Abstract
Despite widespread use of volatile general anesthetics for well over a century, the mechanisms by which they alter specific CNS functions remain unclear. Here, we present evidence implicating the two-pore domain, pH-sensitive TASK-1 channel as a target for specific, clinically important anesthetic effects in mammalian neurons. In rat somatic motoneurons and locus coeruleus cells, two populations of neurons that express TASK-1 mRNA, inhalation anesthetics activated a neuronal K+ conductance, causing membrane hyperpolarization and suppressing action potential discharge. These membrane effects occurred at clinically relevant anesthetic levels, with precisely the steep concentration dependence expected for anesthetic effects of these compounds. The native neuronal K+ current displayed voltage- and time-dependent properties that were identical to those mediated by the open-rectifier TASK-1 channel. Moreover, the neuronal K+ channel and heterologously expressed TASK-1 were similarly modulated by extracellular pH. The decreased cellular excitability associated with TASK-1 activation in these cell groups probably accounts for specific CNS effects of anesthetics: in motoneurons, it likely contributes to anesthetic-induced immobilization, whereas in the locus coeruleus, it may support analgesic and hypnotic actions attributed to inhibition of those neurons.
Keywords: two-pore domain K+ channel, TASK-1, pH-sensitive, volatile anesthetic, motor neuron, locus coeruleus neuron
The mechanisms by which inhalation anesthetics alter the behavior of central neurons have been scrutinized for many years. Early hypotheses based on nonspecific interactions of lipid-soluble anesthetics with membrane bilayers have largely given way to the current idea that membrane-associated proteins, particularly ion channels, are specifically modulated by anesthetics (Franks and Lieb, 1994). Indeed, a number of ion channel targets of anesthetics have been identified, and prominent among these are the inhibitory GABAA and glycine receptor channels (Daniels and Smith, 1993; Franks and Lieb, 1994; Mihic et al., 1997). It now seems certain that enhancement of chloride currents gated by GABA and glycine contributes to effects of inhalation anesthetics.
In addition to the well known potentiation of GABAA and glycine channels, accumulating evidence indicates that neuronal background K+channels are also activated by volatile general anesthetics (Nicoll and Madison, 1982; Takenoshita and Takahashi, 1987; Franks and Lieb, 1988;Sugiyama et al., 1992; Winegar et al., 1996; Sirois et al., 1998;Winegar and Yost, 1998a,b; Ries and Puil, 1999). Anesthetic activation of background K+ channels in central neurons causes membrane hyperpolarization and increases neuronal input conductance, providing an additional inhibitory mechanism that could contribute to the overall central depressant effects of these compounds. However, the molecular identity of neuronal background K+ channels, including those native K+ channels sensitive to volatile anesthetics, has remained obscure.
Over the last few years a new gene family of background K+ channels has been identified. Members of this family exhibit functional properties that suggest their classification as background K+ channels and present structural features that suggest a dimeric arrangement, with each subunit comprising four transmembrane segments and two pore-forming regions (for review, see Goldstein et al., 1998; Lesage and Lazdunski, 1999). TASK-1 (also called KCNK3) is a member of this gene family that generates a pH-sensitive, weakly rectifying K+ current (Duprat et al., 1997; Kim et al., 1998, 1999; Leonoudakis et al., 1998; Lopes et al., 2000); it is called an “open rectifier” because it exhibits no time dependence (and/or extremely fast kinetics; Lopes et al., 2000), and its weak rectification in physiological asymmetric K+ solutions can be accounted for entirely by constant field considerations (Duprat et al., 1997; Kim et al., 1998, 1999; Leonoudakis et al., 1998; Lopes et al., 2000). Importantly, TASK-1 is activated by clinical concentrations of inhalation anesthetics (i.e., 0.1–0.4 mm) after its heterologous expression in mammalian cells (Patel et al., 1999).
We recently found that TASK-1 contributes to a prominent background K+ current with the properties of a pH-sensitive, open-rectifier in rat motoneurons (Talley et al., 2000), and we earlier reported that inhalation anesthetics (i.e., halothane, isoflurane, and sevoflurane) activate a weakly rectifying K+ current in those same neurons (Sirois et al., 1998). Together, these convergent observations suggested that TASK-1 could be an anesthetic-sensitive K+channel in motoneurons. Here, we have taken advantage of its pH- and voltage-dependent properties to demonstrate that TASK-1 represents a native K+ channel activated by clinically appropriate concentrations of inhalation anesthetics in hypoglossal motoneurons (HMs). Moreover, we find a similar anesthetic-sensitive K+ current in locus coeruleus neurons, where TASK-1 transcripts are also expressed (Talley et al., 2000). Activation of TASK-1 in these brainstem neurons could account, in part, for immobilizing and hypnotic effects of anesthetics. Thus, these data provide a molecular identification of a native neuronal and anesthetic-activated background K+ channel that likely contributes to these specific, clinically important anesthetic actions.
MATERIALS AND METHODS
In situ hybridization. In situ hybridization was performed for detection of TASK-1 mRNA, exactly as described (Talley et al., 2000). Briefly, transverse brain sections (fresh–frozen, 10 μm) were obtained from Sprague Dawley rats (Hilltop), thaw-mounted on charged slides, and fixed, dehydrated, and delipidated. Sections were hybridized to a [33P]UTP-labeled cRNA probe transcribed from HindIII-digested rTASK1-pcDNA3 (Leonoudakis et al., 1998; Talley et al., 2000) using SP6 RNA polymerase (these and other enzymes obtained from Promega, Madison, WI) in a hybridization buffer (50 × 106cpm/ml) containing 50% formamide, 4× SSC (1× SSC: 150 mm NaCl and 15 mm sodium citrate, pH 7), 1× Denhardt's solution (0.02% each of Ficoll, polyvinylpyrrolidone, and bovine serum albumin), 10% dextran sulfate, 100 mm DTT, 250 μg/ml yeast tRNA, and 0.5 mg/ml salmon testes DNA. After hybridization, sections were rinsed in SSC and treated with RNase A (Boehringer Mannheim, Indianapolis, IN; 0.1 mg/ml in 10 mm Tris, 500 mm NaCl, and 1 mm EDTA, pH 7, 30 min at 37°C) and exposed to film (Hyperfilm βMAX; Amersham, Arlington Heights, IL) for 4 d. Subsequently, they were dipped in liquid emulsion (NTB-2; Eastman Kodak, Rochester, NY) and exposed for 3 weeks. The specificity of the hybridization signal obtained with this probe has been documented (Talley et al., 2000).
Electrical recordings. Transverse brainstem slices from neonatal rats (7–14 d postnatal) were prepared as described (Sirois et al., 1998; Talley et al., 2000). Rats were anesthetized with ketamine and xylazine, brainstems were removed after rapid decapitation, and transverse slices (200 μm) were cut with a microslicer (DSK 1500E; Dosaka, Tokyo, Japan) in an ice-cold Ringer's solution consisting of (in mm) 260 sucrose, 3 KCl, 5 MgCl2, 1 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, and 1 kynurenic acid. After cutting, slices were incubated for 1 hr at 37°C and subsequently at room temperature in a Ringer's solution of 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose. Ringer's solutions were bubbled with 95% O2 and 5% CO2. Slices were visualized with infrared differential interference optics, and neurons in the hypoglossal nucleus and locus coeruleus (LC) were targeted for recording based on anatomic location and characteristic size and shape (Amaral and Sinnamon, 1977; Viana et al., 1990). Neurons close to the surface of the slice were chosen for recording to minimize effects of endogenous pH buffering within the slice (Voipio and Kaila, 1993;Chesler et al., 1994).
