Abstract
The widespread biological actions of the neurotransmitter dopamine (DA) are mediated by two classes of receptor, the D1 class (D1 and D5) and the D2 class (D2, D3, and D4), which interact synergistically in many paradigms, such as DA agonist-stimulated motor behavior and striatal c-fos expression. Understanding the mechanism(s) of this interaction has been impeded by a controversy regarding the cellular localization of D1 and D2 class receptors. To address this issue from a functional point of view, we elicited striatal Fos by combined administration of a D1 class and a D2 class agonist either in the presence or absence of the fast sodium channel blocker tetrodotoxin (TTX). Striatal Fos elicited by direct D1/D2 stimulation was not reduced by TTX. By contrast, TTX greatly attenuated the Fos response evoked by cocaine or GBR 12909. In separate experiments using antagonists that distinguish among members of the D2 class of receptors, amphetamine-stimulated Fos and motor behavior were attenuated dose-dependently by the selective D2 antagonist L-741,626, but not by the selective D3 antagonist U99194A or the D4-selective antagonist L-745,870. Because Fos expression in the paradigms that were used occurs in enkephalin-negative striatonigral neurons, which show limited coexpression of D1 and D2 receptors, the present findings taken together suggest the intriguing possibility that D1/D2 synergism may be mediated by D1 and D2 receptors residing on separate striatal neurons and interacting in a manner that is not dependent on action potentials.
Keywords: D1 receptors, D2 receptors, D1/D2 synergism, D3receptors, D4 receptors, tetrodotoxin, amphetamine, motor behavior, Fos, striatum
The widespread biological actions of the neurotransmitter dopamine (DA) are mediated by two classes of receptor, the D1 class and the D2 class, which can be distinguished on the basis of second messenger coupling and ligand binding (Kebabian and Calne, 1979; Stoof and Kebabian, 1981). Further molecular distinctions yield five DA receptors that are subsumed into these two classes: the D1 class, composed of the D1 and D5 receptors, and the D2 class, composed of the D2, D3, and D4 receptors (Sibley and Monsma, 1992).
A remarkable feature of normal dopaminergic transmission is that for many behavioral, electrophysiological, and gene-activating influences of DA the concomitant stimulation of D1 class and D2 class receptors is required (Gershanik et al., 1983; Lewis et al., 1983; Braun and Chase, 1986; Walters et al., 1987;LaHoste et al., 1993), a phenomenon we refer to as requisiteD1/D2 synergism. For example, activation of the immediate-early gene c-fos in the striatum occurs after combined administration of direct-acting D1 class and D2 class agonists, but not after either agonist alone (LaHoste et al., 1993). In addition, amphetamine-induced Fos expression in striatum can be blocked by either a D1 class or a D2 class antagonist (Ruskin and Marshall, 1994). In cases of DA agonist-stimulated Fos in striatum, it is specifically the enkephalin-negative striatonigral neurons that are activated (Berretta et al., 1992; Cenci et al., 1992; Ruskin and Marshall, 1994). Similar results indicative of D1/D2 synergism are obtained when agonist-stimulated stereotyped motor behavior is observed (Walters et al., 1987) (for review, see LaHoste and Marshall, 1996). These conclusions regarding D1/D2 synergism are drawn from experiments using pharmacological agents that distinguish well between the D1 and D2classes, but not among members within a class. Thus, it is not clear which member or members of the D1 class interact synergistically with which member or members of the D2 class.
Progress toward elucidating the cellular and molecular mechanisms of D1/D2 synergism has been impeded by controversy regarding the cellular localization of D1 and D2 class receptors. In the striatum, where DA acts to stimulate motor behavior and Fos expression, >90% of neurons are projection neurons comprising the striatonigral and the striatopallidal pathways (Gerfen, 1992). In general, striatonigral neurons, which are the ones that express Fos after DA agonist administration, have been found to express D1 receptor mRNA, whereas striatopallidal neurons have been found to express D2 receptor mRNA. Double in situ hybridization studies of single striatal rat brain sections show segregation of D1 and D2 mRNA-expressing neurons (Gerfen et al., 1990;Gerfen, 1992), and localization of D1 and D2 receptor protein using immunohistochemistry at the electron microscope level also shows no colocalization (Hersch et al., 1995). By contrast, immunohistochemistry at the light microscopy level (Ariano et al., 1995), in situ hybridization of adjacent brain sections (Meador-Woodruff et al., 1991; Lester et al., 1993), and single-cell reverse-transcription PCR (RT-PCR) of dissociated striatal neurons in vitro (Surmeier et al., 1992) provide evidence for at least some cellular colocalization of D1 and D2 mRNA and protein. A partial reconciliation of these discrepancies is provided by more recent single-cell RT-PCR studies indicating that D1/D2 colocalization, at least in enkephalin-negative striatonigral neurons, may be represented more by coexpression of D1 receptor mRNA with D3 or D4 mRNA rather than with D2 mRNA per se (Surmeier et al., 1996).
We have addressed the issue of D1/D2 localization from the perspective of understanding the functional synergism between these two receptor classes. In two series of experiments we have used cellular and behavioral models to address the issue of whether synergistically interacting D1 and D2 class receptors reside on the same or on separate neurons.
