figure4, A and B, illustrates the ability of D890 to block depolarization-induced Ca2+ elevation. The top traces in Figure4B show the Ca2+ signals in the spine and parent dendrite (Fig. 4A) induced by a 500 msec depolarizing voltage-clamp pulse to 0 mV. This signal was detected at 5 min after the onset of whole cell recording (Fig.4B, top). At this time sufficient dye had diffused into the dendrites to make Ca2+detection possible, but the dendritic concentration of D890 was insufficient to block VGCC. Ten minutes later, D890 was in sufficient concentration to block the Ca2+ transient produced by an even longer depolarizing pulse (Fig. 4B, bottom). During this period there was no decrease in synaptic electrical responses. In separate experiments, it was observed that D890 reduces the inward Na+ currents evoked by depolarizing pulses and also prevents action potentials (O. Garaschuk, F. Tempia, and A. Konnerth, unpublished observations). Thus, D890 provides a tool for eliminating VGCC and reducing other voltage-dependent conductances without inhibiting the synaptic response. Figure 4C–E shows that synaptically evoked spine Ca2+ signals can be detected in cells, in which D890 has blocked depolarization-induced Ca2+ entry. Under these conditions, the amplitudes of spine Ca2+ signals were within the normal range (ΔF/F = 86 ± 37%,n = 11 in D890; ΔF/F = 100 ± 46%,n = 8 in control; mean ± SD, Fig.4F). These experiments with D890 strongly argue against a major role of VGCC in generating spine signals during synaptic stimulation.
Fig. 3.
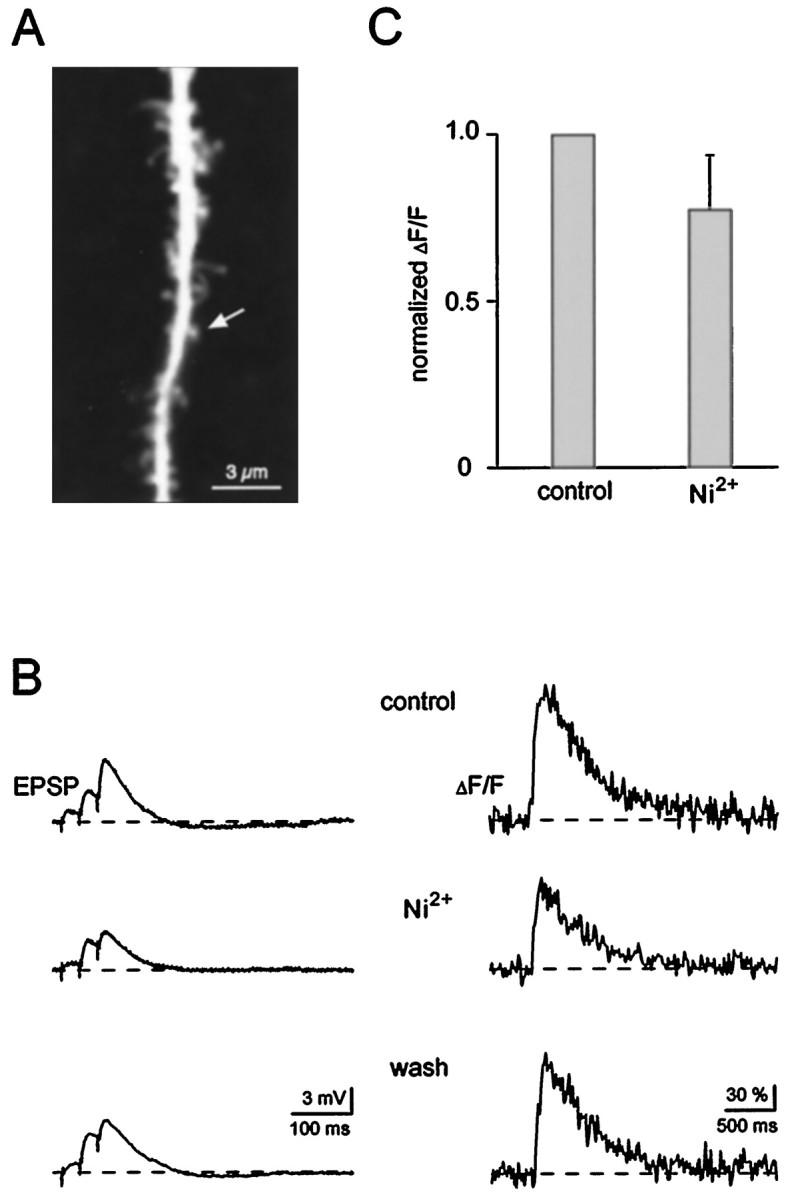
Low-threshold voltage-gated Ca2+ channels are not required for the generation of Ca2+ transients in spines. A,Confocal image of a dendritic segment containing an active spine (arrow). B, EPSPs (left traces) and associated spine Ca2+ signals (ΔF/F, right traces). Afferent fibers were stimulated with a short burst consisting of three stimuli given at 50 Hz. Application of nickel (40 μm), a blocker of T-type voltage-gated Ca2+ channels, reversibly reduced the spine Ca2+ signal only by ∼30%. Note that Ni2+ also slightly reduced the EPSP amplitude. The traces represent averages of three to five individual recordings. Membrane potential was −69 mV. C, Bar graph summarizing the effect of Ni2+ (40–50 μm) on the peak amplitude of the subthreshold Ca2+ responses (n = 10, mean + SD). In each of these experiments, five responses in control conditions and 10 min after wash in of Ni2+ were averaged.
