Abstract
In this study, we highlight a role for the nitric oxide–cGMP-dependent protein kinase (NO–G-kinase) signaling pathway in glial intercellular Ca2+ wave initiation and propagation. Addition of the NO donor molsidomine (100–500 μm) or puffing aqueous NO onto primary glial cell cultures evoked an increase in [Ca2+]iin individual cells and also local intercellular Ca2+ waves, which persisted after removal of extracellular Ca2+. High concentrations of ryanodine (100–200 μm) and antagonists of the NO–G-kinase signaling pathway essentially abrogated the NO-induced increase in [Ca2+]i, indicating that NO mobilizes Ca2+ from a ryanodine receptor-linked store, via the NO–G-kinase signaling pathway. Addition of 10 μm nicardipine to cells resulted in a slowing of the molsidomine-induced rise in [Ca2+]i, and inhibition of Mn2+ quench of cytosolic fura-2 fluorescence mediated by a bolus application of 2 μm aqueous NO to cells, indicating that NO also induces Ca2+ influx in glia. Mechanical stress of individual glial cells resulted in an increase in intracellular NO in target and neighboring cells and intercellular Ca2+ waves, which were NO, cGMP, and G-kinase dependent, because incubating cells with nitric oxide synthase, guanylate cyclase, and G-kinase inhibitors, or NO scavengers, reduced Δ[Ca2+]i and the rate of Ca2+ wave propagation in these cultures. Results from this study suggest that NO–G-kinase signaling is coupled to Ca2+ mobilization and influx in glial cells and that this pathway plays a fundamental role in the generation and propagation of intercellular Ca2+ waves in glia.
Keywords: nitric oxide, glia, calcium waves, mobilization, influx, ryanodine receptors, nitric oxide synthase, DAF-2, phospholipase C, astrocytes
It is becoming increasingly apparent that intercellular Ca2+ waves might be the result of combined contributions of intracellular and extracellular Ca2+ signaling pathways in glial cells. One generally accepted mode of intercellular Ca2+ wave propagation involves the diffusion of Ca2+ mobilizing second messengers, including inositol-1,4,5-trisphosphate (IP3) and Ca2+, across gap junctions (Nedergaard, 1994; Charles, 1998). Extracellular, gap junction-independent modes of Ca2+signaling, involving the release of a diffusible messengers, also appear to operate in this particular system however, with ATP release from cells and P2 receptor-coupled elevation of [Ca2+]i having recently been suggested as a likely candidate (Cotrina et al., 1998;Guthrie et al., 1999). There are however other species that may participate as extracellular messengers in glial intercellular Ca2+ waves. There is increasing evidence that the highly diffusible messenger nitric oxide (NO) can induce Ca2+ mobilization in several cell types (Publicover et al., 1993; Willmott et al., 1995a,b,c; Clementi et al., 1996) either via the cGMP-dependent protein kinase (G-kinase)-coupled activation of ADP-ribosyl cyclase, resulting in an increased synthesis of the potent Ca2+ mobilizing agent cyclic ADP-ribose (Willmott et al., 1996c; Clementi et al., 1996), or via direct nitrosylation of regulatory thiol groups of ryanodine receptors (Stoyanovsky et al., 1997).
Because NO synthesis by constitutive nitric oxide synthase (cNOS) is usually calcium-dependent (Moncada et al., 1991), a rise in [Ca2+]i may serve to amplify NO production as previously reported (Publicover et al., 1993), and a high rate of potentially unrestricted diffusion for NO could also give rise or contribute to Ca2+waves seen in many single cells and tissues (Berridge and Dupont, 1994). Therefore, considering the presence of Ca2+-dependent nitric oxide synthase in glia (Feinstein et al., 1994) and the possibility of cross-talk between NO and Ca2+ in these cells, this study investigated whether the NO–G-kinase signaling pathway is involved in Ca2+ homeostasis in glia and whether a hypothetical mobilization of Ca2+ by NO might initiate regenerative intercellular Ca2+ waves or contribute to intercellular Ca2+ wave propagation induced by mechanical stress of single glial cells in mixed glial-neuron cultures.
MATERIALS AND METHODS
Cell culture. Mixed glial-neuron primary cell cultures were prepared in a similar way as previously described (Goldman et al., 1989). Briefly, four forebrains of 1–2 d postnatal rats were dissected and transferred to Ca/Mg-free HBSS to which an equal volume of 0.25% trypsin–1 mm EDTA solution (Life Technologies, Gaithersburg, MD) had been added. Forebrains were cut into small pieces and were incubated in this medium for 20 min at 37°C in a humidified atmosphere of 95% air and 5% CO2. Forebrain pieces were transferred to a 15 ml conical centrifugation tube (Corning, Corning, NY) and were washed with 4 × 10 ml aliquots of an equal mixture of DMEM and Ham's F-12. Finally pieces were suspended in 3 ml of tissue culture medium consisting of 10% fetal calf serum and 90% of an equal mixture of DMEM and F-12, supplemented with 8 mg/ml d-glucose, 20 U/ml penicillin, and 20 μg/ml streptomycin, and were triturated to homogeneity. Aliquots (100 μl) of the resulting cell suspension were overlaid onto zero thickness glass coverslips in 6-well dishes that had been precoated with poly-l-lysine (2.5 μg/coverslip) and laminin (5 μg/coverslip). After a 3 hr incubation at 37°C in a humidified atmosphere of 95% air and 5% CO2, coverslips were flooded with 2 ml of the above tissue culture medium and were maintained for 1–3 weeks in culture before use. Every 3 d, 1 ml of culture medium was removed from coverslips and replaced with 1 ml of fresh culture medium.
Immunocytochemistry. After 1 week in culture, immunocytochemical stains for glial fibrillary acidic protein (GFAP) and neurofilament protein (NFP) were used to quantify the proportion of astrocytes to neurons on the coverslips. Cultured coverslips were rinsed twice in HBSS (see drugs and solutions) at room temperature and were then fixed with 100% methanol at −20°C for 10 min. After two more washes in HBSS, coverslips were blocked with 10% normal goat serum (Sigma, Poole, UK) in PBS for 20 min at room temperature and were then incubated overnight at 4°C with primary antibody (rabbit IgG anti-GFAP, 1:100, or rabbit anti-neurofilament-200, 1:100; Sigma) in 1% normal goat serum in PBS. Coverslips were then washed three times in PBS at room temperature and were incubated in the secondary antibody in PBS (Oregon Green 488-goat anti-rabbit, 1:50; Molecular Probes, Eugene, OR) for 45 min at room temperature. Coverslips were examined under epifluorescence using a Nikon Diaphot inverted microscope and excitation light of 490 nm. Fluorescence images were captured with Axon Imaging Workbench software (Axon Instruments, Foster City, CA) using an intensified CCD (Prostab) and Axon Image Lightning frame grabber (Axon Instruments). Images were analyzed using Corel Photo-Paint (Corel Corporation).
Measurement of [Ca2+]i in mixed glial-neuron cell cultures. Intracellular and intercellular Ca2+ signaling were assessed in mixed glial-neuron primary cell cultures derived from the forebrains of neonatal rats (1–2 d postnatal). Cell cultures were prepared as above and were maintained for 1–3 weeks in a humidified atmosphere of 95% air and 5% CO2 at 37°C before use. After 1 week, cultures were predominantly composed of astrocytes, as >80% of cells showed cross-reaction with antibody to GFAP. Cells were loaded with Ca2+ indicator by incubation with 2 μm of the AM ester of fura-2 for 1 hr. Intracellular fluorescence was imaged at 22°C in HBSS, using a 40× oil-immersion objective and inverted microscope (Nikon Diaphot), equipped with a xenon arc lamp and excitation filter wheel system (Cairn) and intensified CCD (Prostab) interfaced to a Pentium II computer with an Axon Image Lightning 2000 frame grabber (Axon Instruments), digitizing at a 10-bit grayscale resolution. A low temperature was used for Ca2+ imaging experiments because fura-2 readily compartmentalizes into cellular organelles at higher, physiological temperatures in these cell preparations. Cells were maintained in a static, open chamber throughout experiments. Free cytosolic Ca2+ was quantified by taking the ratio of fluorescence intensities at excitation wavelengths 340 and 380 nm, using an emission wavelength of 510 nm. Pairs of 340 and 380 nm images were captured every 2.5 sec, and ratio images were calculated using Axon Imaging Workbench software (Axon Instruments). Standard CaCl2 solutions were used to calibrate the system for measurements with fura-2, and viscosity corrections were made (Poenie, 1990). Cellular Ca2+ response parameters were compared for statistical significance using Student'st test for unpaired observations.
Assessment of Ca2+ influx by the manganese quench of cytosolic fura-2 fluorescence. Cells were loaded with the AM ester of fura-2 as above. Manganese quench of glial cell cytosolic fura-2 fluorescence was monitored using an excitation wavelength of 365 nm, with a similar microscope-based imaging system as for Ca2+ measurements. Fluorescence images were captured every 8 sec. Mn2+ was added to the cell chamber, and the effect of bolus additions of aqueous NO and/or thapsigargin on Mn2+ quench of cytosolic fura-2 fluorescence was assessed in glial cells.
Monitoring cytosolic nitric oxide production in mixed glial-neuron cell cultures. Changes in cytosolic NO concentration were monitored in glial-neuron primary cell cultures using the recently described single excitation wavelength, fluorescent NO probe DAF-2 (Kojima et al., 1998). Cells were loaded with NO indicator by incubation with 10 μm of DAF-2 DA for 1 hr. Intracellular fluorescence was imaged at 22°C in HBSS, using a 40× oil-immersion objective and inverted microscope (Nikon Diaphot), equipped with a xenon arc lamp and excitation filter wheel system (Cairn), and 12-bit interline CCD (5 MHz Micromax; Princeton Instruments, Princeton, NJ) interfaced to a Pentium II computer. Cells were maintained in a static, open chamber throughout experiments. Free cytosolic NO was monitored by ratioing the change in fluorescence intensity at an excitation wavelength of 490 nm with the initial fluorescence intensity at 490 nm (ΔF/F0), using an emission wavelength of 515 nm. Because DAF-2 is not a quantitative probe, no attempt was made to calibrate DAF-2 fluorescence with standard solutions of aqueous NO. Fluorescence images were captured every 2.5 sec, and ratio images were calculated using Axon Imaging Workbench software (Axon Instruments).