MATERIALS AND METHODS
To assess the role of action potentials in the manifestation of D1/D2 synergism, we performed the following experiment. Adult male Sprague Dawley rats (Charles River, Cambridge, MA) weighing 250–350 gm received bilateral guide cannulae (22 gauge) into the caudate putamen (CPu) under surgical anesthesia and stereotaxic guidance (LaHoste and Marshall, 1991). Coordinates were +0.2 mm anterior to bregma, +3.0 mm lateral to the midsagittal suture, and 3.0 mm ventral to dura mater (26 gauge injectors extended to 5.0 mm ventral to dura; Paxinos and Watson, 1986). Keefe and Gerfen (1995) found that insertion of a dummy cannula (reaching to within 0.5 mm of the injection site) the day before the experimental injection could eliminate nonspecific c-fosmRNA expression caused by mechanical stimulation. We observed the same phenomenon with Fos immunoreactivity and therefore adopted their procedure in the present experiments. At 5–7 d after cannulation surgery and 24 hr after dummy cannula insertion, all rats received an intrastriatal infusion of tetrodotoxin [TTX; 1 μl of a 50 μm solution (=16 ng) in 0.9% saline over 2 min] into the left CPu and received vehicle (0.9% saline) into the right CPu. Fifteen minutes later the DA agonists were administered intrastriatally or systemically as follows: (1) four rats received the combination of the D2 class agonist quinpirole (30 μg) and the D1 class agonist SKF 82526 (10 μg) bilaterally into the CPu (in a volume of 1 μl over 2 min); (2) four rats received intraperitoneal injection of quinpirole (1 mg/kg, i.p.) in combination with the D1 class agonist SKF 82958 (2.5 mg/kg, i.p.); (3) five rats received the selective DA reuptake inhibitor GBR 12909 (20 mg/kg, i.p.); (4) five rats received the monoamine reuptake inhibitor cocaine HCl (40 mg/kg, i.p.); (5) seven rats received the monoamine releaser and reuptake inhibitord-amphetamine sulfate (5 mg/kg, i.p.); (6) five rats received intrastriatal saline; and (7) five rats received systemic saline (1 ml/kg, i.p.).
Then 2 hr after DA agonist administration the rats were anesthetized deeply and perfused transcardially with 4% paraformaldehyde. Fixed brains were prepared for Fos immunoreactivity as described previously (LaHoste et al., 1993). Briefly, fixed frozen brains were cut in the coronal plane at 40 μm thickness and incubated in primary antiserum (1:20,000) raised in rabbit against human Fos peptide (Oncogene Science PC-38, Uniondale, NY). After incubation in biotinylated goat anti-rabbit IgG and conjugation of horseradish peroxidase by avidin–biotin coupling, Fos was visualized by reaction with diaminobenzidine. The number of Fos-immunoreactive nuclei at the intracerebral injection site in each CPu was quantified within a 1 × 1 mm square that was medial and adjacent to the end of the cannula track, using computer-assisted microscopic image analysis (LaHoste et al., 1993) with MCID software from Imaging Research (St. Catherine's, Ontario, Canada).
To determine which member(s) of the D2 class of receptors synergize(s) with D1 class receptors to elicit behavioral activation and striatal Fos immunoreactivity, we used the following selective antagonists (Table1). L-741,626 has a 40-fold selectivity for D2 receptors relative to D3 receptors and a 100-fold selectivity relative to D4 receptors (Kulagowski et al., 1996). U-99194A has a 20-fold selectivity for D3receptors relative to D2 receptors and virtually no affinity for D4 receptors (Waters et al., 1993). L-745,870 has a 2000-fold selectivity for D4 receptors relative to D2receptors in vitro and virtually no affinity for D3 receptors (Kulagowski et al., 1996). All of these agents enter the brain on systemic administration (Waters et al., 1994; Bristow et al., 1997), and all of them lack intrinsic activity at their respective receptors.
Table 1.
Ki (nm) values at cloned receptors
| Drug | D2 | D3 | D4 |
|---|---|---|---|
| L-741,626 | 2.4 | 100 | 220 |
| U-99194A | 1572 | 78 | >2000 |
| L-745,870 | 960 | 2300 | 0.43 |
Receptor selectivity based on in vitro Ki (nm) at cloned dopamine D2, D3, and D4, receptors for L-741,626 (Kulagowski et al., 1996), U-99194A (Waters et al., 1993), and L-745,870 (Kulagowski et al., 1996).
Intact male Sprague Dawley rats (125–175 gm) were prehabituated to 40 × 40 cm Plexiglas observation chambers for 1 hr on each of 2 d preceding the experiment. On the test day each rat was placed into the observation chamber and injected intraperitoneally with one of the following selective antagonists: (1) L-741,626 (3.2 or 10 mg/kg), (2) U-99194A (16 mg/kg), (3) L-745,870 (1 or 10 mg/kg), or (4) vehicle. These doses were chosen on the basis of previously published data (with specific reference to in vivo receptor occupancy when available) and pilot experiments (Waters et al., 1993, 1994; Kulagowski et al., 1996; Bristow et al., 1997). Thirty minutes after antagonist pretreatment one-half of the rats in each antagonist treatment group received d-amphetamine sulfate (5 mg/kg, i.p.) while the other one-half received saline. The number of animals for each antagonist/agonist drug combination was five, except for vehicle/saline and 1 mg/kg of L-745,870/saline, in which cases the number of animals was four per group. L-741,626 and L-745,870 were obtained from Tocris Cookson (Ballwin, MO); U-99194A was obtained from Research Biochemicals (Natick, MA).
Stereotyped motor behavior was recorded on videotape for later observation and quantification. Rearing episodes were counted during the 30 min intervals immediately before and after agonist (amphetamine or saline) injection. The amount of rearing before the agonist was subtracted from the postagonist rearing to provide a total score that took into account any variation in behavior before treatment. Sniffing behavior was quantified during three 1 min intervals at 25, 40, and 55 min after the amphetamine injection. These time points were chosen on the basis of data showing that the average amount of stereotypy observed for the animals in all treatment conditions was maximal during these periods. For each 1 min interval the number of seconds a rat spent sniffing was recorded, with a maximum total score of 180 sec.
Then 2 hr after amphetamine or saline administration the rats were anesthetized deeply and perfused for Fos immunohistochemistry as described above. The number of Fos-immunoreactive nuclei was quantified as indicated above in a region of the central striatum. In addition, Fos induced by U-99194A (vs saline) was quantified in the infralimbic/ventral prelimbic cortex for the purpose of demonstrating that the dose of U-99194A used was neurobiologically efficacious in the present experimental animals.
