Abstract
An event-related functional magnetic resonance imaging study of prefrontal cortex was conducted during which subjects performed a visual “oddball” target detection task. Exemplars of three stimulus categories were presented at a rate of one per 1.5 sec for 10 runs, each consisting of 132 trials. Standards were color squares of varying sizes that were presented on ∼92% of trials. Targets were color circles of varying sizes presented irregularly on ∼4% of trials. Novels were pictures of everyday objects that were also presented irregularly on ∼4% of trials. Ten subjects participated in two separate sessions in which they were required to count mentally or to push a button whenever a target appeared. Targets evoked activation within prefrontal cortex, primarily within the middle frontal gyri (MFG). This MFG activation did not differ as a function of the required response. Novels did not evoke significant activity within this region despite evidence from a separate behavioral and event-related potential study demonstrating their strong influence on processing. In additional imaging sessions with two subjects, the rules were reversed to require a button press whenever an object, but not a circle, appeared. These former novels now evoked activation in the MFG, but the former target circles did not. These experiments indicate that MFG activation is reliably evoked by exemplars from arbitrary stimulus categories that are mapped by experimental rules onto an arbitrary covert or overt response.
Keywords: prefrontal cortex, P300, target detection, novelty, fMRI, visual stimuli
Physiological studies in monkeys have demonstrated dorsolateral prefrontal cortex (dPFC) involvement in processes underlying working memory (Goldman-Rakic, 1987). Neuroimaging studies in humans have supported this assertion by demonstrating dPFC activation during various working memory tasks (Cohen et al., 1994;McCarthy et al., 1994, 1996; Smith et al., 1995; Braver et al., 1997;Belger et al., 1998). These studies demonstrate the sensitivity of dPFC to tasks that engage “on-line” processing; however, the task features most critical for activation are still a matter of interpretation and debate. To illustrate, we have previously used a visual “oddball” target detection task and found that infrequent and irregularly presented targets reliably activated the dPFC, particularly the middle frontal gyrus (MFG) (McCarthy et al., 1997). However, the critical task properties necessary for MFG activation were not established. Thus, MFG activation might be related to differences in probability and/or novelty of the stimuli, to attentional factors, to the nature of the response, or to the maintenance of a mental count.
A virtue of the oddball target detection task is that the number and nature of the stimuli can be easily manipulated, and the rules governing the mapping between stimulus categories and responses can be rapidly changed. In addition, this task has been extensively investigated using event-related potential (ERP) methods. Specific ERP correlates have been well characterized (Squires et al., 1975), and many have been shown to depend on the prefrontal cortex (Knight, 1984;Baudena et al., 1995).
In the present study, we investigated the dependence of MFG activation on these stimulus and response factors using event-related functional magnetic resonance imaging (fMRI). Subjects were engaged in a visual target detection that included equal numbers of task-relevant targets and task-irrelevant novels. ERPs and performance data were recorded to determine their differential effects on processing. To preclude habituation to physical stimulus characteristics, targets and standards were defined by geometric categories, with many exemplars of each generated by varying size and color. Finally, the nature of the response required of targets was varied. In one session subjects mentally counted targets, and in another session subjects made button press responses.
MATERIALS AND METHODS
Subjects. Ten right-handed neurologically normal subjects (5 male and 5 female) ranging from 21 to 44 years in age participated in these studies. All subjects gave informed consent for a protocol approved by the Human Investigation Committee of Yale University School of Medicine. Each subject participated in two to four imaging sessions.
Experimental tasks. There were three stimulus categories: standards, targets, and novels. Each stimulus was presented for 500 msec against a white background that subtended a visual angle of 5.4 × 5.4°. The onset-to-onset interval was 1500 msec. Standards consisted of squares of varying sizes and colors. Targets consisted of circles of varying sizes and colors. Novels consisted of stock photographs of everyday objects (e.g., bicycle and glasses). No individual novel or target was repeated within an imaging session.
Each imaging session consisted of 10 stimulus lists of 132 stimuli each. Targets and novels were pseudorandomly distributed such that they each comprised 3–5% of the stimulus items in a given list. Successive targets and novels were separated by a minimum of eight standards. Subjects participated in a minimum of two imaging sessions differentiated by the response requirements. In the count session, subjects were required to count mentally the number of target circles presented during each list and to report that count at the conclusion of the list. In the button press session, subjects pressed a button whenever a target circle appeared. In both sessions, no overt or covert response was required to either novels or standards.
In addition to these primary tasks, some subjects participated in additional imaging sessions. Two subjects participated in an additional session in which the roles of the circles and object pictures were reversed; i.e., they pressed a button whenever an object picture was presented and ignored the previous target circles. Six subjects participated in an additional session in which they pressed one button for target circles and a second button for both standards and novels.
A computer was used to control stimulus timing and presentation. Visual stimuli were delivered to an active matrix LCD panel and back-projected onto a translucent screen mounted on the patient gurney of the scanner. The subject viewed the stimuli through a mirror mounted in the head coil. A fiber-optic response box was used for the button press sessions, and subjects responded with their dominant hand.
Acquisition of MRI data. Images were acquired using a 1.5 tesla General Electric Signa scanner with a standard quadrature head coil and an advanced nuclear magnetic resonance echoplanar subsystem. The subject's head was positioned along the canthomeatal line and immobilized using a vacuum cushion and a forehead strap. T1-weighted sagittal scans were used to select six contiguous coronal slices through the frontal lobe such that the center of the fourth slice was aligned at the most anterior point of the corpus callosum. Functional images were acquired using a gradient echo echoplanar sequence [repetition time (TR), 1500 msec; echo time (TE), 45 msec; α, 60°; number of excitations (NEX), 1; and voxel size, 3.2 × 3.2 × 7 mm). Each imaging run consisted of 128 images per slice. Four radio-frequency excitations were performed before image acquisition to achieve steady-state transverse relaxation. High-resolution anatomical images for these six slices were acquired using a T1-weighted sequence (TR, 500 msec; TE, 1 msec; NEX, 2; field of view, 24 cm; slice thickness, 7 mm; imaging matrix, 128 × 64).