Human embryonic kidney (HEK) 293 cells were transfected with rTASK1-pcDNA3 by calcium phosphate precipitation. Cells were cotransfected with a modified green fluorescent protein (GFP; pGreenLantern; Life Technologies, Gaithersburg, MD) at a ratio of rTASK-1 to GFP of 6:1. One day after transfection, cells were plated onto glass coverslips; individual transfected cells were visualized using a standard FITC filter set, and those with green fluorescence were chosen for recording.
Electrical recordings of neurons and HEK 293 cells were obtained in a bath solution of (in mm): 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose, with pH adjusted using HCl or NaOH. Tetrodotoxin (TTX, 0.75–1 μm; Calbiochem, La Jolla, CA) was included in the bathing solution for all voltage-clamp experiments in neurons. Bath solutions were bubbled vigorously with a room air gas mixture (21% O2/balance N2); halothane and sevoflurane were added to the perfusate via calibrated vaporizers (Ohmeda, Austell, GA). Solutions equilibrated with anesthetic were covered tightly with parafilm and superfused at ∼2 ml/min. Aqueous concentrations of anesthetic solutions were determined by gas chromatographic analysis from samples collected at the point of presentation to the preparation (Sirois et al., 1998).
Patch electrodes with a DC resistance of 1.8–3.0 MΩ were pulled from borosilicate glass (Warner Instruments) and coated with Sylgard 184 (Dow Corning Corporation). Pipette solution contained (in mm): 120 KCH3SO3; 4 NaCl; 1 MgCl2; 0.5 CaCl2; 10 HEPES; 10 EGTA; 3 MgATP; 0.3 GTP-Tris, with pH buffered to 7.2. To block Ih, ZD 7288 (Tocris Cookson) was included in the pipette solution at concentrations of 12–100 μm. To block GABAA and glycine receptor channels in some experiments, bicuculline (10 μm) and strychnine (30 μm; both from Research Biochemicals International, Natick, MA) were added to the perfusate.
Data acquisition and analysis. Recordings were obtained under whole-cell conditions using an Axopatch 200A amplifier and digitized with a Digidata 1200 analog-to-digital converter (Axon Instruments, Foster City, CA). Series resistance (4–15 MΩ) was compensated by 70–75%, and a liquid junction potential (∼10 mV) was corrected offline. Voltage and current commands were applied, and recordings were made and analyzed with pClamp software (Axon Instruments). In current clamp, membrane potential was adjusted by DC current injection; membrane potential was initially set to −60 mV for determining concentration-dependent effects on membrane potential and input conductance. Input conductance was calculated from voltage responses to constant amplitude current pulses applied at 5–10 sec intervals. Under voltage clamp, cells were held at −60 mV, and membrane currents were recorded at constant intervals of 10–15 sec. Current–voltage (I–V) relationships were obtained in neurons using hyperpolarizing voltage steps (to −130 mV, in 10 mV increments). In HEK 293 cells expressing TASK-1, depolarizing ramps from −130 to + 40 mV (∼0.2 V/sec) were used to obtainI–V curves; slope conductance was determined from linear fits to currents between −60 and −80 mV. Data are presented as mean ± SEM. Curve fitting was accomplished using the least squares method.
RESULTS
Halothane induces a pH-sensitive membrane hyperpolarization in TASK-1-expressing hypoglossal motoneurons
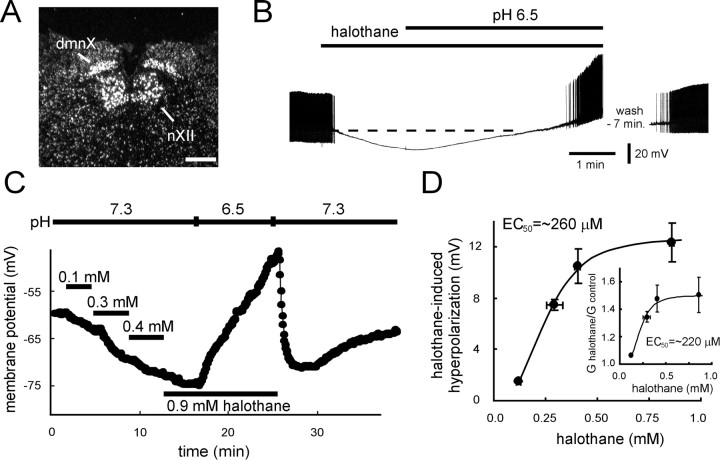
TASK-1 is a pH-sensitive member of the two-pore domain gene family of weakly rectifying neuronal background K+ channels (Duprat et al., 1997; Kim et al., 1998, 1999; Leonoudakis et al., 1998; Lopes et al., 2000) that is activated by inhalation anesthetics and inhibited by extracellular acidification when expressed in mammalian cells (Patel et al., 1999). As shown in the dark-field autoradiograph from an in situhybridization experiment in Figure1A, TASK-1 transcripts are present at high levels in hypoglossal motoneurons (see also Talley et al., 2000). In all hypoglossal motoneurons tested under current clamp, halothane induced a membrane hyperpolarization that was associated with increased input conductance, as reported previously (Sirois et al., 1998). Consistent with the possibility that the acid-sensitive TASK-1 channel mediates these effects of halothane in motoneurons, we found that the hyperpolarization was completely reversed in extracellular solutions acidified to pH 6.5 (Fig.1B). This level of acidification maximally inhibits the pH-sensitive resting K+ conductance in motoneurons (Talley et al., 2000), as it does TASK-1 in heterologous expression systems (Duprat et al., 1997; Leonoudakis et al., 1998;Lopes et al., 2000; Talley et al., 2000).
Fig. 1.