Drugs and solutions. Experiments were performed in HBSS, pH 7.2, containing (in mm): 137 NaCl, 5.4 KCl, 1.3 CaCl2, 0.83 MgSO4, 0.42 Na2HPO4, 0.44 KH2PO4, 4.2 NaHCO3, and 5 glucose. For experiments performed in nominally Ca2+-free medium, CaCl2 was omitted from the above, and 0.5 mm EGTA was added, yielding a free Ca2+ concentration of ∼10 nm. Nitric oxide gas was from Aldrich (Milwaukee, WI). Aqueous nitric oxide was prepared in HBSS, and its concentration was estimated according to a previously described method (Willmott et al., 1996c). HAM's F-12 medium, DMEM, fetal calf serum, penicillin, and streptomycin were from Life Technologies (Glasgow, UK). Fura-2 AM was from Molecular Probes. Rp-8-pCPT-cGMPS was from Biolog Life Science Institute (Hamburg, Germany). LY 83583,NG-methyl-l-arginine (l-NMMA), ryanodine, thapsigargin, DAF-2 DA and 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl 3-oxide (PTIO) were from Calbiochem (La Jolla, CA). All other drugs were from Sigma.
RESULTS
After 1 week in culture, cells were predominantly glial, because >80% cross-reacted with an antibody to GFAP that was tagged with the fluorescent dye Oregon Green (Fig. 1). It was possible to discriminate between glial and neuronal cells in our mixed glial-neuron cultures. Neuronal cells, which cross-reacted with antibody to neurofilament protein, were rounder in shape with fewer processes and appeared phase bright under microscope illumination compared to glia. These differences assisted our selection of cells in mechanical stimulation experiments, in which only individual glial cells were mechanically stressed. In all experiments, uniform cell regions were chosen that were predominantly glial (∼80–90%) and that contained at least 200 cells in a 350 × 300 μm field.
Fig. 1.
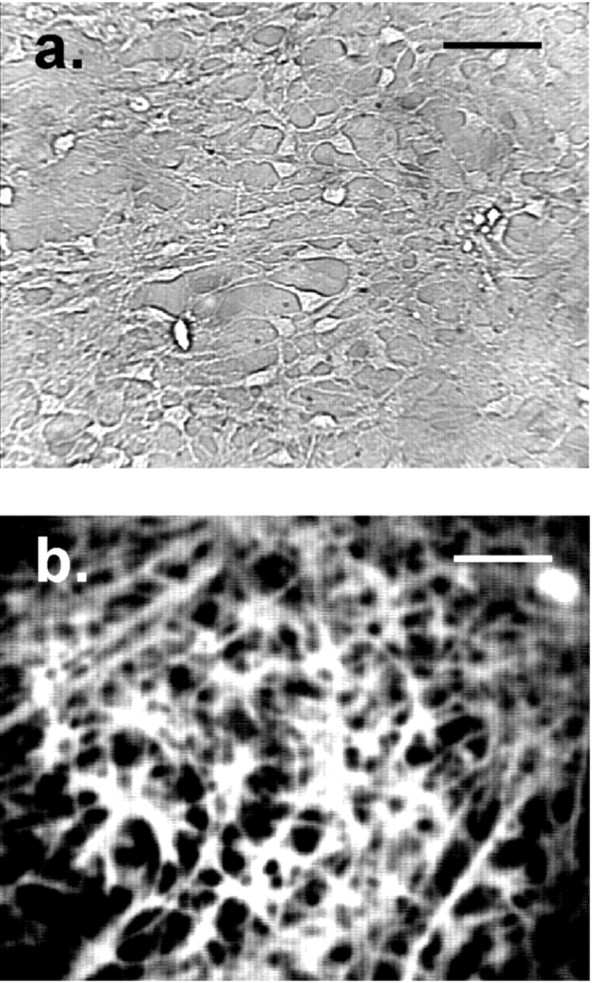
Mixed glial-neuron cultures used in the experiments. a, Typical example of a mixed glial-neuron cell preparation used in the experiments. The image of awas obtained with standard microscope illumination and a 40× oil immersion objective. b, After 1 week in culture, cells were predominantly glial, because >80% cross-reacted with an antibody to GFAP that was tagged with the fluorescent dye Oregon Green. The image of b was acquired using epifluorescence (490 nm excitation). Scale bars, 50 μm.
Nitric oxide induces Ca2+ mobilization in glial cells via the NO–G-kinase signaling pathway
The effect of NO on [Ca2+]i of mixed glial-neuron cultures was assessed. A puff of aqueous NO induced large transient increases in [Ca2+]i of up to 1 μm and often triggered localized intercellular Ca2+ waves involving groups of up to 20 cells (Fig. 2a,b, control sequences of ratio images). In puff experiments, the microinjection pipette containing aqueous NO at a concentration of 35 μm was positioned centrally, at a distance of 150 μm above the cells. Puff duration was 100 msec, resulting in a reproducible delivery of 50 pl (calibrated by measuring injected droplet size in oil). By the same procedure, puffing solely HBSS onto cells did not affect [Ca2+]i. On transferring cells to a nominally Ca2+-free medium (0 Ca2+, 0.5 mm EGTA), a puff of NO induced a smaller increase in [Ca2+]i, which diminished with increasing length of incubation in this medium (Fig.2a,c). This was attributable to intracellular Ca2+ store depletion, because addition of 1 μm ionomycin to cells maintained in this Ca2+-depleted medium for 15 min increased [Ca2+]i by only 150–220 nm (data not shown). These observations indicate that NO induces Ca2+ mobilization in glial cells.
Fig. 2.
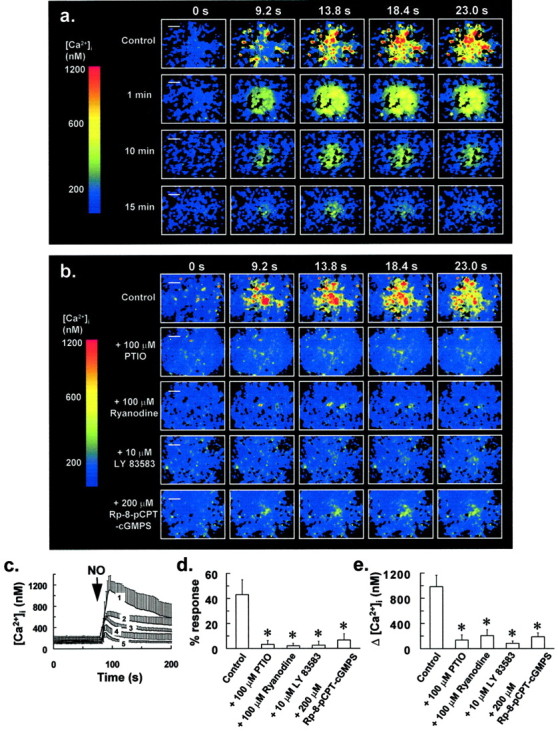
NO induces Ca2+mobilization in glia via the NO–G-kinase signaling pathway.a,Top panel(Control), Fluorescence ratio images showing an increase in [Ca2+]i induced by puffing aqueous NO onto glial cells at t = 0 sec. The pipette concentration of NO was 35 μm, and puff duration was 100 msec, resulting in a reproducible delivery of 50 pl (calibrated by measuring injected droplet size in oil). Bottom panels of a are the same as above, except cells were incubated in a nominally Ca2+-free medium (0 Ca2+, 0.5 mm EGTA) for the indicated times before NO application. This resulted in intracellular Ca2+ store depletion and a gradual reduction in the Δ[Ca2+]i induced by NO (a). Fluorescence ratio image sequences ina are representative of five separate experiments in different coverslips for each treatment. b, Pretreatment of cells with 100 μm PTIO for 5 min, 100 μmryanodine for 30 min, 10 μm LY 83583 for 1 min, or 300 μm Rp-8-pCPT-cGMPS for 30 min resulted in the abrogation of the increase in [Ca2+]i induced by a puff of aqueous NO at t = 0, as ina. Fluorescence ratio image sequences ofb are representative of at least six separate experiments in different coverslips, for untreated control cells and each of the above drug pretreatments. Scale bars, 50 μm. Traces inc were derived from the experiments of aand represent the mean [Ca2+]i for at least 53 responding cells from five separate experiments, which demonstrated an increase in [Ca2+]iafter administration of a puff of NO (arrow) as ina. From top to bottom inc, trace 1 represents the control response to a puff of NO for cells maintained in normal HBSS, whereastraces 2–5 represent responses to a puff of NO after transfer of cells to a nominally Ca2+-free medium for 1, 5, 10, and 15 min, respectively, before NO application.d, Graph derived from the experiments ofb showing a reduction in the percentage of cells in a 350 × 300 μm field that demonstrated a rise in [Ca2+]i to a puff of aqueous NO, after pretreatment of cells with the drugs of b.e, Graph derived from the experiments ofb showing a reduction in Δ[Ca2+]i for cells that responded to a puff of aqueous NO after pretreatment of cells with the drugs of b. Bars in d ande indicate the means of at least six separate estimations. Error bars in c–e indicate SD (*p < 0.05 vs control value; Student'st test for unpaired observations).
Pretreating cells with 100 μm of the cell-permeant NO scavenger PTIO for 5 min essentially abrogated the increase in [Ca2+]i after a puff of NO (Fig. 2b,d,e), underscoring a specific effect for NO on intracellular Ca2+ release in glia. It has previously been reported that NO-induced Ca2+mobilization is ryanodine receptor (RyR)-linked in several cell types (Publicover et al., 1993; Willmott et al., 1995a; Willmott et al., 1996c). To test for the involvement of a RyR-mediated Ca2+ release mechanism, cells were preincubated with an antagonizing concentration of ryanodine before NO application. The binding of ryanodine to RyRs is practically irreversible, and at doses >50 μm, the associated Ca2+ conductance is inhibited (Rousseau et al., 1987; Zimanyi et al., 1992). Because of its slow association kinetics (Pessah et al., 1987), a ryanodine concentration of 100 μm and preincubation of 30 min was used. This treatment had a negligible effect on [Ca2+]i of glial cells in our experiments and did not result in Ca2+ store depletion, because addition of 1 μm ionomycin to cells immediately after transfer to a Ca2+-free medium resulted in a substantial increase in [Ca2+]i of 400–600 nm (data not shown), similar to that of untreated control cells. For glial cells preincubated with this antagonizing concentration of ryanodine, the increase in [Ca2+]i to a puff of NO was almost completely abrogated (Fig.2b,d,e).
Similarly, pretreating cells with the guanylate cyclase inhibitor LY 83583 (10 μm) for 1 min essentially abolished the NO-induced increase in [Ca2+]i, whereas preincubating cells with the G-kinase inhibitor Rp-8-pCPT-cGMPS (200 μm) for 30 min substantially reduced this response, with <10% of cells demonstrating an increase in [Ca2+]i of only 200 nm, after a puff of NO (Fig.2b,d,e). Treating cells with the above agents had no effect on [Ca2+]i and did not cause Ca2+ store depletion during the preincubation period. These data strongly suggest that NO mobilizes Ca2+ from a RyR-linked Ca2+ store via the NO–G-kinase signaling pathway in glial cells.