Functional image analysis. Following the prodecure ofMcCarthy et al. (1997), image time segments consisting of the 6 images preceding and the 10 images following each of the ∼50 targets and 50 novels were excised from the 10 runs constituting each subject's data. Segments synchronized to a random selection of 50 standards were also excised. These images were then averaged by stimulus type, maintaining the order of each image relative to stimulus onset. Thus, each averaged segment consisted of the average of the ∼50 segments for each of the 16 images constituting the segment. The mean of the six prestimulus images was subtracted from each of the 16 images constituting the averaged segment so that deviations in the poststimulus period were relative to a zero baseline.
The averaged segments were then subjected to the following analyses. Because the main hypotheses of the present study were predicated on those of McCarthy et al. (1997), the primary analysis was based on the correlation of the time course of prefrontal event-related activation obtained in that study. Therefore, the smoothed average of the left and right prefrontal time waveforms of McCarthy et al. (their Fig. 3a) was correlated with the time waveform for each voxel in the averaged segments for targets, novels, and standards of the present study. Those voxels significantly positively correlated with the reference waveform (p < 0.01, one-tailedt value uncorrected for multiple comparisons for a correlation based on 16 time points or 14 df; this t value corresponds to r > 0.55) were then superimposed on a high-resolution anatomical image. The number of activated voxels for each subject was tabulated for each of the following anatomical regions in each hemisphere: superior frontal gyrus (SFG), MFG, inferior frontal gyrus (IFG), cingulate gyrus (ACG), and white matter control regions.
Group-averaged images depicting the average of each subject's correlation image were constructed. The correlation images for each subject, slice, stimulus type (target and novel) and response condition (count and button press) were spatially registered to a common image and then averaged. The spatial registration was performed by an operator who translated and scaled each image in the horizontal and vertical axes to match the template image. These group-averaged correlation images were created to visually depict the general tendency of the correlation data.
The analyses described thus far were focused on identifying and measuring voxels with a time course similar to that described byMcCarthy et al. (1997). To explore for the possibility of activated voxels showing a different pattern of activation, group-averaged image segments were similarly constructed for each stimulus and response type. These group-averaged segments were then subjected to region of interest (ROI) analyses in which the average time courses for voxels within the following anatomically circumscribed regions were measured: superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus, anterior cingulate, and white matter. Orbital frontal regions were not circumscribed because of susceptibility artifacts and concomitant signal loss above the eyes.
Event-related potential recording. Subsequent to all of the fMRI sessions, an ERP experiment was conducted using the same task timing and stimulus lists used in the imaging studies. Subjects viewed the stimuli on a computer monitor while seated in a sound-attenuated chamber. Ten subjects (5 male and 5 female; age range, 18–44 years) participated. Of this group, seven subjects were also participants in the fMRI studies reported here, and the additional three subjects also had previous experience with the task. Subjects made speeded choice button press responses to all stimuli with their dominant hand. One button was used to respond to targets (circles), and another button was used for all nontargets (standards and novels).
The electroencephalogram (EEG) was recorded from 10–20 standard sites frontal (Fz), central (Cz), and parietal (Pz). These sites are located on the scalp midline 30, 50, and 70%, respectively, of the distance between the nasion and inion. Each of these scalp electrodes was referenced to an electrode on the chin. A ground electrode was placed on the forehead. A bipolar electrode pair was placed above and over the outer canthus of the right eye to record the electrooculogram (EOG). Impedances of all electrodes were maintained at <5 KΩ.
During the task, the EEG and EOG were continuously digitized at 250 Hz/channel and stored on computer disk. Digital codes synchronized to stimulus onsets and button presses were also stored. Standards, targets, and novels were uniquely coded, as were the standards immediately preceding and immediately following each target and each novel. At the conclusion of the experiment, EEG epochs of 1024 msec duration (100 msec prestimulus, 924 msec poststimulus) associated with each stimulus type were excised from the continuous record. The root mean square voltage of the EOG channel was computed to identify and discard epochs associated with eye movements and blink artifacts. Artifact-free epochs were segregated by stimulus code and averaged by subject. A group average across all 10 subjects was also computed.
RESULTS
ERPs and performance
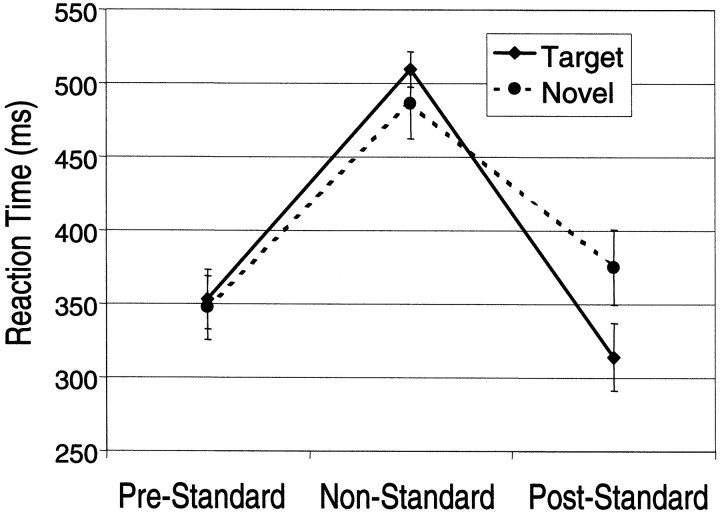
Although acquired subsequent to the imaging sessions, the results from the ERP sessions will be described first to establish the influence of targets and novels on standard measures. The reaction times (RTs) to standards were significantly shorter than the RTs to targets and novels (F(2,18) = 40.22;p < 0.0001), which did not differ significantly. To assess longer-duration effects of target and novel processing, the RTs to the standards immediately preceding and immediately following each target and novel were segregated and averaged and are presented in Figure 1. Repeated measures ANOVA confirmed a strong interaction between the intervening stimulus type (target or novel) on the RTs to the immediately following standard (F(1,9) = 23.46; p < 0.0009). Standards preceding and following both types of nonstandards were compared with post hoc t tests revealing a significantly prolonged RT to standards following novels (p < 0.05, evident in 9 of 10 subjects) and a significantly reduced RT to standards following targets (p < 0.05, evident in 8 of 10 subjects).