RESULTS
Tetrodotoxin infusions
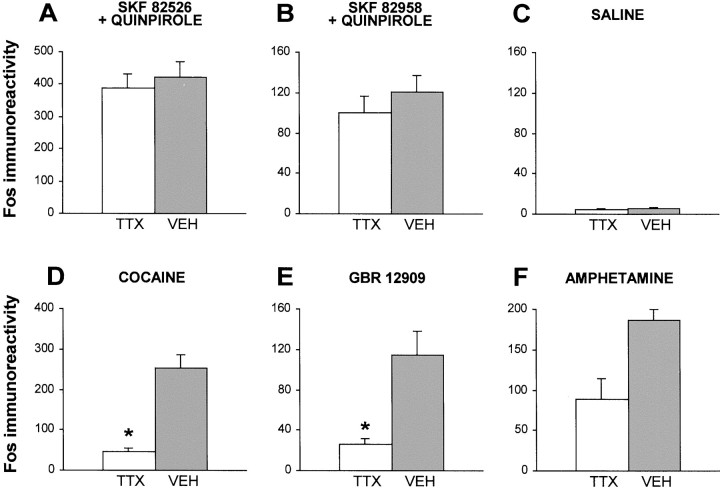
When infused intrastriatally, neither saline nor TTX produced appreciable Fos expression in the striatum (Fig.1C). By contrast, all DA agonist treatments induced significant Fos expression (Figs.1A,B,D–F,2A–C). Striatal Fos induced by the direct D1/D2agonist treatments (either intracerebral quinpirole plus SKF 82526 or intraperitoneal quinpirole plus SKF 82958) was not affected significantly by previous TTX infusion into the striatum (Figs.1A,B, 2A,A′,B,B′). However, striatal Fos induced by the DA reuptake inhibitors GBR 12909 or cocaine was attenuated greatly by TTX (Figs. 1D,E,2B,B′). Amphetamine-induced Fos was blocked partially by TTX (Fig. 1F). ANOVA revealed significant hemispheric differences (i.e., indicative of TTX-induced Fos inhibition) for GBR 12909 (F(1,4) = 12.85; p < 0.025), cocaine (F(1,4) = 32.94; p < 0.005), and amphetamine (F(1,6) = 20.78; p < 0.004), but not for the direct agonists (p > 0.05 in both cases).
Fig. 1.
Striatal Fos expression is induced by direct DA agonists (A, B), saline (C), or various indirect DA agonists (D–F) in vehicle- or TTX-injected hemispheres (see Materials and Methods). Fos immunoreactivity refers to the number of Fos-positive cells per mm2. Statistically significant (*) Fos inhibition by TTX was observed only for the DA reuptake inhibitors cocaine (D) or GBR 12909 (E).
Fig. 2.
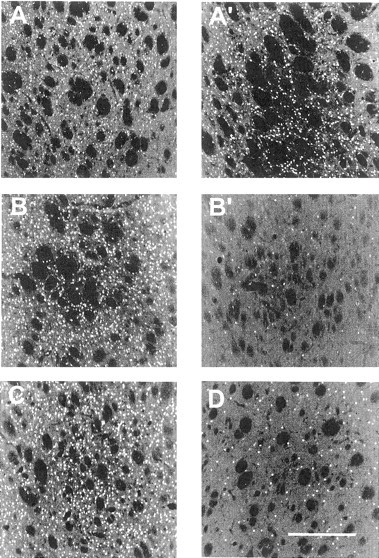
Reverse-image photomicrographs of Fos-like immunoreactivity in TTX- or VEH-treated striata of rats injected systemically with SKF 82526 plus quinpirole (VEH, A; TTX, A′) or cocaine (VEH, B; TTX,B′) and Fos-like immunoreactivity in striata of rats injected systemically with saline plus amphetamine (C) or L-741,626 (10 mg/kg) plus amphetamine (D).
Selective D2 antagonist administration
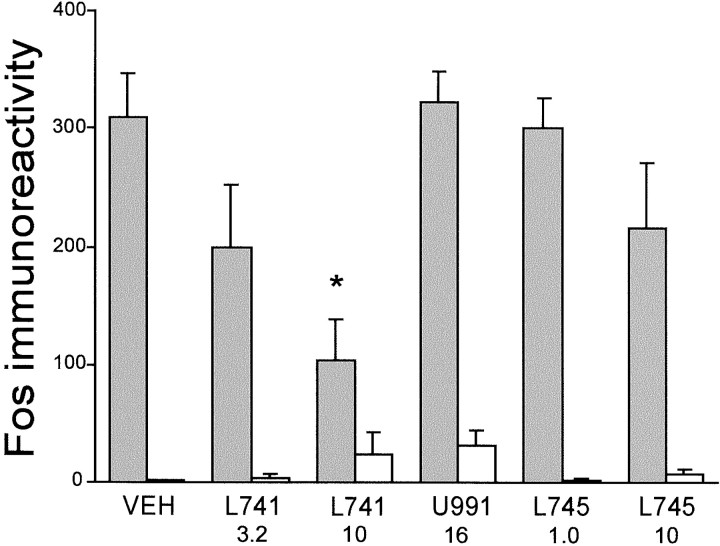
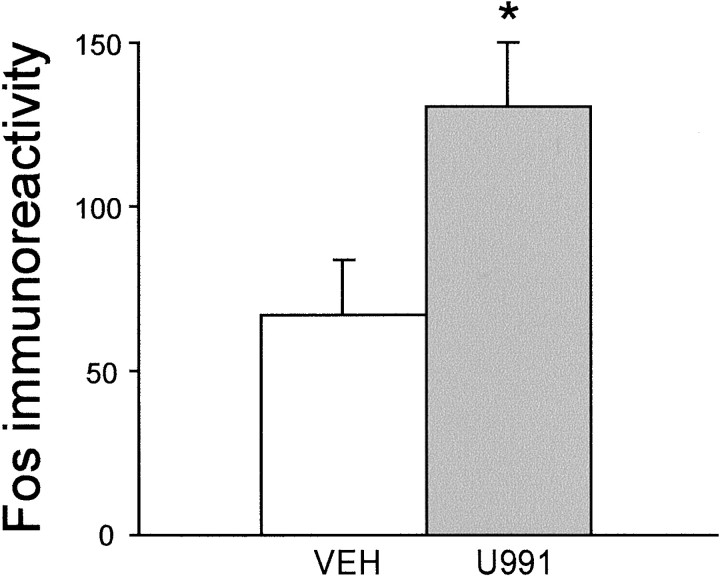
As shown many times, amphetamine injection induced pronounced Fos expression in the striatum. This effect was attenuated by the selective D2 antagonist L-741,626 in a dose-dependent manner (Figs. 2D, 3). By contrast, neither the selective D3 antagonist U-99194A nor the selective D4 antagonist L-745,870 reduced amphetamine-induced Fos in striatum (Fig.3). A two-factor ANOVA (antagonist pretreatment × agonist treatment) yielded significant main effects for antagonist pretreatment (F(5,46) = 3.07; p < 0.05) and agonist treatment (F(1,46) = 137; p < 0.001) as well as a significant interaction (F(5,46) = 3.30; p < 0.05). Post hoc comparisons of amphetamine-treated animals using Dunnett's test revealed that pretreatment with 10 mg/kg of L-741,626 significantly inhibited Fos as compared with vehicle (p < 0.01), U-99194A (p< 0.001), or 1 mg/kg of L-745,870 (p < 0.01), but not compared with 3.2 mg/kg of L-741,626 or 10 mg/kg of L-745,870 (p > 0.05). As previously reported (Merchant et al., 1996), U-99194A alone induced significant Fos expression in the infralimbic/ventral prelimbic cortex as compared with vehicle controls (p < 0.05; Fig.4), demonstrating the neurobiological efficacy of this dose of U99194A in the present study.