Evidence for ryanodine receptor-linked Ca2+release in glia
Bolus application of a low, agonizing concentration of ryanodine (1 μm) onto quiescent glia resulted in intracellular Ca2+ oscillations in 39% of glial cells (Fig. 3a) and localized intercellular Ca2+ waves involving groups of up to 20 cells. These oscillations to 1 μmryanodine were still observed in some cells after transferring cultures to a nominally Ca2+-free medium (Fig.3d), indicating that a low concentration of ryanodine induces Ca2+ mobilization in glia. Data are consistent with a previous study, which suggested the presence of RyR-coupled Ca2+ pools in astrocytes, as a low, agonizing concentration of ryanodine (10 μm) induced Ca2+store depletion in these cells (Giaume and Venance, 1998). In direct contrast, application of an antagonizing concentration of ryanodine (200 μm) onto quiescent cells had a negligible effect on [Ca2+]iof glia, with only 3% of cells demonstrating intracellular Ca2+ oscillations to this treatment over a 20 min time period (Fig. 3b,c). Furthermore, this treatment did not result in a detectable depletion of intracellular Ca2+ stores, because addition of 1 μm ionomycin to cells after transfer to a nominally Ca2+-free medium resulted in a substantial increase in [Ca2+]i of 400–600 nm, comparable to that of untreated control cells.
Fig. 3.
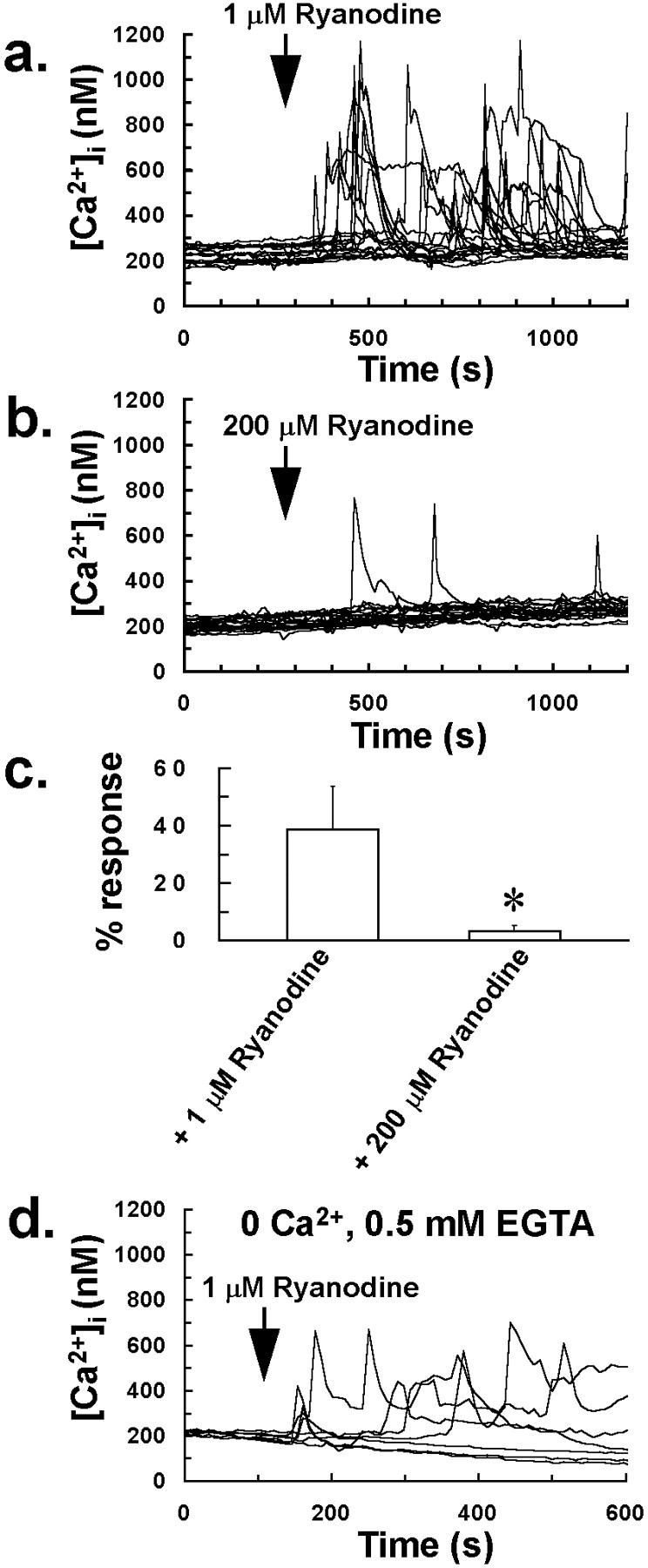
A low concentration of ryanodine induces Ca2+ mobilization and intracellular Ca2+ oscillations in glia. a,Application of a low concentration of ryanodine (1 μm) to mixed glial-neuron cultures induced intracellular Ca2+ oscillations in 39% of cells located in quiescent cell regions, in which there was no random change in [Ca2+]i before ryanodine application.b, In contrast, application of a high, antagonizing concentration of ryanodine (200 μm) to mixed glial-neuron cultures induced intracellular Ca2+ oscillations in only 3% of cells located in quiescent cell regions. Traces ina and b represent [Ca2+]i of 20 cells chosen at random from a 350 × 300 μm field and are typical of four separate experiments for 1 and 200 μm ryanodine treatments.c, Graph showing the percentage of cells in a 350 × 300 μm field that demonstrated a rise in [Ca2+]i to low (1 μm) and high (200 μm) concentrations of ryanodine. Bars inc indicate the mean of four separate estimations, and error bars indicate SD (*p < 0.05 vs 1 μm ryanodine value; Student's t test for unpaired observations). d, Intracellular Ca2+ oscillations to 1 μm ryanodine were still observed in glial cells after transferring cultures to a nominally Ca2+-free medium, indicating that a low concentration of ryanodine induces Ca2+ mobilization from intracellular stores in glia.
Nitric oxide induces Ca2+ influx in glial cells, which is inhibited by nicardipine
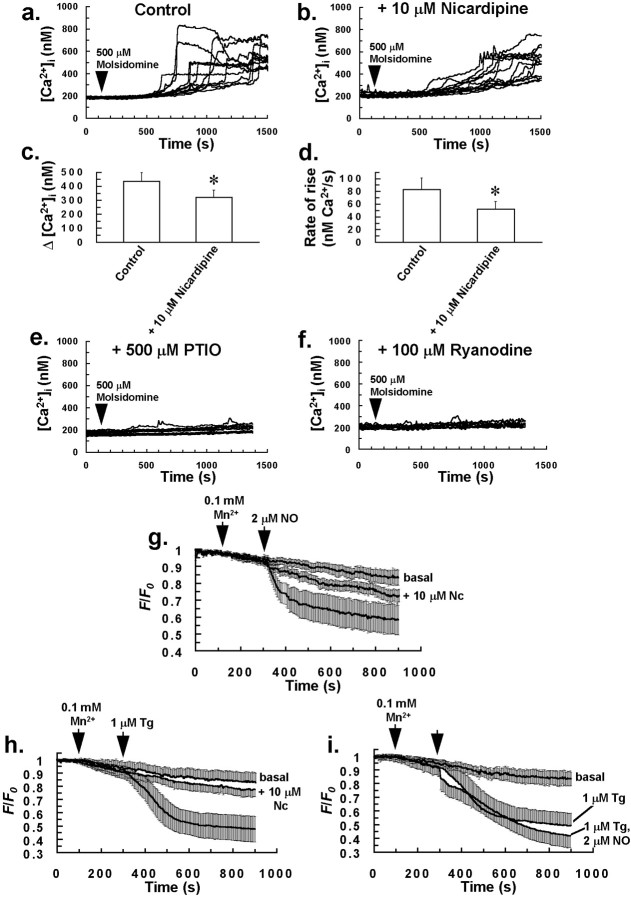
Bolus addition of the NO donor molsidomine (100–500 μm) to the incubation bath resulted in a heterogeneous increase in [Ca2+]i of glial cells and also localized intercellular Ca2+ waves involving groups of 5–15 cells, similar to those observed with puffs of aqueous NO and 1 μm ryanodine. With application of 500 μmmolsidomine, [Ca2+]i started to increase in cells after a latent period of ∼400 sec. Responses consisted of an initial slow increase in [Ca2+]i, followed by a much faster burst phase (Fig.4a), with >90% of glial cells demonstrating an increase in [Ca2+]i to 500 μm molsidomine. This latent effect for molsidomine (Fig. 4a) could possibly be attributable to slow or delayed NO donation. A positive feedback mechanism could also be responsible for the above effect, but considering the lack of a significantly delayed rise in [Ca2+]i of cells after a puff of aqueous NO (Fig. 2c), the former suggestion may seem a more appropriate explanation. The rate of rise for increases in [Ca2+]i induced by molsidomine was reduced by pretreating cells with 10 μm nicardipine for 5 min (Fig.4b,d), with cells demonstrating increases in [Ca2+]i with a prolonged initial slow phase, followed by a burst phase that was often slower and reduced in magnitude compared to control cells (Fig.4b–d). Results suggest that NO induces Ca2+ influx in glia, which is nicardipine-sensitive. As for aqueous NO, molsidomine-induced elevations in [Ca2+]i were essentially abrogated by pretreating cells with 500 μm PTIO for 5 min (Fig. 4e) or 100 μm ryanodine for 30 min (Fig.4f) before molsidomine application.
Fig. 4.
NO induces Ca2+ influx in glia that is nicardipine-sensitive. The rate of rise and magnitude of increases in [Ca2+]i induced by bolus addition of the NO donor molsidomine to the incubation bath (a) were reduced by pretreating cells with 10 μm nicardipine for 5 min (b).c, Graph derived from the experiments ofa and b showing a reduction in Δ[Ca2+]i for cells that responded to 500 μm molsidomine, after pretreatment of cells with 10 μm nicardipine. d, Graph derived from the experiments of a and b showing a reduction in the rate of rise of [Ca2+]i of the final burst phase, for cells that demonstrated an increase in [Ca2+]i to 500 μmmolsidomine, after pretreatment of cells with 10 μmnicardipine. Bars in c and d indicate the means of at least four separate estimations, and error bars indicate SD (*p < 0.05 vs control value; Student'st test for unpaired observations). Molsidomine-induced elevations in [Ca2+]i were completely abrogated by pretreating cells with 500 μm PTIO for 5 min (e) or 100 μm ryanodine for 30 min (f) before molsidomine application (arrow). Traces in a,b, e, and f are from 12 individual cells and are representative of at least four separate experiments for each of the above treatments. Bolus application of 2 μm aqueous NO (g) or 1 μm Tg (h) to cells resulted in Mn2+ quench of cytosolic fura-2 fluorescence (365 nm excitation) that was abrogated by pretreating cells with 10 μm nicardipine (Nc; center traces) for 5 min. There was no significant additive effect on the Mn2+ quench of cytosolic fura-2 fluorescence with a combined application of NO and Tg, compared to Tg alone (i), suggesting that NO acts on the same Ca2+ influx pathway as Tg in glial cells. All traces in g–i represent the mean normalized fluorescence intensity of at least 200 cells from four separate experiments for each of the above treatments. Error bars indicate SD. For measurements of basal Mn2+ quench of cytosolic fura-2 fluorescence (top traces) in g–i, no drugs were added to cells.