Halothane hyperpolarizes motoneurons at clinically relevant concentrations. A, Dark-field photomicrograph of emulsion-dipped section hybridized with a [33P]-labeled cRNA probe specific to TASK-1. Note the high density of silver grains overlaying neurons in the hypoglossal nucleus (nXII); labeling is also apparent in the vagal motor nucleus (dmnX). Scale bar, 250 μm.B, Current-clamp recording of a hypoglossal motoneuron exposed to halothane (1.25 mm) and then to an acidified extracellular solution, pH 6.5, in the continued presence of halothane. Note that halothane caused a membrane hyperpolarization that was reversed by acidosis. The resting potential of the motoneuron was −73 mV; it was induced to fire repetitively under control conditions by current injection (360 pA, DC; action potentials are truncated by the chart recorder). C, Current-clamp recording of membrane potential in a hypoglossal motoneuron exposed to increasing concentrations of halothane. Membrane hyperpolarization was evident even at 0.1 mm and increased further in a concentration-dependent manner. The hyperpolarization induced by halothane was reversed when the acidified bath solution was introduced during halothane exposure. Control membrane potential in this motoneuron (and all cells tested with this protocol) was set at −60 mV by DC current injection. D,Concentration–response curves for membrane effects of halothane. The halothane-induced hyperpolarization from −60 mV was measured in individual cells exposed to multiple concentrations of halothane. Averaged data (± SEM) were plotted as a function of the aqueous concentrations of halothane solution (± SEM), determined from gas chromatographic analysis of replicate samples taken from the point of presentation to the slice. Data were well fitted with the logistic equation: ΔEm = max ΔEm/(1 + ([halothane]/EC50)n), with an EC50 of 260 μm, a Hill coefficient (n) of −2.9, and a maximum hyperpolarization (max ΔEm) of 13 mV.Inset, Input conductance determined at each halothane concentration was normalized to control input conductance (G halothane/G control) and fitted to a logistic equation of similar form, with an EC50 of 220 μm, n of −3.3, and a maximum G halothane/G control of 1.5 (i.e., 50% increase in conductance).
In mammalian cells, activation of recombinant TASK-1 by halothane is concentration-dependent in the clinical range; current was increased by ∼10% at halothane concentrations as low as 0.1 mm, with an apparent EC50 near ∼0.3–0.4 mmand a maximal increase of ∼60% (Patel et al., 1999). Likewise, we found effects of halothane on membrane potential in motoneurons at 0.1 mm, with increasing concentrations of halothane evoking additional hyperpolarization (Fig. 1C). The membrane hyperpolarization and increased conductance induced by halothane were similarly dose-dependent (Fig. 1D), both with EC50 values remarkably close to those expected for anesthetic effects of halothane (∼250 μm;Franks and Lieb, 1994). The fit to these data indicate a maximal hyperpolarization of ∼13 mV from −60 mV, with a 50% increase in input conductance.
Clinically appropriate concentrations of anesthetics activate a pH-sensitive K+ current in hypoglossal motoneurons
Whole-cell voltage-clamp recordings were performed to determine whether the K+ current in HMs has the properties expected of the pH-sensitive TASK-1 channel. To study the anesthetic-sensitive K+ current in relative isolation, ZD 7288 was included in the pipette solution to block the hyperpolarization-activated cation current (Ih) that contributes to anesthetic effects in motoneurons (Sirois et al., 1998) and that may also be modulated by changes in pH (Munsch and Pape, 1999).
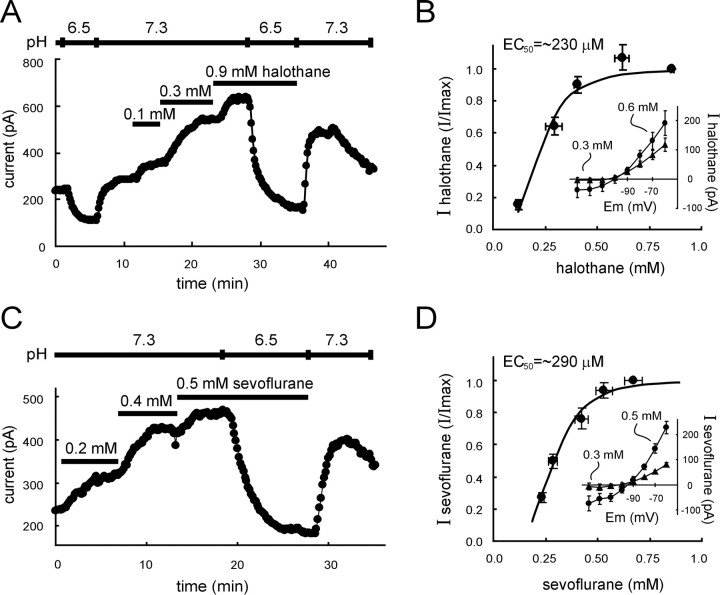
Under these conditions, acidification of the extracellular solution (from pH 7.3 to pH 6.5) induced an inward shift in holding current at −60 mV (Fig. 2A), as expected from block of the pH-sensitive TASK-1 channel in motoneurons (Talley et al., 2000). After wash to normal extracellular pH, increasing concentrations of halothane applied via the perfusate induced stepwise outward shifts in holding current; concordant with current-clamp data, the anesthetic-induced current was completely reversed by bath acidification to pH 6.5 (Fig. 2A). At all concentrations tested, the halothane-induced current was associated with an increase in conductance and displayed a weakly outwardly rectifying current–voltage (I–V) relationship that reversed near EK(Fig. 2B, inset). Activation of this K+ current was steeply dependent on halothane concentration (Hill slope, −3.3) with an EC50 of 230 μm (Fig.2B), similar to that determined for membrane hyperpolarization (Fig. 1D) and again nearly identical to that reported for anesthetic effects of halothane (Franks and Lieb, 1994).
Fig. 2.
Inhalation anesthetics activate a pH-sensitive K+ current at clinically relevant concentrations. A, Voltage-clamp recording of membrane current in a hypoglossal motoneuron held at −60 mV. Bath acidification to pH 6.5 caused an inward shift in holding current. After wash into a neutral pH solution, halothane caused the development of an outward current in a concentration-dependent manner. The current induced by halothane was completely suppressed when acidified bath solution was reintroduced during halothane exposure. B,Concentration–response curve for current activation by halothane. The halothane-induced current at −60 mV was measured in individual cells exposed to multiple concentrations of halothane and normalized to the current obtained with maximal concentrations of halothane (>0.9 mm). Averaged normalized data (± SEM) were plotted as a function of measured aqueous halothane concentrations (± SEM). These concentration–response data were well fitted by using a logistic equation of the formI/Imax = 1/(1 + ([halothane]/EC50)n), with an EC50 of 230 μm and a Hill coefficient (n) of −3.3. Inset, Note that at submaximal and supramaximal halothane concentrations, theI–V relationship of the halothane-induced current rectified weakly in the outward direction, with a reversal potential near EK. C, Voltage-clamp recording of membrane current in a hypoglossal motoneuron held at −60 mV. Sevoflurane caused the development of an outward current in a concentration-dependent manner; the current was suppressed when acidified bath solution was introduced during exposure to the anesthetic. D, Concentration–response curve for current activation by sevoflurane. The sevoflurane-induced current at −60 mV was measured in individual cells exposed to multiple concentrations of sevoflurane and normalized to the current obtained with maximal concentrations of sevoflurane (∼0.7 mm). Averaged normalized data (± SEM) were plotted as a function of the aqueous concentrations of sevoflurane (± SEM). These concentration–response data were well fitted by using a logistic equation of the formI/Imax = 1/(1 + ([sevoflurane]/EC50)n), with an EC50 of 290 μm and a Hill coefficient (n) of −4. Inset, At all concentrations, I–V relationships of the sevoflurane-induced current rectified weakly in the outward direction, with a reversal potential near EK(inset).