Fig. 3.
Effect of selective D2, D3, or D4 antagonists on striatal Fos expression in saline-treated rats (open bars) or amphetamine-treated rats (shaded bars). Fos immunoreactivity refers to the number of Fos-positive cells per mm2. VEH, Vehicle;L741, the selective D2 antagonist L-741,626;U991, the selective D3 antagonist U99194A;L745, the selective D4 antagonist L-745,870. The numbersbelow the abbreviated drug names indicate the dosage (in mg/kg). Statistically significant (*) inhibition of amphetamine-induced Fos was observed only for 10 mg/kg of L-741,626. All other treatments differ significantly from this dose except 3.2 mg/kg of L-741,626.
Fig. 4.
Statistically significant (*) induction of Fos in the infralimbic/ventral prelimbic cortex by the D3antagonist U-99194A demonstrating the neurobiological efficacy of this dose of U99194A in the present study. Fos immunoreactivity refers to the number of Fos-positive cells per mm2. VEH, Vehicle;U991, the selective D3 antagonist U99194A (16 mg/kg).
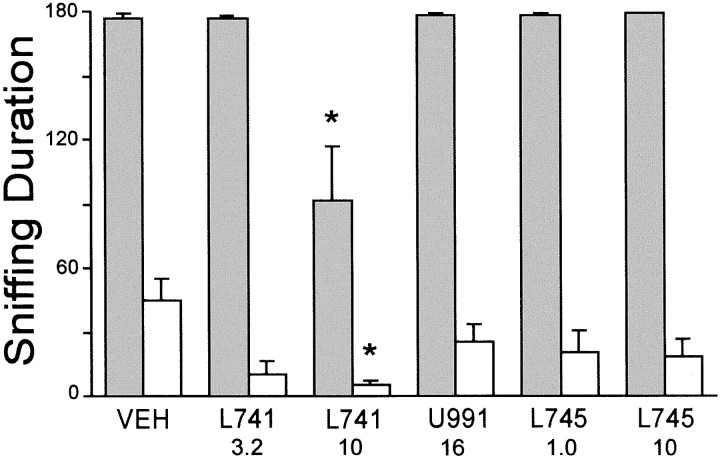
In agreement with the Fos data, L-741,626 greatly attenuated amphetamine-stimulated sniffing behavior (Fig.5) and induced catalepsy on its own (data not shown). Neither U-99194A nor L-745,870 had these effects, although the latter appeared to induce some hindlimb ataxia at the higher dose. A two-factor ANOVA (antagonist pretreatment × agonist treatment) yielded significant main effects for antagonist pretreatment (F(5,46) = 10.66; p < 0.001) and agonist treatment (F(1,46)= 634; p < 0.001) as well as a significant interaction (F(5,46) = 4.76; p < 0.01). Post hoc comparisons of amphetamine-treated animals using Dunnett's test revealed that rats pretreated with 10 mg/kg of L-741,626 displayed significantly less sniffing than any other antagonist pretreatment group (p < 0.05). This dose of L-741,626 also significantly inhibited spontaneous sniffing in saline-treated (i.e., nonamphetamine-treated) animals as compared with vehicle pretreatment (p < 0.05), whereas none of the other pretreatments was effective in this regard.
Fig. 5.
Effect of selective D2, D3, or D4 antagonists on sniffing behavior in saline-treated rats (open bars) or amphetamine-treated rats (shaded bars). Sniffing Duration refers to the number of seconds spent sniffing during three 1 min intervals (see Materials and Methods). For drug name abbreviations and dosages, see the legend to Figure 3. Statistically significant (*) inhibition of amphetamine-stimulated sniffing was observed only for 10 mg/kg of L-741,626, which also significantly inhibited spontaneous sniffing.