Fig. 4.
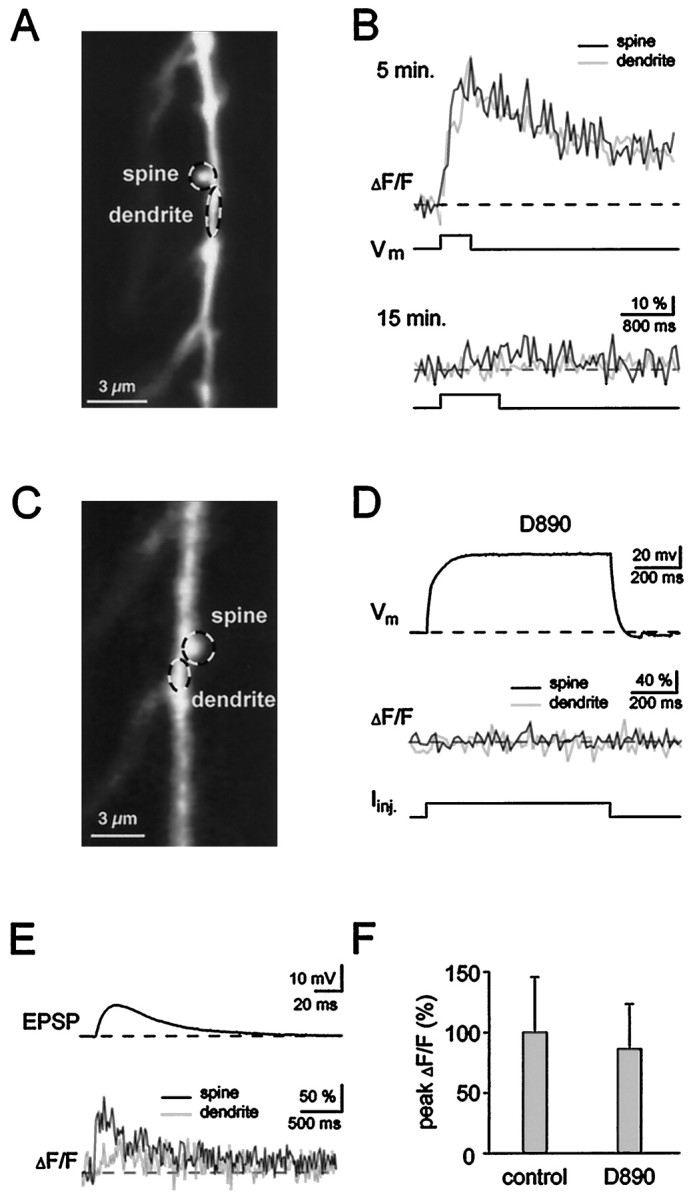
D890 blocks VGCC, but not the spine Ca2+ signal. A, C, confocal images of dendritic segments from two different cells containing active spines. Traces from regions of interest in A andC are displayed in B andD/E, respectively. B, Top traces, Within 5 min of whole-cell recording with a pipette solution containing 1 mm D890, somatic depolarization (from −60 to 0 mV) evoked a large Ca2+ elevation in spines and dendrites. Bottom traces, Ten minutes later, even longer depolarization failed to evoke any Ca2+transient. D, E, Current-clamp recording, otherwise identical conditions as in B. In the presence of D890, a strong somatic current injection failed to induce any Ca2+ transient in the spine or dendrite (D), whereas a single EPSP induced a clear spine Ca2+ signal (E).F, The mean peak amplitude of synaptic spine Ca2+ signals recorded with D890 containing intracellular solution (n = 11, mean + SD) was similar to the mean value obtained in control conditions (n = 8, mean + SD) Responses were evoked by two stimuli given at 50 Hz.