Fig. 1.
Mean reaction times with SE bars are plotted for the standards immediately preceding (Pre-Standard) and following (Post-Standard) nonstandard, i.e., novel (dashed line) or target (solid line). Novels and standards required a button press with the same finger. Targets required a button press with a different finger.
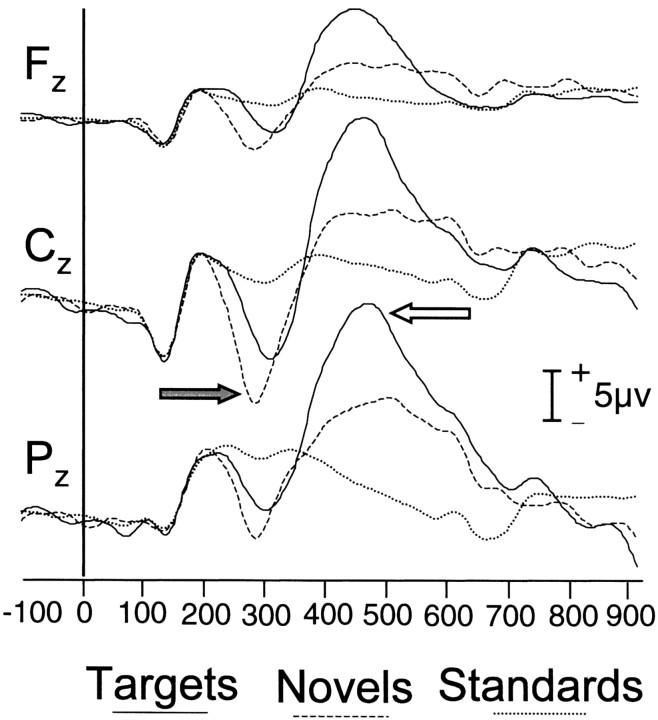
The group-averaged ERPs evoked by standards, targets, and novels are shown in Figure 2. The targets elicited a sharp negative ERP at ∼200 msec (N200 or N2) that was largest in amplitude at Cz and a large late positive ERP (P300 or P3b) at ∼400 msec that was largest at Pz. The novels also elicited a large N2 at Cz but evoked a much smaller P300 than the targets. Standards did not evoke either N2 or P300.
Fig. 2.
ERPs elicited by standards (dotted line), novels (dashed line), and targets (solid line). Recordings were made at midline sites over the frontal (Fz), central (Cz), and posterior (Pz) scalp. The N2 ERP at Cz is indicated by a shaded arrow. The P3b ERP atPz is indicated by an open arrow.
fMRI
The individual subject's data presented in Figure3A typifies the general results. Here, activated voxels to targets identified by correlation with the time function of McCarthy et al. (1997) are shown as color overlays on the subject's anatomical MRI. Activated voxels are clearly evident in the MFG bilaterally, although the activation is greater in the right MFG. The time course of activation is displayed in Figure4A for the voxels encircled in yellow within the right MFG in Figure3A. These voxels were interrogated for the average segments for targets, novels, and standards. A strong effect was obtained for targets that peaked at 6 sec after the target onset. Novels and standards did not activate these voxels. Correlation plots for novels and standards (data not shown) did not show significant activation at these slices for this individual.
Fig. 3.
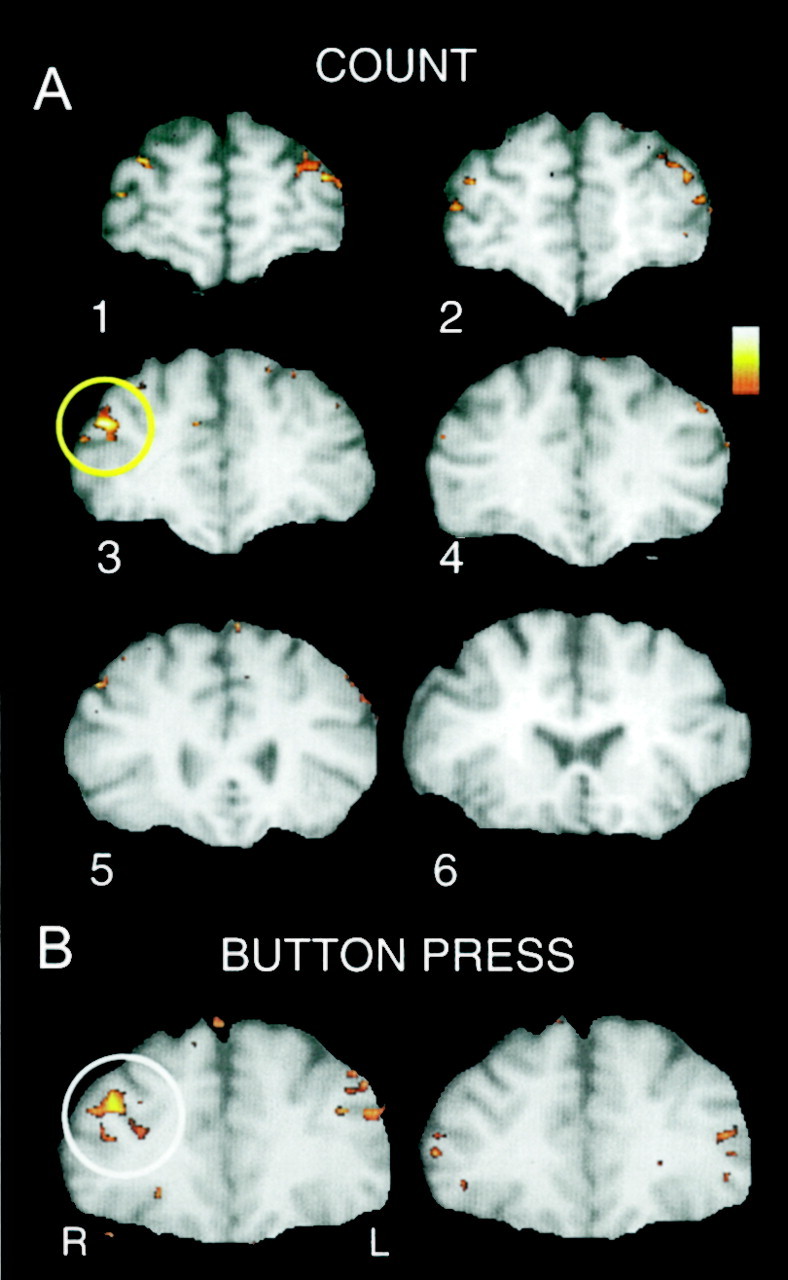
A, Activated voxels evoked by covertly counted targets and identified by correlation with the time function of McCarthy et al. (1997) are shown inred–yellow overlay on T1-weighted anatomical images for the six slices through prefrontal cortex. Activated voxels are clearly evident in the MFG bilaterally, although the activation is greater in the right MFG. This individual's data typify the general pattern of results. B, MFG activation evoked by targets to which the subject made a button press are shown in red–yellowoverlay on T1-weighted anatomical images. As in A, activation is bilateral but greatest in the right hemisphere. In this and all following images, the right hemisphere (R) is depicted on the left, and the left hemisphere (L) is depicted on theright.