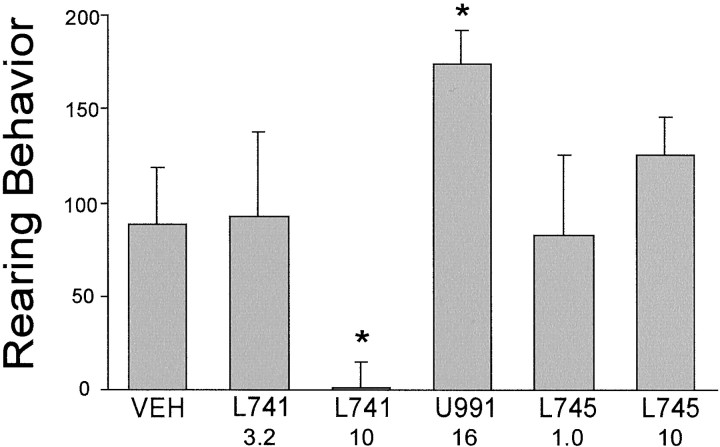
Rearing data were highly variable and therefore were analyzed with the nonparametric Mann–Whitney U test. The results show that amphetamine-induced rearing was decreased significantly only in rats pretreated with 10 mg/kg of L-741,626 (p < 0.05; Fig. 6). U-99194A pretreatment significantly increased amphetamine-induced rearing (p < 0.05), similar to what has been reported earlier for this agent (Waters et al., 1993, 1994).
Fig. 6.
Effect of selective D2, D3, or D4 antagonists on amphetamine-stimulated rearing behavior. Rearing Behavior refers to the number of amphetamine-stimulated rearing episodes during the 30 min poststimulant observation period minus the number of rearing episodes during the 30 min prestimulant observation period (see Materials and Methods). For drug name abbreviations and dosages, see the legend to Figure 3. Statistically significant inhibition of rearing was observed only for 10 mg/kg of L-741,626, whereas U-99194A significantly increased rearing (*significantly different from VEH).
DISCUSSION
The two main findings of the research presented here are that D1/D2 synergism with respect to motor behavior and striatal immediate-early gene expression (1) occurs even under conditions in which action potentials are prevented and (2) depends on agonist stimulation of D2, but not D3 or D4, receptors. Taken together, these findings suggest the intriguing possibility that D1 and D2 receptors reside on separate striatal neurons and interact in a manner that is not dependent on action potentials.
Nondependence on action potentials is demonstrated by the consistent failure of intrastriatal TTX to influence the synergistic actions of combined D1/D2 agonism at the cellular level. This is true regardless of the D1 class agonist that is used or the route of administration. The ineffectiveness of TTX cannot be attributed to nonspecific Fos expression caused by mechanical stimulation during the injection procedure nor to TTX itself because neither saline nor TTX alone induced significant Fos expression. The neurobiological effectiveness of the TTX in blocking action potentials is demonstrated by the appearance of rotation toward the inactivated hemisphere after D1/D2 agonist treatment, similar to that occurring after a unilateral striatal lesion (Barone et al., 1986). Further demonstration of the neurobiological efficacy of TTX is provided by experiments that use DA reuptake inhibitors, for which the effects on synaptic DA are dependent on nigrostriatal action potentials. TTX, which reduces striatal extracellular DA to undetectable levels (Keefe et al., 1993), potently inhibited striatal Fos expression induced by cocaine or GBR 12909. The effect of amphetamine on synaptic DA at the dose that was used is likely to be partially dependent on action potentials and partially independent, because high doses of amphetamine release DA from both vesicular and cytoplasmic stores (Heeringa and Abercrombie, 1995). In the present experiments, amphetamine-induced Fos expression in the striatum was attenuated partially by TTX, presumably because of reduction of the extracellular DA component contributed by vesicular release.
It is possible that, whereas the absolute number of Fos-positive neurons after TTX was not altered significantly in response to direct D1/D2 agonists, there was a change in the phenotype of the neurons expressing Fos immunoreactivity. We have not examined the phenotype of the neurons expressing Fos under normal and TTX conditions.
Most D2 class agonists, including quinpirole, do not distinguish among the D2, D3, and D4 receptors. To determine which of these receptors contributes to the D1/D2 synergism with respect to striatal immediate-early gene expression and motor behavior, we used new antagonists with selectivities for D2, D3, and D4 receptors. In the present experiments the D2-selective antagonist L-741,626 blocked amphetamine-induced motor behavior, blocked amphetamine-induced Fos expression in the striatum, and induced catalepsy when given alone. None of these effects was seen with either the D3 or the D4 antagonists at receptor-selective doses (see below). The probability that L-741,626 exerted its effects by nonselectively blocking D3or D4 receptors is low, given that high receptor–occupancy doses of antagonists selective for these receptors did not produce an effect. Furthermore, the lower dose of L-741,626 is unlikely to have occupied more than a very small proportion of D3 or D4 sites. The present findings using antagonists are consistent with results from studies on gene knock-out mice. D2 knock-out mice are profoundly akinetic (Baik et al., 1995), whereas D3 or D4 knock-out mice show relatively normal motor activity (Accili et al., 1996; Rubinstein et al., 1997). When D1/D2synergism was tested directly in D3 knock-out mice, the mutants were found to be no different from wild types in this regard (Xu et al., 1997). The present data are also consistent with recent findings that the disruptive effects of amphetamine on prepulse inhibition require D2, but not D3 or D4, receptors (Ralph et al., 1999).
It should be noted that the higher dose of the selective D4 antagonist L-745,870 partially attenuated amphetamine-induced motor behavior and striatal Fos expression. This dose, which is estimated to block ∼98% of D4receptors, also can be expected to occupy ∼22% of D2 receptors (Patel et al., 1997). Because no amphetamine-blocking effect was observed at a lower dose of L-745,870 that is estimated to block 97% of D4 receptors but only 2.6% of D2 receptors, it appears likely that this D2 occupancy contributes to the amphetamine-blocking effects at this high dose of L-745,870.