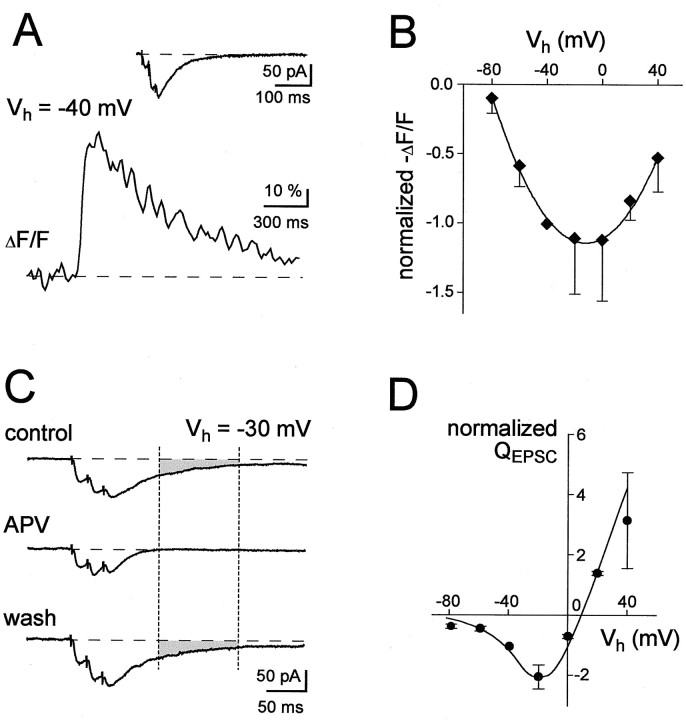
If the subthreshold Ca2+ signals in spines are attributable to NMDARs, the Ca2+signals should be increased by depolarizations that relieve the Mg2+ block and decreased by hyperpolarizations that enhance the Mg2+block. To test this prediction we studied synaptically mediated signals using D890 to block VGCC. To minimize errors caused by dye saturation, we used a low-affinity Ca2+ indicator, Magnesium Green (Kd, ∼6 μm). Furthermore, it was necessary to use a brief burst of stimuli (3 pulses, 50 Hz) to obtain a sufficiently large and reproducible Ca2+ signal (Fig.5A). Despite these precautions, such experiments are difficult to quantify because of several additional potential sources of error [for example, modulations of NMDARs permeability by Ca2+and Na+ accumulations (Rosenmund and Westbrook, 1993; Yu and Salter, 1998) and possible dye nonlinearities]. Thus, although we trust the basic qualitative observation concerning the voltage dependence, one has to be cautious when interpreting the results quantitatively. The main conclusion we draw from Figure 5B is that the spine Ca2+ signal is very large at a holding voltage of ∼0 mV and becomes smaller at more negative and more positive membrane potentials, consistent with the voltage dependence of NMDA receptor-mediated currents (Mayer et al., 1984; Nowak et al., 1984; Garaschuk et al., 1996). The voltage dependence of the synaptically evoked spine Ca2+ signal is similar to that of Ca2+ transients evoked by NMDA receptor activation through agonist application. Thus, at holding voltages of approximately −80 mV, the spine Ca2+ signal was virtually abolished. At very positive holding voltages, the Ca2+signals also become smaller, as expected because of the decrease in the driving force for Ca2+ (Mayer et al., 1987; Schneggenburger et al., 1993; Garaschuk et al., 1996). Under these conditions, we also measured the voltage dependence of the late component of the EPSC (Fig. 5D). This component is blocked by APV (Fig. 5C) and can thus be attributed to the NMDA channel (Fig. 5C). The current–voltage curve is consistent with that reported previously (Hestrin et al., 1990; Keller et al., 1991) and indicates that neither the Ca2+indicator nor D890 has substantially altered the behavior of NMDARs. These results further support the idea that the spine Ca2+ signals are attributable to Ca2+ entry through the NMDARs.
Fig. 5.
Fluorescence–voltage relationships (F–V) of synaptic Ca2+transients in spines. A, Representative example of a transient evoked by a brief burst stimulation recorded atVh = −40 mV. The insetshows the associated EPSC, traces are averages of five individual recordings. The intracellular solution contained D890 (2 mm) and the low-affinity calcium indicator dye Magnesium Green (Kd, ∼6 μm, 300 μm). B,F–V relations of the peak amplitude of the spine Ca2+ signals as measured with Magnesium Green (n = 3–6 measurements from 6 cells). Data were normalized to the values obtained at −40 mV. The line represents a polynomial fit of the data. C, EPSCs recorded atVh = −30 mV. Bath application of APV reversibly abolished a late component (shaded area) that was quantified (D) by integrating the EPSC between 60 and 160 msec (indicated by broken lines) after the last peak of the EPSC. EPSCs are averages of three consecutive responses evoked by triplet stimulation. D,Charge–voltage relationship (Q–V) of the late EPSC component, measured as indicated in C. Data were obtained from the cells that were analyzed in B. Error bars represent SD (n = 3–6 measurements from 6 cells). The intracellular solution contained 2 mm of D890 (A–D). Synaptic responses were evoked by two or three stimuli at 50 Hz.
