Abstract
Circadian rhythms in several species can be phase-shifted by procedures that stimulate locomotor activity (“exercise”) during the usual sleep period. The role of arousal or sleep loss, independent of activity, in this effect has not been adequately resolved. We show here, using the sleep deprivation procedure of gentle handling, that comparably large phase shifts (up to 240 min advances) of the rest–activity cycle can be induced in Syrian hamsters by 3 hr of behavioral arousal, with minimal locomotion, beginning 6 hr before the usual active period. Horizontal distance traveled during the deprivation procedure averaged ∼0.08 km, compared to 2.5 km typical in exercise studies. Hamsters requiring fewer interventions exhibited larger shifts, suggesting that the level or continuity of spontaneous arousal determines shift size. The circadian rhythm of light-induced c-fos expression in the suprachiasmatic nucleus (SCN) was used as a phase marker to further demonstrate that the clock is reset within 1 hr after a 3 hr deprivation. Sleep deprivation mimicked the effects of exercise on basal c-fos expression in two components of the circadian system, suppressing basal Fos immunoreactivity in the SCN, and increasing Fos in the intergeniculate leaflet. Sleep deprivation without exercise in hamsters can rapidly reset the circadian clock and alter gene expression within the circadian system.
Keywords: circadian rhythms, nonphotic entrainment, c-fos, wheel running, phase shifts, suprachiasmatic nucleus
Forty years ago, it was first suggested that an animal's state of arousal or level of locomotor activity might affect properties of its circadian clock (Aschoff, 1960). Convincing evidence followed some 25 years later, when it was demonstrated that running in an activity wheel can alter the period (Yamada et al., 1986) or shift the phase (Reebs and Mrosovsky, 1989) of circadian rhythms in nocturnal rodents. Since then, it has been shown repeatedly, using a range of arousing stimuli applied during the usual rest phase of the circadian sleep–wake cycle, that substantial phase advance shifts (up to 4 hr) are induced if subjects run during or after the stimulus but usually not if running is absent or prevented (Hastings et al., 1998; Mistlberger et al., 2000). Both the magnitude and the direction of these shifts are gated by circadian phase, with maximal phase advance shifts evident when activity is stimulated near the middle of the rest period and small phase delays (≤1 hr) when activity is stimulated during the latter half of the usual wake phase of the circadian cycle (Bobrzynska and Mrosovsky, 1998).
Although these studies implicate high intensity locomotor activity (i.e., exercise) as the behavioral stimulus critical for phase resetting in response to at least some arousing stimuli, the contribution that sleep loss or nonspecific arousal makes to the phase shifting process, independent of locomotion, has not been adequately resolved. Animals that run little after an arousing stimulus may fail to shift because they do not stay awake, whereas the occasional animal that shifts despite little running may do so because it does remain awake. This latter possibility, and potential contributions of nonspecific arousal to shifts induced by nonphotic stimuli, was noted in some of the earliest work on phase resetting by behavioral manipulations (Mrosovsky, 1988; Rusak et al., 1988; Honrado and Mrosovsky, 1989; Turek, 1989). More recently, it has been reported that brief episodes of arousal, induced by a single intraperitoneal injection of saline, can induce phase advance shifts without substantial locomotor activity, although these shifts are much smaller (∼60 min) and exhibit a more constrained circadian phase dependence than those induced by exercise procedures (Mead et al., 1992; Hastings et al., 1998). The possibility that longer episodes of arousal, without intense activity, might reliably produce larger shifts has not been systematically examined. This issue remains of broad interest, because it informs the neurobiological analysis of nonphotic entrainment and has implications for understanding circadian rhythm adaptation in humans after procedures that displace the normal timing of sleep–wake states (e.g., jet travel, shift work rotations, and antidepressant treatments such as early morning light therapy, phase advance of sleep–wake schedule, and short-term sleep deprivation).
We show here, using Syrian hamsters, that the phase-shifting effects of intense running can be fully mimicked by keeping subjects awake by gentle handling, with minimal activity. We used Fos immunocytochemistry (ICC) to further show that phase resetting is rapid (i.e., accomplished within 1 hr of the end of the procedure) and that the deprivation procedure mimics the effects of intense exercise on immediate early gene expression in components of the circadian system.
MATERIALS AND METHODS
Animals and sleep deprivation procedures. Syrian hamsters (male, 90 gm; Charles River, Montreal, Quebec, Canada) were housed under a 14:10 light/dark (LD) cycle (∼30:0 lux) in plastic cages (47 × 26 × 20 cm) equipped with 17.5 cm running wheels connected to an interface and microcomputer to monitor daily activity rhythms. Hamsters (n = 9) were sleep-deprived for 3 hr in their home cages, beginning 6 hr before the usual time of dark onset [zeitgeber time (ZT) 6, during which dark onset is designated ZT12, by convention]. This time was selected because it is normally occupied by sleep (on average, 91% of total time; our unpublished observations) and because continuous exercise at this time reliably induces large phase shifts. Deprivation was accomplished by the method of gentle handling (Tobler and Jaggi, 1987). Briefly, cage tops were removed, and the hamsters were observed continuously. If the hamsters attempted to adopt a sleep posture they were stimulated by a light puff of air, touch of the whiskers, or gentle prod. Running wheels were locked during the procedure, and the lights were dimmed (∼1 lux, red). After deprivation, the animals were left undisturbed for 3 d in constant dark (DD), after which they were re-entrained to LD for 7 d. The sleep deprivation test was conducted in counterbalanced order with a control test, in which the lighting conditions were duplicated without behavioral disturbance. After these tests, the hamsters were subjected at 10 d intervals to a 1 hr sleep deprivation beginning at ZT6 and a second 3 hr sleep deprivation beginning at ZT6, with home cage running wheels locked until ZT12. In replication experiments, 24 additional hamsters were subjected to the 3 hr sleep deprivation and control procedures.
Locomotor activity analysis. To quantify locomotor activity during the 3 hr sleep deprivation procedure, four hamsters used for immunocytochemistry (see below) and two hamsters used for behavioral phase shifting studies were videotaped with an infrared digital camcorder (Handycam; Sony, Tokyo, Japan), and total movement was scored by image analysis. The camera was mounted on a tripod, and aimed directly down over the cages. During playback, animal movements were traced on an acetate slide, which was then scanned into a digital file and loaded into MCID (Imaging Research, St. Catherine's, Ontario, Canada). A module for object area detection was used, and this area was divided by the width of the line giving an approximation of path length.