Although both direct and indirect DA agonists were used in the TTX experiments, only amphetamine was used in the selective antagonist experiments. There is an abundance of behavioral, electrophysiological, and immediate-early gene studies in the literature to support the conclusion that the rules of requisite D1/D2 synergism apply equally to direct and indirect DA agonists. We cite here only two directly relevant references from our laboratories. Ruskin and Marshall (1994) showed that the concomitant stimulation of D1 and D2 class receptors was required for amphetamine-induced Fos in the striatum of neurologically intact rats. LaHoste and colleagues (1993) showed the same effect for striatal Fos elicited by the direct-acting D1 and D2 class agonists SKF 38393 and quinpirole, respectively.
Additionally, although several other studies have reported region-specific Fos expression in the striatum after injection of a nonselective D2 class antagonist, such as haloperidol (Dragunow et al., 1990; Miller, 1990; Nguyen et al., 1992;Robertson et al., 1992), no striatal Fos expression was observed in the present experiment by using a selective D2antagonist at a cataleptogenic dose. This holds true for all striatal regions, not just the 1 mm2 region specified in Materials and Methods (data not shown). The possible contribution of D3 and/or D4 antagonism to the effects on c-fosof nonselective D2 class antagonists may warrant further investigation, although it is possible that the doses of L-741,626 used in the present experiment were not maximal.
Because the D1/D2 synergism in the present studies was not blocked by TTX, one tentative conclusion that could be drawn from the above data is that synergism occurs at the single-cell level via agonist stimulation of D1class and D2 class receptors residing on the same postsynaptic neuron. With respect to DA-stimulated Fos expression in striatum, the manifestation of D1/D2 synergism is restricted to enkephalin-negative striatonigral neurons (Berretta et al., 1992; Cenci et al., 1992; Ruskin and Marshall, 1994). Although virtually all neurons in this subpopulation express abundant levels of D1 mRNA, conventional RT-PCR on single cells showed no colocalization of D2 mRNA (Surmeier et al., 1996). When a second round of PCR was performed, the incidence of D1/D2 colocalization increased from 0 to 19% (Surmeier et al., 1996). Thus, among the striatal neurons that express Fos in response to DA agonists, the percentage of neurons with abundant levels of both D1 and D2 mRNA is low [D2 colocalization with D5receptors, which could be stimulated by nonselective D1 class agonists, does not occur in this subpopulation of neurons (Surmeier et al., 1996)].
An alternative possibility is that D1/D2 synergism occurs at the single-cell level but requires interneuronal communication for its manifestation. A subpopulation of striatal neurons expresses both enkephalin and substance P. Estimates of the relative size of this subpopulation vary between laboratories from 1–2 to 30% (see Surmeier et al., 1996). Using single-cell RT-PCR, Surmeier et al. (1996) found this subpopulation to comprise 17% of striatal neurons. Of importance for the present discussion is that 22–25% of these neurons coexpressed D1 and D2 mRNA after conventional PCR, and 70–80% showed colocalization after a second round of PCR. Thus, these D1/D2-positive striatal neurons may comprise 4–12% of striatal neurons. Because they are enkephalin-positive, it is unlikely that these neurons express Fos after DA stimulation (Berretta et al., 1992). However, it is possible that synergism occurs within these neurons but requires interneuronal communication to be manifested. According to the results of the present experiments, this communication would have to be independent of action potentials.
Although there are several examples of synaptic communication in the striatum that do not require action potentials, none of these withstands the constraints required to serve as a putative mechanism of D1/D2 synergism. An alternative hypothesis to explain TTX-insensitive D1/D2 synergism invokes the concept of direct electrical coupling between adjacent neurons. Electrotonic coupling is believed to occur between medium spiny neurons of the adult rat striatum and to be regulated dynamically by dopaminergic agents (Cepeda et al., 1989; O'Donnell and Grace, 1993;Onn and Grace, 1994). Most of the evidence supporting this view is based on dye coupling, an indirect measure that has been shown to be a good indicator of electrotonic coupling (for a discussion of this point, see Onn and Grace, 1994). Of particular importance to the present discussion is the finding that dye coupling is regulated by DA receptor stimulation. For example, under basal conditions 17% of medium spiny neurons showed coupling to another medium spiny neuron (Onn and Grace, 1994). After concomitant D1/D2 stimulation by apomorphine, 82% of tested medium spiny neurons showed coupling. When a given neuron was coupled, the number of other medium spiny neurons to which it was coupled increased from one, under basal conditions, to three to seven neurons after apomorphine. In addition, the neuronal gap junction protein connexin32 is expressed in rat striatal neurons (Micevych and Abelson, 1991). Moreover, glial cells, which express connexin43 in abundance in adulthood and for which the expression in striatum is modulated by DA (Reuss and Unsicker, 1999), can mediate communication between adjacent neurons via electrotonic coupling (Andrade-Rosental et al., 1999; Ishimatsu and Akasu, 1999). Thus, direct or indirect electrotonic coupling between separate D1- and D2-containing medium spiny neurons could provide a TTX-insensitive mechanism for D1/D2 synergism.
In summary, one can conclude from the TTX experiments that action potentials are not necessary for D1/D2 synergism in the striatum. One also can conclude from the selective antagonist experiments that only D2 receptors interact with striatonigral D1 receptors to give rise to D1/D2 synergism. From previous work on DA receptor colocalization one can conclude that, among the striatal neurons that express Fos in response to DA agonists, the percentage of neurons with abundant levels of both D1 and D2 mRNA is low. Thus, with respect to motor behavior and immediate-early gene expression, D1/D2 synergism in the striatum may be mediated via nonclassical interneuronal communication.