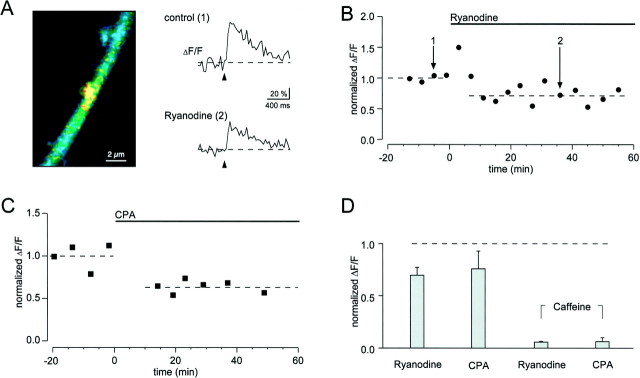
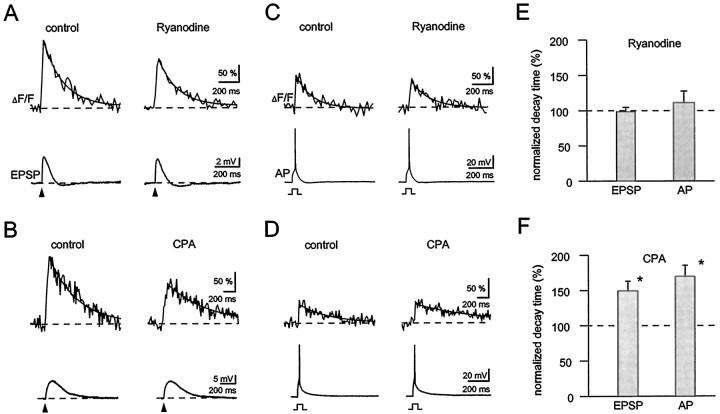
Although the spine signals may be attributable to the NMDARs, it remains possible that the role of actual Ca2+ entry through the channel is to trigger a more massive release of Ca2+from intracellular stores. Indeed, it has recently been proposed that the direct entry through NMDARs is undetectable and that the signals are attributable to Ca2+-induced Ca2+ release (Emptage et al., 1999). This proposal was based on the observation that spine Ca2+ signals are blocked by ryanodine, a drug that has been rigorously shown in skeletal muscle to be an antagonist of Ca2+-induced Ca2+ release from internal stores, and by CPA, a drug that blocks the ATP-dependent uptake into stores and therefore leads to their depletion (Ehrlich et al., 1994). Figure6A–C shows the results of experiments in which we bath-applied either ryanodine or CPA. Neither agent produced a dramatic inhibition of either synaptic transmission (see also Fig. 7) or the size of the spine signals. The summary of experiments in Figure6D shows that, at most, the reduction in Ca2+ signals was 30%. Critical to the interpretation of these experiments is verification of the efficacy of the applied drugs. Two results establish this efficacy. First, in the same set of experiments in which we looked for effect of ryanodine and CPA on spine signals in the outer region of the stratum radiatum, we applied caffeine to more proximal dendritic regions. In CA1 pyramidal cells, caffeine releases Ca2+ from intracellular stores that contain ryanodine receptors (Garaschuk et al., 1997). We locally applied caffeine from a pipette by pressure and were able to detect a local Ca2+ elevation in proximal dendrites. This caffeine-induced signal was almost completely blocked within 10–20 min of either ryanodine or CPA application (Fig. 6D).
Fig. 6.
Contribution of internal Ca2+stores to synaptic Ca2+ signaling in spines.A, EPSP-associated spine Ca2+ signal (color-coded image and top trace) was reduced by ∼30% in the presence of ryanodine (25 μm,bottom trace). B, Time course of the effect of ryanodine (indicated by the bar) on the peak amplitudes of Ca2+ transients. Same experiment as inA. Single-shock stimuli were repeatedly delivered every 4 min, each data point represents a single trial. Note that there was no failure of the Ca2+ signal with such stimulation protocol. The dashed lines represent the values during control conditions (1.0) and in the presence of ryanodine (0.72). Thenumbers point to the individual recordings displayed inA. C, Application of CPA (30 μm, indicated by the bar) reduced the amplitude of synaptic Ca2+ signals in spines by ∼36%. D,Bar graph summarizing the effects of ryanodine (20 or 25 μm) and CPA (30 μm). Data points were normalized to control conditions. The first two bars represent the effect on synaptically evoked Ca2+ signal in spines (n = 7 and 9 cells for ryanodine and CPA, respectively). The last two bars show that both drugs effectively abolish dendritic Ca2+ responses evoked by local application of caffeine (20–40 mm, n = 5 for ryanodine and for CPA). Data points were normalized to the mean control value. Experiments were done at 30°C.
Fig. 7.