Further evidence for NO inducing Ca2+influx in glial cells was derived from Mn2+ quench experiments. It is generally accepted that Mn2+ can enter cells along with Ca2+ via store-operated Ca2+ channels, which open after Ca2+ store depletion (Putney, 1990) and also probably via other Ca2+ or nonselective cation channels. The endoplasmic reticulum (ER) Ca2+-ATPase inhibitor thapsigargin (Tg) is well established as an agent that activates store-operated Ca2+ influx in cells by depleting intracellular Ca2+ stores (Zweifach and Lewis, 1993; Petersen and Berridge, 1994). Bolus application of 2 μm aqueous NO (Fig. 4g), or 1 μm Tg (Fig. 4h), to cells resulted in Mn2+ quench of cytosolic fura-2 fluorescence, which was almost completely prevented by pretreating cells with 10 μm nicardipine (center traces) for 5 min, indicating that both NO and Tg activate a Ca2+ influx mechanism in glia. It is noteworthy that a previous study also demonstrated nicardipine sensitivity for Tg-induced Ca2+ influx in immature monocytes (Willmott et al., 1996b). There was no significant additive effect on Mn2+ quench of cytosolic fura-2 fluorescence with a combined application of NO and Tg, compared to Tg alone (Fig. 4i). These data suggest that NO acts on the same dihydropyridine-sensitive Ca2+ influx pathway as Tg in glial cells. Considering that NO also depletes intracellular Ca2+ stores of glia (see above), it is possible that the nicardipine-sensitive Ca2+ influx activated by Tg and NO may be a store-operated Ca2+ influx mechanism or an influx mechanism activated by an increase in [Ca2+]i. NO-induced Ca2+ influx in glia may also be via cell depolarization and activation of voltage-gated Ca2+ channels, however it was beyond the scope of this study to test whether NO induces glial cell depolarization.
Intercellular Ca2+ waves induced by mechanical stimulation of single glial cells are inhibited by nicardipine and antagonizing concentrations of ryanodine
Mechanical stimulation of a single glial cell evoked an intercellular Ca2+ wave, with a mean propagation rate of 13.9 ± 0.4 μm/sec (Fig.5a,e). This was achieved by slowly lowering a microinjection pipette onto a centrally located, single glial cell. After touching the cell, the pipette was immediately removed. The intercellular wave was characterized by concentric propagation of increased [Ca2+]i through neighboring cells. Although initially regarded as being solely attributable to gap junctional communication between cells, there is increasing evidence that extracellular Ca2+ signaling might also play a role in intercellular Ca2+ waves in glia (Charles, 1998; Cotrina et al., 1998; Guthrie et al., 1999). Indeed in this study, we observed increases in [Ca2+]i (Fig.5f) of cells separated from the mechanically stressed target cell region by a void region (Fig. 5a, bottom sequence of ratio images), suggesting the release of a freely diffusible messenger and an extracellular, gap junction-independent mode of communication for this response.
Fig. 5.
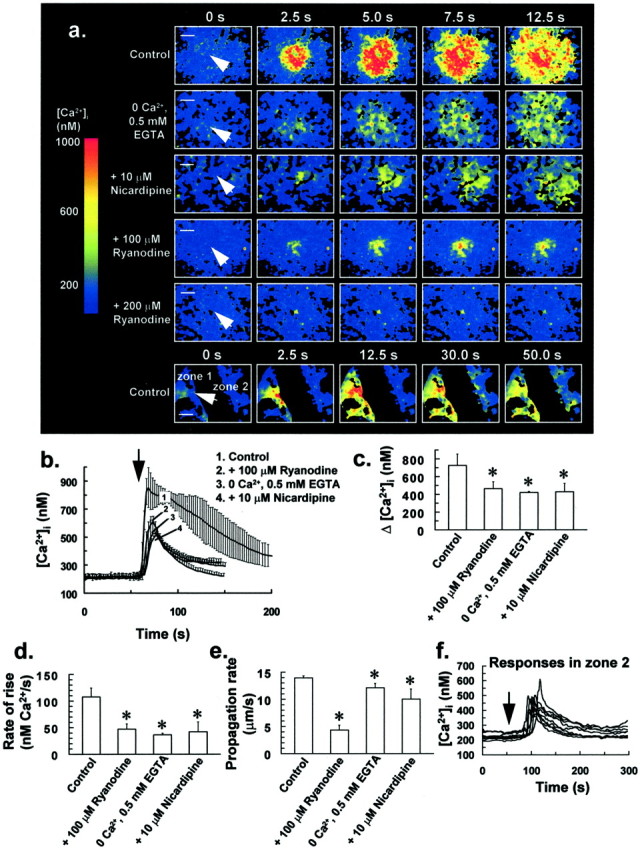
Intercellular Ca2+waves induced by mechanical stress of single glial cells.a,Top panel(control), Mechanical stress of a single glial cell (arrow) evoked an intercellular Ca2+ wave, with a mean propagation rate of 13.9 ± 0.4 μm/sec (n = 5). Bottom panels in a show a reduction in the stress-induced Δ[Ca2+]i after transfer of cells to a nominally Ca2+-free medium (0 Ca2+, 0.5 mm EGTA) for 1 min or by pretreating cells with 10 μm nicardipine for 5 min. High concentrations of ryanodine (100–200 μm) reduced Δ[Ca2+]i and also inhibited Ca2+ wave propagation (a), with a 1 hr incubation of cells with 200 μm ryanodine resulting in an increase in [Ca2+]i in only the mechanically stressed target cell (arrow). Fluorescence ratio image sequences of a are representative of at least six separate experiments for each treatment. Scale bars, 50 μm. b,[Ca2+]i of up to 25 of the closest responding glial cells to the mechanically stressed target cell was averaged for individual experiments. Traces in brepresent the mean of these averaged responses from at least six separate experiments for each of the above treatments, with error bars indicating SD. Similarly, Δ[Ca2+]iand rate of rise of up to 25 of the closest responding glial cells to the target cell were averaged for individual experiments, with bars inc and d indicating the mean of these averaged parameters ± SD from at least six separate experiments for each treatment (*p < 0.05 vs control value; Student's t test for unpaired observations).e, Effect of the above treatments on the stress-induced intercellular Ca2+ wave propagation rate. Bars ine indicate the mean Ca2+ wave propagation rate ± SD from at least six separate experiments (*p < 0.05 vs control value; Student'st test for unpaired observations). f,Traces derived from the bottom sequence of ratio images ofa, showing an increase in [Ca2+]i for cells of zone 2; for two cell regions (zone 1 and zone 2) ∼100 μm apart and separated by a void region (bottom sequence of ratio images ina), mechanical stress of a glial cell in zone 1 (arrow) resulted in an increase in [Ca2+]i in cells located in zone 2 after ∼30 sec. These data suggest that a messenger or messengers are released from cells in zone 1 after an increase in [Ca2+]i, and this messenger or messengers diffuse across the void region and activate cells located in zone 2. The arrow in f denotes the time at which the cell in zone 1 was mechanically stimulated and corresponds to t = 0 sec for the sequence of ratio images ina.
The incubation of cells in a nominally Ca2+-free medium (0 Ca2+, 0.5 mm EGTA) for 1 min or pretreating cells with 10 μm nicardipine for 5 min resulted in a significant reduction in both the stress-induced Δ[Ca2+]i (Fig.5a–c) and rate of rise for increases in [Ca2+]i of individual cells (Fig. 5d). Intercellular Ca2+ wave propagation rate was also slowed by a small, but significant amount by these treatments (Fig.5e). Similarly, high concentrations of ryanodine (100–200 μm) reduced Δ[Ca2+]i (Fig.5a–c), rate of rise of increases in [Ca2+]i (Fig.5d), and also Ca2+ wave propagation rate (Fig. 5e), with a 1 hr incubation of cells with 200 μm ryanodine resulting in an increase in [Ca2+]i in only the mechanically stimulated target cell (Fig. 5a). As for the NO puff experiments, treatment of cells with this concentration of ryanodine did not result in Ca2+ store depletion over the course of drug preincubation. These data suggest that both Ca2+ mobilization and Ca2+ influx, which is nicardipine-sensitive, contribute to stress-induced intercellular Ca2+ waves in glia, with Ca2+ release from a RyR-linked Ca2+ store contributing to the mobilization component. It is therefore noteworthy that the pharmacology of the stress-induced intercellular Ca2+ wave shares similarity with the NO-induced increase in [Ca2+]i, thus suggesting a possible role for the NO–G-kinase signaling pathway in this particular response.
From the data of Figure 5, it may seem unlikely that the propagation rate of the stress-induced Ca2+ wave is dependent on Δ[Ca2+]i of individual cells, because although there was no significant difference in Δ[Ca2+]i for the stress response of cells bathed in a nominally Ca2+-free medium or for cells treated with 100 μm ryanodine or 10 μm nicardipine (Fig.5c), the propagation rate of the Ca2+ wave for ryanodine-treated cells (Fig. 5e) was reduced compared to the other treatments (p < 0.05; Student's t test for unpaired observations).
Intercellular Ca2+ waves induced by mechanical stimulation of single glial cells are inhibited by antagonists of the NO–G-kinase signaling pathway and phospholipase C inhibition
Pretreating cells with inhibitors of the NO–G-kinase signaling pathway (100 μm PTIO for 5 min, 10 mg/ml hemoglobin for 5 min, 300 μml-NMMA for 30 min, 10 μm LY 83583 for 1 min, or 200 μmRp-8-pCPT-cGMPS for 30 min) resulted in a significant reduction in Δ[Ca2+]i (Fig.6a–c), rate of rise of increases in [Ca2+]i of individual cells (Fig. 6d), and also Ca2+ wave propagation rate (Fig.6e) after mechanical stimulation of single glial cells, as above. The extent of intercellular Ca2+wave propagation was also affected by these treatments, with waves usually restricted to coverslip areas of between 1 and 2 × 104 μm2, as opposed to 5 and 6 × 104μm2 for untreated control cells. These agents alone had no effect on [Ca2+]i and did not cause Ca2+ store depletion during the preincubation period. Furthermore, it is unlikely that Ca2+ wave inhibition was caused by some run-down phenomenon, because incubating cells in the absence of drugs for up to 2 hr at 22°C did not significantly affect stress-induced intercellular Ca2+ wave parameters.
Fig. 6.