Footnotes
This work was supported by U.S. Public Health Service Grants MH49690 (G.J.L.) and NS22698 (J.F.M.).
Correspondence should be addressed to Dr. Gerald J. LaHoste, Department of Psychology, University of New Orleans, Lake Front, New Orleans, LA 70148. E-mail: glahoste@uno.edu.
REFERENCES
- 1.Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA. 1996;93:1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade-Rosental AF, Zheng X, Urban F, Chiu F-C, Spray DC, Rosental R. Bidirectional signaling via gap junctions between mammalian hippocampal neurons and astrocytes. Soc Neurosci Abstr. 1999;25:518. doi: 10.1159/000048729. [DOI] [PubMed] [Google Scholar]
- 3.Ariano MA, Larson ER, Noblett KL. Cellular dopamine receptor subtype localization. In: Ariano MA, Surmeier DJ, editors. Molecular and cellular mechanisms of neostriatal function. Landes; Austin, TX: 1995. pp. 59–70. [Google Scholar]
- 4.Baik J-H, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 5.Barone P, Davis TA, Braun AR, Chase TN. Dopaminergic mechanisms and motor function: characterization of D1 and D2 dopamine receptor interactions. Eur J Pharmacol. 1986;123:109–114. doi: 10.1016/0014-2999(86)90694-1. [DOI] [PubMed] [Google Scholar]
- 6.Berretta S, Robertson HA, Graybiel AM. Dopamine and glutamate agonists stimulate neuron-specific expression of Fos-like protein in the striatum. J Neurophysiol. 1992;68:767–777. doi: 10.1152/jn.1992.68.3.767. [DOI] [PubMed] [Google Scholar]
- 7.Braun AR, Chase TN. Obligatory D1/D2 receptor interaction in the generation of dopamine agonist-related behaviors. Eur J Pharmacol. 1986;131:301–306. doi: 10.1016/0014-2999(86)90588-1. [DOI] [PubMed] [Google Scholar]
- 8.Bristow LJ, Collinson N, Cook GP, Curtis N, Freedman SB, Kulagowski JJ, Leeson PD, Patel S, Ragan CI, Ridgill M, Saywell KL, Tricklebank MD. L-745,870, a subtype-selective dopamine D4 receptor antagonist, does not exhibit a neuroleptic-like profile in rodent behavioral tests. J Pharmacol Exp Ther. 1997;283:1256–1263. [PubMed] [Google Scholar]
- 9.Cenci MA, Cambell K, Wictorin K, Björklund A. Striatal c-fos induction by cocaine or apomorphine occurs preferentially in output neurons projecting to the substantia nigra. Eur J Neurosci. 1992;4:376–380. doi: 10.1111/j.1460-9568.1992.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 10.Cepeda C, Walsh JP, Hull CD, Howard SG, Buchwald NA, Levine MS. Dye-coupling in the neostriatum of the rat. I. Modulation by dopamine-depleting lesions. Synapse. 1989;4:229–237. doi: 10.1002/syn.890040308. [DOI] [PubMed] [Google Scholar]
- 11.Dragunow M, Robertson GS, Faull RLM, Robertson HA, Jansen K. D2 dopamine receptor antagonists induce Fos and related proteins in rat striatal neurons. Neuroscience. 1990;37:287–294. doi: 10.1016/0306-4522(90)90399-o. [DOI] [PubMed] [Google Scholar]
- 12.Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 13.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 14.Gershanik O, Heikkila RE, Duvoisin RC. Behavioral correlations of dopamine receptor activation. Neurology. 1983;33:1489–1492. doi: 10.1212/wnl.33.11.1489. [DOI] [PubMed] [Google Scholar]
- 15.Heeringa MJ, Abercrombie ED. Biochemistry of somatodendritic dopamine release in substantia nigra: an in vivo comparison with striatal dopamine release. J Neurochem. 1995;65:192–200. doi: 10.1046/j.1471-4159.1995.65010192.x. [DOI] [PubMed] [Google Scholar]
- 16.Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishimatsu M, Akasu T. Glial gap junctions intermediate among neurons in adult rat locus ceruleus. Soc Neurosci Abstr. 1999;25:1208. [Google Scholar]
- 18.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 19.Keefe KA, Gerfen CR. D1–D2 dopamine receptor synergy in striatum: effects of intrastriatal infusions of dopamine agonists and antagonists on immediate-early gene expression. Neuroscience. 1995;66:903–913. doi: 10.1016/0306-4522(95)00024-d. [DOI] [PubMed] [Google Scholar]
- 20.Keefe KA, Zigmond MJ, Abercrombie ED. In vivo regulation of extracellular dopamine in the neostriatum: influence of impulse activity and local excitatory amino acids. J Neural Transm. 1993;91:223–240. doi: 10.1007/BF01245233. [DOI] [PubMed] [Google Scholar]
- 21.Kulagowski JJ, Broughton HB, Curtis NR, Mawer IM, Ridgill MP, Baker R, Emms F, Freedman SB, Marwood R, Patel S, Patel S, Ragan CI, Leeson PD. 3-[(4-(4-Chlorophenyl)piperazin-1-yl)-methyl]-1H-pyrrolo-2,3-b-pyridine: an antagonist with high affinity and selectivity for the human dopamine D4 receptor. J Med Chem. 1996;39:1941–1942. doi: 10.1021/jm9600712. [DOI] [PubMed] [Google Scholar]
- 22.LaHoste GJ, Marshall JF. Nigral D1 and striatal D2 receptors mediate the behavioral effects of dopamine agonists. Behav Brain Res. 1991;38:233–242. doi: 10.1016/0166-4328(90)90178-h. [DOI] [PubMed] [Google Scholar]
- 23.LaHoste GJ, Marshall JF. Dopamine receptor interactions in the brain. In: Stone TW, editor. CNS neurotransmitters and neuromodulators: dopamine. CRC; Boca Raton, FL: 1996. pp. 107–119. [Google Scholar]