Fig. 4.
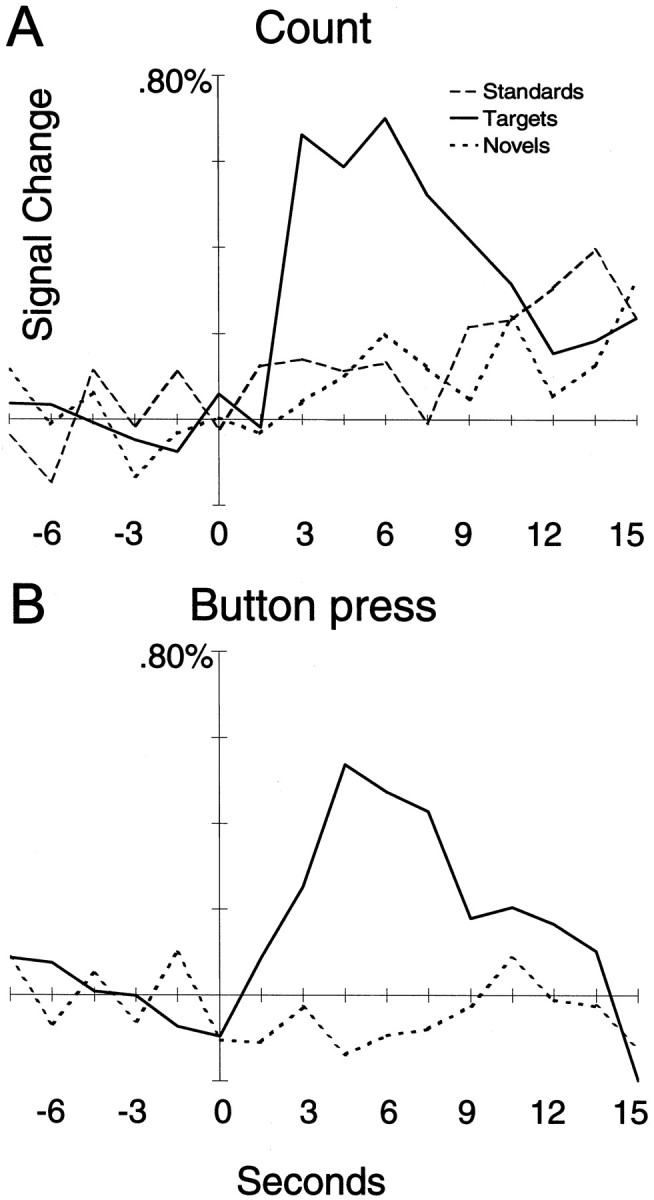
A, Activation time courses for targets (solid line), standards (long-dashed line), and novels (short-dashed line) for the activated voxels in the right MFG encircled in yellowfrom Figure 3A for the covert count task. Only targets show strong activation. B, Activation time courses for targets (solid line) and novels (short-dashed line) for the activated voxels in the right MFG encircled inwhite from Figure 3B for the button press task. As in A, only targets activated these MFG voxels.
Figure 3B shows activation data from one subject in the button press response condition. As in the count condition depicted in Figure 3A, the MFG was strongly activated, particularly the right hemisphere. The time waveforms for the activated voxels encircled in white are shown in Figure 4B and indicate strong activation by targets but not by novels. The correlation images for the novel stimuli for this subject (data not shown) did not reveal activation within the MFG.
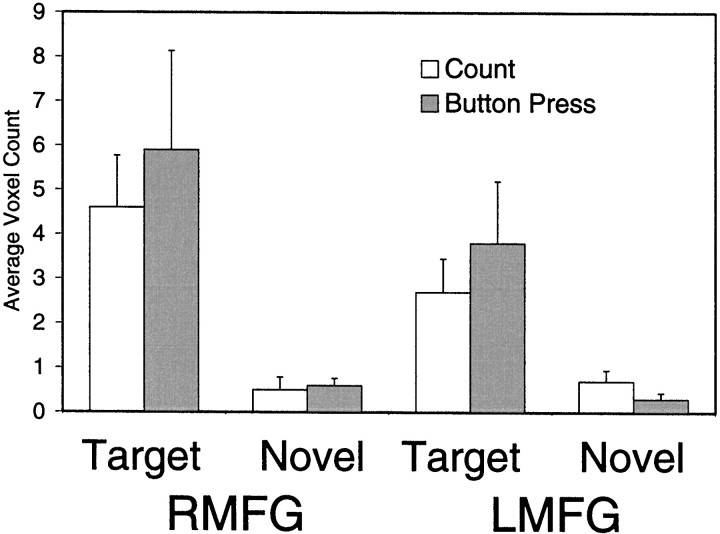
The greatest number of activated voxels occurred within the MFG. A histogram depicting the number of activated voxels within the left and right MFG tabulated across subjects is presented in Figure5. An ANOVA performed on these data indicated a strong stimulus type effect in which targets activated the MFG much greater than novels (F(1,9) = 14.2; p < 0.004) and a strong hemisphere effect in which the right MFG was activated greater than the left MFG (F(1,9) = 5.9; p < 0.01). Although there were numerically more activated voxels for the button press than count conditions, this difference was not statistically significant (F(1,9) = 0.26; p = 0.62).