Immunocytochemistry. To evaluate the rate of clock resetting in response to sleep deprivation, separate groups of hamsters (n = 7) were subjected to either a 3 hr deprivation or the control procedure, and then exposed to a 10 min, 30 lux pulse of light 1 hr later (ZT10). Three additional control hamsters were exposed to light at ZT12. Light pulses during the usual nighttime (ZT12–24) induce heavy fos expression in the suprachiasmatic nucleus (SCN), the site of the primary circadian pacemaker in mammals (Rea, 1989;Kornhauser et al., 1990; Rusak et al., 1990). At ZT10, photic induction of Fos protein in the SCN is minimal. Animals were killed 90 min after the light pulse and processed for Fos ICC.
An additional experiment was run to test whether wheel running from ZT9–10, in the interval between sleep deprivation and light exposure, contributed to the Fos induction observed. Six hamsters were placed into DD at ZT6, exposed to a 10 min light pulse at ZT10, and killed for Fos ICC at ZT11.5. Two of these hamsters were sleep-deprived from ZT6–9, two were not disturbed until ZT9, when they were placed into a novel wheel to stimulate running, and two were not disturbed before the light pulse.
All animals were processed in tandem with their respective controls. Briefly, animals were given an overdose of sodium pentobarbital in the dark with the aid of an infrared camera (Find-R-Scope; FJW Optical, IL) and then blind-folded with aluminum foil to prevent any retinal illumination. Animals were perfused transcardially with 50 ml of cold PBS, pH 7.4, followed by 50 ml of cold 4% paraformaldehyde. Brains were removed and post-fixed in paraformaldehyde for 17 hr, and then cryoprotected in 20% sucrose for 24 hr. Alternate 50 μm sections were collected into PBS-filled wells. Sections were first rinsed in a 0.3% H2O2 in PBSx (0.3% Triton X-100 in PBS) bath to inactivate endogenous peroxidase and then in PBSx (rinsed 3× for 10 min each). The tissue was incubated in 10% normal goat serum (NGS; Vector Laboratories, Burlingame, CA) in PBSx for 90 min followed by 48 hr at 4°C in the primary fosantibody (1:80,000 in 1% NGS-PBSx) raised in rabbits against residues 4–17 of human fos (Oncogene Science; c-fos, AB-5). The tissue was rinsed through PBSx baths (3× for 10 min) and incubated in the secondary goat anti-rabbit antibody (Vectastain Elite kit; Vector Laboratories). After another set of PBSx baths (3× for 10 min), the tissue was incubated in an avidin–biotin complex (ABC, Vectastain Elite kit; Vector Laboratories) bath for 60 min. After a final set of PBSx baths (3× for 10 min), the tissue was developed with a diaminobenzidine (DAB) reaction for 5 min (0.04% DAB in Tris buffer ± 60 μl of 8% NiCl and 10 μl of 30% H2O2). The reaction was quenched in PBSx rinses, and tissue was mounted on slides, dehydrated, and coverslipped. Maintaining the same light column, digital images were captured and flat field-corrected using MCID. Fos-IR in the midcaudal SCN was counted bilaterally using the automatic target counting routine.
To evaluate the effect of sleep deprivation on basal expression of Fos protein within the circadian system, separate groups of hamsters (n = 8) were subjected to either the 3 hr sleep deprivation or control procedures and killed for Fos-ICC at ZT11.5. Quantification of SCN Fos-IR was as above.
Digital pictures were also made of the intergeniculate leaflet (IGL), a retinorecipient thalamic cell group with a major neuropeptide Y (NPY) projection to the SCN that has been implicated in nonphotic phase shifting (Janik and Mrosovsky, 1994; Wickland and Turek, 1994; Marchant et al., 1997; Maywood et al., 1997; Mikkelsen et al., 1998). Fos-positive cells in the IGL were scored visually by two observers, one blind to the treatment conditions, and the average of both scores for each animal was used for analysis. A Pearson product moment correlation was performed to assess inter-rater reliability (r = ±0.976; p = 0.0001)
Data analysis. Activity data were displayed in the form of actograms using Circadia (Behavioral Cybernetics, MA). Phase shifts were measured using a modification of the Aschoff Type II procedure (Aschoff, 1965; Mrosovsky, 1996), i.e., the time of nocturnal activity onset during the second day after sleep deprivation or the control procedure was compared to the average time of activity onset during the previous 5 d in LD. Activity onsets were identified by computer algorithm (the first 10 min bin after light-onset in which running exceeded 50 revolutions after 240 min during which it did not exceed this threshold). Differences between conditions were evaluated by independent t tests or repeated measures ANOVA, withpost hoc pairwise comparisons using the Student–Newman–Keuls' test. Means in the text are reported ± SD.
RESULTS
Sleep deprivation by gentle handling induces large phase-advance shifts
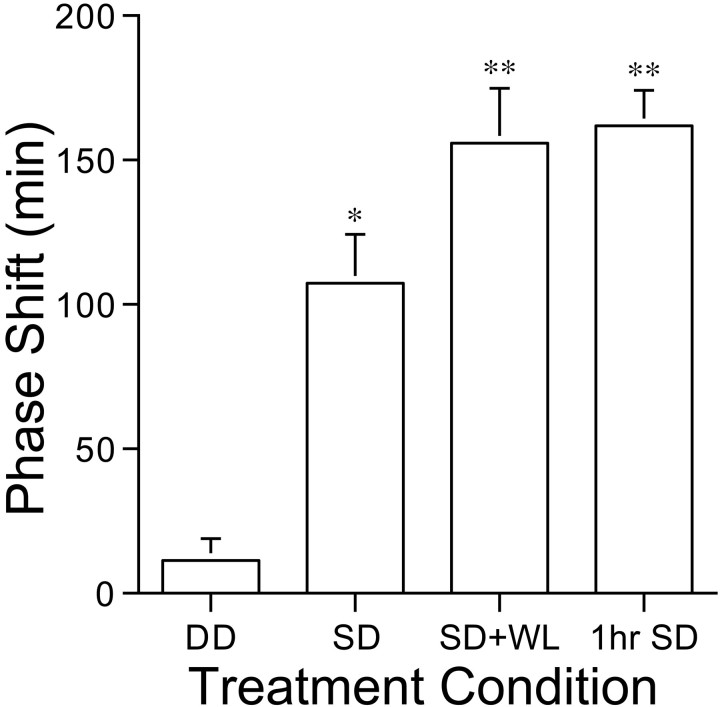
By the second complete day of DD, activity onset was phase-advanced 9 ± 20 min in the control condition, 102 ± 53 min in the first 3 hr sleep deprivation condition (ZT6–9), and 151 ± 48 min in the 1 hr deprivation condition (ZT6–7; F(3,24) = 40.5; p≪ 0.001; Figs. 1A-C,2). Post hoc analysis revealed that phase shifts after sleep deprivation differed significantly from the control condition.