As expected from our previous work with isoflurane and sevoflurane (Sirois et al., 1998), activation of this K+ current was not limited among inhalational anesthetics to halothane. The fluorinated ether anesthetic sevoflurane also induced a concentration-dependent and pH-sensitive outward shift in holding current (Fig. 2C), with weakly rectifying voltage-dependent characteristics (Fig.2D, inset) that were indistinguishable from the halothane-sensitive K+ current. As shown in Figure 2D, and similar to the halothane current, the sevoflurane-induced current activated with steep concentration dependence (Hill slope, −4) and an EC50 of 290 μm, very close to that expected for anesthetic effects of sevoflurane (∼280 μm; Park et al., 1996).
Inhalation anesthetics can enhance chloride currents through GABAA and/or glycine receptor channels, especially at high concentrations (Daniels and Smith, 1993; Franks and Lieb, 1994; Mihic et al., 1997). However, we found no evidence for a contribution from those receptors to observed membrane effects of anesthetics in motoneurons; after blocking GABAAand glycine receptors with bicuculline (10 μm) and strychnine (30 μm), the current induced by high concentrations of halothane (1.25 mm) was not different in magnitude, voltage dependence or reversal potential (n= 7, data not shown).
Extracellular acidification blocks a halothane-induced open-rectifier K+ current in HMs
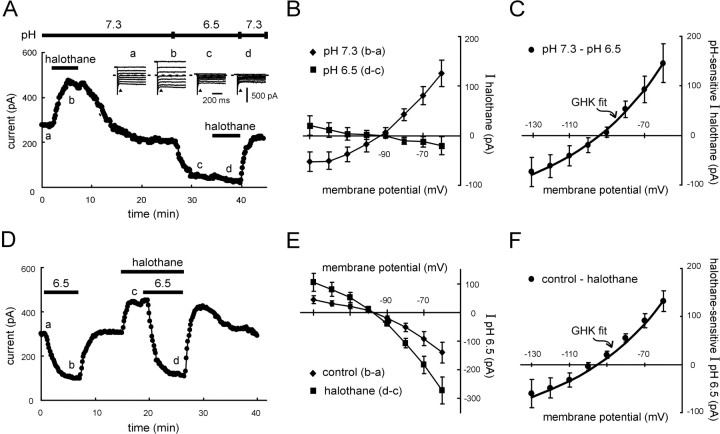
Extracellular acidification induced an inward shift in holding current at −60 mV, and that pH-sensitive current was increased in the continued presence of halothane (Fig. 2A). Because the increase in pH-sensitive current amplitude was essentially the same magnitude as the halothane-induced current itself, it appeared that the same K+ channel activated by halothane was completely blocked by bath acidification. Indeed, even a supramaximal concentration of halothane (1.25 mm), which induced a large outward current under control conditions, was entirely ineffective when delivered with an extracellular solution titrated to pH 6.5 (Fig. 3A). At −60 mV, the current induced by 1.25 mm halothane averaged 160.0 ± 30.7 pA under control conditions and −13.9 ± 13.7 pA in acidified extracellular solution (n = 7;p < 0.005).
Fig. 3.
The pH-sensitive halothane-activated K+ current in motoneurons has the properties of an open-rectifier. A, Voltage-clamp recording of membrane current in a hypoglossal motoneuron held at −60 mV. A supramaximal concentration of halothane (1.25 mm) induced an outward current under control conditions that was completely inhibited after blocking the pH-sensitive background K+ current with an acidified extracellular solution. Inset, Sample current responses to voltage steps (−60 to −130 mV) obtained at the indicated time points; I–V relationships were determined from currents measured immediately after the capacitive transient (arrowhead). B, AveragedI–V relationships (± SEM, n = 5) of the halothane-induced current in motoneurons at pH 7.3 (diamonds) and pH 6.5 (squares).C, Averaged data depicting the I–Vrelationship of the pH-sensitive component of halothane-induced current in motoneurons. The data were derived by subtraction of theI–V data obtained in pH 6.5 from that in pH 7.3 (± SEM, n = 5) and were well fitted by using the GHK constant field equation. D, Voltage-clamp recording of membrane current in a hypoglossal motoneuron held at −60 mV. An acidified solution, pH 6.5, induced an inward shift in current that reversed after wash, whereas halothane (1.25 mm) evoked an outward shift. When retested in the continued presence of halothane, the current induced by acidified solution was enhanced by an amount essentially identical to the size of the halothane current itself.E, Averaged I–V relationships (± SEM,n = 12) of the pH-sensitive currents recorded under control conditions (diamonds) and in the presence of halothane (squares). F, Averaged data depicting the I–V relationship of the halothane-induced component of pH-sensitive current. The I–V data were derived by subtraction of currents induced by acidification under control conditions from those in the presence of halothane (± SEM,n = 12) and were well fitted by using the GHK constant field equation.
In addition to its pH sensitivity, the TASK-1 currents have time- and voltage-dependent properties that characterize it as an “open-rectifier” (Duprat et al., 1997; Kim et al., 1998, 1999;Leonoudakis et al., 1998; Lopes et al., 2000; Talley et al., 2000). That is, TASK-1 currents show instantaneous and/or extremely fast activation kinetics, together with a slight outward rectification under physiological asymmetric K+ concentrations that is predicted by the Goldman–Hodgkin–Katz (GHK) constant field equation (Hille, 1992). We took advantage of the pH sensitivity of the halothane current to determine whether the native anesthetic- and pH-dependent K+ current in hypoglossal motoneurons has the properties of TASK-1 (Fig. 3). I–Vrelationships were obtained from current responses to voltage steps applied at the indicated time points (Fig. 3A,inset); currents evoked by hyperpolarizing voltage steps displayed no appreciable time-dependent activation or inactivation, indicating that they were instantaneous and persistent. TheI–V relationship of the halothane-induced current at pH 7.3 rectified mildly in the outward direction and reversed near the expected EK (Fig. 3B,diamonds); only a small residual halothane-sensitive current remained in pH 6.5 that was not investigated further (Fig.3B, squares). The component of halothane current blocked by acidification was derived by subtracting I–Vcurves from the two pH conditions (Fig. 3C). Those data were well fitted by using the GHK constant field equation, indicating that an open rectifier K+ conductance underlies the pH-sensitive halothane current in hypoglossal motoneurons.
We performed similar I–V analyses, but with a reverse order of application, to reveal the voltage-dependent profile of the component of pH-sensitive current that was enhanced by halothane (Fig.3D–F). I–V relationships of the pH-sensitive current obtained under control conditions (i.e., before halothane application; Fig. 3E, diamonds) and in the presence of halothane (Fig. 3E, squares) indicated that the inward shift in holding current induced by the pH 6.5 solution was associated with a decrease in a weakly rectifying conductance that reversed near EK. Subtracting I–V data in halothane from that in control yielded the halothane-induced component of pH-sensitive current, which was well fitted by using the GHK equation (Fig. 3F), again indicating that halothane activated an open rectifier K+ conductance that was blocked by acidification.