Fig. 5.
The number of activated voxels (and SEM) within the left and right MFG was tabulated across subjects.RMFG, Right middle frontal gyrus; LMFG, left middle frontal gyrus.
This pattern is consistent with the group mean image data presented in Figure 6. Here strong activation of the MFG can be seen for both count and button press conditions in slices 1–4 for targets but not for novels. Some activation by targets of the IFG can be observed in the most posterior slice. As for the MFG, this activation is greater on the right than left. In addition, small activation by novels was observed in posterior slice 5 in the right IFG. No brain region showed a negative correlation with the reference waveform that was consistent across subjects.
Fig. 6.
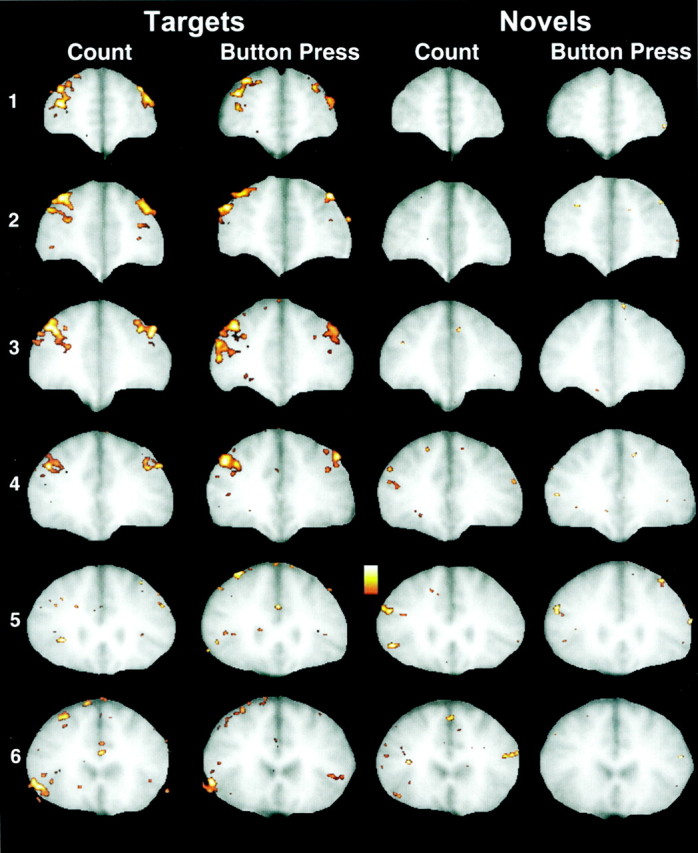
Group mean image data for the six slices (slice1 is most anterior; slice 6 is most posterior). Strong activation of the MFG can be seen for both count and button press conditions in slices 1–4 forTargets but not for Novels. Some activation by Targets of the inferior frontal gyrus bilaterally can be observed in the most posterior slice. As for the MFG, this activation is greater on the right thanleft. Some activation by Novels can be observed in posterior slices 4 and 5 in the right inferior frontal gyrus.
As described in Materials and Methods, two additional control experiments were conducted for a subset of subjects in which different response contingencies were tested. Figure7, A and B, compares two separate button press sessions for one subject. In Figure7A, the standard instructions were in force in which circles were designated as targets and object pictures were task-irrelevant novels. As shown previously, the target circles strongly activated the MFG, whereas the novel object pictures did not. In a subsequent imaging session the rules were switched, and object pictures were designated as targets. As shown in Figure 7B, the picture targets now evoked strong activation within the MFG. The former target circles evoked no such activation. Thus, the MFG activation was evoked by whichever stimulus category was designated target.
Fig. 7.
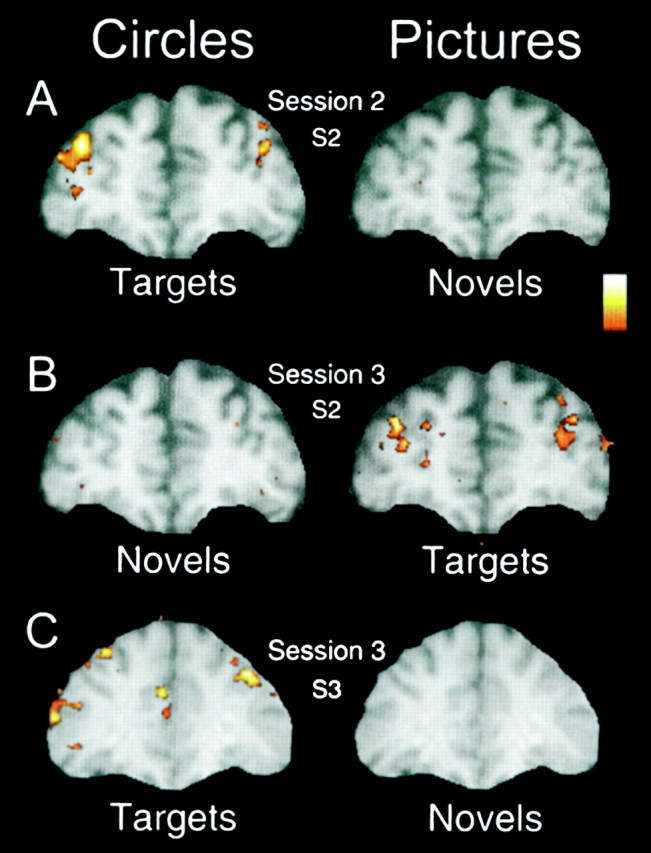
A, Data for the first button press session for subject 2 (overall session 2; session 1 was the covert count condition). Here the circles were targets, and the pictures of everyday objects were task-irrelevant novels. Activation was evoked in the MFG bilaterally (right dominant) for the target circles only.B, Data for the second button press session (overall session 3) for subject 2 in which the response rules were switched. Now the circles were task-irrelevant (novels) and the pictures of everyday objects were the task-relevant targets. Bilateral activation of the MFG was evoked by the picture targets but not by the circle novels. Thus, MFG activation was evoked by whichever stimulus category was designated as target. C, Results for subject 3 depicted for a choice response task in which one button was pressed for both novels and standards and a second button was pressed for targets. Targets evoked strong activation of the MFG, just as they had in this subject's initial session when only a single button press was required for targets. Although now requiring the same button press response as the standards, the novels evoked no such activation.