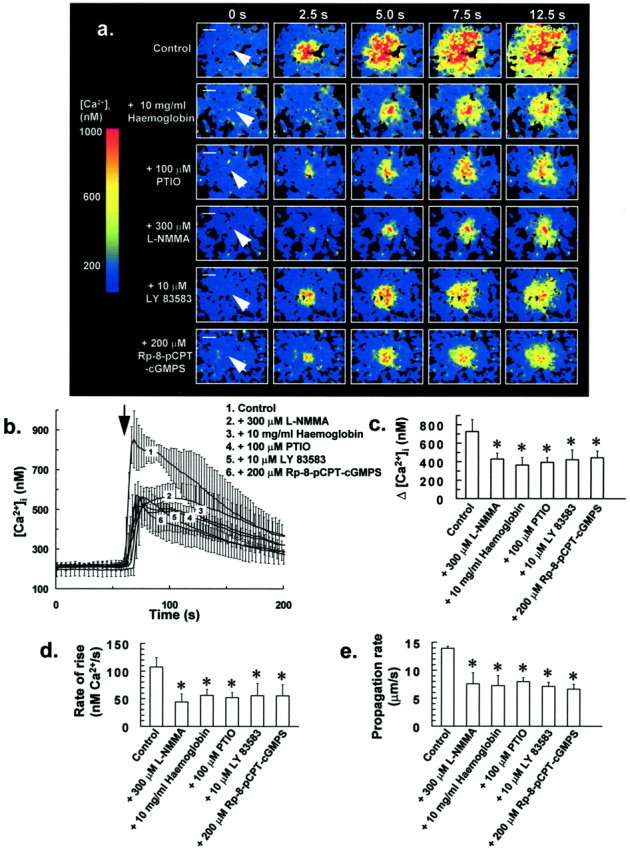
Antagonists of NO–G-kinase signaling inhibit intercellular Ca2+ wave propagation in glia.a, Pretreating cells with NO scavengers or inhibitors of the NO–G-kinase signaling pathway (10 mg/ml hemoglobin for 5 min, 100 μm PTIO for 5 min, 300 μml-NMMA for 30 min, 10 μm LY 83583 for 1 min, or 200 μm Rp-8-pCPT-cGMPS for 30 min) resulted in a reduction in both Δ[Ca2+]i and also Ca2+ wave propagation rate after mechanical stress of single glial cells (arrow). Fluorescence ratio image sequences in a are representative of at least six separate experiments for each treatment. Scale bars, 50 μm.b, [Ca2+]i for 25 of the closest responding glial cells to the mechanically stressed target cell was averaged for individual experiments. Traces inb represent the mean of these averaged responses from at least six separate experiments for each of the above treatments, with error bars indicating SD. Similarly, Δ[Ca2+]i and rate of rise of [Ca2+]i for 25 of the closest responding glial cells to the mechanically stressed target cell were averaged for individual experiments, with bars in c andd representing the mean of these averaged parameters ± SD from at least six separate experiments (*p < 0.05 vs control value; Student'st test for unpaired observations). e,Effect of the above treatments on the stress-induced intercellular Ca2+ wave propagation rate. Bars in erepresent the mean Ca2+ wave propagation rate ± SD from at least six separate experiments (*p < 0.05 vs control value; Student's t test for unpaired observations).
The above agents reduced each of the measured Ca2+ response parameters by ∼50% (Fig.6c–e), suggesting that Ca2+mobilization and influx induced by the NO–G-kinase signaling pathway are fundamental components of stress-induced intercellular Ca2+ waves in glial cells. Because the extracellular NO scavenger hemoglobin (10 mg/ml) was also effective in inhibiting intercellular Ca2+ waves induced by mechanical stress (Fig. 6), it is likely that NO acts as an extracellular messenger in this response, freely diffusing between adjacent cells, and thereby assisting intercellular Ca2+ wave propagation.
Pretreating cells with the phospholipase C inhibitor U 73122 (5 μm) for 10 min or l-NMMA (300 μm) for 30 min and U 73122 (5 μm) for 10 min in combination, resulted in a reduction in both Δ[Ca2+]i (Fig.7a,b), rate of rise of increases in [Ca2+]i of individual glial cells for the combined drug treatment (Fig.7c) and also intercellular Ca2+wave propagation rate (Fig. 7d) after mechanical stress of a single glial cell. For the combined drug treatment, there was a greater reduction in Δ[Ca2+]i and propagation rate of the Ca2+ wave (p < 0.05; Student's t test for unpaired observations) compared to parameters derived from cells that had been treated solely with phospholipase C (PLC) inhibitor for 10 min (Fig. 7) or 300 μml-NMMA for 30 min (Fig. 6) before mechanical stress. This additive inhibitory effect for PLC and nitric oxide synthase inhibitors on the intercellular Ca2+ wave suggests that both IP3 and NO-induced Ca2+ mobilization contribute to the stress-induced Ca2+ response of glia.
Fig. 7.
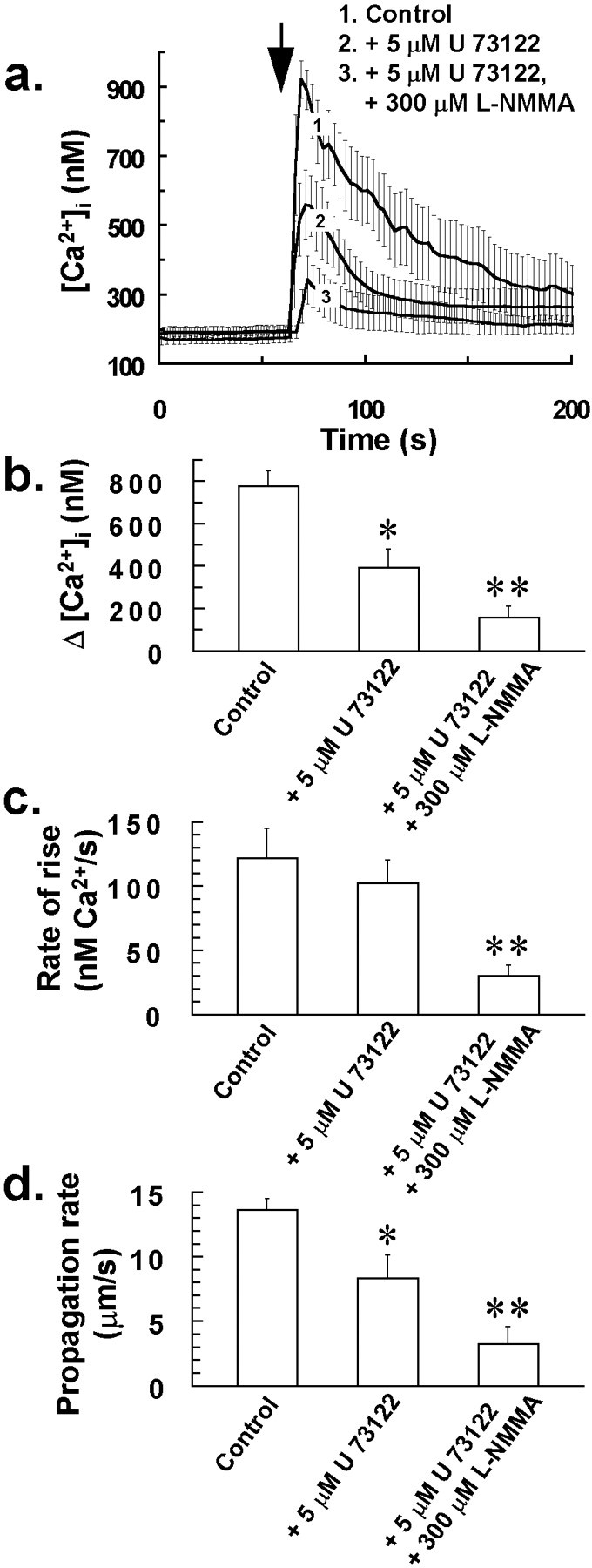
Additive inhibitory effect for PLC and nitric oxide synthase inhibitors on intercellular Ca2+ wave propagation in glia. Pretreating cells with the phospholipase C inhibitor U 73122 (5 μm) for 10 min or l-NMMA (300 μm) for 30 min and U 73122 (5 μm) for 10 min in combination, resulted in a reduction in both Δ[Ca2+]i (b) and also Ca2+ wave propagation rate (d) after mechanical stress of a single glial cell at the time indicated by the arrow ina, with the combined drug treatment having a greater inhibitory effect on the Ca2+ wave compared to solely treating cells with PLC inhibitor. a,[Ca2+]i for 25 of the closest responding glial cells to the mechanically stressed target cell was averaged for individual experiments. Traces in arepresent the mean of these averaged responses from at least four separate experiments for each of the above treatments, with error bars indicating SD. Similarly, Δ[Ca2+]iand rate of rise of [Ca2+]i for 25 of the closest responding glial cells to the mechanically stressed target cell were averaged for individual experiments, with bars inb and c indicating the mean of these averaged parameters ± SD from at least four separate experiments (*p < 0.05 vs control value; **p < 0.05 vs 5 μm U 73122 value; Student's t test for unpaired observations).d, Effect of the above treatments on the stress-induced intercellular Ca2+ wave propagation rate. Bars ind indicate the mean Ca2+ wave propagation rate ± SD from at least four separate experiments (*p < 0.05 vs control value; **p < 0.05 vs 5 μm U 73122 value; Student's t test for unpaired observations).
Increased NO production in glia after mechanical stress of a single glial cell
Further evidence for the participation of NO in stress-induced intercellular Ca2+ waves was derived from experiments using the fluorescent NO probe DAF-2. In these experiments we observed an increase in DAF-2 fluorescence (490 nm excitation) in cells (Fig.8b–d) after treatments that induce an increase in [Ca2+]i presumably independently of cell surface receptor activation (Fig. 8a); these included mechanical stress of a single glial cell, depolarizing cells with 50 mm KCl, and application of 1 μm of the Ca2+ionophore ionomycin to cells. Increases in intracellular DAF-2 fluorescence to these treatments were abrogated by pretreating cells with 300 μml-NMMA for 30 min (Fig. 8c,d), indicating that they were caused by nitric oxide synthase activity and an increase in cytosolic NO. It is especially noteworthy that the profile and time scale of NO responses (Fig. 8c) were similar to the Ca2+ responses (Fig. 8a) induced by the above treatments, possibly suggesting a close coupling between [Ca2+]iand cytosolic NO concentration in cells, and seemingly consistent with a previous study suggesting the presence of Ca2+-dependent nitric oxide synthase in glia (Feinstein et al., 1994). It is also noteworthy that the NO response after mechanical stress of a single glial cell (Fig.8b) shares similar spatiotemporal characteristics as the stress-induced intercellular Ca2+ wave (Figs. 5a, 6a), with cytosolic NO increasing initially in cells close to the target cell, followed by increases in more distant cells after 12.5 sec.
Fig. 8.