Fig. 2.
Group mean phase shifts (± SEM).DD, Control condition in which lights were turned out 6 hr before the usual time of dark onset. SD, 3 hr sleep deprivation, beginning 6 hr before the usual time of dark onset.SD+WL, 3 hr sleep deprivation with home cage running wheel locked for 6 hr. 1hr SD, 1 hr sleep deprivation beginning 6 hr before the usual time of dark onset. *p < 0.05 versus DD; **p < 0.05 versus DD and 3hr SD.
Fig. 1.
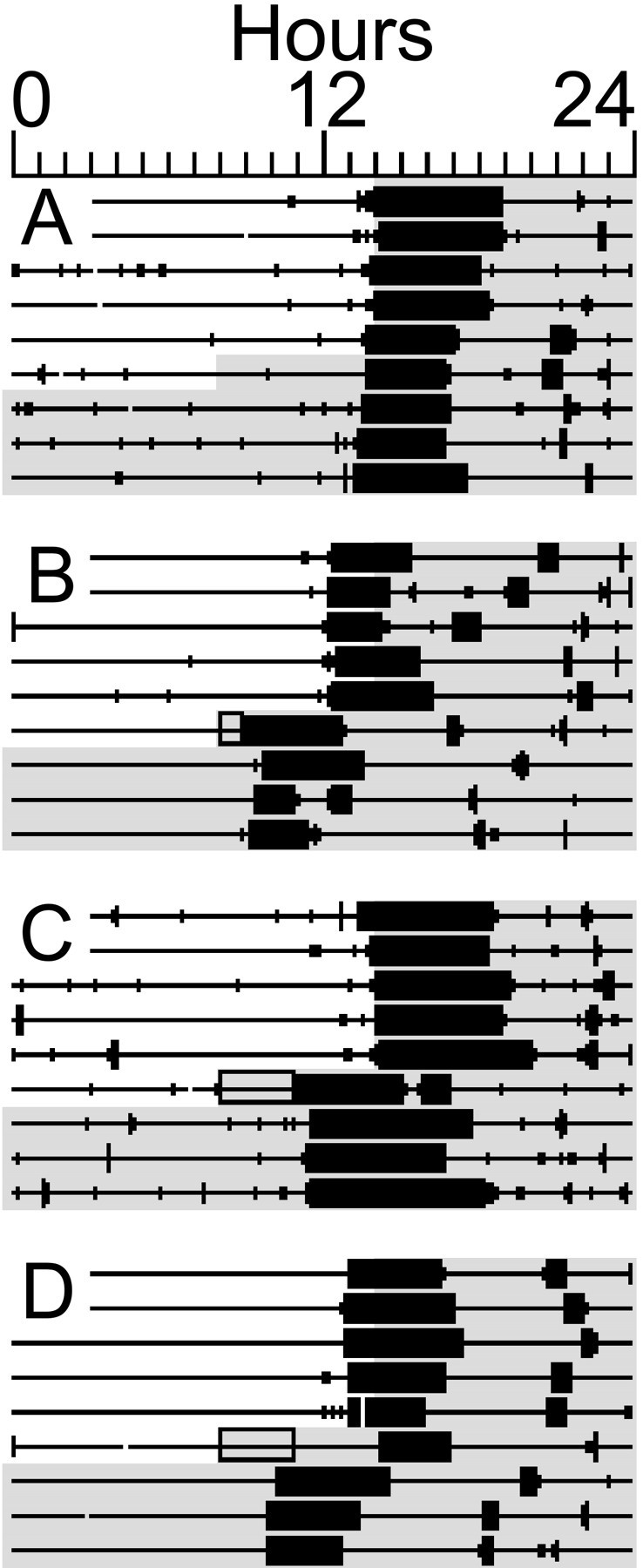
Wheel-running activity records of representative hamsters. Each line represents 24 hr plotted in 10 min bins fromleft to right. Vertical deflections on the line indicate time bins in which wheel-running activity occurred. Shading represents lights-off (LD 14:10). A, Control test, with constant dark beginning 6 hr before usual dark onset and no behavioral disturbance. A 38 min phase advance was evident on the second day after the procedure, relative to the average time of activity onset in LD before the first day of constant dark. B, 1 hr sleep deprivation (shaded rectangle) that induced a 180 min phase advance. C, 3 hr deprivation that induced a 165 min advance. D, 3 hr sleep deprivation with the home cage wheel locked for 6 hr, which induced a 190 min phase advance.
Similar results were obtained in the replication experiments. The mean phase shift to sleep deprivation in the pooled sample (n = 33) was 90 ± 67 min. Twenty-one hamsters shifted by an amount (139 ± 40 min; range, 58–208 min) >2 SDs beyond the mean shift in the control condition, whereas 12 hamsters shifted by an amount (13 ± 12 min; range, −6 to 30 min) that was within 1 SD of the mean control shift.
Clock resetting by sleep deprivation occurs despite low levels of activity
Activity during sleep deprivation was limited to occasional walking, rearing, nest building, and grooming. In a group of six hamsters, the total linear distance traveled within the cage during a 3 hr sleep deprivation averaged 87.2 ± 22.8 m. Four of these hamsters were subsequently processed for Fos ICC, and all four showed suppression of SCN Fos relative to control animals (see below). Behavioral phase shifts were measured in the other two hamsters; one of these moved 65.6 m during the sleep deprivation, and shifted 110 min, whereas the other moved 111 m, and shifted 2 min. The group mean of 87.2 m corresponds to the linear distance traveled by running 158 revolutions in a 17.5-cm-diameter running wheel. By comparison, the typical minimum distance traveled by hamsters that run and phase shift maximally when confined to a novel wheel for 3 hr in the midsubjective day is ∼30 times this distance (∼2.5 km;Bobrzynska and Mrosovsky, 1998). Because running in a wheel is physiologically more demanding than walking on the cage floor, this simple transformation of linear distance into revolutions no doubt understates the difference between these two forms of activity.
Immediately after sleep deprivation, many hamsters did not attempt to sleep, but instead exhibited high levels of wheel running, as if the circadian clock was already advanced by ∼3 hr to the onset of the daily active phase. The group mean latency to running after the 3 hr and 1 hr sleep deprivation tests was 55 ± 69 min and 74± 107 min, respectively, and the mean duration of the first bout of running was 196 ± 72 min and 100 ± 87 min, respectively. To determine whether intense wheel running after sleep deprivation was the stimulus for phase shifting, the initial group of nine hamsters was subjected to a second 3 hr sleep deprivation in which home cage running wheels were locked for 6 hr until ZT12. Phase shifts in this condition averaged 162 ± 53 min, which was significantly greater than the shifts that followed the control and their first 3 hr sleep deprivation tests (Figs. 1D,2). Wheel running immediately after sleep deprivation is clearly not necessary for a full phase shift response.