In the main experiments, positive responses were only required of the targets. Figure 7C presents the results for one subject who was recalled to participate in a third session in which he responded with one button for novels and standards and a second button for targets (the same response requirements as in the ERP and behavioral study). As in his initial session with a single button press (results not shown), the targets evoked strong activation of the MFG. Although now requiring a button press response, the novels evoked no such activation.
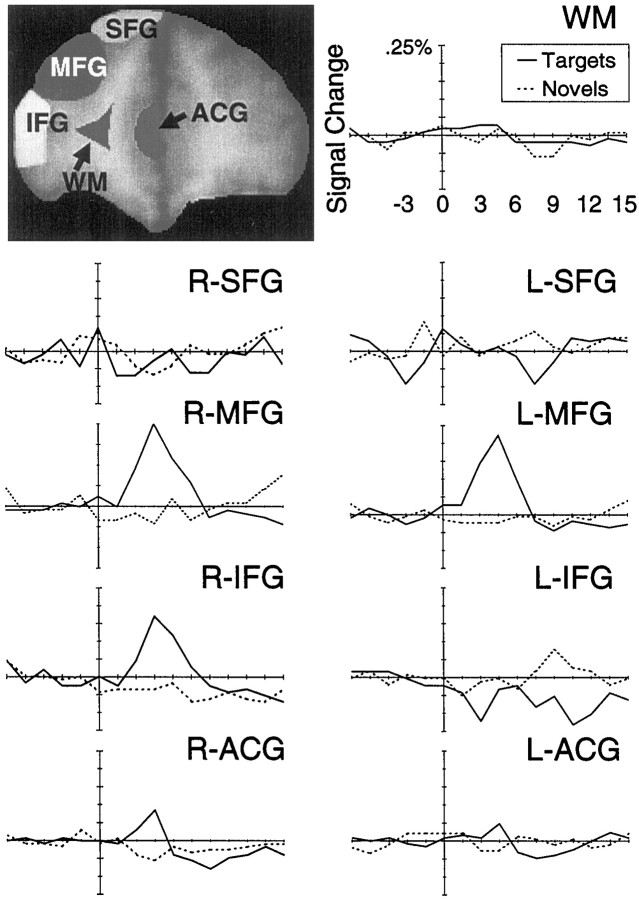
The results reported above were based on the correlation of the time course of each voxel with a reference time course obtained from a previous empirical study of target processing. It is possible that this reference waveform may have missed activation evoked by novels if that activation were manifested in a markedly different time course. We therefore performed an exploratory anatomical region of interest analysis on the group-averaged segments for targets and novels. Figure8 presents the results of the ROI analysis for slice 3. As expected, strong activation of the MFG by targets was noted, and the activation time course was similar to the template used in our correlation analysis. Of particular note here is the essentially flat average time course evoked by novels within the MFG. Thus, the lack of activation evoked by novels observed in our previous correlation analyses was not likely attributable to differences in the shapes of the activation time course for targets and novels.
Fig. 8.
Results of an ROI analysis for slice 3 located 7 mm anterior to the most anterior point of the corpus callosum. Strong bilateral activation of the MFG was evoked by targets (solid line) but not by novels (dashed line). Activation was markedly absent for the superior frontal gyri and the white matter control regions. Some activation evoked by targets was observed in right IFG and cingulate gyrus. WM, White matter.
DISCUSSION
The results confirm those of McCarthy et al. (1997) in that infrequent visual targets evoked transient activation of prefrontal cortex, principally within the right MFG. The MFG activation was not related to a particular physical stimulus, in that there was great variability in the size and color of both standards and targets, but rather to a category of stimuli defined by a differential response. When the experimental rules were redefined to designate the pictures as targets, the activation was evoked by pictures and not by the previous target circles. It is also unlikely that the MFG activation coded for a particular response modality, in that activation did not differ significantly when targets mandated the updating of a mental count or the execution of a button press. Prefrontal activation was not an obligatory consequence of responding per se, because novels did not activate MFG when they required the same dominant button press as standards (see Fig. 7C). Also, MFG activation was greatest in the right hemisphere, ipsilateral to the responding finger.
The MFG activation cannot be explained simply as a reflection of the subject's involuntary attention or orientation to infrequent, irregular events independent of their task relevance. Novels did not evoke MFG activation as measured by fMRI, despite having clear effects on processing as measured by RT and ERPs. RTs to both novels and targets were prolonged relative to standards, even though the response required to novels was the same as that to standards. This latter fact is significant in that RTs to standards following novels were also prolonged, despite the fact that standards always followed novels (i.e., a novel or target cued the subject through experience that a standard would immediately follow) and that subjects made the same motor response during this sequence. Regardless of their experience with the task, subjects reported being momentarily distracted by the appearance of a novel that they also frequently reported mentally naming.