K+ current activation by halothane and alkalization are not occlusive
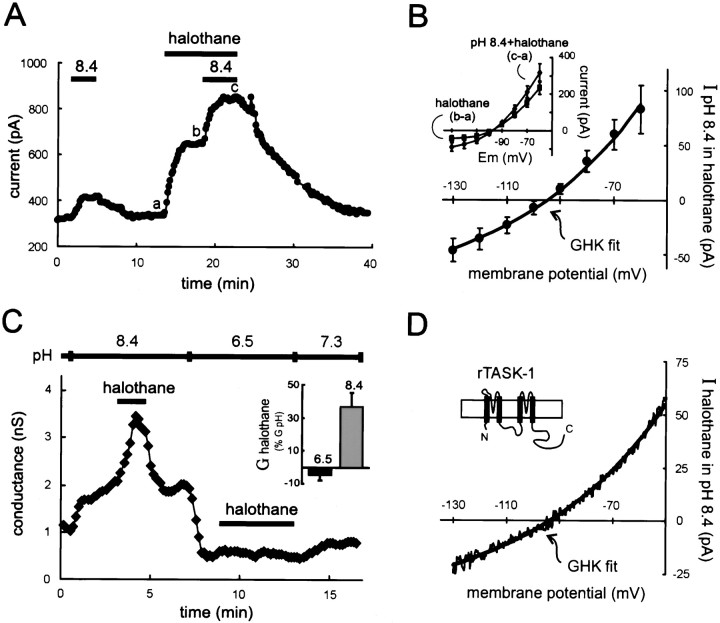
Maximal activation of the motoneuronal K+ current by inhalation anesthetics did not prevent further current activation by extracellular alkalization. As shown in Figure 4A, an outward shift in current was evoked when the pH of the extracellular solution was increased (from pH 7.3 to 8.4). In previous work, we found that further alkalization beyond this level did not cause additional activation of the pH-sensitive K+conductance in motoneurons or of TASK-1 in heterologous expression systems (Talley et al., 2000) (see also Duprat et al., 1997;Leonoudakis et al., 1998; Lopes et al., 2000). Nevertheless, in these experiments, the current induced by halothane (241.2 ± 37.8 pA at −60 mV) was always larger than that evoked by alkalization to pH 8.4 (51.6 ± 9.0 pA; n = 10; p < 0.0005) and, in the continued presence of halothane, alkalization always caused a further increase in holding current that was often enhanced in amplitude (Fig. 4A). The mechanism for this current enhancement was not explored, but it was clear that the current induced by alkalization in the presence of halothane once again had the open-rectifier properties of TASK-1 (Fig.4B).
Fig. 4.
Currents activated maximally by extracellular alkalization and halothane are not occlusive in motoneurons or in rTASK-1-expressing HEK 293 cells. A,Membrane current in a hypoglossal motoneuron held at −60 mV was shifted outward by an alkalized solution, pH 8.4, and by halothane (1.25 mm). In the continued presence of halothane, the current induced by pH 8.4 solution was still evident (and indeed enhanced). B, Averaged data (± SEM,n = 10) depicting the I–Vrelationship of the current induced by alkalized solution in the presence of halothane. The data were derived by subtraction of currents induced by halothane from those obtained in the pH 8.4 solution in the continued presence of halothane (inset). This additional current evoked by extracellular alkalization was well fitted by using the GHK constant field equation. C, The conductance in HEK 293 cells expressing rTASK-1, determined by using ramp voltage commands, was increased by halothane (1.25 mm) when the pH-sensitive current was enabled by an alkalized extracellular solution (pH 8.4), but not after blocking the pH-sensitive conductance with an acidic solution (pH 6.5). Inset, Averaged data (± SEM,n = 6), depicting the halothane-induced conductance (G halothane) as a percentage of the total pH-sensitive conductance (G pH = G8.4 − G6.5), show the complete block of halothane effect in acidified extracellular solution.D, The I–V relationship of the halothane-sensitive current in alkalized solution was derived from ramp voltage commands and was well fitted by using the GHK constant field equation.
Permissive pH conditions are necessary for halothane activation of rTASK-1 in HEK 293 cells
The results presented to this point indicate that the anesthetic-induced K+ channel in motoneurons has the voltage-dependent properties of TASK-1 and is sensitive to extracellular pH. Therefore, we investigated the pH dependence of anesthetic effects on TASK-1 channels expressed heterologously in mammalian cells.
Slow depolarizing voltage ramps were applied to HEK 293 cells transfected with the rat TASK-1 channel, and the slope conductance was determined by linear fit to the resultant I–V curve over the voltage range between −60 and −80 mV. As expected from previous reports with the human TASK-1 channel (Patel et al., 1999), halothane increased conductance in rTASK-1-expressing HEK 293 cells at pH 7.3 (data not shown), confirming the anesthetic sensitivity of the rTASK-1 channel under physiological pH conditions. Alkalization of the extracellular solution induced an increase in conductance, and under these conditions of maximal TASK-1 channel activation by alkalization (Duprat et al., 1997; Leonoudakis et al., 1998; Lopes et al., 2000;Talley et al., 2000), halothane was able to induce an additional increase in conductance (Fig. 4C). So, as with the native current in motoneurons, effects of halothane and alkalization were not occlusive in these rTASK-1-expressing HEK 293 cells. Note that theI–V relationship of the halothane-induced current was well fitted by the overlaid GHK equation (Fig. 4D), reflecting the open rectifier characteristics of the cloned rTASK-1 channel (Leonoudakis et al., 1998). In addition, the rTASK-1 conductance was completely blocked after switching to a pH 6.5 solution (Leonoudakis et al., 1998; Talley et al., 2000), and as in motoneurons, halothane was without any effect under this condition of maximal channel block by extracellular acidification (Fig. 4C).
Thus, rat brainstem motoneurons express TASK-1 and an anesthetic-activated K+ current with time- and voltage-dependent properties essentially identical to TASK-1. Further consistent with a mediating role for TASK-1, we found that both the motoneuronal anesthetic-sensitive K+current and the anesthetic effects on rTASK-1 were similarly dependent on the prevailing pH; in both settings, the anesthetic-sensitive current was blocked in solutions acidified to levels that completely inhibit TASK-1 but enhanced in neutral or alkalized conditions when the TASK-1 channel is enabled. The fact that channel activation by permissive pH conditions and by halothane did not obstruct each other suggests that distinct mechanisms mediate these two forms of modulation, but this issue was not examined in further detail.