Clock resetting by sleep deprivation is rapid
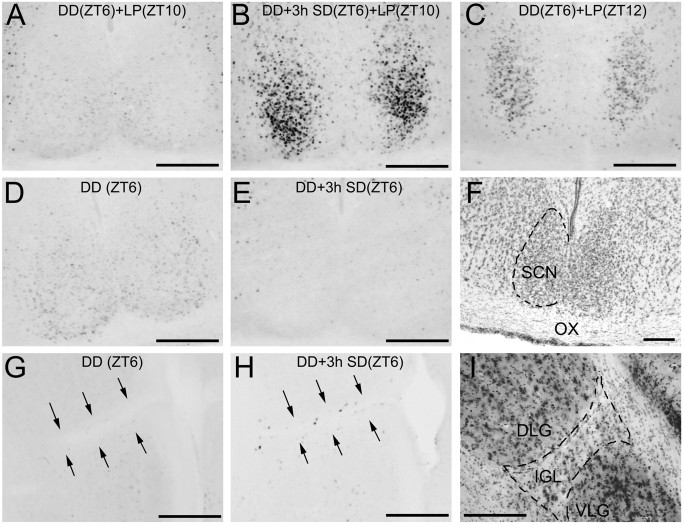
The wheel lock procedure does not prevent hamsters from engaging in other locomotor activities in the home cage after sleep deprivation. However, if the circadian clock is substantially or completely reset by the end of sleep deprivation, then behaviors that occur after the sleep deprivation procedure logically cannot be claimed to cause the phase shift. To estimate the rate of clock resetting more directly, we used ICC to measure light-induced c-fos expression in the SCN. Fos protein in SCN neurons is rapidly induced by brief light pulses only during the “subjective night” (the active phase of the daily rest–activity cycle in nocturnal animals), when light induces phase shifts (Rea, 1989; Kornhauser et al., 1990; Rusak et al., 1990). Fos expression can thus serve as a cellular marker of pacemaker phase in cases in which behavioral assessments are impossible or ambiguous. Separate groups of hamsters were subjected to either 3 hr sleep deprivation or the control procedure at ZT6 and then exposed to a 10 min, 30 lux light pulse at ZT10. SCN from the sleep deprivation group exhibited markedly more Fos-IR cells and more intense labeling than SCN from the control group (t5 = 4.538; p < 0.01; Fig. 3A,B) and from two control hamsters exposed to light at ZT12 (Fig. 3C; visual estimation only). The levels were more similar to those expected after light exposure 1–2 hr later in the active phase (Schwartz et al., 1994). These results indicate that a phase advance of >2 hr in the circadian rhythm of SCN sensitivity to light is accomplished within 1 hr after the sleep deprivation procedure.
Fig. 3.
Fos-IR in the SCN (A–E) and IGL (G, H) from representative hamsters in the following conditions: A, no sleep deprivation, light pulse at ZT10; B, 3 hr deprivation with light pulse at ZT10; C, no deprivation, light pulse at ZT12;D, no deprivation or light pulse; E, 3 hr deprivation, no light pulse; F, Nissl-stained section illustrating SCN. G, No deprivation or light pulse. IGL borders indicated by arrows;H, 3 hr deprivation, no light pulse; I, Nissl-stained section illustrating IGL. Animals were killed at ZT11.5, except for those subjected to a light pulse at ZT12 (C). Scale bars, 200 μm.
To evaluate whether spontaneous activity during the 1 hr interval between the end of the sleep deprivation procedure and the onset of the light pulse contributed to the phase shifts inferred from the photic induction of Fos protein, a control experiment was run in which hamsters were placed into DD at ZT6, and then into a novel wheel for 1 hr at ZT9, before a light pulse at ZT10. The number of Fos-IR cells in these animals (52 ± 18) was virtually identical to the number in control animals run in parallel that received neither sleep deprivation nor stimulated running (52 ± 72). Hamsters run in parallel but subjected to 3 hr sleep deprivation (ZT6–9) before the light pulse (ZT10) showed a substantially higher number of IR cells (233 ± 48), replicating the pattern of light-induced Fos expression reported above.
Sleep deprivation alters c-fos expression in the circadian system
If arousal, independent of exercise, accounts for the phase resetting response to procedures that induce continuous wheel running, then sleep deprivation by gentle handling should also mimic the effects of wheel running on Fos expression in the circadian system. To examine this, Fos-IR in the SCN and IGL was quantified in hamsters subjected to the 3 hr sleep deprivation or control procedures. Compared to hamsters in the control condition, sleep-deprived hamsters exhibited a significant suppression of basal Fos-IR in the SCN (t6 = 4.323; p < 0.01; Fig. 3D–F) and a significant increase in the number of Fos-positive nuclei in the IGL (t6 = 4.07; p < 0.01; Figure 3G–I). These results are very similar to those recently reported for 3 hr of continuous wheel running (Mikkelsen et al., 1998), indicating that arousal with or without exercise has a common effect on immediate early gene expression in the circadian system. These results also demonstrate that the increased levels of SCN Fos evident in sleep-deprived hamsters exposed to light was a response to the light pulse and was not directly evoked by sleep deprivation.
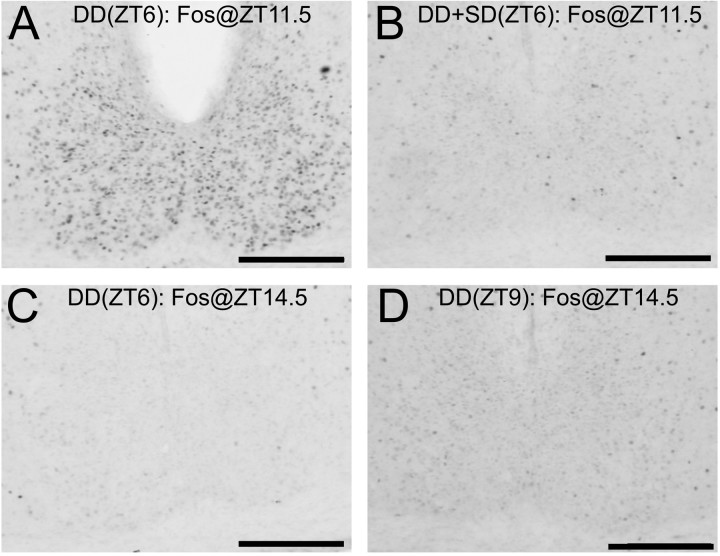
Low levels of SCN Fos after sleep deprivation could represent either a direct cellular response to a nonphotic stimulus or adoption of a new circadian phase. As noted, the magnitude of Fos induction by light after sleep deprivation suggested that SCN pacemaker phase had advanced as much as 3 hr. If Fos levels measured at ZT11.5 after sleep deprivation reflect a new circadian phase, then these levels should be comparable to basal SCN Fos levels in undisturbed control hamsters perfused at ZT14.5. To test this prediction, two hamsters were placed into DD at ZT6 (8.5 hr of darkness before perfusion, but controlling for phase of dark onset) and two at ZT9 (5.5 hr of darkness, matching duration of darkness before perfusion), and then perfused at ZT14.5. These four hamsters were compared with four that were placed into DD at ZT6 and perfused at ZT11.5. Two of this latter group were sleep-deprived from ZT6–9, and two served as undisturbed controls. As illustrated in Figure 4, Fos levels after sleep deprivation were very similar to basal Fos levels at ZT14.5, after either 5.5 or 8.5 hr of darkness (F(2,13) =10.3; p = 0.002).