The present results extend previous fMRI findings concerning the functional role of the dPFC in cognition and the considerable literature on the homologous middle frontal regions in nonhuman primates, particularly area 46. Because the task required attention to all stimuli but responses to only a subset, the present findings are clear that the MFG was activated only by stimuli that required an intended differential response. Neither attention per se nor task-irrelevant novelty was sufficient to engage this region to any significant degree. This finding is harmonious with lesion, imaging, and single-cell recording studies in nonhuman primates. In physiological studies, a single neuron responds during a delay interval only when the response, e.g., an eye movement, is intended; the same neuronal response is not evoked by a spontaneous eye movement made in the dark (Funahashi et al., 1991). Furthermore, the memoranda in working memory tasks need not be visual stimuli; they may be auditory or somatosensory (Bodner et al., 1996). They may also be the previous response of the animal, as in the delayed alternation tasks, in which an animal's present response depends on its previous response (Goldman et al., 1971).
Related to the present finding of the specificity of activation by targets, similarly, prefrontal neurons engaged in mnemonic processes are not affected by the presentation of stimuli other that their preferred targets in their memory fields. Thus, a neuron that exhibits a mnemonic response to a given stimulus presented at 90° is not influenced by the presentation of similar stimuli in other locations (Funahashi et al., 1989) or by the presentation of a different (nonspatial) stimulus (Wilson et al., 1993). These findings also show that attention is not sufficient for MFG activation as subjects attended to the novels and standards, yet these stimuli did not evoke strong activation. Finally, delay-related activation is observed at the single-cell level whether the response is manual or oculomotor (Carlson et al., 1997), indicating that there is flexibility in the nature of the index response, and the memory process can drive more than one effector mechanism similar to our present results in humans with counting and button press.
The present study also required response inhibition on standard and novel trials in the button press conditions. Relevant to these results is that a large fraction of prefrontal neurons can code for the direction of a response, whereas other prefrontal neurons can code for the location of a stimulus (Funahashi et al., 1993). Furthermore, the stimulus coding neurons are activated whether the intended response is to the direction of the stimulus or to a direction opposite to the present stimulus in an antisaccade task, indicating that prefrontal neurons can adjust different responses to the same stimulus on a trial-by-trial basis. Whether arbitrary stimulus– response mapping is trained or instructed, correct target detection requires the subject (human or animal) to be sensitive to changes in context created by recent stimulus and response events and override automatic or prepotent responses. Thus, a unifying feature of tasks that engage the middle frontal areas is that the response to the current stimulus requires remembering response rules, recent past events, and associations. The present study confirms this principle by showing that MFG activation does not require any particular stimulus (pictures, circles, or squares) or any particular response (counting, choice response, or go–no-go responding). The important feature is that the response be goal-directed and that it be guided by a memory process, even an arbitrary one.
As expected, targets evoked a prominent P300 or P3b ERP. This ERP is readily elicited by task-relevant stimuli regardless of the nature of the response required. However, P300 is greatly attenuated or absent when the required response is repeated or predictable (for review, seeDonchin and Coles, 1988). The novels did not evoke a large P300 but did evoke a prominent N200 or N2. N2 has been related to the detection of infrequent stimuli that attract attention regardless of their task relevance, a measure of orienting (Näätänan and Gaillard, 1983). For task-irrelevant novel stimuli, N2 is frequently followed by a more frontally distributed P3a, an ERP that was not clearly evident in the current study.
ERP measurements were included in the present experiment solely to document that novels significantly affected processing. It is nevertheless interesting to note that circumstances that evoked MFG activation (as measured by fMRI) were the same as those that evoked P300 or P3b and not N2–P3a. P3a is diminished by prefrontal lesions, suggesting that prefrontal cortex is critical for orienting or novelty detection (Knight, 1984). This hypothesis is supported by the combined study of ERPs and fMRI (Opitz et al., 1999) that postulated that one of three dipoles for auditory novel P300 was located in the right PFC, where fMRI activation was observed in response to novel sounds. These authors suggested that PFC is engaged in accessing and retrieving semantic concepts related to novel stimuli. The lack of fMRI activation by novels within prefrontal cortex in the present study cannot be easily reconciled with this role.
Tasks that reliably evoke P3a often use stimuli that are characterized by affective attributes, e.g., the sound of a dog barking or telephone ringing (Knight, 1984) among a background of tone pips or electrical shocks among finger vibratory stimuli (Yamaguchi and Knight, 1991). More recent studies from our laboratory have suggested that both auditory and visual stimuli with affective attributes activated more inferior frontal regions (Hinton et al., 1999; Kirino et al., 1999). Thus, it may be that the affective nature of novel stimuli is critical in evoking prefrontal activation. The visual novels presented here were everyday objects with little or no affective content.
Analogous to results described for ERPs (Knight, 1984), it is possible that the first few novels activated the MFG but that the activation rapidly habituated. This early activation might not be evident in the across-runs fMRI average given the unfavorable signal-to-noise ratio. We attempted to examine this possibility by separately averaging the first and last two novels of each run. We did not observe MFG activation evoked by the initial novels; however, such an effect may have been obscured by greater noise evident in these averages based on few trials.