Clearance of spine Ca2+involves internal stores. A, Synaptically evoked Ca2+ signals in spines (top traces) and the underlying EPSPs (bottom traces) during control conditions and in the presence of ryanodine (20 μm). The decay of the Ca2+ transients was not affected by ryanodine. The solid lines represent exponential fits with τ = 196 msec during control and τ = 183 msec in the presence of ryanodine. B, Application of CPA (30 μm) markedly prolonged the decay of synaptic Ca2+ signals (different cell than inA). τ was estimated to be ∼490 msec during control conditions and ∼720 msec in the presence of CPA. C, D,The two drugs showed similar effects on AP-induced Ca2+ signal in spines (same cells as inA and B, respectively). The decay time constants were 155 and 170 msec for ryanodine application and 507 and 1098 msec for CPA application (control and drug application, respectively). E, F, Bar graphs summarizing the effects of ryanodine (E, 20 or 25 μm,n = 7) and CPA (F, 30 μm, n = 9) on the decay time constant of Ca2+ transients induced either by synaptic stimulation (EPSP) or by single AP (AP).Asterisks denote significant changes compared to the control conditions. All traces are averages of four or five consecutive responses. Temperature was 30°C.
A second set of experiments provided further support for the efficacy of CPA and also provided evidence for a role of Ca2+ stores in determining the time course of spine signals. Previous work on dendritic signals in cortical (Markram et al., 1995) neurons indicated that CPA slows the falling phase of the AP-induced Ca2+ signals, consistent with the idea that reuptake into stores contributes strongly to Ca2+ clearance. Figure 7 shows that CPA similarly slows the falling phase of the spine Ca2+ signals caused either by synaptic stimulation (Fig. 7B) or backpropagating action potentials (Fig. 7D) (see also Mainen et al., 1999). The summary of experiments is shown in Figure 7E. In contrast, ryanodine, which does not affect reuptake, did not slow the falling phase of the signals (Fig. 7A,C,E).
DISCUSSION
Mechanism of subthreshold spine Ca2+ signals
Our experiments indicate that the subthreshold Ca2+ signals in dendritic spines are primarily attributable to Ca2+ entry through NMDARs. The signals are blocked by the NMDA receptor antagonist APV and have a voltage dependence of the general form expected of the NMDAR. Although the Ca2+ signals can be enhanced by depolarization that relieves the Mg2+ block of the NMDAR, the signals do not require significant depolarization; they occur even when the EPSP amplitude is only a few millivolts and they are only slightly reduced when the EPSP is blocked with CNQX. It thus appears likely that the signals occur because the NMDARs are in fact not completely blocked by Mg2+ at resting potential. This incompleteness is also apparent by the fact that current through the NMDAR can be detected at resting potential. Thus, when the glutamate from an individual vesicle is released it partially activates NMDARs and thereby generates a significant Ca2+signal in the postsynaptic spine.
Other sources of Ca2+ appear to contribute to subthreshold spine signals in a minor way. For example, hippocampal spines contain VGCC channels (Westenbroek et al., 1995). These could be activated by the EPSP and contribute to the subthreshold spine Ca2+ signals (Schiller et al., 1998). Under our conditions, this component must be small because there is only a small reduction of the spine signals when the EPSP is nearly abolished with CNQX. Furthermore, the size of spine signals is not substantially smaller if VGCC are blocked with Ni2+ or D890. It remains quite possible, however, that T-type Ca2+ channels, which are known to be present in hippocampal neurons (Johnston et al., 1996) and to be sensitive to voltage in the range of resting potential, could contribute significantly to spine signals under other conditions. It is known that these channels are largely inactivated under resting conditions, under hyperpolarized conditions this inactivation would be removed, and they might then contribute significantly to synaptically evoked spine signals (Magee et al., 1995; Magee and Johnston, 1995).
The finding that Ca2+ signals are not significantly reduced by CNQX argues against the possibility that a significant component of the signals is attributable to Ca2+ entry through AMPA channels. This conclusion is in line with earlier observations that CA1 pyramidal cells express AMPA receptor channels with a low Ca2+ permeability (Jonas and Sakmann, 1992), through which the fraction of Ca2+is only one 20th of the Ca2+ charge flowing through NMDARs (Garaschuk et al., 1996). Nevertheless, at extremely hyperpolarized voltages (150–200 mV), the Ca2+ entry through AMPA channels may be significant and become detectable (Yuste et al., 1999).
The contribution of intracellular stores to the spine signals is also small. We find that spine signals are only slightly reduced by ryanodine and CPA, drugs that impair Ca2+release from intracellular stores (Garaschuk et al., 1997). Analysis of the kinetics of the spine signals shows that CPA slows the return of Ca2+ to baseline levels after elevation of Ca2+ by synaptic stimulation or by eliciting a postsynaptic action potential. This slowing would be expected if one of the mechanisms mediating this return was Ca2+ pumping into internal stores (diffusion into the dendrite and extrusion through the plasma membrane are other likely mechanisms). Previous work in cortical neurons has shown that CPA can produce such slowing of the falling phase of the dendritic Ca2+ signal (Markram et al., 1995). Our work shows that this is also true for spine signals (see also Mainen et al., 1999). This slowing is not produced by ryanodine, because pumping of cytoplasmic Ca2+ into internal stores is not abolished by ryanodine.