Locus coeruleus neurons express TASK-1 and a halothane-sensitive current with the properties of TASK-1
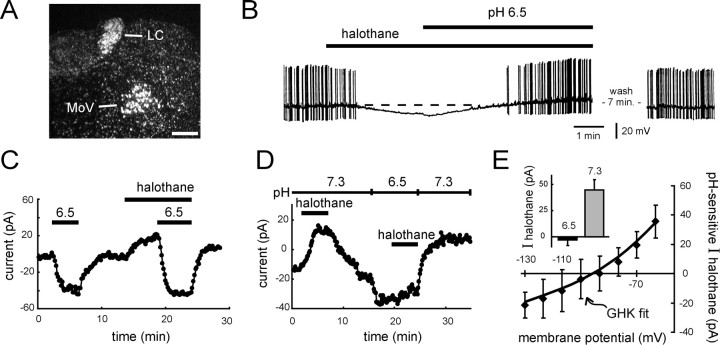
To determine whether TASK-1 expression correlates with effects of inhalation anesthetics on K+ channels in other neurons, we tested effects of halothane in LC neurons, which express moderately high levels of TASK-1 mRNA (Fig.5A). Qualitatively, the results from LC neurons were remarkably similar to those described above in motoneurons, although the maximal anesthetic current in LC neurons was smaller in amplitude. This is consistent with lower levels of TASK-1 expression in LC neurons, as assessed by high power microscopic examination of silver grains overlying individual cells (data not shown; Talley et al., 2000). Despite evoking a smaller current, halothane was nonetheless able to induce a membrane hyperpolarization and decrease the spiking activity in every LC neuron tested (n = 12; Fig. 5B). This inhibition of neuronal firing was observed at clinical concentrations of halothane (i.e., 0.3 and 0.4 mm; n = 4 each) and was not a result of activation of GABAAor glycine receptors because it occurred in the presence of bicuculline and strychnine (10 and 30 μm). Importantly, as in motoneurons, the halothane-induced inhibition was reversed when the extracellular solution was acidified (Fig. 5B).
Fig. 5.
Halothane hyperpolarizes neurons of the locus coeruleus by activating a pH-sensitive, open-rectifier K+ current. A, Dark-field photomicrograph of emulsion-dipped section hybridized with a [33P]-labeled cRNA probe specific to TASK-1. Note the high density of silver grains overlaying neurons in the nucleus locus coeruleus (LC); labeling is also apparent in motoneurons of the trigeminal nucleus (MoV). Scale bar, 250 μm. B, Current-clamp recording of a locus coeruleus neuron exposed to halothane (0.3 mm) and then to an acidified extracellular solution, pH 6.5, in the continued presence of halothane. Halothane caused a membrane hyperpolarization that was reversed by acidosis. Cell was recorded in the continuous presence of bicuculline (10 μm) and strychnine (30 μm), to block GABAA and glycine receptors.C, Voltage-clamp recording of net membrane current in a locus coeruleus cell held at −60 mV. An acidified solution, pH 6.5, induced an inward shift in current that reversed after wash, whereas halothane evoked an outward shift. When retested in the continued presence of halothane, the change in current induced by acidified solution was enhanced. D, Effects of halothane on membrane current in a locus coeruleus neuron held at −60 mV under voltage clamp. Halothane induced an outward current that was completely blocked in solution acidified to pH 6.5. E, Averaged data (± SEM, n = 4) showing the pH sensitivity of halothane-induced current in locus coeruleus neurons.I–V data were derived by subtraction of the halothane current obtained in pH 6.5 from that in pH 7.3, and were well fitted by using the GHK constant field equation. Inset, Halothane current at −60 mV in control, pH 7.3, and acidified, pH 6.5, solutions.
Under voltage clamp, extracellular acidification evoked an inward shift in holding current. Unlike motoneurons, in which this pH-sensitive current was essentially entirely attributable to a K+ conductance with properties of TASK-1 (Fig. 2E) (see also Talley et al., 2000), it appeared that multiple ionic conductances were involved in LC neurons (data not shown). Nevertheless, similar to motoneurons, halothane induced an outward current in LC neurons, and this additional halothane-sensitive current was completely suppressed in acidified bath solution (Fig.5C). This was also apparent when the order of presentation was reversed; the current induced by halothane under control pH conditions was completely blocked in acidified extracellular solutions (Fig. 5D,E, inset). The averaged I–Vrelationships of the pH-sensitive component of halothane current in LC neurons rectified weakly in the outward direction and reversed nearEK (Fig. 5E); again, as in motoneurons and HEK 293 cells expressing rTASK-1, the rectification of the halothane current was well described by the overlaid GHK constant field equation (Fig. 5E).
DISCUSSION
We have characterized a pH-sensitive background K+ current in neurons that is activated by inhalation anesthetics at precisely the concentrations that produce clinical effects of these compounds. This native neuronal K+ current has properties that identify it as TASK-1, or a channel with properties of TASK-1. Its activation in motoneurons and LC neurons suggest that TASK-1 represents a molecular substrate for specific clinical actions of volatile anesthetics mediated by these neurons.
Properties of anesthetic-activated K+ currents in motoneurons and LC neurons are those of TASK-1
In motoneurons and LC neurons, which express native TASK-1, and in HEK 293 cells expressing the cloned rTASK-1 channel, the halothane-sensitive K+ currents were dependent on the prevailing extracellular pH; halothane was able to activate K+ currents in neutral and alkalized conditions, when the pH-sensitive K+ channel was enabled, but was ineffective in solutions acidified to levels that completely block TASK-1. Furthermore, in both native and heterologous systems, the pH-sensitive component of halothane current had the properties of an open-rectifier. Of all K+ channels yet identified by molecular cloning, including other anesthetic-sensitive members of the two-pore domain K+ channel family (see below), the combined properties of pH sensitivity in the physiological range and open rectification are unique to TASK-1 (Duprat et al., 1997; Kim et al., 1998, 1999; Leonoudakis et al., 1998; Lopes et al., 2000; Talley et al., 2000).
It is noteworthy that, of the eight functional mammalian two-pore domain background K+ channels identified to date, three are known to be activated by inhalation anesthetics: TASK-1, TASK-2, and TREK-1 (Patel et al., 1999; Gray et al., 2000). Of these, however, only TASK-1 has the properties of pH sensitivity and open rectification described for the anesthetic-sensitive background K+ current in motoneurons and LC neurons. The recently characterized rat TASK-3 channel is a pH-sensitive open-rectifier, but it has a pK of ∼6.7 (Kim et al., 2000), clearly lower than TASK-1 or the pH-sensitive current we found in motoneurons (pK, ∼7.2–7.4; Duprat et al., 1997; Leonoudakis et al., 1998; Lopes et al., 2000; Talley et al., 2000) and its anesthetic sensitivity has not been reported. The TREK-1 two-pore domain channel is activated by inhalation anesthetics, but only at higher concentrations (Patel et al., 1999). Furthermore, it displays outward rectification (Fink et al., 1996), is sensitive to intracellular rather than extracellular pH and is activated, not inhibited, by acidification (Maingret et al., 1999). The anesthetic-sensitive TASK-2 channel is inhibited by extracellular acidification and activated by alkalization (Gray et al., 2000), but TASK-2 currents have a pK of ∼8.5 (Reyes et al., 1998), much higher than TASK-1 or the pH-sensitive current in motoneurons (Duprat et al., 1997; Leonoudakis et al., 1998; Lopes et al., 2000;Talley et al., 2000). Moreover, TASK-2 generates a time-dependent current that rectifies outwardly (Reyes et al., 1998), unlike TASK-1 and the anesthetic current in motoneurons and LC neurons (Duprat et al., 1997; Kim et al., 1998, 1999; Leonoudakis et al., 1998; Lopes et al., 2000; Talley et al., 2000). Nevertheless, both TREK-1 and TASK-2 are expressed in the mammalian CNS (Fink et al., 1996; Reyes et al., 1998) and it is therefore possible that they could contribute to anesthetic-activated K+ currents described in other neuronal cell groups. Furthermore, given the size of this K+ channel gene family in other species (i.e., >50 genes in Caenorhabditis elegans and 11 inDrosophila melanogaster; Wang et al., 1999; Littleton and Ganetzky, 2000), it is likely that additional members of this gene family will be identified in mammalian species, and some of those could also be anesthetic-sensitive.