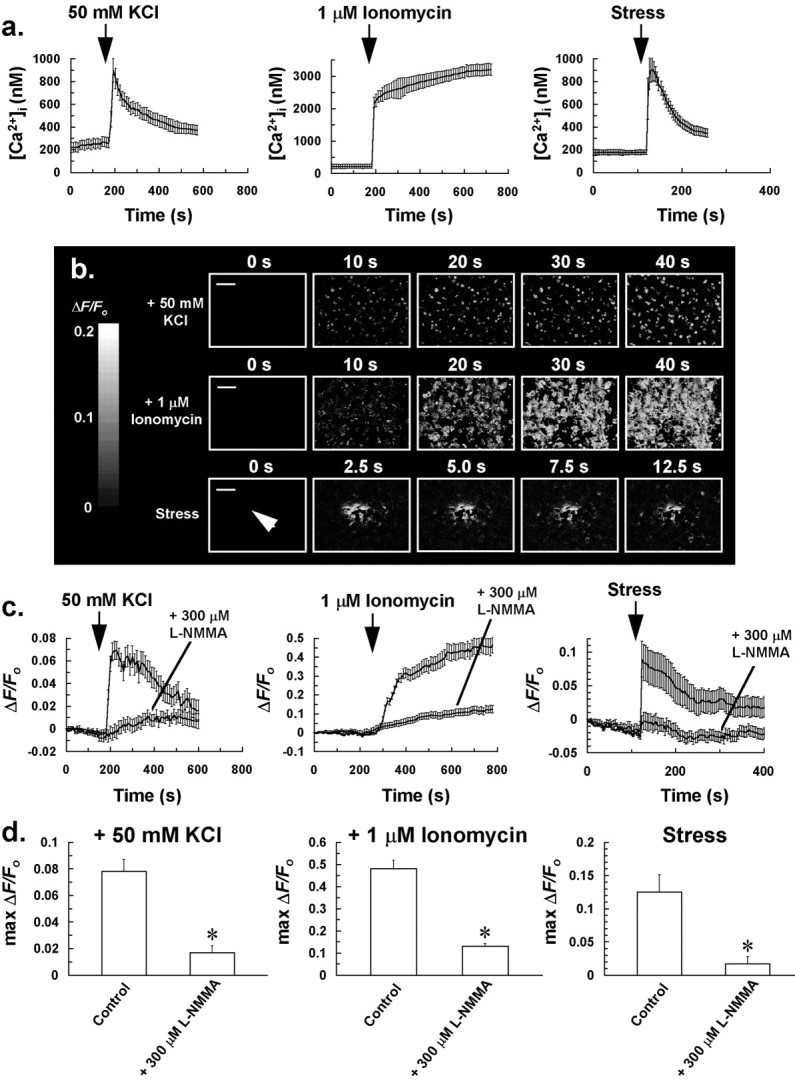
Evidence that mechanical stress and treatments that induce an increase in [Ca2+]iindependently of cell surface receptor activation are coupled to an increase in NO production in glia. a, Increase in [Ca2+]i of glial cells to 50 mm KCl, 1 μm ionomycin, or mechanical stress of a single glial cell. [Ca2+]i of at least 25 responding glial cells (for the stress response, chosen cells were the closest to the mechanically stressed target cell) was averaged for individual experiments. Traces in a represent the mean of these averaged responses from at least four separate experiments for each of the above treatments, with error bars indicating SD. b, Sequences of ratio images (ΔF/Fo) showing an increase in DAF-2 fluorescence in cells after bolus addition of 50 mm KCl or 1 μm ionomycin to the incubation bath, or mechanical stress of a single glial cell (arrow) at t = 0 sec, indicating that these treatments induce an increase in cytosolic NO in cells. Fluorescence ratio image sequences of b are representative of at least four separate experiments for each of the above treatments. Scale bars, 50 μm. c,ΔF/Fo of at least 25 glial cells (for the stress response, chosen cells were the closest to the mechanically stressed target cell) was averaged for individual experiments of b. Traces in c represent the mean of these averaged responses from at least four separate experiments for each of the above treatments, with error bars indicating SD. Pretreatment of cells for 30 min with l-NMMA inhibited the rise in cytosolic NO to the above treatments (c, d). d, Graphs derived from the experiments ofc, showing a reduction in NO production to 50 mm KCl, 1 μm ionomycin, and mechanical stress of a single glial cell, after pretreatment of cells with 300 μml-NMMA for 30 min. The maximum ΔF/Fo value of at least 25 glial cells (for the stress response, chosen cells were the closest to the mechanically stressed target cell) was averaged for individual experiments, with bars in d representing the mean of these averaged parameters ± SD from at least four separate experiments (*p < 0.05 vs control value).
The increase in [Ca2+]i induced by ATP is not mediated via an increased production of NO in glial cells and is not dependent on ryanodine receptor-linked Ca2+ release
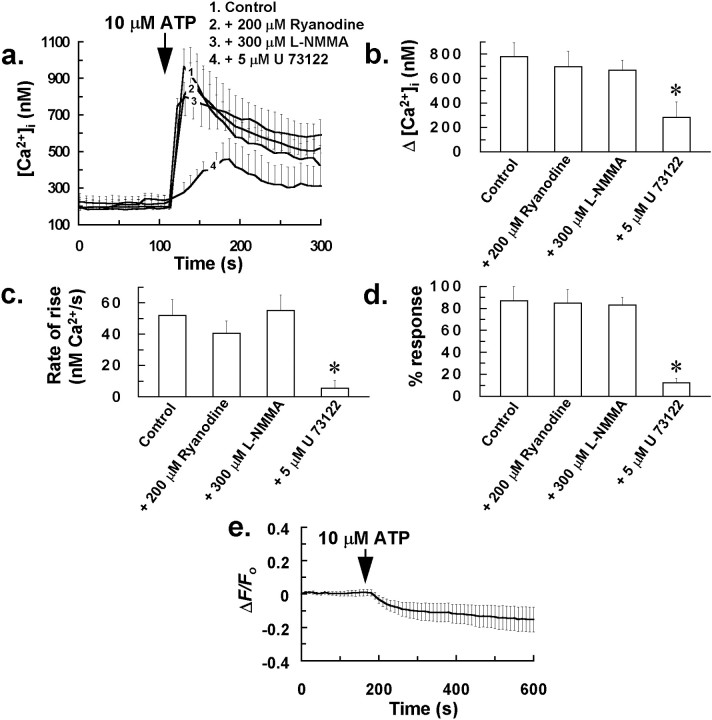
To test whether NO-induced Ca2+mobilization from ryanodine receptor-linked Ca2+ stores might also contribute to a cell surface receptor-linked Ca2+ response that has been proposed to be mediated via the G-protein-coupled activation of PLC and IP3 generation in astrocytes (Centemeri et al., 1997), we examined the effect of an antagonizing concentration of ryanodine (200 μm) andl-NMMA (300 μm) on the increase in [Ca2+]i induced by the P2-receptor agonist ATP. This species has been previously proposed as a candidate for the extracellular messenger involved in mediating intercellular Ca2+waves in glia (Cotrina et al., 1998; Guthrie et al., 1999). In direct contrast to their inhibitory effect on the intercellular Ca2+ wave induced by mechanical stress, the above treatments did not significantly affect the increase in [Ca2+]i induced by bolus application of 10 μm ATP to cells (Fig.9a–d). However, consistent with the study of Centemeri et al. (1997), we did observe significant inhibition of the ATP-induced Ca2+response after pretreatment of glial cells with 5 μm of the PLC inhibitor U 73122 (Fig.9a–d), suggesting that this response is dependent on intracellular IP3 generation and independent of cytosolic NO or RyR-linked Ca2+ release. Further evidence for this notion was derived from experiments with the NO probe DAF-2, in which no increase in DAF-2 fluorescence was observed in any glial cells after administration of 10 μm ATP (Fig. 9e). The absence of any rise in cytosolic NO in response to ATP might explain the apparent lack of RyR-linked Ca2+ release for the above response.
Fig. 9.
P2-receptor activation is not coupled to an increased production of NO in glial cells, and the associated Ca2+ response is not dependent on ryanodine receptor-linked Ca2+ release. a,Effect of pretreating mixed glial-neuron cultures with 200 μm ryanodine for 1 hr, 300 μml-NMMA for 30 min, or 5 μm U 73122 for 10 min on the increase in [Ca2+]i induced by bolus application of 10 μm ATP to the incubation bath. [Ca2+]i for at least 50 responding glial cells was averaged for individual experiments. Traces ina represent the mean of these averaged responses from at least four separate experiments for each of the above treatments, with error bars indicating SD. Similarly, Δ[Ca2+]i and rate of rise of [Ca2+]i for at least 50 responding glial cells were averaged for individual experiments, with bars inb and c representing the mean of these averaged parameters ± SD from at least four separate experiments.d, Percentage of cells in a 350 × 300 μm field that demonstrated a rise in [Ca2+]i to 10 μm ATP, after pretreatment with the above drugs. Bars in d are mean values ± SD from at least four separate experiments (*p < 0.05 vs control value; Student's t test for unpaired observations).e, Decrease in DAF-2 fluorescence in glial cells after application of 10 μm ATP to the incubation bath. ΔF/Fo of at least 50 glial cells was averaged for individual experiments, with the trace ine representing the mean of these averaged responses from at least four separate experiments, with error bars indicating SD.
The P2 receptor antagonist suramin and the ATP-degrading enzyme apyrase do not completely block stress-induced intercellular Ca2+ waves of glia
Pretreating cells with the general P2receptor antagonist suramin (100 μm) or the ATP-degrading enzyme apyrase (40 U/ml; apyrase grade III; Sigma) for 30 min resulted in a reduction in both Δ[Ca2+]i and also Ca2+ wave propagation rate, after mechanical stress of a single glial cell (Fig.10). As for antagonists of NO–G-kinase signaling, the extent of intercellular Ca2+ wave propagation was also affected by pretreating cells with suramin or apyrase, with waves usually restricted to coverslip areas of between 1 and 3 × 104 μm2(involving 15–40 cells), as opposed to coverslip areas of 5 and 6 × 104 μm2(involving > 180 cells) for untreated control cells. These data are essentially similar to the previous observations of Cotrina et al. (1998), who also demonstrated inhibition, but not complete abrogation of the stress-induced intercellular Ca2+wave by suramin and apyrase. This suggests that although ATP release from glia appears to play a role in Ca2+wave propagation, one or more other underlying mechanisms are also likely to contribute to the stress-induced wave.
Fig. 10.