Comparison to other studies on hippocampal and cortical neurons
The study of the mechanism of subthreshold spine signals is in its infancy, and it is of interest to discuss the points of agreement and disagreement in the relatively small number of studies thus far. The one point on which all studies of both hippocampal and cortical spine signals agree is that the signals are blocked by NMDA receptor antagonists. However, there is substantial disagreement about whether the NMDAR can be activated at resting potential and the mechanism by which the NMDAR contributes to the spine signal. With regard to the question of whether the NMDAR can be activated at resting potential, the critical experiment is whether CNQX, which nearly abolishes the EPSP, abolishes the Ca2+ signal. We andKöster et al. (1998) find little effect of CNQX. In contrast,Yuste et al. (1999) found that signals are virtually abolished by CNQX. The reasons for this discrepancy is unclear, but subtle differences in methodological details, such as the holding voltage or the Mg2+ concentration could conceivably be important.
The specific role of VGCCs in generating spine Ca2+ signals in hippocampal cells has been examined by us, by Yuste et al. (1999), and by Emptage et al. (1999), and there is agreement that these channels are not important in generating subthreshold signals. Yuste et al. (1999) omitted ATP from the internal solution and produced “washout” of VGCC, but observed little change in spine signals. In our studies, blocking VGCC from inside with D890 produced little change in the signals. It has been previously argued that local subthreshold signals in dendrites could be blocked by Ni2+, but the experimental methods did not have sufficient resolution to resolve spines (Magee et al., 1995). We and Emptage et al. (1999) have not detected a substantial effect of Ni2+ on spine signals, but this might depend strongly on the size of the EPSP, which was much larger (20 mV) in the study of Magee et al. (1995) than in our work (<10 mV).
Our study and that of Emptage et al. (1999) are the only to specifically examine the role of intracellular Ca2+ release in generating the subthreshold Ca2+ signals, and our results are in complete disagreement. Emptage et al. (1999) argue that the Ca2+ that enters directly through the NMDARs is too small to be measured and that the only detectable signals are ones that have been enormously amplified by rapid intracellular Ca2+ release. We argue that the direct entry is detectable and that it is not substantially amplified. Possible reasons for this discrepancy is the differences in electrical recording methods (patch vs microelectrodes) and differences in slice preparation (acute vs culture). The following rough calculation indicates that even though 95% of the NMDAR conductance is blocked at resting potential, the remaining Ca2+entry should be sufficient to generate a detectable signal. The peak NMDAR conductance during a quanta at +60 mV is 100 pS (estimated from the peak current of 6 pA; Liao et al., 1995). This would yield a current at −60 mV of 4 pA in the absence of Mg2+ block (Spruston et al., 1995) (Fig.4C). From Figure 4, C and F, of the same paper it can be estimated that Mg2+block at −60 mV produces a 94% reduction in current. Given that ∼10% of the current is carried by Ca2+(Garaschuk et al., 1996), taking the spine volume as 0.5 × 10−16 l (Harris and Stevens, 1989) and assuming that 0.5% of the Ca2+ that enters is free (Helmchen et al., 1996), a period of 50 msec of current through the NMDARs would yield a rise of >500 nmfree Ca2+, well about the resting level of 60 nm (Regehr et al., 1989). Although our results indicate that Ca2+ stores are not an important source of Ca2+ during low-frequency synaptic transmission, the stores do have importance as a sink of Ca2+. We found that the return of spine Ca2+ levels to baseline after elevation by either action potentials or subthreshold synaptic events was slowed when the pumping of Ca2+ into intracellular stores was slowed by CPA.
Possible physiological implications of subthreshold Ca2+ signals
Recent work raises the possibility that subthreshold signals may be important in synaptic strengthening processes that occur under certain neuromodulatory conditions. It has been found that activation of the tyrosine kinase src can lead to the upregulation of the NMDAR conductance and to long-term enhancement of AMPA-mediated transmission, an enhancement that occludes with LTP (Lu et al., 1998). Interestingly, this src-induced potentiation can occur without significant synaptic stimulation, the only stimulation being infrequent (subthreshold) test pulses. Nevertheless, this enhancement can be blocked by NMDAR antagonists and by intracellular Ca2+buffers. These results suggest that NMDAR-mediated Ca2+ entry evoked by weak synaptic stimulation may under certain conditions be able to trigger synaptic strengthening.