Clinical relevance of TASK-1 activation by anesthetics in motoneurons and LC neurons
The activation of native neuronal pH-sensitive K+ currents by anesthetics occurs with exactly the concentration dependence expected for anesthetic effects (Franks and Lieb, 1994) and is of particular interest because of the well described physiology of motoneurons and LC neurons and their potential involvement in mediating specific anesthetic effects. TASK-1 transcripts are expressed at high levels in cranial and spinal motoneurons (Talley et al., 2000). Motoneurons directly control muscle activity, and it is therefore likely that TASK-1 activation contributes to the inhibition of motoneuron excitability and immobilization known to accompany inhalation anesthesia (Zhou et al., 1997, 1998). LC neurons are the major source of norepinephrine in the CNS and have long been implicated in control of vigilance and arousal (Aston-Jones et al., 1991). Halothane depresses LC neuronal activity in vivo (Camproux et al., 1996), and in halothane-anesthetized rats, activation of LC neurons converts EEG activity from a sleep-like, large amplitude, slow-wave pattern to the small-amplitude, high-frequency pattern associated with waking (Berridge and Foote, 1991). Moreover, LC neurons mediate hypnotic and supraspinal analgesic effects of α2-adrenoceptor agonist anesthetic compounds such as clonidine and dexmedetomidine (Correa-Sales et al., 1992;Aantaa and Scheinin, 1993; Guo et al., 1996; Lakhlani et al., 1997), which suppress LC firing by activation of a distinct, G-protein-coupled inwardly rectifying K+ (GIRK) conductance (North, 1989; Lakhlani et al., 1997). The inhibition of LC firing that occurs after halothane-induced TASK-1 activation would be expected to produce similar hypnotic and analgesic effects. Furthermore, coactivation in LC neurons of these two different K+ channels—TASK-1 and GIRK—could account, at least in part, for the interactive effects of α2 agonists and inhalation anesthetics [i.e., the ability of clonidine and dexmedetomidine to lower the minimum alveolar concentration (MAC) of halothane; Aantaa and Scheinin, 1993;Lakhlani et al., 1997].
Because TASK-1 is activated by halothane, even in excised patches (Patel et al., 1999), it seems likely that it could contribute to previous reports of anesthetic-sensitive background K+ channels in other neurons (e.g., cerebellar granule neurons, sensory relay neurons of the intralaminar and ventrobasal thalamic nuclei; Sugiyama et al., 1992; Winegar and Yost, 1998a; Ries and Puil, 1999) in which TASK-1 is also expressed (Leonoudakis et al., 1998; Millar et al., 2000; Talley et al., 2000). The intrinsic pH sensitivity of TASK-1 with a pK∼7.2–7.4 (Duprat et al., 1997; Leonoudakis et al., 1998; Lopes et al., 2000; Talley et al., 2000), together with the pH dependence of TASK-1 activation by anesthetics that we have demonstrated, provides a means to determine its contribution to anesthetic-sensitive K+ channels in these other neurons. This pH dependence also suggests that anesthetic effects mediated via this channel in TASK-1-expressing neurons will be enhanced by alkalization and inhibited by acidification in the physiological range. However, interpreting effects of global changes in CSF pH on anesthetic sensitivity solely in the context of TASK-1 modulation is problematic, not only because many other neural processes will potentially be affected by changes in pH, but also because simply lowering pH has narcotic effects on its own (Eisele et al., 1967). This pH-dependent narcosis, which occurs with a MAC at a pH of ∼6.9 (Eisele et al., 1967), would clearly confound any analysis of effects of pH on anesthetic sensitivity caused by TASK-1, which is completely inhibited only at a pH of ∼6.5. Identification of the precise contribution of TASK-1 to effects of anesthetic compounds will likely require experiments using mice with targeted disruptions of TASK-1 or mice that express TASK-1 channels lacking anesthetic sensitivity. Nevertheless, if the presence of TASK-1 indeed predicts inhibitory effects of inhalation anesthetics, as our data indicate, then its differential expression in neurons that subserve unique functional roles provides the potential for specificity in anesthetic actions mediated by TASK-1.
Footnotes
This work was supported by Grant NS33583 (D.A.B.) and Fellowships HL10271 (J.E.S.) and MH12091 (E.M.T.) from the National Institutes of Health. We thank Dr. M. B. Harrison for imaging equipment and support and Dr. A. T. Gray for the gift of TASK-1 cDNA. We also thank Dr. Marcel Durieux for providing helpful comments while this study was ongoing, Ms. Jacqueline Washington for help with gas chromatography, and Dr. Albert Berger for comments on this manuscript.
Correspondence should be addressed to Douglas A. Bayliss, Department of Pharmacology, University of Virginia Health System, P.O. Box 800735, 1300 Jefferson Park Avenue, Charlottesville, VA 22908-0735. E-mail:dab3y@virginia.edu.