- 24.LaHoste GJ, Yu J, Marshall JF. Striatal Fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivity. Proc Natl Acad Sci USA. 1993;90:7451–7455. doi: 10.1073/pnas.90.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lester J, Fink S, Aronin N, DiFiglia M. Colocalization of D1 and D2 dopamine receptor mRNAs in striatal neurons. Brain Res. 1993;621:106–110. doi: 10.1016/0006-8993(93)90303-5. [DOI] [PubMed] [Google Scholar]
- 26.Lewis MH, Widerlov E, Knight DL, Kilts CD, Mailman RB. N-oxides of phenothiazine antipsychotics: effects on in vivo and in vitro estimates of dopaminergic functions. J Pharmacol Exp Ther. 1983;225:539–545. [PubMed] [Google Scholar]
- 27.Meador-Woodruff JH, Mansour A, Healy DJ, Keuhn R, Zhou Q-Y, Bunzow JR, Akil H, Civelli O, Watson SJ., Jr Comparison of the distributions of D1 and D2 dopamine receptor mRNA in rat brain. Neuropsychopharmacology. 1991;5:231–242. [PubMed] [Google Scholar]
- 28.Merchant KM, Figur LM, Evans DL. Induction of c-fos mRNA in rat medial prefrontal cortex by antipsychotic drugs: role of dopamine D2 and D3 receptors. Cereb Cortex. 1996;6:561–570. doi: 10.1093/cercor/6.4.561. [DOI] [PubMed] [Google Scholar]
- 29.Micevych PE, Abelson L. Distribution of mRNAs coding for liver and heart gap junction proteins in the rat central nervous system. J Comp Neurol. 1991;305:96–119. doi: 10.1002/cne.903050110. [DOI] [PubMed] [Google Scholar]
- 30.Miller JC. Induction of c-fos mRNA expression in rat striatum by neuroleptic drugs. J Neurochem. 1990;54:1453–1455. doi: 10.1111/j.1471-4159.1990.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen TV, Kosofsky BE, Birnbaum R, Cohen BM, Hyman SE. Differential expression of c-fos and zif268 in rat striatum after haloperidol, clozapine, and amphetamine. Proc Natl Acad Sci USA. 1992;89:4270–4274. doi: 10.1073/pnas.89.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Donnell P, Grace AA. Dopaminergic modulation of dye coupling between neurons in the core and shell regions of the nucleus accumbens. J Neurosci. 1993;13:3456–3471. doi: 10.1523/JNEUROSCI.13-08-03456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onn S-P, Grace AA. Dye coupling between rat striatal neurons recorded in vivo: compartmental organization and modulation by dopamine. J Neurophysiol. 1994;71:1917–1934. doi: 10.1152/jn.1994.71.5.1917. [DOI] [PubMed] [Google Scholar]
- 34.Patel S, Freedman S, Chapman KL, Emms F, Fletcher AE, Knowles M, Marwood R, Mcallister G, Myers J, Curtis N, Kulagowski JJ, Leeson PD, Ridgill M, Graham M, Matheson S, Rathbone D, Watt AP, Bristow LJ, Rupniak NM, Baskin E, Lynch JJ, Ragan CI. Biological profile of L-745,870, a selective antagonist with high affinity for the dopamine D4 receptor. J Pharmacol Exp Ther. 1997;283:636–647. [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 2nd Ed. Academic; New York: 1986. [Google Scholar]
- 36.Ralph RJ, Varty GB, Kelly MA, Wang YM, Caron MG, Rubinstein M, Grandy DK, Low MJ, Geyer MA. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J Neurosci. 1999;19:4627–4633. doi: 10.1523/JNEUROSCI.19-11-04627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuss B, Unsicker K. Differential effects of dopamine and glutamate on rat astroglial gap junctions. Soc Neurosci Abstr. 1999;25:1507. [Google Scholar]
- 38.Robertson GS, Vincent SR, Fibiger HC. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992;49:285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- 39.Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- 40.Ruskin DN, Marshall JF. Amphetamine- and cocaine-induced Fos in the rat striatum depends on D2 dopamine receptor activation. Synapse. 1994;18:233–244. doi: 10.1002/syn.890180309. [DOI] [PubMed] [Google Scholar]
- 41.Sibley DR, Monsma FJ., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992;13:61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- 42.Stoof JC, Kebabian JW. Opposing roles for D1 and D2 dopamine receptors in efflux of cAMP from rat neostriatum. Nature. 1981;294:366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- 43.Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci USA. 1992;89:10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Surmeier DJ, Song W-J, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walters JR, Bergstrom DA, Carlson JH, Chase TN, Braun AR. D1 dopamine receptor activation required for postsynaptic expression of D2 agonist effects. Science. 1987;236:719–722. doi: 10.1126/science.2953072. [DOI] [PubMed] [Google Scholar]
- 46.Waters N, Svensson K, Haadsma-Svensson SR, Smith MW, Carlsson A. The dopamine D3 receptor: a postsynaptic receptor inhibitory on rat locomotor activity. J Neural Transm. 1993;94:11–19. doi: 10.1007/BF01244979. [DOI] [PubMed] [Google Scholar]
- 47.Waters N, Löfberg L, Haadsma-Svensson S, Svensson K, Sonesson C, Carlsson A. Differential effects of dopamine D2 and D3 receptor antagonists in regard to dopamine release, in vivo receptor displacement and behavior. J Neural Transm. 1994;98:39–55. doi: 10.1007/BF01277593. [DOI] [PubMed] [Google Scholar]
- 48.Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu X-T, White NM, Graybiel AM, White FJ, Tonegawa S. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–848. doi: 10.1016/s0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]