Fig. 4.
Fos-IR in the SCN in hamsters placed into DD at either ZT6 (A–C) or ZT9 (D). A, Control hamster killed at ZT11.5. B, 3 hr sleep deprivation, ZT6–9, killed at ZT11.5. C,D, Control hamsters killed at ZT14.5. Scale bars, 200 μm.
As noted, wheel running induces fos expression in the IGL. To ensure that IGL Fos expression after sleep deprivation was not caused by activity in the interval between sleep deprivation and perfusion, four additional hamsters were sleep-deprived for 3 hr beginning at ZT6 and killed for Fos ICC at ZT9, along with four control hamsters exposed to DD but not disturbed. Fos-positive cells in the IGL averaged 12 ± 5 in the sleep-deprived hamsters, compared to 2 ± 4 in the control hamsters (t6 = 3.04;p < 0.02). These results indicate that the 3 hr period of sleep deprivation is sufficient to account for IGL Fos expression and that 1 hr of activity after sleep deprivation is not necessary.
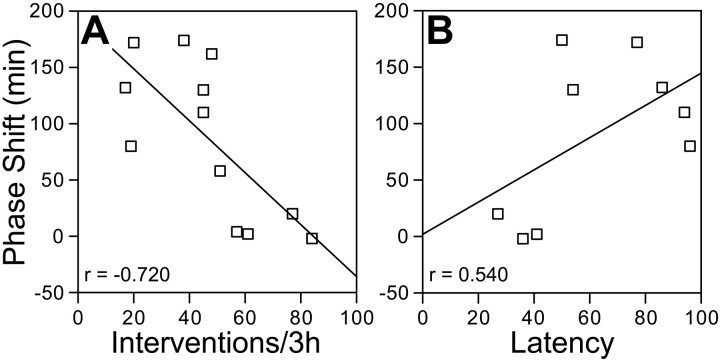
Phase-shift magnitude is inversely related to the number of interventions needed to prevent behavioral sleep
As noted, some hamsters showed small (<30 min) phase shifts in response to sleep deprivation. These animals were observed to be more difficult to keep awake. Two groups of six hamsters (n= 12) were sleep-deprived for 3 hr, and the number of interventions necessary to prevent the sleep posture was recorded as an indirect measure of arousal or sleepiness. There was a strong negative correlation between the total number of interventions and the magnitude of the resulting shift (r = −0.72; p = 0.008; Fig. 5a). On average, 47 ± 22 interventions were needed over the 3 hr sleep deprivation. There was also a weak positive association between the latency to the first intervention and shift magnitude (r = 0.54, p > 0.05; Fig.5b; n = 9, latency data not collected in three cases).
Fig. 5.
Relationship between the phase-shift response to sleep deprivation and the number of interventions required to prevent a sleep posture (A) and the latency to the first intervention (B).
Phase shifts are smaller when sleep deprivation is conducted in the light
Light attenuates arousal in nocturnal rodents (Borbély, 1978; Benca et al., 1998). A group of hamsters (n = 5) were sleep-deprived twice for 3 hr, once under ∼1 lux light, and once under ∼10 lux light. Phase shifts were significantly attenuated in the ∼10 lux condition (88 ± 61 vs 14 ± 12 min; one-tailed paired t4 = 2.36;p < 0.05). The hamsters were also noted to require many more interventions in this condition.
DISCUSSION
It is by now widely recognized that induction of activity in the usual sleep period can shift the phase of the circadian clock in several species. However, it has remained unresolved as to “whether induced activity is the mediator of nonphotic entrainment or … is a correlate of an internal state necessary for resetting” (Hastings et al., 1998). Most work, based largely on studies of Syrian hamsters, has implicated high-intensity locomotor activity as necessary for a shift response, but occasionally hamsters exhibit shifts without substantial running (Antle et al., 1998). Using a gentle procedure applied for 3 hr starting in the middle of the usual sleep period, we have demonstrated here that sleep deprivation that invokes little activity can fully mimic the phase shifting effects of sustained wheel running. Phase shifts previously attributed to exercise may be attributable entirely to the state of aroused waking, or the absence of sleep, associated with activity in the usual rest period. This interpretation is supported by the similar effects of these procedures on Fos expression in both the SCN and the IGL (present study; Mikkelsen et al., 1998). Presumably, animals that fail to run or are prevented from running in response to some arousing stimuli typically fail to shift because they do not stay awake. Conversely, some animals that run little may shift because they remain awake and sufficiently aroused.
Estimating circadian phase by Fos expression
Many hamsters expressed high levels of spontaneous wheel running in the home cage for several hr immediately after the deprivation procedure. At this time of day, hamsters normally sleep much and run little (Tobler and Jaggi, 1987). The running response may reflect continued arousal from the deprivation procedure, and conceivably this could have contributed to the phase shifts evident the next day. However, a probe of circadian phase using photic induction of Fos protein within the SCN demonstrated that the pacemaker was already reset within 1 hr after the deprivation was completed. This is consistent with estimates of clock resetting rates after saline injections late in the day or light pulses at night, also obtained using the phase–response curve for photic induction of SCN c-fos (Mead et al., 1992; Best et al., 1999). Spontaneous activity after sleep deprivation thus likely reflects an advanced onset of the active phase of the circadian cycle, in addition to any residual arousal from the deprivation procedure.
Consistent with this interpretation, basal Fos expression within the SCN, measured 2.5 hr after the sleep deprivation procedure, was significantly suppressed relative to control animals and was comparable to basal levels of Fos expression at ∼ZT14.5 in control animals matched for light history. Fos suppression may be part of the mechanism by which behavioral manipulations in the subjective day induce a phase shift (Wollnik et al., 1995). Alternatively, low Fos levels may be a consequence (i.e., a correlate of a new phase), rather than a cause of phase shifting.