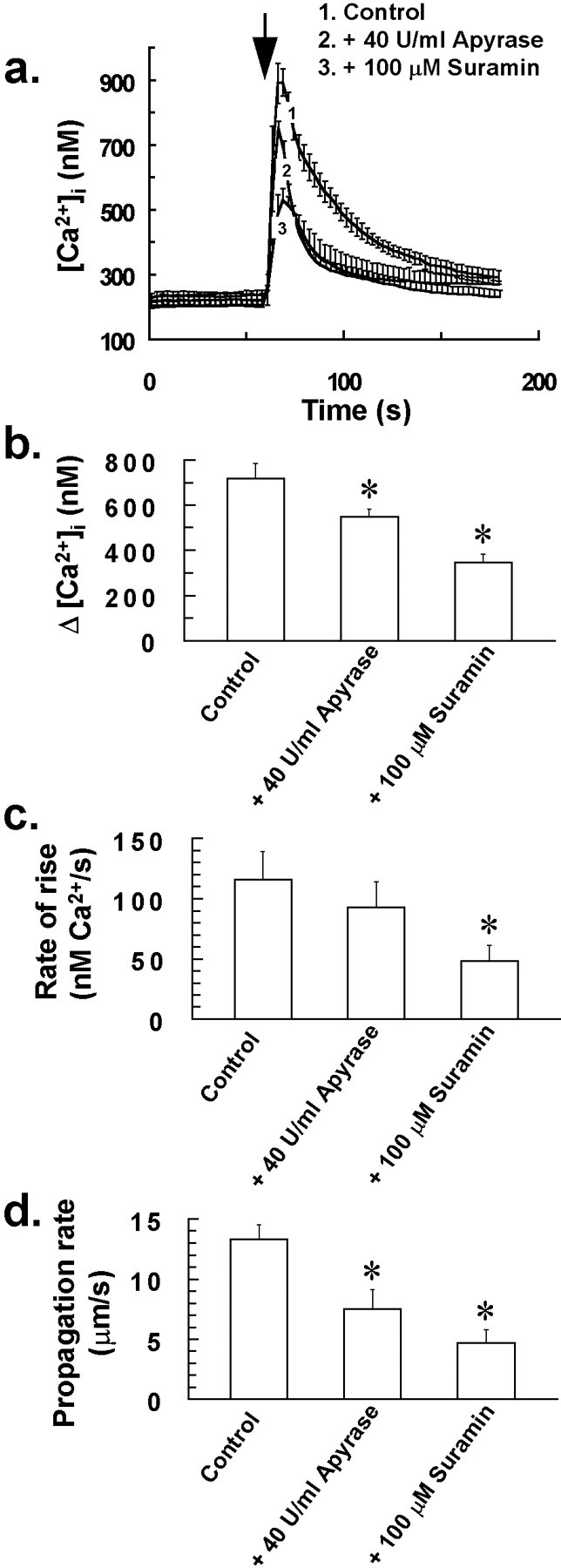
The P2 receptor antagonist suramin and the ATP-degrading enzyme apyrase inhibit the stress-induced intercellular Ca2+ wave of glia. Pretreating cells with the general P2 receptor antagonist suramin (100 μm) or the ATP-degrading enzyme apyrase (40 U/ml) for 30 min resulted in a reduction in both Δ[Ca2+]i (a,b) and Ca2+ wave propagation rate (d) after mechanical stress of a single glial cell at the time indicated by the arrow in a. a,[Ca2+]i for 15 of the closest responding glial cells to the mechanically stressed target cell was averaged for individual experiments. Traces in arepresent the mean of these averaged responses from at least four separate experiments for each of the above treatments, with error bars indicating SD. Similarly, Δ[Ca2+]iand rate of rise of [Ca2+]i for 15 of the closest responding glial cells to the mechanically stressed target cell were averaged for individual experiments, with bars inb and c indicating the mean of these averaged parameters ± SD from at least four separate experiments (*p < 0.05 vs control value; Student'st test for unpaired observations). d,Effect of the above treatments on the stress-induced intercellular Ca2+ wave propagation rate. Bars in dindicate the mean Ca2+ wave propagation rate ± SD from at least four separate experiments (*p < 0.05 vs control value; Student's t test for unpaired observations). The extent of intercellular Ca2+ wave propagation was also affected by pretreating cells with suramin or apyrase, with waves usually restricted to coverslip areas of between 1 and 3 × 104 μm2, as opposed to 5 and 6 × 104μm2 for untreated control cells.
DISCUSSION
Results from this study suggest that NO–G-kinase signaling is coupled to Ca2+ mobilization and influx in glial cells and that this pathway plays a fundamental role in mediating intercellular Ca2+ waves induced by mechanical stimulation of single cells. To our knowledge, this is the first study to highlight a role for the NO–G-kinase signaling pathway and RyR-linked Ca2+ release in intercellular Ca2+ wave generation and propagation in glia. A previous study suggested the presence of RyR-coupled Ca2+ pools in astrocytes, because a low, agonizing concentration of ryanodine (10 μm) induced Ca2+ store depletion in these cells (Giaume and Venance, 1998). However, the effect on intercellular Ca2+ waves of higher, antagonizing ryanodine concentrations, which are known to block the associated Ca2+ conductance of RyRs (Rousseau et al., 1987; Zimanyi et al., 1992), was not assessed byGiaume and Venance (1998).
In our study, we demonstrate that a high concentration of ryanodine (200 μm) is effective in completely blocking the intercellular Ca2+ wave induced by mechanical stress of a single glial cell. This result appears to conflict with data from previous studies that demonstrated abrogation of the stress-induced Ca2+ wave by the IP3 receptor (IP3R) antagonist heparin in retinal glial cells (Newman and Zahs, 1997) and also by the PLC inhibitor U 73122 in astrocytes (Charles et al., 1993;Venance et al., 1997), suggesting that the response was mediated principally by IP3-induced Ca2+ release from IP3R-linked Ca2+stores. However, it is possible that both IP3Rs and RyRs are required in an activated, conducting state to allow the generation and propagation of the Ca2+wave. For example, IP3R-linked Ca2+ release may be required to prime and activate RyR-linked Ca2+ release, possibly via NO production, with the latter mechanism perhaps contributing principally to the propagation of the Ca2+wave. Although the precise reason for the complete sensitivity of the stress-induced Ca2+ wave to either RyR or IP3R/PLC inhibition is not apparent from our study, it nevertheless remains likely that both IP3 and NO -induced Ca2+ release mechanisms contribute to the overall response, because a combined treatment of cells with PLC and NOS inhibitors had an additive inhibitory effect on the Ca2+ wave (Fig. 7). It has previously been reported that treatment of glial cells with U 73122 alone is sufficient to completely abrogate the stress-induced Ca2+ wave, with only an increase in [Ca2+]i being observed in the target cell (Charles et al., 1993; Venance et al., 1997). This is in contrast to the results of our study. For cells treated with up to 10 μm U 73122 for 30 min before mechanical stress, we observed a slowing of Ca2+ wave propagation rate and a reduction of Δ[Ca2+]i, but not complete abolition of the Ca2+ wave, similar to the results of Figure 7.
It is interesting to note that several roles for the NO–G-kinase signaling pathway in the regulation of cellular Ca2+ fluxing have previously been suggested in a few cell types, with certain aspects of intracellular Ca2+ homeostasis having been reported to involve modulation by NO and/or cGMP (Clementi and Meldolesi, 1997). Recent interest has focussed on specific roles for NO–G-kinase signaling in regulating store-operated Ca2+ influx and Ca2+ influx pathways that are dependent on the state of filling of intracellular Ca2+stores (Xu et al., 1994; Bischof et al., 1995; Mathes and Thompson, 1996; Willmott et al., 1996a) and also Ca2+ mobilization via the increased synthesis of the Ca2+ mobilizing agent cyclic ADP-ribose (Clementi et al., 1996; Willmott et al., 1996c). Cyclic ADP-ribose (cADPR) appears to release Ca2+ from ER Ca2+ stores via RyRs and is considered to be a putative modulator of calcium-induced calcium release (Galione et al., 1991). Nitric oxide has also been shown to induce Ca2+ mobilization in pancreatic β cells independently of an increase in cADPR (Willmott et al., 1995a,b), and possibly involving the direct nitrosylation of regulatory thiol groups of the ryanodine receptor (Stoyanovsky et al., 1997). Different modes of intracellular Ca2+ regulation by NO and the NO–G-kinase signaling pathway may reflect fundamental differences in the structure and function and/or expression of participating component enzymes, phosphorylated targets of G-kinase, plasma membrane ion channels, and Ca2+ release receptor isoforms in cells.
Although NO–G-kinase signaling has been shown to increase [Ca2+]i via Ca2+ mobilization and influx in several cell types, its precise contribution to cell surface receptor-coupled increases in [Ca2+]i and possible involvement in intercellular communication via Ca2+ wave initiation and/or propagation are yet to be assessed in most systems. It is also noteworthy that at the present, there is little published information regarding the efficacy of the NO–G-kinase signaling pathway in increasing [Ca2+]i in excitable or neuronal cells, most studies to date having been conducted in nonexcitable cells. In neurosecretory PC12 cells, it has been suggested that NO–G-kinase signaling can evoke Ca2+ mobilization via the increased synthesis of cADPR (Clementi et al., 1996), presenting the possibility that the enzyme responsible for cADPR synthesis, ADP-ribosyl cyclase, might represent a common target for G-kinase in other excitable cells. It is noteworthy that astrocytes can synthesize cADPR extracellularly from NAD, indicating an inherent ADP-ribosyl cyclase activity for this cell type (Pawlikowska et al., 1996). It was beyond the scope of this study however, to determine whether this, or any intracellular cADPR synthesizing activity is upregulated by NO–G-kinase signaling, as in sea urchin eggs (Willmott et al., 1996c) and PC12 cells (Clementi et al., 1996), or whether cADPR generated by astrocytes is effective in releasing Ca2+ from intracellular stores.
Since NO synthesis by cNOS is usually calcium-dependent (Moncada et al., 1991), a NO-induced rise in [Ca2+]i may result in an amplification of NO production in cells, as previously reported (Publicover et al., 1993). Furthermore, considering a high rate of potentially unrestricted diffusion for NO, this cross-talk between NO and Ca2+ could give rise to Ca2+ waves, as seen in many single cells and tissues (Berridge and Dupont, 1994). Data from this study are supportive of such a mechanism operating in glia, because (1) aqueous NO and molsidomine induce an increase in [Ca2+]i in glia and initiate localized intercellular Ca2+waves; (2) mechanical stress of single glial cells evokes an increase in cytosolic NO in the target and neighboring cells; and (3) stress-induced intercellular Ca2+ waves are seemingly reliant on both the free diffusion of NO between cells and a functional NO–G-kinase signaling pathway within cells. Until recently, it was thought that intercellular Ca2+ wave propagation in cultured astrocytes was solely or predominantly via a gap junctional mode of communication. Previously, a role for NO-induced Ca2+ mobilization in intercellular Ca2+ wave propagation had not been considered in glia, although from this study (Fig.5a,f) and other recent observations (Charles, 1998;Cotrina et al., 1998; Guthrie et al., 1999), additional gap junction-independent modes of Ca2+signaling, involving the release of extracellular messengers, are also likely to assist in mediating intercellular Ca2+ waves in these cells. A study supportive of a potential role for NO as an intercellular messenger in glial communication was however reported by Malcolm et al. (1996). In these experiments, NOS inhibition and the extracellular NO scavenger hemoglobin effectively abrogated cGMP synthesis coupled to NMDA receptor activation in mixed neuron–astrocyte cultures. Cross-talk between NO and Ca2+ was also suggested, because extracellular Ca2+ was required for NMDA receptor-coupled cGMP synthesis.