Subthreshold Ca2+ signals may also play a role during higher frequency synaptic events that induce synaptic plasticity. One clear example is long-term depression (LTD) and depotentiation, which can be triggered by subthreshold stimulation (Stevens and Wang, 1994; Stäubli and Ji, 1996) and which are known to be dependent on an activity-dependent rise in intracellular Ca2+ concentration. In most laboratories, LTD can be blocked by NMDA receptor antagonists, suggesting that Ca2+ entry is the trigger for LTD (for review, see Bear and Abraham, 1996). The Ca2+ signals we have detected may thus be the signal that triggers LTD. However, further work is required to establish this point because LTD induction requires repetitive synaptic stimulation in the 1–5 Hz range, whereas we have only examined responses at lower frequencies. Another possible function of subthreshold signals that needs to be considered in view of recent work is a role in receptor “homeostasis”. It has been shown that when all basal glutamatergic synaptic activity is abolished, profound changes in glutamate sensitivity (O'Brien et al., 1998; Turrigiano et al., 1998), the number of glutamate receptors (Rao and Craig, 1997;Lissin et al., 1998), and the number of spines (McKinney et al., 1999) can occur. Thus, it is possible that subthreshold Ca2+ signals in spines are part of a maintenance process of the basal functional properties (e.g., through Ca2+-dependent phosphorylation of receptor channels) at the level of individual synaptic inputs.
Footnotes
This work was supported by grants from Human Frontier Science Program and Deutsche Forschungsgemeinschaft to A.K. and J.E. and National Institutes of Health Grant NS 35083 to J.L. We thank O. Garaschuk for comments on the manuscript and N. Rothgerber and E. Eilers for technical help.
Correspondence should be addressed to A. Konnerth, TU München, Institut für Physiologie, 80802 München, Germany. E-mail:konnerth@physiol.med.tu-muenchen.de.
Dr. Lisman's present address: Department of Biology, Brandeis University, Waltham, MA 02254.
REFERENCES
- 1.Alford S, Frenguelli BG, Schofield JG, Collingridge GL. Characterization of Ca2+ signals induced in hippocampal CA1 neurones by the synaptic activation of NMDA receptors. J Physiol (Lond) 1993;469:693–716. doi: 10.1113/jphysiol.1993.sp019838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen C, Stevens CF. An evaluation of causes for unreliability of synaptic transmission. Proc Natl Acad Sci USA. 1994;91:10380–10383. doi: 10.1073/pnas.91.22.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- 4.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 5.Denk W, Sugimori M, Llinas R. Two types of calcium response limited to single spines in cerebellar Purkinje cells. Proc Natl Acad Sci USA. 1995;92:8279–8282. doi: 10.1073/pnas.92.18.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca(2+)-release channels. Trends Pharmacol Sci. 1994;15:145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 8.Eilers J, Konnerth A. Dendritic signal integration. Curr Opin Neurobiol. 1997;7:385–390. doi: 10.1016/s0959-4388(97)80067-0. [DOI] [PubMed] [Google Scholar]
- 9.Eilers J, Augustine GJ, Konnerth A. Subthreshold synaptic Ca2+ signalling in fine dendrites and spines of cerebellar Purkinje neurons. Nature. 1995;373:155–158. doi: 10.1038/373155a0. [DOI] [PubMed] [Google Scholar]
- 10.Emptage N, Bliss TV, Fine A. Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron. 1999;22:115–124. doi: 10.1016/s0896-6273(00)80683-2. [DOI] [PubMed] [Google Scholar]
- 11.Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 12.Garaschuk O, Schneggenburger R, Schirra C, Tempia F, Konnerth A. Fractional Ca2+ currents through somatic and dendritic glutamate receptor channels of rat hippocampal CA1 pyramidal neurones. J Physiol (Lond) 1996;491:757–772. doi: 10.1113/jphysiol.1996.sp021255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garaschuk O, Yaari Y, Konnerth A. Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones. J Physiol (Lond) 1997;502:13–30. doi: 10.1111/j.1469-7793.1997.013bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 15.Harris KM, Stevens JK. Dendritic spines of CA1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helmchen F, Imoto K, Sakmann B. Ca2+ buffering and action potential-evoked Ca2+ signaling in dendrites of pyramidal neurons. Biophys J. 1996;70:1069–1081. doi: 10.1016/S0006-3495(96)79653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hescheler J, Pelzer D, Trube G, Trautwein W. Does the organic calcium channel blocker D600 act from inside or outside on the cardiac cell membrane? Pflügers Arch. 1982;393:287–291. doi: 10.1007/BF00581411. [DOI] [PubMed] [Google Scholar]
- 18.Hessler NA, Shirke AM, Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- 19.Hestrin S, Nicoll RA, Perkel DJ, Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol (Lond) 1990;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston D, Magee JC, Colbert CM, Cristie BR. Active properties of neuronal dendrites. Annu Rev Neurosci. 1996;19:165–186. doi: 10.1146/annurev.ne.19.030196.001121. [DOI] [PubMed] [Google Scholar]