Conceptual issues: arousal, sleep loss, and stress
Sleep deprivation is a complex stimulus that invokes arousal, displaces sleep, and is probably at least mildly stressful. We hypothesize that clock resetting is induced by neural correlates of nonspecific arousal and that these correlates vary in intensity. Thus, when spontaneous arousal is high, hamsters require fewer interventions to maintain sustained waking for 3 hr, and phase shifts are large. When arousal level is low, because of individual differences or the presence of brighter light (which suppresses activity and promotes sleep in nocturnal rodents; Borbély, 1978; Benca et al., 1998), more interventions are needed, and phase shifts are smaller. In exercise studies, the amount of wheel running may be highly predictive of shifting because running enhances arousal.
Although the concept of nonspecific arousal as necessary and sufficient for nonphotic resetting has intuitive appeal, it is logically possible that phase shifts to sleep deprivation are induced by the absence of sleep, rather than the presence of waking. If so, then sleep from ZT6–9 must normally phase delay the clock, resulting in a phase advance when absent. This conception is given some empirical footing by reports that daytime sleep may induce phase shifts in humans, although the role of dark remains to be further explored (Goichot et al., 1998; Van Cauter et al., 1998; Buxton et al., 2000). Ultimately, neurobiological analyses will identify nonphotic pathways to the circadian pacemaker as “wake on”, “sleep on” or both.
Sleep deprivation procedures are at least mildly stressful, but neuroendocrine stress responses are unlikely to mediate effects on circadian phase or SCN Fos expression. Classic stressors have been shown to induce, rather than suppress Fos protein within the SCN (Sharp et al., 1991; Emmert and Herman, 1999). Moreover, during the usual sleep period, 3 hr of restraint stress in hamsters and 1 hr of social stress in rats do not induce phase shifts (Van Reeth et al., 1991;Meerlo and Daan, 1998). Assuming that hamsters actually remain awake during restraint in the sleep period, these results suggest that sustained stress may impede shifting to nonphotic stimuli.
Generality
Whether nonspecific arousal or sleep loss can explain all modulations of circadian phase or period that have been attributed to locomotor activity remains to be determined. Exercise during the second half of the subjective night, but not the first half, can induce small phase delay shifts in hamsters (Bobrzysnka and Mrosovsky, 1998) and entrainment in mice (Marchant and Mistlberger, 1996). This is as predicted if exercise alters phase by enhancing arousal at a usual time of sleep, because hamsters and mice are spontaneously awake most of the early night (predict no effect of exercise), but run less and sleep more later in the night (predict small effect of exercise). Consolidation of wake bouts by exercise may similarly account for modulations of free-running period in DD (Yamada et al., 1986;Mrosovsky, 1999) and of entrained phase in LD (Mistlberger and Holmes, 2000) that occur when rodents have ad libitum access to a running wheel.
In rats, diurnal ground squirrels, and marmosets, stable entrainment to exercise or arousal schedules typically occurs when the behavioral procedure coincides with either the beginning or the end of the night (Mistlberger, 1991; Hut et al., 1999; Glass et al., 2000). Entrainment may be attributable to consolidation or extension of an aroused wake state, and displacement of sleep, at the transition between the major wake and sleep periods. Less work has been done with humans, but the available evidence indicates that exercise can induce phase shifts during the usual sleep period, but has little effect early in the usual wake period (Van Reeth et al., 1994; Buxton et al., 1997). Control experiments in which arousal levels are matched to those during exercise (e.g., using stimulating video games in place of stationary bikes) have not, to our knowledge, been done. However, truly blind subjects can be entrained by sleep–wake schedules without special exercise procedures (Klerman et al., 1998).
Behavioral states can also be manipulated pharmacologically, but results may be difficult to interpret. In hamsters, caffeine stimulates waking from ZT6–9 without inducing phase shifts. However, caffeine also blocks phase shifts to running in a novel wheel, at doses that do not affect running level (Antle et al., 2000). Drugs may act at some sites to evoke arousal and at other sites to block the effects of arousal on the clock. Other drugs may induce nonphotic-type phase shifts without stimulating arousal (Mistlberger et al., 1991;Biello and Mrosovsky, 1993). These may do so by directly engaging the clockworks or modulating nonphotic input pathways.
In one study, five hamsters that ran 5000–10,000 revolutions (and presumably were awake) during 3 hr in a cold room shifted <60 min (Janik and Mrosovsky, 1993). This implies that some hamsters are insensitive to behavioral zeitgebers. Alternatively, there may be individual differences in the nonphotic phase–response curve, such that a few animals fail to respond at circadian times optimal for most. Either interpretation may explain some of the variability in the present results. If such hamsters are not tested over a range of phases, then the failure to detect a large shift after arousal at one circadian phase is of uncertain significance.
Significance
The broader significance of these results merits consideration. In humans, endogenous depression is often associated with altered circadian timing and can be treated with sleep deprivation (Rosenwasser and Wirz-Justice, 1997). A role for sleep deprivation as a phase-resetting stimulus would be consistent with suggestions that, in certain mood disorders, circadian phase adjustment may have therapeutic value. Phase adjustment is of obvious therapeutic value in recovery from jet lag and shift work malaise, and both of these conditions are commonly associated with sleep loss. We have previously shown that sleep deprivation can attenuate the phase-shifting effects of light pulses (Mistlberger et al., 1997). Given that sleep deprivation can also induce significant clock resetting in at least one animal model, the contributions that short-term sleep deprivation and altered sleep–wake scheduling make to the process of clock adjustment in humans merits continued evaluation.
Footnotes
This work was supported by an operating grant (R.E.M.) and graduate fellowship (M.C.A.) from Natural Sciences and Engineering Research Council, Canada. We thank M. An, O. Antle, G. Arciszewska, L. Dane, M. Guy, M. Holmes, S. Ludgate, M. Pollock, S. Smith, C. Sporer, and A. Wood for assistance. We also thank Dr. Nicholas Mrosovsky and his lab members for a critical reading of this manuscript and Dr. Neil Watson for use of his MCID image analysis system.
Correspondence should be addressed to Dr. Ralph Mistlberger, Department of Psychology, Simon Fraser University, 8888 University Drive, Burnaby BC V5A 1S6 Canada. E-mail: mistlber@sfu.ca.