Although NO–G-kinase signaling and RyR-linked Ca2+ release appear to be fundamentally required for stress-induced intercellular Ca2+ waves of glia, these mechanisms do not appear to play any role in the rise in [Ca2+]i coupled to P2-receptor activation by ATP, which also appears to be involved in intercellular Ca2+ wave propagation (Fig. 10). Although the P2receptor-coupled increase in [Ca2+]i was substantially inhibited by U 73122, suggesting a role for IP3-induced Ca2+release in the response, the increase in [Ca2+]i was not affected by either an antagonizing concentration of ryanodine or byl-NMMA (Fig. 9). Further evidence for a lack of involvement of NO in the rise in [Ca2+]i to ATP was derived from experiments with the NO probe DAF-2, in which we did not observe any increase in DAF-2 fluorescence in glia after treatment of cells with 10 μm ATP (Fig. 9e).
As well as modulating Ca2+ homeostasis in glia, NO appears to regulate gap junction permeability, although its precise mode of action and effect on Ca2+signaling via gap junctions are uncertain. Whether its ultimate effect is to increase or decrease gap junction permeability remains controversial (Bolanos and Medina, 1996; O'Donnell and Grace, 1997).
In conclusion, this study highlights a fundamental role for the NO–G-kinase signaling pathway in mediating intercellular Ca2+ waves in glia, with NO probably acting as an extracellular Ca2+ signaling messenger in this process.
Footnotes
We thank Head First, the Golden Charitable Trust, the National Lottery Charities Board, King's NHS Healthcare Trust, and the Royal Society for supporting this work.
Correspondence should be addressed to Nicholas J. Willmott, Department of Clinical Neuroscience, Rooms E222 and E223, Institute of Psychiatry, King's College London, De Crespigny Park, Denmark Hill, London SE5 8AF. E-mail: spjnnjw@iop.kcl.ac.uk.
REFERENCES
- 1.Berridge MJ, Dupont G. Spatial and temporal signalling by Ca2+. Curr Opin Cell Biol. 1994;6:267–274. doi: 10.1016/0955-0674(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 2.Bischof G, Brenman J, Bredt DS, Machen TE. Possible regulation of capacitative Ca2+ entry into colonic epithelial cells by NO and cGMP. Cell Calcium. 1995;17:250–262. doi: 10.1016/0143-4160(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 3.Bolanos JP, Medina JM. Induction of nitric oxide synthase inhibits gap junction permeability in cultured rat astrocytes. J Neurochem. 1996;66:2091–2099. doi: 10.1046/j.1471-4159.1996.66052091.x. [DOI] [PubMed] [Google Scholar]
- 4.Centemeri C, Bolego C, Abbracchio MP, Cattabeni F, Puglisi L, Burnstock G, Nicosia S. Characterization of the Ca2+ responses evoked by ATP and other nucleotides in mammalian brain astrocytes. Br J Pharmacol. 1997;121:1700–1706. doi: 10.1038/sj.bjp.0701293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles A. Intercellular calcium waves in glia. Glia. 1998;24:39–49. doi: 10.1002/(sici)1098-1136(199809)24:1<39::aid-glia5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Charles AC, Dirksen ER, Merill JE, Sanderson MJ. Mechanisms of intercellular calcium signaling in glial cells studied with dantrolene and thapsigargin. Glia. 1993;7:134–145. doi: 10.1002/glia.440070203. [DOI] [PubMed] [Google Scholar]
- 7.Clementi E, Meldolesi J. The cross-talk between nitric oxide and Ca2+: a story with a complex past and a promising future. Trends Pharmacol Sci. 1997;18:266–269. doi: 10.1016/s0165-6147(97)01087-0. [DOI] [PubMed] [Google Scholar]
- 8.Clementi E, Riccio M, Sciorati C, Nistico G, Meldolesi J. The type 2 ryanodine receptor of neurosecretory PC12 cells is activated by cyclic ADP-ribose. J Biol Chem. 1996;271:17739–17745. doi: 10.1074/jbc.271.30.17739. [DOI] [PubMed] [Google Scholar]
- 9.Cotrina ML, Lin JHC, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signalling. J Neurosci. 1998;18:8794–8804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feinstein DL, Galea E, Cermak J, Chugh P, Lyandvert L, Reis DJ. Nitric oxide synthase expression in glial cells: suppression by tyrosine kinase inhibitors. J Neurochem. 1994;62:811–814. doi: 10.1046/j.1471-4159.1994.62020811.x. [DOI] [PubMed] [Google Scholar]
- 11.Galione A, Lee HC, Busa WB. Ca2+-induced Ca2+-release in sea urchin egg homogenates: modulation by cyclic-ADP-ribose. Science. 1991;253:1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- 12.Giaume C, Venance L. Intercellular Ca2+ signalling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. [PubMed] [Google Scholar]
- 13.Goldman SA, Pulsinelli WA, Clarke WY, Kraig RP, Plum F. The effects of extracellular acidosis on neurons and glia in vitro. J Cereb Blood Flow Metab. 1989;9:471–477. doi: 10.1038/jcbfm.1989.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guthrie PB, Knappenberger J, Segal M, Bennett MVL, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kojima H, Nakatsubo N, Kikuchi K, Urano Y, Higuchi T, Tanaka J, Kudo Y, Nagano T. Direct evidence of NO production in rat hippocampus and cortex using a new fluorescent indicator: DAF-2 DA. NeuroReport. 1998;9:3345–3348. doi: 10.1097/00001756-199810260-00001. [DOI] [PubMed] [Google Scholar]
- 16.Malcolm C, Grieve A, Ritchie L, Schousboe A, Griffiths R. NMDA receptor-mediated cGMP synthesis in primary cultures of mouse cerebellar granule cells appears to involve neuron-astrocyte communication with NO operating as the intercellular messenger. J Neurosci Res. 1996;45:129–142. doi: 10.1002/(SICI)1097-4547(19960715)45:2<129::AID-JNR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 17.Mathes C, Thompson SH. The nitric oxide/cGMP pathway couples muscarinic receptors to the activation of Ca2+ influx. J Neurosci. 1996;16:1702–1709. doi: 10.1523/JNEUROSCI.16-05-01702.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;34:109–142. [PubMed] [Google Scholar]
- 19.Nedergaard M. Direct signalling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 20.Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science. 1997;275:844–845. doi: 10.1126/science.275.5301.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Donnell P, Grace AA. Cortical afferents modulate striatal gap junction permeability via nitric oxide. Neuroscience. 1997;76:1–5. doi: 10.1016/s0306-4522(96)00433-2. [DOI] [PubMed] [Google Scholar]
- 22.Pawlikowska L, Cottrell SE, Harms MB, Li Y, Rosenberg PA. Extracellular synthesis of cADP-ribose from nicotinamide-adenine dinucleotide by rat cortical astrocytes in culture. J Neurosci. 1996;16:5372–5381. doi: 10.1523/JNEUROSCI.16-17-05372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pessah IN, Stambuk RA, Cassida JE. Ca2+-activated ryanodine binding: Mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol Pharmacol. 1987;31:232–238. [PubMed] [Google Scholar]
- 24.Petersen CCH, Berridge MJ. The regulation of capacitative calcium entry by calcium and protein kinase C in Xenopus oocytes. J Biol Chem. 1994;269:32246–32253. [PubMed] [Google Scholar]
- 25.Poenie M. Alteration of intracellular Fura-2 fluorescence by viscosity: a simple correction. Cell Calcium. 1990;11:85–91. doi: 10.1016/0143-4160(90)90062-y. [DOI] [PubMed] [Google Scholar]
- 26.Porter J, McCarthy K. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Publicover NG, Hammond EM, Sanders KM. Amplification of nitric oxide signaling by interstitial cells isolated from canine colon. Proc Natl Acad Sci USA. 1993;90:2087–2091. doi: 10.1073/pnas.90.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 29.Rousseau E, Smith JS, Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987;253:C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- 30.Siesjo B, Zhao Q, Pahlmark K, Siesjo P, Katsura K, Folbergrova J. Glutamate, calcium and free radicals as mediators of ischemic brain damage. Ann Thorac Surg. 1995;59:1316–1320. doi: 10.1016/0003-4975(95)00077-x. [DOI] [PubMed] [Google Scholar]
- 31.Stoyanovsky D, Murphy T, Anno PR, Kim Y-M, Salama G. Nitric oxide activates skeletal and cardiac ryanodine receptors. Cell Calcium. 1997;21:19–29. doi: 10.1016/s0143-4160(97)90093-2. [DOI] [PubMed] [Google Scholar]
- 32.Venance L, Stella N, Glowinski J, Giaume C. Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes. J Neurosci. 1997;17:1981–1992. doi: 10.1523/JNEUROSCI.17-06-01981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willmott NJ, Galione A, Smith PA. Nitric oxide induces Ca2+ mobilization and increases secretion of incorporated 5-hydroxytryptamine in rat pancreatic β-cells. FEBS Lett. 1995a;371:99–104. doi: 10.1016/0014-5793(95)00848-4. [DOI] [PubMed] [Google Scholar]
- 34.Willmott NJ, Galione A, Smith PA. A cyclic-ADP-ribose antagonist does not inhibit secretagogue-, caffeine-, and nitric oxide- induced Ca2+ responses in rat pancreatic β-cells. Cell Calcium. 1995b;18:411–419. doi: 10.1016/0143-4160(95)90056-x. [DOI] [PubMed] [Google Scholar]
- 35.Willmott NJ, Asselin J, Galione A. Calcium store depletion potentiates a phosphodiesterase inhibitor- and dibutyryl cGMP-evoked Ca2+ influx in rat pituitary GH3 cells. FEBS Lett. 1996a;386:39–42. doi: 10.1016/0014-5793(96)00413-9. [DOI] [PubMed] [Google Scholar]
- 36.Willmott NJ, Choudhury Q, Flower RJ. Functional importance of the dihydropyridine-sensitive, yet voltage-insensitive store-operated Ca2+ influx of U937 cells. FEBS Lett. 1996b;394:159–164. doi: 10.1016/0014-5793(96)00939-8. [DOI] [PubMed] [Google Scholar]
- 37.Willmott N, Sethi JK, Walseth TF, Lee HC, White AM, Galione A. Nitric oxide induces calcium mobilization via activation of the cyclic-ADP-ribose pathway. J Biol Chem. 1996c;271:3699–3705. doi: 10.1074/jbc.271.7.3699. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Star RA, Tortorici G, Muallem S. Depletion of intracellular Ca2+ stores activates nitric oxide synthase to generate cGMP and regulate Ca2+ influx. J Biol Chem. 1994;269:12643–12653. [PubMed] [Google Scholar]
- 39.Zimanyi I, Buck E, Abramson JJ, Mack MM, Pessah IN. Ryanodine induces persistent inactivation of the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. Mol Pharmacol. 1992;42:1049–1057. [PubMed] [Google Scholar]
- 40.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]