This experiment was focused on the MFG and guided by our previous studies that have activated this region. However, in addition to the MFG, targets evoked focal activation in the right posterior inferior frontal gyrus, just anterior to the insula. This activation was also evident in the anatomical ROI analysis (Fig. 8). Lesser activation of this region was also noted for novels in Figure 6. These activations were unpredicted and require replication. Our anatomical ROI analysis also revealed that targets evoked a small activation of the ACG (Fig.8), a region proposed as a critical element in an anterior attention system (Posner and Petersen, 1990). Co-activation of the ACG and dPFC has been previously observed by different investigators using different working memory tasks (Petrides et al., 1993; D'Esposito et al., 1995), although exceptions have been noted (Paulesu et al., 1993). Carter et al. (1998) have recently reported that the ACG, but not dPFC, is activated in tasks emphasizing response competition. This latter hypothesis is consistent with the assertion of Pardo et al. (1990) that the ACG is involved in selecting among competing response alternatives and the observation of Paus et al. (1993) that the ACG activation is greater when stimulus and response are incompatible, such as making a saccade away from a visual stimulus. This proposed role for the ACG in selecting among response alternatives is generally compatible with our emphasis on the mapping of stimulus categories to differential responses. This formulation might predict that novels would also activate the ACG to the extent that the delayed RTs for novels observed in our behavioral study reflected increased uncertainty about the correct response. Alternately, if the increased RT reflected longer encoding and naming for pictures, then no ACG activation to novels should be expected. A fuller exploration of the processing stages responsible for this increased RT is necessary to address this issue.
Footnotes
This work was supported by the Department of Veterans Affairs and by National Institute of Mental Health Grants MH-05286 and MH-44866.
Correspondence should be addressed to Dr. Gregory McCarthy, Brain Imaging and Analysis Center, Box 3808, Duke University Medical Center, Durham, NC 27710. E-mail: gregory.mccarthy@duke.edu.
REFERENCES
- 1.Baudena P, Halgren E, Heit G, Clarke JM. Intracerebral potentials to rare target and distractor auditory and visual stimuli. III. Frontal cortex. Electroencephalogr Clin Neurophysiol. 1995;94:251–264. doi: 10.1016/0013-4694(95)98476-o. [DOI] [PubMed] [Google Scholar]
- 2.Belger A, Puce A, Krystal JH, Gore JC, Goldman-Rakic P, McCarthy G. Dissociation of mnemonic and perceptual processes during spatial and nonspatial working memory using fMRI. Hum Brain Mapp. 1998;6:14–32. doi: 10.1002/(SICI)1097-0193(1998)6:1<14::AID-HBM2>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodner M, Kroger J, Fuster J. Auditory memory cells in dorsolateral prefrontal cortex. NeuroReport. 1996;7:1905–1908. doi: 10.1097/00001756-199608120-00006. [DOI] [PubMed] [Google Scholar]
- 4.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 5.Carlson S, Mikami A, Friedman H, Goldman-Rakic PS. Movement and delay related neuronal activity in the posterior cingulate cortex of monkeys performing oculomotor and manual delayed response tasks. In: Sakata H, Mikami A, Fuster JM, editors. The association cortex. Harwood Academic; Amsterdam: 1997. pp. 207–217. [Google Scholar]
- 6.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JD, Forman SD, Braver TS, Casey BJ, Servan-Schreiber D, Noll DC. Activation of prefrontal cortex in a non-spatial working memory task with functional MRI. Hum Brain Mapp. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- 8.D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 9.Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behav Brain Sci. 1988;11:357–374. [Google Scholar]
- 10.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 11.Funahashi S, Bruce CJ, Goldman-Rakic PS. Neuronal activity related to saccadic eye movements in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1991;65:1464–1483. doi: 10.1152/jn.1991.65.6.1464. [DOI] [PubMed] [Google Scholar]
- 12.Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- 13.Goldman PS, Rosvold HE, Vest B, Galkin TW. Analysis of the delayed-alternation deficit produced by dorsolateral prefrontal lesions in the rhesus monkey. J Comp Physiol Psychol. 1971;77:212–220. doi: 10.1037/h0031649. [DOI] [PubMed] [Google Scholar]
- 14.Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, editor. Handbook of physiology, the nervous system, higher functions of the brain. American Physiological Society; Bethesda, MD: 1987. pp. 373–417. [Google Scholar]
- 15.Hinton SC, MacFall JR, McCarthy G. Posterior and frontal activation by auditory targets and novel sounds: an event-related functional magnetic resonance imaging study. Neuroimage. 1999;9:S793. [Google Scholar]
- 16.Kirino E, Takahashi T, McCarthy G. A comparison of prefrontal activation to fearful and non-fearful looming visual stimuli: a functional MRI study. Soc Neurosci Abstr. 1999;25:99. [Google Scholar]
- 17.Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalogr Clin Neurophysiol. 1984;59:9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy G, Blamire AM, Puce A, Nobre AC, Bloch G, Hyder F, Goldman-Rakic P, Shulman RG. Functional magnetic resonance imaging of human prefrontal cortex activation during a spatial working memory task. Proc Natl Acad Sci USA. 1994;91:8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb Cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- 21.Näätänan R, Gaillard AW. The orienting reflex and the N2 deflection of the event-related potential (ERP). In: Gaillard AW, Ritter W, editors. Tutorials in ERP research: endogenous components. North-Holland; Amsterdam: 1983. pp. 119–141. [Google Scholar]
- 22.Opitz B, Mecklinger A, Friederici AD, von Cramon DY. The functional neuroanatomy of novelty processing: integrating ERP and fMRI results. Cereb Cortex. 1999;9:379–391. doi: 10.1093/cercor/9.4.379. [DOI] [PubMed] [Google Scholar]
- 23.Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci USA. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- 25.Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol. 1993;70:453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- 26.Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA. 1993;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Posner MI, Petersen SE. The attentional system of the human brain. In: Cowan WM, Shooter EM, Stevens CF, Thompson RF, editors. Annual review of neuroscience. Annual Reviews; Palo Alto, CA: 1990. pp. 25–42. [DOI] [PubMed] [Google Scholar]
- 28.Smith EE, Jonides J, Koeppe RA, Awh E, Schumacher EH, Minoshima S. Spatial versus object working memory: PET investigations. J Cognit Neurosci. 1995;7:337–356. doi: 10.1162/jocn.1995.7.3.337. [DOI] [PubMed] [Google Scholar]
- 29.Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- 30.Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260:1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi S, Knight RT. P300 generation by novel somatosensory stimuli. Electroencephalogr Clin Neurophysiol. 1991;78:50–55. doi: 10.1016/0013-4694(91)90018-y. [DOI] [PubMed] [Google Scholar]