REFERENCES
- 1.Antle MC, Marchant EG, Niel L, Mistlberger RE. Serotonin antagonists do not attenuate activity-induced phase shifts of circadian rhythms in the Syrian hamster. Brain Res. 1998;813:139–149. doi: 10.1016/s0006-8993(98)01048-8. [DOI] [PubMed] [Google Scholar]
- 2.Antle MC, Steen NM, Mistlberger RE. Rapid circadian clock resetting by sleep deprivation and its inhibition by caffeine. Soc Res Biol Rhythms Abstr. 2000;7:110. [Google Scholar]
- 3.Aschoff J. Cold spring harbor symposium on quantitative biology. XXV. Long Island Biological Association; New York: 1960. Exogenous and endogenous components in circadian rhythms. pp. 11–26. [DOI] [PubMed] [Google Scholar]
- 4.Aschoff J. Response curves in circadian periodicity. In: Aschoff J, editor. Circadian clocks. North-Holland; Amsterdam: 1965. pp. 95–111. [Google Scholar]
- 5.Benca RM, Gilliland MA, Obermeyer WH. Effects of lighting conditions on sleep and wakefulness in albino Lewis and pigmented Brown Norway rats. Sleep. 1998;21:451–460. doi: 10.1093/sleep/21.5.451. [DOI] [PubMed] [Google Scholar]
- 6.Best JD, Maywood ES, Smith KL, Hastings MH. Rapid resetting of the mammalian circadian clock. J Neurosci. 1999;19:828–835. doi: 10.1523/JNEUROSCI.19-02-00828.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biello SM, Mrosovsky N. Circadian phase-shifts induced by chlordiazepoxide without increased locomotor activity. Brain Res. 1993;622:58–62. doi: 10.1016/0006-8993(93)90801-s. [DOI] [PubMed] [Google Scholar]
- 8.Bobrzynska KJ, Mrosovsky N. Phase shifting by novelty-induced running: activity dose-response curves at different circadian times. J Comp Physiol [A] 1998;182:251–258. doi: 10.1007/s003590050175. [DOI] [PubMed] [Google Scholar]
- 9.Borbély AA. Effects of light on sleep and activity rhythms. Prog Neurobiol. 1978;10:1–31. doi: 10.1016/0301-0082(78)90018-7. [DOI] [PubMed] [Google Scholar]
- 10.Buxton OM, Frank SA, L'Hermite-Baleriaux M, Leproult R, Turek FW, Van Cauter E. Roles of intensity and duration of nocturnal exercise in causing phase delays of human circadian rhythms. Am J Physiol. 1997;273:E536–E542. doi: 10.1152/ajpendo.1997.273.3.E536. [DOI] [PubMed] [Google Scholar]
- 11.Buxton OM, L'Hermite-Baleriaux M, Turek FW, van Cauter E. Daytime naps in darkness phase shift the human circadian rhythms of melatonin and thyrotropin secretion. Am J Physiol. 2000;278:R373–R382. doi: 10.1152/ajpregu.2000.278.2.R373. [DOI] [PubMed] [Google Scholar]
- 12.Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res. 1999;845:60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- 13.Glass JD, Tardif SD, Clemens R, Mrosovsky N. Photic and nonphotic circadian phase-resetting responses in a diurnal monkey, the common marmoset. Soc Res Biol Rhythms Abstr. 2000;7:124. [Google Scholar]
- 14.Goichot B, Weibel L, Chapotot F, Gronfier C, Piquard F, Brandenberger G. Effect of the shift of the sleep-wake cycle on three robust endocrine markers of the circadian clock. Am J Physiol. 1998;275:E243–248. doi: 10.1152/ajpendo.1998.275.2.E243. [DOI] [PubMed] [Google Scholar]
- 15.Hastings MH, Duffield GE, Smith EJ, Maywood ES, Ebling FJ. Entrainment of the circadian system of mammals by nonphotic cues. Chronobiol Int. 1998;15:425–445. doi: 10.3109/07420529808998700. [DOI] [PubMed] [Google Scholar]
- 16.Honrado GI, Mrosovsky N. Arousal by sexual stimuli accelerates the re-entrainment of hamsters to phase advanced light-dark cycles. Behav Ecol Sociobiol. 1989;25:57–63. [Google Scholar]
- 17.Hut RA, Mrosovsky N, Daan S. Nonphotic entrainment in a diurnal mammal, the European ground squirrel (Spermophilus citellus). J Biol Rhythms. 1999;14:409–419. doi: 10.1177/074873099129000812. [DOI] [PubMed] [Google Scholar]
- 18.Janik D, Mrosovsky N. Nonphotically induced phase shifts of circadian rhythms in the golden hamster: activity-response curves at different ambient temperatures. Physiol Behav. 1993;53:431–436. doi: 10.1016/0031-9384(93)90135-3. [DOI] [PubMed] [Google Scholar]
- 19.Janik D, Mrosovsky N. Intergeniculate leaflet lesions and behaviorally-induced shifts of circadian rhythms. Brain Res. 1994;651:174–182. doi: 10.1016/0006-8993(94)90695-5. [DOI] [PubMed] [Google Scholar]
- 20.Klerman EB, Rimmer DW, Dijk DJ, Kronauer RE, Rizzo JF, III, Czeisler CA. Nonphotic entrainment of the human circadian pacemaker. Am J Physiol. 1998;274:R991–R996. doi: 10.1152/ajpregu.1998.274.4.r991. [DOI] [PubMed] [Google Scholar]
- 21.Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron. 1990;5:127–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- 22.Marchant EG, Mistlberger RE. Entrainment and shifting of circadian rhythms in mice by forced treadmill running. Physiol Behav. 1996;60:657–663. doi: 10.1016/s0031-9384(96)80045-x. [DOI] [PubMed] [Google Scholar]
- 23.Marchant EG, Watson NV, Mistlberger RE. Both neuropeptide Y and serotonin are necessary for entrainment of circadian rhythms in mice by daily treadmill running schedules. J Neurosci. 1997;17:7974–7987. doi: 10.1523/JNEUROSCI.17-20-07974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maywood ES, Smith E, Hall SJ, Hastings MH. A thalamic contribution to arousal-induced, non-photic entrainment of the circadian clock of the Syrian hamster. Eur J Neurosci. 1997;9:1739–1747. doi: 10.1111/j.1460-9568.1997.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 25.Mead S, Ebling FJ, Maywood ES, Humby T, Herbert J, Hastings MH. A nonphotic stimulus causes instantaneous phase advances of the light-entrainable circadian oscillator of the Syrian hamster but does not induce the expression of c-fos in the suprachiasmatic nuclei. J Neurosci. 1992;12:2516–2522. doi: 10.1523/JNEUROSCI.12-07-02516.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meerlo P, Daan S. Aggressive and sexual social stimuli do not phase shift the circadian temperature rhythm in rats. Chronobiol Int. 1998;15:231–240. doi: 10.3109/07420529808998686. [DOI] [PubMed] [Google Scholar]