REFERENCES
- 1.Aantaa R, Scheinin M. Alpha2-adrenergic agents in anaesthesia. Acta Anaesthesiol Scand. 1993;37:433–448. doi: 10.1111/j.1399-6576.1993.tb03743.x. [DOI] [PubMed] [Google Scholar]
- 2.Amaral DG, Sinnamon HM. The locus coeruleus: neurobiology of a central noradrenergic nucleus. Prog Neurobiol. 1977;9:147–196. doi: 10.1016/0301-0082(77)90016-8. [DOI] [PubMed] [Google Scholar]
- 3.Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- 4.Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci. 1991;11:3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camproux AC, Saunier F, Chouvet G, Thalabard JC, Thomas G. A hidden Markov model approach to neuron firing patterns. Biophys J. 1996;71:2404–2412. doi: 10.1016/S0006-3495(96)79434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesler M, Chen JC, Kraig RP. Determination of extracellular bicarbonate and carbon dioxide concentrations in brain slices using carbonate and pH-selective microelectrodes. J Neurosci Methods. 1994;53:129–136. doi: 10.1016/0165-0270(94)90169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an α2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–952. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Daniels S, Smith EB. Effects of general anaesthetics on ligand-gated ion channels. Br J Anaesth. 1993;71:59–64. doi: 10.1093/bja/71.1.59. [DOI] [PubMed] [Google Scholar]
- 9.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisele JH, Eger EI, Muallem M. Narcotic properties of carbon dioxide in the dog. Anesthesiology. 1967;28:856–865. doi: 10.1097/00000542-196709000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- 12.Franks NP, Lieb WR. Volatile general anaesthetics activate a novel neuronal K+ current. Nature. 1988;333:662–664. doi: 10.1038/333662a0. [DOI] [PubMed] [Google Scholar]
- 13.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein SA, Wang KW, Ilan N, Pausch MH. Sequence and function of the two P domain potassium channels: implications of an emerging superfamily. J Mol Med. 1998;76:13–20. doi: 10.1007/s001090050186. [DOI] [PubMed] [Google Scholar]
- 15.Gray AT, Zhao BB, Kindler CH, Winegar BD, Mazurek MJ, Xu J, Chavez RA, Forsayeth JR, Yost CS. Volatile anesthetics activate the human tandem pore domain baseline K+ channel KCNK5. Anesthesiology. 2000;92:1722–1730. doi: 10.1097/00000542-200006000-00032. [DOI] [PubMed] [Google Scholar]
- 16.Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus coeruleus produces antinociception. Anesthesiology. 1996;84:873–881. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Hille B. Ionic channels of excitable membranes. Sinauer; Sunderland, MA: 1992. [Google Scholar]
- 18.Kim D, Fujita A, Horio Y, Kurachi Y. Cloning and functional expression of a novel cardiac two-pore background K+ channel (cTBAK-1). Circ Res. 1998;82:513–518. doi: 10.1161/01.res.82.4.513. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Bang H, Kim D. TBAK-1 and TASK-1, two-pore K+ channel subunits: kinetic properties and expression in rat heart. Am J Physiol. 1999;277:H1669–H1678. doi: 10.1152/ajpheart.1999.277.5.H1669. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K+ channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- 21.Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, Limbird LE. Substitution of a mutant α2a-adrenergic receptor via “hit and run” gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc Natl Acad Sci USA. 1997;94:9950–9955. doi: 10.1073/pnas.94.18.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonoudakis D, Gray AT, Winegar BD, Kindler CH, Harada M, Taylor DN, Chavez RA, Forsayeth JR, Yost CS. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J Neurosci. 1998;18:868–877. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesage F, Lazdunski M. Potassium channels with two P domains. In: Kurachi Y, Jan LY, Lazdunski M, editors. Potassium channels: molecular structure, function, and diseases. Academic; San Diego: 1999. pp. 199–222. [Google Scholar]
- 24.Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- 25.Lopes CMB, Gallagher PG, Buck ME, Butler MH, Goldstein SAN. Proton block and voltage gating are potassium-dependent in the cardiac leak channel Kcnk3. J Biol Chem. 2000;275:16969–16978. doi: 10.1074/jbc.M001948200. [DOI] [PubMed] [Google Scholar]
- 26.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 27.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 28.Millar JA, Baratt L, Southan AP, Page KM, Fyffe REW, Robertson B, Mathie A. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci USA. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munsch T, Pape H-C. Modulation of the hyperpolarization-activated cation current of rat thalamic relay neurones by intracellular pH. J Physiol (Lond) 1999;519:493–504. doi: 10.1111/j.1469-7793.1999.0493m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicoll RA, Madison DV. General anesthetics hyperpolarize neurons in the vertebrate central nervous system. Science. 1982;217:1055–1057. doi: 10.1126/science.7112112. [DOI] [PubMed] [Google Scholar]
- 31.North RA. Drug receptors and the inhibition of nerve cells. Br J Pharmacol. 1989;98:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park WK, Pancrazio JJ, Suh CK, Lynch C. Myocardial depressant effects of sevoflurane. Mechanical and electrophysiologic actions in vitro. Anesthesiology. 1996;84:1166–1176. doi: 10.1097/00000542-199605000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- 34.Reyes R, Duprat F, Lesage F, Fink M, Salinas M, Farman N, Lazdunski M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J Biol Chem. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- 35.Ries CR, Puil E. Ionic mechanism of isoflurane's actions on thalamocortical neurons. J Neurophysiol. 1999;81:1802–1809. doi: 10.1152/jn.1999.81.4.1802. [DOI] [PubMed] [Google Scholar]
- 36.Sirois JE, Pancrazio JJ, Lynch C, Bayliss DA., III Multiple ionic mechanisms mediate inhibition of rat motoneurones by inhalation anaesthetics. J Physiol (Lond) 1998;512:851–862. doi: 10.1111/j.1469-7793.1998.851bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugiyama K, Muteki T, Shimoji K. Halothane-induced hyperpolarization and depression of postsynaptic potentials of guinea pig thalamic neurons in vitro. Brain Res. 1992;576:97–103. doi: 10.1016/0006-8993(92)90613-e. [DOI] [PubMed] [Google Scholar]
- 38.Takenoshita M, Takahashi T. Mechanisms of halothane action on synaptic transmission in motoneurons of the newborn rat spinal cord in vitro. Brain Res. 1987;402:303–310. doi: 10.1016/0006-8993(87)90037-0. [DOI] [PubMed] [Google Scholar]
- 39.Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 40.Viana F, Gibbs L, Berger AJ. Double- and triple-labeling of functionally characterized central neurons projecting to peripheral targets studied in vitro. Neuroscience. 1990;38:829–841. doi: 10.1016/0306-4522(90)90075-f. [DOI] [PubMed] [Google Scholar]
- 41.Voipio J, Kaila K. Interstitial PCO2 and pH in rat hippocampal slices measured by means of a novel fast CO2/H+-sensitive microelectrode based on a PVC-gelled membrane. Pflügers Arch. 1993;423:193–201. doi: 10.1007/BF00374394. [DOI] [PubMed] [Google Scholar]
- 42.Wang ZW, Kunkel MT, Wei A, Butler A, Salkoff L. Genomic organization of nematode 4TM K+ channels. Ann NY Acad Sci. 1999;868:286–303. doi: 10.1111/j.1749-6632.1999.tb11294.x. [DOI] [PubMed] [Google Scholar]
- 43.Winegar BD, Owen DF, Yost CS, Forsayeth JR, Mayeri E. Volatile general anesthetics produce hyperpolarization of Aplysia neurons by activation of a discrete population of baseline potassium channels. Anesthesiology. 1996;85:889–900. doi: 10.1097/00000542-199610000-00026. [DOI] [PubMed] [Google Scholar]
- 44.Winegar BD, Yost CS. Activation of single potassium channels in rat cerebellar granule cells by volatile anesthetics. Toxicol Lett. 1998a;100–101:287–291. doi: 10.1016/s0378-4274(98)00197-0. [DOI] [PubMed] [Google Scholar]
- 45.Winegar BD, Yost CS. Volatile anesthetics directly activate baseline S K+ channels in Aplysia neurons. Brain Res. 1998b;807:255–262. doi: 10.1016/s0006-8993(98)00687-8. [DOI] [PubMed] [Google Scholar]
- 46.Zhou HH, Mehta M, Leis AA. Spinal cord motoneuron excitability during isoflurane and nitrous oxide anesthesia. Anesthesiology. 1997;86:302–307. doi: 10.1097/00000542-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Zhou HH, Jin TT, Qin BS, Turndorf H. Suppression of spinal cord motoneuron excitability correlates with surgical immobility during isoflurane anesthesia. Anesthesiology. 1998;88:955–961. doi: 10.1097/00000542-199804000-00015. [DOI] [PubMed] [Google Scholar]