- 21.Jonas P, Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol (Lond) 1992;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller BU, Konnerth A, Yaari Y. Patch clamp analysis of excitatory synaptic currents in granule cells of rat hippocampus. J Physiol (Lond) 1991;435:275–293. doi: 10.1113/jphysiol.1991.sp018510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Köster HJ, Sakmann B. Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of back-propagating action potentials and subthreshold excitatory postsynaptic potentials. Proc Natl Acad Sci USA. 1998;95:9596–9601. doi: 10.1073/pnas.95.16.9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 25.Lissin DV, Gomperts SN, Carroll RC, Christine CW, Kalman D, Kitamura M, Hardy S, Nicoll RA, Malenka RC, von Zastrow M. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc Natl Acad Sci USA. 1998;95:7097–7102. doi: 10.1073/pnas.95.12.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu YM, Roder JC, Davidow J, Salter MW. Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science. 1998;279:1363–1367. doi: 10.1126/science.279.5355.1363. [DOI] [PubMed] [Google Scholar]
- 27.Magee JC, Johnston D. Synaptic activation of voltage-gated channels in the dendrites of hippocampal pyramidal neurons. Science. 1995;268:301–304. doi: 10.1126/science.7716525. [DOI] [PubMed] [Google Scholar]
- 28.Magee JC, Christofi G, Miyakawa H, Christie B, Lasser Ross N, Johnston D. Subthreshold synaptic activation of voltage-gated Ca2+ channels mediates a localized Ca2+ influx into the dendrites of hippocampal pyramidal neurons. J Neurophysiol. 1995;74:1335–1342. doi: 10.1152/jn.1995.74.3.1335. [DOI] [PubMed] [Google Scholar]
- 29.Mainen ZF, Malinow R, Svoboda K. Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nature. 1999;399:151–155. doi: 10.1038/20187. [DOI] [PubMed] [Google Scholar]
- 30.Malinow R, Otmakhov N, Blum KI, Lisman J. Visualizing hippocampal synaptic function by optical detection of Ca2+ entry through the N-methyl-D-aspartate channel. Proc Natl Acad Sci USA. 1994;91:8170–8174. doi: 10.1073/pnas.91.17.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markram H, Sakmann B. Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated calcium channels. Proc Natl Acad Sci USA. 1994;91:5207–5211. doi: 10.1073/pnas.91.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markram H, Helm PJ, Sakmann B. Dendritic calcium transients evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. J Physiol (Lond) 1995;485:1–20. doi: 10.1113/jphysiol.1995.sp020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 34.Mayer ML, MacDermott AB, Westbrook GL, Smith SJ, Barker JL. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987;7:3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- 36.Müller W, Connor JA. Dendritic spines as individual neuronal compartments for synaptic Ca2+ responses. Nature. 1991;354:73–76. doi: 10.1038/354073a0. [DOI] [PubMed] [Google Scholar]
- 37.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- 39.Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- 40.Regehr WG, Tank DW. Postsynaptic NMDA receptor-mediated calcium accumulation in hippocampal CA1 pyramidal cell dendrites. Nature. 1990;345:807–810. doi: 10.1038/345807a0. [DOI] [PubMed] [Google Scholar]
- 41.Regehr WG, Connor JA, Tank DW. Optical imaging of calcium accumulation in hippocampal pyramidal cells during synaptic activation. Nature. 1989;341:533–536. doi: 10.1038/341533a0. [DOI] [PubMed] [Google Scholar]
- 42.Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- 43.Schiller J, Schiller Y, Clapham DE. NMDA receptors amplify calcium influx into dendritic spines during associative pre- and postsynaptic activation. Nat Neurosci. 1998;1:114–118. doi: 10.1038/363. [DOI] [PubMed] [Google Scholar]
- 44.Schneggenburger R, Zhou Z, Konnerth A, Neher E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron. 1993;11:133–143. doi: 10.1016/0896-6273(93)90277-x. [DOI] [PubMed] [Google Scholar]
- 45.Spruston N, Jonas P, Sakmann B. Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol (Lond) 1995;482:325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stäubli UV, Ji ZX. The induction of homo- vs. heterosynaptic LTD in area CA1 of hippocampal slices from adult rats. Brain Res. 1996;714:169–176. doi: 10.1016/0006-8993(95)01523-x. [DOI] [PubMed] [Google Scholar]
- 47.Stevens CF, Wang Y. Changes in reliability of synaptic function as a mechanism for plasticity. Nature. 1994;371:704–707. doi: 10.1038/371704a0. [DOI] [PubMed] [Google Scholar]
- 48.Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998;396:757–760. doi: 10.1038/25547. [DOI] [PubMed] [Google Scholar]
- 49.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 50.Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TV, Snutch TP, Catterall WA. Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu XM, Salter MW. Gain control of NMDA-receptor currents by intracellular sodium. Nature. 1998;396:469–474. doi: 10.1038/24877. [DOI] [PubMed] [Google Scholar]
- 52.Yuste R, Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- 53.Yuste R, Majewska A, Cash SS, Denk W. Mechanisms of calcium influx into hippocampal spines: heterogeneity among spines, coincidence detection by NMDA receptors, and optical quantal analysis. J Neurosci. 1999;19:1976–1987. doi: 10.1523/JNEUROSCI.19-06-01976.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Köster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]