- 27.Mikkelsen JD, Vrang N, Mrosovsky N. Expression of Fos in the circadian system following nonphotic stimulation. Brain Res Bull. 1998;47:367–376. doi: 10.1016/s0361-9230(98)00121-x. [DOI] [PubMed] [Google Scholar]
- 28.Mistlberger RE. Effects of daily schedules of forced activity on free-running rhythms in the rat. J Biol Rhythms. 1991;6:71–80. doi: 10.1177/074873049100600108. [DOI] [PubMed] [Google Scholar]
- 29.Mistlberger RE, Holmes MM. Behavioral feedback regulation of circadian rhythm phase angle in light-dark entrained mice. Am J Physiol. 2000;279:R813–R821. doi: 10.1152/ajpregu.2000.279.3.R813. [DOI] [PubMed] [Google Scholar]
- 30.Mistlberger RE, Houpt TA, Moore-Ede MC. The benzodiazepine triazolam phase-shifts circadian activity rhythms in a diurnal primate, the squirrel monkey (Saimiri sciureus). Neurosci Lett. 1991;124:27–30. doi: 10.1016/0304-3940(91)90814-a. [DOI] [PubMed] [Google Scholar]
- 31.Mistlberger RE, Landry GJ, Marchant EG. Sleep deprivation can attenuate light-induced phase shifts of circadian rhythms in hamsters. Neurosci Lett. 1997;238:5–8. doi: 10.1016/s0304-3940(97)00815-x. [DOI] [PubMed] [Google Scholar]
- 32.Mistlberger RE, Antle MC, Glass JD, Miller JD. Behavioral and serotonergic regulation of circadian rhythms. Biol Rhythm Res. 2000;31:240–283. [Google Scholar]
- 33.Mrosovsky N. Phase response curves for social entrainment. J Comp Physiol [A] 1988;162:35–46. doi: 10.1007/BF01342701. [DOI] [PubMed] [Google Scholar]
- 34.Mrosovsky N. Methods of measuring phase shifts: why I continue to use an Aschoff type II procedure despite the skepticism of referees. Chronobiol Int. 1996;13:387–392. doi: 10.3109/07420529609012662. [DOI] [PubMed] [Google Scholar]
- 35.Mrosovsky N. Further experiments on the relationship between the period of circadian rhythms and locomotor activity levels in hamsters. Physiol Behav. 1999;66:797–801. doi: 10.1016/s0031-9384(99)00022-0. [DOI] [PubMed] [Google Scholar]
- 36.Rea MA. Light increases Fos-related protein immunoreactivity in the rat suprachiasmatic nuclei. Brain Res Bull. 1989;23:577–581. doi: 10.1016/0361-9230(89)90204-9. [DOI] [PubMed] [Google Scholar]
- 37.Reebs SG, Mrosovsky N. Effects of induced wheel running on the circadian activity rhythms of Syrian hamsters: entrainment and phase response curve. J Biol Rhythms. 1989;4:39–48. doi: 10.1177/074873048900400103. [DOI] [PubMed] [Google Scholar]
- 38.Rosenwasser AM, Wirz-Justice A. Circadian rhythms and depression: clinical and experimental models. In: Redfern PH, Lemmer B, editors. Handbook of experimental pharmacology: physiology and pharmacology of biological rhythms. Springer; Berlin: 1997. pp. 457–486. [Google Scholar]
- 39.Rusak B, Mistlberger RE, Losier B, Jones CH. Daily hoarding opportunity entrains the pacemaker for hamster activity rhythms. J Comp Physiol [A] 1988;64:165–171. doi: 10.1007/BF00603948. [DOI] [PubMed] [Google Scholar]
- 40.Rusak B, Robertson HA, Wisden W, Hunt SP. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990;248:1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz WJ, Takeuchi J, Shannon W, Davis EM, Aronin N. Temporal regulation of light-induced Fos and Fos-like protein expression in the ventrolateral subdivision of the rat suprachiasmatic nucleus. Neuroscience. 1994;58:573–583. doi: 10.1016/0306-4522(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 42.Tobler I, Jaggi K. Sleep and EEG spectra in the Syrian hamster (Mesocricetus auratus) under baseline conditions and following sleep deprivation. J Comp Physiol [A] 1987;161:449–459. doi: 10.1007/BF00603970. [DOI] [PubMed] [Google Scholar]
- 43.Turek FW. Effects of stimulated physical activity on the circadian pacemaker of vertebrates. J Biol Rhythms. 1989;4:135–147. [PubMed] [Google Scholar]
- 44.Van Cauter E, Moreno-Reyes R, Akseki E, L'Hermite-Baleriaux M, Hirschfeld U, Leproult R, Copinschi G. Rapid phase advance of the 24-h melatonin profile in response to afternoon dark exposure. Am J Physiol. 1998;275:E48–E54. doi: 10.1152/ajpendo.1998.275.1.E48. [DOI] [PubMed] [Google Scholar]
- 45.Van Reeth O, Hinch D, Tecco JM, Turek FW. The effects of short periods of immobilization on the hamster circadian clock. Brain Res. 1991;545:208–214. doi: 10.1016/0006-8993(91)91288-c. [DOI] [PubMed] [Google Scholar]
- 46.Van Reeth O, Sturis J, Byrne MM, Blackman JD, L'Hermite-Baleriaux M, Leproult R, Oliner C, Refetoff S, Turek FW, Van Cauter E. Nocturnal exercise phase delays circadian rhythms of melatonin and thyrotropin secretion in normal men. Am J Physiol. 1994;266:E964–E974. doi: 10.1152/ajpendo.1994.266.6.E964. [DOI] [PubMed] [Google Scholar]
- 47.Wickland C, Turek FW. Lesions of the thalamic intergeniculate leaflet block activity-induced phase shifts in the circadian activity rhythm of the golden hamster. Brain Res. 1994;660:293–300. doi: 10.1016/0006-8993(94)91302-1. [DOI] [PubMed] [Google Scholar]
- 48.Wollnik F, Brysch W, Uhlmann E, Gillardon F, Bravo R, Zimmermann M, Schlingensiepen KH, Herdegen T. Block of c-Fos and JunB expression by antisense oligonucleotides inhibits light-induced phase shifts of the mammalian circadian clock. Eur J Neurosci. 1995;7:388–393. doi: 10.1111/j.1460-9568.1995.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 49.Yamada N, Shimoda K, Takahashi K, Takahashi S. Change in period of free-running rhythms determined by two different tools in blinded rats. Physiol Behav. 1986;36:357–362. doi: 10.1016/0031-9384(86)90029-6. [DOI] [PubMed] [Google Scholar]